Abstract
Background
Atrial fibrillation (AF) is associated with increasing risk of thromboembolic or ischemic stroke. The CHA2DS2‐VASc score is a well‐established predictor of AF stroke. Patients with AF have an increased risk of stroke if they have diabetes. Use of sodium‐glucose cotransporter‐2 inhibitor (SGLT2i) has been shown to be associated with favorable cardiovascular outcomes in patients with diabetes. It was unknown whether use of SGLT2i decreased stroke risk in patients with AF who have diabetes.
Methods and Results
A total of 9116 patients with AF and diabetes from the National Taiwan University historical cohort were longitudinally followed up for 5 years (January 2016–December 2020). The risk of stroke related to SGLT2i use was evaluated by Cox model, adjusting CHA2DS2‐VASc score in the propensity score–matched population with 474 SGLT2i users and 3235 nonusers. Adverse thromboembolic end points during follow‐up were defined as ischemic stroke. The mean age was 73.2±10.5 years, and 61% of patients were men. There were no significant differences of baseline characteristics between users and nonusers of SGLT2i, including CHA2DS2‐VASc score in the propensity score–matched population. The stroke rate was 3.4% (95% CI, 2.8–4.2) patient‐years in SGLT2i users and 4.3% (95% CI, 4.0–4.6) in nonusers (P=0.021). SGLT2i users had a 20% reduction of stroke (hazard ratio, 0.80 [95% CI, 0.64–0.99]; P=0.043) after adjustment for the CHA2DS2‐VASc score.
Conclusions
Use of SGLT2i was associated with a lower stroke risk in patients with diabetes and AF, and it may be considered to escalate SGLT2i to the first‐line treatment in patients with diabetes and AF.
Keywords: atrial fibrillation, diabetes, oral hypoglycemic agent, sodium‐glucose cotransporter‐2 inhibitor, stroke, thromboembolism
Subject Categories: Atrial Fibrillation
Nonstandard Abbreviations and Acronyms
- NTUH
National Taiwan University Hospital
- SGLT2i
sodium‐glucose cotransporter‐2 inhibitor
Clinical Perspective.
What Is New?
This study was the first to investigate the relationship between use of sodium‐glucose cotransporter‐2 inhibitor and embolic events among patients with atrial fibrillation and diabetes.
What Are the Clinical Implications?
The results of our study indicated that sodium‐glucose cotransporter‐2 inhibitor reduced the risk of ischemic stroke in patients with atrial fibrillation and diabetes, and sodium‐glucose cotransporter‐2 inhibitor might be considered as first‐line treatment for those patients.
Second, we did not study the effects of sodium‐glucose cotransporter‐2 inhibitor on stroke outcomes of different stroke subtypes because of limitation on data availability of integrated database.
Atrial fibrillation (AF) is the most common cardiac arrhythmia worldwide. 1 Clinically, AF is associated with increasing risk of ischemic stroke, heart failure, hospitalization, and mortality. 2 Hence, it is critical to modify AF risks and prevent its complications. Currently, the CHADS2VASc score has been used for evaluating the risk of embolic stroke in patients with AF. 2 Among the parameters of the CHADS2VASc score, diabetes is significantly associated with AF occurrence and increasing stroke risk around 2‐fold. 3 It might be attributable to insulin resistance and hyperglycemia status of the patients with diabetes, which engenders inflammation, neurohormonal imbalance, disturbance of reactive oxygen species generation, expansion of epicardial adipose, myocardial remodeling, cardiac fibrosis, atrial structural alteration, and the change of the atrial electrical properties. 1 , 4 , 5
Sodium glucose cotransporter‐2 inhibitor (SGLT2i) is a new class of antihyperglycemic drug. It selectively inhibits transporters, found exclusively in the proximal convoluted tubule of the kidneys, thereby preventing sodium and glucose reabsorption, increases urinary glucose excretion, and decreases blood glucose levels. Several trials, including the EMPA‐REG OUTCOME, 6 CANVAS, 7 DECLARE‐TIMI 58, 8 CREDENCE, 9 DAPA‐HF, 10 and EMPEROR‐Reduced trials, 11 have demonstrated various cardiovascular benefits of SGLT2i on patients with diabetes.
From the animal studies, SGLT2i could decrease oxidative stress in cardiomyocytes, reverse myocardial remodeling, and reduce electronic alterations. 5 These pleiotropic effects of SGLT2i are theoretically beneficial for decreasing AF occurrence and subsequently sequelae. However, none of these studies were particularly designed to evaluate the direct effects of SGLT2i on AF. Whether SGLT2i could decrease the risk of stroke or systemic embolization in patients with diabetes and AF remains unknown. Thus, we hypothesize SGLT2i could prevent AF‐related events among patients with diabetes and clarify this point of view by the present study.
METHODS
The authors declare that all supporting data are available within the article (and its online supplementary files).
Study Population
This was a historical longitudinal follow‐up cohort study using medical data collected from National Taiwan University Hospital (NTUH) Integrative Medical Database. 12 , 13 The NTUH Integrative Medical Database is maintained by the NTUH and contains all records of inpatient, outpatient, and emergency department visits to the NTUH since 2006. The Research Ethics Committee of the NTUH approved the study (200911002R and 202011019RINC). It waived the need for informed consent because of the historical nature of the database study and no patient‐identifying data. All patients with persistent or paroxysmal AF were enrolled in this registry. The diagnosis of AF was determined by taking the patient's history, serial ECG, or ambulatory ECG monitoring.
A total of 29 341 patients with AF were recruited since January 2010. Our central hypothesis in this study was that use of SGLT2i could decrease the risk of ischemic stroke in patients with AF and diabetes. Therefore, among the 29 341 patients with AF, a total of 9116 patients with AF with a concomitant diagnosis of diabetes were selected and followed up since January 2016, when SGLT2i was introduced into the Taiwan market, until the first occurrence of ischemic stroke or until December 2020. After exclusion of patients with incomplete clinical data, follow‐up, and drug information, 6614 patients were included in the analyses (Figure 1). Diabetes was diagnosed by fasting glucose >125 mg/dL and hemoglobin A1c >6.5% or under oral hypoglycemic agent or insulin use.
Figure 1. Consort diagram.
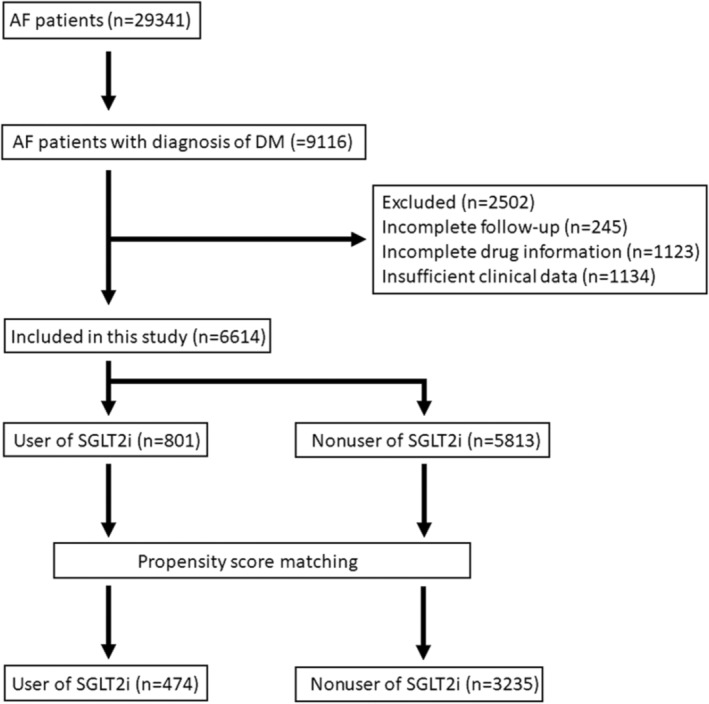
Flowchart of the patients assessed in this study. AF indicates atrial fibrillation; DM, diabetes; and SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
We selected patients with a concomitant diagnosis of diabetes also at the baseline time point to serve as our study population (n=9116). We did not have the medical information before the baseline time point and, therefore, we did not have the information on how near the diagnoses of AF and diabetes were made.
Clinical and Outcome Assessments
All the baseline characteristics were collected as previously described. 14 , 15 , 16 The medical diagnoses of hypertension (blood pressure consistently >140/90 mm Hg or treated with hypertension medication), heart failure (admission for congestive heart failure), and vascular disease (peripheral arterial disease, coronary artery disease, and myocardial infarction) were according to current practice guidelines to ensure medical insurance payment and were defined in the present study based on International Classification of Diseases (ICD) codes. The ICD codes of these diagnoses are provided in Table S1.
The CHA2DS2‐VASc scores were calculated and recorded as measures of stroke risk. The CHA2DS2‐VASc score is based on a point system in which 2 points are assigned for a history of stroke and age >75 years, and 1 point is assigned for presence of hypertension, diabetes, congestive heart failure, and vascular diseases or female sex. 17 We categorized the patients into 2 groups (CHA2DS2‐VASc score ≤2 and >2), because CHA2DS2‐VASc score ≤2 is associated with a lower risk of stroke. 18 , 19 , 20 As mentioned previously, we started to follow up the patients since January 2016 because SGLT2i was introduced to our Taiwan market in 2016. We calculated the CHA2DS2‐VASc score at the start of the follow‐up (January 2016).
The dose for empagliflozin was 25 mg once per day, and the dose for dapagliflozin was 10 mg once per day. Standard therapeutic doses were also given for other diabetes medications. Because we considered a class effect, we lumped diabetes medications of the same class into 1 group. For use of insulin, patients with use of regular insulin, basal insulin, or both were all considered insulin users. As mentioned, all the patients with AF with a concomitant diagnosis of diabetes were enrolled in January 2010, and we started to follow up the patients since January 2016 because SGLT2i was introduced to our Taiwan market in 2016. Therefore, the timing from the diagnosis of diabetes to initiation of SGLT2i was at least 5 years.
Adverse thromboembolic end points during follow‐up were defined as incident episodes of ischemic stroke. Ischemic stroke was defined as sudden‐onset and focal or global neurological deficits that were not explained by other origins, with supporting evidence from imaging studies. Hemorrhagic stroke was excluded from the study because it was generally a complication of use of anticoagulant and not related to AF per se.
Statistical Analysis
Propensity score matching was performed with the nearest neighbor matching method and a caliper of 0.2 SD of the logit of the estimated propensity score to balance the baseline characteristics. The maximum matched control number was 7 for each treatment unit, producing a population of 472 patients with SGLT2i treatment and 3743 without SGLT2i treatment. Propensity scores were calculated by the logistic regression model, with the treatment of SGLT2i as the dependent variable and the following well‐established risk factors and potential confounders of estimating AF‐related stroke as the independent covariates: age, sex, hypertension, diabetes, coronary artery disease, peripheral artery occlusive disease, congestive heart failure, and history of stroke. 21 , 22 , 23
Continuous variables are presented as means±SDs, and categorical variables are presented as percentages. The comparison of data was performed by using the χ2 test (categorical variables) or the Student t‐test (continuous variables). The time to first episode of stroke event was depicted with the Kaplan‐Meier estimate of the survival function. The difference between the survival curves was tested by the log‐rank statistics. The independent effect of variables to predict thromboembolic events was calculated using a Cox proportional hazards regression model. We used the scaled Schoenfeld residuals to evaluate the proportional hazard assumption. Hazard ratios (HRs) and 95% CIs were calculated accordingly.
To account for the correlation between matched individuals in the propensity score–matched population, the robust variance estimator was used to calculate the SE in the Cox regression. We also performed stratified analysis. The matched cases and controls were divided into 4 strata; and in each stratum, the propensity scores of cases were similar with those of controls. The average HR across the 4 strata was calculated to estimate the average treatment effect. P<0.05 was considered statistically significant. Statistical analysis was performed using STATA version 17 for Windows (STATA, Inc, College Station, TX).
RESULTS
Baseline Characteristics
Baseline characteristics of study patients with (n=801) or without (n=5813) the use of SGLT2i are summarized in Table 1. We hypothesized that this was a class effect, and we lumped use of empagliflozin and dapagliflozin together. There were no patients taking canagliflozin during the follow‐up period because it was late in the Taiwan market. Therefore, we did not include the use of canagliflozin in our study.
Table 1.
Baseline Characteristics of Patients With Diabetes and AF in the Original Study Population
| Variable | SGLT2i nonusers | SGLT2i users | P value |
|---|---|---|---|
| (N=5813) | (N=801) | ||
| Age, y | 72.4±11.0 | 67.5±10.5 | <0.0001 |
| <65 | 1364/5813 (23.46) | 300/801 (37.45) | <0.0001 |
| 65–75 | 1720/5813 (29.59) | 290/801 (36.20) | |
| ≥75 | 2729/5813 (46.94) | 211/801 (26.34) | |
| Female sex | 2152/5813 (37.02) | 281/801 (35.08) | 0.286 |
| History of stroke | 997/5813 (17.15) | 129/801 (16.10) | 0.460 |
| CHF | 1619/5813 (27.85) | 332/801 (41.45) | <0.0001 |
| Hypertension | 4362/5813 (75.04) | 621/801 (77.53) | 0.125 |
| Vascular | 2382/5813 (41.00) | 469/801 (58.55) | <0.0001 |
| CAD | 1999/5813 (34.39) | 404/801 (50.44) | <0.0001 |
| PAOD | 383/5813 (6.59) | 65/801 (8.11) | 0.107 |
| CHA2DS2‐VASc score | 3.41±1.81 | 3.13±1.78 | <0.0001 |
Data are given as mean±SD or number/total (percentage). AF indicates atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; PAOD, peripheral arterial occlusion disease; and SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
The mean age was younger in the SGLT2i group. The percentages of patients with congestive heart failure and vascular disease were higher in the SGLT2i group. The mean CHADS2‐VASc score was also lower in the SGTL2i group. To avoid the confounding effect of these comorbidities, we also created a propensity score–matched population with 474 SGLT2i users and 3235 nonusers, and the baseline characteristics of these patients are summarized in Table 2.
Table 2.
Baseline Characteristics of Patients With Diabetes and AF in the Propensity Score–Matched Population
| Variable | SGLT2i nonusers | SGLT2i users | P value |
|---|---|---|---|
| (N=3235) | (N=474) | ||
| Age, y | 73.6±10.2 | 72.9±11.4 | 0.200 |
| <65 | 711/3235 (21.98) | 97/474 (20.46) | 0.585 |
| 65–75 | 1029/3235 (31.81) | 161/474 (33.97) | |
| ≥75 | 1495/3235 (46.21) | 216/474 (45.57) | |
| Female sex | 1288/3235 (39.81) | 182/474 (38.4) | 0.556 |
| History of stroke | 763/3235 (23.59) | 106/474 (22.36) | 0.557 |
| CHF | 1270/3235 (39.3) | 190/474 (40.08) | 0.790 |
| Hypertension | 2393/3235 (73.97) | 357/474 (75.32) | 0.532 |
| Vascular | 1323/3235 (40.9) | 212/474 (44.73) | 0.114 |
| CAD | 1098/3235 (33.94) | 179/474 (37.76) | 0.102 |
| PAOD | 225/3235 (6.96) | 33/474 (6.96) | 0.996 |
| CHA2DS2‐VASc score | 3.44±1.91 | 3.35±1.83 | 0.350 |
Data are given as mean±SD or number/total (percentage). AF indicates atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; PAOD, peripheral arterial occlusion disease; and SGLT2i, sodium‐glucose cotransporter‐2 inhibitor.
CHA2DS2‐VASc Score as a Predictor of Incident Stroke Events
At the end of the follow‐up, 809 patients with AF and diabetes developed ischemic stroke in the propensity score–matched population. The stroke rate was 3.4% (95% CI, 2.8–4.2) patient‐years in SGLT2i users and 4.3% (95% CI, 4.0–4.6) in nonusers (P=0.021). We first investigated whether CHA2DS2‐VASc score was associated with risk of stroke in the propensity score–matched population. 24 , 25 As expected, CHA2DS2‐VASc score was incrementally associated with the risk of stroke. Using the univariate Cox model, we found that there was a 24% increase of risk of stroke with a 1‐point increase of CHA2DS2‐VASc score in this long‐term follow‐up cohort (HR, 1.24 [95% CI, 1.20–1.29]; P<0.001).
The event‐free survival from stroke comparing patients with different levels of CHA2DS2‐VASc score is shown in Figure 2. Patients with a higher CHA2DS2‐VASc score were more likely to develop ischemic stroke than those with a lower CHA2DS2‐VASC score (log‐rank P<0.001).
Figure 2. Kaplan‐Meier curves showing the development of ischemic stroke among patients with different levels of CHA2DS2‐VASc score in the propensity score–matched population.
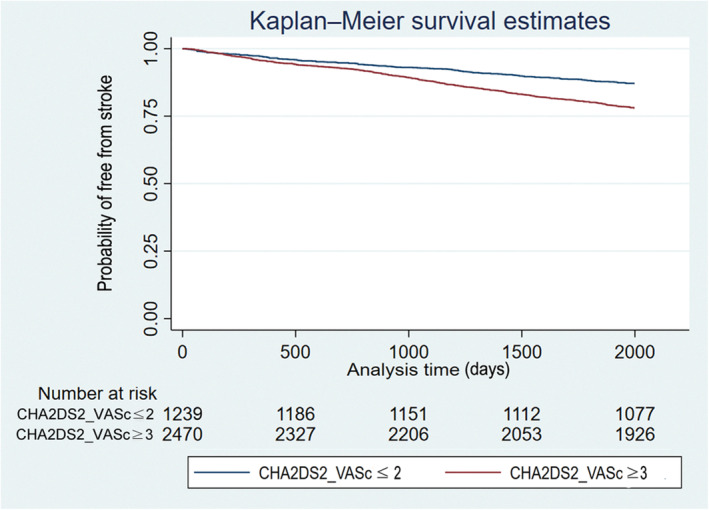
The log‐rank analysis showed a significant difference (P<0.001).
Use of SGLT2i Was Associated With a Lower Risk of Stroke
In the present study, our central hypothesis was to investigate whether the risk of ischemic stroke was decreased in those patients with AF taking SGLT2i independent of the CHA2DS2‐VASc score. In the original study population, we found that use of SGLT2i was associated with a lower risk of stroke. Using the univariable Cox model, there was a 17% decrease in stroke risk (HR, 0.83 [95% CI, 0.70–0.98]; P=0.030). The event‐free survival from stroke comparing patients with or without use of SGLT2i is shown in Figure 3. Patients with use of SGLT2i were less likely to develop ischemic stroke than those not taking SGTL2i (log‐rank P=0.030). After adjusting for the CHA2DS2‐VASc score using the multivariable Cox model, there was a trend that use of SGLT2i was associated with a lower risk of stroke (HR, 0.86 [95% CI, 0.73–1.03]; P=0.094).
Figure 3. Kaplan‐Meier curves showing the development of ischemic stroke among patients with and without use of SGLT2 inhibitor in the original study population.
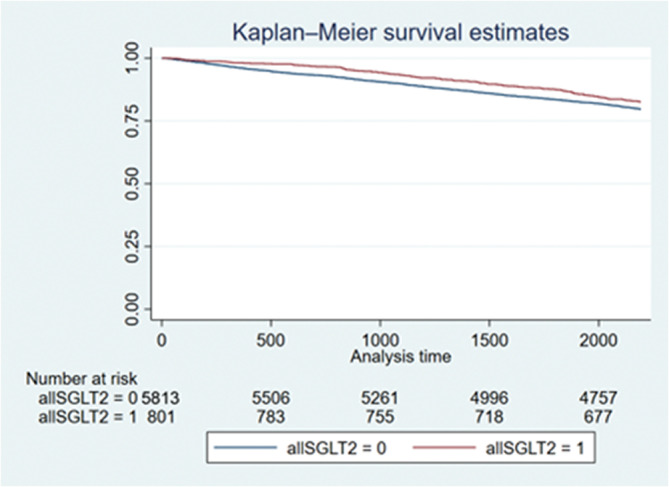
The log‐rank analysis showed significant difference (P=0.030). SGLT2 indicates sodium‐glucose cotransporter‐2.
Because the baseline characteristics and the mean CHA2DS2‐VASc score were significantly different between the SGTL2i users and nonusers, it is possible that the association of SGLT2i use with a lower risk was confounded by the CHA2DS2‐VASc score. Therefore, the association of SGTL2i use with a lower stroke risk was also evaluated in the propensity score–matched population. Using the univariable Cox model, there was a 23% decrease in stroke risk (HR, 0.77 [95% CI, 0.62–0.96]; P=0.023). The event‐free survival from stroke comparing patients with or without use of SGLT2i is shown in Figure 4. Patients with use of SGLT2i were less likely to develop ischemic stroke than those not taking SGTL2i (log‐rank P=0.025).
Figure 4. Kaplan‐Meier curves showing the development of ischemic stroke among patients with and without use of SGLT2i in the propensity score–matched population.
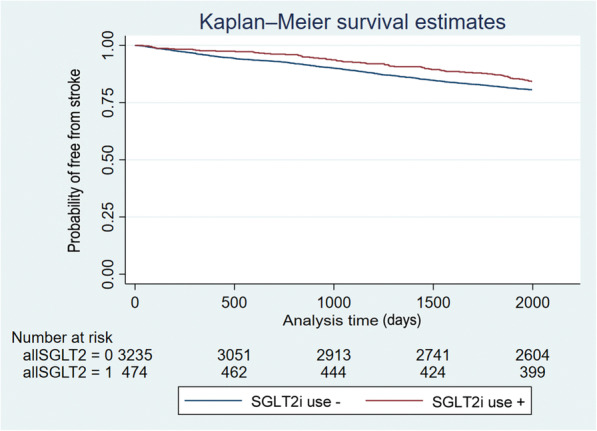
The log‐rank analysis showed significant difference (P=0.025). SGLT2i indicates sodium‐glucose cotransporter‐2 inhibitor.
After adjusting for the CHA2DS2‐VASc score using the multivariable Cox model, use of SGLT2i was still associated with a 20% decrease of risk of stroke (HR, 0.80 [95% CI, 0.64–0.99]; P=0.043). We used the scaled Schoenfeld residuals to evaluate the proportional hazard assumption. The P values for CHA2DS2‐VASc score and SGLT2i use were 0.108 and 0.170, respectively. The P value for the global model was 0.107. These results indicated no violation of the proportional hazard assumption. We also performed stratified analysis based on the propensity scores. There was a 21% decrease in risk of stroke in patients with use of SGLT2i. The average HR across the 4 propensity score strata was 0.79 (95% CI, 0.63–0.98; P=0.035).
DISCUSSION
Main Findings of This Study
This was the first and largest study specifically aiming to investigate the relationship between SGLT2i and embolic events among patients with diabetes and AF. Our results showed that SGLT2i decreased the risk of ischemic stroke after adjustment of CHA2DS2‐VASc score. Therefore, we recommended to upgrade SGLT2i for glycemic control in clinical practice, especially for the patients with diabetes and AF.
Diabetes and Thrombogenic Burden
Diabetes is associated with a 40% higher risk of AF occurrence in the general population. 1 For patients with AF, diabetes is also an independent risk factor of the ischemic events. 1 Thrombotic events and their complications have become the leading cause of death in patients with diabetes. 26 Because diabetes is closely accompanied with various metabolic abnormalities (elevated blood pressure, hyperglycemia, hyperinsulinemia, increased triglyceride, and reduced high‐density lipoprotein), 1 , 27 , 28 it is widely accepted that the pathogenesis of thrombosis among patients with diabetes is multifactorial. 28 These metabolic impairments related to diabetes often induce vascular damage. 29 As for the thrombosis formation, vascular endothelium injury is the early stage, followed by coagulation cascade alternation. 30 For the coagulation abnormality induced by diabetes, inflammation, hyperreactive platelets (overexpressed P‐selectin), upregulation of prothrombotic factors (von Willebrand factor, fibrinogen, tissue factor, and clotting factor VII), and suppression of fibrinolysis (decreased antithrombin‐III and increased plasminogen activator inhibitor‐1 levels) have been noted to be mediated by the presence of insulin resistance and hyperglycemia. 27 , 28 , 31 , 32 , 33 , 34
What is even more, diabetes also impairs nitric oxide synthase activity and enhances the production of reactive oxygen species, thus resulting in diminished nitrogen oxide bioavailability. 30 All of these increase thrombotic events and deteriorate the clinical condition of the patients with diabetes and AF. 30 In consideration of diabetes, AF, and ischemic events all together, it is urgent to evaluate the pleiotropic effects of hypoglycemic agents for the thrombosis prevention as well as glycemic control. That was the reason why we evaluated the antithrombotic effects of SGLT2i in the present study.
Why Is SGLT2i Able to Prevent Embolic Events in Patients With Diabetes and AF?
Our data indicated that SGTL2i was beneficial for reducing ischemic stroke in patients with diabetes and AF. There were many exploratory studies about SGLT2i and its pleiotropic effects. From the literature review, SGTL2i has been noted to have favorable effects on improving blood pressure, body weight, albuminuria, lipid profile, arterial stiffness, and endothelial dysfunction via insulin‐independent mechanisms. These specific characteristics of SGTL2i seem to be capable of reducing both AF occurrence and thrombosis formation and, hence, preventing the embolic events.
Previous studies have defined a proof‐of‐concept and a mechanistic link between SGTL2i and changes of AF occurrence. The post hoc analysis of the DECLARE‐TIMI 58 trial confirmed that dapagliflozin (one kind of the SGLT2i) was superior to placebo in reducing AF incidence of patients with diabetes irrespective of baseline AF (HR, 0.81 [95% CI, 0.68–0.95]). 35 In another word, dapagliflozin reduced the risk of AF by 19% among patients with diabetes in comparison with placebo. 35 There are several potential mechanisms whereby SGLT2i reduces the incidence of AF. First, SGLT2i can prevent glucotoxicity in the heart, improve mitochondrial function, and alleviate atrial remodeling. 36 Second, SGLT2i reduces epicardial adipose tissue and fat mass and thus spares the atrium from proinflammatory signals driving remodeling and fibrosis. 36 Third, SGLT2i is effective to relieve filling pressure by the peculiar diuretic effect and preload reduction. 3 Finally, SGLT2i can reduce sympathetic nervous system activation and counteract AF triggers by modulating cardiomyocyte Na‐H exchange and mitochondrial function. 37 , 38
As for the assumption of the SGLT2i to reduce thrombogenic burden, it is still under debate. SGLT2i has been supposed to be related to reducing blood pressure and increasing hemoconcentration that might amplify the risk of thrombosis formation. 3 However, the study focused on the association of SGLT2i and plasminogen activator inhibitor‐1 showed totally different results. Empagliflozin (another kind of SGLT2i) reduced the plasma plasminogen activator inhibitor‐1 concentration through its synergistic actions of a glucose‐lowering effect, visceral fat area loss, and restoring the adipokine balance. 39 Another meta‐analysis study, including 29 randomized control trials and 56 035 patients with diabetes, found that the incidence of the venous thromboembolism (eg, deep vein thrombosis and pulmonary embolism) was not significantly different between SGLT2i and control group (relative risk [RR], 0.98 [95% CI, 0.75–1.28]). 40
What are the underlying mechanisms of SGLT2i to prevent thrombosis formation? Previously, studies have suggested a link between an active inflammatory process in the heart, especially at left atrial appendages, and thrombosis formation in patients with AF. 41 , 42 From the animal studies, therapy with the SGLT2i ipragliflozin not only improved hyperglycemia, hyperlipidemia, hepatic steatosis, and oxidative stress but also various parameters of inflammation in mice with diabetes. 8 The SGLT2i dapagliflozin also ameliorated glucose homeostasis and decreased markers of inflammation in mice with diabetes. 43 Moreover, studies in experimental models of atherosclerosis have shown that various SGLT2i agents (eg, luseogliflozin, dapagliflozin, and empagliflozin) ameliorated the progression of atherosclerosis by reducing oxidative stress, mitigating vascular inflammation, and preventing platelet activation. 43 , 44 Therefore, possible mechanisms of SGTL2i for reducing thrombosis formation were supposed to reduce inflammation in patients with AF. 5 , 45
Despite these studies, there has been no study examining the direct effect of SGLT2i on patients with diabetes and AF. Therefore, whether SGLT2i deceases the risk of embolic stroke of AF remains unclear. To the best of our knowledge, this was the first and largest observational study to evaluate the preventive effects of SGLT2i of patients with diabetes and AF in the real‐world practice. In the present study, we clearly demonstrated SGLT2i was beneficial in reducing the risk of embolic stroke for patients with diabetes and AF. This effect was consistently significant after adjustment of CHA2DS2‐VASc score.
In the current practice, SGLT2i has been recommended as a second‐ or third‐line agent following metformin or sulphonylurea. 5 In this study, we fist demonstrated the protective effect of SGLT2i in patients with AF was superior to sulphonylurea or insulin. Accordingly, we suggested the place of SGLT2i in glucose‐lowering therapy algorithm should be upgraded in patients with both diabetes and AF. SGLT2i should be used before sulphonylurea therapy for the concerns of primary prevention of embolic stroke in patients with diabetes if they also have AF.
Limitations
There were limitations in the present study. First, this was not a randomized study. However, it may not be feasible to do a randomized study at the current stage because SGLT2i was not primarily used for stroke prevention. The results of our observational study may justify future randomized clinical trials for this purpose. Second, it would be better to study the effects of SGLT2i on stroke outcomes of different stroke subtypes in this study. Third, we only selected individuals with drug use throughout the follow‐up period without change of medication. We did not have the information of the effect of change in medication during the study follow‐up. Finally, we did not provide the real evidence on why SGLT2i could prevent AF‐related stroke.
CONCLUSIONS
In the present study, we explored the role of SLGT2i in preventing thromboembolic events for the patients with diabetes and AF. To sum up, our data were the first to reveal SGLT2i was associated with a lower risk of embolic stroke in comparison with other hypoglycemic therapy. In addition, SGLT2i provided greater protection against the development of thromboembolic complications than would be expected from its effects on glycemic control alone. As patients with diabetes are vulnerable for cardiac arrhythmia and embolic stroke, oral hypoglycemic agents with additional pleiotropic effects on thromboembolic prevention are more suitable for them in clinical use. Our results provided a new direction for the role of SLGT2i in the therapeutic strategy of diabetes.
Sources of Funding
This work was supported by the grants from Ministry of Science and Technology (National Science and Technology Council) (109‐2314‐B‐002‐244‐MY3 and 110‐2314‐B‐002‐201‐MY2).
Disclosures
None.
Supporting information
Table S1
Acknowledgments
We immensely thank the National Taiwan University Hospital Integrative Medical Database facility staff and the support of the National Science and Technology Council. Author contributions: Regarding the contribution of each author, Dr Chang collected the data and worked on the drafting of the manuscript. Drs Chen, Huang, and Wu provided cases and worked for data analysis and data interpretation. Drs Wang and Hwang critically reviewed the manuscript for important intellectual content. Dr Tsai designed the whole study and oversaw the whole program. All authors have read and agreed to the published version of the manuscript.
This article was sent to Kathryn A. Wood, RN, PhD, Guest Editor, for review by expert referees, editorial decision, and final disposition.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.122.027764
For Sources of Funding and Disclosures, see page 8.
REFERENCES
- 1. Ling AW, Chan CC, Chen SW, Kao YW, Huang CY, Chan YH, Chu PH. The risk of new‐onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium glucose cotransporter 2 inhibitors versus dipeptidyl peptidase‐4 inhibitors. Cardiovasc Diabetol. 2020;19:188. doi: 10.1186/s12933-020-01162-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Böhm M, Slawik J, Brueckmann M, Mattheus M, George JT, Ofstad AP, Inzucchi SE, Fitchett D, Anker SD, Marx N, et al. Efficacy of empagliflozin on heart failure and renal outcomes in patients with atrial fibrillation: data from the EMPA‐REG OUTCOME trial. Eur J Heart Fail. 2020;22:126–135. doi: 10.1002/ejhf.1663 [DOI] [PubMed] [Google Scholar]
- 3. Haloot J, Krokar L, Badin A. Effect of SLGT2 inhibitors on patients with atrial fibrillation. J Atr Fibrillation. 2021;14:20200502. doi: 10.4022/jafib.20200502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iyngkaran P, Thomas MC, Neil C, Jelinek M, Cooper M, Horowitz JD, Hare DL, Kaye DM. The heart failure with preserved ejection fraction conundrum‐redefining the problem and finding common ground? Curr Heart Fail Rep. 2020;17:34–42. doi: 10.1007/s11897-020-00454-2 [DOI] [PubMed] [Google Scholar]
- 5. Li D, Liu Y, Hidru TH, Yang X, Wang Y, Chen C, Li KHC, Tang Y, Wei Y, Tse G, et al. Protective effects of sodium‐glucose transporter 2 inhibitors on atrial fibrillation and atrial flutter: a systematic review and meta‐analysis of randomized placebo‐controlled trials. Front Endocrinol (Lausanne). 2021;12:619586. doi: 10.3389/fendo.2021.619586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA‐REG OUTCOME trial. Circulation. 2019;139:1384–1395. doi: 10.1161/circulationaha.118.037778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 8. Wiviott SD, Raz I, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389 [DOI] [PubMed] [Google Scholar]
- 9. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, Agarwal R, Bakris G, Bull S, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 10. Shah AM, Cikes M, Prasad N, Li G, Getchevski S, Claggett B, Rizkala A, Lukashevich I, O'Meara E, Ryan JJ, et al. Echocardiographic features of patients with heart failure and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2019;74:2858–2873. doi: 10.1016/j.jacc.2019.09.063 [DOI] [PubMed] [Google Scholar]
- 11. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. doi: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 12. Chen WC, Hsiao MY, Wang TG. Prognostic factors of functional outcome in post‐acute stroke in the rehabilitation unit. J Formos Med Assoc. 2022;121:670–678. doi: 10.1016/j.jfma.2021.07.009 [DOI] [PubMed] [Google Scholar]
- 13. Hsu CN, Huang K, Lin FJ, Ou HT, Huang LY, Kuo HC, Wang CC, Toh S. Continuity and completeness of electronic health record data for patients treated with oral hypoglycemic agents: findings from healthcare delivery systems in Taiwan. Front Pharmacol. 2022;13:845949. doi: 10.3389/fphar.2022.845949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai CT, Lai LP, Lin JL, Chiang FT, Hwang JJ, Ritchie MD, Moore JH, Hsu KL, Tseng CD, Liau CS, et al. Renin‐angiotensin system gene polymorphisms and atrial fibrillation. Circulation. 2004;109:1640–1646. doi: 10.1161/01.Cir.0000124487.36586.26 [DOI] [PubMed] [Google Scholar]
- 15. Tsai CT, Hsieh CS, Chang SN, Chuang EY, Ueng KC, Tsai CF, Lin TH, Wu CK, Lee JK, Lin LY, et al. Genome‐wide screening identifies a KCNIP1 copy number variant as a genetic predictor for atrial fibrillation. Nat Commun. 2016;7:10190. doi: 10.1038/ncomms10190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang SN, Lai LP, Chiang FT, Lin JL, Hwang JJ, Tsai CT. C‐reactive protein gene polymorphism predicts the risk of thromboembolic stroke in patients with atrial fibrillation: a more than 10‐year prospective follow‐up study. J Thromb Haemost. 2017;15:1541–1546. doi: 10.1111/jth.13735 [DOI] [PubMed] [Google Scholar]
- 17. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 18. Chiang CE, Wu TJ, Ueng KC, Chao TF, Chang KC, Wang CC, Lin YJ, Yin WH, Kuo JY, Lin WS, et al. 2016 guidelines of the Taiwan Heart Rhythm Society and the Taiwan Society of Cardiology for the management of atrial fibrillation. J Formos Med Assoc. 2016;115:893–952. doi: 10.1016/j.jfma.2016.10.005 [DOI] [PubMed] [Google Scholar]
- 19. Tomasdottir M, Friberg L, Hijazi Z, Lindbäck J, Oldgren J. Risk of ischemic stroke and utility of CHA(2) DS(2) ‐VASc score in women and men with atrial fibrillation. Clin Cardiol. 2019;42:1003–1009. doi: 10.1002/clc.23257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen LY, Norby FL, Chamberlain AM, MacLehose RF, Bengtson LGS, Lutsey PL, Alonso A. CHA(2)DS(2)‐VASc score and stroke prediction in atrial fibrillation in whites, blacks, and Hispanics. Stroke. 2019;50:28–33. doi: 10.1161/strokeaha.118.021453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sulzgruber P, Wassmann S, Semb AG, Doehner W, Widimsky P, Gremmel T, Kaski JC, Savarese G, Rosano GMC, Borghi C, et al. Oral anticoagulation in patients with non‐valvular atrial fibrillation and a CHA2DS2‐VASc score of 1: a current opinion of the European Society of Cardiology Working Group on cardiovascular pharmacotherapy and European Society of Cardiology Council on stroke. Eur Heart J Cardiovasc Pharmacother. 2019;5:171–180. doi: 10.1093/ehjcvp/pvz016 [DOI] [PubMed] [Google Scholar]
- 22. Joundi RA, Cipriano LE, Sposato LA, Saposnik G. Ischemic stroke risk in patients with atrial fibrillation and CHA2DS2‐VASc score of 1: systematic review and meta‐analysis. Stroke. 2016;47:1364–1367. doi: 10.1161/strokeaha.115.012609 [DOI] [PubMed] [Google Scholar]
- 23. Aamodt AH, Sandset PM, Atar D, Tveit A, Russell D. Atrial fibrillation and stroke. Tidsskr nor Laegeforen. 2013;133:1453–1457. doi: 10.4045/tidsskr.12.0850 [DOI] [PubMed] [Google Scholar]
- 24. Nagarajarao HS, Penman AD, Taylor HA, Mosley TH, Butler K, Skelton TN, Samdarshi TE, Aru G, Fox ER. The predictive value of left atrial size for incident ischemic stroke and all‐cause mortality in African Americans: the atherosclerosis risk in communities (ARIC) study. Stroke. 2008;39:2701–2706. doi: 10.1161/strokeaha.108.515221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bouzas‐Mosquera A, Broullón FJ, Álvarez‐García N, Méndez E, Peteiro J, Gándara‐Sambade T, Prada O, Mosquera VX, Castro‐Beiras A. Left atrial size and risk for all‐cause mortality and ischemic stroke. CMAJ. 2011;183:E657–E664. doi: 10.1503/cmaj.091688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xin G, Wei Z, Ji C, Zheng H, Gu J, Ma L, Huang W, Morris‐Natschke SL, Yeh JL, Zhang R, et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci Rep. 2016;6:36222. doi: 10.1038/srep36222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grant PJ. Beneficial effects of metformin on haemostasis and vascular function in man. Diabetes Metab. 2003;29:6S44–6S52. doi: 10.1016/s1262-3636(03)72787-6 [DOI] [PubMed] [Google Scholar]
- 28. Markowicz‐Piasecka M, Sadkowska A, Huttunen KM, Podsiedlik M, Mikiciuk‐Olasik E, Sikora J. An investigation into the pleiotropic activity of metformin. A glimpse of haemostasis. Eur J Pharmacol. 2020;872:172984. doi: 10.1016/j.ejphar.2020.172984 [DOI] [PubMed] [Google Scholar]
- 29. Colwell JA. Pathophysiology of vascular disease in diabetes: effects of gliclazide. Am J Med. 1991;90:50s–54s. doi: 10.1016/0002-9343(91)90418-w [DOI] [PubMed] [Google Scholar]
- 30. Lu DY, Huang CC, Huang PH, Chung CM, Lin SJ, Chen JW, Chan WL, Leu HB. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non‐randomized, pair‐matched cohort study. BMC Cardiovasc Disord. 2014;14:187. doi: 10.1186/1471-2261-14-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hess K, Grant PJ. Inflammation and thrombosis in diabetes. Thromb Haemost. 2011;105(suppl 1):S43–S54. doi: 10.1160/ths10-11-0739 [DOI] [PubMed] [Google Scholar]
- 32. Bodary PF, Vargas FB, King SA, Jongeward KL, Wickenheiser KJ, Eitzman DT. Pioglitazone protects against thrombosis in a mouse model of obesity and insulin resistance. J Thromb Haemost. 2005;3:2149–2153. doi: 10.1111/j.1538-7836.2005.01551.x [DOI] [PubMed] [Google Scholar]
- 33. Lim HS, Chong AY, Freestone B, Blann AD, Lip GY. The effect of multi‐factorial intervention on plasma von Willebrand factor, soluble E‐selectin and tissue factor in diabetes mellitus: implications for atherosclerotic vascular disease. Diabet Med. 2005;22:249–255. doi: 10.1111/j.1464-5491.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 34. Perriello G, Pampanelli S, Brunetti P, di Pietro C, Mariz S. Long‐term effects of pioglitazone versus gliclazide on hepatic and humoral coagulation factors in patients with type 2 diabetes. Diab Vasc Dis Res. 2007;4:226–230. doi: 10.3132/dvdr.2007.044 [DOI] [PubMed] [Google Scholar]
- 35. Zelniker TA, Bonaca MP, Furtado RHM, Mosenzon O, Kuder JF, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, et al. Effect of dapagliflozin on atrial fibrillation in patients with type 2 diabetes mellitus: insights from the DECLARE‐TIMI 58 trial. Circulation. 2020;141:1227–1234. doi: 10.1161/circulationaha.119.044183 [DOI] [PubMed] [Google Scholar]
- 36. Shao Q, Meng L, Lee S, Tse G, Gong M, Zhang Z, Zhao J, Zhao Y, Li G, Liu T. Empagliflozin, a sodium glucose co‐transporter‐2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high‐fat diet/streptozotocin‐induced diabetic rats. Cardiovasc Diabetol. 2019;18:165. doi: 10.1186/s12933-019-0964-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Peng X, Li L, Zhang M, Zhao Q, Wu K, Bai R, Ruan Y, Liu N. Sodium‐glucose cotransporter 2 inhibitors potentially prevent atrial fibrillation by ameliorating ion handling and mitochondrial dysfunction. Front Physiol. 2020;11:912. doi: 10.3389/fphys.2020.00912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma A, Verma S. Mechanisms by which glucagon‐like‐peptide‐1 receptor agonists and sodium‐glucose cotransporter‐2 inhibitors reduce cardiovascular risk in adults with type 2 diabetes mellitus. Can J Diabetes. 2020;44:93–102. doi: 10.1016/j.jcjd.2019.09.003 [DOI] [PubMed] [Google Scholar]
- 39. Sakurai S, Jojima T, Iijima T, Tomaru T, Usui I, Aso Y. Empagliflozin decreases the plasma concentration of plasminogen activator inhibitor‐1 (PAI‐1) in patients with type 2 diabetes: association with improvement of fibrinolysis. J Diabetes Complicat. 2020;34:107703. doi: 10.1016/j.jdiacomp.2020.107703 [DOI] [PubMed] [Google Scholar]
- 40. Wang A, Yang K, Wang T, Zhang N, Tang H, Feng X. Effects of sodium‐glucose cotransporter 2 inhibitors on risk of venous thromboembolism in patients with type 2 diabetes: a systematic review and meta‐analysis. Diabetes Metab Res Rev. 2020;36:e3174. doi: 10.1002/dmrr.3174 [DOI] [PubMed] [Google Scholar]
- 41. Hohmann C, Pfister R, Mollenhauer M, Adler C, Kozlowski J, Wodarz A, Drebber U, Wippermann J, Michels G. Inflammatory cell infiltration in left atrial appendageal tissues of patients with atrial fibrillation and sinus rhythm. Sci Rep. 2020;10:1685. doi: 10.1038/s41598-020-58797-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. H. Deng SLW, Xue YM, Zhan XZ. Inflammation was involved in the thrombosis of left atrium in patients with atrial fibrillation. Eur Heart J. 2013;34:5640. [Google Scholar]
- 43. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, Vujacic‐Mirski K, Helmstädter J, Kröller‐Schön S, Münzel T, et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. 2019;2019:7092151. doi: 10.1155/2019/7092151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu Z, Ma X, Ilyas I, Zheng X, Luo S, Little PJ, Kamato D, Sahebkar A, Wu W, Weng J, et al. Impact of sodium glucose cotransporter 2 (SGLT2) inhibitors on atherosclerosis: from pharmacology to pre‐clinical and clinical therapeutics. Theranostics. 2021;11:4502–4515. doi: 10.7150/thno.54498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors: a state‐of‐the‐art review. JACC Basic Transl Sci. 2020;5:632–644. doi: 10.1016/j.jacbts.2020.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


