Abstract
Aims
The randomized, controlled EAST-AFNET 4 trial showed that early rhythm control (ERC) reduces the rate of a composite primary outcome (cardiovascular death, stroke, or hospitalization for worsening heart failure or acute coronary syndrome) by ∼20%. The current study examined the cost-effectiveness of ERC compared to usual care.
Methods and results
This within-trial cost-effectiveness analysis was based on data from the German subsample of the EAST-AFNET 4 trial (n = 1664/2789 patients). Over a 6-year time horizon and from a healthcare payer’s perspective, ERC was compared to usual care regarding costs (hospitalization and medication) and effects (time to primary outcome; years survived). Incremental cost-effectiveness ratios (ICERs) were calculated. Cost-effectiveness acceptability curves were constructed to visualize uncertainty. Early rhythm control was associated with higher costs [+€1924, 95% CI (−€399, €4246)], resulting in ICERs of €10 638 per additional year without a primary outcome and €22 536 per life year gained. The probability of ERC being cost-effective compared to usual care was ≥95% or ≥80% at a willingness-to-pay value of ≥€55 000 per additional year without a primary outcome or life year gained, respectively.
Conclusion
From a German healthcare payer’s perspective, health benefits of ERC may come at reasonable costs as indicated by the ICER point estimates. Taking statistical uncertainty into account, cost-effectiveness of ERC is highly probable at a willingness-to-pay value of ≥€55 000 per additional life year or year without a primary outcome. Future studies examining the cost-effectiveness of ERC in other countries, subgroups with higher benefit from rhythm control therapy, or cost-effectiveness of different modes of ERC are warranted.
Keywords: Early rhythm control, Cost-effectiveness, Atrial fibrillation
Structured Graphical Abstract
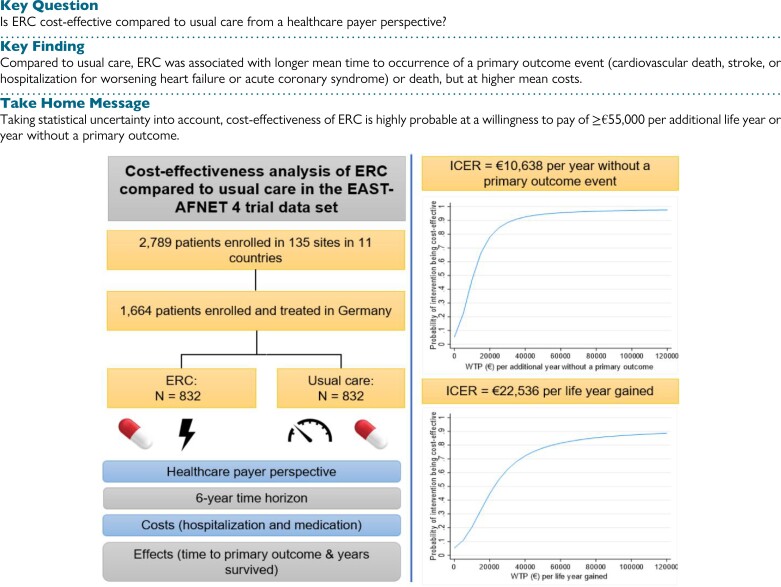
Structured graphical abstract.
Notes: ERC, early rhythm control therapy; ICER, incremental cost-effectiveness ratio; primary outcome event = cardiovascular death, stroke, or hospitalization for stroke or acute coronary syndrome.
What’s new?
While early rhythm control (ERC) has been shown to be clinically effective, its cost-effectiveness has not yet been analysed.
Based on data from the German subsample (n = 1 664) of the EAST-AFNET 4 randomised controlled trial, a cost-effectiveness analysis was conducted using time to first occurrence of a primary outcome event (cardiovascular death, stroke, or hospitalization for worsening heart failure or acute coronary syndrome) and time to death as effect measures.
Compared to usual care, ERC was associated with longer mean time to occurrence of a primary outcome event or death, but at higher mean costs. Taking statistical uncertainty into account, cost-effectiveness of ERC is highly probable at a willingness-to-pay value of ≥€55 000 per additional life year (80%) or per year without a primary outcome event (95%).
Introduction
Atrial fibrillation (AF) is the most common arrhythmia in adults with a lifetime risk of one-third in people of European descent.1 The number of individuals with AF in the European Union is projected to increase to ∼18 million by 2060.2 Affected patients are at higher risk for myocardial infarction, heart failure events, and death.3,4 Additionally, an important proportion of strokes are due to AF.5 Therefore, an increasing prevalence of AF leads to an increasing socioeconomic burden. Currently, the direct cost of AF for the National Health Service (NHS) in the UK is estimated to be between 0.9 and 1.4% of the total NHS expenditure and is projected to increase to 1.35–4.27% over the next 20 years.6 The cost of illness in less-centralized healthcare systems is more difficult to estimate, but in Germany and Sweden, the average annual cost per patient was estimated to be between €5586 and €7241, respectively, in 2005.7 Hospitalizations are the most important driver of cost in patients with AF,8,9 which increased exponentially between 2000 and 2010.10 Similarly, adverse events such as strokes are associated with high costs for treatment and long-term care.11
The current treatment domains in patients comprise anticoagulation, rate and rhythm control, and therapy of concomitant conditions.12,13 The Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) found that systematic early rhythm control (ERC), applied directly after randomization, reduces a composite primary outcome of cardiovascular death, stroke, and hospitalization for worsening heart failure or acute coronary syndrome by ∼20% compared with usual care.14 The main findings could be replicated in large health data sets in the USA15 and in Korea.16 In a cohort of the general population in the UK, over 80% of all patients with newly diagnosed AF were eligible for ERC when the EAST-AFNET 4 criteria were applied.17 Hence, a large proportion of AF patients could potentially benefit from ERC to reduce cardiovascular complications.
Importantly, one concern after the publication of the EAST-AFNET 4 main study was whether the additional treatment would add a reasonable or undue financial burden to healthcare systems.18 The cost-effectiveness of ERC has so far not been evaluated.
The aim of the current study was to examine the cost-effectiveness of ERC in the EAST-AFNET 4 trial over a 6-year period from a German healthcare payer’s perspective.
Methods
The manuscript of this study was prepared in adherence to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) (see Supplementary material online, Table S1).19
Study design and participants
This study was based on data from the EAST-AFNET 4 trial, an international, investigator-initiated, parallel-group, randomized, open, blinded-outcome-assessment strategy trial (EAST-AFNET 4 ISRCTN: ISRCTN04708680; Clinical-Trials.gov: NCT01288352; EudraCT: 2010–021258–20).20 Participants were included if they had early AF (diagnosed ≤12 months before enrolment) and (A) were either older than 75 years or had a previous transient ischemic attack or stroke or (B) had at least two stroke risk factors (>65 years, female sex, heart failure, hypertension, diabetes mellitus, severe coronary artery disease, chronic kidney disease, and left ventricular hypertrophy). Detailed inclusion and exclusion criteria were reported elsewhere.14 A total of 2789 participants from 11 European countries were randomized to either ERC or usual care (stratified randomization according to the study site) between July 2011 and December 2016 and followed up until the end of the trial (6 March 2020). As shown previously, there were differences in treatment patterns in the trial across countries.21 Moreover, different reimbursement practices create large variability in treatment cost between countries and descriptive analyses showed differences, e.g. in the average number of nights spent in hospital. Therefore, and to ensure a consistent and realistic costing approach, the current cost-effectiveness analysis was restricted to data from the German study sites (n = 1664, 60% of the overall sample).
Intervention
In the ERC group, antiarrhythmic drugs, or AF ablation, as well as cardioversion were initiated in all patients early after randomization. The usual care group received rate control therapy initially following guideline recommendations, with rhythm control therapy restricted to patients with uncontrolled AF-related symptoms on adequate rate control therapy.20,21 In the total sample (n = 2789), 65% in the ERC group were still receiving rhythm control therapy at 2 years. Of these, 75% received antiarrhythmic drugs and 25% had been treated with AF ablation. In the usual care group, 85% were still not receiving rhythm control therapy after 2 years. Among the 15% receiving rhythm control therapy, 48% were treated with antiarrhythmic drugs and 52% with AF ablation.14 A more detailed description of treatment patterns, including the proportion of patients who changed from one type of therapy to the other, can be found in Metzner et al.21
Costs and effects
The cost-effectiveness analysis was conducted from a German healthcare payer’s perspective and was restricted to hospitalization (the main driver of AF-related costs7–9,22) and medication costs given the available trial data. Costs were reported in 2021 euros (€), and costs and effects were discounted using the recommended discount rate of 3%.23
All hospitalizations were captured as part of the documentation of serious adverse events (SAEs). Among other information, the type of event, the date of admission and discharge, the interventions performed, and the outcome were specified for each SAE. Additionally, the duration of hospital stays for catheter ablation for AF was recorded. Hospital stays were monetarily valued by assigning diagnosis-related groups (G-DRG catalogue 2021) based on the initial diagnosis that led to admission into a hospital (International Statistical Classification of Diseases and Related Health Problems, 10th revision, German modification, ICD-10-GM) and the performed cardiovascular procedures during the hospital stay (German Operation and Procedure Classification, OPS).24 Furthermore, hospital stays due to events classified as ‘other events’ for which no cardiovascular procedure was performed were monetarily valued based on standardized unit costs, representing the average costs for a night spent in a hospital, inflated to the year 2021 according to the consumer price index.25,26 For ablation visits, the ICD-10 code for AF and the OPS code for catheter ablation were assigned. An overview as well as a more detailed description of the classification of diagnoses and procedures is provided in Supplementary material online, Table S2, and Supplementary material online, Table S3 (see Supplementary material online).
The costs of hospitalization for each participant were derived by calculating the revenue that each hospital receives for each individual hospital stay in the respective DRG, considering the 2021-specific base rate (€3747.98), the DRG-specific cost weight, the surcharges for exceeding the defined maximum length of stay or the deductions for falling below the minimum length of stay, and the daily nursing care revenue.
Medication was assessed at baseline; discharge; 12-month, 24-month, and 36-month follow-up visits; in the context of a SAE; at unscheduled follow-up visits; and at withdrawal. The generic name and the duration of intake (date of administration/discontinuation or current use) were documented for the following medications: antiarrhythmics, anticoagulants, antithrombotic agents, and other drugs administered for AF as well as drugs for cardiovascular concomitant illnesses (including statins and antidiabetics; only chronic treatment). Medication costs were calculated based on the number of days of intake (minus the days spent in hospital), the daily defined dose27 and taking into account the package size and price of the respective generic drug.28
The time to the occurrence of a primary outcome (death from cardiovascular causes, stroke, or hospitalization with worsening of heart failure or acute coronary syndrome) and the time survived in the observation period were used as effect measures in the cost-effectiveness analysis.
Statistical analyses
The statistical analysis plan for the EAST-AFNET 4 trial stated that a health economic evaluation should be conducted but did not contain details. This analysis was conducted considering standard operating procedures of the Department of Health Economics and Health Services Research at the University Medical Center Hamburg-Eppendorf, developed in accordance with international standards in health economic evaluation, and according to the approach recommended by Mutubuki et al. that accounts for the most common statistical challenges in trial-based economic evaluations (e.g. baseline imbalances, skewed costs, correlated costs and effects, and missing data).29
The time horizon for the cost-effectiveness analysis was set to 6 years. Due to the event-driven trial design and the long recruitment period (>4 years), some participants were censored at the end of the study before completing e.g. ≥6 years of participation in the study (40.2%, excluding subjects who died or withdrew before censoring at the end of the study). However, the cost and effect data were available up to the individual time of censoring since the information was collected continuously over the observation period. Missing data were imputed using multiple imputation by chained equations (MICE), where, unless specified otherwise, missing values of each variable are predicted by all other variables in the imputation model (fully conditional specification).30,31 To generate one imputed data set, several cycles are run to stabilize the results. Predictive mean matching was used as the imputation method to ensure that only observed values are imputed, and thus, the true distribution is reflected by the imputed values. To use as much information as possible until study withdrawal or censoring, cost and effect data were imputed in 6-month intervals (e.g. baseline to 6-month follow-up, 6-month follow-up to 12-month follow-up, etc.). The percentage of missing values varied between 0% and 54% across different variables and time points. The ERC group had a higher withdrawal rate but did not affect the outcome in the main analysis.14 Thus, missing data were assumed to be missing at random. In total, 20 imputed data sets were created and used for the analyses. Results were pooled according to Rubin’s Rule.32
Descriptive statistics were calculated for the comparison between ERC and usual care for baseline demographic and clinical characteristics and for unadjusted costs and effects over the observation period. To account for any residual differences between groups despite randomization (especially as the analysis was based on the German subsample), adjusted differences in mean costs and effects between ERC and usual care were calculated using seemingly unrelated regressions.33 In seemingly unrelated regressions, two separate regression models are specified simultaneously, thereby accounting for the correlation between costs and effects while allowing cost and effect differences to be adjusted for different covariates. Candidate covariates were selected from a set of baseline demographic and clinical characteristics (Table 1) and were added to the model if they changed the estimated cost or effect difference between groups by more than 5%. For each effect measure, the seemingly unrelated regression results were used to calculate the incremental cost-effectiveness ratio (ICER) as the ratio of the difference in mean costs and the difference in mean effects between ERC and usual care. Non-parametric bootstrapping (1000 replications; bias-corrected and accelerated bootstrap method) was used to display the uncertainty around the ICER on the cost-effectiveness plane.34 The cost-effectiveness plane consists of a horizontal and a vertical axis, with the intersection of the axes representing the comparator (here: usual care) and the areas above/below or to the right/left of the axes indicating whether the new intervention (here: ERC) is more/less costly or more/less effective than the comparator, respectively. When located in the north-eastern quadrant, the ICER can be interpreted as the additional costs needed for an additional unit of effect (e.g. per additional life year or per year free of a primary outcome event). Furthermore, cost-effectiveness acceptability curves were constructed based on the net benefit approach.35 Cost-effectiveness acceptability curves represent the proportion of bootstrapped ICERs that falls below a certain willingness-to-pay value for an additional unit of effect. As there is no clearly defined willingness-to-pay threshold for a year without a primary outcome or a life year gained, the probability of ERC being cost-effective compared to usual care was calculated for hypothetical willingness-to-pay values between €0 and €120 000. For a detailed explanation on how the uncertainty displayed on the cost-effectiveness plane can be translated into the shape of the cost-effectiveness acceptability curve, interested readers are referred to Fenwick et al.36
Table 1.
Baseline sociodemographic and clinical characteristics of patients with early atrial fibrillation randomized to ERC therapy and usual care. Participants of the EAST-AFNET 4 trial recruited from German study sites (n = 1664)
| Early rhythm control (n = 832) | Usual care (n = 832) | |
|---|---|---|
| Age—mean (SE) | 70.16 (0.30) | 70.87 (0.28) |
| Female sex—n (%) | 393 (47.24) | 390 (46.88) |
| Body mass index—mean (SE) | 29.10 (0.18) | 29.23 (0.18) |
| Mean days since atrial fibrillation diagnosis—mean (SE) | 64.70 (4.79) | 67.71 (6.92) |
| MoCA score—mean (SE) | 25.56 (0.13) | 25.57 (0.13) |
| EQ-5D index—mean (SE) | 0.78 (0.01) | 0.78 (0.01) |
| EQ-VAS—mean (SE) | 70.30 (0.61) | 70.88 (0.63) |
| CHA2DS2-VASc score—mean (SE) | 3.44 (0.05) | 3.48 (0.05) |
| Centre type (D-site)—n (%) | 536 (64.42) | 532 (63.94) |
| Previous stroke or transient ischemic attack—n (%) | 97 (11.66) | 94 (11.30) |
| Atrial fibrillation symptoms—n (%) | 609 (73.24) | 599 (71.89) |
| Arterial hypertension—n (%) | 763 (91.71) | 748 (89.90) |
| Diabetes mellitus—n (%) | 219 (26.32) | 216 (25.96) |
| Severe coronary artery disease (previous myocardial infarction, CABG or PCI)—n (%) | 156 (18.75) | 154 (18.51) |
| Stable heart failure—n (%) | 269 (32.33) | 278 (33.41) |
| Left ventricular hypertrophy on echocardiography (>15 mm wall thickness)—n (%) | 43 (5.17) | 44 (5.29) |
| Chronic kidney disease of MDRD stage 3 or 4—n (%) | 105 (12.62) | 107 (12.86) |
| Peripheral artery disease—n (%) | 49 (5.89) | 34 (4.09) |
| Sinus rhythm at baseline—n (%) | 439 (52.80) | 415 (49.93) |
| Valvular heart disease—n (%) | 357 (42.91) | 382 (45.88) |
| History of syncope—n (%) | 46 (5.47) | 49 (5.89) |
| Chronic obstructive lung disease—n (%) | 59 (7.13) | 72 (8.71) |
| Malignant diseases (with or without currently active disease manifestation)—n (%) | 67 (8.01) | 56 (6.73) |
| First primary outcome events over 72 monthsa—n (%) | 210 (25.23) | 255 (30.59) |
| Cardiovascular deaths over 72 monthsa—n (%) | 67 (8.11) | 84 (10.13) |
| Strokes over 72 monthsa—n (%) | 35 (4.25) | 49 (5.84) |
| Hospitalizations for worsening heart failure over 72 monthsa—n (%) | 128 (15.35) | 146 (17.57) |
| Hospitalizations for acute coronary syndrome over 72 monthsa—n (%) | 39 (4.70) | 50 (5.95) |
| Deaths over 72 monthsa—n (%) | 117 (14.01) | 139 (16.66) |
Abbreviations: SE, standard error; MoCA, Montreal Cognitive Assessment; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; MDRD, Modification of Diet in Renal Disease.
Based on imputed case analysis; non-imputed sample: n = 168 vs. n = 217 first primary outcome events, n = 45 vs. n = 60 cardiovascular deaths, n = 26 vs. n = 40 strokes, n = 95 vs. n = 118 hospitalizations for worsening heart failure, n = 33 vs. n = 43 hospitalizations for acute coronary syndrome, and n = 83 vs. n = 100 deaths.
All analyses were conducted using STATA/SE 16.0 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC). The significance level was set to 0.05.
Sensitivity analyses
Different sensitivity analyses were performed to examine the robustness of base case results. The impact of the chosen discount rate was assessed by varying the discount rate between 0% and 5%.
The data from the EAST-AFNET 4 trial were restricted to costs of hospitalization and medication, while some of the primary outcomes (e.g. stroke or acute coronary syndrome) often also result in the use of rehabilitation and/or formal care services, which generate additional cost to health or long-term care insurances in Germany.37,38 Therefore, the base case analysis was extended by assuming average rehabilitation and care costs after a stroke or acute coronary syndrome (myocardial infarctions) from the literature.39–41 In order to examine the impact of varying cost estimates in the literature, scenario analyses with minimum and maximum reported costs were conducted. In addition, an extreme scenario assuming that every stroke would lead to inpatient care needs and long-term care at the highest level was analysed. This was done by adding the typical care allowance for the highest care level for a person in an inpatient care facility paid by the health and long-term care insurances in Germany to the total costs for each month after a stroke within the 6-year observation period.42
As, for some variables, a relatively high number of values were imputed, and the analyses were repeated by using only participants who were recruited early enough to be observed for the complete 6-year time horizon (n = 852). Missing data due to early withdrawal were imputed.
Additionally, the impact of potential missing not at random values on the results of the base case analysis was examined by modifying the imputed total costs of withdrawals (e.g. +10% costs).43
Finally, the analyses were rerun when all hospital stays were monetarily valued with standardized unit costs per hospital night25 to examine the impact of the costing approach (assigning diagnosis-related groups) on the results.
Results
The 1664 participants from the German study sites were equally randomized (n = 832 each) to ERC and usual care. Overall, baseline characteristics remained balanced between treatment groups in this randomized subsample. The mean age was 70.16 and 70.87 years, respectively, and about 47% were female in both groups (Table 1). Over the follow-up period, fewer first primary outcome events (210 vs. 255) and a lower number of deaths (117 vs. 139) occurred in the ERC compared to the usual care group.
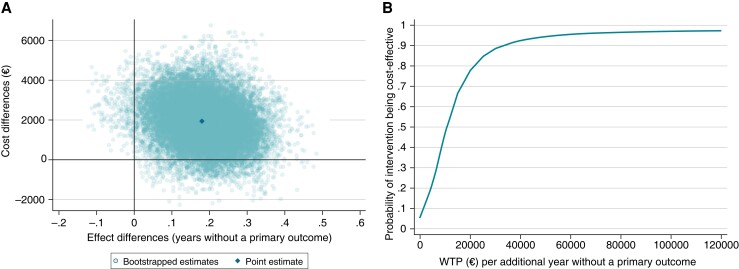
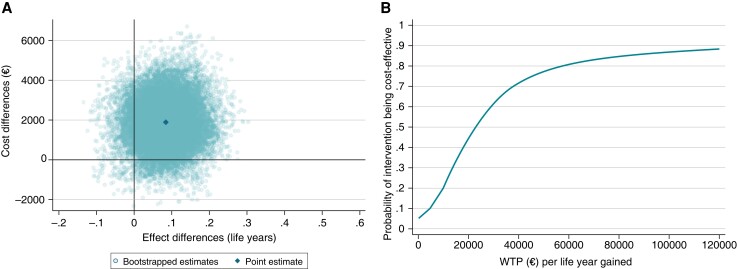
The base case cost-effectiveness results are displayed in Table 2 and Figures 1 and 2. In the unadjusted analysis, ERC was associated with significantly higher mean medication costs [+€1218, 95% CI (€53, €2383)] and non-significantly higher mean hospitalization costs [+€976, 95% CI (−€1025, €2976)]. In the adjusted analysis, the ERC group had non-significantly higher mean total costs [+€1924, 95% CI (−399, 4246)], a significantly longer mean time to the occurrence of a primary outcome event [+0.18 years, 95% CI (0.02, 0.34)], and a non-significantly longer mean survival time than the usual care group [+0.09 years, 95% CI (−0.03, 0.20)]. The ICER was €10 638 per additional year without a primary outcome and €22 536 per life year gained. The cost-effectiveness planes indicated a high degree of uncertainty around the point estimates, with the majority of the bootstrapped ICERs being in the north-eastern quadrant (more costly and more effective than usual care) for both effect measures (Figure 1A and 2A). In terms of time to a primary outcome event, ERC could be considered cost-effective compared to usual care (≥95% probability) at a willingness-to-pay value of ≥€55 000 per additional year without a primary outcome event (Figure 1B). Considering survival time as effect measure, the probability of ERC being cost-effective increased with higher willingness-to-pay values, up to 89% at a willingness-to-pay value of €120 000 per life year gained; ≥80% was reached at a willingness-to-pay value of ≥€55 000 (Figure 2B).
Table 2.
Costs, effects, and incremental cost-effectiveness ratio for ERC (n = 832) vs. usual care (n = 832) in patients with early atrial fibrillation at 6 years follow-up
| Early rhythm control | Usual care | Difference, unadjusted | Difference, adjusteda | |
|---|---|---|---|---|
| Mean | Mean | Mean (95% CIb) | Mean (95% CIb) | |
| Medication | 9862 | 8644 | 1218 (53, 2383) | |
| Hospitalization | 15 693 | 14 718 | 976 (−1025, 2976) | |
| Total costs | 25 556 | 23 362 | 2194 (−152, 4539) | 1924 (−399, 4246) |
| Years to primary outcome | 4.82 | 4.63 | 0.19 (0.03, 0.35) | 0.18 (0.02, 0.34) |
| Years survived | 5.23 | 5.13 | 0.10 (−0.01, 0.22) | 0.09 (−0.03, 0.20) |
| ICER (€ per year without a primary outcome) | 11 736 | 10 638 | ||
| ICER (€ per life year gained) | 21 626 | 22 536 |
Costs were reported in 2021, in euros (€).
Results from seemingly unrelated regressions; cost difference adjusted for peripheral artery disease at baseline; effect difference adjusted for age and peripheral artery disease at baseline (time to primary outcome) or age, peripheral artery disease, chronic obstructive pulmonary disease, malignant diseases, and valvular disease at baseline (years survived).
Based on 1000 bootstrapped replications.
Figure 1.
(A) Cost-effectiveness plane and (B) cost-effectiveness acceptability curves for ERC (n = 832) vs. usual care (n = 832) in patients with early atrial fibrillation at 6 years follow-up. (A) Non-parametric bootstrapping was used to display the uncertainty around the incremental cost-effectiveness ratio. The north-western quadrant means that ERC is less effective and more costly. The south-western quadrant means that ERC is less effective and less costly. The north-eastern quadrant means that ERC is more effective but more costly. The south-eastern quadrant means that ERC is more effective and less costly. (B) The probability that ERC is cost-effective compared to usual care as the willingness to pay for each additional year without a primary outcome is varied from €0–€120 000. WTP, willingness to pay.
Figure 2.
(A) Cost-effectiveness plane and (B) cost-effectiveness acceptability curves for ERC (n = 832) vs. usual care (n = 832) in patients with early atrial fibrillation at 6 years follow-up. (A) Non-parametric bootstrapping was used to display the uncertainty around the incremental cost-effectiveness ratio. The north-western quadrant means that ERC is less effective and more costly. The south-western quadrant means that ERC is less effective and less costly. The north-eastern quadrant means that ERC is more effective but more costly. The south-eastern quadrant means that ERC is more effective and less costly. (B) The probability that ERC is cost-effective compared to usual care as the willingness to pay per life year gained is varied from €0–€120 000. WTP, willingness to pay.
Sensitivity analyses
The results were robust to variation in the discount rate. The ICER point estimates marginally differed from those in the base case analysis assuming a discount rate of 3%, but the cost-effectiveness acceptability curves were almost identical (see Supplementary material online, Table S4, and Supplementary material online, Figure S1).
Including rehabilitation and care costs for stroke and acute coronary syndrome reduced the mean incremental costs between ERC and usual care, but ERC remained more expensive than usual care, and the cost-effectiveness acceptability curves were almost identical to the base case analysis (see Supplementary material online, Table S4, and Supplementary material online, Figure S2).
When performing the analysis with only a subsample of participants recruited early enough to be followed over the complete 6-year time horizon, incremental costs and time without a primary outcome were higher than in the base case analysis, whereas the incremental time survived was lower. This resulted in lower probabilities of cost-effectiveness in the cost-effectiveness acceptability curves compared to the base case analysis, e.g. 88% and 57% were reached at a willingness-to-pay value of €55 000 per additional year without a primary outcome or life year gained (see Supplementary material online, Table S4, and Supplementary material online, Figure S3). The probabilities of ERC being cost-effective remained relatively stable when different missing at random departures were assumed for missing data from study withdrawals (see Supplementary material online, Figure S4).
Monetarily valuing the hospitalization by standardized unit costs reduced the incremental costs and led to slightly higher probabilities of cost-effectiveness at low willingness-to-pay values but generally confirmed the results from the base case analysis (see Supplementary material online, Table S4, Supplementary material online, Table S5, and Supplementary material online, Figure S3).
Discussion
This study examined the cost-effectiveness of systematic, ERC therapy by analysing observed healthcare expenditures in the German subsample of the multicentre randomized EAST-AFNET 4 trial. Early rhythm control reduced the risk of death from cardiovascular causes, stroke, or hospitalization for heart failure or acute coronary syndrome14 but was associated with non-significantly higher costs. The analyses estimated ICERs of €10 638 per additional year without a primary outcome event and €22 536 per life year gained. Based on these point estimates and following common threshold recommendations,44 ERC can be considered economically attractive, even though the cost per quality-adjusted life year (QALY), to which these threshold recommendations refer, is likely to be higher. Statistical uncertainty analyses resulted in high probabilities for cost-effectiveness of ERC if the willingness-to-pay value was ≥€55 000 per year without a primary outcome (95% probability) or per life year gained (80% probability). Sensitivity analyses (e.g. variation of the discount rates, assumption of average rehabilitation and formal care costs following a stroke or myocardial infarction, or monetary valuation of hospitalizations by average costs per night spent in hospital) did not alter the results relevantly.
Drivers for costs incurred for delivering ERC
Costs were higher in patients randomized to ERC, primarily driven by additional medication in our analysis. The majority of patients randomized to ERC received antiarrhythmic drugs, whereas in the control group, these were only administered to mitigate uncontrolled AF-related symptoms despite adequate rate control therapy. Numerically (but not significantly) higher hospitalization costs in patients randomized to ERC can be explained by payments associated with catheter ablations. Consistent with the results of the main study indicating no differences in the nights spent in a hospital between ERC and usual care,14 hospitalization costs were almost equal between both groups when all hospitalizations were monetarily valued by average costs per hospital night (unit costs). Thus, the costs saved by preventing primary outcome events in the ERC group were probably offset by hospitalizations for rhythm control therapy (e.g. ablation or initiation of antiarrhythmic drugs), at least within this 6-year time horizon. This is because the large number of participants receiving (costly) ERC contrasts with a relatively small absolute number of primary outcome events avoided.
Generalizability of ERC and comparability of the results to other studies
The economic analysis of ERC is challenging since it comprises a therapy concept of several treatment options rather than a single treatment. The effectiveness of this therapy concept has been replicated internationally by observational national cohorts in the USA, the UK, and South Korea,15,16 and further trials using ERC to treat AF in different settings are currently pursued in patients with acute stroke or with intracardiac devices (clinicaltrials.gov identifiers: NCT05293080, NCT04612335). Previous studies found that certain patient groups benefited more from rhythm control therapy such as AF patients with heart failure and reduced ejection fraction, or a high comorbidity burden,45–48 who may thus represent a patient group with a more favourable cost-effectiveness of ERC. Moreover, the cost-effectiveness of the ERC therapy concept also depends on the mode used to achieve rhythm control, such as catheter ablation or antiarrhythmic drugs, which are associated with different efficacies, adverse events, and costs.49 For example, the CABANA trial showed that catheter ablation was more effective in improving AF symptoms than antiarrhythmic drug therapy but was also more expensive. The recent cost-effectiveness analysis of CABANA reported an ICER of $57 893 per QALY gained for catheter ablation vs. antiarrhythmic drug therapy in a US setting. However, there was a high degree of uncertainty with only <75% of bootstrap simulation yielding an ICER of <$100 000/QALY.49,50 These results indicate that an increased use of catheter ablations for rhythm control would impact the ICER for ERC compared to usual care. In EAST-AFNET 4, the method of rhythm control therapy was not randomized and ∼25% of the patients still in follow-up at that time received catheter ablation 2 years after randomization. In view of a recent analysis demonstrating that attaining sinus rhythm is the key mediator reducing outcomes in the EAST-AFNET 4 trial,51 it could be speculated that successful maintenance of sinus rhythm, independent of the method of rhythm control, is key to effective and cost-effective delivery of ERC. Recent data support early catheter ablation as a first-line therapy for rhythm control in attaining sinus rhythm and slowing progression to persistent AF.52–54 The recent ESC guidelines for AF did not consider EAST-AFNET 4 and identification of the right patient for rhythm control remains crucial.55 For that, the 4S-AF scheme as described in the recent AF guidelines may be helpful to identify patients who will benefit from rhythm control.56
Examining the cost-effectiveness of ERC in different populations (e.g. patients with AF and heart failure; persistent vs. paroxysmal AF; and older vs. younger AF patients) or different modes of rhythm control (antiarrhythmic drugs or catheter ablation), therefore, represents a future research perspective.
Strengths and limitations
An important strength is the use of empirical data from a large sample (n = 1664) of the randomized, controlled EAST-AFNET 4 trial. Based on these data, costs for the German health insurance system were calculated for observed treatments. This healthcare payer’s perspective is recommended by the German Institute for Quality and Efficiency in Healthcare (IQWiG) for evaluations of medical interventions.57
Restricting the analysis to the German subsample of the trial allowed for an adequate estimation of costs within the German healthcare system but, compared to the almost two-fold bigger main trial, reduced the power to detect differences between groups. The downside of limiting the analysis to the German setting is that the results may not be generalizable to other countries or healthcare systems due to differences between countries in, e.g. the reimbursement of therapies. Comparability of the results with other studies is furthermore limited by the fact that QALYs were not used as effect measure in this study. This was due to the following reasons: (1) health-related quality of life was only assessed at baseline and at 2-year follow-up, where no significant or clinically relevant between-group difference was observed; (2) against the background of the German social legislation and weaknesses of the QALY concept, the IQWiG, which is substantially involved in informing reimbursement decisions in Germany, currently deviates from the international reference scenario for cost-effectiveness analyses by not supporting QALYs as primary measure of benefits;23,58,59 and (3) the EAST-AFNET 4 trial was not powered for between-group differences in quality of life. Instead, the EAST-AFNET 4 primary composite outcome was used. This is in line with the main trial and is medically meaningful. To provide a second reliable and meaningful outcome that can be compared across conditions and therapies, cost-effectiveness was additionally expressed as costs per life year gained.
The long-term effects in preventing cardiovascular events on healthcare utilization were limited in the analysis to the 6 years of follow-up. Within this time horizon, around 40% of the participants were censored earlier, so costs and effects were imputed for the time not observed anymore, which could have introduced additional uncertainty into the results. Larger (modelling) studies across healthcare systems and projected over longer time periods would provide more comprehensive assessments of whether this intervention would be cost-effective in general.
The strong emphasis on empirical data from the RCT and the healthcare payer’s perspective also means a limited cost perspective, where no indirect costs (e.g. productivity losses) or costs of informal care were considered that are relevant from a societal perspective. Productivity losses can only be expected for a relatively small proportion of the EAST-AFNET 4 population, as most patients were at or beyond retirement age at the time of enrolment. There could also be additional costs for the healthcare payer that were not recorded in the study (outpatient costs that could be increased on ERC,60 costs of medications not administered for AF or concomitant cardiovascular diseases, overnight hospital stays only for diagnostic procedures or monitoring, or costs of formal care). The strong contribution of hospitalization costs to overall costs in AF care mitigates these limitations to some extent.7–9,22
Regarding the calculation of hospitalization costs, DRGs were assigned based on cardiovascular events and procedures, whereas other events or non-cardiovascular procedures were not considered for the DRG grouping or were monetarily valued using standardized unit costs. Therefore, a potential over- or underestimation of costs cannot be ruled out, as the length of hospital stays may not always have been attributable to the cardiovascular events or procedures but to other concomitant diagnoses/conditions and procedures that were not the main reason for hospitalization and, thus, were not considered when assigning DRGs.
Conclusion
Estimated costs of delivering ERC therapy in Germany indicate that health benefits of ERC may come at reasonable additional costs. At a willingness-to-pay value of ≥€55 000 per year without a primary outcome or per additional life year, cost-effectiveness of ERC is highly probable (≥95% or ≥80%, respectively). Future studies are needed that adopt a broader cost perspective, evaluate the cost-effectiveness of ERC in different healthcare systems or in subgroups with the highest clinical effectiveness of ERC, or examine the cost-effectiveness of different modes of ERC.
Supplementary Material
Contributor Information
Sophie Gottschalk, Department of Health Economics and Health Services Research, University Medical Center Hamburg-Eppendorf, Hamburg Center for Health Economics, Martinistraße 52 Building W37, 20246 Hamburg, Germany.
Shinwan Kany, Department of Cardiology, University Heart and Vascular Center Hamburg-Eppendorf, Martinistraße 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Germany.
Hans-Helmut König, Department of Health Economics and Health Services Research, University Medical Center Hamburg-Eppendorf, Hamburg Center for Health Economics, Martinistraße 52 Building W37, 20246 Hamburg, Germany.
Harry JGM Crijns, Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM), Universiteitssingel 50, 6200 MD, Maastricht, The Netherlands.
Panos Vardas, European Society of Cardiology Health Policy Unit, European Heart Health Institute, European Heart Agency, 29 square de Meeus, B-1000 Brussels, BELGIUM.
A John Camm, Molecular & Clinical Sciences Research Institute, St George's University of London, Cranmer Terrace, London SW17 0RE, UK.
Karl Wegscheider, Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Christoph-Probst-Weg 1, 20246 Hamburg, Germany.
Andreas Metzner, Department of Cardiology, University Heart and Vascular Center Hamburg-Eppendorf, Martinistraße 52, Hamburg 20246, Germany.
Andreas Rillig, Department of Cardiology, University Heart and Vascular Center Hamburg-Eppendorf, Martinistraße 52, Hamburg 20246, Germany.
Paulus Kirchhof, Department of Cardiology, University Heart and Vascular Center Hamburg-Eppendorf, Martinistraße 52, Hamburg 20246, Germany; German Center for Cardiovascular Research (DZHK), Partner Site Hamburg/Kiel/Lübeck, Germany; College of Medical and Dental Sciences, University of Birmingham, Edgbaston, Birmingham B15 2TT, UK.
Judith Dams, Department of Health Economics and Health Services Research, University Medical Center Hamburg-Eppendorf, Hamburg Center for Health Economics, Martinistraße 52 Building W37, 20246 Hamburg, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
EAST-AFNET 4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) was funded in part by the German Ministry of Education and Research, Berlin, Germany (Grant 01 GI 0204); the German Centre for Cardiovascular Research, Berlin, Germany; the Atrial Fibrillation Network (AFNET); European Heart Rhythm Association; St Jude Medical/Abbott; Sanofi; and the German Heart Foundation. Further support came from the European Union [grant agreement no. 633196 (CATCH ME) to P.K. and AFNET, grant agreement EU IMI 116074 (BigData@Heart) to PK], the British Heart Foundation (FS/13/43/30324, PG/17/30/32961, PG/20/22/35093, and AA/18/2/34218, all to P.K.), and Leducq Foundation to P.K.
Data availability
Data will be made available upon request. Please address your proposals for analysis to info@kompetenznetz-vorhofflimmern.de.
References
- 1. Mou L, Norby FL, Chen LY, O’Neal WT, Lewis TT, Loehr LRet al. Lifetime risk of atrial fibrillation by race and socioeconomic status. Circ Arrhythm Electrophysiol 2018;11:e006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krijthe BP, Kunst A, Benjamin EJ, Lip GYH, Franco OH, Hofman Aet al. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schnabel RB, Yin X, Gona P, Larson MG, Beiser AS, McManus DDet al. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet 2015;386:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Magnussen C, Niiranen TJ, Ojeda FM, Gianfagna F, Blankenberg S, Njolstad Iet al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE consortium (biomarker for cardiovascular risk assessment in Europe). Circulation 2017;136:1588–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke 1991;22:983–8. [DOI] [PubMed] [Google Scholar]
- 6. Burdett P, Lip GYH. Atrial fibrillation in the UK: predicting costs of an emerging epidemic recognizing and forecasting the cost drivers of atrial fibrillation-related costs. Eur Heart J Qual Care Clin Outcomes 2020;8:187–94. [DOI] [PubMed] [Google Scholar]
- 7. Jönsson L, Eliasson A, Kindblom J, Almgren O, Edvardsson N. Cost of illness and drivers of cost in atrial fibrillation in Sweden and Germany. Appl Health Econ Health Policy 2010;8:317–25. [DOI] [PubMed] [Google Scholar]
- 8. McBride D, Mattenklotz AM, Willich SN, Brüggenjürgen B. The costs of care in atrial fibrillation and the effect of treatment modalities in Germany. Value Health 2009;12:293–301. [DOI] [PubMed] [Google Scholar]
- 9. Reinhold T, Rosenfeld S, Müller-Riemenschneider F, Willich SN, Meinertz T, Kirchhof Pet al. [Patients suffering from atrial fibrillation in Germany. Characteristics, resource consumption and costs]. Herz 2012;37:534–42. [DOI] [PubMed] [Google Scholar]
- 10. Deshmukh A, Patel NJ, Pant S, Shah N, Chothani A, Mehta Ket al. In-Hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010. Circulation 2013;128:2104–12. [DOI] [PubMed] [Google Scholar]
- 11. Li X, Tse VC, Au-Doung LW, Wong ICK, Chan EW. The impact of ischaemic stroke on atrial fibrillation-related healthcare cost: a systematic review. Europace 2017;19:937–47. [DOI] [PubMed] [Google Scholar]
- 12. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 13. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CMet al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2023;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan Aet al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 15. Dickow J, Kirchhof P, Van Houten HK, Sangaralingham LR, Dinshaw LHW, Friedman PAet al. Generalizability of the EAST-AFNET 4 trial: assessing outcomes of early rhythm-control therapy in patients with atrial fibrillation. J Am Heart Assoc 2022;11:e024214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim D, Yang PS, You SC, Sung JH, Jang E, Yu HTet al. Treatment timing and the effects of rhythm control strategy in patients with atrial fibrillation: nationwide cohort study. BMJ 2021;373:n991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kany S, Cardoso VR, Bravo L, Williams JA, Schnabel R, Fabritz Let al. Eligibility for early rhythm control in patients with atrial fibrillation in the UK Biobank. Heart 2022;108:1873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzuki T. Early rhythm control in atrial fibrillation. N Engl J Med 2021;384:483–5. [DOI] [PubMed] [Google Scholar]
- 19. Husereau D, Drummond M, Augustovski F, de Bekker-Grob E, Briggs AH, Carswell Cet al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II Good Practices Task Force. Value Health 2022;25:10–31. [DOI] [PubMed] [Google Scholar]
- 20. Kirchhof P, Breithardt G, Camm AJ, Crijns HJ, Kuck KH, Vardas Pet al. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial. Am Heart J 2013;166:442–8. [DOI] [PubMed] [Google Scholar]
- 21. Metzner A, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns Het al. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST - AFNET 4 trial. Europace 2022;24:552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Spyra A, Daniel D, Thate-Waschke IM, Berghaus S, Willich S, Zeymer Uet al. [Atrial fibrillation in Germany: a prospective cost of illness study]. Dtsch Med Wochenschr 2015;140:e142–8. [DOI] [PubMed] [Google Scholar]
- 23. Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen . IQWiG Allgemeine Methoden. Köln: Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen2022.
- 24. DRG-Research Group . Webgrouper. https://www.drg-research-group.de/index.php?option=com_webgrouper&view=webgrouper&Itemid=112lastaccessed).
- 25. Bock JO, Brettschneider C, Seidl H, Bowles D, Holle R, Greiner Wet al. Calculation of standardised unit costs from a societal perspective for health economic evaluation. Gesundheitswesen 2015;77:53–61. [DOI] [PubMed] [Google Scholar]
- 26. OECD . Inflation (CPI) (indicator)2020.
- 27. Scientific Institute of the AOK (WIdO) . German Anatomical Therapeutic Chemical (ATC)-Classification with Defined Daily Doses (DDD): Bonn, Germany: Federal Institute for Drugs and Medical Devices (BfArM); 2021. [Google Scholar]
- 28. Rote Liste Service GmbH . ROTE LISTE 2021: Pharmaceutical Directory for Germany (Including EU Approvals and Certain Medical Devices). Frankfurt/Main: Rote Liste Service GmbH; 2021. [Google Scholar]
- 29. Mutubuki EN, El Alili M, Bosmans JE, Oosterhuis T, Snoek FJ, Ostelo RWJGet al. The statistical approach in trial-based economic evaluations matters: get your statistics together!. BMC Health Serv Res 2021;21:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. StataCorp . Stata Multiple-Imputation Reference Manual. Release 16. College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 31. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley; 1987. [Google Scholar]
- 33. Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461–75. [DOI] [PubMed] [Google Scholar]
- 34. Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990;10:212–4. [DOI] [PubMed] [Google Scholar]
- 35. Löthgren M, Zethraeus N. Definition, interpretation and calculation of cost-effectiveness acceptability curves. Health Econ 2000;9:623–30. [DOI] [PubMed] [Google Scholar]
- 36. Fenwick E, O'Brien BJ, Briggs A. Cost-effectiveness acceptability curves–facts, fallacies and frequently asked questions. Health Econ 2004;13:405–15. [DOI] [PubMed] [Google Scholar]
- 37. Rajsic S, Gothe H, Borba HH, Sroczynski G, Vujicic J, Toell Tet al. Economic burden of stroke: a systematic review on post-stroke care. Eur J Health Econ 2019;20:107–34. [DOI] [PubMed] [Google Scholar]
- 38. Schmid T. Costs of treating cardiovascular events in Germany: a systematic literature review. Health Econ Rev 2015;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Düvel JA, Damm O, Greiner W. Costs of stroke in Germany: a systematic review. Gesundheitsökonomie Qualitätsmanagement 2021;26:40–50. [Google Scholar]
- 40. Brüggenjürgen B, Rupprecht HJ, Willich SN, Spannagl M, Ehlken B, Smala Aet al. Cost of atherothrombotic diseases—myocardial infarction, ischaemic stroke and peripheral arterial occlusive disease—in Germany. J Pub Health 2005;13:216–24. [Google Scholar]
- 41. Schweikert B, Hahmann H, Steinacker JM, Imhof A, Muche R, Koenig Wet al. Intervention study shows outpatient cardiac rehabilitation to be economically at least as attractive as inpatient rehabilitation. Clin Res Cardiol 2009;98:787–95. [DOI] [PubMed] [Google Scholar]
- 42. Bundesministerium für Gesundheit . Pflegeleistungen zum Nachschlagen2022.
- 43. Leurent B, Gomes M, Faria R, Morris S, Grieve R, Carpenter JR. Sensitivity analysis for not-at-random missing data in trial-based cost-effectiveness analysis: a tutorial. Pharmacoeconomics 2018;36:889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJet al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association task force on performance measures and task force on practice guidelines. J Am Coll Cardiol 2014;63:2304–22. [DOI] [PubMed] [Google Scholar]
- 45. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens Let al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med 2018;378:417–27. [DOI] [PubMed] [Google Scholar]
- 46. Rillig A, Magnussen C, Ozga A-K, Suling A, Brandes A, Breithardt Get al. Early rhythm control therapy in patients with atrial fibrillation and heart failure. Circulation 2021;144:845–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PAet al. Ablation versus drug therapy for atrial fibrillation in heart failure. Circulation 2021;143:1377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rillig A, Borof K, Breithardt G, Camm AJ, Crijns HJGM, Goette Aet al. Early rhythm control in patients with atrial fibrillation and high comorbidity burden. Circulation 2022;146:836–47. [DOI] [PubMed] [Google Scholar]
- 49. Chew DS, Li Y, Cowper PA, Anstrom KJ, Piccini JP, Poole JEet al. Cost-effectiveness of catheter ablation versus antiarrhythmic drug therapy in atrial fibrillation: the CABANA Randomized Clinical Trial. Circulation 2022;146:535–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mark DB, Anstrom KJ, Sheng S, Piccini JP, Baloch KN, Monahan KHet al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: the CABANA Randomized Clinical Trial. JAMA 2019;321:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eckardt L, Sehner S, Suling A, Borof K, Breithardt G, Crijns Het al. Attaining sinus rhythm mediates improved outcome with early rhythm control therapy of atrial fibrillation: the EAST - AFNET 4 trial. Eur Heart J 2022;43:4127–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andrade JG, Wells GA, Deyell MW, Bennett M, Essebag V, Champagne Jet al. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med 2021;384:305–15. [DOI] [PubMed] [Google Scholar]
- 53. Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag Vet al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2023;388:105–16. [DOI] [PubMed] [Google Scholar]
- 54. Gunawardene MA, Willems S. Atrial fibrillation progression and the importance of early treatment for improving clinical outcomes. EP Europace 2022;24:ii22–8. [DOI] [PubMed] [Google Scholar]
- 55. Arbelo E, Dagres N. The 2020 ESC atrial fibrillation guidelines for atrial fibrillation catheter ablation, CABANA, and EAST. Europace 2022;24:ii3–7. [DOI] [PubMed] [Google Scholar]
- 56. Malavasi VL, Vitolo M, Colella J, Montagnolo F, Mantovani M, Proietti Met al. Rhythm- or rate-control strategies according to 4S-AF characterization scheme and long-term outcomes in atrial fibrillation patients: the FAMo (Fibrillazione Atriale in Modena) cohort. Intern Emerg Med 2022;17:1001–12. [DOI] [PubMed] [Google Scholar]
- 57. Gerber-Grote A, Sandmann FG, Zhou M, ten Thoren C, Schwalm A, Weigel Cet al. Decision making in Germany: is health economic evaluation as a supporting tool a sleeping beauty? Z Evid Fortbild Qual Gesundhwes 2014; 108: 390–6. [DOI] [PubMed] [Google Scholar]
- 58. Riedel R, Repschläger U, Griebenow R, Breitkopf S, Schmidt S, Guhl A. International standards for health economic evaluation with a focus on the German approach. J Clin Pharm Ther 2013;38:277–85. [DOI] [PubMed] [Google Scholar]
- 59. Schwalm A, Danner M, Seidl A, Volz F, Dintsios CM, Gerber A. IQWiG's methods for the cost-benefit assessment: comparison with an international reference scenario. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2010;53:615–22. [DOI] [PubMed] [Google Scholar]
- 60. Proietti M, Vitolo M, Harrison SL, Lane DA, Fauchier L, Marin Fet al. Real-world applicability and impact of early rhythm control for European patients with atrial fibrillation: a report from the ESC-EHRA EORP-AF Long-Term General Registry. Clin Res Cardiol 2022;111:70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon request. Please address your proposals for analysis to info@kompetenznetz-vorhofflimmern.de.