Physicians with the least clinical training exhibited the highest odds of COVID-19 antibodies early in the pandemic. Prior to COVID-19 vaccine distribution, standard occupational health interventions including increased training and access to personal protective equipment eliminated the excess risk of COVID-19 among vulnerable healthcare workers.
Keywords: COVID-19, SARS-CoV-2, healthcare personnel, healthcare worker, occupational risk factors, infection prevention, prospective cohort
Objective
The aim of the study is to evaluate COVID-19 risk factors among healthcare workers (HCWs) before vaccine-induced immunity.
Methods
We conducted a longitudinal cohort study of HCWs (N = 1233) with SARS-CoV-2 immunoglobulin G quantification by ELISA and repeated surveys over 9 months. Risk factors were assessed by multivariable-adjusted logistic regression and Cox proportional hazards models.
Results
SARS-CoV-2 immunoglobulin G was associated with work in internal medicine (odds ratio [OR], 2.77; 95% confidence interval [CI], 1.05–8.26) and role of physician-in-training (OR, 2.55; 95% CI, 1.08–6.43), including interns (OR, 4.22; 95% CI, 1.20–14.00) and resident physicians (OR, 3.14; 95% CI, 1.24–8.33). Odds were lower among staff confident in N95 use (OR, 0.55; 95% CI, 0.31–0.96) and decreased over the follow-up.
Conclusions
Excess COVID-19 risk observed among physicians-in-training early in the COVID-19 pandemic was reduced with improved occupational health interventions before vaccinations.
LEARNING OUTCOMES
In this large prospective, longitudinal cohort study of healthcare workers (HCWs) in a single metropolitan hospital system, role as a physician-in-training was associated with increased odds of COVID-19 immunoglobulin G antibodies (odds ratio [OR], 2.55; 95% confidence interval [CI], 1.08–6.43) during the early pandemic period from March to May 2020.
Among physicians-in-training, prevalence of COVID-19 immunoglobulin G antibodies exhibited an inverse dose-response relationship to level of training, with highest odds among interns (OR, 4.22; 95% CI, 1.20–14.00), followed by resident physicians (OR, 3.14; 95% CI, 1.24–8.33) and clinical fellows (OR, 1.19; 95% CI, 0.30–4.03) after multivariable adjustment.
Excess risk among physicians-in-training in this cohort was eliminated through standard occupational health interventions, including increased training and access to personal protective equipment, during the first year of the pandemic, before the widespread distribution of vaccines.
LEARNING OUTCOMES
In this large prospective, longitudinal cohort study of healthcare workers (HCWs) in a single metropolitan hospital system, role as a physician-in-training was associated with increased odds of COVID-19 immunoglobulin G antibodies (odds ratio [OR], 2.55; 95% confidence interval [CI], 1.08–6.43) during the early pandemic period from March to May 2020.
Among physicians-in-training, prevalence of COVID-19 immunoglobulin G antibodies exhibited an inverse dose-response relationship to level of training, with highest odds among interns (OR, 4.22; 95% CI, 1.20–14.00), followed by resident physicians (OR, 3.14; 95% CI, 1.24–8.33) and clinical fellows (OR, 1.19; 95% CI, 0.30–4.03) after multivariable adjustment.
Excess risk among physicians-in-training in this cohort was eliminated through standard occupational health interventions, including increased training and access to personal protective equipment, during the first year of the pandemic, before the widespread distribution of vaccines.
The US healthcare workforce experienced more COVID-19–related mortality than that of any other nation in the early pandemic period.1,2 Approximately 40,000 to 60,000 US healthcare workers (HCWs) died from COVID-related illnesses in the first 18 months of the pandemic, according to estimates from the World Health Organization and the Institute for Health Metrics and Evaluation.1,2 During this same period, the Occupational Safety and Health Administration received more than 10,000 COVID-related complaints from the health services sector alone—more than any other sector of the US economy, and an average of one new Occupational Safety and Health Administration complaint per hour through the entire first year of the pandemic.3
Despite these concerns, many recent studies on SARS-CoV-2 risk factors have attributed most HCW infections to community rather than occupational exposures.4–11 In the midst of a pandemic, this conclusion is in fact an expected result, based on the prolonged duration, close proximity, low ventilation rates, and lack of personal protective equipment (PPE) characteristic of most household and community-based exposures. Occupational hazards may persist in healthcare settings irrespective of the population attributable risk, however, and ongoing assessment, communication, and mitigation of modifiable risk factors are critical for infection control and occupational health in the healthcare setting.12
There is substantial evidence that occupational transmission has contributed to the spread of COVID among HCWs,13–17 but recognizing and reducing risk factors for infection in healthcare settings have been challenging because of concomitant community-based transmission. A recent systemic review of HCW risk factors concluded that SARS-CoV-2 infection was associated with direct care of COVID-19 patients and participation in high-risk procedures and mitigated by education and training in infection control, mask use, and use of appropriate PPE. Despite the large number of studies (N > 150) and participants (>200,000 HCWs) summarized in this analysis, however, the quality of the evidence was considered low to moderate because of methodological limitations, heterogeneity, and imprecision.18,19 Most research has also been cross-sectional and insufficient for causal inference, with relatively few longitudinal studies of evolving HCW risk factors and incident infections in the United States.6
In particular, the effect of job role has been inconsistent across studies, with early research showing physicians at higher risk and subsequent studies demonstrating elevated risk among nurses.19 Although research on job role has many challenges—including missing data,20 exposure misclassification, and heterogeneity of job roles19—point estimates for SARS-CoV-2 infection among physicians-in-training have been consistently observed to be higher than attending physicians in the United States.13,21–23 Physicians-in-training may experience elevated occupational risk of SARS-CoV-2 infection due to their level of training as well as their close contact in caring for patients.
To address these gaps in the literature, we sought to provide a more granular analysis of HCW risk factors in a single metropolitan hospital system, including more detailed job classifications that account for physicians-in-training, and longitudinal follow-up to characterize both prevalent and incident infections. This study uses repeated measures of SARS-CoV-2 immunoglobulin G (IgG) and surveys of infectious exposures and health behaviors in a large workforce (N > 1200) to characterize occupational and community risk factors as they evolved during the first year of the pandemic from March 2020 through January 2021. This period also serves as a natural experiment for assessing the effectiveness of occupational health interventions on emerging infections before the development of vaccines, and strategies for reducing occupational infections and improving pandemic preparedness are discussed.
METHODS
Human Subjects
Human subjects older than 18 years who worked at a single, metropolitan academic hospital in Northeastern, United States, were recruited through announcements at regularly scheduled staff meetings and via email beginning in March 2020 with baseline survey and serum collection. Follow-up continued with repeat serology and surveys through January 2021. Recruitment of this volunteer sample and all study methods were approved by the Yale Institutional Review Board and Human Investigations Committee.
Individual and Community Data Sources
Surveys were collected from enrolled participants at 4 time points using the online Qualtrics survey platform, and data were aggregated in a MySQL database (https://www.qualtrics.com). Survey questionnaires captured demographic information and potential occupational and community risk factors associated with COVID-19 transmission. Daily COVID-19 incidence rates per 100,000 individuals for each town were obtained from the Connecticut Department of Health,24 using the standardized case definition of probable and confirmed cases from the Council of State and Territorial Epidemiologists.25 For each participant, community exposure to COVID-19 was calculated as the mean incidence in the participant’s town of residence during the interval between each date of serum collection.
Enzyme-Linked Immunosorbent Assay
Peripheral blood samples were obtained by venipuncture using vacutainer tubes (Becton Dickinson, Falcon Lakes, NJ) with a serum separator, aliquoted and stored at −80°C before use in enzyme-linked immunosorbent assays (ELISAs). All blood samples were handled under biosafety level 2+.
Nunc band Maxisorp™ 96-well microtiter plates from Life Technologies (Grand Island, NY) were coated with 50 μL/well of a 1 μg/mL solution of spike antigen (S1+S2 ECD, YP_009724390.1), extracellular domain (Val-16 to Pro-1213) from Sino Biological (Wayne, PA) in 0.1 M sodium carbonate buffer with pH 9.6, overnight at 4°C. After blocking with 200 μL/well of 3% dry milk/omniblok™ from AmericanBio (Canton, MA) in phosphate-buffered saline, pH 7.4, for 1 hour at room temperature, and 1 hour incubation with 100 μL/well of 1:100 serum, the plates were developed with 75 μL/well of 1:2000 dilution of anti-human IgG-peroxidase, conjugated from Pharmingen (San Diego, CA), clone G18-145 for 1 hour, followed by 100 μL/well 3,3′,5,5′-tetramethylbenzidine substrate as previously described.26–28 Optical density was read at 450 nm, with reference at 650 nm after addition of 2 M HCL.
Optimal cutoffs for binary classification of the continuous optical density scale were established after a receiver operating characteristic analysis with positive control samples obtained from patients documented to have COVID-19 by clinical symptomatology and a positive polymerase chain reaction (PCR) test, and negative controls obtained from prepandemic blood samples. A subset of samples was compared with commercially available serologic assays from EuroImmun, Abbot Architect, and Vitros, with similar results.27 The sensitivity and specificity of the Laboratory Diagnostic Test were estimated to be 96% and 98%, respectively, with an optical density value of 0.345, which was used as the cutoff for this analysis.
Statistical Analysis
Descriptive statistics for categorical and continuous variables were calculated using Fisher exact and Mann-Whitney U tests as appropriate (Table 1). A multivariable-adjusted logistic regression model was constructed for analysis of risk factors of prevalent infections from baseline serologic testing. Department, which was correlated with job position and resulted in models with higher residual deviance, was excluded from adjustment in multivariable models. All other variables included for adjustment exhibited no significant evidence of collinearity. Emergency medicine and nurses were selected as reference categories for department and occupation, respectively, because of diversity of patient exposure, the large sample sizes present in this study, and the low prevalence of infection at baseline.
TABLE 1.
Baseline Characteristics of Study Participants
| Total (N = 1,233) | Seronegative (n = 1,161) | Seropositive (n = 72) | |||
|---|---|---|---|---|---|
| n (%) or Median (IQR) | n (%) or Median (IQR) | n (%) or Median (IQR) | P | ||
| Demographics | |||||
| Sex | 1232 (100) | 1160 (100) | 72 (100) | <0.01 | |
| Female | 810 (65.7) | 775 (66.8) | 35 (48.6) | ||
| Male | 422 (34.3) | 385 (33.2) | 37 (51.4) | ||
| Age | 35 (31.0–45.0) | 35 (31.0–44.2) | 35.5 (31.0–48.5) | 0.41 | |
| Race | |||||
| White | 918 (74.5) | 863 (74.4) | 55 (76.4) | 0.78 | |
| Asian | 219 (17.8) | 206 (17.8) | 13 (18.1) | 1.00 | |
| Black | 48 (3.9) | 45 (3.9) | 3 (4.2) | 0.76 | |
| Ethnicity | 1232 (100) | 1160 (100) | 72 (100) | 0.43 | |
| Latinx | 71 (5.8) | 69 (5.9) | 2 (2.8) | ||
| History of COVID | |||||
| History of COVID symptoms | 196 (15.9) | 163 (14.0) | 33 (45.8) | <0.0001 | |
| Missed work due to illness, d | 0 (0.0–0.0) | 0 (0.0–0.0) | 0 (0.0–5.0) | <0.0001 | |
| COVID PCR+ | 27 (2.2) | 3 (0.3) | 24 (33.3) | <0.0001 | |
| Serology result (optical density) | 0.11 (0.1–0.2) | 0.1 (0.1–0.1) | 0.56 (0.4–0.9) | <0.0001 | |
| Date of enrollment, wk | 5.14 (4.1–6.4) | 5.14 (4.1–6.1) | 5.29 (4.5–6.6) | 0.03 | |
| Environmental exposures | |||||
| Population density (1000 people/mi2) | 2.6 (1.1–9.0) | 2.84 (1.1–9.0) | 1.51 (0.9–9.0) | 0.61 | |
| Household size | 958 (100) | 894 (100) | 64 (100) | 0.61 | |
| 1 person | 189 (19.7) | 174 (19.5) | 15 (23.4) | ||
| 2 people | 331 (34.6) | 308 (34.5) | 23 (35.9) | ||
| 3 or more people | 438 (45.7) | 412 (46.1) | 26 (40.6) | ||
| Adults working outside of home | 958 (100) | 894 (100) | 64 (100) | 0.52 | |
| 1 or none | 542 (56.6) | 503 (56.3) | 39 (60.9) | ||
| 2 or more | 416 (43.4) | 391 (43.7) | 25 (39.1) | ||
| Community COVID incidence | 3 (1.0–4.0) | 3 (1.0–4.0) | 3 (1.8–3.2) | 0.89 | |
| COVID exposure outside of work | 346 (28.1) | 326 (28.1) | 20 (27.8) | 1.00 | |
| Occupational exposures | |||||
| Department | 1232 (100) | 1160 (100) | 72 (100) | 0.15 | |
| Emergency medicine | 228 (18.5) | 222 (19.1) | 6 (8.3) | ||
| Anesthesiology | 161 (13.1) | 150 (12.9) | 11 (15.3) | ||
| General internal medicine | 249 (20.2) | 232 (20.0) | 17 (23.6) | ||
| Internal medicine subspecialty | 280 (22.7) | 266 (22.9) | 14 (19.4) | ||
| Other specialty | 211 (17.1) | 195 (16.8) | 16 (22.2) | ||
| Surgical specialty | 103 (8.4) | 95 (8.2) | 8 (11.1) | ||
| Position | 1232 (100) | 1160 (100) | 72 (100) | 0.29 | |
| Nursing | 265 (21.5) | 255 (22.0) | 10 (13.9) | ||
| Attending physician | 336 (27.3) | 314 (27.1) | 22 (30.6) | ||
| House staff | 367 (29.8) | 340 (29.3) | 27 (37.5) | ||
| Other clinical (eg, APRN, PA) | 172 (14.0) | 165 (14.2) | 7 (9.7) | ||
| Other staff (eg, administrator, medical assistant, technician) | 92 (7.5) | 6 (8.3) | |||
| Direct patient care | 1231 (100) | 1159 (100) | 72 (100) | 0.28 | |
| Less than once a week | 157 (12.8) | 145 (12.5) | 12 (16.7) | ||
| More than once a week | 1074 (87.2) | 1014 (87.5) | 60 (83.3) | ||
| Worked with COVID+ coworker | 187 (15.2) | 177 (15.2) | 10 (13.9) | 0.87 | |
| Exposure to aerosol procedures | 494 (40.1) | 467 (40.2) | 27 (37.5) | 0.71 | |
| Health knowledge and behaviors | |||||
| Confidence in using an N95 | 485 (39.4) | 465 (40.1) | 20 (27.8) | 0.05 | |
| Endorse feeling overworked | 459 (37.3) | 443 (38.2) | 16 (22.2) | <0.01 | |
| Endorse feeling burnout | 913 (74.0) | 870 (74.9) | 43 (59.7) | <0.01 | |
| Concern for COVID at work | 1233 (100) | 1161 (100) | 72 (100) | <0.01 | |
| Less than once a week | 169 (13.7) | 150 (12.9) | 19 (26.4) | ||
| More than once a week | 1064 (86.3) | 1011 (87.1) | 53 (73.6) | ||
APRN, Advanced Practice Registered Nurse; IQR, interquartile range; PA, Physician Assistant; PCR, polymerase chain reaction.
A mixed effects Cox proportional hazards model with the individual subject included as a random effect29 was used to evaluate risk factors for seroconversion in repeated observations during the follow-up period. For missing data, we used a last observation carried forward method. Independent interval-censored data, which can result in biased estimates for time-varying covariates as intervals increase in length,30 was accounted for by multiple imputation31,32 as extended to mixed effects Cox models in R Statistical Software (https://www.r-project.org).33 Community incidence rates and covariates from individual survey data were derived from data corresponding to the imputed survival time; surveys that occurred more than 28 days after the imputed survival time were considered at risk of bias and excluded from analysis for that assessment interval.
Kaplan-Meier curves (Figs. 1, 2) include baseline and follow-up data and illustrate the changing hazards across these 2 periods. All statistical analyses were conducted using R statistical software (version 4.1.3). 34,35
FIGURE 1.
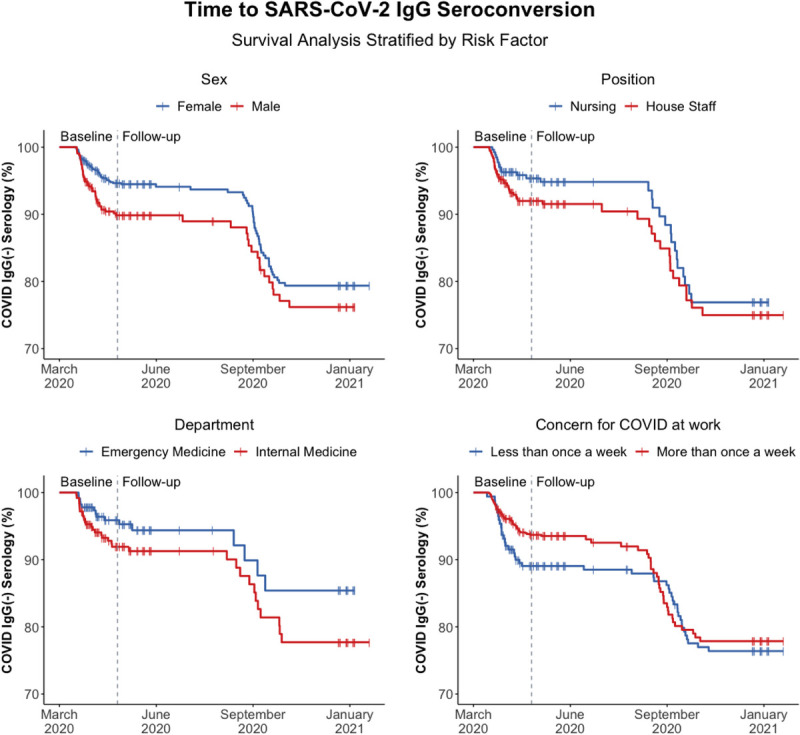
Kaplan-Meier curves for time to SARS-CoV-2 spike antigen-specific IgG seroconversion as stratified by gender, job position, department, and concern for COVID-19 at work, respectively. Prevalent infections recorded during the baseline period of enrollment are designated to the left of vertical dotted line, and incident infections observed during the 9 months of follow-up are designated to the right of the vertical dotted line.
FIGURE 2.
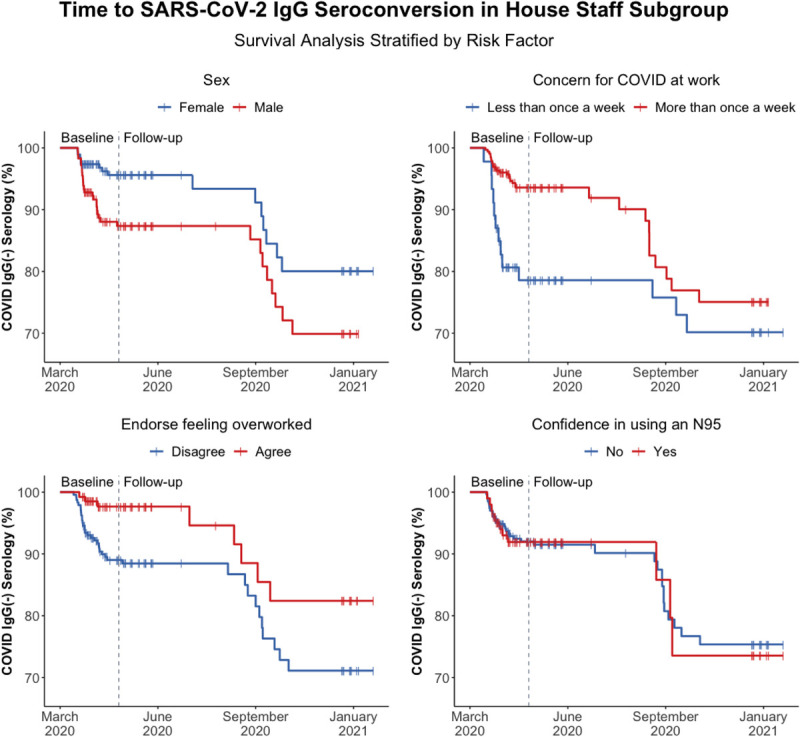
Kaplan-Meier curves for time to SARS-CoV-2 spike antigen-specific IgG seroconversion in a subgroup analysis of house staff physicians as stratified by gender, concern for COVID-19 at work, endorsement of feeling overworked, and confidence in using an N95 respirator, respectively. Prevalent infections recorded during the baseline period of enrollment are designated to the left of vertical dotted line, and incident infections observed during the 9 months of follow-up are designated to the right of the vertical dotted line.
RESULTS
Information on the HCWs (N = 1233) enrolled in the present study is summarized in Table 1, including demographic, environmental, and occupational data relevant to SARS-CoV-2 exposure. The participants were from a large metropolitan hospital in Northeastern, United States, and included hospital staff from general internal medicine (20.2%); emergency medicine (18.5%); internal medicine subspecialties such as pulmonology and infectious disease (22.7%); other specialties such as pediatrics and neurology (17.1%); and anesthesiology (13.1%) and other surgical specialties (8.4%). The population was predominately White (74.5%) and female (65.7%), with a median age of 35, and resided in the vicinity of New Haven, Connecticut (85%). The large majority of participants were house staff officers (29.8%), attending physicians (27.3%), or nurses (21.5%) who were involved in direct patient care more than once a week (87.2%), including exposure to aerosol-generating procedures (40.1%), and reported concern for COVID-19 exposure at work more than once a week (86.3%). Initial enrollment began after the first case of COVID-19 infection was detected in Connecticut on March 8, 2020, and 1223 (99.2%) had baseline serology performed by May 8, 2020. Follow-up with both serology and surveys continued over a 9-month period through January 2021.
SARS-CoV-2 Seroprevalence
Baseline prevalence of SARS-CoV-2 spike antigen-specific IgG detected by ELISA was 72 of participants (5.8%). Prevalence of SARS-CoV-2 IgG was significantly (P < 0.0001) higher in subjects who reported prior positive COVID-19 PCR test result and subjects who reported a history of COVID-19 symptoms. Of those who were seropositive at baseline, 39 individuals (54.2%) reported no history of COVID-19 symptoms. Individuals who used a higher number of sick days and those who enrolled in the study later were also significantly more likely to be seropositive (P < 0.0001 and P = 0.03, respectively). Higher rates of seropositivity at baseline were observed among males, house staff officers, individuals employed in general internal medicine, and those who had less concern for COVID at work and did not report burnout or overwork. Baseline seropositivity was not associated with community-based exposures or other demographic characteristics such as age, race or ethnicity.
Risk Factors for Prevalent Infections
Unadjusted analysis (Table 2) demonstrated significant associations of baseline seropositivity with male gender (odds ratio [OR], 2.13; 95% confidence interval [CI], 1.32–3.44) and departmental affiliation, with higher rates in anesthesiology, general internal medicine, as well as surgical and other specialties when compared with emergency medicine. Analysis of job position demonstrated higher rates among house staff (OR, 2.02; 95% CI, 0.99–4.46) compared with all nurses. The elevated odds among physicians-in-training was attributable to higher odds of seropositivity among residents (OR, 2.44; 95% CI, 1.11–5.64) and interns (OR, 2.51; 95% CI, 0.83–7.02). Odds of seropositivity were not elevated among fellows and chief residents (OR, 1.01; 95% CI, 0.27–3.10).
TABLE 2.
Association of SARS-CoV-2 IgG Serology With HCW Risk Factors for Prevalent and Incident Infections
| Prevalent Infections | Incident Infections | ||||
|---|---|---|---|---|---|
| Total at baseline (n = 1,233) | Total With Follow-up (n = 939) | ||||
| Unadjusted OR (95% CI) | Adjusted* OR (95% CI) | Unadjusted HR (95% CI) | Adjusted* HR (95% CI) | ||
| Demographics | |||||
| Sex | |||||
| Female | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Male | 2.13 (1.32–3.44) | 1.91 (1.13–3.24) | 1.42 (0.39–5.23) | 0.89 (0.38–2.07) | |
| Age | 1.01 (0.99–1.04) | 1.02 (0.99–1.05) | 1.01 (0.97–1.05) | 1.01 (0.97–1.05) | |
| Race | |||||
| White | 1.11 (0.65–2.00) | 1.34 (0.29–5.70) | 0.54 (0.17–1.72) | 0.25 (0.03–2.41) | |
| Asian | 1.02 (0.53–1.84) | 1.18 (0.24–5.09) | 1.52 (0.52–4.48) | 0.50 (0.05–5.15) | |
| Black | 1.08 (0.26–3.05) | 1.42 (0.20–7.76) | 0.89 (0.12–6.63) | 0.17 (0.01–4.31) | |
| Ethnicity | |||||
| Latinx | 0.45 (0.07–1.48) | 0.55 (0.07–2.53) | 1.66 (0.46–5.93) | 0.55 (0.05–5.62) | |
| History of COVID | |||||
| History of COVID symptoms | 5.18 (3.15–8.47) | 5.47 (3.22–9.28) | 1.96 (0.59–6.52) | 1.39 (0.38–5.09) | |
| Missed work due to illness, d | 1.18 (1.13–1.24) | 1.20 (1.14–1.26) | 1.04 (0.90–1.21) | 1.02 (0.89–1.16) | |
| Date of enrollment, wk | 1.09 (0.99–1.20) | 1.26 (1.09–1.44) | 0.67 (0.55–0.82) | 0.67 (0.53–0.85) | |
| Environmental exposures | |||||
| Population density (1000 ppL/mi2) | 0.99 (0.94–1.04) | 0.99 (0.91–1.08) | 1.00 (0.91–1.10) | 0.99 (0.87–1.13) | |
| Household size | |||||
| 1 person | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| 2 people | 0.87 (0.44–1.74) | 0.81 (0.40–1.66) | 0.82 (0.26–2.59) | 0.88 (0.31–2.51) | |
| 3 or more people | 0.73 (0.38–1.45) | 0.62 (0.30–1.32) | 0.58 (0.18–1.85) | 0.61 (0.21–1.80) | |
| Adults working outside of home | |||||
| 1 or none | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| 2 or more | 0.82 (0.49–1.38) | 0.81 (0.47–1.38) | 0.76 (0.31–1.84) | 0.97 (0.45–2.10) | |
| Community COVID incidence | 0.99 (0.80–1.22) | 0.89 (0.71–1.12) | 0.82 (0.54–1.25) | 0.89 (0.57–1.39) | |
| COVID exposure outside of work | 0.99 (0.57–1.65) | 0.93 (0.53–1.58) | 0.55 (0.20–1.49) | 0.67 (0.30–1.49) | |
| Occupational exposures | |||||
| Department | |||||
| Emergency medicine | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Anesthesiology | 2.71 (1.01–8.02) | 2.69 (0.95–8.39) | 0.82 (0.23–2.87) | 0.83 (0.21–3.28) | |
| General internal medicine | 2.71 (1.10–7.63) | 2.77 (1.05–8.26) | 0.82 (0.26–2.56) | 0.86 (0.24–3.13) | |
| Internal medicine subspecialty | 1.95 (0.77–5.58) | 1.67 (0.63–4.96) | 0.73 (0.23–2.30) | 0.86 (0.26–2.80) | |
| Surgical specialty | 3.12 (1.06–9.70) | 2.80 (0.88–9.32) | 0.38 (0.05–2.74) | 0.57 (0.08–4.02) | |
| Other specialty | 3.04 (1.22–8.60) | 2.69 (1.00–8.14) | 0.73 (0.20–2.61) | 0.95 (0.24–3.72) | |
| Position | |||||
| Nursing | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| Attending physician | 1.79 (0.85–4.01) | 1.39 (0.59–3.42) | 1.42 (0.47–4.27) | 0.62 (0.20–1.90) | |
| House staff | 2.02 (0.99–4.46) | 2.55 (1.08–6.43) | 1.32 (0.42–4.14) | 0.59 (0.17–2.07) | |
| Interns | 2.51 (0.83–7.02) | 4.22 (1.20–14.0) | 2.61 (0.56–12.2) | 1.55 (0.31–7.70) | |
| Residents | 2.44 (1.11–5.64) | 3.14 (1.24–8.33) | 0.39 (0.06–2.62) | 0.24 (0.04–1.54) | |
| Fellows and chiefs | 1.01 (0.27–3.10) | 1.19 (0.30–4.03) | 2.40 (0.59–9.81) | 0.87 (0.17–4.47) | |
| Other clinical (eg, APRN, PA) | 1.08 (0.39–2.87) | 1.09 (0.38–3.01) | 1.05 (0.28–4.00) | 0.67 (0.19–2.34) | |
| Other staff (eg, administrator, medical assistant, technician) | 1.78 (0.59–4.94) | 1.38 (0.45–3.91) | 1.60 (0.35–7.25) | 0.96 (0.21–4.41) | |
| Direct patient care | |||||
| Less than once a week | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| More than once a week | 0.71 (0.39–1.42) | 0.93 (0.48–1.92) | 2.44 (0.94–6.31) | 1.72 (0.71–4.17) | |
| Worked with COVID+ coworker | 0.90 (0.43–1.71) | 1.14 (0.53–2.26) | 0.64 (0.16–2.56) | 0.35 (0.07–1.82) | |
| Exposure to aerosol procedures | 0.89 (0.54–1.45) | 1.08 (0.62–1.86) | 1.23 (0.61–2.49) | 0.90 (0.42–1.92) | |
| Health knowledge and behaviors | |||||
| Confidence in using an N95 | 0.57 (0.33–0.96) | 0.55 (0.31–0.96) | 0.63 (0.25–1.59) | 0.68 (0.30–1.53) | |
| Endorse feeling overworked | 0.46 (0.25–0.80) | 0.47 (0.25–0.83) | 0.81 (0.28–2.38) | 0.67 (0.31–1.47) | |
| Endorse feeling burnout | 0.50 (0.31–0.82) | 0.60 (0.36–1.03) | 1.17 (0.43–3.19) | 1.39 (0.57–3.38) | |
| Concern for COVID at work | |||||
| Less than once a week | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | |
| More than once a week | 0.41 (0.24–0.73) | 0.53 (0.30–0.98) | 2.09 (0.71–6.12) | 1.58 (0.67–3.70) | |
CI, confidence interval; HR, hazard ratio; OR, odds ratio.
*All hazard and odds ratios have been adjusted for age, sex, race, ethnicity, community COVID incidence, high-risk COVID exposure outside of work, job position using house staff as a combined category, frequency of direct patient care, exposure to aerosol-generating procedures, and work with coworker who was known or suspected to be COVID+. Baseline odds ratios have also been adjusted for date of enrollment in the study.
Several measures of health knowledge and behaviors were associated with lower odds of SARS-CoV-2 seropositivity, including confidence in using an N95 respirator (OR, 0.57; 95% CI, 0.33–0.96), increased concern for COVID exposure at work (OR, 0.41; 95% CI, 0.24–0.73), endorsement of feeling overworked (OR, 0.46; 95% CI, 0.25–0.80), and experience of burnout (OR, 0.50; 95% CI, 0.31–0.82). Community risk factors, including COVID-19 incidence rates in the town of residence, population density of town of residence, self-reported COVID-19, exposure outside of work, household size, and number of cohabitating adults working outside the household, were not significantly associated with SARS-CoV-2 seropositivity at baseline.
After adjustment for major demographic variables and suspected risk factors—including age, sex, race, ethnicity, date of enrollment, COVID-19 incidence in the town of residence, self-reported COVID-19, exposure outside of work, job position, frequency of direct patient care, exposure to aerosol-generating procedures, and work with coworker who was known or suspected to have COVID—male gender remained highly associated with anti-SARS-CoV-2 IgG with an OR of 1.91 (95% CI, 1.13–3.24). After adjustment, house staff physicians exhibited higher odds of seropositivity (OR, 2.55; 95% CI, 1.08–6.43); the highest odds were observed among interns (OR, 4.22; 95% CI, 1.20–14.00), followed by residents (OR, 3.14; 95% CI, 1.24–8.33). Prevalence of SARS-CoV-2 antibodies were not significantly elevated in fellows and chief residents (OR, 1.19; 95% CI, 0.30–4.03). Subgroup analysis of nurses (not shown) also demonstrated that in comparison with non–intensive care unit nurses, critical care registered nurses (RNs) were also at increased risk of COVID-19 seropositivity (OR, 4.41; 95% CI, 1.16–16.84). Using emergency medicine as a reference, employment in general internal medicine was also associated with an elevated risk of seropositivity (OR, 2.77; 95% CI, 1.05–8.26).
Several measures of health knowledge and behavior remained significantly associated with decreased seropositivity after adjustment, including confidence in using an N95 respirator (OR, 0.55; 95% CI, 0.31–0.96), increased concern for COVID exposure at work (OR, 0.53; 95% CI, 0.30–0.98), and endorsement of feeling overworked (OR, 0.47; 95% CI, 0.25–0.83). Date of enrollment in the study also remained significant after adjustment (OR, 1.26; 95% CI, 1.09–1.44).
Risk Factors for Incident Infections
Of the 1161 individuals who did not test positive at baseline, 939 (81%) returned for antibody testing over a 9-month follow-up, period from April 2020 through January 2021 (302 total person-years of follow-up). Serology demonstrated SARS-CoV-2 IgG in 65 of participants (6.92%) during follow-up, giving an incidence rate of 21.5 new infections per 100 person-years of follow-up.
During the follow-up period, none of the suspected demographic, occupational, and community risk factors were associated with significantly elevated hazard ratios in a mixed effects Cox proportional hazards model. This null finding was unchanged after multivariable adjustment for age, sex, race, ethnicity, COVID-19 incidence in the town of residence, self-reported COVID-19, exposure outside of work, job position, frequency of direct patient care, exposure to aerosol-generating procedures, and work with coworker who was known or suspected to have COVID-19. Date of study enrollment was associated with a lower hazard ratio for seroconversion, which was an expected finding of the study design discussed hereinafter.
Kaplan-Meier curves of time to seropositivity (Fig. 1) include both the prevalent infections detected through the baseline testing period (left of vertical line) and the incident cases detected during 9 months of follow-up (right of vertical line), as stratified by gender, job position, department, and concern for COVID-19. Although offset by the separation observed during the early pandemic period, survival curves are parallel or convergent for incident infections in the follow-up period and reflect nonsignificant differences in hazard ratios between groups.
Kaplan-Meier curves in a subgroup analysis of house staff physicians (Fig. 2) reflect increased rates of seropositivity among males at baseline, but the effect was not sustained during follow-up. Increased concern for COVID-19 at work and endorsement of feeling overworked were both associated with lower rates of seropositivity at baseline, although this effect was attenuated during the follow-up period. Confidence in using an N95 respirator did not seem to have a significant association with COVID-19 seropositivity among house staff at any time point.
DISCUSSION
This report details prevalent and incident SARS-CoV-2 infections from a large longitudinal cohort of HCWs (N = 1233) at a major academic hospital during the first year of the SARS-CoV-2 pandemic. The results demonstrated that a strong association between SARS-CoV-2 seropositivity and occupation during the initial wave of the local pandemic, from March 2020 to May 2020, was ultimately reduced during the follow-up period from May 2020 to January 2021.
Most notably, house staff physicians exhibited a 2- to 3-fold increase in odds (OR, 2.55; 95% CI, 1.08–6.43) of seropositivity that was inversely associated with training. Interns exhibited the highest odds (OR, 4.22; 95% CI, 1.20–14.0), followed by resident physicians (OR, 3.14; 95% CI, 1.24–8.33), and clinical fellows and chief residents (OR, 1.19; 95% CI, 0.30–4.03) after multivariable adjustment. This result is consistent with prior research that disaggregated physicians-in-training from attending physicians13,21–23 but is, to our knowledge, the first report of an inverse dose-response relationship between year of training and COVID-19 seroprevalence among physicians-in-training.
Increased odds of seropositivity was also observed among employees in general internal medicine (OR, 2.77; 95% CI, 1.05–8.26), who staffed both COVID-19 inpatient wards, including intensive care and step-down units, as well as general medicine floors and primary care clinics in the period before widespread availability of COVID-19 PCR testing. In a separate subgroup analysis (data not shown in table because of a different reference level), critical care nurses (CCRNs) who staffed the same intensive care unit and step-down units also exhibited a higher odds of prevalent infections when compared with other nursing staff (OR, 4.41; 95% CI, 1.16–16.84), consistent with prior reseach.36
Increased risk among interns, residents, and CCRNs, especially those affiliated with general internal medicine, likely reflects a common source of exposure or inadequate access to or use of appropriate PPE. In particular, these individuals experienced high contact with patients on both the general medicine floors and the medical intensive care units during a time before the introduction of universal masking for source control and respiratory protection. Interns, residents, and CCRNs are also the most likely to be in close contact with patients during cardiopulmonary resuscitation, a risk factor for viral exposure37–40 that can persist despite N95 respirator use.41–43 The absence of association between seropositivity and aerosol-generating procedures is notable and suggests that most occupational SARS-CoV-2 transmission resulted from unrecognized sources of SARS-CoV-2 exposure, such as unmasked contact with patients not known to have COVID-19 or coworkers sharing workspaces,15,16,44 rather than aerosol-generating procedures performed on known COVID-19 patients. These results are consistent with prior research that demonstrated increased risk among healthcare workers,45 including those HCW who reported no known exposure to COVID-19 patients,14 with the primary risk attributable to the lack of PPE.14,45,46
The current investigation also observed a strong association between SARS-CoV-2 IgG seroprevalence and health knowledge and behaviors during the first wave of the pandemic from March to May 2020. Confidence in using an N95 respirator and concern for occupational transmission of COVID-19 were both associated with reduced odds of SARS-CoV-2 seropositivity and likely reflect the protective effects of a worker’s preventive health knowledge or adherence to PPE use, which is consistent with prior research14,17,19,45,46 Lower odds of seropositivity among those experiencing burnout and endorsing overwork may be related to individual behavioral practices or reflective of lower cumulative occupational exposures, as such workers may be less likely to volunteer for additional shifts. Taken together, the association between training level, and health knowledge and behaviors on COVID-19 prevalence suggests that improved training and awareness of occupational transmission could significantly reduce infections in future pandemics.
The basis for the association between male sex and SARS-CoV-2 IgG seropositivity is uncertain and has been attributed to both behavioral as well as biological differences, such as viral susceptibility and immunogenicity.47,48 In this longitudinal study, the narrowing of seropositivity disparities between sexes over time suggests that sex variation is context sensitive and likely attributable to behavioral differences in the early pandemic period. Gendered differences in preventive behaviors, such as compliance with mask wearing and social distancing, have been previously observed in both the United States49,50 and international contexts.51 The finding from the current study is consistent with a comprehensive longitudinal analysis of sex disparities in COVID-19 outcomes in the United States52 and suggests that public health interventions targeting risk reduction among male HCWs may be beneficial.
The current study did not find a significant relationship between SARS-CoV-2 IgG and demographic risk factors such as race, ethnicity, age, or town of residence. These null findings have been observed in other studies of community risk factors53 and may indicate that community-transmission may have been more limited in the setting of widespread business and school closures that characterized the early pandemic in this area.
In contrast to occupational risk factors identified at baseline, no significant associations were observed for incident infections in mixed-effects Cox proportional hazard models. Resolution of risk over time among house staff physicians, including interns and residents, in a single hospital system may reflect the success of intervening occupational health strategies, which included greater availability of PPE such as N95 respirators; the introduction of universal masking for all patients and hospital staff; greater access to PCR testing in the hospital and the surrounding community; greater knowledge of modes of transmission; and increased awareness of the prevalence of asymptomatic infections. To our knowledge, this is one of the few granular longitudinal studies of HCWs to show that excess risk identified in the early pandemic was successfully controlled with occupational health interventions even before the introduction of COVID-19 vaccinations.
The strengths and weakness of the present study should be recognized when interpreting the findings. The strengths include the research design as a prospective cohort study, the active participation of HCWs, the ability to screen large numbers of participants through high-throughput serology, and the duration of follow-up throughout the first year of the pandemic. A limitation is the focus on a single hospital system, which, although similar to other large academic medical centers, may differ substantially from rural and community hospital settings. Because of differences in the modality and availability of community-based COVID-19, testing, this study could also not determine whether risk of HCW infection during the follow-up period remained in excess of risk in the general population.
CONCLUSIONS
This large-scale prospective cohort study of HCWs at a major academic hospital in the northeast US observed that COVID-19 infection was associated with health knowledge and behaviors consistent with prior research but is the first to identify an inverse dose-response relationship between level of training and COVID-19 seroprevalence among physicians-in-training. Longitudinal analysis demonstrated that this excess risk from the early phase of the pandemic was effectively eliminated with existing occupational health interventions before the introduction of COVID-19 vaccinations. This study indicates that reliable availability and improved training on the use of PPE among healthcare workers, especially those with the least experience and the greatest exposures, can effectively reduce excess infection risk among HCWs in future emerging infections.
ACKNOWLEDGMENTS
The authors thank all of the individuals of the Yale COVID Health Care Worker Study for their active participation.
Footnotes
Ethical Considerations and Disclosure: Yale University human subjects’ institutional review board and human investigation committee approved the current study. Written informed consent was obtained from all participants before enrollment.
Funding source: This study was supported by Yale University institutional funds and the National Institute for Occupational Safety and Health (NIOSH) under Federal Training Grant T03-OH008607. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIOSH.
L.E.F., A.V.W., and C.A.R. planned the study and drafted the manuscript. Q.-A.A., R.F.S., R.S., participated in subject recruitment, and clinical aspects of the study. J.L. and A.V.W. performed ELISAs. L.E.F. performed statistical analyses. All authors read, edited, and approved the final manuscript.
Institution at which the work was performed: Yale University School of Medicine.
Conflict of interest: None declared.
Contributor Information
Louis E. Fazen, Email: louis.fazen@jhmi.edu.
Queenie-Ann Abad, Email: qabad1988@gmail.com.
Richard F. Smith, Email: richard.f.smith@yale.edu.
Romero Santiago, Email: Romero@santiago.us.com.
Jian Liu, Email: jian.liu@yale.edu.
Adam V. Wisnewski, Email: adam.wisnewski@yale.edu.
Carrie A. Redlich, Email: carrie.redlich@yale.edu.
REFERENCES
- 1.Institute for Health Metrics and Evaluation . COVID-19 has caused 6.9 million deaths globally, more than double what official reports show. Updated site last updated on May 6, 2021. Available at: https://www.healthdata.org/news-release/covid-19-has-caused-69-million-deaths-globally-more-double-what-official-reports-show. Accessed October 24, 2022.
- 2.World Health Organization . The impact of COVID-19 on health and care workers: a closer look at deaths. Available at: https://apps.who.int/iris/handle/10665/345300. Accessed October 24, 2022.
- 3.Occupational Safety and Health Administration . Freedom of Information Act: COVID-19 complaint data—previous reports. Available at: https://www.osha.gov/foia/archived-covid-19-data. Accessed October 10, 2022.
- 4.Baker JM Nelson KN Overton E, et al. Quantification of occupational and community risk factors for SARS-CoV-2 seropositivity among health care workers in a large U.S. health care system. Ann Intern Med. 2021;174:649–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dávila-Conn V Soto-Nava M Caro-Vega YN, et al. Seroepidemiology of SARS-CoV-2 in healthcare personnel working at the largest tertiary COVID-19 referral hospitals in Mexico City. PloS One. 2022;17:e0264964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doernberg SB Holubar M Jain V, et al. Incidence and prevalence of COVID-19 within a healthcare worker cohort during the first year of the SARS-CoV-2 pandemic. Clin Infect Dis. 2022;75:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosser JI Röltgen K Dymock M, et al. Severe acute respiratory coronavirus virus 2 (SARS-CoV-2) seroprevalence in healthcare personnel in northern California early in the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2021;42:1053–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wight E Swift M O'Horo JC, et al. COVID-19 infections in health care personnel by source of exposure and correlation with community incidence. J Occup Environ Med. 2022;64:675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimcheff DE Schildhouse RJ Hausman MS, et al. Seroprevalence of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection among veterans affairs healthcare system employees suggests higher risk of infection when exposed to SARS-CoV-2 outside the work environment. Infect Control Hosp Epidemiol. 2021;42:392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lan F-Y Filler R Mathew S, et al. Sociodemographic risk factors for coronavirus disease 2019 (COVID-19) infection among Massachusetts healthcare workers: a retrospective cohort study. Infect Control Hosp Epidemiol. 2021;42:1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganz-Lord FA, Segal KR, Gendlina I, Rinke ML, Weston G. SARS-CoV-2 exposures among healthcare workers in New York city. Occup Med (Lond). 2022;72:248–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Occupational Safety and Health Administration . 12. 1910.132—general requirements. Available at: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.132. Accessed October 20, 2022.
- 13.Barrett ES Horton DB Roy J, et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers in New Jersey, at the onset of the U.S. COVID-19 pandemic. BMC Infect Dis. 2020;20:853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen LH Drew DA Graham MS, et al. Risk of COVID-19 among front-line health-care, workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475–e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataria Y Cole M Duffy E, et al. Seroprevalence of SARS-CoV-2 IgG antibodies and risk factors in health care workers at an academic medical center in Boston, Massachusetts. Sci Rep. 2021;11:9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah VP Breeher LE Alleckson JM, et al. Occupational exposure to severe acute respiratory coronavirus virus 2 (SARS-CoV-2) and risk of infection among healthcare personnel. Infect Control Hosp Epidemiol. 2022;43:1785–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sims MD Maine GN Childers KL, et al. Coronavirus disease 2019 (COVID-19) seropositivity and asymptomatic rates in healthcare workers are associated With job function and masking. Clin Infect Dis. 2021;73(suppl 2):S154–S162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Epidemiology of and risk factors for coronavirus infection in health care workers: a living rapid review. Ann Intern Med. 2020;173:120–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou R, Dana T, Buckley DI, Selph S, Fu R, Totten AM. Update alert 11: epidemiology of and risk factors for coronavirus infection in health care workers. Ann Intern Med. 2022;175:W83–W84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes MM. Update: characteristics of health care personnel with COVID-19—United States, February 12–July 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1364–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.El-Boghdadly K Wong DJN Owen R, et al. Risks to healthcare workers following tracheal intubation of patients with COVID-19: a prospective international multicentre cohort study. Anaesthesia. 2020;75:1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrest CB Xu H Thomas LE, et al. , HERO Registry Research Group . Impact of the early phase of the COVID-19 pandemic on US healthcare workers: results from the HERO registry. J Gen Intern Med. 2021;36:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lepak AJ Buys A Stevens L, et al. COVID-19 in health care personnel: significance of health care role, contact history, and symptoms in those who test positive for SARS-CoV-2 infection. Mayo Clin Proc. 2021;96:2312–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connecticut Department of Public Health . COVID-19 Tests, Cases, and Deaths (By Town) | Connecticut Data. Available at: https://data.ct.gov/Health-and-Human-Services/COVID-19-Tests-Cases-and-Deaths-By-Town-/28fr-iqnx. Accessed August 4, 2021.
- 25.Council of State and Territorial Epidemiologists . Standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). Technical Supplement: Interim-20-ID-01. Available at: https://cdn.ymaws.com/www.cste.org/resource/resmgr/2020ps/Interim-20-ID-01_COVID-19.pdf. Accessed August 4, 2021.
- 26.Amanat F Stadlbauer D Strohmeier S, et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26:1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan S Redlich CA Wisnewski AV, et al. Performance of Abbott Architect, Ortho Vitros, and Euroimmun assays in detecting prior SARS-CoV-2 infection. 2020:2020.07.29.20164343. 2020-07-30. Accessed February 2, 2022.
- 28.Wisnewski AV, Campillo Luna J, Redlich CA. Human IgG and IgA responses to COVID-19 mRNA vaccines. PLoS One. 2021;16:e0249499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Therneau TM. Mixed effects cox models. Updated Janbuary 13, 2020. Available at: https://cran.r-project.org/web/packages/coxme/vignettes/coxme.pdf. Accessed August 1, 2021.
- 30.Therneau T, Crowson C, Atkinson E. Using time dependent covariates and time dependent coefficients in the Cox model. Updated August. 2021;23. Available at: https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf. Accessed October 1, 2021. [Google Scholar]
- 31.Sun J. The Statistical Analysis of Interval-Censored Failure Time Data. Statistics for biology and health. New York, Springer; 2006:302. doi: 10.1007/0-387-37119-2. [DOI] [Google Scholar]
- 32.Sun J Peace KE Rizopoulos D, et al. Interval-censored time-to-event data. 2013:426. [Google Scholar]
- 33.Alarcón‐Soto Y, Langohr K, Fehér C, García F, Gómez G. Multiple imputation approach for interval‐censored time to HIV RNA viral rebound within a mixed effects Cox model. Biom J. 2019;61:299–318. [DOI] [PubMed] [Google Scholar]
- 34.R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2021. Available at: http://www.r-project.org/index.html. Accessed March 27, 2023. [Google Scholar]
- 35.Therneau TM, Grambsch P. Modeling Survival Data: Extending the Cox Model. New York, Springer. 2000;364. doi: 10.1007/978-1-4757-3294-8. [DOI] [Google Scholar]
- 36.Wiggen TD Bohn B Ulrich AK, et al. SARS-CoV-2 seroprevalence among healthcare workers. PloS One. 2022;17:e0266410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christian MD Loutfy M McDonald LC, et al. , SARS Investigation Team . Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis. 2004;10:287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fragkou PC Dimopoulou D Latsios G, et al. Transmission of infections during cardiopulmonary resuscitation. Clin Microbiol Rev. 2021;34:e0001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maron BA Gladwin MT Bonnet S, et al. Perspectives on cardiopulmonary critical care for patients with COVID-19: from members of the American Heart Association Council on cardiopulmonary, critical care, perioperative and resuscitation. J Am Heart Assoc. 2020;9:e017111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PloS One. 2012;7:e35797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barros AJ, Sifri CD, Bell TD, Eby JC, Enfield KB. Effectiveness of elastomeric half-mask respirators vs N95 filtering facepiece respirators during simulated resuscitation: a nonrandomized controlled trial. JAMA Netw Open. 2021;4:e211564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hwang SY Yoon H Yoon A, et al. N95 filtering facepiece respirators do not reliably afford respiratory protection during chest compression: a simulation study. Am J Emerg Med. 2020;38:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin H, Oh J, Lim TH, Kang H, Song Y, Lee S. Comparing the protective performances of 3 types of N95 filtering facepiece respirators during chest compressions: a randomized simulation study. Medicine (Baltimore). 2017;96:e8308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keller SC Pau S Salinas AB, et al. , Centers for Disease Control and Prevention Epicenters Program . Barriers to physical distancing among healthcare workers on an academic hospital unit during the coronavirus disease 2019 (COVID-19) pandemic. Infect Control Hosp Epidemiol. 2022;43:474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Self WH. Seroprevalence of SARS-CoV-2 among frontline health care personnel in a multistate hospital network—13 academic medical centers, April–June 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1221–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rich-Edwards JW Ding M Rocheleau CM, et al. American frontline healthcare personnel's access to and use of personal protective equipment early in the COVID-19 pandemic. J Occup Environ Med. 2021;63:913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;20:442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolfe J Safdar B Madsen TE, et al. Sex- or gender-specific differences in the clinical presentation, outcome, and treatment of SARS-CoV-2. Clin Ther. 2021;43:557–571.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haischer MH Beilfuss R Hart MR, et al. Who is wearing a mask? Gender-, age-, and location-related differences during the COVID-19 pandemic. PLOS ONE Oct 2020;15, 15:e0240785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okten IO, Gollwitzer A, Oettingen G. Gender differences in preventing the spread of coronavirus. Behav Sci Policy. 2020;6:109–122. [Google Scholar]
- 51.Galasso V, Pons V, Profeta P, Becher M, Brouard S, Foucault M. Gender differences in COVID-19 attitudes and behavior: panel evidence from eight countries. Proc Natl Acad Sci U S A. 2020;117:27285–27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Danielsen AC Lee KM Boulicault M, et al. Sex disparities in COVID-19 outcomes in the United States: quantifying and contextualizing variation. Soc Sci Med. 2022;294:114716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Friedman-Klabanoff DJ Fitzpatrick MC Deming ME, et al. Risk of severe acute respiratory syndrome coronavirus 2 acquisition is associated with individual exposure but not community-level transmission. J Infect Dis. 2022;226:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]


