Immune checkpoint inhibitors (ICIs) have revolutionized the treatment of many cancers. Immune checkpoint inhibitors–associated myocarditis (ICI‐M) is one of the most serious complications of this innovant therapy, with reported fatality rate ranging from 25% to 40%, that manifests in 1% to 2% of cases. 1 Management consists of ICI discontinuation and the early introduction of high‐dose corticosteroids. 2 Because diagnosis of ICI‐M is challenging, the International Society of Cardio‐Oncology (IC‐OS) proposed a definition based on pathohistological findings or cardiac troponin (cTn) elevation associated with a combination of clinical criteria, including symptoms/signs, ventricular arrhythmia, conduction disorders, left ventricular dysfunction, other immune related adverse events, and cardiac magnetic resonance (CMR) imaging abnormalities. 3 Although it has been recently endorsed by the European Society of Cardiology Guidelines of Cardio‐Oncology, 2 this definition is empirical and has never been evaluated. We aimed to assess the diagnostic value of the IC‐OS definition and to determine whether addition of new criteria would improve its performance.
Between May 2017 and November 2021, all patients with suspected ICI‐M were consecutively included in a multicenter registry. The study was approved by each institutional review board, and informed consent was obtained. The final diagnosis of ICI‐M was adjudicated from the patient's electronic medical record 1 month after discharge by 2 independent experts. In cases of disagreement, a third expert had to decide. Experts were cardiologist working in the reference cardio‐oncology centers with a skill in cardio‐oncology for >4 years. They were not aware of the IC‐OS definition, because it was published in December 2021, and the last patient in the study was included in November 2021. The presence or absence of each criterion of the IC‐OS definition was retrospectively defined at admission thereafter. As recommended, the definition was fulfilled in case of typical pathohistological findings or when cTn elevation was associated with either 1 major criterion or 1 minor criterion. 3
The data that support the findings of this study are available from the corresponding author upon reasonable request. Clinical characteristics were compared according to the ICI‐M status using classic statistical tests. Diagnostic accuracy of modified versions of the IC‐OS criteria was assessed in comparison with the gold standard. Sensitivity, specificity, positive and negative likelihood ratios, and positive and negative predictive values were estimated with their 95% CIs.
Sixty‐eight patients (69 years of age [interquartile range, 61–76 years]) with suspected ICI‐M were included in the study after excluding 1 with acute coronary syndrome and 3 who did not receive cardiac magnetic resonance imaging. The diagnosis of ICI‐M was met in 45 patients (Figure [A]). The sensitivity, specificity, and positive and negative likelihood ratios of the IC‐OS definition were 93% (95% CI, 82%–99%), 70% (95% CI, 47%–87%), 3.1 (95% CI, 1.7–5.7), and 0.1 (95% CI, 0–0.3), respectively. Between the ICI‐M and no‐ICI‐M groups, there was no difference in sex, with 62% and 74% (P=0.336) men, and in the number of cardiovascular risk factors, with 76% and 61% (P=0.201) with at least 2 cardiovascular risk factors, respectively. Compared with patients without ICI‐M, patients with ICI‐M had more frequently concomitant myositis (P=0.002) or myasthenic syndrome (P=0.023), myocardial edema (P<0.001), nonischemic myocardial injury (P<0.001) on cardiac magnetic resonance imaging, and higher levels of cTn (P<0.001) and creatine kinase (P=0.027) at admission. The median time to onset of first events/symptoms from the initiation of ICI treatment was the only covariate that was significantly associated with the final diagnosis of ICI‐M (P=0.03) and did not fit the IC‐OS definition. The 3‐month cutoff value was significant (P=0.03). Thus, different scenarios, including this covariate as a new minor criterion, were tested to improve the specificity without decreasing the sensitivity of the IC‐OS definition. Adding this criterion to the definition and considering 1 major or 3 minor criteria to define ICI‐M, the specificity increased from 70% to 83%, whereas the sensitivity remained at 93% (Figure [B]). The positive likelihood ratio also increased from 3.1 to 5, whereas the negative likelihood ratio remained at 0.1. The positive predictive value increased from 86% to 91%, and the negative predictive value increased from 85% to 86%.
Figure . Study flowchart (A) and key results (B).
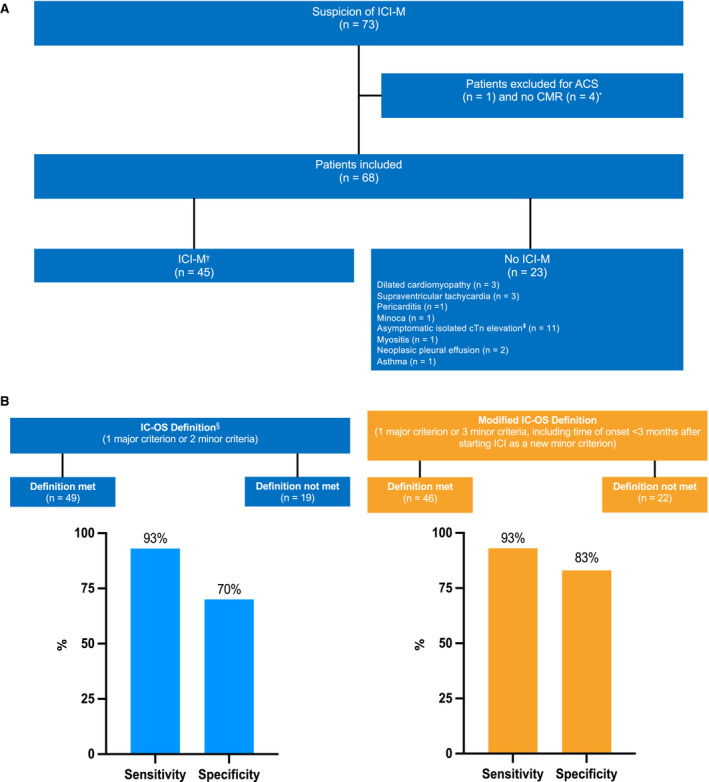
*Patients were excluded in case of infectious myocarditis (since the knowledge of COVID‐19, all patients were tested for COVID‐19 and were negative), acute coronary syndrome confirmed by an acute coronary artery occlusion during angiography, and if CMR could not be performed (because of contraindications, claustrophobia, and image artifact). †To establish this diagnosis, the experts used the recommendations and consensus on myocarditis, and a consensus report specific to myocarditis induced by cancer therapies. ‡Of the 11 patients with asymptomatic isolated cTn elevation, 4 patients had coronary artery disease, and 7 patients did not have coronary artery disease. §Diagnosis was based on cTn elevation with 1 major criterion (modified L‐L Criteria 2/2) or 2 minor criteria among clinical syndromes, decline in cardiac function, ventricular arrhythmia and/or new conduction system disease, other immune‐related adverse events, or suggestive magnetic resonance imaging (modified L‐L criteria 1/2). ACS indicates acute coronary syndrome; CMR, cardiac magnetic resonance; cTn, cardiac troponin; ICI, immune checkpoint inhibitor; ICI‐M, immune checkpoint inhibitor‐associated myocarditis; IC‐OS, International Cardio‐Oncology Society; and Minoca, myocardial infarction with no obstructive coronary artery disease.
Our work shows for the first time that the IC‐OS definition has a high sensitivity but a moderate specificity that may lead to a misdiagnosis of ICI‐M and thus potentially worsen the cancer prognosis due to unnecessary interruption of ICI therapy. Adding time of onset of first events/symptoms <3 months after the initiation of ICIs as a new minor criterion allowed for improving specificity. This criterion is relevant because, in pharmacovigilance analyses, the chronological criteria is a discriminating argument to confirm causality between the intake of a drug and the occurrence of an adverse event. Moreover, in many other studies, most of the IC‐M cases occurred within this time. 1 This study has limitations, including its small sample size and retrospective design. We did not choose endomyocardial biopsy results as the gold standard because its sensitivity remains imperfect (≈60%) and depends on many parameters, especially when myocarditis injuries are minor. 4 Finally, the diagnostic performance of CMR is significantly inferior for ICI‐M compared to other types of myocarditis, and the ideal timing for its utilization in this context remains uncertain. 5
In conclusion, the IC‐OS definition for the diagnosis of ICI‐M showed an excellent sensitivity of 93% but a moderate specificity of 70%, which might be improved by both increasing the number of minor criteria needed to fulfill the definition and adding the time to onset of first events/symptoms <3 months after the initiation of ICIs as a minor criterion. More powerful studies will be needed to confirm the modified IC‐OS definition.
Sources of Funding
This work was supported by the Fondation Coeur et Recheche, Fédération Française de Cardiologie and Assistance Publique–Hôpitaux de Marseille.
Disclosures
F.T. has received support for attending meetings from Astra‐Zeneca and Boerhinger‐Ingelheim. C.D. has received grants and consulting fees from Bioserenity. J.A. has received grants and consulting fees from Bioserenity, Bayer, and Biotronik; consulting fees from Bayer, Novartis, Bristol‐Myers Squibb, Astra‐Zenzca, Servier, Boerhinger‐Ingelheim, Biotronik, and Bioserenity; honoraria for lectures from Bayer, Novartis, and Astra‐Zeneca; and support for attending meetings from Bayer and Bristol‐Myers Squibb. J.C. has received honoraria for lectures from Janssen, Novartis, Pfizer, and Bayer; consulting fees from Boerhinger‐Ingelheim and Janssen; and support for attending meetings from Bayer and Pfizer. The remaining authors have no disclosures to report.
This article was sent to Tochukwu M. Okwuosa, DO, Associate Editor, for review by expert referees, editorial decision, and final disposition.
F. Thuny and J. Cautela contributed equally.
For Sources of Funding and Disclosures, see page 3.
Contributor Information
Franck Thuny, Email: franck.thuny@gmail.com.
Jennifer Cautela, Email: jennifer.cautela@ap-hm.fr.
REFERENCES
- 1. Thuny F, Naidoo J, Neilan TG. Cardiovascular complications of immune checkpoint inhibitors for cancer. Eur Heart J. 2022;43:4458–4468. doi: 10.1093/eurheartj/ehac456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lyon AR, López‐Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler‐Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, et al. 2022 ESC Guidelines on cardio‐oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio‐Oncology Society (IC‐OS). Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244 [DOI] [PubMed] [Google Scholar]
- 3. Herrmann J, Lenihan D, Armenian S, Barac A, Blaes A, Cardinale D, Carver J, Dent S, Ky B, Lyon AR, et al. Defining cardiovascular toxicities of cancer therapies: an International Cardio‐Oncology Society (IC‐OS) consensus statement. Eur Heart J. 2022;43:280–299. doi: 10.1093/eurheartj/ehab674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Palaskas NL, Segura A, Lelenwa L, Siddiqui BA, Subudhi SK, Lopez‐Mattei J, Durand JB, Deswal A, Zhao B, Maximilian Buja L, et al. Immune checkpoint inhibitor myocarditis: elucidating the spectrum of disease through endomyocardial biopsy. Eur J Heart Fail. 2021;23:1725–1735. doi: 10.1002/ejhf.2265 [DOI] [PubMed] [Google Scholar]
- 5. Cadour F, Cautela J, Rapacchi S, Varoquaux A, Habert P, Arnaud F, Jacquier A, Meilhac A, Paganelli F, Lalevee N, et al. Cardiac MRI features and prognostic value in immune checkpoint inhibitor‐induced myocarditis. Radiology. 2022;303:512–521. doi: 10.1148/radiol.211765 [DOI] [PubMed] [Google Scholar]


