Summary
Background
Declining antimicrobial susceptibility to current gonorrhoea antibiotic treatment and inadequate treatment options have raised the possibility of untreatable gonorrhoea. New prevention approaches, such as vaccination, are needed. Outer membrane vesicle meningococcal serogroup B vaccines might be protective against gonorrhoea. We evaluated the effectiveness of a serogroup B meningococcal outer membrane vesicle vaccine (MenB-4C) against gonorrhoea in individuals aged 16–23 years in two US cities.
Methods
We identified laboratory-confirmed gonorrhoea and chlamydia infections among individuals aged 16–23 years from sexually transmitted infection surveillance records in New York City and Philadelphia from 2016 to 2018. We linked gonorrhoea and chlamydia case records to immunisation registry records to determine MenB-4C vaccination status at infection, defined as complete vaccination (two MenB-4C doses administered 30–180 days apart), partial vaccination (single MenB-4C vaccine dose), or no vaccination (serogroup B meningococcal vaccine naive). Using log-binomial regression with generalised estimating equations to account for correlations between multiple infections per patient, we calculated adjusted prevalence ratios (APR) and 95% CIs to determine if vaccination was protective against gonorrhoea. We used individual-level data for descriptive analyses and infection-level data for regression analyses.
Findings
Between Jan 1, 2016, and Dec 31, 2018, we identified 167 706 infections (18 099 gonococcal infections, 124 876 chlamydial infections, and 24 731 gonococcal and chlamydial co-infections) among 109 737 individuals linked to the immunisation registries. 7692 individuals were vaccinated, of whom 4032 (52·4%) had received one dose, 3596 (46·7%) two doses, and 64 (<1·0%) at least three doses. Compared with no vaccination, complete vaccination series (APR 0·60, 95% CI 0·47–0·77; p<0·0001) and partial vaccination series (0·74, 0·63–0·88; p=0·0012) were protective against gonorrhoea. Complete MenB-4C vaccination series was 40% (95% CI 23–53) effective against gonorrhoea and partial MenB-4C vaccination series was 26% (12–37) effective.
Interpretation
MenB-4C vaccination was associated with a reduced gonorrhoea prevalence. MenB-4C could offer cross-protection against Neisseria gonorrhoeae. Development of an effective gonococcal vaccine might be feasible with implications for gonorrhoea prevention and control.
Funding
None.
Introduction
Gonorrhoea is the second most common nationally notifiable sexually transmitted infection (STI) in the USA.1 About 583 000 gonorrhoea cases were reported in 2018, and the reported gonorrhoea incidence in the USA increased by 82% from 98·1 per 100 000 in 2009 to 179·1 per 100 000 in 2018; racial minorities and young people (≤24 years) bore a disproportionate burden.1,2 Gonorrhoea rates in New York City and Philadelphia are among the highest nationally.1,3,4 In 2018, gonorrhoea rates in New York City (303·0 per 100 000) and Philadelphia (472·2 per 100 000) were more than 1·5 times the national rate with persistent racial and age disparities.1,3,4
Effective antibiotic treatment is essential for prevention and control of gonorrhoea. The US Centers for Disease Control and Prevention recommends a single 500 mg intramuscular dose of ceftriaxone for the treatment of uncomplicated gonorrhoea.5 However, Neisseria gonorrhoeae has successively developed resistance to every first-line antimicrobial treatment since the 1930s.6 Declining gonococcal susceptibility to cephalosporins has raised the possibility of untreatable gonorrhoea in the future.5 New approaches, such as vaccination, are needed as long-term strategies to prevent gonorrhoea and address the emerging threat of antimicrobial resistance.7,8
There is no vaccine licensed to prevent gonorrhoea; however, outer membrane vesicle serogroup B meningococcal vaccines have shown some protection against N gonorrhoeae.7 Ecological studies have shown decreases in gonorrhoea rates after mass outer membrane vesicle serogroup B meningococcal vaccination campaigns following epidemics of invasive serogroup B meningococcal disease.9,10 A case–control study that used data from sexual health clinics in New Zealand found that individuals fully vaccinated with a local epidemic strain-specific outer membrane vesicle serogroup B meningococcal vaccine (MeNZB) were less likely than unvaccinated people to be diagnosed with gonorrhoea (vaccine effectiveness of 31%).11 Any cross-protection by outer membrane vesicle serogroup B meningococcal vaccines against N gonorrhoeae might be attributable to the genetic similarities between Neisseria meningitidis and N gonorrhoeae. There is an 80–90% homology in the primary genetic sequences between N meningitidis and N gonorrhoeae, and many antigenic proteins on the outer membrane vesicle of both pathogens are identical.12
There are two serogroup B meningococcal vaccines (MenB-4C and MenB-FHbp) licensed for use against N meningitidis serogroup B in the USA.13 Since 2015, the Advisory Committee on Immunization Practices has recommended vaccination with a serogroup B meningococcal series for adolescents and young adults aged 16–23 years based on shared clinical decision-making to provide short-term protection against serogroup B meningococcal disease.13 MenB-FHbp is not an outer membrane vesicle vaccine. However, MenB-4C is a four-component outer membrane vesicle serogroup B meningococcal vaccine that includes an outer membrane vesicle derived from the New Zealand serogroup B meningococcal epidemic strain plus three recombinant antigenic proteins (neisserial adhesin A [NadA], neisserial heparin binding antigen [NHBA], and factor H binding protein [FHbp]).14 Because MenB-4C contains the same outer membrane vesicle as in MeNZB plus NHBA (which generates anti-gonococcal antibodies),15 MenB-4C could provide cross-protection against N gonorrhoeae. Using population surveillance case data from two US jurisdictions with high gonorrhoea case rates, the objective of this analysis was to estimate the effectiveness of MenB-4C vaccination against gonorrhoea among individuals aged 16–23 years.
Methods
Data source and variables
We obtained data for all reported diagnosed gonorrhoea and chlamydial infections among individuals aged 16–23 years between Jan 1, 2016, and Dec 31, 2018, from the STI surveillance systems of the New York City Department of Health and Mental Hygiene, and the Philadelphia Department of Public Health (figure 1). We classified infections for which an individual was diagnosed with gonorrhoea but not chlamydia as “gonorrhoea”, for which an individual was diagnosed with chlamydia but not gonorrhoea as “chlamydia”, and for which an individual was diagnosed with both gonorrhoea and chlamydia as “gonorrhoea and chlamydia co-infection”. We used the date of specimen collection for gonorrhoea or chlamydia testing (nucleic acid amplification test or culture) as a proxy for diagnosis date.
Figure 1: Data collection.

STI=sexually transmitted infection.
We linked records of these infections to the health department immunisation registry records to obtain data for MenB-4C vaccination status, age at vaccination, dates of vaccination, and number of MenB-4C doses received (figure 1). We then determined whether each infection occurred before or after the MenB-4C vaccination date, or if it had occurred in unvaccinated individuals in order to determine the MenB-4C vaccination status at the time of each infection (figures 1, 2).
Figure 2: Categorisation of sexually transmitted infection and vaccination status.
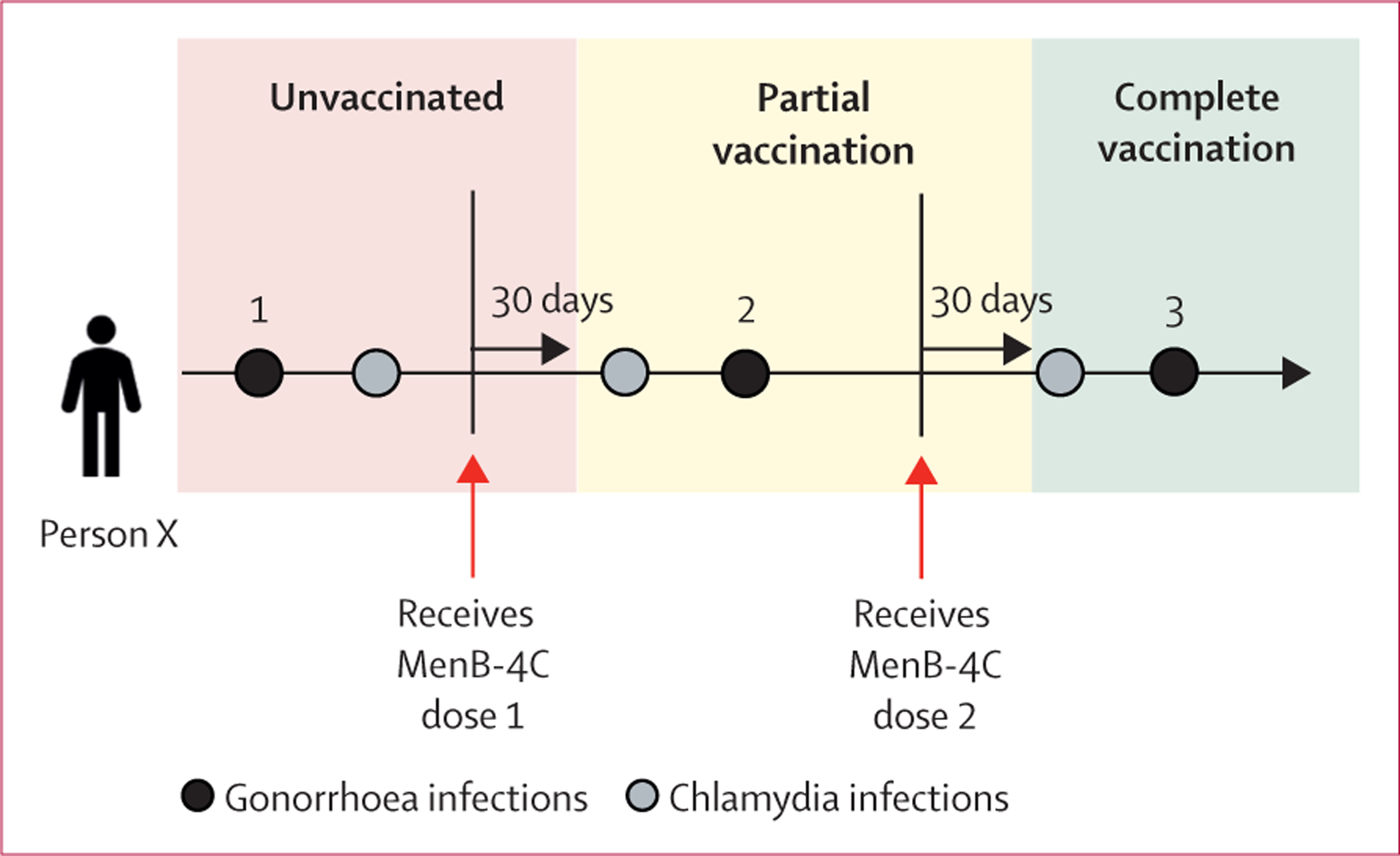
In this example, a first gonorrhoea infection occurs during the unvaccinated period, a second occurs during the partial vaccination period, and a third occurs after complete vaccination has been achieved. Infections that fell within each period of vaccination categories were determined for each individual in the study.
We obtained sociodemographic data at the individual level, and STI and vaccination data at the infection level. Individual-level sociodemographic characteristics were based on characteristics reported at the first diagnosed STI. Because STI and vaccination were obtained at the infection level, we classified vaccination data in relation to each STI. The Advisory Committee on Immunization Practices guidelines recommend two MenB-4C doses administered at least 1 month apart.13 For this analysis, we assumed that post-vaccination immunity started 30 days after receipt of the complete or partial vaccination series.16–19 STIs that occurred at least 30 days after the date of the second MenB-4C dose were categorised as occurring after receipt of the complete vaccination series (figure 2). STIs that occurred at least 30 days after the date of the single MenB-4C dose were categorised as occurring after receipt of the partial vaccination series (figure 2). STIs that occurred before the first vaccine dose (figure 2) or that occurred among serogroup B meningococcal vaccine-naive individuals were categorised as STIs occurring among the unvaccinated population.
The Advisory Committee on Immunization Practices guidelines do not include a maximum interval between the first and second MenB-4C doses. However, because of uncertainty about seroprotection when MenB-4C vaccine doses were given less than 30 days apart or greater than 180 days apart, we excluded vaccine data when first and second MenB-4C vaccine doses were given outside this window.16,17 To allow for post-vaccination immunity to develop, we also excluded STIs and their corresponding data if the STI occurred less than 30 days after receipt of the complete or partial vaccination series. Because MenB-FHbp is not an outer membrane vesicle vaccine, we excluded STI and vaccination date in which only MenB-FHbp was administered or in which both MenB-FHbp and MenB-4C were administered to the same individual.14
The analytic dataset included the vaccination category (complete vaccination, partial vaccination, or unvaccinated) of each individual gonorrhoea, chlamydia, or gonorrhoea and chlamydia co-infection at the time of each specific STI, with infection events as the unit of analysis. Thus, it is possible that during the study period, one individual with multiple diagnosed STIs could have infections associated with an unvaccinated category (if the infection occurred before MenB-4C vaccination) and a vaccination category (complete vaccination or partial vaccination) if the infection occurred after MenB-4C vaccination (figure 2).
Statistical analysis
We calculated frequencies of characteristics using individual-level data to describe the sample. To investigate the potential effectiveness of MenB-4C vaccine against gonorrhoea compared with chlamydia, we calculated vaccine effectiveness by using infection-level data to compare the prevalence of diagnosed gonorrhoea in each vaccination category (complete vaccination and partial vaccination) to the prevalence of diagnosed gonorrhoea in the unvaccinated category (figure 3). We selected chlamydia as the reference group to gonorrhoea because characteristics of individuals diagnosed with chlamydia are similar to individuals diagnosed with gonorrhoea, thus minimising bias.
Figure 3: Calculation of VE.
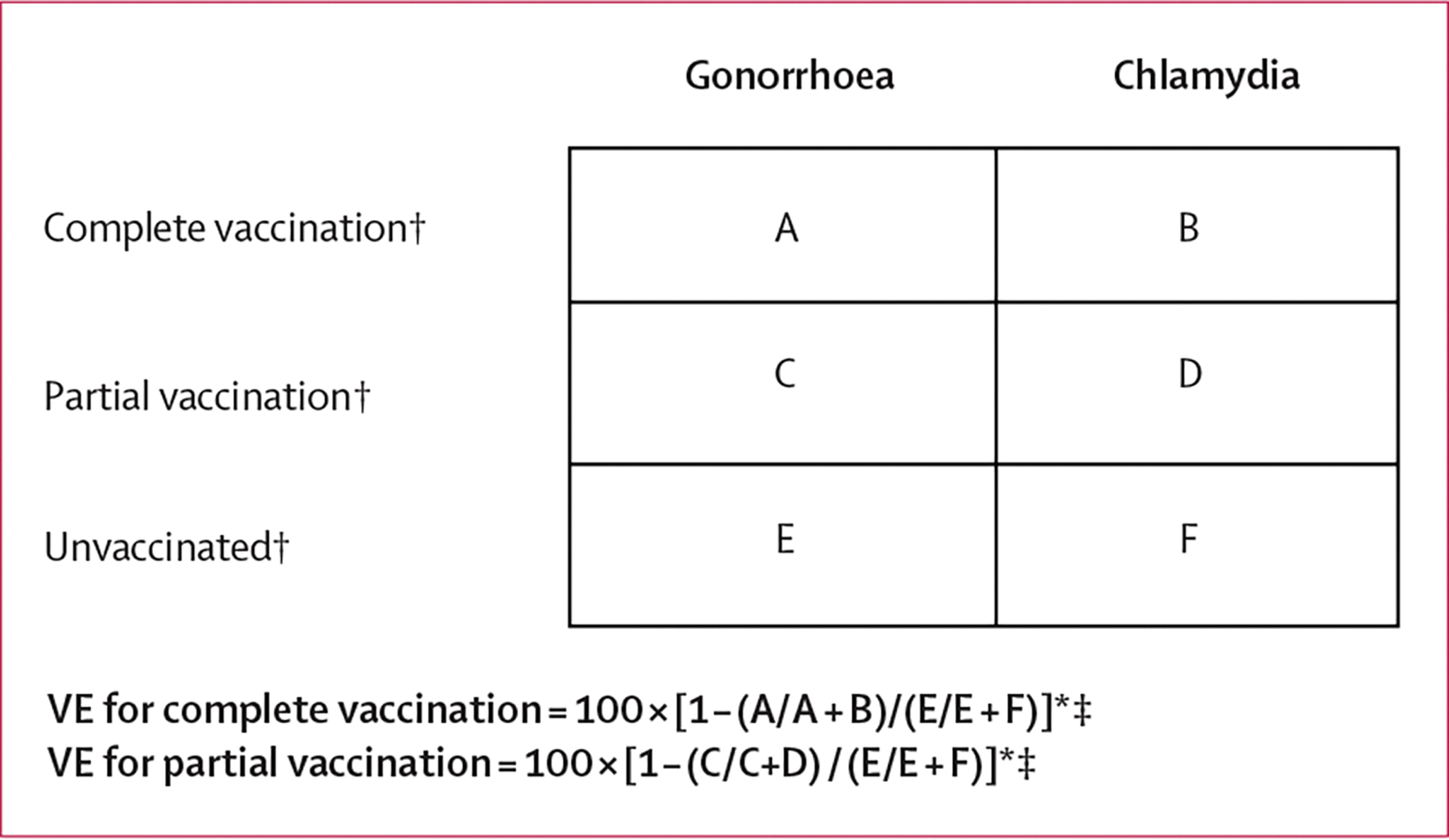
Each cell (A–F) contains the number of infections that occurred within each corresponding vaccination category. The prevalence of gonorrhoea among the complete vaccination category is thus equal to A/A+B. VE=vaccine effectiveness. *Illustrates vaccine effectiveness calculations for 1) gonorrhoea compared with chlamydia and 2) gonorrhoea or chlamydia co-infection compared with chlamydia. †Vaccination status of the patient at the time of the specific gonorrhoea or chlamydia infection. ‡Adjusted for covariates and multiple infections per individual.
In the primary analysis to estimate vaccine effectiveness, we compared the prevalence of gonorrhoea during vaccinated periods to the prevalence of gonorrhoea during unvaccinated periods by conducting bivariate and multivariable analysis to calculate unadjusted prevalence ratios (UPRs) and adjusted prevalence ratios (APRs) and 95% CIs using log-binomial regression with generalised estimating equations to account for correlations between multiple STI infections over time per individual. We included variables that were significant (p<0·05) in bivariate analyses. The multivariable models included MenB-4C vaccination status as the independent variable and race and ethnicity, gender, and jurisdiction as covariates. The dependent variable in the primary analysis was STI (gonorrhoea compared with chlamydia). An APR and 95% CI of less than 1 would indicate that MenB-4C vaccination was associated with a lower prevalence of gonorrhoea compared with non-vaccination, suggesting a protective effect of MenB-4C against gonorrhoea. Vaccine effectiveness was calculated as 100 × (1 − APR).
Because co-infection with gonorrhoea and chlamydia is common, and the immune responses to gonorrhoea and gonorrhoea and chlamydia co-infection can differ, we conducted a secondary analysis to estimate vaccine effectiveness for gonorrhoea and chlamydia co-infections through a multivariable analysis to calculate APRs. The covariates in this model were the same as those used in the primary analysis. The dependent variable in the secondary analysis was STI (gonorrhoea and chlamydia compared with chlamydia).
The duration of MenB-4C vaccine-induced anti-meningococcal immunity could be short-lived and vaccine effectiveness could change over time,17–20 so we decided a priori to conduct sensitivity analyses for the primary and secondary analyses to assess vaccine effectiveness assuming a 6 month or 12 month duration of protection. The definitions of complete vaccination series, partial vaccination series, and unvaccinated were the same for the sensitivity analyses. For analyses that assumed a 6-month duration of protection, STIs that occurred at least 30 days after the date of the second MenB-4C dose and up to 180 days later were categorised as occurring after receipt of the complete vaccination series (appendix p 1). STIs that occurred at least 30 days after the date of the single MenB-4C dose and up to 180 days later were categorised as occurring after receipt of the partial vaccination series. STIs that occurred before the first vaccine dose or that occurred among MenB vaccine-naive individuals were considered as STIs occurring among unvaccinated people. We excluded STIs that were reported after the 180-day observation period for complete or partial vaccination series because the sensitivity analyses assumed a 6-month duration of protection. For analyses that assumed a 12-month duration of protection, we used a 364-day timeframe instead of 180 days (appendix p 2). The definitions of all the analytical variables were the same. We excluded STIs that were reported after the 364-day observation period for complete or partial vaccination series.
Statistical significance was set at p<0·05. All analyses were done using SAS (version 9.4). Human subjects review at the Centers for Disease Control and Prevention and the Institutional Review Board of the New York City Department of Health and Mental Hygiene determined this project was non-research and therefore exempt from further review. The Institutional Review Board of the Philadelphia Department of Public Health reviewed and approved this project.
Role of the funding source
There was no funding source for this study.
Results
We identified 109 737 individuals in the immunisation registries who were aged 16–23 years with a gonorrhoea or chlamydia infection reported to the New York City Department of Health and Mental Hygiene and the Philadelphia Department of Public Health from Jan 1, 2016, to Dec 31, 2018 (table 1). 65·5% of patients were female and 38·7% were non-Hispanic Black; however, race and ethnicity data were missing for a large proportion of individuals (38·2%). 7692 (7·0%) of 109 737 individuals had received at least one dose of MenB-4C and 102 045 (93·0%) of 109 737 individuals had never been vaccinated (MenB vaccine naive). Among 7692 individuals who received at least one dose of MenB-4C, 4032 (52·4%) received one MenB-4C dose, 3596 (46·7%) received two MenB-4C doses, and 64 (<1%) received at least three doses (table 1).
Table 1:
Sociodemographic, vaccination, and STI characteristics among people aged 16–23 years with a diagnosis of gonorrhoea or chlamydia in New York City and Philadelphia (2016–18)
| Number | % | |
|---|---|---|
|
| ||
| Race or ethnicity (n=109737 people) | ||
| Black, non-Hispanic | 42514 | 38.7% |
| Hispanic | 16739 | 15.3% |
| Other* | 2933 | 2.7% |
| White, non-Hispanic | 5645 | 5.1% |
| Missing | 41906 | 38.2% |
| Gender (n=109737 people) | ||
| Male | 37831 | 34.5% |
| Female | 71836 | 65.5% |
| Transgender | 37 | <1.0% |
| Missing | 33 | <1.0% |
| Ever received MenB-4C vaccine (n=109737 people) | ||
| Yes† | 7692 | 7.0% |
| No | 102 045 | 93.0% |
| Number of MenB-4C doses received (n=7692) | ||
| 1 | 4032 | 52.4% |
| 2 | 3596 | 46.7% |
| 3 | 63 | 0.8% |
| 4 | 1 | <1% |
| Jurisdiction (n=109737 people) | ||
| New York City, NY, USA | 72565 | 66.1% |
| Philadelphia, PA, USA | 37172 | 33.9% |
| STIs (infection-level data‡; n=l67706 infections) | ||
| Gonorrhoea | 18099 | 10.8% |
| Chlamydia | 124876 | 74.5% |
| Gonorrhoea and chlamydia co-infection | 24731 | 14.7% |
| MenB-4C vaccination (infection-level data§; n=l67 706 infections) | ||
| Complet¶ | 3058 | 1.8% |
| Partial§ | 6519 | 3.9% |
| Unvaccinated|| | 155330 | 92.6% |
| Other** | 2799 | 1.7% |
STI=sexually transmitted infection.
Asian, American Indian, Native Hawaiian or Pacific Islander, other race, or two or more races.
Receipt of at least one dose of MenB-4C vaccine.
STIs and vaccination data obtained at infection episode-level.
Receipt of one MenB-4C dose.
Receipt of two doses of MenB-4C vaccine separated by 30–180 days.
Never vaccinated against Neisseria meningitidis serogroup B (vaccine naive) or STI occurred before first MenB-4C vaccine dose.
MenB-4C vaccination data where the time interval between first and second doses is less than 30 days or more than 180 days.
Among the 109 737 people who had 167 706 reported STIs, 124 876 (74·5%) of 167 706 reported STIs were chlamydial infections, 18 099 (10·8%) were gonococcal infections, and 24 731 (14·7%) were gonococcal and chlamydial co-infections (table 1). 3058 (1·8%) of 167 706 STIs occurred after the complete vaccination series, 6519 (3·9%) occurred after the partial vaccination series, and 155 330 (92·6%) occurred before the first vaccine dose or among serogroup B meningococcal vaccine-naive individuals (table 1). A small number of STIs (2799 [1·7%] of 167 706) and corresponding vaccination data were excluded because the time interval between the first and second dose of the associated MenB-4C was fewer than 30 days or greater than 180 days.
Compared with being unvaccinated, complete MenB-4C vaccination series (UPR 0·64, 95% CI 0·51–0·79; p<0·0001) and partial MenB-4C vaccination series (0·83, 0·72–0·96; p=0·0204) were protective against gonorrhoea (table 2) in bivariate analyses. After adjusting for race and ethnicity, gender, and jurisdiction, complete vaccination series (APR 0·60, 95% CI 0·47–0·77; p<0·0001) and partial vaccination series (0·74, 0·63–0·88; p=0·0012) remained protective against gonorrhoea compared with being unvaccinated (table 2). Complete MenB-4C vaccination series was thus 40% (95% CI 23–53) effective against gonorrhoea and partial MenB-4C vaccination series was 26% (12–37) effective.
Table 2:
Association between MenB-4C vaccination and gonorrhoea or gonorrhoea and chlamydia co-infection compared with chlamydia
|
Gonorrhoea
|
Gonorrhoea and chlamydia co-infection
|
|||||||
|---|---|---|---|---|---|---|---|---|
| UPR (95% CI) | p value | APR (95% CI) | p value | UPR (95% CI) | p value | APR (95% CI) | p value | |
|
| ||||||||
| MenB-4C vaccination status | ||||||||
| Complete vaccination* | 0.64 (0.51-0.79) | <0.0001 | 0.60 (0.47–0.77) | <0.0001 | 0.90 (0.70–1.17) | 0.44 | 0.85 (0.64–1.13) | 0.28 |
| Partial vaccination† | 0.83 (0.72-0.96) | 0.0204 | 0.74 (0.63–0.88) | 0.0012 | 1.15 (0.97–1.37) | 0.11 | 1.06 (0.88–1.28) | 0.56 |
| Unvaccinated‡ | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Race or ethnicity | ||||||||
| Black, non-Hispanic | 0.94 (0.88–1.01) | 0.11 | 0.81 (0.61–1.09) | 0.17 | 1.40 (1.24–1.57) | <0.0001 | 1.39 (1.23–1.56) | <0.0001 |
| Hispanic | 0.69 (0.63–0.74) | <0.0001 | 0.72 (0.66–0.78) | <0.0001 | 0.86 (0.75–0.97) | 0.0117 | 0.88 (0.77–1.01) | 0.0700 |
| Other§ | 0.97 (0.86–1.08) | 0.54 | 0.97 (0.86–1.09) | 0.60 | 1.32 (1.11–1.59) | 0.0031 | 1.34 (1.11–1.60) | 0.0018 |
| White, non-Hispanic | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Gender | ||||||||
| Male | 2.67 (2.58–2.76) | <0.0001 | 2.64 (2.58-2.80) | <0.0001 | 2.12 (2.01–2.23) | <0.0001 | 2.11 (1.98–2.24) | <0.0001 |
| Female | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Jurisdiction | ||||||||
| New York City, NY, USA | 0.75 (0.72-0.77) | <0.0001 | 1.03 (0.99–1.08) | 0.13 | 0.72 (0.68–0.76) | <0.0001 | 0.99 (0.94–1.06) | 0.91 |
| Philadelphia, PA, USA | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
APR=adjusted prevalence ratio. UPR=unadjusted prevalence ratio.
Receipt of two doses of MenB-4C vaccine separated by 30–180 days.
Receipt of one MenB-4C dose.
Never vaccinated against Neisseria meningitidis serogroup B with MenB-4C or MenB-FHbp (ie, MenB vaccine naive or sexually transmitted infections occurred before first MenB-4C vaccine dose).
Asian, American Indian, Native Hawaiian or Pacific Islander, other race, or two or more races.
Compared with being unvaccinated, complete vaccination series (UPR 0·90, 95% CI 0·70–1·17; p=0·44) or partial vaccination series (1·15, 0·97–1·37; p=0·11) were not protective against gonorrhoea and chlamydia co-infection in bivariate analysis (table 2), which remained non-significant after adjustment (table 2).
Assuming a 6-month duration of vaccine-induced protection, complete vaccination series (APR 0·61, 95% CI 0·49–0·77; p<0·0001) and partial vaccination series (0·66, 0·58–0·76; p<0·0001) were protective against gonorrhoea (table 3). Assuming a 12-month duration of vaccine-induced protection, complete vaccination series (APR 0·56, 95% CI 0·45–0·67; p<0·0001) and partial vaccination series (0·68, 0·60–0·76; p<0·0001) were protective against gonorrhoea (table 4). Neither complete or partial vaccination was protective against gonorrhoea and chlamydia co-infection assuming a 6-month or 12-month duration of protection (tables 3, 4).
Table 3:
Association between MenB-4C vaccination and gonorrhoea or gonorrhoea and chlamydia co-infection compared with chlamydia assuming a duration of vaccine-induced protection of 6 months
|
Gonorrhoea
|
Gonorrhoea and chlamydia co-infection
|
|||||||
|---|---|---|---|---|---|---|---|---|
| UPR (95% CI) | p value | APR (95% CI) | p value | UPR (95% CI) | p value | APR (95% CI) | p value | |
|
| ||||||||
| MenB-4C vaccination status | ||||||||
| Complete vaccination* | 0.58 (0.48-0.71) | <0.0001 | 0.61 (0.49–0.77) | <0.0001 | 0.92 (0.73–1.16) | 0.47 | 0.87 (0.66–1.13) | 0.31 |
| Partial vaccinationt | 0.70 (0.62-0.78) | <0.0001 | 0.66 (0.58–0.76) | <0.0001 | 1.10 (0.95–1.26) | 0.21 | 0.97 (0.83–1.15) | 0.80 |
| Unvaccinated‡ | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Race or ethnicity | ||||||||
| Black, non-Hispanic | 0.95 (0.88–1.02) | 0.26 | 0.94 (0.88–1.02) | 0.17 | 1.38 (1.22–1.54) | <0.0001 | 1.37 (1.22–1.55) | <0.0001 |
| Hispanic | 0.68 (0.63–0.74) | <0.0001 | 0.72 (0.67–0.79) | <0.0001 | 0.84 (0.74–0.96) | 0.011 | 0.88 (0.77–1.01) | 0.071 |
| Other§ | 0.94 (0.84-1.06) | 0.46 | 0.98 (0.87–1.11) | 0.79 | 1.31 (1.08–1.56) | 0.0040 | 1.33 (1.11–1.61) | 0.0018 |
| White, non-Hispanic | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Gender | ||||||||
| Male | 2.68 (2.59–2.78) | <0.0001 | 2.69 (2.58–2.80) | <0.0001 | 2.12 (2.01–2.23) | <0.0001 | 2.10 (1.98–2.23) | <0.0001 |
| Female | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Jurisdiction | ||||||||
| New York City, NY, USA | 0.74 (0.71-0.77) | <0.0001 | 1.01 (0.97–1.06) | 0.41 | 0.71 (0.68–0.75) | <0.0001 | 0.99 (0.93–1.05) | 0.78 |
| Philadelphia, PA, USA | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
APR=adjusted prevalence ratio. UPR=unadjusted prevalence ratio. STI=sexually transmitted infection.
Receipt of two doses of MenB-4C doses separated by 30–180 days.
Receipt of one MenB-4C dose.
Never vaccinated against Neisseria meningitidis serogroup B with MenB-4C or MenB-FHbp (ie, MenB vaccine naive or STI occurred before first MenB-4C vaccine dose).
Asian, American Indian, Native Hawaiian or Pacific Islander, other race, or two or more races.
Table 4:
Association between MenB-4C vaccination and gonorrhoea or gonorrhoea and chlamydia co-infection compared with chlamydia assuming a duration of vaccine-induced protection of 12 months
|
Gonorrhoea
|
Gonorrhoea and chlamydia co-infection
|
|||||||
|---|---|---|---|---|---|---|---|---|
| UPR (95% CI) | p value | APR (95% CI) | p value | UPR (95% CI) | p value | APR (95% CI) | p value | |
|
| ||||||||
| MenB-4C vaccination status | ||||||||
| Complete vaccination* | 0.57 (0.48–0.68) | <0.0001 | 0.56 (0.45–0.67) | <0.0001 | 0.90 (0.74-1.10) | 0.31 | 0.83 (0.65–1.04) | 0.11 |
| Partial vaccination† | 0.72 (0.65–0.80) | <0.0001 | 0.68 (0.60–0.76) | <0.0001 | 1.11 (0.97–1.26) | 0.10 | 0.99 (0.85–1.14) | 0.84 |
| Unvaccinated‡ | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Race or ethnicity | ||||||||
| Black, non-Hispanic | 0.95 (0.87–1.03) | 0.22 | 0.95 (0.88–1.02) | 0.14 | 1.40 (1.24–1.57) | <0.0001 | 1.39 (1.24–1.57) | <0.0001 |
| Hispanic | 0.69 (0.63–0.75) | <0.0001 | 0.73 (0.67-0.79) | <0.0001 | 0.85 (0.75–0.97) | 0.018 | 0.89 (0.78–1.01) | 0.095 |
| Other§ | 0.94 (0.83–1.05) | 0.46 | 0.98 (0.87-1.10) | 0.78 | 1.32 (1.11–1.59) | 0.0025 | 1.34 (1.12–1.61) | 0.0013 |
| White, non-Hispanic | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Gender | ||||||||
| Male | 2.68 (2.59-2.77) | <0.0001 | 2.68 (2.57–2.79) | <0.0001 | 2.12 (2.01–2.23) | <0.0001 | 2.10 (1.98–2.23) | <0.0001 |
| Female | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
| Jurisdiction | ||||||||
| New York City, NY, USA | 0.74 (0.71-0.77) | <0.0001 | 1.02 (0.98–1.06) | 0.43 | 0.71 (0.68–0.76) | <0.0001 | 1.00 (0.94–1.06) | 0.95 |
| Philadelphia, PA, USA | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. | 1 (ref) | .. |
APR=adjusted prevalence ratio. UPR=unadjusted prevalence ratio. STI=sexually transmitted infection.
Receipt of two doses of MenB-4C vaccine separated by 30–180 days.
Receipt of one MenB-4C dose.
Never vaccinated against Neisseria meningitidis serogroup B with MenB-4C or MenB-FHbp (ie, MenB vaccine naive or STI occurred before first MenB-4C vaccine dose).
Asian, American Indian, Native Hawaiian or Pacific Islander, other race, or two or more races.
Discussion
In our analysis of over 165 000 STI infections reported among 109 000 individuals in two US cities, MenB-4C vaccination was associated with a reduced risk of gonorrhoea. Complete MenB-4C vaccination series was 40% (95% CI 23–53) effective against gonorrhoea and partial MenB-4C vaccination series was 26% (12–37) effective. The vaccine effectiveness against gonorrhoea observed in this study is comparable with the 31% effectiveness of MeNZB against gonorrhoea noted in the New Zealand study.11 The cross-protective effect of MenB-4C, an outer membrane vesicle serogroup B meningococcal vaccine, against gonorrhoea in this study is consistent with other studies that showed cross-protection of such vaccines.9–11 MenB-4C vaccination was not protective against gonorrhoea and chlamydia co-infection. Vaccine effectiveness against gonorrhoea using all data from the 3-year study period appeared comparable with the effectiveness of the vaccine assuming 6-month and 12-month periods of protection.
Compared with not being vaccinated, complete MenB-4C vaccination appeared to be more protective against gonorrhoea than partial vaccination. Although the serological correlate of MenB-4C-induced anti-gonococcal protection is unknown, a two-dose MenB-4C vaccination series is more protective against serogroup B meningococcal disease than is a single-dose MenB-4C series.21 Therefore, it is possible that a two-dose MenB-4C vaccine series could stimulate higher and longer-lasting levels of anti-gonococcal antibodies than a single dose, and, therefore, be more protective against gonorrhoea.
MenB-4C was not protective against gonorrhoea and chlamydia co-infection in this study. One previous study observed a slightly protective effect against coinfection (vaccine effectiveness of 14%).11 The absence of protection against co-infection in this study might be attributable to the effect of chlamydia on the host’s immune response to N gonorrhoeae.22–24 Chlamydia downregulates neutrophilic activation and killing, inhibits the formation of neutrophil extracellular traps, and increases the concentration of immune-modulatory chemokines.22,23 These immunological changes provide a more tolerable environment for N gonorrhoeae and are associated with an increased gonococcal colonisation load at the infection site.22–24
The National Institute of Allergy and Infectious Diseases is funding a trial examining the protective efficacy of MenB-4C against genital and extragenital gonorrhoea among men and women aged 18–50 years (NCT04350138) and another trial examining anti-gonococcal immune responses induced by MenB-4C vaccination among individuals aged 18–25 years (NCT04094883). Another trial examining the efficacy of MenB-4C to prevent gonorrhoea in gay and bisexual men in Australia is ongoing (NCT04415424). Even if MenB-4C only shows modest protection against gonorrhoea, findings from these clinical trials will be vital for understanding anatomic site-specific vaccine efficacy, efficacy in the setting of co-infections such as chlamydia and HIV, and the serological correlate and mechanism of anti-gonococcal immune response after vaccination.
An effective vaccine might have a substantial effect on gonorrhoea prevention and control.25–27 Mathematical modelling studies suggest that vaccines of even modest effectiveness and duration of protection can decrease gonorrhoea incidence and prevalence and gonorrhoea-attributable medical costs. Whittles and colleagues25 predicted that vaccinating all gay, bisexual, and other men who have sex with men (MSM) attending sexual health clinics in England with a vaccine of 31% effectiveness for 2–4 years would reduce gonorrhoea incidence by 45–75% in 12 years.25 Another model by Heijne and colleagues26 predicted that vaccinating all sexually active Dutch MSM with a vaccine of 30% effectiveness, a 2-year duration of protection, and vaccination coverage of 20% would reduce gonorrhoea prevalence among MSM by 70% and vaccination coverage of 80% among MSM would reduce gonorrhoea prevalence by 97% over four decades.26 Craig and colleagues27 modelled the effect of the vaccination on gonorrhoea prevalence in adolescents before the first sexual act.27 The model predicted a 90% reduction in gonorrhoea prevalence after 20 years with a non-waning vaccine of 50% efficacy assuming universal vaccine coverage.
Our study has limitations, especially because we used surveillance data. The findings of this study might not be generalisable to all age groups or individuals because data were obtained from individuals aged 16–23 years with a gonorrhoea or chlamydia diagnosis reported to the New York City Department of Health and Mental Hygiene and the Philadelphia Department of Public Health. However, we have no reason to believe that the observed protective effect would be different among other populations or jurisdictions. Because gonorrhoea and chlamydia can be asymptomatic and we did not systematically screen individuals at all potentially infected anatomic sites28 but relied on surveillance data, misclassification bias is possible as infections might have been acquired before vaccination but detected after vaccination. We did not obtain data on anatomic sites of infection, therefore, we are unable to make conclusions about anatomic site-specific vaccine effectiveness. Because we used surveillance data, there might be incomplete or incorrect data from the STI surveillance registry and the immunisation information system that can result in missed or incorrect matches between the STI surveillance registry and the immunisation information system. For example, we were unable to match approximately 15% of the STI data to vaccination data; however, the demographic characteristics between both matched and unmatched cases were similar (data not shown). Providers in New York City are only required to report vaccination data when the vaccinated individual is 18 years and younger or 19 years and older and consents to reporting. Thus, some individuals in the New York City Department of Health and Mental Hygiene STI surveillance registry who were 19 years and older when vaccinated could have been incorrectly misclassified as unvaccinated in our analysis, potentially underestimating vaccine effectiveness. Finally, this is an observational study that assesses vaccine effectiveness and not a randomised control led study that assesses vaccine efficacy.
This study also has its strengths. We used chlamydia cases as controls to reduce bias associated with differences in behaviours and STI health-care access and testing as gonorrhoea and chlamydia have similar risk factors, and screening recommendations and are usually tested together in both jurisdictions. STI surveillance data from both jurisdictions included laboratory-confirmed infections that were obtained by electronic laboratory reporting and these data are largely complete. Public health laws in both jurisdictions mandate health-care providers reporting vaccination data on all vaccinated individuals so these data are also likely to be complete.
These findings suggest that MenB-4C could offer cross-protection against N gonorrhoeae and provide further evidence supporting feasibility of an effective gonococcal vaccine. A vaccine, even of modest effectiveness (30–50%) and duration of protection (2–4 years), might have a substantial effect on gonorrhoea prevention.27–29 Clinical trials to examine MenB-4C vaccine efficacy by anatomic site and in the setting of co-infections such as chlamydia and HIV infections, and to assess the anti-gonococcal immune response to MenB-4C vaccination, are important. These data are essential in informing our understanding of MenB-4C’s anti-gonococcal protective effect and optimising the prospects for a future gonococcal vaccine.
Supplementary Material
Research in context.
Evidence before this study
Declining antimicrobial susceptibility to current gonorrhoea antibiotic treatment and inadequate treatment options have raised the possibility of untreatable gonorrhoea. New prevention approaches, such as vaccination, are needed. We searched PubMed for observational studies of gonorrhoea vaccine candidates published in English on or before Aug 31, 2021. We used the search terms “gonorrhoea” and “vaccine” and did not restrict study dates. We identified four eligible observational studies. Ecological data from Cuba, Norway, and Quebec (Canada) showed a decline in gonorrhoea after vaccination with an outer membrane vesicle meningococcal serogroup B vaccine, and findings from a case-control study in New Zealand showed that MeNZB vaccination was associated with a reduced likelihood of gonorrhoea. These findings suggest that outer membrane vesicle meningococcal serogroup B vaccines might be protective against gonorrhoea.
Added value of this study
Vaccination with MenB-4C, a licensed outer membrane vesicle meningococcal serogroup B vaccine, was associated with a significantly lower likelihood of gonorrhoea compared with not being vaccinated. Prevalence of gonorrhoea also varied by complete MenB-4C and partial MenB-4C vaccination compared with not being vaccinated. These findings show that MenB-4C could offer cross-protection against Neisseria gonorrhoeae and provide further evidence supporting the feasibility of an effective gonococcal vaccine with implications for gonorrhoea prevention and control.
Implications of all the available evidence
A gonococcal vaccine of even moderate effectiveness could have a substantial effect on gonorrhoea prevention and control. MenB-4C vaccination could be effective at reducing gonorrhoea incidence and prevalence and gonorrhoea-attributable medical costs. Clinical trials to examine MenB-4C vaccine efficacy by anatomic site and in the setting of co-infections such as chlamydia and HIV infections, and to assess the anti-gonococcal immune response to MenB-4C vaccination, are important. These data are essential in informing our understanding of MenB-4C’s anti-gonococcal protective effect and optimising the prospects for a future gonococcal vaccine.
Acknowledgments
The findings and conclusions in this study are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
We declare no competing interests.
Contributor Information
Winston E Abara, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Kyle T Bernstein, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Felicia M T Lewis, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA; Philadelphia Department of Public Health, Philadelphia, PA, USA.
Julia A Schillinger, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA; Bureau of STI, New York City Department of Health and Mental Hygiene, New York City, NY, USA.
Kristen Feemster, Philadelphia Department of Public Health, Philadelphia, PA, USA.
Preeti Pathela, Bureau of STI, New York City Department of Health and Mental Hygiene, New York City, NY, USA.
Susan Hariri, Division of Bacterial Diseases, Centers for Disease Control and Prevention, Atlanta, GA.
Aras Islam, Philadelphia Department of Public Health, Philadelphia, PA, USA.
Michael Eberhart, Philadelphia Department of Public Health, Philadelphia, PA, USA.
Iris Cheng, Bureau of Immunization, New York City Department of Health and Mental Hygiene, New York City, NY, USA.
Alexandra Ternier, Bureau of Immunization, New York City Department of Health and Mental Hygiene, New York City, NY, USA.
Jennifer Sanderson Slutsker, Bureau of STI, New York City Department of Health and Mental Hygiene, New York City, NY, USA.
Sarah Mbaeyi, Division of Bacterial Diseases, Centers for Disease Control and Prevention, Atlanta, GA.
Robbie Madera, Division of Bacterial Diseases, Centers for Disease Control and Prevention, Atlanta, GA.
Robert D Kirkcaldy, Division of STD Prevention, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Data sharing
Individual-level data included in this manuscript were part of routine sexually transmitted infection and vaccination surveillance. These data are considered sensitive and will not be shared by the authors.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2018. 2018. https://www.cdc.gov/std/stats18/STDSurveillance2018-full-report.pdf (accessed Jan 16, 2019).
- 2.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2009. 2009. https://www.cdc.gov/std/stats/archive/surv2009-Complete.pdf (accessed Jan 16, 2019).
- 3.New York State Department of Health. Sexually transmitted infections surveillance report New York State 2018. https://www.health.ny.gov/statistics/diseases/communicable/std/docs/sti_surveillance_report_2018.pdf (accessed Jan 16, 2020).
- 4.Philadelphia Department of Public Health. General STD surveillance. https://hip.phila.gov/data-reports-statistics/generalstds/general-std-surveillance-archive/general-std-annual-reports-2013 (accessed Jan 16, 2020).
- 5.St Cyr S, Barbee L, Workowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 1911–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unemo M, Shafer WM. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st century: past, evolution, and future. Clin Microbiol Rev 2014; 27: 587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abara WE, Jerse AE, Hariri S, Kirkcaldy RD. Planning for a gonococcal vaccine: a narrative review of vaccine development and public health implications. Sex Transm Dis 2021; 48: 453–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abara WE, Kirkcaldy RD, Bernstein KB, Zlotorzynska M, Sanchez T. Acceptability of a gonococcal vaccine among sexually active men who have sex with men. Sex Transm Dis 2022; 49: 76–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whelan J, Kløvstad H, Haugen IL, Holle MR, Storsaeter J. Ecologic study of meningococcal B vaccine and Neisseria gonorrhoeae infection, Norway. Emerg Infect Dis 2016; 22: 1137–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Longtin J, Dion R, Simard M, et al. Possible impact of wide-scale vaccination against serogroup B Neisseria meningitidis on gonorrhea incidence rates in one region of Quebec, Canada. Open Forum Infect Dis 2017; 4: S734–35. [Google Scholar]
- 11.Petousis-Harris H, Paynter J, Morgan J, et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet 2017; 390: 1603–10. [DOI] [PubMed] [Google Scholar]
- 12.Tinsley CR, Nassif X. Analysis of the genetic differences between Neisseria meningitidis and Neisseria gonorrhoeae: two closely related bacteria expressing two different pathogenicities. Proc Natl Acad Sci USA 1996; 93: 11109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep 2020; 69: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. Bexsero. 2015. https://www.fda.gov/media/90996/download (accessed Jan 16, 2020).
- 15.Semchenko EA, Tan A, Borrow R, Seib KL. The serogroup B meningococcal vaccine Bexsero elicits antibodies to Neisseria gonorrhoeae. Clin Infect Dis 2019; 69: 1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santolaya ME, O’Ryan M, Valenzuela MT, et al. Persistence of antibodies in adolescents 18–24 months after immunization with one, two, or three doses of 4CMenB meningococcal serogroup B vaccine. Hum Vaccin Immunother 2013; 9: 2304–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santolaya ME, O’Ryan ML, Valenzuela MT, et al. Immunogenicity and tolerability of a multicomponent meningococcal serogroup B (4CMenB) vaccine in healthy adolescents in Chile: a phase 2b/3 randomised, observer-blind, placebo-controlled study. Lancet 2012; 379: 617–24. [DOI] [PubMed] [Google Scholar]
- 18.Giuntini S, Lujan E, Gibani MM, et al. Serum bactericidal antibody responses of adults immunized with the MenB-4C vaccine against genetically diverse serogroup B meningococci. Clin Vaccine Immunol 2017; 24: e00430–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flacco ME, Manzoli L, Rosso A, et al. Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adolescents: a systematic review and meta-analysis. Lancet Infect Dis 2018; 18: 461–72. [DOI] [PubMed] [Google Scholar]
- 20.Lujan E, Winter K, Rovaris J, Liu Q, Granoff DM. Serum bactericidal antibody responses of students immunized with a meningococcal serogroup B vaccine in response to an outbreak on a university campus. Clin Infect Dis 2017; 65: 1112–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladhani SN, Andrews N, Parikh SR, et al. Vaccination of infants with meningococcal group B vaccine (4CMenB) in England. N Engl J Med 2020; 382: 309–17. [DOI] [PubMed] [Google Scholar]
- 22.Rajeeve K, Das S, Prusty BK, Rudel T. Chlamydia trachomatis paralyses neutrophils to evade the host innate immune response. Nat Microbiol 2018; 3: 824–35. [DOI] [PubMed] [Google Scholar]
- 23.Vonck RA, Darville T, O’Connell CM, Jerse AE. Chlamydial infection increases gonococcal colonization in a novel murine coinfection model. Infect Immun 2011; 79: 1566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vonck Stankowski R, Cole JG, Jerse AE. The natural history of incident gonococcal infection in adolescent women: similar observations in a female mouse model of gonococcal and chlamydial coinfection. Sex Transm Dis 2012; 39: 238. [DOI] [PubMed] [Google Scholar]
- 25.Whittles LK, White PJ, Didelot X. Assessment of the potential of vaccination to combat antibiotic resistance in gonorrhea: a modeling analysis to determine preferred product characteristics. Clin Infect Dis 2020; 71: 1912–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heijne JC, Xiridou M, Turner K, et al. The impact of vaccination on Neisseria gonorrhoeae antimicrobial resistance and prevalence in men who have sex with men: a mathematical modelling study. medRxiv 2020; published online Sept 15. 10.1101/2020.09.14.20192062 (preprint). [DOI] [Google Scholar]
- 27.Craig AP, Gray RT, Edwards JL, et al. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine 2015; 33: 4520–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abara WE, Llata EL, Schumacher C, et al. Extragenital gonorrhea and chlamydia positivity and the potential for missed extragenital gonorrhea with concurrent urethral chlamydia among men who have sex with men attending sexually transmitted disease clinics—Sexually Transmitted Disease Surveillance Network, 2015–2019. Sex Transm Dis 2020; 47: 361–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Individual-level data included in this manuscript were part of routine sexually transmitted infection and vaccination surveillance. These data are considered sensitive and will not be shared by the authors.


