Abstract
Background
South Asian individuals are at higher risk for arteriosclerotic cardiovascular disease and diabetes. The factors associated with arteriosclerotic cardiovascular disease severity and their interactions are unknown.
Methods and Results
This is a retrospective cohort study of the first 1162 South Asian participants enrolled in the South Asian Heart Center's AIM to Prevent Program who completed noncontrast coronary computed tomography scans. Using machine‐learning algorithms, we identified and modeled the interaction of predictor variables with coronary artery calcification (CAC) severity in South Asian individuals. Anthropometric, laboratory, demographic, and lifestyle predictor variables were analyzed using continuous boosted regression trees to model the relationship with and in between predictor variables and CAC. Participants with CAC were older, predominately men, had smoking history, had personal histories of diabetes, hypertension, and hypercholesterolemia, and had family histories of coronary artery disease. Insulin, body mass index, blood pressure, fasting blood sugar, hemoglobin A1c, and waist‐to‐height ratio were associated with CAC but not low‐density lipoprotein cholesterol or lipoprotein (a). The arteriosclerotic cardiovascular disease score failed to classify individuals. Only age, body mass index, non–high‐density lipoprotein cholesterol/apolipoprotein B ratio, smoking risk, fasting blood sugar, and diastolic blood pressure were predictive, explaining 30.3% of CAC severity. A non–high‐density lipoprotein cholesterol/apolipoprotein B ratio of 1.4 or less markedly increased coronary calcification.
Conclusions
Our findings highlight factors associated with dysmetabolism and cholesterol‐depleted non–high‐density lipoprotein cholesterol particles with coronary arteriosclerosis, possibly explaining the dual epidemics of diabetes and arteriosclerotic cardiovascular disease in this population. Markers of glucose dysmetabolism and the non–high‐density lipoprotein cholesterol to apolipoprotein B ratio should become the focus of assessment for cardiovascular risk in South Asian individuals, with prevention strategies directed at improving glucose metabolic health.
Keywords: apolipoprotein B, coronary artery calcification, coronary artery disease, diabetes mellitus, dyslipidemia, dysmetabolism, non–HDL‐C to apoB ratio
Subject Categories: Cardiovascular Disease; Epidemiology; Obesity; Diabetes, Type 2; Lifestyle
Nonstandard Abbreviations and Acronyms
- BRT
boosted regression tree
- FBS
fasting blood sugar
- Lp(a)
lipoprotein (a)
Clinical Perspective.
What Is New?
The non–high‐density lipoprotein cholesterol to apolipoprotein B ratio, or the atherogenic cholesterol ratio, identifies the presence of cholesterol‐depleted lipid particles with a ratio <1.4, increasing coronary calcification at any age in South Asian individuals.
Age, body mass index, non–high‐density lipoprotein cholesterol to apolipoprotein B ratio, smoking history, fasting blood sugar, and diastolic blood pressure are associated with coronary arteriosclerosis severity in South Asian individuals. In contrast, low‐density lipoprotein cholesterol, cholesterol to high‐density lipoprotein ratio, and lipoprotein (a) are not.
The pooled arteriosclerotic cardiovascular disease cohort formula fails to classify subjects at risk appropriately.
What Are the Clinical Implications?
The assessment of cardiovascular risk in South Asian individuals should focus on markers of glucose dysmetabolism and dyslipidemia, as measured by the non–high‐density lipoprotein cholesterol to apolipoprotein B ratio.
Prevention and treatment strategies for cardiovascular disease in South Asian individuals should correspondingly be modified to focus on improving glucose metabolic health.
South Asian individuals make up one‐quarter of the world's population and trace their ancestry to the countries of the Indian subcontinent. Compared with other ethnicities, South Asian individuals have at least a 2‐fold increased risk of atherosclerotic cardiovascular disease (ASCVD) and diabetes. 1 , 2 , 3 , 4 , 5 This high prevalence of ischemic heart disease has been observed in native and immigrant populations. 6 , 7 , 8 The Indian subcontinent presently is amid dual epidemics, with cardiovascular disease accounting for 60% of the global burden while having one of the highest incidences of diabetes, with a prevalence of >15% in urban areas. 9 , 10 , 11 In 2006, El Camino Hospital established the South Asian Heart Center as a community‐based nonprofit response, providing diabetes and cardiovascular disease risk assessment, prevention, counseling, and lifestyle coaching to participants through its AIM to Prevent and STOP‐D programs. 12 , 13
Coronary artery calcification (CAC) reflects the actual burden of coronary arteriosclerosis. In contrast, risk factors used to calculate the probability of a cardiac event are insufficient in accurately determining the presence of disease in all populations. 14 , 15 , 16 Studies have shown that traditional risk calculators underestimate the incidence of cardiovascular events in South Asian individuals. 16 , 17 , 18 Attempts at adjusting for anthropometric, clinical, and lifestyle factors have failed to fully explain the increased risk. 16 These factors may have complex, nonlinear interactions that may not be captured with traditional analyses. Machine‐learning algorithms may be useful in capturing these nonlinear relationships. Using boosted regression tree (BRT) analyses, we report the relationship between coronary calcification, a marker for coronary arteriosclerosis, and cardiovascular risk factors, and their interaction in South Asian individuals enrolled in the South Asian Heart Center AIM to Prevent Program.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The study sample consisted of participants enrolled in our cardiovascular disease prevention program who completed noncontrast coronary computed tomography scans from May 2006 to December 2017. The institutional review board of El Camino Hospital, Mountain View, California approved Protocol ECH 06‐26A on November 16, 2019, and waived the requirement for informed consent.
Participants sought preventive health screening as a supplement to their routine health care. They enrolled through physician referrals, self‐referral prompted by word of mouth, outreach events, advertisement in local ethnic newspapers, and radio. All participants were in good health and >18 years of age. The South Asian Heart Center collected demographic, anthropometric, laboratory measurements, medical, family, and medication history, and lifestyle behaviors through face‐to‐face and telephone interviews using a standardized, scripted 30‐minute questionnaire before and no later than 180 days after the heart scan. Trained staff performed all anthropometric measurements, including height, weight, waist circumference, body mass index (BMI), and waist‐to‐height ratio on subjects wearing light clothing and no shoes, as previously reported. 12
Lifestyle measures included type of diet, number of vegetables, fruit, and nut servings per day, sweetened drinks per week, meditation sessions per week, minutes of formal exercise per week, and average sleep hours per night. Laboratory measurements of low‐density lipoprotein cholesterol (LDL‐C), high‐density lipoprotein cholesterol (HDL‐C), triglycerides, total cholesterol, lipoprotein (a) (Lp[a]), apolipoprotein B (apoB), low‐density lipoprotein (LDL) particle size, high‐sensitivity C‐reactive protein (hs‐CRP), fasting blood sugar (FBS), hemoglobin A1c, and insulin were determined using 12‐hour fasting blood samples using methodology reported in Data S1.
Hypertension was defined as systolic blood pressure (BP) ≥140 mm Hg or diastolic BP ≥90 mm Hg, or previous history of hypertension, or self‐reported use of antihypertensive medications. 19 Diabetes was defined as FBS ≥126 mg/dL or pharmacological treatment of diabetes or prior history of diabetes. Family history of coronary artery disease (CAD) was considered positive if a coronary event had occurred in a male first‐degree relative before the age of 55 years or a female first‐degree relative before the age of 65 years. Family history of diabetes was considered positive if diabetes was present in any first‐degree relative. Current or prior tobacco use was considered smoking risk.
Coronary calcium scans were recommended to men ≥40 years of age and women ≥45 years of age. A noncontrast, high‐resolution, ECG‐synchronized heart computed tomography examination was obtained using cardiac gating. An aggregate Agatston score was calculated using Siemens's software. We also generated a categorical variable to differentiate subjects with 0 and non‐0 scores, respectively.
We defined dyslipidemia by assessing total cholesterol, LDL‐C, HDL‐C, total cholesterol to HDL‐C ratio, triglycerides, apoB, non‐HDL‐C/apoB ratio, LDL particle size distribution, and Lp(a). We defined dysmetabolism by assessing FBS, insulin, BMI, waist circumference, waist‐to‐height ratio, hemoglobin A1c, and a personal history of diabetes. We evaluated the role of inflammation with hs‐CRP and incorporated familial predisposition by including family history of CAD, hypertension, and diabetes.
Statistical Analysis
For reporting and analysis, we followed the Strengthening the Reporting of Observational Studies in Epidemiology guidelines. 20 Tables 1 and 2 and Table S1 list the 50 predictor variables evaluated. We used nonparametric Spearman rank correlations to identify relationships between the continuous predictor variables and CAC scores. Likewise, we used Mann‐Whitney U tests to identify univariate relationships between the categorical predictor variables and CAC scores. We used BRTs to model the relationship between the predictor variables described in Tables 1 and 2, Tables S2 and S3, and calcium score because of the limitations of multivariate and univariate analyses. 21 However, the ASCVD score, being a metric composed of variables already in the BRT analysis, was excluded so as not to overshadow the impact of the individual variables. We selected BRTs for this analysis because of their ability to handle numerous predictor variables, handle missing observations, accommodate collinear predictor variables, model nonlinear relationships, and automatically model interactions between predictor variables. 21 , 22 , 23 Moreover, growing literature documents the improved predictive ability of machine‐learning algorithms such as BRTs over more traditional linear and logistic regression approaches. 22
Table 1.
Demographic and Clinical Characteristics Based on Coronary Calcium Score
| Characteristic | Sample size* | Total | 0 Ca score | Non‐0 Ca score | χ2 | P value |
|---|---|---|---|---|---|---|
| Age, y | 1162/694/468 | 49.84±0.22 | 47.81±0.24 | 52.85±0.38 | 101 156 | <0.001 |
| Sex | 1162/694/469 | 19.99 | <0.001 | |||
| Men | 933 (80%) | 527 (76%) | 406 (87%) | |||
| Women | 229 (20%) | 167 (24%) | 62 (13%) | |||
| Birthplace | 1073/632/441 | 0.18 | 0.915 | |||
| India | 951 (89%) | 559 (88%) | 392 (89%) | |||
| Other | 90 (8%) | 53 (8%) | 37 (8%) | |||
| USA | 32 (3%) | 20 (3%) | 12 (3%) | |||
| Postgraduate degree | 1085/642/443 | 847 (78%) | 488 (76%) | 359 (81%) | 3.58 | 0.059 |
| Smoking risk | 1086/644/442 | 225 (21%) | 104 (16%) | 121 (27%) | 19.43 | <0.001 |
| Physical exercise, min | 973/572/401 | 37.85±0.53 | 36.65±0.72 | 39.56±0.79 | 102 892 | 0.005 |
| Vegetarian | 1086/644/442 | 453 (42%) | 280 (43%) | 173 (39%) | 1.85 | 0.173 |
| Personal history | ||||||
| Diabetes | 1087/644/443 | 175 (16%) | 82 (13%) | 93 (21%) | 12.65 | <0.001 |
| Hypertension | 1087/644/443 | 369 (34%) | 149 (23%) | 220 (50%) | 81.17 | <0.001 |
| High cholesterol | 1091/645/446 | 932 (85%) | 535 (83%) | 397 (89%) | 7.32 | 0.007 |
| Family history | ||||||
| Diabetes | 1060/631/429 | 585 (55%) | 358 (57%) | 227 (53%) | 1.36 | 0.244 |
| Hypertension | 1066/632/434 | 757 (71%) | 457 (72%) | 300 (69%) | 1.12 | 0.290 |
| High cholesterol | 1058/628/430 | 474 (45%) | 285 (45%) | 189 (44%) | 0.16 | 0.692 |
| Coronary artery disease | 1055/626/429 | 320 (30%) | 175 (28%) | 145 (34%) | 3.84 | 0.050 |
| Stroke | 1052/620/432 | 178 (17%) | 94 (15%) | 84 (19%) | 3.02 | 0.082 |
| Heart failure | 1080/641/439 | 62 (6%) | 39 (6%) | 23 (5%) | 0.21 | 0.650 |
| Medication for | ||||||
| Diabetes | 955/542/413 | 113 (12%) | 48 (9%) | 65 (16%) | 9.99 | 0.002 |
| Hypertension | 955/542/413 | 255 (27%) | 99 (18%) | 156 (38%) | 44.58 | <0.001 |
| Hypercholesterolemia | 955/542/413 | 376 (39%) | 144 (27%) | 232 (56%) | 84.83 | <0.001 |
Categorical values are shown as n (% of column total), and continuous variables are shown as mean±1 standard error. One hundred eight subjects were excluded due to incomplete cardiometabolic data for Spearman rank correlations and Mann‐Whitney U tests in the univariate analyses.
Sample size (n for total, 0, and non‐0 scores).
Table 2.
Clinical Characteristics According to Coronary Calcium Score Adjusted for Medication Use
| Characteristic | Sample size* | All | 0 Ca score | Non‐0 Ca score | W statistic | P value |
|---|---|---|---|---|---|---|
| Lipids | ||||||
| Total cholesterol, mg/dL | 706/493/213 | 198.5±1.29 | 197.95±1.51 | 199.78±2.5 | 51 743 | 0.759 |
| LDL‐C, mg/dL | 701/491/210 | 124.15±1.11 | 123.36±1.29 | 126±2.15 | 49 341 | 0.367 |
| HDL‐C, mg/dL | 706/493/213 | 45.62±0.42 | 45.8±0.51 | 45.2±0.76 | 54 048 | 0.535 |
| Triglycerides, mg/dL | 706/493/213 | 145.81±2.94 | 145.08±3.45 | 147.5±5.58 | 52 619 | 0.963 |
| TC/HDL‐C ratio | 706/493/213 | 4.55±0.04 | 4.53±0.05 | 4.61±0.08 | 49 922 | 0.299 |
| Triglycerides/HDL‐C ratio | 706/493/213 | 3.59±0.11 | 3.57±0.14 | 3.66±0.18 | 51 756 | 0.764 |
| Non–HDL‐C/apoB | 701/489/212 | 1.54±0 | 1.55±0.01 | 1.53±0.01 | 54 071 | 0.364 |
| apoB, mg/dL | 701/489/212 | 99.17±0.77 | 98.43±0.9 | 100.89±1.48 | 48 646 | 0.195 |
| Inflammation | ||||||
| hs‐CRP, mg/L | 648/449/199 | 2.04±0.12 | 2.05±0.15 | 2.01±0.18 | 43 132 | 0.482 |
| BP | ||||||
| Systolic BP, mm Hg | 764/501/263 | 119.57±0.54 | 117.53±0.62 | 123.46±0.99 | 52 271 | <0.001 |
| Diastolic BP, mm Hg | 764/501/263 | 75.47±0.34 | 74.42±0.39 | 77.46±0.62 | 55 161 | <0.001 |
| Pulse pressure | 764/501/263 | 44.09±0.4 | 43.1±0.47 | 45.97±0.71 | 58 288 | 0.001 |
| Genetic risk factors | ||||||
| Lipoprotein (a), mg/dL | 581/341/240 | 30.51±1.27 | 31.94±1.73 | 28.47±1.84 | 43 517 | 0.192 |
| Lipoprotein (a), nmol/L | 490/294/196 | 78.26±2.22 | 78.95±2.82 | 77.22±3.6 | 29 466 | 0.670 |
| Metabolic risk factors | ||||||
| Fasting blood sugar | 956/585/371 | 92.61±0.34 | 91.59±0.42 | 94.23±0.58 | 93 070 | <0.001 |
| Insulin, μIU/mL† | 477/284/193 | 11.25±0.29 | 10.75±0.31 | 12.03±0.55 | 31 342 | 0.064 |
| Insulin, μIU/mL‡ | 539/328/211 | 83.02±1.99 | 84.61±2.54 | 80.67±3.19 | 28 625 | 0.410 |
| HbA1c | 562/346/216 | 5.74±0.01 | 5.71±0.02 | 5.8±0.02 | 31 586 | 0.002 |
| Biometric risk factors | ||||||
| Body mass index, kg/m2 | 999/591/408 | 26.01±0.12 | 25.68±0.15 | 26.48±0.18 | 105 668 | 0.001 |
| Waist, cm | 924/541/383 | 91.92±0.33 | 90.72±0.44 | 93.61±0.51 | 86 213 | <0.001 |
| Waist‐to‐height ratio | 923/540/383 | 0.54±0 | 0.54±0 | 0.55±0 | 90 377 | <0.001 |
| ASCVD risk score | 684/478/206 | 4.10±0.19% | 3.13±0.14% | 6.327±0.50% | 22 978 | <0.001 |
Values are shown as mean±1 standard error. apoB indicates apolipoprotein B; ASCVD, arteriosclerotic cardiovascular disease; BP, blood pressure; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; hs‐CRP, high‐sensitivity C‐reactive protein; LDL‐C, low‐density lipoprotein cholesterol; Non–HDL‐C/apoB, non–high‐density lipoprotein cholesterol to apolipoprotein B ratio; TC/HDL‐C, total cholesterol to high‐density lipoprotein cholesterol ratio; and Triglycerides/HDL‐C, triglycerides to high‐density lipoprotein cholesterol ratio.
Sample size (n for total, 0, and non‐0 scores).
Siemens Centaur immunoassay.
Beckman immunoassay.
The continuous BRT evaluates the severity of CAC as a function of the predictor values. We used the Dismo package in R to run our BRT models, varying the tree complexity, learning rate, and bag fraction parameters to find the combination that yielded the lowest cross‐validated deviance and highest cross‐validated correlation. 24 We then ran a second BRT model on the reduced set of predictor variables, using the results of this model to examine interactions between variables via pairwise interaction plots (see Data S1, Boosted Regression Tree Methodology).
This study was funded by community philanthropic support through El Camino Health Foundation, Mountain View, California. The funders had no role in study design, data collection, data analysis, interpretation, or writing of this article.
Results
Table 1 shows demographics and clinical characteristics based on coronary calcification. Table S1 presents the demographics and clinical characteristics by sex for the 1162 participants who had computed tomography scans. Forty‐two percent of the subjects were vegetarian, and 19% had tobacco exposure or smoking risk. Diabetes was present in 16%. Non‐0 calcium score participants were older, predominately men, had smoking risk, exercised slightly longer, had personal histories of diabetes (21% versus 13%), had hypertension (50% versus 23%), and had hypercholesterolemia (89% versus 83%), were taking medications for diabetes, hypertension, and hypercholesterolemia, and had family histories of CAD (Table 1). BMI in the group with CAC was higher (26.48±0.18) than in those without (25.68±0.15). Systolic (123.46±0.99 versus 117.53±0.62) and diastolic (77.46±0.62 versus 74.42±0.39) BP were also higher in those with non‐0 calcium scores, as was pulse pressure.
Two hundred fifty‐five participants were on BP‐lowering medications, 376 participants were on cholesterol‐lowering medications, and 113 participants were on diabetes medication. Table 2 presents the means for the predictor variables adjusted for medication use. After controlling for medication use, lipids, apoB, LDL particle size, insulin risk, hs‐CRP, and Lp(a) risk showed no significant differences between the 2 groups (Table 2 and Table S3). In contrast, FBS, hemoglobin A1c, BMI, waist circumference, and waist‐to‐height ratio were higher in the non‐0 calcium score group (Table 1). The ASCVD risk score was significantly higher in the non‐0 group (6.4%±0.5% versus 3.1%±0.1%, P≤0.001). Of the 684 participants who met the criteria to measure an ASCVD score, 142 of 558 (21%) had a non‐0 CAC score and an ASCVD score of <7.5%. Conversely, 62 of 126 participants (49%) with an ASCVD score ≥7.5% had a CAC score of 0. Furthermore, 9 out of the 15 participants excluded from the ASCVD analysis for LDL >189 had CAC scores of 0.
The continuous BRT model showed that age is the most important, followed by BMI, the ratio of non‐HDL‐C to apoB, smoking risk, blood glucose, and diastolic BP (Table 3). The cross‐validated deviance explained by our model (a measure of model fit akin to the R 2 of linear regression) was 30.3%. The correlation between predicted and observed calcium scores was 0.422.
Table 3.
Results of a Continuous Boosted Regression Tree Analysis on Calcium Score
| Predictor variable | Relative influence |
|---|---|
| Age | 38.1 |
| Body mass index | 15.9 |
| Non–HDL‐C/apoB | 14.0 |
| Smoking risk | 12.1 |
| Fasting blood sugar | 10.4 |
| Diastolic blood pressure | 9.4 |
| Cross‐validated correlation | 0.422 |
| Cross‐validated deviance explained | 30.3% |
Non–HDL‐C/apoB indicates non–high density lipoprotein cholesterol to apolipoprotein B ratio.
The relationships between each predictor variable and CAC severity are presented using partial dependence plots that adjust for the effects of all other variables while plotting the relationship between a focal predictor and calcium score effect (Figure 1). The amount of calcification shows no association with age until 50 years of age, at which point it increases until about 70 years of age (Figure 1A). There is no relationship between calcium score and BMI until a BMI of 30, at which point it increases up to a BMI of 35 (Figure 1B). A ratio of non‐HDL‐C to apoB below 1.4 is associated with an elevated calcium score (Figure 1C). Smoking risk increases CAC (Figure 1D). Blood sugar level above 85 is associated with CAC severity (Figure 1E). There is a slight increase in CAC with diastolic BP between 80 and 90 mm Hg, at which point it sharply increases until 100 mm Hg, where it levels off (Figure 1F).
Figure 1. Continuous boosted regression tree partial dependence plots ordered by relative influence illustrating the relationship between predicted calcium score and (A) age, (B) BMI, (C) non–HDL cholesterol to apoB ratio, (D) smoking risk, (E) blood glucose, and (F) diastolic BP, while controlling for all the other variables.
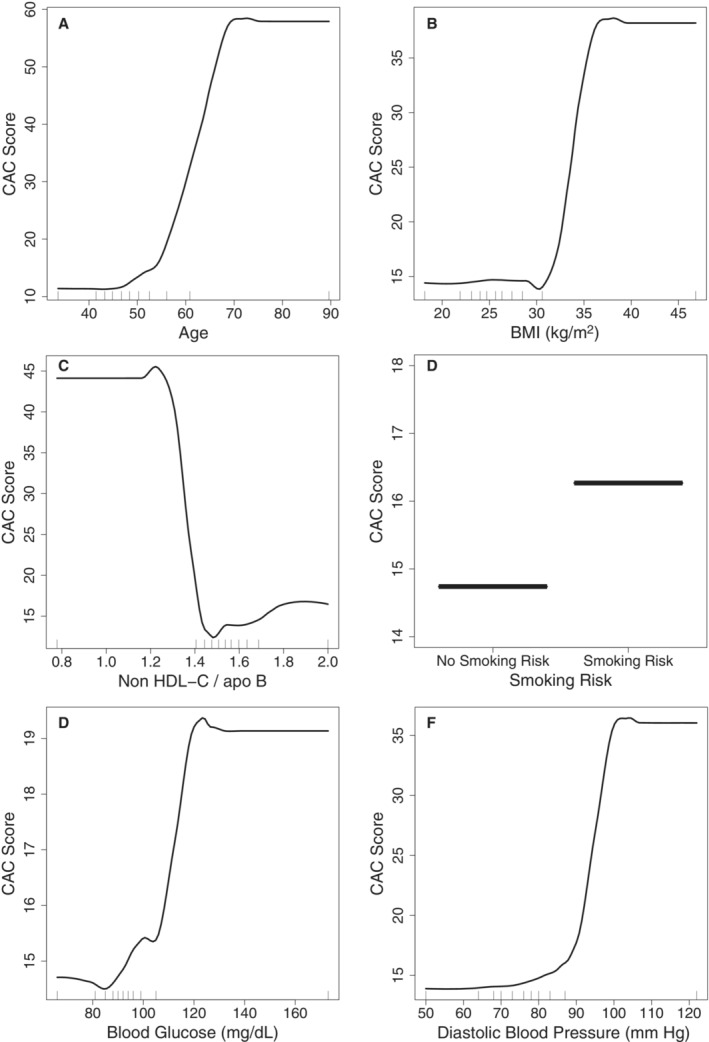
BMI indicates body mass index; CAC score, coronary artery calcium score; and non–HDL‐C/ApoB, non‐high density lipoprotein cholesterol to apolipoprotein B ratio.
Table S4 presents the interaction strength for the 6 variables in the continuous BRT model. Of these, the interaction between age and non‐HDL‐C to apoB ratio, age and BMI, age and diastolic BP, age and smoking risk, and diastolic BP and BMI are the strongest. There is a strong interaction between diastolic BP and BMI. Figure 2 illustrates the interactions between the variables according to interaction strengths. Figure 2A shows the interaction between non‐HDL‐C/apoB to age and CAC. At any age, CAC is higher at ratios <1.4. A ratio of ≥1.4 attenuates the impact of age on CAC. Figure 2B demonstrates the interactive effects of BMI and age on CAC. At any age, CAC is higher when BMI is >30. A BMI above 30 augmented the impact of age on CAC at all ages, with the largest effect above 60 years of age (Figure 2B). Furthermore, a diastolic BP above 80 mm Hg amplified the impact of age, particularly after 60 years of age (Figure 2C). Figure 2D displays the interaction between diastolic BP and BMI. At any BMI, there is a higher effect for a diastolic BP >80. The influence is most pronounced after a BMI of 35. Figure 2E demonstrates the interaction between smoking risk, age, and CAC. After 55 years of age, there is a higher score in subjects with smoking risk.
Figure 2. Continuous boosted regression tree interaction plots ordered by interaction strength between (A) the ratio of non‐HDL cholesterol to apoB and age, (B) BMI and age, (C) diastolic BP and age, (D) BMI and diastolic BP, and (E) smoking risk and age.
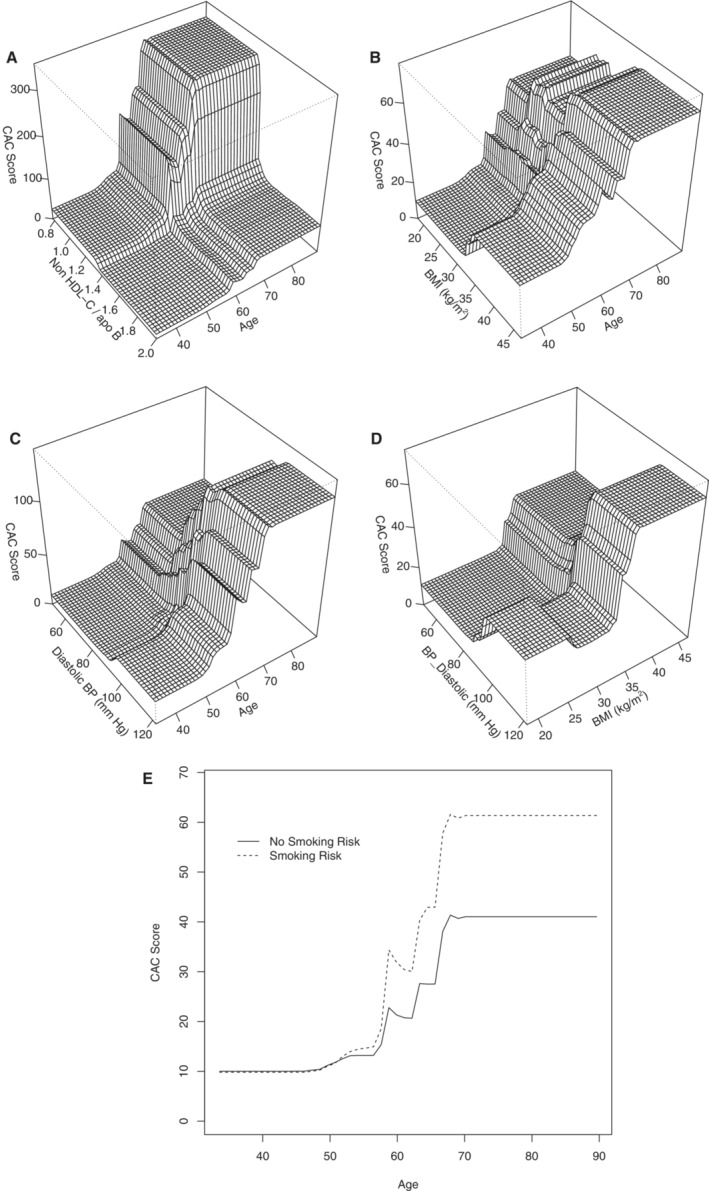
BMI indicates body mass index; BP, blood pressure; CAC score, coronary calcium score; and non–HDL‐C/apoB, non–high density lipoprotein cholesterol to apolipoprotein B ratio.
Discussion
To our knowledge, this is the largest cohort study of South Asian individuals in North America. Our subjects represent the typical self‐referred patient who attends a physician's office for preventive medical services. Coronary arteriosclerosis, as diagnosed by CAC in South Asian individuals, is poorly predicted by traditional risk factors. Only 30.3% of the variability of CAC severity is explained by 6 of the 50 predictor factors evaluated with BRT analysis. Furthermore, our analysis highlights the relationship between dysmetabolism in contrast to elevated LDL‐C, non–HDL‐C, and Lp(a) levels and coronary arteriosclerosis. FBS, insulin, hemogolbin A1c, BMI, waist circumference, and waist‐to‐height ratio were associated with coronary calcification.
In contrast, none of the components of the typical lipid panel predicted coronary calcification, suggesting dysmetabolism and cholesterol‐depleted atherogenic particles may better explain residual non–age‐related coronary calcification. BMI is the most significant factor after age in the continuous BRT analysis. BMI, FBS, and elevated diastolic BP accounted for 35.8% of the explained variability in coronary calcification. These are 3 of the 5 factors that define metabolic syndrome. 25 This relationship with metabolic syndrome may help explain the dual epidemic of CAD and diabetes afflicting South Asian individuals because the factors associated with increased incidence of diabetes (BMI, FBS, and BP) explain the majority of coronary calcification after the effect of age. Individuals with metabolic syndrome have an increased risk for cardiovascular disease and diabetes. Risk increases with the number of metabolic syndrome components. 25 These results are consistent with the hypothesis that insulin resistance underlies the high coronary risk in South Asian individuals, reinforcing the evidence for a fundamental role of dysmetabolism in the cause of coronary heart disease. 26 , 27
hs‐CRP did not demonstrate an association with the presence of coronary calcification. However, hs‐CRP levels in South Asian individuals were significantly higher than those reported in the Third National Health and Nutrition Examination Survey. 28 In our study, hs‐CRP is not an independent risk factor for coronary calcification. Other studies have demonstrated that hs‐CRP identifies individuals who benefit from statin therapy but does not predict CAC. 29 , 30 , 31 , 32 In the Rotterdam trial, Oei et al reported age, sex, BMI, BP, diabetes, and smoking correlated with coronary calcification, whereas there was no association with lipid levels when concurrently measured at the time of scanning. Furthermore, 29% of the men and 15% of the women without risk factors had a high calcium score. 33 In the MESA (Multi‐Ethnic‐Study of Arteriosclerosis), the presence of CAC was associated only in the subset of subjects with mixed hyperlipidemia and hypercholesterolemia, with both groups being defined by an LDL‐C >160. 34 Furthermore, Hecht et al found no correlation between LDL‐C levels and coronary calcium scores in a non–South Asian population. 35
Lp(a) has been proposed to be a strong determinant of cardiovascular risk. 36 , 37 Enas et al have suggested that elevated Lp(a) levels explain the high incidence of malignant CAD in South Asian individuals. 5 , 38 We report elevated Lp(a) levels but no association with coronary calcification. 39 Previously, we reported that Lp(a) levels diminished as the number of metabolic syndrome factors increased. 40 Because these metabolic risk factors best explain the severity of coronary calcification, the absence of a correlation between Lp(a) and coronary calcification is not unexpected. Furthermore, the MASALA (Mediators of Arteriosclerosis in South Asians Living in America) and MESA studies did not find an association of Lp(a) with CAC, nor with enhanced ASCVD events in the UK Biobank Prospective Cohort Study. 16 , 39 , 41 The lack of association between LDL‐C, non–HDL‐C, and apoB plasma concentrations with CAC should not be interpreted as suggesting that lipids are irrelevant for arteriosclerosis but that the presence of cholesterol‐depleted atherogenic particles is the significant lipid abnormality driving coronary calcification in South Asian individuals. Variation in atherogenic particle cholesterol content has been reported in patients with metabolic syndrome. 42 Studies have identified a wide variation in the cholesterol content of atherogenic particles using discordance analysis. Individuals with cholesterol‐depleted apoB particles have a higher incidence of metabolic syndrome, hypertension, diabetes, obesity, and CAD. 43 , 44 , 45 Cantey and Wilkins recently reviewed the literature and identified 10 studies evaluating discordance between atherogenic particle cholesterol content to atherogenic lipid particle number. 46 , 47 The discordance favoring cholesterol‐depleted particles enhanced the increased risk of cardiovascular disease and events. 45 Because no biological level previously defined a lipid particle as cholesterol‐depleted or cholesterol‐enriched, many studies have used different discordance cutoffs. Most investigators use percentile, quintile, and the difference between expected and measured values calculated by regression analysis to define discordance. The different approaches to identify discordance make this analysis cumbersome and not easily applicable in the clinical setting.
Non–HDL‐C is the total cholesterol within LDL, intermediate‐density lipoprotein, Lp(a), very low‐density lipoprotein, chylomicron, and remnant particles. 48 , 49 apoB is the main apoprotein in non–HDL‐C. 50 There is 1 apoB molecule in each atherogenic lipid particle. 50 , 51 , 52 , 53 The apoB assay measures both apo B100, found in non–HDL‐C, and apo B48, found in chylomicrons and chylomicron remnants, but in fasting samples, >95% of apoB in plasma is apo B100. Therefore, apoB provides a direct measure of the number of atherogenic lipoprotein particles in circulation.
Our study simplified the question by calculating the average cholesterol concentration in the non–HDL‐C fraction by dividing non–HDL‐C in milligrams per deciliter by apoB in milligrams per deciliter to identify the physiologically relevant ratio distinguishing cholesterol‐depleted and cholesterol‐enriched particles and the severity of coronary calcification. The non–HDL‐C to apoB ratio explained 14% of the variability in coronary calcification following age and BMI. A ratio of non–HDL‐C to apoB below 1.4 is associated with an elevated calcium score at any age (Figure 2A). Calcification increases with age for all participants, but the increase is amplified in those with a low ratio or cholesterol‐depleted apoB. Our study confirms that cholesterol‐depleted particles are more atherogenic than cholesterol‐enriched particles. Furthermore, this is the first study reporting an easy‐to‐calculate ratio identifying cholesterol‐depleted particles. A non–HDL‐C to apoB ratio of <1.4 appears to be the cutoff for increased coronary arteriosclerosis at any age.
There is an important interaction between BMI and age (Figure 2B) and diastolic BP and age (Figure 2C) to the severity of coronary calcification. BMI, diastolic BP, FBS, and non–HDL‐C to apoB ratio of <1.4 identify subjects most at risk of coronary arteriosclerosis and suggest that weight‐related metabolic dysfunction may be the driver of increased arteriosclerosis in South Asian individuals (Figure 2 and Table S4).
The American College of Cardiology/American Heart Association published the ASCVD risk formula to calculate the 10‐year risk for atherosclerotic cardiovascular events and advised statin therapy for those with a risk of ≥7.5%. 54 The group with coronary calcification had an average ASCVD score of 6.4% versus 3.1% in the group without (Table 3). However, an ASCVD threshold of <7.5% missed 21% of participants with non‐0 scores, who may have benefitted from statin therapy, and misclassified 49% of participants with a CAC score of 0 but with an ASCVD score ≥7.5%, who would have received no benefit from statin therapy. 55 Furthermore, 9 of the 15 participants excluded from the ASCVD analysis because of an LDL ≥190 had CAC scores of 0. Therefore, the ASCVD score appears to be an unreliable tool in South Asian individuals.
We found that the ability to predict coronary arteriosclerosis is poor. This is clinically relevant, because it demonstrates the inability of risk factors to predict coronary arteriosclerosis, which is the primary concern in patients seeking cardiovascular preventive care. Therefore, risk factor–based approaches fall significantly short of a coronary heart scan.
Study Limitations
Although the importance of prospective clinical studies is uncontested, there is also value in reporting observational community‐based findings. Our findings are clinically relevant, because our cohort represents the typical self‐referred patient who attends a physician's office for preventive medical services. However, sampling bias may be a potential concern, because all participants were enrolled from a self‐referred screening program. The use of statins may have masked the role of LDL and non–HDL‐C in CAC. hs‐CRP, LDL‐C, apoB, and non–HDL‐C to apoB ratio are affected by statins. Statins are also known to accelerate coronary calcification. 56 Therefore, the analysis had to be adjusted for medication use. It is difficult to eliminate the possibility that statin therapy or its adjustment skewed our results. Statin therapy was significantly associated with a non‐0 CAC score but was not an independent variable in the continuous BRT analysis predicting the severity of CAC. In other words, the use of statins did not predict the severity of coronary calcification. Furthermore, our attempts to adjust for the changes in the insulin and Lp(a) assays and reference ranges and cholesterol pattern distribution from gradient gel to ion mobility throughout the study may have affected our results and their relationship to CAC. Finally, coronary atherosclerosis was evaluated by measuring CAC, so we cannot extend our findings to the prevalence of noncalcified coronary plaque.
Conclusions
We present a simple formula, the non–HDL‐C to apoB ratio, or the atherogenic cholesterol ratio, identifying the presence of cholesterol‐depleted lipid particles. A ratio of non–HDL‐C to apoB <1.4 increases coronary calcification and shows the best association with CAC compared with all other tested lipoproteins. The presence of cholesterol‐depleted particles may explain some of the unaccounted increased risk of CAD in South Asian individuals. Furthermore, using machine‐learning algorithms, our findings highlight the relationship between the factors associated with diabetes and coronary arteriosclerosis, possibly explaining the dual epidemic of diabetes and CAD in this population. Therefore, the assessment of cardiovascular risk in South Asian individuals should focus on markers of glucose dysmetabolism and dyslipidemia, as measured by the non–HDL‐C to apoB ratio. Prevention and treatment strategies for cardiovascular disease in South Asian individuals should correspondingly be modified to focus on improving glucose metabolic health.
Sources of Funding
This study was funded by community philanthropic support through El Camino Health Foundation, Mountain View, California.
Disclosures
Dr Molina reports the following fees: June 2020 to May 2020, Consultant to BCG Digital Venture Group: Consulted on the relationship between lifestyle and cardiovascular disease and diabetes. October 2020 to November 2020, Movano Corporation: Assisted in the writing of blood pressure assessment protocols and an application to an institutional review board for a technology company interested in developing an electronic wrist blood pressure meter. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S4
References 57,58
Acknowledgments
The authors are grateful to the study participants and the dedicated coaching and research personnel who undertook outreach and follow‐up and who make the South Asian Heart Center work as a resource to the community. C.R.M., A.M., C.S., A.S., and L.K. had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. C.R.M. and A.M. were responsible for the study concept. C.R.M., A.M., C.S., and A.S. were responsible for data curation. All authors were responsible for protocol writing and investigation. C.R.M., C.S., and A.M. conducted data analysis. All authors interpreted the data. C.R.M. and C.S. drafted the original article, which was reviewed and edited by all authors. C.S. was responsible for data visualization. A.M. acquired the funding for the study. C.R.M. and A.M. supervised the project. C.R.M. was responsible for the decision to submit the article. All authors gave final approval of the version to be published.
This article was sent to Peter W. Wilson, MD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027697
For Sources of Funding and Disclosures, see page 10.
REFERENCES
- 1. Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases. Circulation. 2001;104:2855–2864. doi: 10.1161/hc4701.099488 [DOI] [PubMed] [Google Scholar]
- 2. Jose PO, Frank AT, Kapphahn KI, Goldstein BA, Eggleston K, Hastings KG, Cullen MR, Palaniappan LP. Cardiovascular disease mortality in Asian Americans. J Am Coll Cardiol. 2014;64:2486–2494. doi: 10.1016/j.jacc.2014.08.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gupta M, Brister S, Verma S. Is South Asian ethnicity an independent cardiovascular risk factor? Can J Cardiol. 2006;22:193–197. doi: 10.1016/S0828-282X(06)70895-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 5. Enas EA, Mehta J. Malignant coronary artery disease in young Asian Indians: thoughts on pathogenesis, prevention, and therapy. Clin Cardiol. 1995;18:131–135. doi: 10.1002/clc.4960180305 [DOI] [PubMed] [Google Scholar]
- 6. Enas EA, Yusuf S, Mehta JL. Prevalence of coronary artery disease in Asian Indians. Am J Cardiol. 1992;70:945–949. doi: 10.1016/0002-9149(92)90744-J [DOI] [PubMed] [Google Scholar]
- 7. McKeigue PM, Miller G, Marmot M. Coronary heart disease in South Asians overseas: a review. J Clin Epidemiol. 1989;42:597–609. doi: 10.1016/0895-4356(89)90002-4 [DOI] [PubMed] [Google Scholar]
- 8. Gupta M, Singh N, Verma S. South Asians and cardiovascular risk. Circulation. 2006;113:e924–e929. doi: 10.1161/CIRCULATIONAHA.105.583815 [DOI] [PubMed] [Google Scholar]
- 9. Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, Bhansali A, Joshi SR, Joshi PP, Yajnik CS, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research‐India DIABetes (ICMR‐INDIAB) study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5 [DOI] [PubMed] [Google Scholar]
- 10. Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, Adhikari P, Rao PV, Saboo B, Kumar A, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR‐INDIAB population‐based cross‐sectional study. Lancet Diabetes Endocrinol. 2017;5:585–596. doi: 10.1016/s2213-8587(17)30174-2 [DOI] [PubMed] [Google Scholar]
- 11. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726 [DOI] [PubMed] [Google Scholar]
- 12. Sathe A, Flowers E, Mathur A, Garcia DM, Kotrys J, Gandhi R, Molina C, Mathur A. A culturally specific health coaching program targeting cardiovascular disease risk in South Asians: rationale, design, and baseline data. Ethn Dis. 2013;23:304–309. [PubMed] [Google Scholar]
- 13. Volgman AS, Palaniappan LS, Aggarwal NT, Gupta M, Khandelwal A, Krishnan AV, Lichtman JH, Mehta LS, Patel HN, Shah KS, et al. Atherosclerotic cardiovascular disease in South Asians in the United States: epidemiology, risk factors, and treatments: a scientific statement from the American Heart Association. Circulation. 2018;138:e1–e34. doi: 10.1161/CIR.0000000000000580 [DOI] [PubMed] [Google Scholar]
- 14. Rodriguez‐Granillo GA, Carrascosa P, Bruining N. Progression of coronary artery calcification at the crossroads: sign of progression or stabilization of coronary atherosclerosis? Cardiovasc Diagn Ther. 2016;6:250–258. doi: 10.21037/cdt.2016.03.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, et al. Progression of coronary artery calcium predicts all‐cause mortality. JACC Cardiovasc Imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018 [DOI] [PubMed] [Google Scholar]
- 16. Patel AP, Wang M, Kartoun U, Ng K, Khera AV. Quantifying and understanding the higher risk of atherosclerotic cardiovascular disease among South Asian individuals. Circulation. 2021;144:410–422. doi: 10.1161/CIRCULATIONAHA.120.052430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhopal R, Unwin N, White M, Yallop J, Walker L, Alberti KGMM, Harland J, Patel S, Ahmad N, Turner C, et al. Heterogeneity of coronary heart disease risk factors in Indian, Pakistani, Bangladeshi, and European origin populations: cross sectional study. BMJ. 1999;319:215–220. doi: 10.1136/bmj.319.7204.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Findlay SG, Kasliwal RR, Bansal M, Tarique A, Zaman A. A comparison of cardiovascular risk scores in native and migrant South Asian populations. SSM–Population Health. 2020;11:100594. doi: 10.1016/j.ssmph.2020.100594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo J, Joseph L, Jones DW, Materson BJ, Oparil S, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2571. doi: 10.1001/jama.289.19.2560 [DOI] [PubMed] [Google Scholar]
- 20. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; Initiative ftS . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18:800–804. doi: 10.1097/EDE.0b013e3181577654 [DOI] [PubMed] [Google Scholar]
- 21. Zar JH. Biostatistical Analysis. Prentice Hall; 2010. [Google Scholar]
- 22. Elith J, Leathwick JR, Hastie T. A working guide to boosted regression trees. J Anim Ecol. 2008;77:802–813. doi: 10.1111/j.1365-2656.2008.01390.x [DOI] [PubMed] [Google Scholar]
- 23. De'ath G. Boosted trees for ecological modeling and prediction. Ecology. 2007;88:243–251. doi: 10.1890/0012-9658(2007)88[243:Btfema]2.0.Co;2 [DOI] [PubMed] [Google Scholar]
- 24. Hijmans RJ, Phillips S, Leathwick J, Elith J. dismo: Species distribution modeling.
- 25. Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C. Definition of metabolic syndrome. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6 [DOI] [PubMed] [Google Scholar]
- 26. McKeigue PM, Ferrie JE, Pierpoint T, Marmot MG. Association of early‐onset coronary heart disease in South Asian men with glucose intolerance and hyperinsulinemia. Circulation. 1993;87:152–161. doi: 10.1161/01.CIR.87.1.152 [DOI] [PubMed] [Google Scholar]
- 27. Rossello X, Raposeiras‐Roubin S, Oliva B, Sánchez‐Cabo F, García‐Ruíz JM, Caimari F, Mendiguren JM, Lara‐Pezzi E, Bueno H, Fernández‐Friera L, et al. Glycated hemoglobin and subclinical atherosclerosis in people without diabetes. J Am Coll Cardiol. 2021;77:2777–2791. doi: 10.1016/j.jacc.2021.03.335 [DOI] [PubMed] [Google Scholar]
- 28. Wong ND, Pio J, Valencia R, Thakal G. Distribution of C‐reactive protein and its relation to risk factors and coronary heart disease risk estimation in the National Health and Nutrition Examination Survey (NHANES) III. Prev Cardiol. 2001;4:109–114. doi: 10.1111/j.1520-037x.2001.00570.x [DOI] [PubMed] [Google Scholar]
- 29. Mehta A, Patel J, Al Rifai M, Ayers CR, Neeland IJ, Kanaya AM, Kandula N, Blaha MJ, Nasir K, Blumenthal RS, et al. Inflammation and coronary artery calcification in South Asians: the mediators of atherosclerosis in South Asians living in America (MASALA) study. Atherosclerosis. 2018;270:49–56. doi: 10.1016/j.atherosclerosis.2018.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646 [DOI] [PubMed] [Google Scholar]
- 31. Collaboration TERF . C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang NC, Matthews KA, Barinas‐Mitchell EJM, Chang C‐CH, El Khoudary SR. Inflammatory/hemostatic biomarkers and coronary artery calcium progression in women at midlife (from the study of Women's health across the nation, heart study). Am J Cardiol. 2016;118:311–318. doi: 10.1016/j.amjcard.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Oei H‐HS, Vliegenthart R, Hofman A, Oudkerk M, Witteman JCM. Risk factors for coronary calcification in older subjects: the Rotterdam coronary calcification study. Eur Heart J. 2004;25:48–55. doi: 10.1016/j.ehj.2003.10.008 [DOI] [PubMed] [Google Scholar]
- 34. Paramsothy P, Knopp RH, Bertoni AG, Blumenthal RS, Wasserman BA, Tsai MY, Rue T, Wong ND, Heckbert SR. Association of combinations of lipid parameters with carotid intima‐media thickness and coronary artery calcium in the MESA (multi‐ethnic study of atherosclerosis). J Am Coll Cardiol. 2010;56:1034–1041. doi: 10.1016/j.jacc.2010.01.073 [DOI] [PubMed] [Google Scholar]
- 35. Hecht HS, Superko HR, Smith LK, McColgan BP. Relation of coronary artery calcium identified by electron beam tomography to serum lipoprotein levels and implications for treatment. Am J Cardiol. 2001;87:406–412. doi: 10.1016/S0002-9149(00)01392-8 [DOI] [PubMed] [Google Scholar]
- 36. Nordestgaard BG, Chapman MJ, Ray K, Borén J, Andreotti F, Watts GF, Ginsberg H, Amarenco P, Catapano A, Descamps OS, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gudbjartsson DF, Thorgeirsson G, Sulem P, Helgadottir A, Gylfason A, Saemundsdottir J, Bjornsson E, Norddahl GL, Jonasdottir A, Jonasdottir A, et al. Lipoprotein(a) concentration and risks of cardiovascular disease and diabetes. J Am Coll Cardiol. 2019;74:2982–2994. doi: 10.1016/j.jacc.2019.10.019 [DOI] [PubMed] [Google Scholar]
- 38. Enas EA, Varkey B, Dharmarajan TS, Pare G, Bahl VK. Lipoprotein(a): an underrecognized genetic risk factor for malignant coronary artery disease in young Indians. Indian Heart J. 2019;71:184–198. doi: 10.1016/j.ihj.2019.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Huffman MD, Kandula NR, Baldridge AS, Tsai MY, Prabhakaran D, Kanaya AM. Evaluating the potential association between lipoprotein(a) and atherosclerosis (from the mediators of atherosclerosis among South Asians living in America cohort). Am J Cardiol. 2019;123:919–921. doi: 10.1016/j.amjcard.2018.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Prasad M, Flowers E, Divakaruni M, Molina C, Mathur A, Assimes TL, Aouizerat BE, Sathe A, Malhotra D, Turakhia MP. The relationship of lipoprotein(a), C‐reactive protein and homocysteine with metabolic syndrome in South Asians. J Indian Coll Cardiol. 2014;4:208–213. doi: 10.1016/j.jicc.2014.08.004 [DOI] [Google Scholar]
- 41. Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: the multi‐ethnic study of atherosclerosis. J Cardiovasc Comput Tomogr. 2021;15:154–160. doi: 10.1016/j.jcct.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tehrani DM, Zhao Y, Blaha MJ, Mora S, Mackey RH, Michos ED, Budoff MJ, Cromwell W, Otvos JD, Rosenblit PD, et al. Discordance of low‐density lipoprotein and high‐density lipoprotein cholesterol particle versus cholesterol concentration for the prediction of cardiovascular disease in patients with metabolic syndrome and diabetes mellitus (from the multi‐ethnic study of atherosclerosis [MESA]). Am J Cardiol. 2016;117:1921–1927. doi: 10.1016/j.amjcard.2016.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC Jr. Clinical implications of discordance between low‐density lipoprotein cholesterol and particle number. J Clin Lipidol. 2011;5:105–113. doi: 10.1016/j.jacl.2011.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sniderman AD, St‐Pierre AC, Cantin B, Dagenais GR, Després J‐P, Lamarche B. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. Am J Cardiol. 2003;91:1173–1177. doi: 10.1016/S0002-9149(03)00262-5 [DOI] [PubMed] [Google Scholar]
- 45. Mora S, Buring JE, Ridker PM. Discordance of low‐density lipoprotein (LDL) cholesterol with alternative LDL‐related measures and future coronary events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cantey EP, Wilkins JT. Discordance between lipoprotein particle number and cholesterol content: an update. Curr Opin Endocrinol Diabetes Obes. 2018;25:130–136. doi: 10.1097/med.0000000000000389 [DOI] [PubMed] [Google Scholar]
- 47. Cao J, Nomura SO, Steffen BT, Guan W, Remaley AT, Karger AB, Ouyang P, Michos ED, Tsai MY. Apolipoprotein B discordance with low‐density lipoprotein cholesterol and non‐high‐density lipoprotein cholesterol in relation to coronary artery calcification in the multi‐ethnic study of atherosclerosis (MESA). J Clin Lipidol. 2020;14:109–121.e105. doi: 10.1016/j.jacl.2019.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Emerging Risk Factors Collaboration , Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. doi: 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brunner FJ, Waldeyer C, Ojeda F, Salomaa V, Kee F, Sans S, Thorand B, Giampaoli S, Brambilla P, Tunstall‐Pedoe H, et al. Application of non‐HDL cholesterol for population‐based cardiovascular risk stratification: results from the multinational cardiovascular risk consortium. Lancet. 2019;394:2173–2183. doi: 10.1016/S0140-6736(19)32519-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bayly GR. CHAPTER 37—lipids and disorders of lipoprotein metabolism. In: Marshall WJ, Lapsley M, Day AP, Ayling RM, eds. Clinical Biochemistry: Metabolic and Clinical Aspects (Third Edition). Churchill Livingstone; 2014:702–736. doi: 10.1016/B978-0-7020-5140-1.00037-7 [DOI] [Google Scholar]
- 51. Sniderman AD, Lamarche B, Contois JH, de Graaf J. Discordance analysis and the gordian knot of LDL and non‐HDL cholesterol versus apoB. Curr Opin Lipidol. 2014;25:461–467. doi: 10.1097/MOL.0000000000000127 [DOI] [PubMed] [Google Scholar]
- 52. Sniderman AD, Pencina M, Thanassoulis G. Limitations in the conventional assessment of the incremental value of predictors of cardiovascular risk. Curr Opin Lipidol. 2015;26:210–214. doi: 10.1097/mol.0000000000000181 [DOI] [PubMed] [Google Scholar]
- 53. Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd‐Jones DM. Discordance between apolipoprotein B and LDL‐cholesterol in young adults predicts coronary artery calcification: the CARDIA study. J Am Coll Cardiol. 2016;67:193–201. doi: 10.1016/j.jacc.2015.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stone NJ, Robinson JG, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [PubMed] [Google Scholar]
- 55. Mitchell JD, Fergestrom N, Gage BF, Paisley R, Moon P, Novak E, Cheezum M, Shaw LJ, Villines TC. Impact of statins on cardiovascular outcomes following coronary artery calcium scoring. J Am Coll Cardiol. 2018;72:3233–3242. doi: 10.1016/j.jacc.2018.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. Coronary artery calcification and its progression: what does it really mean? Vol 11. JACC: Cardiovascular Imaging; 2018:127–142. doi: 10.1016/j.jcmg.2017.10.012 [DOI] [PubMed] [Google Scholar]
- 57. Caulfield MP, Li S, Lee G, Blanche PJ, Salameh WA, Benner WH, Reitz RE, Krauss RM. Direct determination of lipoprotein particle sizes and concentrations by ion mobility analysis. Clin Chem. 2008;54:1307–1316. doi: 10.1373/clinchem.2007.100586 [DOI] [PubMed] [Google Scholar]
- 58. Soykan CU, Eguchi T, Kohin S, Dewar H. Prediction of fishing effort distributions using boosted regression trees. Ecol Appl. 2014;24:71–83. doi: 10.1890/12-0826.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S4
References 57,58


