Abstract
Background
Early vascular aging (EVA) is associated with higher risk of adverse cardiovascular events and can be estimated noninvasively by assessing arterial hemodynamics. Women with a history of preeclampsia have increased risk of cardiovascular disease, but underlying mechanisms are incompletely understood. We hypothesized that women with a history of preeclampsia display persistent arterial abnormalities and EVA in the postpartum period.
Methods and Results
We performed a comprehensive, noninvasive arterial hemodynamic evaluation in women with a history of preeclampsia (n=40) and age‐matched controls with previous normotensive pregnancies (n=40). We used validated methods integrating applanation tonometry with transthoracic echocardiography to obtain measures of aortic stiffness, steady and pulsatile arterial load, central blood pressure, and arterial wave reflections. Presence of EVA was defined as aortic stiffness higher than that predicted from reference values based on the participant's age and blood pressure. The association of preeclampsia with arterial hemodynamic variables was assessed with multivariable linear regression, and the association of severe preeclampsia with EVA was assessed with multivariable logistic regression, adjusted for confounders. We found that women with a history of preeclampsia had greater aortic stiffness, steady arterial load, central blood pressure, and arterial wave reflections when compared with controls. We observed a dose–response relationship, with the greatest abnormalities observed in subgroups with severe, preterm, or recurrent preeclampsia. Women with severe preeclampsia had 9.23 times greater odds of having EVA as compared with controls (95% CI, 1.67–51.06, P=0.011) and 7.87 greater odds of EVA as compared with women with nonsevere preeclampsia (95% CI, 1.29–47.77, P=0.025).
Conclusions
Our study comprehensively characterizes arterial hemodynamic abnormalities after preeclampsia and suggests that specific subgroups of women with a history of preeclampsia exhibit greater alterations in arterial hemodynamics related to arterial health. Our findings have important implications for understanding potential links between preeclampsia and cardiovascular events, and suggest women with severe, preterm, or recurrent preeclampsia as subgroups who may deserve intensification of efforts for prevention and early detection of cardiovascular disease.
Keywords: arterial health, arterial stiffness, early vascular aging, hypertension, preeclampsia
Subject Categories: Women, Preeclampsia, Hypertension
Nonstandard Abbreviations and Acronyms
- cfPWV
carotid‐femoral pulse wave velocity
- DBP
diastolic blood pressure
- EVA
early vascular aging
- PAC
proximal aortic compliance
- SBP
systolic blood pressure
Clinical Perspective.
What Is New?
Women with a history of preeclampsia display significant abnormalities in arterial health and early vascular aging, especially in measures of aortic stiffness, central blood pressure, steady arterial load, and arterial wave reflections.
Alterations in arterial health parameters postpartum exhibit a dose–response relationship, with the greatest abnormalities observed in women who experienced severe, preterm, or recurrent preeclampsia.
The presence of any preeclampsia added an estimated 6 additional years to a woman's expected arterial age, while preterm and recurrent preeclampsia added an equivalent of 7 to 8 years, and severe preeclampsia added ≈11 years to arterial age.
What Are the Clinical Implications?
Given the known independent associations of measures of arterial stiffness and central hemodynamics with future cardiovascular events, our findings suggest a mechanistic link connecting preeclampsia to worse arterial health and early vascular aging, and thus, cardiovascular disease.
Since the worst abnormalities in arterial health were observed among women with severe, preterm, or recurrent preeclampsia, our study suggests that clinical and scientific efforts to target risk stratification and primary prevention may focus on preeclamptic women with these features in hopes of maximizing mitigation of cardiovascular risk.
Hypertensive disorders of pregnancy are the leading causes of maternal morbidity and mortality, and are often associated with a woman's higher risk of cardiovascular disease later in life. 1 Preeclampsia is a hypertensive disorder of pregnancy that occurs after 20 weeks of gestation, with a prevalence of 11.5 per 1000 deliveries in Canada, 2 7% to 10% of all pregnancies in the United States, 3 and a 4.6% incidence in women worldwide. 4 Existing data suggest that women who have had preeclampsia have a 4‐ to 8‐fold higher risk of developing cardiovascular disease, and are 6 times more likely to die of cardiovascular complications when compared with nonaffected women. 3 , 5 , 6 Although the pathophysiology predisposing some preeclamptic women to future cardiovascular disease is not fully understood, some theories postulate that, despite clinical resolution of preeclampsia after delivery, structural changes in central arteries persist leading to hemodynamic modifications that increase the overall cardiovascular risk. 5 , 7
Early vascular aging (EVA) is defined by adverse structural and biomechanical aortic modifications leading to aortic wall stiffening in an accelerated fashion, manifesting years or decades earlier than the expected normal vascular aging. These alterations in arterial structure are demonstrated by increased carotid–femoral pulse wave velocity (cfPWV), which expresses aortic stiffness and is the hallmark of EVA. 8 Abnormally high cfPWV for age correlates with inability to properly repair vascular insults 9 and leads to impairment of aortic pressure‐buffering function, which may in turn predispose to subclinical target organ damage, 10 left ventricular remodeling, 11 , 12 and overt cardiovascular disease. 8 , 13 , 14 , 15 The toxemic status to which women with preeclampsia are exposed during pregnancy adversely affects the endothelium and arterial function, 5 , 9 , 16 which are the primordial mechanisms in a cascade of adverse events that eventually culminate in cardiovascular disease. 16 , 17 , 18 , 19 , 20 Furthermore, women with a history of preeclampsia who have more adverse obstetric characteristics such as severe, 21 preterm, 22 or recurrent 23 , 24 preeclampsia experience higher risk of cardiovascular events than their nonsevere, nonpreterm, or nonrecurrent counterparts. As such, understanding arterial hemodynamics and arterial aging after preeclampsia, particularly as it relates to severity, timing, and recurrence, may help elucidate links to future target organ damage and cardiovascular events. 25
Based on this knowledge, we hypothesized that women with obstetric history of preeclampsia would display worse aortic stiffness and arterial hemodynamics consistent with EVA, and that that aortic stiffness and arterial load would be highest among women with severe, preterm, or recurrent preeclampsia. To address these hypotheses, we performed a comprehensive, noninvasive arterial hemodynamic evaluation in women with a history of preeclampsia and age‐matched controls with previous normotensive pregnancies.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Participants and Design
We conducted a cross‐sectional study of adult women with previous history of preeclampsia (n=40) and age‐matched controls with previous normotensive pregnancies (n=40) within 6 months to 6 years postpartum since their last pregnancy. The choice of this timeframe was based on our intention to study arterial hemodynamics after the hemodynamics of pregnancy were no longer present (≥6 months postpartum), but not so late that cardiovascular disease would have already set in (since the average time lapse between a maternal placental syndrome and the incidence of first cardiovascular event is 10 years). 21 Preeclampsia was defined according to American College of Obstetricians and Gynecologists guidelines 26 as the new onset of hypertension after 20 weeks' gestation associated or not with proteinuria in a previously normotensive woman. In the absence of proteinuria, preeclampsia was diagnosed when multi‐organ involvement occurred in addition to hypertension (ie, new onset of renal, or liver injury, or evidence of placental insufficiency). Term preeclampsia was defined as onset of the disease at a gestational age of 37+0 weeks or later, and preterm preeclampsia if onset any time before then. Severe preeclampsia was defined as disease associated with end‐organ dysfunction (evidence of neurologic, cardiovascular, hematological, renal, hepatic, and/or uteroplacental injury). Recurrent preeclampsia was defined as 2 or more previous pregnancies affected by the disease. Exclusion criteria were a history of surgical repair of the aorta, previous aortic valve replacement, more than mild aortic stenosis, more than moderate aortic regurgitation, and permanent atrial fibrillation or flutter, because these may confound the arterial hemodynamic assessment.
Participants were recruited between February 2017 and October 2021 from Level 2 and 3 Obstetric Centres or the CardioPrevent Postpartum program in Ottawa, ON, Canada and invited for a single research encounter for clinical and hemodynamic assessment. All research visits were conducted in the Non‐invasive Arterial Hemodynamics Research Laboratory at University Ottawa Heart Institute in ON, Canada. All participants completed a standardized questionnaire including past medical, gynecological, and obstetrical history, current medication use, and details regarding the index pregnancy evolution and outcomes. Race was self‐reported based on categories from Statistics Canada. 27 Brachial blood pressure (BP) was measured 3 times, 2 minutes apart, with participants in supine position using an electronic sphygmomanometer (Non‐Invasive Hemodynamics, Cardiovascular Engineering Inc., Norwood, MA) according to established protocols for this arterial hemodynamic assessment 28 , 29 ; the average measure was used for analyses. Brachial pulse pressure was calculated as brachial systolic (SBP) – diastolic (DBP) blood pressures. Mean arterial pressure was calculated as [SBP+ (2×DBP)]/3. Chronic hypertension was defined as current use of antihypertensives or physician diagnosis. Diabetes was defined as physician diagnosis or use of insulin or oral hypoglycemic agents. Smoking history was defined as having smoked ≥100 cigarettes in their lifetime. Anthropometric measurements were collected during the study visit, and body mass index was calculated in kg/m2. A blood sample was drawn for fasting lipids, hemoglobin A1c, and serum creatinine. Lifetime risk of cardiovascular disease (to age 95 years) was calculated for each participant according to the QRisk‐lifetime model. 30 The study was conducted according to the principles from the Declaration of Helsinki. This protocol was approved by the University of Ottawa Heart Institute's Research Ethics Board and all participants provided written informed consent.
Noninvasive Arterial Hemodynamic Assessment
Arterial tonometry integrated with echocardiography was performed to comprehensively characterize the arterial hemodynamics of each participant. All assessments were performed by 1 of 3 trained cardiac sonographers with substantial experience in arterial tonometry and the study protocol. All data were digitized during the primary acquisition and analyzed using a custom‐built software capable of analysis of the pressure and flow data obtained (Cardiovascular Engineering Inc., Norwood, MA).
The noninvasive hemodynamic assessment has been previously described by our group 28 , 31 , 32 and is summarized in Data S1. Variables were obtained to assess 5 main elements of arterial hemodynamics: aortic stiffness, central BP, pulsatile and steady arterial loads, and arterial wave reflections. For each participant, we measured (1) cfPWV, which is the criterion standard measure of aortic stiffness; (2) steady arterial load (systemic vascular resistance, which is determined by the resistance imposed by peripheral arteries and arterioles); (3) pulsatile arterial load (aortic characteristic impedance, representing the pressure/flow relationship in the proximal aorta in early systole; proximal aortic and total arterial compliances; and the amplitude of the forward pressure wave, which is generated by the interaction of the beating left ventricle with the elastic properties of the proximal aorta); (4) arterial wave reflections (amplitude of the reflected pressure wave, global reflection coefficient, representing the amplitude of the reflected pressure wave relative to amplitude of the forward pressure wave; and the augmentation index, representing the proportion of the central pulse pressure attributed to augmented pressure); and (5) central systolic and diastolic pressures, and central pulse pressure calculated as central systolic BP – central diastolic BP. EVA was defined as having a measured cfPWV higher than the estimated cfPWV based on each person's mean arterial pressure and age, as in previously published reference values. 33 , 34
Statistical Analysis
Continuous data are presented as mean±SD if close to being normally distributed, or as median and interquartile range if skewed. Nominal data are presented as n (%). Characteristics of participants were compared using a t test for normally distributed continuous variables, a Wilcoxon rank‐sum test for skewed continuous variables, the χ2 for nominal variables, and the Fisher exact test for nominal variables with low counts (≤5). The independent association of preeclampsia with arterial hemodynamics was assessed with multivariable linear regression models progressively adjusted for: age (Model 1); age, body mass index, serum creatinine, and history of hypertension (Model 2); and age, body mass index, serum creatinine, gravidity, history of hypertension, hyperlipidemia, diabetes, and smoking (Model 3). In subgroup analyses, we repeated the multivariable linear regression models, this time subdividing the preeclampsia group based on preeclampsia severity (preeclampsia with and without severe features), timing of onset (preterm versus term), and recurrence (single preeclamptic episode versus recurrent preeclampsia). For these subgroup analyses, a new variable with 3 levels was used in the models instead (ie, severe preeclampsia, nonsevere preeclampsia and controls; preterm preeclampsia, term preeclampsia and controls; recurrent preeclampsia, single preeclampsia and controls), always using the control group as reference. Results were presented as the adjusted mean difference for each hemodynamic variable among women with preeclampsia as compared with controls, and its 95% CI. Lastly, we performed multivariable logistic regression models to predict EVA based on nonsevere versus severe preeclampsia status, while adjusting the models for the same covariates as in the linear regression models above. A 2‐sided P value <0.05 was considered statistically significant. Statistical analyses were executed with the software JMP version 13 (SAS Institute Inc, Cary, NC). The corresponding author had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Our sample included 40 women with previous preeclampsia and 40 age‐matched controls with previous normotensive pregnancies, with a mean age 35.8±3.9 years for the whole sample. A summary of our study's design and results is depicted in Figure 1. None of the participants had fetal demise or pregnancy loss with the index pregnancy. Participant characteristics are summarized in Table 1 and compared between preeclampsia and control groups. Both groups were similar in age, gravidity, number of living children, history of smoking, renal function, and family history of coronary artery disease or hypertensive disorders of pregnancy. Women with a history of preeclampsia had a greater burden of cardiometabolic abnormalities, demonstrated by a higher body mass index, larger waist circumference, higher brachial SBP, DBP, and mean arterial pressure, hemoglobin A1c, triglycerides, total and low‐density lipoprotein cholesterol, and higher prevalence of prepregnancy hypertension and diabetes as compared with controls. Because of this, women with preeclampsia had a higher estimated lifetime cardiovascular risk than controls (33.2% versus 25.5% to age 95 years, respectively, P=0.0007). Six (15%) of the 40 women with previous preeclampsia were still taking antihypertensive medications at the time of study participation, which were withheld 12 hours before the assessment as per the protocol of the study.
Figure 1. The EVA study.
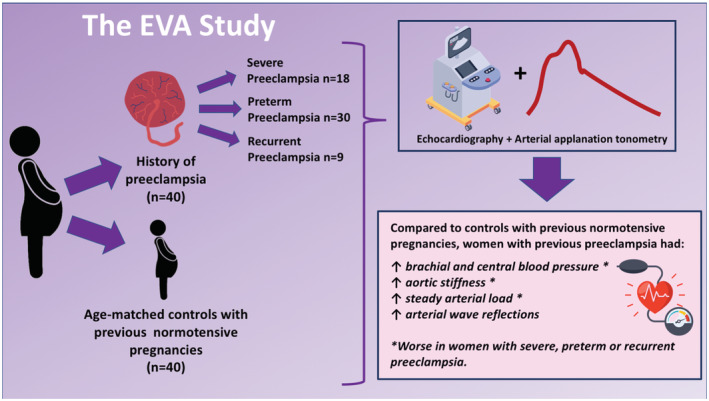
EVA indicates early vascular aging.
Table 1.
Participant Characteristics
| Variable | Preeclampsia (n=40) | Controls (n=40) | P value |
|---|---|---|---|
| Age, y | 35.7±4.5 | 35.9±3.1 | 0.863* |
| Race and ethnicity (n=67) | |||
| White, n (%) | 29 (88%) | 31 (91%) | 0.514 |
| Black, n (%) | 0 | 1 (3%) | |
| Hispanic/Latino, n (%) | 1 (3%) | 2 (6%) | |
| Filipino, n (%) | 2 (6%) | 0 | |
| Other, n (%) | 1 (3%) | 0 | |
| Weight, kg | 81.7±25.6 | 71.2±14.9 | 0.029* |
| Height, cm | 161.9±7.6 | 165±5.4 | 0.039* |
| BMI (kg/cm2) – median (IQR) | 27.8 (24.1–36.8) | 24.7 (21.8–29.5) | 0.020‡ |
| Waist circumference, cm | 98.8±20.2 | 86.2±13.3 | 0.002* |
| Brachial SBP mm Hg – median (IQR) | 109 (102–125) | 101 (95–106) | <0.0001‡ |
| Brachial DBP mm Hg – median (IQR) | 62 (57–72) | 54 (52–60) | <0.0001‡ |
| Brachial PP, mm Hg | 49.5±12.1 | 46±8.2 | 0.139* |
| MAP mm Hg – median (IQR) | 82 (76–93) | 72 (69–75) | <0.0001‡ |
| Gravidity | 0.841† | ||
| 1, n (%) | 12 (30%) | 10 (25%) | |
| 2, n (%) | 14 (35%) | 17 (43%) | |
| 3, n (%) | 6 (15%) | 7 (17%) | |
| >3, n (%) | 8 (20%) | 6 (15%) | |
| Living children | 0.101§ | ||
| 0, n (%) | 1 (3%) | 0 | |
| 1, n (%) | 18 (45%) | 12 (30%) | |
| 2, n (%) | 19 (47%) | 23 (57%) | |
| 3, n (%) | 0 | 4 (10%) | |
| >3, n (%) | 2 (5%) | 1 (3%) | |
| Time between last pregnancy and research study, y | 2.0±1.2 | 2.6±1.2 | 0.030* |
| Gestational diabetes, n (%) | 4 (10%) | 0 | 0.112§ |
| Onset of preeclampsia, GA in wks | 33.7±5.0 | … | … |
| Preterm preeclampsia, n (%) | 30 (75%) | … | … |
| Severe preeclampsia, n (%) | 18 (45%) | … | … |
| Recurrent preeclampsia, n (%) | 9 (22.5%) | … | … |
| History of smoking, n (%) | 13 (32.5%) | 9 (22.5%) | 0.316† |
| Pregestational hypertension, n (%) | 4 (10%) | 0 | 0.116§ |
| Pregestational diabetes, n (%) | 3 (7.5%) | 0 | 0.241§ |
| Total cholesterol, mmol/L | 5.0±0.9 | 4.5±1.0 | 0.041* |
| HDL cholesterol, mmol/L | 1.6±0.4 | 1.6±0.3 | 0.207* |
| LDL cholesterol, mmol/L | 2.9±0.7 | 2.4±0.8 | 0.012* |
| Triglycerides (mmol/L) – median (IQR) | 1.0 (0.6–1.5) | 0.8 (0.6–0.9) | 0.017‡ |
| Hemoglobin A1c, % | 5.3±0.4 | 5.1±0.3 | 0.010* |
| Serum creatinine, mmol/L | 62±8 | 62±8.9 | 0.742* |
| Estimated cardiovascular risk to age 95 y, %|| | 33.2±2.0 | 25.5±8.0 | 0.0009* |
| Family history of CAD, n (%) | 16 (40%) | 11 (27.5%) | 0.236† |
| Family history of HDP, n (%) | 6 (15%) | 7 (17.5%) | 0.762† |
BMI indicates body mass index; CAD, coronary artery disease; DBP, diastolic blood pressure; GA, gestational age; HDL, high‐density lipoprotein; HDP, hypertensive disorders of pregnancy; IQR, interquartile range; LDL, low‐density lipoprotein; MAP, mean arterial pressure; PP, pulse pressure; and SBP, systolic blood pressure.
Comparison made using a t test.
Comparison made using a χ2 test.
Comparison made using Wilcoxon rank‐sum test.
Comparison made using Fisher exact test.
Based on estimation from the Qrisk‐lifetime model. 30
Unadjusted comparisons of arterial hemodynamic measures between groups are depicted in Table 2 and Figure 2. Women with a history of preeclampsia had greater aortic stiffness, lower proximal aortic compliance and total arterial compliance, higher steady arterial load, and arterial wave reflections than controls, resulting in higher central BP in the preeclampsia group. Table S1 (Models 1–3) and Table 3 (fully adjusted Model 3 only) summarize the results of the multivariable linear regression models, demonstrating that women with a history of preeclampsia continued to exhibit significantly higher aortic stiffness, brachial and central BPs, steady arterial load, and arterial wave reflections than controls, despite adjustment for confounders. Since brachial and central SBP and DBP, mean arterial pressure, systemic vascular resistance, and reflected pressure wave amplitude were not normally distributed, we performed sensitivity analyses after removing the highest 5% values in each hemodynamic variable's distribution, and found that all variables except reflected pressure wave amplitude retained statistical significance (analyses not shown). These findings demonstrate the presence of significant arterial dysfunction affecting multiple hemodynamic domains in young women with a history of preeclampsia.
Table 2.
Unadjusted Comparison of Arterial Hemodynamics Between Preeclampsia and Controls
| Variable | Preeclampsia (n=40) mean±SD | Controls (n=40) mean±SD | P value |
|---|---|---|---|
| Aortic stiffness | |||
| cfPWV, m/s | 6.3±1.0 | 5.7±0.7 | 0.001* |
| e‐aPWV (m/s) – median (IQR) | 6.4 (5.9–7.0) | 5.9 (5.6–6.1) | <0.0001‡ |
| EVA, n (%) | 14 (45%) | 3 (18%) | 0.049† |
| Pulsatile arterial load | |||
| Zc, dyne × s × m−5 | 186.0±53.8 | 175.0±4.3.5 | 0.320* |
| Pf, mm Hg | 39±11 | 37±9 | 0.323* |
| PAC (10−6 cm4/dyne) – median (IQR) | 8.9 (6.9–10.4) | 10.0 (8.7–12.0) | 0.032‡ |
| TAC, mL/mm Hg | 1.5±0.5 | 1.7±0.4 | 0.049* |
| Central blood pressure | |||
| Central SBP, mm Hg | 110 (97–122) | 94 (90–100) | <0.0001‡ |
| Central DBP, mm Hg | 62 (58–72) | 54 (52–60) | <0.0001‡ |
| Central PP, mm Hg | 46±14 | 39±8 | 0.010* |
| Steady arterial load | |||
| SVR, dyne×s×cm−5 | 1881.0 (1639.0–2133.0) | 1571.5 (1397.5–1692.5) | <0.0001‡ |
| Arterial wave reflections | |||
| Pb, mm Hg | 13 (10–17) | 11 (10–13) | 0.031‡ |
| AIx, % | 10.1±9.6 | 1.0±8.6 | <0.0001* |
| GRC | 0.35±0.07 | 0.32±0.06 | 0.015* |
AIx indicates augmentation index; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; e‐aPWV, estimated aortic pulse wave velocity calculated based on age and mean arterial pressure; EVA, early vascular aging; GRC, global reflection coefficient; IQR, interquartile range; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; PP, pulse pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAC, total arterial compliance; and Zc, aortic characteristic impedance.
Comparison made using a t test.
Comparison made using a χ2 test.
Comparison made using Wilcoxon rank‐sum test.
Figure 2. Unadjusted comparisons of arterial hemodynamics between women with history of preeclampsia and controls with previous normotensive pregnancies.
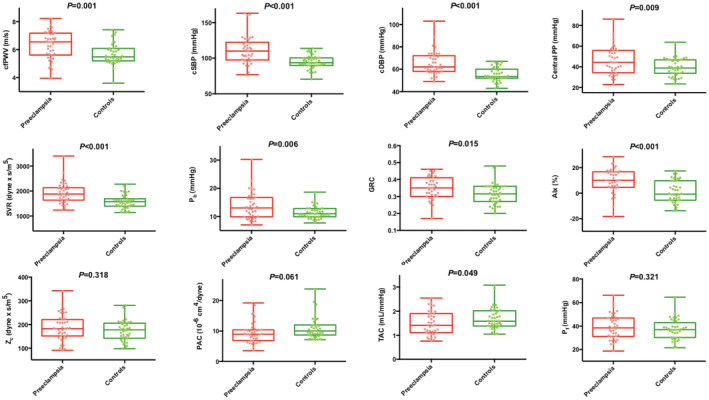
Top row: measures of aortic stiffness and central blood pressure. Middle row: measures of steady arterial load and arterial wave reflections. Bottom row: measures of pulsatile arterial load. P value for the ANOVA is indicated in each graphic. AIx indicates augmentation index; cfPWV, carotid‐femoral pulse wave velocity; cDBP, central diastolic blood pressure; cSBP, central systolic blood pressure; Central PP, central pulse pressure; GRC, global reflection coefficient; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; SVR, systemic vascular resistance; TAC, total arterial compliance; and Zc, aortic characteristic impedance.
Table 3.
Summary of Multivariable Linear Regression Models Comparing Arterial Hemodynamics Between Women With History of Preeclampsia and Controls
| Hemodynamic variable | Preeclampsia vs controls, aMD (95% CI) | P value |
|---|---|---|
| Brachial blood pressure | ||
| SBP, mm Hg | +7.2 (1.1 to 13.3) | 0.021 |
| DBP, mm Hg | +6.9 (2.8 to 10.9) | 0.001 |
| MAP, mm Hg | +8.0 (3.2 to 12.8) | 0.001 |
| Brachial PP, mm Hg | +0.4 (−4.7 to 5.4) | 0.888 |
| Central blood pressure | ||
| Central SBP, mm Hg | +10.0 (3.0 to 17.2) | 0.006 |
| Central DBP, mm Hg | +7.0 (2.9 to 11.01) | 0.001 |
| Central PP, mm Hg | +3.2 (−2.4 to 8.6) | 0.260 |
| Aortic stiffness | ||
| cfPWV, m/s | +0.42 (0.007 to 0.82) | 0.047 |
| Steady arterial load | ||
| SVR, dyne×s/m5 | +326.4 (170.2 to 482.6) | <0.0001 |
| Pulsatile arterial load | ||
| Zc,, dyne×s/m5 | −1.8 (−26.0 to 22.4) | 0.881 |
| PAC, 10−6 cm4/dyne | −0.35 (−2.13 to 1.44) | 0.701 |
| TAC, mL/mm Hg | −0.12 (−0.35 to 0.10) | 0.290 |
| Pf, mm Hg | −1.4 (−6.2 to 3.4) | 0.558 |
| Arterial wave reflections | ||
| Pb, mm Hg | +1.8 (0.2 to 3.5) | 0.033 |
| GRC | +0.05 (0.02 to 0.08) | 0.0004 |
| AIx, % | +8.7 (4.6 to 12.8) | <0.0001 |
Results are presented as the aMD for each hemodynamic measure between women with preeclampsia and controls, after adjustment for confounders in the multivariable model. The reported P value refers to the comparison of each hemodynamic measure between the two groups. AIx indicates augmentation index; aMD, adjusted mean difference; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; GRC, global reflection coefficient; MAP, mean arterial pressure; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; PP, pulse pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAC, total arterial compliance; and Zc, aortic characteristic impedance.
Subgroup Analyses Based on Preeclampsia Severity, Time of Onset, and Recurrence
Unadjusted differences in arterial hemodynamics across subgroups are depicted in Figures S1‐S3. Results of the multivariable linear regression models comparing arterial hemodynamics based on preeclampsia severity are reported in Table 4. There was a demonstrable gradient of worsening arterial abnormalities from controls to nonsevere preeclampsia, to severe preeclampsia. While both nonsevere and severe preeclampsia were independently associated with higher brachial and central SBP and DBP, and steady arterial load, the magnitude of these abnormalities (as compared with controls) was greater among women with severe preeclampsia. In addition, women with severe preeclampsia also exhibited greater aortic stiffness than controls, while women with nonsevere preeclampsia did not. Conversely, the extent of abnormalities in arterial wave reflections was greater among women with nonsevere preeclampsia, which can be explained by the greater aortic stiffness in severe preeclampsia, and consequent decreased wave reflections in that subgroup.
Table 4.
Summary of Multivariable Linear Regression Models Comparing Arterial Hemodynamics Among Women With History of Preeclampsia With and Without Severe Features, and Controls
| Variable | Nonsevere preeclampsia vs controls, aMD (95% CI) | Severe preeclampsia vs controls, aMD (95% CI) |
|---|---|---|
| Brachial blood pressure | ||
| SBP, mm Hg | +4.2 (−3.7, 12.05), P=0.293 | +9.1 (2.3, 15.9), P=0.010 |
| DBP, mm Hg | +6.2 (1.6–10.8), P=0.009 | +7.9 (2.7–13.2), P=0.004 |
| MAP, mm Hg | +8.9 (2.7–13.6), P=0.004 | +7.9 (1.7–14.2), P=0.014 |
| Brachial PP, mm Hg | +3.0 (−2.7, 8.6), P=0.295 | +3.8 (−10.2, 2.7), P=0.249 |
| Central blood pressure | ||
| Central SBP, mm Hg | +8.5 (−0.7, 17.7), P=0.070 | +11.2 (3.1–19.2), P=0.008 |
| Central DBP, mm Hg | +6.3 (1.6–11.0), P=0.010 | +8.0 (2.7–13.3), P=0.004 |
| Central PP, mm Hg | +5.0 (−1.4, 11.2), P=0.121 | +0.6 (−6.5, 7.6), P=0.882 |
| Aortic stiffness | ||
| cfPWV m/s | +0.19 (−0.26, 0.63), P=0.414 | +0.78 (0.27–1.29), P=0.003 |
| Steady arterial load | ||
| SVR, dyne×s/m5 | +290.4 (111.5–469.2), P=0.002 | +378.6 (178.0–579.4), P=0.0004 |
| Pulsatile arterial load | ||
| Zc, dyne×s/m5 | −1.67 (−29.53, 26.2), P=0.905 | −2.05 (−33.31, 29.22), P=0.896 |
| PAC, 10−6 cm4/dyne | −0.23 (−1.80, 2.26), P=0.820 | −1.20 (−3.47, 1.09), P=0.300 |
| TAC, mL/mm Hg | −0.09 (−0.35, 0.17), P=0.497 | −0.17 (−0.47, 0.13), P=0.256 |
| Pf, mm Hg | +0.1 (−5.3, 5.6), P=0.964 | −3.6 (−9.8, 2.5), P=0.240 |
| Arterial wave reflections | ||
| Pb, mm Hg | +2.7 (0.8–4.6), P=0.006 | +0.6 (−1.3, 2.7), P=0.593 |
| GRC | +0.06 (0.03–0.09), P=0.0003 | +0.04 (0.004–0.07), P=0.030 |
| AIx, % | +9.5 (4.9–14.2), P=0.001 | +7.5 (2.3–12.7), P=0.006 |
Results are presented as the aMD for each hemodynamic measure between women with preeclampsia and controls, after adjustment for confounders in the multivariable model. The reported P value refers to the comparison of each hemodynamic measure between the 2 groups. AIx indicates augmentation index; aMD, adjusted mean difference; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; GRC, global reflection coefficient; MAP, mean arterial pressure; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; PP, pulse pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAC, total arterial compliance; and Zc, aortic characteristic impedance.
Table 5 shows the results of the multivariable linear regression models based on the time of onset of preeclampsia. Women with preterm preeclampsia had significantly higher brachial and central BP, aortic stiffness, steady arterial load, arterial wave reflections, and lower total arterial compliance than controls. When preeclampsia was diagnosed after 37 weeks, abnormalities in steady arterial load, albeit to a lower magnitude than that observed in preterm preeclampsia, were noted. Additionally, arterial wave reflections were also higher in women with term preeclampsia than in controls. These findings support the notion that preterm preeclampsia may lead to a greater degree of arterial health damage, demonstrated by the worse arterial abnormalities observed in that group.
Table 5.
Summary of Multivariable Linear Regression Models Comparing Arterial Hemodynamics Among Women With a History of Preterm Preeclampsia, Term Preeclampsia, and Controls
| Variable | Term PE vs controls, aMD (95% CI) | Preterm PE vs controls, aMD (95% CI) |
|---|---|---|
| Brachial blood pressure | ||
| SBP, mm Hg | +1.5 (−7.4 to 10.2), P=0.749 | +10.1 (3.3 to 16.8), P=0.004 |
| DBP , mm Hg | +3.4 (−2.5 to 9.3), P=0.249 | +8.6 (4.1 to 13.1), P=0.003 |
| MAP, mm Hg | +1.9 (−4.9 to 8.7), P=0.581 | +11.1 (5.8 to 16.3), P<0.0001 |
| Brachial PP, mm Hg | −2.0 (−9.4 to 5.4), P=0.592 | +1.6 (−4.2 to 7.3), P=0.599 |
| Central blood pressure | ||
| Central SBP, mm Hg | +0.7 (−10.318 to 9.043), P=0.896 | +15.7 (8.03 to 23.3), P=0.0001 |
| Central DBP, mm Hg | +3.5 (−2.5 to 9.4), P=0.245 | +8.8 (4.2 to 13.5), P=0.0003 |
| Central PP, mm Hg | −4.1 (−11.7 to 3.6), P=0.286 | +6.9 (0.9 to 12.9), P=0.025 |
| Aortic stiffness | ||
| cfPWV, m/s | +0.17 (−0.42 to 0.76), P=0.574 | +0.52 (0.08 to 0.10), P=0.023 |
| Steady arterial load | ||
| SVR, dyne×s/m5 | +237.42 (12.41 to 462.44), P=0.039 | +372.75 (195.3 to 550.2), P<0.0001 |
| Pulsatile arterial load | ||
| Zc, dyne×s/m5 | +−32.27 (−65.93 to 1.34), P=0.060 | +14.05 (−12.5 to 2.14), P=0.295 |
| PAC, 10−6 cm4/dyne | +2.0 (−0.45 to 4.45), P=0.108 | −1.57 (−3.50 to 0.38), P=0.112 |
| TAC, mL/mm Hg | +0.16 (−0.16 to 0.48), P=0.320 | −0.27 (−0.51 to −0.02), P=0.036 |
| Pf, mm Hg | −7.83 (−14.43 to − 1.23), P=0.021 | +1.94 (−3.27 to 7.15), P=0.460 |
| Arterial wave reflections | ||
| Pb, mm Hg | +0.2 (−2.16 to 2.56), P=0.865 | +2.66 (0.80 to 4.52), P=0.006 |
| GRC | +0.07 (0.03 to 0.11), P=0.001 | +0.04 (0.01 to 0.07), P=0.008 |
| AIx, % | +9.73 (3.84 to 15.61), P=0.002 | +8.15 (3.51 to 12.8), P=0.0008 |
Results are presented as the aMD for each hemodynamic measure between women with preeclampsia and controls, after adjustment for confounders in the multivariable model. The reported P value refers to the comparison of each hemodynamic measure between the 2 groups. AIx indicates augmentation index; aMD, adjusted mean difference; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; GRC, global reflection coefficient; MAP, mean arterial pressure; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; PE, preeclampsia; PP, pulse pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAC, total arterial compliance; and Zc, aortic characteristic impedance.
Table 6 summarizes results of the multivariable linear regression models based on recurrence of the disease (1 versus 2 previous pregnancies affected with preeclampsia). Women who had recurrent preeclampsia exhibited significantly higher brachial and central BP, aortic stiffness, steady arterial load, and arterial wave reflections than controls. Conversely, women who had only 1 preeclamptic pregnancy had higher brachial and central BP, systemic vascular resistance, and arterial wave reflections compared with controls, albeit to a lower magnitude than in women with 2 preeclamptic pregnancies. This suggests cumulative insults to arterial health with each affected pregnancy, culminating with more extensive deteriorations in arterial health.
Table 6.
Summary of Multivariable Linear Regression Models Comparing Arterial Hemodynamics Among Women With History of Preeclampsia Recurrence, Single Preeclampsia, and Controls
| Variable | 1 pregnancy affected with preeclampsia vs controls, aMD (95% CI) | 2 pregnancies affected with preeclampsia vs controls, aMD (95% CI) |
|---|---|---|
| Brachial blood pressure | ||
| SBP, mm Hg | +5.9 (−0.6 to 12.6), P=0.074 | +11.0 (1.3 to 20.7), P=0.027 |
| DBP, mm Hg | +4.8 (0.6 to 9.1), P=0.024 | +13.0 (6.8 to 19.2) P<0.0001 |
| MAP, mm Hg | +5.9 (0.9 to 11.0), P=0.021 | +14.3 (6.8 to 21.7), P=0.0003 |
| Brachial PP, mm Hg | +1.1 (−4.3 to 6.5), P=0.683 | +2.0 (−10.1 to 6.1), P=0.623 |
| Central blood pressure | ||
| Central SBP, mm Hg | +8.2 (0.5 to 15.9), P=0.036 | +15.6 (4.4 to 26.7), P=0.007 |
| Central DBP, mm Hg | +4.5 (0.5 to 9.2), P=0.027 | +13.0 (6.8 to 19.3), P<0.0001 |
| Central PP, mm Hg | +3.4 (−2.7 to 9.2), P=0.273 | +2.5 (−6.1 to 11.3), P=0.558 |
| Aortic stiffness | ||
| cfPWV, m/s | +0.36 (−0.08 to 0.79), P=0.101 | +0.58 (−0.06 to 1.2), P=0.077 |
| Steady arterial load | ||
| SVR, dyne×s/m5 | +280.28 (112.49 to 448.1), P=0.001 | +462.18 (217.65 to 706.71), P=0.0003 |
| Pulsatile arterial load | ||
| Zc, dyne×s/m5 | −1.65 (−27.94 to 24.85), P=0.908 | +2.65 (−41.11 to 35.81), P=0.892 |
| PAC, 10−6 cm4/dyne | −0.06 (−1.99 to 1.87), P=0.949 | +1.18 (−3.98 to 1.62), P=0.403 |
| TAC, mL/mm Hg | −0.07 (−0.31 to 0.17), P=0.550 | −0.26 (−0.061 to 0.09), P=0.151 |
| Pf, mm Hg | −2.4 (−9.98 to 5.19), P=0.531 | −1.07 (−6.28 to 4.13), P=0.682 |
| Arterial wave reflections | ||
| Pb, mm Hg | +1.84 (−0.8 to 4.5), P=0.168 | +1.80 (−0.007 to 3.62), P=0.051 |
| GRC | +0.05 (0.02 to 0.08), P=0.001 | +0.05 (0.01 to 0.09), P=0.016 |
| AIx, % | +8.42 (4.0 to 12.9), P=0.0003 | +9.47 (3.03 to 15.92), P=0.005 |
Results are presented as the aMD for each hemodynamic measure between women with preeclampsia and controls, after adjustment for confounders in the multivariable model. The reported P value refers to the comparison of each hemodynamic measure between the 2 groups. AIx indicates augmentation index; aMD, adjusted mean difference; cfPWV, carotid‐femoral pulse wave velocity; DBP, diastolic blood pressure; GRC, global reflection coefficient; MAP, mean arterial pressure; PAC, proximal aortic compliance; Pb, reflected pressure wave amplitude; Pf, forward pressure wave amplitude; PP, pulse pressure; SBP, systolic blood pressure; SVR, systemic vascular resistance; TAC, total arterial compliance; and Zc, aortic characteristic impedance.
To enhance the interpretation of our findings, we compared the cfPWV of each study group with published reference values for cfPWV for people in their 30s (similar age to our participants). 34 Based on the average increase in cfPWV of 0.7 m/s per decade, and the adjusted mean difference of 0.42 m/s between any preeclampsia and controls, 0.78 m/s between severe preeclampsia and controls, 0.52 m/s between preterm preeclampsia and controls, and 0.58 m/s between recurrent preeclampsia and controls, these values represented an additional 6 years in vascular age for any preeclampsia, additional 7 to 8 years of vascular age for preterm or recurrent preeclampsia, and additional 11 years of vascular age for severe preeclampsia, as compared with controls.
Lastly, in multivariable logistic regression models, the presence of severe preeclampsia was associated with 9.23 (95% CI, 1.67–51.06, P=0.011) greater odds of EVA as compared with controls and 7.87 (95% CI, 1.29–47.77, P=0.025) greater odds of EVA as compared with women with nonsevere preeclampsia. The nonsevere preeclampsia group had similar odds of EVA as compared with controls (odds ratio: 1.17, 95% CI, 0.29–4.77, P=0.824).
Discussion
Our study has several novel findings: (1) women with a history of preeclampsia display significant abnormalities in multiple domains of arterial hemodynamics as compared with age‐and sex‐matched controls; (2) alterations in arterial health and hemodynamics are more pronounced in severe, preterm, and recurrent preeclampsia as compared with their nonsevere, term, and nonrecurrent counterparts, suggesting a dose–response mechanism; (3) in this young age group (35.8±3.9 years), arterial health abnormalities seen after preeclampsia were restricted to brachial and central BP, aortic stiffness, steady arterial load and arterial wave reflections, without independent effects on pulsatile arterial load; (4) having had preeclampsia added ≈6 years to the vascular age of affected women; and (5) EVA was significantly more likely to be present in women with severe preeclampsia than in nonsevere preeclampsia or controls. In conjunction, our findings highlight that preeclampsia independently correlates with worse arterial health and EVA postpartum, with a dose–response relationship based on preeclampsia severity, time of onset, and recurrence.
To our knowledge, our study is the first to comprehensively characterize multiple domains of arterial hemodynamics after preeclampsia, and to extend this characterization to subgroups. Importantly, our study identifies women with previous preeclampsia who display the greatest abnormalities in arterial health and EVA (severe, preterm, or recurrent preeclampsia) and who may benefit most from early, targeted efforts at risk stratification and primary prevention of cardiovascular disease.
Preeclampsia and Arterial Health
One potential mechanism for higher BP in preeclampsia would be reduced ability of a dysfunctional kidney to participate in long‐term BP control. However, in our study renal function was not statistically different between preeclampsia and control groups. Furthermore, our findings were independent of serum creatinine, suggesting that the abnormalities in arterial hemodynamics found among women with preeclampsia were independent of renal function. Previous studies demonstrate adverse effects of preeclampsia on endothelial function and arterial hemodynamics, even after clinical signs of the disease have resolved. 35 , 36 Mechanistic links between preeclampsia and vascular damage eventually culminating with cardiovascular disease remain incompletely understood. Various theories have postulated that preeclampsia is a consequence of endothelial dysfunction that leads to vasoconstriction, hypoxic–ischemic damage, and oxidative stress at the vascular level. 5 , 16 , 36 , 37 In most preeclamptic women, the impaired placentation fails to accomplish vascular remodeling of spiral arteries during early pregnancy, with consequent tissue hypoxia that triggers a release of toxic antiangiogenic factors such as soluble fms‐like tyrosinekinase‐1, soluble endoglin, and other inflammatory mediators, which enter the maternal circulation and induce endothelial dysfunction. 16 , 38 Soluble fms‐like tyrosinekinase‐1 is believed to be the main factor released in the pathogenesis of preeclampsia. 39 It primarily counteracts the function of pro‐angiogenic factors that usually reduce microvascular resistance, including vascular endothelial growth factor and placental growth factor, by binding to them and preventing their ability to act on the endothelium. 38 These angiogenic factors have been associated with cardiovascular disease and heart failure in the postpartum period. 3 , 16
Previous studies have also shown low availability of NO, a potent vasodilator, in women with preeclampsia. 3 , 40 Such changes seen during pregnancy and in the postpartum period are linked to alterations in arterial flow‐mediated dilatation, implying endothelial functional abnormalities in those women. Additional mechanisms have been linked to preeclampsia and cardiovascular disease, as the inherent endothelial hypersensitivity to the angiotensin II receptor that leads to increased vasoconstriction in women with preeclampsia. Additionally, the increased sympathetic nervous system activity associated with the disease leads to microvascular dysfunction and impaired vasodilatation, which is directly associated with a higher risk of cardiovascular disease. 3 , 16 , 38
EVA and Preeclampsia: Cause or Consequence?
Despite the substantial evidence linking preeclampsia to poor maternal arterial health and premature cardiovascular disease, there remains a knowledge gap regarding the temporality of these associations. Previous studies have suggested that women with underlying poor arterial health are predisposed to preeclampsia. 20 , 41 In this scenario, pregnancy would serve as a “natural stress test” leading to unmasking of underlying vascular abnormalities and identification of women at high risk of cardiovascular disease. This proposition defines preeclampsia as a marker, rather than a cause, of suboptimal cardiovascular health. Conversely, other studies have suggested that the oxidative stress caused by the ischemic placenta permanently impairs the mother's arterial health, 7 , 16 , 36 which in turn initiates a series of arterial structural and functional insults that culminate in cardiovascular disease later in life. This proposition defines preeclampsia as a causative agent for cardiovascular disease in affected women. As a parallel observation, the same frameworks can be transposed to the realm of arterial hemodynamics and EVA: Are women with underlying EVA and worse arterial hemodynamics, who are perhaps predestined to have cardiovascular disease, more likely to develop preeclampsia when pregnant? Or do the toxemic effects of preeclampsia start a process of vascular damage in previously healthy women, leading to worse arterial hemodynamics and EVA months to years later? Although our study significantly advances our understanding of arterial health and function after preeclampsia, its cross‐sectional nature prevents us from unequivocally concluding the sequence of events. To definitively answer this question, longitudinal studies that compare arterial hemodynamics preconception, throughout pregnancy, and then postpartum are necessary.
Severe, Preterm, and Recurrent Preeclampsia: Greater Threats to the Arterial Health of Women
Our subgroup analyses revealed that women with severe, preterm, and recurrent preeclampsia exhibited worse arterial hemodynamics than controls, translated into greater aortic stiffness, systemic vascular resistance, and arterial wave reflections, and contributed to higher central and brachial BPs. Importantly, we found a dose–response gradient of arterial abnormalities because women with severe, preterm, or recurrent preeclampsia had worse arterial hemodynamics than women with nonsevere, term, or nonrecurrent preeclampsia, who in turn had worse arterial health than women with previously normotensive pregnancies. Furthermore, the greatest susceptibility to EVA was seen among women with severe forms of preeclampsia. Our findings are in concordance to those from Benschop et al, who defined EVA based on normative values for carotid intima‐media thickness and demonstrated that women with severe preeclampsia who had worse cardiovascular health scores also had older vascular age. 42
Previous studies support that the longer women are exposed to preeclampsia (as observed in preterm and recurrent preeclampsia), the more pronounced are the hemodynamic insults to the arterial tree. 5 , 17 , 36 In this context, it is plausible that greater magnitudes of cardiovascular disruptions of more adverse subgroups of preeclampsia would translate into a more significant dysregulation of arterial health and dysfunction in the postpartum period.
These findings have important clinical implications, because although preeclampsia affects many pregnancies and increases risk of cardiovascular disease, most preeclamptic women do not develop cardiovascular events. As such, public health policies mandating intense, annual cardiovascular risk stratification and screening of all reproductive‐age women with history of preeclampsia for many decades are likely unfeasible and potentially not cost‐beneficial. Thus, it is of critical importance to identify subgroups of women with preeclampsia with the highest risk of future cardiovascular events, in order to focus screening and preventative efforts on those who are most likely to benefit from them. Based on our findings, we identified women with severe, preterm, or recurrent preeclampsia as those exhibiting the greatest burden of arterial health abnormalities. Given the known associations of EVA and arterial hemodynamic alterations with future cardiovascular events, 8 , 14 , 15 these subgroups of women may represent focused targets for enhanced cardiovascular risk assessment and preventative strategies, which remains amenable to testing in future longitudinal studies and clinical trials.
Strengths and Limitations
The main strengths of our study are the most extensive noninvasive arterial evaluation described to date in women with preeclampsia, and the ability to demonstrate a dose–response effect by comprehensively characterizing arterial health in subgroups of women with worsening features of preeclampsia. Furthermore, all participants were assessed 6 months postpartum or later, when the physiological hormonal and hemodynamic modifications of pregnancy are resolved.
Our study is not without limitations. First, invasive measurement of arterial hemodynamics would be considered criterion standard. However, our noninvasive protocol has been previously validated and shown to correlate well with invasive measures, 43 in addition to having high reproducibility. 44 , 45 Importantly, the noninvasive nature of the assessment avoids the inherent risks of arterial catheterization. We did not have information on advanced biochemical markers such as free fatty acids, which remains amenable to evaluation in future studies. Lastly, the cross‐sectional nature of our analyses does not allow us to determine the causality or temporality of the associations found.
Conclusions and Future Directions
Women with a history of preeclampsia display significant alterations in aortic stiffness, central BP, steady arterial load, and arterial wave reflections compared with age‐matched women with previously normotensive pregnancies, confirming insults to arterial health that culminate in EVA in this population. In addition, we observed a dose–response effect, with worse alterations to arterial health observed among women with severe, preterm, or recurrent preeclampsia. The presence of preeclampsia alone added the equivalent of 6 additional years of vascular age to a woman, while early and recurrent preeclampsia added the equivalent of 7 to 8 years, and severe preeclampsia amounted to an additional 11 years of arterial age to affected women. These findings robustly confirm preeclampsia as an independent determinant of worse arterial health and EVA, and highlight women with early, recurrent, or severe preeclampsia as potentially higher risk individuals deserving focused scientific and clinical attention to mitigate their future risk of cardiovascular events. Our study serves as a solid foundation for future (1) longitudinal studies aimed at establishing the association of aortic stiffness and arterial hemodynamic measures with future incidence of cardiovascular events, (2) cross‐sectional studies to establish normative values for these measures in young women, and (3) randomized controlled trials using measures of aortic stiffness and arterial hemodynamics as therapeutic targets after preeclampsia. This ongoing investigation is necessary for incorporation of these hemodynamic measures in clinical practice after preeclampsia.
Sources of Funding
This study was funded by an internal, peer‐reviewed Heart Team grant from the University of Ottawa Heart Institute Foundation.
Disclosures
TC is supported by the Chair in Women's Cardiovascular Health at the University of Ottawa Heart Institute Foundation. AP is supported by doctoral/fellowship scholarships from the Canadian Institutes for Health Research (Canada), Fonds de recherche du Québec – Santé (Québec, Canada), Association des Cardiologues du Québec (Québec, Canada), Laval University Dean's Scholarship (Québec, Québec, Canada), and Réseau de recherche en cardiométabolique, diabète et obésité (Québec, Canada). AW: None.
Supporting information
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.028116
For Sources of Funding and Disclosures, see page 12.
References
- 1. Magee LA, Smith GN, Bloch C, Côté A‐M, Jain V, Nerenberg K, von Dadelszen P, Helewa M, Rey E. Guideline No. 426: hypertensive disorders of pregnancy: diagnosis, prediction, prevention, and management. J Obstet Gynaecol Canada. 2022;44:547–571.e1. doi: 10.1016/j.jogc.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 2. Canadian Institute for Health Information DAD (DAD) . Health Canada. 2014. Accessed August 20, 2022. https://www.canada.ca/en/public‐health/services/publications/healthy‐living/maternal‐hypertension‐canada.html.
- 3. Stanhewicz AE. Residual vascular dysfunction in women with a history of preeclampsia. Am J Physiol Integr Comp Physiol. 2018;315:R1062–R1071. doi: 10.1152/ajpregu.00204.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170:1–7. doi: 10.1016/j.ejogrb.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 5. Yinon Y, Kingdom JCP, Odutayo A, Moineddin R, Drewlo S, Lai V, Cherney DZI, Hladunewich MA. Vascular dysfunction in women with a history of preeclampsia and intrauterine growth restriction. Circulation. 2010;122:1846–1853. doi: 10.1161/CIRCULATIONAHA.110.948455 [DOI] [PubMed] [Google Scholar]
- 6. Lin Y‐S, Tang C‐H, Yang C‐YC, Wu L‐S, Hung S‐T, Hwa H‐L, Chu P‐H. Effect of pre‐eclampsia–eclampsia on major cardiovascular events among peripartum women in Taiwan. Am J Cardiol. 2011;107:325–330. doi: 10.1016/j.amjcard.2010.08.073 [DOI] [PubMed] [Google Scholar]
- 7. Ghossein‐Doha C, Khalil A, Lees CC. Maternal hemodynamics: a 2017 update. Ultrasound Obstet Gynecol. 2017;49:10–14. doi: 10.1002/uog.17377 [DOI] [PubMed] [Google Scholar]
- 8. Bruno RM, Nilsson PM, Engström G, Wadström BN, Empana J‐P, Boutouyrie P, Laurent S. Early and supernormal vascular aging. Hypertension. 2020;76:1616–1624. doi: 10.1161/HYPERTENSIONAHA.120.14971 [DOI] [PubMed] [Google Scholar]
- 9. Usselman CW, Adler TE, Coovadia Y, Leone C, Paidas MJ, Stachenfeld NS. A recent history of preeclampsia is associated with elevated central pulse wave velocity and muscle sympathetic outflow. Am J Physiol Circ Physiol. 2020;318:H581–H589. doi: 10.1152/ajpheart.00578.2019 [DOI] [PubMed] [Google Scholar]
- 10. Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovasc Imaging. 2011;4:754–761. doi: 10.1016/j.jcmg.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coutinho T, Pellikka PA, Bailey KR, Turner ST, Kullo IJ. Sex differences in the associations of hemodynamic load with left ventricular hypertrophy and concentric remodeling. Am J Hypertens. 2016;29:73–80. doi: 10.1093/ajh/hpv071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zamani P, Bluemke DA, Jacobs DR, Duprez DA, Kronmal R, Lilly SM, Ferrari VA, Townsend RR, Lima JA, Budoff M, et al. Resistive and pulsatile arterial load as predictors of left ventricular mass and geometry. Hypertension. 2015;65:85–92. doi: 10.1161/HYPERTENSIONAHA.114.04333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cunha PG, Boutouyrie P, Nilsson PM, Laurent S. Early vascular ageing (EVA): definitions and clinical applicability. Curr Hypertens Rev. 2017;13:8–15. doi: 10.2174/1573402113666170413094319 [DOI] [PubMed] [Google Scholar]
- 14. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen C‐H, Cruickshank JK, et al. Aortic pulse wave velocity improves cardiovascular event prediction. J Am Coll Cardiol. 2014;63:636–646. doi: 10.1016/j.jacc.2013.09.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with arterial stiffness. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061 [DOI] [PubMed] [Google Scholar]
- 16. Hausvater A, Giannone T, Sandoval Y‐HG, Doonan RJ, Antonopoulos CN, Matsoukis IL, Petridou ET, Daskalopoulou SS. The association between preeclampsia and arterial stiffness. J. Hypertens. 2012;30:17–33. doi: 10.1097/HJH.0b013e32834e4b0f [DOI] [PubMed] [Google Scholar]
- 17. Franz MB, Burgmann M, Neubauer A, Zeisler H, Sanani R, Gottsauner‐Wolf M, Schiessl B, Andreas M. Augmentation index and pulse wave velocity in normotensive and pre‐eclamptic pregnancies. Acta Obstet Gynecol Scand. 2013;92:960–966. doi: 10.1111/aogs.12145 [DOI] [PubMed] [Google Scholar]
- 18. Endemann DH, Schiffrin E. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–1992. doi: 10.1097/01.ASN.0000132474.50966.DA [DOI] [PubMed] [Google Scholar]
- 19. Kinlay S, Ganz P. Role of endothelial dysfunction in coronary artery disease and implications for therapy. Am. J. Cardiol. 1997;80:11I–16I. doi: 10.1016/S0002-9149(97)00793-5 [DOI] [PubMed] [Google Scholar]
- 20. Thilaganathan B, Ghossein‐Doha C, Khalil A, Lees CC, Franz MB, Burgmann M, Neubauer A, Zeisler H, Sanani R, Gottsauner‐Wolf M, et al. Arterial stiffness in women previously with preeclampsia from a semi‐rural region of South Africa. Ultrasound Obstet Gynecol. 2017;49:S287–S288. doi: 10.1016/j.preghy.2014.10.145 [DOI] [Google Scholar]
- 21. Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population‐based retrospective cohort study. Lancet. 2005;366:1797–1803. doi: 10.1016/S0140-6736(05)67726-4 [DOI] [PubMed] [Google Scholar]
- 22. Dall'Asta A, D'Antonio F, Saccone G, Buca D, Mastantuoni E, Liberati M, Flacco ME, Frusca T, Ghi T. Cardiovascular events following pregnancy complicated by pre‐eclampsia with emphasis on comparison between early‐ and late‐onset forms: systematic review and meta‐analysis. Ultrasound Obstet Gynecol. 2021;57:698–709. doi: 10.1002/uog.22107 [DOI] [PubMed] [Google Scholar]
- 23. Auger N, Fraser WD, Schnitzer M, Leduc L, Healy‐Profitós J, Paradis G. Recurrent pre‐eclampsia and subsequent cardiovascular risk. Heart. 2017;103:235–243. doi: 10.1136/heartjnl-2016-309671 [DOI] [PubMed] [Google Scholar]
- 24. Brouwers L, van der Meiden‐van RA, Savelkoul C, Vogelvang T, Lely A, Franx A, van Rijn B. Recurrence of pre‐eclampsia and the risk of future hypertension and cardiovascular disease: a systematic review and meta‐analysis. BJOG An Int J Obstet Gynaecol. 2018;125:1642–1654. doi: 10.1111/1471-0528.15394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Najjar SS, Scuteri A, Lakatta EG. Arterial aging. Hypertension. 2005;46:454–462. doi: 10.1161/01.HYP.0000177474.06749.98 [DOI] [PubMed] [Google Scholar]
- 26. Roberts J, August P, Bakris G, Barton J, Bernstein I, Druzin M, Gaiser R, Granger J, Jeyabalan A, Johnson D, et al. Hypertension in pregnancy. Obstet Gynecol. 2013;122:1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88 [DOI] [PubMed] [Google Scholar]
- 27. Visible Minority and Population Group Reference Guide . Census of Population, 2021, Statistics Canada (Statistique Canada). 2022. Accessed February 20, 2023. https://www12.statcan.gc.ca/census‐recensement/2021/ref/98‐500/006/98‐500‐x2021006‐eng.cfm.
- 28. Coutinho T, Borlaug BA, Pellikka PA, Turner ST, Kullo IJ. Sex differences in arterial stiffness and ventricular‐arterial interactions. J Am Coll Cardiol. 2013;61:96–103. doi: 10.1016/j.jacc.2012.08.997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hippisley‐Cox J, Coupland C, Robson J, Brindle P. Derivation, validation, and evaluation of a new QRISK model to estimate lifetime risk of cardiovascular disease: cohort study using QResearch database. BMJ. 2010;341:c6624. doi: 10.1136/bmj.c6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boczar KE, Boodhwani M, Beauchesne L, Dennie C, Chan KL, Wells GA, Coutinho T. Aortic stiffness, central blood pressure, and pulsatile arterial load predict future thoracic aortic aneurysm expansion. Hypertension. 2021;77:126–134. doi: 10.1161/HYPERTENSIONAHA.120.16249 [DOI] [PubMed] [Google Scholar]
- 32. Rooprai J, Boodhwani M, Beauchesne L, Chan K, Dennie C, Nagpal S, Messika‐Zeitoun D, Coutinho T. Thoracic aortic aneurysm growth in bicuspid aortic valve patients: role of aortic stiffness and pulsatile hemodynamics. J Am Heart Assoc. 2019;8:8. doi: 10.1161/JAHA.118.010885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Diaz A, Zocalo Y, Bia D, Wray S, Cabrera DE. Reference intervals and percentiles for carotid‐femoral pulse wave velocity in a healthy populaion aged between 9 and 87 years. J Clin Hypertens. 2018;20:659–671. doi: 10.1111/jch.13251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattace‐Raso F, Hofman A, Verwoert G, Wittemana J, WIlkinson I, Dolejsova M. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values.’ Eur Heart J. 2010;31:2338–2350. doi: 10.1093/eurheartj/ehq165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Amaral L, Cunningham MW Jr, Cornelius D, LaMarca B. Preeclampsia: long‐term consequences for vascular health. Vasc Health Risk Manag. 2015;11:403–415. doi: 10.2147/VHRM.S64798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orabona R, Sciatti E, Vizzardi E, Bonadei I, Valcamonico A, Metra M, Frusca T. Endothelial dysfunction and vascular stiffness in women with previous pregnancy complicated by early or late pre‐eclampsia. Ultrasound Obstet Gynecol. 2017;49:116–123. doi: 10.1002/uog.15893 [DOI] [PubMed] [Google Scholar]
- 37. Palei AC, Spradley FT, Warrington JP, George EM, Granger JP. Pathophysiology of hypertension in pre‐eclampsia: a lesson in integrative physiology. Acta Physiol. 2013;208:224–233. doi: 10.1111/apha.12106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kirollos S, Skilton M, Patel S, Arnott C. A systematic review of vascular structure and function in pre‐eclampsia: non‐invasive assessment and mechanistic links. Front Cardiovasc Med. 2019;6:6. doi: 10.3389/fcvm.2019.00166/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maison SG, Pilote L, Okano M, Landry T, Dayan N. Markers of vascular dysfunction after hypertensive disorders of pregnancy. A systematic review and meta‐analysis. Hypertension. 2016;68:1447–1458. doi: 10.1161/HYPERTENSIONAHA.116.07907 [DOI] [PubMed] [Google Scholar]
- 40. Breetveld NM, Ghossein‐Doha C, van Neer J, Sengers MJJM, Geerts L, van Kuijk SMJ, van Dijk AP, van der Vlugt MJ, Heidema WM, Brunner‐La Rocca HP, et al. Decreased endothelial function and increased subclinical heart failure in women several years after pre‐eclampsia. Ultrasound Obs Gynecol. 2018;52:196–204. doi: 10.1002/uog.17534 [DOI] [PubMed] [Google Scholar]
- 41. Thilaganathan B. Placental syndromes: getting to the heart of the matter. Ultrasound Obstet Gynecol. 2017;49:7–9. doi: 10.1002/uog.17378 [DOI] [PubMed] [Google Scholar]
- 42. Benschop L, Schelling SJ, Duvekot JJ, Roeters van Lennep JE. Cardiovascular health and vascular age after severe preeclampsia: a cohort study. Atherosclerosis. 2020;292:136–142. doi: 10.1016/j.atherosclerosis.2019.11.023 [DOI] [PubMed] [Google Scholar]
- 43. Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-V [DOI] [PubMed] [Google Scholar]
- 44. Mitchell GF, Lacourcière Y, Ouellet J‐P, Izzo JL, Neutel J, Kerwin LJ, Block AJ, Pfeffer MA. Determinants of elevated pulse pressure in middle‐aged and older subjects with uncomplicated systolic hypertension. Circulation. 2003;108:1592–1598. doi: 10.1161/01.CIR.0000093435.04334.1F [DOI] [PubMed] [Google Scholar]
- 45. Mitchell GF, Tardif J‐C, Arnold JMO, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433–1439. doi: 10.1161/hy1201.098298 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


