Abstract
Aims
Insights into subclinical atrial fibrillation (AF) development are warranted to inform the strategies of screening and subsequent clinical management upon AF detection. Hence, this study sought to characterize the onset and progression of subclinical AF with respect to 12-lead electrocardiogram (ECG) parameters.
Methods and results
We included AF-naïve individuals aged 70–90 years with additional stroke risk factors who underwent implantable loop recorder (ILR) monitoring in the LOOP Study. Using data from daily ILR recordings and the computerized analysis of baseline ECG, we studied empirically selected ECG parameters for AF detection (≥6 min), cumulative AF burden, long-lasting AF (≥24 h), and AF progression. Of 1370 individuals included, 419 (30.6%) developed AF during follow-up, with a mean cumulative AF burden of 1.5% [95% CI: 1.2–1.8]. Several P-wave-related and ventricular ECG parameters were associated with new-onset AF and with cumulative AF burden in AF patients. P-wave duration (PWD), P-wave terminal force in Lead V1, and interatrial block (IAB) further demonstrated significant associations with long-lasting AF. Among AF patients, we observed an overall reduction in cumulative AF burden over time (IRR 0.70 [95% CI: 0.51–0.96]), whereas IAB was related to an increased risk of progression to AF ≥24 h (HR 1.86 [95% CI: 1.02–3.39]). Further spline analysis also revealed longer PWD to be associated with this progression in AF duration.
Conclusion
We identified several ECG parameters associated with new-onset subclinical AF detected by ILR. Especially PWD and IAB were robustly related to the onset and the burden of AF as well as progression over time.
Keywords: Atrial fibrillation, Electrocardiography, P-wave, Cardiac arrhythmias, Stroke
Graphical Abstract
Graphical Abstract.
What’s new?
Both P-wave-related and ventricular parameters from 12-lead electrocardiogram were associated with the cumulative burden of new-onset atrial fibrillation (AF) detected by long-term continuous monitoring.
Individuals with shorter and longer P-wave duration (PWD), with greater P-wave terminal force in Lead V1, and with interatrial block (IAB) were at increased risk of long-lasting AF.
Individuals with device-detected AF tended to experience an overall reduction in cumulative AF burden over time.
Longer PWD and IAB were associated with progression to longer AF duration.
Introduction
Atrial fibrillation (AF) is a well-known and treatable risk factor for stroke but often remains asymptomatic and thus undiagnosed.1–4 This has stimulated a substantial interest in screening for subclinical AF. Mounting evidence further indicates that a greater burden of subclinical AF is associated with increased stroke risk.1–5 Therefore, insights into the onset and the progression of subclinical AF are warranted to inform screening strategy and to guide subsequent clinical management.
The pathogenesis of AF is thought to be closely related to structural and functional changes in the atria that are encompassed in a newly proposed entity, the so-called atrial cardiomyopathy.6 These changes would be reflected in electrical abnormalities and can, therefore, be detected by a standard 12-lead electrocardiogram (ECG). Indeed, various ECG parameters have already been demonstrated to predict clinical AF in large epidemiological studies.6–17 There is also a growing body of evidence on the applicability of artificial intelligence (AI) algorithm based on sinus rhythm ECGs in AF risk prediction, and a recent study further showed that AI-based risk stratification could help to increase the yield for AF detection by screening.6,18 However, data on the underlying relationships between ECG parameters and subclinical or screen-detected AF are sparse. In this post hoc analysis of the LOOP Study (Atrial Fibrillation detected by Continuous ECG Monitoring using Implantable Loop Recorder to prevent Stroke in High-risk Individuals), we aimed to identify 12-lead ECG parameters associated with the onset and progression of subclinical AF detected by long-term continuous monitoring.
Methods
Study design
The LOOP Study was a randomized, controlled trial to investigate continuous AF screening with an implantable loop recorder (ILR; Reveal LINQ, Medtronic). The trial was registered at Clinical-Trials.gov (NCT02036450) and approved by the Regional Scientific Ethics Committee for the Capital Region of Denmark (H-4-2013-025). Oral and written informed consents were obtained from all participants. A detailed description of the trial design and the primary reporting of the LOOP Study have been published previously.19,20 In brief, AF-naïve individuals aged 70–90 years and with any history of hypertension, diabetes, stroke, or heart failure were randomized to either ILR monitoring or usual care. At baseline, all participants underwent a 12-lead ECG. For ILR participants, continuous ECG monitoring was performed via the device during follow-up and automated remote transmissions were reviewed daily by an experienced physician. Any new-onset ILR-detected AF episode lasting ≥6 min was independently evaluated by at least two senior cardiologists, while subsequent ILR-detected AF ≥24 h was adjudicated by at least one experienced physician.
In the present analysis, we included the LOOP participants with available ILR recordings and with a baseline 12-lead ECG suitable for the measurement of ECG parameters.
Electrocardiogram measurement
All digital 12-lead ECGs were processed by the Marquette 12SL ECG Analysis Program (version 23) to obtain relevant ECG measurements (see Supplementary Methods for more details). Using this analysis program, we excluded the ECGs with non-sinus rhythm or other findings unsuitable for the measurement of ECG parameters, including ectopic atrial rhythm, junctional rhythm, ventricular rhythm, undetermined rhythm, second- and third-degree atrioventricular block, and delta-wave.
We assessed the following ECG parameters as potential AF predictors: PR interval (abnormal interval defined as <120 or >200 ms); P-wave duration (PWD, abnormal duration defined as >120 ms); P-wave voltage in Lead I (PWV, abnormal voltage defined as <100 µV); P-wave axis (PWA, abnormal axis defined as axis deviation outside the range 0–75°); P-wave terminal force in Lead V1 (PTF, abnormal terminal force defined as >4000 ms × µV); the presence of interatrial block (IAB, defined as PWD ≥120 ms combined with biphasic P-wave in any inferior lead); heart rate-corrected QT (QTc) interval (abnormal interval defined as >450 ms); QRS duration (abnormal duration defined as >120 ms); and QRS-T angle (abnormal angle defined as ≥100°).
Outcomes and follow-up
The primary outcome was time to first AF detection (≥6 min). Secondary outcomes were: (i) time to first long-lasting AF episode ≥24 h; (ii) cumulative AF burden—defined as cumulative duration of all ILR-detected AF episodes ≥6 min from the first adjudicated episode, divided by the total monitoring duration; (iii) time from first AF detection to first AF episode ≥24 h; and (iv) progression in cumulative AF burden over time. Cumulative AF burden progression was assessed in the timespan starting from the first adjudicated AF episode to censoring, which was divided into two equal periods. Hence, the progression in cumulative AF burden was estimated by comparing cumulative AF duration in the first half-period with the second half. The study participants were right-censored at end of device service or death, whichever came first.
Statistical analysis
For baseline characteristics, continuous variables are presented as mean with standard deviation (SD), whereas categorical variables are presented as frequency with proportion. The distributions are compared by t-test and χ2 test, respectively.
The risks of AF detection (≥6 min) and AF episode ≥24 h were assessed with the time-to-first-event principle. Crude event rates were calculated with Poisson regression and are presented as events per 100 person-years [95% confidence interval (CI)]. The relative risks were determined in the multivariate, cause-specific Cox regression models accounting for death as competing risk and are presented as hazard ratio (HR) [95% CI]. Additionally, to enhance the statistical power and to provide more flexibility in our analyses, ECG parameters were also evaluated as continuous variables using the restricted cubic spline regression, where HR was estimated with the median value as the reference for each of the relevant parameters. The Cox proportional-hazards assumption was tested with scaled Schoenfeld residuals and no violations were detected.
Crude rates of cumulative AF burden were calculated in the negative binomial regression model using cumulative AF duration (in minutes) as count data and the total monitoring time (in minutes) as offset and are presented as percentage [95% CI]. The relative risks according to the ECG parameters were estimated as incidence rate ratio (IRR) for cumulative AF duration in the multivariate negative binomial model using the total monitoring time (offset) to adjust for interindividual differences in the monitoring duration. Moreover, progression in cumulative AF burden over time was assessed in the multivariate generalized linear mixed model with negative binomial distribution for cumulative AF duration (in minutes) in each half-period as the outcome variable and the corresponding monitoring duration (in minutes) as the offset.
The multivariate models were adjusted for sex, age, baseline comorbidities (including hypertension, diabetes, previous stroke, heart failure, valvular heart disease, ischaemic heart disease, and peripheral artery disease), baseline antiarrhythmic treatment (including beta-blockers and non-dihydropyridine calcium channel blockers), QRS duration, Pend-Q interval (only for P-wave parameters except PR interval), and left ventricular hypertrophy (only for ventricular ECG parameters); see Supplementary Methods for definitions of these ECG covariates. The generalized linear mixed model was additionally adjusted for time from ILR implantation to the first adjudicated AF episode. The statistical analysis was performed using R (version 4.1.0) and a two-sided P-values ≤0.05 defined the statistical significance.
Results
In the LOOP Study, 1420 (94.6%) of the 1501 participants assigned to the ILR group had received an ILR. Among them, 9 (0.6%) were excluded from the present analysis due to un-retrievable ILR recordings and further 41 (2.9%) were excluded due to missing baseline ECG or ECG with findings unsuitable for measurement. The final study population consisted of 1370 participants, with a mean ILR monitoring time of 3.19 years (SD, 0.52). Table 1 summarizes the baseline characteristics. The study participants had a mean age of 74.7 (SD, 4.1) years and 53.4% of them were male. For baseline medications, a total of 354 (25.8%) participants received treatment with either beta-blockers or non-dihydropyridine calcium channel blockers. IAB was observed in 91 (6.7%) participants: 67 (6.4%) among the participants without beta-blocker treatment vs. 24 (7.5%) among those treated with beta-blockers. During follow-up, 419 (30.6%) developed AF and 14 (1.0%) died. The baseline prevalence of IAB was 10.1% among the participants who developed AF during follow-up and 5.2% among those who did not.
Table 1.
Overview of baseline characteristics
| The study population (n = 1370) | |
|---|---|
| Male sex (%) | 731 (53.4) |
| Age, years (SD) | 74.7 (4.1) |
| Alcohol consumption, standard units per week (SD) | 7.4 (8) |
| Smoking pack years (SD) | 16.7 (22.9) |
| Body-mass index, kg/m2 (SD) | 27.8 (4.6) |
| CHA2DS2-VASc score (SD) | 3.7 (1.2) |
| Comorbidities (%) | |
| Hypertension arterialis | 1254 (91.5) |
| Diabetes mellitus | 382 (27.9) |
| Congestive heart failure | 57 (4.2) |
| Previous stroke | 236 (17.2) |
| Chronic ischaemic heart disease | 157 (11.5) |
| Valvular heart disease | 59 (4.3) |
| Peripheral artery disease | 34 (2.5) |
| Concomitant medications (%) | |
| Beta-blockers | 319 (23.3) |
| Calcium channel blockers | 512 (37.4) |
| Non-dihydropyridine calcium channel blocker | 40 (2.9) |
| Renin-angiotensin system inhibitors | 898 (65.5) |
| Diuretics | 449 (32.8) |
| Statins | 802 (58.5) |
| Insulins | 111 (8.1) |
| Other antidiabetic drugs | 297 (21.7) |
| ECG parameters | |
| PR interval, ms (SD) | 170.7 (31.7) |
| P-wave duration, ms (SD) | 94.5 (21.8) |
| P-wave voltage in Lead I, µV (SD) | 65.7 (36.1) |
| P-wave axis, degree (SD) | 47.3 (24) |
| P-wave terminal force in Lead V1, ms × µV (SD) | 1813.4 (2248.7) |
| Interatrial block (%) | 91 (6.7) |
| QTc interval, ms (SD) | 421.2 (22.2) |
| QRS duration, ms (SD) | 92.3 (19) |
| QRS-T angle, degree (SD) | 42.4 (35.4) |
Interatrial block was defined as P-wave duration ≥120 ms combined with the presence of biphasic P-wave (positive–negative) in any inferior lead. QTc interval was estimated using the Framingham formula.
Missing observations: PR interval, n = 3; P-wave duration, n = 4; P-wave voltage in Lead I, n = 7; P-wave axis, n = 28, P-wave terminal force in Lead V1, n = 7; interatrial block, n = 7.
Abbreviations: AF, atrial fibrillation; ECG, electrocardiogram; QTc, heart rate-corrected QT interval; SD, standard deviation.
The risk of atrial fibrillation
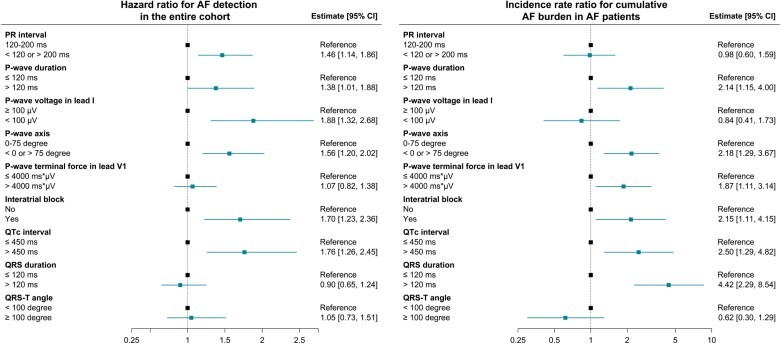
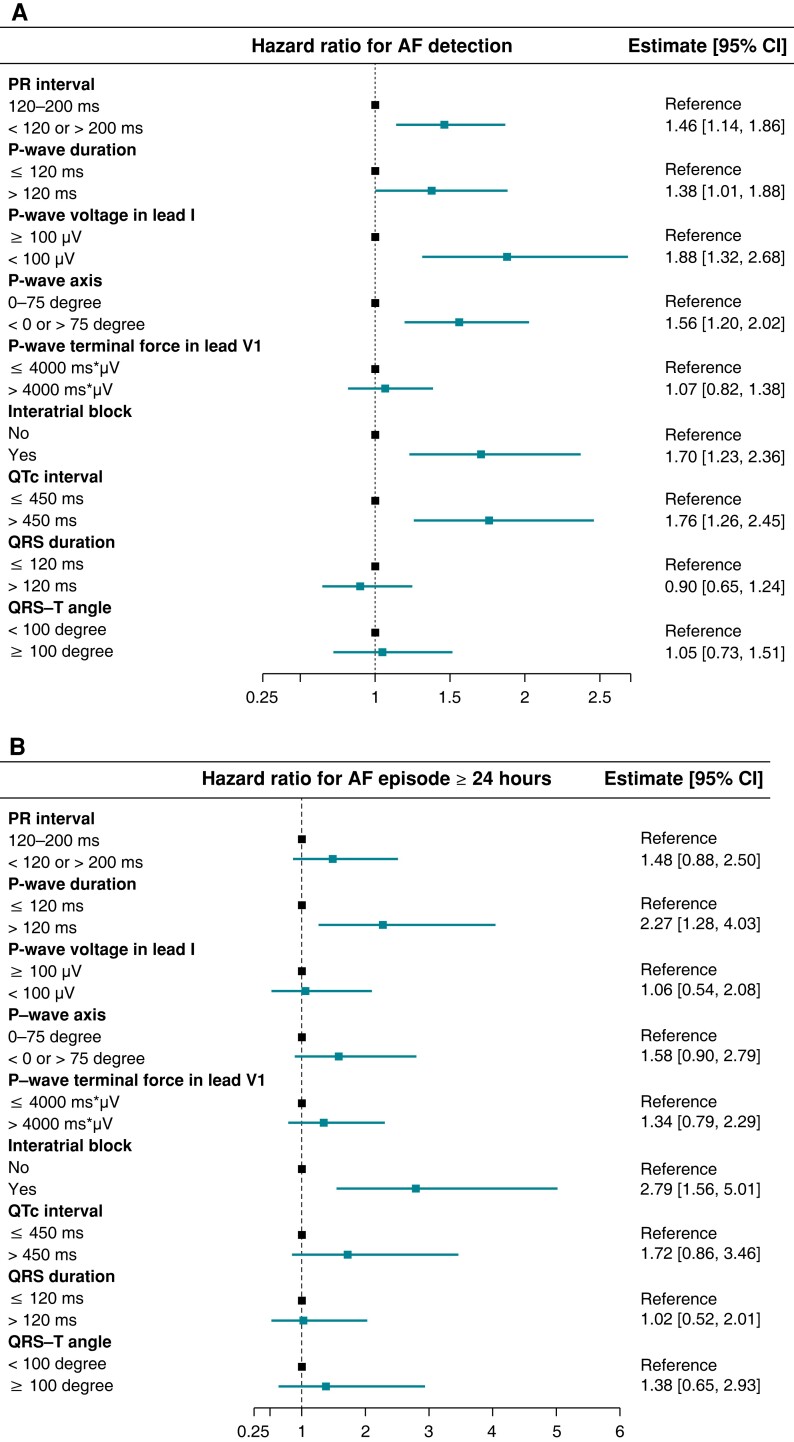
The time-to-first-event analysis revealed that abnormal PR interval, PWD, PWV, PWA, and QTc interval as well as the presence of IAB were associated with an increased risk of AF detection (HR 1.46 [1.14–1.86], 1.38 [1.01–1.88], 1.88 [1.32–2.68], 1.56 [1.20–2.02], 1.76 [1.26–2.45], and 1.70 [1.23–2.36], respectively; Figure 1A). For long-lasting AF, 83 participants experienced AF episodes ≥24 h during follow-up. Only abnormal PWD and IAB were significantly associated with the risk of AF episodes ≥24 h (HR 2.27 [1.28–4.03] and 2.79 [1.56–5.01], respectively; Figure 1B). Event rates and HRs according to dichotomized ECG parameters are listed in Supplementary material online, Table S1.
Figure 1.
Hazard ratio for detection of any AF and for first AF episode ≥24 h. The figure shows the relative risk of AF detection (A) and AF episode ≥24 h (B) according to dichotomized ECG parameters. Interatrial block was defined as P-wave duration ≥120 ms combined with biphasic (positive–negative) P-wave in any inferior lead. QTc interval was estimated using the Framingham formula. Hazard ratios were determined in multivariate Cox models with death as competing risk. Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiogram; QTc, heart rate-corrected QT interval.
Supplementary material online, Figures S1 and S2 illustrate the restricted cubic spline analysis assessing ECG parameters as continuous variables. For AF detection, the risk appeared to be higher in patients with longer PR interval, lower PWV, and longer QTc interval. PWD demonstrated a U-shaped relationship with AF detection and AF episode ≥24 h, where the incidences were increased both for shorter and longer durations. Albeit no significant association with AF risk, greater PTF did confer a higher risk of AF duration ≥24 h. Further exploration with a cut-off of PTF >6000 ms × µV revealed a remarkably increased risk of AF episode ≥24 h, as indicated by HR 2.28 [1.11–4.67].
Cumulative atrial fibrillation burden among atrial fibrillation patients
Among the 419 patients who developed AF during follow-up, the total number of AF episodes was 38,579, with a mean cumulative AF burden of 1.5% [1.2–1.8%]. The average time from ILR implantation to the first adjudicated AF episode was 1.01 (SD, 0.98) years. Table 2 presents AF characteristics according to ECG parameters. Among the ECG parameters assessed in the present study, abnormal PWD, PWA, PTF, QTc interval, and QRS duration as well as the presence of IAB were related to a significant increase in cumulative AF burden (Figure 2).
Table 2.
AF characteristics according to ECG parameters among 419 AF patients
| Cumulative AF burden, % [95% CI] | Number of AF episodes (SD) | Mean episode duration, hour (SD) | Maximum episode duration, hour (SD) | Any episode ≥24 h (%) | ||
|---|---|---|---|---|---|---|
| PR interval | 120–200 ms (n = 332) | 1.5 [1.2–1.9] | 80.5 (209.3) | 6.9 (21.3) | 72.4 (321.7) | 63 (19.0) |
| <120 or >200 ms (n = 85) | 1.6 [1.0–2.4] | 118.5 (167.8) | 5.6 (18.6) | 54.4 (248.8) | 17 (20.0) | |
| P-wave duration | ≤120 ms (n = 368) | 1.4 [1.1–1.7] | 82.9 (190.1) | 5.8 (19.3) | 64.2 (309.2) | 65 (17.7) |
| >120 ms (n = 48) | 2.4 [1.4–4.4] | 130.8 (276.6) | 13.2 (29) | 105 (303.2) | 15 (31.2) | |
| P-wave voltage in Lead I | ≥100 µV (n = 34) | 2.2 [1.1–4.5] | 51.1 (95) | 9.4 (20.5) | 132.9 (465) | 9 (26.5) |
| <100 µV (n = 380) | 1.5 [1.2–1.8] | 90.9 (208.1) | 6.5 (20.8) | 63.5 (291.4) | 71 (18.7) | |
| P-wave axis | 0–75° (n = 332) | 1.5 [1.2–1.9] | 77.4 (192.3) | 7.1 (22.4) | 63.8 (287) | 63 (19.0) |
| <0° or >75° (n = 77) | 1.8 [1.1–2.8] | 142.2 (242.9) | 5.5 (12.6) | 94.6 (399.4) | 15 (19.5) | |
| P-wave terminal force in Lead V1 | ≤4000 ms × µV (n = 340) | 1.5 [1.2–1.9] | 92.7 (214.7) | 6.2 (20.4) | 58.6 (268.5) | 62 (18.2) |
| >4000 ms × µV (n = 74) | 1.7 [1.0–2.7] | 64.3 (122.2) | 8.8 (22.6) | 118.1 (450.4) | 18 (24.3) | |
| IAB | No (n = 374) | 1.4 [1.1–1.7] | 83.2 (189.2) | 5.9 (19.3) | 64 (306.9) | 66 (17.6) |
| Yes (n = 42) | 2.6 [1.4–4.8] | 135.3 (291.9) | 13.9 (30.1) | 112.1 (322.2) | 14 (33.3) | |
| QTc interval | ≤450 ms (n = 367) | 1.4 [1.1–1.7] | 75.8 (163) | 6.4 (19.9) | 67.5 (311.3) | 71 (19.3) |
| >450 ms (n = 52) | 2.3 [1.3–4.0] | 188.4 (375.6) | 8.3 (25.5) | 75.1 (280.7) | 9 (17.3) | |
| QRS duration | ≤120 ms (n = 376) | 1.4 [1.1–1.7] | 90.3 (211.4) | 6.4 (20.1) | 62.6 (298.7) | 73 (19.4) |
| >120 ms (n = 43) | 2.6 [1.4–4.8] | 84.8 (132.5) | 8.6 (25.5) | 119.3 (374.5) | 7 (16.3) | |
| QRS-T angle | <100° (n = 383) | 1.6 [1.3–1.9] | 90.9 (209) | 6.8 (21.3) | 69.2 (312.7) | 73 (19.1) |
| ≥100° (n = 36) | 1 [0.5–1.9] | 77.7 (151.1) | 5.3 (13) | 59.9 (245.9) | 7 (19.4) |
Crude cumulative AF burden was calculated as cumulative AF duration in the negative binomial model with the total monitoring duration as offset. IAB was defined as P-wave duration ≥120 ms combined with the presence of biphasic P-wave (positive–negative) in any inferior lead. QTc interval was estimated using the Framingham formula.
Missing observations: PR interval, n = 2; P-wave duration, n = 3; P-wave voltage in Lead I, n = 5; P-wave axis, n = 10, P-wave terminal force in Lead V1, n = 5; IAB, n = 3.
Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiogram; IAB, interatrial block; QTc, heart rate-corrected QT interval; SD, standard deviation.
Figure 2.
Incidence rate ratio for cumulative AF burden among 419 AF patients. The figure shows the incidence rate ratio for cumulative AF burden according to dichotomized ECG parameters among 419 patients who developed AF. The interatrial block was defined as P-wave duration ≥120 ms combined with biphasic (positive–negative) P-wave in any inferior lead. QTc interval was estimated using the Framingham formula. Incidence rate ratios were determined in multivariate negative binomial regression models. Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiogram; QTc, heart rate-corrected QT interval.
Atrial fibrillation progression among atrial fibrillation patients
Among 419 AF patients, an overall reduction in cumulative AF burden was seen in the second half-period compared with the first half of the timespan from the first adjudicated AF episode to censoring (IRR 0.70 [0.51–0.96]). No significant interactions were detected between the ECG parameters and the development in cumulative AF burden over time (see Supplementary material online, Table S2).
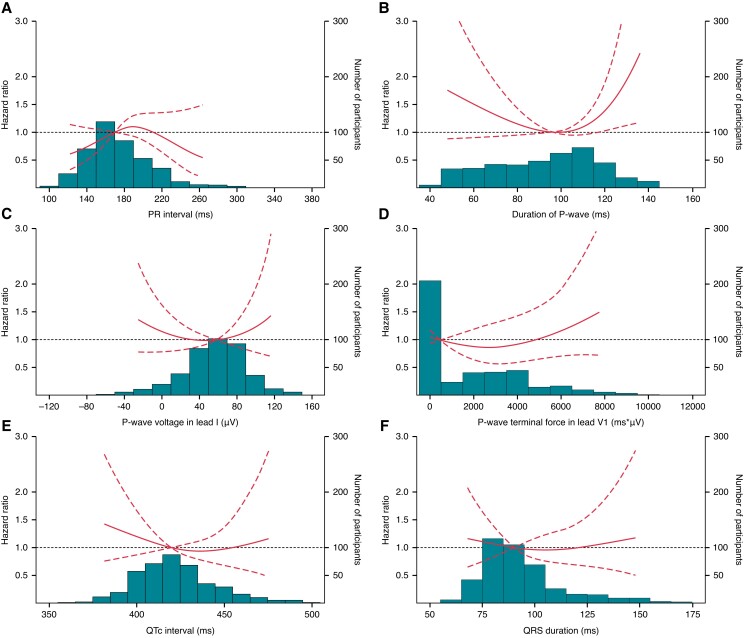
During follow-up, one (0.2%) of the 419 AF patients debuted with AF episode ≥24 h. Among the remaining 418 patients with shorter AF at first detection, the presence of IAB was associated with progression to AF duration ≥24 h in the time-to-first-event analysis using the date of first AF episode as index (HR 1.86 [1.02–3.39]; Figure 3). Additionally, with ECG parameters treated as continuous variables, also longer PWD demonstrated a significant association with this progression in AF episode duration (Figure 4).
Figure 3.
Hazard ratio for progression to AF episode ≥24 h. The figure shows the risk of AF progression to the first episode ≥24 h according to dichotomized ECG parameters, among 418 patients debuted with shorter AF duration. The interatrial block was defined as P-wave duration ≥120 ms combined with biphasic (positive–negative) P-wave in any inferior lead. QTc interval was estimated using the Framingham formula. Hazard ratios were determined in a multivariate Cox model with death as competing risk. Abbreviations: AF, atrial fibrillation; CI, confidence interval; ECG, electrocardiogram; QTc, heart rate-corrected QT interval.
Figure 4.
The relationships between ECG parameters as a continuous variable and the risk of progression to AF episode ≥24 h. The figure shows the risk of AF progression to the first episode ≥24 h as a function of PR interval (A), P-wave duration (B), P-wave voltage in Lead I (C), P-wave terminal force in Lead V1 (D), QTc interval estimated using the Framingham formula (E), and QRS duration (F) among 418 patients debuted with shorter AF duration, superimposed on a histogram of the distribution of each of the ECG parameters. Hazard ratios were estimated by using the respective medians as the references in multivariate Cox models with death as a competing risk. The dashed lines represent 95% confidence intervals. Abbreviations: AF, atrial fibrillation; ECG, electrocardiogram; QTc, heart rate-corrected QT interval.
Discussion
This post hoc analysis of the LOOP Study investigated the relationships between ECG parameters and new-onset AF detected by long-term continuous monitoring in elderly individuals with additional stroke risk factors. The key findings were (i) several ECG parameters—mainly those related to P-wave—were associated with new-onset AF; (ii) both shorter and longer PWD, greater PTF, and the presence of IAB demonstrated associations with an increased risk of long-lasting AF; (iii) abnormal PWD, PWA, PTF, QTc interval, and QRS duration as well as the presence of IAB were associated with higher cumulative AF burden; and (iv) longer PWD and IAB were related to an increased risk of AF progression.
Atrial fibrillation detection
Previous research has identified various ECG parameters as predictors of incident AF as well as AF recurrence after ablation.6–17,21,22 Especially the use of P-wave parameters has been suggested by the recent consensus document from the International Society of Electrocardiology for detection of pathological changes in atria and thereby AF risk stratification.6 Indded, through an AI-based algorithm, a non-randomized, interventional trial also confirmed the utility of sinus rhythm ECG features in the selection of patients with high AF risk for screening.18 In line herewith, our study found several ECG parameters to be associated with new-onset ILR-detected AF, with abnormal PWV showing the most remarkable association (Figure 1A). This inverse correlation between PWV and AF is supported by a retrospective study of patients undergoing AF ablation wherein Park et al.7 observed a higher incidence of AF recurrence in patients with lower PWV, along with displaced interatrial conduction. Given the axis of Lead I running in parallel to Bachmann’s bundle, a reduction of PWV might, therefore, represent impaired conduction in Bachmann’s region, similarly to the proposed mechanism underlying IAB that has been linked to incident AF in previous studies.15,21,22 For PWD and PR interval, Nielsen et al.11,17 demonstrated a U-shaped relationship between both parameters and incident AF in the Copenhagen ECG Study of nearly 300 000 subjects. Our data portrayed a similar trend for PWD, but not for PR interval which was related to AF detection only in the longer range (see Supplementary material online, Figure S1). However, it could be speculated that a heritability of shortened PR interval predisposing to enhanced AF susceptibility23 might potentially also have led to early AF diagnosis and thereby exclusion from the LOOP Study. It is also possible that a larger sample size and thus greater statistical power were needed to identify such an AF risk increment for shortened PR interval, considering that the community-based Framingham Heart Study neither showed an association of AF with PR shortening.9 These two explanations might also apply to the inconsistency between our results and that of the Copenhagen ECG study with respect to QTc interval. Indeed, Nielsen et al.12 reported QTc interval to confer a higher risk of clinical AF both in the shorter and the longer range, whereas our study did not detect a significantly increased AF rate for shortened QTc interval (Figure 1A and Supplementary material online, Figure S1) in agreement with an analysis of three US population-based cohorts.8 However, these inconsistent findings also indicate the complexity of the associations between ECG parameters and AF, and hence, more evidence is needed to establish the clinically relevant reference values for the respective parameters.6
Atrial fibrillation burden
One of the main issues in AF screening raised by the recent position paper from the European Heart Rhythm Association is the lack of a practical, cost-effective screening strategy.1 Indeed, we have learned from the primary reporting of the LOOP Study that not all AF are worth being screened for. There is a need for risk stratification tools to better identify the high-risk subpopulation more likely to benefit from AF screening. In the present study, we attempted to investigate 12-lead ECG parameters for the burden of new-onset subclinical AF, as numerous studies have ascertained a dose–response relationship between AF burden and stroke risk.2–5 Given the fact that widely varying definitions of AF burden have been applied in previous research,2,5,24 the burden of ILR-detected AF was, therefore, examined in our study both as the risk of continuous AF episode ≥24 h and as the cumulative AF duration divided by the total monitoring duration.
For the risk of long-lasting AF, we demonstrated PWD and IAB to be associated with AF episodes ≥24 h, with PWD exhibiting a similar U-shaped association pattern as for AF detection (Figure 1B and Supplementary material online, Figure S2). More interestingly, although greater PTF was not related to higher incidence of AF detection, it did appear to be associated with an increased risk of AF duration ≥24 h. However, the statistical significance was not reached at the pre-specified cut-off of >4000 ms × µV, but first at >6000 ms × µV. This seemingly accords with results from a large epidemiological study in Finland showing an increased AF risk only in subjects with PTF >6000 ms × µV, but not those with 4000–6000 ms × µV.10
With AF burden assessed as the percentage of the total monitoring time, the mean cumulative burden among AF patients was estimated to be 1.5% [1.2–1.8%] in the present study, which was lower than the previously reported 3.0% among paroxysmal AF patients with cardiac implantable electronic device (CIED).25 However, this difference comes as no surprise, since our study population exclusively comprised AF-naïve subjects at baseline, with an average time of 1.01 (SD, 0.98) years to first AF detection among AF patients. Our study reported several ECG parameters to be significantly associated with cumulative AF burden, with abnormal QRS duration showing the largest increase (Figure 2). This is in alignment with Aeschbacher et al.13 who found an association between longer QRS and increased risk of clinical AF in the Atherosclerosis Risk in Communities Study. Given the lacking associations between QRS duration and the risks of AF detection and long-lasting AF, our results may suggest that individuals with longer QRS duration tend to experience more short-lasting AF episodes. However, further studies are needed to elucidate the clinical impact of a high cumulative AF burden in the absence of long-lasting episodes.
Atrial fibrillation progression
For the development of ILR-detected AF, we observed an overall reduction of cumulative AF burden over time in AF patients (see Supplementary material online, Table S2). This is in line with a previous sub-analysis of the ILR participants by Diederichsen et al.26 showing a cumulative burden reduction over time, even when censoring for initiation of AF ablation, Direct-Current cardioversion, or the initiation of Class I/III antiarrhythmics. These results may thus imply that subclinical AF is a disease of highly heterogeneous nature, with the majority emerging only transiently and diminishing over time spontaneously afterwards. Therefore, it is particularly important to identify in advance the patients who tend to experience persistence and even progression of their subclinical AF. Indeed, these patients would be more prone to AF-related complications and may constitute the appropriate patient population more likely to benefit from AF treatment including anticoagulation. In our study, although no ECG parameters succeeded in predicting the course of cumulative AF burden over time, we found PWD and IAB to be significantly associated with progression from shorter AF episode to ≥24 h (Figures 3 and 4). These findings are indeed consistent with the observed trend towards persistent cumulative AF burden over time in AF patients with PWD >120 ms and IAB, albeit no statistical significance was reached (see Supplementary material online, Table S2). More importantly, both parameters demonstrated significant associations with this progression in AF episode duration, independent of conventional risk factors such as sex and comorbidities which have been linked to transition to longer AF duration in previous studies of CIED patients.27 Therefore, these P-wave parameters may potentially also contribute to better risk characterization of patients with known AF for further intensification of management strategies, as the conventional comorbidity-based risk stratification tools have shown to predict the residual stroke risk in these patients receiving guideline-directed AF treatment.28 Moreover, it is noteworthy that only patients with longer PWD were at increased risk of transition to longer AF duration in our study. The lack of significant association with P-wave shortening could be partly due to the insufficient power of this study and partly due to the predominantly short-term effect of shorter PWD on AF as observed in the Copenhagen ECG Study.17
Perspectives
According to current guidelines from the European Society of Cardiology, opportunistic AF screening is recommended in patients aged ≥65 years.2 However, with a number of 110 needed to screen for AF detection in the general population, this strategy does not seem to be realistic and cost-effective.1 Therefore, better risk stratification tools to define the high-risk patient population for AF screening are urgently demanded to optimize the cost-effectiveness and inform screening strategy. In this regard, the easily accessible 12-lead ECG may be useful, as particularly P-wave parameters are found to be risk factors for incident AF and AF-related complications such as stroke, heart failure, dementia, and death.6 Indeed, our study suggests several ECG parameters to be associated with the onset and the cumulative burden of subclinical AF. PWD and IAB have further been demonstrated to be effective at identifying individuals with a higher risk of long-lasting AF and AF progression. This might imply that these two P-wave parameters could serve as early risk markers of atrial cardiomyopathy that acts as the underlying cause of AF and cardioembolic stroke.1,2,6 However, further studies are needed to assess whether these parameters could predict the benefits of subclinical AF detection and treatment.6
Limitations
Our study has several limitations. First, our study might be underpowered to detect small, but relevant associations between ECG parameters and subclinical AF, although the study has a very well-characterized population with adjudicated outcomes. Secondly, the participant recruitment outside the hospital setting, which is highly relevant for screening, could have introduced healthy user bias, and further, these results might not be extrapolated outside the Caucasian population. Thirdly, we do not have data on the anatomy, function, and fibrosis of the atria which are also associated with subclinical AF. However, we were able to adjust for several baseline cardiovascular comorbidities and risk factors that may be closely related to the atrial morphology.
Conclusions
Several ECG parameters were associated with new-onset subclinical AF detected by ILR, and especially P-wave parameters demonstrated robust associations with both the onset, the burden and the progression of AF over time. Hence, P-wave parameters may help to identify patients more prone to AF-related complications and thus more likely to benefit from screening. However, further studies are needed to assess whether ECG parameters could predict the benefits of subclinical AF detection and treatment.
Supplementary Material
Acknowledgements
We thank Christian Kronborg (University of Southern Denmark, Denmark) for his contribution to the trial steering committee of the LOOP Study. We thank Dan Atar (Oslo University Hospital Ullevål, Norway), Gregory Y. H. Lip (The University of Liverpool, UK), and Mårten Rosenqvist (Karolinska Institutet and Danderyd Hospital, Sweden) for their contribution in the international advisory committee of the LOOP Study. We thank our colleagues at Rigshospitalet, Bispebjerg and Frederiksberg Hospital, Zealand University Hospital, and Odense University Hospital who assisted with the conduct of the LOOP Study.
Contributor Information
Lucas Yixi Xing, Department of Cardiology, Copenhagen University Hospital – Rigshospitalet, Inge Lehmanns Vej 7, 2100 Copenhagen, Denmark; Department of Cardiology, Zealand University Hospital Roskilde, 4000 Roskilde, Denmark.
Søren Zöga Diederichsen, Department of Cardiology, Copenhagen University Hospital – Rigshospitalet, Inge Lehmanns Vej 7, 2100 Copenhagen, Denmark; Department of Cardiology, Bispebjerg Hospital, Copenhagen University Hospital, 2400 Copenhagen, Denmark.
Søren Højberg, Department of Cardiology, Bispebjerg Hospital, Copenhagen University Hospital, 2400 Copenhagen, Denmark.
Derk W Krieger, Department of Neurology, Mediclinic City Hospital, Dubai, United Arabic Emirates; Department of Neuroscience, Mohammed Bin Rashid University of Medicine and Health Science, Dubai, United Arabic Emirates.
Claus Graff, Department of Health Science and Technology, Aalborg University, 9220 Aalborg, Denmark.
Morten S Olesen, Department of Cardiology, Copenhagen University Hospital – Rigshospitalet, Inge Lehmanns Vej 7, 2100 Copenhagen, Denmark; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Jonas Bille Nielsen, Department of Cardiology, Copenhagen University Hospital – Rigshospitalet, Inge Lehmanns Vej 7, 2100 Copenhagen, Denmark.
Axel Brandes, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, 5000 Odense C, Denmark; Department of Cardiology, Odense University Hospital, 5000 Odense, Denmark; Department of Cardiology, University Hospital of Southern Denmark Esbjerg, 6700 Esbjerg, Denmark.
Lars Køber, Department of Cardiology, Copenhagen University Hospital – Rigshospitalet, Inge Lehmanns Vej 7, 2100 Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Ketil Jørgen Haugan, Department of Cardiology, Zealand University Hospital Roskilde, 4000 Roskilde, Denmark.
Jesper Hastrup Svendsen, Department of Cardiology, Copenhagen University Hospital – Rigshospitalet, Inge Lehmanns Vej 7, 2100 Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
The LOOP Study was supported by Innovation Fund Denmark [grant number 12-1352259], The Research Foundation for the Capital Region of Denmark, The Danish Heart Foundation [grant number 11-04-R83-A3363-22625], Aalborg University Talent Management Program, Arvid Nilssons Fond, Skibsreder Per Henriksen, R og Hustrus Fond, Horizon 2020 [grant number 847770 to the AFFECT-EU consortium], Læge Sophus Carl Emil Friis og hustru Olga Doris Friis’ Legat, and an unrestricted grant from Medtronic. The employment of the first author, L.Y.X., is funded by the AFFECT-EU consortium and thereby the Horizon 2020 programme.
Data availability
The data underlying this article cannot be shared publicly for ethical reasons, but the methodology will be shared on reasonable request to the corresponding author (J.H.S.).
References
- 1. Kalarus Z, Mairesse GH, Sokal A, Boriani G, Średniawa B, Arroyo RCet al. . Searching for atrial fibrillation: looking harder, looking longer, and in increasingly sophisticated ways. An EHRA position paper. Europace Published online October 18,2022. 10.1093/europace/euac144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GAet al. . 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci Aet al. . Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–9. [DOI] [PubMed] [Google Scholar]
- 4. Carrington M, Providência R, Chahal CAA, Ricci F, Epstein AE, Gallina Set al. . Clinical applications of heart rhythm monitoring tools in symptomatic patients and for screening in high-risk groups. Europace 2022;24:1721–9. [DOI] [PubMed] [Google Scholar]
- 5. Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar Det al. . Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018;39:1407–15. [DOI] [PubMed] [Google Scholar]
- 6. Chen LY, Ribeiro ALP, Platonov PG, Cygankiewicz I, Soliman EZ, Gorenek Bet al. . P wave parameters and indices: a critical appraisal of clinical utility, challenges, and future research—a consensus document endorsed by the International Society of Electrocardiology and the International Society for Holter and Noninvasive Electrocardiology. Circ Arrhythmia Electrophysiol 2022;15:E010435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park JK, Park J, Uhm JS, Joung B, Lee MH, Pak HN. Low P-wave amplitude (<0.1 mV) in lead I is associated with displaced inter-atrial conduction and clinical recurrence of paroxysmal atrial fibrillation after radiofrequency catheter ablation. Europace 2016;18:384–91. [DOI] [PubMed] [Google Scholar]
- 8. Mandyam MC, Soliman EZ, Alonso A, Dewland TA, Heckbert SR, Vittinghoff Eet al. . The QT interval and risk of incident atrial fibrillation. Heart Rhythm 2013;10:1562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cheng S, Keyes MJ, Larson MG, McCabe EL, Newton-Cheh C, Levy Det al. . Long-term outcomes in individuals with prolonged PR interval or first-degree atrioventricular block. JAMA 2009;301:2571–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eranti A, Aro AL, Kerola T, Anttonen O, Rissanen HA, Tikkanen JTet al. . Prevalence and prognostic significance of abnormal P terminal force in lead V1 of the ECG in the general population. Circ Arrhythmia Electrophysiol 2014;7:1116–21. [DOI] [PubMed] [Google Scholar]
- 11. Nielsen JB, Pietersen A, Graff C, Lind B, Struijk JJ, Olesen MSet al. . Risk of atrial fibrillation as a function of the electrocardiographic PR interval: results from the Copenhagen ECG study. Heart Rhythm 2013;10:1249–56. [DOI] [PubMed] [Google Scholar]
- 12. Nielsen JB, Graff C, Pietersen A, Lind B, Struijk JJ, Olesen MSet al. . J-shaped association between QTc interval duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. J Am Coll Cardiol 2013;61:2557–64. [DOI] [PubMed] [Google Scholar]
- 13. Aeschbacher S, O′Neal WT, Krisai P, Loehr L, Chen LY, Alonso Aet al. . Relationship between QRS duration and incident atrial fibrillation. Int J Cardiol 2018;266:84–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jogu HR, O′Neal WT, Broughton ST, Shah AJ, Zhang ZM, Soliman EZ. Frontal QRS-T angle and the risk of atrial fibrillation in the elderly. Ann Noninvasive Electrocardiol 2017;22(2): e12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skov MW, Ghouse J, Kühl JT, Platonov PG, Graff C, Fuchs Aet al. . Risk prediction of atrial fibrillation based on electrocardiographic interatrial block. J Am Heart Assoc 2018;7:e008247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maheshwari A, Norby FL, Soliman EZ, Koene R, Rooney M, O′Neal WTet al. . Refining prediction of atrial fibrillation risk in the general population with analysis of P-wave axis (from the atherosclerosis risk in communities study). Am J Cardiol 2017;120:1980–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nielsen JB, Kühl JT, Pietersen A, Graff C, Lind B, Struijk JJet al. . P-wave duration and the risk of atrial fibrillation: results from the Copenhagen ECG study. Heart Rhythm 2015;12:1887–95. [DOI] [PubMed] [Google Scholar]
- 18. Noseworthy PA, Attia ZI, Behnken EM, Giblon RE, Bews KA, Liu Set al. . Artificial intelligence-guided screening for atrial fibrillation using electrocardiogram during sinus rhythm: a prospective non-randomised interventional trial. Lancet 2022;400:1206–12. [DOI] [PubMed] [Google Scholar]
- 19. Diederichsen SZ, Haugan KJ, Køber L, Højberg S, Brandes A, Kronborg Cet al. . Atrial fibrillation detected by continuous electrocardiographic monitoring using implantable loop recorder to prevent stroke in individuals at risk (the LOOP study): rationale and design of a large randomized controlled trial. Am Heart J 2017;187:122–32. [DOI] [PubMed] [Google Scholar]
- 20. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg Cet al. . Implantable loop recorder detection of atrial fibrillation to prevent stroke (the LOOP study): a randomised controlled trial. Lancet 2021;398:1507–16. [DOI] [PubMed] [Google Scholar]
- 21. Intzes S, Zagoridis K, Symeonidou M, Spanoudakis E, Arya A, Dinov Bet al. . P-wave duration and atrial fibrillation recurrence after catheter ablation: a systematic review and meta-analysis. Europace 2013;6:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Skrebelyte-Strøm L, Rønning OM, Dahl FA, Steine K, Kjekshus H. Prediction of occult atrial fibrillation in patients after cryptogenic stroke and transient ischaemic attack: PROACTIA. Europace 2022;24:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pfeufer A, Van Noord C, Marciante KD, Arking DE, Larson MG, Smith AVet al. . Genome-wide association study of PR interval. Nat Genet 2010;42:153–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Go AS, Reynolds K, Yang J, Gupta N, Lenane J, Sung SHet al. . Association of burden of atrial fibrillation with risk of ischemic stroke in adults with paroxysmal atrial fibrillation: the KP-RHYTHM study. JAMA Cardiol 2018;3:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charitos EI, Pürerfellner H, Glotzer T V, Ziegler PD. Clinical classifications of atrial fibrillation poorly reflect its temporal persistence: insights from 1,195 patients continuously monitored with implantable devices. J Am Coll Cardiol 2014;63:2840–8. [DOI] [PubMed] [Google Scholar]
- 26. Diederichsen SZ, Haugan KJ, Brandes A, Lanng MB, Graff C, Krieger Det al. . Natural history of subclinical atrial fibrillation detected by implanted loop recorders. J Am Coll Cardiol 2019;74:2771–81. [DOI] [PubMed] [Google Scholar]
- 27. Boriani G, Glotzer T V, Ziegler PD, De Melis M, Mangoni di S, Stefano Let al. . Detection of new atrial fibrillation in patients with cardiac implanted electronic devices and factors associated with transition to higher device-detected atrial fibrillation burden. Heart Rhythm 2018;15:376–83. [DOI] [PubMed] [Google Scholar]
- 28. Ding WY, Blomström-Lundqvist C, Fauchier L, Marin F, Potpara TS, Boriani Get al. . Contemporary management of atrial fibrillation and the predicted vs. Absolute risk of ischaemic stroke despite treatment: a report from ESC-EHRA EORP-AF long-term general registry. Europace Published online November2022;25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly for ethical reasons, but the methodology will be shared on reasonable request to the corresponding author (J.H.S.).