Abstract
Background
Transthyretin amyloid cardiomyopathy (ATTR‐CM) is an important cause of heart failure in older individuals. Misfolding and deposition of transthyretin or prealbumin protein causes ATTR‐CM in the context of a normal (wild‐type) or variant TTR sequence. Variant ATTR‐CM is most commonly caused by the substitution of valine for isoleucine at position 122 in transthyretin (Val122Ile or pV142I, almost exclusively observed in individuals of West African ancestry), demonstrated in 3.4% of self‐identified Black individuals in the United States with an estimated 1.5 million carriers. Despite the large number of known pV142I carriers, the proportion of older Black patients with heart failure attributable to ATTR‐CM remains unknown.
Methods
To address this knowledge gap, the SCAN‐MP (Screening for Cardiac Amyloidosis with Nuclear Imaging in Minority Populations) study was funded by the National Institutes of Health/National Heart, Lung, and Blood Institute (R01HL139671) to enroll a targeted population of self‐identified, community‐dwelling Black or Caribbean Hispanic patients (many of whom are of West African ancestry) >60 years of age with heart failure and identify ATTR‐CM by noninvasive nuclear imaging. The principal objective of SCAN‐MP is to determine the prevalence of ATTR‐CM in this population. Secondary objectives will explore TTR genotype, demographics, progression of variant versus wild‐type ATTR‐CM, and biochemical mechanisms of transthyretin amyloid fibril formation.
Conclusions
The SCAN‐MP study is the largest, prospective study of cardiac amyloidosis in Black and Hispanic individuals. Both wild‐type and variant ATTR‐CM are now treatable with the US Food and Drug–approved drug tafamidis. The insights gained from SCAN‐MP are likely to improve those at risk for or afflicted with ATTR‐CM.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03812172.
Keywords: amyloidosis, cardiomyopathy, disparities, precision medicine
Subject Categories: Cardiomyopathy, Nuclear Cardiology and PET, Disparities, Precision Medicine
Nonstandard Abbreviations and Acronyms
- AL
light‐chain amyloidosis
- ATTR‐CM
transthyretin amyloid cardiomyopathy
- ATTR V122I
transthyretin amyloid cardiomyopathy owing to the V122I transthyretin gene variant
- ATTRwt
ATTR wild type
- HMDP
technetium‐99m hydroxymethylene diphosphonate imaging
- PYP
technetium‐99m‐pyrophosphate imaging
- RBP4
retinol binding protein‐4
- SCAN‐MP
Screening for Cardiac Amyloidosis with Nuclear Imaging in Minority Populations
- TTR
transthyretin or prealbumin protein
- pV142I
valine to isoleucine substitution at position 122 of the transthyretin protein (also known as V122I or Val122Ile)
Amyloid cardiomyopathy represents the cardiac manifestation of systemic amyloid diseases characterized by the misfolding and aggregation of amyloidogenic proteins in and around the heart. One aggregate type, cross‐β‐sheet amyloid fibrils, accumulate between cardiac myocytes, impairing myocardial compliance and precipitating direct cellular injury that further degrades heart function. 1 The vast majority of cases of amyloid cardiomyopathy are caused by misfolding of 1 of 2 precursor proteins: immunoglobulin light‐chain (for which the cardiomyopathy is known as AL‐CM) or transthyretin amyloid cardiomyopathy (ATTR‐CM). While AL amyloidosis is a rare disease with an estimated annual incidence of 1 in 75 000 to 100 000 people, and estimated prevalence of 1 in 25 000 or 12 000 adults living with AL amyloidosis in 2015, 2 increasing evidence now suggests that ATTR amyloidosis is likely considerably more common. ATTR amyloidosis occurs in the context of normal (wild‐type) or an abnormal (hereditary or variant) TTR genetic sequence in heterozygotes. ATTR amyloid deposits increase with aging according to autopsy studies, suggesting that up to 25% of people >80 years of age have demonstrable amyloid deposits, 3 increasing in proportion in men and those with heart failure. 4 Recent community‐dwelling cohort data suggest that among patients >60 years of age with heart failure having increased left ventricular wall thickness, the overall prevalence of ATTR‐CM is 6% in a predominantly White population, with a prevalence that increases to 10% in men. 5 Similarly, clinically important ATTR prevalence of 15% to 20% has also been reproducibly observed among patients with severe aortic stenosis undergoing valve replacement. 6 Thus, ATTR‐CM is likely a commonly encountered, but usually undiagnosed cause of heart failure. 7
The most common TTR variant associated with hereditary ATTR‐CM is the valine for isoleucine substitution at position 122 of transthyretin or prealbumin protein (TTR) (Val122Ile, V122I, or pV142I). 8 This variant has been reproducibly identified in 3.4% of self‐identified Black people in the United States corresponding to ≈1.5 million carriers of the allele when extrapolating from the US Black population of 47 million. Genotyping of populations in Africa has shown that the Val122Ile variant is common in individuals of West African ancestry. 8 TTR is a 4 exon gene (coding a homo‐tetrameric protein) that resides on chromosome 18. ATTR‐CM follows an autosomal dominant inheritance pattern. While the variant is present since birth, the clinical manifestations of ATTR‐CM are most commonly recognized over the age of 60 years (perhaps 5–10 years younger in rare homozygotes 9 ). That said, there is uncertainty regarding the phenotypic penetrance of ATTR‐CM in heterozygotes among those who carry the pV142I allele, with 1 prior study suggesting a low clinical penetrance of 7% by echocardiographic measures. 10 While this study and others demonstrated that the pV142I allele was associated with an increased risk of heart failure, 11 , 12 ATTR‐CM ascertainment relied on echocardiographic wall thickness and likely underestimates the true clinical penetrance, which undoubtedly increases with age. But even at this low estimate, population estimates yield >100 000 undiagnosed cases in the United States.
Establishment of the diagnosis of ATTR‐CM previously required tissue biopsy with demonstration of TTR amyloid deposits by histology. Congo red is the classically applied stain pathognomonic for amyloid deposits that yields green birefringence under polarized light microscopy. Immunohistochemistry, or now more preferably laser capture tandem mass spectrometry, is required to define the protein that composes the amyloid deposits. In 2016, a seminal multicenter collaborative study was reported wherein diagnosis of ATTR‐CM could be established with 100% specificity using a combination of technetium‐99m‐labeled nuclear tracers (including Tc‐99m‐PYP or pyrophosphate, Tc‐99m‐DPD or 3,3‐diphosphono‐1,2‐propanodicarboxylic acid, and Tc‐99m‐HMDP or hydroxymethylene diphosphonate) and blood and urine testing to exclude a monoclonal protein (and thus AL amyloidosis). 13 Subsequent studies began to apply these radiotracers to identify ATTR‐CM in various populations 5 , 6 , 14 ; however, it is important to note that the vast majority of patients included those identified as White race.
Previously treatable only by solid organ (heart and/or liver) transplantation, most patients with ATTR‐CM are of advanced age or have limiting comorbidities that preclude transplantation. In 2018, the randomized, placebo‐controlled, Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR‐ACT) demonstrated the efficacy of the orally administered TTR stabilizer tafamidis in the reduction of death and cardiovascular hospitalizations in wild‐type and hereditary ATTR‐CM. 15 On the strength of these data, the US Food and Drug Administration in 2019 approved tafamidis for the treatment of ATTR‐CM, and other agents are presently in late‐stage ATTR‐CM clinical trials. Thus, over a course of only 5 years, ATTR‐CM became a treatable, easily diagnosable, and appreciated as a likely relatively common cause of heart failure that remains largely underrecognized, especially in minority populations.
The mechanisms by which ATTR‐CM develops, particularly in the context of wild‐type TTR protein, remain unclear. Tetramer dissociation is the rate‐limited step for TTR aggregation, including amyloid fibril formation. 1 A subunit exchange assay is available that can measure the TTR dissociation rate in plasma that quantifies TTR kinetic stability, lending insight into factors that precipitate TTR misfolding. 16 One known important contributor to TTR kinetic stability is the stoichiometry of the TTR‐holo retinol binding protein 4 (RBP4) complex, whose formation kinetically stabilizes the TTR tetramer. It has been demonstrated that lower circulating RBP4 is a feature of ATTR‐CM in the context of the pV142I variant, 17 suggesting its utility as both a risk marker and plausible mechanistic factor in ATTR‐CM development.
Rationale and Objectives of the SCAN‐MP Study
The SCAN‐MP (Screening for Cardiac Amyloidosis with Nuclear Imaging in Minority Populations) study was designed to leverage noninvasive nuclear imaging to actively ascertain cases of ATTR‐CM in older, self‐identifying Black or Caribbean Hispanic patients with heart failure. Given the uncertainty in the burden of disease comprising ATTR‐CM as a cause of heart failure, the study's principal objective is to define the prevalence of ATTR‐CM in the recruited cohort of older community‐dwelling patients with heart failure, with secondary objectives to explore differences between hereditary versus wild‐type disease, distribution by sex, and prognosis. The study will also explore the mechanism of TTR misfolding through direct measurement of TTR stability and stabilization of TTR by protein ligand binding, principally holo‐RBP4, known to bind TTR and stabilize the molecule.
Overall Study Design
SCAN‐MP (ClinicalTrials.gov identifier NCT03812172) is a multisite prospective, noninterventional cohort study that commenced in May 2019 and is funded by an NHLBI R01 award (HL139671) with an Investigational New Drug application (IND 133555) issued for the nuclear radiotracer Tc‐99m‐PYP. The study sites include Columbia University Irving Medical Center and Harlem Hospital (New York City, NY), Boston Medical Center (Boston, MA), and as of April 2022, Yale New Haven Hospital (New Haven, CT). Enrolled participants complete baseline study procedures that include nuclear imaging to identify ATTR‐CM and genotyping to identify TTR variants. Owing to the national shortage of Tc‐99m‐PYP in 2022, the study protocol was amended in June 2022 to permit imaging with Tc‐99m‐HMDP when Tc‐99m‐PYP was not available. Participants with suspected ATTR‐CM by imaging or a TTR variant are referred to the relevant amyloidosis specialists at the associated site for follow‐on standard of care testing to confirm ATTR‐CM and initiate treatment. These participants return for protocol‐driven 6‐month and 12‐month follow‐up visits as detailed below. Participants without a variant and no ATTR‐CM by scintigraphy are followed by phone follow‐up only at 6 and 12 months with additional relevant hospitalization or survival data collected by the electronic health record. The overall design and study outcomes are illustrated in the Figure, and further details can be found at the study website: https://www.bumc.bu.edu/scan‐mp/
Figure 1. Graphical overview of SCAN‐MP design.
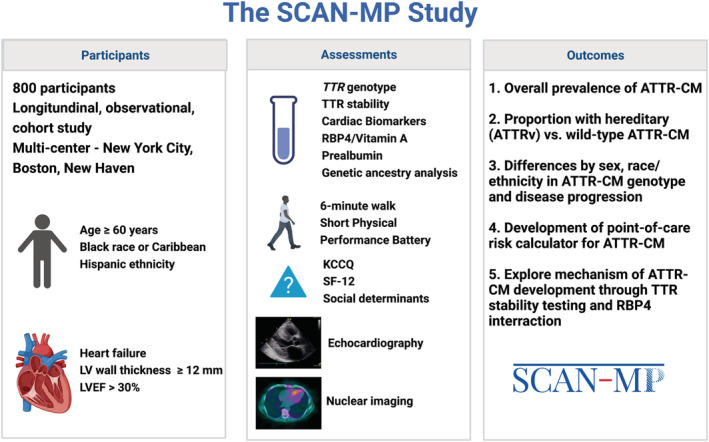
Assessments are performed at baseline for all participants, and again at 6‐ and 12 months for those with a Tc‐99m‐PYP scan diagnostic of ATTR‐CM and for all with the pV142I variant irrespective of Tc‐99m‐PYP scan result. Tc‐99m‐PYP scan, TTR genotype, and genetic ancestry analysis are performed at baseline only. ATTR‐CM indicates transthyretin amyloid cardiomyopathy; ATTRv, transthyretin amyloidosis owing to any transthyretin gene variant; KCCQ, Kansas City Cardiomyopathy Questionnaire; LV, left ventricular; LVEF, left ventricular ejection fraction; RBP4, retinol binding protein–4; SCAN‐MP, Screening for Cardiac Amyloidosis with Nuclear Imaging in Minority Populations; SF‐12, Short Form–12; Tc‐99m‐PYP, technetium‐99m‐pyrophosphate; and TTR, transthyretin. Created with BioRender.com.
Study Participants
Participant enrollment will be secured by categorizing the proposed 800 subjects into age (≤75 and >75 years), sex, and self‐identified race and ethnicity bins with derived targets and then enrolling subjects until the particular bin has reached target. The final proposed self‐identified race and ethnicity targets are 600 Black and 200 Caribbean Hispanic participants. Inclusion and exclusion criteria can be found in the Table, 18 , 19 and the specific age, race, and ethnicity recruitment target bins can be found in Table S1. SCAN‐MP is approved by the Western Institutional Review Board (IRB) in a single‐IRB model. All subjects have provided written informed consent.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
|
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AA indicates secondary amyloidosis; AL, light‐chain amyloidosis; eGFR, estimated glomerular filtration rate; and NHANES, National Health and Nutrition Examination Survey.
Study Assessments
Baseline Assessments
Medical history and medication inventory
Physical examination including vital signs, height, and weight
12‐lead ECG
- Laboratory testing including:
- Basic metabolic panel, hepatic function panel, and prealbumin
- 4 exon TTR gene sequencing (performed at Boston University)—performed at baseline only
- Cardiac biomarkers including B‐type natriuretic peptide, NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), hsTnI (high‐sensitivity troponin I), hs‐TnT (high‐sensitivity troponin T), and galectin‐3 (measured through a research collaboration with Abbott Laboratories)
- Vitamin A (retinol) and RBP4 in plasma and urine (performed at Columbia University)
- TTR subunit exchange (TTR stability testing, performed at The Scripps Research Institute)
- Genetic ancestry (performed at Columbia University)—performed at baseline only
- Functional testing including:
- Short Physical Performance Battery
- 6‐minute walk duration
- Patient‐reported outcomes/quality of life assessments including:
- Kansas City Cardiomyopathy Questionnaire
- Short‐Form survey 12
Transthoracic echocardiogram including full Doppler interrogation and speckle tracking global longitudinal strain, left atrial strain, and right ventricular strain analyses
Scintigraphy using Tc‐99m‐PYP (or Tc‐99m‐HMDP when PYP unavailable) by planar imaging, and gated single photon emitted computed tomography with computed tomography registration acquired if there is uptake consistent with ATTR‐CM after review of the planar images, performed at baseline only
Six‐ and 12‐Month Assessments
For those participants with ATTR‐CM or pV142I TTR variant without ATTR‐CM at baseline, at 6 months repeat testing includes repeat history/physical examination, ECG, laboratory testing (with the exception of TTR gene sequencing and TTR kinetic stability), functional testing, and quality of life/patient‐reported assessments. At 12 months after baseline, participants return for testing that includes all baseline testing (including echocardiography) except scintigraphy, genetic ancestry analysis, and TTR genetic testing.
Study End Points
The primary objective of SCAN‐MP is to improve the identification of ATTR‐CM among self‐identified Black and Caribbean Hispanic patients and in so doing enhance the understanding of genotype–phenotype associations among those with variant or wild‐type ATTR. The primary hypothesis is that the prevalence of ATTR‐CM will be ≥10% in the SCAN‐MP population and that significant differences in pV142I transthyretin amyloidosis owing to any transthyretin gene variant and ATTR wild type (ATTRwt) will be seen between the Black and Hispanic participants. Secondary analyses will explore (1) differences in ATTR‐CM prevalence as varies by age (60–75 years versus >75 years), sex, and race and ethnicity, (2) differences in the rate of disease progression between variant and wild‐type ATTR as measured by decrement in 6‐minute walk duration, mortality, and heart failure hospitalization incidence, (3) the performance of a risk prediction model to identify ATTR‐CM that will include RBP4 concentration, as well as other circulating and imaging markers of ATTR‐CM, and (4) the associations between TTR kinetic stability and RBP4 concentration in the plasma and urine among prospectively identified cases with ATTR‐CM versus nonamyloid controls.
ATTR‐CM will be defined by diagnostic (Perugini) grade 2 or grade 3 uptake by scintigraphy with verification of single photon emitted computed tomography with computed tomography registration imaging that the observed uptake is myocardial, consistent with consensus recommendations. 23 All participants with positive scintigraphy will be tested for the presence of a monoclonal gammopathy (to exclude AL amyloidosis) by serum free light chain assay and serum immunofixation electrophoresis through standard‐of‐care testing. Those with abnormal testing will be referred to amyloidosis specialty providers at the respective site for endomyocardial biopsy confirmation of amyloid type. It is anticipated that all identified participants with ATTR‐CM will be offered tafamidis as an ATTR‐specific therapy through standard clinical practice. Participant mortality, all cause, and heart failure hospitalizations will be determined by 6‐month follow‐up phone calls from the study team with verification from electronic health record review when possible. A prescribed hierarchical composite study end point of mortality at 1 year, number of heart failure hospitalizations, and 30% decline in 6‐minute walk duration is specified to compare progression of wild‐type and variant ATTR‐CM. Disease progression at baseline and 1 year will also be associated with changes in the biochemical profile (including cardiac‐specific biomarkers) and echocardiography. Genetic ancestry and degree of admixture will be determined by principal component analysis using a 500K SNP array. Cardiac‐specific biomarker analysis will be performed through a research agreement with Abbott Laboratories permitting a more complete characterization and comparisons of the different assays results.
Statistical Analysis
The sample size of SCAN‐MP was calculated to achieve a desirable level of precision (ie, half‐width of 95% CI) for estimating the prevalence of ATTR‐CM with adequate CIs, while also permitting accrual of adequate numbers of cases of ATTR‐CM to test the hypotheses exploring disease progression and mechanism. Specifically, from 800 participants we anticipate 80 cases of ATTR‐CM and 720 controls, which will be age, race, and sex matched. Anticipating that there may be a significant difference in ATTR‐CM prevalence between the different ethnicities enrolled, an adaptive design was proposed at the outset of the study such that after enrollment of 250 participants, the relative overall recruitment targets by race and ethnicity could be adjusted. Indeed, at 250 participants, noting a very low prevalence of ATTR‐CM in the Hispanic participants, the targeted enrollment was adjusted from an even distribution of Black and Hispanic participants to 600 Black and 200 Hispanic participants overall. The planned sample size produces 2‐sided 95% CIs with a precision (half‐width) of maximum 0.024 for a true prevalence of ATTR‐CM between 10% and 15%. In the ATTR‐CM group, we will compare a composite time‐to‐first‐event end point at 1 year of death, heart failure hospitalization, or 30% decline in 6‐minute hall walk between transthyretin amyloidosis owing to any transthyretin gene variant and ATTRwt participants, using 2‐sided log‐rank test. An overall sample size of 80 subjects achieves 80% power to detect a minimum difference of 22% (hazard ratio of 0.47) in the probability of not having attained the composite end point at 1 year between the 2 groups, assuming a 50% proportion will be positive for the end point in the ATTR‐CM group. The Kaplan–Meier method will be used to estimate the probabilities of the composite end point and Cox regression analysis will be performed to generate the hazard ratio (95% CI), and to allow adjustment for other covariates such as sex or race and ethnicity, or explore interaction with other variables including cardiac biomarkers, echocardiographic parameters, or qualify‐of‐life assessments. Alternatively, if event rates are low, we will apply the unmatched Finkelstein‐Schoenfeld method and matched win‐ratio method to afford appropriate priority to the more clinically important event (eg, mortality). The risk prediction model will be developed by variable selection/model construction based on weights from a multivariable regression model for ATTR‐CM identification, with prediction ability quantified by receiver operator characteristic analysis and goodness‐of‐fit by Hosmer‐Lemeshow testing.
Study Oversight
An observational study monitoring board consisting of a panel of experts with extensive experience in heart failure, cardiac imaging, and amyloidosis meets twice yearly to systematically review study procedures and progress. The Charter of the SCAN‐MP observational study monitoring board stipulates that its purpose is “safeguarding the interests of study participants, assessing the safety and efficacy of study procedures, ensuring data quality, and for monitoring the overall conduct of the study.” Representatives from the National Heart, Lung, and Blood Institute program also attend observational study monitoring board meetings but are nonvoting members. At the conclusion of the study, anonymized data and materials will been made publicly available through a National Heart, Lung, and Blood Institute data repository.
Discussion
Heart failure with preserved ejection fraction (HFpEF) accounts for more than half of the cases of heart failure identified in the past decade and is expected to increase as the population ages. Therapies for HFpEF are beginning to emerge as our understanding of the underlying pathophysiology becomes clearer. Among the large cohort of patients with HFpEF, ATTR‐CM has emerged as an important cause, often unrecognized, with effective therapy that improves outcomes relevant to both patients and their caregivers. Identification of affected patients, however, and in particular those early in the course of their illness, remains challenging. This is in part because HFpEF disproportionately impacts older and minority individuals who have a higher burden of cardiovascular risk factors such as hypertension, diabetes, and obesity that can contribute to a clinical phenotype similar to that of cardiac amyloidosis. Accordingly, providers face significant challenges in identifying minority individuals who are affected by ATTR‐CM. Thus, identifying and treating patients from underserved minority HF communities with personalized and precise medical approaches is urgently needed.
Protein aggregation including amyloid infiltration of the myocardium remains an underappreciated cause of HFpEF. Among the types of cardiac amyloidosis, ATTR‐CM accounts for the vast majority of affected patients. While Val122Ile is the most common variant worldwide and has been shown to almost exclusively affect individuals who are of West African ancestry, wild‐type transthyretin cardiac amyloidosis is even more common. To date, affected individuals with ATTRwt are disproportionately White and male. This is corroborated by recent evidence from a screening study from Rochester, MN in which 6% of patients with HFpEF, nearly all of whom were White individuals, had ATTRwt cardiac amyloidosis but none had variant disease. 5 That said, there is no biologic reason to explain this disparity in ATTRwt disease prevalence by race or ethnicity, and likely a significant portion of older adult minority patients of both sexes are affected by ATTRwt‐CM.
By carefully phenotyping individuals with an increased wall thickness and a clinical syndrome of heart failure with an EF >30% (a cut point selected because on average at presentation a significant percentage of those with the Val122Ile mutation have HFrEF), who do not have obvious causes such as ischemic or valvular disease, we can leverage the robust and multimodal data being collected in SCAN‐MP including demographic information, clinical data, laboratory/biochemical, electrocardiographic, and echocardiographic parameters to develop a risk calculator. This can be used to inform providers at the point of care regarding the need for further specific evaluation for ATTR‐CM. Data from SCAN‐MP can be pooled and combined with other programs of active ascertainment for transthyretin cardiac amyloidosis to provide the most accurate and robust method for early identification of affected individuals. In addition to general laboratory data including various cardiac biomarkers (eg, natriuretic peptides, troponins, and galactin‐3), SCAN‐MP is exploring the capacity of prealbumin itself as well as several novel biomarkers that are involved in transthyretin biology including RBP4 and vitamin A (in both the urine and plasma) and their ability to identify affected individuals. Additionally, we are exploring the kinetic stability of tetrameric TTR assessed in a plasma‐based subunit exchange assay to determine whether instability of TTR could serve as a diagnostic test.
Early diagnosis is essential to best capitalize on the efficacy of emerging therapies that predominantly address new amyloid fibril formation but do not address existing deposits. Identification of affected individuals is also essential to avoid institution of heart failure therapies that have potential harm, particularly in the management of atrial dysrhythmias. As an example, case reports suggest that nondihydropyridine calcium channel blockers are poorly tolerated in patients with cardiac amyloidosis and, by expert consensus opinion, are avoided. 24 Additionally, emerging data suggest that standard heart failure therapy, especially β‐blockers, may need to be avoided, de‐prescribed, or at least not given in high dosages in ATTR‐CM.
The natural history of TTR cardiac amyloidosis was quite poor just a decade ago, with studies showing declines in 6‐minute hall walk distance of ≈25 m along with increases in NT‐proBNP of >1800 picograms/mL every 6 months and very high mortality in 2 to 3 years. 25 However, there has been tremendous progress in the ability to identify patients at an earlier stage of disease, at a time when pharmacological therapies have the potential to have the greatest benefit. This is in part driven by the capacity of scintigraphy to establish a diagnosis of ATTR‐CM without a biopsy and accordingly, those identified with ATTR‐CM by scintigraphy appear to be diagnosed at a much earlier state of disease. Ironically, even though the most common cardiovascular question prompting Tc‐99m‐PYP scintigraphy is for diagnosis of ATTR‐CM, the radiopharmaceutical is not approved by the US Food and Drug Administration for this indication. SCAN‐MP is operating under an Investigational New Drug application from the US Food and Drug Administration and will provide essential data on the interobserver reproducibility of Tc‐99m‐PYP and it its diagnostic utility that could be used to expand its labeled indication.
Recruitment of the targeted population in SCAN‐MP requires significant engagement with community resources including faith‐based organizations and health care institutions that predominantly provide care to a minority and often underserved population. Accordingly, SCAN‐MP has focused on sites that have not only the available population but also the relationships with needed collaborators, especially committed health care teams including health educators, who can effectively communicate the importance of participating in this prospective epidemiological cohort study. Additionally, given the known mobility limitations among older adults with heart failure, we have provided a means for individual transportation to study sites for clinical phenotyping using commercial ride‐sharing companies. This coupled with appropriate reimbursement for participant time and effort will be important to achieve our enrollment targets. While the objective of SCAN‐MP is to identify cases of ATTR‐CM, it is expected that the majority of participants will have nonamyloid HF and neither harbor the pV142I variant nor have ATTR‐CM by Tc‐99m‐PYP imaging. In anticipation of this, collected data will be made available to the scientific community at the conclusion of the study by means of a National Heart, Lung, and Blood Institute data repository so that interested investigators will be able to leverage the rich phenotyping of SCAN‐MP to pursue future amyloid or nonamyloid HF questions.
Trial Status
SCAN‐MP is actively enrolling at all 3 sites with anticipated completion of accrual in Q2 2024.
SUMMARY
SCAN‐MP will be one of the largest prospectively recruited community‐dwelling older adult cohorts with heart failure and the only one that is exclusively focusing on minority individuals at risk for ATTR‐CM. With the advent of noninvasive approaches to accurately diagnose ATTR‐CM without the need for biopsy and the emergence of an effective disease‐modifying therapy that significantly prolongs life, slows functional decline, and blunts reductions in health‐related quality of life, the timing of SCAN‐MP is opportune to provide data that will change the approach to transthyretin cardiac amyloidosis by screening and early diagnosis.
Sources of Funding
SCAN‐MP is funded by National Institutes of Health/National Heart, Lung and Blood Institute‐R01 HL139671 and HL139671‐S1.
Disclosures
The cardiac biomarkers analysis will be performed by Abbott Laboratories through a separate in‐kind research grant. FLR has research grant support from Pfizer, Alnylam Pharmaceuticals, and Ionis/Akcea Therapeutics and consulting income from Attralus. MSM has consulting income from Eidos, Prothena, Ionis and Alnylam, Novo‐Nordisck and Intellia as well as institutional support in the form of clinical trial funding from Pfizer, Ionis, Eidos, and Alnylam. AJE has speaker fees from Ionetix; has received consulting fees from W. L. Gore & Associates; has received authorship fees from Wolters Kluwer Healthcare – UpToDate; and has received grants or grants pending to his institution from Attralus, Canon Medical Systems, Eidos Therapeutics, GE Healthcare, Pfizer, Roche Medical Systems, W. L. Gore & Associates, and XyloCor Therapeutics. EJM has served as a consultant to Bracco, Pfizer, GE Healthcare, and Alnylam; he has received grant support from Bracco, Eidos, Alnylam, and Pfizer. The remaining authors have no disclosures to report.
Supporting information
Table S1
Acknowledgments
The SCAN‐MP research team is deeply indebted to study participants and their families. SCAN‐MP is grounded in community‐engaged research practice and in so doing has constructed a community advisory board (CAB) that will meet twice yearly to review study progress and obtain feedback. CAB members will include patients with ATTR‐CM, participants in SCAN‐MP, community leaders, academics, and patient advocates.
This article was sent to Marc A. Simon, MD, MS, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028534
For Sources of Funding and Disclosures, see page 8.
References
- 1. Ruberg FL, Grogan M, Hanna M, Kelly JW, Maurer MS. Transthyretin amyloid cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:2872–2891. doi: 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quock TP, Yan T, Chang E, Guthrie S, Broder MS. Epidemiology of AL amyloidosis: a real‐world study using US claims data. Blood Adv. 2018;2:1046–1053. doi: 10.1182/bloodadvances.2018016402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tanskanen M, Peuralinna T, Polvikoski T, Notkola IL, Sulkava R, Hardy J, Singleton A, Kiuru‐Enari S, Paetau A, Tienari PJ, et al. Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2‐macroglobulin and tau: a population‐based autopsy study. Ann Med. 2008;40:232–239. doi: 10.1080/07853890701842988 [DOI] [PubMed] [Google Scholar]
- 4. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, et al. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Heart failure. 2014;2:113–122. doi: 10.1016/j.jchf.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. AbouEzzeddine OF, Davies DR, Scott CG, Fayyaz AU, Askew JW, McKie PM, Noseworthy PA, Johnson GB, Dunlay SM, Borlaug BA, et al. Prevalence of transthyretin amyloid cardiomyopathy in heart failure with preserved ejection fraction. JAMA Cardiol. 2021;6:1267–1274. doi: 10.1001/jamacardio.2021.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maurer MS, Bokhari S, Damy T, Dorbala S, Drachman BM, Fontana M, Grogan M, Kristen AV, Lousada I, Nativi‐Nicolau J, et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Heart Fail. 2019;12:e006075. doi: 10.1161/CIRCHEARTFAILURE.119.006075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Buxbaum JN, Ruberg FL. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age‐dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19:733–742. doi: 10.1038/gim.2016.200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reddi HV, Jenkins S, Theis J, Thomas BC, Connors LH, Van Rhee F, Highsmith WE Jr. Homozygosity for the V122I mutation in transthyretin is associated with earlier onset of cardiac amyloidosis in the African American population in the seventh decade of life. J Mol Diagn. 2014;16:68–74. doi: 10.1016/j.jmoldx.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 10. Quarta CC, Buxbaum JN, Shah AM, Falk RH, Claggett B, Kitzman DW, Mosley TH, Butler KR, Boerwinkle E, Solomon SD. The amyloidogenic V122I transthyretin variant in elderly black Americans. N Engl J Med. 2015;372:21–29. doi: 10.1056/NEJMoa1404852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Damrauer SM, Chaudhary K, Cho JH, Liang LW, Argulian E, Chan L, Dobbyn A, Guerraty MA, Judy R, Kay J, et al. Association of the V122I hereditary transthyretin amyloidosis genetic variant with heart failure among individuals of African or Hispanic/Latino ancestry. JAMA. 2019;322:2191–2202. doi: 10.1001/jama.2019.17935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parcha V, Malla G, Irvin MR, Armstrong ND, Judd SE, Lange LA, Maurer MS, Levitan EB, Goyal P, Arora G, et al. Association of transthyretin Val122Ile variant with incident heart failure among black individuals. JAMA. 2022;327:1368–1378. doi: 10.1001/jama.2022.2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez‐Lopez E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐Del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, et al. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. doi: 10.1093/eurheartj/ehv338 [DOI] [PubMed] [Google Scholar]
- 15. Maurer MS, Sultan MB, Rapezzi C. Tafamidis for transthyretin amyloid cardiomyopathy. N Engl J Med. 2019;380:196–197. doi: 10.1056/NEJMc1814074 [DOI] [PubMed] [Google Scholar]
- 16. Rappley I, Monteiro C, Novais M, Baranczak A, Solis G, Wiseman RL, Helmke S, Maurer MS, Coelho T, Powers ET, et al. Quantification of transthyretin kinetic stability in human plasma using subunit exchange. Biochemistry. 2014;53:1993–2006. doi: 10.1021/bi500171j [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arvanitis M, Koch CM, Chan GG, Torres‐Arancivia C, LaValley MP, Jacobson DR, Berk JL, Connors LH, Ruberg FL. Identification of transthyretin cardiac amyloidosis using serum retinol‐binding protein 4 and a clinical prediction model. JAMA Cardiol. 2017;2:305–313. doi: 10.1001/jamacardio.2016.5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806 [DOI] [PubMed] [Google Scholar]
- 19. Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4 [DOI] [PubMed] [Google Scholar]
- 20. Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, Hale FA. Quick assessment of literacy in primary care: the newest vital sign. Ann Fam Med. 2005;3:514–522. doi: 10.1370/afm.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sternthal MJ, Slopen N, Williams DR. Racial disparities in health: how much does stress really matter? Du Bois Rev. 2011;8:95–113. doi: 10.1017/S1742058X11000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson LA, Dedrick RF. Development of the trust in physician scale: a measure to assess interpersonal trust in patient‐physician relationships. Psychol Rep. 1990;67:1091–1100. doi: 10.2466/pr0.1990.67.3f.1091 [DOI] [PubMed] [Google Scholar]
- 23. Dorbala S, Ando Y, Bokhari S, Dispenzieri A, Falk RH, Ferrari VA, Fontana M, Gheysens O, Gillmore JD, Glaudemans A, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: part 1 of 2‐evidence base and standardized methods of imaging. Circ Cardiovasc Imaging. 2021;14:e000029. doi: 10.1161/HCI.0000000000000029 [DOI] [PubMed] [Google Scholar]
- 24. Kittleson MM, Maurer MS, Ambardekar AV, Bullock‐Palmer RP, Chang PP, Eisen HJ, Nair AP, Nativi‐Nicolau J, Ruberg FL; American Heart Association Heart F and Transplantation Committee of the Council on Clinical C . Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American Heart Association. Circulation. 2020;142:e7–e22. doi: 10.1161.CIR.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 25. Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, Falk RH, Cheung KN, Patel AR, Pano A, et al. Prospective evaluation of the morbidity and mortality of wild‐type and V122I mutant transthyretin amyloid cardiomyopathy: the transthyretin amyloidosis cardiac study (TRACS). Am Heart J. 2012;164:222–228. doi: 10.1016/j.ahj.2012.04.015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


