Abstract
Aims
Atrial fibrillation (AF) progression is associated with adverse outcome, but the role of the circadian or diurnal pattern of AF onset remains unclear. We aim to assess the association between the time of onset of AF episodes with the clinical phenotype and AF progression in patients with self-terminating AF.
Methods and results
The Reappraisal of AF: Interaction Between Hypercoagulability, Electrical Remodelling, and Vascular Destabilization in the Progression of AF study included patients with self-terminating AF who underwent extensive phenotyping at baseline and continuous rhythm monitoring with an implantable loop recorder (ILR). In this subanalysis, ILR data were used to assess the development of AF progression and the diurnal pattern of AF onset: predominant (>80%) nocturnal AF, predominant daytime AF, or mixed AF without a predominant diurnal AF pattern. The median follow-up was 2.2 (1.6–2.8) years. The median age was 66 (59–71) years, and 117 (42%) were women. Predominant nocturnal (n = 40) and daytime (n = 43) AF onset patients had less comorbidities compared to that of mixed (n = 195) AF patients (median 2 vs. 2 vs. 3, respectively, P = 0.012). Diabetes was more common in the mixed group (12% vs. 5% vs. 0%, respectively, P = 0.031), whilst obesity was more frequent in the nocturnal group (38% vs. 12% vs. 27%, respectively, P = 0.028). Progression rates in the nocturnal vs. daytime vs. mixed groups were 5% vs. 5% vs. 24%, respectively (P = 0.013 nocturnal vs. mixed and P = 0.008 daytime vs. mixed group, respectively).
Conclusion
In self-terminating AF, patients with either predominant nocturnal or daytime onset of AF episodes had less associated comorbidities and less AF progression compared to that of patients with mixed onset of AF.
Clinical trial registration
Keywords: Self-terminating atrial fibrillation, Rhythm monitoring, Atrial fibrillation progression, Time of onset
Graphical Abstract
Graphical Abstract.
What’s new?
We classified comprehensively phenotyped patients with self-terminating atrial fibrillation (AF) according to time of onset, based on their onset of AF being either nocturnal, daytime, or mixed, using long-term continuous rhythm monitoring.
We showed that patients with either only nocturnal or only daytime onset of AF had less associated comorbidities and less AF progression compared to that of patients with a mixed onset of AF.
Assessment of the timepoint of onset of AF episodes may guide therapies: patients without a clear circadian pattern of AF episodes may warrant more aggressive therapies as compared to that of those with predominant nocturnal or daytime onsets of AF episodes.
Furthermore, it is unknown whether various antiarrhythmic and rate–control drugs have differential effects, depending on the pattern of AF onset, which requires future investigations.
Introduction
Atrial fibrillation (AF) progression is associated with adverse outcome.1‐4 Predictors of AF progression include age, hypertension, heart failure, obesity, and possibly sex.5‐7 Some patients present with a typical circadian or diurnal pattern of AF episodes. Atrial fibrillation can either start predominantly during night-time (nocturnal AF) or predominantly during activities and stress (adrenergically or daytime AF).8,9 In clinical practice, the definite onset of AF is often difficult to assess because not all episodes are symptomatic and continuous rhythm monitoring is not always available.10 The limited data available show prevalences of nocturnal AF ranging from 6 to 27% and of daytime AF from 7 to 16%.11‐13 Differences in clinical phenotype and outcome between these groups have rarely been investigated.
In the Reappraisal of AF: Interaction Between Hypercoagulability, Electrical Remodelling, and Vascular Destabilization in the Progression of AF (RACE V) study, all participants underwent extensive phenotyping and received a continuous rhythm monitoring,7 which offers a unique opportunity to carefully evaluate the diurnal pattern of AF by the assessment of the onset of individual AF episodes in addition to AF progression.
We aim to study the differences in the clinical profile of self-terminating AF patients included in the RACE V trial based on the time of onset of AF and the role time of onset has on AF progression.
Methods
Study design
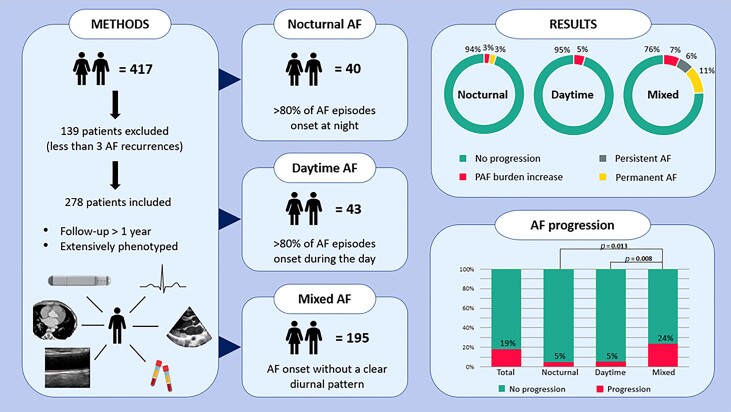
Patients with self-terminating AF included in the Reappraisal of AF: Interaction Between Hypercoagulability, Electrical Remodelling, and Vascular Destabilization in the Progression of AF (RACE V) study were analysed. The RACE V study focuses on mechanisms of AF and determinants of AF progression as previously described.7,14 This subanalysis contains 417 patients, all having a follow-up duration of ≥1 year. Eligible participants were patients who had at least two documented episodes of self-terminating AF or one documented episode combined with ≥2 symptomatic episodes suspected of being AF; did not have a history of persistent AF, pulmonary vein isolation (PVI), intention to undergo PVI or current amiodarone treatment; and were willing to undergo implantation of Medtronic (Minneapolis, USA) Reveal LINQ® implantable loop recorder. Patients with Medtronic pacemakers were also eligible if atrial high rate episodes (AHRE) of >190 b.p.m. lasting >6 min as these were qualified as AF episodes. Patients with different types of pacemakers, defibrillators, or cardiac resynchronization therapy devices were not eligible to participate due to differences in AHRE algorithm or incompatibility of the device with the type of home monitoring. Inclusion and exclusion criteria are described in more detail by Nguyen et al.7
Clinical assessment
Patients were extensively phenotyped at baseline. Clinical history, symptomatology, current medication, physical examination, a 12-lead electrocardiogram (ECG), echocardiography, blood samples, vascular assessment, and cardiac computed tomography (CT) were all assessed as previously described by Nguyen et al.7
Classification of onset of atrial fibrillation
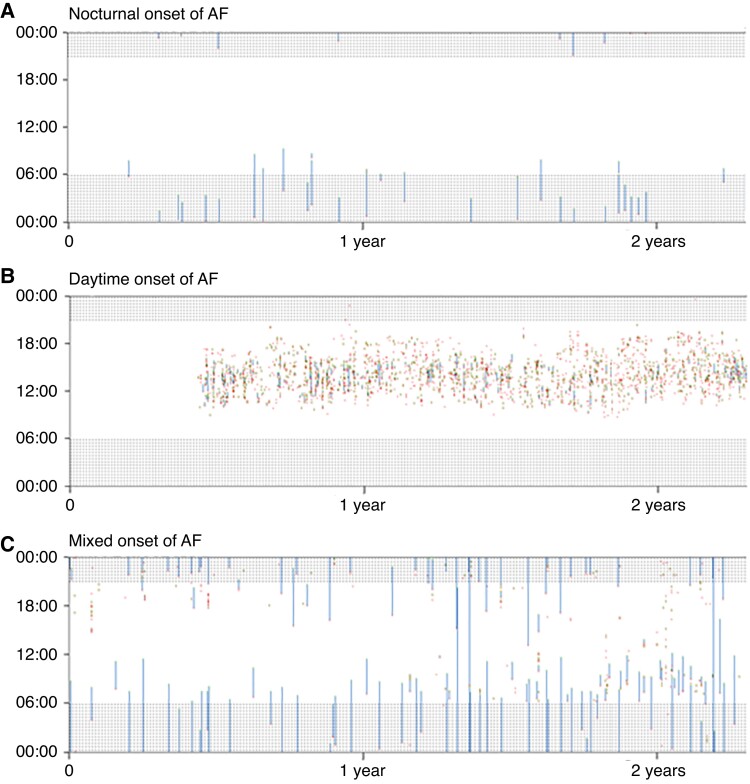
To adequately define the role of the time of onset of AF, only patients with ≥3 self-terminating AF episodes were included. Implantable loop recorder (ILR) data were used to assess whether AF was mainly started at night (>80% of episodes starting during night time, from 21:00 p.m. to 06:00 a.m., nocturnal AF) or mainly started during daytime (>80% starting during daytime, 06:00 a.m. to 21:00 p.m., daytime AF). If patients could not be identified as either nocturnal or daytime onset of AF, they were classified as patients without a clear diurnal pattern (mixed AF). Figure 1 shows examples of the three types of AF onset patients.
Figure 1.
Patients with either nocturnal or daytime or atrial fibrillation (AF) onset without a clear diurnal pattern (mixed group). Examples of patients with either nocturnal AF onset (A), daytime AF onset (B), or mixed AF onset (C) with 2.5 years of follow-up. White indicates no AF is present, and blue represents episodes of AF. Atrial fibrillation initiations are shown in red and AF terminations are shown in green. Shaded areas indicate nightly hours. The x-axis represents follow-up in years, and the y-axis is the time of day. AF, atrial fibrillation.
Atrial fibrillation progression
As described before to examine AF progression, all corrected AF episodes were visualized by a custom-made software using Microsoft Visual Basic. Initially, assessment of AF progression was done visually by six physicians (B.O.N.; I.C.V.G.; V.W.; M.R.; D.L.; H.J.G.M.C.). Atrial fibrillation progression was defined as 1 the development of persistent or permanent AF during follow-up or 2 an increase of >3% AF burden over the first 6 months or total follow-up as explained before.7 The duration of monitoring for current analysis lasted until 1 May 2020, until the last available rhythm monitoring for patients that died after >1 year of continuous rhythm monitoring, until the date of PVI, or in case of a successful PVI.
Statistical analysis
Baseline characteristics are presented as mean ± standard deviation (SD) for normally distributed data, as median and interquartile range for non-normally distributed continuous data. Categorical data are presented as numbers with percentages. Whether there is a difference in characteristics, treatment or outcome between the three groups with vagal, adrenergic, and mixed trigger will be analysed by one-way ANOVA in normally distributed data, Kruskal–Wallis test in non-normally distributed data, or X2 in categorical data. Logistic regression was used to study the association of time of onset (nocturnal, daytime, and mixed onset of AF) and AF progression. Four different models were performed: (1) univariately, (2) sex adjusted, (3) sex and age adjusted, and (4) RACE V clinical risk (which was developed to predict AF progression including PR interval, female sex, waist circumference, mitral valve regurgitation, and left atrial strain).7 Hosmer and Lemeshow tests were used to assess the model’s goodness of fit. Analyses were performed using software R v 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Circadian atrial fibrillation pattern and clinical characteristics
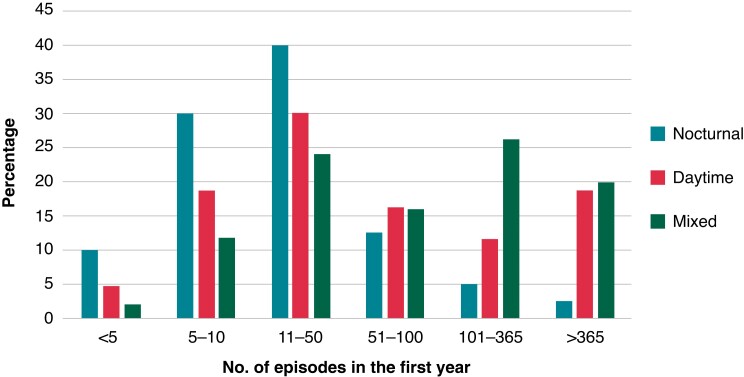
Of a total of 417 patients, 278 had ≥3 AF episodes; all other 139 patients had <3 AF episodes and were excluded from the present analysis (Graphical Abstract). The median age was 66 (59–71) years, and 117 (42%) were women (Table 1). The median follow-up of continuous rhythm monitoring was 2.2 (1.6–2.8) years. Nocturnal AF onset was observed in 40 (14%) patients and daytime AF onset in 43 (15%) patients, and 195 (70%) patients had onset of AF episodes throughout the day without a clear diurnal pattern (mixed type). Nocturnal AF patients had on average 124 AF episodes in the first year, daytime AF patients had 317 AF episodes, and the AF patients without a clear diurnal pattern had on average 323 AF episodes. The distribution of each group is displayed in Figure 2.
Table 1.
Patient characteristics
| Characteristic | Total population (n = 278) | Nocturnal AF (n = 40) | Daytime AF (n = 43) | Mixed AF (n = 195) | P value |
|---|---|---|---|---|---|
| Age (years) | 66 (59–71) | 64 (58–70) | 67 (61–72) | 66 (59–71) | 0.383 |
| Female sex | 117 (42%) | 21 (52%) | 17 (39%) | 79 (41%) | 0.351 |
| Total history AF (years) | 2.6 (0.8–5.0) | 3.1 (1.4–5.1) | 2.5 (0.9–4.7) | 2.5 (0.7–5.0) | 0.559 |
| HFrEF | 7 (3%) | 1 (3%) | 0 (0%) | 6 (3%) | 0.506 |
| HFpEF | 49 (18%) | 7 (18%) | 11 (26%) | 31 (16%) | 0.618 |
| Hypertension | 227 (82%) | 34 (85%) | 37 (86%) | 156 (80%) | 0.546 |
| Diabetes mellitus | 25 (9%) | 2 (5%) | 0 (0%) | 23 (12%) | 0.031 |
| Coronary artery disease | 36 (13%) | 3 (8%) | 5 (12%) | 28 (14%) | 0.481 |
| Atherosclerosis* | 134 (48%) | 22 (55%) | 22 (51%) | 90 (46%) | 0.544 |
| Ischemic stroke | 11 (4%) | 0 (0%) | 3 (7%) | 8 (4%) | 0.260 |
| Number of comorbidities** | 3 (2–3) | 2 (2–3) | 2 (2–3) | 3 (2–4) | 0.012 |
| CHA2DS2-VASc score | 3 (2–4) | 2 (2–3) | 2 (2–3) | 3 (2–4) | 0.328 |
| EHRA class | 0.879 | ||||
| I | 28 (10%) | 2 (5%) | 5 (12%) | 21 (11%) | |
| IIa | 87 (31%) | 12 (30%) | 12 (28%) | 63 (32%) | |
| IIb | 112 (40%) | 20 (50%) | 19 (44%) | 73 (37%) | |
| III | 50 (18%) | 6 (15%) | 7 (16%) | 37 (19%) | |
| IV | 1 (0%) | 0 (0%) | 0 (0%) | 1 (1%) | |
| BMI (kg/m2) | 27 (24–30) | 27 (24–32) | 26 (24–28) | 27 (25–30) | 0.157 |
| Obesity (BMI > 30) | 72 (26%) | 15 (38%) | 5 (12%) | 52 (27%) | 0.028 |
| Waist circumference (cm) | 102 (94–110) | 97 (86–113) | 101 (89–105) | 102 (95–110) | 0.072 |
| Systolic blood pressure (mmHg) | 135 (127–146) | 136 (130–146) | 135 (130–146) | 135 (125–147) | 0.687 |
| Diastolic blood pressure (mmHg) | 80 (73–86) | 80 (75–88) | 80 (74–86) | 80 (73–85) | 0.369 |
| eGFR (mL/min*1.73m2) | 79 (68–88) | 84 (73–90) | 82 (71–95) | 78 (67–87) | 0.018 |
| PR interval (ms) | 168 (152–189) | 164 (153–178) | 160 (146–182) | 172 (154–192) | 0.073 |
| Left atrial volume indexed (mL/m2) | 30 (25–38) | 30 (25–38) | 28 (25–37) | 31 (24–39) | 0.729 |
| Left ventricular ejection fraction (%) | 59 (8) | 58 (5) | 59 (6) | 59 (6) | 0.448 |
| Class I antiarrhythmic drugs | 63 (23%) | 16 (40%) | 16 (37%) | 31 (16%) | < 0.001 |
| Class III antiarrhythmic drugs | 15 (5%) | 1 (3%) | 0 (0%) | 14 (7%) | 0.113 |
| β-blocker | 146 (53%) | 19 (48%) | 23 (54%) | 104 (54%) | 0.775 |
| Verapamil/diltiazem | 53 (19%) | 8 (20%) | 12 (28%) | 33 (17%) | 0.256 |
| Digoxin | 5 (2%) | 0 (0%) | 0 (0%) | 5 (3%) | 0.336 |
| ACE-inhibitor | 52 (19%) | 6 (15%) | 4 (9%) | 42 (22%) | 0.138 |
| Angiotensin receptor blocker | 58 (21%) | 11 (28%) | 10 (23%) | 37 (19%) | 0.452 |
| Statin | 105 (38%) | 17 (43%) | 16 (37%) | 72 (37%) | 0.811 |
| Diuretic | 45 (16%) | 5 (13%) | 8 (19%) | 32 (17%) | 0.742 |
| Vitamin K antagonist | 35 (13%) | 6 (15%) | 1 (2%) | 28 (14%) | 0.086 |
| NOAC | 165 (60%) | 23 (58%) | 30 (70%) | 112 (58%) | 0.333 |
Data are presented as mean ± standard deviation, number of patients (%), or median (interquartile range).
Abbreviations: ACE = angiotensin-converting enzyme; BMI = body mass index; EHRA = European Heart Rhythm Association; eGFR = estimated glomerular filtration rate; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; NOAC = non-vitamin K anticoagulation.
*Atherosclerosis is defined as the presence of history of myocardial infarction, percutaneous coronary intervention, coronary artery bypass graft, ischemic cerebral infarction, peripheral vascular disease, Agatston score > 400 or plaque.
**The number of comorbidities was calculated by awarding 1 point for the presence of hypertension, heart failure, age > 65 years, diabetes mellitus, coronary artery disease, BMI > 25 kg/m2, moderate or severe mitral valve regurgitation, and kidney dysfunction (eGFR < 60).
Figure 2.
Distribution plot for the number of episodes in the first year for the nocturnal AF, daytime AF, and mixed AF groups. The distribution of episodes in the first recorded for the nocturnal, daytime, and mixed groups. The x-axis represents the number of AF episodes in the first year, and the y-axis represents the percentage of patients in each group. AF, atrial fibrillation.
Patients with predominantly nocturnal or daytime AF onset had less comorbidities compared to that of mixed AF onset patients (median 2 vs. 2 vs. 3, respectively, P = 0.012). Patients with predominantly nocturnal or daytime AF onset had a slightly better estimated glomerular filtration rate as compared to that of the mixed AF group (median 84 vs. 82 vs. 78 mL/min*1.73m2 in the nocturnal and daytime groups vs. mixed, respectively, P = 0.018), and diabetes was more common (12% vs. 5% vs. 0% in the mixed vs. the nocturnal vs. daytime AF onset groups, respectively, P = 0.031). Obesity was most often present in nocturnal AF onset patients (38% vs. 12% vs. 27%, in the nocturnal vs. daytime vs. mixed AF onset patients, respectively P = 0.028) (Table 1).
There is a significant association between daily AF pattern and Class I antiarrhythmic drugs with nocturnal vs. mixed odds ratio (OR) 3.3 (95% CI 1.6–7.1 P = 0.001) and daytime vs. mixed OR 3.0 (95% CI 1.5–6.2 P = 0.003)
Circadian atrial fibrillation pattern and atrial fibrillation progression
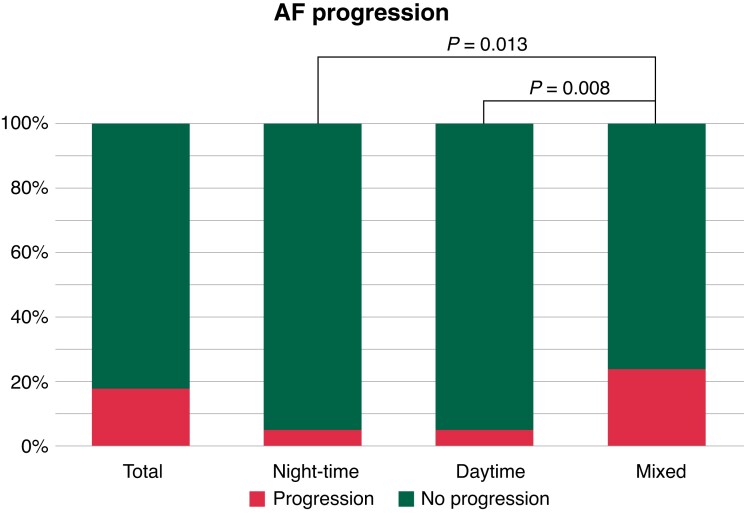
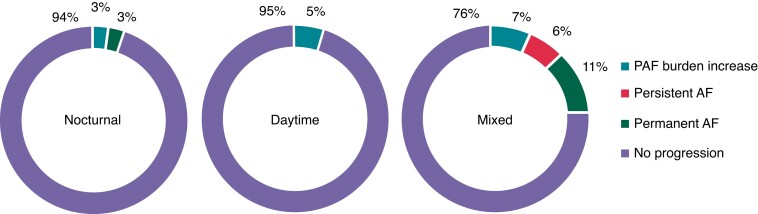
In the total group, AF progression occurred in 51 cases (19%). Atrial fibrillation progression rates in the nocturnal vs. daytime vs. mixed AF onset groups were 5% vs. 5% vs. 24%, respectively (P = 0.013 nocturnal vs. mixed and P = 0.008 daytime vs. mixed groups, respectively) (Figure 3). Atrial fibrillation progression in the nocturnal group occurred in two (5%) patients, in one due to self-terminating AF burden increase and in the other one because of progression to permanent AF. Atrial fibrillation progression in the daytime group also occurred in two (5%) patients, in both cases because of an increase of AF burden whilst still having paroxysmal AF. Atrial fibrillation progression in the mixed AF onset group without a clear diurnal pattern occurred in 47 (24%) patients, in 13 (5%) patients due to paroxysmal AF with an increase in AF burden whilst still having paroxysmal AF, in 12 patients due to progression to persistent AF, and in 22 patients due to progression to permanent AF (Figure 4). Univariate analysis revealed that patients with either nocturnal or daytime AF onset had a lower risk of AF progression vs. those with mixed onset of AF. When adjusting for age and sex, the association between time of onset and AF progression persisted. When adjusted for the RACE V clinical risk prediction model for AF progression, the OR for the nocturnal vs. mixed was 0.20 (0.05–0.89); the OR for daytime vs. mixed was 0.18 (0.04–0.79) (Table 2).
Figure 3.
Atrial fibrillation (AF) progression in the nocturnal AF, daytime AF, and mixed AF onset groups. Atrial fibrillation progression for all patients and the three respective groups based on the time of onset of AF. The x-axis represents the AF group based on the time of onset, and the y-axis AF progression is displayed as a percentage. AF, atrial fibrillation.
Figure 4.
Type of AF progression according to time of atrial fibrillation onset. Atrial fibrillation (AF) progression in the nocturnal, daytime, and mixed AF onset groups. Blue represents AF progression due to a burden increase of > 3%. Red represents progression to persistent AF. Green represents progression to permanent AF. AF, atrial fibrillation; PAF, paroxysmal atrial fibrillation.
Table 2.
Logistic regression
| Odds ratio | 95% CI | P value | ||
|---|---|---|---|---|
| Time of onseta | Nocturnal | 0.17 | 0.04–0.71 | 0.02 |
| Daytime | 0.15 | 0.04–0.66 | 0.01 | |
| Time of onsetb | Nocturnal | 0.18 | 0.04–0.76 | 0.02 |
| Daytime | 0.15 | 0.03–0.65 | 0.01 | |
| Time of onsetc | Nocturnal | 0.18 | 0.04–0.79 | 0.02 |
| Daytime | 0.15 | 0.03–0.65 | 0.01 | |
| Time of onsetd | Nocturnal | 0.20 | 0.05–0.89 | 0.04 |
| Daytime | 0.18 | 0.04–0.79 | 0.02 | |
| Time of onsete | Nocturnal | 0.18 | 0.04–0.78 | 0.02 |
| Daytime | 0.16 | 0.04–0.70 | 0.02 |
Univariatly adjusted.
Sex adjusted.
Sex and age adjusted.
RACE V clinical score (PR time, female sex, waist circumference, mitral valve regurgitation, and left atrial strain) adjusted.
Antiarrhythmic drug use adjusted. Mixed onset of AF is used as a reference.
Discussion
The aim of this study was to assess the role of time of onset of AF on clinical profile and AF progression in patients with self-terminating AF included in the RACE V trial. First, we showed that patients with predominant nocturnal and daytime onset of AF had less comorbidities as compared to the that of the mixed AF onset group. Second, and in line with the latter observation, patients with either nocturnal or daytime onset of AF had lower progression rates as compared to that of the mixed AF onset group.
Circadian pattern of atrial fibrillation onset, comorbidities, and atrial fibrillation progression
Previous studies proposed a circadian pattern of AF episodes and showed that in patients with frequent self-terminating AF, most AF episodes originated during the night.15,16 This is in contrast with our data based on continuous rhythm monitoring. Although part of our patients had a predominant nocturnal onset of AF, the majority, however, had AF episodes starting throughout the day. It may well be that time of onset may differ between studies depending on the clinical phenotype of the patients and the severity of the atrial cardiomyopathy, but also depending on the type of rhythm monitoring.
We observed that patients in the mixed AF onset group had more comorbidities in comparison to that of those in the nocturnal and daytime AF onset groups. In line with the presence of more comorbidities, the mixed AF onset patients showed the highest AF progression rates. Multiple factors are associated with AF progression as outlined by many studies including atherosclerosis, inflammation, and hypercoagubility.6,7 The more comorbidities are present, the worse the outcome.17 Therefore, early identification and treatment of risk factors and comorbidities in order to improve sinus rhythm maintenance and outcome are essential.6,7,18
It is well known that AF almost never comes alone and is connected with comorbidities. The development of atrial remodelling and atrial cardiomyopathy occurs due to these comorbidities and to AF itself.1,18 During time and depending on the number of comorbidities and progression of AF, atrial remodelling becomes more severe.
The reason why in the presence of more comorbidities patients deteriorate into a profile of having both nocturnal as well as daytime start of AF episodes is difficult to explain. It may well be that due to a more severe atrial cardiomyopathy, AF develops more often due to structural conditions present throughout the day. In contrast, AF episodes in patients with predominant nocturnal or daytime AF patterns may be more likely triggered by intermittent arrhythmogenic factors not related to permanent structural atrial alterations transiently increasing AF risk predominantly during day or night-time in line with a dynamic AF substrate.19 Treating comorbidities is important for improving cardiovascular outcome in AF patients.20,21
Antiarrhythmic drugs were more often instituted in patients in the daytime and night-time groups in comparison to the mixed group. Since institution of antiarrhythmic drugs was left to the discretion of the treating physician, it is difficult to exactly know the reason for this; however, patients in the night-time and daytime groups may have been more suitable for starting Class I antiarrhythmic drugs because of the lower number of comorbidities. As we learned only recently, rate–control drugs may affect AF progression.22
There is a need to categorize AF patients based on their phenotypes, for differentiating treatment options and personalized medicine.14,23 The EAST–AFNET 4 showed early rhythm control improved cardiovascular outcome regardless of AF pattern classification based on the first episode of AF, paroxysmal AF, or persistent. Nevertheless, patients with a first episode of AF had a higher risk of hospitalization and acute coronary syndrome.24 Early approaches to AF rhythm management can improve outcomes in patients with AF.23 Perhaps an improved phenotype classification, including diurnal distribution of AF episodes, may contribute to improving outcome in AF patients.
Strengths and limitations
Our study has several strengths, which include the extensively phenotyped patients and the long-term continuous rhythm monitoring all patients received. However, there are also a few limitations that need to be considered. First, in the RACE V study, treatment was at the discretion of the treating physician, which may have influenced AF progression. Sample size with 278 patients is modest and therefore limits statistical power. Moreover, patients were classified based on the onset of AF, but no information was recorded on whether the patients were actual sleeping or whether they were post-prandial or at rest during the day. We did not assess temporary conditions which could have a proarrhythmic effect, and this could impact AF progression numbers. Lastly, as is the case in clinical trials, the RACE V population is a selective population, in this case relatively healthy AF patients. Therefore, real-world data could be different.
Conclusions
We assessed the role of time of AF onset on clinical differences and AF progression in patients with self-terminating AF included in the RACE V trial. Our main findings include that the nocturnal and daytime AF onset groups had less AF progression as compared to that of those patients who suffered from AF both starting at night and during daytime. This may be associated with the presence of less comorbidities being present in those two groups. Whether knowledge on time of onset will impact clinical decision-making needs further study.
Contributor Information
Martijn E van de Lande, Department of Cardiology, University of Groningen University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Rajiv S Rama, Department of Cardiology, University of Groningen University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Tim Koldenhof, Department of Cardiology, Martini Hospital, Van Swietenplein 1, 9728 NT Groningen, The Netherlands.
Vicente Artola Arita, Department of Cardiology, University of Groningen University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Bao-Oanh Nguyen, Department of Cardiology, University of Groningen University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Colinda van Deutekom, Department of Cardiology, University of Groningen University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Vanessa Weberndorfer, Department of Cardiology, Maastricht University Medical Centre+, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 632, 6229 ER Maastricht, The Netherlands.
Harry J G M Crijns, Department of Cardiology, Maastricht University Medical Centre+, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 632, 6229 ER Maastricht, The Netherlands.
Martin E W Hemels, Department of Cardiology, Rijnstate Hospital, Wagnerlaan 55, 6815 AD Arnhem, The Netherlands.
Robert G Tieleman, Department of Cardiology, Martini Hospital, Van Swietenplein 1, 9728 NT Groningen, The Netherlands.
Mirko de Melis, Medtronic Bakken Research Centre, Endepolsdomein 5, 6229 GW Maastricht, The Netherlands.
Ulrich Schotten, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 632, 6229 ER Maastricht, The Netherlands; Department of Physiology, University of Maastricht, Debyelaan 25, 6229 HX Maastricht, The Netherlands.
Dominik Linz, Department of Cardiology, Maastricht University Medical Centre+, P. Debyelaan 25, 6229 HX Maastricht, The Netherlands; Cardiovascular Research Institute Maastricht (CARIM), Maastricht University, Universiteitssingel 50, 632, 6229 ER Maastricht, The Netherlands.
Isabelle C Van Gelder, Department of Cardiology, University of Groningen University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Michiel Rienstra, Department of Cardiology, University of Groningen University Medical Centre Groningen, Hanzeplein 1, 9713 GZ Groningen, The Netherlands.
Funding
This study was funded from the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014-9: Reappraisal of Atrial Fibrillation: interaction between hyperCoagulability, Electrical remodelling and Vascular destabilization in the progression of AF (RACE V).
Data availability
The data underlying this article are available on request from the principle investigator.
References
- 1. Nattel S, Guasch E, Savelieva I, Cosio FG, Valverde I, Halperin JLet al. . Early management of atrial fibrillation to prevent cardiovascular complications. Eur Heart J 2014;35:1448–56. [DOI] [PubMed] [Google Scholar]
- 2. Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJet al. . Higher risk of death and stroke in patients with persistent vs. Pparoxysmal atrial fibrillation: results from the ROCKET-AF trial. Eur Heart J 2015;36:288–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ganesan AN, Chew DP, Hartshorne T, Selvanayagam JB, Aylward PE, Sanders Pet al. . The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J 2016;37:1591–602. [DOI] [PubMed] [Google Scholar]
- 4. Dudink EAMP, Erküner Ö, Berg J, Nieuwlaat R, de Vos CB, Weijs Bet al. . The influence of progression of atrial fibrillation on quality of life: a report from the euro heart survey. Europace 2018;20:929–34. [DOI] [PubMed] [Google Scholar]
- 5. de Vos CB, Pisters R, Nieuwlaat R, Prins MH, Tieleman RG, Coelen RJet al. . Progression from paroxysmal to persistent atrial fibrillation clinical correlates and prognosis. J Am Coll Cardiol 2010;55:725–31. [DOI] [PubMed] [Google Scholar]
- 6. Blum S, Aeschbacher S, Meyre P, Zwimpfer L, Reichlin T, Beer JHet al. . Incidence and predictors of atrial fibrillation progression. J Am Heart Assoc 2019;8:e012554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nguyen BO, Weberndorfer V, Crijns HJ, Geelhoed B, Ten Cate H, Spronk Het al. . Prevalence and determinants of atrial fibrillation progression in paroxysmal atrial fibrillation [published online ahead of print, 2022 Jul 20]. Heart. 2022; heartjnl-2022-321027. doi: 10.1136/heartjnl-2022-321027. [DOI] [PMC free article] [PubMed]
- 8. Coumel P. Cardiac arrhythmias and the autonomic nervous system. J Cardiovasc Electrophysiol 1993;4:338–55. [DOI] [PubMed] [Google Scholar]
- 9. Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone?. Eur Heart J 1994;15:9–16. [DOI] [PubMed] [Google Scholar]
- 10. Linz D, Elliott AD, Hohl M, Malik V, Schotten U, Dobrev Det al. . Role of autonomic nervous system in atrial fibrillation. Int J Cardiol 2019;287:181–8. [DOI] [PubMed] [Google Scholar]
- 11. de Vos CB, Nieuwlaat R, Crijns HJ, Camm AJ, LeHeuzey JY, Kirchhof CJet al. . Autonomic trigger patterns and anti-arrhythmic treatment of paroxysmal atrial fibrillation: data from the euro heart survey. Eur Heart J 2008;29:632–9. [DOI] [PubMed] [Google Scholar]
- 12. Rosso R, Sparks PB, Morton JB, Kistler PM, Vohra JK, Halloran Ket al. . Vagal paroxysmal atrial fibrillation: prevalence and ablation outcome in patients without structural heart disease. J Cardiovasc Electrophysiol 2010;21:489–93. [DOI] [PubMed] [Google Scholar]
- 13. Oral H, Chugh A, Scharf C, Hall B, Cheung P, Veerareddy Set al. . Pulmonary vein isolation for vagotonic, adrenergic, and random episodes of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol 2004;15:402–6. [DOI] [PubMed] [Google Scholar]
- 14. De With RR, Erküner Ö, Rienstra M, Nguyen BO, Körver FWJ, Linz Det al. . Temporal patterns and short-term progression of paroxysmal atrial fibrillation: data from RACE V. Europace 2020;22:1162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shusterman V, Warman E, London B, Schwartzman D. Nocturnal peak in atrial tachyarrhythmia occurrence as a function of arrhythmia burden. J Cardiovasc Electrophysiol 2012;23:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sopher SM, Hnatkova K, Waktare JE, Murgatroyd FD, Camm AJ, Malik M. Circadian variation in atrial fibrillation in patients with frequent paroxysms. Pacing Clin Electrophysiol PACE 1998;21:2445–9. [DOI] [PubMed] [Google Scholar]
- 17. Kloosterman M, Crijns HJGM, Mulder BA, Groenveld HF, Van Veldhuisen DJ, Rienstra Met al. . Sex-related differences in risk factors, outcome, and quality of life in patients with permanent atrial fibrillation: results from the RACE II study. Europace 2020;22:1619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunawardene MA, Willems S. Atrial fibrillation progression and the importance of early treatment for improving clinical outcomes. Europace 2022;24:ii22–8. PMID: 35661866. [DOI] [PubMed] [Google Scholar]
- 19. Heijman J, Linz D, Schotten U. Dynamics of atrial fibrillation mechanisms and comorbidities. Annu Rev Physiol 2021;83:83–106. [DOI] [PubMed] [Google Scholar]
- 20. Patel SM, Palazzolo MG, Murphy SA, Antman EM, Braunwald E, Lanz HJet al. . Evaluation of the atrial fibrillation better care pathway in the ENGAGE AF-TIMI 48 trial. Europace 2022;24:1730–8. [DOI] [PubMed] [Google Scholar]
- 21. Proietti M, Lip GYH, Laroche C, Fauchier L, Marin F, Nabauer Met al. . ESC-EORP Atrial fibrillation general long-term registry investigators group. Relation of outcomes to ABC (atrial fibrillation better care) pathway adherent care in European patients with atrial fibrillation: an analysis from the ESC-EHRA EORP atrial fibrillation general long-term (AFGen LT) registry. Europace 2021;23:174–83. [DOI] [PubMed] [Google Scholar]
- 22. Koldenhof T, Wijtvliet PEPJ, Pluymaekers NAHA, Rienstra M, Folkeringa RJ, Bronzwaer Pet al. . Rate control drugs differ in the prevention of progression of atrial fibrillation. Europace 2022;24:384–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CMet al. . Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goette A, Borof K, Breithardt G, Camm AJ, Crijns HJGM, Kuck KHet al. . Presenting pattern of atrial fibrillation and outcomes of early rhythm control therapy. J Am Coll Cardiol 2022;80:283–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available on request from the principle investigator.