Abstract
Aims
The SMART Pass™ (SP) algorithm is a high-pass filter that aims to reduce inappropriate therapy (IT) in subcutaneous internal cardiac defibrillator (S-ICD), but SP can deactivate due to low amplitude sensed R waves or asystole. The association between IT and SP deactivation and management strategies were evaluated, hypothesizing SP deactivation increases the risk of IT and device re-programming, or lead/generator re-positioning could reduce this risk.
Methods and results
Retrospective single-centre audit of Emblem™ S-ICD devices implanted 2016 to 2020 utilizing health records and remote monitoring data. Cox regression models evaluated associations between SP deactivation and IT. A total of 348 patients (27 ± 16.6 months follow-up) were studied: 73% primary prevention. Thirty-eight patients (11.8%) received 83 shocks with 27 patients (7.8%) receiving a total of 44 IT. Causes of IT were oversensing (98%) and aberrantly conducted atrial fibrillation (2%). SP deactivation occurred in 32 of 348 patients (9%) and was significantly associated with increased risk of IT (hazard ratio 5.36, 95% CI 2.37-12.13). SP deactivation was due to low amplitude R waves (94%), associated with a higher defibrillation threshold at implant and presence of arrhythmogenic right ventricular cardiomyopathy. No further IT occurred 16 ± 15.5 months after corrective interventions, with changing the sensing vector being successful in 59% of cases.
Conclusion
To reduce the risk of IT, the cause of the SP deactivation should be investigated, and appropriate reprogramming, device, or lead modifications made. Utilizing the alert for SP deactivation and electrograms could pro-actively prevent IT.
Keywords: Subcutaneous ICD, S-ICD, Oversensing, TWOS, Defibrillation, Programming, Inappropriate shocks
What’s new?
SMART Pass™ (SP) algorithm deactivation has a hazard ratio of 5.36 (95% CI 2.37-12.13) associated with inappropriate subcutaneous implantable cardioverter defibrillator (S-ICD) therapy.
SP algorithm deactivation is more likely to occur in patients with higher defibrillation threshold at implant, patients with arrhythmogenic right ventricular cardiomyopathy (ARVC) and devices programmed with S-ECG gain “X2”.
Active management after inappropriate therapy (IT) by reprogramming the sensing vector is the most successful approach or intervention for preventing further IT.
Timely management of SP deactivation could significantly reduce IT in S-ICD patients with a pro-active alert of automatic deactivation and application of a systematic management strategy.
Introduction
The subcutaneous implantable cardioverter defibrillator (S-ICD) is being increasingly utilized in everyday practice for prevention of sudden cardiac death. S-ICD implantation is now recommended in the Heart Rhythm Society (HRS) and the European Society of Cardiology (ESC) guidelines and following the Prospective, Randomized Comparison of Subcutaneous and Transvenous Implantable Cardioverter-Defibrillator Therapy (PRAETORIAN trial).1-4 A concern remains regarding the burden of inappropriate therapy (IT), which affected 9.6% of patients over a total 4-year follow-up period in the PRAETORIAN randomized controlled trial, and in the most recent update of the evaluated impacting clinical outcome and cost effectiveness of the S-ICD registry (EFFORTLESS), IT burden increased to 16.9% at 5 years.5 Importantly, both PRAETORIAN and EFFORTLESS were conducted using older-generation devices without the SMART Pass™ (SP) algorithm installed. SP (Boston Scientific Corporation, Natick, MA) is a high-pass filter that has been shown to reduce IT in simulations, single-centre studies, and multi-centre registries.6-11 Indeed, in the UNTOUCHED study where SP was enabled, SP reduced IT to 3.1% over 1 year predominantly through reduction in T wave oversensing (TWOS).12 However, a key under-investigated feature is the algorithm’s ability to automatically deactivate itself, and the device is unable to reactivate this setting without in-clinic device interrogation. We aimed to assess: (i) the clinical factors associated with SP deactivation; (ii) why the device deactivates SP; and (iii) how to manage this scenario in a large, single-centre study. We hypothesized that the SP algorithm’s automatic deactivation would increase the risk of IT, and this could provide an early warning to enable pro-active IT prevention.
SMART Pass
The SP algorithm is a high-pass filter designed to reduce cardiac signal oversensing.10 It is a first-order high-pass filter with a corner frequency between 8 and 9 Hz and a roll-off rate of 20 db/decade. This allows for gradual reduction in lower frequency, therefore preserving signals at higher frequencies (>10 Hz). T waves are usually at a frequency of <9 Hz, so the algorithm increases the R:T ratio. The SP algorithm can disable itself in the instance of a small amplitude QRS complex (<0.25 mV) with two long intervals >1.4 s or due to periods of asystole (>10 s). SP can only reactivate with a face-to-face device interrogation with a device programmer and an automatic or manual setup conducted. The reason the S-ICD deactivates SP is to prevent undersensing of ventricular arrhythmias which could be ongoing at the time of the SP deactivation. Inadvertently, the SP algorithm acts as a ‘small R wave’ and ‘pause’ alert within the S-ICD device. When deactivated due to small R waves, oversensing is significantly more likely due to the removal of T wave suppression, therefore decreasing in the R:T ratio and ‘double counting’ of the R and T waves. At the time of SP deactivation, the device records a standard surface electrocardiogram (S-ECG) that is transmitted to the LATITUDE™ remote monitoring system. This is, at time of writing, is not commercially available for clinicians. It is only available to technical services from the manufacturer.
Methods
Study design
A retrospective audit of all Emblem S-ICD devices (A209 and A219) implanted from 2016 to 2020 was completed utilizing information gained from electronic pacing and health records and the LATITUDE™ remote monitoring system. Baseline clinical characteristics, S-ICD programming parameters, and SP status were recorded at implant and throughout follow-up. Shock impedance was assessed from the implant defibrillation threshold test (DFT). Follow-up data were collected from emergency and scheduled appointments plus remote monitoring follow-up. A locally developed S-ICD programming protocol was used for all devices, with a conditional zone set at 200 b.p.m. and a non-conditional zone at 250 b.p.m. Electrograms were reviewed independently by two International Board of Heart Rhythm Examiners (IBHRE)–accredited cardiac scientists to confirm accurate analysis, and any debatable recordings were reviewed by a third independent expert for final decision. Events were classified by different causes of inappropriate shocks and oversensing episodes including supraventricular tachycardias; P, R, and T wave oversensing; myopotentials; air oversensing; and baseline wander. SP deactivation recordings were obtained retrospectively from the LATITUDE™ remote monitoring system. Patients transferred to other centres or lost to follow-up were included up to their last device interrogation. The audit was registered with an internal clinical effectiveness unit for ethical approval prior to data collection.
Statistical methods
Continuous variables are presented as mean ± SD. Categorical data are shown as number (%). Differences between patients who had SP deactivated and the rest were assessed using Wilcoxon rank sum test and Fisher exact test for continuous and binary variables, respectively. The rhythm triggering the first inappropriate shock was used for calculating incidence of IT because changes were commonly made after the first IT. SP was evaluated using the status at the time of implant, date of deactivation during follow-up, and at time of the episode, either ON or OFF. The association between SP status (ON or OFF) and first IT was assessed using proportional hazard regression (Cox) models, with time to event starting at the date of implant. Uni-variable Cox regression analysis was conducted for 16 variables that have been previously associated with a higher rate of IT including age, aetiology, LV function, S-ECG gain, programmed vector, QRS duration, programming zones, and shock impedance at DFT. A multi-variable Cox analysis was performed on significant uni-variable variables. A P value of <0.05 was considered statistically significant. Analyses were conducted using MATLAB 2019b and R 4.2.
Results
The study cohort consisted of 348 patients with a follow-up duration of 27 ± 16.6 months. The mean age of patients was 45.7 ± 13.6 years with a mean QRS duration of 96 ± 15.9 ms. DFT shock impedance was recorded in 241 patients with a mean of 73 ± 20 ohms. Study cohort patient demographics are shown in Table 1. Thirty-eight (11.8%) of patients received total of 83 shocks. Twenty-seven (7.8%) patients received IT, with a total of 44 inappropriate shocks. Aberrantly conducted atrial fibrillation (AF) caused one inappropriate shock; the remaining 43 were due to oversensing. A breakdown of oversensing causes is seen in Supplementary material online, Figure S1. No patient had a ventricular arrhythmia undersensing with the SP filter being ON or OFF.
Table 1.
Patient cohort demographics
| Patient characteristics | n = 348 |
|---|---|
| Mean age, year | 45.7 ± 13.6 |
| Women, n (%) | 109 (31.3) |
| Ischaemic heart disease, n (%) | 67 (19.3) |
| Previous valve surgery, n (%) | 2 (0.57) |
| History of AF, n (%) | 12 (3.4) |
| Normal EF >55%, n (%) | 219 (62.9) |
| Moderate EF 36–55%, n (%) | 46 (13.2) |
| Poor EF <35%, n (%) | 83 (23.9) |
| NYHA class II/III, n (%) | 148 (42.5) |
| Mean QRS duration, ms | 96 ± 15.9 |
| Primary prevention, n (%) | 250 (71.8) |
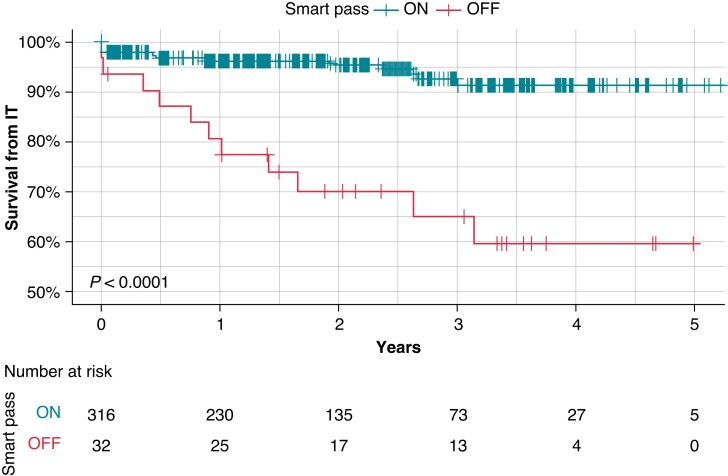
Patients with SP ON during the entire study (n=316) and those who experienced a SP deactivation (n=32) is shown in Table 2, which describes the clinical parameters associated with SP deactivation. SP deactivation was more common in ARVC (6.6% ON vs. 21.9% OFF, P < 0.005); a device with programmed S-ECG gain X2 (programmable X1 or X2) (2.5% ON vs. 21.9% OFF P ≤ 0.0001); and higher DFT impedance (87, 70–98 vs. 68, 59–81, median interquartile range P ≤ 0.001). During follow-up, the incidence of appropriate therapy was similar across the two SP status groups (4.2% vs. 6.2%, P = 0.57) but the incidence of IT was greater in patients with SP OFF than in patients with SP ON (34.4% vs. 5.1%, P < 0.001). IT occurred in 34.4% of patients with the average time to SP deactivation from implant was 15 ± 13 months and the average time from SP deactivation to IT was 4.6 ± 6 months. From the time of SP deactivation, patients whose SP was turned OFF were at a significantly higher risk of IT than patients whose SP was constantly ON (Figure 1).
Table 2.
Clinical and programming variables comparing SMART Pass™ on and off
| All (n = 348) | SP ON (n = 316) | SP OFF (n = 32) | P | |
|---|---|---|---|---|
| Age | 47.0 (35.0–55.0) | 53.0 (37.0–59.5) | 46.0 (35.0–54.0) | 0.079 |
| Male | 239 (68.7%) | 221 (69.9%) | 18 (56.2%) | 0.11 |
| Previous atrial fibrillation | 12 (3.4%) | 10 (3.2%) | 2 (6.2%) | 0.36 |
| Hypertrophic cardiomyopathy | 108 (31.0%) | 102 (32.3%) | 6 (18.8%) | 0.11 |
| Ischaemic heart disease | 67 (19.3%) | 60 (19.0%) | 7 (21.9%) | 0.69 |
| Dilated cardiomyopathy | 56 (16.1%) | 52 (16.5%) | 4 (12.5%) | 0.56 |
| Arrhythmogenic cardiomyopathy | 28 (8.0%) | 21 (6.6%) | 7 (21.9%) | <0.005 |
| Left ventricular ejection fraction <35% | 83 (27.5%) | 75 (27.6%) | 8 (26.7%) | 0.92 |
| Secondary prevention | 98 (28.2%) | 90 (28.5%) | 8 (25.0%) | 0.68 |
| S-ECG x2 gain | 15 (4.3%) | 8 (2.5%) | 7 (21.9%) | ≤ 0.0001 |
| Primary vector | 178 (51.1%) | 164 (51.9%) | 14 (43.8%) | 0.38 |
| Secondary vector | 128 (36.8%) | 117 (37.0%) | 11 (34.4%) | 0.77 |
| Alternate vector | 42 (12.1%) | 35 (11.1%) | 7 (21.9%) | 0.074 |
| QRS (ms) | 96 (86–100) | 98 (90–104) | 95 (85–100) | 0.16 |
| Conditional zone (b.p.m.) | 200 (200–220) | 200 (200–220) | 200 (200–220) | 0.75 |
| Non-conditional zone (b.p.m.) | 70 (61–81) | 87 (70–98) | 68 (59–81) | 0.4 |
| DFT shock impedance (ohms) | 250 (230–250) | 250 (230–250) | 250 (230–250) | <0.001 |
| Appropriate therapy | 15 (4.3%) | 13 (4.1%) | 2 (6.2%) | 0.57 |
| Inappropriate therapy | 27 (7.8%) | 16 (5.1%) | 11 (34.4%) | ≤ 0.0001 |
Figure 1.
Kaplan–Meier survival curve for inappropriate therapy with SMART Pass™ ON and SMART Pass™ OFF. Timelines start from date of implant.
Uni-variate and multi-variate predictors of SMART Pass™ deactivation
Sixteen different variables were assessed individually in a uni-variate analysis (see Supplementary material online, Figure S2). Only two were significantly associated with increased risk of IT: SP deactivation and S-ECG gain programmed X2. SP deactivation has a hazard ratio for IT of 6.05 (95% CI: 2.8 to 13.09) while the hazard ratio for S-ECG gain X2 was 3.83 (95% CI: 1.32 to 11.07). In a multi-variate analysis, SP deactivation remained significantly associated with IT, with a hazard rate of 5.36 (CI: 2.36 to 12.13, P < 0.001), whereas gain X2 was no longer significantly associated with IT, with a hazard ratio of 1.86 (CI: 0.60 to 5.74, P = 0.28).
Reasons for automatic SMART Pass deactivation
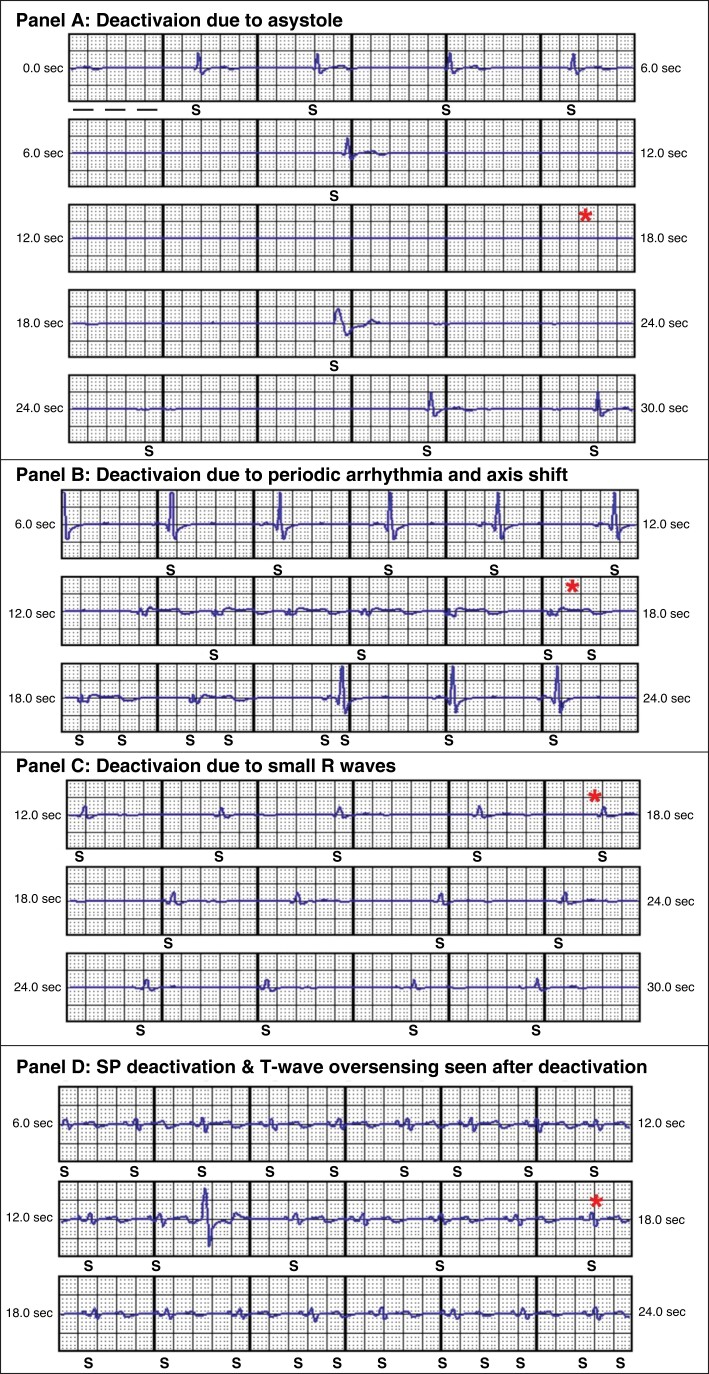
During follow-up, 32 patients had SP disabled. Only one of these patients had SP disabled at implant, due to poor R wave amplitude later receiving IT due to air in pocket. For two patients, SP was unavailable as they received shocks prior to SP software instillation. SP deactivation recordings were available for 17 of the 30 patients from LATITUDE™ remote monitoring. We categorized these deactivations into three groups: small R waves, arrhythmia with periodic axis shift, and sustained pause. Examples of these SP deactivation S-ECG recordings are shown in Figure 2. In one patient, shown in Panel A, SP disabled due to a 10 s sinus pause who was subsequently upgraded to a transvenous device. The remaining SP deactivations were due to low amplitude R waves (94%) with 11 (65%) patients had SP disabled due to a drop in R wave amplitude or QRS change, which could be caused by positional or transient morphology changes with periods of bradycardia. Periodic arrhythmias with QRS axis shift caused five deactivations (29%) with a variety of arrhythmias: non-sustained ventricular tachyarrhythmias, idioventricular rhythms, or aberrant supra-ventricular arrhythmias. No patient symptoms correlated with change of ECG morphology to warn them of a possible change in device sensing.
Figure 2.
SMART Pass™ deactivation recordings. * Highlights point at which SP deactivates.
Management changes to reduce further events
The interventions made to devices after IT to prevent future events were reviewed; the mean follow-up time after these interventions was 15.7 ± 16.4 months. Table 3 summarizes the programming, hardware, or drug changes made. Changing the sensing vector resolved sensing issues in 16 patients (59%) with or without SP disablement. Lead re-positioning was performed in two patients, one had macro lead displacement due to a reel-mechanism, and one was re-positioned to the right side of the sternum after successful screening for alternative vectors. For two patients with IT due to air in the sensing circuit, temporary deactivation for 1 week was successful without changing the sensing vector. One patient with IT due to rapidly conducted atrial fibrillation and aberrancy was treated with medical management (addition of beta-blocker) to prevent further IT. All changes resulted in continuous SP enablement and were successful at preventing further IT.
Table 3.
Management changes for patients after inappropriate therapy and implications on further events (SMART Pass™ = SP), total 27 patients
| Management action | SP enabled at time of IT (n = 16) | SP disabled at time of IT (n = 11) | Further IT after change (16 ± 15.5 months) |
|---|---|---|---|
| Change in sensing vector | 10 | 6 | 0 |
| Increase device detection rate (conditional zone from 200 to 220 b.p.m.) | 1 | 0 | 0 |
| Lead re-positioning | 1 | 1 | 0 |
| Temporary deactivation (1 week) | 0 | 2 | 0 |
| No changes | 3 | 0 | 2 |
| Reactivate SP and increase gain | 0 | 1 | 0 |
| Medical management (beta-blockers) | 1 | 0 | 0 |
| Transvenous device implant | 0 | 1 | 0 |
Discussion
This study investigates the S-ICD automatic deactivation of SP algorithm and its association with IT. The main findings of the study were that SP was associated with a higher burden of IT, with 34.4% patients after a follow-up duration of 15 ± 13 months receiving IT, and a hazard ratio of 5.36, which is substantially higher than what reported in previous studies.9,10 This risk of IT was also reported in a recent analysis of the Understanding Outcomes With the S-ICD in Primary Prevention Patients With Low Ejection Fraction, UNTOUCHED study, with intermittent SP deactivation causing IT in 10.3% of cases over 549 days post-implant.13
To understand the greater significance of this automatic SP deactivation, the causes need to be fully delineated as if acted upon early, this could prevent IT.
The importance of the SP in reducing IT is now well-recognized from several studies as our cohort illustrates. Theuns et al. retrospectively reviewed a large, multi-centre cohort of patients with S-ICDs from the LATITUDE™ remote monitoring system and found a reduction of IT by 68%, with no impact on appropriate therapies.10 An important difference from our cohort is that this trial was conducted reviewing whether SP was enabled at implant, rather than for the episode itself. This potentially underestimates the importance of the algorithm as this disabling function could be an opportunity to identify and rectify the cause to reduce IT. Ninni et al. produced a multi-centre registry which had 63% of patients with the SP algorithm enabled, similarly this was recorded after implant.7 Despite this, SP was shown to be an independent predictor of IT with a hazard ratio of 3.3. Indeed, the most contemporary data from the UNTOUCHED primary prevention trial showed that generation 3 devices which had SP enabled at implant had IT rates of 2.9% [95% upper confidence limit (UCL), 4.4%] compared to 6.0% (UCL, 8.5%) for generation 2 devices.11
SMART Pass recordings
We were able to review 53% (17/32) of the SP deactivation recordings due to some devices having manual interrogation and not presenting through LATITUDE™ remote monitoring. The most common cause for SP deactivation was small amplitude R waves and undersensing (65%), which is likely due to patient position or a combination of chronically small R waves and sustained bradycardia, causing the >1.4 s interval duration as seen in Figure 2 (panel A).13 We were unable to find any link between the cause for deactivation and occurrence of IT; however, we hypothesize that the observation of isolated bradycardias and pauses (>10 s) is unlikely to correlate with IT as the R wave amplitude is unlikely to be compromised. The observation of TWOS immediately after the SP deactivation (Figure 2 panel D) episode occurred in patients who went on to receive IT should be noted as this could indicate that the appearance of TWOS after SP being switched off may be the most significant predictor of IT. This should be formally assessed in a larger cohort.
Therefore, SP deactivation is predominantly a marker of R wave size and/or variability, which can change over time. This is likely to be the reason for more arrhythmogenic right ventricular cardiomyopathy (ARVC) patients being in the SP deactivation arm, as the disease progression could cause R wave amplitude changes. Patients with SP deactivated were more likely to have S-ECG programmed gain 2 × on the device, as clinicians would increase the gain to view the electrogram more clearly. Furthermore, SP-deactivation patients were also more likely to have a higher DFT shock impedance, with a median 19 ohms higher than the average 87 ohms. This suggests that both implant position and shock impedance are related to R wave amplitude size, which warrants further investigation in larger cohorts to evaluate not only positioning but the amount of subcutaneous tissue beneath the lead/generator. These are easily rectifiable factors to optimize R wave sensing at implant.
At the end of 2022, an alert for the deactivation for SP on remote monitoring was released, and SP recordings made available to clinicians, both remotely and on the device programmer. The average time for SP to deactivate was at 15 ± 13 months post-implant, which suggests that this is unlikely to be predicted at implant. This alert could prove to be imperative as the mean time from SP deactivation to IT was 142 ± 184 days, providing ample time to reduce the risk of IT. With SP deactivation, the question remains, what should be done about it?
Proposed management strategy for automatic SMART Pass deactivation
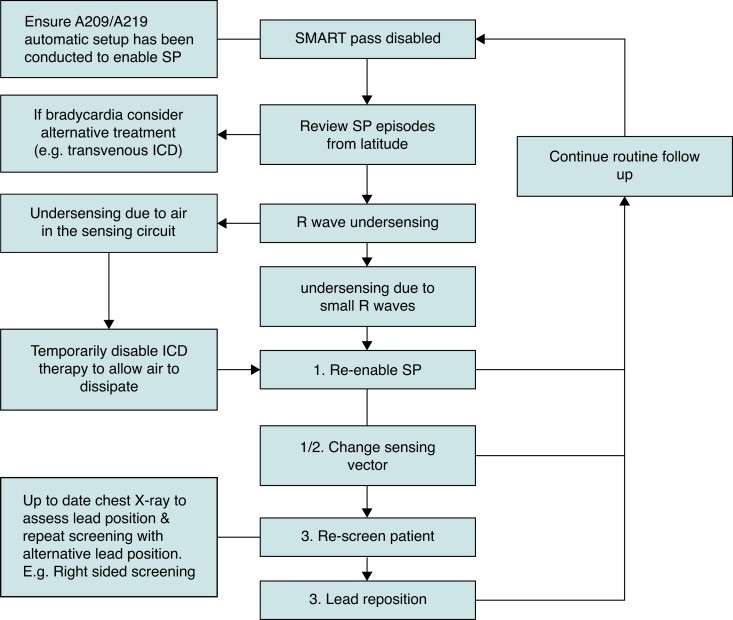
We have developed a flow chart of how to troubleshoot SP disablement, to aid implanting centres to reduce IT in their S-ICD patients (Figure 3). The first action when SP is disabled should be to assess the SP deactivation recording to determine the cause of deactivation. This is available on the Latitude™ remote monitoring platform and updated device programmers. If bradyarrhythmia is the cause, symptoms should be assessed, and suitability of S-ICD reviewed by the clinician. For arrhythmia with periodic changes in morphology, there is an option to re-enable SP in the same vector in clinic but this may not prevent further deactivations if future arrhythmias occur. If SP has disabled or inappropriate shocks have occurred due to air in the sensing circuit, temporary deactivation of the device is already known to be successful in preventing further events in our cohorts and in case reports—this gives time for the air to dissipate.14,15 If air in the sensing circuit is suspected, a review of the S-ECG is required as well as post-procedure chest radiograph.14,15 SP deactivation could also be caused by lead position changes over time; we previously reported a case of S-ICD lead macro-displacement presenting with SP deactivation and inappropriate AF detections.16 Comparisons of the S-ECG and all sense vectors can highlight lead migration with a chest radiograph to confirm this.
Figure 3.
Proposed management guide for SMART Pass™ disablement.
Preventing inappropriate therapy
From our cohort, the most successful solution to avoid future inappropriate shocks was to change the sensing vector (60%). The QRS amplitude and morphology can be different in each S-ICD sensing vector and depending on R:T wave amplitude ratio of the remaining sensing vectors, programming a different vector could solve the issue. This requires a manual interrogation with the device programmer which, with the recent update on the S-ICD auto setup, allows the clinician to determine which other vectors could be suitable and select a new vector if required. This raises the question as to what is best option if the patient has no alternative sensing vector? Or if, in the future, SP disables in the second vector? At this point, re-screening should be considered including sternal and right-sided lead positions, with a joint patient–clinician discussion regarding the risks and benefits of a lead re-positioning procedure. We believe the community should consider a lower threshold for lead re-positioning, since the risk of systemic infection or longer-term complications is significantly lower with maintenance of S-ICD implantation vs. conversion to a transvenous system.
Changes in the non-conditional and conditional rate zones were successful in one patient; however, with the limited evidence of high-rate programming with S-ICDs, this should be used with extreme caution to ensure undersensing of ventricular arrhythmias does not occur. Other programming solutions, such as gain increase, should be investigated further, particularly for devices with myopotential oversensing. Medical management for arrhythmia that causes deactivation of SP could also be considered at the discretion of the follow-up physician.
Study limitations
Our study has some limitations. Firstly, since this is a retrospective audit, the management plan is formulated using the strategies that were used to troubleshoot individual cases which have not been assessed prospectively. Furthermore, the nature of a single-centre study is prone to selection biases and programming settings may not be generalized for other settings. Due to the fact there is no pro-active alert for SP deactivation, this was only noted after events had occurred, hence not allowing for optimal pre-emptive actions to address these sensing issues. Further assessment of risk factors of individual characteristics should be investigated to improve statistical power.
Conclusion
SP deactivation is a significant predictor of inappropriate shocks. If the SP filter is deactivated, this is likely to suggest low amplitude R waves, either periodically or continuously. To reduce the risk of inappropriate shocks, the cause of the automatic SP deactivation should be investigated, and sensing vector changes should be strongly considered. If the SP algorithm is unable to sustain activation, lead re-positioning should be considered, akin to a transvenous right ventricular ICD lead for poor sensing. With the recent addition of a remote monitoring yellow alert for SP deactivation, this could show to be significantly beneficial in reducing the rate of S-ICD IT, warranting prospective investigation.
Supplementary Material
Contributor Information
Christopher Monkhouse, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Amy Wharmby, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Zoe Carter, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Ross Hunter, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Mehul Dhinoja, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Anthony Chow, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Antonio Creta, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Shohreh Honarbakhsh, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Syed Ahsan, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK.
Michele Orini, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK; Institute of Cardiovascular Science, University College London (UCL), 62 Huntley Street, London EC1A 7BE, UK.
Pier D Lambiase, Barts Heart Centre, West Smithfield, EC1A 7BE, London, UK; Institute of Cardiovascular Science, University College London (UCL), 62 Huntley Street, London EC1A 7BE, UK.
Supplementary material
Supplementary material is available at Europace online.
Funding
None declared.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis ABet al. . 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation 2018;138:e272–391. [DOI] [PubMed] [Google Scholar]
- 2. Knops RE, Olde Nordkamp LRA, Delnoy PPHM, Boersma LVA, Kuschyk J, El-Chami MFet al. . Subcutaneous or transvenous defibrillator therapy. N Engl J Med 2020;383:526–36. [DOI] [PubMed] [Google Scholar]
- 3. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm Jet al. . 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015;36:2793–867. [DOI] [PubMed] [Google Scholar]
- 4. Lambiase PD, Theuns DA, Murgatroyd F, Barr C, Eckardt L, Neuzil Pet al. . Subcutaneous implantable cardioverter-defibrillators: long-term results of the EFFORTLESS study. Eur Heart J 2022;43:2037–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willy K, Reinke F, Rath B, Ellermann C, Wolfes J, Bögeholz Net al. . Pitfalls of the S-ICD therapy: experiences from a large tertiary centre. Clin Res Cardiol 2021;110:861–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ninni S, Echivard M, Marquié C, Ortmans S, Labreuche J, Drumez Eet al. . Predictors of subcutaneous implantable cardioverter-defibrillator shocks and prognostic impact in patients with structural heart disease. Can J Cardiol 2021;37:400–6. [DOI] [PubMed] [Google Scholar]
- 7. Gold MR, Weiss R, Theuns DAMJ, Smith W, Leon A, Knight BPet al. . Use of a discrimination algorithm to reduce inappropriate shocks with a subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2014;11:1352–8. [DOI] [PubMed] [Google Scholar]
- 8. Brisben AJ, Burke MC, Knight BP, Hahn SJ, Herrmann KL, Allavatam Vet al. . A new algorithm to reduce inappropriate therapy in the S-ICD system. J Cardiovasc Electrophysiol 2015;26:417–23. [DOI] [PubMed] [Google Scholar]
- 9. Theuns DAMJ, Brouwer TF, Jones PW, Allavatam V, Donnelley S, Auricchio Aet al. . Prospective blinded evaluation of a novel sensing methodology designed to reduce inappropriate shocks by the subcutaneous implantable cardioverter-defibrillator. Heart Rhythm 2018;15:1515–22. [DOI] [PubMed] [Google Scholar]
- 10. Gold MR, Lambiase PD, El-Chami MF, Knops RE, Aasbo JD, Bongiorni MGet al. . Primary results from the understanding outcomes with the S-ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation 2021;143:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karimianpour A, John L, Gold MR. The subcutaneous ICD: a review of the UNTOUCHED and PRAETORIAN trials. Arrhythm Electrophysiol Rev 2021;10:108–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boersma LVA, Aasbo J, Knops RE, Lambiase PD, Bongiorni MG, Deharo JCet al. . The impact of SMARTpass algorithm status on inappropriate shock rates in the UNTOUCHED study. Europace 2022;24:euac053.391. [Google Scholar]
- 13. Adams MG, Drew BJ. Body position effects on the ECG: implication for ischemia monitoring. J Electrocardiol 1997;30:285–91. [DOI] [PubMed] [Google Scholar]
- 14. Yang YC, Aung TT, Bailin SJ, Rhodes TE. Air entrapment causing inappropriate shock from a subcutaneous implantable cardioverter defibrillator. Cardiol Res 2019;10:128–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yap SC, Bhagwandien RE, Szili-Torok T, Theuns DAMJ. Air entrapment causing early inappropriate shocks in a patient with a subcutaneous cardioverter-defibrillator. HeartRhythm Case Rep Elsevier 2015;1:156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vyas S, Monkhouse C, Srinivasan N, Lambiase PD. Subcutaneous implantable cardioverter defibrillator lead displacement. Pacing Clin Electrophysiol 2021;44:723–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.