Abstract
Aims
The field of conduction system pacing (CSP) is evolving, and our aim was to obtain a contemporary picture of European CSP practice.
Methods and results
A survey was devised by a European CSP Expert Group and sent electronically to cardiologists utilizing CSP. A total of 284 physicians were invited to contribute of which 171 physicians (60.2%; 85% electrophysiologists) responded. Most (77%) had experience with both His-bundle pacing (HBP) and left bundle branch area pacing (LBBAP). Pacing indications ranked highest for CSP were atrioventricular block (irrespective of left ventricular ejection fraction) and when coronary sinus lead implantation failed. For patients with left bundle branch block (LBBB) and heart failure (HF), conventional biventricular pacing remained first-line treatment. For most indications, operators preferred LBBAP over HBP as a first-line approach. When HBP was attempted as an initial approach, reasons reported for transitioning to utilizing LBBAP were: (i) high threshold (reported as >2 V at 1 ms), (ii) failure to reverse bundle branch block, or (iii) > 30 min attempting to implant at His-bundle sites. Backup right ventricular lead use for HBP was low (median 20%) and predominated in pace-and-ablate scenarios. Twelve-lead electrocardiogram assessment was deemed highly important during follow-up. This, coupled with limitations from current capture management algorithms, limits remote monitoring for CSP patients.
Conclusions
This survey provides a snapshot of CSP implementation in Europe. Currently, CSP is predominantly used for bradycardia indications. For HF patients with LBBB, most operators reserve CSP for biventricular implant failures. Left bundle branch area pacing ostensibly has practical advantages over HBP and is therefore preferred by many operators. Practical limitations remain, and large randomized clinical trial data are currently lacking.
Keywords: CSP, HBP, LBBAP, Survey
What’s new?
The highest ranked pacing indications for CSP are atrioventricular block (irrespective of left ventricular ejection fraction) and when coronary sinus lead implantation failed.
Conventional biventricular pacing remains first-line treatment for patients with LBBB and HF.
For most indications, operators prefer LBBAP over HBP as a first-line approach.
When HBP was attempted as an initial approach, reasons reported for transitioning to utilizing LBBAP were: (i) high threshold (reported as >2 V at 1 ms), (ii) failure to reverse bundle branch block, or (iii) > 30 min attempting to implant at His-bundle sites.
Backup right ventricular lead use for HBP is low (median 20%) and predominated in pace-and-ablate scenarios.
Introduction
Permanent His-bundle pacing (HBP) was first reported over 20 years ago1 as a means of providing physiological ventricular activation and currently features in major societal guidelines.2–7 More recently, left bundle branch area pacing (LBBAP) using a transseptal approach was described8,9 as an approach for delivering physiological pacing in cases where HBP could not be delivered. Its use though has expanded, and it is now often reported as a first-line approach for providing physiological pacing.10,11 Left bundle branch area pacing is currently however, not included in either the European or US guidelines for pacing therapy. This is perhaps because much of the published data were not available when the guidelines were being prepared. Most of the data regarding the safety and efficacy of conduction system pacing (CSP) come from observational studies, with only small numbers of patients included in randomized studies to date.
His-bundle pacing and LBBAP are both promising pacing options as they might prevent or mitigate the ventricular dyssynchrony and mechanical adverse remodelling associated with right ventricular (RV) pacing or even biventricular pacing when utilized in patients with bradycardia.2–5 His-bundle pacing and LBBAP may also have a role for patients currently indicated for traditional biventricular pacing [LBBB >150 ms and heart failure (HF)]12 as CSP can frequently correct bundle branch block, resulting in more rapid ventricular activation times.13
The objective of this study was to conduct and report a survey from a sample of European device implanters with a range of experience in CSP. Our aim was to gather information regarding current CSP practice as well as desired developments in the field. We looked to better understand: (i) patient selection, (ii) implant techniques, (iii) physician preference for HBP vs. LBBAP, (iv) backup lead utilization, (v) patient follow-up approaches, and (vi) desired developments in the field.
Methods
A group of 20 experienced device implanters from 14 European countries, with expertise in both HBP and LBBAP, was gathered with the responsibility of (i) developing a detailed questionnaire on CSP, (ii) identifying and sending the questionnaire to physicians performing CSP, and (iii) reviewing and interpreting the analyses.
A literature search first identified relevant publications, consensus documents and guidelines to determine reported potential indications for CSP. A total of nine2–6,14–17 publications reporting recommendations on the application of CSP in selected patients were considered and used as a base for developing the first round of the questionnaire. Table 1 reports the identified indications for CSP (i.e. HBP).
Table 1.
Comparison of cardiovascular society guidelines indications for CSP
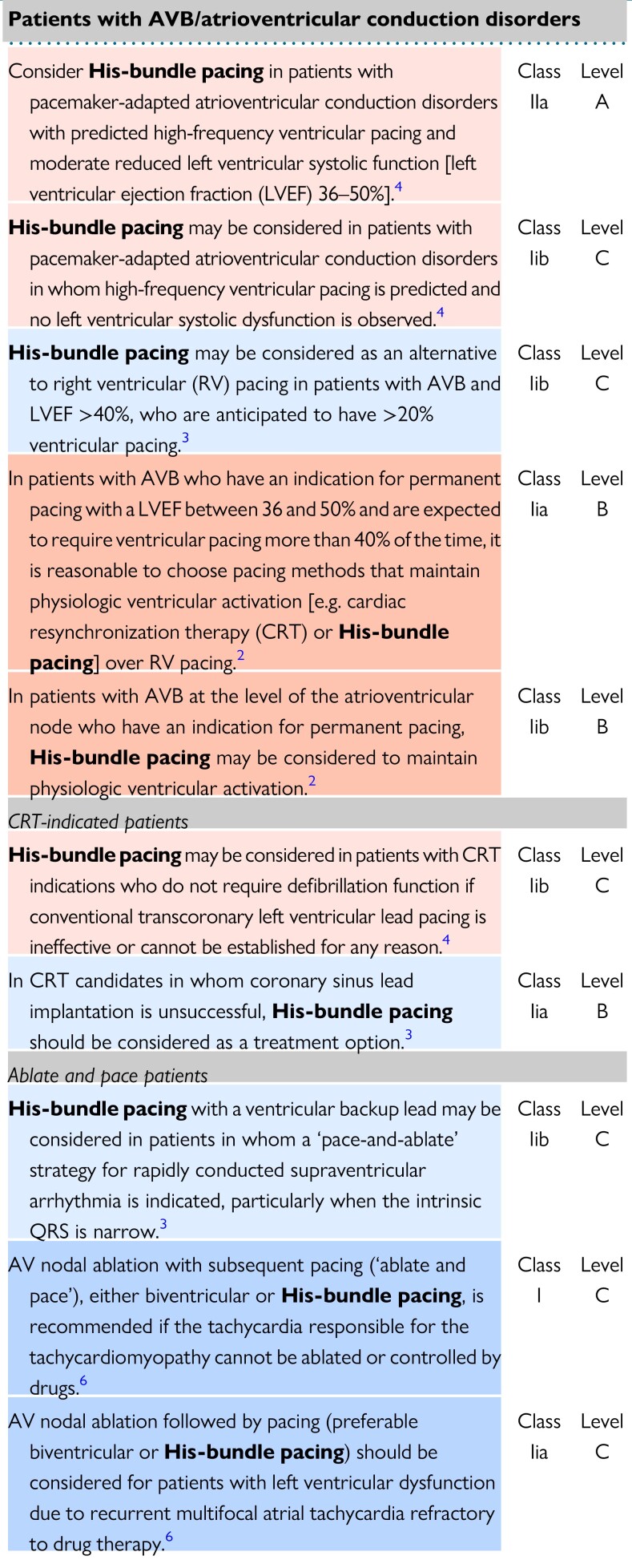
|
Light Blue shading indicates recommendations from ESC 2021 Pacing guideline, Dark Blue shading indicates recommendations from ESC 2019 SVT guideline, Light Red shading indicates recommendations from 2021 Annual JCS/JHRS guideline, Red shading indicates recommendations from ACC/AHA 2018 Pacing guideline.
The results of the literature search were discussed by the steering group leading to the formation of the questions to include within the proposed survey (see Supplementary material online, Appendix S1 details the survey questions in full). The survey consisted of a total of 35 questions: 8 were introductory questions aimed at stratifying the sample of respondents, 5 focused on patient selection, 20 on clinical practice (12 were related to the implant procedure, 5 to the follow-up management, and 3 to the use of backup leads), the last 2 questions aimed to explore potential areas for improving CSP.
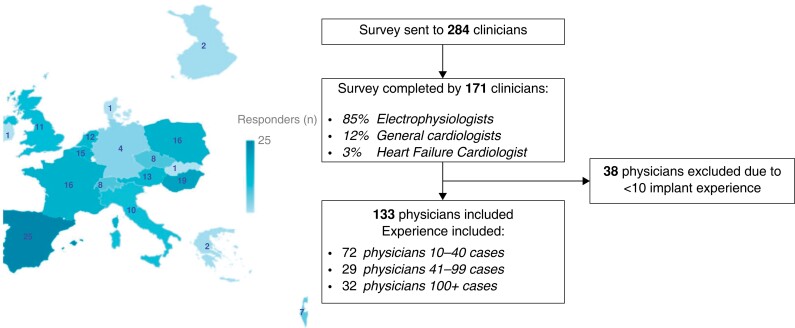
Each member of the steering group sent the on-line survey to at least 10 physicians with expertise in CSP in their geographical area. A total of 284 invitations were sent and 171 European physicians completed the survey anonymously via Qualtrics electronic platform18 (response rate 60.2%). We only considered survey responses from physicians with at least a 10-patient experience in CSP. The subsequent surveys were reviewed and discussed by the steering group.
Statistical analysis
Descriptive statistics were used to summarize respondents’ characteristics and survey results. This includes fractions and percentages for categorical variables and median (Q1–Q3) for continuous variables. One sample proportion test was used to evaluate any difference in preference between HBP and LBBAP. No imputation was used. A two-sided P-value of <0.05 was considered to indicate statistical significance. All statistical analysis was performed using R Statistical Software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Physicians surveyed
The survey was completed by 171 physicians after being sent to 284 clinicians from 18 European countries (response rate 60.2%). The role, location, and experience of those surveyed are displayed in Figure 1 and Supplementary material online, Appendix S2. Responses from 133 physicians were included in the analysis; the responses from 38 physicians were not included in the main analysis as they did not meet the CSP implantation experience (>10 cases) which was a prespecified criteria.
Figure 1.
Summary of respondent’s profile.
The majority of respondents (77%) had experience with both HBP and LBBAP techniques. Of the physicians with experience in both techniques, just 8.7% currently perform mainly HBP when attempting CSP in clinical practice, while 43% mostly perform LBBAP, the remaining 48.3% perform both techniques. From the remaining 23% with experience of just one technique, 13% perform HBP and 10% LBBAP.
Understanding physician decision-making
Ninety-nine per cent of physicians surveyed believe that CSP approaches have clinical advantages over alternative pacing approaches. The main reported advantages were its ability to prevent HF (99% of respondents) and to improve patient quality of life (72% of respondents). For these reasons, implanters consider its role for patients (i) likely to have a high burden of ventricular pacing or (ii) who have left ventricular (LV) impairment prior to pacemaker implantation.
Surveyed implanters felt that experience and training (i.e. ‘need for several procedures before being confident’) was the most substantial barrier to greater adoption of the technique compared with other pacing techniques. When compared with conventional biventricular (BiV) pacing, 37% of responders felt that lack of implant kit limited the approach. Very few surveyed physicians felt that the lack of an electrophysiology (EP) recording system was a limiting factor (only 10% of those surveyed).
Indications for conduction system pacing
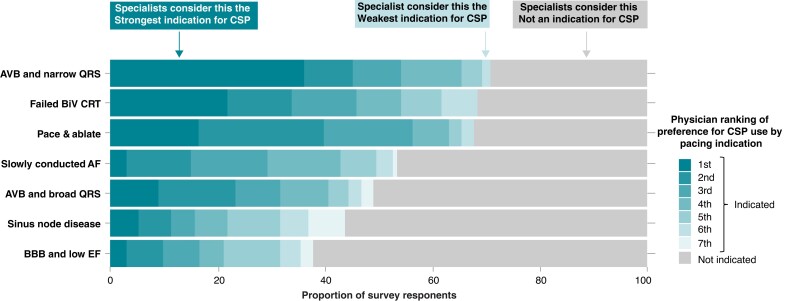
The three most frequently selected appropriate indications for CSP included patients with (i) atrioventricular block (AVB) and narrow QRS, (ii) failed biventricular cardiac resynchronization therapy (CRT), and (iii) atrial fibrillation (AF) with planned AV node ablation (Figure 2). This practice seems to be in line with current Guideline recommendations.2–4 Atrioventricular block with broad QRS was also a frequent first choice indication in the surveyed physicians (17% first rank). Conversely, few physicians who were surveyed favoured using CSP as the first-line CRT approach for patients with HF and bundle branch block (6% first rank) and few ranked sinus node disease highly as an indication for CSP.
Figure 2.
Survey answers on CSP indications. Reports the ranking of respondent’s preferences for CSP for different pacing indications. Patients with AVB and narrow QRS were considered the strongest indication for CSP conversely. Patients with BBB and low ejection fraction (EF) were most frequently considered not indicated for CSP. AVB, atrioventricular block; BBB, bundle branch block; CSP, conduction system pacing.
His bundle pacing, left bundle pacing, or biventricular pacing?
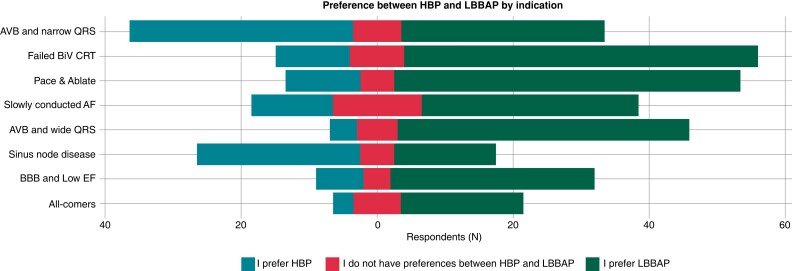
Across all pacing indications, LBBAP was the preferred approach in most cases. His-bundle pacing preference was limited to patients with (i) AVB and narrow QRS (47.1 vs. 42.9%; conduction disease possibly more proximal) and (ii) patients with sinus node disease (54.5 vs. 34.1%; no manifest evidence of AV conduction disease).
Surveyed physicians reported ‘conduction disease location’ to be a key consideration when selecting a CSP approach with likely distal disease being a negative factor for HBP but not LBBAP.
For patients with bundle branch block and severely impaired LV function, BiV pacing was the preferred pacing option in 75% of surveyed physicians (median value). If this failed though left bundle area pacing was the second-choice option, rather than HBP, in the overwhelming majority of respondents (Figure 3).
Figure 3.
Preferences between HBP and LBBAP by indication. HBP, His-bundle pacing; LBBAP, left bundle branch area pacing.
Implant approach considerations
Tools used
Fixed curve sheaths were the preferred first choice delivery approach for both HBP and LBBAP in 88% of surveyed physicians, despite the availability of more novel deflectable sheaths. Deflectable sheaths were preferred by just 12% of those surveyed. In ∼60%, the preferred lead type was the lumen-less lead (3830 Medtronic). For the remaining physicians, there was a greater preference for stylet driven leads when utilizing LBBAP compared with HBP (29 vs. 14%, P = 0.02) this is perhaps because they felt it to be easier to deploy a stylet driven lead into the interventricular septum compared with at the His-bundle location.
Mapping the His bundle
When planning to perform HBP, most respondents (78%) report that they identify the His bundle using the pacing lead in unipolar fashion. EP mapping catheters (90% of responders) or 3D mapping systems (95% of responders) are infrequently deemed to be necessary. About one-third of operators map the His in all their LBBAP procedures (33%) and 30% map the His in some cases.
Acceptable capture response
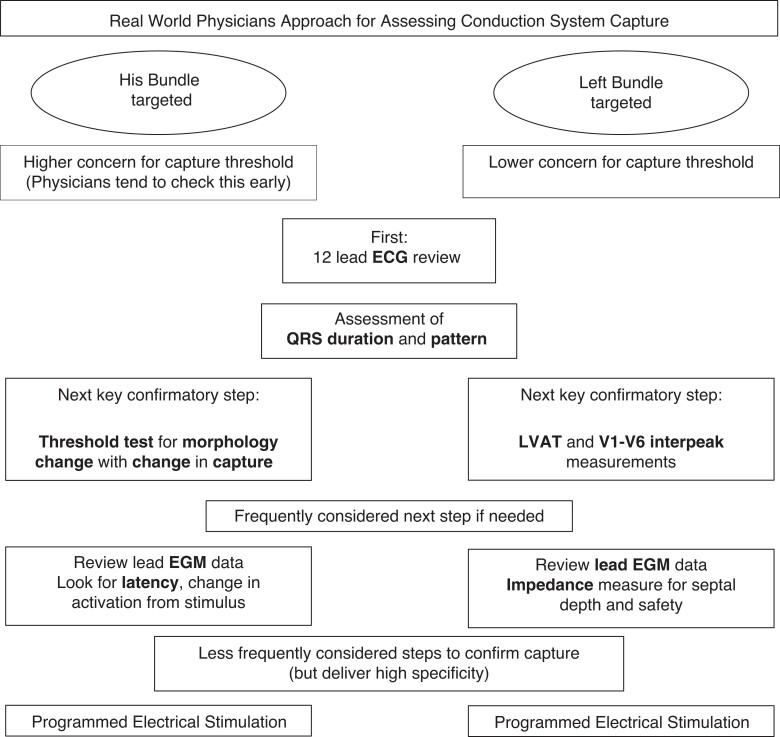
Following lead deployment at the His-bundle position, acceptable capture threshold was felt to be the key priority to check (88% of respondents) followed next by the observation of a change in electrocardiogram (ECG) morphology during threshold testing, in order to confirm His-bundle capture.
Conversely, thresholds were reported to be less of a priority at the left bundle location (perhaps as there is an assumption that they are likely to be lower and therefore less of a clinical problem) and the top priority was the paced ECG response (96%) to confirm capture type. This was followed by lead impedance, which is often used as a marker for progression through the septum and therefore safety (low impedance suggests potential septal perforation).
For both techniques, the paced ECG response (and paced QRS duration) were deemed to be the most useful and important technique for confirming optimal lead position (∼95% of surveyed physicians). For HBP, lead electrogram recordings and the assessment of any latency period were the next most important features, these characteristics can help determine whether His-bundle capture has been achieved and specifically whether it is selective or non-selective. For LBBAP however, the additional markers considered important were LV activation time (LVAT; typically measured from pacing stimulus to R-wave peak in V5 or V6) and the R-wave interpeak time (typically measured as the V6–V1 interpeak interval measured from the paced R-wave peak in V5/6 to R-wave peak in V119). These are widely cited markers to help identify the likely presence of left bundle capture. Assessing for change in morphology with HBP was more frequently reported than for LBBAP to confirm capture (Figure 4).
Figure 4.
How surveyed physicians approach their assessment of conduction system capture. ECG: electrocardiogram; EGM: electrogram; LVAT: left ventricular activation time measured from pacing stimulus to R-wave peak in V5 or V6; V1–V6 interpeak measurement: R-wave peak time (measured as the V6–V1 interpeak interval measured from R-wave peak in V5/6 to the R-wave peak in V1).
Switching from His-bundle pacing to left bundle branch area pacing or abandoning an implant attempt
In HBP implants, failure to capture the conduction system or a high pacing threshold (indicated as >2 V at 1 ms by the most of respondents) were considered the main factors to give up an HBP approach and/or to switch to LBBAP. Other reported reasons included (i) failure of bundle branch recruitment in BBB, (ii) sensing issues, or (iii) if the His lead positioning was taking longer than 30 min.
Time constraints were considered less of a limitation for LBBAP. The two main reasons reported to abandon a left bundle pacing attempt were failure to reverse bundle branch block and difficulties fixating the lead deep within the septum.
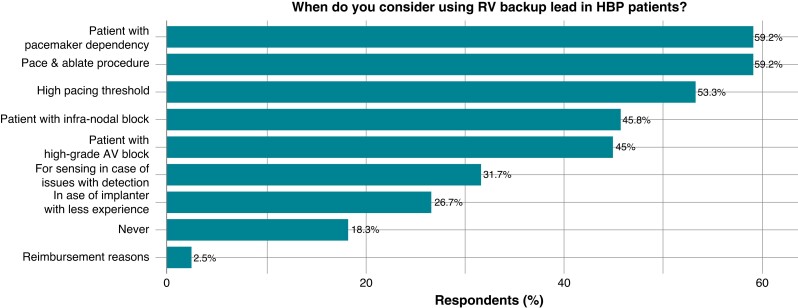
Backup lead use
The minority (18%) of physicians stated they would never consider a backup lead when utilizing an HBP strategy. In contrast, the overwhelming majority (78%) of surveyed physicians reported that they did not consider a backup lead to be necessary with LBBAP.
The most frequently cited reason for considering a backup lead across both pacing approaches was pacemaker dependency, due to either (i) AV node ablation (20% of physicians for left bundle pacing vs. 59% for HBP) or (ii) advanced conduction system disease. For HBP cases, the additional reasons for backup lead use include (i) high threshold at implant (>2 V) or (ii) poor sensing (Figure 5).
Figure 5.
Questions and answers of the survey on backup lead use.
Follow-up
Most physicians (62%) see patients with CSP devices in person every 6 months, this is in keeping with ESC pacing guidelines.3 Twenty-three per cent review annually, whereas a much smaller minority see quarterly.
Of the 95 physicians that utilize remote monitoring (RM) for CSP patient follow-up, spread was variable for how often these virtual visits are performed: 3 monthly 54%, 6 monthly 36%, and annually 11%. Thirty-two physicians reported not using RM for follow-up at all.
Automated device-based threshold testing is not designed to determine capture type with CSP and therefore relying on capture management algorithms for HBP is generally not recommended—particularly in the presence of selective HBP.
For optimal follow-up care, surveyed physicians (>90%) stressed the value of a 12-lead ECG which is different to follow-up of other cardiac implantable electrical devices. To confirm CSP capture, survey respondents mainly evaluate QRS morphology, coupled with threshold transitions for HBP patients and with LVAT for LBBAP patients. Only infrequently (24%) did physicians report resorting to more involved or complex assessments like programmed electrical stimulation to confirm definitively the presence or absence of conduction system capture.
Future perspectives
Finally, the survey explored physician’s opinions regarding the priorities for future development within CSP. The most selected technological developments by surveyed physicians were: (i) new delivery sheaths designed to assist with implantation in patients with challenging anatomies, (ii) leads with a longer screw length, (iii) dedicated capture management algorithms, and (iv) batteries with longer life expectancy particularly for HBP in keeping with its relatively higher capture thresholds.
Discussion
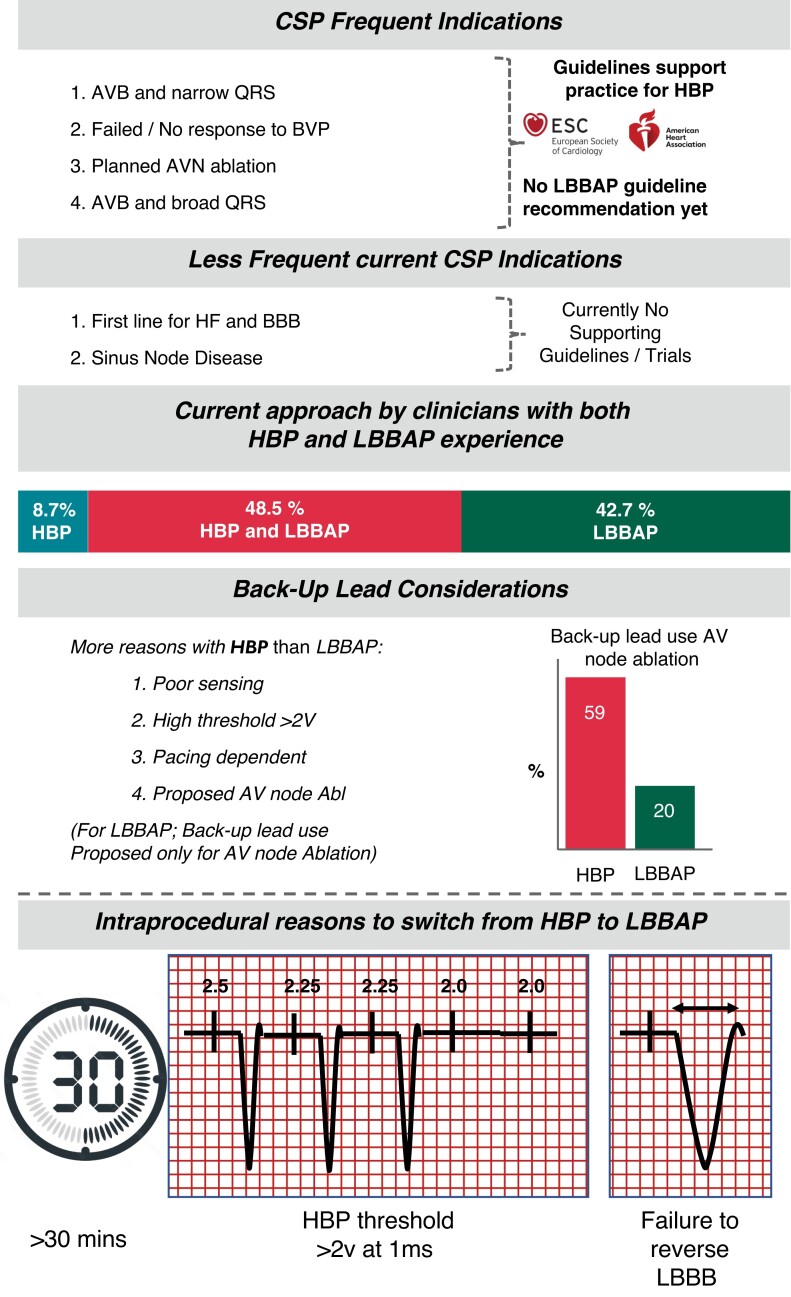
This survey provides a contemporary picture of European CSP practice. Summary findings are detailed in Figure 6.
Figure 6.
Conduction system pacing survey summary of findings.
Main findings
Our findings suggest that European conduction system implanters practice is consistent with guidelines recommendations for CSP, and with similar findings to other surveys conducted.20 It is predominately utilized for patients with ventricular rather than atrial pacing indications but it is not currently replacing conventional biventricular pacing as a first-line therapy for patients with LV impairment and bundle branch block. It is possible that this practice may change in future as more data are obtained from randomized clinical trials (RCTs).
Despite the 2021 European guidelines, only giving recommendations for HBP (without covering LBBAP), the surveyed physicians show a greater utilization and adoption of LBBAP for most indications. That said, HBP is still frequently used particularly for patients with sinus node disease and also those with AV block and a narrow QRS—perhaps as in these cases the conduction system disease is presumed to be proximal in location.
This survey interestingly for the first time defines what prompts intra-procedural transition from one approach to the other.
Physicians have simplified implant approaches, and this survey highlights that clinicians no longer consider the need for femoral placed mapping catheters or even in some cases an EP system to guide the implant and have in general moved away from utilizing backup leads except in the situation of proposed AV node ablation. Backup lead usage is greater with HBP than for left bundle pacing.
Pacing indications
Both ACC/AHA2 and ESC guidelines3,7 make recommendations for the use of HBP. Neither society at present provides recommendations for LBBAP, although an update from the American guidelines is expected shortly which is likely to include this treatment modality.
Our survey suggests that current clinical practice in Europe for conduction system implanters is largely in line with guideline recommendations for patient selection with respect to CSP, but that many are increasingly using LBBAP rather than HBP. For patients with LBBB and a reduced EF, conventional biventricular pacing remains currently the preferred first-line treatment approach.
In contrast to guidelines recommendations, clinicians prefer to implant far more left bundle leads rather than His-bundle leads. This may lead some to consider the published guidelines already outdated although only recently published. This could be because left bundle pacing is a relatively new approach and the appraisal of publications prior to guideline writing may well predate much of the available data. That said, the hesitancy around left bundle pacing in the recent ESC pacing guidelines must be acknowledged. Left bundle pacing did not at the time have as much longer term data as that which exists for His pacing (hence why His is included) and unfortunately despite physician enthusiasm both approaches currently lack large adequately powered RCT data supporting their widespread use. Large RCTs are therefore needed to confirm the role that these physiological promising techniques offer.
It is worth noting, although low in number, physicians will utilise CSP for patients with sinus node disease. These patients may benefit from CSP if they develop AV block, have tachy-brady syndromes requiring AV blocking drugs or may potentially be candidates for future AV node ablation. That said, these patients may not necessarily have much to gain initially from CSP pacing approaches but the physician might progress more rapidly along their personal learning curve with very little cost/risk to the patient. It is well known there is a learning curve associated with both His and left bundle implants.11,21
Which pacing approach?
His-bundle pacing provides the closest ventricular activation to how nature intended by harnessing the exact same electrical pathways thus providing the most physiological activation possible. Unfortunately, with the current tools, techniques and devices, HBP cannot always be delivered either due to failure to engage the conduction system or due to pacing parameters being unacceptable e.g. high thresholds or low sensing. Furthermore, it is apparent that 7–13% of His lead implants will require lead revision or inactivation, prompting safety concerns to be raised by some.11,21–24 Left bundle pacing was first described over 5 years ago and seemingly offers a robust alternative to HBP with minimal compromise on providing physiological ventricular activation. For these reasons, many physicians appear to have transitioned from utilizing His-bundle approaches to the left bundle approach and with some new CSP implanters only learning the left bundle approach. This is because left bundle pacing, although not seen as the physiological ideal by some, may be perceived as being quicker to implant with overall less concerns regarding lead threshold or sensing. However, follow-up data for this approach is limited both by time and by number of patients and therefore vigilance is required assessing for complications with this approach.11,21,22 The His-bundle approach should not be abandoned, with many skilled operators reporting excellent patient outcomes with very satisfactory long-term parameters. What may be of use is further investment in improvements in implantation techniques, patient selection, lead design and device technology to improve the feasibility and safety of HBP which serves as the physiological pacing ideal.
With the current RCT level of evidence supporting biventricular pacing for patients with LV impairment and LBBB, it is of no surprise that biventricular pacing remains the recommended first line in guidelines for these patients and this recommendation is commonly followed by physicians. Conduction system pacing does however offer an alternative when needed. Its use as a first-line approach though requires further study; the current small studies which have been conducted focused mainly on echo parameters and frequently report no difference between the approaches, but no study has yet been conducted looking even for non-inferiority for the much-needed hard endpoints of hospitalization and mortality.
Backup lead utilization
Backup lead use is far more likely in patients with His pacing systems rather than with left bundle leads. This relates to the concerns, regarding sensing and capture issues with His and as such for those that use them, the backup lead fulfils an important safety role.
In the context of proposed AV node ablation, both His and LBBAP approaches had high backup lead utilization. The rationale for backup lead use in LBBAP is not clear cut. It could be because a backup lead is recommended in ESC guidelines3 for patients receiving His pacing in the context of pace and ablate and this has been extrapolated by physicians for their left bundle lead practice or could be because of genuine clinical concern amongst operators although this is not observed in other situations where patients are also pacing dependent. Further research is needed here to help understand this. What is known, however, is that additional hardware has both a financial cost and could lead to greater device related complications due to more leads being implanted, and as such should only be implanted if and when needed.
When to change approach
This survey for the first time has captured physician’s views on what prompts a change in pacing strategy from HBP to left bundle pacing. The reported criteria included (i) a threshold of >2 V at 1 ms, (ii) time longer than 30 min to achieve His-bundle capture, and (iii) failure to reverse LBBB when this was being targeted. Whether these prompts were different prior to the availability of the left bundle pacing is unknown but in the current era seem pragmatic for those engaging in a strategy that targets the His-bundle first. It is possible that when LBBAP is attempted, even if true left bundle pacing has not been achieved many operators may choose to accept LV septal pacing which can frequently be obtained in this scenario.25 This has not been readily shown to have detrimental effects for those with bradycardia indications9 but may be less effective for those patients with impaired LV function and LBBB.26
Assessing pacing response and device follow-up
For CSP to be a true replacement for the current approaches to deliver cardiac pacing (particularly to replace RV pacing in bradycardia indicated patients), it must evolve into a simple and quick enough procedure with simple yet accurate enough steps to accurately confirm appropriate conduction system capture. The practice of surveyed physicians suggests this is already occurring, with reliance on 12-lead ECG markers being the primary method in both pacing techniques to determine the type of pacing being delivered.
This is important as a 12-lead ECG is readily available in all pacing catheter laboratories the world over. For both His and LBBAP cases, the paced QRS was felt to be key, coupled for His pacing approaches with the observation of QRS transitions during threshold testing. For LBBAP additional key measurements of LVAT sometimes referred to as R wave peak time (RWPT) measured as the time from pacing stimulus to R wave in v5/v6 and the V6–V1 interpeak intervals were keys for physicians to assess the type of capture delivered. Validation of these simplified methods alone to enable confirmation of type of capture must be performed to enable reliable pacing delivery while ensuring physicians are not expected to be burdened with more complex testing manoeuvres when not necessary.
Limitations
The present survey reflects the experience of those physicians (60.2%) that choose to respond to our request and does not correspond to the experience of all contacted physicians. As for any anonymous survey based on voluntary participation, we can presume that willingness to participate, together with the removal of responses from those with <10 CSP implants, may identify healthcare professionals with a specific interest on the topic of CSP and with specific knowledge on most recent technologies for cardiac pacing. This may limit the possibility to extrapolate the findings to other healthcare professionals with a lower degree of knowledge or confidence with CSP. The reported data were not verified which could prompt bias with over or under reporting of actual data but with such a survey we need to rely on the data entered by respondents much like in many observational studies. Because of the risk of significant bias towards under reporting of complications, we did not attempt to collect complication rates in this survey.
Conclusions
This large survey of practicing physicians provides a picture of the implementation of CSP in Europe. Conduction system pacing use is increasing, particularly for bradycardia indications in keeping with the guidelines, but does not yet replace the evidence based approach of BiV pacing in HF patients with LBBB. Left bundle branch area pacing ostensibly has practical advantages over HBP and is therefore preferred in many situations. Practical limitations remain for which solutions are sought and notably large RCT data are currently missing.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Acknowledgements
The authors thank all the participating physicians from all over Europe for completing the survey. Additionally, the authors thank Teodora Bellone, Lorenza Mangoni, Alphons Vincent, Sergio Cavaglia, and Cecile Menard from Medtronic for their technical and methodological support to the project.
Contributor Information
Daniel Keene, National Heart and Lung Institute, Imperial College London, Du Cane Road, London W12, UK.
Frédéric Anselme, Department of Cardiology, Centre Hospitalier Universitaire de Rouen Charles Nicolle, Rouen, France.
Haran Burri, Department of Cardiology, University Hospital of Geneva, Geneva, Switzerland.
Óscar Cano Pérez, Unidad de Arritmias, Servicio de Cardiología, Hospital Universitario y Politécnico La Fe, Valencia, Spain; Department of Cardiology, Centro de Investigaciones Biomédicas en RED en Enfermedades Cardiovasculares, Madrid, Spain.
Karol Čurila, Department of Cardiology, Third Faculty of Medicine, Charles University and University Hospital Kralovske Vinohrady, Prague, Czech Republic.
Michael Derndorfer, Department of Internal Medicine 2 with Cardiology, Angiology and Intensive Care, Ordensklinikum Linz Elisabethinen, Linz, Austria.
Paul Foley, Wiltshire Cardiac Centre, Great Western Hospitals NHS Foundation Trust, Swindon, UK.
László Gellér, Semmelweis University, Cardiovascular Center, Budapest, Hungary.
Michael Glikson, Department of Cardiology, Shaare Zedek Medical Center and Hebrew University faculty of medicine, Jerusalem, Israel.
Wim Huybrechts, Department of Cardiology, University Hospital Antwerp, Edegem, Belgium.
Marek Jastrzebski, First Department of Cardiology, Interventional Electrocardiology and Hypertension, Jagiellonian University Medical College, Kraków, Poland.
Krzysztof Kaczmarek, Electrocardiology Department, Medical University of Lodz, Lodz, Poland.
Grigorios Katsouras, Department of Cardiology, ‘F. Miulli’ Hospital, Acquaviva delle Fonti, Bari, Italy.
Jonathan Lyne, Cardiology Department, Beacon Hospital, Dublin, Ireland.
Pablo Peñafiel Verdú, Arrhythmia Unit, Department of Cardiology, Virgen de la Arrixaca University Hospital, Murcia, Spain.
Christian Restle, Division of Cardiology and Angiology II, University Heart Center Freiburg-Bad Krozingen, Bad Krozingen, Germany.
Sergio Richter, Department of Cardiology, Heart Center Dresden, University Hospital, Technische Universität Dresden, Dresden, Germany.
Stefan Timmer, Department of Cardiology, Northwest Clinics, Alkmaar, The Netherlands.
Kevin Vernooy, Department of Cardiology, Cardiovascular Research Institute Maastricht (CARIM), Maastricht University Medical Center, Maastricht, The Netherlands.
Zachary Whinnett, National Heart and Lung Institute, Imperial College London, Du Cane Road, London W12, UK.
Funding
All authors have received honoraria from Medtronic in relation to this project.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Deshmukh P, Casavant D, Romanyshyn M, Anderson K. Permanent, direct His-bundle pacing: a novel approach to cardiac pacing in patients with normal His-Purkinje activation. Circulation 2000;101:869–77. [DOI] [PubMed] [Google Scholar]
- 2. Kusumoto FM, Schoenfeld MH, Barrett C, Edgerton JR, Ellenbogen KA, Gold MRet al. 2018 ACC/AHA/HRS guideline on the evaluation and management of patients with bradycardia and cardiac conduction delay: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm. J Am Coll Cardiol 2019;74:932–87. [DOI] [PubMed] [Google Scholar]
- 3. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2022;43:4229–361. [DOI] [PubMed] [Google Scholar]
- 4. Kurita T, Nona A, Kimura M, Grassland K, Aiya M, Rigid S. 2021 Annual JCS/JHRS guideline focus update version nondrug therapy for arrhythmia JCS/JHRS 2021 guideline focused update on non-pharmacotherapy of cardiac arrhythmias. Circ J 2021;86:337–63. [DOI] [PubMed] [Google Scholar]
- 5. Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OAet al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2013;34:1070–118. [DOI] [PubMed] [Google Scholar]
- 6. Brugada J, Katritsis DG, Arbelo E, Arribas F, Bax JJ, Blomström-Lundqvist Cet al. 2019 ESC guidelines for the management of patients with supraventricular tachycardia. Eur Heart J 2020;41:655–720. [DOI] [PubMed] [Google Scholar]
- 7. Glikson M, Nielsen JC, Kronborg MB, Michowitz Y, Auricchio A, Barbash IMet al. 2021 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Europace 2022;24:71–164. [DOI] [PubMed] [Google Scholar]
- 8. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou Xet al. A novel pacing strategy with low and stable output: pacing the left bundle branch immediately beyond the conduction block. Can J Cardiol 2017;33:1736.e1–.e3. [DOI] [PubMed] [Google Scholar]
- 9. Mafi-Rad M, Luermans JG, Blaauw Y, Janssen M, Crijns HJ, Prinzen FWet al. Feasibility and acute hemodynamic effect of left ventricular septal pacing by transvenous approach through the interventricular septum. Circ Arrhythmia Electrophysiol 2016;9:e003344. [DOI] [PubMed] [Google Scholar]
- 10. Padala S, Ellenbogen K. Left bundle branch pacing is the best approach to physiological pacing. Hear Rhythm 2020;1:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jastrzebski M, Kiełbasa G, Cano O, Curila K, Heckman L, De Pooter Jet al. Left bundle branch area pacing outcomes: the multicentre European MELOS study. Eur Heart J 2022;43:4161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shan QJ, Xu H, Zhou XJ, Chang Q, Ji L, Chen C Cet al. Effects of permanent left bundle branch area pacing on QRS duration and short-term cardiac function in pacing-indicated patients with left bundle branch block. Chin Med J 2021;134:1101–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lin J, Chen K, Dai Y, Sun Q, Li Y, Jiang Yet al. Bilateral bundle branch area pacing to achieve physiological conduction system activation. Circ Arrhythm Electrophysiol 2020;13:781–91. [DOI] [PubMed] [Google Scholar]
- 14. Huang WJ, Huang DJ, Zhang S. Chinese Society of Pacing and Electrophysiology and Chinese Society of Arrhythmias, “Chinese expert consensus on His-Purkinje conduction system pacing.” Chin J Cardiac Arrhyth 2021;25:10–36. [Google Scholar]
- 15. Vijayaraman P, Dandamudi G, Zanon F, Sharma PS, Tung R, Huang Wet al. Permanent his bundle pacing: recommendations from a multicenter His bundle pacing collaborative working group for standardization of definitions, implant measurements, and follow-up. Hear Rhythm 2018;15:460–8. [DOI] [PubMed] [Google Scholar]
- 16. Vijayaraman P, Chung MK, Dandamudi G, Upadhyay GA, Krishnan K, Crossley Get al. His bundle pacing. J Am Coll Cardiol 2018;72:927–47. [DOI] [PubMed] [Google Scholar]
- 17. Bogale N, Witte K, Priori S, Cleland J, Auricchio A, Gadler Fet al. The European Cardiac Resynchronization Therapy Survey: comparison of outcomes between de novo cardiac resynchronization therapy implantations and upgrades. Eur J Heart Fail 2011;13:974–83. [DOI] [PubMed] [Google Scholar]
- 18. Qualtrics Surveys—Qualtrics XM Software. https://www.qualtrics.com(7 July 2021, date last accessed).
- 19. Jastrzębski M, Burri H, Kiełbasa G, Curila K, Moskal P, Bednarek Aet al. The V6-V1 interpeak interval: a novel criterion for the diagnosis of left bundle branch capture. Europace 2022;24:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kircanski B, Boveda S, Prinzen F, Sorgente A, Anic A, Conte Get al. Conduction system pacing in everyday clinical practice: EHRA physician survey. Europace 2022:euac201, EPUB ahead of print: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keene D, Arnold AD, Jastrzębski M, Burri H, Zweibel S, Crespo Eet al. His bundle pacing, learning curve, procedure characteristics, safety, and feasibility: insights from a large international observational study. J Cardiovasc Electrophysiol 2019;30:1984–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su L, Cai M, Wu S, Wang S, Xu T, Vijayaraman Pet al. Long-term performance and risk factors analysis after permanent His-bundle pacing and atrioventricular node ablation in patients with atrial fibrillation and heart failure. Europace 2020;22:ii19–26. [DOI] [PubMed] [Google Scholar]
- 23. Teigeler T, Kolominsky J, Vo C, Shepard RK, Kalahasty G, Kron Jet al. Intermediate-term performance and safety of His-bundle pacing leads: a single-center experience. Hear Rhythm 2021;18:743–9. [DOI] [PubMed] [Google Scholar]
- 24. Oates C, Kawamura I, Turagam MK, Langan MN, McDonaugh M, Whang Wet al. A single-center experience with early adoption of physiologic pacing approaches. J Cardiovasc Electrophysiol 2022;33:308–14. [DOI] [PubMed] [Google Scholar]
- 25. Wu S, Sharma P, Huang W. Novel left ventricular cardiac synchronization: left ventricular septal pacing or left bundle branch pacing? Europace 2020;22:ii10–8. [DOI] [PubMed] [Google Scholar]
- 26. Jastrzębski M, Moskal P, Huybrechts W, Curila K, Sreekumar P, Rademakers LMet al. Left bundle branch-optimized cardiac resynchronization therapy (LOT-CRT): results from an international LBBAP collaborative study group. Hear Rhythm 2022;19:13–21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.