Abstract
Aims
To evaluate the efficacy of oesophageal cooling in the prevention of oesophageal injury in patients undergoing atrial fibrillation (AF) catheter ablation.
Methods and results
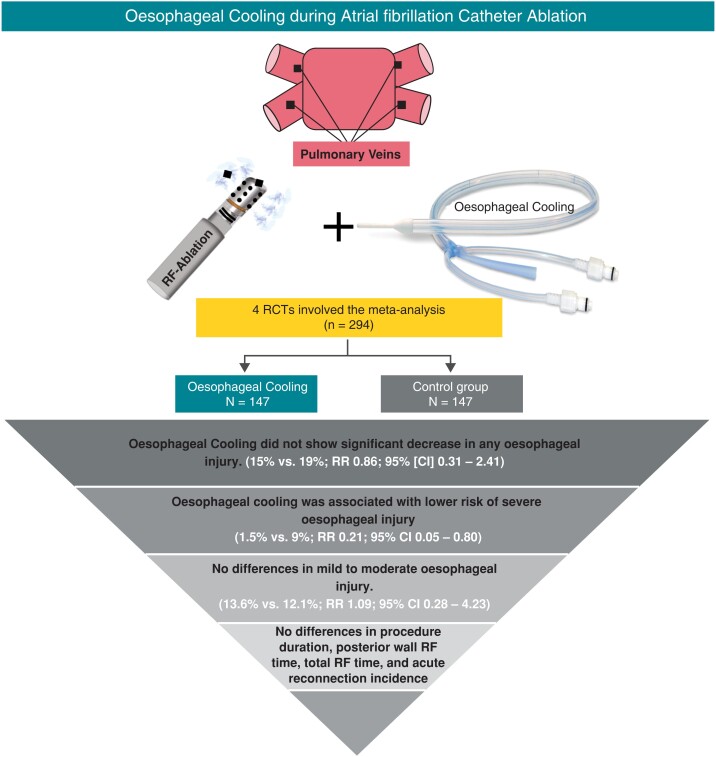
Comprehensive search of MEDLINE, EMBASE, and Cochrane databases through April 2022 for randomized controlled trials (RCTs) evaluating the role of oesophageal cooling compared with control in the prevention of oesophageal injury during AF catheter ablation. The study primary outcome was the incidence of any oesophageal injury. The meta-analysis included 4 RCTs with a total of 294 patients. There was no difference in the incidence of any oesophageal injury between oesophageal cooling and control [15% vs. 19%; relative risk (RR) 0.86; 95% confidence interval (CI) 0.31–2.41]. Compared with control, oesophageal cooling showed lower risk of severe oesophageal injury (1.5% vs. 9%; RR 0.21; 95% CI 0.05–0.80). There were no significant differences among the two groups in mild to moderate oesophageal injury (13.6% vs. 12.1%; RR 1.09; 95% CI 0.28–4.23), procedure duration [standardized mean difference (SMD) −0.03; 95% CI −0.36–0.30], posterior wall radiofrequency (RF) time (SMD 0.27; 95% CI −0.04–0.58), total RF time (SMD −0.50; 95% CI −1.15–0.16), acute reconnection incidence (RR 0.93; 95% CI 0.02–36.34), and ablation index (SMD 0.16; 95% CI −0.33–0.66).
Conclusion
Among patients undergoing AF catheter ablation, oesophageal cooling did not reduce the overall risk of any oesophageal injury compared with control. Oesophageal cooling might shift the severity of oesophageal injuries to less severe injuries. Further studies should evaluate the long-term effects after oesophageal cooling during AF catheter ablation.
Keywords: Atrial fibrillation, Catheter ablation, Radiofrequency ablation, Oesophageal cooling, Oesophageal injury
Graphical Abstract
Graphical Abstract.
What’s New?
This current meta-analysis of randomized controlled trials (RCTs) evaluated the role of oesophageal cooling compared with control in the prevention of oesophageal injury during AF catheter ablation.
Our study demonstrated that oesophageal cooling did not reduce the overall risk of any oesophageal injury compared with control.
Oesophageal cooling might shift the severity of oesophageal injuries to less severe and non-ulcerous injuries.
Our findings suggested that oesophageal cooling did not affect the acute outcomes including the duration or success of the ablation process.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting almost 60 million adults worldwide.1,2 The role of AF catheter ablation is expanding in this population group, although safety of the procedure is an important consideration. Complications from catheter ablation include stroke, pericardial effusion, cardiac tamponade, oesophageal injury, atrio-oesophageal fistula (AEF), phrenic nerve injury, and vascular complications and usually occur in less than <4% of the cases.3–5 During both radiofrequency (RF) ablation and cryoablation, energy delivery can extend beyond the myocardium affecting adjacent structures including the oesophagus, which lies next to the left atrial posterior wall. Thermal injury could lead to the development of AEF, a rare yet lethal complication.6,7 Oesophageal ulceration could be the initial injury that can lead to AEF formation and usually present within hours or days following catheter ablation.8–10,11 Endoscopic evaluations have reported the incidence of oesophageal lesions to occur in 2–47% of cases post ablation.12–14 Importantly, clinical data suggest that severe oesophageal injuries are the most concerning, as 9.6% progress to AEF.9
Multiple preventive strategies have been introduced including luminal oesophageal temperature monitoring, pre-procedural assessment of oesophagus position, tagging of oesophagus and intra-procedural real-time visualization of its course, mechanical oesophageal displacement, reducing ablation energy to the posterior wall of the left atrium, and gastric acid suppression. Yet, none of these methods have shown efficacy in preventing oesophageal injuries that is a precursor to AEF.8,11,15–19 Oesophageal cooling for oesophageal protection during RF ablation has been investigated in a few randomized controlled trials (RCTs), which have yielded mixed results.20–23 Importantly, these studies were not adequately powered to detect true differences with oesophageal cooling strategies. In this study, we performed a systemic review and meta-analysis of RCTs that assessed the role of oesophageal cooling on the incidence of oesophageal injury and their degree of severity during AF catheter ablation.
Methods
Data sources and search strategy
A comprehensive search of MEDLINE, EMBASE, and Cochrane databases through April 2022 for RCTs evaluating the role of oesophageal cooling compared with control in the prevention of oesophageal injury during AF catheter ablation was performed. The following keywords were used separately and in combination: ‘esophageal’ OR ‘oesophageal’ OR ‘cooling’ OR ‘Ablation’ OR ‘Catheter ablation’. In addition, further screening of Clinicaltrials.gov and prior meta-analyses were performed to retrieve previously published RCTs that did not appear in the initial search. Our study was conducted in accordance with PRISMA (Preferred Reporting Items for Systemic Reviews and Meta-Analyses) guidelines24 (see Supplementary material online, Table S1). Details of the systematic review were submitted for registration in PROSPERO with ID 329145.
Selection criteria
We included RCTs that compared the protective role of oesophageal cooling vs. control on the prevention of oesophageal injury during AF catheter ablation. We only included studies that assessed oesophageal injury by performing post-ablation endoscopy. The control group in the included studies should have employed oesophageal temperature probes to monitor the oesophageal temperature during RF ablation. We only included studies that were published in English. Conference abstracts, review articles, case reports, case series, case–control, cohort, and non-randomized trials were all excluded.
Data extraction
Data were extracted by two investigators independently (M.H. and S.E.) and included various study features, baseline characteristics, and outcomes. Any disagreements between investigators were settled by consensus.
Outcomes
Our study’s primary outcome was the incidence of any oesophageal injury (i.e. irrespective of the severity of oesophageal injury). The secondary outcomes included the incidence of severe oesophageal injury, the incidence of mild to moderate oesophageal injury, procedural duration, posterior wall RF time, total RF time, acute reconnection incidence, and ablation index. The definition of any oesophageal injury and the degree of severity were adopted as per each study. Ablation index, a marker of ablation lesion quality, is a weighted formula that incorporates power, duration, and contact force. These outcomes were reported for the patients with the longest follow-up period till after endoscopic evaluation and on an intention-to-treat basis.
Assessment of the quality of the included studies
The included studies’ quality was evaluated using the Cochrane risk assessment for RCTs, which includes various criteria such as random sequence generation and allocation concealment for selection bias, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Based on the aforementioned criteria, studies were classified as having a low risk of bias, a high risk of bias, or an unclear risk of bias25 (see Supplementary material online, Table S3).
Statistical analysis
Data were pooled by a random-effects model to overcome anticipated heterogeneity among the included studies. To evaluate statistical heterogeneity between the included studies, we used I2 statistics; values < 25%, 25% to 50%, and >50% were considered to be a low, moderate, and a high degree of heterogeneity, respectively.26 For continuous variables, standardized mean difference (SMD) were used, and for categorical variables, risk ratios (RR) were used. Since our meta-analysis included a small number of studies, publication bias was not assessed. The P-values for all analyses were considered significant if <0.05. Statistical analyses of the study data were conducted using RevMan 5.0 software (Cochrane Collaboration, Oxford, UK).
Results
Included studies
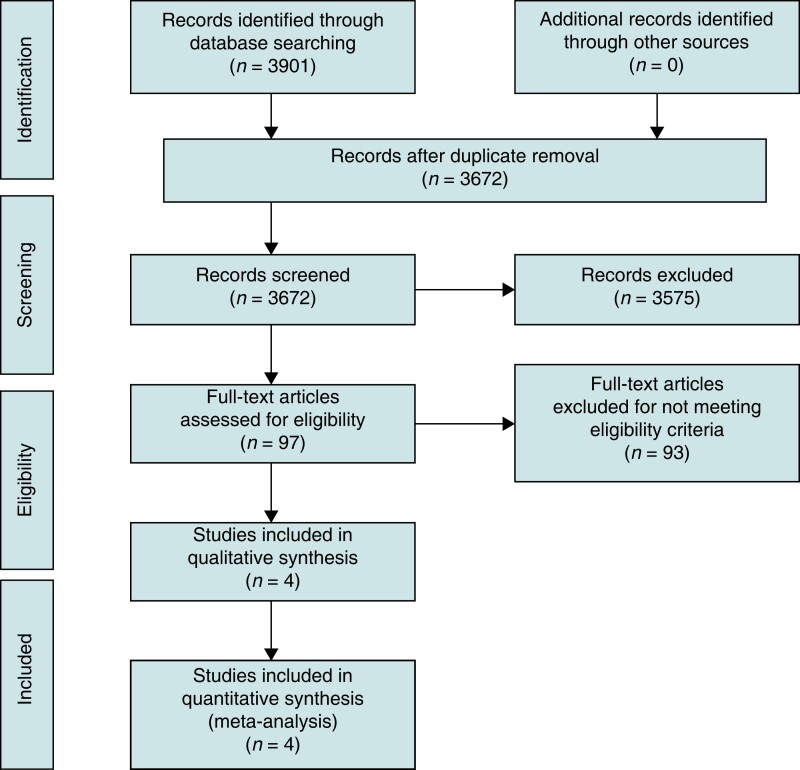
The study selection process was outlined in Figure 1. The final analysis included 4 RCTs which involved a total of 294 patients: 147 in the oesophageal cooling group and 147 in the control group.20–23 The characteristics of the included studies and patient population appear in Table 1 and Table 2. The weighted mean age was 62.1 years, while the proportion of men was 70.1%. The included studies used various methods of oesophageal cooling. Kuwahara et al.20 used ice water (0°C) that was injected through a gastric tube into oesophagus. Oliveira et al. used a catheter balloon filled with cold saline solution and continuously irrigated the oesophageal wall with the catheter balloon.22 Both Leung et al.21 and Tschabrunn et al.23 used the ensoETM device for oesophageal cooling with oesophageal temperature maintained at 4°C. EnsoETM is an FDA-approved device which is a medical-grade silicone tube through which consistent high volume of distilled water in a closed-loop irrigation system that can be used for cooling or warming.21,23 All control groups used oesophageal temperature sensor probes (either single sensor or multi-sensor) to monitor oesophageal temperature and ablation was stopped in case of rising temperature in the oesophagus.20–23 Oesophageal injury was assessed by using endoscopy with different protocols among the included studies. Kuwahara et al. used endoscopy 1 day after ablation, while Tschabrunn et al. used endoscopy within 48 h after the ablation procedure. In Oliveira et al.’s study, endoscopy and endoscopic ultrasound were done within 3 days after the procedure. Leung et al. used endoscopy 7 days after the ablation procedure. The definition of oesophageal thermal injury and the degree of severity of thermal injury in the included studies are reported in Supplementary material online, Table S2. All involved studies used RF AF catheter ablation and were single-centred studies.20–23 The quality of the included studies appears in Supplementary material online, Table S3. All studies were double-blinded studies except Kuwahara et al., which was a single-blinded study as operators were unblinded.20 The remaining risk of bias was considered to be low among all studies.
Figure 1.
Study flowsheet.
Table 1.
Characteristics of the included studies
| Study | Publication year | No. of centres | Group 1 (oesophageal cooling) | Group 2 (control) | Oesophageal cooling method | Oesophageal temperature monitoring in control group | Follow-up endoscopy | Type of AF ablation and catheter used | Study objective |
|---|---|---|---|---|---|---|---|---|---|
| Tschabrunn | 2022 | Single centre | 22 | 22 | Active oesophageal cooling (EnsoETM device) | Standard linear and non-deflectable single-sensor probe (Smiths Medical ASD Inc., Keene, NH) | Within 48 h following the ablation procedure | RF (Smart-Touch Surround Flow Thermocool, Biosense Webster Inc.). | Number and percentage of participants with oesophageal thermal injury |
| Leung (IMPACT) | 2021 | Single centre | 60 | 60 | Active oesophageal cooling (EnsoETM device) | Single-sensor temperature probe (Oesophageal Temperature Probe, Smiths Medical, Minneapolis, MN, USA) | 7 days after ablation | RF; using irrigated contact force sensing catheters (STSF or Qdot Micro, Biosense Webster, Johnson and Johnson, Diamond Bar, CA, USA) | The incidence of endoscopically detected oesophageal mucosal lesions and/or gastroparesis |
| Oliveira | 2020 | Single centre | 15 | 15 | Continuous irrigation of the oesophageal wall with a catheter balloon filled with cold saline solution | Single-thermocouple oesophageal temperature probe (Braile Biomedica) | Endoscopy plus EUS within 3 days after the procedure | RF generator (Stockert EP-Shuttle Generator System; Biosense Webster) | To compare the difference in prevalence of post-AF ablation oesophageal and peri-oesophageal injuries |
| Kuwahara | 2014 | Single centre | 50 | 50 | Injecting ice water through gastric tube into the oesophagus under oesophageal temperature monitoring | Multi-thermocouple temperature probe (Sensitherm, St. Jude Medical) | One day after the AF catheter ablation session | RF (Thermocool, Biosense Webster Inc.) | To elucidate whether oesophageal cooling with the ice water could prevent the incidence of oesophageal lesions complicating the catheter ablation of AF |
EUS, endoscopic ultrasound; AF, atrial fibrillation; RF, radiofrequency.
Table 2.
Baseline characteristics of the study population
| Tschabrunn | Leung (IMPACT) | Oliveira | Kuwahara | ||
|---|---|---|---|---|---|
| Age in years (mean ± SD) | Cooling | 62.8 ± 9.6 | 65 ± 10 | 47.8 ± 13.3 | 62 ± 9 |
| Control | 63.6 ± 9.3 | 65 ± 9 | 53.3 ± 9.4 | 64 ± 10 | |
| Male % | Cooling | 64 | 60 | 66.7 | 76 |
| Control | 73 | 61.7 | 86.7 | 84 | |
| BMI (kg/m2) | Cooling | 30.5 ± 7.3 | 28.5 ± 5.3 | 28.7 ± 4.8 | 24 ± 3 |
| Control | 31.0 ± 5.1 | 29.8 ± 6.98 | 30.4 ± 3.3 | 24 ± 3 | |
| Paroxysmal AF % | Cooling | 50 | 45 | 86.7 | 70 |
| Control | 64 | 50 | 73.3 | 58 | |
| Diabetes % | Cooling | 5 | – | 0 | – |
| Control | 9 | – | 13.3 | – | |
| Hypertension % | Cooling | 55 | – | 46.7 | – |
| Control | 59 | – | 66.7 | – | |
| EF % | Cooling | 54.7 ± 11.4 | 55 ± 9 | 63.5 ± 3.9 | 64 ± 8 |
| Control | 55.8 ± 9.4 | 52 ± 8 | 62.9 ± 4.8 | 64 ± 7 | |
| LAD (cm) | Cooling | – | 4.1 ± 0.9 | 4.05 ± 0.48 | 4.0 ± 0.5 |
| Control | – | 4.2 ± 0.6 | 4.17 ± 0.37 | 4.0 ± 0.6 | |
SD, standard deviation; kg, kilogram; m, meter; EF, ejection fraction; LAD, left atrial diameter; cm, centimetre.
Primary outcome
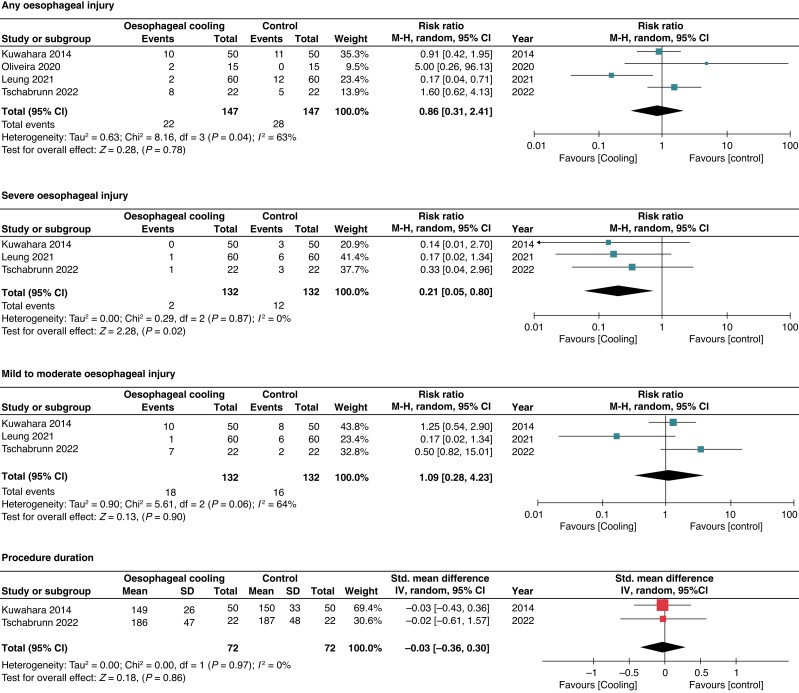
The primary outcome was reported in all included RCTs.20–23 Oesophageal cooling did not show a significant decrease in any oesophageal injury compared to control [15% vs. 19%; RR 0.86; 95% confidence interval (CI) 0.31–2.41; I2 = 63%] (Figure 2). We performed stepwise sensitivity analyses to evaluate the source of heterogeneity by excluding one study at a time. After excluding the study with the highest contribution to heterogeneity (Leung et al.), sensitivity analysis showed similar results (23% vs. 18.4%; RR 1.20; 95% CI 0.67–2.15) although heterogeneity decreased significantly (I2 = 0%).20,22,23 Several other sensitivity analyses were conducted as follows: including studies using the most commonly cooling method (i.e. ensoETM device) (RR 0.55; 95% CI 0.05–5.60; I2 = 86%),21,23 including studies using consistent temperature monitoring method (i.e. single-sensor probe) (RR 0.90; 95% CI 0.14–5.87; I2 = 76%)21–23 and including studies performing endoscopy in the first 3 days after the ablation procedure (RR 1.20; 95% CI 0.67–2.15; I2 = 0%),20,22,23 which all showed similar results to the primary analysis (see Supplementary material online, Figure S1).
Figure 2.
Forrest plot for any oesophageal injury, severe oesophageal injury, mild–moderate oesophageal injury, and procedural duration.
Secondary outcomes
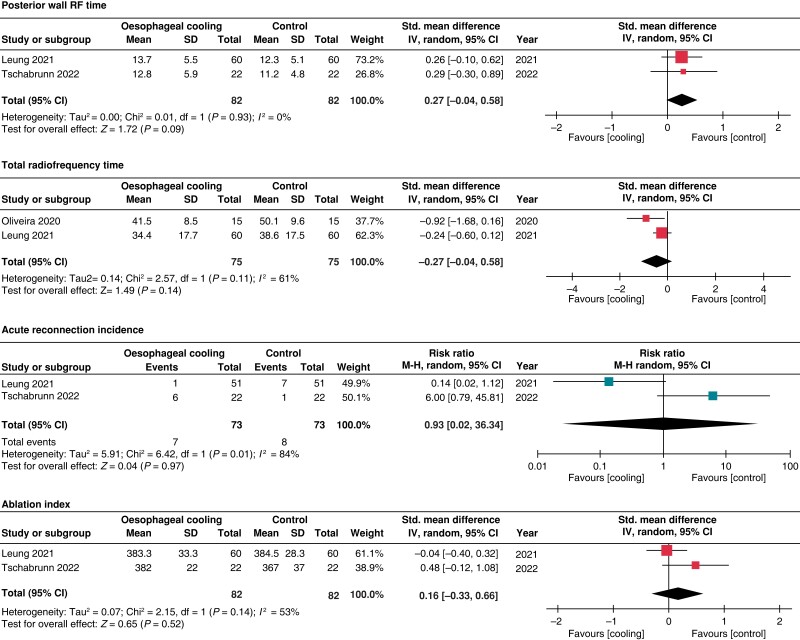
The risk of severe oesophageal injury was reported in 3 studies; however, the definitions varied among these studies (see Supplementary material online, Table S2). Oesophageal cooling was associated with a lower risk of severe oesophageal injury (1.5% vs. 9%; RR 0.21; 95% CI 0.05–0.80) with low heterogeneity (I2 = 0%). Sensitivity analysis excluding the study with the highest contribution to heterogeneity (i.e. Leung et al.), demonstrated no significant difference in the rate of severe oesophageal lesions between both groups (RR 0.25; 95% CI 0.04–1.42, I2 = 0) (see Supplementary material online, Figure S1). There were no differences among the oesophageal cooling and control groups in mild to moderate oesophageal injury (13.6% vs. 12.1%; RR 1.09; 95% CI 0.28–4.23; I2 = 64%), procedure duration (SMD −0.03; 95% CI −0.36–0.30; I2 = 0%), posterior wall RF time (SMD 0.27; 95% CI −0.04–0.58; I2 = 0%), total RF time (SMD −0.50; 95% CI −1.15–0.16; I2 = 61%), acute reconnection incidence (RR 0.93; 95% CI 0.02–36.34; I2 = 84%), and ablation index (SMD 0.16; 95% CI −0.33–0.66; I2 = 53%). (Figures 2 and 3)
Figure 3.
Forrest plot for posterior wall RF time, total radiofrequency time, acute reconnection incidence, and ablation index.
Discussion
In this meta-analysis of 4 RCTs, including 294 patients, we evaluated the efficacy and safety of oesophageal cooling in the prevention of oesophageal injuries in patients with AF undergoing catheter ablation. The salient findings of this study are as follows: (i) there was no significant difference in the incidence of any oesophageal injury in patients undergoing oesophageal cooling compared with the control group; (ii) patients undergoing oesophageal cooling might have lower risk of severe oesophageal injury; however, the definition of severe oesophageal injuries varied among included studies; and (iii) there was no significant difference between both groups in mild to moderate oesophageal injury, posterior wall RF time, total procedure duration, total RF time, acute reconnection incidence, and ablation index. Importantly, the current analysis only evaluated the impact of oesophageal cooling on acute efficacy and safety of AF ablation, while there were no data regarding the long-term recurrence risk of AF after oesophageal cooling.
AEF from RF ablation is a rare, but a feared complication as it can be fatal in up to 80% of cases9–11,1,5. Although the mechanism of AEF formation is not well understood, there is general agreement that thermal injury is a precursor.9 Thermal injury alters the microvasculature of oesophageal tissue leading to ischaemic necrosis of the mucosal layers.27,28 Importantly, higher-grade thermal injury has a higher risk of progression to AEF.9
Few RCTs and multiple observational studies have evaluated the role of oesophageal cooling in AF catheter ablation and shown variable results.20–23,29–32 Leung et al. demonstrated that oesophageal cooling significantly reduces oesophageal thermal injury,21 while Kuwahara et al. and Tschabrunn et al. showed that oesophageal cooling did not reduce the incidence of oesophageal injury; however, it could reduce the severity.20,23 In contrast, Oliveira et al. showed a higher incidence of oesophageal injuries in oesophageal cooling relative to control22, which might have been attributed to the higher RF energy needed in the cooling group. Prior meta-analyses were conducted to evaluate the role of oesophageal cooling on the incidence of oesophageal injury, and their results suggested no significant difference on the incidence of oesophageal injury.33,34 However, these meta-analyses were mainly comprehensive of observational studies and only one RCT. The current meta-analysis of RCTs demonstrated no significant difference on the incidence of any oesophageal injury. Our study involved a variety of methods used in oesophageal cooling including ice water cooling, cold saline solution, or ensoETM device. Exploratory analysis did not reveal an interaction between the study results and the cooling method used. Importantly, we analysed the different grades of oesophageal injuries in the study groups, to provide deeper insight into the potential degree of oesophageal protection with cooling methods. Severe oesophageal injuries are strongly correlated with the development of AEF and carry significant morbidity and mortality.9–11,1,5 However, our analysis was limited by the lack of homogenous definition for the severity of oesophageal injuries among the included studies. In 2 of the 3 studies reporting the severity of oesophageal injuries,21,23 severe injuries were defined mainly by the presence of ulcerous lesions, while in the last study, severe injuries were qualitatively defined by the colour and extent of the injury.20 Furthermore, exploratory analysis excluding the study with the highest contribution to heterogeneity showed no significant difference in the risk of severe oesophageal injury among both groups. Our analysis suggests that oesophageal cooling does not reduce the incidence of oesophageal injuries but may shift the severity of injuries to less severe (i.e. non-ulcerous) injuries.
Several ablation techniques and tools could impact the degree of thermal injury with AF ablation procedures. For example, the use of contact force technology can provide real-time feedback for catheter tissue contact and, hence, could contribute to better safety and effectiveness of ablation.35 Also, the use of techniques such as high-power/short-duration ablation can achieve superior ablation lesions, without increasing the risk of thermal injury.36,37 Our analysis was limited by the variation in ablation techniques among the included studies, and further clinical trials are warranted to evaluate the role of oesophageal cooling among various ablation techniques. In addition, there was variability in the oesophageal temperature probes among the included studies (i.e. single sensor vs. multi-sensor probes). Prior studies suggested a superior thermodynamic profile with multi-sensor vs. single-sensor probes; however, differences in clinical thermal injuries according to the type of temperature probe are yet to be proven.11 Relevantly, our exploratory analysis including studies using single-sensor temperature probes only showed consistent results, similar to the primary analysis.
The suggested reduced risk of severe oesophageal injury with cooling methods could be multifactorial. Data suggest that cooling could have a protective effect leading to reduction in lesion thickness.38 Burn studies suggest that there is a relationship between cooling and thermal injury, as cooling after thermal insults can prevent the progression of thermal injury.39 Relevantly, although oesophageal cooling might reduce the severity of thermal injuries, it did not alter the ablation process in the acute outcomes, as it did not show any significant difference in the procedure duration, posterior wall duration, total RF time, acute reconnection incidence, or ablation index.40,41
This meta-analysis includes the totality of randomized data evaluating the role of oesophageal cooling in AF catheter ablation. Our findings demonstrated that the use of oesophageal cooling in patients undergoing AF catheter ablation had no effect on the incidence of any oesophageal injury. Oesophageal cooling might shift the severity of oesophageal injuries to less severe, non-ulcerous injuries. Yet, given the small sample size of available trials, larger randomized trials are still warranted to further characterize the role of oesophageal cooling in the prevention of oesophageal injuries in patients undergoing AF catheter ablation. Furthermore, standardized definitions for the severity of oesophageal injuries should be adopted in future trials.
Limitations
This meta-analysis had some limitations. First, the few numbers of available RCTs in the study topic have still limited the power of our analysis. Based on the observed rates of oesophageal lesions in both groups, a sample size of 2800 patients would be needed to achieve a study power of 0.80 to answer the hypothesis. Second, some of the study outcomes had a considerable degree of heterogeneity. Moreover, we have employed a random effects model in our analysis to mitigate the between-study heterogeneity. Furthermore, we performed a secondary analysis to explore the sources of heterogeneity in the primary study outcome, and similar results were obtained after excluding the study with the highest contribution to heterogeneity of the primary outcome. Third, there were variabilities among the included studies regarding the oesophageal cooling methods, temperature monitoring methods, the timing of oesophageal endoscopic assessment, and the ablation strategies and tools. To further explore such variabilities, we aimed to perform several exploratory sensitivity analyses for the primary study outcome and demonstrated consistent study results. Fourth, our analysis only evaluated the efficacy of ablation in the acute outcomes with the lack of long-term follow-up regarding arrythmia free data. Fifth, the lack of patient-level data precluded more granular analyses.
Conclusion
In this meta-analysis of randomized trials, oesophageal cooling in patients undergoing AF ablation did not reduce the incidence of any oesophageal thermal injury, compared with the control. Oesophageal cooling might shift the severity of oesophageal injuries to less severe and non-ulcerous injuries. There were no significant differences between the two groups in the acute outcomes regarding posterior wall RF time, total procedure duration, total RF time, acute reconnection incidence, and ablation index. Further larger randomized trials are needed to better characterize the short- and long-term effects of oesophageal cooling among patients undergoing AF catheter ablation.
Supplementary Material
Contributor Information
Mohamed Hamed, Department of Internal Medicine, Florida Atlantic University, 777 Glades Road BC-71, Boca Raton, FL 33431, USA.
Sheref A Elseidy, Department of Internal Medicine, Rochester General Hospital, 1425 Portland Ave, Rochester, NY 14621, USA.
Mohamed Abdelazeem, Department of Internal Medicine, St. Elizabeth’s Medical Center, 736 Cambridge St, Brighton, MA 02135, USA.
Ramez Morcos, Division of Cardiology, Florida Atlantic University, 777 Glades Road BC-71, Boca Raton, FL 33431, USA.
Ahmed Abdallah, Division of Cardiology, East Tennessee State University, 1276 Gilbreath Dr, Johnson City, TN 37614, USA.
Yasser Sammour, Division of Cardiology, Houston Methodist Hospital, 6565 Fannin St, Houston, TX 77030, USA.
Amr F Barakat, UPMC Heart and Vascular Institute, University of Pittsburgh, 3550 Terrace St, Pittsburgh, PA 15213, USA.
Wissam Khalife, Division of Cardiology, University of Texas Medical Branch, 1302 Mechanic St, Galveston, TX 77550, USA.
Vijay Ramu, Division of Cardiology, East Tennessee State University, 1276 Gilbreath Dr, Johnson City, TN 37614, USA.
Mamas A Mamas, Keele Cardiovascular Research Group, Keele University, Keele, Newcastle ST5 5BG, UK.
Ayman Elbadawi, Division of Cardiology, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd, Dallas, TX 75390, USA.
Supplementary material
Supplementary material is available at Europace online.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Author contributions
All authors contributed to the study conception and design, material preparation, data collection, statistical analysis, writing the article, critical revision of the article, and final approval of the article.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data availability
The data underlying this article are available in the article and in its online Supplementary material.
References
- 1. Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WPet al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation 2006;114:119–25. [DOI] [PubMed] [Google Scholar]
- 2. Essien UR, Kornej J, Johnson AE, Schulson LB, Benjamin EJ, Magnani JW. Social determinants of atrial fibrillation. Nat Rev Cardiol 2021;18:763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corrigendum to: 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS). Eur Heart J 2021;42:546–7. [DOI] [PubMed] [Google Scholar]
- 4. Pappone C, Vicedomini G, Augello G, Manguso F, Saviano M, Baldi Met al. Radiofrequency catheter ablation and antiarrhythmic drug therapy: a prospective, randomized, 4-year follow-up trial: the APAF study. Circ Arrhythm Electrophysiol 2011;4:808–14. [DOI] [PubMed] [Google Scholar]
- 5. Nielsen J, Kragholm KH, Christensen SB, Johannessen A, Torp-Pedersen C, Kristiansen SBet al. Periprocedural complications and one-year outcomes after catheter ablation for treatment of atrial fibrillation in elderly patients: a nationwide Danish cohort study. J Geriatr Cardiol 2021;18:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2018;20:e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pappone C, Oral H, Santinelli V, Vicedomini G, Lang CC, Manguso Fet al. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation 2004;109:2724–6. [DOI] [PubMed] [Google Scholar]
- 8. Dagres N, Anastasiou-Nana M. Prevention of atrial-esophageal fistula after catheter ablation of atrial fibrillation. Curr Opin Cardiol 2011;26:1–5. [DOI] [PubMed] [Google Scholar]
- 9. Halbfass P, Pavlov B, Muller P, Nentwich K, Sonne K, Barth Set al. Progression from esophageal thermal asymptomatic lesion to perforation complicating atrial fibrillation ablation: a single-center registry. Circ Arrhythm Electrophysiol 2017;10. [DOI] [PubMed] [Google Scholar]
- 10. Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation 2017;136:1247–55. [DOI] [PubMed] [Google Scholar]
- 11. Tschabrunn CM, Silverstein J, Berzin T, Ellis E, Buxton AE, Josephson MEet al. Comparison between single- and multi-sensor oesophageal temperature probes during atrial fibrillation ablation: thermodynamic characteristics. Europace 2015;17:891–7. [DOI] [PubMed] [Google Scholar]
- 12. Martinek M, Meyer C, Hassanein S, Aichinger J, Bencsik G, Schoefl Ret al. Identification of a high-risk population for esophageal injury during radiofrequency catheter ablation of atrial fibrillation: procedural and anatomical considerations. Heart Rhythm 2010;7:1224–30. [DOI] [PubMed] [Google Scholar]
- 13. Di Biase L, Saenz LC, Burkhardt DJ, Vacca M, Elayi CS, Barrett CDet al. Esophageal capsule endoscopy after radiofrequency catheter ablation for atrial fibrillation: documented higher risk of luminal esophageal damage with general anesthesia as compared with conscious sedation. Circ Arrhythm Electrophysiol 2009;2:108–12. [DOI] [PubMed] [Google Scholar]
- 14. Marrouche NF, Guenther J, Segerson NM, Daccarett M, Rittger H, Marschang Het al. Randomized comparison between open irrigation technology and intracardiac-echo-guided energy delivery for pulmonary vein antrum isolation: procedural parameters, outcomes, and the effect on esophageal injury. J Cardiovasc Electrophysiol 2007;18:583–8. [DOI] [PubMed] [Google Scholar]
- 15. Pappone C, Vicedomini G, Santinelli V. Atrio-esophageal fistula after AF ablation: pathophysiology, prevention &treatment. J Atr Fibrillation 2013;6:860–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muller P, Dietrich JW, Halbfass P, Abouarab A, Fochler F, Szollosi Aet al. Higher incidence of esophageal lesions after ablation of atrial fibrillation related to the use of esophageal temperature probes. Heart Rhythm 2015;12:1464–9. [DOI] [PubMed] [Google Scholar]
- 17. Oral H, Siontis KC. Prevention of atrioesophageal fistula after catheter ablation: if the esophagus cannot stand the heat (cold), can it be moved to the sidelines? JACC Clin Electrophysiol 2017;3:1155–7. [DOI] [PubMed] [Google Scholar]
- 18. Palaniswamy C, Koruth JS, Mittnacht AJ, Miller MA, Choudry S, Bhardwaj Ret al. The extent of mechanical esophageal deviation to avoid esophageal heating during catheter ablation of atrial fibrillation. JACC Clin Electrophysiol 2017;3:1146–54. [DOI] [PubMed] [Google Scholar]
- 19. Tran VN, Kusa S, Smietana J, Tsai WC, Bhasin K, Teh Aet al. The relationship between oesophageal heating during left atrial posterior wall ablation and the durability of pulmonary vein isolation. Europace 2017;19:1664–9. [DOI] [PubMed] [Google Scholar]
- 20. Kuwahara T, Takahashi A, Okubo K, Takagi K, Yamao K, Nakashima Eet al. Oesophageal cooling with ice water does not reduce the incidence of oesophageal lesions complicating catheter ablation of atrial fibrillation: randomized controlled study. Europace 2014;16:834–9. [DOI] [PubMed] [Google Scholar]
- 21. Leung LWM, Bajpai A, Zuberi Z, Li A, Norman M, Kaba RAet al. Randomized comparison of oesophageal protection with a temperature control device: results of the IMPACT study. Europace 2021;23:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. de Oliveira BD, Oyama H, Hardy CA, de Melo SL, Pisani CF, Chokr MOet al. Comparative study of strategies to prevent esophageal and periesophageal injury during atrial fibrillation ablation. J Cardiovasc Electrophysiol 2020;31:924–33. [DOI] [PubMed] [Google Scholar]
- 23. Tschabrunn CM, Attalla S, Salas J, Frankel DS, Hyman MC, Simon Eet al. Active esophageal cooling for the prevention of thermal injury during atrial fibrillation ablation: a randomized controlled pilot study. J Interv Card Electrophysiol 2022;63:197–205. [DOI] [PubMed] [Google Scholar]
- 24. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman ADet al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Halm U, Gaspar T, Zachaus M, Sack S, Arya A, Piorkowski Cet al. Thermal esophageal lesions after radiofrequency catheter ablation of left atrial arrhythmias. Am J Gastroenterol 2010;105:551–6. [DOI] [PubMed] [Google Scholar]
- 28. Martinek M, Bencsik G, Aichinger J, Hassanein S, Schoefl R, Kuchinka Pet al. Esophageal damage during radiofrequency ablation of atrial fibrillation: impact of energy settings, lesion sets, and esophageal visualization. J Cardiovasc Electrophysiol 2009;20:726–33. [DOI] [PubMed] [Google Scholar]
- 29. Tsuchiya T, Ashikaga K, Nakagawa S, Hayashida K, Kugimiya H. Atrial fibrillation ablation with esophageal cooling with a cooled water-irrigated intraesophageal balloon: a pilot study. J Cardiovasc Electrophysiol 2007;18:145–50. [DOI] [PubMed] [Google Scholar]
- 30. John J, Garg L, Orosey M, Desai T, Haines DE, Wong WS. The effect of esophageal cooling on esophageal injury during radiofrequency catheter ablation of atrial fibrillation. J Interv Card Electrophysiol 2020;58:43–50. [DOI] [PubMed] [Google Scholar]
- 31. Scanavacca M, Pisani C, Neto S, Tamaki W, Santo sR GC, Oyama Het al. Cooled intra-esophageal balloon to prevent thermal injury of esophageal wall during radiofrequency ablation. Eur Soc Cardiol Cong 2007. 1–5 September: September 1–5 2007; Vienna, Austria. 2007. [Google Scholar]
- 32. Sohara H, Satake S, Takeda H, Yamaguchi Y, Nagasu N. Prevalence of esophageal ulceration after atrial fibrillation ablation with the hot balloon ablation catheter: what is the value of esophageal cooling? J Cardiovasc Electrophysiol 2014;25:686–92. [DOI] [PubMed] [Google Scholar]
- 33. Leung LW, Gallagher MM, Santangeli P, Tschabrunn C, Guerra JM, Campos Bet al. Esophageal cooling for protection during left atrial ablation: a systematic review and meta-analysis. J Interv Card Electrophysiol 2020;59:347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ha FJ, Han HC, Sanders P, Teh AW, O'Donnell D, Farouque Oet al. Prevalence and prevention of oesophageal injury during atrial fibrillation ablation: a systematic review and meta-analysis. Europace 2019;21:80–90. [DOI] [PubMed] [Google Scholar]
- 35. Geczy T, Ramdat Misier NL, Szili-Torok T. Contact-force-sensing-based radiofrequency catheter ablation in paroxysmal supraventricular tachycardias (COBRA-PATH): a randomized controlled trial. Trials 2020;21:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kotadia ID, Williams SE, O'Neill M. High-power, short-duration radiofrequency ablation for the treatment of AF. Arrhythm Electrophysiol Rev 2020;8:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halbfass P, Wielandts J-Y, Knecht S, Le Polain de Waroux J-B, Tavernier R, De Wilde Vet al. Safety of very high-power short-duration radiofrequency ablation for pulmonary vein isolation: a two-centre report with emphasis on silent oesophageal injury. EP Europace 2021;24:400–5. [DOI] [PubMed] [Google Scholar]
- 38. Montoya MM, Mickelsen S, Clark B, Arnold M, Hanks J, Sauter Eet al. Protecting the esophagus from thermal injury during radiofrequency ablation with an esophageal cooling device. J Atr Fibrillation 2019;11:2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Griffin BR, Frear CC, Babl F, Oakley E, Kimble RM. Cool running water first aid decreases skin grafting requirements in pediatric burns: a cohort study of two thousand four hundred ninety-five children. Ann Emerg Med 2020;75:75–85. [DOI] [PubMed] [Google Scholar]
- 40. Gianni C, Atoui M, Mohanty S, Trivedi C, Bai R, Al-Ahmad Aet al. Difference in thermodynamics between two types of esophageal temperature probes: insights from an experimental study. Heart Rhythm 2016;13:2195–200. [DOI] [PubMed] [Google Scholar]
- 41. Koranne K, Basu-Ray I, Parikh V, Pollet M, Wang S, Mathuria Net al. Esophageal temperature monitoring during radiofrequency ablation of atrial fibrillation: a meta-analysis. J Atr Fibrillation 2016;9:1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online Supplementary material.