Abstract
Trimesic acid-functionalized chitosan (Cs/ECH-TMA) material was prepared through a simple procedure by using inexpensive and commercially available chitosan (Cs), epichlorohydrin (ECH) linker and trimesic acid (TMA). The obtained bio-based Cs/ECH-TMA material was characterized using energy-dispersive X-ray (EDX) and Fourier-transform infrared (FTIR) spectroscopy, field emission scanning electron microscopy (FESEM) and X-ray diffraction (XRD) analysis. The Cs/ECH-TMA material was successfully used, as a multifunctional heterogeneous and sustainable catalyst, for efficient and expeditious synthesis of medicinally important polyhydroquinoline (PHQ) and polyhydroacridinedione (PHA) scaffolds through the Hantzsch condensation in a one-pot reaction. Indeed, the heterogeneous Cs/ECH-TMA material can be considered as a synergistic multifunctional organocatalyst due to the presence of a large number of acidic active sites in its structure as well as hydrophilicity. Both PHQs and PHAs were synthesized in the presence of biodegradable heterogeneous Cs/ECH-TMA catalytic system from their corresponding substrates in EtOH under reflux conditions and high to quantitative yields. The Cs/ECH-TMA catalyst is recyclable and can be reused at least four times without significant loss of its catalytic activity.
Keywords: Modified biopolymers, Heterogeneous organocatalysis, Biodegradable catalyst, Solid acid, Multicomponent reactions (MCRs), Heterocycles, Green and sustainable chemistry
1. Introduction
The use of agricultural, fishery and industrial biopolymeric by-products have become very attractive in designing of new heterogeneous catalytic systems mainly as appropriate supports in the recent years. Indeed, these biodegradable supports are simply extracted from endless non-toxic biological resources of nature as well as demonstrate proper synergistic catalytic activity with the active catalytic centers and biodegradability [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11]]. The development of new heterogeneous catalytic systems has become a major research area in recent decades due to their ability to minimize of contamination in the organic synthesis methodologies. In addition, there are several positive aspects for using of heterogeneously-catalyzed processes including simplification of procedures, assistance to decreasing trend of the waste production, and easy separation and recycling of the catalysts [[12], [13], [14]]. Interestingly, these biomaterials are environmentally-friendly alternative supports for other materials such as diverse synthetic organic polymers [[15], [16], [17], [18]], metal organic frameworks (MOFs) [19,20], graphene-oxide or its reduced form [[21], [22], [23]], alumina [24], silica, zeolites or their magnetic composites [16,[25], [26], [27], [28], [29]], which can be used to stabilize active catalytic agents onto the surface of the supports as well as tune desired catalytic activities.
Chitosan is one of the best biopolymeric substrates which is easily obtained from natural sources and can be used for a variety of applications in numerous industrial areas [[30], [31], [32]]. It is easily derived by the random N-deacetylation of chitin, a by-product of the fishing industry, under alkaline conditions. Also, chitosan is a linear biopolymer with special features including hydrophilicity, crystallinity, ionic conductivity, high viscosity and nitrogen richness that make it to stand out from other biopolymeric materials [33,34]. Due to the presence of both amino and hydroxyl groups with proper geometry on the chitosan backbone, it has a great ability to form coordination interactions and covalent bonds with a variety of metals and organic compounds, respectively [[35], [36], [37], [38]]. Hence, chitosan has been widely studied as an appropriate precursor or ingredient in various research areas [32]. Interestingly, chitosan has been used as a heterogeneous catalyst alone in some organic transformations [39]. In other cases, chitosan after post-modification with proper ligands or metallic species has also been used along with nanomaterials to proceed different catalytic reactions [11,[40], [41], [42], [43], [44], [45], [46], [47], [48], [49]]. Furthermore, the non-toxic nature, presence of proper functional groups and hydrophilicity of chitosan or its derivatives make them an appropriate choice for drug delivery, anti-bacterial wound dressing materials, bioelectronics, sensors, adsorbents, hydrogels, etc [[50], [51], [52], [53], [54], [55], [56], [57], [58], [59]]. Hence, designing, preparation, and exploring the applications of new chitosan-derived materials in various research fields is in high demand.
Trimesic acid (TMA) is an organic compound with a complementary functional groups, which can easily be used for various applications such as the synthesis of self-assembled dendrimers or architectures [60,61], coordination polymers and metal-organic framework [[62], [63], [64]], nonporous materials [65], polyamides [66], and absorbance of toxic substances and metals [67,68]. TMA can act as a hydrogen donor or receptor by creating a variety of resonance structures [69]. Due to the structural symmetry and multiplicity of the acidic functional groups of TMA, we were interested to use it by grafting onto the chitosan backbone for preparing a cost-effective catalytic system with proper catalytic efficiency.
Literature survey shows that multi-component reactions (MCRs) are widely used to synthesize many pharmaceutical nuclei, new surfaces with proper functionalities, laser dyes or chemosensors as well as biomimetic asymmetric transfer hydrogenation and electron donors and proton sources in photoredox catalyzed processes including Hantzsch esters as a subdivision of heterocyclic compounds [[70], [71], [72], [73], [74], [75], [76], [77], [78]]. One of the well-known methods for synthesis of polyhydroquinolines (PHQs) and polyhydroacridinediones (PHAs), as two scaffolds containing 1,4-dihydropyridines (1,4-DHP) moiety, is the Hantzsch condensation. For example, amlodipine, felodipine, nicardipine and nifedipine which are classified as economic drugs, obtained from this method. These pharmaceuticals are considered as L-type Ca2+ channels (LTCC) blockers and mainly applied for the treatment of hypertension and angina [79]. Therefore, due to significant and wide applications of 1,4-DHPs in different areas [80] as well as efficiency and low cost of the Hantzsch method for synthesis of these compounds, the multicomponent reaction of 1,3-dicarbonyl compounds, aldehydes and an amine source is still considered as the first choice. Hence, different homogeneous or heterogeneous acidic and basic catalytic systems have been introduced to proceed Hantzsch condensation more efficiently. Some recent catalytic systems for promoting of PHQs and PHAs synthesis are sulfonic acid supported γ-Fe2O3 [81], Zn [(L)proline]2 [82], 3,4,5-trifluorobenzeneboronic acid in ionic liquid [83] Co3O4–CNT nanocomposites, platinum nanoparticles supported with reduced graphene oxide [84], nickel containing ionic liquid based ordered nanoporous organosilica, 1,3,5-tris(2-hydroxyethyl) isocyanurate covalently functionalized MCM-41, urease enzyme [85], layered double hydroxides and PPh3 [86], and alginic acid [79,87]. It is obvious that each of these catalytic systems has simultaneously their own advantages and disadvantages for catalyzing of Hantzsch's reaction. Hence, there is still much room for designing and exploring of new and more efficient catalytic systems with tailored functional groups, which address green and sustainable chemistry principles. In continuation of our interest to develop natural biopolymers or their modified products, as efficient catalytic systems for different organic transformation, we wish herein to report trimesic acid-functionalized chitosan (Cs/ECH-TMA) material for efficient synthesis of PHQs and PHAs in EtOH/H2O under reflux conditions (Scheme 1). Indeed, avoiding the use of toxic substances and transition metals in the preparation of Cs/ECH-TMA catalyst is one of the advantages of this work. This was achieved by the use of commercially available and biodegradable chitosan polysaccharide and epichlorohydrin.
Scheme 1.
Schematic preparation of the Cs/ECH-TMA (1), as a heterogeneous organocatalyst, for the synthesis of polyhydroquinoline 6 and acridinedione 7 derivatives.
2. Experimental section
2.1. Reagents and apparatus
All chemicals and reagents were purchased from Merck and Aldrich and used without further purification, except for benzaldehyde, which was used as a freshly distilled sample. Chitosan (MW = 100,000–300,000 Da) was purchased from Acros Organics. Melting points were determined using a digital Electrothermal 9100 capillary apparatus and are uncorrected. Fourier-transform infrared spectroscopy (FTIR) spectra were recorded, as KBr pellets, on a Shimadzu FT-IR-8400S spectrometer. 1H NMR spectra (500 MHz) were obtained using a Bruker DRX-500 Avance spectrometer. All 1H NMR spectra were run in CDCl3 solution, relative to TMS (0.00 ppm), at ambient temperature. Analytical thin layer chromatography (TLC) was performed using Merck 0.2 mm silica gel 60 F-254 Al-plates for monitoring of reactions. All products were characterized by spectroscopic methods (FTIR and 1H NMR spectra) and melting points.
2.2. General procedure for the preparation of heterogeneous Cs/ECH-TMA organocatalyst (1)
In a 100 ml round button flask, chitosan (MW = 100,000–300,000 Da, Acros Organics, 1.0 g) was suspended in EtOH/H2O (1:1, 50 ml). Then, epichlorohydrin (d = 1.183 g ml−1, 1.0 ml, 12.8 mmol) was added to the obtained mixture, stirred and heated under reflux conditions for 3 h. After that, the obtained solid was filtered off and suspended in EtOH (3 × 2 ml) for 10 min and then filtered by vacuum filtration. The solid was dried in an oven at 60 °C for 8 h to afford the epichlorohydrine-grafted chitosan (Cs-ECH). In the next step, Cs-ECH (0.5 g) and triethylamine (TEA, 2.0 ml) were suspended in DMSO (30 ml) and stirred for 30 min at room temperature. Then, trimesic acid (TMA, 2.4 mmol, 0.5 g) was added to the obtained mixture and stirred at 100 °C for 4 h. The obtained solid was filtered and washed with acetone (3 × 2 ml) and soaked in HCl solution (1.0 M) for 3 h. Then, the obtained deep cream powder was washed with deionized water (3 × 5 ml) and dried in oven to afford the catalyst 1.
General procedure for the synthesis of PHQ 6a-n and PHA 7a-l derivatives catalyzed by the Cs/ECH-TMA nanomaterial (1)
In a 10 ml round-bottom flask equipped with a magnetic stirring bar and reflux condenser, aldehyde (2a-l, 1.0 mmol), dimedone (3, 1.0 mmol) and ethyl acetoacetate (5, 1.0 mmol), NH4OAc (5, 1.2 mmol), and Cs/ECH-TMA (1, 10 mg) were mixed in EtOH (96%, 2 ml). The reaction mixture was stirred and heated under reflux conditions for the reaction times indicated in Table 3. The progress of the reaction was monitored by TLC. After completion of the reaction, EtOH (96%, 2 ml) was added to dissolve any solid crude product 6 and remain the solid catalyst 1 insoluble. The mixture was separated by vacuum filtration, allowing the filtrate solution to cool over time to obtain the desired polyhydroquinolines (PHQs, 6) crystals. The products were finally collected by vacuum filtration, washed with EtOH (96%, 2 ml) and dried at 50 °C for 1 h. In the case of polyhydroacridinediones (PHAs, 7), aldehyde (2, 1.0 mmol), dimedone (3, 2.0 mmol), NH4OAc (4, 1.2 mmol) and Cs/ECH-TMA (1, 10 mg) were mixed in EtOH (96%, 2 ml) by following the same described procedure.
Table 3.
Synthesis of PHA derivatives 5a-l from different aldehydes 2a-l catalyzed by the Cs/ECH-TMA organocatalyst (1) under optimized conditions.a
| Entry | Aldehyde 2 | Product 7 | Time (min) | Yield (%) | M.P/Obs. (oC) | M.P/Rep. (oC) |
|---|---|---|---|---|---|---|
| 1 | 4-Chlorobenzaldehyde (2a) | 5a | 25 | 95 | 306–308 | 303 ‒ 305 [98] |
| 2 | 2,4-Dichlorobenzaldehyde (2b) | 5b | 25 | 94 | 319–322 | 321 [99] |
| 3 | 4-Nitrobenzaldehyde (2c) | 5c | 40 | 84 | 301–303 | 302 ‒ 305 [100] |
| 4 | 4-Hydroxy-3-methoxybenzaldehyde (2d) | 5d | 35 | 88 | 293–296 | 295 ‒ 298 [101] |
| 5 | 3-Nitrobenzaldehyde (2e) | 5e | 40 | 80 | 292–294 | 294 ‒ 296 [102] |
| 6 | 4-Florobenzaldehyde (2f) | 5f | 25 | 96 | 246–248 | 246 ‒ 248 [103] |
| 7 | Benzaldehyde (2g) | 5g | 25 | 92 | 190–191 | 189 ‒ 191 [103] |
| 8 | 2-Chlorobenzaldehyde (2h) | 5h | 25 | 92 | 222–224 | 221 ‒ 222 [104] |
| 9 | 4-Methoxybenzaldehyde (2i) | 5i | 25 | 86 | 295–297 | 296 ‒ 298 [105] |
| 10 | 4-Hydorxybenzaldehyde (2j) | 5j | 25 | 87 | 303–305 | >300 [106] |
| 11 | 4-Methylbenzaldehyde (2k) | 5k | 25 | 89 | 300–302 | >300 [106] |
| 12 | 2-Bromobenzaldehyde (2l) | 5l | 25 | 90 | 253–255 | 252 ‒ 254 [107] |
bAll products are known and their structures were established from their spectral data and melting points compared to authentic samples or literature values.
cIsolated yields.
Reaction conditions: Dimedone (3, 2.0 mmol), aryl aldehydes (2, 1.0 mmol), NH4OAc (5, 1.2 mmol) and the Cs/ECH-TMA (1, 10 mg) in EtOH under reflux conditions.
3. Results and discussion
3.1. Preparation of the Cs/ECH-TMA organocatalyst (1)
The trimesic acid-functionalized chitosan (Cs/ECH-TMA) material was simply prepared by grafting of the trimesic acid (TMA) to the chitosan (Cs) backbone using epichlorohydrin (ECH), as an applicable and inexpensive linker, under mild conditions (Scheme 1). For this purpose, several paths have been reported. However, the EtOH/H2O (1:1) solvent was chosen, which demonstrated to be more efficient for reaction of the amino group of chitosan with the epoxide moiety of ECH than its chloride group compared to other solvents [108]. Due to the higher reactivity of the epoxide ring of ECH, induced by hydrogen bonding between ECH and EtOH/H2O, the amine group opens the ring instead of the substitution of chlorine. In the next step, the aprotic and polar DMSO solvent was used in the presence of triethylamine (TEA) organic base to activate the carboxylic acid group of TMA and completion of the chloride group substitution and its grafting to the chitosan backbone. It should be noted that due to the existence of three acidic functional groups of trimesic acid, different ammonium carboxylate salts with triethylamine can be formed. Hence, after preparing and washing the catalyst 1, it was soaked in dilute HCl solution for several hours to recover the remaining carboxylic acid functional groups of TMA moiety.
3.2. Characterization of the Cs/ECH-TMA organocatalyst (1)
In order to investigate the surface functionalization of Cs with TMA moieties, several appropriate techniques such as Fourier transform infrared (FTIR) spectroscopy, field emission scanning electron microscopy (FESEM), thermal gravimetric analysis (TGA), energy dispersive X-ray (EDX) spectroscopy and X-ray powder diffraction (XRD) were used. Fig. 1a - c shows the FTIR spectra of chitosan (a), Cs/ECH (b) and Cs/ECH-TMA (c), respectively. As can be seen, the signal related to the asymmetric and symmetric stretching vibration of N–H bond at ∼3500 is observed in all spectra (a - c). Of course, the broad adsorption band at 3450 ‒ 3150 cm−1 can be attributed to the stretching vibrations of the hydroxyls groups of both Cs and ECH linker as well as TMA. Furthermore, the strong absorption band at 1725 cm−1 is as assigned to the stretching vibration of carbonyl group (C O) and confirms the presence of trimesic acid in the structure of Cs/ECH-TMA (1, spectrum c). On the other hand, two absorption bands near 1240 and 1370 cm−1 are related to the in-plane bending vibrations of hydroxyl groups, respectively [109].
Fig. 1.
FTIR spectra of the commercial chitosan (a), chitosan containing epichlorohydrin linker (Cs/ECH, b) and the Cs/ECH-TMA material (1, c).
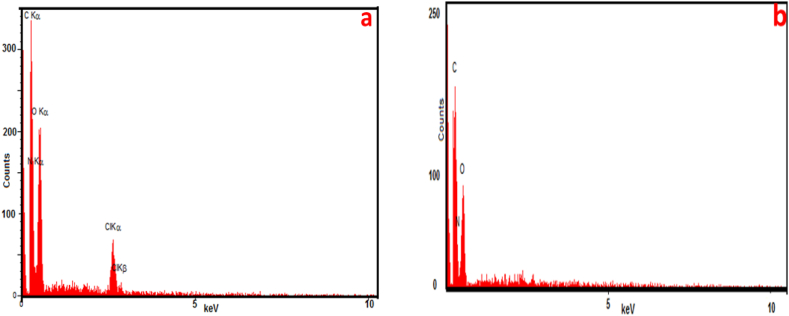
The presence of chlorine element in the EDX analysis of Cs/ECH (Fig. 2a) confirms that the first step in the preparation of the catalyst 1 was successfully performed with proper regioselectivity towards the epoxide moiety of ECH. Subsequent elimination of the chlorine element in the EDX analysis of Cs/ECH-TMA (Fig. 2b) is a clear indication of its substitution with the carboxylic acid functional groups of TMA by a covalent binding to the chitosan backbone through the ECH linker (Fig. 2a, b).
Fig. 2.
Energy dispersive spectroscopy (EDX) analysis of the Cs/ECH (a) and the Cs/ECH-TMA material (1, b).
Field emission scanning electron microscopy (FESEM) images show the morphology of Cs/ECH-TMA material compared to the commercial chitosan (Fig. 3a–d). The surface of the commercial chitosan is almost smooth and free of cross-linked groups including ECH and TMA (Fig. 3aandb). However, additional particles have spread across the surface of the Cs support and demonstrating its uniform functionalization by the TMA units grafted by the ECH linker. The fine and regular aggregation of these particles is probably related to the formed hydrogen bonding and self-assembly of TMA moieties (Fig. 3c and d).
Fig. 3.
FESEM images of the commercial chitosan (a - b) and the Cs/ECH-TMA material (1, c - d).
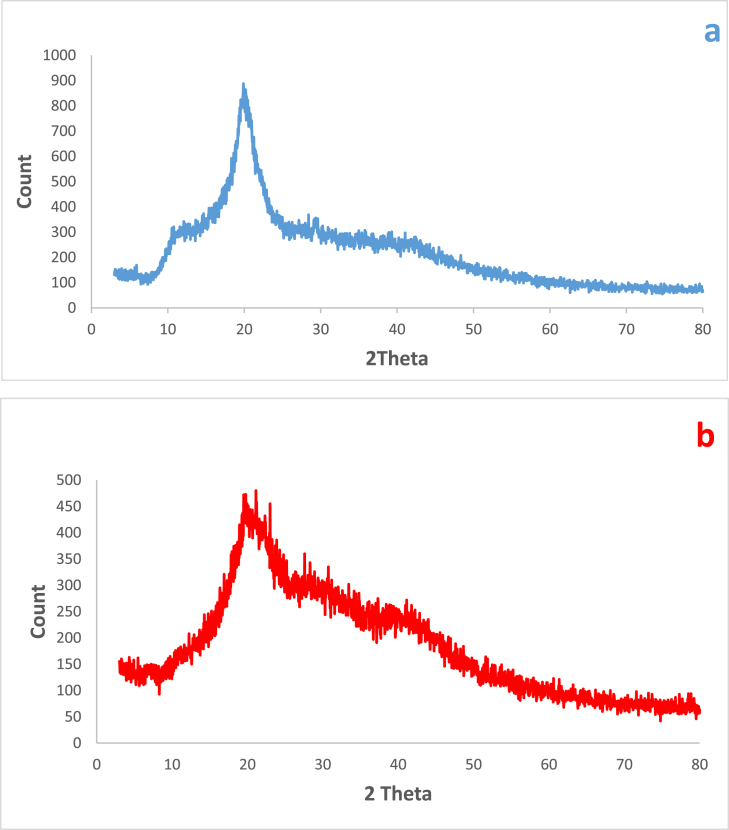
The XRD spectra of the commercial chitosan and Cs/ECH-TMA material was also studied (Fig. 4a, b). The characteristic peaks at about 2ϴ = 10° and 20° are related to the structure of pristine chitosan (4a). After grafting of TMA on the chitosan backbone, its crystalline structure changes and new signals appear at 21.2°, 23.0°, 28.3°, 30.7° and 41.8° (4b). Furthermore, observation of broad peaks in the spectrum (4b) indicates lower crystallinity of the catalyst 1 than the pristine chitosan which can be attributed to the linkage of TMA on the surface of chitosan.
Fig. 4.
XRD pattern of the commercial chitosan (a) and the Cs/ECH-TMA material (1, b).
3.3. Investigation of the catalytic activity of Cs/ECH-TMA for the synthesis of PHQ and PHA derivatives
In order to evaluate the catalytic activity of Cs/ECH-TMA and find the optimized conditions for the synthesis of polyhydroquinolines (PHQs) and polyhydroacridinediones (PHAs), different factors and parameters including solvent, temperature and time in the presence of the variable amount of Cs/ECH-TMA catalyst (1) were examined (Table 1). For this purpose, the four-component condensation of ethyl acetoacetate (3, 1.0 mmol), dimedone (4, 1.0 mmol), 4-chlorobenzaldehyde (2a, 1.0 mmol) and NH4OAc (5, 1.2 mmol), was investigated as the model reaction. The results have been summarized in Table 1.
Table 1.
Optimization of the Hantzsch four-component reaction for the synthesis of 6a catalyzed by Cs/ECH-TMA (1).a
| Entry | Catalyst loading (mg) | Solventb | Temperature (0C) | Time (min) | Yield (%)b |
|---|---|---|---|---|---|
| 1 | – | EtOH | Reflux | 180 | 25 |
| 2 | 20c | EtOH | Reflux | 120 | 30 |
| 3 | 20 | EtOH | Reflux | 30 | 97 |
| 4 | 15 | EtOH | Reflux | 30 | 97 |
| 5 | 10 | EtOH | Reflux | 35 | 96 |
| 6 | 7 | EtOH | Reflux | 35 | 88 |
| 7 | 10 | EtOH | Reflux | 25 | 82 |
| 8 | 10 | H2O | 50 | 90 | 74 |
| 9 | 10 | EtOH | rt | 100 | 35 |
| 10 | 10 | H2O | Reflux | 50 | 75 |
| 11 | 10 | – | 110 | 50 | 70 |
| 12 | 10 | EtOH/H2O (1:1) | 50 | 80 | 78 |
| 13 | 10 | CH3CN | Reflux | 90 | 65 |
| 14 | 10 | DMSO | 100 | 70 | 67 |
| 15 | 10 | EtOAc | Reflux | 80 | 54 |
| 16 | 10 | THF | Reflux | 100 | 60 |
| 17 | 10 | – | 60 | 110 | 45 |
| 18 | 10 | EtOH/H2O (1:1) | Reflux | 50 | 86 |
Reaction conditions: Dimedone (3, 1.0 mmol), ethyl acetoacetate (4, 1.0 mmol), 4-chlorobenzaldehyde (2a, 1.0 mmol), NH4OAc (5, 1.2 mmol), Cs/ECH-TMA (1), and solvent (2.0 ml) unless otherwise stated.
Isolated yields.
Commercial chitosan (MW = 100,000–300,000 Da) without any post-modification was used as catalyst.
Initially, the reaction was performed without any catalyst, and the reaction yield for the synthesis of desired product, 4-(4-chlorophenyl)-2,7,7-trimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-3-carboxylate (6a), was lower than 30% after 3 h (entry 1). Then, commercial chitosan was used, as a catalyst, in the model reaction which did not afford a high yield of the desired product 6a (entry 2). To determine the optimized conditions, different catalyst loadings were used (entries 3–6). The best result was observed when 10.0 mg catalyst loading was used in EtOH under reflux conditions (entry 5). The results clearly confirm the high impact of Cs/ECH-TMA organocatalyst to proceed the model reaction. Investigation of the solvent effects showed that polar protic solvents (entries 5, 10 and 18) demonstrate higher efficiency than polar aprotic solvents (entries 13–16). On the other hand, solvent-free conditions afforded moderate yields of the desired products at both 60 and 110 °C (entries 11 and 17).
To show the general scope of the reaction, different aromatic aldehydes bearing electron-withdrawing or electron-donating substituents 2a-n were investigated under optimized conditions (10 mg Cs/ECH-TMA loading in EtOH under reflux conditions). The results have been summarized in Table 2. Indeed, the catalyst 1 demonstrated a high activity for the synthesis of corresponding PHQs and the desired products 6a-n were obtained in high to excellent yields and short reaction time. According to the literature survey, ethyl acetoacetate in the presence of dimedone only leads to the formation of the corresponding polyhydroquinoline (PHQ), and the competitive polyhydroacridinedione (PHA) is not formed as a by-product [110]. As shown in Table 2, aldehydes with electron-withdrawing groups on the aromatic ring (entries 1–2, 6, 8 and 12) afforded higher yields of the desired products in shorter reaction times compared to those ones bearing electron-donating groups. This can be attributed to higher susceptibility of the carbonyl group in aldehydes with electron-withdrawing groups for nucleophilic addition of the enolic components of Hantzsch reaction in order to form the corresponding Knoevenagel intermediates. However, aldehydes bearing nitro groups (entries 3 and 5) afforded lower yields of their corresponding PHQ derivatives compared to other electron-withdrawing groups. This behavior may be relevant to simultaneous formation of the corresponding imines of nitro derivatives by reaction with NH3 demonstrating lower activity as well as redox disproportionation of their PHQs [11]. In general, other aldehydes have been well activated by the catalyst 1 to afford desired products under optimized conditions in high to excellent yields.
Table 2.
Synthesis of PHQ derivatives 6a-n from different aldehydes 2a-n catalyzed by the Cs/ECH-TMA solid acid (1) under optimized conditions.a
| Entry | Aldehyde 2 | Product 6b | Time (min) | Yield (%) | M.P/Obs. (oC) | M.P/Rep. (oC) |
|---|---|---|---|---|---|---|
| 1 | 4-Chlorobenzaldehyde (2a) | 6a | 35 | 96 | 241–243 | 244 ‒ 246 [88] |
| 2 | 2,4-Dichlorobenzaldehyde (2b) | 6b | 35 | 94 | 240–242 | 240 ‒ 243 [89] |
| 3 | 4-Nitrobenzaldehyde (2c) | 6c | 45 | 82 | 240–242 | 240 ‒ 242 [90] |
| 4 | 4-Hydroxy-3-methoxybenzaldehyde (2d) | 6d | 45 | 90 | 210–212 | 210 ‒ 212 [91] |
| 5 | 3-Nitrobenzaldehyde (2e) | 6e | 50 | 77 | 178–180 | 177 ‒ 178 [91] |
| 6 | 4-Florobenzaldehyde (2f) | 6f | 35 | 96 | 183–184 | 183 ‒ 185 [92] |
| 7 | Benzaldehyde (2g) | 6g | 30 | 92 | 202–204 | 203 ‒ 204 [93] |
| 8 | 2-Chlorobenzaldehyde (2h) | 6h | 35 | 90 | 206–208 | 210 ‒ 212 [94] |
| 9 | 4-Methoxybenzaldehyde (2i) | 6i | 35 | 84 | 250–252 | 253 ‒ 256 [89] |
| 10 | 4-Hydorxybenzaldehyde (2j) | 6j | 35 | 86 | 228–230 | 229 ‒ 232 [95] |
| 11 | 4-Methylbenzaldehyde (2k) | 6k | 40 | 90 | 260–263 | 258 ‒ 260 [91] |
| 12 | 4-Bromobenzaldehyde (2l) | 6l | 35 | 92 | 253–255 | 252 ‒ 254 [91] |
| 13 | Furfural (2m) | 6m | 35 | 94 | 244–246 | 246 ‒ 248 [96] |
| 14 | Formaldehyde (2n) | 6n | 35 | 95 | 173–175 | 173 ‒ 174 [97] |
bAll products are known compounds and their structures were established from their spectral data and melting points as compared with authentic samples or literature values.
Reaction conditions: Ethyl acetoacetate (4, 1.0 mmol), aldehyde (2, 1.0 mmol), NH4OAc (5, 1.2 mmol), dimedone (3, 1.0 mmol) and Cs/ECH-TMA (1, 10 mg) in EtOH (2 ml) under reflux conditions.
In addition, the catalytic activity of the Cs/ECH-TMA (1) was investigated under optimized conditions for the production of 3,3,6,6-tetramethyl-3,4,6,7,9,10-hexahydro-2H,5H-1,8-acridinedione derivatives (PHAs, 7a-l) from pseudo-four-component condensation of dimedone (4), aromatic aldehydes 2a-l and NH4OAc (5). As shown in Table 3, shorter reaction times were required for the synthesis of PHA derivatives 7 than PHQs ones 6 under the same conditions. Aldehydes with electron-withdrawing groups on the aromatic ring, except nitro groups (entries 1–2, 6, 8 and 12), demonstrated more reactivity than aldehydes bearing electron-releasing groups under optimized conditions.
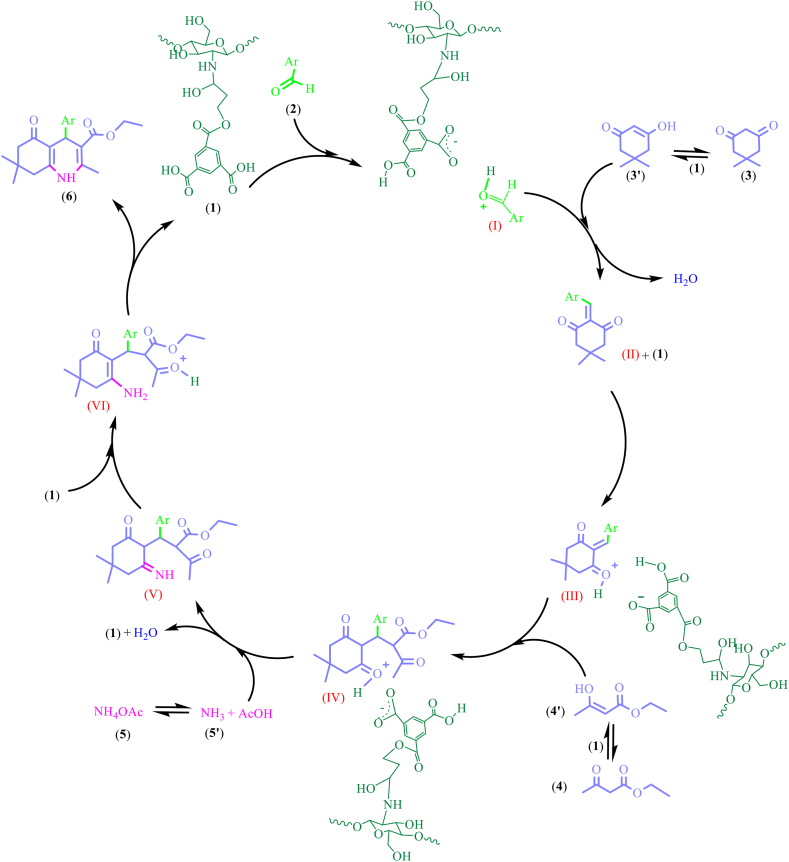
According to the obtained results, the mechanism presented in Scheme 2 can be proposed for the synthesis of PHQs 6a-n and PHAs 7a-l catalyzed by Cs/ECH-TMA solid acid (1). In the first step, acidic protons of the trimesic acid moiety of the catalyst 1 cause activation of the carbonyl functional group of aldehydes 2 to react with the enolic form of dimedone (3′) and affording the corresponding Knoevenagel intermediates (II). This α,β-unsaturated carbonyl is then activated by the Cs/ECH-TMA (1) and react with the enol form of β-dicarbonyls, 3′ or 4’, to form the Michael adduct intermediate (IV). In the next step, the intermediate (IV) reacts on one of its ketone functional group with the ammonia source (5) and forms imine intermediate (V). After tautomerization of the imine intermediate (V) and its conversion to the corresponding enamine intermediate (VI), the intramolecular reaction between enamine (VI) and remaining ketone functional group in this intermediate leads to ring closure. Eventually, by elimination of the third H2O molecule, PHQ 6 or PHA 7 derivatives are produced. Furthermore, due to the presence of a large numbers of hydroxyl groups in the chitosan backbone and the ECH linker of the Cs/ECH-TMA catalyst (1), water molecules can be adsorbed on its surface to promote the Hantzsch condensations smoothly. Moreover, this hydroxyl groups contribute to more activation of carbonyl groups by development of hydrogen bonding [38,39,79,[111], [112], [113]]. Hence, the heterogeneous Cs/ECH-TMA material can be considered as a synergistic multifunctional organocatalyst due to the presence of a large number of acidic active sites in its structure as well as hydrophilicity.
Scheme 2.
Feasible mechanism for the Hantzsch four-component reaction of different aldehydes 2, β-dicarbonyls 3 and 4, and NH4OAc (5) catalyzed by the synergistic multifunctional Cs/ECH-TMA organocatalyst (1).
Due to the importance of heterogeneous catalyst recycling for industrial applications, this aspect of the Cs/ECH-TMA solid acid catalyst was also investigated. Therefore, after completion of the reaction, the Cs/ECH-TMA material (1) was separated from the reaction mixture and washed three times with acetone solvent. Then, the separated catalyst was dried in an oven at 60 °C for 6 h to be used for the next run. As shown in Fig. 5, the activity of catalyst 1 for synthesis of desired product 6a was still significant after five consecutive uses.
Fig. 5.
Reusability of the Cs/ECH-TMA catalyst (1) for synthesis of PHQ derivative 6a from 4-chlorobenzaldehyde (2a), dimedone (3), ethyl acetoacetate (4) and NH4OAc (5).
Finally, in order to further clarify the high efficiency of Cs/ECH-TMA organocatalyst (1) and the merits of the present protocol for the synthesis of polyhydroquinolines and acridinediones, several previously reported methods are listed in Table 4 for comparison. As can be seen, the present work is indeed preferable to several of the others in terms of the use of low loading of a biodegradable catalyst, higher isolated yield, short reaction time, elimination of toxic transition metals and solvents to accelerate synthesis of PHQs and PHAs.
Table 4.
Comparison of the catalytic efficiency of Cs/ECH-TMA solid acid (1) with other heterogeneous or homogeneous catalysts for the synthesis of 6a.
| Entry | Catalyst | Catalyst loading | Solvent | Temp. oC | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Co3O4-CNTs | 30 mg | EtOH | Reflux | 35 | 97 | [114] |
| 2 | Vitamin-B1 | 20 mol% | EtOH | Reflux | 60 | 90 | [115] |
| 3 | Fe(HSO4)3/SiO2 | 10 mol% | EtOH | 80 | 60 | 95 | [116] |
| 4 | FeF3 | 5 mol% | EtOH | Reflux | 60 | 92 | [117] |
| 5 | Al2(SO4)3 | 10 mol% | EtOH | Reflux | 300 | 87 | [118] |
| 6 | Cs/ECH-TMA | 10 mg | EtOH | Reflux | 35 | 96 | This Work |
4. Conclusions
In summary, novel trimesic acid-functionalized chitosan (Cs/ECH-TMA) material was prepared through a simple procedure and properly characterized. The Cs/ECH-TMA material was used, as a reusable and solid acidic organocatalyst, for the multicomponent reactions in synthesis of polyhydroquinolines (PHQs) and polyhydroacridinediones (PHAs) under mild and sustainable conditions. The heterogeneous Cs/ECH-TMA material was demonstrated to act as synergistic multifunctional nanocatalyst due to the presence of a large number of acidic active sites in its structure as well as hydrophilicity. Indeed, the use of a metal-free and low loading catalyst with facile separation from the reaction mixture and its reusability as well as commercially available biomaterial precursor and short reaction time in a green solvent are important advantages of this new methodology.
Author contribution statement
Rahman Beiranvand: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Mohammad G. Dekamin: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful for the financial support from The Research Council of Iran University of Science and Technology (IUST), Tehran, Iran (Grant No: 160/22061). The partial financial support of The Iran Nanotechnology Initiative Council (INIC) is also gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16315.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Chen Y., Hung S.-T., Chou E., Wu H.-S. Review of polyhydroxyalkanoates materials and other biopolymers for medical applications. Mini-Reviews Org. Chem. 2018;15:105–121. [Google Scholar]
- 2.Baran T., Menteş A. Construction of new biopolymer (chitosan)-based pincer-type Pd (II) complex and its catalytic application in Suzuki cross coupling reactions. J. Mol. Struct. 2017;1134:591–598. [Google Scholar]
- 3.Kosera V.S., Cruz T.M., Chaves E.S., Tiburtius E.R. Triclosan degradation by heterogeneous photocatalysis using ZnO immobilized in biopolymer as catalyst. J. Photochem. Photobiol. Chem. 2017;344:184–191. [Google Scholar]
- 4.Guan Q., Yang C., Wang S., He L., Kong Z., Chai X., Xin H., Ning P. Reactive metal–biopolymer interactions for semihydrogenation of acetylene. ACS Catal. 2019;9:11146–11152. [Google Scholar]
- 5.Chaka K.T. Extraction of cellulose nanocrystals from agricultural by-products: a review. Green Chem. Lett. Rev. 2022;15:582–597. [Google Scholar]
- 6.Shen C., Xu J., Yu W., Zhang P. A highly active and easily recoverable chitosan@ copper catalyst for the C–S coupling and its application in the synthesis of zolimidine. Green Chem. 2014;16:3007–3012. [Google Scholar]
- 7.Patil P.G., Sehlangia S., More D.H. Chitosan-SO3H (CTSA) an efficient and biodegradable polymeric catalyst for the synthesis of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ol) and α-amidoalkyl-β-naphthol’s. Synth. Commun. 2020;50:1696–1711. [Google Scholar]
- 8.Dashti M., Nikpassand M., Mokhtary M., Zare Fekri L. Polycyclic Aromatic Compounds; 2022. Fe3O4@SP@Chitosan@Fe3O4 Nanocomposite: A Catalyst with Double Magnetite Parts for Sustainable Synthesis of Novel Azo-Linked 4-Benzylidene-2-Phenyloxazol-5-Ones; pp. 1–17. [Google Scholar]
- 9.Rostami N., Dekamin M.G., Valiey E., Fanimoghadam H. Chitosan-EDTA-Cellulose network as a green, recyclable and multifunctional biopolymeric organocatalyst for the one-pot synthesis of 2-amino-4H-pyran derivatives. Sci. Rep. 2022;12:8642. doi: 10.1038/s41598-022-10774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alirezvani Z., Dekamin M.G., Valiey E. Cu(II) and magnetite nanoparticles decorated melamine-functionalized chitosan: a synergistic multifunctional catalyst for sustainable cascade oxidation of benzyl alcohols/Knoevenagel condensation. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-53765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dekamin M.G., Kazemi E., Karimi Z., Mohammadalipoor M., Naimi-Jamal M.R. Chitosan: an efficient biomacromolecule support for synergic catalyzing of Hantzsch esters by CuSO4. Int. J. Biol. Macromol. 2016;93:767–774. doi: 10.1016/j.ijbiomac.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Lanzafame P., Perathoner S., Centi G., Gross S., Hensen E. Grand challenges for catalysis in the Science and Technology Roadmap on Catalysis for Europe: moving ahead for a sustainable future. Catal. Sci. Technol. 2017;7:5182–5194. [Google Scholar]
- 13.Lucarelli C., Vaccari A. Examples of heterogeneous catalytic processes for fine chemistry. Green Chem. 2011;13:1941–1949. [Google Scholar]
- 14.Liang J., Liang Z., Zou R., Zhao Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv. Mater. 2017;29 doi: 10.1002/adma.201701139. [DOI] [PubMed] [Google Scholar]
- 15.Lu J., Toy P.H. Organic polymer supports for synthesis and for reagent and catalyst immobilization. Chem. Rev. 2009;109:815–838. doi: 10.1021/cr8004444. [DOI] [PubMed] [Google Scholar]
- 16.Karami S., Dekamin M.G., Valiey E., Shakib P. DABA MNPs: a new and efficient magnetic bifunctional nanocatalyst for the green synthesis of biologically active pyrano[2,3-c]pyrazole and benzylpyrazolyl coumarin derivatives. New J. Chem. 2020;44:13952–13961. [Google Scholar]
- 17.Akbarzadeh A., Dekamin M.G. A facile and environmentally benign polyethylene glycol 600-mediated method for the synthesis of densely functionalized 2-aminothiophene derivatives under ultrasonication. Green Chem. Lett. Rev. 2017;10:315–323. [Google Scholar]
- 18.Dekamin M.G., Alikhani M., Javanshir S. Organocatalytic clean synthesis of densely functionalized 4 H-pyrans by bifunctional tetraethylammonium 2-(carbamoyl) benzoate using ball milling technique under mild conditions. Green Chem. Lett. Rev. 2016;9:96–105. [Google Scholar]
- 19.Chen Y.-Z., Zhang R., Jiao L., Jiang H.-L. Metal–organic framework-derived porous materials for catalysis. Coord. Chem. Rev. 2018;362:1–23. [Google Scholar]
- 20.Ghasemzadeh M.A., Abdollahi-Basir M.H., Mirhosseini-Eshkevari B. Multi-component synthesis of spiro [diindeno [1, 2-b: 2′, 1′-e] pyridine-11, 3′-indoline]-triones using zinc terephthalate metal-organic frameworks. Green Chem. Lett. Rev. 2018;11:47–53. [Google Scholar]
- 21.Shan C., Wang L., Li Z., Zhong X., Hou Y., Zhang L., Shi F. Graphene oxide enhanced polyacrylamide-alginate aerogels catalysts. Carbohyd. Poly. 2019;203:19–25. doi: 10.1016/j.carbpol.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 22.Hasanzadeh Banakar S., Dekamin M.G., Yaghoubi A. Selective and highly efficient synthesis of xanthenedione or tetraketone derivatives catalyzed by ZnO nanorod-decorated graphene oxide. New J. Chem. 2018;42:14246–14262. [Google Scholar]
- 23.Adeel M., Bilal M., Rasheed T., Sharma A., Iqbal H.M.N. Graphene and graphene oxide: functionalization and nano-bio-catalytic system for enzyme immobilization and biotechnological perspective. Int. J. Biol. Macromol. 2018;120:1430–1440. doi: 10.1016/j.ijbiomac.2018.09.144. [DOI] [PubMed] [Google Scholar]
- 24.Pines H., Manassen J. The mechanism of dehydration of alcohols over alumina catalysts. Adv. Cataly. 1966:49–93. Elsevier. [Google Scholar]
- 25.Bhatia S. CRC press; 1989. Zeolite Catalysts: Principles and Applications. [Google Scholar]
- 26.Zebardasti A., Dekamin M.G., Doustkhah E. The isocyanurate-carbamate-bridged hybrid mesoporous organosilica: an exceptional anchor for Pd nanoparticles and a unique catalyst for nitroaromatics reduction. Catalysts. 2021;11:621. [Google Scholar]
- 27.Zebardasti A., Dekamin M.G., Doustkhah E., Assadi M.H.N. Carbamate-isocyanurate-bridged periodic mesoporous organosilica for van der Waals CO2 capture. Inorg. Chem. 2020;59:11223–11227. doi: 10.1021/acs.inorgchem.0c01449. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X., He X., Zhao S. Preparation of a novel Fe3O4@ SiO2@ propyl@ DBU magnetic core–shell nanocatalyst for Knoevenagel reaction in aqueous medium. Green Chem. Lett. Rev. 2021;14:85–98. [Google Scholar]
- 29.Eslami M., Dekamin M.G., Motlagh L., Maleki A. MCM-41 mesoporous silica: a highly efficient and recoverable catalyst for rapid synthesis of α-aminonitriles and imines. Green Chem. Lett. Rev. 2018;11:36–46. [Google Scholar]
- 30.Antony R., Arun T., Manickam S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019;129:615–633. doi: 10.1016/j.ijbiomac.2019.02.047. [DOI] [PubMed] [Google Scholar]
- 31.Islam S., Bhuiyan M.R., Islam M. Chitin and chitosan: structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017;25:854–866. [Google Scholar]
- 32.Rinaudo M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006;31:603–632. [Google Scholar]
- 33.Yuan Y., Chesnutt B.M., Haggard W.O., Bumgardner J.D. Deacetylation of chitosan: material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials. 2011;4:1399–1416. doi: 10.3390/ma4081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zargar V., Asghari M., Dashti A. A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. ChemBioEng Rev. 2015;2:204–226. [Google Scholar]
- 35.Dohendou M., Dekamin M.G., Namaki D. Pd@L-Asparagine-EDTA-Chitosan: a highly effective and reusable bio-based and biodegradable catalyst for Heck cross-coupling reaction under mild conditions. Nanoscale Adv. 2023;5:2621–2638. doi: 10.1039/d3na00058c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dohendou M., Pakzad K., Nezafat Z., Nasrollahzadeh M., Dekamin M.G. Progresses in chitin, chitosan, starch, cellulose, pectin, alginate, gelatin and gum based (nano)catalysts for the Heck coupling reactions: a review. Int. J. Biol. Macromol. 2021;192:771–819. doi: 10.1016/j.ijbiomac.2021.09.162. [DOI] [PubMed] [Google Scholar]
- 37.Valiey E., Dekamin M.G., Bondarian S. Sulfamic acid grafted to cross-linked chitosan by dendritic units: a bio-based, highly efficient and heterogeneous organocatalyst for green synthesis of 2,3-dihydroquinazoline derivatives. RSC Adv. 2023;13:320–334. doi: 10.1039/d2ra07319f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rostami N., Dekamin M.G., Valiey E. Chitosan-EDTA-Cellulose bio-based network: a recyclable multifunctional organocatalyst for green and expeditious synthesis of Hantzsch esters. Carbohydr. Poly. Techn. Appl. 2023;5 [Google Scholar]
- 39.Dekamin M.G., Azimoshan M., Ramezani L. Chitosan: a highly efficient renewable and recoverable bio-polymer catalyst for the expeditious synthesis of α-amino nitriles and imines under mild conditions. Green Chem. 2013;15:811–820. [Google Scholar]
- 40.El Kadib A. Chitosan as a sustainable organocatalyst: a concise overview. ChemSusChem. 2015;8:217–244. doi: 10.1002/cssc.201402718. [DOI] [PubMed] [Google Scholar]
- 41.Macquarrie D.J., Hardy J.J. Applications of functionalized chitosan in catalysis. Ind. Eng. Chem. Res. 2005;44:8499–8520. [Google Scholar]
- 42.Valiey E., Dekamin M.G., Alirezvani Z. Melamine-modified chitosan materials: an efficient and recyclable bifunctional organocatalyst for green synthesis of densely functionalized bioactive dihydropyrano[2,3-c]pyrazole and benzylpyrazolyl coumarin derivatives. Int. J. Biol. Macromol. 2019;129:407–421. doi: 10.1016/j.ijbiomac.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 43.Alirezvani Z., Dekamin M.G., Valiey E. Cu (II) and magnetite nanoparticles decorated melamine-functionalized chitosan: a synergistic multifunctional catalyst for sustainable cascade oxidation of benzyl alcohols/Knoevenagel condensation. Sci. Rep. 2019;9:1–12. doi: 10.1038/s41598-019-53765-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan K., Siddiqui Z.N. An efficient synthesis of tri- and tetrasubstituted imidazoles from benzils using functionalized chitosan as biodegradable solid acid catalyst. Ind. Eng. Chem. Res. 2015;54:6611–6618. [Google Scholar]
- 45.Ahmed N., Siddiqui Z.N. Cerium supported chitosan as an efficient and recyclable heterogeneous catalyst for sustainable synthesis of spiropiperidine derivatives. ACS Sustain. Chem. Eng. 2015;3:1701–1707. [Google Scholar]
- 46.Zhao Z.-S., Zhang Y., Fang T., Han Z.-B., Liang F.-S. Chitosan-Coated metal–organic-framework nanoparticles as catalysts for tandem deacetalization–knoevenagel condensation reactions. ACS Appl. Nano Mater. 2020;3:6316–6320. [Google Scholar]
- 47.Tang Z., Xiao J., Li F., Ma Z., Wang L., Niu F., Sun X. Cobalt-tetraamide-phthalocyanine immobilized on Fe3O4/chitosan microspheres as an efficient catalyst for baeyer–villiger oxidation. ACS Omega. 2020;5:10451–10458. doi: 10.1021/acsomega.0c00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng X., Zhao J., Xu M., Zeng M. Preparation of porous chitosan/reduced graphene oxide microspheres supported Pd nanoparticles catalysts for Heck coupling reactions. Carbohyd. Poly. 2020;230 doi: 10.1016/j.carbpol.2019.115583. [DOI] [PubMed] [Google Scholar]
- 49.Jirawutthiwongchai J., Krause A., Draeger G., Chirachanchai S. Chitosan-oxanorbornadiene: a convenient chitosan derivative for click chemistry without metal catalyst problem. ACS Macro Lett. 2013;2:177–180. doi: 10.1021/mz400006j. [DOI] [PubMed] [Google Scholar]
- 50.García M.C., Aldana A.A., Tártara L.I., Alovero F., Strumia M.C., Manzo R.H., Martinelli M., Jimenez-Kairuz A.F. Bioadhesive and biocompatible films as wound dressing materials based on a novel dendronized chitosan loaded with ciprofloxacin. Carbohydrate Polym. 2017;175:75–86. doi: 10.1016/j.carbpol.2017.07.053. [DOI] [PubMed] [Google Scholar]
- 51.Kedir W.M., Abdi G.F., Goro M.M., Tolesa L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: a review. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh, et al. Preparation and properties of highly soluble chitosan–l-glutamic acid aerogel derivative. Carbohyd. Poly. 2009;76:188–195. [Google Scholar]
- 53.Sheng Z., Guo A., Wang J., Chen X. Preparation, physicochemical properties and antimicrobial activity of chitosan from fly pupae. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e11168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang C., Yokota T., Someya T. Natural biopolymer-based biocompatible conductors for stretchable bioelectronics. Chem. Rev. 2021;121:2109–2146. doi: 10.1021/acs.chemrev.0c00897. [DOI] [PubMed] [Google Scholar]
- 55.Wang R., Zhu J., Jiang G., Sun Y., Ruan L., Li P., Cui H. Forward wound closure with regenerated silk fibroin and polylysine-modified chitosan composite bioadhesives as dressings. ACS Appl. Bio Mater. 2020;3:7941–7951. doi: 10.1021/acsabm.0c01064. [DOI] [PubMed] [Google Scholar]
- 56.Herdiana Y., Wathoni N., Shamsuddin S., Muchtaridi M. Drug release study of the chitosan-based nanoparticles. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2021.e08674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cui C., Shao C., Meng L., Yang J. High-strength, self-adhesive, and strain-sensitive chitosan/poly (acrylic acid) double-network nanocomposite hydrogels fabricated by salt-soaking strategy for flexible sensors. ACS Appl. Mater. Interfaces. 2019;11:39228–39237. doi: 10.1021/acsami.9b15817. [DOI] [PubMed] [Google Scholar]
- 58.Morikawa K., Masubuchi Y., Shchipunov Y., Zinchenko A. DNA-chitosan hydrogels: formation, properties, and functionalization with catalytic nanoparticles. ACS Appl. Bio Mater. 2021;4:1823–1832. doi: 10.1021/acsabm.0c01533. [DOI] [PubMed] [Google Scholar]
- 59.Unuabonah E.I., Adewuyi A., Kolawole M.O., Omorogie M.O., Olatunde O.C., Fayemi S.O., Günter C., Okoli C.P., Agunbiade F.O., Taubert A. Disinfection of water with new chitosan-modified hybrid clay composite adsorbent. Heliyon. 2017;3 doi: 10.1016/j.heliyon.2017.e00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nath K.G., Ivasenko O., Miwa J.A., Dang H., Wuest J.D., Nanci A., Perepichka D.F., Rosei F. Rational modulation of the periodicity in linear hydrogen-bonded assemblies of trimesic acid on surfaces. J. Am. Chem. Soc. 2006;128:4212–4213. doi: 10.1021/ja0602896. [DOI] [PubMed] [Google Scholar]
- 61.Wendland M.S., Zimmerman S.C. Synthesis of cored dendrimers. J. Am. Chem. Soc. 1999;121:1389–1390. [Google Scholar]
- 62.Song J., Luo Z., Britt D.K., Furukawa H., Yaghi O.M., Hardcastle K.I., Hill C.L. A multiunit catalyst with synergistic stability and reactivity: a polyoxometalate–metal organic framework for aerobic decontamination. J. Am. Chem. Soc. 2011;133:16839–16846. doi: 10.1021/ja203695h. [DOI] [PubMed] [Google Scholar]
- 63.Cheng D., Khan M.A., Houser R.P. Coordination polymers composed of copper(II), trimesic acid, and imidazole: 3D architecture stabilized by hydrogen bonding. Inorg. Chem. 2001;40:6858–6859. doi: 10.1021/ic015609v. [DOI] [PubMed] [Google Scholar]
- 64.Chui S.S.-Y., Lo S.M.-F., Charmant J.P., Orpen A.G., Williams I.D. A chemically functionalizable nanoporous material [Cu3 (TMA) 2 (H2O) 3] n. Science. 1999;283:1148–1150. doi: 10.1126/science.283.5405.1148. [DOI] [PubMed] [Google Scholar]
- 65.Dmitriev A., Lin N., Weckesser J., Barth J., Kern K. Supramolecular assemblies of trimesic acid on a Cu (100) surface. J. Phys. Chem. B. 2002;106:6907–6912. [Google Scholar]
- 66.Jikei M., Chon S.-H., Kakimoto M.-a., Kawauchi S., Imase T., Watanebe J. Synthesis of hyperbranched aromatic polyamide from aromatic diamines and trimesic acid. Macromolecules. 1999;32:2061–2064. [Google Scholar]
- 67.Herbstein F., Kapon M., Reisner G. Catenated and non-catenated inclusion complexes of trimesic acid. J. Inclusion Phenom. 1987;5:211–214. [Google Scholar]
- 68.Li H., Li Y., Zhou Y., Li B., Liu D., Liao H. Efficient removal of uranium using a melamine/trimesic acid-modified hydrothermal carbon-based supramolecular organic framework. J. Colloid Interface Sci. 2019;544:14–24. doi: 10.1016/j.jcis.2019.02.079. [DOI] [PubMed] [Google Scholar]
- 69.Nikooei N., Dekamin M.G., Valiey E. Benzene-1,3,5-tricarboxylic acid-functionalized MCM-41 as a novel and recoverable hybrid catalyst for expeditious and efficient synthesis of 2,3-dihydroquinazolin-4(1H)-ones via one-pot three-component reaction. Res. Chem. Intermed. 2020;46:3891–3909. [Google Scholar]
- 70.Domling A., Wang W., Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Afshari R., Shaabani A. Materials functionalization with multicomponent reactions: state of the art. ACS Comb. Sci. 2018;20:499–528. doi: 10.1021/acscombsci.8b00072. [DOI] [PubMed] [Google Scholar]
- 72.Rathee G., Kohli S., Singh N., Awasthi A., Chandra R. Calcined layered double hydroxides: catalysts for xanthene, 1,4-dihydropyridine, and polyhydroquinoline derivative synthesis. ACS Omega. 2020;5:15673–15680. doi: 10.1021/acsomega.0c01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Q.-A., Chen M.-W., Yu C.-B., Shi L., Wang D.-S., Yang Y., Zhou Y.-G. Biomimetic asymmetric hydrogenation: in situ regenerable hantzsch esters for asymmetric hydrogenation of benzoxazinones. J. Am. Chem. Soc. 2011;133:16432–16435. doi: 10.1021/ja208073w. [DOI] [PubMed] [Google Scholar]
- 74.Wang P.-Z., Chen J.-R., Xiao W.-J. Hantzsch esters: an emerging versatile class of reagents in photoredox catalyzed organic synthesis. Org. Biomol. Chem. 2019;17:6936–6951. doi: 10.1039/c9ob01289c. [DOI] [PubMed] [Google Scholar]
- 75.Etivand N., Khalafy J., Dekamin M.G. Fast and efficient green procedure for the synthesis of benzo[5,6]chromene derivatives and their sulfur analogues in water by organocatalyst potassium phthalimide-N-oxyl. Synthesis. 2020;52:1707–1718. [Google Scholar]
- 76.Alirezvani Z., Dekamin M.G., Valiey E. New hydrogen-bond-enriched 1,3,5-Tris(2-hydroxyethyl) isocyanurate covalently functionalized MCM-41: an efficient and recoverable hybrid catalyst for convenient synthesis of acridinedione derivatives. ACS Omega. 2019;4:20618–20633. doi: 10.1021/acsomega.9b02755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Katkar S.S., Mohite P.H., Gadekar L.S., Arbad B.R., Lande M.K. ZnO-beta zeolite: as an effective and reusable heterogeneous catalyst for the one-pot synthesis of polyhydroquinolines. Green Chem. Lett. Rev. 2010;3:287–292. [Google Scholar]
- 78.Patel A., Patel S., Mehta M., Patel Y., Patel R., Shah D., Patel D., Shah U., Patel M., Patel S., Solanki N., Bambharoliya T., Patel S., Nagani A., Patel H., Vaghasiya J., Shah H., Prajapati B., Rathod M., Bhimani B., Patel R., Bhavsar V., Rakholiya B., Patel M., Patel P. A review on synthetic investigation for quinoline- recent green approaches. Green Chem. Lett. Rev. 2022;15:337–372. [Google Scholar]
- 79.Dekamin M.G., Karimi Z., Latifidoost Z., Ilkhanizadeh S., Daemi H., Naimi-Jamal M.R., Barikani M. Alginic acid: a mild and renewable bifunctional heterogeneous biopolymeric organocatalyst for efficient and facile synthesis of polyhydroquinolines. Int. J. Biol. Macromol. 2018;108:1273–1280. doi: 10.1016/j.ijbiomac.2017.11.050. [DOI] [PubMed] [Google Scholar]
- 80.Blazquez-Barbadillo C., González J.F., Porcheddu A., Virieux D., Menéndez J.C., Colacino E. Benign synthesis of therapeutic agents: domino synthesis of unsymmetrical 1,4-diaryl-1,4-dihydropyridines in the ball-mill. Green Chem. Lett. Rev. 2022:1–12. [Google Scholar]
- 81.Koukabi N., Kolvari E., Zolfigol M.A., Khazaei A., Shaghasemi B.S., Fasahati B. A magnetic particle‐supported sulfonic acid catalyst: tuning catalytic activity between homogeneous and heterogeneous catalysis. Adv. Synth. Catal. 2012;354:2001–2008. [Google Scholar]
- 82.Sivamurugan V., Kumar R.S., Palanichamy M., Murugesan V. Synthesis of hantzsch 1, 4‐dihydropyridines under solvent‐free condition using zn [(L) proline] 2 as lewis acid catalyst. J. Heterocycl. Chem. 2005;42:969–974. [Google Scholar]
- 83.Sridhar R., Perumal P.T. A new protocol to synthesize 1, 4-dihydropyridines by using 3, 4, 5-trifluorobenzeneboronic acid as a catalyst in ionic liquid: synthesis of novel 4-(3-carboxyl-1H-pyrazol-4-yl)-1, 4-dihydropyridines. Tetrahedron. 2005;61:2465–2470. [Google Scholar]
- 84.Aday B., Yıldız Y., Ulus R., Eris S., Sen F., Kaya M. One-pot, efficient and green synthesis of acridinedione derivatives using highly monodisperse platinum nanoparticles supported with reduced graphene oxide. New J. Chem. 2016;40:748–754. [Google Scholar]
- 85.Zhu G., Li Y. Molecular Diversity; 2020. Urease: a Highly Efficient Biocatalyst for Synthesis of Polyhydroquinolines and Polyhydroacridines from the Ammonia Formed in Situ. [DOI] [PubMed] [Google Scholar]
- 86.Debache A., Ghalem W., Boulcina R., Belfaitah A., Rhouati S., Carboni B. An efficient one-step synthesis of 1, 4-dihydropyridines via a triphenylphosphine-catalyzed three-component Hantzsch reaction under mild conditions. Tetrahedron Lett. 2009;50:5248–5250. [Google Scholar]
- 87.Dekamin M.G., Ilkhanizadeh S., Latifidoost Z., Daemi H., Karimi Z., Barikani M. Alginic acid: a highly efficient renewable and heterogeneous biopolymeric catalyst for one-pot synthesis of the Hantzsch 1,4-dihydropyridines. RSC Adv. 2014;4:56658–56664. [Google Scholar]
- 88.Dondoni A., Massi A., Minghini E., Bertolasi V. Multicomponent Hantzsch cyclocondensation as a route to highly functionalized 2-and 4-dihydropyridylalanines, 2-and 4-pyridylalanines, and their N-oxides: preparation via a polymer-assisted solution-phase approach. Tetrahedron. 2004;60:2311–2326. [Google Scholar]
- 89.Nasr-Esfahani M., Hoseini S.J., Montazerozohori M., Mehrabi R., Nasrabadi H. Magnetic Fe3O4 nanoparticles: efficient and recoverable nanocatalyst for the synthesis of polyhydroquinolines and Hantzsch 1, 4-dihydropyridines under solvent-free conditions. J. Mol. Catal. Chem. 2014;382:99–105. [Google Scholar]
- 90.Maheswara M., Siddaiah V., Damu G.L.V., Rao C.V. An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using a heterogeneous catalyst under solvent-free conditions. ARKIVOC (Gainesville, FL, U. S.) 2006;2:201–206. [Google Scholar]
- 91.Salehi H., Guo Q.X. Synthesis of substituted 1, 4‐dihydropyridines in water using phase‐transfer catalyst under microwave irradiation. Synth. Commun. 2004;34:4349–4357. [Google Scholar]
- 92.Breitenbucher J.G., Figliozzi G. Solid-phase synthesis of 4-aryl-1, 4-dihydropyridines via the Hantzsch three component condensation. Tetrahedron Lett. 2000;41:4311–4315. [Google Scholar]
- 93.Ko S., Sastry M., Lin C., Yao C.-F. Molecular iodine-catalyzed one-pot synthesis of 4-substituted-1, 4-dihydropyridine derivatives via Hantzsch reaction. Tetrahedron Lett. 2005;46:5771–5774. [Google Scholar]
- 94.Kumar S., Sharma P., Kapoor K.K., Hundal M.S. An efficient, catalyst-and solvent-free, four-component, and one-pot synthesis of polyhydroquinolines on grinding. Tetrahedron. 2008;64:536–542. [Google Scholar]
- 95.Loev B., Snader K.M. The Hantzsch reaction. I. Oxidative dealkylation of certain dihydropyridines. J. Org. Chem. 1965;30:1914–1916. [Google Scholar]
- 96.Donelson J.L., Gibbs R.A., De S.K. An efficient one-pot synthesis of polyhydroquinoline derivatives through the Hantzsch four component condensation. J. Mol. Catal. Chem. 2006;256:309–311. [Google Scholar]
- 97.Stankevich E., Grinshtein E., Dubur G.Y. Structures of the products of the reaction of β-aminovinylcarbonyl compounds, a β-diketone, and an aldehyde. Chem. Heterocycl. Compd. 1975;11:196–198. [Google Scholar]
- 98.Zolfigol M.A., Karimi F., Yarie M., Torabi M. Catalytic application of sulfonic acid‐functionalized titana‐coated magnetic nanoparticles for the preparation of 1, 8‐dioxodecahydroacridines and 2, 4, 6‐triarylpyridines via anomeric‐based oxidation. Appl. Organomet. Chem. 2018;32 [Google Scholar]
- 99.Rostamizadeh S., Amirahmadi A., Shadjou N., Amani A.M. MCM‐41‐SO3H as a nanoreactor for the one‐pot, solvent‐free synthesis of 1, 8‐dioxo‐9‐aryl decahydroacridines. J. Heterocycl. Chem. 2012;49:111–115. [Google Scholar]
- 100.Yü S.-J., Wu S., Zhao X.-M., Lü C.-W. Green and efficient synthesis of acridine-1, 8-diones and hexahydroquinolines via a KH 2 PO 4 catalyzed Hantzsch-type reaction in aqueous ethanol. Res. Chem. Intermed. 2017;43:3121–3130. [Google Scholar]
- 101.Jin T.-S., Zhang J.-S., Guo T.-T., Wang A.-Q., Li T.-S. Synthesis, 2004; 2004. One-pot Clean Synthesis of 1, 8-Dioxo-Decahydroacridines Catalyzed by P-Dodecylbenezenesulfonic Acid in Aqueous Media; pp. 2001–2005. [Google Scholar]
- 102.Fan X., Li Y., Zhang X., Qu G., Wang J. An efficient and green preparation of 9‐arylacridine‐1, 8‐dione derivatives, Heteroatom Chemistry. Int. J. Main Group Elements. 2007;18:786–790. [Google Scholar]
- 103.Zhu A., Liu R., Du C., Li L. Betainium-based ionic liquids catalyzed multicomponent Hantzsch reactions for the efficient synthesis of acridinediones. RSC Adv. 2017;7:6679–6684. [Google Scholar]
- 104.Patil D., Chandam D., Mulik A., Patil P., Jagadale S., Kant R., Gupta V., Deshmukh M. Novel brønsted acidic ionic liquid ([CMIM][CF 3 COO]) prompted multicomponent hantzsch reaction for the eco-friendly synthesis of acridinediones: an efficient and recyclable catalyst. Catal. Lett. 2014;144:949–958. [Google Scholar]
- 105.Ziarani G.M., Badiei A., Hassanzadeh M., Mousavi S. Synthesis of 1, 8-dioxo-decahydroacridine derivatives using sulfonic acid functionalized silica (SiO2-Pr-SO3H) under solvent free conditions. Arab. J. Chem. 2014;7:335–339. [Google Scholar]
- 106.Wang G.-W., Xia J.-J., Miao C.-B., Wu X.-L. Environmentally friendly and efficient synthesis of various 1, 4-dihydropyridines in pure water. Bull. Chem. Soc. Jpn. 2006;79:454–459. [Google Scholar]
- 107.Tiwari K.N., Uttam M.R., Kumari P., Vatsa P., Prabhakaran S. Efficient synthesis of acridinediones in aqueous media. Synth. Commun. 2017;47:1013–1019. [Google Scholar]
- 108.Galhoum A.A., Atia A.A., Mahfouz M.G., Abdel-Rehem S.T., Gomaa N.A., Vincent T., Guibal E. Recovery from dilute solutions using magnetic-chitosan nano-based particles grafted with amino acids. J. Mater. Sci. 2015;50:2832–2848. [Google Scholar]
- 109.Zheng C., Ren H., Cui Z., Chen F., Hong G. Synthesis and characterization of nano-scale Terbium (III)-trimesic acid (TMA)-1, 10-phenanthroline (phen) luminescent complex. J. Alloys Compd. 2009;477:333–336. [Google Scholar]
- 110.Undale K., Shaikh T., Gaikwad D., Pore D. One-pot multi-component synthesis of polyhydroquinolines at ambient temperature. Compt. Rendus Chem. 2011;14:511–515. [Google Scholar]
- 111.Rostami N., Dekamin M.G., Valiey E., FaniMoghadam H. l-Asparagine–EDTA–amide silica-coated MNPs: a highly efficient and nano-ordered multifunctional core–shell organocatalyst for green synthesis of 3,4-dihydropyrimidin-2(1H)-one compounds. RSC Adv. 2022;12:21742–21759. doi: 10.1039/d2ra02935a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dekamin M.G., Karimi Z., Farahmand M. Tetraethylammonium 2-(N-hydroxycarbamoyl)benzoate: a powerful bifunctional metal-free catalyst for efficient and rapid cyanosilylation of carbonyl compounds under mild conditions. Catal. Sci. Technol. 2012;2:1375–1381. [Google Scholar]
- 113.Dekamin M.G., Sagheb-Asl S., Reza Naimi-Jamal M. An expeditious synthesis of cyanohydrin trimethylsilyl ethers using tetraethylammonium 2-(carbamoyl)benzoate as a bifunctional organocatalyst. Tetrahedron Lett. 2009;50:4063–4066. [Google Scholar]
- 114.Zarnegar Z., Safari J., Mansouri-Kafroudi Z. Environmentally benign synthesis of polyhydroquinolines by Co3O4–CNT as an efficient heterogeneous catalyst. Catal. Commun. 2015;59:216–221. [Google Scholar]
- 115.Gholap S., Gunjal N. Thiamine hydrochloride (Vit-B1): an optimized green alternative for the synthesis of polyhydroquinoline derivatives. Iran. J. Catal. 2016;6:147–152. [Google Scholar]
- 116.Waghmare A.S., Patil T., Kadam K., Pandit S.S. SFHS: reusable catalyst for the synthesis of polyhydroquinoline derivatives and its molecular docking studies against tyrosine protein kinase. Iran. J. Catal. 2015;5:1–8. [Google Scholar]
- 117.Surasani R., Kalita D., Rao A.D., Yarbagi K., Chandrasekhar K. FeF3 as a novel catalyst for the synthesis of polyhydroquinoline derivatives via unsymmetrical Hantzsch reaction. J. Fluor. Chem. 2012;135:91–96. [Google Scholar]
- 118.Kulkarni P. Al2 (SO4) 3 is an efficient and mild acid catalyst for the one-pot, four-component synthesis of polyhydroquinoline. J. Chil. Chem. Soc. 2014;59:2319–2321. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.