Abstract
Aims
Limited data compared antiarrhythmic drugs (AADs) with concomitant non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients, hence the aim of the study.
Methods and results
National health insurance database were retrieved during 2012–17 for study. We excluded patients not taking AADs, bradycardia, heart block, heart failure admission, mitral stenosis, prosthetic valve, incomplete demographic data, and follow-up <3 months. Outcomes were compared in Protocol 1, dronedarone vs. non-dronedarone; Protocol 2, dronedarone vs. amiodarone; and Protocol 3, dronedarone vs. propafenone. Outcomes were acute myocardial infarction (AMI), ischaemic stroke/systemic embolism, intracranial haemorrhage (ICH), major bleeding, cardiovascular death, all-cause mortality, and major adverse cardiovascular event (MACE) (including AMI, ischaemic stroke, and cardiovascular death). In Protocol 1, 2298 dronedarone users and 6984 non-dronedarone users (amiodarone = 4844; propafenone = 1914; flecainide = 75; sotalol = 61) were analysed. Dronedarone was associated with lower ICH (HR = 0.61, 95% CI = 0.38–0.99, P = 0.0436), cardiovascular death (HR = 0.24, 95% CI = 0.16–0.37, P < 0.0001), all-cause mortality (HR = 0.33, 95% CI = 0.27–0.42, P < 0.0001), and MACE (HR = 0.56, 95% CI = 0.45–0.70, P < 0.0001). In Protocol 2, 2231 dronedarone users and 6693 amiodarone users were analysed. Dronedarone was associated with significantly lower ICH (HR = 0.53, 95%=CI 0.33–0.84, P = 0.0078), cardiovascular death (HR = 0.20, 95% CI = 0.13–0.31, P < 0.0001), all-cause mortality (HR 0.27, 95% CI 0.22–0.34, P < 0.0001), and MACE (HR = 0.53, 95% CI = 0.43–0.66, P < 0.0001), compared with amiodarone. In Protocol 3, 812 dronedarone users and 2436 propafenone users were analysed. There were no differences between two drugs for primary and secondary outcomes.
Conclusion
The use of dronedarone with NOACs was associated with cardiovascular benefits in an Asian population, compared with non-dronedarone AADs and amiodarone.
Keywords: Dronedarone, Amiodarone, Propafenone, Atrial fibrillation, Non-vitamin K antagonist oral anticoagulants
Graphical Abstract
Graphical Abstract.
Comparison of primary study outcomes of acute myocardial infarction, ischaemic stroke/systemic embolism, intracranial haemorrhage, major bleeding, cardiovascular death, and all-cause mortality in patients with atrial fibrillation using dronedarone vs. non-dronedarone antiarrhythmic drugs.
What’s new?
In this retrospective Asian nationwide cohort study, when concomitantly used with non-vitamin K antagonist oral anticoagulants (NOACs), dronedarone was associated with reduced risks in intracranial haemorrhage, cardiovascular death, all-cause mortality, and major adverse cardiovascular events, compared to non-dronedarone anti-arrhythmic drugs (AADs) as a group or directly to amiodarone.
Dabigatran, rivaroxaban, or edoxaban were used at lower doses, while apixaban was used at standard dose in patients with atrial fibrillation concomitantly taking AADs in real-world setting.
NOAC doses were similar between groups and should not be factors that affected the outcomes.
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia with a high disease burden for subsequent ischaemic stroke, heart failure (HF), and mortality.1 Guideline-directed therapy for AF comprises antiarrhythmic drugs (AADs), anticoagulants and catheter ablation. Traditional AADs, such as amiodarone, have side effects that concern physicians and limit their widespread use. Dronedarone is a benzofuran derivative with pharmacological properties similar to amiodarone, but with reduced thyroid and other end-organ adverse effects.2 Dronedarone is therefore a welcome addition for cardiologists.
Dronedarone offers cardiovascular benefits for appropriately selected AF patients, as demonstrated in several trials.2 In the ATHENA trial, dronedarone reduced the primary composite outcome of mortality or first cardiovascular hospitalization in patients with paroxysmal or persistent AF.3 Notably, the difference in this composite outcome is driven by the first cardiovascular hospitalization. The efficacy of dronedarone in maintaining sinus rhythm was also demonstrated in the EURIDIS/ADONIS trials.4 However, there are also major safety concerns for selected AF patients. The ANDROMEDA or PALLAS trial raised safety concerns regarding the use of dronedarone in patients with decompensated HF or permanent AF, respectively.5,6 Therefore, dronedarone is indicated for AF patients in sinus rhythm with a history of paroxysmal or persistent AF and contraindicated in patients with permanent AF, New York Heart Association functional class IV HF or recent decompensation. The 2020 ESC guidelines reinforce this, in that dronedarone is not recommended in patients with NYHA class III or IV or unstable HF.1
The AF management requires a holistic and integrated approach, proposed as the Atrial fibrillation Better Care pathway. Stroke prevention is part of the holistic AF management through the use of anticoagulants.1,7 It is difficult to achieve the balance of thromboembolic events and bleeding among Asian patients, especially with the use of warfarin.8,9 Asians have a higher risk of intracranial haemorrhage even when the international normalized ratios (INR) are maintained between 2 and 3. However, a lower INR target range increases thromboembolism in Asian patients. Therefore, non-vitamin K antagonist oral anticoagulants (NOACs) are increasingly replacing warfarin for stroke prevention in AF due to better safety profile and similar or higher efficacy.10 Nonetheless, there is still a higher risk of intracranial bleeding in Asian patients than in non-Asian patients when treated with the same dose of NOAC.9 When managing AF in a holistic manner, the drug–drug interaction of AADs and NOACs may further affect the outcomes in Asian patients. However, there are limited data regarding the efficacy and safety profile of the concomitant use of AADs and NOACs, especially in real-world clinical practice and among the Asian population.
We aimed to compare the risks of cardiovascular outcomes and major bleeding among different AADs in Asian patients with paroxysmal or persistent AF concomitantly treated with NOACs.
Methods
Data source
The data of this study were obtained from the universal health insurance claims database provided by the National Health Insurance (NHI) Administration and managed by Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare of Taiwan. The Taiwan’s NHI Program started in 1995 and provides over 99.5% coverage for the 23 million residents. The NHI Research Database (NHIRD) is a claim-based administration dataset, which provides inpatient, outpatient, and emergency department services, diagnoses, prescriptions, examinations, operations, and expenditures. To protect the privacy of patients, all the identifier numbers are encrypted in the NHIRD’s claims database under HWDC, analyses must be conducted on-site (official website), and only the result reports were allowed to be taken out. The Institutional Review Board of Chang Gung Memorial Hospital Linkou Branch approved this study (No. 202000065B0C501).
Study patients and outcomes
By searching claims of medical records from the database between 1 June 2012 and 31 December 2017, we retrieved patients with a discharge diagnosis of AF, defined as having two outpatient diagnoses or one inpatient diagnosis in the previous year, and were undergoing anticoagulation therapy. We excluded patients who took AADs within 3 months before index date, did not take AADs during the study period, with bradycardia, heart block, history of admission for heart failure, mitral stenosis, prosthetic valve, incomplete demographic data, and follow-up less than 3 months (index date after 30 September 2017).
The primary outcomes were acute myocardial infarction (AMI), ischaemic stroke/systemic embolism (IS/SE), intracranial haemorrhage (ICH), major bleeding, cardiovascular death, and all-cause mortality. The secondary outcome was major adverse cardiovascular events (MACE), which was a composite of AMI, ischaemic stroke, and cardiovascular death. The definition of major bleeding was based on the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and ICD-10-CM diagnostic code (see Supplementary material online, Table S1). Comorbidities included hypertension, myocardial infarction, congestive HF, peripheral vascular disease, cerebrovascular disease, ischaemic stroke, diabetes mellitus, chronic pulmonary obstructive disease, moderate or severe liver disease, chronic kidney disease, anemia, rheumatic disease, malignancy, and history of bleeding. The comorbidity was defined as having at least two times of outpatient or inpatient diagnosis. Similarly, usage of medication was retrieved based on claims data within 3 months before the index date. Patients were followed from 1 June 2012 till 31 December 2017, with at least 3 months of follow-up period.
Protocols
In Protocol 1, we compared the primary and secondary outcomes of patients with AF who were treated with dronedarone vs. non-dronedarone AADs, which included amiodarone, propafenone, flecainide, and sotalol. In Protocol 2, we compared the primary and secondary outcomes of patients treated with dronedarone vs. amiodarone. In Protocol 3, we compared the primary and secondary outcomes of patients treated with dronedarone vs. propafenone.
Statistical analyses
Baseline characteristics between the groups were reported in mean ± standard deviation and number with percentage. Propensity score-matched (i) dronedarone vs. non-dronedarone; (ii) dronedarone vs. amiodarone; (iii) dronedarone vs. propafenone users were compared for study outcomes. Cox proportional hazards model was used to calculate the adjusted hazard ratio. Patients who switched AADs or NOACs during the treatment of atrial fibrillation were censored. Events which occurred within the 7 days after the switch were considered to be related to the drug before the switch.
In Protocol 1, each patient in the dronedarone group was matched with a counterpart in the non-dronedarone group. The covariates used to calculate the propensity score (the predicted probability to be dronedarone group derived from logistic regression) included demographics (sex and age), residence level (urban, suburban, and rural), occupation (five categories), income (five quintiles), comorbidities (16 of them, as mentioned earlier), risk scores (CHA2DS2-VASc score, HAS-BLED score, Charlson Comorbidity Index (CCI), and medications (30 in total). In Protocol 2, each patient in the dronedarone group was matched with a counterpart in the amiodarone group. In Protocol 3, each patient in the dronedarone group was matched with a counterpart in the propafenone group. The matching was processed using a greedy nearest neighbour algorithm with a caliper of 0.2.
The balance of covariates between the groups before and after propensity score matching was checked using the absolute value of standardized mean difference between the groups, where a value less than 0.1 was considered negligible difference and a value ranged from 0.1 to 0.2 was considered a small difference. Statistical significance was set at P < 0.05. All statistical operations were performed using the SAS® 9.4 version.
Results
The study population
From 1 June 2012 to 31 December 2017, 97 947 patients with AF undergoing anticoagulation therapy were retrieved. After exclusion based on pre-specified exclusion criteria, there remained 30 093 patients eligible for analysis. There were 2304 dronedarone users and 27 789 non-dronedarone AADs users (amiodarone, 20 530; propafenone, 6860; flecainide, 225; and sotalol, 174).
Protocol 1: dronedarone vs. non-dronedarone
After 1:3 matching, there were 2298 dronedarone users and 6984 non-dronedarone users (amiodarone, 4844; propafenone, 1914; flecainide, 75; and sotalol, 61) eligible for analysis (Figure 1). There were 49.96% male in dronedarone group and 48.84% in non-dronedarone group, with mean age 75.95 in dronedarone group and 75.96 in non-dronedarone group (Table 1). Mean CHA2DS2-VASc score was 4.20 in dronedarone group and 4.18 in non-dronedarone group. Mean HAS-BLED score was 3.01 in dronedarone group and 2.94 in non-dronedarone group. Mean CCI was 3.18 in dronedarone group and 3.18 in non-dronedarone group.
Figure 1.
Study design and flow chart for the enrollment of patients with AF using dronedarone vs. non-dronedarone antiarrhythmic drugs.
Table 1.
Summary of baseline characteristics of study patients: dronedarone vs. non-dronedarone
| Demographics | Before PSM | ASMD | After PSM (1:3) | ASMD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dronedarone | Non-dronedarone | Dronedarone | Non-dronedarone | |||||||
| (n = 2304) | (n = 27789) | (n = 2298) | (n = 6894) | |||||||
| n | % | n | % | n | % | n | % | |||
| Male | 1150 | 49.91% | 15111 | 54.38% | 0.0895 | 1148 | 49.96% | 3367 | 48.84% | 0.0223 |
| Age (mean ± SD) | 75.98 | 8.1 | 74.08 | 10.22 | 0.2066 | 75.95 | 8.09 | 75.96 | 9.57 | 0.0011 |
| Comorbidities | ||||||||||
| Hypertension | 1978 | 85.85% | 23618 | 84.99% | 0.0244 | 1972 | 85.81% | 5915 | 85.80% | 0.0004 |
| Myocardial infarction | 90 | 3.91% | 1405 | 5.06% | 0.0556 | 89 | 3.87% | 259 | 3.76% | 0.0061 |
| Congestive heart failure | 629 | 27.30% | 10519 | 37.85% | 0.2266 | 629 | 27.37% | 1923 | 27.89% | 0.0117 |
| Peripheral vascular disease | 204 | 8.85% | 2624 | 9.44% | 0.0204 | 204 | 8.88% | 639 | 9.27% | 0.0136 |
| Cerebrovascular disease | 859 | 37.28% | 12109 | 43.57% | 0.1285 | 856 | 37.25% | 2526 | 36.64% | 0.0126 |
| Ischaemic stroke | 523 | 22.70% | 8533 | 30.71% | 0.1817 | 522 | 22.72% | 1531 | 22.21% | 0.0122 |
| Transient ischaemic attack | 241 | 10.46% | 2779 | 10.00% | 0.0152 | 240 | 10.44% | 697 | 10.11% | 0.0110 |
| Diabetes mellitus | 811 | 35.20% | 10372 | 37.32% | 0.0442 | 805 | 35.03% | 2387 | 34.62% | 0.0085 |
| COPD | 780 | 33.85% | 9791 | 35.23% | 0.029 | 776 | 33.77% | 2387 | 34.62% | 0.0180 |
| Peptic ulcer disease | 1096 | 47.57% | 11937 | 42.96% | 0.0928 | 1092 | 47.52% | 3296 | 47.81% | 0.0058 |
| Chronic liver disease | 4 | 0.17% | 74 | 0.27% | 0.0198 | <5 | <0.22% | 17 | 0.25% | 0.0158 |
| Chronic kidney disease | 523 | 22.70% | 6448 | 23.20% | 0.012 | 521 | 22.67% | 1575 | 22.85% | 0.0042 |
| Anaemia | 309 | 13.41% | 3391 | 12.20% | 0.0362 | 306 | 13.32% | 930 | 13.49% | 0.0051 |
| Rheumatic disease | 131 | 5.69% | 1455 | 5.24% | 0.0198 | 129 | 5.61% | 401 | 5.82% | 0.0087 |
| Malignancy | 225 | 9.77% | 2713 | 9.76% | 0.0001 | 224 | 9.75% | 668 | 9.69% | 0.0020 |
| History of bleeding | 54 | 2.34% | 963 | 3.47% | 0.0668 | 54 | 2.35% | 169 | 2.45% | 0.0066 |
| Risk Scores | ||||||||||
| CHA2DS2-VASc score (mean ± SD) | 4.21 | 1.64 | 4.26 | 1.77 | 0.0333 | 4.20 | 1.64 | 4.18 | 1.68 | 0.0161 |
| HAS-BLED score (mean ± SD) | 3.01 | 1.1 | 2.98 | 1.17 | 0.0282 | 3.01 | 1.10 | 2.94 | 1.11 | 0.0609 |
| Charlson Comorbidity Index (mean ± SD) | 3.18 | 2.25 | 3.43 | 2.31 | 0.1112 | 3.18 | 2.25 | 3.18 | 2.22 | 0.0036 |
| Medications | ||||||||||
| Aspirin | 897 | 38.93% | 11546 | 41.55% | 0.0534 | 894 | 38.90% | 2742 | 39.77% | 0.0178 |
| Clopidogrel | 288 | 12.50% | 3269 | 11.76% | 0.0226 | 285 | 12.40% | 842 | 12.21% | 0.0057 |
| Ticlopidine | 68 | 2.95% | 724 | 2.61% | 0.0211 | 68 | 2.96% | 213 | 3.09% | 0.0076 |
| Ticagrelor | 14 | 0.61% | 411 | 1.48% | 0.0858 | 14 | 0.0061 | 44 | 0.64% | 0.0037 |
| Bisoprolol | 643 | 27.91% | 10350 | 37.24% | 0.2002 | 643 | 27.98% | 1922 | 27.88% | 0.0023 |
| Digoxin | 149 | 6.47% | 4417 | 15.89% | 0.3026 | 149 | 6.48% | 443 | 6.43% | 0.0024 |
| Diltiazem | 436 | 18.92% | 7260 | 26.13% | 0.1730 | 436 | 18.97% | 1323 | 19.19% | 0.0055 |
| Metoprolol | 29 | 1.26% | 434 | 1.56% | 0.0257 | 29 | 1.26% | 100 | 1.45% | 0.0163 |
| Labetalol | 40 | 1.74% | 1091 | 3.93% | 0.1323 | 40 | 1.74% | 137 | 1.99% | 0.0182 |
| Propranolol | 392 | 17.01% | 4845 | 17.43% | 0.0112 | 392 | 17.06% | 1190 | 17.26% | 0.0054 |
| Atorvastatin | 307 | 13.32% | 4165 | 14.99% | 0.0477 | 306 | 13.32% | 962 | 13.95% | 0.0186 |
| Fluvastatin | 50 | 2.17% | 511 | 1.84% | 0.0236 | 50 | 2.18% | 165 | 2.39% | 0.0146 |
| Pravastatin | 51 | 2.21% | 528 | 1.90% | 0.0221 | 50 | 2.18% | 153 | 2.22% | 0.0030 |
| Pitavastatin | 49 | 2.13% | 614 | 2.21% | 0.0057 | 49 | 2.13% | 135 | 1.96% | 0.0123 |
| Irbesartan | 156 | 6.77% | 1551 | 5.58% | 0.0494 | 154 | 6.70% | 457 | 6.63% | 0.0029 |
| Losartan | 223 | 9.68% | 2947 | 10.60% | 0.0307 | 223 | 9.70% | 704 | 10.21% | 0.0170 |
| Olmesartan | 140 | 6.08% | 2070 | 7.45% | 0.0547 | 140 | 6.09% | 430 | 6.24% | 0.0060 |
| NSAIDs | 529 | 22.96% | 7439 | 26.77% | 0.0882 | 528 | 22.98% | 1582 | 22.95% | 0.0007 |
| Warfarin | 420 | 18.23% | 4860 | 17.49% | 0.0193 | 419 | 18.23% | 1249 | 18.12% | 0.0030 |
| NOACs | 0.4073 | 0.0427 | ||||||||
| Dabigatran | 479 | 20.79% | 10906 | 39.25% | 479 | 20.84% | 1408 | 20.42% | ||
| Rivaroxaban | 1305 | 56.64% | 12518 | 45.05% | 1304 | 56.74% | 3937 | 57.11% | ||
| Apixaban | 345 | 14.97% | 3367 | 12.12% | 345 | 15.01% | 1058 | 15.35% | ||
| Edoxaban | 175 | 7.60% | 998 | 3.59% | 170 | 7.40% | 491 | 7.12% | ||
ASMD, absolute standardized mean difference; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; PSM, propensity score matching.
In terms of primary outcomes, the use of dronedarone was associated with significantly lower ICH [hazard ratio (HR) 0.61, 95% confidence interval (CI) 0.38–0.99, P = 0.0436], cardiovascular death (HR 0.24, 95% CI 0.16–0.37, P < 0.0001), and all-cause mortality (HR 0.33, 95% CI 0.27–0.42, P < 0.0001), when used concomitantly with NOACs (Table 2). There was no significant difference between the use of dronedarone and non-dronedarone AADs in AMI (P = 0.9104), IS/SE (P = 0.2324), and major bleeding (P = 0.2008). In terms of secondary outcome, the use of dronedarone was associated with significantly lower MACE (HR 0.56, 95% CI 0.45–0.70, P < 0.0001), when used concomitantly with NOACs (Table 2). Kaplan-Meier survival analyses also showed significant lower events with dronedarone in ICH (P = 0.0398), cardiovascular death (P < 0.0001), all-cause mortality (P < 0.0001), and MACE (P < 0.0001), compared to non-dronedarone AADs, when used concomitantly with NOACs (Graphical Abstract and Figure 2).
Table 2.
Study outcomes: dronedarone vs. non-dronedarone
| Before PSM | |||||
|---|---|---|---|---|---|
| No. of events/Person years | Incidence rate [95%CI] | No. of events/Person years | Incidence rate [95%CI] | Hazard ratio [95%CI]; P-value | |
| (per 100 person-years) | (per 100 person-years) | ||||
| Dronedarone | Non-dronedarone | Dronedarone vs. Non-dronedarone | |||
| Acute myocardial infarction | 14/3168.42 | 0.44 [0.21–0.67] | 184/42984.02 | 0.43 [0.37–0.49] | 1.01 [0.58–1.73]; P = 0.9849 |
| Ischaemic stroke/systemic embolism | 67/3115.76 | 2.15 [1.64–2.67] | 1257/41629 | 3.02 [2.85–3.19] | 0.69 [0.54–0.89]; P = 0.0036 |
| Intracranial haemorrhage | 20/3169.14 | 0.63 [0.35–0.91] | 433/42727.61 | 1.01 [0.92–1.11] | 0.62 [0.39–0.97]; P = 0.0349 |
| Major bleeding | 58/3132.02 | 1.85 [1.38–2.33] | 928/42212.72 | 2.20 [2.06–2.34] | 0.83 [0.63–1.08]; P = 0.1626 |
| Cardiovascular death | 24/3177.17 | 0.76 [0.45–1.06] | 1692/43142.91 | 3.92 [3.73–4.11] | 0.19 [0.12–0.28]; P < 0.0001 |
| All-cause mortality | 87/3177.17 | 2.74 [2.16–3.31] | 4523/43142.91 | 10.48 [10.18–10.79] | 0.25 [0.20–0.31]; P < 0.0001 |
| MACE | 98/3107.06 | 3.15 [2.53–3.78] | 2787/41643.73 | 6.69 [6.44–6.94] | 0.46 [0.37–0.56]; P < 0.0001 |
| After PSM | |||||
|---|---|---|---|---|---|
| No. of events/Person years | Incidence rate [95%CI] | No. of events/Person years | Incidence rate [95%CI] | Hazard ratio [95%CI]; P-value | |
| (per 100 person-years) | (per 100 person-years) | ||||
| Dronedarone | Non-dronedarone | Dronedarone vs. Non-dronedarone | |||
| Acute myocardial infarction | 14/3165 | 0.44 [0.21–0.67] | 43/10213.59 | 0.42 [0.3–0.55] | 1.04 [0.56–1.91]; P = 0.9104 |
| Ischaemic stroke/systemic embolism | 66/3112.5 | 2.12 [1.61–2.63] | 245/9971.67 | 2.46 [2.15–2.76] | 0.84 [0.64–1.11]; P = 0.2324 |
| Intracranial haemorrhage | 20/3165.72 | 0.63 [0.35–0.91] | 105/10163.68 | 1.03 [0.84–1.23] | 0.61 [0.38–0.99]; P = 0.0436 |
| Major bleeding | 58/3128.6 | 1.85 [1.38–2.33] | 224/10051.59 | 2.23 [1.94–2.52] | 0.83 [0.61–1.11]; P = 0.2008 |
| Cardiovascular death | 24/3173.75 | 0.76 [0.45–1.06] | 313/10249.24 | 3.05 [2.72–3.39] | 0.24 [0.16–0.37]; P < 0.0001 |
| All-cause mortality | 87/3173.75 | 2.74 [2.17–3.32] | 830/10249.24 | 8.10 [7.55–8.65] | 0.33 [0.27–0.42]; P < 0.0001 |
| MACE | 97/3103.8 | 3.13 [2.50–3.75] | 542/9965.11 | 5.44 [4.98–5.90] | 0.56 [0.45–0.70]; P < 0.0001 |
MACE, major adverse cardiovascular event, a composite of myocardial infarction, ischaemic stroke or cardiovascular death; PSM, propensity score matching.
Figure 2.
Comparison of secondary study outcome of major adverse cardiovascular event (MACE), which was a composite of acute myocardial infarction, ischaemic stroke, and cardiovascular death in patients with AF using dronedarone vs. non-dronedarone antiarrhythmic drugs.
The mean daily doses of AADs at index date were dronedarone 736.38 ± 146.69 mg, amiodarone 256.32 ± 114.67 mg, propafenone 326.31 ± 93.50 mg, flecainide 184.58 ± 43.83 mg, and sotalol 280.68 ± 88.35 mg (Table 3). When used concomitantly with dronedarone, the mean daily dose of NOACs were dabigatran 204.67 ± 50.49 mg, rivaroxaban 13.45 ± 3.66 mg, apixaban 9.59 ± 1.38 mg, and edoxaban 44.17 ± 15.02 mg. When used concomitantly with non-dronedarone AADs, the mean daily doses of NOACs after matching were dabigatran 214.37 ± 41.19 mg, rivaroxaban 13.17 ± 3.54 mg, apixaban 9.76 ± 1.08 mg, and edoxaban 47.84 ± 14.93 mg (Table 3). Therefore, the NOACs doses were similar between groups. Switching of medications and adjustment of medication dose are shown in Supplementary material online, Tables S2 and S3, respectively. Rates of switching AAD were on the order of sotalol (63.93%) > dronedarone (39.25%) > flecainide (30.67%) > propafenone (32.45%) > amiodarone (13.81%). Rates of adjustment of AAD dose were on the order of amiodarone (26.71%) > propafenone (20.98%) > sotalol (15.25%) > dronedarone (9.15%) > flecainide (7.58%).
Table 3.
Mean daily AAD and NOAC dose at index and during follow-up: dronedarone vs. Non-Dronedarone
| Before PSM | After PSM (1:3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Index, mean ± SD |
Follow-up, mean ± SD |
Index, mean ± SD |
Follow-up, mean ± SD |
||||||||
| Mean Daily AAD Dose | Mean Daily AAD Dose | ||||||||||
| Dronedarone | Dronedarone | 736.2 | ±146.86 | 733.09 | ±140.69 | Dronedarone | Dronedarone | 736.38 | ±146.69 | 733.27 | ±140.5 |
| Non-dronedarone | Amiodarone | 260.04 | ±118.02 | 240.98 | ±90.4 | Non-dronedarone | Amiodarone | 256.32 | ±114.67 | 235.39 | ±84.91 |
| Propafenone | 324.53 | ±91.86 | 320.5 | ±85.71 | Propafenone | 326.31 | ±93.50 | 322.2 | ±88.54 | ||
| Flecainide | 192.93 | ±37.03 | 190.85 | ±37.83 | Flecainide | 184.58 | ±43.83 | 183.21 | ±45.39 | ||
| Sotalol | 286.39 | ±78.84 | 281.49 | ±79.22 | Sotalol | 280.68 | ±88.35 | 273.07 | ±90.92 | ||
| Mean Daily NOAC Dose | Mean Daily NOAC Dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dronedarone | Dabigatran | 204.67 | ±50.49 | 205.15 | ±47.97 | Dronedarone | Dabigatran | 204.67 | ±50.49 | 205.15 | ±47.97 |
| Rivaroxaban | 13.45 | ±3.66 | 13.14 | ±3.25 | Rivaroxaban | 13.45 | ±3.66 | 13.15 | ±3.25 | ||
| Apixaban | 9.59 | ±1.38 | 9.62 | ±1.23 | Apixaban | 9.59 | ±1.38 | 9.62 | ±1.23 | ||
| Edoxaban | 44.11 | ±15.02 | 43.69 | ±14.83 | Edoxaban | 44.17 | ±15.02 | 43.74 | ±14.84 | ||
| Non-dronedarone | Dabigatran | 217.4 | ±42.02 | 216.69 | ±39.12 | Non-dronedarone | Dabigatran | 214.37 | ±41.19 | 212.93 | ±39.2 |
| Rivaroxaban | 13.97 | ±3.68 | 13.7 | ±3.16 | Rivaroxaban | 13.71 | ±3.54 | 13.46 | ±3.12 | ||
| Apixaban | 9.78 | ±1.03 | 9.78 | ±0.93 | Apixaban | 9.76 | ±1.08 | 9.75 | ±0.98 | ||
| Edoxaban | 47.51 | ±14.94 | 47.15 | ±14.69 | Edoxaban | 47.84 | ±14.93 | 47.3 | ±14.59 | ||
AAD, anti-arrhythmic drug; NOAC, non-vitamin K antagonist oral anticoagulant; PSM, propensity-score matching.
Protocol 2: dronedarone vs. amiodarone
After 1:3 matching, there were 2231 dronedarone users and 6693 amiodarone users eligible for analysis (Figure 3). There were 50.34% male in dronedarone group and 50.52% in amiodarone group, with mean age 75.88 in dronedarone group and 75.74 in amiodarone group (Table 4). Mean CHA2DS2-VASc score was 4.21 in dronedarone group and 4.17 in amiodarone group. Mean HAS-BLED score was 3.01 in dronedarone group and 2.94 in amiodarone group. Mean CCI was 3.20 in dronedarone group and 3.17 in amiodarone group.
Figure 3.
Study design and flow chart for the enrollment of patients with AF using dronedarone vs. amiodarone.
Table 4.
Summary of baseline characteristics of study patients: dronedarone vs. amiodarone
| Demographics | Before PSM | After PSM (1:3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dronedarone | Amiodarone | ASMD | Dronedarone | Amiodarone | ASMD | |||||
| (n = 2304) | (n = 20530) | (n = 2231) | (n = 6693) | |||||||
| n | % | n | % | n | % | n | % | |||
| Male | 1150 | 49.91% | 11326 | 55.17% | 0.1054 | 1123 | 50.34% | 3381 | 50.52% | 0.0036 |
| Age (mean ± SD) | 75.98 | 8.1 | 74.63 | 10.27 | 0.1463 | 75.88 | 8.07 | 75.74 | 9.56 | 0.0150 |
| Comorbidities | ||||||||||
| Hypertension | 1978 | 85.85% | 17626 | 85.85% | 0.0001 | 1910 | 85.61% | 5736 | 85.70% | 0.0026 |
| Myocardial infarction | 90 | 3.91% | 1253 | 6.10% | 0.1009 | 88 | 3.94% | 273 | 4.08% | 0.0069 |
| Congestive heart failure | 629 | 27.30% | 8586 | 41.82% | 0.3090 | 626 | 28.06% | 1886 | 28.18% | 0.0027 |
| Peripheral vascular disease | 204 | 8.85% | 2045 | 9.96% | 0.0379 | 202 | 9.05% | 607 | 9.07% | 0.0005 |
| Cerebrovascular disease | 859 | 37.28% | 9445 | 46.01% | 0.1776 | 843 | 37.79% | 2538 | 37.92% | 0.0028 |
| Ischaemic stroke | 523 | 22.70% | 6837 | 33.30% | 0.2378 | 519 | 23.26% | 1558 | 23.28% | 0.0004 |
| Transient ischaemic attack | 241 | 10.46% | 2042 | 9.95% | 0.0170 | 234 | 10.49% | 703 | 10.50% | 0.0005 |
| Diabetes mellitus | 811 | 35.20% | 7957 | 38.76% | 0.0738 | 786 | 35.23% | 2319 | 34.65% | 0.0122 |
| COPD | 780 | 33.85% | 7487 | 36.47% | 0.0548 | 760 | 34.07% | 2275 | 33.99% | 0.0016 |
| Peptic ulcer disease | 1096 | 47.57% | 8744 | 42.59% | 0.1002 | 1050 | 47.06% | 3137 | 46.87% | 0.0039 |
| Chronic liver disease | 4 | 0.0017 | 60 | 0.29% | 0.0246 | <5 | <0.22% | 13 | 0.19% | 0.0035 |
| Chronic kidney disease | 523 | 22.70% | 5132 | 25.00% | 0.0539 | 508 | 22.77% | 1511 | 22.58% | 0.0046 |
| Anaemia | 309 | 13.41% | 2619 | 12.76% | 0.0194 | 299 | 13.40% | 907 | 13.55% | 0.0044 |
| Rheumatic disease | 131 | 5.69% | 1046 | 5.09% | 0.0262 | 122 | 5.47% | 402 | 6.01% | 0.0231 |
| Malignancy | 225 | 9.77% | 2023 | 9.85% | 0.0030 | 221 | 9.91% | 652 | 9.74% | 0.0055 |
| History of bleeding | 54 | 2.34% | 826 | 4.02% | 0.0958 | 54 | 2.42% | 163 | 2.44% | 0.0010 |
| Risk Scores | ||||||||||
| CHA2DS2-VASc score (mean ± SD) | 4.21 | 1.64 | 4.42 | 1.78 | 0.1221 | 4.21 | 1.65 | 4.17 | 1.67 | 0.0260 |
| HAS-BLED score (mean ± SD) | 3.01 | 1.1 | 3.05 | 1.17 | 0.0326 | 3.01 | 1.11 | 2.94 | 1.11 | 0.0666 |
| Charlson Comorbidity Index (mean ± SD) | 3.18 | 2.25 | 3.60 | 2.33 | 0.1836 | 3.20 | 2.26 | 3.17 | 2.17 | 0.0137 |
| Medications | ||||||||||
| Aspirin | 897 | 38.93% | 8748 | 42.61% | 0.0749 | 869 | 38.95% | 2662 | 39.77% | 0.0168 |
| Clopidogrel | 288 | 12.50% | 2695 | 13.13% | 0.0188 | 276 | 12.37% | 807 | 12.06% | 0.0096 |
| Ticlopidine | 68 | 2.95% | 521 | 2.54% | 0.0253 | 63 | 2.82% | 190 | 2.84% | 0.0009 |
| Ticagrelor | 14 | 0.0061 | 375 | 1.83% | 0.1113 | 14 | 0.0063 | 36 | 0.54% | 0.0118 |
| Bisoprolol | 643 | 27.91% | 8014 | 39.04% | 0.2375 | 639 | 28.64% | 1905 | 28.46% | 0.0040 |
| Digoxin | 149 | 6.47% | 3774 | 18.38% | 0.3673 | 149 | 6.68% | 455 | 6.80% | 0.0048 |
| Diltiazem | 436 | 18.92% | 5805 | 28.28% | 0.2216 | 433 | 19.41% | 1306 | 19.51% | 0.0026 |
| Metoprolol | 29 | 1.26% | 312 | 1.52% | 0.0223 | 29 | 1.30% | 80 | 1.20% | 0.0094 |
| Labetalol | 40 | 1.74% | 956 | 4.66% | 0.1666 | 40 | 1.79% | 125 | 1.87% | 0.0056 |
| Propranolol | 392 | 17.01% | 3291 | 16.03% | 0.0265 | 380 | 17.03% | 1168 | 17.45% | 0.0111 |
| Atorvastatin | 307 | 13.32% | 3153 | 15.36% | 0.0580 | 299 | 13.40% | 873 | 13.04% | 0.0106 |
| Fluvastatin | 50 | 2.17% | 386 | 1.88% | 0.0206 | 47 | 2.11% | 134 | 2.00% | 0.0074 |
| Pravastatin | 51 | 2.21% | 374 | 1.82% | 0.0279 | 48 | 2.15% | 140 | 2.09% | 0.0041 |
| Pitavastatin | 49 | 2.13% | 464 | 2.26% | 0.0091 | 48 | 2.15% | 161 | 2.41% | 0.0170 |
| Irbesartan | 156 | 6.77% | 1092 | 5.32% | 0.0609 | 145 | 6.50% | 439 | 6.56% | 0.0024 |
| Losartan | 223 | 9.68% | 2194 | 10.69% | 0.0333 | 219 | 9.82% | 657 | 9.82% | 0.0000 |
| Olmesartan | 140 | 6.08% | 1585 | 7.72% | 0.0649 | 138 | 6.19% | 417 | 6.23% | 0.0019 |
| NSAIDs | 529 | 22.96% | 5677 | 27.65% | 0.1081 | 521 | 23.35% | 1601 | 23.92% | 0.0134 |
| Warfarin | 420 | 18.23% | 3651 | 17.78% | 0.0116 | 407 | 18.24% | 1186 | 17.72% | 0.0136 |
| NOACs | 0.4216 | 0.0478 | ||||||||
| Dabigatran | 479 | 20.79% | 7949 | 38.72% | 479 | 21.47% | 1451 | 21.68% | ||
| Rivaroxaban | 1305 | 56.64% | 9507 | 46.31% | 1283 | 57.51% | 3862 | 57.70% | ||
| Apixaban | 345 | 14.97% | 2386 | 11.62% | 337 | 15.11% | 974 | 14.55% | ||
| Edoxaban | 175 | 7.60% | 688 | 3.35% | 132 | 5.92% | 406 | 6.07% | ||
ASMD, absolute standardized mean difference; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; PSM, propensity score matching.
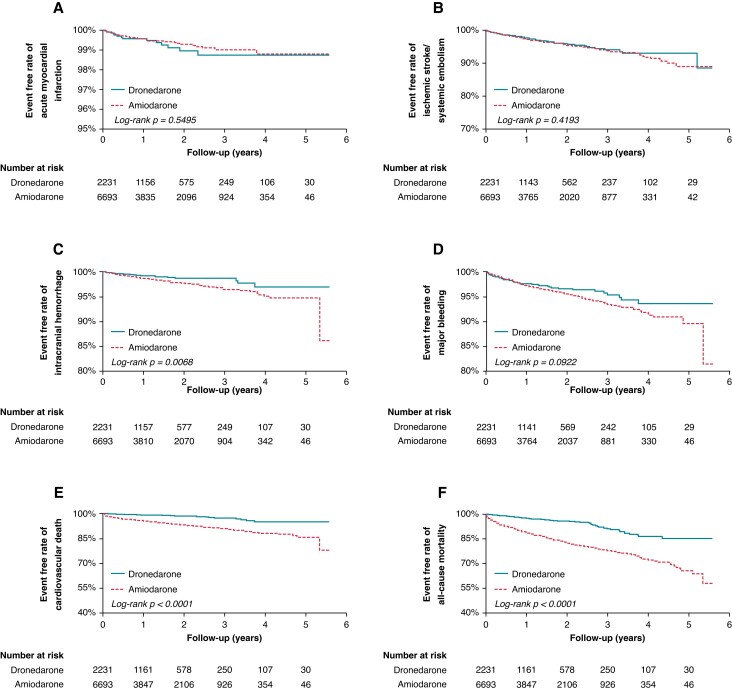
In terms of primary outcomes, the use of dronedarone was associated with significantly lower ICH (HR 0.53, 95% CI 0.33–0.84, P = 0.0078), cardiovascular death (HR 0.20, 95% CI 0.13–0.31, P < 0.0001), and all-cause mortality (HR 0.27, 95% CI 0.22–0.34, P < 0.0001), compared to the use of amiodarone, when used concomitantly with NOACs (Table 5). There was no significant difference between the use of dronedarone and amiodarone in AMI (P = 0.5552), IS/SE (P = 0.4200), and major bleeding (P = 0.0918). In terms of the secondary outcome, the use of dronedarone was associated with a significant reduction in MACE (HR 0.53, 95% CI 0.43–0.66, P < 0.0001), compared to amiodarone, when used concomitantly with NOACs (Table 5). Kaplan-Meier survival analyses showed significant lower events with dronedarone in ICH (P = 0.0068), cardiovascular death (P < 0.0001), all-cause mortality (P < 0.0001), and MACE (P < 0.0001), compared to amiodarone, when used concomitantly with NOACs (Figures 4 and 5).
Table 5.
Study outcomes: dronedarone vs. amiodarone
| Before PSM | |||||
|---|---|---|---|---|---|
| No. of events/Person years | Incidence rate [95%CI] | No. of events/Person years | Incidence rate [95%CI] | Hazard ratio [95%CI]; P-value | |
| (per 100 person-years) | (per 100 person-years) | ||||
| Dronedarone | Amiodarone | Dronedarone vs. Amiodarone | |||
| Acute myocardial infarction | 14/3168.42 | 0.44 [0.21–0.67] | 143/32218.6 | 0.44 [0.37–0.52] | 0.96 [0.56–1.67]; P = 0.8979 |
| Ischaemic stroke/systemic embolism | 67/3115.76 | 2.15 [1.64–2.67] | 1039/31078.76 | 3.34 [3.14–3.55] | 0.62 [0.49–0.8]; P = 0.0002 |
| Intracranial haemorrhage | 20/3169.14 | 0.63 [0.35–0.91] | 342/31993.54 | 1.07 [0.96–1.18] | 0.58 [0.37–0.92]; P = 0.0193 |
| Major bleeding | 58/3132.02 | 1.85 [1.38–2.33] | 739/31581.28 | 2.34 [2.17–2.51] | 0.78 [0.59–1.01]; P = 0.0630 |
| Cardiovascular death | 24/3177.17 | 0.76 [0.45–1.06] | 1603/32348.02 | 4.96 [4.71–5.20] | 0.15 [0.10–0.22]; P < 0.0001 |
| All-cause mortality | 87/3177.17 | 2.74 [2.16–3.31] | 4280/32348.02 | 13.23 [12.83–13.63] | 0.20 [0.16–0.24]; P < 0.0001 |
| MACE | 98/3107.06 | 3.15 [2.53–3.78] | 2470/31103.81 | 7.94 [7.63–8.25] | 0.38 [0.31–0.47]; P < 0.0001 |
| After PSM | |||||
|---|---|---|---|---|---|
| No. of events/Person years | Incidence rate [95%CI] | No. of events/Person years | Incidence rate [95%CI] | Hazard ratio [95%CI]; P-value | |
| (per 100 person-years) | (per 100 person-years) | ||||
| Dronedarone | Amiodarone | Dronedarone vs. Amiodarone | |||
| Acute myocardial infarction | 14/3096.92 | 0.45 [0.22–0.69] | 38/10329.84 | 0.37 [0.25–0.48] | 1.21 [0.65–2.24]; P = 0.5552 |
| Ischaemic stroke/systemic embolism | 65/3046.32 | 2.13 [1.62–2.65] | 237/10094.4 | 2.35 [2.05–2.65] | 0.89 [0.68–1.18]; P = 0.4200 |
| Intracranial haemorrhage | 20/3097.64 | 0.65 [0.36–0.93] | 124/10250.81 | 1.21 [1.00–1.42] | 0.53 [0.33–0.84]; P = 0.0078 |
| Major bleeding | 58/3060.52 | 1.90 [1.41–2.38] | 241/10121.91 | 2.38 [2.08–2.68] | 0.78 [0.59–1.04]; P = 0.0918 |
| Cardiovascular death | 23/3105.67 | 0.74 [0.44–1.04] | 370/10359.67 | 3.57 [3.21–3.94] | 0.20 [0.13–0.31]; P < 0.0001 |
| All-cause mortality | 85/3105.67 | 2.74 [2.16–3.32] | 1008/10359.67 | 9.73 [9.13–10.33] | 0.27 [0.22–0.34]; P < 0.0001 |
| MACE | 95/3037.62 | 3.13 [2.50–3.76] | 581/10085.53 | 5.76 [5.29–6.23] | 0.53 [0.43–0.66]; P < 0.0001 |
MACE, major adverse cardiovascular event, a composite of myocardial infarction, ischaemic stroke or cardiovascular death.
Figure 4.
Comparison of primary study outcomes of acute myocardial infarction, ischaemic stroke/systemic embolism, intracranial haemorrhage, major bleeding, cardiovascular death, and all-cause mortality in patients with AF using dronedarone vs. amiodarone.
Figure 5.
Comparison of secondary study outcome of major adverse cardiovascular event (MACE), which was a composite of acute myocardial infarction, ischaemic stroke, and cardiovascular death in patients with atrial fibrillation using dronedarone vs. amiodarone.
The mean daily doses of dronedarone at index date were 736.28 ± 146.79 mg and amiodarone 256.28 ± 83.6 mg (Table 6). When used concomitantly with dronedarone, the mean daily doses of NOACs were dabigatran 204.67 ± 50.49 mg, rivaroxaban 13.45 ± 3.66 mg, apixaban 9.59 ± 1.37 mg, and edoxaban 44.52 ± 15.05 mg. When used concomitantly with amiodarone, the mean daily doses of NOACs were dabigatran 217.04 ± 41.84 mg, rivaroxaban 13.75 ± 3.62 mg, apixaban 9.78 ± 1.02 mg, and edoxaban 47.54 ± 14.86 mg (Table 6). Switching of medications and adjustment of medication dose are shown in Supplementary material online, Tables S4 and S5, respectively. Rates of switching AAD was dronedarone (39.71%) > amiodarone (13.94%). Rates of adjustment of AAD dose were amiodarone (25.68%) > dronedarone (9.22%).
Table 6.
Mean daily AAD and NOAC dose at index and during follow-up: dronedarone vs. Amiodarone
| Before PSM | After PSM (1:3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Index, mean ± SD |
Follow-up, mean ± SD |
Index, mean ± SD |
Follow-up, mean ± SD |
||||||||
| Mean Daily AAD Dose | Mean Daily AAD Dose | ||||||||||
| Dronedarone | Dronedarone | 736.20 | ±146.86 | 733.09 | ±140.69 | Dronedarone | Dronedarone | 736.28 | ±146.79 | 732.79 | ±140.94 |
| Amiodarone | Amiodarone | 260.04 | ±118.02 | 240.98 | ±90.4 | Amiodarone | Amiodarone | 256.28 | ±112.96 | 236.43 | ±85.22 |
| Mean Daily NOAC Dose | Mean Daily NOAC Dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dronedarone | Dabigatran | 204.67 | ±50.49 | 205.15 | ±47.97 | Dronedarone | Dabigatran | 204.67 | ±50.49 | 205.15 | ±47.97 |
| Rivaroxaban | 13.45 | ±3.66 | 13.14 | ±3.25 | Rivaroxaban | 13.45 | ±3.66 | 13.15 | ±3.26 | ||
| Apixaban | 9.59 | ±1.38 | 9.62 | ±1.23 | Apixaban | 9.59 | ±1.37 | 9.63 | ±1.23 | ||
| Edoxaban | 44.11 | ±15.02 | 43.69 | ±14.83 | Edoxaban | 44.52 | ±15.05 | 44.06 | ±14.83 | ||
| Amiodarone | Dabigatran | 216.85 | ±43.20 | 215.88 | ±40.2 | Amiodarone | Dabigatran | 217.04 | ±41.84 | 215.42 | ±39.36 |
| Rivaroxaban | 13.99 | ±3.72 | 13.72 | ±3.17 | Rivaroxaban | 13.75 | ±3.62 | 13.52 | ±3.13 | ||
| Apixaban | 9.80 | ±0.99 | 9.8 | ±0.9 | Apixaban | 9.78 | ±1.02 | 9.81 | ±0.87 | ||
| Edoxaban | 47.63 | ±15.00 | 46.82 | ±14.65 | Edoxaban | 47.54 | ±14.86 | 46.85 | ±14.37 | ||
AAD, anti-arrhythmic drug; NOAC, non-vitamin K antagonist oral anticoagulant; PSM, propensity-score matching.
Protocol 3: dronedarone vs. propafenone
After 1:3 matching, there were 812 dronedarone users and 2436 propafenone users eligible for analysis (Figure 6). There were 52.34% male in dronedarone group and 53.04% in amiodarone group, with mean age 72.11 in dronedarone group and 72.11 in amiodarone group (Table 7). Mean CHA2DS2-VASc score was 3.77 in dronedarone group and 3.65 in amiodarone group. Mean HAS-BLED score was 2.79 in dronedarone group and 2.70 in amiodarone group. Mean CCI was 2.91 in dronedarone group and 2.08 in amiodarone group.
Figure 6.
Study design and flow chart for the enrollment of patients with AF using dronedarone vs. propafenone.
Table 7.
Baseline characteristics of study patients: dronedarone vs. propafenone
| Demographics | Before PSM | ASMD | After PSM (1:3) | ASMD | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dronedarone | Propafenone | Dronedarone | Propafenone | |||||||
| (n = 2304) | (n = 6860) | (n = 812) | (n = 2436) | |||||||
| n | % | n | % | n | % | n | % | |||
| Male | 1150 | 49.91% | 3573 | 52.08% | 0.0434 | 425 | 52.34% | 1292 | 53.04% | 0.0140 |
| Age (mean ± SD) | 75.98 | 8.1 | 72.6 | 9.91 | 0.3741 | 72.11 | 8.28 | 72.11 | 8.28 | 0.1144 |
| Comorbidities | ||||||||||
| Hypertension | 1978 | 85.85% | 5668 | 82.62% | 0.0886 | 652 | 80.30% | 1971 | 80.91% | 0.0156 |
| Myocardial infarction | 90 | 3.91% | 142 | 2.07% | 0.1080 | 7 | 0.86% | 35 | 1.44% | 0.0539 |
| Congestive heart failure | 629 | 27.30% | 1817 | 26.49% | 0.0183 | 232 | 28.57% | 638 | 26.19% | 0.0534 |
| Peripheral vascular disease | 204 | 8.85% | 551 | 8.03% | 0.0296 | 66 | 8.13% | 176 | 7.22% | 0.0339 |
| Cerebrovascular disease | 859 | 37.28% | 2526 | 36.82% | 0.0095 | 306 | 37.68% | 860 | 35.30% | 0.0495 |
| Ischaemic stroke | 523 | 22.70% | 1622 | 23.64% | 0.0224 | 197 | 24.26% | 562 | 23.07% | 0.0280 |
| Transient ischaemic attack | 241 | 10.46% | 690 | 10.06% | 0.0132 | 90 | 11.08% | 246 | 10.10% | 0.0320 |
| Diabetes mellitus | 811 | 35.20% | 2282 | 33.27% | 0.0408 | 257 | 31.65% | 819 | 33.62% | 0.0420 |
| COPD | 780 | 33.85% | 2182 | 31.81% | 0.0436 | 264 | 32.51% | 758 | 31.12% | 0.0300 |
| Peptic ulcer disease | 1096 | 47.57% | 3019 | 44.01% | 0.0715 | 356 | 43.84% | 1022 | 41.95% | 0.0382 |
| Moderate or severe liver disease | 4 | 0.17% | 14 | 0.20% | 0.0070 | <5 | <0.62% | 6 | 0.25% | 0.0000 |
| Chronic kidney disease | 523 | 22.70% | 1234 | 17.99% | 0.1172 | 129 | 15.89% | 381 | 15.64% | 0.0068 |
| Anaemia | 309 | 13.41% | 728 | 10.61% | 0.0862 | 80 | 9.85% | 198 | 8.13% | 0.0603 |
| Rheumatic disease | 131 | 5.69% | 384 | 5.60% | 0.0038 | 49 | 6.03% | 137 | 5.62% | 0.0175 |
| Malignancy | 225 | 9.77% | 647 | 9.43% | 0.0113 | 84 | 10.34% | 234 | 9.61% | 0.0247 |
| History of bleeding | 54 | 2.34% | 132 | 1.92% | 0.0290 | 11 | 1.35% | 36 | 1.48% | 0.0104 |
| Risk Scores | ||||||||||
| CHA2DS2-VASc score (mean ± SD) | 4.21 | 1.64 | 3.84 | 1.68 | 0.2206 | 3.77 | 1.67 | 3.65 | 1.69 | 0.0683 |
| HAS-BLED score (mean ± SD) | 3.01 | 1.1 | 2.80 | 1.12 | 0.1966 | 2.79 | 1.15 | 2.7 | 1.12 | 0.0821 |
| Charlson Comorbidity Index (mean ± SD) | 3.18 | 2.25 | 2.96 | 2.17 | 0.1006 | 2.91 | 2.16 | 2.86 | 2.08 | 0.0226 |
| Medications | ||||||||||
| Aspirin | 897 | 38.93% | 2689 | 39.20% | 0.0055 | 319 | 39.29% | 962 | 39.49% | 0.0042 |
| Clopidogrel | 288 | 12.50% | 537 | 7.83% | 0.1551 | 55 | 6.77% | 136 | 5.58% | 0.0495 |
| Ticlopidine | 68 | 2.95% | 187 | 2.73% | 0.0136 | 20 | 2.46% | 70 | 2.87% | 0.0255 |
| Ticagrelor | 14 | 0.61% | 33 | 0.48% | 0.0172 | <5 | 0.22% | 10 | 0.41% | 0.0066 |
| Bisoprolol | 643 | 27.91% | 2209 | 32.20% | 0.0937 | 281 | 34.61% | 868 | 35.63% | 0.0215 |
| Digoxin | 149 | 6.47% | 608 | 8.86% | 0.0902 | 94 | 11.58% | 244 | 10.02% | 0.0503 |
| Diltiazem | 436 | 18.92% | 1375 | 20.04% | 0.0283 | 189 | 23.28% | 486 | 19.95% | 0.0809 |
| Metoprolol | 29 | 1.26% | 114 | 1.66% | 0.0336 | 12 | 1.48% | 51 | 2.09% | 0.0465 |
| Labetalol | 40 | 1.74% | 130 | 1.90% | 0.0119 | 20 | 2.46% | 46 | 1.89% | 0.0394 |
| Propranolol | 392 | 17.01% | 1489 | 21.71% | 0.119 | 210 | 25.86% | 591 | 24.26% | 0.0369 |
| Atorvastatin | 307 | 13.32% | 948 | 13.82% | 0.0144 | 102 | 12.56% | 357 | 14.66% | 0.0611 |
| Fluvastatin | 50 | 2.17% | 120 | 1.75% | 0.0304 | 16 | 1.97% | 39 | 1.60% | 0.0279 |
| Pravastatin | 51 | 2.21% | 147 | 2.14% | 0.0048 | 15 | 1.85% | 54 | 2.22% | 0.0262 |
| Pitavastatin | 49 | 2.13% | 132 | 1.92% | 0.0144 | 11 | 1.35% | 46 | 1.89% | 0.0423 |
| Irbesartan | 156 | 6.77% | 432 | 6.30% | 0.0192 | 51 | 6.28% | 159 | 6.53% | 0.0101 |
| Losartan | 223 | 9.68% | 708 | 10.32% | 0.0214 | 92 | 11.33% | 246 | 10.10% | 0.0398 |
| Olmesartan | 140 | 6.08% | 456 | 6.65% | 0.0234 | 54 | 6.65% | 178 | 7.31% | 0.0258 |
| NSAIDs | 529 | 22.96% | 1662 | 24.23% | 0.0299 | 191 | 23.52% | 634 | 26.03% | 0.0580 |
| Warfarin | 420 | 18.23% | 1129 | 16.46% | 0.0468 | 122 | 15.02% | 399 | 16.38% | 0.0372 |
| NOACs | 0.4476 | 0.0417 | ||||||||
| Dabigatran | 479 | 20.79% | 2844 | 41.46% | 430 | 52.96% | 1251 | 51.35% | ||
| Rivaroxaban | 1305 | 56.64% | 2821 | 41.12% | 247 | 30.42% | 763 | 31.32% | ||
| Apixaban | 345 | 14.97% | 920 | 13.41% | 120 | 14.78% | 389 | 15.97% | ||
| Edoxaban | 175 | 7.60% | 275 | 4.01% | 15 | 1.85% | 33 | 1.35% | ||
ASMD, absolute standardized mean difference; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; NOAC, non-vitamin K antagonist oral anticoagulant; NSAID, non-steroidal anti-inflammatory drug; PSM, propensity score matching.
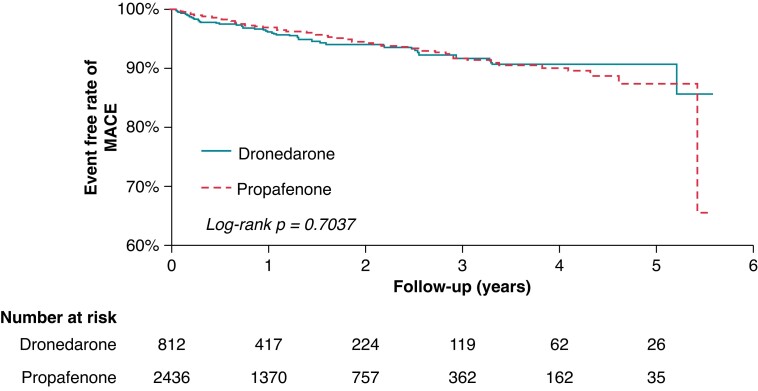
In terms of primary outcomes, there was no significant difference between the use of dronedarone and propafenone in AMI (P = 0.1135), IS/SE (P = 0.6402), ICH (P = 0.5521), major bleeding (P = 0.5645), cardiovascular death (P = 0.0908), and all-cause mortality (P = 0.6452), when used concomitantly with NOACs (Table 8). In terms of the secondary outcome, there was also no significant difference between the use of dronedarone and propafenone in MACE (P = 0.7068), when used concomitantly with NOACs (HR 0.38, 95% CI 0.31–0.47, P < 0.0001) (Table 8). Kaplan-Meier survival analyses showed no difference between the use of dronedarone and propafenone in both primary and secondary outcomes (Figures 7 and 8).
Table 8.
Study outcomes: dronedarone vs. propafenone
| Before PSM | |||||
|---|---|---|---|---|---|
| No. of events/Person years | Incidence rate [95%CI] | No. of events/Person years | Incidence rate [95%CI] | Hazard ratio [95%CI]; P-value | |
| (per 100 person-years) | (per 100 person-years) | ||||
| Dronedarone | Propafenone | Dronedarone vs. Propafenone | |||
| Acute myocardial infarction | 14/3168.42 | 0.44 [0.21–0.67] | 39/10236.06 | 0.38 [0.26–0.50] | 1.14 [0.62–2.11]; P = 0.6670 |
| Ischaemic stroke/systemic embolism | 67/3115.76 | 2.15 [1.64–2.67] | 210/10023.41 | 2.10 [1.81–2.38] | 1.00 [0.76–1.32]; P = 0.9950 |
| Intracranial haemorrhage | 20/3169.14 | 0.63 [0.35–0.91] | 86/10206.4 | 0.84 [0.66–1.02] | 0.75 [0.46–1.22]; P = 0.2419 |
| Major bleeding | 58/3132.02 | 1.85 [1.38–2.33] | 182/10107.67 | 1.80 [1.54–2.06] | 1.01 [0.75–1.36]; P = 0.9293 |
| Cardiovascular death | 24/3177.17 | 0.76 [0.45–1.06] | 82/10263.69 | 0.80 [0.63–0.97] | 0.95 [0.61–1.50]; P = 0.8400 |
| All-cause mortality | 87/3177.17 | 2.74 [2.16–3.31] | 231/10263.69 | 2.25 [1.96–2.54] | 1.23 [0.96–1.57]; P = 0.1010 |
| MACE | 98/3107.06 | 3.15 [2.53–3.78] | 303/10014.92 | 3.03 [2.68–3.37] | 1.02 [0.82–1.29]; P = 0.8362 |
| After PSM | |||||
|---|---|---|---|---|---|
| No. of events/Person years | Incidence rate [95%CI] | No. of events/Person years | Incidence rate [95%CI] | Hazard ratio [95%CI]; P-value | |
| (per 100 person-years) | (per 100 person-years) | ||||
| Dronedarone | Propafenone | Dronedarone vs. Propafenone | |||
| Acute myocardial infarction | 9/1205.18 | 0.75 [0.34–1.42] | 15/3828.01 | 0.39 [0.19–0.59] | 1.96 [0.85–4.52]; P = 0.1135 |
| Ischaemic stroke/systemic embolism | 27/1186.81 | 2.27 [1.42–3.13] | 75/3759.25 | 2.00 [1.54–2.45] | 1.11 [0.71–1.73]; P = 0.6402 |
| Intracranial haemorrhage | 7/1208.56 | 0.58 [0.23–1.19] | 29/3824.08 | 0.76 [0.48–1.03] | 0.78 [0.34–1.78]; P = 0.5521 |
| Major bleeding | 21/1191.43 | 1.76 [1.01–2.52] | 57/3800.35 | 1.50 [1.11–1.89] | 1.16 [0.70–1.94]; P = 0.5645 |
| Cardiovascular death | <5 | 0.33 [0.09–0.84] | 31/3840.67 | 0.81 [0.52–1.09] | 0.42 [0.15–1.15]; P = 0.0908 |
| All-cause mortality | 23/1212.32 | 1.9 [1.12–2.67] | 81/3840.67 | 2.11 [1.65–2.57] | 0.90 [0.57–1.42]; P = 0.6452 |
| MACE | 38/1179.67 | 3.22 [2.2–4.25] | 111/3750.3 | 2.96 [2.41–3.51] | 1.07 [0.74–1.56]; P = 0.7068 |
MACE, major adverse cardiovascular event, a composite of myocardial infarction, ischaemic stroke or cardiovascular death.
Figure 7.
Comparison of primary study outcomes of acute myocardial infarction, ischaemic stroke/systemic embolism, intracranial haemorrhage, major bleeding, cardiovascular death, and all-cause mortality in patients with AF using dronedarone vs. propafenone.
Figure 8.
Comparison of secondary study outcome of major adverse cardiovascular event (MACE), which was a composite of acute myocardial infarction, ischaemic stroke, and cardiovascular death in patients with atrial fibrillation ( using dronedarone vs. propafenone.
The mean daily doses of dronedarone at index date were 737.80 ± 145.04 mg and propafenone 325.10 ± 91.49 mg (Table 9). When used concomitantly with dronedarone, the mean daily doses of NOACs were dabigatran 206.97 ± 48.39 mg, rivaroxaban 13.55 ± 3.34 mg, apixaban 9.79 ± 1.02 mg, and edoxaban 54.00 ± 12.42 mg. When used concomitantly with propafenone, the mean daily doses of NOACs were dabigatran 216.61 ± 38.80 mg, rivaroxaban 14.18 ± 3.35 mg, apixaban 9.80 ± 0.97 mg, and edoxaban 47.42 ± 15.05 mg (Table 9). Switching of medications and adjustment of medication dose are shown in Supplementary material online, Tables S6 and S7, respectively. Rates of switching AAD were dronedarone (45.81%) > propafenone (33.91%). Rates of adjustment of AAD dose were propafenone (22.09%) > dronedarone (10.24%).
Table 9.
Mean daily AAD and NOAC dose at index and during follow-up: dronedarone vs. Propafenone
| Before PSM | After PSM (1:3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Index, mean ± SD |
Follow-up, mean ± SD |
Index, mean ± SD |
Follow-up, mean ± SD |
||||||||
| Mean Daily AAD Dose | Mean Daily AAD Dose | ||||||||||
| Dronedarone | Dronedarone | 736.20 | ±146.86 | 733.09 | ±140.69 | Dronedarone | Dronedarone | 737.8 | ±145.04 | 733.16 | ±139.19 |
| Propafenone | Propafenone | 324.53 | ±91.86 | 302.59 | ±133.67 | Propafenone | Propafenone | 325.1 | ±91.49 | 297.31 | ±130.16 |
| Mean Daily NOAC Dose | Mean Daily NOAC Dose | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dronedarone | Dabigatran | 204.67 | ±50.49 | 205.15 | ±47.97 | Dronedarone | Dabigatran | 206.97 | ±48.39 | 208.11 | ±44.65 |
| Rivaroxaban | 13.45 | ±3.66 | 13.14 | ±3.25 | Rivaroxaban | 13.55 | ±3.34 | 13.34 | ±2.89 | ||
| Apixaban | 9.59 | ±1.38 | 9.62 | ±1.23 | Apixaban | 9.79 | ±1.02 | 9.83 | ±0.71 | ||
| Edoxaban | 44.11 | ±15.02 | 43.69 | ±14.83 | Edoxaban | 54 | ±12.42 | 52.75 | ±12.71 | ||
| Propafenone | Dabigatran | 218.69 | ±38.96 | 218.71 | ±36.14 | Propafenone | Dabigatran | 216.61 | ±38.8 | 216.37 | ±35.91 |
| Rivaroxaban | 13.9 | ±3.55 | 13.65 | ±3.16 | Rivaroxaban | 14.18 | ±3.35 | 14 | ±2.9 | ||
| Apixaban | 9.72 | ±1.15 | 9.74 | ±1.01 | Apixaban | 9.8 | ±0.97 | 9.87 | ±0.72 | ||
| Edoxaban | 48.21 | ±14.76 | 48.26 | ±14.77 | Edoxaban | 47.42 | ±15.05 | 46.77 | ±15.04 | ||
AAD, anti-arrhythmic drug; NOAC, non-vitamin K antagonist oral anticoagulant; PSM, propensity-score matching.
Discussion
This is the first nationwide retrospective study that compared the use of dronedarone with non-dronedarone AADs, the use of dronedarone with amiodarone, and the use of dronedarone with propafenone, in patients with AF, to assess cardiovascular events and bleeding risks, when used concomitantly with NOACs. Our findings showed that: (1) The use of dronedarone was associated with lower risks of ICH, cardiovascular death, all-cause mortality, and MACE, but a similar risk of AMI, IS/SE, and major bleeding compared to the use of non-dronedarone AADs, when used concomitantly with NOACs. (2) The use of dronedarone was associated with lower risks of ICH, cardiovascular death, all-cause mortality, and MACE but similar risks of AMI, IS/SE, and major bleeding, compared to amiodarone, when used concomitantly with NOACs. (3) The use of dronedarone had similar risks of AMI, IS/SE, ICH, major bleeding, cardiovascular death, all-cause mortality, and MACE, compared to propafenone, when used concomitantly with NOACs. (4) Our study revealed the doses of NOACs used in clinical practice, which was lacking in previous large observational studies, were similar between groups and thus are not likely to be factors that affected the outcomes.
The ATHENA trial and its post hoc analyses demonstrated the cardiovascular benefits of dronedarone including reducing the risk of stroke or first acute coronary syndrome.2 In EAST-AFNET 4 study, early rhythm-control strategy was associated with reduced risk in the composite outcome of cardiovascular mortality, stroke, HF hospitalization, or acute coronary syndrome hospitalization, when compared to usual care, in patients with recently diagnosed AF.11 Notably, the difference in this composite outcome is driven by the reduction in cardiovascular mortality or stroke. This study has prompted the use of rhythm control therapies on top of usual care which include anticoagulation for early AF patients. However, NOACs were not available in the early studies of dronedarone, and only about half of patients used NOACs in EAST-AFNET 4.12
Several retrospective studies that compared the efficacy of dronedarone against other AADs have been reported. In a Swedish registry study of 300 000 patients with AF, dronedarone or flecainide was associated with a lower all-cause mortality than sotalol (HR 0.44, 95% CI 0.34–0.57 or HR 0.55, 95% CI 0.44–0.68, respectively).13 Additionally, dronedarone was the only AAD associated with a lower risk of ventricular arrhythmia vs. sotalol (HR 0.58, 95% CI 0.37–0.90). In a German retrospective study of 3498 patients using dronedarone and 17 724 patients using other AADs (amiodarone, flecainide, propafenone, or sotalol), dronedarone was associated with lower risks of myocardial infarction (HR 0.78, 95% CI 0.63–0.96; P = 0.020) or stroke (HR 0.84, 95% CI 0.71–0.99; P = 0.043).14 Another retrospective study in the USA analysed 10 455 patients, and found that when compared to dronedarone, amiodarone was associated with higher risks of cardiovascular event (HR 1.7, 95% CI 1.1–2.4) or stroke (HR 2.0, 95% CI 1.33–2).15 However, the proportion of NOAC users in all these studies ranged from 3–30% or was unknown, and NOAC doses were unknown. Thus, it remains to be determined if similar observations could be made when all the patients concomitantly used NOACs. A small-scale Taiwanese retrospective study compared rivaroxaban alone, and with concomitant amiodarone, dronedarone, or propafenone.16 Their study showed that the concomitant use of rivaroxaban and dronedarone was associated with the lowest risk of all-cause mortality (P = 0.013), and there was no difference in the safety endpoint of both major and minor bleedings among the four groups (P = 0.892).
In this study, in terms of efficacy outcomes, we showed that dronedarone was associated with lower ICH, cardiovascular death, all-cause mortality, and MACE, when compared to non-dronedarone AADs as a group or to amiodarone only, in addition to NOACs. Our study showed that there was no advantage of AMI or IS/SE, for use of dronedarone over non-dronedarone AADs nor directly to amiodarone. Our study results differed in terms of AMI outcome as the German study,13 and did not show the beneficial stroke outcome with the use of dronedarone vs. amiodarone as in the US study.15 In terms of all-cause mortality, our result is consistent with the Taiwanese retrospective study.16 Overall, the efficacy outcomes of our studies were generally consistent with previous studies. On the other hand, our study showed that the use of dronedarone was not associated with difference in cardiovascular events compared to the use of propafenone. There were little data regarding the comparison of the cardiovascular outcomes using dronedarone vs. propafenone in patients with AF, except Korean studies that showed they have similar efficacy in maintenance of sinus rhythm in patients with AF after electrical cardioversion.16,17
In terms of safety outcomes, dronedarone was associated with significantly lower ICH, compared to non-dronedarone AADs and amiodarone, when used concomitantly with NOACs. Our results that showed no significant difference in major bleeding between dronedarone and amiodarone was consistent with the Taiwanese retrospective study, although it is worth mentioning that their study combined both major and minor bleeding in their safety endpoint.18 Another retrospective study in Taiwan by Chang S.H. and colleagues assessed the bleeding risks of concomitant use of AADs and NOACs vs. NOACs alone.19 The study found that the concomitant use of dronedarone with NOACs was not associated with an increased major bleeding, when compared to NOACs alone [rate ratio (RR) 0.89, 99% CI 0.71–1.13]. However, the concomitant use of amiodarone with NOACs was associated with an increased major bleeding (RR 1.37, 99% CI 1.25–1.5).19 A US retrospective study found that the concomitant use of dronedarone with apixaban was not associated with increased bleeding when compared with apixaban alone.20 However, the concomitant use of dronedarone with dabigatran or rivaroxaban was associated with increased gastrointestinal bleeding when compared to the NOAC alone. We could not determine if these observations could be made in our study since our study did not compare the concomitant use of AADs and NOACs vs. NOACs alone, and that our study did not evaluate gastrointestinal bleeding specifically. On the other hand, our study also showed that the use of dronedarone was not associated with difference in major bleeding events compared to the use of propafenone.
In this study, we found that the mean daily doses of dabigatran and rivaroxaban were 204.67–214.37 mg and 13.45–13.71 mg, respectively. These doses were lower than the ‘reduced dose’ recommended in the ESC guidelines 2020.1 The mean daily dose of edoxaban was 44.17–47.84 mg, which was in between the recommended standard and lower dose. In contrast, apixaban was used at a mean daily dose of 9.59–9.76 mg, which meant most prescriptions were given at the standard dose. Generally, physicians prefer to prescribe lower doses of anticoagulants for preventative purposes and for safety concerns. The J-ROCKET AF study found that the pharmacokinetic profile in Japanese patients treated with 15-mg rivaroxaban was similar to White patients treated with 20-mg rivaroxaban.21 The Asian subgroup analysis of RE-LY study found that low-dose dabigatran was associated with a reduced risk of major bleeding compared to warfarin.22 These studies may have prompted the use of lower-dose rivaroxaban or dabigatran in Asian patients. In contrast, the Asian subgroup analysis of ARISTOTLE study showed that standard-dose apixaban was associated with a significantly lower risk of major bleeding compared with warfarin.23 This study may have prompted the use of standard-dose apixaban in Taiwan. To date, there is no clinical trial or observational study that investigates systemically the use of different AADs concomitantly with the four NOACs available on market for their combined pharmacokinetics and pharmacodynamics performance. As such, there is limited clinical data supporting the recommendations given in the EHRA guidelines.10 As part of the holistic management of AF shifts from better symptom control to better rhythm management, patients with newly diagnosed AF are recommended to receive rhythm control earlier on, on top of anticoagulants and management of comorbidities.24 Our study showed that when used with NOACs, dronedarone was at least as effective and safe as non-dronedarone AADs in terms of AMI, IS/SE, and major bleeding, and was more effective than non-dronedarone AADs in reducing the risk of ICH, cardiovascular death, and all-cause mortality. Dronedarone has less adverse effects than amiodarone such as pulmonary fibrosis and thyroid dysfunction.25 There is a trend in favour of dronedarone when compared to propafenone in cardiovascular death, even though the difference is not significant. This may be explained by the favourable safety profile of dronedarone in patients with structural heart disease, in contrast to class 1c drugs which may lead to proarrhythmia in these patients.1 These reasons may contribute to the better outcomes in the use of dronedarone when compared with non-dronedarone AADs. Previous similar large studies lack information of NOAC doses, which may be imbalanced between groups. Therefore, it is difficult for physicians to make use of such studies when deciding the NOAC doses to be used with AAD. It is even more difficult for Asian patients who are more prone to bleeding risk than non-Asian patients with the use of anticoagulants.9 Our study revealed that the concomitant use of dronedarone with reduced doses of dabigatran, rivaroxaban or edoxaban, or standard dose of apixaban, was associated with cardiovascular benefits and no increased bleeding risk, when compared with non-dronedarone AADs. Therefore, our study provides data for physicians to consider when prescribing NOACs concomitantly with AADs, while taking in mind the limitations of this study.
Limitations
Our study has several limitations. First, patient screening using ICD-9-CM and ICD-10 codes may lead to missing cases when the conditions or diagnoses were not coded correctly. Second, in ICD-9-CM, atrial fibrillation was not coded in terms of paroxysmal, persistent, and permanent as in ICD-10. Nonetheless, it is assumed that physicians did not prescribe dronedarone for permanent AF. Third, the retrospective nature of the study made the causality of medications and outcome event not definitively established. Fourth, we did not compare the outcomes of patients treated with dronedarone directly with other individual AADs such as propafenone, flecainide, or sotalol. Fifth, multiplicity of P values was not accounted for. Sixth, this study was conducted in a homogenous ethnic background. Seventh, the timing of drug intake for dronedarone and NOACs is unknown, which may have affected the NOAC exposure according to a previous study.26 Eighth, the study could not capture residual confounders. An example is alcohol use, which is independently associated with bleeding. Ninth, the covariates used in propensity score matching do not contain information about the severity of diseases. For example, the New York Heart Association classification of HF is not known and may be imbalanced between groups.
Conclusions
The use of dronedarone with NOACs was associated with cardiovascular benefits in an Asian population with AF, compared with non-dronedarone AADs and amiodarone. The NOAC doses were similar between groups in both protocols and thus were likely not factors that drove the outcomes.
Supplementary Material
Acknowledgements
The authors thank the statistical assistance and the support of the Maintenance Project of the Center for Big Data Analytics and Statistics at Chang Gung Memorial Hospital for study design and monitor, data analysis, and interpretation. This study is based in part on data from the NHIRD provided by the NHI Administration and managed by Health and Welfare Data Science Center, Ministry of Health and Welfare. However, the interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital, NHI Administration and Ministry of Health and Welfare.
Contributor Information
Victor Chien-Chia Wu, Division of Cardiology, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan.
Chun-Li Wang, Division of Cardiology, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan.
Yu-Chang Huang, Division of Cardiology, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan.
Hui-Tzu Tu, Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan.
Yu-Tung Huang, Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan.
Chien-Hao Huang, Department of Gastroenterology and Hepatology, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan City, Taiwan.
Shao-Wei Chen, Department of Cardiothoracic and Vascular Surgery, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan City, Taiwan.
Chang-Fu Kuo, Division of Rheumatology, Allergy and Immunology, Department of Internal Medicine, Chang Gung Memorial Hospital, Linkou Medical Center, Taoyuan City, Taiwan; Division of Rheumatology, Orthopaedics and Dermatology, School of Medicine, University of Nottingham, Nottingham, UK.
Kuo-Chun Hung, Division of Cardiology, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan.
Shang-Hung Chang, Division of Cardiology, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan; Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou Medical Center, No. 5 Fuxing Street, Guishan District, Taoyuan City 33305, Taiwan; Graduate Institute of Nursing, Chang Gung University of Science and Technology, Taoyuan City, Taiwan.
Supplementary material
Supplementary material is available at Europace online.
Funding
This investigator-sponsored study received funding from Sanofi. Victor Chien-Chia Wu, Chun-Li Wang, and Shang-Hung Chang received grants and support from Sanofi. The remaining authors have nothing to disclose.
Data Availability
The data is available to all under reasonable request.
Authors’ Contributions
Study conception and design: VCW, CLW, YCH.
Acquisition of data: HTT, YTH.
Analysis and interpretation of data: VCW, HTT, CHH, SWC, CFK, KCH.
Drafting of manuscript: VCW, CLW.
Critical revision: YTH, SHC.
References
- 1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 2. Boriani G, Blomström-Lundqvist C, Hohnloser SH, Bergfeldt L, Botto GL, Capucci A, et al. Safety and efficacy of dronedarone from clinical trials to real-world evidence: implications for its use in atrial fibrillation. Europace 2019;21:1764–75. [DOI] [PubMed] [Google Scholar]
- 3. Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med 2009;360:668–78. [DOI] [PubMed] [Google Scholar]
- 4. Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, et al. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med 2007;357:987–99. [DOI] [PubMed] [Google Scholar]
- 5. Kober L, Torp-Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, et al. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med 2008;358:2678–87. [DOI] [PubMed] [Google Scholar]
- 6. Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, et al. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med 2011;365:2268–76. [DOI] [PubMed] [Google Scholar]
- 7. Chao TF, Joung B, Takahashi Y, Lim TW, Choi EK, Chan YH, et al. 2021 Focused update consensus guidelines of the Asia pacific heart rhythm society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost 2022;122:20–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol 2007;50:309–15. [DOI] [PubMed] [Google Scholar]
- 9. Gorog DA, Gue YX, Chao TF, Fauchier L, Ferreiro JL, Huber K, et al. Assessment and mitigation of bleeding risk in atrial fibrillation and venous thromboembolism: executive summary of a European and Asia-pacific expert consensus paper. Thromb Haemost 2022;122:1625–52. [DOI] [PubMed] [Google Scholar]
- 10. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace 2021;23:1612–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–16. [DOI] [PubMed] [Google Scholar]
- 12. Metzner A, Suling A, Brandes A, Breithardt G, Camm AJ, Crijns HJ, et al. Anticoagulation, therapy of concomitant conditions, and early rhythm control therapy: a detailed analysis of treatment patterns in the EAST—AFNET 4 trial. Europace 2022;24:552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Friberg L. Ventricular arrhythmia and death among atrial fibrillation patients using anti-arrhythmic drugs. Am Heart J 2018;205:118–27. [DOI] [PubMed] [Google Scholar]
- 14. Ehrlich JR, Look C, Kostev K, Israel CW, Goette A. Impact of dronedarone on the risk of myocardial infarction and stroke in atrial fibrillation patients followed in general practices in Germany. Int J Cardiol 2019;278:126–32. [DOI] [PubMed] [Google Scholar]
- 15. Gao S, Dai W, Zhang L, Juhaeri J, Wang Y, Caubel P. Risk of cardiovascular events, stroke, congestive heart failure, interstitial lung disease, and acute liver injury: dronedarone versus amiodarone and other antiarrhythmics. J Atr Fibrillation 2013;6:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chun KJ, Byeon K, Im SI, Park KM, Park SJ, Kim JS, et al. Efficacy of dronedarone versus propafenone in the maintenance of sinus rhythm in patients with atrial fibrillation after electrical cardioversion. Clin Ther 2014;36:1169–75. [DOI] [PubMed] [Google Scholar]
- 17. Gwag HB, Chun KJ, Hwang JK, Park SJ, Kim JS, Park KM, et al. Which antiarrhythmic drug to choose after electrical cardioversion: A study on non-valvular atrial fibrillation patients. PLoS One 2018;13:e0197352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chiou WR, Huang CC, Lin PL, Chuang JY, Liu LY, Su MI, et al. Safety and effectiveness of rivaroxaban in combination with Various antiarrhythmic drugs in patients with non-permanent atrial fibrillation. Am J Cardiovasc Drugs 2021;21:459–69. [DOI] [PubMed] [Google Scholar]
- 19. Chang SH, Chou IJ, Yeh YH, Chiou MJ, Wen MS, Kuo CT, et al. Association between use of non-vitamin K oral anticoagulants with and without concurrent medications and risk of Major bleeding in nonvalvular atrial fibrillation. JAMA 2017;318:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gandhi SK, Reiffel JA, Boiron R, Wieloch M. Risk of Major bleeding in patients with atrial fibrillation taking dronedarone in combination with a direct acting oral anticoagulant (from a U.S. Claims database). Am J Cardiol 2021;159:79–86. [DOI] [PubMed] [Google Scholar]
- 21. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. Warfarin in Japanese patients with atrial fibrillation—the J-ROCKET AF study. Circ J 2012;76:2104–11. [DOI] [PubMed] [Google Scholar]
- 22. Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke 2013;44:1891–6. [DOI] [PubMed] [Google Scholar]
- 23. Goto S, Zhu J, Liu L, Oh BH, Wojdyla DM, Aylward P, et al. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial. Am Heart J 2014;168:303–9. [DOI] [PubMed] [Google Scholar]
- 24. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckleyet CM, et al. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2022;25:6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Santangeli P, Di Biase L, Pelargonio G, Burkhardt JD, Natale A. The pharmaceutical pipeline for atrial fibrillation. Ann Med 2011;43:13–32. [DOI] [PubMed] [Google Scholar]
- 26. Drug interaction study with dabigatran etexilate and dronedarone in healthy subjects. ClinicalTrials.govIdentifier: NCT01306162https://clinicaltrials.gov/ct2/show/results/NCT01306162. Accessed March 31 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available to all under reasonable request.