Abstract
Aims: Diffuse intrinsic pontine glioma (DIPG) is a childhood brainstem tumor with a median overall survival of eleven months. Lack of chemotherapy efficacy may be related to an intact blood-brain barrier (BBB). In this study we aim to investigate the neurovascular unit (NVU) in DIPG patients.
Methods: DIPG biopsy (n = 4) and autopsy samples (n = 6) and age-matched healthy pons samples (n = 20) were immunohistochemically investigated for plasma protein extravasation, and the expression of tight junction proteins claudin-5 and zonula occludens-1 (ZO-1), basement membrane component laminin, pericyte marker PDGFR-β, and efflux transporters P-gp and BCRP. The mean vascular density and diameter were also assessed.
Results: DIPGs show a heterogeneity in cell morphology and evidence of BBB leakage. Both in tumor biopsy and autopsy samples, expression of claudin-5, ZO-1, laminin, PDGFR-β and P-gp was reduced compared to healthy pontine tissues. In DIPG autopsy samples, vascular density was lower compared to healthy pons. The density of small vessels (<10 µm) was significantly lower (P<0.001), whereas the density of large vessels (≥10 µm) did not differ between groups (P = 0.404). The median vascular diameter was not significantly different: 6.21 µm in DIPG autopsy samples (range 2.25-94.85 µm), and 6.26 µm in controls (range 1.17-264.77 µm).
Conclusion: Our study demonstrates evidence of structural changes in the NVU in DIPG patients, both in biopsy and autopsy samples, as well as a reduced vascular density in end-stage disease. Adding such a biological perspective may help to better direct future treatment choices for DIPG patients.
Keywords: Diffuse intrinsic pontine glioma (DIPG), Neurovascular unit (NVU), Blood-brain barrier (BBB), Tight junctions, Brainstem, Pons
Introduction
Diffuse intrinsic pontine gliomas (DIPGs) are rare and aggressive childhood malignancies of the brainstem. These tumors are characterized by a diffuse growth pattern closely interwoven within white matter tracts and grey matter structures, and an intrinsic nature, uttered by hypertrophy of the brainstem often encasing the basilar artery [1],[2]. With a median overall survival of eleven months, and a two-year survival rate of 10%, DIPGs are the leading cause of brain tumor-related deaths in children [3],[4],[5],[6],[7]. In the recent World Health Organization (WHO) classification of tumors of the central nervous system (CNS), DIPGs were reclassified as H3K27M mutated Diffuse Midline Gliomas (DMG H3K27M) [8].
Though much research has been dedicated to DIPG, its poor outcome has remained unchanged for the past 40 years [9]. To date, radiotherapy remains the only (temporarily) effective, albeit palliative treatment, and no chemotherapy regimens prolonging survival have been identified yet. Since in vitro and in vivo drug testing on patient-derived tumor cells has shown sensitivity to conventional cytotoxic agents and novel drugs, the lack of efficacy in patients is hypothesized to be related to ineffective drug delivery due to an intact blood-brain barrier (BBB) [10],[11],[12].
The BBB is formed by endothelial cells interconnected and sealed by tight junctions. The abluminal surface of the endothelium is covered by a basement membrane in which pericytes are embedded. Pericytes control the cerebral blood flow by regulating capillary diameter and vessel stability. The basement membrane is enclosed by astrocyte end-feet, also important for brain homeostasis. Together, pericytes and astrocyte end-feet induce and maintain the integrity of the BBB [13],[14]. The BBB regulates transport of essential nutrients to the brain through active transport mechanisms, such as glucose transporters of the GLUT-family. The efflux of waste products and exogenous compounds is mediated through efflux transporters of the ATP-binding cassette family (e.g., P-gp, BCRP, MRP-1) [14],[15]. Additionally, the paracellular barrier capacities of the tight junctions limit transport of circulating monoamines and drugs across the BBB [14],[15],[16]. The intimate contact and interaction of the BBB complex, formed by endothelial cells, tight junctions, pericytes and astrocyte end-feet, with neurons and perivascular microglia form a dynamic functional unit, called the neurovascular unit (NVU) [13],[14],[16].
Some studies report different expression of tight junction proteins throughout the brain, suggestive of regional heterogeneity in BBB permeability [17],[18]. However, little research has been done on the BBB and NVU in the brainstem and particularly in the pons. Yet, better insight into the BBB and the NVU at these sites is needed to develop new treatment strategies for pediatric brainstem tumors. This especially holds true for DIPG, where the BBB is thought to be a major contributor to therapeutic inefficacy. In this study, we aim at determining and comparing the histological and immunohistochemical characteristics of the NVU of the pons in children with DIPG and age-matched controls.
Patients and Methods
Patients and Samples
DIPG pre-treatment biopsy samples (n = 4) were obtained from the Biobank of the Princess Máxima Center for Pediatric Oncology, Utrecht, the Netherlands, and processed as formalin-fixed paraffin-embedded tissue. End-stage disease DIPG autopsy samples (n = 6) were obtained from the ‘VUmc Brain autopsy in children with DIPG’ study [19]. This study was approved by the institutional review board of Amsterdam UMC, location VUmc (METc VUmc, study number: VUMC2009/237) and the Scientific Committee of the Dutch Childhood Oncology Group (DCOG). In this study, brain tissue was obtained within a post-mortem interval of less than six hours for Dutch patients and less than nine hours for patients from abroad, and was processed as formalin-fixed paraffin-embedded tissue or snap frozen. Biopsy samples were MRI-guided and taken from the tumor area displaying the highest hyper-intensity on T2-weighted image (Figure 1A). Autopsy samples were obtained from the non-necrotic tumor core in the pons (Figure 1B).
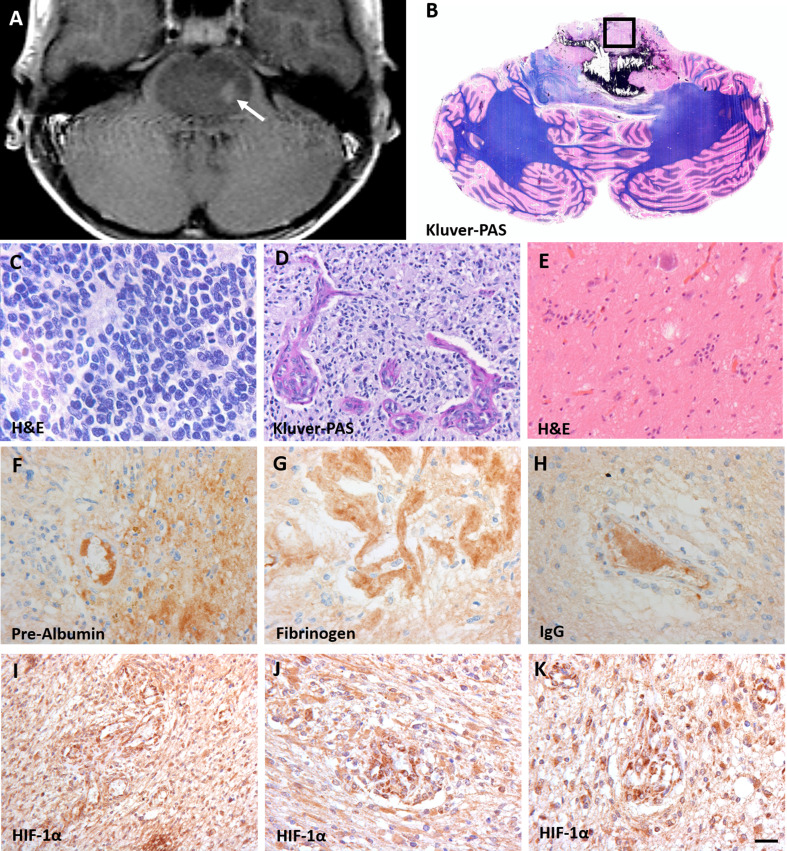
Figure 1. Characteristics of diffuse intrinsic pontine glioma (DIPG).
A: T2-weighted MRI-image of a DIPG patient showing an expanded tumor at the basis of the pons. The arrow indicates the biopsy sampling area; B: Gross axial section of the pons and cerebellum showing the presence of a diffuse infiltrating tumor in the pons, reaching the middle cerebellar peduncles. The box indicates the sampling location of autopsy tissue at the non-necrotic tumor site; C-E: hematoxylin and eosin (H&E) staining of the vital tumor bulk showing morphologic heterogeneity compatible with WHO grade IV tumors (C and D) and grade I tumors (E); F-H: stains against intravascular plasma proteins pre-albumin (F), fibrinogen (G) and IgG (H) showing extravasation of these protein into the DIPG tumor parenchyma; I-K: hypoxia inducible factor-1α (HIF-1α) staining demonstrating a high expression of HIF-1α. (scale bar: 5 µm)
Age-matched, healthy pontine tissue samples (n = 20) where obtained from the NIH NeuroBio-Bank, Maryland, United States. Samples were selected based on (i) brain region (pons), (ii) clinical brain diagnosis (unaffected control/sudden deaths), (iii) post-mortem interval (<17 hours), and (iv) presence of formalin-fixed tissue and frozen tissue.
Table 1 shows patient and treatment characteristics of the DIPG patients. Median age at diagnosis was 7.7 years (range 1.3-17.0 years). All patients had a H3K27M mutated DIPG. All autopsy patients, except for the youngest, received radiotherapy at diagnosis. Of these, at disease progression, three out of six received different chemotherapy regimens and two patients did not proceed to further treatment. Median overall survival of patients that were autopsied was 19.5 months (range 5.5-24.0 months). Supplementary Table 1 shows the characteristics of the control group. Median age was 7.0 years (range 1.0-19.0 years). All controls were healthy and had an accidental death.
Table 1. DIPG patient characteristics.
F: female; M: male; y: year; DMG H3K27M: H3K27M mutated diffuse midline glioma; WHO: World Health Organization; RTx: radiotherapy; Chemo-RTx: radiotherapy combined with chemotherapy; OS (mo.): overall survival (months); n.a.: not applicable.
| Case | Gender | Age at diagnosis (y) | Type of sample | Genetics (WHO grading) | First-line treatment | Second-line treatment | OS (mo.) |
|---|---|---|---|---|---|---|---|
| 1 | F | 3.5 | Biopsy | DMG H3K27M (WHO IV) | n.a. | n.a. | n.a. |
| 2 | F | 6.9 | Biopsy | DMG H3K27M (WHO IV) | n.a. | n.a. | n.a. |
| 3 | M | 7.9 | Biopsy | DMG H3K27M (WHO IV) | n.a. | n.a. | n.a. |
| 4 | F | 13.6 | Biopsy | DMG H3K27M (WHO IV) | n.a. | n.a. | n.a. |
| 5 | M | 14.4 | Autopsy | DMG H3K27M (WHO IV) | RTx | Chemo | 18.7 |
| 6 | F | 11.4 | Autopsy | DMG H3K27M (WHO IV) | RTx | Chemo | 24.0 |
| 7 | F | 17.0 | Autopsy | DMG H3K27M (WHO IV) | Chemo-RTx | Chemo | 24.7 |
| 8 | F | 1.3 | Autopsy | DMG H3K27M (WHO IV) | Chemo | None | 10.6 |
| 9 | F | 4.0 | Autopsy | DMG H3K27M (WHO IV) | RTx | Chemo-RTx | 20.2 |
| 10 | M | 7.5 | Autopsy | DMG H3K27M (WHO IV) | RTx | None | 5.5 |
Immunohistochemistry
Air-dried five-μm-thick cryosections were fixed in 2% formaldehyde for 10 min at room temperature (RT). Aldehyde groups were blocked in 0.1 g glycine in 100 ml distilled water for 10 min at RT. Sections were incubated overnight at RT with a primary antibody: (i) tight junction protein claudin-5 (1:50, Invitrogen, Carlsbad, CA, USA); (ii) tight junction protein ZO-1 (1:50, Invitrogen, Carlsbad, CA, USA); (iii) basement membrane component laminin (1:500, Novus Biologicals, Abingdon UK); or (iv) pericyte marker PDGFR-β (1:500, Abcam, Cambridge, UK). The sections were co-stained with glial fibrillary acidic protein (GFAP; 1:1000, Merck, Darmstadt, Germany). The following day, the sections were incubated with Alexa Fluor®-labelled secondary antibodies, background was quenched with 0.1% Sudan black B, and sections were mounted in mounting medium (Vectashield with 4',6-diamidino-2-fenylindool (DAPI); Vector Laboratories Inc., Burlingame, CA, USA).
Five-μm-thick formalin‐fixed paraffin-embedded tissue sections were routinely stained with hematoxylin & eosin (H&E). A gross axial section through the pons and cerebellum was stained with Luxol fast blue-periodic acid-Schiff. For immunohistochemistry, sections were deparaffinized using xylene, and rehydrated through descending alcohol concentrations. Endogenous peroxidase activity was blocked by incubating the slides for 30 min in phosphate buffered saline (PBS) containing 0.3% H2O2. Heat-induced antigen retrieval was performed in 0.01 M citrate buffer (pH 6.0). After washing in PBS, the slides were incubated overnight at RT with primary antibodies against P-gp (1:20; Millipore, CA, USA), BCRP (1:40; Abcam, Cambridge, UK), pre-albumin (1:50,000, Dako, Glostrup, Denmark), fibrinogen (1:1,600, Dako, Glostrup, Denmark), IgG (1:800, Dako, Glostrup, Denmark) and hypoxia-inducible factor 1α (HIF-1α; 1:40, Cayman Chemical, Michigan, USA). The next day, slides were incubated with ready-to-use EnVision™-HRP (Dako, Glostrup, Denmark) for 1 hour at RT and visualized with 3,3'Diaminobenzidine (DAB+ DAKO; 1:50, Glostrup, Denmark) for 10 min. The slides were counterstained with hematoxylin for 1 min and mounted with Quick-D mounting medium (Klinipath, Duiven, The Netherlands).
Data analysis
Sections were imaged using a Leica DM6000B microscope (400x magnification; Leica Microsystems BV, Rijswijk, The Netherlands). From each tissue slide, ten images were made. A semi-quantitative analysis of the BBB staining, comparing DIPG samples with control samples, was done by two independent reviewers (FE and RH) using the Leica Application Suite X: LAS X version 3.1.5.16308. The vascular density was assessed on claudin-5-stained tissue sections by counting the number of blood vessels per mm². The luminal diameter of the blood vessels was measured with the Leica Application Suite X: LAS X version 3.1.5.16308.
Statistics
Data were analyzed using an independent samples t-test (p-value = 0.05) using IBM SPSS Statistics version 26. The Levene’s Test of Equality of Variance was used to first test the assumption of homogeneity or variance between the groups (p-value = 0.05). When equal variances were assumed, pooled estimates were used for the independent t-test statistics. When equal variances were not assumed, unpooled data and an adjustment to the degree of freedom (df) were used for the independent t-test statistics.
Results
Immunohistochemistry
Figure 1 shows typical DIPG MRI features with enlargement of the pons and contrast enhancement. Gross inspection confirms the presence of a partly necrotic and hemorrhagic tumor center in the pons. Microscopic examination shows variability of tumor cell morphology, ranging from grade I to grade IV according to the 2016 WHO classification of CNS tumors. Tumor areas were recognized based on cell density, the presence of (atypical) mitotic figures and features of high-grade glioma, including necrosis and microvascular proliferation. In these tumors, the integrity of the BBB is compromised, as demonstrated by extravasation of pre-albumin, fibrinogen and IgG. This corresponds with expression of HIF-1α, indicating tumor hypoxia. Notably, as expected for a heterogeneous tumor such as DIPG, the density of GFAP-expressing astrocytes varied throughout the tumor [20]. Additionally, tumor cells were differentiated from pre-existing astrocytes by their higher expression levels of GFAP, conceivably also related to their less differentiated state [20].
Immunohistochemical staining of claudin-5, ZO-1, laminin, PDGFR-β, P-gp and BCRP were evaluable in all samples. Expression of tight junction proteins claudin-5 and ZO-1 was lower at inspection in all DIPG biopsy and autopsy samples compared to control samples (Figure 2). The expression of basement membrane protein laminin was lower at the glial basement membrane in DIPG biopsy and autopsy samples. Interestingly, this was observed in both pre-existent vessels within the tumor cells and in neovascular proliferation (Figure 3). Expression of pericyte marker PDGFR-β was also reduced in both DIPG biopsy and autopsy samples (Figure 4). Efflux transporter P-gp expression was lower in DIPG biopsy and autopsy samples, whereas the expression of BCRP was not different in DIPG compared to controls (Figure 5).
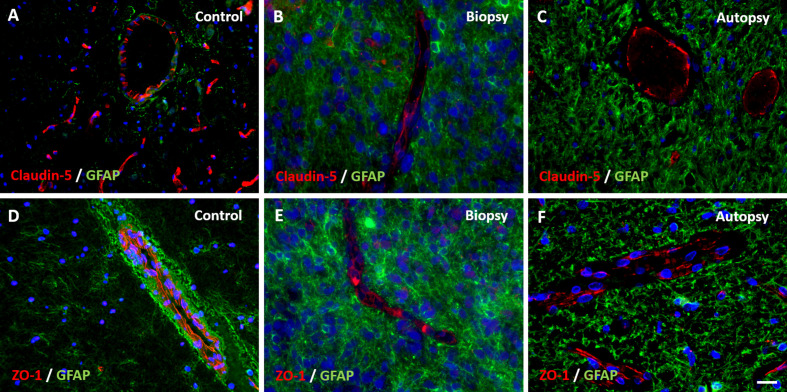
Figure 2. Expression of tight junction proteins claudin-5 and zonula occludens-1 (ZO-1) in DIPG pre-treatment biopsy and post-mortem autopsy samples.
In controls, claudin-5 and ZO-1 are sharply defined and have a segmented pattern (A and D). Claudin-5 and ZO-1 show reduced expression in DIPG samples (B, C, E, F). Please note the non-activated state of GFAP-expressing astrocytes in control tissue. (blue: nuclei; green: astrocytes; red: claudin-5 or ZO-1; scale bar: 5 µm)
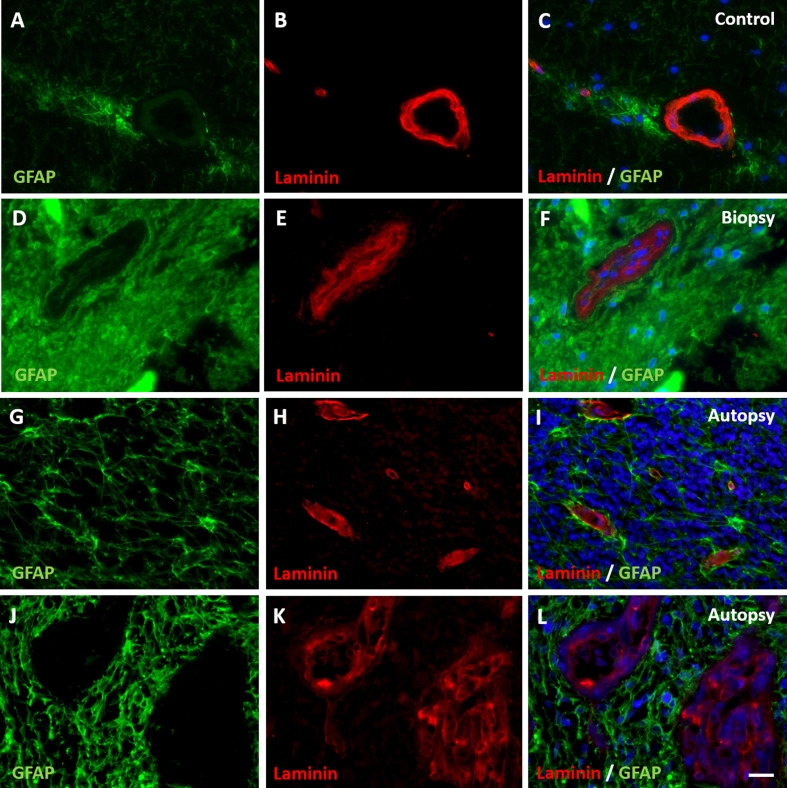
Figure 3. Expression of basement membrane component laminin in DIPG pre-treatment biopsy and post-mortem autopsy samples.
In controls (A-C), laminin shows a continuous pattern. Laminin expression was reduced at the glial basement membrane in both DIPG biopsy (D-F) and autopsy samples (G-I). This was also observed in neovascularization in autopsy samples (J-L). Of note: neovascular proliferation was not detected in biopsy samples. (blue: nuclei; green: astrocytes; red: laminin; scale bar: 5 µm)
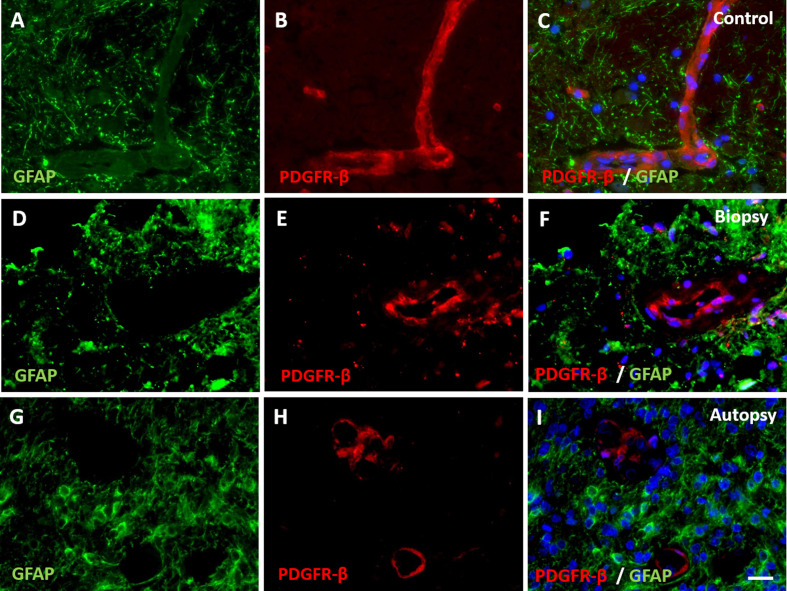
Figure 4. Expression of pericyte marker PDGFR-β in DIPG pre-treatment biopsy and post-mortem autopsy samples.
In controls (A-C), PDGFR-β shows a continuous pattern. Expression of PDGFR-β was reduced in both DIPG biopsy (D-F) and autopsy samples (G-I). (blue: nuclei; green: astrocytes; red: PDGFR-β; scale bar: 5 µm).
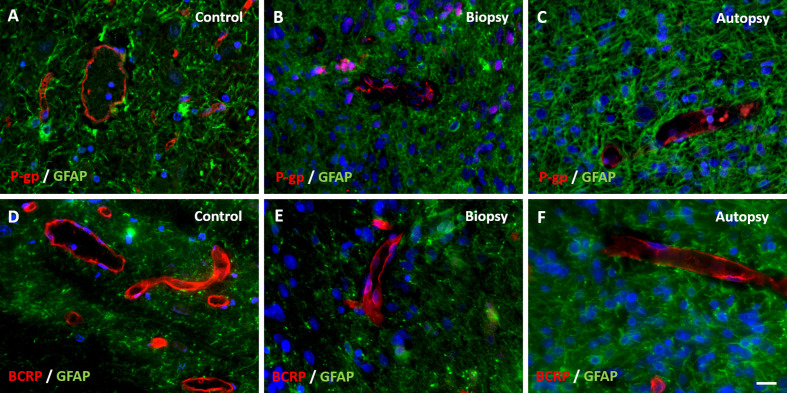
Figure 5. Expression of efflux transporters P-gp and BCRP in DIPG pre-treatment biopsy and post-mortem autopsy samples.
P-gp and BCRP are sharply defined and have a segmented pattern in controls (A and D). Expression of P-gp was reduced in both DIPG samples (B and C). BCRP expression was unchanged in DIPG samples (E and F). (blue: nuclei; green: astrocytes; red: P-gp or BCRP; scale bar: 5 µm)
Vascular density
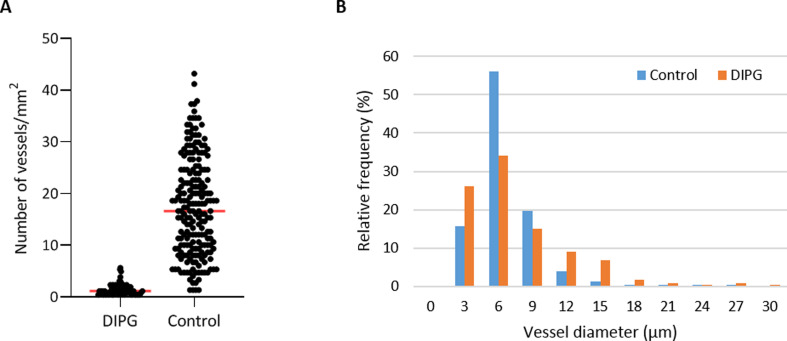
Vascular density per mm² was measured in non-necrotic biopsy and autopsy tissue. It was significantly reduced in DIPG autopsy samples compared to controls (1.5±1.2/mm² versus 17.5±9.5/mm², respectively; t113,890 = 6.831, p-value <0.001; Figure 6A). Notably, the density of small blood vessels (<10 µm) was significantly lower in DIPG autopsy samples than in controls (t180,609 = -4.303, p-value <0.001), whereas the density of large blood vessels (≥10 µm) did not differ between groups (t597 = -0.835, p-value = 0.404). Most blood vessels in DIPG autopsy and control samples had a diameter smaller than 10 µm. The median vascular diameter was 6.21 µm in DIPG autopsy samples (range 2.25-94.85 µm), versus 6.26 µm in controls (range 1.17-264.77 µm; Figure 6B). Due to the very small size of the biopsy samples, it was not possible to statistically analyze the vascular density and dia-meter in these tissue samples. Visual inspection of three patients, however, showed a mean vascular density of 7.5 vessels per mm², and a median vascular diameter of 8.23 µm (data not shown).
Figure 6. Vascular density and diameter in DIPG post-mortem autopsy and healthy control samples.
A: vascular density in DIPG post-mortem autopsy samples and healthy control samples. Mean vascular density was 1.5±1.2/mm² in DIPG versus 17.5±9.5/mm² in controls (red line). B: Vascular size distribution in DIPG post-mortem samples and healthy control samples.
Discussion
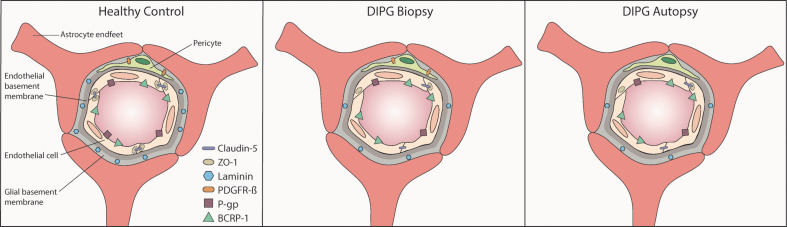
Little research has been done to identify the NVU in DIPG, while it is hypothesized that treatment failure is caused by an intact BBB. As summarized by Figure 7, our study demonstrates structural changes in the NVU of DIPG patients that are already present at diagnosis, suggesting these to be tumor-related and not only due to treatment.
Figure 7. Graphical overview of the structural capillary changes observed in DIPG pre-treatment biopsy and post-mortem autopsy samples:
lower expression of tight junction proteins claudin-5 and ZO-1, basement membrane component laminin, pericyte marker PDGFR-β and efflux transporter P-gp in DIPG biopsy and autopsy samples; unchanged expression of efflux transporter BCRP-1 in DIPG biopsy and autopsy samples.
All studied DIPG patients harbored a H3K27M mutation, thus fulfilling the diagnosis of DMG H3K27M according to the revised WHO classification [8]. Up to 85% of DIPG patients harbor this mutation [21],[22]. Since three out of six DIPG autopsy patients were long-term survivors, the median overall survival of the patients in this group was longer than known from literature, 19.5 months versus 11 months, respectively [7]. Whether neuropathological grading (WHO II-IV), tumor location or the presence of a H3K27M mutation have an impact on survival is still not clear [22],[23],[24].
The barrier properties of the NVU strongly depend on the complex interaction between endothelial cells and their tight junctions, pericytes, basement membranes and astrocytes. In physiological conditions, tight junctions are formed by inter-endothelial connections between transmembrane proteins of the claudin-family (claudin-1, 3, 5, and 12), which regulate the function of these tight junctions[25],[26]. Claudins are anchored into the endothelial cells by proteins from the zonula occludens-family (ZO-1, 2, and 3) that regulate adherens junctions and influence cytoskeletal organization, angioge- nic potential and cell migration [27]. Moreover, ZO-1 is responsible for the spatial organization of claudin-5 by linking it to the actin cytoskeleton [25]. Downregulation of ZO-1 can lead to tight junction disruption and a larger intercellular distance between endothelial cells and thus pathologically increased paracellular transport [27]. Claudin-5 is most abundant in brain vessels (600-times higher expression than other claudins), where it has a heterogeneous distribution [26]. The highest claudin-5 expression is seen in capillaries and small post-capillary venules [25],[28]. In a claudin-5 knockout mouse model, an increased leakage of molecules up to 800 Da was observed [29], whereas the permeability of normal BBB only allows passage of molecules up to 500 Da [30],[31]. When additional tight junction proteins are downregulated, a size-dependent increase in paracellular transport is seen of molecules with a size up to 10,000 Da [29],[32]. In our study, a reduced expression of claudin-5 and ZO-1 was observed in DIPG patients both pre-treatment and post-mortem, indicating a barrier defect, and increasing paracellular transport across the BBB [27]. Nevertheless, there are more tight junction proteins of the zonula occludens and claudin-family expressed by brain endothelial cells. Whether possible downregulation of claudin-5 and ZO-1 is compensated by overexpression of other tight junction proteins remains unknown.
Endothelial cells are surrounded by a basement membrane that contains laminin produced by pericytes and astrocytes [33],[34]. Laminin is essential for basement membrane assembly and maintenance of NVU integrity [35]. In our study, employing a pan- laminin antibody, we found that expression of laminin was lower at the glial basement membrane in both DIPG biopsy and autopsy samples. This was observed in pre-existent vessels amongst the tumor cells and in neovascular proliferation. Our results suggest a pathological involvement of pericytes and astrocytes in DIPG that could have consequences on the behavior of the endothelial cells, thus also disrupting the integrity of the NVU [34]. Immunohistochemistry showed also a lower expression of PDGFR-β in DIPG biopsy and autopsy samples, suggesting a reduction in pericytic coverage in DIPG NVU. Besides contributing to secretion of basement membrane components [33],[34], pericytes are essential for regula-ting capillary diameter and vessel stability [13]. The possible reduction of pericytic coverage observed in our study may explain the possible downregulation of laminin at the glial basement membrane in DIPG patients.
Under physiological conditions, P-gp and BCRP are the most dominantly expressed efflux transpor-ters in de BBB [36],[37]. Our study shows a decreased P-gp expression and unchanged BCRP expression in DIPG pre-treatment and post-mortem samples. This is in line with previous work showing a “moderate expression” of P-gp and intense staining of BCRP in DIPG tumor vasculature [10].
Overall, our results show alterations of the NVU in DIPG patients, which could result in or reflect a more leaky NVU. This hypothesis of a leaky NVU is supported by the demonstrated extravasation of some intravascular proteins, such as pre-albumin, fibrinogen and IgG. Theoretically, this might positively influence influx of chemotherapeutic agents into the tumor, based on passive diffusion. Clinical data, however, do not support this possibility [38],[39]. A possible explanation for this discrepancy might be the markedly reduced vascular density in DIPG that could overrule the effects of a leaky NVU. Whether the reduction of vascular density is also present at diagnosis remains to be investigated.
Lack of therapy efficacy in DIPG has been linked to an intact BBB. Here, we demonstrated structural changes of the NVU together with a lower vascular density in these tumors. These findings have consequences for drug administration, since coverage of the whole tumor, including the migrating/diffusely growing tumor cells, is essential. Our findings suggest that drug administration techniques that mostly rely on vascular density for drug distribution, including conventional systemic administration and microbubble mediated focused ultrasound, might show limited efficacy in DIPG [12]. In contrast, convection-enhanced delivery might be a more suitable technique, in which drug distribution across the tumor relies on a positive pressure gradient instead of passive diffusion [12],[40],[41]. Adding such a biological NVU perspective may help to better direct treatment choices for DIPG patients in the future.
Acknowledgement
We thank the employees of the Expertise Center for Post-mortem Diagnostics of the Amsterdam University Medical Centers, location VUmc, for assisting with the autopsies of the DIPG patients. We also thank the NIH NeuroBiobank for providing the control tissue. We would also like to thank the Semmy Foundation (Stichting Semmy) for financially supporting DIPG research in the Amsterdam University Medical Centers, location VUmc.
Ethical Approval
The study was approved by the Medical ethical committee of the Amsterdam University Medical Center, location VUmc (METc VUmc, study number VUMC2009/237). This study was conducted in accordance to the declaration of Helsinki.
Data Sharing and Data Accessibility
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
References
- Intrinsic brain-stem tumors of childhood: surgical indications. Epstein F, McCleary E. Journal of Neurosurgery. 1986 Jan;64(1) doi: 10.3171/jns.1986.64.1.0011. [DOI] [PubMed] [Google Scholar]
- Clinicopathological study of diffuse type brainstem gliomas: Analysis of 40 autopsy cases. Yoshimura J, Onda K, Tanaka R, Takahashi H. Neurologia medico-chirurgica. 2003;43(8) doi: 10.2176/nmc.43.375. [DOI] [PubMed] [Google Scholar]
- Supratentorial high-grade astrocytoma and diffuse brainstem glioma: Two challenges for the pediatric oncologist. Broniscer A, Gajjar A. The Oncologist. 2004 Apr 01;9(2) doi: 10.1634/theoncologist.9-2-197. [DOI] [PubMed] [Google Scholar]
- Diffuse brainstem glioma in children: Critical review of clinical trials. Hargrave D, Bartels U, Bouffet E. The Lancet Oncology. 2006 Mar;7(3) doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Jansen M, Veldhuijzen van Zanten S, Sanchez Aliaga E, Heymans M, Warmuth-Metz M, Hargrave D, Hoeven E, Gidding C, Bont E, Eshghi O, Reddingius R, Peeters C, Schouten-van Meeteren A, Gooskens R, Granzen B, Paardekooper G, Janssens G, Noske D, Barkhof F, Kramm C, Vandertop W, Kaspers G, Vuurden D. Neuro-Oncology. 2015 Jan;17(1) doi: 10.1093/neuonc/nou104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. Veringa S, Biesmans D, Vuurden D, Jansen M, Wedekind L, Horsman I, Wesseling P, Vandertop W, Noske D, Kaspers G, Hulleman E. Ulasov I, editor. PLoS ONE. 2013 Apr 29;8(4) doi: 10.1371/journal.pone.0061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of diffuse intrinsic pontine glioma (dipg): A collaborative report from the international and european society for pediatric oncology DIPG registries. Hoffman L, Veldhuijzen van Zanten S, Colditz N, Baugh J, Chaney B, Hoffmann M, Lane A, Fuller C, Miles L, Hawkins C, Bartels U, Bouffet E, Goldman S, Leary S, Foreman N, Packer R, Warren K, Broniscer A, Kieran M, Minturn J, Comito M, Broxson E, Shih C, Khatua S, Chintagumpala M, Carret A, Escorza N, Hassall T, Ziegler D, Gottardo N, Dholaria H, Doughman R, Benesch M, Drissi R, Nazarian J, Jabado N, Boddaert N, Varlet P, Giraud G, Castel D, Puget S, Jones C, Hulleman E, Modena P, Giagnacovo M, Antonelli M, Pietsch T, Gielen G, Jones D, Sturm D, Pfister S, Gerber N, Grotzer M, Pfaff E, Bueren A, Hargrave D, Solanki G, Jadrijevic Cvrlje F, Kaspers G, Vandertop W, Grill J, Bailey S, Biassoni V, Massimino M, Calmon R, Sanchez E, Bison B, Warmuth-Metz M, Leach J, Jones B, Vuurden D, Kramm C, Fouladi M. Journal of Clinical Oncology. 2018 Jul 01;36(19) doi: 10.1200/JCO.2017.75.9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Louis D, Perry A, Reifenberger G, Deimling A, Figarella-Branger D, Cavenee W, Ohgaki H, Wiestler O, Kleihues P, Ellison D. Acta Neuropathologica. 2016 Jun;131(6) doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- Convection enhanced delivery of carmustine to the murine brainstem: A feasibility study. Sewing A, Caretti V, Lagerweij T, Schellen P, Jansen M, Vuurden D, Idema S, Molthoff C, Vandertop W, Kaspers G, Noske D, Hulleman E. Journal of Neuroscience Methods. 2014 Dec;238 doi: 10.1016/j.jneumeth.2014.09.020. [DOI] [PubMed] [Google Scholar]
- In vitro drug response and efflux transporters associated with drug resistance in pediatric high grade glioma and diffuse intrinsic pontine glioma. Veringa S, Biesmans D, Vuurden D, Jansen M, Wedekind L, Horsman I, Wesseling P, Vandertop W, Noske D, Kaspers G, Hulleman E. Ulasov I, editor. PLoS ONE. 2013 Apr 29;8(4) doi: 10.1371/journal.pone.0061512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Functionally defined therapeutic targets in diffuse intrinsic pontine glioma. Grasso C, Tang Y, Truffaux N, Berlow N, Liu L, Debily M, Quist M, Davis L, Huang E, Woo P, Ponnuswami A, Chen S, Johung T, Sun W, Kogiso M, Du Y, Qi L, Huang Y, Hütt-Cabezas M, Warren K, Le Dret L, Meltzer P, Mao H, Quezado M, Vuurden D, Abraham J, Fouladi M, Svalina M, Wang N, Hawkins C, Nazarian J, Alonso M, Raabe E, Hulleman E, Spellman P, Li X, Keller C, Pal R, Grill J, Monje M. Nature Medicine. 2015 Jun;21(6) doi: 10.1038/nm.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overview of current drug delivery methods across the blood–brain barrier for the treatment of primary brain tumors. Haumann R, Videira J, Kaspers G, Vuurden D, Hulleman E. CNS Drugs. 2020 Nov;34(11) doi: 10.1007/s40263-020-00766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Development, maintenance and disruption of the blood-brain barrier. Obermeier B, Daneman R, Ransohoff R. Nature Medicine. 2013 Dec;19(12) doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anatomy and physiology of the blood–brain barrier. Serlin Y, Shelef I, Knyazer B, Friedman A. Seminars in Cell & Developmental Biology. 2015 Feb;38 doi: 10.1016/j.semcdb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrocyte–endothelial interactions at the blood–brain barrier. Abbott N, Rönnbäck L, Hansson E. Nature Reviews Neuroscience. 2006 Jan 01;7(1) doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Functional morphology of the blood–brain barrier in health and disease. Liebner S, Dijkhuizen R, Reiss Y, Plate K, Agalliu D, Constantin G. Acta Neuropathologica. 2018 Mar;135(3) doi: 10.1007/s00401-018-1815-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heterogeneity of the blood-brain barrier. Wilhelm I, Nyúl-Tóth Á, Suciu M, Hermenean A, Krizbai I. Tissue Barriers. 2016 Jan 02;4(1) doi: 10.1080/21688370.2016.1143544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The evolving concept of the Blood Brain Barrier (BBB): From a single static barrier to a heterogeneous and dynamic relay center. Villabona-Rueda A, Erice C, Pardo C, Stins M. Frontiers in Cellular Neuroscience. 2019 Sep 20;13 doi: 10.3389/fncel.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Implementation of a multi-institutional diffuse intrinsic pontine glioma autopsy protocol and characterization of a primary cell culture: DIPG autopsy protocol and post mortem primary culture. Caretti V, Jansen M, Vuurden D, Lagerweij T, Bugiani M, Horsman I, Wessels H, Valk P, Cloos J, Noske D, Vandertop W, Wesseling P, Wurdinger T, Hulleman E, Kaspers G. Neuropathology and Applied Neurobiology. 2013 Jun;39(4) doi: 10.1111/j.1365-2990.2012.01294.x. [DOI] [PubMed] [Google Scholar]
- Deceptive morphologic and epigenetic heterogeneity in diffuse intrinsic pontine glioma. Bugiani M, Zanten S, Caretti V, Schellen P, Aronica E, Noske D, Vandertop W, Kaspers G, Vuurden D, Wesseling P, Hulleman E. Oncotarget. 2017 Sep 01;8(36) doi: 10.18632/oncotarget.19726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Castel D, Philippe C, Calmon R, Le Dret L, Truffaux N, Boddaert N, Pagès M, Taylor K, Saulnier P, Lacroix L, Mackay A, Jones C, Sainte-Rose C, Blauwblomme T, Andreiuolo F, Puget S, Grill J, Varlet P, Debily M. Acta Neuropathologica. 2015 Dec;130(6) doi: 10.1007/s00401-015-1478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Karremann M, Gielen G, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M, Bison B, Claviez A, Vuurden D, Bueren A, Gessi M, Kühnle I, Hans V, Benesch M, Sturm D, Kortmann R, Waha A, Pietsch T, Kramm C. Neuro-Oncology. 2018 Jan 10;20(1) doi: 10.1093/neuonc/nox149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treatment options in childhood pontine gliomas. Wagner S, Warmuth-Metz M, Emser A, Gnekow A, Sträter R, Rutkowski S, Jorch N, Schmid H, Berthold F, Graf N, Kortmann R, Pietsch T, Sörensen N, Peters O, Wolff J. Journal of Neuro-Oncology. 2006 Sep;79(3) doi: 10.1007/s11060-006-9133-1. [DOI] [PubMed] [Google Scholar]
- A suggestion to introduce the diagnosis of “diffuse midline glioma of the pons, H3 K27 wildtype (WHO grade IV)”. Bueren A, Karremann M, Gielen G, Benesch M, Fouladi M, Vuurden D, Zanten S, Hoffman L, Kramm C. Acta Neuropathologica. 2018 Jul;136(1) doi: 10.1007/s00401-018-1863-6. [DOI] [PubMed] [Google Scholar]
- Claudin-5: gatekeeper of neurological function. Greene C, Hanley N, Campbell M. Fluids and Barriers of the CNS. 2019 Dec;16(1) doi: 10.1186/s12987-019-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The role of claudin-5 in blood-brain barrier (BBB) and brain metastases (Review) Jia W, Lu R, Martin T, Jiang W. Molecular Medicine Reports. 2014 Mar;9(3) doi: 10.3892/mmr.2013.1875. [DOI] [PubMed] [Google Scholar]
- ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. Tornavaca O, Chia M, Dufton N, Almagro L, Conway D, Randi A, Schwartz M, Matter K, Balda M. Journal of Cell Biology. 2015 Mar 16;208(6) doi: 10.1083/jcb.201404140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel 3D analysis of Claudin-5 reveals significant endothelial heterogeneity among CNS microvessels. Paul D, Cowan A, Ge S, Pachter J. Microvascular Research. 2013 Mar;86 doi: 10.1016/j.mvr.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Size-selective loosening of the blood-brain barrier in claudin-5–deficient mice. Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Journal of Cell Biology. 2003 May 12;161(3) doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effective drug delivery in diffuse intrinsic pontine glioma: A theoretical model to identify potential candidates. El-Khouly F, Vuurden D, Stroink T, Hulleman E, Kaspers G, Hendrikse N, Veldhuijzen van Zanten S. Frontiers in Oncology. 2017 Oct 30;7 doi: 10.3389/fonc.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PET radiotracers: crossing the blood–brain barrier and surviving metabolism. Pike V. Trends in Pharmacological Sciences. 2009 Aug;30(8) doi: 10.1016/j.tips.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autoregulated paracellular clearance of amyloid-β across the blood-brain barrier. Keaney J, Walsh D, O’Malley T, Hudson N, Crosbie D, Loftus T, Sheehan F, McDaid J, Humphries M, Callanan J, Brett F, Farrell M, Humphries P, Campbell M. Science Advances. 2015 Sep 04;1(8) doi: 10.1126/sciadv.1500472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The role of pericytic laminin in blood brain barrier integrity maintenance. Gautam J, Zhang X, Yao Y. Scientific Reports. 2016 Dec;6(1) doi: 10.1038/srep36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astrocytic laminin regulates pericyte differentiation and maintains blood brain barrier integrity. Yao Y, Chen Z, Norris E, Strickland S. Nature Communications. 2014 May;5(1) doi: 10.1038/ncomms4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laminin-induced signaling in tumor cells. Givant-Horwitz V, Davidson B, Reich R. Cancer Letters. 2005 Jun;223(1) doi: 10.1016/j.canlet.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Proteomic quantification of human blood–brain barrier SLC and ABC Transporters in healthy individuals and dementia patients. Al-Majdoub Z, Al Feteisi H, Achour B, Warwood S, Neuhoff S, Rostami-Hodjegan A, Barber J. Molecular Pharmaceutics. 2019 Mar 04;16(3) doi: 10.1021/acs.molpharmaceut.8b01189. [DOI] [PubMed] [Google Scholar]
- Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors: Protein levels of transporters at human BBB. Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, Terasaki T. Journal of Neurochemistry. 2011 Apr;117(2) doi: 10.1111/j.1471-4159.2011.07208.x. [DOI] [PubMed] [Google Scholar]
- Diagnostics and treatment of diffuse intrinsic pontine glioma: where do we stand? El-Khouly F, Veldhuijzen van Zanten S, Santa-Maria Lopez V, Hendrikse N, Kaspers G, Loizos G, Sumerauer D, Nysom K, Pruunsild K, Pentikainen V, Thorarinsdottir H, Rutkauskiene G, Calvagna V, Drogosiewicz M, Dragomir M, Deak L, Kitanovski L, Bueren A, Kebudi R, Slavc I, Jacobs S, Jadrijevic-Cvrlje F, Entz-Werle N, Grill J, Kattamis A, Hauser P, Pears J, Biassoni V, Massimino M, Lopez Aguilar E, Torsvik I, Joao Gil-da-Costa M, Kumirova E, Cruz-Martinez O, Holm S, Bailey S, Hayden T, Thomale U, Janssens G, Kramm C, Vuurden D. Journal of Neuro-Oncology. 2019 Oct;145(1) doi: 10.1007/s11060-019-03287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- A twenty-year review of diagnosing and treating children with diffuse intrinsic pontine glioma in The Netherlands. Veldhuijzen van Zanten S, Jansen M, Sanchez Aliaga E, Vuurden D, Vandertop W, Kaspers G. Expert Review of Anticancer Therapy. 2015 Feb;15(2) doi: 10.1586/14737140.2015.974563. [DOI] [PubMed] [Google Scholar]
- A novel implantable catheter system with transcutaneous port for intermittent convection-enhanced delivery of carboplatin for recurrent glioblastoma. Barua N, Hopkins K, Woolley M, O’Sullivan S, Harrison R, Edwards R, Bienemann A, Wyatt M, Arshad A, Gill S. Drug Delivery. 2016 Jan 02;23(1) doi: 10.3109/10717544.2014.908248. [DOI] [PubMed] [Google Scholar]
- Chronic, intermittent convection-enhanced delivery devices. Lewis O, Woolley M, Johnson D, Rosser A, Barua N, Bienemann A, Gill S, Evans S. Journal of Neuroscience Methods. 2016 Feb;259 doi: 10.1016/j.jneumeth.2015.11.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.