Abstract
Background
Treatment for breast cancer (BC) frequently involves radiotherapy. Guidelines recommend screening for cardiac adverse events starting 10 years after radiotherapy. The rationale for this interval is unclear.
Methods and Results
We aimed to study cardiovascular event rates in the first decade following curative radiotherapy for BC. We compared mortality and cardiovascular event rates with an age‐ and risk factor‐matched control population. We included 1095 patients with BC (mean age 56±12 years). Two hundred and eighteen (19.9%) women died. Cancer and cardiovascular mortality caused 107 (49.1%) and 22 (10.1%) deaths, respectively. A total of 904 cases were matched to female FLEMENGHO (Flemish Study on Environment, Genes and Health Outcomes) participants. Coronary artery disease incidence was similar (risk ratio [RR], 0.75 [95% CI, 0.48–1.18]), yet heart failure (RR, 1.97 [95% CI, 1.19–3.25]) and atrial fibrillation/flutter (RR, 1.82 [95% CI, 1.07–3.08]) occurred more often in patients with BC. Age (hazard ratio [HR], 1.033 [95% CI, 1.006–1.061], P=0.016), tumor grade (HR, 1.739 [95% CI, 1.166–2.591], P=0.007), and neoadjuvant treatment setting (HR, 2.782 [95% CI, 1.304–5.936], P=0.008) were risk factors for mortality. Risk factors for major adverse cardiac events were age (HR, 1.053 [95% CI, 1.013–1.093]; P=0.008), mean heart dose (HR, 1.093 [95% CI, 1.025–1.167]; P=0.007), history of cardiovascular disease (HR, 2.386 [95% CI, 1.096–6.197]; P=0.029) and Mayo Clinic Cardiotoxicity Risk Score (HR, 2.664 [95% CI, 1.625–4.367]; P<0.001).
Conclusions
Ten‐year mortality following curative treatment for unilateral BC was mainly cancer related, but heart failure and atrial fibrillation/flutter were already common in the first decade following irradiation. Mean heart dose, pre‐existing cardiovascular diseases, and Mayo Clinic Cardiotoxicity Risk Score were risk factors for cardiac adverse events. These results suggest a need for early dedicated cardio‐oncological follow‐up after radiotherapy.
Keywords: breast cancer, cardio‐oncology, cardiovascular diseases, epidemiology, radiation therapy, risk factors
Subject Categories: Cardio-Oncology, Cardiotoxicity
Nonstandard Abbreviations and Acronyms
- BC
breast cancer
- FLEMENGHO
Flemish Study on Environment, Genes and Health Outcomes
- HER2
human epidermal growth factor receptor 2
- MACE
major adverse cardiac event
Clinical Perspective.
What Is New?
This study focused on major adverse cardiac events (cardiovascular mortality, coronary events, heart failure, atrial fibrillation/flutter) in the first decade after radiotherapy in a curatively treated breast cancer population.
Heart failure and atrial fibrillation/flutter were more prevalent in the first 10 years as compared with age‐ and cardiovascular risk factor‐matched controls.
What Are the Clinical Implications?
These results encourage early dedicated cardio‐oncological follow‐up after radiotherapy.
In women, breast cancer (BC) is the most common malignancy and the leading cause of cancer death worldwide. 1 The multidisciplinary management of BC involves chemotherapy, targeted agents, hormone therapy, and radiotherapy and reduces recurrence and mortality in early and locally advanced disease. 2 , 3 Consequently, the focus of the management of BC shifted toward maintaining the quality of life of women treated for BC by overcoming treatment‐related adverse events, in particular cardiovascular mortality and morbidity. 4 Systemic therapy potentially entails a decline in left ventricular function, arrhythmias, and vascular toxic effects. Thoracic irradiation can cause peri‐ and myocardial fibrosis, rhythm and conduction abnormalities, valvular heart disease, heart failure (HF), and ischemic heart disease, often after a long latency period. 5 , 6 Cardiac irradiation has previously been associated with HF, more specifically HF with preserved ejection fraction (EF). 7 Current guidelines issued by cardiological and oncological societies recommend screening for delayed cardiovascular adverse events in BC survivors exposed to irradiation, typically from 10 years after therapy onwards. 6 , 8 , 9
Little is known about cardiac side effects in the first 10 years after radiotherapy. Our aim was to investigate cardiac side effects in the first 10 years after radiotherapy. Therefore, our objective was to compare the rates of mortality and major adverse cardiac events (MACEs) in the first decade following contemporary radiation therapy as part of the curative treatment regimen for unilateral BC with those observed in age‐ and risk factor‐matched controls. We hypothesized that radiotherapy of the chest might entail higher mortality and morbidity related to cardiac radiation damage.
We identified excess cardiovascular event rates in patients with BC compared with controls. In order to be able to identify patients at risk, our secondary aim was to identify in the cohort with BC tumor‐ and treatment‐related factors and comorbidities predictive of (cardiac) adverse events, including the Mayo Clinic Cardiotoxicity Risk Score. 10 The Mayo Clinic Cardiotoxicity Risk Score (Figure S1) integrates patient‐related risk factors (previous cardiovascular disease, classic cardiovascular risk factors, previous radiation therapy or anthracycline exposure, age, sex) and medication‐related risk factors (cardiotoxicity profile of administered chemotherapeutics) and was introduced to guide screening, monitoring, and initiation of preventive treatment for patients undergoing chemotherapy. 10
METHODS
The data underlying this article will be shared on reasonable request to the corresponding author.
Design of the Study
We performed a monocentric, retrospective study selecting all women with unilateral BC who received radiotherapy as part of their curative treatment in 2007 or 2008 from the BC registry of the University Hospitals Leuven (Figure 1). We compared mortality and cardiovascular event rates during the first decade with an age‐ and risk factor‐matched control population (FLEMENGHO [Flemish Study on Environment, Genes and Health Outcomes]). Second, we explored tumor‐ and treatment‐related factors and comorbidities, predictive of (cardiac) adverse events, including the Mayo Clinic Cardiotoxicity Risk Score.
Figure 1. Study design.
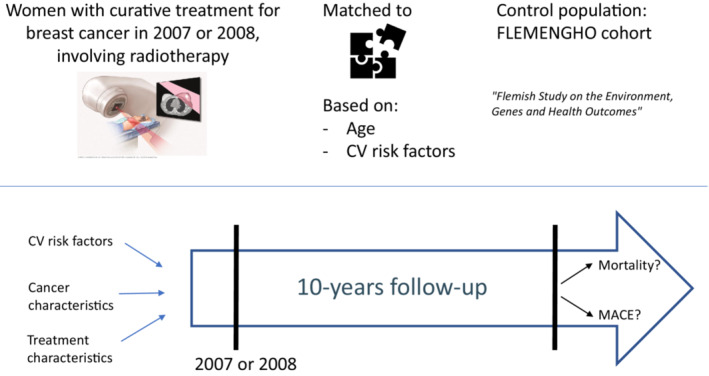
CV indicates cardiovascular; FLEMENGHO, Flemish Study on Environment, Genes and Health Outcomes; and MACE, major adverse cardiac event.
Cohort With Breast Cancer
Since 2000, patients with BC visiting the Leuven Multidisciplinary Breast Cancer Center (University Hospitals Leuven, Belgium) have systematically been entered into a clinical database, containing extensive patient‐, tumor‐, and treatment‐related data as well as follow‐up data and blood samples. Collection of patient data and blood analysis were approved by the local ethics committee (S63773, University Hospitals Leuven). All patients included in the study gave written informed consent.
For this study we selected all women with unilateral early and locally advanced BC, treated at the University Hospitals Leuven with external beam radiation in the years 2007 or 2008 from the clinical BC registry. Additional chemotherapy administration, if needed, and follow‐up were done at the University Hospitals Leuven or external. The study protocol complied with the Declaration of Helsinki. The Ethics Committee of the University Hospitals Leuven (approval number S61741) approved the study and in view of the retrospective design waived the requirement for informed consent.
Between January 1, 2007 and December 31, 2008, 1338 patients were diagnosed with breast cancer at our institution and treated with curative intent. We studied women diagnosed with unilateral BC and with radiation therapy as part of their anticancer treatment, thus excluding 12 men, 38 women with bilateral BC, and 193 women without external‐beam radiation therapy (Figure 2). Consequently, the studied cohort with BC consisted of 1095 patients who were followed until November 1, 2018 (censoring date).
Figure 2. Consolidated Standards of Reporting Trials (CONSORT) diagram of the studied populations.
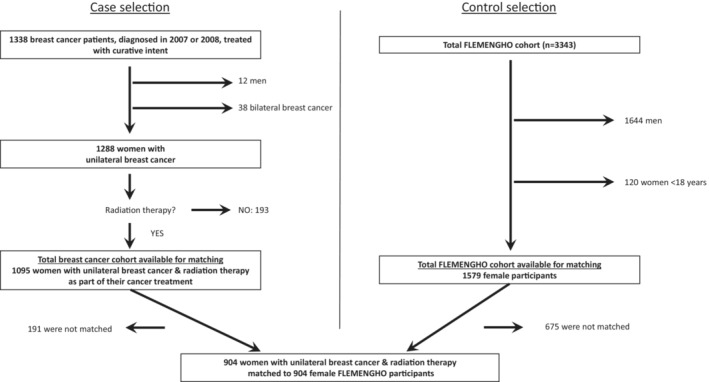
FLEMENGHO indicates Flemish Study on Environment, Genes and Health Outcomes.
Their electronic hospital records were retrospectively analyzed for tumor characteristics, received anticancer treatment, oncological parameters, and the occurrence of cardiac events before and after the BC therapy. HF was defined as symptoms of HF (dyspnea, orthopnea, edema) or decline of EF <45%. Fatal HF was defined as HF as main cause of death. Cause of death was determined by checking the electronic hospital records and classified as either oncological, cardiovascular, infectious, other, or unknown. When death was classified as cardiovascular, it was further subclassified as either acute coronary syndrome, HF, stroke, pulmonary embolism, sudden cardiac death, other, or unknown.
The predefined end points were total, cardiovascular, noncardiovascular, and cancer mortality, as well as MACE. MACE was defined as cardiovascular mortality, fatal and nonfatal coronary events, fatal and nonfatal HF, and atrial fibrillation or flutter (AF). Cardiovascular risk factors, such as hypertension, diabetes, and smoking status were extracted from the hospital records. History of cardiovascular disease was defined as a history of coronary artery disease, HF, stroke, or AF.
Radiotherapy Dosimetry
Radiotherapy to the chest invariably leads to a radiation dose to the heart. Depending on the target volume, different radiation techniques can be applied, resulting in variable mean heart doses. We evaluated the mean heart dose depending on the regions involved in the irradiation. In our studied cohort of 1095 patients, 307 patients received only radiotherapy to the breast or thoracic wall. A total of 788 patients received radiotherapy to the parasternal and subclavian nodes (cumulative dose of 50 Gray [Gy] in 2 Gy fractions), with an average mean heart dose of 2.3 Gy for right‐sided versus 6.7 Gy for left‐sided BC (Figure S2).
The patients receiving radiotherapy on parasternal and subclavian nodes were further subcategorized depending on the additional regions involved, resulting in different radiation techniques.
In a subgroup of 384 patients, the target volume consisted of the breast and parasternal and subclavian lymph nodes. The most commonly used radiation modality was an oblique parasternal photon technique (data available for 264 patients). The average mean heart dose was 1.7 Gy for right‐sided versus 6.4 Gy for left‐sided BC.
In 74 patients, the target volume included only the parasternal and subclavian volumes. All patients were irradiated with a mixed beam technique (data available for 69 patients). The average mean heart dose was 4.7 Gy for right‐sided versus 7.8 Gy for left‐sided BC.
A total of 330 patients received radiotherapy to the thoracic wall and parasternal and subclavian nodes. A technique of electron beam for the thoracic wall and mixed photon/electron beam for the nodal volumes was used in almost all cases (95.9%). In these patients the mean heart doses were not routinely calculated. To estimate mean heart doses, these were calculated in a subset of 20 patients (10 left‐sided BC, 10 right‐sided BC) based on the previously drafted plans. The average mean heart dose was 9.4 Gy for right‐sided versus 14.3 Gy for left‐sided BC.
Control Population and Matching
FLEMENGHO is a family‐based epidemiological study. 11 , 12 Participants are representative of the population of a geographically defined area in northern Belgium. The University Hospitals Leuven are the main health care referral center for the FLEMENGHO catchment area. Recruitment of FLEMENGHO participants started in 1985 and continued until 2007. The FLEMENGHO study is registered with the Belgian Data Protection Agency (ethics approval number, S58373). At annual intervals the vital status of FLEMENGHO participants was ascertained via the National Population Registry and the cause of death established via the Flemish Death Certificate Registry, Ministry of the Flemish Community, Brussels. The cause of death was validated against the medical records of general practitioners and hospitals. To collect nonfatal outcomes, at the yearly contacts, standardized questionnaires were completed to collect information about each participant's personal and familial medical history, use of medication, and smoking. If participants declined a physical contact at the local examination center in the catchment area, the questionnaires were completed by telephone interview. Arterial hypertension was an office systolic blood pressure of ≥140 mm Hg or diastolic blood pressure of ≥90 mm Hg, or use of antihypertensive medication. Diabetes was defined as the use of antidiabetic drugs and a fasting or random blood glucose of ≥126 mg/dL or ≥200 mg/dL, respectively. Dyslipidemia was a low‐density lipoprotein cholesterol of ≥115 mg/dL or use of lipid‐lowering drugs. All fatal and nonfatal outcomes were validated via consultation of the medical records of general practitioners, specialists, and regional hospitals.
The total FLEMENGHO population consists of 3343 patients. We excluded male patients and patients younger than 18 years. We had a total of 1579 FLEMENGHO participants available for matching (Figure 2). Using a Greedy matching algorithm, the patients with BC were matched individually in a 1:1 ratio with FLEMENGHO participants according to age (within 5 years), smoking status. and the presence versus absence of hypertension and diabetes. A total of 191 patients with BC could not be matched (due to higher median age in the breast cancer population versus the control population) and were therefore excluded from the case–control analysis. Matching was checked and was optimal. Matching results can be found in Figure S3.
We had a total matched population of 904 patients with BC and 904 FLEMENGHO participants available for further analysis.
Statistical Analysis
Continuous variables are expressed as means with SD. Normality was assessed by the Kolmogorov–Smirnov test. Categorical variables are presented as numbers and percentages. For between‐group comparison of means, the large‐sample Z test or a nonparametric test, as appropriate, were applied, and for comparison of proportions Fisher's exact test. Rates were expressed per 1000 person‐years and 95% CI were computed as , where R and N are the rate and the number of individuals used to compute the rate. We plotted cumulative incidence rates and estimated 10‐year absolute risks from the Fine and Gray proportional subdistribution hazards model, taking into account competing mortality from causes other than the event of interest. 13 The association of health outcomes in the cohort with BC was assessed by univariable and multivariable Cox regression analysis. The covariates analyzed in these models are age, tumor characteristics, treatment characteristics, history of cardiovascular disease, and Mayo Clinic Cardiotoxicity Risk score (details can be found in Table S1). The multivariable models were adjusted for smoking status, arterial hypertension, and diabetes. Multivariable models were first run as a complete model including all variables, because of convergence problems the Cox multivariable analyses for AF and HF were subsequently run as reduced models including only variables significant in univariable analysis.
In the multivariable models, estrogen receptor positivity and hormonal treatment caused strong multicollinearity, so for model purposes only estrogen receptor positivity could be included. Proportional hazard assumptions were calculated using Schoenfeld residuals for all Cox multivariable models and can be found in Table S2 and Figure S4. A 2‐sided P value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC, USA), STATA 14.2 (StataCorp, College Station, TX, USA) and GraphPad Prism 8 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Characteristics of the Population With Breast Cancer
Baseline characteristics of the cohort with BC are described in Table 1.
Table 1.
Baseline Characteristics of the Cohort With Breast Cancer
| Cohort with breast cancer | |
|---|---|
| No. of patients | 1095 |
| Age, y, mean±SD | 56.3±12.3 |
| Cardiovascular risk profile | |
| Smoking, n (%) | 337 (30.8%) |
| Arterial hypertension, n (%) | 318 (29.0%) |
| Diabetes, n (%) | 68 (6.2%) |
| Hypercholesterolemia, n (%) | 209 (19.1%) |
| Obesity, n (%) | 150 (13.7%) |
| History of cardiovascular disease, n (%) | 65 (5.9%) |
| Mayo Clinic Cardiotoxicity Risk score, mean±SD | 5±2 |
| Oncological history, n (%) | 56 (5.1%) |
| Including chemotherapy, n (%) | 11 (1.0%) |
| Including radiotherapy, n (%) | 14 (1.3%) |
| Timing of start systemic therapy | |
| Neoadjuvant, n (%) | 108 (9.9%) |
| Adjuvant, n (%) | 987 (90.1%) |
| Laterality of breast cancer | |
| Right, n (%) | 544 (49.7%) |
| Left, n (%) | 551 (50.3%) |
| Tumor stage | |
| Neoadjuvant systemic therapy | |
| II a/b, n (%) | 6 (5.6%)/17 (15.7%) |
| III a/b/c, n (%) | 28 (25.9%)/35 (32.4%)/18 (16.7%) |
| Unknown, n (%) | 4 (3.7%) |
| Adjuvant systemic therapy | |
| I a/b, n (%) | 349 (35.4%)/37 (3.8%) |
| II a/b, n (%) | 266 (27.0%)/186 (18.8%) |
| III a/b/c, n (%) | 89 (9.0%)/8 (0.8%)/38 (3.9%) |
| Unknown, n (%) | 14 (1.4%) |
| Cancer treatment | |
| Irradiation median subclavian and parasternal nodes, n (%) | 788 (72.0%) |
| Hormonal therapy, n (%) | 900 (82.2%) |
| Chemotherapy, n (%) | 569 (52.0%) |
| Antracyclines, n (%) | 532 (48.5%) |
| Human epidermal growth factor receptor 2 (HER2)‐targeted therapy, n (%) | 135 (12.3%) |
Mortality and Adverse Cardiovascular Events Following Curative Treatment for Unilateral Breast Cancer
During the first decade of follow‐up after BC therapy, 218 women died (19.9%); the death rate per 1000 patient years was 20.9 (95% CI 18.2–23.7). Cancer death occurred in 107 women (49.1%); the corresponding death rate per 1000 patient years was 10.3 (95% CI 8.3–12.2). Twenty‐two women (10.1%) died of a cardiovascular death (5 strokes, 7 sudden deaths, 8 HF, 2 endocarditis); the death rate per 1000 patient years was 2.1 (95% CI, 1.2–3.0). Ischemic heart disease developed in 30 women (2.7%; incidence rate per 1000 patient years: 2.9 [95% CI, 1.9–3.9]). Nonfatal and combined fatal and nonfatal HF occurred in 51 (4.7%) and 56 (5.1%) patients respectively (incidence rates per 1000 patient years: 5.0 [95% CI, 3.6–6.4] and 5.5 [95% CI, 4.0–6.9]). Out of the 56 HF cases, 29 were HF with preserved EF and 27 HF with reduced EF. The mean time from radiotherapy to development of HF was 5.4±3.1 years. There was no significant time difference between the occurrence of HF with preserved EF and HF with reduced EF (5.5±3.1 years versus 5.3±3.3 years; Mann–Whitney P=0.84). AF developed in 41 women (3.7%); the corresponding incidence rate per 1000 patient years was 5.9 (95% CI, 4.1–7.6).
Mortality and Cardiovascular Event Rates in Patients With Breast Cancer and Age‐ and Risk‐Matched FLEMENGHO Women
In order to adjust for the difference in baseline risk factors between the 2 cohorts, patients with BC were matched to FLEMENGHO participants for age (within 5 years), smoking status, hypertensive status, and the presence of diabetes. As a result, 904 patients with BC could be matched to 904 female FLEMENGHO participants. Mean age was 56.4±12.8 years in the patients with BC and 56.3±12.9 years in the corresponding controls. Mean difference in age between cases and controls was 0.17 years (5th to 95th percentile range: −0.74 to 2.21). The prevalence of smoking, hypertension, and diabetes was 35.2%, 35.0%, and 6.7% respectively. Characteristics of the matched cohorts can be found in Table 2.
Table 2.
Baseline Characteristics of Matched Cohort
| Cohort with breast cancer | FLEMENGHO cohort | P value | |
|---|---|---|---|
| No. of patients | 904 | 904 | |
| Age, y, mean±SD | 56.4±12.8 | 56.3±12.9 | 0.78 |
| Cardiovascular risk profile | |||
| Smoking, n (%) | 318 (35.2) | 318 (35.2) | NA: matched |
| Arterial hypertension, n (%) | 316 (35.0) | 316 (35.0) | NA: matched |
| Diabetes, n (%) | 61 (6.75) | 61 (6.75) | NA: matched |
| Hypercholesterolemia, n (%) | 187 (20.7) | 393 (43.5) | P<0.001 |
| Obesity, n (%) | 129 (14.7) | 151 (16.7) | 0.15 |
| History of cardiovascular disease, n (%) | 41 (4.54) | 36 (3.98) | 0.34 |
FLEMENGHO indicates Flemish Study on the Environment, Genes and Health Outcomes; NA, not applicable.
In patients with BC, total, noncardiovascular and cancer mortality was significantly increased, whereas cardiovascular mortality was significantly lower during the first decade of follow‐up (Figures 3A and 4). The incidence of ischemic heart disease did not differ between patients with BC and matched controls, yet nonfatal and combined fatal and nonfatal HF events occurred significantly more often in the studied patients with cancer (Figures 3B and 4). Finally, irradiated survivors with BC were more likely to develop AF during 10 years of follow‐up (Figure 4).
Figure 3. Incidence of mortality and heart failure in patients and controls.
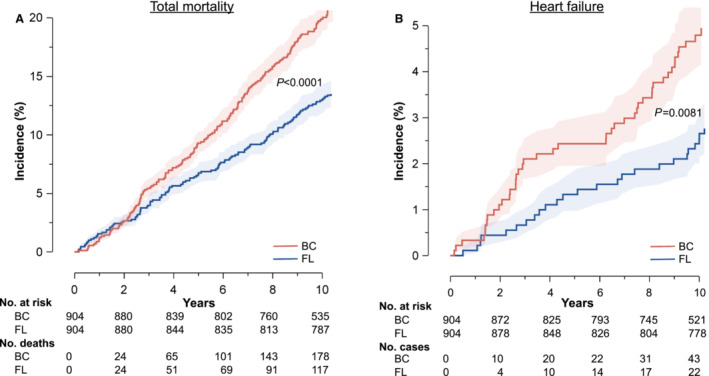
Cumulative incidence of all‐cause mortality (A) and heart failure (B) in 904 women enrolled in the cohort with breast cancer (BC) and 904 matched population controls selected from the Flemish Study on the Environment, Genes and Health Outcomes (FL). Patients and controls were matched for age, smoking status, hypertension, and diabetes. Shaded areas indicate the 95% confidence bands of the inverse survival function. The P values denote the significance of the difference between patients and controls. The tables list the number of women at risk at 2‐year intervals in both cohorts.
Figure 4. Comparison of 10‐year risks in patients and controls.
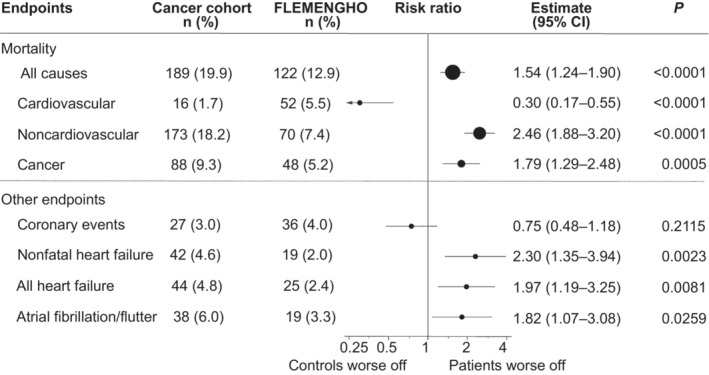
Forest plot comparing the 10‐year risks in 904 women enrolled in the breast cancer cohort and 904 matched population controls selected from FLEMENGHO (Flemish Study on the Environment, Genes and Health Outcomes). Patients and controls were matched for age, smoking status, hypertension, and diabetes. Estimates were derived by proportional hazard regression. Estimates for fatal cause‐specific outcomes accounted for the competing risk of alternative causes of death. Dots representing the point estimates have a size proportional to the number of events in the analysis. The horizontal lines denote the 95% CI. BC indicates breast cancer.
Risk Factors for Mortality and Cardiovascular Events Following Curative Treatment for Unilateral Breast Cancer
Because of markedly increased event rates in patients with BC compared with age and cardiovascular risk factor matched control subjects, we sought to identify patient‐, tumor‐, and treatment‐related factor predictive of mortality and MACE. In univariable analysis for unilateral BC (Table S3): age (1.057 [95% CI, 1.030–1.084]; P<0.001); tumor size, stage, and grade; nodal status; estrogen and progesterone receptor positivity; neoadjuvant treatment setting; mean heart dose; treatment with hormonal therapy and HER2 (human epidermal growth factor receptor 2) inhibitors; previous cardiovascular disease; and Mayo Clinic Cardiotoxicity Risk Score were associated with early mortality. Of note, left‐ or right‐sided BC and treatment with chemotherapy were not. In multivariable analysis (Table 3), only age (1.033 [95% CI, 1.006–1.061], P=0.016), tumor grade, and neoadjuvant treatment setting were associated with early mortality.
Table 3.
Multivariable Cox Regression for Survival, MACE, AF, and HF
| Tumor characteristics | Overall mortality | P value | MACE | P value | AF | P value | HF | P value |
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | Hazard ratio (95% CI) | |||||
| Left‐ versus right‐sided breast cancer | 0.881 (0.561–1.385) | 0.584 | 0.775 (0.428–1.404) | 0.401 | … | … | … | … |
| Tumor size | 0.722 (0.455–1.171) | 0.187 | 0.847 (0.432–1.661) | 0.629 | … | … | 0.611 (0.239–1.564) | 0.304 |
| Nodal status | 1.237 (0.866–1.767) | 0.242 | 1.594 (0.937–2.710) | 0.085 | 1.765 (0.717–4.344) | 0.216 | 1.460 (0.736–2.895) | 0.279 |
| Tumor stage | 1.370 (0.715–2.627) | 0.343 | 0.788 (0.322–1.931) | 0.603 | 0.973 (0.258–3.671) | 0.968 | 1.134 (0.388–4.445) | 0.661 |
| Tumor grade | 1.739 (1.166–2.591) | 0.007 | 1.306 (0.811–2.103) | 0.273 | … | … | 1.936 (0.955–3.924) | 0.067 |
| Estrogen receptor positive | 0.854 (0.410–1.780) | 0.674 | 2.053 (0.533–7.904) | 0.296 | … | … | … | … |
| Progesterone receptor positive | 0.591 (0.322–1.084) | 0.890 | 1.891 (0.741–4.829) | 0.183 | … | … | … | … |
| HER2 receptor positive | 0.921 (0.219–3.862) | 0.910 | 0.098 (0.002–6.248) | 0.273 | … | … | … | … |
| Treatment characteristics | ||||||||
| Treatment setting (neoadjuvant vs adjuvant) | 2.782 (1.304–5.936) | 0.008 | 2.754 (0.815–9.306) | 0.103 | 3.032 (0.510–18.020) | 0.223 | … | … |
| Irradiation mediastinal and subclavian nodes | 0.764 (0.224–2.616) | 0.669 | 0.379 (0.082–1.747) | 0.214 | … | … | … | … |
| Mean heart dose | 1.048 (0.996–1.103) | 0.072 | 1.093 (1.024–1.167) | 0.007 | 1.113 (0.999–1.240) | 0.053 | 1.100 (0.978–1.237) | 0.113 |
| Treatment with hormonal therapy | … | … | … | … | … | … | … | … |
| Treatment with chemotherapy | 0.702 (0.151–3.264) | 0.651 | 0.062 (0.008–0.484) | 0.007 | … | … | … | … |
| Treatment with HER2 inhibitors | 0.404 (0.082–1.981) | 0.264 | 0.880 (0.013–58.724) | 0.952 | … | … | … | … |
| Cardiovascular risk factors | ||||||||
| History of cardiovascular disease | 1.182 (0.547–2.554) | 0.670 | 2.386 (1.096–5.197) | 0.029 | 1.783 (0.351–9.064) | 0.486 | 3.205 (1.006–10.208) | 0.049 |
| Mayo Clinic Cardiotoxicity Risk score | 1.116 (0.772–1.614) | 0.559 | 2.664 (1.625–4.367) | 0.001 | … | … | 1.305 (0.980–1.738) | 0.069 |
Multivariable Cox regression for overall survival, MACE, AF, and HF in 1095 patients with unilateral breast cancer treated with radiation therapy in 2007 or 2008. AF indicates atrial fibrillation/flutter; HER2, human epidermal growth factor receptor 2; HF, heart failure; and MACE, major adverse cardiac event.
For the composite end point of cardiovascular mortality and MACE (Table S4), age (1.065 [95% CI, 1.046–1.085]; P<0.001), tumor size and stage, nodal status, mean heart dose, treatment with HER2 inhibitors, and history of cardiovascular disease were risk factors in univariable analysis. In multivariable analysis (Table 3), age (1.053 [95% CI, 1.013–1.093]; P=0.008), mean heart dose, history of cardiovascular disease, and Mayo Clinic Cardiotoxicity Risk Score were associated with MACE. Chemotherapy emerged as a protective factor, yet this was because patients treated with chemotherapy were significantly younger than those without (51.7±11.2 compared with 61.1±11.6; P<0.001). Mortality and adverse cardiovascular event rates did not differ between patients treated for left‐sided versus right‐sided BC (Table S5). Patients receiving anthracycline containing chemotherapy were more likely to die from cancer and less likely from cardiovascular causes compared with patients receiving chemotherapy that did not contain anthracycline. In addition, AF was less likely to occur following anthracycline chemotherapy whereas nonfatal and combined fatal and nonfatal HF rates were similar in both subgroups (Table S6).
Age (1.072 [95% CI, 1.037–1.107]; P<0.001), tumor stage, nodal status, neoadjuvant treatment, mean heart dose, and history of cardiovascular disease were associated with the development of new‐onset AF in univariable analysis (Table S7), yet none remained predictive of new‐onset AF in multivariable analysis (Table 3). There was a trend toward higher AF incidence with higher mean heart doses (P for trend 0.021 and 0.017 with mean heart dose respectively divided in tertiles and quantiles).
For HF, age (1.057 [95% CI, 1.030–1.084]; P<0.001); tumor size, stage, and grade; nodal status; mean heart dose; history of cardiovascular disease; and Mayo Clinic Cardiotoxicity Risk Score were associated with its occurrence in univariable analysis (Table S8), yet only history of cardiovascular disease remained associated with HF in multivariable analysis (Table 3).
DISCUSSION
Consistent with prior studies, our study confirmed that cancer‐associated death is the most common cause of death during the first 10 years following BC treatment (49.1%), whereas cardiovascular death was the second most common retrievable cause of death, albeit much less frequent (10.1%). 14 , 15 Apart from age, histological tumor grade and neoadjuvant treatment setting were risk factors. We observed a significantly lower cardiovascular mortality in the cohort with BC as compared with the FLEMENGHO controls (P<0.001). We hypothesize that this is attributable to a competing risk with oncological death combined with a short follow‐up period, as previously reported, in the cohort with BC. 15 , 16 , 17
Aside from assessing (cardiovascular) mortality, this study also focused on MACE after radiotherapy within the first 10 years of follow‐up. Cardiovascular morbidity is an important end point for survivors of cancer, because of its impact on quality of life as well as on additional future oncologic treatment possibilities. 8 Our most important finding included a significantly greater risk of developing HF and AF within the early postirradiation period, commonly regarded as a time window low in cardiovascular complications. The higher incidence of HF is in line with the recently published results of the Pathways Heart Study. 18 BC is highly prevalent and has a major impact on society. Our findings suggest that cardiovascular morbidity in the first decade after radiotherapy might be more common than currently appreciated. This suggests that early dedicated cardiological follow‐up of patients after radiotherapy might be useful. Chemotherapy and thoraco‐abdominal surgery for cancer have previously been reported as a risk factor for the development of AF 5 ; our data also implicate thoracic irradiation as a risk factor. Given the burden of disease associated with AF, our findings deserve validation in other cohorts. 19 Its occurrence beyond active treatment argue for atrial fibrosis rather than inflammation as a causative mechanism, yet mechanistic studies are essential to elucidate its pathophysiology and eventual therapeutic approaches. For now, our data indicate the need for active surveillance for AF. As a consequence, clinicians should probably have a lower threshold for advanced rhythm monitoring using state‐of‐the‐art wearables or remote monitoring technology within the first decade following radiation therapy for BC. The risk of coronary artery disease was not increased in our patients with BC compared with a control population and is in contrast with an earlier report of increased risk of ischemic heart disease in women after radiation therapy, beginning within 5 years after exposure. 20 Additional subgroup analyses in our study showed similar rates of coronary artery disease, HF, and AF when irradiated for left‐sided versus right‐sided BC. Previous research on radiotherapy, ischemic heart disease, and tumor laterality produced mixed results beyond 10 years of follow‐up, whereas HF was shown to be unaffected by tumor laterality in up to 15 years of follow‐up. 21 , 22 , 23
Although age is a common and strong risk factor for cardiovascular diseases and cancer in the general population, it has already previously been withheld as a risk factor for the development of cardiovascular diseases following successful cancer treatment. 24 , 25 , 26 Previous cardiovascular disease also emerged as a risk factor for MACE in our study. 27 Cancer treatment is gradually being considered a new, albeit nonclassical, bonafide cardiovascular risk factor, and the preferential occurrence of new MACE in patients already affected by cardiovascular disease is in line with previous research. 24 , 28
The success of newly developed cancer therapies has led to an increased age of survivors of cancer with a parallel increase in age‐related cardiovascular diseases. In addition, cancer treatments can induce debilitating cardiovascular side effects. Hence, screening for synchronic and metachronic cardiovascular diseases in patients with cancer and survivors has become an integral part of state‐of‐the‐art cancer care. 8 Guidance on whom to focus for screening efforts is nowadays broadly defined in guidelines and position papers from both cardiological and oncological societies yet urgently needs more specific corroborating data, such as ours. 29 Our study is the first to show an association between the Mayo Clinic Cardiotoxicity Risk score and the occurrence of MACE in a cohort with BC following radiation therapy. Furthermore, our data confirm the usefulness of this score beyond the active cancer treatment window, at least during the early follow‐up phase (<10 years) following irradiation. Further research is required to assess its lasting cardiovascular risk prediction potential for midterm and long‐term survivors of cancer.
We recognize the limitations of our study. First, included patients were derived from 1 tertiary care cancer referral center. Whereas uniform patient and data management are advantages of this approach, this might have introduced bias, in particular deviation of the studied sample from the general population. Yet, the clinical characteristics of studied patients are consistent with earlier studies, supporting the generalizability and reliability of our results. 30 Second, radiation techniques are continuously evolving, minimizing exposure of healthy tissues to ionizing radiation. We intentionally analyzed the patient cohort irradiated in 2007 or 2008, to include 10 years of follow‐up and state‐of‐the‐art radiation techniques. Third, we could not reproduce certain risk factors reported by others, including the higher propensity to develop ischemic heart disease when irradiated for left‐sided versus right‐sided BC, 31 , 32 which may be accounted for by the shorter follow‐up time of our study. Fourth, AF incidence rate was derived from a subset of survivors of cancer, as electrocardiograms and long‐term electrocardiographic recordings were not available for all patients, which might have introduced bias. Fifth, despite the higher incidence of AF in irradiated cancer survivors, they suffered significantly less from stroke. These results should be interpreted with caution given the low overall incidence rate of stroke and the relatively short follow‐up time of our study. Sixth, matching with a cohort of healthy controls might have introduced bias, such as a lower detection rate of new onset AF and HF, owing to a lower number of visits to health care providers. Finally, proportional hazard assumptions were respected for all variables, except for estrogen receptor positivity in the Cox multivariable model for mortality (see Figure S4).
CONCLUSIONS
Mortality within 10 years following curative treatment including radiation therapy for unilateral BC was mainly attributable to cancer death. Despite the competing risk of cancer death, HF and AF were already common within the first decade of follow‐up. This should encourage early dedicated cardiological surveillance and timely introduction of targeted anticoagulant, antiarrhythmic, and evidence‐based HF therapies.
Sources of Funding
None.
Disclosures
Stefan Janssens is holder of a named chair at the University of Leuven, financed by AstraZeneca. Lucas Van Aelst received the KULeuven Clinical Research and Training Board starting grant in 2018. Lucas Van Aelst and Johanna Jacobs received research grants from the Foundation for Cardiac Surgery (2021) and Belgian Heart Foundation (2022). Johanna Jacobs received a travel grant from Research Foundation–Flanders to present these data at the European Society of Cardiology congress 2022. Joerg Herrmann is holder of a grant from the National Institutes of Health/National Cancer Institute (CA233610), and receives royalties from book sales from Elsevier and consulting fees from Pfizer. He also has a pending patent for artificial intelligence‐ECG for the detection of myocarditis. He is part of the International Cardio‐Oncology Society. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S8
Figures S1–S4
Acknowledgments
The Non‐Profit Research Association Alliance for the Promotion of Preventive Medicine (APPREMED) received a nonbinding grant from Omron Healthcare, Ltd., Co., Kyoto, Japan. Lutgarde Thijs and Lucas N. L. Van Aelst designed the tables and figures. Johanna E. J. Jacobs, Joerg Herrmann, Jan A. Staessen, Stefan Janssens, and Lucas N. L. Van Aelst contributed to study design. Johanna E. J. Jacobs, Wouter L'Hoyes, Lieselotte Lauwens, Hans Wildiers, Patrick Neven, and Caroline Weltens contributed to data collection. Johanna E. J. Jacobs, Lieselotte Lauwens, Lutgarde Thijs, Jan A. Staessen, and Lucas N. L. Van Aelst contributed to data analysis. Jan A. Staessen initiated and coordinated the FLEMENGHO study. Lutgarde Thijs did the annual updates of the FLEMENGHO database. Caroline Weltens, Joerg Herrmann, Lutgarde Thijs, Jan A. Staessen, Stefan Janssens, and Lucas N. L. Van Aelst contributed to data interpretation. Johanna E. J. Jacobs, Jan A. Staessen, Stefan Janssens, and Lucas N. L. Van Aelst wrote the article. Johanna E. J. Jacobs, Marius Brusselmans, and Yu‐Ling Yu performed additional analyses for the revision. All authors contributed to critical review of the article, both in the original version as well as the revision.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027855
For Sources of Funding and Disclosures, see page 10.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Thompson MK, Poortmans P, Chalmers AJ, Faivre‐Finn C, Hall E, Huddart RA, Lievens Y, Sebag‐Montefiore D, Coles CE. Practice‐changing radiation therapy trials for the treatment of cancer: where are we 150 years after the birth of Marie Curie? Br J Cancer. 2018;119:389–407. doi: 10.1038/s41416-018-0201-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haussmann J, Corradini S, Nestle‐Kraemling C, Bölke E, Djiepmo Njanang FJ, Tamaskovics B, Orth K, Ruckhaeberle E, Fehm T, Mohrmann S, et al. Recent advances in radiotherapy of breast cancer. Radiat Oncol. 2020;15:71. doi: 10.1186/s13014-020-01501-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 1. J Am Coll Cardiol. 2017;70:2536–2551. doi: 10.1016/j.jacc.2017.09.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol. 2020;17:474–502. doi: 10.1038/s41569-020-0348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, Aboyans V, Asteggiano R, Galderisi M, Habib G, Lenihan DJ, Lip GYH, Lyon AR, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 7. Saiki H, Petersen IA, Scott CG, Bailey KR, Dunlay SM, Finley RR, Ruddy KJ, Yan E, Redfield MM. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, Herrmann J, Porter C, Lyon AR, Lancellotti P, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand JB, Ewer M, et al. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400 [DOI] [PubMed] [Google Scholar]
- 10. Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio‐oncology. Mayo Clin Proc. 2014;89:1287–1306. doi: 10.1016/j.mayocp.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stolarz‐Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, et al. Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA. 2011;305:1777–1785. doi: 10.1001/jama.2011.574 [DOI] [PubMed] [Google Scholar]
- 12. Zhang ZY, Marrachelli VG, Yang WY, Trenson S, Huang QF, Wei FF, Thijs L, Van Keer J, Monleon D, Verhamme P, et al. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a population study. Eur J Prev Cardiol. 2019;26:22–32. doi: 10.1177/2047487318797395 [DOI] [PubMed] [Google Scholar]
- 13. Fine JP, Gray RJ. A proportional hazards model for the sub distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 14. Patnaik JL, Byers T, DiGuiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253 [DOI] [PubMed] [Google Scholar]
- 16. Roychoudhuri R, Robinson D, Putcha V, Cuzick J, Darby S, Møller H. Increased cardiovascular mortality more than fifteen years after radiotherapy for breast cancer: a population‐based study. BMC Cancer. 2007;7:9. doi: 10.1186/1471-2407-7-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harris EE. Cardiac mortality and morbidity after breast cancer treatment. Cancer Control. 2008;15:120–129. doi: 10.1177/107327480801500204 [DOI] [PubMed] [Google Scholar]
- 18. Greenlee H, Iribarren C, Rana JS, Cheng R, Nguyen‐Huynh M, Rillamas‐Sun E, Shi Z, Laurent CA, Lee VS, Roh JM, et al. Risk of cardiovascular disease in women with and without breast cancer: the Pathways Heart Study. J Clin Oncol. 2022;40:1647–1658. doi: 10.1200/JCO.21.01736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio‐Thoracic Surgery (EACTS). Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 20. Darby SC, Ewertz M, McGale P, Bennet AM, Blom‐Goldman U, Brønnum D, Correa C, Cutter D, Gagliardi G, Gigante B, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 21. Doyle JJ, Neugut AI, Jacobson JS, Wang J, McBride R, Grann A, Grann VR, Hershman D. Radiation therapy, cardiac risk factors, and cardiac toxicity in early‐stage breast cancer patients. Int J Radiat Oncol Biol Phys. 2007;68:82–93. doi: 10.1016/j.ijrobp.2006.12.019 [DOI] [PubMed] [Google Scholar]
- 22. Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, Solin LJ. Late cardiac mortality and morbidity in early‐stage breast cancer patients after breast‐conservation treatment. J Clin Oncol. 2006;24:4100–4106. doi: 10.1200/JCO.2005.05.1037 [DOI] [PubMed] [Google Scholar]
- 23. Patt DA, Goodwin JS, Kuo YF, Freeman JL, Zhang DD, Buchholz TA, Hortobagyi GN, Giordano SH. Cardiac morbidity of adjuvant radiotherapy for breast cancer. J Clin Oncol. 2005;23:7475–7482. doi: 10.1200/JCO.2005.13.755 [DOI] [PubMed] [Google Scholar]
- 24. Abdel‐Qadir H, Austin PC, Lee DS, Amir E, Tu JV, Thavendiranathan P, Fung K, Anderson GM. A population‐based study of cardiovascular mortality following early‐stage breast cancer. JAMA Cardiol. 2017;2:88–93. doi: 10.1001/jamacardio.2016.3841 [DOI] [PubMed] [Google Scholar]
- 25. Derks MGM, Van de Velde CJH, Giardiello D, Seynaeve C, Putter H, Nortier JWR, Dirix LY, Bastiaannet E, Portielje JEA, Liefers GJ. Impact of comorbidities and age on cause‐specific mortality in postmenopausal patients with breast cancer. Oncologist. 2019;24:e467–e474. doi: 10.1634/theoncologist.2018-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blaes A, Prizment A, Koene RJ, Konety S. Cardio‐oncology related to heart failure: common risk factors between cancer and cardiovascular disease. Heart Fail Clin. 2017;13:367–380. doi: 10.1016/j.hfc.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dhindsa DS, Sandesara PB, Shapiro MD, Wong ND. The evolving understanding and approach to residual cardiovascular risk management. Front Cardiovasc Med. 2020;7:88. doi: 10.3389/fcvm.2020.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hershman DL, Till C, Shen S, Wright JD, Ramsey SD, Barlow WE, Unger JM. Association of cardiovascular risk factors with cardiac events and survival outcomes among patients with breast cancer enrolled in SWOG clinical trials. J Clin Oncol. 2018;36:2710–2717. doi: 10.1200/JCO.2017.77.4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shelburne N, Adhikari B, Brell J, Davis M, Desvigne‐Nickens P, Freedman A, Minasian L, Force T, Remick SC. Cancer treatment‐related cardiotoxicity: current state of knowledge and future research priorities. J Natl Cancer Inst. 2014;106:1–9. doi: 10.1093/jnci/dju232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Poortmans PM, Weltens C, Fortpied C, Kirkove C, Peignaux‐Casasnovas K, Budach V, van der Leij F, Vonk E, Weidner N, Rivera S, et al. Internal mammary and medial supraclavicular lymph node chain irradiation in stage I‐III breast cancer (EORTC 22922/10925): 15‐year results of a randomised, phase 3 trial. Lancet Oncol. 2020;21:1602–1610. doi: 10.1016/S1470-2045(20)30472-1 [DOI] [PubMed] [Google Scholar]
- 31. Bouillon K, Haddy N, Delaloge S, Garbay JR, Garsi JP, Brindel P, Mousannif A, Lê MG, Labbe M, Arriagada R, et al. Long‐term cardiovascular mortality after radiotherapy for breast cancer. J Am Coll Cardiol. 2011;57:445–452. doi: 10.1016/j.jacc.2010.08.638 [DOI] [PubMed] [Google Scholar]
- 32. Darby SC, McGale P, Taylor CW, Peto R. Long‐term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: prospective cohort study of about 300,000 women in US SEER cancer registries. Lancet Oncol. 2005;6:557–565. doi: 10.1016/S1470-2045(05)70251-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S8
Figures S1–S4


