Abstract
Purpose:
Tolvaptan, a selective vasopressin type-2 antagonist, has been shown to increase serum sodium (Na) and urine output in hyponatremic left ventricular assist device (LVAD) patients in retrospective studies. In this prospective randomized pilot study, we aimed to assess the efficacy of tolvaptan in this population.
Methods:
We conducted a prospective, randomized, non-blinded pilot study of LVAD recipients with post-operative hyponatremia (Na < 135 mEq/L) (NCT05408104). Eligible participants were randomized to receive tolvaptan 15 mg daily in addition to usual care versus usual care alone. The primary outcome was a change in Na level and estimated glomerular filtration rate (eGFR), from the first post-operative day of hyponatremia (the day of randomization) to discharge.
Results:
A total of 33 participants were enrolled, and 28 underwent randomization (median age 55 [IQR 50–62]), 21% women, 54% Black, 32% ischemic cardiomyopathy, median baseline Na 135 (IQR 134–138). Fifteen participants were randomized to tolvaptan (TLV) and 13 were randomized to usual care alone (No-TLV). Mean change in Na from randomization to discharge in the TLV group was 2.7 mEq/L (95%CI 0.7–4.7, p = 0.013) and 1.8 (95%CI 0.5–4.0, p = 0.11) in the No- TLV group, though baseline and final Na levels were similar between groups. The mean change in eGFR was 2.6 ml/min/1.73 m2 (95%CI 10.1–15.3, p = 0.59) in TLV versus 7.5 ml/min/1.73 m2 (95%CI 5.2–20.2, p = 0.15) in No-TLV. TLV participants had significantly more urine output than No-TLV patients during their first 24 h after randomization (3294 vs 2155 ml, p = 0.043).
Conclusion:
TLV significantly increases urine output, with nominal improvement in Na level, in hyponatremic post-operative LVAD patients without adversely impacting renal function.
Keywords: diuretics, heart failure, hyponatremia, LVAD
1 |. INTRODUCTION
Hyponatremia has been associated with worse in-hospital, 1-year, and 5-year survival outcomes in heart failure patients.1 Tolvaptan is a vasopressin-2 antagonist that inhibits vasopressin type 2 receptors located on the collecting duct of the kidney, which in turn prevents reabsorption of free water via the aquaporin system, leading to an increase in urinary free water excretion.2 Tolvaptan has been studied as adjunctive therapy in both acute decompensated heart failure and chronic heart failure, resulting in increased net fluid loss, reduced weight, and improved dyspnea.3,4 While tolvaptan has not shown an effect on mortality in acutely decompensated heart failure patients in general, prospective and retrospective studies have suggested a benefit in those with hyponatremia.5,6 Furthermore, patients with right ventricular dilation, suggesting elevated right-sided filling pressures and right ventricular failure, have been shown to be associated with tolvaptan responsiveness. One subset of patients that may derive particular benefit from tolvaptan is those with right ventricular failure, due to the frequent association of right ventricular failure with hyponatremia, diuretic resistance, and improved clinical response to tolvaptan.7–10
Left ventricular assist device (LVAD) implantation is becoming increasingly common for patients with advanced heart failure.11 Right ventricular failure is a frequent occurrence following LVAD implantation, particularly in the early postoperative period, and requires aggressive volume management to maintain appropriate preload.12 These patients often require escalating doses of diuretics. Furthermore, persistent hyponatremia after LVAD implantation is associated with increased heart failure hospitalizations, regardless of pre- operative sodium level.13 However, there are limited data on the efficacy of tolvaptan use in patients with LVADs. A previous retrospective study showed that tolvaptan use was safe following LVAD implantation, and was associated with improvement in serum Na levels and increased urine output, and the need for a prospective, randomized assessment of tolvaptan in LVAD patients was highlighted.14,15
In this pilot study, we prospectively assessed the efficacy of tolvaptan in LVAD patients in a randomized study. We hypothesized that patients treated with tolvaptan during the post- operative period would have higher serum sodium (Na) levels and improved urine output than patients not receiving tolvaptan without adverse effects on renal function.
2 |. METHODS
We conducted a prospective, randomized, non-blinded pilot study of LVAD recipients with post-operative hyponatremia at our institution. The study protocol was approved by the Institutional Review Board at the University of Chicago and registered on ClinicalTrials.gov (NCT05408104). Patients 18 years or older undergoing durable LVAD implantation at our institution were screened for enrollment in this study. We excluded patients undergoing LVAD exchange, as well as Jehovah’s Witnesses, who do not receive daily phlebotomy per hospital protocol. All participants provided informed, written consent prior to LVAD implantation. Participants who developed acute kidney injury, defined as the need for renal replacement therapy, after enrollment but prior to randomization were withdrawn from the study.
2.1 |. Randomization and study protocol
Enrolled participants were randomized on the day that they developed hyponatremia, defined as Na < 135 mEq/L. Participants were randomized in a 1:1 fashion to the TLV group (tolvaptan plus usual care) or the No-TLV group (usual care). Tolvaptan was administered at a dose of 15 mg daily and could be increased to 30 mg daily at the discretion of the treating physician every day that the participant was hyponatremic. Participants in the No-TLV group could cross over to the TLV group if Na ≤ 125 mEq/L and they were determined to be hypervolemic. Tolvaptan could be held if the participant was deemed to be hypovolemic. Diuretic dosing was not specified in the study protocol and was administered at the treating physician’s discretion. Tolvaptan was not continued beyond the index hospitalization.
Baseline characteristics including laboratory, echocardiographic, and hemodynamic data were recorded. The most recent data available prior to LVAD implantation were used as baseline data. Daily Na levels and creatinine were recorded from the time of randomization until the time of discharge. Estimated glomerular filtration rate (eGFR) was calculated according to the Modified Diet in Renal Disease equation. Urine output was measured over the 24-h period prior to randomization and for each 24-h period from randomization until the correction of hyponatremia or discharge. The daily dose of loop diuretics was recorded as equivalents of oral furosemide (Table S1).
Length of stay in the cardiothoracic intensive care unit and for the entire post-operative index hospitalization were recorded. Right ventricular failure was defined as per the 2013 INTERMACS definition.16 During the six-month surveillance period, patients had to have clinical signs of right heart failure hospital admission for intravenous diuresis, vasodilators, vasoactive/inotropic support, and/or right-sided mechanical circulatory support.16 Participants were followed for 6- months post-operatively for the assessment of hemocompatibility-related adverse events (HRAE). HRAE were defined as previously described and a hemocompatibility score was constructed.17 Hospitalizations for heart failure were also recorded.
The primary efficacy outcome was the change in Na from randomization to discharge. The primary safety outcome was the change in eGFR from the randomization to discharge. Secondary outcomes included change in urine output, the length of stay in the cardiothoracic intensive care unit, and the length of stay for the index hospitalization. Exploratory outcomes included the number of heart failure hospitalizations and the hemocompatibility score in the two groups at 6 months follow-up.
2.2 |. Statistical analysis
Participants were assessed via an intention-to-treat methodology. Continuous variables were expressed as median (interquartile range) and compared between the groups using the Kruskal-Wallis test. Categorical variables were expressed as number and percentage and compared between the groups using Fisher’s exact test. Trends of continuous variables were assessed using Wilcoxon signed-rank test.
We estimated a difference of Na at the time of endpoint as 2.0 mEq with 2.5 mEq/L of standard deviation in both groups. The alpha is defined as 0.05, the effect size as 0.8, and 1-β as 0.8. We estimated that we would need 26 patients in each group. Two-sided p-values < 0.05 were considered statistically significant. Statistical analyses were performed using SPSS Statistics 22 (SPSS Inc, Armonk, IL, USA).
3 |. RESULTS
3.1 |. Patient selection
Between May 2019 and August 2020 46 patients underwent durable LVAD implantation at our institution. Eight patients were excluded (6 LVAD exchanges, 1 patient < 18 years old, 1 Jehovah’s Witness), three patients were unable to consent due to the need for emergency surgery, and one patient declined to participate. One patient consented but did not undergo subsequent LVAD implantation and was therefore withdrawn from the study. Due to restrictions on research due to the COVID-19 pandemic, this study was terminated before full enrollment. In total, 33 patients were enrolled in the study.
Of the 33 patients enrolled in the study, 3 did not develop hyponatremia post-operatively and, therefore, were not randomized. Of the remaining 30 participants, 17 were randomized to the TLV arm and 13 to the No-TLV arm. However, two of the patients randomized to TLV were excluded due to acute kidney injury requiring hemodialysis prior to the development of hyponatremia. In total, 15 TLV and 13 No-TLV participants were included in the final analysis.
3.2 |. Baseline characteristics
Of these 28 eligible participants, the median age was 54.5 (IQR 49.8–61.5), 21% were women, 54% were Black, and 68% had underlying non-ischemic cardiomyopathy. Twenty-five (89%) patients had a HeartMate 3 LVAD (Abbott, Abbott Park, IL) and 3 (11%) had a HeartWare HVAD LVAD (Medtronic, Minneapolis, MN) (Table 1). There were no significant differences in surgical approaches, concomitant procedures, or repeat sternotomies (Table 2). 31- French Protek Duo (LivaNova Inc, Houston, TX) percutaneous right ventricular assist devices (RVAD) were placed in seven total patients, four in the TLV arm and three in the No-TLV arm. Six of the seven were implanted intra-operatively at the time of LVAD implantation, while one patient in the TLV arm had the RVAD placed after randomization, on post-operative day #9, due to the development of cardiogenic shock with oliguric renal failure. The median day of randomization was post-operative day 4 (IQR 2–4 ) for the TLV group and post-o perative day 3 (IQR 2–3) for the No- TLV group (p = 0.49). Baseline characteristics are listed in Table 1. Patients in the TLV group were less likely to be Black (33% vs 77%, p = 0.021) and more likely to have a history of stroke (33% vs 0%, p = 0.031). There were no other significant differences in baseline characteristics, baseline serum laboratory levels, or pre-operative invasive hemodynamic measurements (Table 1).
TABLE 1.
Baseline characteristics
| All (n = 28) | Tolvaptan (n = 15) | No tolvaptan (n = 13) | p value | |
|---|---|---|---|---|
| Post-operative day of randomization, median (IQR) | 3 (2–5) | 4 (2–4) | 3 (2–3) | 0.49 |
| Age, median (IQR) | 55 (50–62) | 54 (50–62) | 55 (50–61) | 0.89 |
| Female, n (%) | 6 (21) | 3 (20) | 3 (23) | 0.84 |
| Race | ||||
| Black, n (%) | 15 (54) | 5 (33) | 10 (77) | 0.021 |
| White/Non- Hispanic, n (%) | 9 (32) | 7 (47) | 2 (15) | 0.077 |
| Other, n (%) | 4 (14) | 3 (20) | 1 (8) | 0.35 |
| Past medical history | ||||
| Non- ischemic cardiomyopathy (n, %) | 19 (68) | 8 (53) | 11 (85) | 0.086 |
| Hypertension, n (%) | 14 (50) | 8 (53) | 6 (46) | 0.50 |
| Diabetes, n (%) | 13 (46) | 7 (47) | 6 (46) | 0.64 |
| Chronic Kidney Disease, n (%) | 9 (32) | 4 (29) | 5 (38) | 0.40 |
| Stroke, n (%) | 5 (18) | 5 (33) | 0 (0) | 0.031 |
| Peripheral arterial disease, n (%) | 5 (18) | 4 (29) | 1 (8) | 0.21 |
| Pre-operative laboratory values | ||||
| Na (mEq/L), median (IQR) | 135 (134–138) | 136 (135–139) | 134 (132–136) | 0.12 |
| Blood urea nitrogen (mg/dl), median (IQR) | 22.5 (19.3–30.5) | 24 (19.5–35) | 53 (41–70) | 0.47 |
| Creatinine (mg/dl), median (IQR) | 1.2 (1.0–1.6) | 1.1 (0.9–1.6) | 1.3 (1.1–1.6) | 0.56 |
| Estimated glomerular filtration rate (ml/min/1.73 m2), median (IQR) | 62.5 (44.0–75.0) | 69 (47– 79) | 53 (41– 70) | 0.33 |
| Albumin (g/dl), median (IQR) | 3.7 (3.3–3.9) | 3.6 (3.2–3.9) | 3.7 (3.5–4.0) | 0.72 |
| Total Bilirubin (mg/dl), median (IQR) | 0.6 (0.4–1.4) | 0.5 (0.4–0.7) | 1.5 (0.5–1.9) | 0.12 |
| White blood cell count (103/μl), median (IQR) | 6.7 (5.7–8.4) | 7.8 (6.4–8.5) | 5.9 (5.4–7.1) | 0.13 |
| Hemoglobin (g/dl), median (IQR) | 11.1 (9.7–12.7) | 11 (9.5–12.8) | 11.8 (9.7–12.6) | 0.93 |
| Platelets (103/μl), median (IQR) | 214 (179–250) | 211 (196–247) | 216 (160–260) | 1.0 |
| Post-operative day of randomization laboratory values | ||||
| Na (mEq/L), median (IQR) | 133 (132–134) | 133 (132–134) | 133 (132–134) | 0.65 |
| Blood urea nitrogen (mg/dl), median (IQR) | 21 (17–33) | 19 (17–44) | 27 (17–31) | 0.89 |
| Creatinine (mg/dl), median (IQR) | 1.0 (0.8–1.4) | 1.0 (0.9–1.4) | 1.0 (0.7–1.3) | 0.65 |
| Estimated glomerular filtration rate (ml/min/1.73 m2), median (IQR) | 76 (51–106) | 76 (51–98) | 78 (51–106) | 0.44 |
| White blood cell count (103/μl), median (IQR) | 14.9 (11.4–16.1) | 14.9 (13.5–15.9) | 13 (8.8–19.7) | 0.56 |
| Hemoglobin (g/dl), median (IQR) | 9.2 (8.5–9.9) | 9.3 (8.4–10.0) | 9.0 (8.5–9.8) | 0.93 |
| Platelets (103/μl), median (IQR) | 173 (107–203) | 188 (118–203) | 128 (96–207) | 0.50 |
| Aspartate aminotransferase U/L, median (IQR) | 35 (22–48) | 27 (24–53) | 35 (21–48) | 0.65 |
| Alanine aminotransferase U/L, median (IQR) | 28 (15–49) | 26 (15–70) | 31 (16–41) | 0.56 |
| Alkaline Phosphatase U/L, median (IQR) | 96 (68–117) | 93 (70–118) | 98 (65–100) | 0.84 |
| Total Bilirubin mg/dl, median (IQR) | 0.6 (0.4–1.5) | 0.5 (0.4–0.7) | 1.5 (0.5–1.9) | 0.34 |
| Pre-operative right heart catheterization | ||||
| Pre-operative day of right heart catheterization (days) (median, IQR) | −12 (9–16) | −10 (6–16) | 0.76 | |
| Right atrial pressure (mm Hg) (median, IQR) | 13 (8–17) | 13 (9–18) | 15 (7–16) | 1.0 |
| Right ventricular diastolic pressure (mm Hg) (median, IQR) | 13 (9–17) | 15 (7–21) | 0.65 | |
| Pulmonary arterial saturation (%) (median, IQR) | 54.5 (50.0–62.5) | 50.1 (39.9–65.7) | 0.55 | |
| Fick cardiac output (L/min) (median, IQR) | 4.0 (3.2–5.4) | 4.0 (3.5–5.4) | 4.1 (2.6–5.4) | 0.94 |
| Pulmonary artery pulsatility index (median, IQR) | 1.8 (1.0–2.8) | 1.8 (1–2.8) | 1.9 (1.3–2.6) | 0.13 |
| Central venous pressure: Pulmonary capillary wedge pressure ratio (median, IQR) | 0.5 (0.4–0.6) | 0.5 (0.4–0.6) | 0.4 (0.4–0.6) | 0.65 |
TABLE 2.
Index hospitalization six-month clinical outcomes
| Tolvaptan (n = 15) | No tolvaptan (n = 13) | p-value | |
|---|---|---|---|
| Surgical characteristics | |||
| Repeat sternotomy, n (%) | 3 (20%) | 4 (31%) | 0.59 |
| Concomitant mitral valve repair, n (%) | 3 (20%) | 5 (38%) | 0.28 |
| Concomitant aortic valve replacement, n (%) | 1 (7%) | 2 (15%) | 0.46 |
| Concomitant tricuspid valve repair, n (%) | 1 (7%) | 4 (31%) | 0.096 |
| Concomitant atrial septal defect/patent foramen ovale closure, n (%) | 2 (13%) | 2 (15%) | 0.88 |
| Concomitant left atrial appendage ligation, n (%) | 3 (20%) | 3 (23%) | 0.84 |
| Bypass time (minutes), median (IQR) | 126 (88–149) | 174 (125–192) | 0.11 |
| Intraoperative volume intake (ml), median (IQR) | 1300 (850–2028) | 1500 (982–1923) | 0.55 |
| Net intraoperative intake/output (ml), median (IQR) | 385 (−328–1128) | 698 (55–1075) | 0.45 |
| Total number intraoperative blood product transfusions, median (IQR) | 0 (0–2) | 0 (0–4) | 0.76 |
| Post-operative RVAD use, n (%) | 4 (27%) | 3 (23%) | 0.83 |
| Duration of post-operative RVAD use (days), median (IQR) | 7 (6–11) | 10 (13–23) | 0.83 |
| Index hospitalization outcomes | |||
| Post- operative time on inotropes, days (median, IQR) | 8 (6–16) | 9 (5–10) | 0.15 |
| Post- operative intensive care unit LOS, days (median, IQR) | 9 (6–11) | 8 (5–10) | 0.84 |
| Postoperative hospitalization LOS, days (median, IQR) | 19 (15–24) | 20 (17–24) | 0.93 |
| Early right ventricular failure, n (%) | 9 (60%) | 9 (69%) | 0.46 |
| Duration of the first post-operative hyponatremic episode (median, IQR) | 2 (1–3) | 2 (2– 9) | 0.50 |
| Discharge laboratory values | |||
| Discharge Na (mEq/L), median (IQR) | 135 (134–137) | 135 (132–137) | 0.53 |
| Discharge estimated glomerular filtration rate (ml/min/1.73 m2), median (IQR) | 80 (66–87) | 106 (56–117) | 0.12 |
| Discharge aspartate aminotransferase U/L, median (IQR) | 25 (24–32) (n = 9) | 34 (20–39) (n = 10) | 0.38 |
| Discharge alanine aminotransferase U/L, median (IQR) | 19 (13–35) (n = 9) | 27 (17–37) (n = 10) | 0.91 |
| Discharge alkaline phosphatase U/L, median (IQR) | 120 (109–127) (n = 9) | 113 (95–137) (n = 10) | 0.26 |
| Discharge total bilirubin mg/dl, median (IQR) | 0.7 (0.5–0.8) (n = 9) | 1.2 (0.6–1.9) (n = 10) | 0.55 |
| Six-month outcomes | |||
| Aspartate aminotransferase U/L, median (IQR) | 30 (18–37) (n = 9) | 35 (26–43) (n = 10) | 0.65 |
| Alanine aminotransferase U/L, median (IQR) | 22 (12–29) (n = 9) | 30 (22–41) (n = 10) | 0.46 |
| Alkaline phosphatase U/L, median (IQR) | 99 (88–111) (n = 9) | 130 (107–165) (n = 10) | 0.48 |
| Total bilirubin mg/dl, median (IQR) | 0.3 (0.3–0.7) (n = 9) | 0.8 (0.6–0.9) (n = 10) | 0.27 |
| Heart failure hospitalizations | 1 | 0 | 0.54 |
| Total HRAE | 3 | 6 | 0.24 |
| Hemocompatibility score | 6 | 13 | 0.29 |
Abbreviations: IQR, interquartile range; LOS, length of stay.
The median duration of the first post-operative hyponatremia episode was 2 days (IQR 1–3 days) in the TLV arm and 2 days (IQR 2–9 days) in the No-TLV arm, p = 0.50. All 15 participants randomized to the TLV arm received at least one dose of tolvaptan 15 mg. The median number of doses was 3.0 (IQR 2.5–3.0, minimum 2, maximum 13). Two of the 13 (15%) participants in the No-TLV received a 15 mg dose of tolvaptan for Na ≤ 125 mEq/L, as per the study protocol. One of these two participants also received a dose of tolvaptan outside of the study protocol. The other 11 of the 13 participants (85%) in the No-TLV arm did not receive any doses of tolvaptan.
3.3 |. Changes in sodium and eGFR
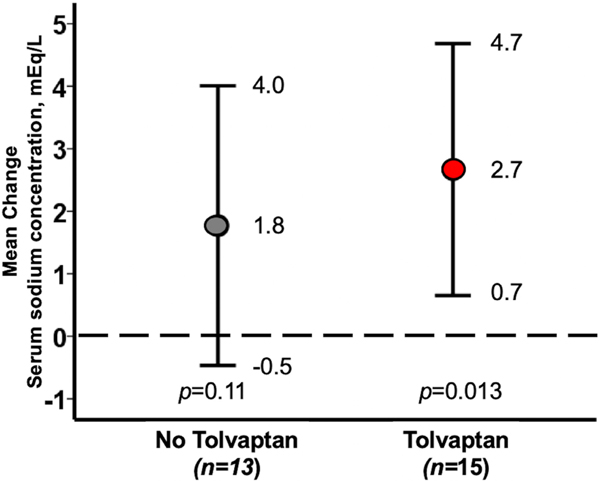
Participants in the TLV arm had a significant increase in Na from randomization to index hospitalization discharge with a change of 2.7 mEq/L (95% CI 0.7–4.7, p = 0.013) (Figure 1). Median Na was 133 (IQR 132–134) on the day of randomization and increased to 135 mEq/L (IQR 134–137) on discharge, p = 0.018 (Figure S1). Participants in the No-TLV arm did not have a significant change in Na from randomization to index hospitalization discharge with a mean change of 1.8 mEq/L (95% CI 0.5–4.0, p = 0.11) (Figure 1). Median Na was 133 (IQR 132–134) on the day of randomization and increased to 135 mEq/L (IQR 132–137) on discharge, p = 0.13 (Figure S1). Notably, despite the significant change in Na in the TLV arm, the median baseline and final Na values in the two groups were the same.
FIGURE 1.
Mean change in serum sodium from randomization to discharge
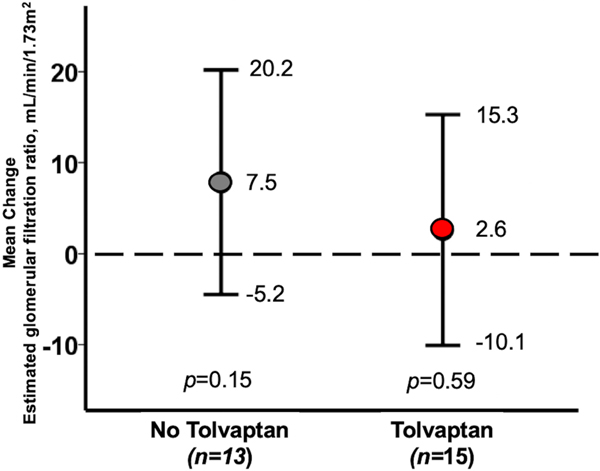
There were no significant differences in renal function in either arm of the study. In the TLV arm, eGFR remained unchanged with a mean change of 2.6 ml/min/1.73 m2 (95% CI −10.1–15.3, p = 0.59) (Figure 2). The median eGFR on day of randomization was 76 (IQR 51–99) ml/min/1.73 m2 and increase to 80 (IQR 66–90) ml/min/1.73 m2, on discharge, p = 0.87 (Figure S2). Similarly, in the No-TLV arm eGFR also remained unchanged with a mean change of 7.5 ml/min/1.73 m2 (95%CI 5.2–20.2, p = 0.15) (Figure 2). Median eGFR was 78 (IQR 56–115) ml/min/1.73 m2 on the day of randomization and increased to 106 (IQR 56–117) ml/min/1.73 m2, on discharge, p = 0.24 (Figure S2).
FIGURE 2.
Mean change in glomerular filtration rate from randomization to discharge
3.4 |. Urine output and diuretic use
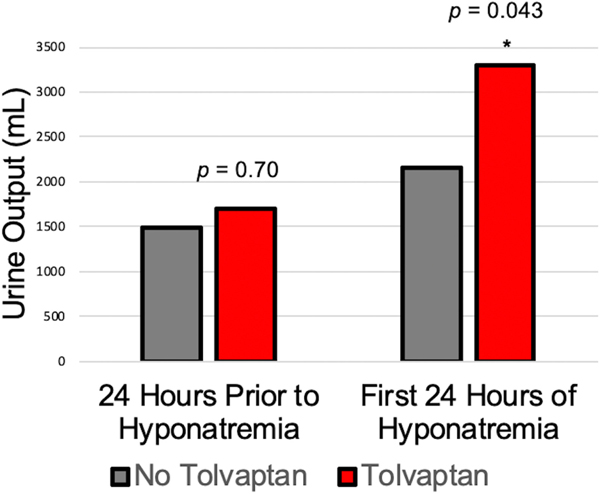
There were no differences in urine output between the two groups during the 24 h prior to randomization. However, participants in the TLV group had significantly more urine output than those in the No- TLV group during their first 24 h of post- operative hyponatremia (median 3294 vs 2155 ml, p = 0.043) (Central Illustration, Figure 3).
FIGURE 3.
Median daily urine output peri-hyponatremia
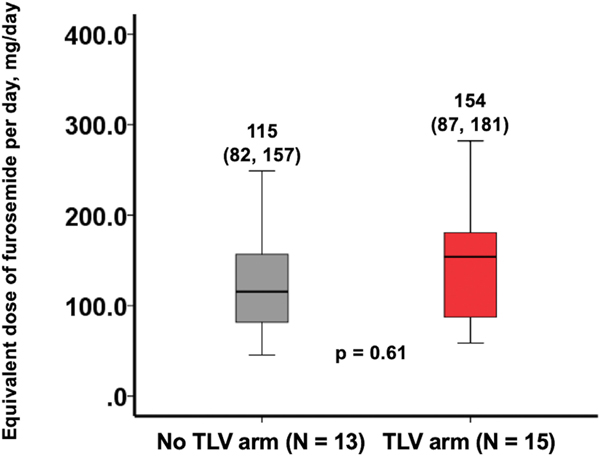
There were no significant differences in the median daily dose of oral furosemide equivalent loop diuretic between randomization and index hospitalization discharge: TLV 154 mg (IQR 87–181 mg) versus No-TLV 115 mg (IQR 82–157 mg), p = 0.61 (Figure 4). In the TLV group, in addition to tolvaptan, 4 (27%) patients had their loop diuretic dose increased on the day of randomization, 4 (27%) had their dose decreased, and 7 (47%) did not have any change in their loop diuretic dosing. In the No-TLV group, 1 (8%) patient had their loop diuretic dose increased, 4 (31%) had their dose decreased, and 8 (62%) did not have any changes made to their loop diuretic dose.
FIGURE 4.
Median equivalent dose of furosemide dosing per day from randomization through discharge
3.5 |. Clinical outcomes
There were no significant differences between the two groups in the incidence of right ventricular failure, the length of stay in the cardiac intensive care unit, or the length of the index hospitalization (Table 2). At six months follow-up, the hemocompatibility scores were 6 in the TLV group and 13 in the No TLV group, p = 0.29 (Table 2). Tolvaptan is associated with a risk for hepatotoxicity; there were no differences in liver function between the two groups pre-operatively, at index hospitalization discharge, or 6-month follow-up.
4 |. DISCUSSION
This pilot study is the first prospective, randomized-controlled trial of tolvaptan use in LVAD patients with hyponatremia. Our main findings are as follows: (1) there was a greater increase in sodium in the TLV group than in the No- TLV group, though baseline and final Na levels were similar between groups, (2) urine output was significantly increased in the TLV group compared to the No- TLV group, and (3) there was no significant change in renal function in either group.
Tolvaptan has been shown to increase urine output and decrease body weight for patients admitted with acute decompensated heart failure.2,3,18,19–21 Our study confirms that tolvaptan can augment diuresis and increase sodium levels in LVAD patients during the postoperative period. Vasopressin antagonists may have particular a benefit in patients with right ventricular dysfunction.10 This is highly relevant for the LVAD population where right ventricular dysfunction is prevalent due to the chronic, advanced nature of heart failure in these patients. The post-operative period is associated with abnormal right ventricular hemodynamics, as the improved cardiac output following LVAD implantation often overloads a right ventricle, that is accustomed to lower filling pressures. Increased preload leads to right ventricular dilation, which increases right ventricular wall stress, and often leads to tricuspid regurgitation, which further exacerbates right ventricular failure.12 Our group has previously shown an interaction between abnormal hemodynamics (i.e., right atrial pressure > 12 mm Hg, pulmonary capillary wedge pressure > 18 mm Hg) in LVAD patients and subsequent HRAE.22 Further studies are warranted to evaluate this interaction.
Similar to the retrospective analysis of tolvaptan use in LVADs, renal function was not significantly affected by tolvaptan use in this prospective trial.14 This confirms the safety of tolvaptan use in this patient population. Notably, urine output was significantly increased in hyponatremic patients following tolvaptan use, and this increased urine output continued through to discharge, despite similar daily doses of loop diuretics. Therefore, increased urine output can be correlated with tolvaptan use, and the increased decongestion is the most likely reason for the significant improvement in hyponatremia in this arm of the study.
Unfortunately, despite the improved Na level with tolvaptan, this did not translate into any significant difference in clinical outcomes. Retrospective data have indicated more frequent heart failure hospitalizations with post-operative hyponatremia, though patients were considered hyponatremic at 1-month following implantation, while our study evaluated hyponatremia during the post-operative index hospitalization.13 Tolvaptan has not been associated with improved clinical outcomes in heart failure patients without LVADs, though the sub-group of the EVEREST trial with Na < 130 mEq did have improved cardiovascular mortality with tolvaptan use.6 In our study, there were no significant differences in clinical outcomes, HRAEs, or hemocompatibility scores over a 6-month follow-u p, thus, but our study was not powered to detect such differences. We demonstrated the safety and efficacy of tolvaptan to correct hyponatremia and increase urine output in LVAD patients, however, further studies are warranted to explore if those effects will lead to clinical benefit.
4.1 |. Limitations
This is a pilot study that was underpowered to show improvements in sodium and urine output. We did not mandate diuretic regimens during the study period, and thus some of the effects seen may have been due to adjustment of diuretics other than tolvaptan. Additionally, there were no specified protocols instituted for “usual care,” including targets for net fluid balance, as this was left to the discretion of the treating physicians. We did not have routine hemodynamic assessment post- hyponatremia, as many participants no longer had a pulmonary artery catheter in place, and therefore assessment of the effect of tolvaptan on hemodynamics was not possible. Finally, there was limited exposure to tolvaptan in the TLV arm of the study, as the median number of doses given was three, which may explain similar baseline and final Na levels in the two study groups.
5 |. CONCLUSION
In post-operative LVAD participants with hyponatremia, tolvaptan significantly increases urine output, with nominal improvement in Na level, and without adversely affecting renal function. Larger clinical studies are still needed to further elucidate hemodynamic and clinical outcomes.
Supplementary Material
Footnotes
CONFLICT OF INTEREST
Mark N. Belkin, Teruhiko Imamura, Daniel Rodgers, Anthony J. Kanelidis, Michael P. Henry, Takeo Fujino, Viktoriya Kagan, Karen Meehan, Justin Okray, Shana Creighton, Colleen LaBuhn, Tae Song, Takeyoshi Ota, Ann B. Nguyen, Ben B. Chung, Bryan A. Smith, Sara Kalantari, Jonathan Grinstein, Nitasha Sarswat, Sean P. Pinney, Gene Kim: None. Valluvan Jeevanandam: Data saftey monitoring board for Thoratec Abbott. Gabriel Sayer: Consulting Fees from Abbott. Nir Uriel: Advisory board member Leviticus, Livemetric.
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122:857–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Felker GM, Mentz RJ, Cole RT, Adams KF, Egnaczyk GF, Fiuzat M, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol. 2017;69:1399–406. [DOI] [PubMed] [Google Scholar]
- 3.Konstam MA, Gheorghiade M, Burnett JC Jr, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST outcome trial. JAMA. 2007;297:1319–31. [DOI] [PubMed] [Google Scholar]
- 4.Matsue Y, Suzuki M, Torii S, Yamaguchi S, Fukamizu S, Ono Y, et al. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail. 2016;22:423–32. [DOI] [PubMed] [Google Scholar]
- 5.Shirakabe A, Hata N, Yamamoto M, Kobayashi N, Shinada T, Tomita K, et al. Immediate administration of tolvaptan prevents the exacerbation of acute kidney injury and improves the mid-term prognosis of patients with severely decompensated acute heart failure. Circ J. 2014;78:911–21. [DOI] [PubMed] [Google Scholar]
- 6.Hauptman PJ, Burnett J, Gheorghiade M, Grinfeld L, Konstam MA, Kostic D, et al. Clinical course of patients with hyponatremia and decompensated systolic heart failure and the effect of vasopressin receptor antagonism with tolvaptan. J Card Fail. 2013;19:390–7. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovitz A, Raiszadeh F, Zolty R. Association of hyponatremia and outcomes in pulmonary hypertension. J Card Fail. 2013;19:550–6. [DOI] [PubMed] [Google Scholar]
- 8.Forfia PR, Mathai SC, Fisher MR, Housten-Harris T, Hemnes AR, Champion HC, et al. Hyponatremia predicts right heart failure and poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2008;177:1364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kramer BK, Schweda F, Riegger GA. Diuretic treatment and diuretic resistance in heart failure. Am J Med. 1999;106:90–6. [DOI] [PubMed] [Google Scholar]
- 10.Nonin S, Iwata S, Ito A, Tamura S, Kitada R, Kawai Y, et al. Right ventricular enlargement predicts responsiveness to tolvaptan in congestive heart failure patients with reduced ejection fraction. Int J Cardiol Heart Vasc. 2018;21:69–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldstein DJ, Meyns B, Xie R, Cowger J, Pettit S, Nakatani T, et al. Third annual report from the ISHLT mechanically assisted circulatory support registry: a comparison of centrifugal and axial continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2019;38:352–63. [DOI] [PubMed] [Google Scholar]
- 12.Romano MA, Cowger J, Aaronson KD, Pagani FD. Diagnosis and management of right-sided heart failure in subjects supported with left ventricular assist devices. Curr Treat Options Cardiovasc Med. 2010;12:420–30. [DOI] [PubMed] [Google Scholar]
- 13.Kanelidis AJIT, Yang B, Miller T, Bharmal M, Kim GH, Sayer GT, et al. The clinical importance of hyponatremia in patients with left ventricular assist devices. ASAIO J. 2020. Accepted. [DOI] [PubMed]
- 14.Fujino T, Imamura T, Nguyen A, Chung B, Raikhelkar J, Rodgers D, et al. Short- term efficacy and safety of tolvaptan in patients with left ventricular assist devices. ASAIO J. 2020;66:253–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birks EJ, Kolodziej A. Pumps and the new pills. ASAIO J. 2020;66:258–60. [DOI] [PubMed] [Google Scholar]
- 16.INTERMACS Executive Committee. INTERMACS adverse event definitions: adult and pediatric patients. STS Intermacs Database. University of Alabama at Birmingham; 2013. [Google Scholar]
- 17.Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, et al. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation. 2017;135:2003–12. [DOI] [PubMed] [Google Scholar]
- 18.Udelson JE, Bilsker M, Hauptman PJ, Sequeira R, Thomas I, O’Brien T, et al. A multicenter, randomized, double-blind, placebo-controlled study of tolvaptan monotherapy compared to furosemide and the combination of tolvaptan and furosemide in patients with heart failure and systolic dysfunction. J Card Fail. 2011;17:973–81. [DOI] [PubMed] [Google Scholar]
- 19.Kinugawa K, Sato N, Inomata T. Effects of tolvaptan on volume overload in patients with heart failure. Int Heart J. 2018;59:1368–77. [DOI] [PubMed] [Google Scholar]
- 20.Vidic A, Shuster JE, Goff ZD, Godishala A, Joseph SM, Chibnall JT, et al. Vasopressin antagonism for decompensated right-sided heart failure. Int J Cardiol. 2019;274:245–7. [DOI] [PubMed] [Google Scholar]
- 21.Konstam MA, Kiernan M, Chandler A, Dhingra R, Mody FV, Eisen H, et al. Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol. 2017;69:1409–19. [DOI] [PubMed] [Google Scholar]
- 22.Imamura T, Nguyen A, Kim G, Raikhelkar J, Sarswat N, Kalantari S, et al. Optimal haemodynamics during left ventricular assist device support are associated with reduced haemocompatibility-related adverse events. Eur J Heart Fail. 2019;21:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.