Abstract
The pituitary gland regulates growth, metabolism, reproduction, the stress response, uterine contractions, lactation, and water retention. It secretes hormones in response to hypothalamic input, end organ feedback, and diurnal cues. The mechanisms by which pituitary stem cells are recruited to proliferate, maintain quiescence, or differentiate into specific cell types, especially thyrotropes, are not well understood. We used single-cell RNA sequencing in juvenile P7 mouse pituitary cells to identify novel factors in pituitary cell populations, with a focus on thyrotropes and rare subtypes. We first observed cells coexpressing markers of both thyrotropes and gonadotropes, such as Pou1f1 and Nr5a1. This was validated in vivo by both immunohistochemistry and lineage tracing of thyrotropes derived from Nr5a1-Cre; mTmG mice and demonstrates that Nr5a1-progenitors give rise to a proportion of thyrotropes during development. Our data set also identifies novel factors expressed in pars distalis and pars tuberalis thyrotropes, including the Shox2b isoform in all thyrotropes and Sox14 specifically in Pou1f1-negative pars tuberalis thyrotropes. We have therefore used single-cell transcriptomics to determine a novel developmental trajectory for thyrotropes and potential novel regulators of thyrotrope populations.
Keywords: pituitary, single-cell, thyrotrope, development, pars tuberalis
The pituitary gland plays a critical central role in the hypothalamic-pituitary neuroendocrine axes, which control many aspects of mammalian physiology, and its dysfunction, due to disease or trauma, is implicated in many disorders. Understanding the origins of pituitary cell types and the gene regulatory networks that drive their specification is therefore essential to understand and develop treatments for these disorders. Each cell type in the pituitary has a different relative abundance, and this can vary throughout the animal's lifetime. The 5 anterior pituitary endocrine cell types are thought to developmentally derive from separate lineages (1). Growth hormone (GH)-producing somatotropes, prolactin (PRL)-producing lactotropes, and thyrotropin (TSH)-producing thyrotropes arise from precursors expressing the transcription factor POU1F1. Homozygous mutations in POU1F1 in humans and mice cause GH, PRL, and TSH deficiencies (2-4). Gonadotropes produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH) and are derived from precursor cells expressing the transcription factor NR5A1 (also known as steroidogenic factor 1/SF1), and Nr5a1-mutant mice lack gonadotropes (5, 6). Gonadotropes are not reduced in Pou1f1-deficient mouse models (4) and thyrotropes are not reduced in Nr5a1-deficient models (5), suggesting they are transcription factor determinants of separate lineages. Cellular coexpression of POU1F1 and NR5A1 has not been described in normal cells, although this has been reported in pituitary tumors (7).
Thyrotropes are one of the smallest proportion of anterior pituitary (pars distalis) endocrine cell types, comprising about 5% of total pituitary cells (8). TSH induces thyroid hormone synthesis and secretion to regulate metabolism, protein synthesis, neural maturation, and postnatal growth. The hypothalamic-pituitary-thyroid axis is regulated by the circadian pacemaker in the suprachiasmatic nucleus, and maintenance of daily TSH secretion profiles is important for normal metabolism (9). Newborn screening for hypothyroidism is essential to allow prompt treatment to prevent permanent intellectual disability, and abnormal levels of TSH are not uncommon in the population (10). Research into the drivers of thyrotrope fate is important for novel insights to combat hypothyroid and hyperthyroid states (11). The TSH hormone is a heterodimeric protein containing the TSH β subunit (TSHB) and the glycoprotein hormones α subunit (CGA, chorionic gonadotropin α), which is common to TSH, LH, and FSH. Thyrotropes and gonadotropes also share expression of the transcription factors Gata2, Isl1, and Foxl2 (12-14). The coexpression of Pou1f1 and Gata2 within the same cell is thought to be a major driver of thyrotrope specification (13), while Isl1 is also an important transcription factor for thyrotrope cell fate (15). The mechanisms that drive pituitary progenitor differentiation into the thyrotrope fate rather than other fates are not fully understood.
The pars tuberalis also contains thyrotropes together with gonadotropes and forms a partial collar around the infundibular stalk (4). The Tshb transcript and peptide produced in the ovine pars tuberalis is identical in sequence to that produced by pars distalis thyrotropes (16). Pars tuberalis thyrotropes differ from pars distalis thyrotropes in that they do not respond to hypothalamic TSH-releasing hormone (TRH) or thyroid hormone. Rather, they modulate seasonal reproduction through TSH expression in response to photoperiodic input and melatonin (16, 17). Pars tuberalis thyrotropes express Gata2 but do not express Pou1f1, which is required for Tshb expression in the pars distalis (3, 4). Previous studies have demonstrated that long photoperiods in quails, sheep, and mice can induce Tshb transcription through upregulation of transcription factors including Eya3, Six1, and Tef (18-20). Humans exhibit seasonal changes in metabolism and hormone secretion, but the role of pars tuberalis-derived TSH in these processes is not known (9).
Single-cell RNA sequencing (scRNAseq) has become a powerful technique to study gene expression patterns and identify novel regulators of cell populations, and has aided in determining temporal gene expression dynamics, cellular developmental trajectories, and gene regulatory networks of the hypothalamus, pituitary, and other organs (8, 21-26). In this study, we have used single-cell transcriptomics and lineage-tracing to identify and validate a novel endocrine subpopulation in the juvenile pituitary gland, including a new developmental trajectory giving rise to thyrotropes. Thyrotropes are relatively rare and do not always form a distinct cell cluster in single-cell studies (26, 27), therefore we enriched for thyrotropes in our data set using P7 Tg:Tshb-Cre; R26R-Eyfp mice to increase the thyrotrope frequency from approximately 3% in the unsorted sample to more than 57% after fluorescence-activated cell sorting (FACS). Although our data set and interpretations were limited by the pooling of male and female samples together, we were able to validate several of our findings in vivo both in males and females. We identified the short Shox2b isoform in all thyrotropes and Sox14 in pars tuberalis thyrotropes as potential candidates for regulating thyrotrope subpopulation specification and/or hormone expression in mice.
A web-based interactive version of this data set is available on the Broad Single Cell Portal https://singlecell.broadinstitute.org/single_cell/study/SCP2110/single-cell-transcriptomics-of-p7-mouse-pituitary-cells-including-enriched-thyrotropes.
Materials and Methods
Mouse Models
Use of mice at the University of Michigan was approved by the University of Michigan's Animal Care and Use Program and Institutional Animal Care and Use Committee. Use of mice at the Crick Institute was approved by the Crick Institute Ethical Review Body, and use of animals at King's College London were approved by the King's College London Ethical Committee. Use of mice at the University of Calgary was approved by its Life and Environmental Sciences Animal Care Committee. Mice were housed under standard light/dark conditions and provided food ad libitum. All mice used in this study have been previously published. Tg:Tshb-Cre mice (subsequently Tshb-Cre) carry a transgenic bacterial artificial chromosome (BAC) containing the Tshb locus and flanking regions with a Cre cassette inserted at the transcription start site of Tshb (28). Rosa26LSL-stop-Eyfp mice (R26-Eyfp) mice carry a lox-Stop-lox-Eyfp cassette knocked in to the genomic Rosa26 locus (29). Tg:Nr5a1-Crehigh (Nr5a1-Cre) mice carry a transgenic BAC containing the Nr5a1 locus and flanking regions with a Cre cassette replacing the BAC:Nr5a1 coding sequence (30). Tg:Pou1f1-Cre mice (Pou1f1-Cre) carry a transgene containing the Pou1f1 upstream element and Cre-recombinase (31). Rosa26mTmG (R26-mTmG) mice carry a cassette containing tandem floxed-Tomato and eGFP sequences knocked in to the genomic Rosa26 locus (32). Shox2LacZ mice carry a LacZ expression cassette inserted into the Shox2 genomic locus at the start of the first exon of Shox2a, and acts as a knockout or severe hypomorph (33). The effect on Shox2b expression, if any, is unclear. Sox14Egfp mice (MGI 3836003) carry an Egfp expression cassette replacing the genomic Sox14 coding sequence (34). Foxl2CreERT2 mice carry the Cre recombinase-estrogen receptor fusion protein sequence knocked in to the genomic Foxl2 locus and were obtained from the Jackson Laboratories (JAX strain No. 015854). Prop1−/− mice (MGI 3521736) have a deletion from the start codon through the first exon and intron and the beginning of exon 2 (35).
LacZ Staining
Whole-mount embryonic staining for beta-galactosidase (LacZ) activity was performed according to standard techniques as previously described (36). To ensure access of staining solution to the pituitary, the brain was removed before staining the skull base and attached pituitary.
Single-Cell RNA Libraries Preparation
We dissected pituitary glands from 3 male and 1 female Tshb-Cre; R26-Eyfp mouse (2 males from 1 litter and 1 male and 1 female from a second litter) and dispersed cells for FACS using EYFP fluorescence in one pool. We also prepared single-cell RNA libraries from 2 male R26-Eyfp control mice (1 from each corresponding litter of the Cre-positive samples), which were dispersed but did not undergo FACS, as a second pool for our analyses to include all pituitary cell types. Pituitary tissue was dissected and dispersed using an enzymatic mix for 4 hours and prepared for scRNAseq as previously performed (27, 37). Single-cell RNA libraries were prepared by the University of Michigan's Advanced Genomics Core under a standardized pipeline on the 10x Genomics Chromium platform and controller, following the manufacturer's instructions for Single Cell 3' v3 reagents, and sequenced on the NovaSeq6000 platform with an S4 chip.
Computational Analyses of Single-Cell RNA Sequencing Data
Samples were demultiplexed with Cellranger Single Cell Software Suite 3.1.0 and fastq files aligned to a modified University of California Santa Cruz mm10 (GRCm38) reference genome containing amended annotation for the Prop1 3' untranslated region (27) and the Eyfp sequence (https://www.ncbi.nlm.nih.gov/nuccore/LC171744.1, positions 2933-3640). Binary sequence alignment maps were converted into the loom format using velocyto.py (38), which were then analyzed in R using the Seurat 3.1.2 package (39, 40). Core Seurat functions were used to read in loom files, integrate samples, generate uniform manifold approximation and projection (UMAP) plots, calculate clusters, perform differential gene expression analyses, and generate plots of expression data. Cell filtering was performed by retaining only cells with more than 500 detected genes, between 3000 and 30 000 unique molecular identifiers (ie, unique transcripts), and less than 10% mitochondrial content (Supplementary Fig. S1 (41)). A total of 10% was chosen as the threshold using the lower value of 2 SDs above mean in either sample (10.32% in R26-Eyfp sample and 18.11% in Tshb-Cre; R26-Eyfp sample). Some plots were graphically modified with ggplot2 options. Expression plots used Nebulosa 3.16 to display gene-weighted density estimation (42); in those plots, low expression is dark blue and high expression is yellow. Single-cell regulatory network inference and clustering (SCENIC) was performed using the SCENIC R package (43). Counts of cells expressing genes above and below the calculated threshold was calculated using Seurat and graphed in Microsoft Excel or Graphpad Prism 8. Differential gene expression calculations in Seurat that returned an adjusted P value of 0 were converted to 1E-305 to allow calculable conversion into -log10(adj. P-val). Exported plots were compiled using Adobe Photoshop CC 2023. Sequencing data are available on the National Center for Biotechnology Information Gene Expression Omnibus under accession number GSE205418, and expression data are available on the Broad Single Cell Portal at SCP2110.
Immunofluorescence
Head tissue from 3 P1 Nr5a1-Cre; R26-mTmG mice were fixed in 37% formaldehyde overnight, paraffin embedded, and sectioned in the coronal orientation at 5 μm. Four P6 wild-type pituitary glands were dissected, fixed in 10% neutral buffered formalin for a minimum of 5 hours, and embedded in optimal cutting temperature compound after cryoprotection. For Sox14Egfp mice, whole mouse heads were collected at P0 and skin removed for overnight fixation with 4% paraformaldehyde, then washed with phosphate-buffered saline for at least 24 hours at 4 °C before they were cryoprotected in 30% sucrose, frozen on dry ice, and cryosectioned with bone intact at 20-μm coronal sections. Immunofluorescence was performed by conventional protocols previously described (37, 44, 45). Primary antibodies used were rabbit anti-GFP (Abcam ab6556 lot GR3216572-1) at 1:1000; goat anti-GFP (Abcam ab5450) at 1:1000; chicken anti-GFP (Abcam ab13970) at 1:5000; guinea pig anti-TSHB (National Hormone and Peptide Program [NHPP] AFP967793) at 1:100; rabbit anti-TSHB (NHPP AFP-1274789) at 1:1600; rabbit anti-POU1F1 (generously provided by Dr Simon Rhodes, University of North Florida) at 1:100, rabbit anti-NR5A1 (commercially custom generated (46)) at 1:500; rat anti-NR5A1 (TransGenic Inc/Cosmo Bio Co Ltd KO610 clone 1B1F10) at 1:2000; guinea pig anti-LHB (NHPP) at 1:100; and monkey anti-GH (NHPP AFP411S) at 1:200. Directly conjugated secondary antibodies used were donkey anti-rat AlexFluor 594 (Invitrogen A21206) at 1:500; donkey anti-rabbit-AlexaFluor 555 (Invitrogen A32794) at 1:500; donkey anti-guinea pig AlexaFluor 488 (Jackson ImmunoResearch 706-545-148) at 1:500; goat anti-human AlexaFluor 488 (Invitrogen A11013); donkey anti-goat AlexaFluor 555 (Invitrogen A21432); donkey anti-rabbit fluorescein isothiocyanate (FITC) (Jackson ImmunoResearch 711-095-152); donkey anti-rabbit-AlexaFluor 568 (Invitrogen A10042) at 1:500; and donkey anti-chicken-AlexaFluor 488 (Invitrogen SA1-72000) at 1:500. Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Nonconfocal imaging was performed on a Leica Leitz DMB microscope and Leica DFC310 FX camera. Confocal imaging was performed using a Leica TSC SPE. Images were compiled in Adobe Photoshop CC 2019 with minimal adjustments to brightness, contrast, levels, and/or curves made for visual clarity. Some images originally captured in red fluorescence were hue-adjusted to purple for accessibility. Adjustments are applied across the entirety of each image.
Fluorescent Messenger RNA Detection
Paraffin sections from control P2 and e15.5 mice were used for fluorescent messenger RNA (mRNA) detection using the RNAscope Multiplex Fluorescent Detection Kit V2 (Advanced Cell Diagnostics 323110) and Efna2 probe (Mm-Efna2-C2 507481-C2) following the manufacturer's protocol.
Hormone Cell Counts
In Nr5a1-Cre mice, TSHB/GFP coimmunofluorescence was performed on 3 separate sections spaced approximately 75 μm or more apart, and images captured from both lateral wings and the medial portion beneath the posterior lobe per section. TSHB-positive cells and TSHB/GFP double-positive cells were counted per image and totaled per mouse (ie, 3 images per section × 3 sections per mouse × 3 mice). LH/GFP and GH/GFP costaining was counted using 1 section and totaled per mouse (ie, 3 images per section × 1 section per mouse × 3 mice). In P6 wild-type mice, 5 images were taken each from 1 male and 1 female FVB/NJ mouse and 1 male and 1 female C57BL6 mouse and counted for NR5A1/POU1F1/TSHB/DAPI.
Quantitative Polymerase Chain Reaction
mRNA was extracted from dissected mouse pituitary glands using the Qiagen RNeasy Mini kit (Qiagen No. 74104) and complementary DNA made using the SuperScript First-Strand Synthesis System (Invitrogen No. 11904018) following the manufacturer's protocols. We used a combination of wild-type and heterozygous Prop1+/− mice (which have no known phenotype) as controls. Quantitative polymerase chain reaction (qPCR) was performed using the SYBR Green PCR Master Mix (Applied Biosystems 4309155) on an Applied Biosystems 7500 Real Time PCR System. Primers included the following: Shox2b F: GAGGAATTGAGCCAGCGACT; Shox2b R: CGCCTGAACCTGCTGAAATG; Shox2a F: GAGCTGGACATGGGAGCC; Shox2a R: AGCGTCTGGATAGTGGGTCT; Hprt F: GCTGGTGAAAAGGACCTCT; Hprt R: CACAGGACTAGAACACCTGC. We used the ΔΔCT method to calculate comparative expression values.
Protein Structure Prediction
The Alphafold Protein Structure Database was used to generate predicted protein structures for mouse SHOX2A (P70390) and SHOX2B (A0A140T8S9). PyMOL was used to overlay predicted protein structures (PyMOL Molecular Graphics System, version 2.3.2 Schrödinger, LLC).
Statistical Analyses
Comparisons of cell counts were calculated using unpaired t tests in Prism 8 and R. Statistical analyses of single-cell data were performed by the Seurat and base R libraries.
Results
Cells Expressing Both Thyrotrope and Gonadotrope Markers in Single-Cell RNA Sequencing Data Set of Enriched Thyrotropes
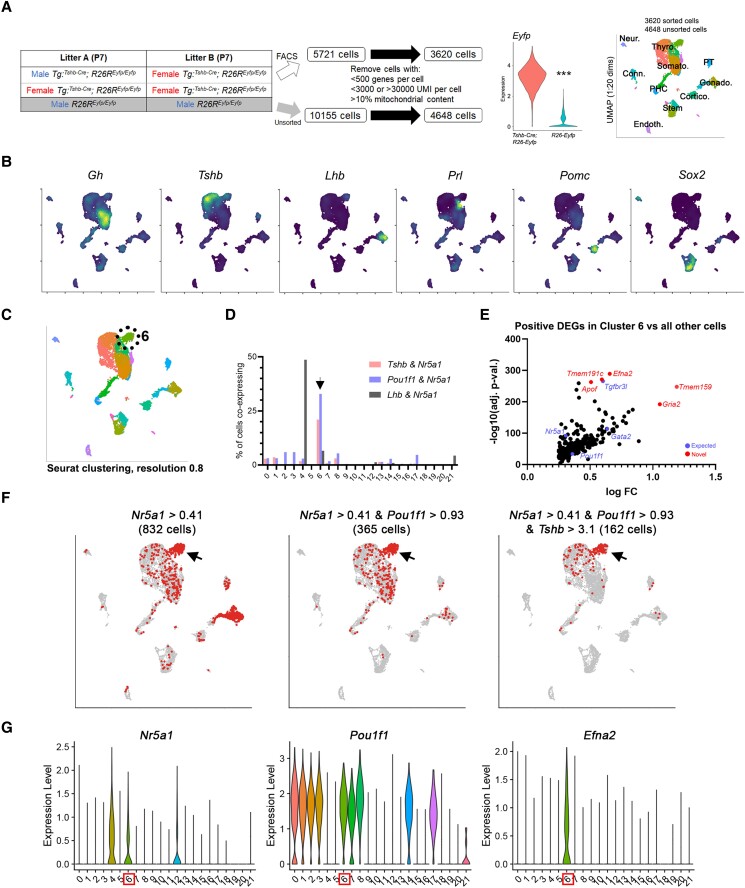
We performed scRNAseq of 7-day-old (P7) murine pituitary cells in 2 concurrently processed pools using Tshb-Cre; Rosa26-Eyfp FACS-purified pituitary cells combined with control (Cre-negative) unsorted total pituitary cells to capture all pituitary populations with increased proportion of rare Tshb-lineage populations. We also selected P7 pituitary glands to capture increased proportions of stem cells, which are more abundant at young ages. After filtering to remove sequenced cells with particularly high or low unique molecular identifier counts, low number of detected genes, and high mitochondrial content (Supplementary Fig. S1 (41)), the EYFP-sorted pool contained 3620 cells and the unsorted pool contained 4648 cells (Fig. 1A). Expression of the transgenic Eyfp transcript was significantly higher in Tshb-Cre; R26-Eyfp cells than control R26-Yfp cells (3.23 ± 0.74 vs 0.29 ± 0.41; P < 2.2e-16), indicating FACS captured Tshb-lineage traced cells with high accuracy. Cells were computationally clustered using a shared nearest neighbor modularity optimization-based algorithm using Seurat, and broad population identities were assigned based on expression of known endocrine and stem cell markers (Fig. 1B).
Figure 1.
Single-cell RNA sequencing (scRNAseq) of whole pituitary and enriched thyrotropes identified cells expressing markers of both gonadotropes and thyrotropes. A, Outline of experimental design. Pituitary glands from P7 Tshb-Cre; R26-Eyfp mice were dissected, dispersed, and fluorescence-activated cell sorted. Littermate Cre-negative control pituitaries were dispersed but not sorted. Cells were processed for scRNAseq on the 10x Chromium platform, sequenced, and analyzed in R. Eyfp expression is significantly higher in Tshb-Cre; R26-Eyfp cells (P = 2.2e-16). Cells were clustered on uniform manifold approximation and projection and assigned identities based on known markers shown next. B, Expression of known pituitary markers. Dark cells have lower expression and bright cells have higher expression. C and D, Computationally calculated clusters showed a population with far higher proportion of cells coexpressing markers of thyrotropes and gonadotropes together, for example, Tshb and Nr5a1, and Pou1f1 and Nr5a1 (arrowhead). Typical gonadotropes coexpress Lhb and Nr5a1. E, Differential expression analysis in this population shows enrichment of both known thyrotrope and gonadotrope factors, such as Tgfbr3l, Nr5a1, and Pou1f1. F, Binarized indication of cells expressing combinations of gonadotrope and thyrotrope genes above the indicated Otsu threshold. Red cells are those positive for the indicated genes and gray are those that are negative. G, Violin plots of expression showing expression of both Nr5a1 and Pou1f1 in these cells, as well as unique enrichment of factors such as Efna2.
We detected a population of cells coexpressing genes characteristic of gonadotropes and thyrotropes, such as the gonadotrope transcription factor Nr5a1 and the somatotrope/lactotrope/thyrotrope marker Pou1f1, as well as expression of Tshb in Nr5a1-expressing cells (Fig. 1C and 1D). Differential gene expression analysis of cells in this cluster against all other cells demonstrated enriched expression of genes characteristic of both gonadotropes and thyrotropes, as well as other novel factors (Fig. 1E), such as Tmem191c and ephrin A2 (Efna2). Total transcripts per cell per cluster were similar in this population and thyrotropes and gonadotropes, suggesting they were not gonadotrope-thyrotrope doublet artifacts. We found that individual cells in this cluster coexpressed thyrotropes and gonadotrope markers together such as Pou1f1 and Nr5a1 (Fig. 1F). While gonadotrope markers such as the inhibin B co-receptor Tgfbr3L were detected in gonadotropes and this novel population, we observed that Efna2 and other genes such as Tmem191c were enriched in this population alone, further indicating they are a novel population (Fig. 1G). Efna2 mRNA detection by RNAscope found expression in the embryonic colliculus and juvenile P2 pituitary and hypothalamic subventricular zone (Supplementary Fig. S2 (41)), although signal in the pituitary was detected in more cells than observed by scRNAseq (Fig. 1G) or frequency of Pou1f1/Nr5a1-double-positive cells in vivo (Fig. 2A and 2B). The reason for this is unclear, as the probe appears specific because it stains the embryonic colliculus in a similar pattern to public data from Allen Brain Atlas and Genepaint (Supplementary Fig. S2A (41)).
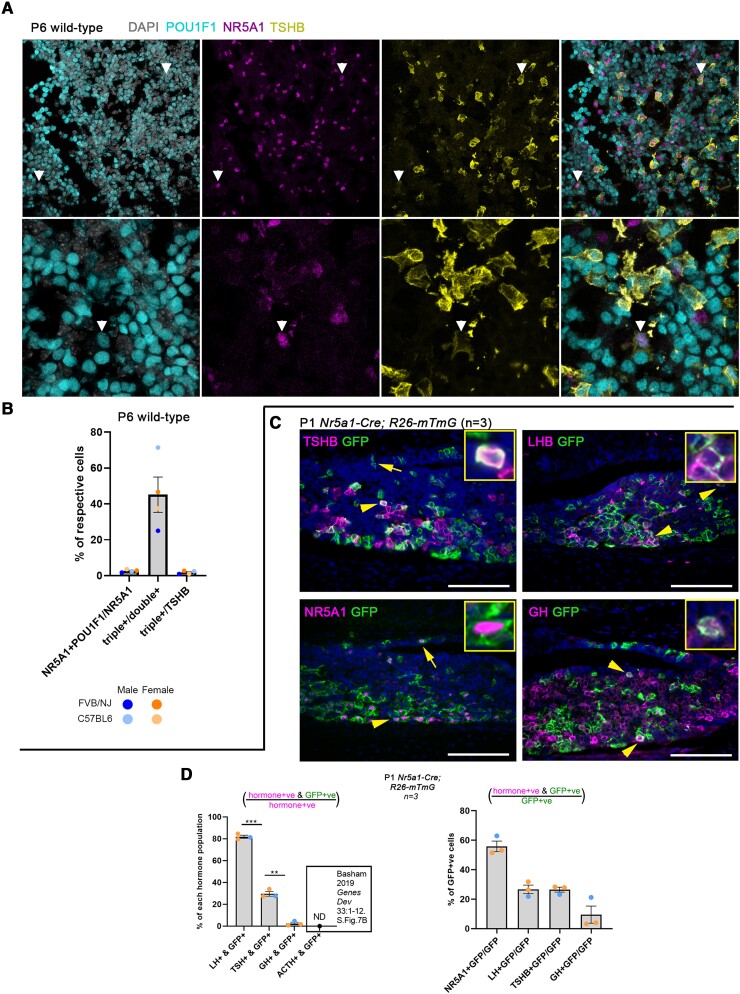
Figure 2.
In vivo detection of Pou1f1/Nr5a1 double-positive cells and Nr5a1-Cre lineage traces 30% of thyrotropes. A, Immunofluorescence for POU1F1 (cyan), NR5A1 (magenta), and TSH β subunit (TSHB; yellow) counterstained with 4′,6-diamidino-2-phenylindole (DAPI; gray-scale) in 4 wild-type P6 mouse pituitaries. At this age many cells express POU1F1 at varying intensities, but POU1F1-negative cells are clear. Rare POU1F1/NR5A1 double-positive cells are observed, of which some also express TSHB (white arrowheads). B, Cell counts of Fig 2A showed that 2.6% of NR5A1+ cells are also POU1F1+; of which 45% also express TSHB. A total of 1.7% of thyrotropes express both POU1F1 and NR5A1. C, Nr5a1-Cre; R26-mTmG lineage tracing at P1 showed high coexpression of NR5A1 or LHB with green fluorescent protein (GFP), some overlap of TSHB and GFP, and very little overlap of growth hormone (GH) and GFP. D, Cell counts from sections of 3 P1 Nr5a1-Cre; R26-mTmG mice showed approximately 30% of thyrotropes are Nr5a1-Cre lineage traced, while fewer than 5% of somatotropes were lineage traced. Note that these percentages are calculated per hormone population and therefore cannot be totaled. There was no detectable Nr5a1-Cre lineage tracing in 6-week-old female adrenocorticotropin (ACTH_-expressing corticotropes (46). Of Nr5a1-Cre lineage-traced (GFP+) cells, 55% are NR5A1+, 25% are luteinizing hormone positive (LH+), 25% are TSHB+, and 9% are GH+. These percentages do not necessarily total 100% because these markers can be coexpressed by the same cell.
In Vivo Detection of Cells pou1f1/Nr5a1 Double-Positive Cells, and Nr5a1-Cre Lineage Traces 30% of Thyrotropes
To determine whether POU1F1/NR5A1 double-positive and NR5A1/POU1F1/TSHB triple-positive cells are observed in vivo, we performed coimmunofluorescence for POU1F1, NR5A1, and TSHB in P6 wild-type mouse pituitary glands. We observed cells that coexpressed POU1F1/NR5A1/TSHB in vivo (Fig. 2A). We also observed thyrotropes that were NR5A1 positive but POU1F1 negative (see Fig. 2A). Cell counts indicated that 2.6% of NR5A1+ cells also express POU1F1, and 45% of double-positive cells also express TSHB (ie, triple-positive). A total of 1.7% of thyrotropes expressed both NR5A1 and POU1F1 (Fig. 2B). We also performed lineage tracing to determine whether Nr5a1-expressing cells give rise to thyrotropes during development. P1 Nr5a1-Cre; R26-mTmG pituitary sections were costained with GFP and TSHB, LHB, NR5A1, or GH (Fig. 2C). We observed cells coexpressing TSHB and GFP, indicating that Nr5a1-expressing cells during pituitary organogenesis give rise to a proportion of thyrotropes by P1. Cell counts of GFP and hormone coexpression demonstrated that approximately 30% of thyrotropes were derived from Nr5a1-expressing cells during pituitary development (Fig. 2D). The majority (∼80%) of LHB- and NR5A1-expressing gonadotropes were labeled with GFP as expected. In contrast, few GH-expressing somatotropes were lineage-traced with GFP (∼2.5%). A previous study found no observable Nr5a1-Cre lineage tracing in proopiomelanocortin (POMC)-expressing corticotropes (46). A total of 55% of Nr5a1-Cre lineage-traced (GFP-expressing) cells were NR5A1+, 25% were LHB+, 25% were TSHB+, and 9% were GH+, noting that cells can coexpress these markers and therefore may not total 100%. We therefore validated our scRNAseq data in 2 ways, by demonstrating that NR5A1/POU1F1 coexpressing cells reside in the juvenile pituitary, and by lineage tracing, which showed a novel developmental trajectory giving rise to thyrotropes.
Expression of Shox2b Isoform in Pituitary Thyrotropes
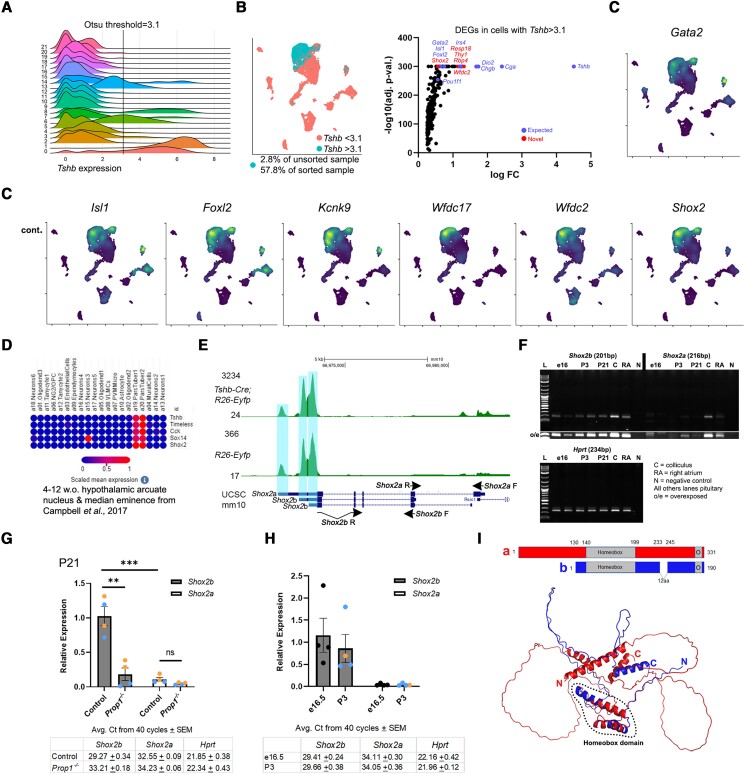
We used Otsu's method to determine a threshold for identifying cells with high Tshb expression (47) (Fig. 3A). As expected, the proportion of cells with high Tshb expression in the Tshb-Cre–sorted sample increased to 57.8% from 2.8% in the unsorted whole pituitary sample, demonstrating the benefit of our FACS approach to enrich for rare thyrotropes (Fig. 3B). We performed differential expression comparison between Tshb-high and Tshb-low (above and below Otsu's threshold, respectively) to determine expression of genes associated with high Tshb expression. This analysis confirmed known thyrotrope transcription factor genes such as Isl1 and Foxl2, and found correlative expression of the transcription factor short stature homeobox 2 (Shox2) (see Fig. 3B and 3C). Shox2 was also detected in an scRNAseq data set of the adult hypothalamus and arcuate nucleus, which included pars tuberalis thyrotropes (Broad Single Cell portal SCP97 (48)) (Fig. 3D). We verified from the sequence alignment maps of the 2 scRNAseq samples that sequencing peaks were appropriately centered around different Shox2 transcript variants and that there were no overlapping genes that could cause incorrect mapping of reads to Shox2 (Fig. 3E). qPCR using Shox2a- and Shox2b-specific primers showed consistent expression of the shorter Shox2b isoform in pituitary developmental and early postnatal life (Fig. 3F). One of the Shox2b-specific primers spans its unique exon-exon junction between the last and penultimate exons to amplify only the Shox2b isoform (see Fig. 3E, Supplementary Fig. S3A (41)). Shox2a is the longer isoform and was not strongly detected in pituitary tissue. qPCR of P21 Prop1-mutant pituitary glands, which lack thyrotropes as well as somatotropes and lactotropes, showed strong reduction in Shox2b and no change to Shox2a, which in controls is much lower than Shox2b (Fig. 3G). qPCR in controls at e16.5 and P3 showed similar expression of Shox2b and low expression of Shox2a (Fig 3H). A Shox2aLacZ knockin mouse strain (33) did not show strong expression of Shox2a in the pituitary (Supplementary Fig. S3B (41)), and Shox2aLacZ/LacZ mutant mice have normal thyrotrope specification at e15.5 (Supplementary Fig. S3C (41)). The SHOX2B protein sequence is shorter than SHOX2A but both contain the homeobox and otp, aristaless, and rax (OAR) domains and there are no other known structural domains in either isoform (Fig. 3I).
Figure 3.
Identification of Shox2b expression in thyrotropes. A, The Otsu method was used to determine a threshold for high Tshb expression and is shown on a ridge plot of Tshb expression distribution across Seurat-calculated clusters. Some clusters are clearly Tshb-high or -low. B, Cells in our data set were separated into two groups based on high or low Tshb expression (above/below Otsu's threshold) and differential expression analysis was performed. Known thyrotrope markers were found such as Isl1 and Foxl2. Novel expression associated with high Tshb expression included the transcription factor Shox2. D, Broad Single Cell Portal data set (SCP97) of adult hypothalamus arcuate nucleus also observed Shox2 enrichment in (pars tuberalis) thyrotropes (48). E, Sequence alignment map of reads from the sorted and unsorted samples show appropriate peaks at the ends of Shox2 transcript variants and no overlapping genes that could cause alignment issues. F, Quantitative polymerase chain reaction (qPCR) using Shox2a- and Shox2b-specific primers shows consistent expression of the Shox2b isoform in pituitary tissue from embryonic and early postnatal ages. No stringent band is observed for Shox2a even when the image was overexposed. G, qPCR of Prop1-mutant mice showed large reduction in Shox2b. H, qPCR of controls showed Shox2b expression is relatively similar from development to early postnatal and Shox2a is virtually undetected. I, Shox2a and Shox2b predicted protein structures both retain the homeobox and otp, aristaless, and rax (OAR; O) domains with no other domains identified (Alphafold).
Expression of Sox14 in Pou1f1-negative Pars Tuberalis Thyrotropes
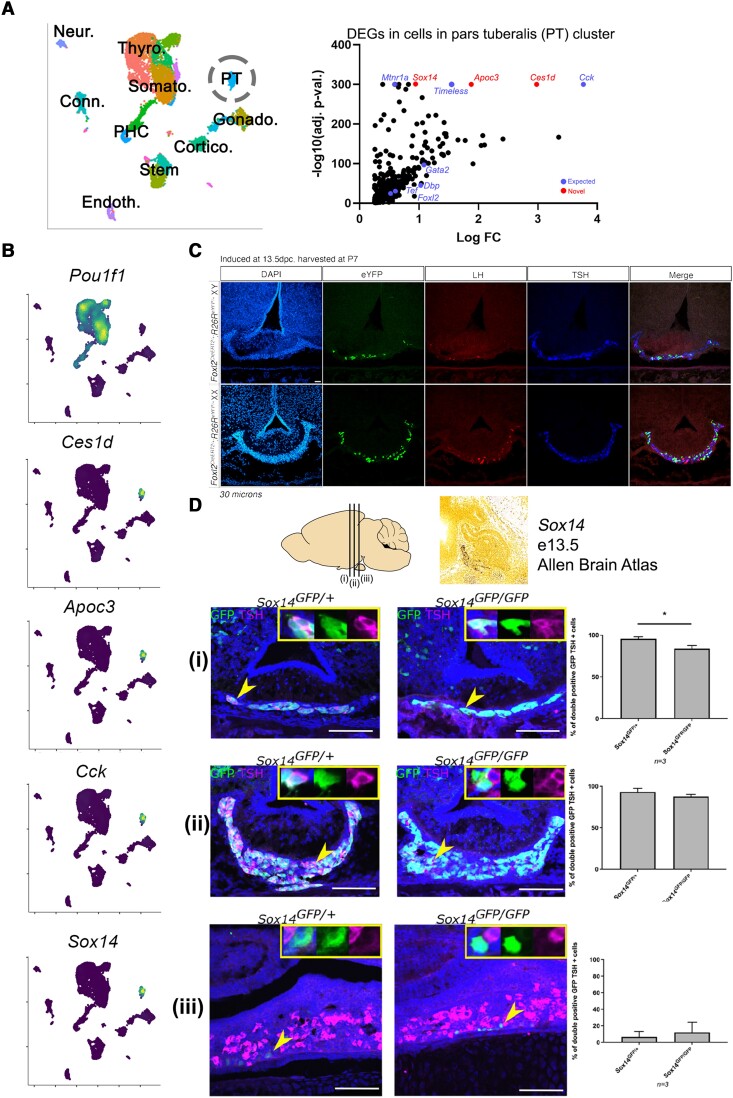
Our data set also contained pars tuberalis thyrotropes, which express Tshb but are negative for Pou1f1 (4). We observed distinct TRH receptor (Trhr) expression in pars distalis thyrotropes and TSH receptor (Tshr) in pars tuberalis thyrotropes (Supplementary Fig. S4B (41)). Thyroid hormone receptor α and β (Thra/Thrb) were detected in both thyrotrope populations, and gonadotropin-releasing hormone receptor (Gnrhr) was detected only in gonadotropes. The pars tuberalis also contains gonadotropes in various species including humans, rats, and mice (49-51) although we were not able to differentiate gonadotrope populations in this analysis, possibly because of the rarity of pars tuberalis gonadotropes that were not enriched by the Tshb-Cre cell sorting. Pars tuberalis thyrotropes were expressed the brain-gut peptide hormone cholecystokinin (Cck) as well as Timeless (Fig. 4A and 4B), both previously reported in the pars tuberalis (48). We identified several novel markers of this population, in particular high specificity of the transcription factor Sox14 (Fig. 4B). Since Sox14 is also expressed in the developing rostral tip of the Rathke pouch, we performed tamoxifen-inducible Foxl2-CreERT2 lineage tracing at e13.5, after the specification of Pou1f1-independent rostral tip thyrotropes but before the specification of Pou1f1-dependent Rathke pouch thyrotropes. We observed GFP tracing in the postnatal pars tuberalis of animals induced at e13.5 (Fig. 4C), demonstrating that the Foxl2-positive embryonic rostral tip gives rise to the postnatal pars tuberalis. We investigated the role of Sox14 in the pars tuberalis by studying Sox14Egfp/Egfp knockout mice (34, 52), which contain an EGFP-expressing cassette replacing the single exon. We validated our scRNAseq finding and found Sox14-Egfp cells in the pars tuberalis lining the median eminence of the anterior and medial hypothalamus in P0 mice (Fig. 4D). Coimmunofluorescence with TSHB demonstrates that almost all SOX14-expressing cells also express TSHB. In homozygote Sox14Egfp/Egfp mice, we found a small reduction in the proportion of EGFP/TSHB double-positive cells in the anterior hypothalamic region, but most EGFP-positive cells continue to express TSHB (Fig. 4D). Therefore, we showed that Sox14 is strongly expressed from early pituitary development but that it is not required for pars tuberalis thyrotrope specification.
Figure 4.
Expression of Sox14 in Pou1f1-negative pars tuberalis (PT) thyrotropes. A, PT cells identified in our single-cell RNA sequencing (scRNAseq) data set and differential gene analysis found several known PT genes such as Cck and Timeless. B, Expression of known and novel genes in the PT, including high specificity of the transcription factor Sox14. C, Foxl2CreERT2 lineage tracing after induction at e13.5 showed EGFP-traced cells in the PT, indicating that the embryonic rostral tip of the Rathke pouch gives rise to the postnatal PT. D, Sox14 is expressed in the PT lining the median eminence in the anterior and medial hypothalamus, but the number of TSH β subunit (TSHB)-expressing cells in Sox14Egfp/Egfp knockout mice remains similar to heterozygous controls. Arrowheads indicate cells in the inset. n = 3 per genotype. Murine brain schematic and (i/ii/iii) notation indicates approximate plane of section.
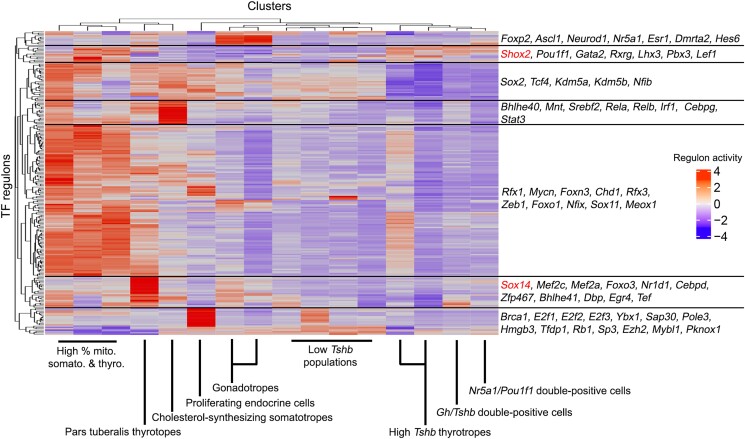
Computationally Predicted Activation of Shox2 and Sox14 Regulons
We performed single-cell regulatory network inference and clustering (SCENIC) (43) on cell clusters that contained Eyfp-expressing (ie, Tshb-Cre-derived) cells (Supplementary Fig. S4, Supplementary Table S1 (41)). The Sox14 regulon was predicted to be active in pars tuberalis thyrotropes, together with known factors such as Tef and Dbp. Shox2 activation was found in high Tshb-expressing clusters together with other known thyrotrope factors such as Pou1f1 and Gata2 (Fig. 5).
Figure 5.
Single-cell regulatory network inference and clustering (SCENIC) (43) on Tshb-Cre; Eyfp-traced cell populations.
Discussion
In this study, we have used a genetic mouse model to enrich for pituitary thyrotropes to gain higher resolution of thyrotrope heterogeneity and rare cell states. Although our data set contains RNA libraries prepared from pooled male and female samples, we were able to validate Shox2b expression and Nr5a1/Pou1f1 double-positive cells in both sexes in vivo.
Discovery of Novel Pou1f1/Nr5a1 Double-positive Cells and Subpopulation of Nr5a1-derived Thyrotropes
Conventional understanding of pituitary developmental transcriptional cascades considers that thyrotropes and gonadotropes are derived from 2 separate, unrelated lineages (1). POU1F1 mutations in humans and mice cause pituitary hormone deficiencies of GH, PRL, and TSH (2, 4, 53), and NR5A1 mutations in humans and mice can cause partial LH/FSHB deficiencies in hypogonadotropic hypogonadism (6, 54, 55). In this study, we have characterized a novel developmental cell state with concurrent expression of both Nr5a1 and Pou1f1 through scRNAseq and validated this by coimmunofluorescence in vivo. We demonstrated through Nr5a1-Cre lineage tracing that 30% of thyrotropes are derived from gonadotrope progenitors by P1, indicating that these cells were born during development, but it is unclear whether Nr5a1-lineage thyrotropes differ in biological function from those derived from the Pou1f1 progenitors. We continue to observe Nr5a1/Pou1f1 double-positive cells by single-cell sequencing and immunofluorescence at P6-P7, although we have not examined later ages to determine whether these cells are maintained postnatally. Unique expression of double-positive-specific genes such as Efna2 and Tmem191c will need to be further validated and functionally tested. Tshb-Cre lineage-traced gonadotropes may represent the proportion that are derived from this dual-identity precursor population. While we ultimately cannot rule out nonspecific CRE expression in our mouse models and thus question their origin and fate (28, 31), the presence of NR5A1; POU1F1 double-positive cells in vivo together with the lack of similar Nr5a1-Cre lineage tracing in somatotropes and corticotropes strongly support the existence of Nr5a1-derived thyrotropes during development. A low proportion of gonadotropes have also been observed to be traced by the Tg:Pou1f1-Cre mouse model (56), which has previously been attributed to nonspecific CRE expression. We performed cell counts with limited samples (n = 2) and observed 25% of gonadotropes were Tg:Pou1f1-Cre lineage traced. Intermediate thyrotrope-gonadotrope cells expressing FSH and TSH have also been observed in early mouse development (57). Immortalization of these dual-identity precursors may be the source of plurihormonal adenomas that express both TSH and gonadotropins in humans (7, 58, 59).
Expression of Shox2b in Thyrotropes
Shox2 is implicated in craniofacial, brain, heart, and limb development (60, 61). Mutations in SHOX2 in humans can cause heart defects such as atrial fibrillation (62-65). SHOX2 is lowly expressed in the human adult pituitary (Human Protein Atlas [proteinatlas.org] and GTEx [gtexportal.org]). We detected Shox2 expression in pars distalis thyrotropes, as well as pars tuberalis thyrotropes, where transcripts are elevated in the night relative to day if melatonin receptor 1 is functional (66). Bone morphogenetic protein 4 (Bmp4) is a direct target of SHOX2 in the developing heart (60), and BMPs including BMP4 are produced in the ventral diencephalon and are required for the initial induction of oral ectoderm into the pituitary primordium called the Rathke pouch (67-69). Mice lacking the BMP2/4 antagonist Noggin have pituitary induction defects but endocrine specification, including into the thyrotrope lineage, is not affected in later development (69). Islet 1 (Isl1) is a direct transcriptional target of Shox2 in the developing heart (70), and an Isl1, Shox2, Tbx3, and Hox gene program regulates sinoatrial node development (71-73). Isl1 is an important regulator of thyrotrope specification (14, 15) and Shox2 may therefore be a novel regulator of thyrotrope specification by acting through Isl1 and other genes. It is also possible that it plays a role in the daily oscillations in Tshb transcription in pars distalis thyrotropes by acting together with Nr1d1 (Rev-Erbα) (74).
We detected Shox2 enrichment in thyrotropes in our single-cell data, and subsequently found that it is the shorter Shox2b isoform that is expressed in the developmental and early postnatal pituitary. Shox2a appears to be the more widely expressed, essential isoform given that Shox2–/– genomic mutants, with both Shox2a and Shox2b deleted, and compound heterozygote Shox2LacZ/– mice both died at birth and showed similar limb and palate defects (33). Shox2a and Shox2b are both expressed in the embryonic heart (75). The 2 Shox2 isoforms have different transcriptional start sites and first exons leading to an additional 129 N-terminal amino acids encoded by Shox2a, as well as a 12–amino acid extension of the last exon of Shox2a (Fig. 3I) (61, 76). However, both isoforms contain the homeobox domain and otp, aristaless, and rax (OAR) domains and the C-terminal portions of both isoforms (after the homeobox domain) (see Fig. 3I) can act as transcriptional activators or repressors depending on cell line (77, 78). The 2 isoforms can homodimerize or heterodimerize (79). The N-terminal portion of Shox2a interacts with B56 regulatory δ isoform (Ppp2r5d) of protein phosphatase 2a and is phosphorylated at Ser92 and Ser110, which is required to bind to the promoter of Nkx2.5 (80), an important cardiac transcription factor (81). The specific expression of Shox2b in the pituitary may therefore be a mechanism to prevent activation of Shox2a target genes.
Sox14 Expression in Pars Tuberalis Thyrotropes
In contrast to some studies suggesting rostral tip thyrotropes are transient in development (4, 82), we used the inducible Foxl2CreERT2 to demonstrate that rostral tip cells tamoxifen-induced at e13.5 give rise to the pars tuberalis postnatally. Pars tuberalis thyrotropes regulate photoperiodicity and seasonal breeding (in eg, sheep) in response to photoperiod-dependent melatonin input from the pineal gland (83-86). Long photoperiods in mammals and birds have been found to alter gene expression in the pars tuberalis such as Nr1d1 and Dbp (20), and studies have previously identified factors including Eya3, Six1, and Tef as photoperiod-induced transcription factors that directly drive Tshb expression (18, 20). Sox14 was a highly specific transcription factor expressed in the pars tuberalis, but the number of TSHB-positive thyrotropes in the pars tuberalis of Sox14Egfp/Egfp knockout mice was similar, indicating that Sox14 was not required for pars tuberalis thyrotrope specification during development. Sox14 is expressed in the brain subcortical regions, cerebellum, thalamus, and hypothalamus (44, 52, 87-90), and it is required for robust synchronization of circadian-regulated behaviors with the day-night light cycle (52). Future studies will examine whether Sox14 is important for circadian regulation of gene expression in pars tuberalis thyrotropes. GATA factors have been implicated as part of the Sox14 regulatory network. SOX14 regulates GATA3 in the dorsal midbrain (91) while in the diencephalon Gata2 appears to be upstream of Sox14 (92). Thus, Sox14 may act downstream of Gata2 in pars tuberalis thyrotropes.
Summary
We performed scRNAseq using control pituitary cells combined with FACS cells from P7 Tg(Tshb-Cre); R26Eyfp/Eyfp mice to increase the proportion of thyrotrope-related populations within our data set. We found transcriptomic and in vivo evidence of a novel cell population concurrently expressing Nr5a1 and Pou1f1, and a novel developmental trajectory for Nr5a1-derived thyrotropes. Our single-cell data set provides a resource for further dissection of neonatal thyrotrope and pituitary transcriptomics. Future studies will examine the role of top candidates of thyrotropes such as Shox2 and Sox14 in day-night oscillations in gene expression.
Acknowledgments
We thank the University of Michigan Advanced Genomics Core, in particular Dr Olivia Koues, Judith Opp, and Dr Robert Lyons, for help in performing single-cell RNA sequencing. We thank Prof Sue Hammoud and Dr Paul Le Tissier for input to data analyses and manuscript preparation. We thank Dr Cynthia Andoniadou for sharing the TSHB antibody.
Glossary
Abbreviations
- ACTH
adrenocorticotropin
- BAC
bacterial artificial chromosome
- BMP
bone morphogenetic protein
- DAPI
4′,6-diamidino-2-phenylindole
- FACS
fluorescence-activated cell sorting
- FSH
follicle-stimulating hormone
- GH
growth hormone
- LH
luteinizing hormone
- mRNA
messenger RNA
- OAR
otp, aristaless, and rax
- P7
aged 7 days
- PRL
prolactin
- qPCR
quantitative polymerase chain reaction
- scRNAseq
single-cell RNA sequencing
- TRH
TSH-releasing hormone
- TSH
thyrotropin
- TSHβ
TSH β subunit
Contributor Information
Leonard Y M Cheung, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109, USA.
Lucy Menage, School of Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London SE5 8AF, UK.
Karine Rizzoti, Laboratory of Stem Cell Biology and Developmental Genetics, The Francis Crick Institute, London NW1 1AT, UK.
Greg Hamilton, Department of Biological Sciences, University of Calgary, Calgary AB T2N 1N4, Canada.
Typhanie Dumontet, Training Program in Organogenesis, Center for Cell Plasticity and Organ Design, University of Michigan, Ann Arbor, MI 48109, USA; Department of Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, MI 48109, USA.
Kaitlin Basham, Department of Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Current affiliation: Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA.
Alexandre Z Daly, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109, USA; Current affiliation is Vanguard, Valley Forge, PA 19482, USA.
Michelle L Brinkmeier, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109, USA.
Bailey E Masser, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109, USA.
Mathias Treier, Max Delbrϋck Center for Molecular Medicine (MDC), 13092 Berlin, Germany; Charité-Universitätsmedizin Berlin, 10117 Berlin, Germany.
John Cobb, Department of Biological Sciences, University of Calgary, Calgary AB T2N 1N4, Canada.
Alessio Delogu, School of Neuroscience, Institute of Psychiatry, Psychology and Neuroscience, King's College London, London SE5 8AF, UK.
Robin Lovell-Badge, Laboratory of Stem Cell Biology and Developmental Genetics, The Francis Crick Institute, London NW1 1AT, UK.
Gary D Hammer, Department of Internal Medicine, Division of Metabolism, Endocrinology, and Diabetes, University of Michigan, Ann Arbor, MI 48109, USA; Endocrine Oncology Program, Rogel Cancer Center, University of Michigan, Ann Arbor, MI 48109, USA.
Sally A Camper, Department of Human Genetics, University of Michigan, Ann Arbor, MI 48109, USA.
Funding
This work was supported by the National Institutes of Health (R01HD034283 and R01HD097096 to S.A.C.); Natural Sciences and Engineering Research Council of Canada (RGPIN-2019-04812 to J.C.); Training program in organogenesis, Dean's Non-Traditional Fellowship (to T.D.), University of Michigan; International Fund Congenital Adrenal Hyperplasia (to G.D.H.); National Institutes of Health (DK062027 to G.D.H.); Biotechnology and Biological Sciences Research Council (BB/R007020/1 to A.D.); Medical Research Council, UK (U117512772, U117562207 and U117570590) and the Francis Crick Institute core funding from Cancer Research UK (FC001107), the Medical Research Council, United Kingdom (FC001107), and the Wellcome Trust (FC001107 to R.L.B.).
Author Contributions
Conceptualization, L.Y.M.C., K.R., R.L.B., G.D.H., S.A.C.; methodology, L.Y.M.C., A.Z.D.; software, L.Y.M.C., A.Z.D.; Validation, L.Y.M.C., K.R., G.H., L.M.; formal analysis, L.Y.M.C., L.M., K.R., G.H.; investigation, L.Y.M.C., L.M., K.R., G.H.; resources, L.Y.M.C., K.R., T.D., K.B., B.E.M., M.T., R.L.B., G.D.H., S.A.C., G.H., J.C., L.M., A.D.; data curation, L.Y.M.C., K.R., G.H., L.M.; writing—original draft, L.Y.M.C., S.A.C.; writing—review/editing, L.Y.M.C., L.M., K.R., T.D., K.B., A.Z.D., M.L.B., M.T., R.L.B., G.D.H., S.A.C.; visualization, L.Y.M.C.; supervision, L.Y.M.C., A.D., J.C., R.L.B., G.D.H., S.A.C.; project administration, R.L.B., G.D.H., S.A.C., J.C., A.D.; funding acquisition, R.L.B., G.D.H., S.A.C., J.C., A.D.
Disclosures
The authors have nothing to disclose.
Data Availability
Sequencing data are available on the National Center for Biotechnology Information Gene Expression Omnibus under accession number GSE205418 and expression data are available on the Broad Single Cell Portal at SCP2110. Some data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Current Affiliation
Alexandre Z. Daly’s current affiliation is Vanguard, Valley Forge, PA 19482, USA.
References
- 1. Prince KL, Walvoord EC, Rhodes SJ. The role of homeodomain transcription factors in heritable pituitary disease. Nat Rev Endocrinol. 2011;7(12):727‐737. [DOI] [PubMed] [Google Scholar]
- 2. Radovick S, Nations M, Du Y, Berg LA, Weintraub BD, Wondisford FE. A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science. 1992;257(5073):1115‐1118. [DOI] [PubMed] [Google Scholar]
- 3. Li S, Crenshaw EB, Rawson EJ III, Simmons DM, Swanson LW, Rosenfeld MG. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene Pit-1. Nature. 1990;347(6293):528‐533. [DOI] [PubMed] [Google Scholar]
- 4. Lin SC, Li S, Drolet DW, Rosenfeld MG. Pituitary ontogeny of the snell dwarf mouse reveals Pit-1-independent and Pit-1-dependent origins of the thyrotrope. Development. 1994;120(3):515‐522. [DOI] [PubMed] [Google Scholar]
- 5. Ingraham HA, Lala DS, Ikeda Y, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8(19):2302‐2312. [DOI] [PubMed] [Google Scholar]
- 6. Shinoda K, Lei H, Yoshii H, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995;204(1):22‐29. [DOI] [PubMed] [Google Scholar]
- 7. Tordjman KM, Greenman Y, Ram Z, et al. Plurihormonal pituitary tumor of Pit-1 and SF-1 lineages, with synchronous collision corticotroph tumor: a possible stem cell phenomenon. Endocr Pathol. 2019;30(1):74‐80. [DOI] [PubMed] [Google Scholar]
- 8. Zhang S, Cui Y, Ma X, et al. Single-cell transcriptomics identifies divergent developmental lineage trajectories during human pituitary development. Nat Commun. 2020;11(1):5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikegami K, Refetoff S, Van Cauter E, Yoshimura T. Interconnection between circadian clocks and thyroid function. Nat Rev Endocrinol. 2019;15(10):590‐600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado Thyroid Disease Prevalence Study. Arch Intern Med. 2000;160(4):526‐534. [DOI] [PubMed] [Google Scholar]
- 11. Daly AZ, Dudley LA, Peel MT, Liebhaber SA, Parker SCJ, Camper SA. Multi-omic profiling of pituitary thyrotropic cells and progenitors. BMC Biol. 2021;19(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellsworth BS, Egashira N, Haller JL, et al. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol. 2006;20(11):2796‐2805. [DOI] [PubMed] [Google Scholar]
- 13. Dasen JS, O’Connell SM, Flynn SE, et al. Reciprocal interactions of Pit1 and GATA2 mediate signaling gradient-induced determination of pituitary cell types. Cell. 1999;97(5):587‐598. [DOI] [PubMed] [Google Scholar]
- 14. Brinkmeier ML, Bando H, Camarano AC, et al. Rathke's cleft-like cysts arise from Isl1 deletion in murine pituitary progenitors. J Clin Invest. 2020;130(8):4501‐4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Castinetti F, Brinkmeier ML, Mortensen AH, et al. ISL1 is necessary for maximal thyrotrope response to hypothyroidism. Mol Endocrinol. 2015;29(10):1510‐1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bockmann J, Böckers TM, Winter C, et al. Thyrotropin expression in hypophyseal pars tuberalis-specific cells is 3,5,3'-triiodothyronine, thyrotropin-releasing hormone, and Pit-1 independent. Endocrinology. 1997;138(3):1019‐1028. [DOI] [PubMed] [Google Scholar]
- 17. Shinomiya A, Shimmura T, Nishiwaki-Ohkawa T, Yoshimura T. Regulation of seasonal reproduction by hypothalamic activation of thyroid hormone. Front Endocrinol (Lausanne). 2014;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dardente H, Wyse CA, Birnie MJ, et al. A molecular switch for photoperiod responsiveness in mammals. Curr Biol. 2010;20(24):2193‐2198. [DOI] [PubMed] [Google Scholar]
- 19. Nakao N, Nakagawa K, Sasaki A, Yamaguchi A, Tsushima N, Tanaka M. Photoperiod-specific expression of eyes absent 3 splice variant in the pars tuberalis of the Japanese quail. J Poult Sci. 2021;58(1):64‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masumoto KH, Ukai-Tadenuma M, Kasukawa T, et al. Acute induction of Eya3 by late-night light stimulation triggers TSHβ expression in photoperiodism. Curr Biol. 2010;20(24):2199‐2206. [DOI] [PubMed] [Google Scholar]
- 21. Kim DW, Washington PW, Wang ZQ, et al. The cellular and molecular landscape of hypothalamic patterning and differentiation from embryonic to late postnatal development. Nat Commun. 2020;11(1):4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shami AN, Zheng X, Munyoki SK, et al. Single-cell RNA sequencing of human, macaque, and mouse testes uncovers conserved and divergent features of mammalian spermatogenesis. Dev Cell. 2020;54(4):529‐547.e512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green CD, Ma Q, Manske GL, et al. A comprehensive roadmap of murine spermatogenesis defined by single-cell RNA-Seq. Dev Cell. 2018;46(5):651‐667.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ruf-Zamojski F, Zhang Z, Zamojski M, et al. Single nucleus multi-omics regulatory landscape of the murine pituitary. Nat Commun. 2021;12(1):2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Z, Zamojski M, Smith GR, et al. Single nucleus transcriptome and chromatin accessibility of postmortem human pituitaries reveal diverse stem cell regulatory mechanisms. Cell Rep. 2022;38(10):110467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mayran A, Sochodolsky K, Khetchoumian K, et al. Pioneer and nonpioneer factor cooperation drives lineage specific chromatin opening. Nat Commun. 2019;10(1):3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cheung LYM, George AS, McGee SR, et al. Single-cell RNA sequencing reveals novel markers of male pituitary stem cells and hormone-producing cell types. Endocrinology. 2018;159(12):3910‐3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Castinetti F, Brinkmeier ML, Gordon DF, et al. PITX2 and PITX1 regulate thyrotroph function and response to hypothyroidism. Mol Endocrinol. 2011;25(11):1950‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bingham NC, Verma-Kurvari S, Parada LF, Parker KL. Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis. 2006;44(9):419‐424. [DOI] [PubMed] [Google Scholar]
- 31. Cheung L, Le Tissier P, Goldsmith SG, Treier M, Lovell-Badge R, Rizzoti K. NOTCH activity differentially affects alternative cell fate acquisition and maintenance. Elife. 2018;7:e33318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593‐605. [DOI] [PubMed] [Google Scholar]
- 33. Rosin JM, Kurrasch DM, Cobb J. Shox2 is required for the proper development of the facial motor nucleus and the establishment of the facial nerves. BMC Neurosci. 2015;16(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crone SA, Quinlan KA, Zagoraiou L, et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60(1):70‐83. [DOI] [PubMed] [Google Scholar]
- 35. Nasonkin IO, Ward RD, Raetzman LT, et al. Pituitary hypoplasia and respiratory distress syndrome in Prop1 knockout mice. Hum Mol Genet. 2004;13(22):2727‐2735. [DOI] [PubMed] [Google Scholar]
- 36. Nagy A, Gertsenstein M, Vintersten K, Behringer R. Staining whole mouse embryos for {beta}-galactosidase (lacZ) activity. CSH Protoc. 2007;2007:pdb.prot4725. [DOI] [PubMed] [Google Scholar]
- 37. Cheung LYM, Camper SA. PROP1-dependent retinoic acid signaling regulates developmental pituitary morphogenesis and hormone expression. Endocrinology. 2020;161(2):bqaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. La Manno G, Soldatov R, Zeisel A, et al. RNA velocity of single cells. Nature. 2018;560(7719):494‐498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stuart T, Butler A, Hoffman P, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888‐1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheung LYM, Menage L, Rizzoti K, et al. Supplementary data for “Novel candidate regulators and developmental trajectory of pituitary thyrotropes.” Figshare 2023. Uploaded April 26, 2023. 10.6084/m9.figshare.22700050.v1 [DOI] [PMC free article] [PubMed]
- 42. Alquicira-Hernandez J, Powell JE. Nebulosa recovers single cell gene expression signals by kernel density estimation. Bioinformatics. 2021;37(16):2485‐2487. [DOI] [PubMed] [Google Scholar]
- 43. Aibar S, González-Blas CB, Moerman T, et al. SCENIC: single-cell regulatory network inference and clustering. Nat Methods. 2017;14(11):1083‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jager P, Moore G, Calpin P, et al. Dual midbrain and forebrain origins of thalamic inhibitory interneurons. Elife. 2021;10:e59272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moncho-Amor V, Chakravarty P, Galichet C, Matheu A, Lovell-Badge R, Rizzoti K. SOX2 is required independently in both stem and differentiated cells for pituitary tumorigenesis in p27-null mice. Proc Natl Acad Sci U S A. 2021;118(7):e2017115118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Basham KJ, Rodriguez S, Turcu AF, et al. A ZNRF3-dependent Wnt/β-catenin signaling gradient is required for adrenal homeostasis. Genes Dev. 2019;33(3-4):209‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fletcher PA, Smiljanic K, Maso Prévide R, et al. Cell type- and sex-dependent transcriptome profiles of rat anterior pituitary cells. Front Endocrinol (Lausanne). 2019;10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Campbell JN, Macosko EZ, Fenselau H, et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci. 2017;20(3):484‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Asa SL, Kovacs K, Bilbao JM. The pars tuberalis of the human pituitary. A histologic, immunohistochemical, ultrastructural and immunoelectron microscopic analysis. Virchows Arch A Pathol Anat Histopathol. 1983;399(1):49‐59. [DOI] [PubMed] [Google Scholar]
- 50. Gross DS. The mammalian hypophysial pars tuberalis: a comparative immunocytochemical study. Gen Comp Endocrinol. 1984;56(2):283‐298. [DOI] [PubMed] [Google Scholar]
- 51. Alim Z, Hartshorn C, Mai O, et al. Gonadotrope plasticity at cellular and population levels. Endocrinology. 2012;153(10):4729‐4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Delogu A, Sellers K, Zagoraiou L, et al. Subcortical visual shell nuclei targeted by ipRGCs develop from a Sox14+-GABAergic progenitor and require Sox14 to regulate daily activity rhythms. Neuron. 2012;75(4):648‐662. [DOI] [PubMed] [Google Scholar]
- 53. Fang Q, George AS, Brinkmeier ML, et al. Genetics of combined pituitary hormone deficiency: roadmap into the genome era. Endocr Rev. 2016;37(6):636‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zhao L, Bakke M, Parker KL. Pituitary-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol. 2001;185(1-2):27‐32. [DOI] [PubMed] [Google Scholar]
- 55. Lin L, Philibert P, Ferraz-de-Souza B, et al. Heterozygous missense mutations in steroidogenic factor 1 (SF1/Ad4BP, NR5A1) are associated with 46,XY disorders of sex development with normal adrenal function. J Clin Endocrinol Metab. 2007;92(3):991‐999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gaston-Massuet C, Andoniadou CL, Signore M, et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc Natl Acad Sci U S A. 2011;108(28):11482‐11487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Wen S, Ai W, Alim Z, Boehm U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc Natl Acad Sci U S A. 2010;107(37):16372‐16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tritos NA, Eppakayala S, Swearingen B, et al. Pathologic and clinical features of pituitary adenomas showing TSH immunoreactivity. Pituitary. 2013;16(3):287‐293. [DOI] [PubMed] [Google Scholar]
- 59. Bermingham J, Haenel LC. Hyperthyroidism with an FSH-and TSH-secreting pituitary adenoma. J Am Osteopath Assoc. 1989;89(12):1560‐1566. [PubMed] [Google Scholar]
- 60. Puskaric S, Schmitteckert S, Mori AD, et al. Shox2 mediates Tbx5 activity by regulating Bmp4 in the pacemaker region of the developing heart. Hum Mol Genet. 2010;19(23):4625‐4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Blaschke RJ, Monaghan AP, Schiller S, et al. SHOT, a SHOX-related homeobox gene, is implicated in craniofacial, brain, heart, and limb development. Proc Natl Acad Sci U S A. 1998;95(5):2406‐2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li N, Wang ZS, Wang XH, et al. A SHOX2 loss-of-function mutation underlying familial atrial fibrillation. Int J Med Sci. 2018;15(13):1564‐1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hoffmann S, Paone C, Sumer SA, et al. Functional characterization of rare variants in the SHOX2 gene identified in sinus node dysfunction and atrial fibrillation. Front Genet. 2019;10:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hoffmann S, Clauss S, Berger IM, et al. Coding and non-coding variants in the SHOX2 gene in patients with early-onset atrial fibrillation. Basic Res Cardiol. 2016;111(3):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sumer SA, Hoffmann S, Laue S, et al. Precise correction of heterozygous SHOX2 mutations in hiPSCs derived from patients with atrial fibrillation via genome editing and sib selection. Stem Cell Reports. 2020;15(4):999‐1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Unfried C, Burbach G, Korf HW, von Gall C. Melatonin receptor 1-dependent gene expression in the mouse pars tuberalis as revealed by cDNA microarray analysis and in situ hybridization. J Pineal Res. 2010;48(2):148‐156. [DOI] [PubMed] [Google Scholar]
- 67. Treier M, Gleiberman AS, O’Connell SM, et al. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12(11):1691‐1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125(6):1005‐1015. [DOI] [PubMed] [Google Scholar]
- 69. Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305(1):145‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hoffmann S, Berger IM, Glaser A, et al. Islet1 is a direct transcriptional target of the homeodomain transcription factor Shox2 and rescues the Shox2-mediated bradycardia. Basic Res Cardiol. 2013;108(2):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Galang G, Mandla R, Ruan H, et al. ATAC-seq reveals an Isl1 enhancer that regulates sinoatrial node development and function. Circ Res. 2020;127(12):1502‐1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Eif VWW, Protze S, Bosada FM, et al. Genome-wide analysis identifies an essential human TBX3 pacemaker enhancer. Circ Res. 2020;127(12):1522‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. van Eif VWW, Stefanovic S, van Duijvenboden K, et al. Transcriptome analysis of mouse and human sinoatrial node cells reveals a conserved genetic program. Development. 2019;146(8):dev173161. [DOI] [PubMed] [Google Scholar]
- 74. Aninye IO, Matsumoto S, Sidhaye AR, Wondisford FE. Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J Biol Chem. 2014;289(24):17070‐17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Blaschke RJ, Hahurij ND, Kuijper S, et al. Targeted mutation reveals essential functions of the homeodomain transcription factor Shox2 in sinoatrial and pacemaking development. Circulation. 2007;115(14):1830‐1838. [DOI] [PubMed] [Google Scholar]
- 76. Rovescalli AC, Asoh S, Nirenberg M. Cloning and characterization of four murine homeobox genes. Proc Natl Acad Sci U S A. 1996;93(20):10691‐10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu L, Liu H, Yan M, et al. Shox2 is required for chondrocyte proliferation and maturation in proximal limb skeleton. Dev Biol. 2007;306(2):549‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Liu H, Chen CH, Espinoza-Lewis RA, et al. Functional redundancy between human SHOX and mouse Shox2 genes in the regulation of sinoatrial node formation and pacemaking function. J Biol Chem. 2011;286(19):17029‐17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Aza-Carmona M, Barca-Tierno V, Hisado-Oliva A, et al. NPPB and ACAN, two novel SHOX2 transcription targets implicated in skeletal development. PLoS One. 2014;9(1):e83104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu H, Chen CH, Ye W, et al. Phosphorylation of Shox2 is required for its function to control sinoatrial node formation. J Am Heart Assoc. 2014;3(3):e000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Benson DW, Silberbach GM, Kavanaugh-McHugh A, et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104(11):1567‐1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gleiberman AS, Michurina T, Encinas JM, et al. Genetic approaches identify adult pituitary stem cells. Proc Natl Acad Sci U S A. 2008;105(17):6332‐6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dardente H, Wood S, Ebling F, Sáenz de Miera C. An integrative view of mammalian seasonal neuroendocrinology. J Neuroendocrinol. 2019;31(5):e12729. [DOI] [PubMed] [Google Scholar]
- 84. Sáenz de Miera C, Bothorel B, Jaeger C, Simonneaux V, Hazlerigg D. Maternal photoperiod programs hypothalamic thyroid status via the fetal pituitary gland. Proc Natl Acad Sci U S A. 2017;114(31):8408‐8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wood S, Loudon A. The pars tuberalis: the site of the circannual clock in mammals? Gen Comp Endocrinol. 2018;258:222‐235. [DOI] [PubMed] [Google Scholar]
- 86. Wood SH. How can a binary switch within the pars tuberalis control seasonal timing of reproduction? J Endocrinol. 2018;239(1):R13‐R25. [DOI] [PubMed] [Google Scholar]
- 87. Huisman C, Cho H, Brock O, et al. Single cell transcriptome analysis of developing arcuate nucleus neurons uncovers their key developmental regulators. Nat Commun. 2019;10(1):3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Prekop HT, Kroiss A, Rook V, et al. Sox14 is required for a specific subset of cerebello-olivary projections. J Neurosci. 2018;38(44):9539‐9550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hargrave M, Karunaratne A, Cox L, Wood S, Koopman P, Yamada T. The HMG box transcription factor gene Sox14 marks a novel subset of ventral interneurons and is regulated by sonic hedgehog. Dev Biol. 2000;219(1):142‐153. [DOI] [PubMed] [Google Scholar]
- 90. Jager P, Ye Z, Yu X, et al. Tectal-derived interneurons contribute to phasic and tonic inhibition in the visual thalamus. Nat Commun. 2016;7(1):13579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Makrides N, Panayiotou E, Fanis P, Karaiskos C, Lapathitis G, Malas S. Sequential role of SOXB2 factors in GABAergic neuron specification of the dorsal midbrain. Front Mol Neurosci. 2018;11:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Virolainen SM, Achim K, Peltopuro P, Salminen M, Partanen J. Transcriptional regulatory mechanisms underlying the GABAergic neuron fate in different diencephalic prosomeres. Development. 2012;139(20):3795‐3805. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Sequencing data are available on the National Center for Biotechnology Information Gene Expression Omnibus under accession number GSE205418 and expression data are available on the Broad Single Cell Portal at SCP2110. Some data sets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.