Abstract
Background
Quality of Life (QoL) is a prognostic factor in heart failure (HF) of patients with acquired cardiac disease. The aim of this study was to determine the predictive value of QoL on outcomes in adults with congenital heart disease (ACHD) and HF.
Methods and Results
Quality of life of 196 adults with congenital heart disease with clinical heart failure (HF) (mean age: 44.3±13.8 years; 51% male; 56% with complex congenital heart disease; 47% New York Heart Association class III/IV) included in the prospective multicentric registry FRESH‐ACHD (French Survey on Heart Failure−Adult with Congenital Heart Disease) was assessed using the 36‐Item Short Form Survey (SF‐36), a patient‐reported survey. The primary end point was defined by all‐cause death, HF‐related hospitalization, heart transplantation, and mechanical circulatory support. At 12 months, 28 (14%) patients reached the combined end point. Patients with low quality of life experienced major adverse events more frequently (logrank P=0.013). On univariate analysis, lower score at physical functioning (hazard ratio [HR], 0.98 [95% CI, 0.97–0.99]; P=0.008), role limitations related to physical health (HR, 0.98 [95% CI, 0.97–0.99]; P=0.008), and general health dimensions of the SF‐36 (HR, 0.97 [95% CI, 0.95–0.99]; P=0.002) were significantly predictive of cardiovascular events. However, after multivariable analysis, SF‐36 dimensions were no longer significantly associated with the primary end point.
Conclusions
Patients with congenital heart disease with HF and poor quality of life experience severe events more frequently, making quality of life assessment and rehabilitation programs essential to alter their trajectory.
Keywords: congenital heart disease, heart failure, quality of life
Subject Categories: Congenital Heart Disease, Heart Failure, Quality and Outcomes
Clinical Perspective.
What Is New?
We determined the predictive value of quality of life on outcomes among adult patients with congenital heart disease and heart failure.
At 12 months of follow‐up, patients with low quality of life (SF‐36 score <30) experienced major adverse cardiovascular events more frequently, which was predominantly heart failure–related rehospitalization.
After adjustment for confounding factors (eg, chronic renal failure, pulmonary arterial hypertension, New York Heart Association class III‐IV, and brain natriuretic peptide >360 ng/L), quality of life scores were no longer significantly associated with the primary end point.
What Are the Clinical Implications?
Quality of life should be considered as a surrogate end point of heart failure treatment in adult patients with congenital heart disease and in trials involving patients with congenital heart disease and heart failure.
Nonstandard Abbreviations and Acronyms
- CCTIRS
Comité Consultatif sur le Traitement de l'Information en matière de Recherche dans le domaine de la Santé
- CNIL
Commission National Informatique et Liberté
- FRESH‐ACHD cohort
French Survey on Heart Failure – Adult with Congenital Heart Disease
- RLPE
role limitations due to emotional problems
- RLPH
role limitations due to physical health
As a result of major advances in the management of children with congenital heart disease (CHD) over the past 5 decades, most children with CHD reach adulthood, and the population of adults with congenital heart disease (ACHD) is exponentially growing. 1 Heart failure (HF) is the most common complication in ACHD and has become the leading cause of premature death, accounting for 17% to 26% of all deaths. 2 , 3
Some predictors such as brain natriuretic peptide (BNP), New York Heart Association (NYHA) class, and CHD lesion characteristics have been already identified as predictors of HF‐related adverse outcomes. 4 HF is also known to have a negative impact on quality of life (QoL), 5 , 6 and several studies have demonstrated a significant association between poor QoL and survival among patients with HF in acquired cardiovascular disease. 7 , 8 Moreover, a recent prospective study found that improvement in QoL was independently associated with decreased mortality and a reduced risk of HF hospitalization. 9
A potential association between QoL and adverse outcomes has never been investigated in ACHD with HF. However, QoL is an important determinant in this population and can be improved by a variety of medical, surgical, and nonpharmacological interventions, including exercise training 10 , 11 and cardiac rehabilitation. 12 Therefore, we sought to assess clinical characteristics of patients with low QoL and to determine the predictive value of QoL on outcomes in patients with ACHD and HF.
Methods
The data will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
Data were collected from the FRESH‐ACHD cohort (French Survey on Heart Failure – Adult with Congenital Heart Disease; NCT01956539). FRESH‐ACHD is a multicenter, prospective observational study on HF in ACHD. Between 2017 and 2019, 339 patients from 14 French tertiary centers were enrolled and were followed for 1 year after their inclusion. Inclusion criteria were the following: patient ≥18 years old, with underlying CHD, and affected by acute or chronic HF. Acute HF was defined as hospitalization for clinical HF, or introduction/augmentation of diuretic therapy for clinical signs and symptoms of HF. 13 Chronic HF was defined by the association of 2 of the following criteria: NYHA class ≥II or significant decrease in peak oxygen consumption at cardiopulmonary exercise test, significant increase in BNP or NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), 14 and decreased systemic ventricular ejection fraction <50%. Fontan circulatory failure with respect to the recent consensual definition 15 was also qualified as chronic HF.
The study complies with the Declaration of Helsinki, and ethical or research committee approval was obtained in each contributing center. All patients were included after information and signature of the consent. The study protocol was approved by the CNIL (Commission National Informatique et Liberté) and by the CCTIRS (Comité Consultatif sur le Traitement de l'Information en matière de Recherche dans le domaine de la Santé).
Clinical Data
Data were independently collected at each participating center using uniform methodology. The patients’ medical records were reviewed to collect demographic information, medical, and surgical details. Baseline data including clinical data, 12‐lead electrocardiography, echocardiography, cardiopulmonary exercise test, QoL score were obtained at patient inclusion. Standard biological markers (blood cell count, ionogram, urea, and creatinine), and BNP levels were measured at baseline.
CHD complexity was classified according to the classification reported in the European guideline on management of ACHD. 16 , 17 Chronic renal failure was defined as the presence of kidney damage or an estimated glomerular filtration rate <60 mL/min per 1.73 m2, persisting for ≥3 months. Pulmonary arterial hypertension (PAH) was noted in the presence of Eisenmenger syndrome or when precapillary pulmonary hypertension was invasively confirmed according to European Society of Cardiology (ESC) guidelines. 18 Systemic ventricular systolic function was assessed by transthoracic echocardiography following consensus recommendations. 19 Systemic left ventricular ejection fraction was assessed quantitatively by the biplane Simpson method, systemic right ventricle by fractional area change, tricuspid annular plane systolic excursion, tissue Doppler velocity of the basal free wall (S′), and qualitatively by visual assessment from multiple views. The final conclusion was made taking into account both visual assessment and quantitative parameters. Reduced ejection fraction was considered when left ventricular ejection fraction <40% and when systemic right ventricle or single ventricle function was estimated to be moderately to severely impaired.
QoL was assessed at baseline by 36‐Item Short Form scoring (SF‐36), with the scaling system of the MOS (Medical Outcome Study) SF‐36. The SF‐36 questionnaire was completed by the patients at the outpatient clinic on a paper form. SF‐36 is a self‐report questionnaire of general health status with 36‐item questionnaires. A higher score indicates a better health‐related QoL. 20 All but the second of the 36 items in the SF‐36 are aggregated into 8 multi‐item scales: physical functioning, role limitations because of physical health, role limitations because of emotional problems, energy, emotional well‐being, social functioning, pain, and general health. In the present study, the 0 to 100 scoring algorithm was used.
The primary composite end point was defined by all‐cause mortality, rehospitalization for acute HF, heart transplantation, and mechanical circulatory support implantation during the follow‐up.
Statistical Analysis
Continuous data were presented as mean values and SD if normally distributed or median with interquartile range (25%–75%) if skewed. Categorical variables were presented as frequencies and percentages.
The internal consistency of the SF‐36 questionnaire was evaluated by measurement of Cronbach's alpha and its 95% CI. Patient characteristics were compared between tertiles of QoL‐score such as in the Hoekstra et al study, 8 which evaluated QoL association with long‐term mortality in a well‐defined HF population. Groups were defined as group with low (SF‐36 score <30), mid (SF‐36 score 33 to 55), and high (SF‐36 score >55) QoL. Continuous variables of these 3 groups were compared using 1‐way ANOVA or Kruskal–Wallis nonparametric test. Unpaired comparisons were performed with the use of the χ2 test or the Fisher exact test for categorical data, as appropriate.
Cardiovascular events (defined by all‐cause mortality, rehospitalization for acute HF, heart transplantation, and mechanical circulatory support implantation) were plotted with the Kaplan–Meier method and compared between low, mid, and high QoL groups using the logrank test. Patients lost to follow‐up were censored. Univariate analysis with Cox proportional hazards model was used to investigate the association between QoL at inclusion as continuous variable, as well as each of the 8 dimensions of SF‐36, and the primary end point. We repeated analysis, adjusting for confounding variables, which were significant and associated with the primary end point (P<0.05) on univariate analysis; they were: previous renal failure, PAH, NYHA III/IV, and BNP >360 ng/L. The assumption of proportionality was examined for each variable using the Schoenfeld residuals test.
All statistical analysis was 2‐tailed, and a P value <0.05 was considered statistically significant. Statistical analyses were performed using STATA software (StataCorp. 2021. Stata: Release 17. Statistical Software, College Station, TX: StataCorp LLC).
Results
Study Population
Among 339 patients included in the FRESH‐ACHD study, 196 patients (57.8%) were assessed with the SF‐36 questionnaire at inclusion and constituted our study population. Characteristics of patients with and without SF‐36 completion were not significantly different (Table S1) except for HF treatment, which was significantly more often reported in the group with available SF‐36 data. Cronbach's alpha was estimated at 0.86 (95% CI, 0.82–0.89), suggesting a good internal consistency for assessment of QoL among patients with ACHD and HF. Characteristics at baseline are summarized in Table 1. Congenital heart defects were mainly moderate (35%) and complex (56%). NYHA functional class was >II in 88 (47%) patients, systemic ejection fraction was reduced (<40%) in 54 (28%), and 35 (18%) patients had a failing Fontan.
Table 1.
Patients Characteristics at Baseline and Major Clinical Events During 1‐Year of Follow‐Up
| Total (n=196) | Low QoL (<30) (n=53) | Mid QoL (30–55) (n=95) | High QoL (>55) (n=48) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y | 44.3 (±13.8)* | 46.5 (±14.0) | 43.4 (±13.6) | 43.6 (±14.1) | 0.397 |
| Male, n (%) | 100 (51) | 23 (43) | 48 (51) | 29 (60) | 0.230 |
| Medical history | |||||
| Congenital heart disease | |||||
| Simple | 17 (9) | 4 (8) | 10 (11) | 3 (6) | 0.595 |
| Moderate | 69 (35) | 23 (43) | 29 (31) | 17 (35) | |
| Complex | 109 (56) | 26 (49) | 55 (58) | 28 (58) | |
| Previous cardiac surgery | 167 (85) | 46 (87) | 81 (85) | 40 (83) | 0.887 |
| Renal failure | 9 (5) | 5 (9) | 4 (4) | 0 (0) | 0.065 |
| COPD | 8 (4) | 4 (8) | 1 (1) | 3 (6) | 0.069 |
| PAH | 35 (18) | 13 (25) | 15 (16) | 5 (15) | 0.327 |
| Diabetes | 9 (5) | 5 (9) | 3 (3) | 1 (2) | 0.195 |
| History of heart failure hospitalization | 61 (54) | 20 (67) | 28 (51) | 13 (48) | 0.284 |
| Depression | 17 (9) | 9 (17) | 6 (7) | 2 (4) | 0.054 |
| Clinical characteristics | |||||
| BMI, kg/m2 | 24.8 (±6.0) | 25.4 (±6.0) | 24.5 (±5.7) | 24.6 (±6.5) | 0.632 |
| NYHA class | p for III/IV vs others: | <0.001 | |||
| I | 23 (12) | 2 (4) | 12 (13) | 9 (19) | |
| II | 77 (41) | 14 (27) | 38 (42) | 25 (53) | |
| III | 70 (37) | 27 (53) | 32 (36) | 11 (23) | |
| IV | 18 (10) | 8 (16) | 8 (9) | 2 (4) | |
| Systolic blood pressure, mm Hg | 117 (±17) | 116 (±17) | 116 (±18) | 118 (±17) | 0.856 |
| Diastolic blood pressure, mm Hg | 67 (±12) | 65 (±13) | 68 (±12) | 68 (±12) | 0.153 |
| Heart rate, bpm | 77 (±19) | 76 (±18) | 78 (±17) | 77 (±22) | 0.773 |
| Sodium, mmol/L | 139 (±3) | 139 (±3) | 139 (±2) | 138 (±3) | 0.482 |
| BNP, ng/L | 220 [129–507]† | 274 [150–619] | 203 [115–463] | 212 [127–413] | 0.285 |
| GFR, MDRD | 90 (±32) | 86 (±33) | 92 (±32) | 92 (±32) | 0.469 |
| Hemoglobin | 14.0 (±2.7) | 13.8 (±2.7) | 14.1 (±2.9) | 14.1 (±2.5) | 0.818 |
| VEF, % | 46.6 (±11.6) | 44.2 (±10.4) | 46.6 (±12.4) | 48.7 (±11.1) | 0.286 |
| Medication at the time of the inclusion | |||||
| Loop diuretics | 113 (59) | 34 (64) | 55 (60) | 24 (50) | 0.323 |
| ACE inhibitors | 82 (43) | 18 (34) | 42 (46) | 22 (46) | 0.318 |
| β‐blockers | 120 (63) | 32 (60) | 60 (66) | 28 (58) | 0.633 |
| 1‐y events | |||||
| Rehospitalization for heart failure | 18 (9) | 10 (19) | 6 (6) | 2 (4) | 0.023 |
| Death | 9 (5) | 4 (8) | 5 (5) | 0 (0) | 0.176 |
| Heart transplantation | 2 (1) | 0 (0) | 2 (2) | 0 (0) | 0.498 |
| Mechanical circulatory support implantation | 1 (1) | 0 (0) | 1 (1) | 0 (0) | 1.000 |
| Composite end point‡ | 28 (14) | 13 (25) | 13 (14) | 2 (4) | 0.014 |
ACE indicates angiotensin‐conversion enzyme; BMI, body mass index; BNP, brain natriuretic peptide; bpm, beats per minute; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; GFR, glomerular filtration rate; HF, heart failure; MDRD, modification of diet in renal disease; NYHA, New York Heart Association; QoL, quality of life; PAH, pulmonary arterial hypertension; and VEF, ventricular ejection fraction.
Mean (±SD).
Median [interquartile range].
Death or transplantation or HF hospitalization or circulatory support.
Patients characteristics at baseline were not significantly different among the 3 groups of QoL, except NYHA functional class, which was significantly higher in patients with low QoL: NYHA functional class was III/IV in 69% of patients with low QoL, 45% in patients with mid QoL, and 27% in patients with high QoL (P<0.001, Table 1).
QoL and Prognosis Factors
At 12 months, the follow‐up data were complete for all the patients included, except for 20 who were lost to follow‐up (3 patients had no follow‐up data available, 6 were lost to follow‐up after 8 or 9 months of follow‐up, and 11 were lost to follow‐up during the 11th month). The survival curve with censored data is shown in Figure S1). Twenty‐eight (14%) patients reached the primary end point. Rehospitalization for HF was the most common event, accounting for 64% of primary end points. Cumulative incidence of mortality at 1 year was 5%. Patients with low QoL experienced severe cardiovascular events more frequently (Figure, logrank P=0.013), which was predominantly HF‐related rehospitalization (Table 1). Table 2 shows associations between baseline characteristics and primary end points. Chronic renal failure, PAH, high NYHA functional classes, and high BNP were predictive factors of the composite end point (P<0.04; Table 2).
Figure . Freedom from the primary end point defined by all‐cause mortality, rehospitalization for acute heart failure, heart transplantation, and mechanical circulatory support implantation.
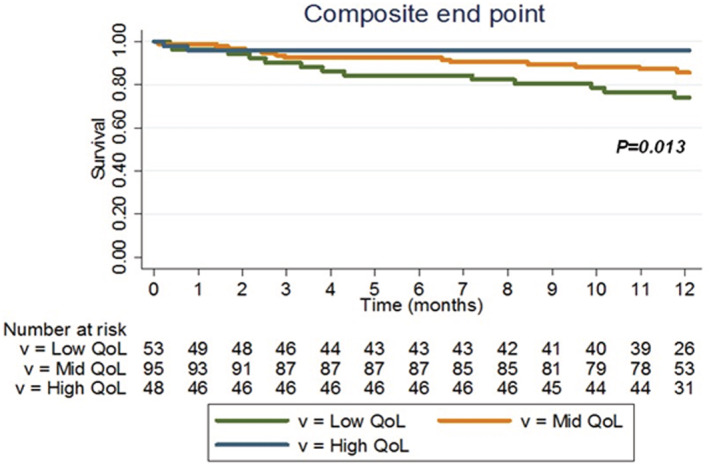
Kaplan–Meier survival curves for freedom from the primary end point are plotted and compared according to the group of quality of life. Patients with low QoL experienced severe cardiovascular events more frequently (logrank P=0.013). QoL indicates quality of life.
Table 2.
Factors Associated With the Primary End Point
| Univariate analysis | Multivariable analysis | |||||
|---|---|---|---|---|---|---|
| Crude HR | 95% CI | P value | HR | 95% CI | P value | |
| Demographics | ||||||
| Age (y) | 1.04 | 1.01–1.06 | 0.006 | |||
| Male, % | 1.28 | 0.61–2.71 | 0.514 | |||
| Medical history | ||||||
| Complex congenital heart disease | 0.57 | 0.27–1.20 | 0.140 | |||
| Previous cardiac surgery | 0.79 | 0.30–2.08 | 0.631 | |||
| Previous renal failure | 5.35 | 2.03–14.12 | 0.001 | 3.02 | 1.10–8.34 | 0.033 |
| COPD | 1.75 | 0.42–7.39 | 0.444 | |||
| PAH | 2.63 | 1.19–5.82 | 0.017 | 2.77 | 1.21–6.33 | 0.016 |
| Diabetes | 0.71 | 0.10–5.20 | 0.733 | |||
| History of heart failure hospitalization | 0.83 | 0.31–2.21 | 0.712 | |||
| Depression | 1.26 | 0.38–4.16 | 0.709 | |||
| Clinical characteristics | ||||||
| BMI, kg/m2 | 1.00 | 0.93–1.06 | 0.898 | |||
| NYHA class III‐IV | 7.36 | 2.55–21.30 | < 0.001 | 5.20 | 1.76–15.41 | 0.003 |
| Systolic blood pressure, mm Hg | 0.99 | 0.97–1.01 | 0.428 | |||
| Diastolic blood pressure, mm Hg | 0.99 | 0.96–1.02 | 0.661 | |||
| Heartbeat, bpm | 1.01 | 0.99–1.03 | 0.136 | |||
| Sodium, mmol/L | 0.86 | 0.78–0.96 | 0.007 | |||
| GFR, mL/min per 1.73 m2, MDRD | 0.98 | 0.96–0.99 | 0.003 | |||
| BNP > 360 ng/L (tertile 3) | 3.29 | 1.53–7.10 | 0.002 | 2.51 | 1.14–5.53 | 0.022 |
| Hemoglobin | 0.89 | 0.77–1.04 | 0.139 | |||
| Systemic ventricular EF, % | 0.99 | 0.95–1.02 | 0.448 | |||
| Medication at the time of the inclusion | ||||||
| Loop diuretics | 3.40 | 1.29–8.99 | 0.013 | |||
| ACE inhibitors | 0.55 | 0.24–1.25 | 0.151 | |||
| β‐blockers | 0.53 | 0.25–1.13 | 0.102 | |||
ACE indicates angiotensin‐conversion enzyme; BMI, body mass index; BNP, brain natriuretic peptide; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; GFR, glomerular filtration rate; HR, hazard ratio; MDRD, modification of diet in renal disease; NYHA, New York Heart Association; and PAH, pulmonary arterial hypertension.
Among SF‐36 dimensions, lower physical functioning (hazard ratio [HR], 0.98 [95% CI, 0.97–0.99]; P=0.008), role physical (HR, 0.98 [95% CI, 0.97–0.99]; P=0.008), and general health scores (HR, 0.97 [95% CI, 0.95–0.99]; P=0.002) were significantly predictive of primary end points on univariate analysis but were not after adjustment for confounding factors, which were chronic renal failure, PAH, NYHA III‐IV, and BNP >360 ng/L (Table 3).
Table 3.
Univariate and Multivariable Analysis of Quality of Life Scores and Primary End Point
| Univariate analysis | P value | Multivariable analysis | P value | |
|---|---|---|---|---|
| HR (95% CI) | Adjusted HR (95% CI) | |||
| SF‐36 | ||||
| Physical | 0.98 (0.97–0.99) | 0.008 | 1.00 (0.98–1.01) | 0.715 |
| RLPH | 0.98 (0.97–0.99) | 0.008 | 0.99 (0.97–1.00) | 0.136 |
| RLEP | 1.00 (0.99–1.01) | 0.445 | 1.00 (0.99–1.01) | 0.699 |
| Energy | 0.99 (0.97–1.00) | 0.139 | 1.00 (0.98–1.03) | 0.725 |
| Emotion | 1.01 (0.99–1.03) | 0.418 | 1.02 (1.00–1.04) | 0.103 |
| Social | 1.00 (0.99–1.01) | 0.876 | 1.01 (1.00–1.03) | 0.124 |
| Pain | 0.99 (0.98–1.00) | 0.156 | 1.00 (0.99–1.02) | 0.691 |
| General | 0.97 (0.95–0.99) | 0.002 | 0.99 (0.97–1.01) | 0.195 |
For multivariable analysis, quality of life scores were adjusted for patient characteristics found to be significant and related to mortality (see Table 2); they were history of renal failure, history of pulmonary arterial hypertension, NYHA class III‐IV, and BNP >360 ng/L. BNP indicates brain natriuretic peptide; HR, hazard ratio; NYHA, New York Heart Association; RLEP, role limitations due to emotional problems; RLPH, role limitations due to physical health; and SF‐36, 36‐Item Short Form.
Discussion
To our knowledge, this is the first study that evaluates QoL in ACHD patients with HF. We showed that worse events occurred in patients with low QoL scores. Low levels of physical functioning and general health dimensions were significantly associated with 1‐year adverse events in patients with ACHD and HF, including death, rehospitalization for acute HF, heart transplantation, and mechanical circulatory support implantation. Indeed, at 1 year, a significant proportion of patients reached the combined end point (14%), essentially patients rehospitalized for acute HF. Poor QoL was associated with worse outcomes; however, patient comorbidities remained the most important risk factors for severe cardiac events in CHD‐related HF.
In patients with HF and acquired heart disease, health‐related QoL, notably poor physical health status, predicts mortality and congestive HF–related hospitalizations. 5 , 6 , 21 The prognostic value of QoL in ACHD was rarely investigated, except in the Blok et al study that showed decrease in SF‐36–Physical component summary, following initiation of PAH–specific therapy in patients with PAH associated with CHD, which was a determinant of mortality. 22 Consequently, it is crucial to consider QoL as a surrogate end point of HF treatment, and cardiac rehabilitation programs may positively impact outcomes in patients with CHD and HF. Indeed, if their general condition improves, as illustrated by QoL improvement, we might expect a decrease in mortality and morbidities in these patients.
Since the past 2 decades, clinical trials in congestive HF are increasingly demonstrating that cardiac rehabilitation improves results in functional capacity and reduces clinical severity. 22 , 23 , 24 In contrast, in the CHD spectrum, only 2 randomized studies evaluated cardiac rehabilitation efficiency on QoL. Dulfer et al showed that aerobic exercise improved health‐related QoL in patients with Tetralogy of Fallot or Fontan circulation, but this clinical trial included only children. 25 Novakovic et al demonstrated in a randomized trial that continuous exercise training improved cardiac autonomic function and QoL in adults with a tetralogy of Fallot repair. 26 Moreover, several observational studies also showed that implementation of a cardiac rehabilitation program improve physical capacity, exercise tolerance, and QoL in patients with CHD including patients with complex CHD. 11 , 27 , 28 Interestingly, CHD complexity did not contribute to the variability in QoL, which is consistent with other studies. 29 , 30 However, all of these studies did not include any patients with ACHD and HF.
A variety of medical, surgical, and nonpharmacological interventions can help to maintain or improve QoL in patients with HF. Among nonpharmacological interventions, besides exercise training and cardiac rehabilitation, 10 , 11 , 12 self‐care interventions and treatment of depression may improve QoL in ACHD with HF. Indeed, depression is associated with a higher risk of death or rehospitalization in patients affected by HF 31 and ACHD, who have have a higher probability of experiencing symptoms consistent with mood and anxiety disorders, and depression. 32 Standardized screening pathways for depression in patients with CHD‐related HF may offer the potential for early identification and optimal management of depression to improve QoL and outcomes.
Patients with impaired QoL had a worse NYHA functional class. Nevertheless, we noted that 27% of patients with high QoL scores were in class III/IV NYHA despite statistical significance. This reveals how assessing NYHA but also perception of QoL, even with a questionnaire, may be subjective in ACHD.
After adjustment for confounding variables, QoL was no more significantly predictive of primary outcomes. The severity of the patient's condition influences the baseline scores of most investigated components of QoL, mainly with regard to the physical components, which may explain the significant relationship between physical functioning and role physical with the primary end point on univariate analysis. However, the magnitude of changes for QoL in patients with HF‐related CHD is moderate 33 and may explain why QoL had a lower association with major adverse clinical events compared with markers of organ failure such as PAH, NYHA functional class, renal failure, and BNP. Our results showed baseline characteristics did not allow the identification of patients with worse QoL, except NYHA functional class. Indeed, objective markers such as BNP, renal function, and PAH did not significantly differ between the 3 groups of QoL. This may also explain why QoL was not found to be significantly associated with primary outcomes on multivariable analysis.
CHD is, by definition, present from birth, and patients adapt daily activities to their ability. As a consequence, ACHD often have abnormal baseline cardiovascular function and are subject to lifelong adaptation to their cardiac physiological derangement. Therefore, QoL evaluation in adults with CHD‐related HF may be quite different from those with HF because of acquired heart disease. These nuances highlight the need for serial evaluation of QoL in ACHD at risk of HF and to develop adapted questionnaire including this chronic constitutive cardiac condition.
Nevertheless, QoL is becoming an essential HF‐related outcome measures in many randomized controlled trials, 34 , 35 and is considered a key treatment purpose in European guidelines for HF. 13 Our results suggest that QoL could be used as a surrogate in trials involving patients with CHD and HF.
This study has several limitations. The period of follow‐up was only 1 year, which is a very short time for predicting long‐term events. Our study was conducted in a relatively small number of patients (196 patients), related to missing data for SF‐36 questionnaires at inclusion, which may induce some bias and reduce the power of the study. The study included only patients followed in expert centers and may create center bias. Another limitation was that peak VO2 was not used in univariate and multivariable analyses as a confounding factor because 83% of data were missing. Peak VO2 (or maximum [max] rate [V] of oxygen [O2]) is known to be correlated with QoL in CHD. 11 Moreover, several studies showed that peak VO2 was also a significant predictor of survival in congestive HF as well as in ACHD. 36 , 37 , 38
Conclusions
In our study, clinical and biological factors, such as PAH, NYHA functional class, renal dysfunction, and BNP, were significant predictors of HF worsening and death in ACHD‐related HF. However, patients with a low QoL more frequently experienced severe cardiovascular events. For this reason, QoL is an interesting marker that should be part of the clinical evaluation of ACHD patients with HF. The evaluation of QoL could be considered as a therapeutic target of rehabilitation programs in these patients.
Sources of Funding
This work was supported by the Fédération Française de Cardiologie and promoted by the French Society of Cardiology.
Disclosures
Sebastien Hascoet reports proctoring activity and consultant fees from Abbott and a research grant from Edwards Lifesciences outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Table S1
Figure S1
Acknowledgments
We thank Mrs A. Boubrit, a French research technician from the reference center of complex congenital heart disease M3C, who helped in recording data. We thank the French Society of Cardiology for its help in the management of the FRESH‐ACHD registry, notably Mrs N. Naccache for the coordination, Mrs E. Drouet and Mr T Simon for the monitoring (URC‐EST, AP‐HP, Sorbonne Université‐Paris 06), and the informatics support Clinigrid.
For Sources of Funding and Disclosures, see page 8.
References
- 1. Mulder BJM. Epidemiology of adult congenital heart disease: demographic variations worldwide. Neth Heart J. 2012;20:505–508. doi: 10.1007/s12471-012-0335-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Engelings CC, Helm PC, Abdul‐Khaliq H, Asfour B, Bauer UMM, Baumgartner H, Kececioglu D, Körten D, Diller GP, Tutarel O. Cause of death in adults with congenital heart disease–an analysis of the German National Register for Congenital Heart Defects. Int J Cardiol. 2016;211:31–36. doi: 10.1016/j.ijcard.2016.02.133 [DOI] [PubMed] [Google Scholar]
- 3. Yu C, Moore BM, Kotchetkova I, Cordina RL, Celermajer DS. Causes of death in a contemporary adult congenital heart disease cohort. Heart. 2018;104:1678–1682. doi: 10.1136/heartjnl-2017-312777 [DOI] [PubMed] [Google Scholar]
- 4. Wang F, Harel‐Sterling L, Cohen S, Liu A, Brophy JM, Paradis G, Marelli AJ. Heart failure risk predictions in adult patients with congenital heart disease: a systematic review. Heart. 2019;105:1661–1669. doi: 10.1136/heartjnl-2019-314977 [DOI] [PubMed] [Google Scholar]
- 5. Lupón J, Gastelurrutia P, de Antonio M, González B, Cano L, Cabanes R, Urrutia A, Diez C, Coll R, Altimir S, et al. Quality of life monitoring in ambulatory heart failure patients: temporal changes and prognostic value. Eur J Heart Fail. 2013;15:103–109. doi: 10.1093/eurjhf/hfs133 [DOI] [PubMed] [Google Scholar]
- 6. Apers S, Kovacs AH, Luyckx K, Thomet C, Budts W, Enomoto J, Sluman MA, Wang JK, Jackson JL, Khairy P, et al. Quality of life of adults with congenital heart disease in 15 countries: evaluating country‐specific characteristics. J Am Coll Cardiol. 2016;67:2237–2245. doi: 10.1016/j.jacc.2016.03.477 [DOI] [PubMed] [Google Scholar]
- 7. Mommersteeg PMC, Denollet J, Spertus JA, Pedersen SS. Health status as a risk factor in cardiovascular disease: a systematic review of current evidence. Am Heart J. 2009;157:208–218. doi: 10.1016/j.ahj.2008.09.020 [DOI] [PubMed] [Google Scholar]
- 8. Hoekstra T, Jaarsma T, van Veldhuisen DJ, Hillege HL, Sanderman R, Lesman‐Leegte I. Quality of life and survival in patients with heart failure. Eur J Heart Fail. 2013;15:94–102. doi: 10.1093/eurjhf/hfs148 [DOI] [PubMed] [Google Scholar]
- 9. Greene SJ, Butler J, Spertus JA, Hellkamp AS, Vaduganathan M, DeVore AD, Albert NM, Duffy CI, Patterson JH, Thomas L, et al. Comparison of New York Heart Association class and patient‐reported outcomes for heart failure with reduced ejection fraction. JAMA Cardiol. 2021;6:522–531. doi: 10.1001/jamacardio.2021.0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Piepoli MF, Davos C, Francis DP, Coats AJS; ExTraMATCH Collaborative . Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328:189. doi: 10.1136/bmj.328.7441.711-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amedro P, Gavotto A, Bredy C, Guillaumont S. Cardiac rehabilitation for children and adults with congenital heart disease. Presse Med. 2017;46:530–537. doi: 10.1016/j.lpm.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 12. Opotowsky AR, Rhodes J, Landzberg MJ, Bhatt AB, Shafer KM, Yeh DD, Crouter SE, Tikkanen AU. A randomized trial comparing cardiac rehabilitation to standard of care for adults with congenital heart disease. World J Pediatr Congenit Heart Surg. 2018;9:185–193. doi: 10.1177/2150135117752123 [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592 [DOI] [PubMed] [Google Scholar]
- 14. Eindhoven JA, van den Bosch AE, Jansen PR, Boersma E, Roos‐Hesselink JW. The usefulness of brain natriuretic peptide in complex congenital heart disease: a systematic review. J Am Coll Cardiol. 2012;60:2140–2149. doi: 10.1016/j.jacc.2012.02.092 [DOI] [PubMed] [Google Scholar]
- 15. Alsaied T, Rathod RH, Aboulhosn JA, Budts W, Anderson JB, Baumgartner H, Brown DW, Cordina R, D'udekem Y, Ginde S, et al. Reaching consensus for unified medical language in Fontan care. ESC. Heart Fail. 2021;8:3894–3905. doi: 10.1002/ehf2.13294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ombelet F, Goossens E, Van De Bruaene A, Budts W, Moons P. Newly developed adult congenital heart disease anatomic and physiological classification: first predictive validity evaluation. J Am Heart Assoc. 2020;9:e014988. doi: 10.1161/JAHA.119.014988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Houyel L, Khoshnood B, Anderson RH, Lelong N, Thieulin AC, Goffinet F, Bonnet D; EPICARD Study group . Population‐based evaluation of a suggested anatomic and clinical classification of congenital heart defects based on the International Paediatric and Congenital Cardiac Code. Orphanet J Rare Dis. 2011;6:64. doi: 10.1186/1750-1172-6-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ruperti‐Repilado FJ, Thomet C, Schwerzmann M. 2020 ESC guidelines on treatment of adult congenital heart disease (ACHD). Herz. 2021;46:14–27. doi: 10.1007/s00059-020-05003-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li W, West C, McGhie J, van den Bosch AE, Babu‐Narayan SV, Meijboom F, Mongeon FP, Khairy P, Kimball TR, Beauchesne LM, et al. Consensus recommendations for echocardiography in adults with congenital heart defects from the International Society of Adult Congenital Heart Disease (ISACHD). Int J Cardiol. 2018;272:77–83. doi: 10.1016/j.ijcard.2018.07.058 [DOI] [PubMed] [Google Scholar]
- 20. Bratt EL, Moons P. Forty years of quality‐of‐life research in congenital heart disease: temporal trends in conceptual and methodological rigor. Int J Cardiol. 2015;195:1–6. doi: 10.1016/j.ijcard.2015.05.070 [DOI] [PubMed] [Google Scholar]
- 21. Bundgaard JS, Thune JJ, Gislason G, Fosbøl EL, Torp‐Pedersen C, Aagaard D, Nielsen JC, Haarbo J, Thogersen AM, Videbaek L, et al. Quality of life and the associated risk of all‐cause mortality in nonischemic heart failure. Int J Cardiol. 2020;305:92–98. doi: 10.1016/j.ijcard.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 22. Blok IM, van Riel ACMJ, Schuuring MJ, Duffels MG, Vis JC, van Dijk APJ, Hoendermis ES, Mulder BJM, Bouma BJ. Decrease in quality of life predicts mortality in adult patients with pulmonary arterial hypertension due to congenital heart disease. Neth Heart J. 2015;23:278–284. doi: 10.1007/s12471-015-0666-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johansson I, Joseph P, Balasubramanian K, McMurray JJV, Lund LH, Ezekowitz JA, Kamath D, Alhabib K, Bayes‐Genis A, Budaj A, et al. Health‐related quality of life and mortality in heart failure: the global congestive heart failure study of 23 000 patients from 40 countries. Circulation. 2021;143:2129–2142. doi: 10.1161/CIRCULATIONAHA.120.050850 [DOI] [PubMed] [Google Scholar]
- 24. Piotrowicz E, Stepnowska M, Leszczyńska‐Iwanicka K, Piotrowska D, Kowalska M, Tylka J, Piotrowski W. Quality of life in heart failure patients undergoing home‐based telerehabilitation versus outpatient rehabilitation – a randomized controlled study. Eur J Cardiovasc Nurs. 2015;14:256–263. doi: 10.1177/1474515114537023 [DOI] [PubMed] [Google Scholar]
- 25. Dulfer K, Duppen N, Kuipers IM, Schokking M, van Domburg RT, Verhulst FC, Helbing WA, Utens EMWJ. Aerobic exercise influences quality of life of children and youngsters with congenital heart disease: a randomized controlled trial. J Adolesc Health. 2014;55:65–72. doi: 10.1016/j.jadohealth.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 26. Novaković M, Prokšelj K, Rajkovič U, Vižintin Cuderman T, Janša Trontelj K, Fras Z, Jug B. Exercise training in adults with repaired tetralogy of Fallot: a randomized controlled pilot study of continuous versus interval training. Int J Cardiol. 2018;255:37–44. doi: 10.1016/j.ijcard.2017.12.105 [DOI] [PubMed] [Google Scholar]
- 27. Gierat‐Haponiuk K, Haponiuk I, Chojnicki M, Jaworski R, Bakuła S. Exercise capacity and the quality of life late after surgical correction of congenital heart defects. Kardiol Pol. 2011;69:810–815. [PubMed] [Google Scholar]
- 28. Gierat‐Haponiuk K, Haponiuk I, Szalewska D, Chojnicki M, Jaworski R, Niedoszytko P, Leszczynska K, Bakula S. Effect of complex cardiac rehabilitation on physical activity and quality of life during long‐term follow‐up after surgical correction of congenital heart disease. Kardiol Pol. 2015;73:267–273. doi: 10.5603/KP.a2014.0206 [DOI] [PubMed] [Google Scholar]
- 29. LeMond L, Mai T, Broberg CS, Muralidaran A, Burchill LJ. Heart failure in adult congenital heart disease: nonpharmacologic treatment strategies. Cardiol Clin. 2015;33:589–598. doi: 10.1016/j.ccl.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 30. Jackson JL, Hassen L, Gerardo GM, Vannatta K, Daniels CJ. Medical factors that predict quality of life for young adults with congenital heart disease: what matters most? Int J Cardiol. 2016;202:804–809. doi: 10.1016/j.ijcard.2015.09.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jani BD, Mair FS, Roger VL, Weston SA, Jiang R, Chamberlain AM. Comorbid depression and heart failure: a community cohort study. PLoS One. 2016;11:e0158570. doi: 10.1371/journal.pone.0158570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kovacs AH, Saidi AS, Kuhl EA, Sears SF, Silversides C, Harrison JL, Ong L, Colman J, Oechslin E, Nolan RP. Depression and anxiety in adult congenital heart disease: predictors and prevalence. Int J Cardiol. 2009;137:158–164. doi: 10.1016/j.ijcard.2008.06.042 [DOI] [PubMed] [Google Scholar]
- 33. Lu CW, Wang JK, Yang HL, Kovacs AH, Luyckx K, Ruperti‐Repilado FJ, Van De Bruaene A, Enomoto J, Sluman MA, Jackson JL, et al. Heart failure and patient‐reported outcomes in adults with congenital heart disease from 15 countries. J Am Heart Assoc. 2022;11:e024993. doi: 10.1161/JAHA.121.024993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Altenberger J, Parissis JT, Ulmer H, Poelzl G, Investigators LR. Rationale and design of the multicenter randomized trial investigating the efficacy and safety of pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep study). Eur J Heart Fail. 2010;12:186–192. doi: 10.1093/eurjhf/hfp189 [DOI] [PubMed] [Google Scholar]
- 35. de Vries AE, de Jong RM, van der Wal MHL, Jaarsma T, van Dijk RB, Hillege HL. The value of INnovative ICT guided disease management combined with telemonitoring in OUtpatient clinics for Chronic Heart failure patients. Design and methodology of the IN TOUCH study: a multicenter randomised trial. BMC Health Serv Res. 2011;11:167. doi: 10.1186/1472-6963-11-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sarullo FM, Fazio G, Brusca I, Fasullo S, Paterna S, Licata P, Novo G, Novo S, Di Pasquale P. Cardiopulmonary exercise testing in patients with chronic heart failure: prognostic comparison from peak VO2 and VE/VCO2 slope. Open Cardiovasc Med J. 2010;4:127–134. doi: 10.2174/1874192401004010127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.CIR.83.3.778 [DOI] [PubMed] [Google Scholar]
- 38. Kempny A, Dimopoulos K, Uebing A, Moceri P, Swan L, Gatzoulis MA, Diller GP. Reference values for exercise limitations among adults with congenital heart disease. Relation to activities of daily life‐‐single centre experience and review of published data. Eur Heart J. 2012;33:1386–1396. doi: 10.1093/eurheartj/ehr461 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Figure S1


