Following the emergence of the coronavirus disease 2019 (COVID-19) pandemic in early 2020, many countries implemented containment measures to curb the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These containment measures were associated with a substantial reduction in the activity of other respiratory viruses as well as bacterial disease such as pneumococci [1, 2]. Consequently, as containment measures were gradually withdrawn, a rebound of respiratory syncytial virus (RSV) disease in children [3] and increases in pneumococcal diseases were observed [4]. However, little is known about how increasing population immunity against SARS-CoV-2, the circulation of new SARS-CoV-2 variants and the withdrawal of containment measures have impacted the microbiology of community-acquired pneumonia (CAP) over time. We describe the aetiology of hospitalised CAP among adults in Germany during 2021, the second year of the COVID-19 pandemic. In 2021, Germany had implemented strict COVID-19 containment measures, which were gradually withdrawn over the year and re-implemented before 2022 [5].
Short abstract
In Germany, the proportion of community-acquired pneumonia due to Streptococcus pneumoniae rebounded to a near-pandemic level in the second half of 2021. Vaccination uptake against respiratory pathogens, including S. pneumoniae, should be strengthened. https://bit.ly/3JMlwFt
To the Editor:
Following the emergence of the coronavirus disease 2019 (COVID-19) pandemic in early 2020, many countries implemented containment measures to curb the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). These containment measures were associated with a substantial reduction in the activity of other respiratory viruses as well as bacterial disease such as pneumococci [1, 2]. Consequently, as containment measures were gradually withdrawn, a rebound of respiratory syncytial virus (RSV) disease in children [3] and increases in pneumococcal diseases were observed [4]. However, little is known about how increasing population immunity against SARS-CoV-2, the circulation of new SARS-CoV-2 variants and the withdrawal of containment measures have impacted the microbiology of community-acquired pneumonia (CAP) over time. We describe the aetiology of hospitalised CAP among adults in Germany during 2021, the second year of the COVID-19 pandemic. In 2021, Germany had implemented strict COVID-19 containment measures, which were gradually withdrawn over the year and re-implemented before 2022 [5].
This ongoing, prospective, population-based surveillance study has been conducted since 4 January 2021, at one community hospital and two large tertiary care hospitals in Thuringia, Germany, serving a population of ∼280 000 inhabitants. Adult patients with suspicion of lower respiratory tract infection admitted to study hospitals were screened for eligibility, and individuals with documented or clinically suspected pneumonia were enrolled. Patients included in this analysis 1) were enrolled between 1 January and 31 December 2021; 2) had radiologically confirmed CAP diagnosed within 48 h of hospital admission; and 3) had information on hospital discharge disposition available. Study staff collected data on patient characteristics, medical history, hospital course and standard-of-care microbiological testing. Nasopharyngeal swabs were tested by PCR for 10 respiratory viruses (SARS-CoV-2, RSV, influenza virus, parainfluenza virus, human metapneumovirus, rhinovirus, human endemic coronavirus, adenovirus, enterovirus, bocavirus). Urine samples were tested using the commercially available pneumococcal urinary antigen test (PUAT; BinaxNOW Streptococcus pneumoniae) and with proprietary serotype-specific urinary antigen detection (UAD) assays [6, 7]. UAD assays detect all serotypes included in currently licensed vaccines. All data were descriptively summarised. The association of S. pneumoniae co-infection among COVID-19 patients with severe outcomes adjusted for patient age was analysed using the Mantel–Haenszel method.
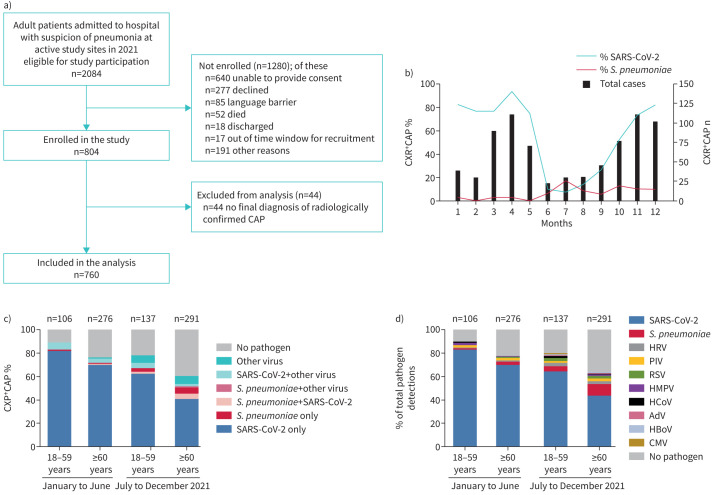
Out of 2084 patients with study-qualifying pneumonia, 804 were enrolled, and 760 were included in this analysis (figure 1a). The median age of patients with radiologically confirmed CAP was 67 years (interquartile range 58–79 years), and 57.6% were male. Approximately three-quarters (75.9%) of patients had at least one comorbidity. The uptake for same-season influenza vaccine, cumulative uptake of 23-valent pneumococcal polysaccharide vaccine (PPV23), and the 13-valent pneumococcal conjugate vaccine (PCV13) were 23.4%, 25.0% and 3.0%, respectively. The uptake for one or more doses of a COVID-19 vaccine was 14.3% in the first half of 2021 and increased to 50.6% in the second half. The median hospital length of stay was 8 days; 122 (16.1%) patients were admitted to the intensive care unit (ICU); 43.7% had a pneumonia severity index (PSI) grade IV or V; and in-hospital case-fatality rate was 8.4%.
FIGURE 1.
Pathogen detection among adults with community-acquired pneumonia (CAP) requiring hospitalisation in Thuringia, Germany in 2021. For all patients enrolled in the study, nasopharyngeal swabs and urine were collected and multiplex PCR for 10 respiratory viruses and urinary antigen tests for the C-wall polysaccharide (BinaxNOW Streptococcus pneumoniae) and serotype antigens (urinary antigen detection assay) were performed. Results from the per-protocol and standard-of-care testing are shown. a) Eligibility, enrolment and analysis of patients with CAP. b) Total number of enrolled adults with radiologically confirmed hospitalised CAP, and proportion of patients with detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and S. pneumoniae between 4 January 2021 and 31 December 2021, by month. c) Proportion of CAP due to SARS-CoV-2, S. pneumoniae or other respiratory viruses, by age group and study period. d) Proportion of pathogens detected, according to age group and study period. n indicates the total number of pathogen detections. CXR: chest radiography; HRV: human rhinovirus; PIV: parainfluenza virus 1–4; RSV: respiratory syncytial virus A and B; HMPV: human metapneumovirus; HCoV: human coronavirus (229E, OC43, NL63); AdV: adenovirus; HBoV: human bocavirus; CMV: cytomegalovirus.
Adherence to the diagnostic testing protocol was high throughout the study period and did not differ between the first and second halves of 2021. Overall, 96% of all CAP cases were tested for SARS-CoV-2; 91% for other respiratory viruses; and 80% for S. pneumoniae using UAD and PUAT. Additionally, the proportion of CAP cases with prior antibiotic treatment did not differ between the first and second halves of 2021 (7.8% and 8.0%, respectively). A respiratory pathogen was detected in 553 (72.8%) patients with CAP. The most frequent pathogen was SARS-CoV-2 (n=498, 68.2%), followed by S. pneumoniae (n=40, 6.4%), rhinovirus and parainfluenza virus (n=16, 2.3% each), and RSV (n=14, 2.0%) (figure 1d). Other viruses were infrequent (≤1.0% each), and no cases of influenza were identified. Out of the 40 pneumococcal CAP cases, 30 had a positive UAD test; PUAT was positive in 17; and S. pneumoniae grew from culture in eight (blood n=6, sputum and tracheal aspirate n=1 each). The percentage of CAP patients with SARS-CoV-2 detection was higher in patients aged 18–59 years (n=176, 79.3%) than those aged ≥60 years (n=322, 63.4%). Conversely, S. pneumoniae was more frequently detected in patients aged ≥60 years (n=33, 7.8%) than in patients aged 18–59 years (n=7, 3.6%). Out of the 40 pneumococcal CAP cases, PCV13-, PCV15-, PCV20- and PPV23-including serotypes accounted for 42.5% (n=17), 45.0% (n=18), 70.0% (n=28) and 73% (n=29), respectively. The most frequent serotypes were 3 (n=5), 8 (n=4) and 19A/6A/11A (n=3 each).
The aetiology of CAP changed substantially throughout 2021 (figure 1). The monthly proportion of CAP cases associated with SARS-CoV-2 ranged from 76.7% to 93.7% between January and May, dipped to 7.1% in July and then rose continuously to 82.4% until December (figure 1b). S. pneumoniae caused 0.0–2.9% of CAP cases between January and May and accounted for 5.9–16.7% afterwards (figure 1b). The proportion of CAP cases associated with SARS-CoV-2 decreased from the first to the second half of 2021 both in older adults (from 74.7% to 51.4%) and younger adults (from 88.0% to 72.1%) (figure 1c). By contrast, the proportion of CAP cases associated with S. pneumoniae increased between the first and second half of 2021 in older adults from 2.5% to 12.4%, and in younger adults from 1.1% to 5.6% (figure 1c).
Similar to S. pneumoniae, between the first and second half of 2021, respiratory viruses other than SARS-CoV-2 increased between from 5.3% to 11.3%. Co-infection of SARS-CoV-2 and S. pneumoniae occurred in two (0.7%) out of 283 patients in the first half of 2021, but in 13 (6.0%) out of 215 adults aged ≥18 years and 11 (8.7%) out of 127 adults aged ≥60 years in the second half of 2021 (figure 1c). 13 (38.5%) out of 34 pneumococcal detections in the second half of 2021 were in patients with COVID-19 (figure 1c). We did not find evidence for an association of S. pneumoniae co-infection in COVID-19 patients with death after pneumonia (age-adjusted odds ratio (aOR) 1.22, 95% CI 0.26–5.72; p=0.802), admission to ICU (aOR 1.13, 95% CI 0.31–4.15; p=0.854) or need for mechanical ventilation (aOR 1.55, 95% CI 0.33–7.24; p=0.575). However, due to the relatively low numbers of cases, our analysis was not powered to exclude such an association. Co-infections of SARS-CoV-2 with other respiratory viruses remained unchanged at ∼5% over the year 2021.
In this pneumonia surveillance study, we describe changes in CAP aetiology in Germany during 2021, covering the time of the spread of the COVID-19 Wuhan strain, and the subsequent waves of alpha and delta variants [8]. SARS-CoV-2 was the predominant cause of CAP in 2021, and the percentage of CAP due to S. pneumoniae rose from very low levels in early 2021 to near pre-pandemic levels among older patients in the second half of 2021 [9], with 12.4% of CAP cases caused by S. pneumoniae and 78.5% of these due to PCV20 serotypes. Previous studies reported that S. pneumoniae co-infection was rare among COVID-19 patients, but if present, associated with high case-fatality [10, 11]. In the second half of 2021, S. pneumoniae was detected in 8.7% of older adults with COVID-19, a co-infection rate higher than recently reported for influenza [12]. The re-emergence of S. pneumoniae may be related to several factors, including changes in social mixing with the relaxation of containment measures and healthcare-seeking behaviour, and differences in the interference of individual SARS-CoV-2 variants with other respiratory pathogens [13]. Underestimation of pneumococcal CAP by previous studies may be related to the low sensitivity of the standard-of-care culture methods compared to urinary antigen testing used in this study.
The main strength of this study lies in the high testing rates of 80–96% for 11 respiratory pathogens. A limitation of the study is that only 39% of eligible patients were enrolled. The most common reason for nonenrolment was a lack of capacity to provide consent, possibly reducing the generalisability of our results to severely ill, debilitated patients. However, the proportion of patients with severe disease as measured by PSI grade IV or V in our cohort is similar to that reported in other recent CAP surveillance studies [14, 15]. Additionally, the roll-out of the COVID-19 vaccination campaign in 2021 preferentially targeted older adults, and this may bias comparisons across age groups.
S. pneumoniae, the leading bacterial cause of CAP prior to the COVID-19 pandemic, was reduced during the early months of 2021, but rebounded to near pre-pandemic levels after the summer of 2021, affecting both COVID-19 and non-COVID-19 CAP patients in Germany. This rebound occurred in the absence of influenza circulation. With a more diverse spectrum of CAP aetiologies emerging, it will be critical to strengthen vaccination uptake, not only for SARS-CoV-2, but also for the currently re-emerging influenza [16] and S. pneumoniae [17]. Finally, studies with larger sample sizes should re-evaluate the impact of dual infection of SARS-CoV-2 and S. pneumoniae on severe clinical outcomes. This study shows that clinicians should be aware that co-infection with SARS-CoV-2 and pneumococci, especially in patients aged >60 years, is not uncommon, and may continue to increase.
Acknowledgements
We would like to thank the research team at the University Medical Center Jena, including Sebastian Weis, Christina Bahrs, Anne Moeser, Bettina Löffler and Steffi Kolanos; the research team at the SRH Klinik Gera, including Dagmar Täuscher, Nancy Schmidt, Nicole Haupt, Sabine Sell, Anne Meinzenbach, Melissa Rohde, Romy Anger, Mandy Michaelis, Mandy Wießner, Kristin Zoeger and Julia Schulze; and the research team at the SRH Klinik Suhl, including Mohamed Ahmed El Sebai, Cristian-Marian Andrei, Anastasia Mihali, Mara-Viviana Crasnic, Nicole Gerlach, Gabriela Günther, Nadine Marr and Stefanie Resiger for their support with the study. Medical writing support was provided by Qi Yan (Pfizer Inc.).
Provenance: Submitted article, peer reviewed.
Conflict of interest: C. Theilacker, K. Pan, L. Wang, C. Schwarz, C. von Eiff and B.D. Gessner are full-time employees of Pfizer Vaccines, and hold stock and/or stock options. M.W. Pletz reports research support to his institution from BioNTech, MSD, Roche, Pfizer, Bayer, GSK, Thermo Fisher, AstraZeneca and Novartis; personal fees from MSD, Roche, Pfizer, Bayer, GSK, Thermo Fisher, AstraZeneca and Novartis; support for attending meetings and/or travel from Pfizer and MSD; patents planned, issued or pending for “Biological Glass, CO-releasing woven”; participation on a data safety monitoring or advisory board with Inflarx with payment to his institution; and is on the board of directors of CAPNETZ, Paul Ehrlich Society, and WHO Guidance Group COVID-19 with no payments. S. Hagel reports payments or honoraria from Pfizer, MSD, Infectopharm, Philips, Advanz, Beckman Coulter, Shionogi, Tillots, and Thermo Fisher; support for attending meetings and/or travel from Pfizer, MSD, Infectopharm, Philips, Advanz, Beckman Coulter, Thermo Fisher, Shionogi and Tillots; and participation on a data safety monitoring or advisory board with Advanz and Shionogi. J. Ankert has no declarations.
Support statement: The study was funded by Pfizer Inc. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Olsen SJ, Winn AK, Budd AP, et al. Changes in influenza and other respiratory virus activity during the COVID-19 pandemic – United States, 2020–2021. MMWR Morb Mortal Wkly Rep 2021; 70: 1013–1019. doi: 10.15585/mmwr.mm7029a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brueggemann AB, Jansen van Rensburg MJ, Shaw D, et al. Changes in the incidence of invasive disease due to Streptococcus pneumoniae, Haemophilus influenzae, and Neisseria meningitidis during the COVID-19 pandemic in 26 countries and territories in the Invasive Respiratory Infection Surveillance Initiative: a prospective analysis of surveillance data. Lancet Digit Health 2021; 3: e360–e370. doi: 10.1016/S2589-7500(21)00077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danino D, Ben-Shimol S, van der Beek BA, et al. Decline in pneumococcal disease in young children during the coronavirus disease 2019 (COVID-19) pandemic in Israel associated with suppression of seasonal respiratory viruses, despite persistent pneumococcal carriage: a prospective cohort study. Clin Infect Dis 2022; 75: e1154–e1164. doi: 10.1093/cid/ciab1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertran M, Amin-Chowdhury Z, Sheppard CL, et al. Increased incidence of invasive pneumococcal disease among children after COVID-19 pandemic, England. Emerg Infect Dis 2022; 28: 1669–1672. doi: 10.3201/eid2808.220304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Our World in Data . Oxford Coronavirus Government Response Tracker (OxCGRT) Stringency Index. 2022. https://ourworldindata.org/covid-stringency-index. Date last accessed: 27 October 2022.
- 6.Pride MW, Huijts SM, Wu K, et al. Validation of an immunodiagnostic assay for detection of 13 Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Vaccine Immunol 2012; 19: 1131–1141. doi: 10.1128/CVI.00064-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalina WV, Souza V, Wu K, et al. Qualification and clinical validation of an immunodiagnostic assay for detecting 11 additional Streptococcus pneumoniae serotype-specific polysaccharides in human urine. Clin Infect Dis 2020; 71: e430–e438. doi: 10.1093/cid/ciaa158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Institute for Infectious Diseases and Infection Control, University Hospital Jena . Monthly SARS-CoV-2 Lineages (Thuringia). Available from: https://charts.mongodb.com/charts-routine-sequencing-sars-c-amykg/public/dashboards/e9453286-1dce-4202-9423-a8459e3962f8 Date last accessed: 27 October 2022. [Google Scholar]
- 9.Bahrs C, Kesselmeier M, Kolditz M, et al. A longitudinal analysis of pneumococcal vaccine serotypes in pneumonia patients in Germany. Eur Respir J 2022; 59: 2102432. doi: 10.1183/13993003.02432-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langford BJ, So M, Raybardhan S, et al. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin Microbiol Infect 2020; 26: 1622–1629. doi: 10.1016/j.cmi.2020.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin-Chowdhury Z, Aiano F, Mensah A, et al. Impact of the coronavirus disease 2019 (COVID-19) pandemic on invasive pneumococcal disease and risk of pneumococcal coinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): prospective national cohort study, England. Clin Infect Dis 2020; 72: e65–e75. doi: 10.1093/cid/ciaa1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartley PS, Deshpande A, Yu PC, et al. Bacterial coinfection in influenza pneumonia: rates, pathogens, and outcomes. Infect Control Hosp Epidemiol 2022; 43: 212–217. doi: 10.1017/ice.2021.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyams C, Begier E, Garcia Gonzalez M, et al. Incidence of acute lower respiratory tract disease hospitalisations, including pneumonia, among adults in Bristol, UK, 2019, estimated using both a prospective and retrospective methodology. BMJ Open 2022; 12: e057464. doi: 10.1136/bmjopen-2021-057464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torres A, Menéndez R, España PP, et al. The evolution and distribution of pneumococcal serotypes in adults hospitalized with community-acquired pneumonia in Spain using a serotype-specific urinary antigen detection test: the CAPA study, 2011–2018. Clin Infect Dis 2021; 73: 1075–1085. doi: 10.1093/cid/ciab307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isturiz RE, Ramirez J, Self WH, et al. Pneumococcal epidemiology among US adults hospitalized for community-acquired pneumonia. Vaccine 2019; 37: 3352–3361. doi: 10.1016/j.vaccine.2019.04.087 [DOI] [PubMed] [Google Scholar]
- 16.Australian Government Department of Health and Aged Care . Australian Influenza Surveillance Report - No. 14, 2022, reporting fortnight: 26 September to 09 October 202. 2022. www.health.gov.au/sites/default/files/documents/2022/10/aisr-fortnightly-report-no-14-26-september-to-9-october-2022.pdf Date last accessed: 27 October 2022.
- 17.The Academy of Medical Sciences . COVID-19: Preparing for the Future. Looking Ahead to Winter 2021/22 and Beyond. 2021. https://acmedsci.ac.uk/policy/policy-projects/covid-19-looking-ahead-to-winter-2021-22-and-beyond Date last accessed: 10 August 2021.