Abstract
Deficits in physical function that occur with aging contribute to declines in quality of life and increased mortality. There has been a growing interest in examining associations between physical function and neurobiology. Whereas high levels of white matter disease have been found in individuals with mobility impairments in structural brain studies, much less is known about the relationship between physical function and functional brain networks. Even less is known about the association between modifiable risk factors such as body mass index and functional brain networks. The current study examined baseline functional brain networks in 192 individuals from the BNET study, an ongoing longitudinal, observational study in community-dwelling adults aged 70 and older. Physical function and BMI were found to be associated with sensorimotor and dorsal attention network connectivity. There was a synergistic interaction such that high physical function and low BMI were associated with the highest network integrity. White matter disease did not modify these relationships. Future work is needed to understand the causal direction of these relationships.
1. Introduction
Mobility disability in older adults contributes to declines in quality of life (Groessl, Kaplan et al. 2007; Fagerstrom and Borglin 2010), challenges with activities of daily living (ADLs)(Albert, Bear-Lehman et al. 2015), social isolation (Savikko, Routasalo et al. 2005; Cohen-Mansfield, Hazan et al. 2016), and even mortality (Majer, Nusselder et al. 2011). Decades of research has identified several modifiable risk factors associated with poor mobility including body mass index (BMI), hypertension, physical activity, and diabetes. These same risk factors are important in brain health (Livingston, Sommerlad et al. 2017). To date, it is not known whether the combined association of varying levels of physical function with modifiable risk factors are related to functional brain networks. This knowledge is critical to advance the mechanistic understanding of physical disability and lead to innovation in the design of intervention research. Using cross sectional data, this study aims to model the relationships between varying levels of mobility in older adults in combination with major risk factors on functional brain networks.
There is a rapidly growing body of research examining with the relationship of poor physical function to neural mechanisms (Rosano, Rosso et al. 2014; Sorond, Cruz-Almeida et al. 2015). Both white matter lesions and subclinical measures of white matter health derived from diffusion imaging are reliably associated with declining mobility (Nadkarni, Studenski et al. 2013; Nadkarni, Perera et al. 2015; Rosario, Rosso et al. 2016) suggesting that degraded communication between brain regions contributes to mobility disability. Unfortunately, most white matter changes detected using structural brain imaging are irreversible. However, it is likely that poorer mobility is related to disruptions in brain connectivity because white matter represents connections between brain regions. Analysis of functional brain networks using functional MRI has the specific advantage that it can identify disruptions in communication between brain areas before those disruptions are irreversible.
It is only in the past few years that studies have begun to examine how varying levels of physical function in older adults are related to brain connectivity. In an early pilot study (Hugenschmidt, Burdette et al. 2014), we demonstrated that community structure of the sensorimotor network (SMN) was degraded in older adults with poor physical function as measured with the short physical performance battery (SPPB). Subsequent work has shown that poor gait stability is associated with low efficiency of resting-state connectivity in the SMN in older adults (Di Scala, Dupuy et al. 2019). Similarly, slow gait speed is associated with low levels of resting-state connectivity in the basal ganglia (Karim, Rosso et al. 2020). Recently, it was demonstrated (Samogin, Rueda Delgado et al. 2022) that older adults with better performance on manual visual-motor task have stronger connectivity within the SMN and dorsal attention network (DAN). Our most recent work demonstrated that DAN connectivity is enhanced during a motor imagery task compared to rest in older adults (Neyland, Hugenschmidt et al. 2021), but associations with physical function have yet to be investigated. Much of the emerging work examining the neural correlates of mobility decline has been in people with mild cognitive impairment (MCI). It has been found that slow gait speed has been shown to be associated with high connectivity between the SMN and the fronto-parietal network (Hsu, Best et al. 2019) whereas high connectivity between the SMN and default-mode network is associated with postural sway (Crockett, Hsu et al. 2019; Hsu, Crockett et al. 2020).
While there are a growing number of studies examining associations between physical function and brain connectivity in older adults (Hsu, Best et al. 2017; Crockett, Hsu et al. 2019; Di Scala, Dupuy et al. 2019; Hsu, Best et al. 2019; Hsu, Crockett et al. 2020; Karim, Rosso et al. 2020), brain network signatures that distinguish high functioning from low functioning older adults are still not known. Most existing studies used small samples, were pilot or exploratory, or focused on individuals with cognitive impairment. Here, we report baseline data from the Brain Networks and Mobility (B-NET) study, a longitudinal study (n=192) examining relationships between physical function and brain networks in cognitively unimpaired older adults who have no history of CNS disease. The purpose of this analysis is to: 1) assess the replicability of associations between physical function and resting-state SMN community structure observed in our small pilot study (Hugenschmidt, Burdette et al. 2014), 2) determine if physical function-brain network associations are modified during the performance of a motor imagery task, 3) identify associations between physical function and DAN connectivity at rest and during motor imagery, and 4) examine the combined association of physical function and modifiable risk factors including body mass index (BMI), hypertension, physical activity, and diabetes on SMN and DAN connectivity. Comparisons between older and younger adults were performed to serve as a referent for the four main outcomes. The direction of differences between the group of younger adults with high physical function compared to the older adults was used for interpretation purposes.
We hypothesized that using advanced regression analyses the association between physical function and SMN community structure would be replicated in this larger study. It was further anticipated that any observed associations in SMN would be strongest for resting state and the DAN associations would be strongest during motor imagery based on prior findings with motor imagery (Neyland, Hugenschmidt et al. 2021). We also hypothesized that the main effects of physical function and modifiable risk factors would each be associated with the integrity of brain network community structure in both networks. Specifically, lower levels of physical function would be associated with lower community structure. Similarly, low physical activity, high BMI, and the presence of hypertension or diabetes would all be associated with lower community structure. The modifiable risk factors were an essential aspect of the original study design as they could serve as potential future targets for intervention. Our initial analysis plan was focused on the main effects of these risk factors, but we decided to explore the risk factors as potential moderators of eSPPB on brain organization using models with interaction terms. The interaction results are presented in the primary study outcomes because main effects cannot readily be interpreted in the presence of interactions.
2. Methods
2.1. B-NET Study Design
B-NET (NCT03430427) is an ongoing longitudinal, observational study of community-dwelling older adults aged 70 and older recruited from Forsyth County, NC and the surrounding regions. Participants were recruited through direct mailings, posted flyers, and the community newsletter from the Sticht Center for Healthy Aging and Alzheimer’s Prevention at Wake Forest University School of Medicine, as well as through word of mouth. Participants were asked to complete a baseline study visit along with follow-ups visits at 6, 18, and 30 months. Visits included extensive heath histories, cognitive testing, physical function testing, and brain MRIs. The current study evaluated a single timepoint (baseline) with MRI scans, physical function measures, and demographic/heath data all being collected at baseline. The longitudinal data collection is ongoing at the time of writing this report and will be reported later.
2.2. Participants
The complete BNET enrollment included 192 participants who were 70 years of age or greater. All older participants are participating in this 30-month longitudinal study. We also enrolled 30 younger adults (22–36 years of age) who only completed baseline measures. The objective of including the younger population was to provide a reference group for expected community structure in healthy young individuals. Exclusion criteria included being a single or double amputee, having musculoskeletal implants severe enough to impede functional testing (e.g. joint replacements), or dependency on a walker or another person to ambulate. Neurological/psychiatric exclusions included: unwilling or unable to complete a brain MRI scan, clinical diagnosis of neurologic disease affecting mobility (including Parkinson’s disease, Amyotrophic Lateral Sclerosis, and multiple sclerosis), prior traumatic brain injury with residual deficits, history of brain tumor, seizures within the last year, diagnosis of schizophrenia, bipolar, or other psychotic disorders, alcoholism (>21 drink per week), or evidence of impaired cognitive function. Cognitive impairment was defined based on scores on the Montreal Cognitive Assessment (MoCA). MoCA scores from 21–25 were reviewed by the study neuropsychologist to determine eligibility and scores of 20 or lower on were considered impaired and exclusionary (Ciesielska, Sokolowski et al. 2016; Carson, Leach et al. 2018). Mobility declines with age regardless of the presence of cognitive impairment or CNS disease. These conditions may affect mobility on their own and through pathways other than those due to the age-related loss. Therefore, and in accordance with a formative workshop in this area, we excluded those with CI or any diagnosis of a CNS disease to gain a clear picture of age-related change (Rosso, Studenski et al. 2013).
Participants were also excluded if they had undergone surgery or hospitalization within the past 6 months; serious or uncontrolled chronic disease (stage 3 or 4 cancer, stage 3 or 4 heart failure, liver failure or cirrhosis of the liver, uncontrolled angina, respiratory disease requiring the use or oxygen, renal failure requiring dialysis), major uncorrected hearing or vision problems, plans to relocate within the next two years, or active participation in a behavioral intervention trial. All participants gave written informed consent in this study as approved by the Institutional Review Board (IRB) of the Wake Forest School of Medicine (IRB protocol #IRB00046460; approval date: 08/27/2020).
2.3. Study Measurements
2.3.1. Baseline Study Visit:
The current study includes data collected at BNET’s baseline, an extensive evaluation over two study visits. Most measures were collected in the first visit, and the second visit was primarily focused on the MRI scan with visits typically within one month of each other. Demographic variables in the analyses included self-reported age, sex, and education. Height and weight were measured at the baseline visit using a wall-mounted stadiometer and calibrated scale. Diabetes was defined as anyone on a current diabetes medication or fasting serum glucose >130. Systolic hypertension was defined as anyone on hypertension medication and systolic blood pressure (SBP) > 130 or SBP > 150. BMI was calculated using kg/m2 based on height and weight. Self-reported physical activity was assessed using the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire (Stewart, Mills et al. 2001). Scores on the CHAMPS were divided into four groups based on levels of moderate aerobic activity: 1) No Activity, 2) <= 60m/week, 3) 60m/week < and <= 135m/week, and 4) >135m/week. Only a small fraction of (n=13) of our participants were current smokers. MRI scans (protocol described below) were collected at a separate visit that typically occurred within one month of the baseline visit.
2.3.2. Expanded Short Physical Performance Battery (eSPPB):
Physical function was assessed using the eSPPB (Simonsick, Newman et al. 2001). The expanded Short Physical Performance Battery (eSPPB) was adapted from the test described by Guralnik et al. (Guralnik, Simonsick et al. 1994) in order to address ceiling effects that could limit the value of the traditional SPPB in a well-functioning cohort such as BNET. There are four components to the eSPPB that are believed to measure different dimensions of physical function. For balance, participants are asked to hold a side-by-side posture for 10 seconds, and the semi-tandem, tandem, and one-leg position for 30 seconds each. Gait measures include the usual 4-m gait speed (m/sec) as well as a pace on a narrow walk wherein participants are required to keep their steps in between 2 parallel lines marked 20 cm apart. The final measure is the time it takes to stand up from a seated position five times. Scores for each subcomponent are then calculated based on the ratio to the best possible score. The resulting overall eSPPB score is continuous and ranges from 0–4, rather than the traditional 12-point right-skewed categorical score distribution of the SPPB. Higher values represent better performance. There were two participants with missing data preventing the calculation of eSPPB scores. All analyses using eSPPB were restricted to the 190 participants with complete data.
2.3.3. Brain Imaging and analyses:
Brief methodological details are presented here with full imaging protocol and image analysis details included in the Supplemental Methods (Sections S1.1–1.3). All brain images were collected on a Siemens 3T Skyra MRI Scanner equipped with a 32-channel head coil. Each scan session lasted approximately one hour. An anatomical image was collected using a T1-weighted 3D volumetric MPRAGE for image warping. To assess white matter health, fluid-attenuated inversion recovery (FLAIR) and diffusion tensor imaging (DTI) were used to calculate white matter lesion volume and fractional anisotropy, respectively. Functional MRI (fMRI) data were collected using blood oxygenation level-dependent (BOLD) imaging (Ogawa, Lee et al. 1990) at rest and during motor imagery tasks, For the resting-state scan a fixation cross was displayed on the monitor. For the visual imagery task, continuous feed videos adapted from the Mobility Assessment Tool – short form, or MAT-sf (Rejeski, Marsh et al. 2013; Rejeski, Rushing et al. 2015) were played on the monitor as previously detailed (Neyland, Hugenschmidt et al. 2021).
Structural image segmentation was completed using Statistical Parametric Mapping version 12 (SPM12, http://www.fil.ion.ucl.ac.uk/spm). The gray matter and white matter segmented images were summed and any voxel with ≥0.5 probability value was retained as a mask of brain parenchyma with non-brain tissue and cerebral spinal fluid (CSF) excluded. Structural images were masked and spatially normalized to the Montreal Neurological Institute (MNI) template using Advanced Normalization Tools (ANTs, (Avants, Epstein et al. 2008)). White matter hyperintensity lesions were identified on T2 FLAIR images using the lesion prediction algorithm implemented in the Lesion Segmentation Toolbox (LST) version 2.0.15 for SPM12. All intracranial lesion volumes (ICLV) used in regression analyses were scaled by the total intracranial volume. See Supplement Section 1.3.2 for more details on processing anatomical images for measures of white matter lesions and integrity.
Functional images went to standard preprocessing steps including distortion correction, slice time correction, realignment, coregistration to the native-space anatomical images, and warping to MNI space using the transformation derived from ANTs. Head motion was corrected using the motion scrubbing procedure (Power, Barnes et al. 2012). Images were band-pass filtered (0.009 – 0.08 Hz) and a regression was used to remove signal from total white matter, total gray matter, total CSF, and the 6 rigid-body motion parameters generated during the realignment process. The first 10 volumes of the BOLD images were dropped to allow for signal normalization.
2.3.4. Brain network generation:
A voxel-wise cross-correlation was performed where the preprocessed time series from each voxel was correlated with each and every other voxel. A threshold was then applied to the matrix with values above the threshold were set to 1, indicating the presence of a connection, 0 otherwise. The threshold (S) was empirically determined (Hayasaka and Laurienti 2010) and ensured that the density of connections was comparable across all participants with S = log (N)/log (K) and S=2.5 and K = average number of connections per node.
A dynamic Markov process (Delvenne, Yaliraki et al. 2010) was used to identify the network community partition that maximized modularity or Q (Newman 2006). The result of the partitioning is that each individual’s brain network is broken into a set of categorical communities. To perform group analyses, the communities from each participant are compared to a priori templates for the SMN and DAN generating a vector of scaled inclusivity (SI) values from all image voxels. The SI value in each voxel quantifies the spatial alignment of the communities with the templates (SMN or DAN). SI values for the templates from each participant were used in the regression analyses described below.
2.3.5. Statistical analyses:
Baseline descriptive statistics (i.e., mean, standard deviation, proportions) were calculated separately for younger and older cohorts. T-tests were used to compare means between groups for continuous variables, and chi-square tests were used for proportions.
Analyses examining associations between predictor variables and brain network community structure used a distance regression. This method was developed specifically to assess relationships between brain networks and continuous and or categorical phenotypes while controlling for potentially confounding and/or nuisance variables (Tomlinson, Laurienti et al. 2022). Separate models were run for each condition (rest and task) and network (SMN and DAN) combination. Further details can be found in section S1.4 in Supplemental Methods. For the current study, a 3-dimensional SI brain map and a list of continuous/categorical variables were the input for each study participant. The distance between the SI community structure maps was quantified using the Jaccard distance (See S1.4 in Supplemental Methods). For the independent variables, the distance was an absolute difference between participants. The distances were computed between every subject pair to generate a distance matrix for each variable in the model. The community structure distance was regressed against the predictor variable distances using a linear statistical model with individual-level fixed effects (Tomlinson, Laurienti et al. 2022). Statistical significance was set at p ≤ 0.05 for all analyses. For each risk factor an adapted false discovery rate (Benjamini and Hochberg 2000) was used to correct interaction p-values to account for the four independent models that were evaluated. Fully adjusted models included age, sex, education, intracranial white matter lesion volume, and number of brain volumes removed due to head motion.
The premise behind the distance regression begins with the underlying hypothesis that spatial patterns of brain connectivity are correlated with demographic measures or health behaviors (Figure 1). The regression models used distance scores between participants for the spatial mappings of the SMN-CS and DAN-CS as the dependent variables. The independent variables were the absolute difference scores, or distances (δ), between participant pairs. The hypothesis being tested is that as distances between independent variables increase, distance between the spatial patterns of the community structure between the same people will also increase. On the other hand, people with similar independent measures will have similar spatial patterns of the community structure. It is important to note that the model cannot determine if higher (or lower) independent variable scores are associated with higher (or lower) community structure. Rather, the model can only determine if differences in the independent variables are associated with differences in community structure and the direction of that relationship between the distances. Upon finding a significant distance regression outcome, the actual community structure was subsequently evaluated to determine if higher independent variables of interest were associated with higher or lower community structure. Community structure brain maps were generated by averaging voxel-wise SI values in each condition (e.g. SMN during rest) for upper and lower tertiles of the independent variable of interest (e.g. eSPPB) and mapping those mean values back into brain space.
Figure 1.
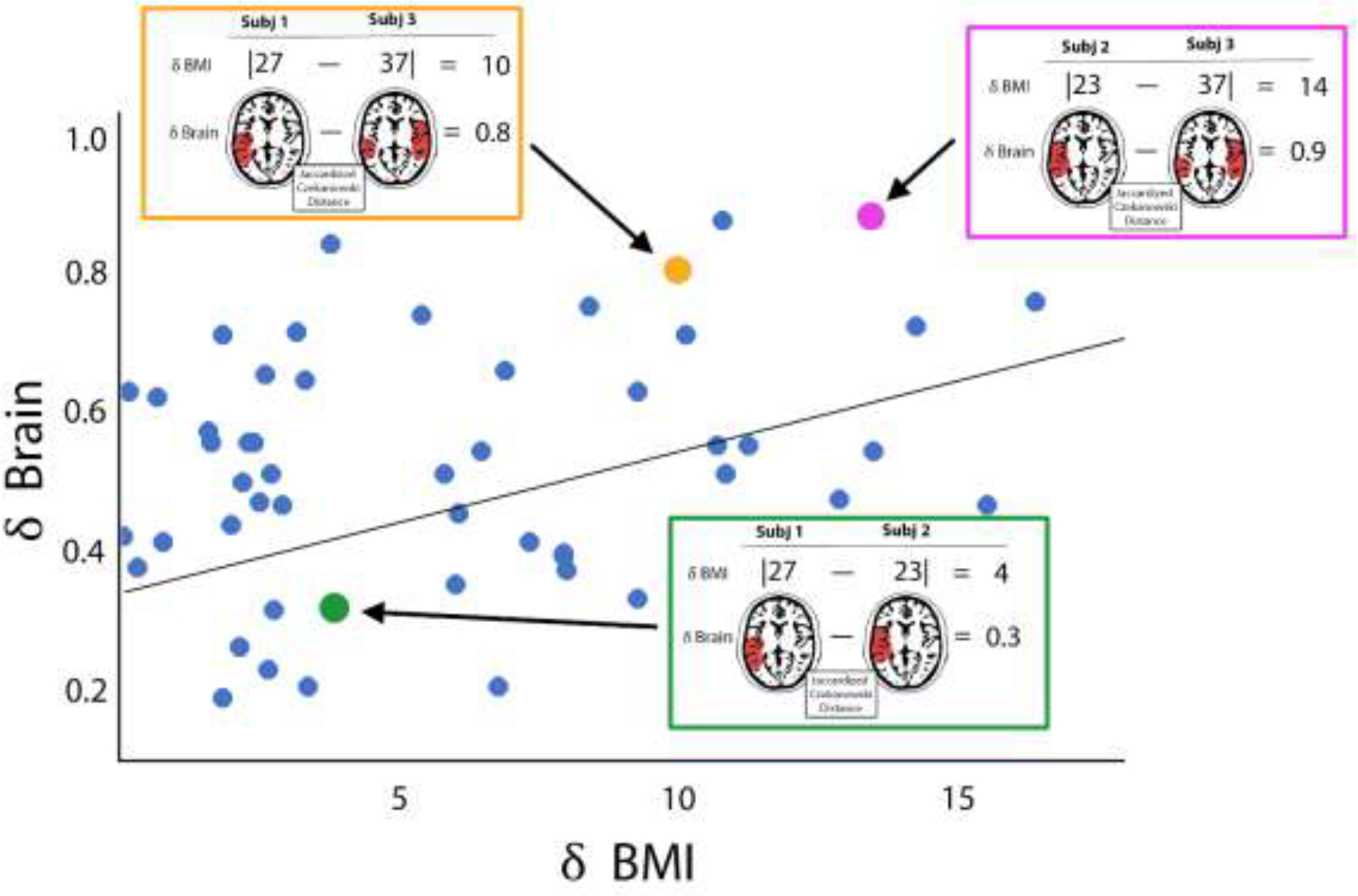
Schematic cartoon depicting the distance regression method. In this cartoon, the brain maps and BMI from three simulated participants from a larger group are shown. Each participant is compared to each other participant using distance measures. For numeric variables (the independent variables in this study), the absolute difference between participant is computed. This distance is the X value in the regression model. For the brain maps of network community structure (the dependent variable in this study), the weighted Jaccard distance is computed. This metric assesses spatial similarity of the two brain maps. The Jaccard distance (δBrain) is the Y variable in the regression. Note that subject 1 and subject 2 both have low BMIs, the δBMI is low (green box). They also have very similar brain maps resulting in low δBrain. When these two participants are compared to subject 3 with a higher BMI (red and blue boxes), the δBMI values are also higher. Since BMI and brain network organization are correlated in this cartoon example, the higher δBMIs are associated with larger δBrain values. Once the distances between all participants pairs is computed (simulated blue data points), a regression is performed. This cartoon shows a simple linear regression but the reality is that all data points for an individual participant compared to each of the remaining participants are correlated. Thus, the regression model is performed using an F test with individual level effects (ILE). This more complex model is not easily depicted in cartoon form, but the premise of the distance regression is the same.
2.3.6. Model building with eSPPB and modifiable risk factors:
The analyses examining community structure relationships with eSPPB, BMI, diabetes, hypertension, and self-reported physical activity were restricted to the older adults. The dependent variables were always the community structure for SMN or DAN at rest or during motor imagery. Each full model (Interaction models) included eSPPB, the modifiable risk factor of interest, and the interaction between eSPPB and the modifiable risk factor as the primary covariates of interest. All models also included age, sex, education, number of motion volumes removed, and ICLV as covariates. Although our original study design did not include interaction terms between eSPPB and the risk factors, we decided to explore these interactions as potential moderating relationships. As one cannot readily interpret main effects of independent variables in the presence of an interaction, the interaction models are presented first in the results. For models without a significant interaction, reduced models (Main effects models) were run after removing the interaction term to examine main effects of eSPPB and the modifiable risk factor.
3. Results
3.1. Participant characteristics
Table 1 shows demographic details for the older and younger adult samples. Group comparisons revealed significant differences for all variables except for sex and motor white matter volume. Although there were racial differences between groups, both groups were predominantly Caucasian/White. Three younger and two older adults reported Hispanic/Latino ethnicity. Many of the group differences were anticipated given known age associations including higher incidence of hypertension and diabetes, lower cognitive function scores, higher white matter lesion load, lower FA, and lower physical function scores. The self-reported physical activity levels had differences at the upper and lower ends of activity, with all younger adults reporting moderate levels of activity. As anticipated, the older adults had white matter measures indicative of poorer white matter health.
Table 1.
Baseline Characteristics of Participants By Age Cohort
| Younger (n=30) |
Older (n=192) |
P-value | |
|---|---|---|---|
|
| |||
| Age | 29.99 (3.83) | 76.43 (4.72) | <.0001 |
| Race/Ethnicity | <.0001 | ||
| Caucasian/White | 18 (60.0) | 173 (89.1) | |
| African American/Black | 4 (13.3) | 18 (9.4) | |
| Asian | 3 (10.0) | 1 (1.0) | |
| Multiracial/other | 5 (16.7) | 0 (0.5) | |
| Sex | 0.4659 | ||
| Men | 11 (36.7) | 84 (43.8) | |
| Women | 19 (63.3) | 108 (56.3) | |
| Years of Education | 16.93 (2.05) | 15.68 (2.45) | 0.0085 |
| BMI | 25.52 (5.05) | 28.39 (5.63) | 0.0091 |
| Diabetes | 0.0058 | ||
| No | 30 (100) | 152 (79.2) | |
| Yes | 0 (0.0) | 40 (20.8) | |
| Hypertension | <.0001 | ||
| No | 26 (86.7) | 92 (47.9) | |
| Yes | 4 (13.3) | 100 (52.1) | |
| Self-Reported Moderate Aerobic Activity Level | <.0001 | ||
| No Activity Reported | 0 (0.0) | 33 (17.2) | |
| Low <= 60min/week | 0 (0.0) | 45 (23.4) | |
| 60m/week < Mid <= 135m/week | 30 (100) | 60 (31.3) | |
| High > 135min/week | 0 (0.0) | 54 (28.1) | |
| MRI measures | |||
| Fractional anisotropy in the white matter for the motor region | 0.25 (0.01) (n=29) | 0.23 (0.02) (n=188) | <.0001 |
| Volume of lesions within the motor ROI in CC | 0.01 (0.01) | 1.02 (2.37) (n=191) | 0.0208 |
| Intracranial lesion volume CC | 0.15 (0.10) | 6.25 (9.14) (n=191) | 0.0003 |
| Intracranial volume CC | 1556.8 (217.60) | 1632.9 (178.71) (n=191) | 0.0368 |
| Volume of the motor ROI in CC | 88.26 (12.95) | 84.71 (9.81) | 0.0794 |
| Cognitive measures | |||
| MOCA Adjusted Score | 27.00 (5.44) | 25.64 (2.20) | 0.0153 |
| DSST | 81.20 (10.60) (n=25) | 55.18 (12.20) | <.0001 |
| Physical Function Measures | |||
| eSPPB | 2.81 (0.25) | 2.00 (0.52) (n=190) | <.0001 |
| 400m walk (m/sec) | 1.57 (0.21) | 1.27 (0.43) | 0.0002 |
Values are mean (SD) for continuous measures and counts (% within cohort) for categorical measures. CC = cubic centimeters.
3.2. Age-related differences in community structure
Community structure of both the SMN and DAN (SMN-CS and DAN-CS, respectively) was compared between the older and younger adults during the resting-state and visual imagery tasks to identify age-related differences. These models were on the main group effect using two-sample tests without covariates. Figure 2 shows that the younger population had more consistent community structure in both the SMN and the DAN during both rest and task. The regression analyses clearly demonstrate statistically that the distances observed in both networks were significantly different between older and younger adults at rest and during the task (Table 2). In this two-group regression, the estimates indicated the magnitude of the mean community structure distance between groups. Thus, while there is no simple direct interpretation of the individual estimates, the larger the estimate the more the community structure maps differ between older and younger adults. As reflected in the images and verified by the estimates, the distances between older and younger adults were smaller for the DAN than the SMN.
Figure 2.
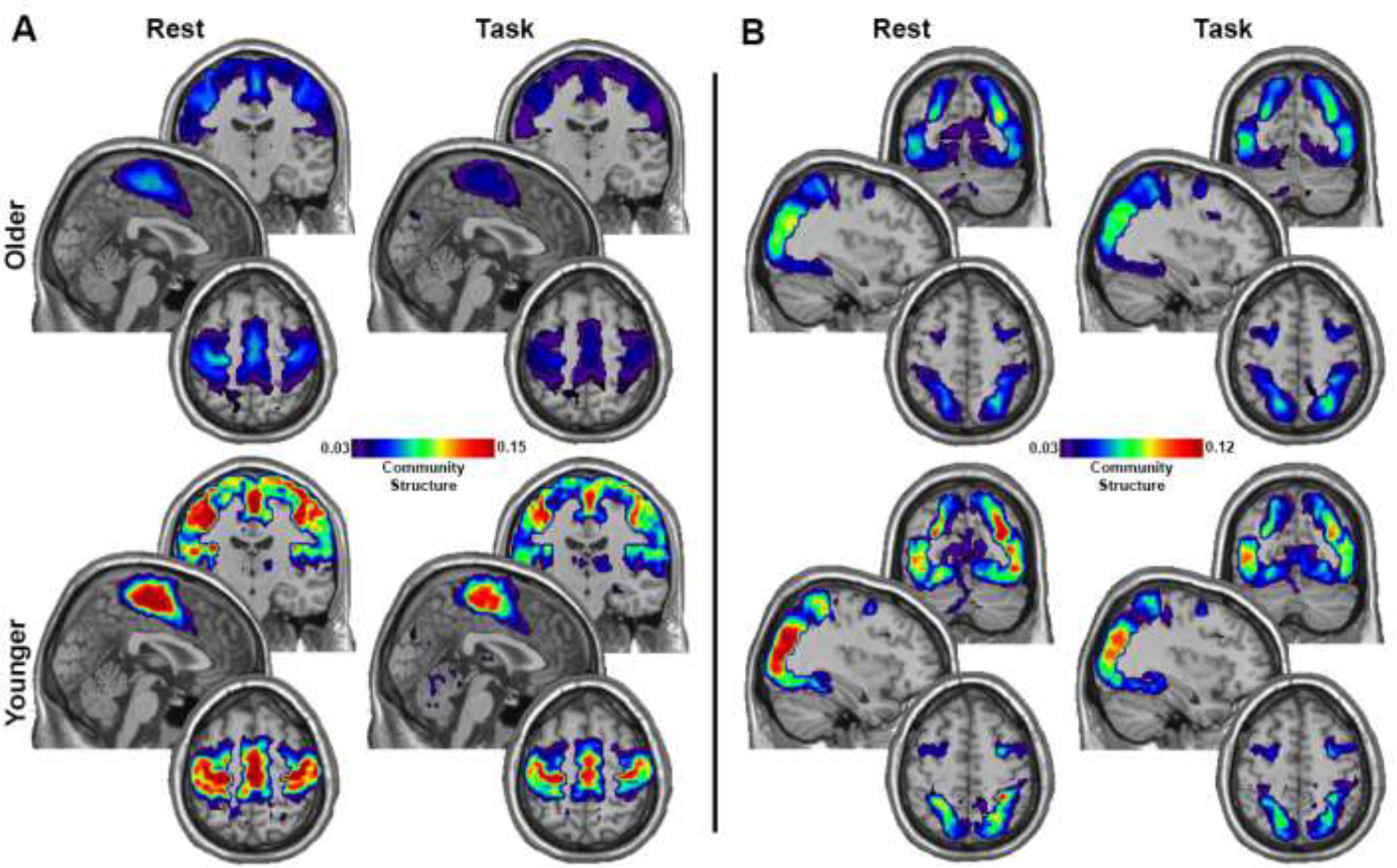
Community structure maps for the younger and older adults. A) Maps for the SMN. B) Maps for the DAN. Each map is an average across all participants in each group for the condition (Rest and Task) and network (SMN and DAN). Brain regions with hotter colors were more frequently part of the community across participants, indicating greater spatial consistency. The color scale represents the average SI value across each group/condition and has been scaled the min and max and applies to all images within each figure section (A and B). Each image collage contains a coronal slice at the top (y: SMN=−15, DAN=−70), a sagittal slice in the middle (x: SMN=2, DAN 36), and an axial slice on the bottom (z: SMN=58, DAN= 54).
Table 2.
Tests of community structure differences by age
| Network/Condition | Estimate | SE | T score | p-Value |
|---|---|---|---|---|
|
| ||||
| SMN /Rest |
0.0233 | 0.0009 | 25.3235 | <0.0001 |
| SMN /Task |
0.0211 | 0.0008 | 26.7307 | <0.0001 |
| DAN /Rest |
0.0064 | 0.0009 | 7.0410 | <0.0001 |
| DAN /Task | 0.0051 | 0.0008 | 6.5081 | <0.0001 |
The full sample of older (n = 192) and younger (n=30) adults were included in these analyses. SE = standard error. Positive estimates indicate higher community structure in younger adults.
3.2. Associations of eSPPB and modifiable risk factors with community structure
The primary finding of the interaction models with eSPPB and each of the modifiable risk factors was that only BMI exhibited a consistent interaction with eSPPB across conditions and networks. There were minimal associations of the other three risk factors with either SMN-CS or DAN-CS. Those findings are summarized in Section 3.3.4 with details presented in the Supplemental Information.
3.3.1. BMI*eSPPB model results (Table 3):
Table 3.
Full model for eSPPB interacting with BMI in the population of older adults
| Network/Condition | Variable | Estimate | SE | T score | p-Value |
|---|---|---|---|---|---|
|
| |||||
| SMN/Rest | eSPPB | 0.0006 | −0.0008 | 0.6989 | 0.4846 |
| BMI | 0.0002 | −0.0001 | 2.2613 | 0.0238 | |
| eSPPB*BMI | 0.0002 | −0.0001 | 2.1179 | 0.0342 | |
| Sex | 0.0016 | −0.0004 | 3.5963 | 0.0003 | |
| Motion | 0.0001 | 0.0000 | 1.6138 | 0.1066 | |
| Education | 0.0001 | −0.0001 | 0.8034 | 0.4218 | |
| ICLV | 0.0332 | −0.0920 | 0.3612 | 0.7179 | |
| Age | 0.0000 | −0.0001 | −0.5553 | 0.5787 | |
|
| |||||
| SMN/Task | eSPPB | −0.0006 | 0.0007 | −0.8282 | 0.4076 |
| BMI | 0.0000 | 0.0001 | −0.5975 | 0.5502 | |
| eSPPB*BMI | 0.0001 | 0.0001 | 1.8072 | 0.0707 | |
| Sex | 0.0008 | 0.0004 | 2.0821 | 0.0373 | |
| Motion | 0.0001 | 0.0000 | 4.2480 | <0.0001 | |
| Education | −0.0001 | 0.0001 | −1.2545 | 0.2097 | |
| ICLV | 0.0210 | 0.0765 | 0.2749 | 0.7834 | |
| Age | 0.0000 | 0.0001 | 0.5045 | 0.6139 | |
|
| |||||
| DAN/Rest | eSPPB | −0.0014 | −0.0009 | −1.4561 | 0.1454 |
| BMI | 0.0004 | −0.0001 | 4.1527 | <0.0001 | |
| eSPPB*BMI | 0.0003 | −0.0001 | 3.5018 | 0.0005 | |
| Sex | 0.0037 | −0.0005 | 7.5611 | <0.0001 | |
| Motion | 0.0001 | 0.0000 | 2.6325 | 0.0085 | |
| Education | −0.0002 | −0.0001 | −1.4610 | 0.1440 | |
| ICLV | 0.1228 | −0.1025 | 1.1979 | 0.2310 | |
| Age | 0.0001 | −0.0001 | 1.6730 | 0.0943 | |
|
| |||||
| DAN/Task | eSPPB | −0.0011 | −0.0008 | −1.3719 | 0.1701 |
| BMI | 0.0003 | −0.0001 | 3.5128 | 0.0004 | |
| eSPPB*BMI | 0.0004 | −0.0001 | 4.2871 | <0.0001 | |
| Sex | 0.0008 | −0.0004 | 1.9822 | 0.0475 | |
| Motion | 0.0003 | 0.0000 | 9.6182 | <0.0001 | |
| Education | 0.0000 | −0.0001 | −0.1739 | 0.8620 | |
| ICLV | 0.1034 | −0.0884 | 1.1702 | 0.2419 | |
| Age | 0.0000 | −0.0001 | 0.6894 | 0.4906 | |
All models included eSPPB and BMI as the main predictor variables, the interaction between eSPPB and BMI, as well as the remaining covariates. Due to 2 missing eSPPB scores and 1 missing ICLV, 189 of the total 192 older adults were included in the analyses. Motion = number of brain volumes removed during motion correction. SE = standard error
In the resting state, there was a significant interaction for both the SMN (p=0.0342) and DAN (p = 0.0005). For the motor imagery task the interaction was significant in the DAN (p < 0.0001) but fell short of significance in SMN (p = 0.0707). Correcting p-values for the interaction terms based on running four BMI models did not meaningfully change the statistical outcomes (SMN-rest p = 0.0456, SMN-task p = 0.0707, DAN-rest p = 0.001, and DAN-task p = 0.0004). Sex was significant in both resting state models and in the DAN during the task. Head motion was significant in both DAN models but not in the SMN. None of the other covariates were significant. In the reduced model for the SMN for task (Supplemental Table 1), the main effects for eSPPB and BMI were not significant. Details concerning associations between the independent variables are included in Supplemental Tables 2 and 3.
3.3.2. BMI*eSPPB plots:
The overall relationship across significant models was that the eSPPB distances (δeSPPB) interacted with the BMI distances (δBMI) in a synergistic manner as indicated by the positive estimates. Thus, community structure distances were consistently the largest in when δeSPPB and δBMI were both maximal. For visualization, two interaction plots were generated (Figure 3). Panel A shows δeSPPB as a continuous measure on the x-axis and ten evenly spaced, discrete values of δBMI. Panel B shows δBMI as a continuous measure on the x-axis and ten evenly spaced, discrete values of δeSPPB. The plots show that the interaction effect resulted in SMN-CS and DAN-CS distances being the largest when both - δeSPPB and δBMI were maximal. The interaction was more pronounced in the DAN compared to the SMN. If participants performed equivalently on the eSPPB (δeSPPB=0), community structure distances still varied by δBMI as indicated by spread between the y intercept values in Panel A and the positive slopes of the bottom yellow line in Panel B. That is, participants who had different BMIs but the same eSPPB score had different brain community structure. However, when δBMI=0 the association between δeSPPB and SMN-CS was minimal (bottom yellow line in Panel A and y intercepts in Panel B). It is interesting to note that the relationship between δeSPPB and DAN-CS actually flipped but was quite small in magnitude when δBMI was approximately 5 kg/m^2. See Supplemental Methods, Section S1.4, for details concerning outlier removal for generating of these plots.
Figure 3.
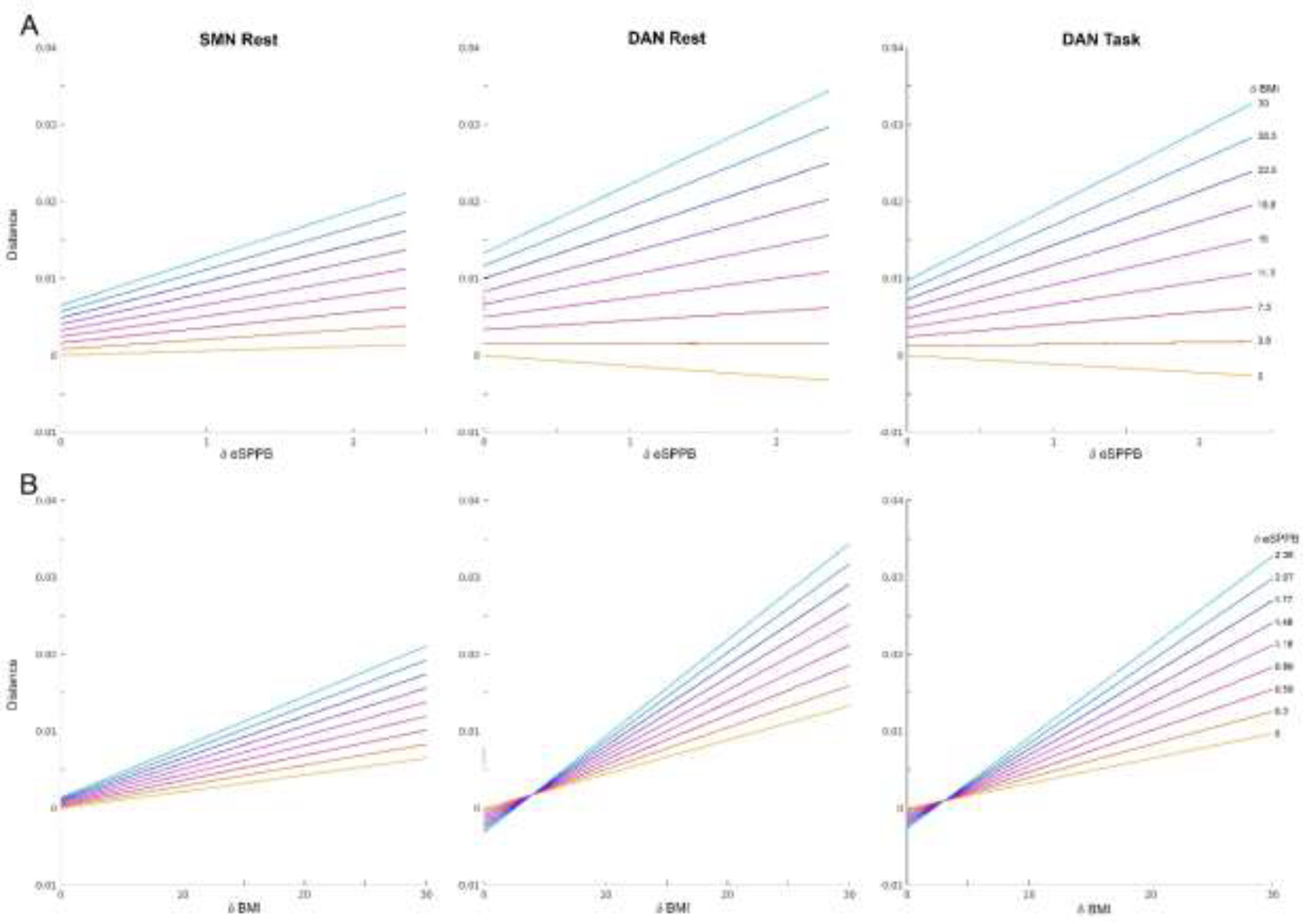
Plots showing how δeSPPB and δBMI interact in their effects on community structure. A) The colored lines represent the relationships between eSPPB distances (δeSPPB) on the x-axis and community structure distances on the y-axis for ten evenly spaced, discrete BMI distances (δBMI). The bottom yellow line represents the relationship between δeSPPB and community structure distance when the BMI distance is 0 and the top light blue line shows a δBMI of 30 kg/m2. The y-intercept shows the effect of δBMI when eSPPB scores are the same (δeSPPB=0). Note that there are systematic increases in community structure distance with increases in δBMI even between individuals with the comparable eSPPB scores. B) Colored lines represent the relationship between δBMI between participants on the x-axis and community structure distance on the y-axis for ten evenly spaced, discrete δeSPPB values. Similarly to panel A, the bottom yellow line represents the relationship between δBMI and community structure distance when there is no difference in eSPPB between participants and the y-intercept shows the effect of δeSPPB when BMI scores are the same. For the SMN at rest there are no meaningful effects of eSPPB in individuals with the same BMI. For the DAN at rest and during task, there is actually a decrease, albeit quite small, in distance with greater δeSPPB in individuals with the same BMI.
3.3.3. BMI and eSPPB community structure maps:
While the regression results clearly demonstrate significant associations of community structure distances with δeSPPB and δBMI, the regression models do not indicate the direction of the between-participant distances in community structure. In other words, with large between-subject differences in BMI (δBMI) and eSPPB (δeSPPB), there are large distances in community structure, but it is not known if higher community structure is associated with higher or lower eSPPB or BMI. Brain maps were generated for upper and lower tertiles of eSPPB and BMI in order to assess if the measures were positively or negatively associated with the spatial patterns of community structure. As stated above, the largest distances between community structure were found when comparing individuals where δeSPPB and δBMI were both maximal. The figures give a sense of the difference in the integrity of the community structure for groups with large differences in eSPPB and BMI. As shown in Figure 4A, individuals with higher eSPPB score compared to lower showed more consistent SMN-CS spatial localization, indicating a positive association between eSPPB and SMN-CS. Similar findings were observed for maps of DAN-CS during rest (Figure 4B) and during the motor imagery task (Figure 4B). Figure 4D–F shows findings for BMI. Those with lower BMI had higher consistency in community structure spatial localization compared to those individuals with higher BMI for SMN-CS and DAN-CS at rest and DAN-CS during motor imagery, indicating a negative association between BMI and community structure. It is important to note that the scale changed relative to the age-group comparison shown in Figure 2, such that older adults in the upper tertiles for eSPPB or lower tertile for BMI remained lower than the younger adults.
Figure 4.
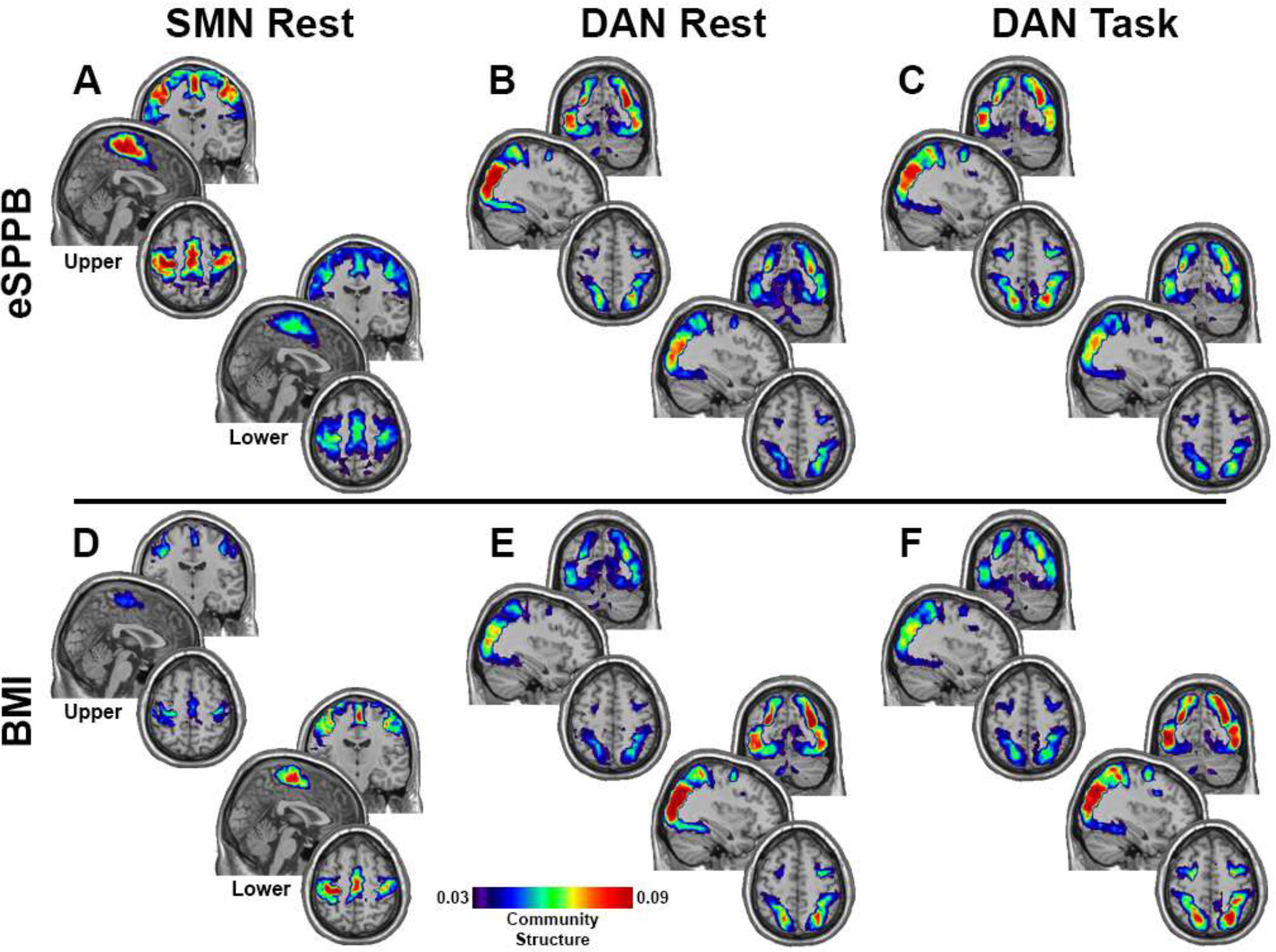
Community structure maps for the groups in the upper and lower tertiles (N=54 in each group) of eSPPB and BMI. For eSPPB, the upper tertile had greater community spatial overlap across participants for all three conditions/networks (A-C). BMI exhibited the opposite relationship with community structure with the lower tertile having the higher spatial consistency (D-F). Brain regions with hotter colors were more frequently part of the community across participants, indicating greater spatial consistency. The color bar applies to all images and is scaled as in Figure 2. Slice locations are the same as in Figure 2.
3.3.4. Model results for hypertension, CHAMPS, and diabetes:
There was a significant interaction between hypertension and eSPPB (p = 0.0335) in the SMN at rest (Table S4). This interaction between hypertension and eSPPB did not remain significant after correcting for multiple comparisons using a FDR (p = 0.1340). No other interaction models were significant for hypertension. Given that the interaction was found in only one network/condition model and did not survive correction, it was not explored in further detail. The interaction models for CHAMPS and diabetes with eSPPB were not significant for either network or condition (Tables S6 and S8, respectively). Thus, correcting interaction p-values for multiple comparisons had no meaningful effect on the outcomes. In the main effects models for hypertension (Table S5), CHAMPS (Table S7), and diabetes (Table S9), multiple consistent findings were evident. After adjusting for hypertension, CHAMPS, and diabetes, significant effects of eSPPB on DAN-CS were found during the task in each of the separate models. In models that adjusted for CHAMPS and diabetes, there were significant effects of eSPPB on SMN-CS at rest but not during the task. Sex and motion were significant in the majority of the main effects models. The presence of diabetes was the only significant risk factor main effect, and this was observed only in the SMN for task.
4. Discussion
The current study examined associations for physical function and modifiable risk factors with functional brain network community structure in older adults. The main findings were that significant interactions existed between eSPPB and BMI in associations with brain network community structure for both the SMN and DAN. The interactions were such that they amplified each other’s associations and brain network community structure was lowest in people with low physical function combined with high BMI. The lower community structure in these individuals is a network manifestation of lower regional interconnectivity in the SMN and DAN, circuits known to be important to physical functioning. It is intuitive that the SMN is essential for physical function as this circuit contains the primary motor neurons that initiate movement. The DAN is a spatial attention circuit, and it has been shown that community structure of this circuit is particularly important during motor imagery tasks (Hetu, Gregoire et al. 2013; Neyland, Hugenschmidt et al. 2021). Consistent with the current findings, our prior preliminary work found that SMN-CS was higher in those with “high” (11–12) SPPB scores compared those with “low” (7–9) SPPB scores (Hugenschmidt, Burdette et al. 2014). Thus, this work replicates our prior findings and expands the study to address prior study limitations. That initial work lacked some of the key components of the current study including: 1) the more sensitive, continuously scaled eSPPB test, 2) the use of resting state and a motor imagery task, 3) assessment of DAN-CS in addition to SMN-CS, 4) the use of a regression model allowing for the inclusion of covariates and the examination of interactions between eSPPB and modifiable risk factors, and 5) the exclusion of those with cognitive impairment or clinically diagnosed CNS disease.
Most research examining brain-based contributors to physical function has examined structural brain changes that occur with age. White matter disease that is likely irreversible is the most replicated brain pathology associated with poor physical function. White matter disease is also diseases such as type 2 diabetes and cardiovascular disease. In the current study, rankings for white matter damage differed significantly by age, but only accounted for about 7% of the variance in the ranked eSPPB scores (Supplemental Table 2) and did not account for a significant amount of the community structure variance when included in full models. These findings, in the context of the robust literature supporting an association between WMH and gait, suggest that the associations of eSPPB and BMI with brain network organization are occurring prior to structural damage to brain white matter tracts. It is possible that the relatively healthy BNET sample had not developed white matter disease to the degree that it affects physical function or brain network organization. The lack of an association between white matter disease and brain network community structure may also explain why neither hypertension nor diabetes (both major risk factors for white matter disease) had associations with community structure to the degree observed for BMI. Longitudinal data could help resolve these uncertainties as BNET participants develop more extensive structural brain changes.
Previous work on aging and physical function shows that those older adults with obesity (BMI ≥ 30) have poorer physical function (Lang, Llewellyn et al. 2008; Riebe, Blissmer et al. 2009). Also, a large multi-center trial of weight loss in people with type 2 diabetes, including older adults, found following 4-years of intervention that those randomized to weight loss as compared to a support group had a relative reduction of 48% in the risk of self-reported loss of mobility, effects mediated by both weight loss and change in fitness as assessed by a treadmill test (Rejeski, Ip et al. 2012). It is known that people with obesity have lower levels of physical activity, increased fat mass and reduced muscle mass (Jankowski, Gozansky et al. 2008), as well as higher levels of multi-site pain (Nur, Sertkaya et al. 2018). Emerging evidence from the field of obesity shows that in younger adults, obesity is associated with reduced functional network connectivity in the sensorimotor cortex (Geha, Cecchi et al. 2017; Lee, Kwon et al. 2022) and that structural sensorimotor ‘brain age’ was improved after bariatric surgery (Zeighami, Dadar et al. 2022).
Several other studies have found associations between physical function and connectivity of the SMN. Low efficiency of local connectivity in the SMN in older adults was associated with poor gait stability (Di Scala, Dupuy et al. 2019) whereas low levels of connectivity in the basal ganglia were associated with slow gait speed (Karim, Rosso et al. 2020). Slow gait speed has been shown to be associated with high interconnectivity between the SMN and the fronto-parietal network (FPN) in older adults with mild cognitive impairment (Hsu, Best et al. 2019). Furthermore, an aerobic intervention in older adults with mild vascular cognitive impairment decreased connectivity between SMN and FPN and the change was associated with improved mobility (Hsu, Best et al. 2017). There have also been findings that high connectivity between the SMN and default-mode network (DMN) is associated with postural sway in adults with MCI and a history of falls (Crockett, Hsu et al. 2019) and in MCI patients with low mobility assessed using the Life Space Assessment (Hsu, Crockett et al. 2020).
The current findings make important contributions to emerging literature on associations between physical function, BMI, and brain organization. Specifically, this study showed that low physical function combined with obesity is associated with deficits in the community structure of the SMN in a resting state. The visual imagery task allowed us to examine the integrity of the DAN while participants imagined themselves ambulating through space. The motor imagery task was based on the MAT-sf (Rejeski, Marsh et al. 2013; Rejeski, Rushing et al. 2015), a task that requires participants to actively engage computer animated video clips that range in difficulty from slow walking, to ascending stairs, negotiating environmental barriers, and walking outdoors on a slight incline with an uneven surface. The finding that poor physical function was associated with lower DAN community structure, particularly during the motor imagery task, suggests that the embodiment of actively engaging with the environment is compromised. Given that the DAN is important for visuospatial attentional processing, participants with low physical functioning likely have challenges effectively imagining themselves performing a task that they likely were once able to do.
Although causation cannot be determined in the current work, the findings are consistent with the idea that disuse of sensorimotor or spatial attention circuits could result in degradation of the network connectivity. There was no association between brain network community structure and self-reported aerobic activity. Given the self-reported nature of the assessment, it is possible that our measure of activity is not accurate enough to identify associations. Unfortunately, we do not have measures of activity or fitness, such as accelerometry or treadmill tests, to further investigate ideas about disuse. It may be useful to evaluate how longitudinal changes in self-reported activity are associated with changes in SMN and DAN connectivity in future research. It is important to study functional brain networks within the context of physical function because functional brain networks retain plasticity with aging, making them potential targets for novel interventions. There is supporting evidence that increasing physical function in older adults can enhance brain network community structure (Petrie, Rejeski et al. 2017). As people age, increased attention is being given to modifying the design of physical activity interventions. Specifically, promoting multiple components of fitness (e.g., balance, strength, and agility) performed in varied, challenging physical environments. Also, new technologies, such as virtual reality animation, are being used in the context of physical rehabilitation. Perhaps this technology could be used more broadly as an adjunct to physical activity programs for older adults, facilitating the strengthening of community structure in the SMN and DAN.
The current study is not without weaknesses. First, this is a cross-sectional analysis examining associations between physical function and brain networks across participants. Although the findings were quite robust, causation cannot be inferred. It is possible that declines in physical function and BMI synergistically result in disuse of brain motor circuits causing declines in community structure. It is also possible that declines in brain network community structure precede and are responsible for declines in physical function and increases in BMI. One major step toward addressing these possibilities is to examine longitudinal changes in physical function, BMI, and brain networks. Indeed, the BNET study is actively collecting longitudinal data on these very participants for future analyses directed at addressing the directionality of the relationships via change. Another limitation is that the study sample was fairly homogeneous, relatively healthy and high functioning, and not particularly representative of the general population. It will be important to perform these analyses in a diverse population to determine if the findings generalize beyond our study sample. While the main objective of this study was to assess associations between physical function and brain networks, we did compare brain network community structure between the older adults and a cohort of younger adults. However, the young adult sample was quite small, differed from the older adults on many measures, and the groups were not balanced. The findings nevertheless can serve as reference for “ideal” brain network organization from a group of younger adults with high physical function as high physical functioning in this sample of older adults was not associated with compensatory reorganization of their brain networks. Finally, the duration of our scans was relatively short. There is yet to be universal agreement on ideal scan durations (Whitlow, Casanova et al. 2011; Birn, Molloy et al. 2013), but the shorter scans are certainly more susceptible to noise and potentially have lower reliability. As work in this area expands, future studies should examine the effects of scan duration on brain network-physical function associations.
5. Conclusions
This study clearly demonstrated strong and synergistic associations of physical function and BMI with brain network organization. Better understanding the direction of causality in the observed associations could help direct future interventions. If disuse is causing network degradations, then behavioral interventions can be designed and utilized to increase activity to improve network organization. The interaction may provide a unique opportunity to personalize interventions to target sedentary behavior, weight loss, or both, as appropriate for the given individual. The synergistic associations mean that sedentary individuals with obesity have the greatest to gain with a dual intervention. If causality is directed from brain networks to behavior, then interventions will need to identify therapeutics or possibly brain stimulation methods to improve network organization. Such interventions could improve physical function and lead to weight loss, two changes that would interact and facilitate positive intervention outcomes. We hypothesize that there may be a circular causal relationship with disuse resulting in network declines leading to further disuse, resulting in spiraling declines in physical function and brain health. That said, we did not see associations between self-reported physical activity and brain network organization. Our conclusion that disrupted spatial organization in the SMN and DAN is detrimental is based on the observed association with poor physical function and high BMI in this study population. It is important to note that it is theoretically possible that preservation of physical function could occur through compensatory changes in brain connectivity that result in decreased spatial consistency of the community structure, as observed in this study. Given the critical importance of physical function to overall health and quality of life in older adults, the observed associations between low physical function/high BMI and brain network community structure deserve considerable attention in future mechanistic and intervention studies.
Supplementary Material
Highlights.
Sensorimotor and dorsal attention networks are important for physical function
Brain network community structure is lower in older compared to younger adults
Physical function was positively associated with community structure
Higher body mass index (BMI) was negatively associated community structure
Level of physical function and BMI interacted synergistically
6. Acknowledgements
We would like to thank the entire BNET study team that contributed to the participant recruitment, data collection, and data curation/analyses. We would also like to thank the BNET participants that contributed substantial amounts of time and effort toward participation. This work was supported by the National Institute on Aging (AG047422, AG047422–05S1, and P30 AG021332).
Footnotes
Declarations of interest: none
CRediT authorship contribution statement
Paul J Laurienti – Conceptualization, Methodology, Resources, Writing - Original Draft, Visualization, Supervision, Funding acquisition, Michael E Miller – Conceptualization, Formal analysis, Validation, Data Curation, Writing - Review & Editing, Robert G Lyday – Software, Formal analysis, Data Curation, Visualization, Madeline C. Boyd – Investigation, Formal analysis, Alexis Tanase – Investigation, Formal analysis, Jonathan H Burdette – Conceptualization, Writing - Review & Editing, Christina E Hugenschmidt – Conceptualization, Writing - Original Draft, W Jack Rejeski – Conceptualization, Writing - Review & Editing, Sean L. Simpson – Methodology, Writing - Review & Editing, Laura D. Baker- Writing – Investigation, Review & Editing, Chal E. Tomlinson – Methodology, Software, Stephen B Kritchevsky – Conceptualization, Resources, Writing - Original Draft, Supervision, Funding acquisition.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
7. References
- Albert SM, Bear-Lehman J and Anderson SJ (2015). “Declines in mobility and changes in performance in the instrumental activities of daily living among mildly disabled community-dwelling older adults.” J Gerontol A Biol Sci Med Sci 70(1): 71–77. DOI: 10.1093/gerona/glu088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M and Gee JC (2008). “Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain.” Med Image Anal 12(1): 26–41. DOI: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y and Hochberg Y (2000). “On the adaptive control of the false discovery fate in multiple testing with independent statistics.” Journal of Educational and Behavioral Statistics 25(1): 60–83. DOI: 10.3102/10769986025001060. [DOI] [Google Scholar]
- Birn RM, Molloy EK, Patriat R, Parker T, Meier TB, Kirk GR, Nair VA, Meyerand ME and Prabhakaran V (2013). “The effect of scan length on the reliability of resting-state fMRI connectivity estimates.” Neuroimage 83: 550–558. DOI: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson N, Leach L and Murphy KJ (2018). “A re-examination of Montreal Cognitive Assessment (MoCA) cutoff scores.” Int J Geriatr Psychiatry 33(2): 379–388. DOI: 10.1002/gps.4756. [DOI] [PubMed] [Google Scholar]
- Ciesielska N, Sokolowski R, Mazur E, Podhorecka M, Polak-Szabela A and Kedziora-Kornatowska K (2016). “Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis.” Psychiatr Pol 50(5): 1039–1052. DOI: 10.12740/PP/45368. [DOI] [PubMed] [Google Scholar]
- Cohen-Mansfield J, Hazan H, Lerman Y and Shalom V (2016). “Correlates and predictors of loneliness in older-adults: a review of quantitative results informed by qualitative insights.” Int Psychogeriatr 28(4): 557–576. DOI: 10.1017/S1041610215001532. [DOI] [PubMed] [Google Scholar]
- Crockett RA, Hsu CL, Best JR, Beauchet O and Liu-Ambrose T (2019). “Head over heels but I forget why: Disruptive functional connectivity in older adult fallers with mild cognitive impairment.” Behavioural Brain Research 376. DOI: ARTN 112104 10.1016/j.bbr.2019.112104. [DOI] [PubMed] [Google Scholar]
- Delvenne J-C, Yaliraki SN and Barahona M (2010). “Stability of graph communities across time scales.” Proceedings of the National Academy of Sciences 107(29): 12755–12760. DOI: 10.1073/pnas.0903215107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Scala G, Dupuy M, Guillaud E, Doat E, Barse E, Dillhareguy B, Jean FAM, Audiffren M, Cazalets JR and Chanraud S (2019). “Efficiency of Sensorimotor Networks: Posture and Gait in Young and Older Adults.” Exp Aging Res 45(1): 41–56. DOI: 10.1080/0361073X.2018.1560108. [DOI] [PubMed] [Google Scholar]
- Fagerstrom C and Borglin G (2010). “Mobility, functional ability and health-related quality of life among people of 60 years or older.” Aging Clin Exp Res 22(5–6): 387–394. DOI: 10.1007/BF03324941. [DOI] [PubMed] [Google Scholar]
- Geha P, Cecchi G, Todd Constable R, Abdallah C and Small DM (2017). “Reorganization of brain connectivity in obesity.” Hum Brain Mapp 38(3): 1403–1420. DOI: 10.1002/hbm.23462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groessl EJ, Kaplan RM, Rejeski WJ, Katula JA, King AC, Frierson G, Glynn NW, Hsu FC, Walkup M and Pahor M (2007). “Health-related quality of life in older adults at risk for disability.” Am J Prev Med 33(3): 214–218. DOI: 10.1016/j.amepre.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA and Wallace RB (1994). “A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission.” J Gerontol 49(2): M85–94. DOI: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- Hayasaka S and Laurienti PJ (2010). “Comparison of characteristics between region-and voxel-based network analyses in resting-state fMRI data.” Neuroimage 50(2): 499–508. DOI: 10.1016/j.neuroimage.2009.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetu S, Gregoire M, Saimpont A, Coll MP, Eugene F, Michon PE and Jackson PL (2013). “The neural network of motor imagery: an ALE meta-analysis.” Neurosci Biobehav Rev 37(5): 930–949. DOI: 10.1016/j.neubiorev.2013.03.017. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Best JR, Voss MW, Handy TC, Beauchet O, Lim C and Liu-Ambrose T (2019). “Functional Neural Correlates of Slower Gait Among Older Adults With Mild Cognitive Impairment.” Journals of Gerontology Series a-Biological Sciences and Medical Sciences 74(4): 513–518. DOI: 10.1093/gerona/gly027. [DOI] [PubMed] [Google Scholar]
- Hsu CL, Best JR, Wang S, Voss MW, Hsiung RGY, Munkacsy M, Cheung W, Handy TC and Liu-Ambrose T (2017). “The Impact of Aerobic Exercise on Fronto-Parietal Network Connectivity and Its Relation to Mobility: An Exploratory Analysis of a 6-Month Randomized Controlled Trial.” Front Hum Neurosci 11: 344. DOI: 10.3389/fnhum.2017.00344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CL, Crockett R, Chan P, ten Brinke L, Doherty S and Liu-Ambrose T (2020). “Functional connectivity underpinning changes in life-space mobility in older adults with mild cognitive impairment: A 12-month prospective study.” Behavioural Brain Research 378. DOI: 10.1016/j.bbr.2019.112216. [DOI] [PubMed] [Google Scholar]
- Hugenschmidt CE, Burdette JH, Morgan AR, Williamson JD, Kritchevsky SB and Laurienti PJ (2014). “Graph theory analysis of functional brain networks and mobility disability in older adults.” J Gerontol A Biol Sci Med Sci 69(11): 1399–1406. DOI: 10.1093/gerona/glu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski CM, Gozansky WS, Van Pelt RE, Schenkman ML, Wolfe P, Schwartz RS and Kohrt WM (2008). “Relative contributions of adiposity and muscularity to physical function in community-dwelling older adults.” Obesity (Silver Spring) 16(5): 1039–1044. DOI: 10.1038/oby.2007.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim HT, Rosso A, Aizenstein HJ, Bohnen NI, Studenski S and Rosano C (2020). “Resting state connectivity within the basal ganglia and gait speed in older adults with cerebral small vessel disease and locomotor risk factors.” Neuroimage-Clinical 28. DOI: 10.1016/j.nicl.2020.102401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang IA, Llewellyn DJ, Alexander K and Melzer D (2008). “Obesity, physical function, and mortality in older adults.” J Am Geriatr Soc 56(8): 1474–1478. DOI: 10.1111/j.1532-5415.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- Lee H, Kwon J, Lee JE, Park BY and Park H (2022). “Disrupted stepwise functional brain organization in overweight individuals.” Commun Biol 5(1): 11. DOI: 10.1038/s42003-021-02957-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbaek G, Teri L and Mukadam N (2017). “Dementia prevention, intervention, and care.” Lancet 390(10113): 2673–2734. DOI: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Majer IM, Nusselder WJ, Mackenbach JP, Klijs B and van Baal PH (2011). “Mortality risk associated with disability: a population-based record linkage study.” Am J Public Health 101(12): e9–15. DOI: 10.2105/AJPH.2011.300361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni NK, Perera S, Studenski SA, Rosano C, Aizenstein HJ and VanSwearingen JM (2015). “Callosal hyperintensities and gait speed gain from two types of mobility interventions in older adults.” Arch Phys Med Rehabil 96(6): 1154–1157. DOI: 10.1016/j.apmr.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni NK, Studenski SA, Perera S, Rosano C, Aizenstein HJ, Brach JS and Van Swearingen JM (2013). “White matter hyperintensities, exercise, and improvement in gait speed: does type of gait rehabilitation matter?” J Am Geriatr Soc 61(5): 686–693. DOI: 10.1111/jgs.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MEJ (2006). “Modularity and community structure in networks.” Proceedings of the National Academy of Sciences 103(23): 8577–8582. DOI: 10.1073/pnas.0601602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyland BR, Hugenschmidt CE, Lyday RG, Burdette JH, Baker LD, Rejeski WJ, Miller ME, Kritchevsky SB and Laurienti PJ (2021). “Effects of a Motor Imagery Task on Functional Brain Network Community Structure in Older Adults: Data from the Brain Networks and Mobility Function (B-NET) Study.” Brain Sci 11(1). DOI: 10.3390/brainsci11010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nur H, Sertkaya BS and Tuncer T (2018). “Determinants of physical functioning in women with knee osteoarthritis.” Aging Clin Exp Res 30(4): 299–306. DOI: 10.1007/s40520-017-0784-x. [DOI] [PubMed] [Google Scholar]
- Ogawa S, Lee TM, Kay AR and Tank DW (1990). “Brain magnetic resonance imaging with contrast dependent on blood oxygenation.” Proc Natl Acad Sci U S A 87(24): 9868–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie M, Rejeski WJ, Basu S, Laurienti PJ, Marsh AP, Norris JL, Kim-Shapiro DB and Burdette JH (2017). “Beet Root Juice: An Ergogenic Aid for Exercise and the Aging Brain.” J Gerontol A Biol Sci Med Sci 72(9): 1284–1289. DOI: 10.1093/gerona/glw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL and Petersen SE (2012). “Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion.” Neuroimage 59(3): 2142–2154. DOI: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Ip EH, Bertoni AG, Bray GA, Evans G, Gregg EW, Zhang Q and Look ARG (2012). “Lifestyle change and mobility in obese adults with type 2 diabetes.” N Engl J Med 366(13): 1209–1217. DOI: 10.1056/NEJMoa1110294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Marsh AP, Anton S, Chen SH, Church T, Gill TM, Guralnik JM, Glynn NW, King AC, Rushing J, Ip EH and Group LR (2013). “The MAT-sf: clinical relevance and validity.” J Gerontol A Biol Sci Med Sci 68(12): 1567–1574. DOI: 10.1093/gerona/glt068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejeski WJ, Rushing J, Guralnik JM, Ip EH, King AC, Manini TM, Marsh AP, McDermott MM, Fielding RA, Newman AB, Tudor-Locke C, Gill TM and Group LS (2015). “The MAT-sf: identifying risk for major mobility disability.” J Gerontol A Biol Sci Med Sci 70(5): 641–646. DOI: 10.1093/gerona/glv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riebe D, Blissmer BJ, Greaney ML, Garber CE, Lees FD and Clark PG (2009). “The relationship between obesity, physical activity, and physical function in older adults.” J Aging Health 21(8): 1159–1178. DOI: 10.1177/0898264309350076. [DOI] [PubMed] [Google Scholar]
- Rosano C, Rosso AL and Studenski SA (2014). “Aging, brain, and mobility: progresses and opportunities.” J Gerontol A Biol Sci Med Sci 69(11): 1373–1374. DOI: 10.1093/gerona/glu159. [DOI] [PubMed] [Google Scholar]
- Rosario BL, Rosso AL, Aizenstein HJ, Harris T, Newman AB, Satterfield S, Studenski SA, Yaffe K, Rosano C and Health ABCS (2016). “Cerebral White Matter and Slow Gait: Contribution of Hyperintensities and Normal-appearing Parenchyma.” J Gerontol A Biol Sci Med Sci. DOI: 10.1093/gerona/glv224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Studenski SA, Chen WG, Aizenstein HJ, Alexander NB, Bennett DA, Black SE, Camicioli R, Carlson MC, Ferrucci L, Guralnik JM, Hausdorff JM, Kaye J, Launer LJ, Lipsitz LA, Verghese J and Rosano C (2013). “Aging, the central nervous system, and mobility.” J Gerontol A Biol Sci Med Sci 68(11): 1379–1386. DOI: 10.1093/gerona/glt089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samogin J, Rueda Delgado L, Taberna GA, Swinnen SP and Mantini D (2022). “Age-Related Differences of Frequency-Dependent Functional Connectivity in Brain Networks and Their Link to Motor Performance.” Brain Connect. DOI: 10.1089/brain.2021.0135. [DOI] [PubMed] [Google Scholar]
- Savikko N, Routasalo P, Tilvis RS, Strandberg TE and Pitkala KH (2005). “Predictors and subjective causes of loneliness in an aged population.” Arch Gerontol Geriatr 41(3): 223–233. DOI: 10.1016/j.archger.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, Newman AB, Nevitt MC, Kritchevsky SB, Ferrucci L, Guralnik JM, Harris T and Health ABCSG (2001). “Measuring higher level physical function in well-functioning older adults: expanding familiar approaches in the Health ABC study.” J Gerontol A Biol Sci Med Sci 56(10): M644–649. DOI: 10.1093/gerona/56.10.m644. [DOI] [PubMed] [Google Scholar]
- Sorond FA, Cruz-Almeida Y, Clark DJ, Viswanathan A, Scherzer CR, De Jager P, Csiszar A, Laurienti PJ, Hausdorff JM, Chen WG, Ferrucci L, Rosano C, Studenski SA, Black SE and Lipsitz LA (2015). “Aging, the Central Nervous System, and Mobility in Older Adults: Neural Mechanisms of Mobility Impairment.” J Gerontol A Biol Sci Med Sci 70(12): 1526–1532. DOI: 10.1093/gerona/glv130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D and Ritter PL (2001). “CHAMPS physical activity questionnaire for older adults: outcomes for interventions.” Med Sci Sports Exerc 33(7): 1126–1141. DOI: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Tomlinson CE, Laurienti PJ, Lyday RG and Simpson SL (2022). “A regression framework for brain network distance metrics.” Netw Neurosci 6(1): 49–68. DOI: 10.1162/netn_a_00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlow CT, Casanova R and Maldjian JA (2011). “Effect of resting-state functional MR imaging duration on stability of graph theory metrics of brain network connectivity.” Radiology 259(2): 516–524. DOI: 10.1148/radiol.11101708. [DOI] [PubMed] [Google Scholar]
- Zeighami Y, Dadar M, Daoust J, Pelletier M, Biertho L, Bouvet-Bouchard L, Fulton S, Tchernof A, Dagher A, Richard D, Evans A and Michaud A (2022). “Impact of weight loss on brain age: Improved brain health following bariatric surgery.” Neuroimage 259: 119415. DOI: 10.1016/j.neuroimage.2022.119415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


