Abstract
Background
Detection of appropriate indicators is valuable for preventing incidental osteoporotic fractures. We statistically evaluated the significance of serum cystatin C‐to‐creatinine ratio (CysC/Cr) as a surrogate marker for incident major osteoporotic fractures (MOF) prediction.
Methods
Eligible patients with simultaneous measurement of CysC/Cr and bone mineral density in the lumbar spine and proximal femur were selected, and their fracture histories until 5 years after baseline were observed in the retrospective area cohort data. Patients who were followed up until termination or the first osteoporotic fracture were included, and loss of follow‐up or death was excluded. Candidate risk factors for osteoporotic fractures were tested for risk ratios using a cox regression analysis. Receiver operating characteristic tests were performed on factors with significantly higher risk ratios and evaluated with Kaplan‐Meier survival analysis to determine the hazard ratios of the factors.
Results
A total of 175 patients of whom 28 had incident MOF, 38 men, and 137 women, were enrolled. The mean age was 70.2 years. A significantly higher risk ratio was shown in the presence of prevalent MOF, hyper fall‐ability, lifestyle‐related diseases, chronic kidney diseases ≥ Grade3a, and higher CysC/Cr. All parameters had cutoff indices and showed significantly higher hazard ratios.
Conclusions
These results suggested that CysC/Cr may be a predictive marker of incident osteoporotic fractures. It might work as a screening tool for MOF risk.
Keywords: creatinine, cystatin C, fracture, osteoporosis, risk ratio, surrogate marker
A risk weight of serum cystatin C‐to‐creatinine ratio (CysC/Cr) for incident major osteoporotic fracture was investigated using a retrospective case‐control study. CysC/Cr had a significantly high risk ratio (Hazard ratio: 6.32) using Kaplan‐Meier survival analysis with the cutoff index of 1.345. We conclude that CysC/Cr would be a surrogate marker of osteoporotic fracture prediction.
1. INTRODUCTION
Osteoporotic fracture is the biggest social pressure factor worldwide, 1 especially in Japan, which is facing an aging society, 2 and preventing this outbreak is one of the major missions for medical care. 3 , 4 Identifying risk factors for osteoporotic fractures, sharing them, and having them recognized as a common knowledge will be very beneficial in preventing the occurrence of osteoporotic fractures, and the significance will make a great clinical contribution. Known risk factors include aging, gender, smoking, alcohol use, low bone density, rheumatoid arthritis, lifestyle‐related diseases such as diabetes and chronic obstructive pulmonary disease, and steroid use. 5 , 6 , 7
Serum creatinine‐to‐cystatin C ratio (Cr/CysC) has attracted attention in recent years as a surrogate marker of muscle mass. Although serum CysC and Cr levels are indicators of renal function, CysC is distributed to all nucleated cells of the body, while Cr is produced only in the striated muscle, and therefore, Cr/CysC levels can be considered a sarcopenia index 8 , 9 , 10 , 11 , 12 because it indirectly reflects the skeletal muscle mass. Cr/CysC has also been attracted as a marker of osteoporosis. A cross‐sectional study was conducted to investigate the association between Cr/CysC and fractures, including fragility fractures, in 285 patients with type 2 diabetes. A high BMI with low Cr/CysC was associated with a relative increase in fat mass and an increased risk of fracture. These authors also suggested that Cr/CysC could be used as a screening tool for fragility fractures, using an optimal cutoff value of 0.90 and a hazard ratio of 2.60 in postmenopausal women. 13 Another retrospective longitudinal study of the relationship between body weight and risk of fragility fractures in patients with type 2 diabetes showed that decreased BMI increased the risk of fragility fractures. 14 In addition, another study from the same registry examined the association between Cr/CysC and bone fragility fractures and found that the incidence of bone fragility fractures was significantly higher in the lowest quartile of patients with Cr/CysC. 15 Moreover, a novel index of osteoporosis that represented as T‐score ≤ −2.5, which constituted the combined criteria of age, Cr/CysC, and tartrate‐resistant acid phosphatase‐5b (TRACP‐5b), was promoted recently. 16
Although these studies were limited in diabetic patients, correlation between Cr/CysC and osteoporotic fracture would be suggested. 17 Therefore, we hypothesized that serum cystine C‐to‐creatine ratio (CysC/Cr), that is, the reciprocal of Cr/CysC, might function as a surrogate marker for expecting incident osteoporotic fractures in elderly population, and tested this hypothesis in a retrospective cohort study.
2. MATERIALS AND METHODS
Institute where this study was performed is the one clinic; however, one osteoporosis control center in the community where the clinic is located. Patients who had relatively higher risk were followed up every 3‐month interval. Risk factors adopted in the institute are female gender, age no <50 in female or no <70 in male, low body mass index (BMI) ≤18 or high BMI >30, presence of history of fractures of patient's parent, prevalent major osteoporotic fracture of the patient (MOF; pr‐MOF), comorbidities such as lifestyle‐related diseases (LSD), hyper fall‐ability (Fall‐ability), chronic kidney diseases ≥ Grade3a (CKD), cognitive impairment (C‐I), rheumatoid arthritis (RA), and low bone mineral density (BMD) with the T‐score ≤ −2.0. These patients were judged as high‐risk patient and got blood tests simultaneously. BMDs were measured using dual‐energy X‐ray absorptiometry in the lumbar spine and femoral neck, and item of the blood test included CysC/Cr. MOF included vertebral fractures, proximal femur fractures, proximal humerus fractures, and distal radius fractures. These fractures were picked up in an interview performed by nurses and physicians and confirmed using X‐ray pictures, of what X‐ray of the spine was routinely performed especially in order to confirm morphological fracture. LSD included comorbidities such as type 2 diabetes mellitus, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic heart failure, and insomnia. Fall‐ability included comorbidities such as musculoskeletal ambulation disability complex, osteoarthritis of the lower extremities, joint contractures of the trunk or lower extremities, disuse syndrome, parkinsonism, and neuromuscular disorders. The diagnoses of these comorbidities were made by the authors, who are specialists certified by the Japanese Society of Internal Medicine, the Japanese Orthopedic Association, and the Japanese College of Rheumatology.
Patients who were judged as high‐risk patients from November 2010 to December 2015 were picked up and had been followed up. The dates of when judged as a high‐risk patient were set as baseline. Their clinical data were in follow‐up until 5 years after baseline data were collected. Inclusion criteria of subjects' age were 65 years or older in female, 70 years or older in male, and 50 years or older to whom oral glucocorticoid (GCS) was thrown. Patients who had lost to follow‐up, faced death, hospitalized due to serious comorbidities such as pneumonia, stroke, and cardiovascular events were extruded from the study. As one more extrude criterion, patients who had CKD ≥ Grade 3b were extruded, because of inaccuracy of measuring the creatinine. The primary outcomes were incident MOF. Follow‐up was continued until the first fracture occurred or was completed (Figure 1).
FIGURE 1.
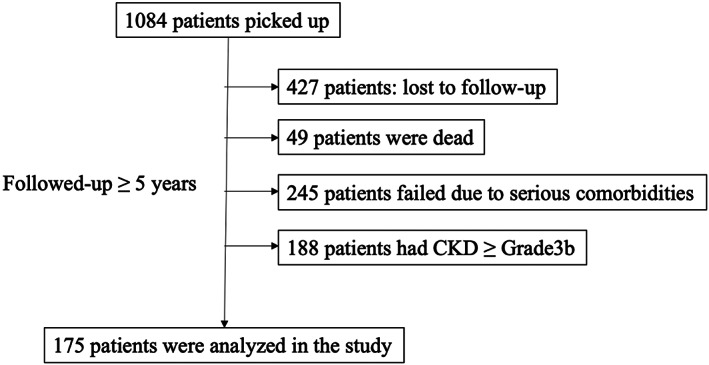
Flowchart of the study. CKD, chronic kidney disease.
In these patients, the relationship between incident MOF and variants at baseline was investigated using a cox regression analysis. The variants were set as candidate risk factors for incident MOF, which included female gender, older age, lower BMD in the LS and in the FN, higher CysC/Cr, presence of LSD, Fall‐ability, CKD ≥ Grade3a (CKD), C‐I, RA, pr‐MOF, drug administration of anti‐osteoporotic drug (OPD), vitamin‐D, GCS, and administration of polypharmacy ≥6 kinds of tablets/capsules (Polypharmacy), and presence of habitat such as smoking and alcohol drinking.
After identifying the risk factors using univariate models, multivariate model was undergone in order to relative higher risk in the factors. Receivers operating characteristic analyses (ROC) were conducted in order to determine the cutoff index (COI) of significant factors demonstrated in the univariate model. Kaplan‐Meier survival analysis was tested in order to determine hazard ratio for presenting these risk factors. Statistical significance was lower than 5%. All statistical analyses were performed using StatPlus:mac® (AnalystSoft, Inc.).
The research was approved by the IRB of the authors. In addition, informed consent has been obtained from all patient and relevant persons after explaining anonymity was ensured for all patients and their families who participated in this study, and no names and/or addresses were issued that could help identify these individuals.
3. RESULTS
A total of 1084 patients were recruited. In these, 427 had lost to follow‐up in whom almost of them had admitted in nursing homes, 49 had dead, 245 had failed to follow‐up because of serious comorbidities, and 188 had CKD ≥ Grade3B. Afterall, 175 patients in whom 38 men and 137 women and 28 incident MOF were included in the dataset (Figure 1). The mean age at baseline was 70.2 years, ranged from 50 to 98 years, and the mean time length after baseline to incident MOR was 15.8 months. Mean BMD at baseline was 0.734 and 0.659 g/cm2 (T‐score: −2.21 and −2.04) in the lumbar spine and femoral neck, respectively, and mean CysC/Cr at baseline was 1.49. Fall‐ability, LSD, and pr‐MOF were present in, 59, 113, and 77, respectively. Administration of OPD, vitamin‐D, GCS, and polypharmacy were present in 92, 170, 12, and 47, respectively (Table 1).
TABLE 1.
Demographic characteristics and results of Cox regression analysis in the dataset.
| Dataset (n = 175) | MOF (n = 28) | Cox regression analysis | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| p‐Value | Risk ratio (95%CI) | p‐Value | Risk ratio (95%CI) | |||
| Female gender | 137 (78.3) | 23 (82.1) | 0.61 | |||
| Age, year‐old | 70.2 (14.6) | 74.6 (10.3) | 0.10 | |||
| Follow‐up length, months | 52.9 (16.9) | 15.8 (12.3) | <0.001 | |||
| Presence of incident MOF | 28 (16.0) | 28 (100) | – | |||
| BMI, kg/m2 | 22.2 (4.1) | 22.8 (3.1) | 0.33 | |||
| Number of BMI < 18 | 23 (13.1%) | 2 (7.1%) | 0.16 | |||
| Number of BMI > 30 | 0 (0%) | 0 (0%) | – | |||
| BMD in LS, g/cm2 | 0.734 (0.215) | 0.782 (0.121) | 0.19 | |||
| BMD in FN, g/cm2 | 0.659 (0.152) | 0.617 (0.119) | 0.14 | |||
| T‐score in LS | −2.21 (1.75) | −2.70 (0.98) | 0.14 | |||
| T‐score in FN | −2.04 (1.23) | −2.39 (0.97) | 0.12 | |||
| CysC/Cr | 1.49 (0.29) | 1.59 (0.22) | <0.05 | 4.15 (1.09–15.80) | 0.39 | 1.94 (0.42–8.87) |
| Presence of lifestyle‐related diseases | 113 (64.6) | 24 (85.7) | <0.05 | 3.60 (1.25–10.40) | <0.05 | 2.92 (1.00–8.54) |
| Presence of hyper fall‐ability | 59 (33.7) | 19 (67.9) | <0.001 | 4.90 (2.21–10.86) | <0.01 | 3.93 (1.73–8.95) |
| Presence of CKD ≥ Grade3a | 49 (28.3) | 10 (37.0) | <0.05 | 2.57 (1.21–5.43) | 0.33 | 1.46 (0.68–3.17) |
| Presence of cognitive impairment | 12 (6.9) | 3 (10.7) | 0.77 | |||
| Presence of rheumatoid arthritis | 54 (30.9) | 11 (39.3) | 0.70 | |||
| Presence of prevalent MOF | 77 (44.0) | 21 (75.0) | <0.001 | 5.25 (1.58–17.39) | 0.06 | 2.46 (0.68–6.32) |
| Antiosteoporotic drug administration | 92 (52.6) | 19 (67.9) | 0.27 | |||
| Vitamin D supplementation | 170 (97.1) | 22 (78.6) | 0.18 | |||
| Glucocorticoid steroid administration | 12 (6.9) | 2 (7.1) | 0.89 | |||
| Presence of polypharmacy | 47 (26.9) | 10 (35.7) | 0.46 | |||
| Current smoking habitat | 7 (4.0) | 2 (7.1) | 0.29 | |||
| Alcohol drinking habitat | 12 (6.9) | 4 (14.3) | 0.11 | |||
Note: The values are presented as mean (SD) unless indicated otherwise. In the other, number of cases and percentage are presented.
Abbreviations: BMD, bone mineral density; BMI, body mass index; CKD, chronic kidney diseases; CysC/Cr, serum cystatin C‐to‐creatinine ratio; FN, femoral neck; LS, lumbar spine; MOF, major osteoporotic fracture.
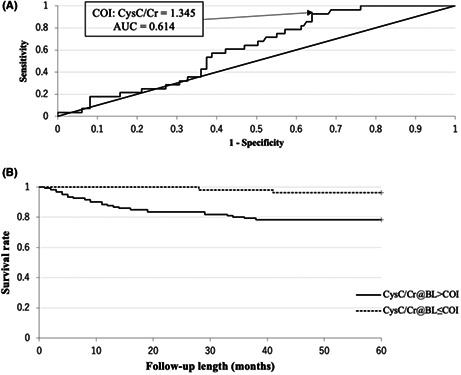
In cox regression analysis, the presence of pr‐MOF, fall‐ability, LSD, CKD ≥ Grade3a, and higher CysC/Cr had significant higher risk ratios with univariate models. In these, the presence of LSD and fall‐ability had significant higher risk ratios with multivariate model (Table 1). The COI of ROC for binary factors was the presence of each event. CysC/Cr also had COI with 1.345 (Figure 2A). Thus, all factors showed statistically significant COI (Table 2 and Figure 2A).
FIGURE 2.
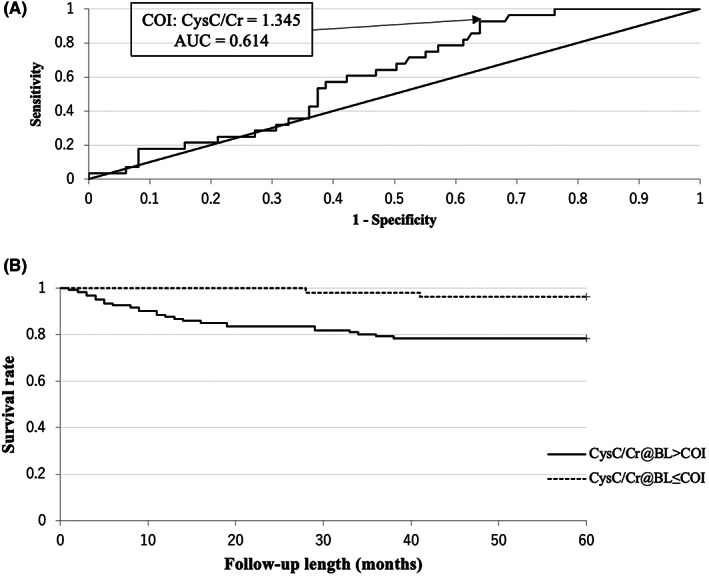
ROC curve and Kaplan‐Meier survival curve in regard to CysC/Cr for incident MOF. (A) ROC curve is shown. The cutoff index (COI) is 1.345, and the area under the curve (AUC) is 0.614. (B) Kaplan‐Meier survival curve is shown. The hazard ratio of CysC/Cr > 1.345 is 6.32 (p < 0.01).
TABLE 2.
Results of receiver operation characteristics (ROC) and Kaplan‐Meier survival analysis (K‐M) for each factor.
| Factor | ROC | K‐M | |||
|---|---|---|---|---|---|
| Cut‐off index | Area under the curve | p‐Value | Hazard ratio (95%CI) | p‐Value | |
| CysC/Cr | >1.345 | 0.614 | <0.01 | 6.32 (2.87–13.92) | <0.01 |
| LSD | Present | 0.626 | <0.01 | 3.60 (1.67–7.73) | <0.05 |
| Fall‐ability | Present | 0.703 | <0.001 | 4.83 (2.16–10.21) | <0.001 |
| CKD | Present | 0.612 | <0.05 | 2.56 (1.06–6.20) | <0.05 |
| pr‐MOF | Present | 0.685 | <0.001 | 4.81 (2.08–9.39) | <0.001 |
Note: Hazard ratios are calculated as present or more than cut‐off index/absent or no more than cut‐off index.
Abbreviations: CKD, presence of chronic kidney diseases with estimated glomerular filtration ratio <45 ml/1.73 m2; CysC/Cr, serum cystatin C‐to‐creatinine ratio; Fall‐ability, presence of hyper fall‐ability; LSD, presence of lifestyle‐related diseases; pr‐MOF, presence of prevalent major osteoporotic fracture.
In the Kaplan‐Meier survival analysis, CysC/Cr > 1.345, presence of fall‐ability, presence of pr‐MOF, presence of LSD, and presence of CKD were significantly higher in descending order of hazard ratio (6.32, 4.83, 4.81, 3.60, 2.56, respectively) (Table 2 and Figure 2B).
4. DISCUSSION
This is a cohort study evaluating the association between CysC/Cr and the presence of incident MOF in clinical practice. In selecting candidate risk factors, commonly established risk factors such as female gender, older age, and low BMD were included. Recently focused risk factor for MOF, namely presenting diabetes mellitus, chronic obstructive pulmonary diseases, chronic kidney disfunction, and insomnia, was included together in the presence of LSD. Adding to them, hyper fall‐ability was included because the more fall‐ability increases, the more fracture risk increase is expected. 18 Drug factors such as antiosteoporotic drug and glucocorticoid were also included. However, we did not include family history of MOF because almost of patients were elderly person so that their memory was vague and unreliable. BMD > 30 was not also included because there was no case whose BMD > 30. Preference habitat such as current smoking and drinking alcohol were also included because there were very few number of subjects in the study.
Cystatin C is an endogenous cysteine proteinase inhibitor belonging to the type 2 cystatin superfamily. Its molecular weight is 13,343–13,359 Da, containing four characteristic disulfide‐paired cysteine residues. Human cystatin C is encoded by the CST3 gene, ubiquitously expressed at moderate levels. 19 , 20 The main catabolic site of cystatin C is the kidney: more than 99% of the protein is cleared from the circulation by glomerular ultrafiltration and tubular reabsorption. The diagnostic value of cystatin C as a marker of kidney dysfunction has been extensively investigated in multiple clinical studies on adults, children, and in the elderly, 20 and even for race free studies. 21 Equations that include cystatin C predict GFR more accurately than serum creatinine in children, adults, and older adults with larger effects among persons who are acutely ill. 22 Not only illness but also drug influences were diminished by using cystatin C that improves the safety and efficacy of medications that have narrow therapeutic windows. 23
Based on these characteristics of cystatin C, mixed studies with creatinine are applicable to clinical use, such as measurement of drug trough levels, 22 detection of immune disorders, 24 diagnosis of shrunken pore syndrome, 25 diagnosis of sarcopenia, 10 , 11 , 12 , 13 , 26 , 27 , 28 , 29 surrogate marker of prognosis prediction in chronic pulmonary diseases, 30 and cancer. 28 , 29 , 31
Cr/CysC is a novel surrogate marker of osteoporosis. It might reflect the lean muscle mass, which may correlate with fall tendency and bone fragility. Thus, the reciprocal of Cr/CysC equal to CysC/Cr should correlate with the incidence of bone fragility fracture. These results suggest that CysC/Cr may be a strong candidate surrogate marker for MOF occurrence or a strong risk factor when it exceeds the COI. This value is the reciprocal of Cr/CysC, and if Cr/CysC reflects the lean muscle mass, CysC/Cr is assumed to reflect the low relative muscle mass. It is supposed that this is also affected by gender difference and age. When the parameters associated with CysC/Cr in the present dataset were examined by linear regression analysis, the presence of female gender, older age, and pr‐MOF was significantly associated with CysC/Cr (Table 3). Thus, CysC/Cr is included as a risk factor for osteoporotic and preexisting fractures in women and the elderly. This suggests that the risk of osteoporosis can be estimated by blood examination even in clinics without radiographic facilities. CysC/Cr did not demonstrate significant higher risk factor with Cox regression analysis using multivariate model; however, it demonstrated significantly higher hazard ratio with Kaplan‐Meier analysis. Therefore, CysC/Cr might be suggested as a primary screening index for incident MOF.
TABLE 3.
Correlation between CysC/Cr and variants using linear regression analysis.
| Variant | Univariate models | Multivariate model (R = 0.68) | ||
|---|---|---|---|---|
| p‐Value | R | p‐Value | c.c. (95%CI) | |
| Female gender | <0.001 | 0.44 | <0.001 | 0.20 (0.12–0.29) |
| Older age | <0.001 | 0.58 | <0.001 | 0.01 (0.005–0.011) |
| BMD in LS | 0.16 | 0.14 | ||
| BMD in FN | 0.08 | 0.17 | ||
| T‐score in LS | 0.12 | 0.14 | ||
| T‐score in FN | 0.11 | 0.16 | ||
| Presence of lifestyle‐related diseases | 0.17 | 0.11 | ||
| Presence of hyper fall‐ability | 0.10 | 0.12 | ||
| Presence of CKD ≥ Grade3a | <0.001 | 0.16 | 0.12 | −0.07 (−0.15–0.02) |
| Presence of cognitive impairment | <0.05 | 0.17 | 0.6 | −0.04 (−0.18–0.10) |
| Antiosteoporotic drug administration | <0.001 | 0.44 | 0.94 | −0.003 (−0.09–0.08) |
| Vitamin D supplementation | 0.75 | 0.03 | ||
| Glucocorticoid steroid administration | 0.63 | 0.04 | ||
| Presence of polypharmacy | <0.05 | 0.16 | 0.20 | 0.05 (−0.03–0.12) |
| Presence of prevalent MOF | <0.001 | 0.48 | <0.05 | 0.10 (0.02–0.19) |
Abbreviations: BMD, bone mineral density; c.c., correlation coefficients; CKD, chronic kidney disease; CysC/Cr, serum cystatin C‐to‐creatinine ratio; FN, femoral neck; LS, lumbar spine; MOF, major osteoporotic fracture.
However, there was no significant correlation with BMD. In one previous cross‐sectional study examining an association between BMD and Cr/CysC, there was no significant correlation between them, 16 and there was a significant correlation by age and combination with one of the bone resorption markers, TRACP‐5b. 16 Cr/CysC and BMD are influenced by several common factors, such as women and older age, but are likely to be influenced by other factors. For example, BMD may be influenced by the involvement of inflammatory cytokines, the presence of autoantibodies such as anticitrullinated polypeptide antibodies, blood calcium and phosphorus levels, and blood parathyroid hormone levels, and Cr/CysC may be influenced by muscle training, exercise habits, and vitamin D intake.
In any case, there seems to be a correlation between MOF and CysC/Cr. Early measurement of CysC/Cr facilitates screening for fractures. It would be recommended that initial BMD measurement and CysC/Cr calculation should simultaneously be set as osteoporosis screening, as soon as the old fracture including distal radius or unrecognized vertebral body fracture is observed. As a result, it may be easier to implement fracture prevention programs such as drug interventions such as OPD and exercise habit guidance.
There is one caveat. The measurement accuracy of CysC and Cr lowers, when the renal function lowers over the fixed. 32 In view of this, creatinine clearance and creatinine estimated glomerular filtration rate (eGFR) should be used as reference indices. Patients with eGFR <45 ml/1.73 m2 were also excluded from the dataset for analysis in this study.
This study has various limitations. Because of the small number of cases of MOF, younger patients are at disproportionate risk, particularly in single‐center studies, where observation was short and CKD ≥ Grade3b or higher was excluded. And one more thing, cystatin C is modified by comorbidities such as hyperthyroidism, hypothyroidism, malignant tumor, posttransplantation status, or HIV infection. These conditions were checked before entry; however, it cannot be said that potential malignancy or thyroid disfunction was denied. Nevertheless, evidence for CysC/Cr suggests that this index may be a surrogate marker of MOF development. CysC/Cr > 1.345 would have more than sixfold high risk than no more than that value. This information would be helpful for elderly osteoporosis candidate persons.
AUTHOR CONTRIBUTIONS
IY: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Roles/Writing—original draft; Visualization. NS: Data curation; Validation; Roles/Writing—review & editing. TC: Data curation; Validation; Roles/Writing—review & editing.
CONFLICT OF INTEREST STATEMENT
Ichiro Yoshii, Naoya Sawada, and Tatsumi Chijiwa declare that they have no conflict of interest. And their families have nothing to declare for this study.
ETHICS APPROVAL STATEMENT
This study was approved by Yoshii Hospital ethics committee (approval number: Y‐OP‐2020‐3) in accordance with the ethical standards laid down in 1964 Declaration of Helsinki and its later amendments.
PATIENT CONSENT STATEMENT
Patient and his/her family were informed that anonymity was ensured for all patients and their families who participated in this study, and no names and/or addresses were issued that could help identify these individuals.
ACKNOWLEDGMENTS
Authors would like to thank Saori Tamura for the enthusiastic DXA and BMD measurements and Kaoru Kuwabara, Sayori Masuoka, Eri Morichika, and Aoi Yoshida for their dedicated data collection. The authors would also like to thank Enago (www.enago.jp) for the enthusiastic English language review.
Yoshii I, Sawada N, Chijiwa T. Clinical significance of serum cystatin C‐to‐creatinine ratio as a surrogate marker for incident osteoporotic fracture predictions. J Gen Fam Med. 2023;24:178–184. 10.1002/jgf2.618
DATA AVAILABILITY STATEMENT
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Curtis EM, Moon RJ, Harvey NC, Cooper C. Reprint of: the impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone. 2017;104:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chapter 2 Population . Statistical handbook of Japan. [Internet]. Statistics Bureau: Ministry of Internal Affairs and Communication [cited 2021 Jan 16]. Available from: https://www.stat.go.jp/english/data/handbook/index.html (in Japanese)
- 3. Tsukutani Y, Hagino H, Ito Y, Nagashima H. Epidemiology of fragility fractures in Sakaiminato, Japan: incidence, secular trends, and prognosis. Osteoporos Int. 2015;26:2249–55. [DOI] [PubMed] [Google Scholar]
- 4. Iihara N, Ohara E, Bando Y, Yoshida T, Ohara M, Kirino Y. Fragility fractures in older people in Japan based on the National Health Insurance Claims Database. Bio Pharm Bull. 2019;42:778–85. [DOI] [PubMed] [Google Scholar]
- 5. Orimo H, Igi M, Ishibashi H, Itoi E, Itoh M, Inaba M, et al. Guideline for prevention and treatment of osteoporosis. Committee of Guideline for the Prevention and Treatment of Osteoporosis in Japan. [Internet]. [cited 2020 Dec 28]. Available from: http://www.josteo.com/ja/guideline/doc/15_1.pdf (in Japanese)
- 6. Soen S, Fukunaga M, Sugimoto T, Sone T, Fujiwara S, Endo N, et al. Japan osteoporosis society joint review Committee for the Revision of the diagnostic criteria for primary osteoporosis. Diagnostic criteria for primary osteoporosis: year 2012 revision. J Bone Miner Metab. 2013;31:247–57. [DOI] [PubMed] [Google Scholar]
- 7. Sugimoto T, Sato M, Dehle FC, Brnabic AJM, Weston A, Burge R. Lifestyle‐related metabolic disorders, osteoporosis, and fracture risk in Asia: a systematic review. Value Health Reg Issues. 2016;9:49–56. [DOI] [PubMed] [Google Scholar]
- 8. Tetsuka S, Morita M, Ikeguchi K, Nakano I. Creatinine/cystatin C ratio as a surrogate marker of residual muscle mass in amyotrophic lateral sclerosis. Neurol Clin Neurosci. 2013;1:32–7. [Google Scholar]
- 9. Suzuki K, Furuse H, Tsuda T, Masaki Y, Okazawa S, Kambara K, et al. Utility of creatinine/cystatin C ratio as a predictive marker for adverse effects of chemotherapy in lung cancer: a retrospective study. J Int Med Res. 2015;43:573–82. [DOI] [PubMed] [Google Scholar]
- 10. Harada H, Kai H, Shibata R, Niiyama H, Nishiyama Y, Murohara T, et al. New diagnostic index for sarcopenia in patients with cardiovascular diseases. PLoS One. 2017;12:e0178123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kashani K, Sarvottam K, Pereira NL, Barreto EF, Kennedy CC. The sarcopenia index: a novel measure of muscle mass in lung transplant candidates. Clin Transplant. 2018;32:e13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Osaka T, Hamaguchi M, Hashimoto Y, Ushigome E, Tanaka M, Yamazaki M, et al. Decreased the creatinine to cystatin C ratio is a surrogate marker of sarcopenia in patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;139:52–8. [DOI] [PubMed] [Google Scholar]
- 13. Compston JE, Flahive J, Hosmer DW, Watts NB, Siris ES, Silverman S, et al. GLOW investigators. Relationship of weight, height, and body mass index with fracture risk at different sites in postmenopausal women: the global longitudinal study of osteoporosis in women (GLOW). J Bone Miner Res. 2014;29:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komorita Y, Iwase M, Fujii H, Ohkuma T, Ide H, Jodai‐Kitamura T, et al. Impact of body weight loss from maximum weight on fragility bone fractures in Japanese patients with type 2 diabetes: the Fukuoka diabetes registry. Diabetes Care. 2018;41:1061–7. [DOI] [PubMed] [Google Scholar]
- 15. Komorita Y, Iwase M, Fujii H, Ide H, Ohkuma T, Jodai‐Kitamura T, et al. The serum creatinine to cystatin C ratio predicts bone fracture in patients with type 2 diabetes: the Fukuoka diabetes registry. Diabetes Res Clin Pract. 2018;146:202–10. [DOI] [PubMed] [Google Scholar]
- 16. Yoshii I, Chijiwa T, Sawada N. Screening osteoporotic femoral neck without measuring bone mineral density with the use of tartrate resistant acid phosphatase‐5b and serum‐creatinine‐to‐cystatin C ratio in Japanese postmenopausal women. J Orthop Sci. 2020;25:671–6. [DOI] [PubMed] [Google Scholar]
- 17. Yoshii I, Sawada N, Chijiwa T. AB1007 clinical significance of serum creatinine‐to‐cystatin C ratio as a surrogate marker for incident osteoporotic fracture. London, UK: BMJ Publishing Group Ltd; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshii I, Chijiwa T, Sawada N, Kokei S. Musculoskeletal ambulation disability symptom complex as a risk factor of incident bone fragility fracture. Osteoporos Sarcopenia. 2021;7:115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman DJ, Cystatin C. Cystatin C. Ann Clin Biochem. 2002;39:89–104. [DOI] [PubMed] [Google Scholar]
- 20. Mussap M, Plebani M. Biochemistry and clinical role of human Cystatin C. Crit Rev Clin Lab Sci. 2004;41:467–550. [DOI] [PubMed] [Google Scholar]
- 21. Inker LA, Eneanya ND, Coresh J, Tighiouart H, Wang D, Sang Y, et al. New creatinine‐ and Cystatin C–based equations to estimate GFR without race. New Engl J Med. 2021;385:1737–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ebert N, Shlipak MG. Cystatin C is ready for clinical use. Curr Opin Nephrol Hypertens. 2020;29:591–8. [DOI] [PubMed] [Google Scholar]
- 23. Barreto EF, Rule AD, Murad MH, Kashani KB, Lieske JC, Erwin PJ, et al. Prediction of the renal elimination of drugs with Cystatin C vs creatinine: a systematic review. Mayo Clin Proc. 2019;94:500–14. [DOI] [PubMed] [Google Scholar]
- 24. Zi M, Xu Y. Involvement of cystatin C in immunity and apoptosis. Immunol Lett. 2018. Apr;196:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhou H, Yang M, He X, Xu N. eGFR, cystatin C and creatinine in shrunken pore syndrome. Clin Chim Acta. 2019;498:1–5. [DOI] [PubMed] [Google Scholar]
- 26. Tabara Y, Kohara K, Okada Y, Ohyagi Y, Igase M. Creatinine‐to‐cystatin C ratio as a marker of skeletal muscle mass in older adults: J‐SHIPP study. Clin Nutr. 2020;39:1857–62. [DOI] [PubMed] [Google Scholar]
- 27. Nishida K, Hashimoto Y, Kaji A, Okamura T, Sakai R, Kitagawa N, et al. Creatinine/(cystatin C × body weight) ratio is associated with skeletal muscle mass index. Endocr J. 2020;67:733–40. [DOI] [PubMed] [Google Scholar]
- 28. Ulmann G, Kaï J, Durand J‐P, Neveux N, Jouinot A, De Bandt J‐P, et al. Creatinine‐to‐cystatin C ratio and bioelectrical impedance analysis for the assessment of low lean body mass in cancer patients: comparison to L3–computed tomography scan. Nutrition. 2021;81:110895. [DOI] [PubMed] [Google Scholar]
- 29. Zheng C, Wang E, Li J‐S, Xie K, Luo C, Ge Q‐Y, et al. Serum creatinine/cystatin C ratio as a screening tool for sarcopenia and prognostic indicator for patients with esophageal cancer. BMC Geriatr. 2022;22:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amado CA, García‐Unzueta MT, Lavin BA, Guerra AR, Agüero J, Ramos L, et al. The ratio serum creatinine/serum Cystatin C (a surrogate marker of muscle mass) as a predictor of hospitalization in chronic obstructive pulmonary disease outpatients. Respiration. 2019;97:302–9. [DOI] [PubMed] [Google Scholar]
- 31. Jung C‐Y, Kim HW, Han SH, Yoo T‐H, Kang S‐W, Park JT. Creatinine–cystatin C ratio and mortality in cancer patients: a retrospective cohort study. J Cachexia Sarcopenia Muscle. 2022;13:2064–72. 10.1002/jcsm13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roos JF, Doust J, Tett SE, Kirkpatrick CMJ. Diagnostic accuracy of cystatin C compared to serum creatinine for the estimation of renal dysfunction in adults and children—a meta‐analysis. Clin Biochem. 2007;40:383–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


