ABSTRACT
The incidence of heart failure and chronic kidney disease is increasing, and many patients develop both diseases. Angiotensin receptor‐neprilysin inhibitor (ARNI) is a promising therapeutic candidate for both diseases. ARNI has demonstrated superior cardioprotective effects compared with renin–angiotensin system inhibitors (RAS‐Is) in large clinical trials such as the PARADIGM‐HF (Prospective Comparison of ARNI With ACEI [Angiotensin‐Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial. It has also been suggested that ARNI can provide renoprotective effects beyond those of RAS‐Is in patients with HF. ARNI might have beneficial effects on the kidneys because of its ability to improve cardiac function in patients with heart failure and affect renal hemodynamics by enhancing the effects of hormones such as natriuretic peptide. In contrast, in the PARADIGM‐HF trial, ARNI was associated with more albuminuria compared with RAS‐I; thus, it is unclear whether long‐term ARNI therapy has renoprotective effects. Additionally, ARNI did not provide renoprotective effects beyond RAS‐I in patients with chronic kidney disease in the UK HARP‐III (United Kingdom Heart and Renal Protection‐III) trial. In other words, the patient population in which ARNI is more renoprotective than RAS‐I might be limited. Collectively, ARNI may have renoprotective effects in addition to cardioprotective effects, but the evidence to date is applicable only to heart failure. Theoretically, given the molecular mechanism of ARNI, it could also be renoprotective in conditions such as nephrosclerosis, which has low risks of albuminuria and reduced kidney perfusion, but the evidence for such effects is lacking. Further research is needed to clarify whether ARNI therapy is an acceptable treatment strategy for renal protection.
Keywords: albuminuria, angiotensin receptor‐neprilysin inhibitor, chronic kidney disease, heart failure, kidney‐protective effect, renin–angiotensin system inhibitors
Subject Categories: Heart Failure, ACE/Angiotension Receptors/Renin Angiotensin System, Hypertension
Nonstandard Abbreviations and Acronyms
- ARNI
angiotensin receptor‐neprilysin inhibitor
- AT1R
angiotensin II type 1 receptor
- ESKD
end‐stage kidney disease
- NEP‐I
neutral endopeptidase inhibitor
- NP
natriuretic peptide
- RAS‐I
renin–angiotensin system inhibitor
- UACR
urinary albumin/creatinine ratio
The heart and kidneys are closely related and interdependent. 1 The incidence of chronic kidney disease (CKD) and heart failure (HF) is increasing, and in many cases, patients have both diseases. 2 , 3 Angiotensin receptor‐neprilysin inhibitors (ARNIs) are established treatments for HF. ARNIs are also expected to exert renoprotective effects, but a consensus on such effects has not been reached. This review aims to summarize the current evidence on renoprotection by ARNIs, along with blood pressure (BP) and cardiovascular protection, and to identify areas in which more evidence is required for the renoprotective effects of ARNIs. We also discuss the potential and concerns regarding the renoprotective effects of ARNIs compared with renin–angiotensin system inhibitors (RAS‐Is) alone, including the results of previous randomized controlled trials (RCTs) and the mechanisms involved in the renoprotective effects of ARNIs.
RAS‐Is and Cardiorenal Protective Effects
RAS‐Is have long been used for cardiorenal protection. 4 Two types of RAS‐Is have been widely used in clinical practice, angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs), and direct renin inhibitors are also available. 5 Renin secreted from the kidneys produces angiotensin I from angiotensinogen synthesized in the liver. 5 , 6 Angiotensin I is converted to angiotensin II by ACE. 6 Angiotensin II exerts a strong hypertensive effect by constricting peripheral blood vessels and increasing water and sodium reabsorption via enhanced angiotensin II type 1 receptor and aldosterone secretion. 6 Additionally, angiotensin II is involved in remodeling and fibrosis in the heart, blood vessels, and kidneys. 6 , 7 ARBs specifically block angiotensin II type 1 receptor, and ACEIs inhibit ACE, which is required to convert angiotensin I to angiotensin II. 8 RAS‐Is are believed to exhibit cardiorenal protective effects in addition to antihypertensive effects by inhibiting circulating RAS and local tissue RAS. ACEIs and ARBs have been demonstrated to be cardioprotective in patients with HF and CKD in several RCTs. 4 , 9 , 10 However, because ACEIs inhibit the degradation of bradykinin, they have been reported to increase the risks of angioedema and cough. 11
Angiotensin Receptor‐Neprilysin Inhibitors
A new class of cardiovascular agents, termed ARNIs, was introduced in the late 2000s. ARNIs combine an ARB and a neutral endopeptidase inhibitor (NEP‐I). 12 , 13 Sacubitril/valsartan (also named LCZ696) was the first agent in the ARNI class. Because neprilysin degrades NPs (natriuretic peptides), including ANP (atrial NP), BNP (B‐type NP), and C‐type NP. 14 NEP‐Is enhance the effects of active NPs. NPs improve myocardial relaxation and reduce hypertrophy through cyclic guanosine monophosphate dependent pathways. 14 , 15 Additionally, NPs promote natriuresis, dilate blood vessels, and potentially have antifibrosis and sympathoinhibitory effects. 14 , 16 , 17 The targets of neprilysin include glucagon, glucagon‐like peptide‐1, bradykinin, substance P, endothelin, amyloid‐beta, and NPs. 18 , 19 Omapatrilat, which combines an ACE‐I and NEP‐I, exhibited better antihypertensive effects than enalapril and reduced the risks of HF all‐cause mortality and hospitalizations. 20 , 21 However, because bradykinin is a target of NEP, increased bradykinin levels due to NEP and ACE inhibition increased angioedema events. 18 , 20 , 21 , 22 The next agent to be developed, sacubitril/valsartan, combined an NEP‐I with an ARB instead of an ACEI, and therefore, it was associated with good hemodynamics with no cough or angioedema in early trials. 23 , 24
Greater Antihypertensive Effect of ARNIs
Ruilope et al. reported that for patients with hypertension, sacubitril/valsartan was associated with significantly greater reductions in the mean sitting diastolic BP versus the appropriate comparator dose of valsartan (mean reduction, −2.17 mm Hg [95% CI, −3.28 to −1.06]; P<0.001). 24 The PARAMETER (Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin Receptor Blocker Measuring Arterial Stiffness in the Elderly) trial of older hypertensive patients with arterial stiffness and increased pulse pressure revealed the superiority of sacubitril/valsartan to olmesartan based on its ability to lower BP by −3.7 mm Hg (95% CI, −6.4 to −0.9), and it was also associated a better nighttime reduction in BP. 25 ARNI therapy also displayed excellent antihypertensive effects in hypertensive patients with HF and CKD. In the PARADIGM‐HF trial of patients with reduced left ventricular ejection fraction (LVEF), the mean systolic BP (SBP) at 8 months was 3.2 ± 0.4 mm Hg lower in the sacubitril/valsartan group than in the enalapril group (P<0.001). 26 In the PARAGON‐HF (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction) trial of patients with preserved LVEF, the mean SBP at 8 months was 4.5 mm Hg (95% CI, 3.6–5.4) lower in the sacubitril–valsartan group than in the valsartan group. 27 In the UK HARP‐III study of patients with CKD, the mean SBP was 5.4 (95% CI, −7.4 to −3.4) mm Hg lower in the sacubitril/valsartan group than in the irbesartan group. 28
Effects of ARNIs on the Heart: Evidence for Cardioprotection
The characteristics of each RCT and subgroup that reported cardiovascular and renal outcomes are described in Table 1.
Table 1.
Definitions of Terms in Each Study
| Study type | Treatment | Dose, mg | No., male sex, % | Age, y | Race, % | Hypertension, % | Diabetes, % | HHF, % | MI, % | EF (%) | Systolic blood pressure, mm Hg | NT‐proBNP, pg/mL | eGFR, mL/min/1.73 m2 | UACR, mg/mmol | RAS‐I *, % | Inclusion/exclusion criteria for major cardiac and renal function | Results of cardiovascular outcomes in S/V group; HR or difference (vs RAS‐I) | Results of kidney outcomes in S/V group; HR or difference (vs RAS‐I) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PARADIGM‐HF | ||||||||||||||||||
| Overall RCT 26 | S/V | 400 |
4187 (79) |
64 |
White 66 Black 5 Asian 18 Other 11 |
71 | 35 | 62 | 43 | 30 | 122 | 1631 | 70 | 1.0 | 100 |
Inclusion: age ≥ 18 years; NYHA class II–IV; LVEF ≤35%; plasma BNP ≥150 pg/mL (or NT‐proBNP ≥600 pg/mL) at the screening visit or a BNP ≥100 pg/mL (or NT‐proBNP ≥400 pg/mL) and a HHF within the past 12 months Exclusion: current acute decompensated HF, eGFR <30 mL/min/1.73 m2 at screening, ACS, stroke, transient ischemic attack, cardiac, carotid, or other major cardiovascular surgery, PCI, or carotid angioplasty within the 3 months before screening |
Primary outcome (cardiovascular death + HHF), 0.8 (0.73–0.87); cardiovascular death, 0.8 (0.71–0.89); HHF 0.79 (0.71–0.89) | Prespecified composite renal outcome†, 0.86 (0.65–1.13); ≥50% decrease in eGFR, 0.75 (0.48–1.19); >30 mL/min/1.73 m2 decrease in eGFR to <60, 1.11 (0.80–1.53); ESRD 0.50 (0.21–1.16); Post hoc composite renal outcome (≥50% reduction in eGFR or ESRD), 0.63 (0.42–0.95); the rate of decrease in the eGFR, S/V, −1.61 mL/min/1.73 m2/year (95% CI, −1.77 to −1.44) vs enalapril, −2.04 mL/min/1.73 m2/year (95% CI, −2.21 to −1.88; P<0.001); the UACR was significantly higher in the S/V group compared with the enalapril group (P<0.001) |
| Enalapril | 20 |
4212 (77) |
64 |
White 66 Black 5 Asian 18 Other 11 |
71 | 35 | 63 | 43 | 29 | 121 | 1594 | 100 | ||||||
| Subgroup (eGFR<60) 26 , 29 | S/V vs enalapril |
2745 (76) |
70 |
White 73 Black 3 Asian 14 Other 10 |
78 | 39 | 63 | 50 | 30 | 129 | 1209 | 49 | 1.6 | 100 | Primary outcome (cardiovascular death + HHF), 0.79 (0.69–0.90); cardiovascular death, 0.76 [0.63–0.90]; HHF 0.79 (0.67–0.95) | Prespecified composite renal outcome†, 0.64 (0.37–1.08); ≥50% decrease in eGFR, 0.73 (0.35–1.54); >30 mL/min/1.73 m2 decrease in eGFR to <60, 0.62 (0.28–1.35); ESRD 0.70 (0.25–1.95); Post hoc composite renal outcome (≥50% reduction in eGFR or ESRD), 0.64 (0.34–1.19) | ||
| Subgroup (eGFR≥60) 26,29 | S/V vs enalapril |
5654 (79) |
79 |
White 63 Black 6 Asian 20 Other 11 |
67 | 32 | 63 | 40 | 29 | 128 | 761 | 81 | 1.0 | 100 | Primary outcome (cardiovascular death + HHF), 0.81 (0.73–0.91); cardiovascular death, 0.84 (0.72–0.96); HHF 0.81 (0.70–0.94) | Prespecified composite renal outcome†, 0.97 (0.70–1.34); ≥50% decrease in eGFR, 0.77 (0.43–1.39); >30 mL/min/1.73 m2 decrease in eGFR to <60, 1.25 (0.87–1.79); ESRD 0.28 (0.06–1.32); post hoc composite renal outcome (≥50% reduction in eGFR or ESRD), 0.63 (0.36–1.10) | ||
| Subgroup (UACR≥3.5) 26,29 | S/V vs enalapril |
441 (84) |
68 |
White 93 Black 4 Asian 1 Other 2 |
86 | 55 | 63 | 50 | 30 | 127 | 1235 | 65 | 7.55 | 100 | Primary outcome (cardiovascular death + HHF), 0.94 (0.67–1.31); cardiovascular death, 1.12 [0.70–1.80]; HHF 0.88 (0.59–1.32) | Prespecified composite renal outcome†, 0.94 (0.40–2.21); ≥50% decrease in eGFR, 2.05 (0.38–1.12); >30 mL/min/1.73 m2 decrease in eGFR to <60, 1.02 (0.38–2.72); ESRD 0.56 (0.05–6.17); Post hoc composite renal outcome (≥50% reduction in eGFR or ESRD), 1.05 (0.26–4.21) | ||
| Subgroup (UACR<3.5) 26,29 | S/V vs enalapril |
1431 (80) |
67 |
White 96 Black 3 Asian 0 Other 2 |
75 | 34 | 59 | 49 | 30 | 122 | 837 | 68 | 0.8 | 100 | Primary outcome (cardiovascular death + HHF), 0.77 (0.61–0.97); cardiovascular death, 0.88 (0.64–1.21); HHF 0.68 (0.51–0.91) | Prespecified composite renal outcome†, 0.54 (0.26–1.14); ≥50% decrease in eGFR, 0.79 (0.24–2.59); >30 mL/min/1.73 m2 decrease in eGFR to <60, 0.56 (0.24–1.29); ESRD 0.47 (0.04–5.18); post hoc composite renal outcome (≥50% reduction in eGFR or ESRD), 0.59 (0.19–1.80) | ||
| PARAGON‐HF | ||||||||||||||||||
| Overall RCT 27 | S/V | 400 | 2407 (48) | 73 |
White 82 Black 2 Asian 12 Other 4 |
96 | 44 | 47 | 23 | 58 | 131 | 904 | 63 | NA | 86 |
Inclusion: signs and symptoms of HF, NYHA class II–IV, EF ≥45 within the previous 6 months, elevated level of natriuretic peptides Exclusion: current acute decompensated HF, eGFR <30 mL/min/1.73 m2 at screening, any prior echocardiographic measurement of LVEF <40%; ACS (including MI), cardiac surgery, other major cardiovascular surgery, or urgent PCI within the 3 months before screening |
Primary outcome (cardiovascular death + HHF), 0.87 (0.75–1.01); cardiovascular death, 0.95 (0.79–1.16); HHF 0.85 (0.72–1.00) | Renal composite outcome‡, 0.50 (0.33–0.77); ≥50% decrease in eGFR, 0.44 (0.28–0.69); ESRD, 0.58 (0.23–1.47); the rate of decrease in the eGFR, S/V, 2.0 mL/min/1.73 m2/year (95% CI, −2.2 to −1.9) vs enalapril, −2.7 mL/min/1.73 m2/year (95% CI, −2.8 to −2.5; P<0.001) |
| Valsartan | 320 | 2389 (48) | 73 |
White 81 Black 2 Asian 13 Other 4 |
95 | 43 | 49 | 22 | 58 | 131 | 915 | 62 | NA | 86 | ||||
|
Subgroup |
S/V | 1164 (42) | 75 |
White 83 Black 2 Asian 11 Other 4 |
96 | 44 | 47 | 23 | 58 | 129 | 1060 | 47 | NA | 85 | Primary outcome (cardiovascular death + HHF), 0.79 (0.66–0.95) | Renal composite outcome‡, 0.50 (0.28–0.92); ≥50% decrease in eGFR, 0.39 (0.20–0.79); ESRD, 0.51 (0.19–1.35) | ||
| Valsartan | 1177 (45.2) | 75 |
White 83 Black 2 Asian 12 Other 3 |
96 | 46 | 50 | 22 | 58 | 130 | 1025 | 47 | NA | 85 | |||||
|
Subgroup (eGFR≥60) 27,30 |
S/V | 1211 (54) | 71 |
White 80 Black 2 Asian 13 Other 4 |
95 | 43 | 47 | 24 | 57 | 132 | 764 | 77 | NA | 88 | Primary outcome (cardiovasculardeath + HHF), 1.01 (0.80–1.27) | Renal composite outcome‡, 0.51 (0.29–0.93); ≥50% decrease in eGFR, 0.48 (0.27–0.88); ESRD, NA. | ||
| Valsartan | 1243 (51) | 70 |
White 80 Black 2 Asian 14 Other 4 |
95 | 40 | 48 | 22 | 57 | 131 | 780 | 77 | NA | 88 | |||||
| UK HARP‐III | ||||||||||||||||||
| Overall RCT 28 | S/V | 400 |
207 (71) |
62 |
White 90 Black 3 Asian 11 Other 7 |
NA | 39 | 4 | 10* | NA | 146 | 255 (ng/L) | 35 | 52 | 84 |
Inclusion: eGFR of ≥45 and <60 mL/min/1.73 m2 and UACR >20 mg/mmol (177 mg/g), or eGFR of ≥20 and < 45 mL/min/1.73 m2. Exclusion: currently nephrotic syndrome, ACS in 3 months before screening |
NT‐proBNP concentrations were 18% (−25 to −11%) lower and troponin I levels were 16% (−23% to −8%) lower in S/V group compared with IRB |
At 12 months, the mean (SE) measured GFR § was 29.8 (0.5) mL/min/1.73 m2 in S/V group compared with 29.9 (0.5) mL/min/1.73 m2 in irbesartan, a nonsignificant difference of 0.1 (0.7) mL/min/1.73 m2 (P=0.86). UACR was 9% (95% CI, −18% to 1%, P=0.08) lower in S/V group compared with irbesartan group |
| Irbesartan | 300 |
207 (72) |
64 |
White 92 Black 2 Asian 3 Other 2 |
NA | 40 | 3 | 16* | NA | 146 | 251 (ng/L) | 36 | 56 | 80 | ||||
Total of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers.
Prespecified kidney composite outcome (first occurrence of any of the following): (1) 50% decline in eGFR relative to baseline; (2) >30 mL/min/1.73 m2 decrease in eGFR relative to baseline to <60 mL/min/1.73 m2; and (3) ESRD or the following individual end points: (1) 50% decrease in eGFR relative to baseline; (2) >30 mL/min/1.73 m2 decrease in eGFR relative to baseline to <60 mL/min/1.73 m2; and (3) reaching ESRD.
A composite of the following: (1) ≥50% decrease in eGFR relative to baseline; (2) development of end‐stage kidney disease; and (3) death attributable to kidney causes.
GFR was measured in the study centers using 51Cr‐EDTA, 99mTc‐diethylenetriaminepentaacetic acid, or iohexol methods depending on local practices (with each center using the same method at baseline and 12 months).
ACS indicates acute coronary syndrome; BNP, B‐type natriuretic peptide; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESRD, end‐stage renal disease; HF, heart failure; HHF, hospitalization for heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NA, not available; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; PARADIGM‐HF, Prospective Comparison of ARNI [Angiotensin Receptor‐Neprilysin Inhibitor] With ACEI [Angiotensin‐Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure; PARAGON‐HF, Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction; PCI, percutaneous coronary intervention; RAS‐I, renin–angiotensin system inhibitor; RCT, randomized controlled trial; S/V, sacubitril/valsartan; and UACR, urinary albumin/creatinine ratio.
The PARADIGM‐HF trial was a double‐blind RCT that compared the efficacy and safety of sacubitril/valsartan with those of enalapril in 8399 patients with New York Heart Association class II to IV HF and reduced LVEF (≤35%). In this trial, 4187 patients who were randomly assigned to sacubitril/valsartan exhibited a 20% (hazard ratio [HR], 0.80 [95% CI, 0.73–0.87]) reduction in the primary composite end point of cardiovascular death or hospitalization attributable to HF versus enalapril. 26 Although this study excluded patients with estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, a subgroup analysis of patients with mild kidney impairment (eGFR ≥30 mL/min/1.73 m2 and <60 mL/min/1.73 m2) revealed a 21% lower risk of the primary composite end point of cardiovascular death or hospitalization for HF (HR, 0.79 [95% CI, 0.69–0.90]) and a 24% lower risk of cardiovascular death (HR, 0.76; [95% CI, 0.63–0.90]) in the sacubitril/valsartan group. 29 In patients without albuminuria (urinary albumin/creatinine ratio [UACR] ≥3.5 mg/mmol), the risk of the primary composite end point was lower in the sacubitril/valsartan group than in the enalapril group (HR, 0.77 [95% CI, 0.61–0.97]), but in patients with albuminuria (UACR <3.5 mg/mmol), no difference in risk was identified between the groups (HR, 0.94 [95% CI, 0.67–1.31]). 29
The PARAGON‐HF (Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction) trial was a double‐blind RCT that compared the efficacy and safety of sacubitril/valsartan with those of valsartan in 4796 patients with New York Heart Association class II to IV HF and preserved LVEF (>45%). In this trial, sacubitril/valsartan reduced the risk of the primary composite end point of cardiovascular death or hospitalization for HF by 13% (HR, 0.87 [95% CI, 0.75–1.01]) versus valsartan, although the result was not statistically significant. In a subgroup analysis of patients with mildly reduced LVEF (LVEF ≤57%), valsartan reduced the risk of the primary end point by 22% (HR, 0.78 [95% CI, 0.64–0.95]). 27 A subgroup analysis of patients with mild kidney impairment (eGFR ≥30 mL/min/1.73 m2 and <60 mL/min/1.73 m2) revealed a 21% reduction in the composite end point of cardiovascular death or hospitalization (HR, 0.79 [95% CI, 0.66–0.95]). 27 However, there was no difference between sacubitril/valsartan and valsartan in patients with eGFR ≥60 mL/min/1.73 m2. No subgroup analysis based on the presence of albuminuria was performed.
The UK HARP‐III trial compared the therapeutic effects of sacubitril/valsartan with irbesartan, an ARB, in patients with CKD and eGFR=45–60 mL/min/1.73 m2 and UACR >20 mg/mmol (177 mg/g creatinine) or eGFR=20–45 mL/min/1.73 m2. In this study, the primary outcome of eGFR change did not differ between the 2 treatments, but sacubitril/valsartan significantly reduced cardiac biomarker levels, such as N‐terminal proBNP and troponin I, compared with irbesartan. Specifically, N‐terminal proBNP and troponin I levels were reduced by 18% (95% CI, 11%–25%) and 16% (95% CI, 8%–23%), respectively, in the sacubitril/valsartan group. 28
Effects of ARNIs on the Kidneys: Evidence of Renoprotection
In the PARADIGM‐HF trial, there was no difference in the prespecified kidney composite outcome, first occurrence of any of the following: (1) a 50% decrease in eGFR relative to baseline; (2) a >30 mL/min/1.73 m2 decrease in eGFR relative to the baseline to <60 mL/min/1.73 m2; or (3) reaching end‐stage kidney disease (ESKD) between the sacubitril/valsartan and enalapril groups (HR, 0.86 [95% CI, 0.65–1.13]). 29 However, the risk of the new composite kidney outcome added during the post hoc analysis (ESKD or a ≥50% decrease in eGFR from baseline) was reduced by 37% (HR, 0.63 [95% CI, 0.42–0.95]) in the sacubitril/valsartan group. 29 The decrease in eGFR during the study was smaller for sacubitril/valsartan than for enalapril (−1.61 mL/min/1.73 m2/year [95% CI, −1.77 to −1.44] versus −2.04 mL/min/1.73 m2/year [95% CI, −2.21 to −1.88], P<0.001). 29 However, sacubitril/valsartan was associated with an increased risk of albuminuria compared with enalapril, and UACR was significantly higher at 1 and 8 months after treatment in the sacubitril/valsartan group than in the enalapril group. 29 For patients with eGFR <60 mL/min/1.73 m2 (N=2754), sacubitril/valsartan displayed a positive trend for both the prespecified kidney composite outcome and the new postanalysis kidney composite outcome, but it did not demonstrate a statistical advantage over enalapril for either outcome (HR, 0.64 [95% CI, 0.37–1.08]; HR, 0.64 [95% CI, 0.34–1.19], respectively). 29 There was also no difference between the groups regarding the kidney composite outcome in the baseline UACR ≥3.5 mg/mmol (HR, 0.94 [95% CI, 0.40–2.21] and UACR <3.5 mg/mmol groups (HR, 1.05 [95% CI, 0.26–4.21]). 29
The PARAMOUNT (Prospective Comparison of ARNI With ARB on Management of Heart Failure With Preserved Ejection Fraction) trial, which included patients with preserved EF, similarly found an increase in albuminuria in the sacubitril/valsartan versus valsartan despite the greater antihypertensive effect in the former group. 17 , 31
In an exploratory analysis in the PARAGON‐HF trial, sacubitril/valsartan reduced the risk of composite kidney events (≥50% decrease in eGFR relative to baseline; ESKD development; or death attributable to kidney causes; HR, 0.50 [95% CI, 0.33–0.77]) and the individual event of a ≥ 50% decrease in eGFR from baseline (HR, 0.44 [95% CI, 0.28–0.69]) compared with valsartan. 30 Additionally, sacubitril/valsartan was linked to a reduction in eGFR decline compared with valsartan (adjusted mean difference, 0.6 [95% CI, 0.4–0.9]), but there was no difference in the risk of progression to ESKD. 30 Among patients with CKD (eGFR <60 mL/min/1.73 m2 [N=2341]), sacubitril/valsartan reduced the risks of the kidney composite end point and the events associated with a > 50% eGFR reduction but not that of progression to ESKD, compared with valsartan. 30 Data on albuminuria were not presented.
In the UK HARP‐III trial, there was no difference in the primary outcome of measured GFR between sacubitril/valsartan and irbesartan (measured GFR [SE], sacubitril/valsartan, 29.8 mL/min/1.73 m2 [0.5] versus irbesartan, 29.9 mL/min/1.73 m2 [0.5]). 28 Additionally, there was no difference between sacubitril/valsartan and irbesartan for the primary outcome in any of the following subgroup analyses: baseline UACR >30 mg/mmol, UACR ≤30 mg/mmol, measured GFR >30 mL/min/1.73 m2, and measured GFR ≤30 mL/min/1.73 m2. Sacubitril/valsartan was associated with a nonsignificant 9% reduction (−18% to 1%, P=0.08) in UACR compared with irbesartan, and this reduction was associated with a reduction in BP. 28
Relationship Between ARNI Outcomes and BP
Summaries of the treatment effects of ARNI and RAS‐I are summarized in Table 2. In the PARADIGM‐HF and PARAGON‐HF trials, ARNI therapy provided superior cardiorenal protection in patients with reduced or preserved EF. 26 , 27 However, it should be noted that the greater antihypertensive effect in the ARNI group might have influenced this organ protection. Strict BP control has been reported to improve the outcomes of patients with HF. 32 , 33 Post hoc analyses of the PARADIGM‐HF and PARAGON‐HF trials reported that the organ‐protective effects of ARNIs were independent of their antihypertensive effects. 34 , 35 However, ARNI treatment reduced BP more strongly during the night, 25 and, likely, these effects were not captured by daytime BP measurements in these trials. Therefore, it is unclear whether the actual antihypertensive and organ‐protective effects of ARNIs are unrelated. In addition, the baseline BP of the patients should be considered with caution. The PARAGON‐HF trial recorded a greater reduction in the risk of renal events than the PARADIGM‐HF trial, consistent with the presence or absence of CKD. Notably, PARAGON‐HF had 20% more patients with hypertension than the PARADIGM‐HF trial, and the mean SBP was nearly 10 mm Hg higher in this trial. 26 , 27 In the PARADIGM‐HF trial, it was reported that patients with a higher baseline SBP received a greater benefit from ARNI treatment. 34 Also, in general, lower BP often results in lower GFR. Although the organ‐protective effect of ARNIs within the PARAGON‐HF trial was reported to be independent of its antihypertensive effect, 35 it is possible that the baseline BP of the included patients, in addition to differences in cardiac contractility, led to different results in the PARADIGM‐HF and PARAGON‐HF trials.
Table 2.
Summary of Results for Each Study
| Study | Patients | Subgroup | Treatment | Summary for BP‐lowering effect | Summary for cardiovascular protection | Summary for kidney protection |
|---|---|---|---|---|---|---|
| Ruilope LM et al, 2010 24 | Mild‐to‐moderate hypertension patients | S/V vs valsartan |
Sitting diastolic BP: S/V better Systolic BP: S/V better |
NA | NA | |
| PARAMOUNT 17 , 31 | HF with preserved EF patients (EF ≥45%) | S/V vs valsartan | Systolic BP: S/V better | NT‐proBNP reduction: S/V better |
eGFR preservation: S/V better UACR reduction: valsartan better |
|
| PARAMETER 25 | Systolic hypertension patients | S/V vs olmesartan |
Central aortic systolic pressure: S/V > valsartan 24‐hour brachial systolic BP: S/V better (significantly lower during sleep) |
NA | NA | |
| PARADIGM‐HF 26 , 29 | HF with reduced EF patients (EF ≤35%) | S/V vs enalapril | Systolic BP: S/V better | Composite outcome: S/V better |
Post hoc composite outcome: S/V better eGFR preservation: S/V better UACR reduction: valsartan better |
|
| + eGFR <60 | S/V vs enalapril | NA | Composite outcome: S/V better | Post hoc composite outcome: comparable | ||
| + eGFR ≥60 | S/V vs enalapril | NA | Composite outcome: S/V better | Post hoc composite outcome: comparable | ||
| + UACR ≥3.5 | S/V vs enalapril | NA | Composite outcome: comparable | Post hoc composite outcome: comparable | ||
| + UACR <3.5 | S/V vs enalapril | NA | Composite outcome: S/V better | Post hoc composite outcome: comparable | ||
| PARAGON‐HF 27 , 30 | HF with preserved EF patients (EF ≥45%) | S/V vs valsartan | Systolic BP: S/V better | Composite outcome: S/V better (trend) |
Composite outcome: S/V better eGFR preservation: S/V better |
|
| + eGFR <60 | S/V vs valsartan | NA | Composite outcome: S/V better | Composite outcome: S/V better | ||
| + eGFR ≥60 | S/V vs valsartan | NA | Composite outcome: comparable | Composite outcome: S/V better | ||
| UK HARP‐III 28 | Chronic kidney disease patients | S/V vs irbesartan | Systolic BP: S/V better |
NT‐proBNP reduction: S/V better Troponin I reduction: S/V better |
eGFR preservation: comparable UACR reduction: comparable |
BP indicates blood pressure; EF, ejection fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; NA, not available; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PARADIGM‐HF, Prospective Comparison of ARNI [Angiotensin Receptor‐Neprilysin Inhibitor] With ACEI [Angiotensin‐Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure; PARAGON‐HF, Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction; PARAMETER, Prospective Comparison of Angiotensin Receptor Neprilysin Inhibitor With Angiotensin Receptor Blocker Measuring Arterial Stiffness in the Elderly; PARAMOUNT, Prospective Comparison of ARNI With ARB [Angiotensin Receptor Blocker] on Management of Heart Failure With Preserved Ejection Fraction; S/V, sacubitril/valsartan; UACR, urinary albumin/creatinine ratio; and UK HARP‐III, United Kingdom Heart and Renal Protection‐III.
Areas With Insufficient Evidence for ARNI Treatment Outcomes
For CKD patients with advanced GFR decline or high albuminuria, ARNIs did not exert a stronger renoprotective effect than ARBs, although the results suggested a possible cardioprotective effect. 28 In post hoc analysis of the PARADIGM‐HF trial, ARNI had no cardiorenal protective effect versus enalapril in patients with HF and albuminuria. 29 Figure 1 presents the patient populations evaluated for the renoprotective effects of ARNIs. There is insufficient evidence in current RCTs for some patient populations. For example, the renal impact of ARNIs in patients without HF and albuminuria and with reduced GFR (eg, nephrosclerosis) is unclear. In addition, ARNIs did not have different renoprotective effects than RAS‐Is in patients with HF and albuminuria, although it should be noted that the number of eligible patients was small (N=441). 29 Furthermore, patients with eGFR<30 mL/min/1.73 m2 were excluded in the PARADIGM‐HF and PARAGON‐HF trials, 26 , 27 which showed positive effects of ARNIs on the heart and kidneys, and the cardiorenal protective effects of ARNIs in patients with HF and severe renal dysfunction or overt albuminuria are unclear.
Figure 1. Patient populations evaluated for the renoprotective effects of ARNIs in major RCTs.

Boxes with light blue background described in red letters are populations for which there is insufficient evidence on ARNI treatment to date. ARNI indicates angiotensin receptor‐neprilysin inhibitor; EF, ejection fraction; GFR, glomerular filtration rate; HF, heart failure; PARADIGM‐HF, Prospective Comparison of ARNI With ACEI [Angiotensin‐Converting Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure; PARAGON‐HF, Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction; RCT, randomized controlled trial; UACR, urinary albumin/creatinine ratio; and UK HARP‐III, United Kingdom Heart and Renal Protection‐III.
Meanwhile, the racial demographics of these RCTs differed. Most patients were White, and a few Asian or Black patients were included. 26 , 27 , 28 Racial differences might affect the sensitivity of the kidneys to treatment, and additional evidence is needed for racial and ethnic minority groups in these trials. 36 It should also be noted that the results revealing the superior renoprotection of ARNIs versus RAS‐Is were only obtained as secondary end points or in post hoc analyses, and kidney protection beyond RAS‐Is as a primary outcome was not demonstrated.
Potential Mechanism of the Renoprotective Effects of ARNIs
There are several possible reasons why ARNIs display renoprotective effects in patients with HF. First, renal blood flow and perfusion gradients decrease with decreased cardiac output in HF, exacerbating renal hemodynamic changes. 37 The worsening renal prognosis in patients with HF and preserved LVEF is not much different from that in patients with HF and reduced LVEF, and a similar mechanism may be involved. 38 ARNIs have been reported to improve cardiac function in patients with HF, especially in reduced LVEF. 39 Therefore, the improvement in cardiac function in patients with HF following ARNI treatment might contribute to renal protection by increasing renal perfusion. In addition, hemodynamic differences between ARNIs and RAS‐Is in the kidneys might contribute to differences in renal protection. RAS‐Is have been reported to potentially decrease intraglomerular pressure via dilation of the efferent artery 19 , 40 (Figure 2). This decrease in intraglomerular pressure prevents glomerular hyperfiltration and improves albuminuria. 40 Generally, this mechanism might explain the long‐term kidney‐protective effects of RAS‐Is. However, this is also a risk factor for rapid GFR reduction and hyperkalemia under conditions such as reduced kidney blood flow attributable to HF. 1 , 41 , 42 NEP‐Is contained in ARNIs increase kidney blood flow by enhancing NP activity, leading to increased intraglomerular pressure via predominant afferent artery dilation 19 , 43 and increased GFR via mesangial cell relaxation and an increased filtration coefficient 43 (Figure 2). This effect might have improved reduced renal perfusion in HF pathology and exerted a renoprotective effect, including an increase in GFR. Conversely, in the UK HARP‐III trial of patients with CKD, an increase in GFR following ARNI treatment as observed in other trials (in the first 3 months) 29 , 30 did not record. 28 In patients with CKD, some nephrons are sclerotic, whereas the residual nephrons are hyperfiltered. It is possible that patients with CKD already had hyperfiltration of residual nephrons, and therefore, they did not have the additional capacity to increase GFR after ARNI administration.
Figure 2. Kidney hemodynamics under hyperfiltration conditions and the influence of RAS‐Is and ARNIs on the kidneys.
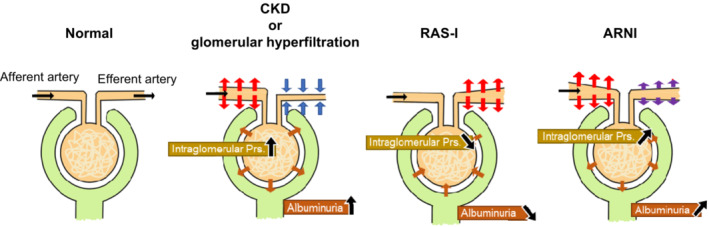
ARNI indicates angiotensin receptor‐neprilysin inhibitor; CKD, chronic kidney disease; Prs., pressure; and RAS‐I, renin–angiotensin system inhibitor.
Concerns About Renal Stress Induced by ARNIs
In patients with advanced GFR decline or severe albuminuria, ARNIs might be stressful to the kidneys. Increased intraglomerular pressure and increased GFR coupled with the direct effects of NPs could explain the increased incidence of albuminuria in several trials. 8 , 44 , 45 , 46 Elevated intraglomerular pressure might lead to glomerular damage and albuminuria. In addition, ANP and BNP might increase albumin efflux into the tubular lumen more strongly because of increased membrane permeability. 44 , 46 An elevated level of endothelin‐1, the degradation of which is inhibited by NEP‐I, was also reported to be involved in glomerular inflammation and damage to glomerular leg cells, thereby promoting proteinuria and glomerulosclerosis. 19 , 47 In addition to direct glomerular damage attributable to increased intraglomerular pressure, long‐term albuminuria can cause glomerulosclerosis, tubular damage, and interstitial fibrosis. 8 It is unclear whether long‐term ARNI administration is protective of the kidneys, even in patients with HF, when increased albuminuria is present.
In the PARAGON‐HF trial, which found that ARNIs preserved eGFR, the slope of the eGFR decrease in the ARNI group in the second half of the study appeared to be similar to that in the RAS‐I group. 30 Furthermore, a subgroup analysis of patients with diabetes revealed a steeper eGFR reduction in the late stage of the study period in the ARNI group than in the RAS‐I group. 48 In these studies, sacubitril/valsartan resulted in a smaller eGFR decline from baseline over the study period because of the increase of eGFR during the initial study period, but the aforementioned findings cast doubt over whether sacubitril/valsartan has the long‐term ability to preserve kidney function. There was no information available on albuminuria.
ARNIs in Basic Animal Studies
Basic science studies reported several ARNI's cardiorenal mechanisms and potential effects. In animal studies, combination treatment with NEP‐Is and ARBs prevented cardiac damage (ie, fibrosis, inflammation, and apoptosis) in rats with streptozotocin‐induced diabetes, and it was also associated with normalization of histone acetylation and histone acetyltransferase levels. 49 In experiments using Zucker obese rats, which exhibit hereditary obesity with insulin resistance and impaired glucose tolerance, and db/db (+Leprdb/+Leprdb) mice, which exhibit genetic obesity and type 2 diabetes attributable to leptin receptor abnormalities, ARNIs exerted protective effects on glomerular podocytes and reduced the risk of proteinuria. 50 , 51 These mechanisms might be advantageous for patients with CKD and diabetes in clinical trials. However, another study using Zucker obese rats and rats with 5/6 nephrectomy‐induced CKD found no effect of ARNIs on albuminuria, 52 , 53 suggesting that the renal effects of ARNI may vary by pathology and model. ARNI was also reported to reduce tubular damage in a cyclophosphamide‐induced nephrotoxicity model 54 and in an abdominal aortic ligation model. 55 However, only positive results are likely to be published (publication bias), and the existence of negative results is unclear. Clinical trials have often found that ARNI treatment results in more albuminuria than RAS‐Is therapy, but there might be a discrepancy between the results of many published animal studies and clinical trials. Additionally, research to clarify the missing pieces of ARNI clinical trial evidence is also currently insufficient (eg, HF pathology with overt albumin). We attempted to address one of these gaps by investigating the molecular mechanisms of the renoprotective effects of ARNI using cardiorenal syndrome model mice with overt albuminuria (data not presented; studies ongoing).
PERSPECTIVES: Strategy for the Renoprotective Effects of ARNIs
Based on previous findings, ARNIs can provide superior cardiovascular and kidney‐protective effects compared with those of RAS‐Is in patients with HF and low albuminuria. ARNIs might also have a positive effect on the kidneys in patients with decreased renal perfusion and GFR with mild albuminuria, as in nephrosclerosis. Conversely, for patients without HF who have albuminuria, the evidence of the renoprotective effects of ARNIs is limited. Whereas ARNIs can increase GFR, tubular and glomerular damage attributable to albuminuria might also develop. Furthermore, if there is no residual nephron reserve, as in advanced CKD, ARNIs might not induce an increase in GFR. In patients with moderate‐to‐severe albuminuria, sodium–glucose cotransporter 2 inhibitors and mineral corticoid antagonists used in addition to RAS‐Is have been demonstrated to have superior cardiovascular and renoprotective effects. 56 , 57 For such populations, the combination of sodium–glucose cotransporter 2 inhibitors, mineral corticoid antagonists, and RAS‐Is may be a better choice. These patients should be monitored for a sudden reduction in kidney perfusion and hyperkalemia, and if these occur, the option of switching to ARNIs, which increase kidney perfusion, should be considered. The renoprotective effects of ARNIs in populations with high albuminuria and HF or with low albuminuria without HF remain controversial. Furthermore, most evidence of the renoprotective effects of ARNIs was obtained from post hoc analyses, and high‐quality RCTs with kidney outcomes as the primary end point are needed. It is also important to caution that most renal events including UACR are measured on a creatinine basis, which may not be an appropriate indicator depending on renal function. Creatinine and albuminuria are affected by tubular secretion and reabsorption. Additionally, because of the differences in the doses of ARNI and RAS‐Is used in each trial, it is impossible to assess the extent to which RAS was suppressed in each trial, which limits the interpretation of the overall results. Further trials, including basic experiments, are needed to overcome these problems.
Conclusions
ARNIs have potential renoprotective effects in addition to their cardioprotective and antihypertensive effects. However, optimal ARNI use remains controversial. Further research is needed to determine the conditions under which ARNIs are renoprotective and whether ARNIs have stronger renoprotective effects than RAS‐Is even when used for longer periods.
Sources of Funding
This work was supported by grants from the Yokohama Foundation for Advancement of Medical Science, the Uehara Memorial Foundation, and the Japan Society for the Promotion of Science.
Disclosures
None.
Acknowledgments
We thank Joe Barber Jr., PhD, from Edanz (https://jp.edanz.com/ac) for editing a draft of this article.
This article was sent to Sula Mazimba, MD, MPH, Associate Editor, for review by expert referees, editorial decision, and final disposition.
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Banerjee D, Rosano G, Herzog CA. Management of heart failure patient with CKD. Clin J Am Soc Nephrol. 2021;16:1131–1139. doi: 10.2215/CJN.14180920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. House AA, Wanner C, Sarnak MJ, Piña IL, McIntyre CW, Komenda P, Kasiske BL, Deswal A, de Filippi CR, Cleland JGF, et al. Heart failure in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2019;95:1304–1317. doi: 10.1016/j.kint.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 3. Maddox TM, Januzzi JL Jr, Allen LA, Breathett K, Butler J, Davis LL, Fonarow GC, Ibrahim NE, Lindenfeld J, Masoudi FA, et al. 2021 update to the 2017 acc expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology solution set oversight committee. J Am Coll Cardiol. 2021;77:772–810. doi: 10.1016/j.jacc.2020.11.022 [DOI] [PubMed] [Google Scholar]
- 4. Sarafidis P, Papadopoulos CE, Kamperidis V, Giannakoulas G, Doumas M. Cardiovascular protection with sodium‐glucose cotransporter‐2 inhibitors and mineralocorticoid receptor antagonists in chronic kidney disease: a milestone achieved. Hypertension. 2021;77:1442–1455. doi: 10.1161/HYPERTENSIONAHA.121.17005 [DOI] [PubMed] [Google Scholar]
- 5. Cruz‐López EO, Ye D, Wu C, Lu HS, Uijl E, Mirabito Colafella KM, Danser AHJ. Angiotensinogen suppression: a new tool to treat cardiovascular and renal disease. Hypertension. 2022;79:2115–2126. doi: 10.1161/HYPERTENSIONAHA.122.18731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karimi F, Maleki M, Nematbakhsh M. View of the renin‐angiotensin system in acute kidney injury induced by renal ischemia‐reperfusion injury. J Renin Angiotensin Aldosterone Syst. 2022;2022:9800838–9800810. doi: 10.1155/2022/9800838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mezzano SA, Ruiz‐Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–638. doi: 10.1161/hy09t1.094234 [DOI] [PubMed] [Google Scholar]
- 8. Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23:1917–1928. doi: 10.1681/ASN.2012040390 [DOI] [PubMed] [Google Scholar]
- 9. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- 10. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161 [DOI] [PubMed] [Google Scholar]
- 11. Messerli FH, Bangalore S, Bavishi C, Rimoldi SF. Angiotensin‐converting enzyme inhibitors in hypertension: to use or not to use? J Am Coll Cardiol. 2018;71:1474–1482. doi: 10.1016/j.jacc.2018.01.058 [DOI] [PubMed] [Google Scholar]
- 12. Campbell DJ. Long‐term neprilysin inhibition–implications for Arnis. Nat Rev Cardiol. 2017;14:171–186. doi: 10.1038/nrcardio.2016.200 [DOI] [PubMed] [Google Scholar]
- 13. Azizi M, Rossignol P, Hulot JS. Emerging drug classes and their potential use in hypertension. Hypertension. 2019;74:1075–1083. doi: 10.1161/HYPERTENSIONAHA.119.12676 [DOI] [PubMed] [Google Scholar]
- 14. Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: hormones secreted from the heart. Peptides. 2019;111:18–25. doi: 10.1016/j.peptides.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 15. Zhang X, Zhou Y, Ma R. Potential effects and application prospect of angiotensin receptor‐neprilysin inhibitor in diabetic kidney disease. J Diabetes Complications. 2022;36:108056. doi: 10.1016/j.jdiacomp.2021.108056 [DOI] [PubMed] [Google Scholar]
- 16. Gardner DG, Chen S, Glenn DJ, Grigsby CL. Molecular biology of the natriuretic peptide system: implications for physiology and hypertension. Hypertension. 2007;49:419–426. doi: 10.1161/01.HYP.0000258532.07418.fa [DOI] [PubMed] [Google Scholar]
- 17. Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher‐Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, et al. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double‐blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6 [DOI] [PubMed] [Google Scholar]
- 18. D'Elia E, Iacovoni A, Vaduganathan M, Lorini FL, Perlini S, Senni M. Neprilysin inhibition in heart failure: mechanisms and substrates beyond modulating natriuretic peptides. Eur J Heart Fail. 2017;19:710–717. doi: 10.1002/ejhf.799 [DOI] [PubMed] [Google Scholar]
- 19. Yamamoto K, Rakugi H. Angiotensin receptor‐neprilysin inhibitors: comprehensive review and implications in hypertension treatment. Hypertens Res. 2021;44:1239–1250. doi: 10.1038/s41440-021-00706-1 [DOI] [PubMed] [Google Scholar]
- 20. Rouleau JL, Pfeffer MA, Stewart DJ, Isaac D, Sestier F, Kerut EK, Porter CB, Proulx G, Qian C, Block AJ. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356:615–620. doi: 10.1016/S0140-6736(00)02602-7 [DOI] [PubMed] [Google Scholar]
- 21. Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 22. Cruden NL, Fox KA, Ludlam CA, Johnston NR, Newby DE. Neutral endopeptidase inhibition augments vascular actions of bradykinin in patients treated with angiotensin‐converting enzyme inhibition. Hypertension. 2004;44:913–918. doi: 10.1161/01.HYP.0000146483.78994.56 [DOI] [PubMed] [Google Scholar]
- 23. Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi‐Molessa A, Zhang Y, Gotou H, Lefkowitz M, Zhang J. Efficacy and safety of LCZ696, a first‐in‐class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double‐blind, placebo‐controlled study. Hypertension. 2014;63:698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002 [DOI] [PubMed] [Google Scholar]
- 24. Ruilope LM, Dukat A, Böhm M, Lacourcière Y, Gong J, Lefkowitz MP. Blood‐pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin ii receptor and neprilysin: a randomised, double‐blind, placebo‐controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8 [DOI] [PubMed] [Google Scholar]
- 25. Williams B, Cockcroft JR, Kario K, Zappe DH, Brunel PC, Wang Q, Guo W. Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension. 2017;69:411–420. doi: 10.1161/HYPERTENSIONAHA.116.08556 [DOI] [PubMed] [Google Scholar]
- 26. McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077 [DOI] [PubMed] [Google Scholar]
- 27. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, et al. Angiotensin‐neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381:1609–1620. doi: 10.1056/NEJMoa1908655 [DOI] [PubMed] [Google Scholar]
- 28. Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, Bowman L, Brunskill N, Cockwell P, Hill M, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138:1505–1514. doi: 10.1161/CIRCULATIONAHA.118.034818 [DOI] [PubMed] [Google Scholar]
- 29. Damman K, Gori M, Claggett B, Jhund PS, Senni M, Lefkowitz MP, Prescott MF, Shi VC, Rouleau JL, Swedberg K, et al. Renal effects and associated outcomes during angiotensin‐neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6:489–498. doi: 10.1016/j.jchf.2018.02.004 [DOI] [PubMed] [Google Scholar]
- 30. Mc Causland FR, Lefkowitz MP, Claggett B, Anavekar NS, Senni M, Gori M, Jhund PS, McGrath MM, Packer M, Shi V, et al. Angiotensin‐neprilysin inhibition and renal outcomes in heart failure with preserved ejection fraction. Circulation. 2020;142:1236–1245. doi: 10.1161/CIRCULATIONAHA.120.047643 [DOI] [PubMed] [Google Scholar]
- 31. Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, et al. Renal effects of the angiotensin receptor neprilysin inhibitor lcz696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015;17:510–517. doi: 10.1002/ejhf.232 [DOI] [PubMed] [Google Scholar]
- 32. Pareek M, Vaduganathan M, Byrne C, Mikkelsen AD, Kristensen AMD, Biering‐Sørensen T, Kragholm KH, Omar M, Olsen MH, Bhatt DL. Intensive blood pressure control in patients with a history of heart failure: the Systolic Blood Pressure Intervention Trial (SPRINT). Eur Heart J Cardiovasc Pharmacother. 2022;8:E12–e14. doi: 10.1093/ehjcvp/pvab085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Böhm M, Young R, Jhund PS, Solomon SD, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Swedberg K, et al. Systolic blood pressure, cardiovascular outcomes and efficacy and safety of sacubitril/valsartan (LCZ696) in patients with chronic heart failure and reduced ejection fraction: results from PARADIGM‐HF. Eur Heart J. 2017;38:1132–1143. doi: 10.1093/eurheartj/ehw570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Selvaraj S, Claggett BL, Böhm M, Anker SD, Vaduganathan M, Zannad F, Pieske B, Lam CSP, Anand IS, Shi VC, et al. Systolic blood pressure in heart failure with preserved ejection fraction treated with sacubitril/valsartan. J Am Coll Cardiol. 2020;75:1644–1656. doi: 10.1016/j.jacc.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chu CD, Powe NR, McCulloch CE, Crews DC, Han Y, Bragg‐Gresham JL, Saran R, Koyama A, Burrows NR, Tuot DS. Trends in chronic kidney disease care in the US by race and ethnicity, 2012‐2019. JAMA Netw Open. 2021;4:e2127014. doi: 10.1001/jamanetworkopen.2021.27014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Damman K, Testani JM. The kidney in heart failure: an update. Eur Heart J. 2015;36:1437–1444. doi: 10.1093/eurheartj/ehv010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jung S, Bosch A, Kolwelter J, Striepe K, Kannenkeril D, Schuster T, Ott C, Achenbach S, Schmieder RE. Renal and intraglomerular haemodynamics in chronic heart failure with preserved and reduced ejection fraction. ESC Heart Fail. 2021;8:1562–1570. doi: 10.1002/ehf2.13257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin‐receptor neprilysin inhibitor on cardiac reverse remodeling: meta‐analysis. J Am Heart Assoc. 2019;8:e012272. doi: 10.1161/JAHA.119.012272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wenzel RR. Renal protection in hypertensive patients: selection of antihypertensive therapy. Drugs. 2005;65(Suppl 2):29–39. doi: 10.2165/00003495-200565002-00005 [DOI] [PubMed] [Google Scholar]
- 41. Raebel MA. Hyperkalemia associated with use of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers. Cardiovasc Ther. 2012;30:e156–e166. doi: 10.1111/j.1755-5922.2010.00258.x [DOI] [PubMed] [Google Scholar]
- 42. Palmer SC, Mavridis D, Navarese E, Craig JC, Tonelli M, Salanti G, Wiebe N, Ruospo M, Wheeler DC, Strippoli GF. Comparative efficacy and safety of blood pressure‐lowering agents in adults with diabetes and kidney disease: a network meta‐analysis. Lancet. 2015;385:2047–2056. doi: 10.1016/S0140-6736(14)62459-4 [DOI] [PubMed] [Google Scholar]
- 43. Theilig F, Wu Q. ANP‐induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Renal Physiol. 2015;308:F1047–F1055. doi: 10.1152/ajprenal.00164.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jacobs EM, Vervoort G, Branten AJ, Klasen I, Smits P, Wetzels JF. Atrial natriuretic peptide increases albuminuria in type I diabetic patients: evidence for blockade of tubular protein reabsorption. Eur J Clin Invest. 1999;29:109–115. doi: 10.1046/j.1365-2362.1999.00422.x [DOI] [PubMed] [Google Scholar]
- 45. Moore KB, McKenna K, Osman M, Tormey WP, McDonald D, Thompson CJ. Atrial natriuretic peptide increases urinary albumin excretion in people with normoalbuminuric type‐2 diabetes. Ir J Med Sci. 2007;176:67–73. doi: 10.1007/s11845-007-0030-1 [DOI] [PubMed] [Google Scholar]
- 46. Axelsson J, Rippe A, Rippe B. Transient and sustained increases in glomerular permeability following ANP infusion in rats. Am J Physiol Renal Physiol. 2011;300:F24–F30. doi: 10.1152/ajprenal.00347.2010 [DOI] [PubMed] [Google Scholar]
- 47. Barton M, Sorokin A. Endothelin and the glomerulus in chronic kidney disease. Semin Nephrol. 2015;35:156–167. doi: 10.1016/j.semnephrol.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peikert A, Vaduganathan M, Mc Causland F, Claggett BL, Chatur S, Packer M, Pfeffer MA, Zannad F, Lefkowitz MP, Pieske B, et al. Effects of sacubitril/valsartan versus valsartan on renal function in patients with and without diabetes and heart failure with preserved ejection fraction: insights from PARAGON‐HF. Eur J Heart Fail. 2022;24:794–803. doi: 10.1002/ejhf.2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malek V, Gaikwad AB. Telmisartan and thiorphan combination treatment attenuates fibrosis and apoptosis in preventing diabetic cardiomyopathy. Cardiovasc Res. 2019;115:373–384. doi: 10.1093/cvr/cvy226 [DOI] [PubMed] [Google Scholar]
- 50. Habibi J, Aroor AR, Das NA, Manrique‐Acevedo CM, Johnson MS, Hayden MR, Nistala R, Wiedmeyer C, Chandrasekar B, DeMarco VG. The combination of a neprilysin inhibitor (sacubitril) and angiotensin‐ii receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker obese rat. Cardiovasc Diabetol. 2019;18:40. doi: 10.1186/s12933-019-0847-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Myakala K, Jones BA, Wang XX, Levi M. Sacubitril/valsartan treatment has differential effects in modulating diabetic kidney disease in db/db mice and KKAy mice compared with valsartan treatment. Am J Physiol Renal Physiol. 2021;320:F1133–f1151. doi: 10.1152/ajprenal.00614.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gray EA, Patel SN, Doris PA, Hussain T. Combining neprilysin inhibitor with at(2)r agonist is superior to combination with at(1)r blocker in providing reno‐protection in obese rats. Front Pharmacol. 2021;12:778953. doi: 10.3389/fphar.2021.778953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jing W, Vaziri ND, Nunes A, Suematsu Y, Farzaneh T, Khazaeli M, Moradi H. LCZ696 (sacubitril/valsartan) ameliorates oxidative stress, inflammation, fibrosis and improves renal function beyond angiotensin receptor blockade in CKD. Am J Transl Res. 2017;9:5473–5484. [PMC free article] [PubMed] [Google Scholar]
- 54. Abbas SS, Schaalan MF, Gebril SM, Hassan FE, Mahmoud MO, Hassanin SO. LCZ696 (sacubitril/valsartan) protects against cyclophosphamide‐induced nephrotoxicity in adult male rats: up‐regulation of Apelin‐13/ACE2, miR‐200, and down‐regulation of TGF‐β/SMAD2/3 and miR‐192. Life Sci. 2022;306:120850. doi: 10.1016/j.lfs.2022.120850 [DOI] [PubMed] [Google Scholar]
- 55. Li Y, Kang L, Rong K, Zhang Y, Suo Y, Yuan M, Bao Q, Shao S, Tse G, Li R, et al. Renal protective effects and mechanisms of the angiotensin receptor‐neprilysin inhibitor LCZ696 in mice with cardiorenal syndrome. Life Sci. 2021;280:119692. doi: 10.1016/j.lfs.2021.119692 [DOI] [PubMed] [Google Scholar]
- 56. Tsukamoto S, Morita R, Yamada T, Urate S, Azushima K, Uneda K, Kobayashi R, Kanaoka T, Wakui H, Tamura K. Cardiovascular and kidney outcomes of combination therapy with sodium‐glucose cotransporter‐2 inhibitors and mineralocorticoid receptor antagonists in patients with type 2 diabetes and chronic kidney disease: a systematic review and network meta‐analysis. Diabetes Res Clin Pract. 2022;194:110161. doi: 10.1016/j.diabres.2022.110161 [DOI] [PubMed] [Google Scholar]
- 57. Ruilope LM, Agarwal R, Anker SD, Filippatos G, Pitt B, Rossing P, Sarafidis P, Schmieder RE, Joseph A, Rethemeier N, et al. Blood pressure and cardiorenal outcomes with finerenone in chronic kidney disease in type 2 diabetes. Hypertension. 2022;79:2685–2695. doi: 10.1161/HYPERTENSIONAHA.122.19744 [DOI] [PMC free article] [PubMed] [Google Scholar]


