ABSTRACT
Racial disparities in cardiovascular disease are unjust, systematic, and preventable. Social determinants are a primary cause of health disparities, and these include factors such as structural and overt racism. Despite a number of efforts implemented over the past several decades, disparities in cardiovascular disease care and outcomes persist, pervading more the outpatient rather than the inpatient setting, thus putting racial and ethnic minority groups at risk for hospital readmissions. In this article, we discuss differences in care and outcomes of racial and ethnic minority groups in both of these settings through a review of registries. Furthermore, we explore potential factors that connote a revolving door phenomenon for those whose adverse outpatient environment puts them at risk for hospital readmissions. Additionally, we review promising strategies, as well as actionable items at the policy, clinical, and educational levels aimed at locking this revolving door.
Keywords: cardiovascular disease, racial, disparities
Subject Categories: Cardiovascular Disease, Race and Ethnicity, Disparities, Health Equity, Social Determinants of Health
Nonstandard Abbreviations and Acronyms
- ADHERE
Acute Decompensated Heart Failure National Registry
- ARTEMIS
Affordability and Real‐World Antiplatelet Treatment Effectiveness After Myocardial Infarction Study
- CHAMP‐HF
Change the Management of Patients With Heart Failure
- FAITH
Faith‐Based Approaches in the Treatment of Hypertension
- FL‐PR CReSD
Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities
- GWTG
Get With The Guidelines
- HRRP
Hospital Readmissions Reduction Program
- MESA
Multi‐Ethnic Study of Atherosclerosis
- MI‐FREE
Full Coverage for Preventive Medications After Myocardial Infarction
- PINNACLE
Practice innovation and clinical excellence
- REACH
Reduction of Atherothrombosis for Continued Health
- SDOH
social determinants of health
- SOL
Study of Latinos
Scope of the Problem: The Revolving Door
It has been nearly 4 decades since the landmark Heckler report highlighted the yearly excess deaths, predominantly in Black people, and emphasized the elimination of health care disparities as a national priority. 1 In 2003, the Institute of Medicine Report “Unequal Treatment Confronting Racial and Ethnic Disparities in Health Care” defined disparities as “racial or ethnic differences in the quality of health care that are not due to access‐related factors, or clinical needs, preferences, and appropriateness of intervention”. 2 This definition highlights that disparities in care across race and ethnicity are not caused by inherent biological differences but instead result from social determinants of health (SDOH) and systemic structural racism, making them both avoidable and unnecessary.
In 2011, the Centers for Disease Control reported that the life expectancies for Black men and women were more than 4 and 3 years shorter, respectively, when compared with those of White men and women; further analysis showed that the greatest contributor to this racial mortality gap was cardiovascular disease (CVD). 3 Paradoxically, the past 40 years have witnessed an explosive growth in the medical armamentarium against CVD, especially in heart failure (HF), myocardial infarction (MI), and stroke care. Treatments once considered advanced, such as percutaneous coronary interventions, coronary artery bypass surgery, implantation of permanent pacemakers, and defibrillators are widespread and newer technologies have now become the standard of care. In parallel with these advances, public health campaigns have led to impressive reductions in certain behaviors that increase CVD risk such as tobacco use, which in turn have translated into a decline in CVD in the past few decades. However, these benefits are not equally shared among all Americans, and the COVID‐19 pandemic has harshly exposed this reality.
A number of efforts have been implemented with varying degrees of success since the aforementioned Institute of Medicine Report documented differences in CVD care across race and ethnic groups. 2 These efforts have ranged from implementation of prospective registries across practices emphasizing the importance of race and ethnicity data collection, system of care platforms with clinical decision support tools, attainment of specific benchmarks, and dissemination of quality improvement and education tools through various medical societies and organizations. One example of this was the American College of Cardiology coalition to reduce racial and ethnic disparities in CVD outcomes (Credo), which launched several initiatives built on evidence‐based principles such as performance measure–based quality improvement, provider cultural competency training, team‐based care, and patient education. 4
The implementation of prospective registries across practices, albeit imperfect, has allowed us to analyze patterns of care and adherence to evidence‐based benchmarks over time and across racial and ethnic groups. A signal towards narrowing of disparities of CVD care and outcomes in racial and ethnic minority groups compared with White patients has unfolded over the past decade. This is more evident in inpatient registries, though important gaps still remain. Unfortunately, outpatient registries remain sparse in comparison to inpatient ones and show persisting CVD care disparities without clear narrowing among the groups experiencing disproportionate impact. 5 , 6 , 7 The 3 to 4 times higher readmission rates for HF and the widened gap in 30‐day post‐MI rehospitalization rates affecting Black patients is a testament that significant disparities remain and these extend beyond hospital walls.
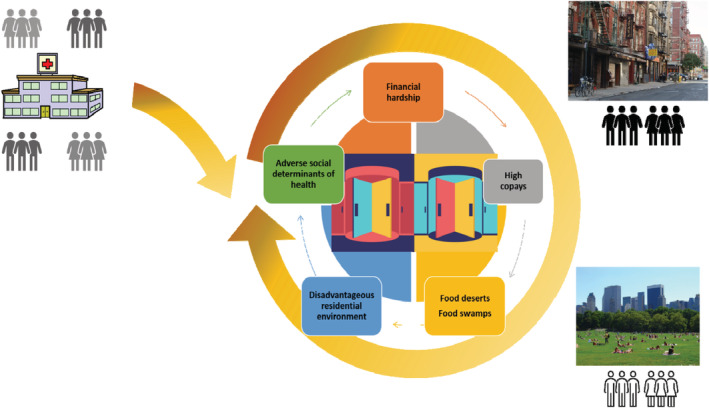
This review describes disparities in CVD care and outcomes using a historical and holistic framework. We review the landscape of the racial and ethnic groups affected, highlight the heterogeneity across groups, examine the results of inpatient and outpatient registries, highlighting where disparity care gaps are closing and contrasting with areas where significant gaps remain. As we review these data, from the care, outcomes, and rehospitalization perspective, a disturbing “revolving door” concept emerges: that while gaps in CVD care of our groups that have been marginalized are narrowing in the inpatient setting, they widen once more in the outpatient one. The environment from which our diverse patients experiencing disadvantage come may not determine their future CVD outcome so much as the one they return to (Figure 1). Locking this “revolving door” or stopping this vicious cycle of inequality will require an integrative, multipronged, interdisciplinary approach that utilizes promising strategies aimed at closing clinical, research, and policy gaps.
Figure 1. The revolving door concept: while cardiovascular disparities in the inpatient setting have narrowed, they widen after crossing the hospital door.
Landscape of Racial and Ethnic Groups Affected
Well‐established waves of migration have been recognized across North American history, including well before the founding of the United States. The history of individual racial and ethnic group immigration and their subsequent minoritization (and sometimes deminoritization) have shaped the nation's public health context, one that is fluid and constantly changing. Herein we review this public health context, which has broad implications for affected racial and ethnic groups.
After centuries of persecution and discrimination, Indigenous people face significant social and economic barriers, with 1 in 4 being below the poverty line; this is the highest poverty rate of any major racial group. 8 Per the 2020 US Census Bureau, Indigenous people (categorized as American Indian and Alaska Native) represent 9.7 million (2.9% of the population). Despite contemporary acknowledgement of treaties including the provision of health care by the federal US government in exchange for land, mistrust, lack of intercultural integration, and underfunding of Indian Health Services among other factors have affected the CVD care of Indigenous people. They face substantially higher CVD risk burden for tobacco, obesity, and hypertension and are also 50% more likely to be diagnosed with coronary heart disease than non‐Hispanic White people. 8
Black people were forced to immigrate to the United States as slaves more than 5 centuries ago, and after being set free experienced segregation, lack of rights to property, voting, or education, in turn affecting their health care and outcomes. Black people represent 13.3% of the US population and have a significantly earlier onset of HF, stroke, and peripheral vascular disease, mostly attributable to hypertension, which affects up to 44% of this population, representing one of the highest rates in the world. 9
In the past 5 decades, the US immigrant population rose from 9.6 million in 1970 to 45 million in 2015. This considerable growth has been largely attributed to the immigration from Latin America and Asia. Today over half of all US immigrants are from Latin America and another 28% are from Asia. Among new immigrant arrivals, Asian immigrants outnumber Hispanic/Latino immigrants and are projected to become the largest immigrant group in the United States by 2055. 10
Foreign‐born US residents often display better health and lower mortality relative to US‐born groups of similar race and ethnicity, a finding known as the “immigrant advantage.” 11 Contributors to this may include positive health selection of immigrants from originating populations, negative health selection of ailing ones back to their countries of origin, supportive familial and social networks, and cultural differences in health‐associated behaviors among immigrant groups. 11 However, this advantage disappears over time as demonstrated in the MESA (Multi‐Ethnic Study of Atherosclerosis) immigrant cohort, where immigrants with shorter US residences (<10 years), despite better baseline CVD health and lower CVD event incidence than US‐born residents of similar race and ethnicity, experience the largest CVD health decline over time. A more pronounced cardiovascular health decline is seen among those <65 years of age at baseline and among Hispanic/Latino participants compared with Chinese ones. 12 This could be explained by acculturation, the process by which new cultural elements and engagement in specific behaviors, including lifestyle, diet, beliefs, and values of a new country, are acquired. This process has been associated with moderate and extreme obesity, increased smoking, hypertension, and diabetes, among Hispanic individual, 13 , 14 , 15 smoking and hypertension among Asian American individuals, 14 and poor or intermediate cardiovascular health among Guyanese and Haitian Afro‐Caribbean immigrants. 16
Heterogeneity in CVD Risk Burden Within Groups
Heterogeneity within racial and ethnic groups is often much greater than that between racial and ethnic groups. This is particularly relevant to Hispanic/Latino and Asian populations, 2 of the largest racial and ethnic groups in the United States.
The Hispanic/Latino population is composed of a mixture of subgroups, such as White, Afro‐Caribbean, and Mestizo, all of whom may self‐identify as Hispanic/Latino because of country of origin, language, culture, and value system preferences. This may predispose to dilution of biological differences, when taking all of them as a group, whereas focusing on 1 particular subgroup (eg, Mexican individuals, as most US studies have done in the past) may fail to capture the full burden of disease among these groups and result in false generalizations.
Two American Heart Association Scientific Advisory Statements have highlighted the importance of disaggregation within diverse Asian and Hispanic/Latino populations. 17 , 18 These groups differ vastly in atherosclerotic cardiovascular disease risk profiles, socioeconomic status, and acculturation. In turn, these differences result in highly variable rates of CVD and mortality that are often not considered in heart disease statistics. 19 Recent efforts to characterize some of these differences among Hispanic/Latino subgroups have demonstrated differences according to country of origin, with Mexicans having the highest prevalence of diabetes, and Puerto Ricans and Cubans having the highest prevalence of smoking. 20 , 21 Similarly, higher mortality rates for ischemic and hypertensive heart disease has been seen In Puerto Ricans, whereas Mexicans experience higher rates of cerebrovascular disease deaths. 19
The Asian‐American and Pacific Islander population is also incredibly diverse, with more than two‐thirds of them tracing their roots to more than 20 countries, each with unique languages, culture, and other characteristics. 22 South Asian populations, such as those from Bangladesh, Bhutan, India, Pakistan, Sri Lanka, and Nepal, have an early‐onset high CVD risk profile and proportional mortality when compared with other Asian groups and non‐Hispanic White groups. 23 , 24 While most studies in the United States focus on the Asian Indian subgroup, other subgroups at risk include Filipino‐American people, who have the highest prevalences of hypertension among the Asian‐American population, and elderly Chinese and Korean American individuals, who are thought to have poorer health statuses than national norms. Data on Asian‐American subgroups often overlook its diversity when this group is not disaggregated. 22
This heterogeneity within and between racial and ethnic groups highlights the importance of disaggregation by region or country of origin. The role of and interactions between acculturation, socioeconomic status, and also sex are critical to develop targeted preventive interventions.
Factors Contributing to CVD Care Disparities
Biologic Factors
Cardiovascular diseases are unquestionably affected by biologic, environmental, and societal factors; however, the contribution of these factors varies significantly among different populations. 25 It is important to recognize that overemphasis on biology as a major explanatory factor in health disparities can lead to an underestimation of other, far more substantial and plausible factors that contribute to disparities. Race is a socially constructed concept and not a biological factor, and reliance on biology also reinforces racial stereotyping, which may contribute to health disparities in the first place. 26 Traditional risk factors, namely diet, cigarette smoking, diabetes, and hypertension, are the strongest risk factors for coronary artery disease and peripheral arterial disease, 27 but statistical adjustment for these and other traditional risk factors does not completely eliminate the excess prevalence in Black and Hispanic/Latino populations when compared with White populations. 28 A plausible explanation for the inability to elucidate the excess prevalence of CVD in minority populations is an inability to completely account for the difficult‐to‐quantify adverse lived experiences that may contribute to negative health outcomes. These outcomes are more often present in marginalized communities that are more often made up of those from racial and ethnic minority backgrounds.
Clinical studies that have genotyped different groups have identified correlations between populations and CVD biomarkers, including inflammation, thrombosis, hypertension, lipid profiles, arrhythmia, and left ventricular mass. 29 , 30 Recent studies have performed genome‐wide association studies of Black populations for genetic relationships to cardiac risk factors including hypertension and biomarkers of inflammation. 29 , 30 Furthermore, the heritability of hypertension documented in adoption, twin, and family studies suggests that 15% to 35% of the correlation may be genetic. 31 For instance, Black boys and girls have higher blood pressure levels and a higher prevalence of hypertension (13.8% in Black versus 8.4% in non‐Hispanic White and 10.4% in Hispanic/Latino populations). 32 Findings from the Bogalusa Heart Study indicate that higher blood pressure levels during childhood track into elevated blood pressure in adults. 33 These differences persist into older ages, as evidenced by the MESA study, in which the odds of hypertension were 1.5 times higher in Black than in White people through age 75 years. 34
In the MESA study, the adjusted odds for incident peripheral arterial disease were 1.67 times higher in Black compared with White subgroups. 35 The San Diego Population Study included markers of inflammation in multivariable models to evaluate whether they explained the residual excess risk in Black compared with White people, but found that, although the relative risk for peripheral artery disease between Black and White people were further attenuated, they remained statistically significantly higher, ranging from 1.5 to 2.0. 36 Findings from the SOL (Study of Latinos) showed that Hispanic/Latino groups are different with respect to the presence of peripheral arterial disease. When compared with the Mexican American population, the Cuban American population had a more than 3‐fold higher odds for the presence of peripheral arterial disease, and this was independent of sociodemographic and traditional cardiovascular risk factors. 37
The aforementioned studies must be understood within the previously discussed concept that the lived experiences of individuals are incompletely captured within a narrow set of biological factors or biomarkers. The future of disparity research seeks to move beyond observations that increased density of biologic factors associated with race explain disparities in outcomes and into an era where the adversity of lived experiences is part of the research methods (ie, by including SDOH as explanatory variables).
Societal and Environmental Factors
The societal and cultural environment within which racial and ethnic minority groups live can accentuate disparities in CVD care, prevalence, and outcomes. Scientific societies have published statements addressing the social determinants of risk and outcomes of CVD. These recommendations (Figure 2) provide a comprehensive review of factors outside of genetics that contribute to cardiovascular health and directly affect the well‐being of groups that have been marginalized. Unfortunately, Black, Hispanic/Latino, and other racial and ethnic minority groups face an overabundance of adverse structural, social, and environmental characteristics in the United States. Approximately 18.8% of Black and 15.7% of Hispanic populations live in poverty compared with 7.3% of non‐Hispanic White population and 10.5% of the overall population. Furthermore, the median family income is lower in Black and Hispanic/Latino households than in White ones. 38 As a result, preventive health resources such as healthy foods, safe spaces for physical activity, and psychological stability resulting from occupational stability, to name a few, are not as widely available to racial and ethnic minority groups.
Figure 2. Findings and actionable items from the American Heart Association scientific statement on social determinants of risk and outcomes for cardiovascular disease.
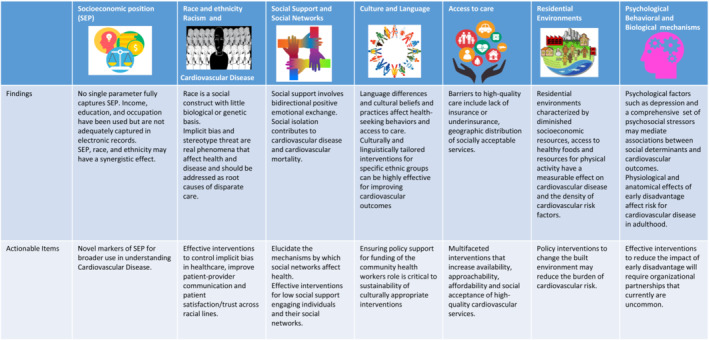
The multiple and unique sources of stress faced by groups that have been marginalized may help explain the persistent disparities in care and outcomes across the socioeconomic ranges among populations. Neighborhood‐related safety fears, job stress, socioeconomic concerns, and major life events combine with other salient sources of stress for the Black population—such as discrimination—to contribute to these disparities. 39 Perceived racial discrimination is known to be associated with all aspects of health, including hypertension, weight gain and obesity, persistent inflammation, and other subclinical processes, as well as incident cardiovascular events. 40 The potential for interventions that promote positive psychological health to reduce stress levels may be relevant in improving the health behaviors and ultimately health outcomes of racial and ethnic minority groups.
Bias may represent overt prejudice on the part of health providers or could be the result of subconscious perceptions. Several studies have shown that the race and sex of patients independently influence physicians' recommendations for the management and treatment of CVD, including referrals for cardiac procedures as well as advanced heart failure therapies. 41 In particular, Black women were significantly less likely to be referred for cardiac catheterization than White men, and Black race negatively influenced the decision‐making process for heart transplants. 41
The Revolving Door Foundations: Differences Between Inpatient and Outpatient Realities Through Registries
Inpatient Registries
The American Heart Association created the national Get With The Guidelines (GWTG) database in 2000, to serve not only as a prospective observational registry but also as a quality improvement initiative through which national advances in evidence‐based medicine might be measured. Participating hospitals submit clinical information on the history, care, and outcomes of cardiac inpatients via a standardized data set. This results in an ever‐growing, curated patient cohort that sheds light on temporal trends in evidence‐based care. 42 Subdivisions have appeared within the database to allow for disease‐specific analyses; these divisions include GWTG‐CAD (GWTG‐Coronary Artery Disease), as well as GWTG‐HF (GWTG‐Heart Failure).
From a racial and ethnic perspective, the GWTG databases hold particular value in understanding the applicability of, and national adherences to evidence‐based care across different groups. They also monitor outcomes within racial and ethnic minority inpatient populations in the United States. Hence, a report using the GWTG‐CAD database from 2002 to 2007 showed that among 142 953 patients with ST‐segment elevation–myocardial infarctions (STEMI) and non–ST‐segment‐elevation–myocardial infarctions. Black patients experienced the greatest delays in thrombolytic administration as well as the greatest median door‐to‐balloon time. These gaps decreased with time, with the last quarter of the registry analyzed revealing no significance at all in defect‐free care among Black, Hispanic/Latino, and non‐Hispanic White patients. 43 This is particularly notable given that Black and Hispanic/Latino patients are overall younger than non‐Hispanic White patients; and Black, Hispanic/Latino, and Asian patients have higher rates of hypertension, smoking, prior stroke, diabetes, obesity, or renal insufficiency. 43 , 44 , 45 Similarly, in the Acute Coronary Treatment and Intervention Outcomes Network‐GWTG database from 2007 to 2009, among 46 245 patients with STEMI, Hispanic/Latino patients experienced the same overall rates of in‐hospital evidence‐based interventions and received the same discharge therapies compared with non‐Hispanic White patients. 45 Using the GWTG‐CAD database from 2008 to 2011, few differences in acute medication use or invasive therapies among different racial and ethnic groups were found in 175 370 patients. 42 Other cardiovascular registries that stand apart from GWTG such as the ADHERE (Acute Decompensated Heart Failure National Registry) from 2001 to 2004 demonstrated that out of 135 734 patients, Black patients had lower in‐hospital mortality with shorter intensive care unit and overall hospital lengths of stay than non‐Hispanic White patients. They also received higher rates of angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, and β‐blocker prescriptions at discharge than non‐Hispanic Whites. 5
A subset of the GWTG‐Stroke registry termed the FL‐PR CReSD (Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities), which includes Florida and Puerto Rico hospitals that participate in the voluntary stroke registry, has shown findings similar to the other GWTG registries in terms of closing gaps of care and outcomes in the inpatient setting. 6 From 2010 to 2013, out of 44 013 patients with ischemic strokes, no racial disparities for in‐hospital mortality were found, yet Black patients had higher 30‐day readmission rates than non‐Hispanic White patients. 6 Similarly, from 2010 to 2014, out of 58 864 patients with ischemic stroke, Black patients were least likely to arrive to the hospital within 2 hours of symptom onset and demonstrated, as previously discussed, higher rates of chronic conditions such as hypertension and diabetes. 46 Initial racial and ethnic disparities in anti‐thrombotic use and smoking cessation counseling were no longer significant in the last year of follow‐up, indicating improvement in hospital adherence to evidence‐based guidelines. 46
Though these databases illustrate signals towards a narrowing of disparities, a few continue to persist. Thus, Black women receive lower rates of invasive/interventional procedures than White women when presenting with non–ST‐segment‐elevation–myocardial infarctions. 42 Though these differences were not associated with higher in‐hospital mortality or adverse clinical events, such a dramatic difference remains troubling. In comparison to the highly protocol‐driven STEMI management, non–ST‐segment‐elevation–myocardial infarctions management allows for more provider judgment, suggesting the possibility that inherent biases within the medical system may cause providers to serve a greater role as gatekeepers to therapy in patients with non–ST‐segment‐elevation–myocardial infarction. More recently, in an analysis of the National Inpatient Sample from 2006 to 2015, significant racial, ethnic, and sex differences exist in procedural utilization and clinical outcomes in patients with STEMI and cardiogenic shock, with women, Black, and Hispanic/Latino patients being less likely to undergo invasive cardiac procedures including revascularization and mechanical circulatory support and higher likelihood of death compared with White men. 7
Other areas of racial and ethnic disparity can be seen in the GWTG‐HF registry, where there was a lower β‐blocker prescription and smoking cessation counseling at discharge after acute coronary events in Black patients 47 and lower rates of cardiac rehabilitation prescription affecting mostly Asian patients. 48 In the same database, Black and Hispanic/Latino patients were also less likely to have implantable cardioverter defibrillators implanted despite robust evidence demonstrating that implantable cardioverter defibrillators result in up 30% reductions in hazard of death compared with conventional medical therapy. 49 Asian patients with HF with preserved ejection fraction were also found to have the highest inpatient mortality rate 44 among all of the racial and ethnic groups, a concerning departure—that is still poorly understood—from the generalized trend of lower in‐hospital mortality among racial and ethnic minority groups. The above may be indicative of systemic biases with CAD and HF treatments, which become clinically less pronounced by rigorous protocols but may re‐emerge whenever provider or system judgments play significant gatekeeping roles.
Outpatient Registries
The findings highlighted above provide a glimpse of improvement in equitable care in the inpatient environment. When it comes to the outpatient setting, worlds of difference exist outside of hospital doors that are difficult to measure, account for, and modify. Racial and ethnic minority groups remain chronically disadvantaged from lower health literacy, lack of safe environments for physical activity, and access to healthy foods. 43 , 48 , 50 They also experience greater difficulty in affording treatment, be it acute or ongoing, than non‐Hispanic White patients because of lower rates of insurance; and higher number of life‐saving delays such as longer time to arrival to the emergency room, leading to reperfusion delays beyond recommended windows. 45 For all of the efforts directed at inpatient guideline adherence, even the most optimal of health care will be unable to fully eliminate racial disparities in long‐term morbidity and mortality if the SDOH remain unchanged outside the hospital walls.
Unfortunately, outpatient registries remain sparse in comparison to inpatient ones. The PINNACLE (Practice Innovation and Clinical Excellence) registry, a database formulated by the American College of Cardiology, is the largest cardiology outpatient quality improvement registry that captures outpatient encounters from cardiovascular clinics across the United States. 51 Despite the magnitude of PINNACLE and robust number of publications coming from this data set, few have analyzed racial and ethnic differences or disparities in depth. One study analyzing compliance rates with performance measures among the first 14 000 outpatients enrolled in the PINNACLE program found no substantial racial or sex differences in compliance rates for key performance measures for 3 cardiac conditions (CAD, HF, and atrial fibrillation). 52 One of the few analyses that includes Asian people found that they were younger than non‐Hispanic White patients and had higher proportions of persistent/permanent atrial fibrillation as well as valvular atrial fibrillation, along with higher rates of diabetes, and chronic liver and kidney dysfunction. Despite the higher rates of valvular atrial fibrillation, Asian people were overall less likely to use warfarin and also less likely to undergo catheter ablation than White patients, despite guidelines recommending such ablation for younger patients.
Another outpatient registry, CHAMP‐HF (Change the Management of Patients with Heart Failure), found that Black patients had worse health status scores than non‐Hispanic White patients, and that Hispanic/Latino patients had worse health status scores even after multivariable adjustment. 53 Similarly, in the REACH (Reduction of Atherothrombosis for Continued Health) registry, an analysis of 2168 patients with peripheral arterial disease from 2003 to 2004 showed that Black patients had lower rates of statin use than non‐Hispanic White patients at study initiation, with differences disappearing at follow‐up at both year 1 and 2. 54 Black and Hispanic/Latino patients were found to be twice as likely to be incapacitated and unable to work, and also had higher rates of diabetes, obesity, and hypertension compared with non‐Hispanic White patients. 54 Finally, a retrospective study of Medicare fee‐for‐service patients with CAD, cared for at outpatient practices participating in the PINNACLE registry from January 2010 to 2015, found that practices that serve patients experiencing the highest socioeconomic disadvantage perform worse on some clinical outcomes, despite providing similar guideline‐recommended care as other practices. 55
Registries have limitations relating to completeness of the data they collect and variability in reporting and in quality. Limitations notwithstanding, registries can provide useful information by collecting real‐world data. They help estimate actual disease treatment patterns and outcomes, and also answer research questions that are not easily addressed by randomized clinical trials. These data are particularly important for generating research hypotheses.
Strict adherence to inpatient guidelines and evidence‐based protocols may further reduce disparities remaining among racial and ethnic groups with regard to CAD, HF, and stroke management. However, efforts to address the far starker disparities that exist within the community remain the biggest challenge. Improvements in outcomes noted in the past 2 decades are a clear indication that these processes and protocols are feasible. However, significant work remains to be done in both the inpatient and outpatient domains. The quest for equitable care must re‐focus on secondary prevention and continuity of care, along with directed interventions to reduce the burden of chronic conditions and cardiovascular risk factors that lead to hospitalization in the first place. Furthermore, hospitals that are able to participate in guideline‐based quality improvement are often larger and better‐resourced than smaller community hospitals. Hospitals that perform less well on health care quality measures tend to serve higher percentages of patients from underresourced communities, as highlighted in the study by Hanain‐Wynia et al, which showed that only 20% of patients in top‐performing hospitals versus 70% of patients in lower‐performing facilities were from racial and ethnic minority groups or groups experiencing disadvantages. 56 Already under‐resourced hospitals that struggle to meet guidelines can be further penalized by the unintended side effects of initiatives such as the Hospital Readmissions Reduction Program (HRRP), 57 which further reduces resources to already struggling institutions. This leads to a vicious cycle of hospital penalization and chronic under‐resourcing for the neediest of hospitals. 58 As demonstrated here, one of the greatest barriers to prioritizing prevention and reducing the gaps in communities that are underserved is our own health care system. The lack of reimbursement for prevention is linked to the societal focus on revenue‐generating procedural interventions in treating late‐stage disease. To make prevention a priority, and reduce disparities, we must re‐evaluate the current model of reimbursement for medical services and shift towards adequately reimbursing preventive services at primordial and primary prevention levels. 59 The ongoing cycle of increased CVD readmissions and mortality within racial and ethnic minority groups will not be solved until these revolving doors of unequal environments are addressed in a systematic comprehensive and multidimensional way.
Locking the Revolving Door: Promising Strategies
In this section we summarize a variety of strategies, including prospective studies and randomized clinical trials that have demonstrated the potential to reduce CVD care disparities in the outpatient setting. Despite this existing evidence, specific next steps are needed along a continuum of action based on prioritization of primary prevention and elimination of structural racism (Figure 3).
Figure 3. Promising strategies to reduce cardiovascular disease disparities and next steps.
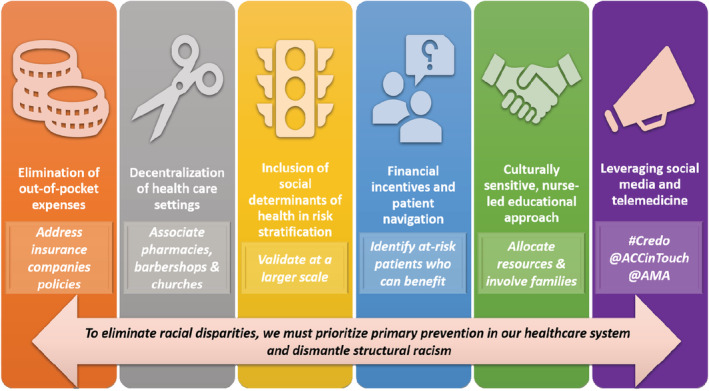
Elimination of Out‐of‐Pocket Expenses
Financial limitations constitute a significant barrier for medication adherence and affect long‐term outcomes even in insured populations. The first randomized clinical trial that addressed this issue was the MI‐FREE (Full Coverage for Preventive Medications After Myocardial Infarction) trial. This study demonstrated that elimination of copayments for statins, β‐blockers, angiotensin‐converting‐enzyme inhibitors, or angiotensin‐receptor blockers increased medication adherence, reduced total major vascular events, and decreased patient spending without increasing overall health costs. 60 This led Aetna to launch a value‐based insurance program reducing copayments for all secondary prevention medications among insured beneficiaries with prior myocardial infarction. 61 The contemporary ARTEMIS (Affordability and Real‐world Antiplatelet Treatment Effectiveness After Myocardial Infarction Study) randomized clinical trial demonstrated that among patients with myocardial infarction, the provision of a co‐payment voucher for a P2Y12 inhibitor increased patient‐reported medication adherence (defined as continued treatment without gap in use of 30 days or longer), though it did not lead to a significant reduction in 1‐year major adverse cardiovascular events. 62 This higher self‐reported P2Y12 inhibitor adherence was confirmed with pharmacy fill data as well as serum drug metabolite levels in a subset of patients. An important observation of this study is that within the intervention group, 28% of patients did not use the voucher. They were more likely to be unemployed, less educated, and have higher rates of medication nonpersistence and major adverse cardiovascular events than those who used the voucher at least once. This highlights the multifactorial causes that are associated with medication nonadherence, including knowledge, attitudes, complexity of prescribed regimens, and difficulties accessing medications (transportation, etc).
Decentralization of Health Care Settings
Decentralizing medical interventions from the physician's office and installing them in the community could decrease access barriers, thus improving adherence and outcomes. Thus, the use of nontraditional health care settings has been proposed to reduce disparities in CVD care. In this regard, health promotion by barbers coupled with drug therapy prescribed by specialty‐trained pharmacists (under a collaborative practice agreement with participants' doctors) led to a significant reduction in blood pressure among Black male barbershop patrons with uncontrolled hypertension. 63 The mean reduction in systolic blood pressure was large (21.6 mm Hg) and was consistent across clusters. More importantly, these effects were sustained at 12 months with fewer in‐person pharmacist visits, supporting the need for broad‐scale implementation studies. 64
Additionally, in the FAITH (Faith‐Based Approaches in the Treatment of Hypertension) study, a therapeutic lifestyle change associated with motivational interviewing sessions delivered by lay health advisors at churches reduced systolic blood pressure at 6 months among Black patients with uncontrolled hypertension. 65 The net reduction was 5.8 mm Hg in systolic blood pressure and remained marginally significant at the 9‐month follow‐up. Unlike the barbershop study, most participants were Black women and significant challenges were encountered during recruitment and course of the study, leading to a 30% attrition rate. Weight loss interventions have also been tried in church‐based settings, achieving modest effects. A recent community intervention study used not only educational resources but also church vegetable and fruit gardens, cooking and nutrition classes, community mapping of food and physical activity environments, and daily mobile messaging, achieving 5% weight loss among participants. 66 The addition of a high‐intensity, lifestyle‐based program delivered by a health coach in primary care clinics has been demonstrated to achieve 5% weight reduction at 24 months in an underserved population according to the recent PROPEL (Promoting Successful Weight Loss in Primary Care in Louisiana) study. 67
Inclusion of Social Determinants of Health in Risk Stratification
A “SDOH score” based on several socioeconomic parameters (place of birth, education, financial strain, health literacy, stress level, living arrangements, social isolation, delay in care, census‐based income, and racial and ethnic minority background) has demonstrated a dose effect on cardiovascular risk factors such as systolic blood pressure, Framingham risk score, glycated hemoglobin, and smoking in a primary care cohort of 2876 patients in Miami, FL. 68 Similarly, having multiple affected areas of the SDOH has been associated with incident stroke in individuals younger than 75 years. 69 If this score is successfully validated in prospective cohorts of different CVDs, it could open a potential system‐level applicability to help identify those at the highest risk for CVD, and subsequently guide appropriate interventions.
Financial Incentives and Patient Navigation
These 2 interventions were combined to increase smoking cessation at a large safety‐net hospital in Boston, Massachusetts. In this study, among patients who smoked on average 15 cigarettes per day, patient navigation along with financial incentives increased the rates of biochemically confirmed smoking cessation at 12 months. 70 The intervention was particularly more beneficial among women, non‐White patients, the elderly, and those with household incomes <$20 000. 71 The sustainability of this approach will need to be further addressed in multicentric studies.
Culturally Sensitive, Nurse‐Led Educational Approach
Project Dulce evaluated the effectiveness of a culturally sensitive, nurse case management/peer education approach to improving diabetes care and health status among underserved primarily Hispanic/Latino participants. The nurse case management component consisted of a nurse‐led team with a registered nurse/certified diabetes educator, bilingual/bicultural medical assistant, and bilingual/bicultural dietitian who traveled to a different clinic site each day to see patients. Peer education was provided via twelve 2‐hour classes given to individuals with diabetes who exemplified the traits of a “natural leader” identified from the clinic's patient population and trained initially using a program developed by the Latino Health Access Program. Significant reductions in hemoglobin A1c, total cholesterol, low‐density lipoprotein cholesterol, triglycerides, and systolic and diastolic blood pressure were seen. 72
Leveraging Social Media and Telemedicine
Social media is a valid platform for addressing SDOH, with the potential to improve awareness surrounding these issues. 73 Awareness leads to investigation, research, education, advocacy, and eventually legislation. Social media groups focused on specific areas of SDOH could strategically increase the awareness of patient‐focused information and allow small groups to form in communities that can actively address the issues, share information, and collaborate to propose solutions. 74 Each community faces different issues and there will not be a one‐size‐fits‐all solution.
Telemedicine has been widely used during the pandemic; however, this has not been homogeneously seen across all communities. When connectivity issues, language barriers, and other challenges arise, telemedicine is likely to compound existing disparities in health care access, cost, and quality, harming patients with the fewest resources. 75 Changes needed to ensure equitable telemedicine practices include increasing broadband access (National Digital Inclusion Alliance maintains a list of nationwide broadband plans that are <$20/month), having available language interpreters, minimizing barriers to access visits (enrollment in patient portals can be done with a family member or with an administrative team member), and deploying digital health tools. 76
Actionable Clinical, Educational, and Policy Items
Diversification of the Clinical Workforce
Now more than ever, there is a pressing need for cardiology as a field to address diversity. The health crisis brought by the COVID‐19 pandemic along with social unrest, racial, and political polarization have unmasked glaring inequities and disparities in our institutions and public health. The urgency of the moment demands that cardiology as a profession assume the challenge to diversify our workforce to meet the current and future demands of our nation.
Racial and ethnic disparities in cardiovascular care can be attributed in part to a lack of diversity in the cardiovascular physician workforce, both in practitioners and trainees. 77 Physicians from races and ethnicities under‐represented in medicine bring unique outlooks to the bedside and provide enhanced care to a multicultural patient population, increasing patient satisfaction and potentially reducing racial health care disparities. 78
A recent update from the Accreditation Council for Graduate Medical Education states that residency and fellowship programs should include an assessment of their efforts to recruit and retain a diverse workforce in their annual evaluations. 79 Translating these efforts into success requires deep engagement from leadership and recruitment committees. A variety of strategies have been shown to increase diversity among trainees. 77 , 80 Some of those used in cardiology programs are listed in Table 1. 77 , 80 Other strategies used in family, emergency, and internal medicine residency programs have included scholarship‐based externship programs, funded second‐look events, increased involvement of underrepresented in medicine faculty in the interview and recruitment process, 81 minimization of bias during interviews (blinding scores and standardizing interview encounters), and analysis of recruitment data. 82 Additional successful experiences from the American Society of Nephrology shown to increase recruitment into their specialty include mentor‐mentee online curriculum and the Kidney STARS program, a team‐based learning experience where medical students and residents with interest in the specialty get paired with leading nephrologists at their Annual Scientific Sessions. 83 The adoption of some of these strategies may change the current face of cardiology, where physicians from Hispanic/Latino, Black, and American Indian or Alaska Native origin constitute 4.4%, 4.1%, and <0.2% of CVD fellows enrolled in Accreditation Council for Graduate Medical Education–accredited programs, respectively. 84
Table 1.
Strategies to Increase Diversity in Cardiology Training Programs
|
Finally, it is important to remark that under‐represented in medicine physicians who come from low‐income families, lack generational wealth or physician legacy, and/or have higher educational debt are disproportionately affected by financial hardship during training. Current trainees' salaries are often insufficient to support a family, which often includes childcare and housing in expensive cities. Urging Accreditation Council for Graduate Medical Education, hospital systems and training programs to provide additional funding and resources to cover these unmet needs is almost compulsory if we truly want to increase diversity within cardiology (Table 2).
Table 2.
Diversity Pearls
|
The involvement and partnership of medical societies and professional groups, such as the American College of Cardiology (both national and local chapters), American Heart Association, American Medical Women Association, and American Medical Association, is critical to foment this change. They can provide tools, best practice models, platforms for professional advancement and mentoring, and policy statements, such as what has been done to enhance recruitment, retention, and career advancement for women in cardiology. 85
Diversification of Research Participants
A big step toward reducing disparities in cardiovascular health is the inclusion of culturally and linguistically diverse people in clinical trials. Underrepresentation of racial and ethnic minority groups in CVD research results in gaps in our understanding of race‐related differences in disease pathobiology, diet and lifestyle, and drug responses. 86 In order to address the critical barriers to participation of patients from racial and ethnic minority groups in clinical trials and make research more inclusive, investigators must engage with multiple stakeholders (eg, primary provider, community leaders, patients and care partners). Key factors to creating inclusive clinical trials include building trust, common understanding of the goal, clinical trial process awareness, optimizing the role of the program coordinator, and addressing resource and time constraints. 86 Another major step is standardizing how we collect race, ethnicity, and primary language data in health care settings. 87 A lack of understanding of how best to collect this information from patients results in fragmented information within and across organizations. 88
Policy
The historical model of identifying risk factors for disease and focusing on individual behavioral changes has failed to resolve health inequities among groups that have been economically and socially marginalized. We must reorganize and prioritize new models that incorporate policymakers, legislators, housing administrators, food distribution and manufacturing, urban planning, adequate health insurance, and access to high‐quality health care to achieve health equity.
In 2020, the COVID‐19 pandemic and social injustices further exposed the severity of disparities that co‐occur in the context of broader inequality. They brought attention to the call for criminal justice reform, economic wealth gaps, disproportionate educational financing, and other structural and systemic racist components of our society. Clinician and clinician investigators find ourselves at a pivotal moment during which we are uniquely poised to offer solutions for health inequities through evidence‐informed policy. Although changes in the health care sector alone will be insufficient, they can act as a vanguard of a multifaceted, multisector, policy‐driven approach to addressing and resolving these issues. 89 Critical areas needing our attention include the roles of (1) the built environment and (2) health care organizations.
The Built Environment Structural and Systemic Inequities
The built environment surrounding health risk behaviors includes differential concentrations of food resources (eg, fast food, food deserts), toxic chemicals, pollution, education, health access, and others. 89 These factors undoubtedly contribute to differential health outcomes. 90 Adequately addressing these structural and systemic inequities requires upstream improvements that disrupt disparate built environments in education, income, social and welfare services, jobs, transportation, access to healthy foods, and safe community environments. This will require intersectional collaboration among several levels of potential stakeholders (eg, health care organizations, urban planners, regulators, and policymakers). This more comprehensive approach challenges the foundation that supports continued health disparities by embracing health from cradle to grave. Positive early life experiences create a higher likelihood of adopting healthy lifestyle behavior, improved economic and health status, and translates to intergenerational health benefits. Early childhood intervention programs can positively impact individuals across their lifespans by addressing these SDOH underlying chronic diseases. Examples of early childhood intervention programs that address education and parental support demonstrate the potential for improving health outcomes. 91 Similarly, nutrition policy is an important part of the built environment that needs addressing to work toward health equity, given that diet is the leading contributing factor to premature death because of CVD in the United States. 27 Americans underconsume vegetables, fruits, grains, nuts, seeds, and seafood, and overconsume sodium, sugary products, and meats. 92 Despite mild improvements in Americans' overall dietary quality over the past 2 decades, persistent and widening disparities remain across incomes, education levels, as well as race and ethnicity. 92 Evidence‐based nutrition policies offer opportunities for increased health equity. For example, since socioeconomically marginalized individuals have the highest burden of sugary‐sweetened beverage–related diseases, they could end up benefiting the most from policies that reduce sugary‐sweetened beverage consumption. Potential nutrition policy interventions deserving further research to determine their ability to improve health equity include taxes on foods that disproportionately affect socioeconomically marginalized individuals (eg, sugary‐sweetened beverages), changes in advertising rules, nutrition labeling, education, the local built environment (eg, grocery stores in food deserts), and incentives/disincentives to shift food purchases toward healthier items. 93 Additionally, policy considerations for government programs such as the US Supplemental Nutrition Assistance Program, which served 1 in 8 (≈46 million) Americans in 2019, can directly impact the health of socioeconomically marginalized individuals. 94 There is a critical need for studying ways to reorient the US Supplemental Nutrition Assistance Program toward treating modern malnutrition (both lack of access and lack of access to healthy food), such as by adding incentives for healthy foods, disincentives for unhealthy foods, restricting unhealthy item purchases, increasing benefit allotments, and determining optimal use of online grocery purchasing platforms. 95 These programmatic changes have the potential to shift US Supplemental Nutrition Assistance Program consumer practices and decrease the incidence of CVD. With diet as the top contributor to premature mortality and cardiovascular mortality, it is imperative to explore these and other ways to maximize nutrition policy to support socioeconomically marginalized individuals, but to do so in a way that does not further marginalize already socioeconomically marginalized individuals.
In order to achieve these benefits, and disrupt the impact of social determinants from an early age, adequate financial resources need to be allocated in school systems that are located in socioeconomically deprived areas along with commitment from urban planners to contribute to safe environments that minimize environmental stressors and toxins. This requires the commitment of local and state policymakers and health care organizations at every level and the establishment of best practices that include health as a core tenet. By addressing the SDOH care, we can accept the challenges that confront us and stimulate the multigenerational, intersectional, and governmental collaboration that is needed. 91
Role of Health Care Organizations, Local and State Government, and Communities
The United States has the world's highest per capita investment in health care, but ranks low in quality measures compared with other industrialized nations. 96 This is more profound in racial and ethnic minority populations with a resultant disparity in health care access and outcomes. Several initiatives seek to address this disparity, including (1) improved patient education and health literacy, (2) patient‐held records, (3) improving proximity of primary health care, (4) increased rural outreach clinics, (5) the patient‐centered medical home, and (6) payment and insurance reforms. These initiatives have a high level of support but lack data. Large successful system models, including Kaiser Permanente and Veterans Administration, are exemplary in these domains for consistent benefits for patients while reducing disparities. 97
We have a well‐developed, poorly integrated, fragmented health care system that was not developed to address SDOH. It is imperative that we redirect health care's evolution toward strategies that benefit population health. The Affordable Care Act, which focuses on population health management and newer care models such as the accountable care organizations and the Patient‐Centered Medical home, have been created to improve the overall health of the nation. These programs have potential for efficacy among patient groups that face the most barriers, including women and children and recognizing the symbiosis between primary care and public health. However, health care access in insurance exchanges invariably creates a distinctive group of insured and uninsured, leading to health disparities. 98 Additional policy innovation is necessary to address these barriers in care among populations experiencing socioeconomic disadvantages.
At the clinical level, there are things that we can begin to do today. 90 First, we must recognize and commit to improving the health outcomes of our populations experiencing disproportionate impact. Our literature is full of analyses detailing descriptors of populations disproportionally affected, but solutions to disparate health outcomes are just now on the horizon. Second, we must address the environmental, psychosocial, and community barriers to health care. The inverse relationship between socioeconomic status and unhealthy behaviors such as tobacco use, physical inactivity, and poor nutrition are well demonstrated. Women, children, and patients from low socioeconomic status face more barriers while seeking health care, and are less likely to seek routine health screening and more likely to utilize emergency medical services. Social issues must be part of our patient encounters. It is important that clinicians begin to explore problems of employment, food insecurity, violence, sexual abuse, psychosocial trauma, economic instability, education, transportation, and other issues that may create barriers to adequate health care. Third, we must familiarize ourselves with social agencies and welfare networks that can provide support for patients who have been socially and economically marginalized, including identifying community support services and facilitating access. Promoting health equity should be incorporated into every aspect of our society. To achieve this aim we must identify resources to train health care workers and patients to manage the social determinants for promoting health and health equity.
Conclusions
Racial CVD disparities in care and outcomes persist in our nation despite significant advancements in evidence‐based CVD care. The revolving door concept emerges when racially diverse patients with CVD achieve similar outcomes in the inpatient setting, but those of color invariably return to an outpatient setting charged with adverse social determinants of health that prevents them from attaining cardiovascular health, putting them at risk for rehospitalization. We are at an inflection point where we must look back at our gains and rescue effective measures, broaden and implement them, and address actionable items. Our health care system is yearning for a reform that necessitates a multisector intervention to vigorously address the environment that feeds these inequities.
Sources of Funding
None.
Disclosures
None.
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Sources of Funding and Disclosures, see page 14.
References
- 1. Heckler, M . United States Department of Health and Human Services . Report of the Secretary's Task Force on Black and Minority Health. Washington DC: Department of Health and Human Services. National Library of Medicine; 1985. Accessed March 28, 2021. https://minorityhealth.hhs.gov/assets/pdf/checked/1/ANDERSON.pdf [Google Scholar]
- 2. Smedley BD, Stith AY, Nelson AR, eds. Study charge and committee assumptions ‐ defining racial and ethnic health care disparities. In: Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 3. Bernstein A, Bilheimer LT, Makuc DM. Health, United States, 2011; with special feature on socioeconomic status and health. National Center for Health Statistics (U.S.); 2012. DHHS publication; no. 2012‐1232. Accessed February 27, 2023. https://stacks.cdc.gov/view/cdc/13680 [PubMed] [Google Scholar]
- 4. Yancy CW, Wang TY, Ventura HO, Pina IL, Vijayaraghavan K, Ferdinand KC, Hall LL, Credo Advisory Group . The coalition to reduce racial and ethnic disparities in cardiovascular disease outcomes (credo): why credo matters to cardiologists. J Am Coll Cardiol. 2011;57:245–252. doi: 10.1016/j.jacc.2010.09.027 [DOI] [PubMed] [Google Scholar]
- 5. Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med. 2008;168:1152–1158. doi: 10.1001/archinte.168.11.1152 [DOI] [PubMed] [Google Scholar]
- 6. Gardener H, Leifheit EC, Lichtman JH, Wang Y, Wang K, Gutierrez CM, Ciliberti‐Vargas MA, Dong C, Oluwole S, Robichaux M, et al. Racial/ethnic disparities in mortality among medicare beneficiaries in the FL ‐ PR CR eSD study. J Am Heart Assoc. 2019;8:e009649. doi: 10.1161/JAHA.118.009649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ya'qoub L, Lemor A, Dabbagh M, O'Neill W, Khandelwal A, Martinez SC, Ibrahim NE, Grines C, Voeltz M, Basir MB. Racial, ethnic, and sex disparities in patients with STEMI and cardiogenic shock. JACC Cardiovasc Interv. 2021;14:653–660. doi: 10.1016/j.jcin.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control . Summary health statistics: National Health Interview Survey: 2018. Accessed March 28, 2021. https://www.cdc.gov/nchs/nhis/shs.htm
- 9. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. doi: 10.1161/CIR.0000000000000534 [DOI] [PubMed] [Google Scholar]
- 10. Argeseanu Cunningham S, Ruben JD, Venkat Narayan KM. Health of foreign‐born people in the United States: a review. Health Place. 2008;14:623–635. doi: 10.1016/j.healthplace.2007.12.002 [DOI] [PubMed] [Google Scholar]
- 11. Koya DL, Egede LE. Association between length of residence and cardiovascular disease risk factors among an ethnically diverse group of United States immigrants. J Gen Intern Med. 2007;22:841–846. doi: 10.1007/s11606-007-0163-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Le‐Scherban F, Albrecht SS, Bertoni A, Kandula N, Mehta N, Diez Roux AV. Immigrant status and cardiovascular risk over time: results from the Multi‐Ethnic Study of Atherosclerosis. Ann Epidemiol. 2016;26:429–435 e421. doi: 10.1016/j.annepidem.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Isasi CR, Ayala GX, Sotres‐Alvarez D, Madanat H, Penedo F, Loria CM, Elder JP, Daviglus ML, Barnhart J, Siega‐Riz AM. Is acculturation related to obesity in Hispanic/Latino adults? Results from the Hispanic community health study/study of Latinos. J Obes. 2015;2015:186276. doi: 10.1155/2015/186276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodriguez F, Echeverria SE, Pentakota SR, Amadi C, Hastings KG, Palaniappan LP. Comparison of ideal cardiovascular health attainment and acculturation among Asian Americans and Latinos. Ethn Dis. 2019;29:287–296. doi: 10.18865/ed.29.2.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Brien MJ, Alos VA, Davey A, Bueno A, Whitaker RC. Acculturation and the prevalence of diabetes in US Latino adults, National Health and Nutrition Examination Survey 2007‐2010. Prev Chronic Dis. 2014;11:E176. doi: 10.5888/pcd11.140142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thomas SC, Umer A, Commodore‐Mensah Y, Davidov D, Abildso CG. Length of residence and cardiovascular health among Afro‐Caribbean Immigrants in New York City. J Racial Ethn Health Disparities. 2019;6:487–496. doi: 10.1007/s40615-018-00547-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. doi: 10.1161/CIR.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palaniappan LP, Araneta MRG, Assimes TL, Barrett‐Connor EL, Carnethon MR, Criqui MH, Fung GL, Narayan KV, Patel H, Taylor‐Piliae RE. Call to action: cardiovascular disease in Asian Americans: a science advisory from the American Heart Association. Circulation. 2010;122:1242–1252. doi: 10.1161/CIR.0b013e3181f22af4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rodriguez F, Hastings KG, Boothroyd DB, Echeverria S, Lopez L, Cullen M, Harrington RA, Palaniappan LP. Disaggregation of cause‐specific cardiovascular disease mortality among Hispanic subgroups. JAMA Cardiol. 2017;2:240–247. doi: 10.1001/jamacardio.2016.4653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, Fujimoto WY, Imperatore G. Prevalence of diabetes by race and ethnicity in the United States, 2011‐2016. JAMA. 2019;322:2389–2398. doi: 10.1001/jama.2019.19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaplan RC, Bangdiwala SI, Barnhart JM, Castañeda SF, Gellman MD, Lee DJ, Pérez‐Stable EJ, Talavera GA, Youngblood ME, Giachello AL. Smoking Among U.S. Hispanic/Latino adults: the Hispanic Community Health Study/Study of Latinos. Am J Prev Med. 2014;46:496–506. doi: 10.1016/j.amepre.2014.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim G, Chiriboga DA, Jang Y, Lee S, Huang CH, Parmelee P. Health status of older Asian Americans in California. J Am Geriatr Soc. 2010;58:2003–2008. doi: 10.1111/j.1532-5415.2010.03034.x [DOI] [PubMed] [Google Scholar]
- 23. Volgman AS, Palaniappan LS, Aggarwal NT, Gupta M, Khandelwal A, Krishnan AV, Lichtman JH, Mehta LS, Patel HN, Shah KS. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018;138:e1–e34. doi: 10.1161/CIR.0000000000000580 [DOI] [PubMed] [Google Scholar]
- 24. Kalra D, Vijayaraghavan K, Sikand G, Desai NR, Joshi PH, Mehta A, Karmally W, Vani A, Sitafalwalla SJ, Puri R, et al. Prevention of atherosclerotic cardiovascular disease in South Asians in the US: a clinical perspective from the National Lipid Association. J Clin Lipidol. 2021;15:402–422. doi: 10.1016/j.jacl.2021.03.007 [DOI] [PubMed] [Google Scholar]
- 25. Bonow RO, Grant AO, Jacobs AK. The cardiovascular state of the union. Circulation. 2005;111:1205–1207. doi: 10.1161/01.CIR.0000160705.97642.92 [DOI] [PubMed] [Google Scholar]
- 26. Sankar P, Cho MK, Condit CM, Hunt LM, Koenig B, Marshall P, Lee SS‐J, Spicer P. Genetic research and health disparities. JAMA. 2004;291:2985–2989. doi: 10.1001/jama.291.24.2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. US Burden of Disease Collaborators ; Mokdad AH, Ballestros K, Echko M, Glenn S, Olsen HE, Mullany E, Lee A, Khan AR, Ahmadi A, et al. The state of US health, 1990‐2016: burden of diseases, injuries, and risk factors among US states. JAMA. 2018;319:1444–1472. doi: 10.1001/jama.2018.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng Z‐J, Sharrett AR, Chambless LE, Rosamond WD, Nieto FJ, Sheps DS, Dobs A, Evans GW, Heiss G. Associations of ankle‐brachial index with clinical coronary heart disease, stroke and preclinical carotid and popliteal atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis. 1997;131:115–125. doi: 10.1016/S0021-9150(97)06089-9 [DOI] [PubMed] [Google Scholar]
- 29. Ellis J, Lange EM, Li J, Dupuis J, Baumert J, Walston JD, Keating BJ, Durda P, Fox ER, Palmer CD. Large multiethnic Candidate Gene Study for C‐reactive protein levels: identification of a novel association at CD36 in African Americans. Hum Genet. 2014;133:985–995. doi: 10.1007/s00439-014-1439-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kromhout D. C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Somes GW, Harshfield GA, Alpert BS, Goble MM, Schicken RM. Genetic influences on ambulatory blood pressure patterns: the Medical College of Virginia Twin Study. Am J Hypertens. 1995;8:474–478. doi: 10.1016/0895-7061(95)00017-J [DOI] [PubMed] [Google Scholar]
- 32. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 33. Bao W, Threefoot SA, Srinivasan SR, Berenson GS. Essential hypertension predicted by tracking of elevated blood pressure from childhood to adulthood: the Bogalusa Heart Study. Am J Hypertens. 1995;8:657–665. doi: 10.1016/0895-7061(95)00116-7 [DOI] [PubMed] [Google Scholar]
- 34. Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle‐aged and older adults. Hypertension. 2011;57:1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Allison MA, Criqui MH, McClelland RL, Scott JM, McDermott MM, Liu K, Folsom AR, Bertoni AG, Sharrett AR, Homma S. The effect of novel cardiovascular risk factors on the ethnic‐specific odds for peripheral arterial disease in the Multi‐Ethnic Study of Atherosclerosis (MESA). J Am Coll Cardiol. 2006;48:1190–1197. doi: 10.1016/j.jacc.2006.05.049 [DOI] [PubMed] [Google Scholar]
- 36. Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans. J Am Coll Cardiol. 2008;51:2347–2354. doi: 10.1016/j.jacc.2008.03.022 [DOI] [PubMed] [Google Scholar]
- 37. Allison MA, Gonzalez F, Raij L, Kaplan R, Ostfeld RJ, Pattany MS, Heiss G, Criqui MH. Cuban Americans have the highest rates of peripheral arterial disease in diverse Hispanic/Latino communities. J Vasc Surg. 2015;62:665–672. doi: 10.1016/j.jvs.2015.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DeNavas‐Walt C, Proctor BD. Income and poverty in the United States: 2013. US Government Printing Office; 2014. [Google Scholar]
- 39. Lewis TT, Cogburn CD, Williams DR. Self‐reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psychol. 2015;11:407–440. doi: 10.1146/annurev-clinpsy-032814-112728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Everson‐Rose SA, Lutsey PL, Roetker NS, Lewis TT, Kershaw KN, Alonso A, Diez Roux AV. Perceived discrimination and incident cardiovascular events: the Multi‐Ethnic Study of Atherosclerosis. Am J Epidemiol. 2015;182:225–234. doi: 10.1093/aje/kwv035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Breathett K, Yee E, Pool N, Hebdon M, Crist JD, Yee RH, Knapp SM, Solola S, Luy L, Herrera‐Theut K, et al. Association of gender and race with allocation of advanced heart failure therapies. JAMA Netw Open. 2020;3:e2011044. doi: 10.1001/jamanetworkopen.2020.11044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Edmund Anstey D, Li S, Thomas L, Wang TY, Wiviott SD. Race and sex differences in management and outcomes of patients after ST‐elevation and non‐ST‐elevation myocardial infarct: results from the NCDR. Clin Cardiol. 2016;39:585–595. doi: 10.1002/clc.22570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cohen MG, Fonarow GC, Peterson ED, Moscucci M, Dai D, Hernandez AF, Bonow RO, Smith SC Jr. Racial and ethnic differences in the treatment of acute myocardial infarction: findings from the Get With the Guidelines‐Coronary Artery Disease program. Circulation. 2010;121:2294–2301. doi: 10.1161/CIRCULATIONAHA.109.922286 [DOI] [PubMed] [Google Scholar]
- 44. Ziaeian B, Heidenreich PA, Xu H, DeVore AD, Matsouaka RA, Hernandez AF, Bhatt DL, Yancy CW, Fonarow GC. Race/ethnic differences in outcomes among hospitalized Medicare patients with heart failure and preserved ejection fraction. JACC Heart Fail. 2017;5:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guzman LA, Li S, Wang TY, Daviglus ML, Exaire J, Rodriguez CJ, Torres VI, Funk M, Saucedo J, Granger C, et al. Differences in treatment patterns and outcomes between Hispanics and non‐Hispanic Whites treated for ST‐segment elevation myocardial infarction: results from the NCDR ACTION Registry‐GWTG. J Am Coll Cardiol. 2012;59:630–631. doi: 10.1016/j.jacc.2011.10.882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sacco RL, Gardener H, Wang K, Dong C, Ciliberti‐Vargas MA, Gutierrez CM, Asdaghi N, Burgin WS, Carrasquillo O, Garcia‐Rivera EJ, et al. Racial‐ethnic disparities in acute stroke care in the Florida‐Puerto Rico Collaboration to Reduce Stroke Disparities Study. J Am Heart Assoc. 2017;6:e004073. doi: 10.1161/JAHA.116.004073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thomas KL, Hernandez AF, Dai D, Heidenreich P, Fonarow GC, Peterson ED, Yancy CW. Association of race/ethnicity with clinical risk factors, quality of care, and acute outcomes in patients hospitalized with heart failure. Am Heart J. 2011;161:746–754. doi: 10.1016/j.ahj.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 48. Li S, Fonarow GC, Mukamal KJ, Liang L, Schulte PJ, Smith EE, DeVore A, Hernandez AF, Peterson ED, Bhatt DL. Sex and race/ethnicity‐related disparities in care and outcomes after hospitalization for coronary artery disease among older adults. Circ Cardiovasc Qual Outcomes. 2016;9:S36–S44. doi: 10.1161/CIRCOUTCOMES.115.002621 [DOI] [PubMed] [Google Scholar]
- 49. Hess PL, Hernandez AF, Bhatt DL, Hellkamp AS, Yancy CW, Schwamm LH, Peterson ED, Schulte PJ, Fonarow GC, Al‐Khatib SM. Sex and race/ethnicity differences in implantable cardioverter‐defibrillator counseling and use among patients hospitalized with heart failure: findings from the Get With The Guidelines‐Heart Failure Program. Circulation. 2016;134:517–526. doi: 10.1161/CIRCULATIONAHA.115.021048 [DOI] [PubMed] [Google Scholar]
- 50. Fang Zhang F, Liu J, Rehm CD, Wilde P, Mande JR, Mozaffarian D. Trends and disparities in diet quality among US adults by Supplemental Nutrition Assistance Program participation status. JAMA Netw Open. 2018;1:e180237. doi: 10.1001/jamanetworkopen.2018.0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gu K, Mahtta D, Kaneria A, Sharedalal P, Dennis B, Song Y, Wei X, Khalid U, Hess P, Cho SH, et al. Racial disparities among Asian Americans with atrial fibrillation: an analysis from the NCDR® PINNACLE Registry. Int J Cardiol. 2021;329:209–216. [DOI] [PubMed] [Google Scholar]
- 52. Chan PS, Oetgen WJ, Buchanan D, Mitchell K, Fiocchi FF, Tang F, Jones PG, Breeding T, Thrutchley D, Rumsfeld JS, et al. Cardiac performance measure compliance in outpatients: the American College of Cardiology and National Cardiovascular Data Registry's PINNACLE (Practice Innovation And Clinical Excellence) program. J Am Coll Cardiol. 2010;56:8–14. doi: 10.1016/j.jacc.2010.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Khariton Y, Nassif ME, Thomas L, Fonarow GC, Mi X, DeVore AD, Duffy C, Sharma PP, Albert NM, Patterson JH, et al. Health status disparities by sex, race/ethnicity, and socioeconomic status in outpatients with heart failure. JACC Heart Fail. 2018;6:465–473. doi: 10.1016/j.jchf.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meadows TA, Bhatt DL, Hirsch AT, Creager MA, Califf RM, Ohman EM, Cannon CP, Eagle KA, Alberts MJ, Goto S, et al. Ethnic differences in the prevalence and treatment of cardiovascular risk factors in US outpatients with peripheral arterial disease: insights from the reduction of atherothrombosis for continued health (REACH) registry. Am Heart J. 2009;158:1038–1045. doi: 10.1016/j.ahj.2009.09.014 [DOI] [PubMed] [Google Scholar]
- 55. Wadhera RK, Bhatt DL, Kind AJH, Song Y, Williams KA, Maddox TM, Yeh RW, Dong L, Doros G, Turchin A, et al. Association of outpatient practice‐level socioeconomic disadvantage with quality of care and outcomes among older adults with coronary artery disease: implications for value‐based payment. Circ Cardiovasc Qual Outcomes. 2020;13:e005977. doi: 10.1161/CIRCOUTCOMES.119.005977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hasnain‐Wynia R, Baker DW, Nerenz D, Feinglass J, Beal AC, Landrum MB, Behal R, Weissman JS. Disparities in health care are driven by where minority patients seek care: examination of the hospital quality alliance measures. Arch Intern Med. 2007;167:1233–1239. doi: 10.1001/archinte.167.12.1233. [DOI] [PubMed] [Google Scholar]
- 57. Centers for Medicare and Medicaid Services. Hospital Readmissions Reduction Program (HRRP). Accessed December 15, 2021. https://www.cms.gov/Medicare/Medicare‐Fee‐for‐Service‐Payment/AcuteInpatientPPS/Readmissions‐Reduction‐Program
- 58. Aggarwal R, Hammond JG, Joynt Maddox KE, Yeh RW, Wadhera RK. Association between the proportion of Black patients cared for at hospitals and financial penalties under value‐based payment programs. JAMA. 2021;325:1219–1221. doi: 10.1001/jama.2021.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Agarwala A, Goldberg AC, Ballantyne CM, Guyton JR. JCL roundtable. Making prevention a priority. J Clin Lipidol. 2021;15:530–537. doi: 10.1016/j.jacl.2021.08.004 [DOI] [PubMed] [Google Scholar]
- 60. Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913 [DOI] [PubMed] [Google Scholar]
- 61. Aetna. Aetna launching value‐based program that improves medication adherence, cost and outcomes for members who have suffered from heart attacks. 2011. Accessed March 21, 2021. https://news.aetna.com/news‐releases/aetna‐launching‐value‐based‐program‐that‐improves‐medication‐adherence‐cost‐and‐outcomes‐for‐members‐who‐have‐suffered‐from‐heart‐attacks.
- 62. Wang TY, Kaltenbach LA, Cannon CP, Fonarow GC, Choudhry NK, Henry TD, Cohen DJ, Bhandary D, Khan ND, Anstrom KJ, et al. Effect of medication co‐payment vouchers on P2Y12 inhibitor use and major adverse cardiovascular events among patients with myocardial infarction: the ARTEMIS Randomized Clinical Trial. JAMA. 2019;321:44–55. doi: 10.1001/jama.2018.19791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx‐Drew D, et al. A cluster‐randomized trial of blood‐pressure reduction in Black barbershops. N Engl J Med. 2018;378:1291–1301. doi: 10.1056/NEJMoa1717250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Victor RG, Blyler CA, Li N, Lynch K, Moy NB, Rashid M, Chang LC, Handler J, Brettler J, Rader F, et al. Sustainability of blood pressure reduction in black barbershops. Circulation. 2019;139:10–19. doi: 10.1161/CIRCULATIONAHA.118.038165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schoenthaler AM, Lancaster KJ, Chaplin W, Butler M, Forsyth J, Ogedegbe G. Cluster randomized clinical trial of FAITH (Faith‐Based Approaches in the Treatment of Hypertension) in Blacks. Circ Cardiovasc Qual Outcomes. 2018;11:e004691. doi: 10.1161/CIRCOUTCOMES.118.004691. [DOI] [PubMed] [Google Scholar]
- 66. Derose KP, Williams MV, Florez KR, Griffin BA, Payan DD, Seelam R, Branch CA, Hawes‐Dawson J, Mata MA, Whitley MD, et al. Eat, pray, move: a pilot cluster randomized controlled trial of a multilevel church‐based intervention to address obesity among African Americans and Latinos. Am J Health Promot. 2019;33:586–596. doi: 10.1177/0890117118813333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Katzmarzyk PT, Martin CK, Newton RL Jr, Apolzan JW, Arnold CL, Davis TC, Price‐Haywood EG, Denstel KD, Mire EF, Thethi TK, et al. Weight loss in underserved patients ‐ a cluster‐randomized trial. N Engl J Med. 2020;383:909–918. doi: 10.1056/NEJMoa2007448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Palacio A, Mansi R, Seo D, Suarez M, Garay S, Medina H, Tang F, Tamariz L. Social determinants of health score: does it help identify those at higher cardiovascular risk? Am J Manag Care. 2020;26:e312–e318. doi: 10.37765/ajmc.2020.88504 [DOI] [PubMed] [Google Scholar]
- 69. Reshetnyak E, Ntamatungiro M, Pinheiro LC, Howard VJ, Carson AP, Martin KD, Safford MM. Impact of multiple social determinants of health on incident stroke. Stroke. 2020;51:2445–2453. doi: 10.1161/STROKEAHA.120.028530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lasser KE, Quintiliani LM, Truong V, Xuan Z, Murillo J, Jean C, Pbert L. Effect of patient navigation and financial incentives on smoking cessation among primary care patients at an urban safety‐net hospital: a randomized clinical trial. JAMA Intern Med. 2017;177:1798–1807. doi: 10.1001/jamainternmed.2017.4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Di Palo KE, Patel K, Assafin M, Pina IL. Implementation of a patient navigator program to reduce 30‐day heart failure readmission rate. Prog Cardiovasc Dis. 2017;60:259–266. doi: 10.1016/j.pcad.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 72. Philis‐Tsimikas A, Walker C, Rivard L, Talavera G, Reimann JO, Salmon M, Araujo R, Project D. Improvement in diabetes care of underinsured patients enrolled in Project Dulce: a community‐based, culturally appropriate, nurse case management and peer education diabetes care model. Diabetes Care. 2004;27:110–115. doi: 10.2337/diacare.27.1.110 [DOI] [PubMed] [Google Scholar]
- 73. Cawcutt KA, Erdahl LM, Englander MJ, Radford DM, Oxentenko AS, Girgis L, Migliore LL, Poorman JA, Silver JK. Use of a coordinated social media strategy to improve dissemination of research and collect solutions related to workforce gender equity. J Womens Health (Larchmt). 2019;28:849–862. doi: 10.1089/jwh.2018.7515 [DOI] [PubMed] [Google Scholar]
- 74. Ndumbe‐Eyoh S, Mazzucco A. Social media, knowledge translation, and action on the social determinants of health and health equity: a survey of public health practices. J Public Health Policy. 2016;37:249–259. doi: 10.1057/s41271-016-0042-z [DOI] [PubMed] [Google Scholar]
- 75. Eberly LA, Khatana SAM, Nathan AS, Snider C, Julien HM, Deleener ME, Adusumalli S. Telemedicine outpatient cardiovascular care during the COVID‐19 pandemic: bridging or opening the digital divide? Circulation. 2020;142:510–512. doi: 10.1161/CIRCULATIONAHA.120.048185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Scheerens C, Gilissen J, Volow AM, Powell JL, Ferguson CM, Farrell D, Li B, Berry C, Sudore RL. Developing eHealth tools for diverse older adults: lessons learned from the PREPARE for Your Care Program. J Am Geriatr Soc. 2021;69:2939–2949. doi: 10.1111/jgs.17284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Crowley AL, Damp J, Sulistio MS, Berlacher K, Polk DM, Hong RA, Weissman G, Jackson D, Sivaram CA, Arrighi JA, et al. Perceptions on diversity in cardiology: a survey of cardiology fellowship training program directors. J Am Heart Assoc. 2020;9:e017196. doi: 10.1161/JAHA.120.017196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Capers Q, Sharalaya Z. Racial disparities in cardiovascular care: a review of culprits and potential solutions. J Racial Ethn Health Disparities. 2014;1:171–180. doi: 10.1007/s40615-014-0021-7 [DOI] [Google Scholar]
- 79. ACGME . ACGME Common Program Requirements (Residency). 2020:55. Accessed May 20, 2021. https://www.acgme.org/globalassets/PFAssets/ProgramRequirements/cprresidency2020.pdf
- 80. Auseon AJ, Kolibash AJ Jr, Capers Q. Successful efforts to increase diversity in a cardiology fellowship training program. J Grad Med Educ. 2013;5:481–485. doi: 10.4300/JGME-D-12-00307.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Tunson J, Boatright D, Oberfoell S, Bakes K, Angerhofer C, Lowenstein S, Zane R, King R, Druck J. Increasing resident diversity in an emergency medicine residency program: a pilot intervention with three principal strategies. Acad Med. 2016;91:958–961. doi: 10.1097/ACM.0000000000000957 [DOI] [PubMed] [Google Scholar]
- 82. Aibana O, Swails JL, Flores RJ, Love L. Bridging the gap: holistic review to increase diversity in graduate medical education. Acad Med. 2019;94:1137–1141. doi: 10.1097/ACM.0000000000002779 [DOI] [PubMed] [Google Scholar]
- 83. Nair D, Pivert KA, Baudy A, Thakar CV. Perceptions of nephrology among medical students and internal medicine residents: a national survey among institutions with nephrology exposure. BMC Nephrol. 2019;20:146. doi: 10.1186/s12882-019-1289-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. ACGME . Chapter C. Residents characteristics. In: Data Resource Book, Academic Year 2019‐2020. ACGME, ed.; 20; 2020:47–89. [Google Scholar]
- 85. Sharma G, Sarma AA, Walsh MN, Hayes SN, Sahni S, Brown SA, Singh T, Harrington RA, Douglas PS, Duvernoy CS, et al. 10 Recommendations to enhance recruitment, retention, and career advancement of women cardiologists. J Am Coll Cardiol. 2019;74:1839–1842. doi: 10.1016/j.jacc.2019.08.016 [DOI] [PubMed] [Google Scholar]
- 86. Clark LT, Watkins L, Pina IL, Elmer M, Akinboboye O, Gorham M, Jamerson B, McCullough C, Pierre C, Polis AB, et al. Increasing diversity in clinical trials: overcoming critical barriers. Curr Probl Cardiol. 2019;44:148–172. doi: 10.1016/j.cpcardiol.2018.11.002 [DOI] [PubMed] [Google Scholar]
- 87. Bhalla R, Yongue BG, Currie BP. Standardizing race, ethnicity, and preferred language data collection in hospital information systems: results and implications for healthcare delivery and policy. J Healthc Qual. 2012;34:44–52. doi: 10.1111/j.1945-1474.2011.00180.x [DOI] [PubMed] [Google Scholar]
- 88. Hasnain‐Wynia R, Baker DW. Obtaining data on patient race, ethnicity, and primary language in health care organizations: current challenges and proposed solutions. Health Serv Res. 2006;41:1501–1518. doi: 10.1111/j.1475-6773.2006.00552.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kris‐Etherton PM, Petersen KS, Velarde G, Barnard ND, Miller M, Ros E, O'Keefe JH, Williams K Sr, Horn LV, Na M, et al. Barriers, opportunities, and challenges in addressing disparities in diet‐related cardiovascular disease in the United States. J Am Heart Assoc. 2020;9:e014433. doi: 10.1161/JAHA.119.014433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Andermann A, Collaboration C . Taking action on the social determinants of health in clinical practice: a framework for health professionals. CMAJ. 2016;188:E474–E483. doi: 10.1503/cmaj.160177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thornton RL, Glover CM, Cene CW, Glik DC, Henderson JA, Williams DR. Evaluating strategies for reducing health disparities by addressing the social determinants of health. Health Aff (Millwood). 2016;35:1416–1423. doi: 10.1377/hlthaff.2015.1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rehm CD, Penalvo JL, Afshin A, Mozaffarian D. Dietary intake among US adults, 1999‐2012. JAMA. 2016;315:2542–2553. doi: 10.1001/jama.2016.7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. doi: 10.1161/CIRCULATIONAHA.115.018585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Supplemental Nutrition Assistance Program Participation and Costs. USDA Food and Nutrition Service. 2021. Accessed May 17, 2021. https://fns‐prod.azureedge.net/sites/default/files/resource‐files/SNAPsummary‐1.pdf
- 95. Mozaffarian D, Liu J, Sy S, Huang Y, Rehm C, Lee Y, Wilde P, Abrahams‐Gessel S, de Souza Veiga Jardim T, Gaziano T, et al. Cost‐effectiveness of financial incentives and disincentives for improving food purchases and health through the US Supplemental Nutrition Assistance Program (SNAP): a microsimulation study. PLoS Med. 2018;15:e1002661. doi: 10.1371/journal.pmed.1002661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Davis K, Stremikis K, Squires D, Schoen C. Mirror, Mirror on the Wall: How the Performance of the U.S. Health Care System Compares Internationally. The Commonwealth Fund; 2014. Update. Accessed February 27, 2023. http://www.commonwealthfund.org/ [Google Scholar]
- 97. Kanter MH, Lindsay G, Bellows J, Chase A. Complete care at Kaiser Permanente: transforming chronic and preventive care. Jt Comm J Qual Patient Saf. 2013;39:484–494. doi: 10.1016/S1553-7250(13)39064-3 [DOI] [PubMed] [Google Scholar]
- 98. Koh HK, Piotrowski JJ, Kumanyika S, Fielding JE. Healthy people: a 2020 vision for the social determinants approach. Health Educ Behav. 2011;38:551–557. doi: 10.1177/1090198111428646 [DOI] [PubMed] [Google Scholar]


