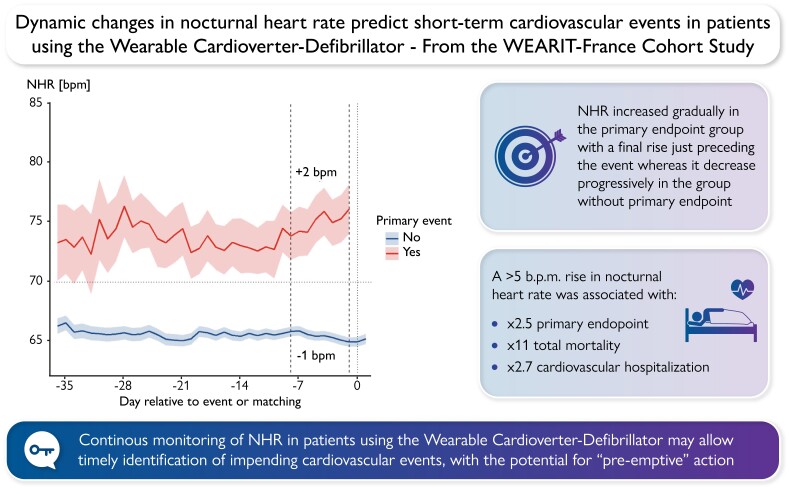
Abstract
Aims
While elevated resting heart rate measured at a single point of time has been associated with cardiovascular outcomes, utility of continuous monitoring of nocturnal heart rate (NHR) has never been evaluated. We hypothesized that dynamic NHR changes may predict, at short term, impending cardiovascular events in patients equipped with a wearable cardioverter-defibrillator (WCD).
Methods and results
The WEARIT-France prospective cohort study enrolled heart failure patients with WCD between 2014 and 2018. Night-time was defined as midnight to 7 a.m. NHR initial trajectories were classified into four categories based on mean NHR in the first week (High/Low) and NHR evolution over the second week (Up/Down) of WCD use. The primary endpoint was a composite of cardiovascular death and heart failure hospitalization. A total of 1013 [61 (interquartile range, IQR 53–68) years, 16% women, left ventricular ejection fraction 26% (IQR 22–30)] were included. During a median WCD wear duration of 68 (IQR 44–90) days, 58 patients (6%) experienced 69 events. After considering potential confounders, High-Up NHR trajectory was significantly associated with the primary endpoint compared to Low-Down [adjusted hazard ratio (HR) 6.08, 95% confidence interval (CI) 2.56–14.45, P < 0.001]. Additionally, a rise of >5 bpm in weekly average NHR from the preceding week was associated with 2.5 higher composite event risk (HR 2.51, 95% CI 1.22–5.18, P = 0.012) as well as total mortality (HR 11.21, 95% CI 3.55–35.37, P < 0.001) and cardiovascular hospitalization (HR 2.70, 95% CI 1.51–4.82, P < 0.001).
Conclusion
Dynamic monitoring of NHR may allow timely identification of impending cardiovascular events, with the potential for ‘pre-emptive’ action.
Registration number
Clinical Trials.gov Identifier: NCT03319160
Keywords: Wearable cardioverter-defibrillator, Heart rate, Remote monitoring, Pre-emptive action, Heart failure
Graphical Abstract
Graphical Abstract.
What’s new?
In patients equipped with wearable cardioverter-defibrillator, dynamic changes in nocturnal heart rate (NHR) were demonstrated to correlate in a temporal fashion with hard clinical endpoints such as cardiovascular death and hospitalization for heart failure.
These observations suggest that longitudinal monitoring of NHR could be a valuable addition to the risk assessment arsenal for prediction of cardiovascular events, thereby opening avenues for near-term prevention.
Introduction
The past two decades have witnessed growing evidence for a strong relationship between high heart rate and adverse cardiovascular events.1,2 The resting heart rate has often been inferred from ECGs done randomly at different times during the day, which may be subject to some variability, thereby introducing some deviation from a true resting state.3 On the other hand, nocturnal heart rate (NHR) during sleep, being a state of true physiological rest, is likely to be a more accurate marker of resting heart rate, but has not been well studied as a prognostic risk marker. In addition, studies have traditionally relied on heart rate assessments at one fixed time point, often early in the course, to predict long-term outcomes.4 However, similar to other risk markers, dynamic measurements of heart rate changes over time could improve the specificity of this marker and also potentially allow timely, specific pre-emptive action.
Using data from a large, nationwide cohort of patients with heart failure equipped with a wearable cardioverter-defibrillator (WCD) capable of recording all heart beats over the entire duration of use, we assessed whether dynamic monitoring of NHR allows timely identification of adverse cardiovascular events.
Methods
Study design and patient population
The WEARIT-France study (Clinical Trials.gov Identifier: NCT03319160) is a prospective nationwide cohort study assessing the use of the WCD in patients with heart failure across 88 French cardiology centres. The complete methodology has been described before.5 This study complies with the Declaration of Helsinki and an ethics committee approved the research protocol. All patients who agreed to participate were entered into the study after having given their informed consent.
The wearable cardioverter-defibrillator
The WCD technology used in the WEARIT-France study is a commercially available external defibrillator (LifeVest, ZOLL Cardiac Management Solutions, PA), guided by an algorithm to detect ventricular tachyarrhythmia events.6,7 The functioning of WCD has already been described8,9 and current indications are summarized in the last guidelines.10,11 During the index hospitalization when WCD therapy was initiated, the treating physician systematically assessed the appropriateness of WCD prescription and educated the patient regarding the transient risk for sudden cardiac death, functioning of the WCD, and benefits expected from the device. Additionally, just before discharge, a technical expert from the WCD company imparted 2 h of practical education to the patient, encompassing the nature of the disease, indication for WCD, alarm management, and remote transmission. The local remote monitoring team monitored daily wear duration on a regular basis.
Collected data and study end points
At the time of enrolment, medical history, comorbidities, symptoms, and other baseline characteristics were collected in addition to the indication for WCD. The WCD is equipped with four electrodes allowing the calculation of heart rate based on the R–R intervals, which is computed as the mean of all the heart rate provided every 5 min. The WCD prescription period is 90 days in France and the observation period was therefore limited to this period.
The telemonitoring platform also allows for heart rate monitoring. We specifically focused on NHR, which was defined as the mean heart rate from midnight to 7 a.m. because of lower heart rate variability (see Supplementary material online, Figure S1). In addition, we monitored NHR changes over time. To analyse mid-term predictive value of initial NHR trajectories over the WCD use period, patients were classified into four groups (High-Up, High-Down, Low-Up, Low-Down) based on NHR trajectory using a cut-off mean of 70bpm during the first seven nights of use (High if ≥70 bpm or Low if <70 bpm) and then according to an increase (Up) or decrease (Down) of NHR between Week 1 and Week 2 (see Supplementary material online, Figure S2). Similar to previous publications,1 mean cut-off of 70 bpm was determined because mean NHR was 68.8 (10.3) in this population. To assess the short-term dynamics of NHR, we defined ΔNHR as the difference between the weekly average NHR and that of the preceding week for each subject.
The primary endpoint was a composite of cardiovascular death and heart failure-related hospitalization. Endpoints were centrally adjudicated by an independent clinical events committee composed of three experts who adjudicated the events, by analysing the medical records/electrogram information, independent of each other and blinded to NHR and any additional information.
Statistical analysis
Preparation of this report was carried out in accordance with the STrengthening the Reporting of Observational studies in Epidemiology (STROBE) statement.12 Descriptive statistics were used to report major clinical characteristics and frequency of events. Continuous variables are presented as mean (standard deviation) or median and interquartile range (IQR) where appropriate and compared with Welch’s t-test or Wilcoxon–Mann–Whitney test. Nominal variables were expressed as number and percentage and compared using the Pearson’s χ2 test.
The time to event for each individual was defined from the first day of WCD wear to the day of first primary event, censoring, or end of follow-up (90 days), whichever came first. Cumulative incidence curves stratified by the initial NHR trajectories (High-Up, High-Down, Low-Up, Low-Down) were calculated by one minus the Kaplan–Meier estimator. Difference was assessed by the log-rank statistic. A multivariable Cox regression model with subjects’ baseline characteristics as covariates was used to estimate the hazard ratios (HRs) of NHR initial trajectories for primary endpoint, adjusting on age, sex, body mass index, New York Heart Association class, left ventricular ejection fraction, prior hospitalization for heart failure, history of atrial fibrillation or kidney disease, and beta-blocker use.
Nocturnal heart rate dynamics were plotted in the primary endpoint and the non-primary endpoint groups to evaluate changes in trajectory over time. A nested case–control methodology was used matching 1 case for 7–9 controls to remove the effect of time since start of wear. In this way, on the day of an event (e.g. Day 14), a case is compared to a matched control of the same sex and on the same day of WCD wear.
To evaluate short-term dynamics of NHR (i.e. weekly changes in NHR), we used a prospective approach computing ΔNHR independently from the event. ΔNHR was defined as the difference between the weekly average NHR from 2 weeks back (W-2) and the week before (W-1) for each subject. Since a previous publication showed a significant HR increase in the last 10 days before an adverse cardiovascular event,13 we assumed that a week represented adequate duration to reflect a clinically relevant change, but was not too long to miss any significant events. The ΔNHRs were updated weekly until the week just preceding the week of the event or until the end of observation period in case of no event (see Supplementary material online, Figure S3). ΔNHR was modelled as a continuous and a categorical covariate. The association between each endpoint and ΔNHR (W-1 minus W-2) as continuous covariates (per 5 bpm increase from W-2 to W-1) andcategorical covariate (change > 5 bpm from W-2 to W-1) was assessed by the Cox model14,15 with adjustment for baseline NHR (2 weeks back: W-2) and for the confounders: age, sex, body mass index, New York Heart Association class, left ventricular ejection fraction, prior hospitalization for heart failure, history of atrial fibrillation or kidney disease, and beta-blocker use. The relationship between ΔNHR as a continuous variable and the HR for the primary endpoint was also examined by cubic spline curve with three knots, using a reference value of 60 bpm.
We performed sensitivity analysis among both genders, patients without history of atrial fibrillation and patients with New York Heart Association Class I and II, with respect to both mid-term NHR trajectories and short-term dynamics of NHR.
Analyses were performed using R software (version 4.1.3). All statistical tests performed were two-sided. A P-value of <0.05 was considered statistically significant. The proportional hazards assumption was tested and found satisfied.
Results
Baseline patient characteristics
Among 1157 patients enrolled in the WEARIT-France study, 1013 (88%) wore the WCD more than 2 weeks and were analysed. Clinical characteristics of the patients are listed in Table 1. The median age was 61 (IQR 53–68) years, 167 (16%) were females and median left ventricular ejection fraction was 26% (IQR 22–30). New York Heart Association status was Class I or II in 723 (71%) patients. A total of 76 (8%) patients had renal disease requiring therapy, 103 (10%) patients had history of atrial fibrillation, 71 (7%) had previous stroke. Regarding medical therapies, 905 (89%) patients were prescribed beta-blockers, 879 (87%) angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, and 160 (16%) amiodarone.
Table 1.
Clinical characteristics of patients at baseline (N = 1013)
| All population (N = 1013) | No event (N = 955) | Event (N = 58) | P-value | |
|---|---|---|---|---|
| Age, yrs | 61 (53, 68) | 61 (53, 68) | 62 (54, 69) | 0.505 |
| Male sex, N (%) | 846 (84) | 800 (84) | 46 (79) | 0.374 |
| BMI, kg/m2 | 25.6 (23.1, 28.7) | 25.6 (23.1, 28.7) | 25.6 (23.4, 27.4) | 0.720 |
| NYHA class, N (%) | 0.005 | |||
| I and II | 723 (71%) | 691 (72%) | 32 (55%) | |
| III and IV | 290 (29%) | 264 (28%) | 26 (45%) | |
| Left ventricular ejection fraction, (%) | 26 (22, 30) | 26 (23, 30) | 25 (20, 29) | 0.104 |
| Medical history, N (%) | ||||
| Myocardial infarction | 830 (82%) | 780 (82%) | 50 (86%) | 0.512 |
| Valvular disease | 129 (13%) | 119 (12%) | 10 (17%) | 0.386 |
| Atrial fibrillation | 103 (10%) | 95 (9.9%) | 8 (14%) | 0.347 |
| Renal disease | 76 (7.5%) | 67 (7.0%) | 9 (16%) | 0.034 |
| Stroke | 71 (7.0%) | 64 (6.7%) | 7 (12%) | 0.117 |
| Medical therapy, N (%) | ||||
| Beta-blockers | 905 (89%) | 854 (89%) | 51 (88%) | 0.721 |
| Diuretics | 817 (81%) | 767 (80%) | 50 (86%) | 0.270 |
| ACE-I/ARBs | 879 (87%) | 837 (88%) | 42 (72%) | <0.001 |
| Amiodarone | 160 (16%) | 147 (15%) | 13 (22%) | 0.155 |
Data are presented as n (%) or mean ± SD.
ACE-I/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; NYHA, New York Heart Association.
Follow-up and primary endpoint evaluation
Median WCD wear time period was 68 (IQR 44–90) days in the overall patient population, with 58 (7%) patients experiencing events: 10 deaths (including 5 cardiovascular deaths) and 97 patients with cardiovascular hospitalizations (including 58 patients with heart failure-related hospitalizations) (Table 2).
Table 2.
Summary of events
| Patients, N = 1013 | Events | |
|---|---|---|
| Death | 10 (1.0) | 10 |
| - Cardiovascular cause | 5 (0.5) | 5 |
| - Non-cardiovascular cause | 5 (0.5) | 5 |
| Hospitalization | 133 (13) | 160 |
| - Cardiovascular hospitalization | 97 (9.6) | 112 |
| Hospitalization for heart failure | 58 (5.6) | 64 |
| Other cardiovascular hospitalization | 44 (4.3) | 48 |
| - Non-cardiovascular hospitalization | 45 (4.4) | 48 |
Lines in bold are event categories. Lines preceded by “ - ” (such as “ - Cardiovascular cause ”) are subcategories of the event in bold.
At the end of wear time period, 548 (54.1%) received an implantable cardioverter-defibrillator, and left ventricular ejection fraction improved in 343 (33.9%). When comparing patients with and without event, 49.5 vs. 53.8%, respectively had an implantable cardioverter-defibrillator implantation and 4.1 vs. 35.9% had improved left ventricular ejection fraction (global P-value ≤ 0.001).
Initial nocturnal heart rate trajectories
Considering the whole population, the mean NHR was 68 ± 11 bpm during the first 2 weeks of WCD use and 64 ± 11 bpm during the last 2 weeks of WCD use (P < 0.001).
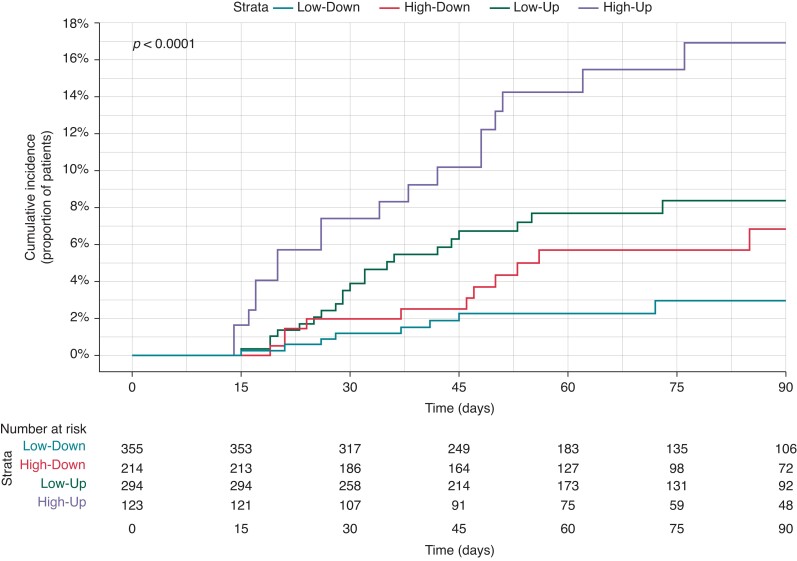
Looking at initial NHR trajectories classified into 4 groups (High-Up, High-Down, Low-Up, Low-Down), 123 (12%) patients were in the High-Up, 214 (22%) in the High-Down, 294 (30%) in the Low-Up, and 355 (36%) in the Low-Down group (27 patients were not classified because of missing NHR). The primary endpoint rate in the High-High group was significantly higher compared to the other groups (Log Rank P < 0.001; Figure 1). In multivariate Cox analysis, High-Up trajectory remained significantly associated with worse outcome [adjusted HR 6.08, 95% confidence interval (CI) 2.56–14.45; Low-Down as reference, P < 0.001] along with history of heart failure hospitalization (HR 2.26, 95% CI 1.25–4.10, P = 0.007), whereas angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use was associated with lower occurrence of primary endpoint (HR 0.30, 95% CI 0.16–0.57, P < 0.001). In sensitivity analyses, the risk associated with a High-Up trajectory was confirmed in both sexes, in patients without history of atrial fibrillation, and in patients with New York Heart Association Class I and II [HR 95% CI 6.09 (2.25–16.50), P < 0.001 for males, 8.85 (1.21–64.63), P = 0.032 for females; 5.58 (2.22–14.05), P < 0.001 for patients without atrial fibrillation, 8.75 (2.28–33.62), P = 0.002 for New York Heart Association Class I and II].
Figure 1.
Primary endpoint cumulative incidence according to initial NHR trajectories. NHR, nocturnal heart rate.
Short-term dynamics of nocturnal heart rate
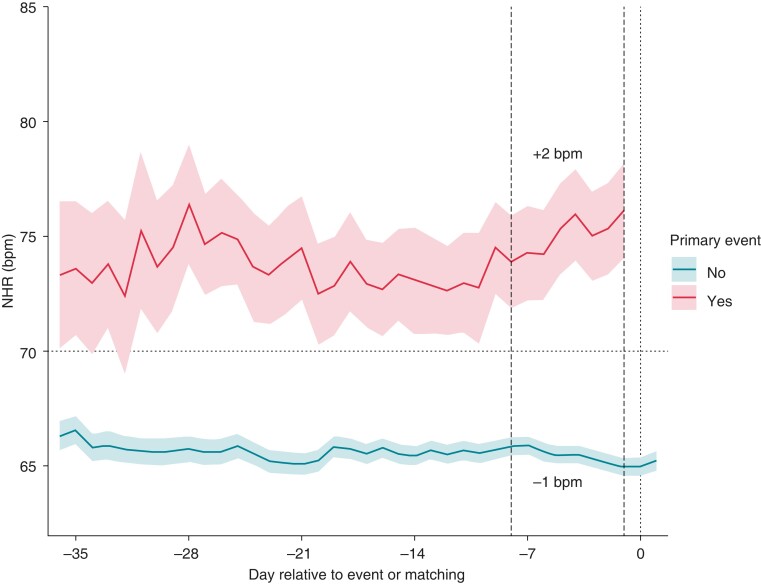
Heart rate dynamics according to the primary endpoint are represented in Figure 2. In the group with cardiovascular events, NHR increased starting 7 to14 days before the event, whereas it decreased progressively in the group without primary endpoint. In the last 7 days before the event (from −8 to −1 day), NHR increased by +2 bpm (IQR −3; + 6) in the group with primary endpoint, whereas it decreased by −1 (IQR −1; −1) in the non-event group (Figure 2).
Figure 2.
NHR dynamics among patients with and without primary event. NHR, nocturnal heart rate.
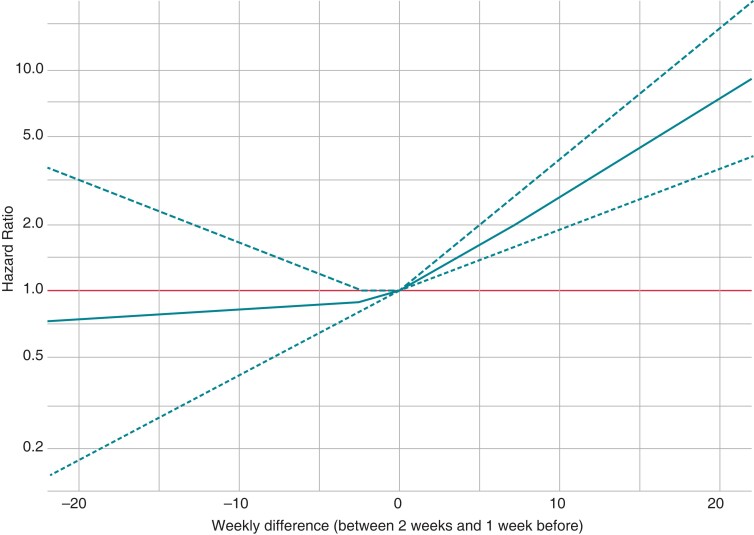
The distribution of ΔNHR is graphically shown in Supplementary material online, Figure S4. Most patients had only minimal change in NHR from the preceding week [median ΔNHR—0.44 bpm (IQR −2.44; 1.53)] during the WCD wear period. The restricted cubic spline model showed that increase in ΔNHR correlated linearly with higher risk for primary endpoint (Figure 3).
Figure 3.
Association between ΔNHR and primary endpoint occurrence. NHR, nocturnal heart rate.
The association between individual components of the primary endpoint and NHR at the first night of WCD use as well as ΔNHR is summarized in Table 3. As a continuous covariate, both NHR at first night and ΔNHR were associated with total mortality and cardiovascular hospitalization. When considering ΔNHR as a continuous variable, each 5 bpm increase in ΔNHR was associated with a 41% higher risk of adverse events. This association was even stronger when considering ΔNHR as a categorical variable; an increase of ΔNHR > 5 bpm was associated with a 2.5-fold higher risk of primary endpoint (HR 2.51, 95% CI 1.22–5.18, P = 0.012), an 11-fold higher risk of death (HR 11.21, 95% CI 3.55–35.37, P < 0.001), and an almost three-fold higher risk of cardiovascular hospitalization (HR 2.70, 95% CI 1.51–4.82, P < 0.01). When considering subgroup analysis, similar results were obtained in males and females. In patients without history of atrial fibrillation, an increase of ΔNHR > 5 bpm was associated with two-fold higher risk of primary endpoint (HR 2.51; 95% CI 1.16–5.40, P = 0.019). Regarding patients with New York Heart Association Class I and II, an increase of NHR ≥ 5 as compared to the previous week was associated with point estimates for HR > 2 but was not significant due to loss of power.
Table 3.
Association between NHR covariates and outcomes
| Continuous analysis | Continuous analysis | Categorical analysis | ||||
|---|---|---|---|---|---|---|
| NHR at first night of WCD use (per 5 bpm increase) | Δ NHR (per 5 bpm increase) | Δ NHR increase > 5 bpm | ||||
| HR (95%CI) | P-value | HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Cardiovascular death or heart failure hospitalization | 1.23 (1.13–1.35) | <0.001 | 1.41 (1.13–1.77) | 0.003 | 2.51 (1.22–5.18) | 0.012 |
| All death | 1.28 (1.04–1.56) | 0.023 | 1.64 (1.15–2.36) | 0.007 | 11.21 (3.55–35.37) | <0.001 |
| Cardiovascular hospitalization | 1.14 (1.05–1.23) | 0.015 | 1.42 (1.18–1.70) | <0.001 | 2.70 (1.51–4.82) | <0.001 |
bpm, beats per minute; HR, hazard ratio; NHR, nocturnal heart rate; WCD, wearable cardioverter-defibrillator.
Discussion
In this study, we found that NHR was associated with adverse cardiovascular events in a heart failure population within a short- to mid-term timeframe. Moreover, dynamic changes in NHR were demonstrated to correlate in a temporal fashion with hard clinical endpoints such as cardiovascular death and hospitalization for heart failure. Weekly rise of NHR > 5 bpm was especially associated with high risk, suggesting potentially actionable cut-offs for clinical application. While needing further validation in future studies, these observations suggest that NHR, especially with longitudinal monitoring, could be a valuable addition to the risk assessment arsenal for prediction of cardiovascular events.
Prediction of cardiovascular events
Because of the high rate of re-hospitalizations, high mortality, poor quality of life, and the substantial cost sustained by national healthcare systems, much effort has been made to identify the parameters/risk factors that can effectively contribute to prediction and prevention of decompensation events and hospitalizations in patients with heart failure.16 Previous studies showed that heart rate was another parameter associated with adverse outcome in different settings, especially in heart failure.17 Nevertheless, prediction of cardiovascular events with ‘static’ heart rate assessment, reflecting one-time status, has limitations as it can be affected by a number of factors, potentially affecting specificity. In addition, event risk is a dynamic, time-varying phenomenon; therefore, it makes sense that continuous measurements would be preferable to a single one.18 In this regard, dynamic monitoring holds promise, wherein each patient serves as his/her own control and changes over time may yield higher sensitivity as well as specific risk assessment. Vazir et al.4 reported that, compared to the previous visit, an increase >5 bpm in resting heart rate was associated with 1.06 times higher risk of cardiovascular death or hospitalization for heart failure. However, HR assessment was irregular as it was evaluated at any time from every 2 weeks to every 4 months. A closer and automated measurement of heart rate over time, as in this study, could identify events with better accuracy and in a timely manner to avoid hospitalization or death. We assessed the mean NHR weekly and found that an increase of > 5 bpm was associated with a more than two-fold risk of cardiovascular death or hospitalization for heart failure.
Remote monitoring and connected devices
Telemedicine can allow for remote monitoring and management of patients with chronic cardiovascular diseases, making it possible to assess medication adherence and detecting early signs of decompensation before it results in additional complications or hospital readmission. Even though this has mostly been done with invasive devices,19 advances in technology now make it possible to use non-invasive solutions.20,21 Moreover, the large population of patients with implanted and wearable devices (such as implantable cardioverter-defibrillator, cardiac resynchronization therapy and WCD) with rapid expansion of remote monitoring technology presents an important opportunity, which needs to be leveraged to improve risk prediction.
To the best of our knowledge, this work is the first to use heart rate collected in an automatic and continuous manner. Indeed, in prior work attempting to predict cardiovascular events, heart rate was measured manually or derived from a single 12 lead ECG.4,13,22 While we studied a selected population of patients equipped with a WCD, signal acquisition is becoming easier with the recent development of a wide range of connected devices,23 which have become deeply entrenched in our daily lives. Despite the promise of remote patient monitoring, this technology has thus far remained relatively underutilized. In the era of artificial intelligence, remote and increasingly personalized patient care, one can imagine that heart rate could be monitored with a simple connected watch, greatly expanding the applicability of this concept.24 With such connected devices, continuous data acquisition has the potential to open up avenues for near-term prevention, where dynamic changes in monitored parameters can be used to take corrective, ‘pre-emptive’ action, avoiding adverse events.
Near-term prevention
Long-term risk prediction is often disappointing with imperfections in risk assessment as well as solutions (for instance, an implantable cardioverter-defibrillator along with its side effects).25,26Near-term prevention, which relies upon prompt action in response to warning signs, could allow timely intervention to avoid the adverse outcome but without the inconvenience of ‘permanent’ therapy.27 In fact, cardiovascular risk is dynamic and modulated by a variety of environmental factors, seasonal variations, and circadian rhythms.28 In the present study, we have demonstrated that dynamic monitoring of NHR has the potential to be not only a reliable predictor of cardiovascular events in patients with heart failure but could also pave the way towards near-term prevention of cardiovascular events. Underlying mechanisms for heart rate increase before the event remain to be fully elucidated and compensatory tachycardia in response to volume overload could be one of the possible mechanisms. Nonetheless, the important point is that the NHR rise preceded major events such as hospitalization or death by a time period which appears reasonably sufficient for timely clinical intervention. One can imagine that in the future, combining clinical characteristics and remote monitoring will allow to identify specific groups at risk of coronary event or heart failure acutization or maybe sudden cardiac arrest. Therefore, we will be able to pre-empt these events and take specific measures.
Limitations
In this study, we have presented novel findings that may help refine use of heart rate as a marker to eventually improve short-term prediction and survival in heart failure populations; however, we need to acknowledge some limitations. First, this work should be viewed as a proof-of-concept study, as heart rate was collected using a WCD with limited follow-up, and may be not applicable to the entire heart failure population. However, as already mentioned, similar information is obtainable for other implanted devices and wearable sensors; as a result, this approach can be further tested and expanded in the future. Our study population consisted mainly in patients with ischaemic cardiomyopathy, so caution has to be exercised in extrapolating results to other causes of heart failure. Moreover, a high proportion of the patients were on beta-blockers; whether the magnitude of risk associations would be different in patients not on heart rate modulating drugs warrants further evaluation. Finally, further work is needed to confirm the associations reported in this study as well as the effectiveness of a strategy based on NHR monitoring to reduce hospitalization and mortality.
Conclusions
In patients with WCD, continuous dynamic monitoring of NHR helps to predict adverse cardiovascular events. It holds promise as a means of improving risk prediction prior to timely pre-emptive action, enabling reduction of adverse outcomes in populations at risk.
Supplementary Material
Acknowledgements
We deeply thank all the investigators of the WEARIT-France Study: Laurence Guedon Moreau, Mohanad Mahfoud, Philippe Ritter, Jean Marc Dupuis, Arab Yalioua, Antoine Dompnier, Marc Goralski, Saïda Cheggour, Christophe Leclercq, Eloi Marijon, Jérôme Bouet, Clémentine André, Fabrice Extramiana, Florent Briand, Anne Rollin, Gilles Lande, Philippe Chevalier, Jean-Luc Pasquié, Nicolas Lellouche, Bruno Degand, Yves Cottin, François Jourda, Jacques Mansourati, Angeline Martin, Antoine Da Costa, Olivier Billon, Isabelle Cheradame, Jean-Sylvain Hermida, Julien Bayard, Romain Eschalier, Benoit Guy Moyat, Vladimir Manenti, Hervé Gorka, Nicolas Combes, Antoine Milhem, Olivier Piot, Yves Guyomar, Renaud Fouché, Elisabeth Somody Litoux, Anne Quentin, Jean Baptiste Berneau, Thierry Tibi, Aurélien Miralles, Fiorella Salerno, Peggy Jacon, Laurentin Nitu, Damien Brunet, Hugues Bader, Arnaud Savoure, Pascal Sagnol, Frédéric Sebag, Sebastien Buffler, Joël Fedida, Julien Pineau, Guillaume De Geeter, Adlane Zemmoura, Ahmed Salhi, Eric Verbrugge, Sophie Pynn, Jean Pierre Gueffet, Walid Amara, Aurélien Seemann, Pierre Winum, Nicolas Johnson, Claire Vanesson, Alexis Mechulan, Vincent Hugon, Xavier Marchand, Jean-Claude Deharo, Sylvain Reuter, Raphael Sandras, Damien Legalloi, Olivier Garrier, Gaël Jauvert, Jérémy Descoux, Hugues Blangy, Alain Lebon, Pierre Sultan, Franck Sibellas, Samer Mousi, Gabriel Laurent, Maxime Pons, Halim Marzak, Jérôme Clerc, Hassan Barake, Aurélie Guiot, Vincent Algalarrondo, Laurent Palud, Denis Raguin, Frédéric Treguer, Pierre Le Franc, Isabelle Lecardonnel, Frederic Fossati, Ghassan Moubarak, Omar Bilel Mokrani, Pascal Chavernac. We gratefully thank Nobutaka Murata and Nicole Bianco of ZOLL for providing the technical assistance for data analysis.
Contributor Information
Rodrigue Garcia, Department of Cardiology, University Hospital of Poitiers, 86021 Poitiers, France; Centre d'Investigation Clinique CIC1402, CHU Poitiers, 86000, Poitiers, France.
Peder Emil Warming, Department of Cardiology, Copenhagen University Hospital, Rigshospitalet, Denmark.
Kumar Narayanan, Department of Cardiology, Medicover Hospitals, Hyderabad, Telangana 500081, India; Université Paris Cité, Inserm, PARCC, F-75015 Paris, France.
Pascal Defaye, Department of Cardiology, University Hospital Grenoble Alpes, Grenoble 38043, France.
Laurence Guedon-Moreau, Heart and Lung Institute, University Hospital of Lille, Lille 59000, France.
Hugues Blangy, Department of Cardiology, University Hospital of Nancy, Vandoeuvre-Lès-Nancy 54500, France.
Olivier Piot, Department of Cardiology, Cardiology Center of Nord, Saint Denis 93200, France.
Christophe Leclercq, Department of Cardiology, University Hospital Pontchaillou, Rennes 35000, France.
Eloi Marijon, Department of Cardiology, European Georges Pompidou Hospital, Paris Cedex 15, 75908, France; Université Paris Cité, Inserm, PARCC, F-75015 Paris, France.
the WEARIT-France Investigators:
Laurence Guedon Moreau, Mohanad Mahfoud, Philippe Ritter, Jean Marc Dupuis, Arab Yalioua, Antoine Dompnier, Marc Goralski, Saïda Cheggour, Christophe Leclercq, Eloi Marijon, Jérôme Bouet, Clémentine André, Fabrice Extramiana, Florent Briand, Anne Rollin, Gilles Lande, Philippe Chevalier, Jean-Luc Pasquié, Nicolas Lellouche, Bruno Degand, Yves Cottin, François Jourda, Jacques Mansourati, Angeline Martin, Antoine Da Costa, Olivier Billon, Isabelle Cheradame, Jean-Sylvain Hermida, Julien Bayard, Romain Eschalier, Benoit Guy Moyat, Vladimir Manenti, Hervé Gorka, Nicolas Combes, Antoine Milhem, Olivier Piot, Yves Guyomar, Renaud Fouché, Elisabeth Somody Litoux, Anne Quentin, Jean Baptiste Berneau, Thierry Tibi, Aurélien Miralles, Fiorella Salerno, Peggy Jacon, Laurentin Nitu, Damien Brunet, Hugues Bader, Arnaud Savoure, Pascal Sagnol, Frédéric Sebag, Sebastien Buffler, Joël Fedida, Julien Pineau, Guillaume De Geeter, Adlane Zemmoura, Ahmed Salhi, Eric Verbrugge, Sophie Pynn, Jean Pierre Gueffet, Walid Amara, Aurélien Seemann, Pierre Winum, Nicolas Johnson, Claire Vanesson, Alexis Mechulan, Vincent Hugon, Xavier Marchand, Jean-Claude Deharo, Sylvain Reuter, Raphael Sandras, Damien Legalloi, Olivier Garrier, Gaël Jauvert, Jérémy Descoux, Hugues Blangy, Alain Lebon, Pierre Sultan, Franck Sibellas, Samer Mousi, Gabriel Laurent, Maxime Pons, Halim Marzak, Jérôme Clerc, Hassan Barake, Aurélie Guiot, Vincent Algalarrondo, Laurent Palud, Denis Raguin, Frédéric Treguer, Pierre Le Franc, Isabelle Lecardonnel, Frederic Fossati, Ghassan Moubarak, Omar Bilel Mokrani, and Pascal Chavernac
Supplementary material
Supplementary material is available at Europace online.
Funding
This work was supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and Zoll Medical Corporation.
Data availability
The data underlying this article will be shared on reasonable request to ZOLL.
References
- 1. Fox K, Ford I, Steg PG, Tendera M, Robertson M, Ferrari Ret al. Heart rate as a prognostic risk factor in patients with coronary artery disease and left-ventricular systolic dysfunction (BEAUTIFUL): a subgroup analysis of a randomised controlled trial. Lancet 2008;372:817–21. [DOI] [PubMed] [Google Scholar]
- 2. Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand Jet al. Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol 2017;69:2885–96. [DOI] [PubMed] [Google Scholar]
- 3. Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 2009;296:H847–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vazir A, Claggett B, Jhund P, Castagno D, Skali H, Yusuf Set al. Prognostic importance of temporal changes in resting heart rate in heart failure patients: an analysis of the CHARM program. Eur Heart J 2015;36:669–75. [DOI] [PubMed] [Google Scholar]
- 5. Garcia R, Combes N, Defaye P, Narayanan K, Guedon-Moreau L, Boveda Set al. Wearable cardioverter-defibrillator in patients with a transient risk of sudden cardiac death: the WEARIT-France cohort study. Europace 2021;23:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kutyifa V, Moss AJ, Klein H, Biton Y, McNitt S, MacKecknie Bet al. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation 2015;132:1613–9. [DOI] [PubMed] [Google Scholar]
- 7. Wäßnig NK, Günther M, Quick S, Pfluecke C, Rottstädt F, Szymkiewicz SJet al. Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation 2016;134:635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung MK, Szymkiewicz SJ, Shao M, Zishiri E, Niebauer MJ, Lindsay BDet al. Aggregate national experience with the wearable cardioverter-defibrillator: event rates, compliance, and survival. J Am Coll Cardiol 2010;56:194–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mueller-Leisse J, Brunn J, Zormpas C, Hohmann S, Hillmann HAK, Eiringhaus Jet al. Extended follow-up after wearable cardioverter-defibrillator period: the PROLONG-II study. ESC Heart Fail 2021;8:5142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm Met al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–726. [DOI] [PubMed] [Google Scholar]
- 11. Zeppenfeld K, Tfelt-Hansen J, de Riva M, Winkel BG, Behr ER, Blom NAet al. 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 2022;43:3997–4126. [DOI] [PubMed] [Google Scholar]
- 12. Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJet al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805–35. [DOI] [PubMed] [Google Scholar]
- 13. Vazir A, Claggett B, Pitt B, Anand I, Sweitzer N, Fang Jet al. Prognostic importance of temporal changes in resting heart rate in heart failure and preserved ejection fraction: from the TOPCAT study. JACC Heart Fail 2017;5:782–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 1999;20:145–57. [DOI] [PubMed] [Google Scholar]
- 15. Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KLet al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107:2932–7. [DOI] [PubMed] [Google Scholar]
- 16. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O’Connor CM, She Let al. Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA 2006;296:2217–26. [DOI] [PubMed] [Google Scholar]
- 17. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama Aet al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–85. [DOI] [PubMed] [Google Scholar]
- 18. Messerli FH, Hofstetter L, Rimoldi SF, Rexhaj E, Bangalore S. Risk factor variability and cardiovascular outcome: JACC review topic of the week. J Am Coll Cardiol 2019;73:2596–603. [DOI] [PubMed] [Google Scholar]
- 19. Gibson CM, Holmes D, Mikdadi G, Presser D, Wohns D, Yee MKet al. Implantable cardiac alert system for early recognition of ST-segment elevation myocardial infarction. J Am Coll Cardiol 2019;73:1919–27. [DOI] [PubMed] [Google Scholar]
- 20. Lee SP, Ha G, Wright DE, Ma Y, Sen-Gupta E, Haubrich NRet al. Highly flexible, wearable, and disposable cardiac biosensors for remote and ambulatory monitoring. NPJ Digital Medicine 2018;1:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hillmann HAK, Hohmann S, Mueller-Leisse J, Zormpas C, Eiringhaus J, Bauersachs Jet al. Feasibility and first results of heart failure monitoring using the wearable cardioverter-defibrillator in newly diagnosed heart failure with reduced ejection fraction. Sensors (Basel) 2021;21:7798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vazir A, Claggett B, Cheng S, Skali H, Shah A, Agulair Det al. Association of resting heart rate and temporal changes in heart rate with outcomes in participants of the atherosclerosis risk in communities study. JAMA Cardiol 2018;3:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McConnell MV, Turakhia MP, Harrington RA, King AC, Ashley EA. Mobile health advances in physical activity, fitness, and atrial fibrillation: moving hearts. J Am Coll Cardiol 2018;71:2691–701. [DOI] [PubMed] [Google Scholar]
- 24. Narayan SM, Wang PJ, Daubert JP. New concepts in sudden cardiac arrest to address an intractable epidemic: JACC state-of-the-art review. J Am Coll Cardiol 2019;73:70–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nielsen JC, Dagres N. How can we assess the risk for sudden cardiac death to decide for primary prophylactic implantable cardioverter-defibrillator in patients with heart failure in 2022? Europace 2022;24:1199–200. [DOI] [PubMed] [Google Scholar]
- 26. Kahle A-K, Jungen C, Alken F-A, Scherschel K, Willems S, Pürerfellner Het al. Management of ventricular tachycardia in patients with ischaemic cardiomyopathy: contemporary armamentarium. Europace 2022;24:538–51. [DOI] [PubMed] [Google Scholar]
- 27. Marijon E, Garcia R, Narayanan K, Karam N, Jouven X. Fighting against sudden cardiac death: need for a paradigm shift-adding near-term prevention and pre-emptive action to long-term prevention. Eur Heart J 2022;43:1457–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arntz HR, Willich SN, Schreiber C, Brüggemann T, Stern R, Schultheiss HP. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. Eur Heart J 2000;21:315–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to ZOLL.