Abstract
Background:
We assessed the utility of EndoPAT, a device that measures reactive hyperemia index (RHI) as a clinical screening tool for identifying low coronary flow reserve (CFR). Distinguishing normal from low CFR aids assessment for coronary microvascular dysfunction (CMD) or large vessel coronary artery disease (CAD).
Methods:
From June 2014-May 2019, in a convenience sample, we measured RHI in adults undergoing clinically indicated cardiac Rubidium-82 positron emission tomography/computed tomography (PET/CT) at a single center. Exclusion criteria were inability to consent, lack of English proficiency, and physical limitation. We defined low RHI as <1.67 and low CFR as <2.5. Distribution of RHI was skewed so we used its natural logarithm (LnRHI) to calculate Pearson correlation and area under the curve (AUC).
Results:
Of 265 patients with PET/CT, we enrolled 131, and 100 had adequate data. Patients had a mean age of 61 years (SD = 12), 46% were female, 29% non-white. Thirty-six patients had low RHI, and 60 had depressed CFR. LnRHI did not distinguish patients with low from normal CFR (AUC=0.53; 95% Cl, 0.41–0.64) and did not correlate with CFR (r=−0.021, p=0.83). Low RHI did not distinguish patients with traditional CAD risk factors, presence of calcification, or perfusion defect (p >0.05). Conversely, mean augmentation index, a measure of arterial stiffness, was higher with low RHI (p=0.005) but not CFR (p=0.625). RHI was lower in patients we identified as CMD (low CFR, no perfusion defect and calcium score of 0) (1.88 versus 2.21; p=0.35) although we were underpowered (n=12) to meet statistical significance.
Conclusions:
Peripheral RHI is insufficient as a clinical screening tool for low CFR as measured by cardiac PET/CT. Differences in vascular pathology assessed by each method may explain this finding.
Keywords: coronary flow reserve, myocardial perfusion reserve, peripheral flow, ischemic heart disease, microcirculation, arterial tonometry
Introduction
Chest pain is the second-most common presentation to the emergency department (ED) across the United States.(Rui P, 2017) Timely identification of coronary ischemia in over 6 million chest pain ED patients remains a priority given the adverse outcomes associated with ischemic heart disease. However a majority of patients presenting to EDs with chest pain do not have acute coronary syndrome and the decision whether to pursue advanced cardiac imaging or observation among low-risk patients remains a clinical challenge(Safdar et al., 2020) (Hess et al., 2016).
While the highest mortality is associated with ischemia from obstructive coronary artery disease (CAD), ischemia can also occur from alternate sources such as coronary microvascular dysfunction (CMD) and has been linked with recurrent symptoms and adverse cardiac outcomes.(Bairey Merz et al., 2017) CMD incorporates a heterogenous group of causes of ischemia such as endothelial dysfunction, vascular smooth muscle cell dysfunction, microembolization, and coronary slow flow.(Safdar et al., 2014) Diagnosis of CMD rarely occurs in the ED setting, as it requires either invasive coronary angiography with vasoreactivity testing or sophisticated tests such as positron emission tomography (PET), cardiac magnetic resonance imaging (MRI), or Doppler echocardiography available at select centers.(Crea et al., 2014) The non-invasive modalities measure flow reserve within coronary vessels, a decrease in which in the absence of CAD is one of the diagnostic criteria for CMD(Camici et al., 2015).
Coronary flow reserve (CFR) as measured by PET/Computed Tomography (CT) (also described as myocardial flow reserve), is a composite measure of the vasodilatory function of coronary epicardial and microvascular vessels(Camici et al., 2015). Regardless of presence or absence of obstructive CAD, abnormal CFR measured by PET/CT is associated with adverse cardiovascular outcomes and return hospital visits for chest pain.(Safdar et al., 2020; Taqueti et al., 2017) (Green et al., 2019). PET/CT is a specialized test not readily available in all EDs. Thus, there is an acute need to identify bedside screening tests that can help distinguish ED patients with chest pain who have normal CFR from abnormal CFR to route them with appropriate follow ups.
Reactive hyperemia index (RHI) measured using peripheral arterial tonometry (PAT) is a noninvasive, point-of-care method of assessing this function and is readily available in clinical settings. Attenuated peripheral vasodilatory function is associated with adverse cardiovascular outcomes.(Matsuzawa et al., 2015) It is believed that RHI primarily measures endothelial function, but other mechanisms may also be involved.(Nohria et al., 2006) In this study we investigated whether RHI could be used as a clinical screening measure to differentiate patients with abnormal CFR from those with normal flows as measured by PET/CT. We also examined the association between RHI, CFR, and traditional cardiovascular risk factors in patients undergoing PET/CT.
Material and Methods
Study Design and Setting:
We conducted a cross-sectional study in a convenience sample of patients undergoing cardiac 82Rb PET/CT as part of their routine clinical evaluation at a single hospital system between June 2014-May 2019. Clinical indications for 82Rb PET/CT perfusion imaging included chest discomfort or angina equivalent symptoms, or preoperative clearance. All patients gave informed consent to participate in the study. The Institutional Review Board at Yale School of Medicine approved this study.
Participants:
We recruited adult patients scheduled for cardiac 82Rb PET/CT at Yale New Haven Hospital. Exclusion criteria included patients unable to read or understand English, those with conditions that impeded interview such as cognitive or communication impairment, and those in police custody. Additionally, we excluded patients who were physically incapable of completing the EndoPAT, such as those with tremors affecting the hand or arm or injuries to the arm or hand. All symptomatic patients were ruled out for acute myocardial infarction using serial troponin.
Variables:
We collected health and sociodemographic history from patient interviews including: age, sex, race, ethnicity, education experience, marital status, employment status, insurance status, traditional cardiac risk factors, smoking status, height, weight, body mass index (BMI), history of chest pain prior to the current encounter, previous stress test, family history of dementia or premature cardiac death, menarche/menopause (if applicable). A patient was defined as an ever smoker if they answered positively to smoking greater than 100 lifetime cigarettes. Medical record review for full patient medication lists, comorbidities, and results of metabolic testing supplemented the interviews.
Physiological measures:
All measurements were taken under standardized protocols. Patients abstained from caffeine and vasoactive medications for more than six hours prior to testing.
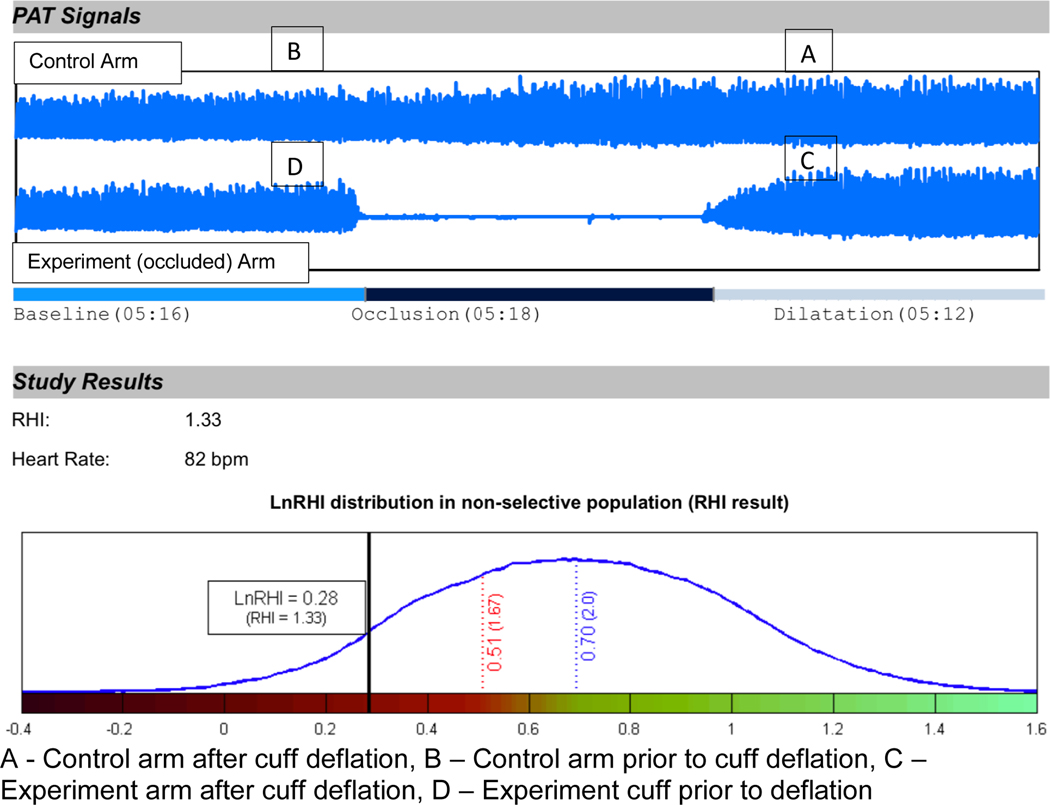
Peripheral reactive blood flow. We measured RHI with the Endo-PAT2000 (Itamar Medical, Israel). This non-invasive device measures endothelial function by detecting plethysmographic changes at rest and during recovery from blood flow restriction through pressure-sensitive probes placed on one finger of each hand. Patients laid supine in bed or in reclining hospital chair unless this position impaired their breathing. After a five-minute equilibration period, we inflated a blood pressure cuff on the experiment arm to at least 60 mmHg above the patient’s systolic blood pressure (between 200 mmHg and 300 mmHg) and held for a five-minute occlusion period. The pressure was released and reactive hyperemia was measured for a subsequent five minutes. The reactive hyperemia index data is digitally analyzed (EndoPAT2000 software version 3.0.4). It reflects the extent of reactive hyperemia and is calculated as the ratio of the average amplitude of PAT signal over 1 min starting 1.5 min after cuff deflation (control arm, A; occluded arm, C) divided by the average amplitude of PAT signal of a 2.5-min time period before cuff inflation (baseline) (control arm, B; occluded arm, D). Thus, the software calculates RH-PAT index using the formula RH-PAT index =(C/D)/(A/B) x baseline correction(Matsuzawa et al., 2010). Figure 1 is an example of the EndoPAT software report for RHI.
Augmentation Index (AI). The EndoPAT2000 software calculated augmentation index, which is an indirect measurement of arterial stiffness. The higher the AI, greater the stiffness. Since AI is influenced by heart rate, the software also calculated the heart-rate adjusted augmentation index to 75 beats per minute (AI@75).
Figure 1.
Representative EndoPAT report for RHI
PET Imaging and Analysis:
Dynamic rest-stress 82Rb PET myocardial perfusion imaging was performed on a hybrid PET 64-slice CT scanner (Discovery 690, GE Healthcare) as previously described.(Feher et al., 2020) Briefly, rest PET/CT images were acquired in list mode over 7 minutes after intravenous (IV) injection of 82Rb. After the rest PET scan, patients underwent pharmacological stress with regadenoson, which was administered as a slow bolus (0.4 mg over 40 seconds). At peak stress, 82Rb was administered IV and PET images were acquired in list mode. A low dose CT scan was acquired for attenuation correction of PET images. Heart rate and rhythm 12-lead electrocardiogram and noninvasive blood pressure were recorded at rest, at peak stress and in recovery.
82Rb PET/CT Data Analysis:
PET images were reconstructed with attenuation correction on system software creating a dynamic series of PET images that were reoriented and processed using Invia Corridor 4DM v2017 (Ann Arbor, MI). Regional and global rest and peak stress myocardial blood flow (MBF) were calculated by fitting the 82Rb time-activity curves to a one-compartment tracer kinetic model as described previously (Feher et al., 2020). Rest and stress flows were corrected for the rate pressure product (RPP) (heart rate x systolic blood pressure) as follows: rest and stress flows were multiplied by the respective rest or peak stress RPPs and then divided by the reference RPP (9000). CFR was calculated as the ratio of stress to rest MBF. We report findings using non-RPP corrected data in this manuscript, as at our institution, non-RPP corrected data is reported clinically. RPP-corrected data is included in the supplementary material. Intra-class correlation for CFR measurement was 0.95 (95% CI, 0.93–0.97).
Outcomes:
The primary outcome was RHI as measured by EndoPAT2000. Our secondary outcome was augmentation index.
Statistical methods:
We defined low RHI as <1.67 and for CFR, we defined normal as ≥2.5, depressed CFR as 1.5<CFR≤2.5, and severely depressed CFR as ≤1.5. We assessed the relationship between RHI and patient characteristics including sex, age, hypertension, smoking, diabetes, dyslipidemia, presence of CAD, BMI, and history of chest pain. Given the skewed distribution, we used the natural logarithm of RHI (LnRHI) to calculate Spearman correlation and area under curve (AUC). We evaluated the correlation of RHI and CFR with Spearman correlation and receiver operating characteristic curve. We performed exploratory analysis for the potential relationships between patient characteristics and RHI. We used simple linear regression for continuous variables and analysis of variance or t-test and fisher’s exact test or chi-square test for categorical variables as appropriate. To address the heterogeneity of our population, we repeated a sensitivity analysis by repeating the primary correlation analysis within the subgroups of patients with CAD, those with depressed CFR in absence of coronary artery disease, and in patients with neither CFR nor CAD. We completed all statistical analyses using the software R(Team, 2010) via RStudio version 1.1.456(Team, 2016).
Sample size:
For RHI to distinguish patients with normal versus abnormal CFR on 82Rb PET/CT (AUC > 65%), we calculated the sample size as 100.
Results
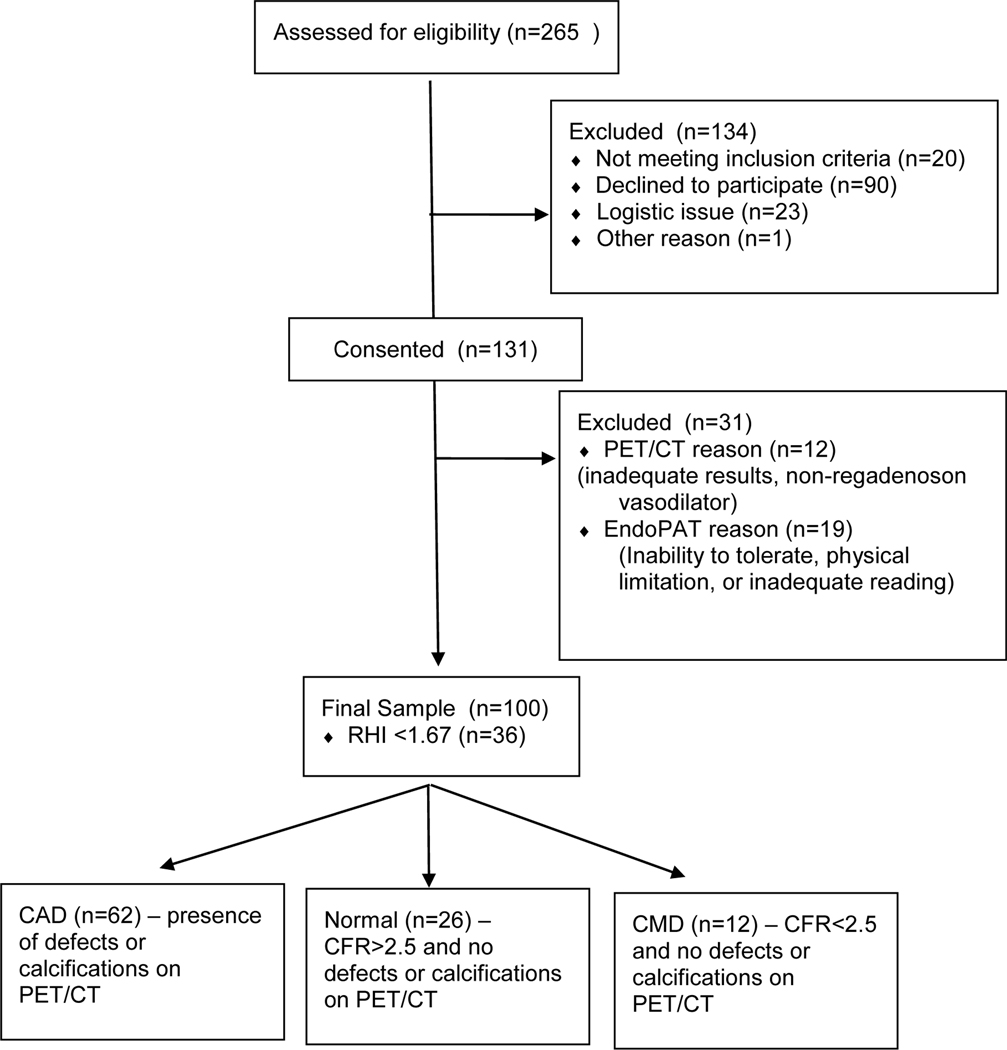
We approached 265 patients undergoing PET/CT over the study period, of which we enrolled 131 patients, and had 100 with adequate data for analysis (Figure 2). Forty five patients were enrolled from the emergency department, 24 as inpatients, and 31 as outpatients. The most common cause of screen failure was refusal, representing 67% of cases, followed by logistic issue in 17%, language barrier 12%, cognitive impairment 3%, and other 1%.
Figure 2.
Flow chart for study recruitment and enrollment
Table I provides the baseline characteristics of our study population by CFR with mean age of 61 years (SD =12), 46% female, and 29% self-identified as a non-white. Most patients had at least one traditional risk factor for cardiovascular disease, including hypertension (83%), dyslipidemia (67%), diabetes mellitus (40%) and history of ever smoking (58%), or were previously diagnosed with CAD (32%). Mean RHI for the cohort was 2.05 (SD = 0.75) and mean CFR was 2.32 (SD = 0.65). A tenth had prior revascularization and did not differ by RHI or CFR. Sixty percent of patients had depressed CFR and 36% had low RHI.
Table I.
Baseline characteristics of the study population, stratified by CFR category.
| Total | CFR ≤1.5 | 1.5<CFR≤2.5 | CFR > 2.5 | P Value | |
|---|---|---|---|---|---|
|
| |||||
| N = 10 | N = 50 | N = 40 | |||
|
| |||||
| Sociodemographics | |||||
| Age (yrs), mean (SD) | 60.61 (12.49) | 72.70 (10.39) | 62.16 (11.72) | 55.65 (11.52) | <0.001 |
| Female, n (%) | 46 (46.0) | 5 (50.0) | 20 (40.0) | 21 (52.5) | 0.480 |
| Non-white race, n (%) | 29 (29.0) | 2 (20.0) | 12 (24.0) | 15 (37.5) | 0.301 |
| Hispanic, n (%) | 7 (7.0) | 0.00 (0.00) | 3 (6.0) | 4 (10.0) | 0.501 |
| Married/Living Together, n (%) | 52 (52.0) | 5 (50.0) | 21 (42.0) | 26 (65.0) | 0.145 |
| Employed, n (%) | 32 (32.3) | 1 (10.0) | 18 (36.7) | 13 (32.5) | .087 |
| Clinical Profile | |||||
| Diabetes, n (%) | 40 (40.0) | 3 (30.0) | 26 (52.0) | 11 (27.5) | 0.049 |
| Insulin-dependent, n (%) | 16 (16.0) | 2 (20.0) | 12 (24.0) | 2 (5.0) | 0.047 |
| Hypertension, n (%) | 83 (83.0) | 7 (70.0) | 46 (92.0) | 30 (75.0) | 0.053 |
| Ever smoker, n (%) | 58 (58.0) | 5 (50.0) | 32 (64.0) | 28 (45) | 0.049 |
| Dyslipidemia, n (%) | 67 (67.0) | 10 (100.0) | 37 (74.0) | 20 (50.0) | 0.004 |
| Known CAD, n (%) | 32 (32.0) | 6 (60.0) | 20 (40.0) | 6 (15.0) | 0.006 |
| History of CABG or PCI, n (%) | 10 (10.1) | 2 (20.0) | 5 (10.0) | 3 (7.5) | 0.499 |
| Family History of Premature MI (N=96), | 27 (28.1) | 3 (33.3) | 17 (34.7) | 7 (18.4) | 0.230 |
| Prior chest pain (N=87), n (%) | 55 (63.2) | 5 (11.0) | 29 (58) | 21 (38.2) | .879 |
| Height (in), mean (SD) | 66.96 (4.08) | 65.73 (4.96) | 66.94 (4.05) | 67.30 (3.92) | 0.557 |
| Weight (lb), mean (SD) | 240.42 (70.66) | 194.33 (70.27) | 239.66 (75.27) | 252.89 (60.93) | 0.062 |
| BMI (kg/m2), mean (SD) | 37.47 (10.07) | 31.30 (9.99) | 37.30 (10.45) | 39.23 (9.19) | 0.082 |
| Laboratory Data | |||||
| Total Cholesterol (N=75), mean (SD) | 162 (39.16) | 141 (34.77) | 165 (42.22) | 163 (36.28) | 0.388 |
| LDL (N=74), mean (SD) | 87 (30.54) | 71 (21.71) | 87 (34.74) | 90 (26.43) | 0.379 |
| HDL (N=75), mean (SD) | 49 (18.42) | 51 (13.85) | 47 (15.45) | 50 (22.05) | 0.707 |
| Triglycerides (N=75), mean (SD) | 138 (91.83) | 99 (48.50) | 161 (115.78) | 120 (57.33) | 0.105 |
| Creatinine (N=76), mean (SD) | 1.73 (5.40) | 2.71 (4.95) | 1.06 (0.50) | 0.88 (0.26) | 0.0153 |
| Glucose (N=79), mean (SD) Medications | 125 (46.43) | 107 (29.98) | 144 (53.06) | 107 (32.04) | 0.001 |
| Aspirin, n (%) | 46 (46.0) | 3 (30.0) | 25 (50.0) | 18 (45.0) | 0.504 |
| Ace-I or ARB n, (%) | 43 (43.0) | 4 (40.0) | 26 (52.0) | 13 (32.5) | 0.175 |
| Beta Blockers, n (%) | 53 (53.0) | 6 (60.0) | 33 (66.0) | 14 (35.0) | 0.012 |
| Ca2+ Channel Blocker, n (%) | 31 (31.0) | 2 (20.0) | 18 (36.0) | 11 (27.5) | 0.502 |
| Lipid lowering medication, (%) | 58 (58.0) | 4 (40.0) | 33 (66.0) | 21 (52.5) | 0.208 |
| Antidepressants, n (%) | 26 (26.0) | 2 (20.0) | 14 (28.0) | 10 (25.0) | 0.856 |
| Imaging data | |||||
| PET Defects Present, n (%) | 33 (33.6) | 6 (60.0) | 22 (44.0) | 6 (15.0) | 0.002 |
| PET/CT Calcifications Present, n (%) | 55 (55.6) | 8 (80.0) | 33 (66.0) | 14 (35.9) | 0.003 |
| CFR (rest), mean (SD) | 2.44 (0.83) | 1.36 (0.43) | 2.16 (0.62) | 2.06 (0.66) | N/A |
| CFR (stress), mean (SD) | 1.89 (0.64) | 1.25 (0.25) | 1.69 (0.56) | 2.29 (0.55) | N/A |
| Baseline HR, mean (SD) | 72 (12.14) | 71 (14.50) | 72 (11.96) | 72 (12.07) | 0.947 |
| Baseline SBP, mean (SD) | 131 (21.14) | 131 (18.12) | 134 (24.47) | 127 (16.79) | 0.336 |
| Baseline DBP, mean (SD) | 67 (10.54) | 64 (13.87) | 67 (10.43) | 67 (9.92) | 0.632 |
| Average Stress HR, mean (SD) | 88 (14.15) | 78 (15.65) | 86 (13.25) | 92 (13.62) | 0.010 |
| Average Stress SBP, mean (SD) (N=99) | 132 (22.26) | 124 (20.33) | 134 (24.17) | 131 (20.27) | 0.486 |
| Average Stress DBP, mean (SD) (N=99) | 66 (9.64) | 63 (10.83) | 66 (10.53) | 68 (8.04) | 0.325 |
| EndoPAT AI, mean (SD) | 12.33 (17.98) | 14.40 (11.93) | 13.62 (20.58) | 10.19 (15.73) | 0.625 |
| EndoPAT AI@75, mean (SD) | 10.04 (15.92) | 10.70 (10.60) | 11.52 (18.68) | 8.02 (13.13) | 0.583 |
| RHI, median (IQR) | 1.855 (1.02) | 1.76 (1.08) | 1.94 (1.12) | 1.895 (0.84) | |
| lnRHI, mean (SD) | 0.66 (0.34) | 0.64 (0.33) | 0.66 (0.36) | 0.66 (0.33) | 0.982 |
Note: Bold text indicates significance at p<0.05. ANOVA performed for continuous variables, chi-square or fisher’s exact test for categorical.
CAD = coronary artery disease, BMI = body mass index, LDL = low density lipoprotein, HDL = high density lipoprotein, ACE-I = angiotensin converting enzyme-inhibitor, ARB = angiotensin II receptor blocker, HR = heart rate, SBP = systolic blood pressure, DBP = diastolic blood pressure
Primary Outcome:
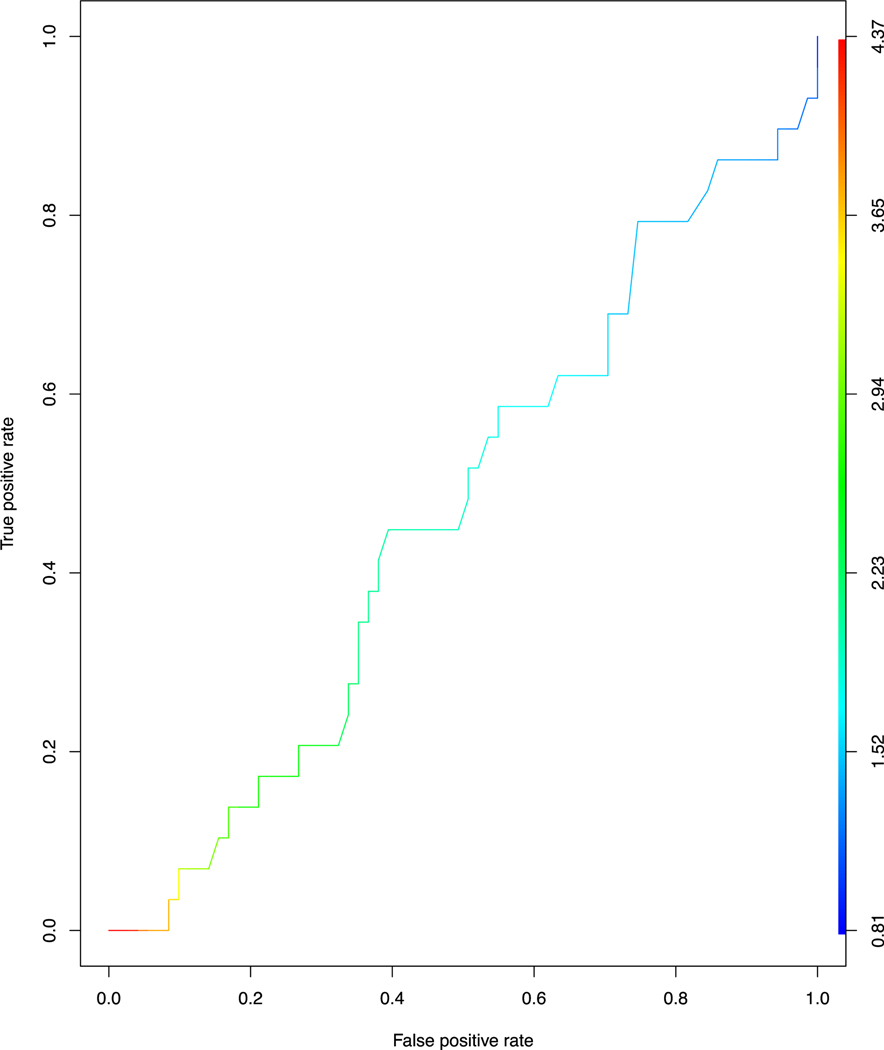
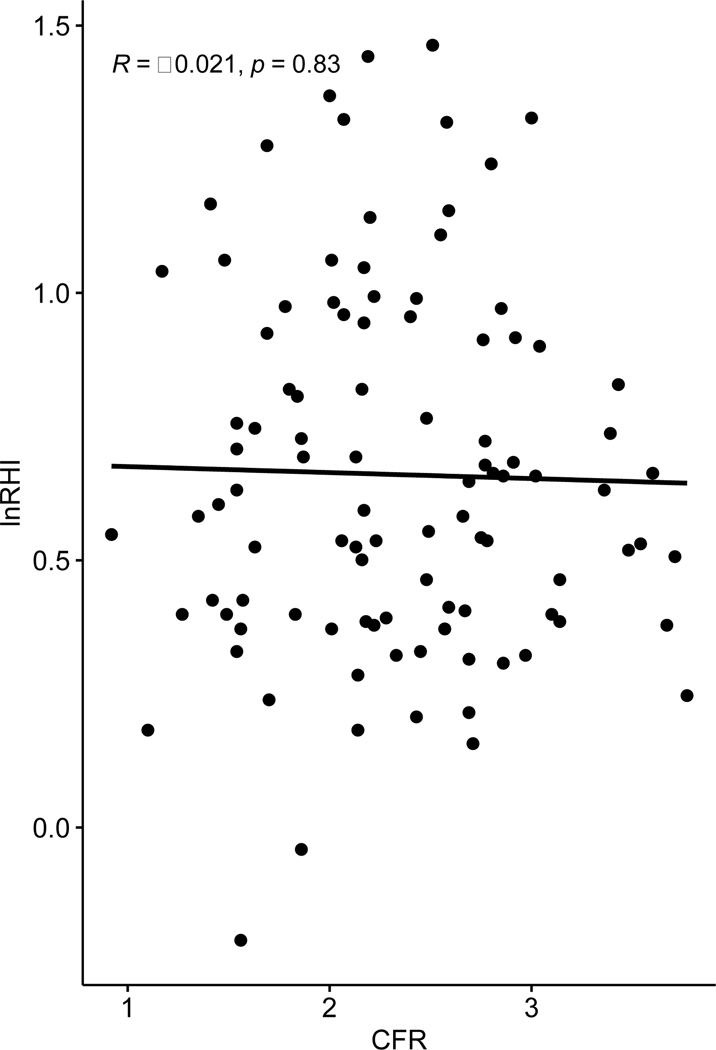
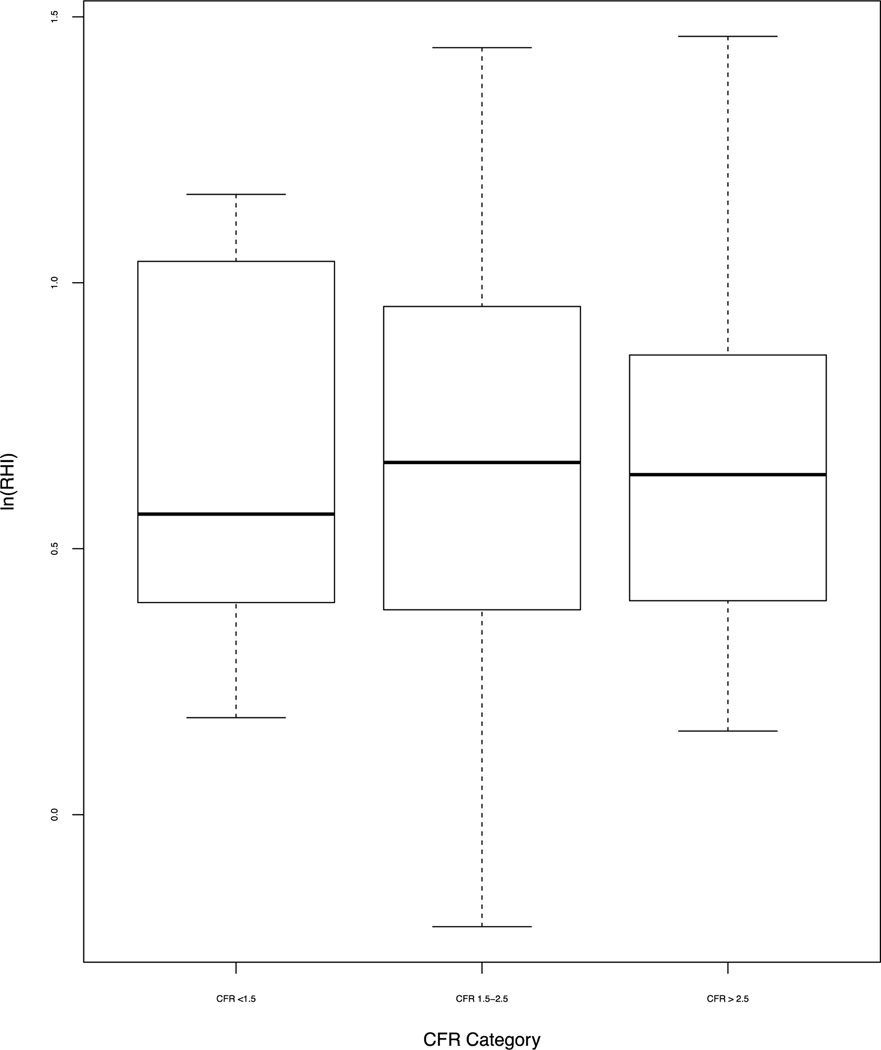
To investigate our primary hypothesis, we compared log transformed RHI (LnRHI) with CFR ≥2.0. Figure 3 shows that LnRHI did not distinguish patients with low and normal CFR, with an AUC=0.53 (95% Cl, 0.41–0.64). Figure 4 shows that LnRHI also did not correlate with CFR (r=−0.039, p=0.7). Figure 5 shows mean LnRHI by each category of CFR and again showed no statistical difference between the three groups (mean LnRHI 0.66, 0.66 and 0.64 by decreasing CFR category; p= 0.982). The lack of association between RHI and CFR was maintained in sensitivity analysis (not shown). There was a trend for lower RHI in patients we identified as CMD (low CFR, no perfusion defect and calcium score of 0) (1.88 versus 2.21; p=0.35) although we were underpowered (n=12) to meet statistical significance.
Figure 3.
Receiver Operating Characteristic curve for LnRHI and CFR threshold of 2.0
Figure 4.
Simple Regression of lnRHI vs. CFR utilizing Pearson correlation
Figure 5.
Mean LnRHI by CFR category.
Mean LnRHI for CFR<1.5 = 0.641 (SD=0.33); Mean RHI for CFR 1.5–2.5 = 0.663 (SD=0.36), Mean RHI for CFR >2.5 = 0.662 (SD =.33); p=0.965
Distribution of risk factors:
Table I provides the clinical profile of patients by CFR and shows a different distribution of risk factors by RHI as shown in Table II. Patients with low CFR had higher proportions of traditional risk profile such as older age, diabetes, hypertension, smoking, dyslipidemia, known CAD, higher creatinine, and family history of CAD than patients with normal CFR. Accordingly, the use of beta blockers was also higher in patients with low CFR.
Table II.
Characteristics of patients with normal and low RHI
| Total | RHI >1.67 | RHI<1.67 | P value | |
|---|---|---|---|---|
|
| ||||
| Sociodemographics | N = 64 | N = 36 | ||
| Age (yrs), mean (SD) | 60.61 (12.49) | 60.39 (13.32) | 61.00 (11.04) | 0.816 |
| Female, n (%) | 46 (46.0) | 29 (45.3) | 17 (47.2) | 1.000 |
| Non-white race, n (%) | 29 (29.0) | 17 (26.6) | 12 (33.3) | 0.626 |
| Hispanic, n (%) | 7 (7.0) | 3 (4.7) | 4 (11.1) | 0.248 |
| Married/Living Together | 52 (52.0) | 31 (48.4) | 21 (58.3) | 0.623 |
| Employed | 32 (32.3) | 20 (31.7) | 12 (33.3) | 0.571 |
| Clinical Profile | ||||
| Diabetes, n (%) | 40 (40.0) | 24 (37.5) | 16 (44.4) | 0.640 |
| Insulin-dependent, n (%) | 16 (16.0) | 10 (15.6) | 6 (16.7) | 1.000 |
| Hypertension, n (%) | 83 (83.0) | 54 (84.4) | 29 (80.6) | 0.833 |
| Ever smoker, n (%) | 58 (58.0) | 41 (64.1) | 17 (47.2) | 0.154 |
| Dyslipidemia, n (%) | 67 (67.0) | 43 (67.2) | 24 (66.7) | 1.000 |
| Known CAD, n (%) | 32 (32.0) | 18 (28.1) | 14 (38.9) | 0.377 |
| History of CABG or PCI, n (%) | 10 (10.0) | 4 (6.2) | 6 (16.7) | 0.187 |
| Family History of Premature MI (N=96), n (%) | 27 (28.1) | 18 (29.0) | 9 (26.5) | 0.976 |
| Prior chest pain (N=87), n (%) | 55 (63.2) | 34 (59.6) | 21 (70.0) | 0.473 |
| Height (in), mean (SD) | 66.96 (4.08) | 66.76 (4.02) | 67.33 (4.21) | 0.509 |
| Weight (lb), mean (SD) | 240.42 (70.66) | 242.59 (75.57) | 236.56 (61.81) | 0.684 |
| BMI (kg/m2), (mean (SD) | 37.47 (10.07) | 38.07 (11.04) | 36.41 (8.13) | 0.432 |
| Laboratory data | ||||
| Total Cholesterol (N=75), mean (SD) | 162 (39.16) | 160 (37.86) | 165 (41.64) | 0.593 |
| LDL (N=74), mean (SD) | 87 (30.54) | 86 (31.28) | 88 (29.82) | 0.812 |
| HDL (N=75), mean (SD) | 49 (18.42) | 47 (15) | 52 (22.82) | 0.274 |
| Triglycerides (N=75), mean (SD) | 138 (91.83) | 142 (104.56) | 132 (68.19) | 0.648 |
| Creatinine (N=76), mean (SD) | 1.73 (5.40) | 1.30 (2.04) | 0.90 (0.24) | 0.321 |
| Glucose (N=79), mean (SD) | 125 (46.43) | 121 (47.61) | 131 (44.52) | 0.358 |
| Medications | ||||
| Aspirin, n (%) | 46 (46.0) | 28 (43.8) | 18 (50.0) | 0.694 |
| Ace-I or ARB, n (%) | 43 (43.0) | 28 (43.8) | 15 (41.7) | 1.000 |
| Beta Blockers, n (%) | 53 (53.0) | 33 (51.6) | 20 (55.6) | 0.861 |
| Ca2+ Channel Blocker, n (%) | 31 (31.0) | 20 (31.2) | 11 (30.6) | 1.000 |
| Lipid lowering medication, n (%) | 58 (58.0) | 35 (54.7) | 23 (63.9) | 0.494 |
| Antidepressants, n (%) | 26 (26.0) | 18 (28.1) | 8 (22.2) | 0.683 |
| Imaging data | ||||
| PET Defects, n (%) | 33 (33.7) | 21 (32.8) | 13 (37.1) | 0.909 |
| PET/CT Calcifications, n (%) | 55 (55.6) | 36 (56.1) | 19 (54.3) | 0.900 |
| EndoPAT AI, mean (SD) | 12.33 (17.98) | 16.06 (18.08) | 5.69 (15.96) | 0.005 |
| EndoPAT AI@75, mean (SD) | 10.04 (15.92) | 12.66 (16.42) | 5.38 (14.05) | 0.028 |
| CFR, mean (SD) | 2.32 (0.65) | 2.32 (0.63) | 2.31 (0.69) | 0.915 |
| CFR (rest), mean (SD) | 2.44 (0.83) | 2.47 (0.86) | 2.38 (0.78) | 0.587 |
| CFR (stress), mean (SD) | 1.89 (0.64) | 1.88 (0.62) | 1.90 (0.68) | 0.875 |
| Baseline HR, mean (SD) | 72 (12.14) | 72 (12.47) | 73 (11.67) | 0.628 |
| Baseline SBP, mean (SD) | 131 (21.14) | 133 (20.16) | 127 (22.62) | 0.197 |
| Baseline DBP, mean (SD) | 67 (10.54) | 67 (10.34) | 66 (10.99) | 0.541 |
| Average Stress HR, mean (SD) | 88 (14.15) | 87 (15.48) | 89 (11.54) | 0.540 |
| Average Stress SBP, mean (SD) (N=99) | 132 (22.26) | 133 (20.87) | 129 (24.74) | 0.444 |
| Average Stress DBP, mean (SD) (N=99) | 66 (9.64) | 67 (9.71) | 65 (9.55) | 0.373 |
Note: Bold text indicates significance at p<0.05. ANOVA performed for continuous variables, chi-square or fisher’s exact test for categorical.
CAD = coronary artery disease, BMI = body mass index, LDL = low density lipoprotein, HDL = high density lipoprotein, ACE-I = angiotensin converting enzyme-inhibitor, ARB = angiotensin II receptor blocker, HR = heart rate, SBP = systolic blood pressure, DBP = diastolic blood pressure
In contrast, patients with low RHI had similar clinical profiles as patients with normal RHI. LnRHI was not significantly associated with patient age, sex, smoking, presence of diabetes, hyperlipidemia, history of CAD, BMI, or use of lipid- lowering medication (all p>0.05). Mean LnRHI based on select clinical characteristics is displayed in Table III.
Table III.
Secondary Outcomes: Mean rate-adjusted Augmentation Index (AI@75) and Natural Logarithm of Reactive Hyperemia Index (LnRHI) based on clinical characteristics
| Augmentation Index | ||||
|---|---|---|---|---|
|
| ||||
| Yes | No | T statistic | P Value | |
|
| ||||
| Known Coronary | 8.31 | 10.85 | 0.73 | 0.47 |
| Artery Disease Coronary Artery Calcifications | 12.95 | 6.48 | −2.11 | 0.037 |
| Perfusion Defects | 12.47 | 8.79 | −1.03 | 0.30 |
|
| ||||
| LnRHI | ||||
| Yes | No | T statistic | P Value | |
|
| ||||
| Coronary Artery Calcifications | 0.639 | 0.686 | 0.68 | 0.5 |
| Perfusion Defects | 0.616 | 0.683 | 0.97 | 0.34 |
| Prior Chest Pain | 0.598 | 0.762 | 2.16 | 0.036 |
Note: Bold text indicates significance at p<0.05.
Secondary measures:
We compared AI, a surrogate for arterial stiffness, in patients with and without previously diagnosed CAD, 82Rb PET/CT results suggesting CAD (i.e., perfusion defects and coronary artery calcifications) and low RHI. Results are shown in Table III.
Discussion
In a cohort of patients with cardiac symptoms or cardiac risk factors representative of care seeking patients at one U.S. hospital system, we did not find peripheral measurement of reactive hyperemia using EndoPAT2000 to correlate with CFR as measured by cardiac 82Rb PET/CT. This could be explained by the difference in clinical profile seen in patients with low CFR compared to those with low RHI. Patients with low CFR were older and had higher proportions of traditional cardiac risk factors and known CAD compared with patients with normal flows, indicating its role in detection of CAD as well as CMD. RHI, on the other hand, did not have a significant association with traditional cardiovascular risk factors in this patient cohort. However, low RHI was correlated with prior history of recurrent chest pain. Given that CMD is not always correlated with traditional atherosclerotic factors, it is possible that RHI and CFR are measuring changes in different vascular beds and endothelial function pathways and may provide different information about microvascular health.
Previous studies have suggested that male sex (Hamburg et al., 2011; Konttinen et al., 2013; Schnabel et al., 2011), history of smoking(Hamburg et al., 2011; Konttinen et al., 2013; Kuvin et al., 2003; Michelsen et al., 2016), diabetes(Hamburg et al., 2011; Konttinen et al., 2013; Michelsen et al., 2016), and dyslipidemia(Hamburg et al., 2011; Schnabel et al., 2011) may be associated with decreased RHI, and data is mixed on the relationship between RHI and diagnosis of CAD(Kuvin et al., 2003; Matsuzawa et al., 2010; Venturi et al., 2016; Venuraju et al., 2019). Our population was older (mean 61 years), had a much higher BMI (mean 37.47), and more cardiovascular risk factors than other populations in which the EndoPAT has been studied, including 83% with hypertension, 58% ever smokers, and 40% with diabetes. “Reverse epidemiology,” might also play a role, with high-risk patients receiving appropriate medications that attenuate their risk.(Venturi et al., 2016) In our population, most were on at least one cardiovascular medication, including 58% on lipid lowering agents, 53% on beta blockers, 46% on aspirin, 43% on ACE inhibitors or Angiotensin II receptor blockers, and 31% on calcium channel blockers. Results from studies that examined relationships between the use of lipid-lowering medications and RHI have been mixed(Hamburg et al., 2011; Konttinen et al., 2013; Venturi et al., 2016). An important future direction of research would be to compare RHI in patients with cardiovascular risk factors receiving optimal medical management to those who are not.
A proposed mechanism for the lack of correlation between RHI and CFR may also be due to the difference in methods of measuring endothelial function.(Orbaek et al., 2017) Chemical stress agents can cause vasodilation in both endothelium-dependent and endothelium-independent manners. Regadenoson is a selective A2A adenosine receptor agonist. Activation of A2A adenosine receptors on the endothelium results in increased nitric oxide (NO) production,(Arsyad and Dobson, 2016; Fahim et al., 2001) as well as vascular smooth muscle cells to open adenosine triphosphate-dependent and voltage-gated potassium channels, resulting in cell hypopolarization and vasorelaxation.(Goodwill et al., 2017; Hein et al., 1999) Thus, the CFR measured by regadenoson may be dependent on both the function of the endothelium (endothelium-dependent) and vascular smooth muscle (endothelium-independent) vasodilation.(Buus et al., 2001; Mustafa et al., 2009) On the other hand, the pathways contributing to RHI measurement through EndoPAT are not completely understood.(Takase et al., 2018) Impaired NO production post-occlusion and ensuing endothelial dysfunction and could be partly responsible for this measurement. However, other mechanisms may be also involved. In prior studies, inhibition of NO synthesis by NG-nitro-L-arginine methyl ester reduced but did not completely abolish vasodilation in patients undergoing EndoPAT measurement.(Nohria et al., 2006) These could include other endothelium-dependent factors such as prostaglandins(Axtell et al., 2010) or non-endothelium dependent mechanisms(Hamburg et al., 2011) and α-adrenergic tone contributing to vascular response in RHI.(Allan et al., 2013; Lee et al., 2012) This theory is supported by a recent study that compared the biochemical profile for markers of endothelial function, including NO breakdown products among participants who underwent EndoPAT, and found no significant differences between the group with low RHI compared to normal.(Jakubowski et al., 2020)
Our results add to the literature that similarly showed poor correlation between EndoPAT-RHI and central measures of endothelial function.(Daniel et al., 2015; Michelsen et al., 2016; Orbaek et al., 2017) While a previous study found reduced RHI in patients with decreased vasodilation in response to acetylcholine (a measure of conduit artery function), there was no significant difference in CFRs measured in response to adenosine (a measure of microcirculatory function) in their low RHI group versus normal.(Bonetti et al., 2004) Our results agree with findings from a study by Ørbaek et al which found no correlation between RHI and CFR as measured by 82Rb PET/CT in a group of 48 HIV positive patients receiving anti-retroviral therapy.(Orbaek et al., 2017) Moreover, our study addressed some important limitations in the Ørbaek study by including a larger sample size, a more heterogenous patient population with cardiovascular risk factors, and same day PET and EndoPAT measures (the prior study had a mean duration of 3.3 years between PET and Endo-PAT measurements).
A recent study that investigated correlation of RHI with different types of invasive coronary microvascular testing found that RHI was correlated with resistance and flow responses with dobutamine but not with responses to adenosine provocation.(Nardone et al., 2020) These findings are consistent with an earlier study comparing RHI to coronary flow velocity reserve (CFVR) with dipyridamole infusion shat showed no correlation between CFVR and RHI and no difference in mean RHI among 3 stratified CVFR categories.(Michelsen et al., 2016) Dipyridamole increases bioavailability of adenosine by inhibiting its uptake. Thus, our findings extend the theory that adenosine-based central measures of endothelial function may not correlate with RHI. Future work in larger populations are needed to confirm our findings.
Our results should be interpreted in light of their limitations. First, this was an exploratory study from a convenience sample and our results should be considered hypothesis-generating as opposed to hypothesis-testing. Second, we only measured cross-sectional correlation between central and peripheral flows. It is possible that RHI and CFR correlates better for assessing serial changes in longitudinal studies. Third, we did not control for some potential confounders for RHI measurement such as time of the day or time of patient’s last meal, as these could affect endothelial function.(Truschel et al., 2009) Fourth, our study population had a higher mean BMI and more cardiovascular risk factors than other studies on this subject, which might limit the generalizability of our results to healthy individuals or those with lower BMIs. One of the indications for cardiac 82Rb PET/CT is increased BMI, thus our sample is inherently skewed to those with higher BMIs. Repeat studies in patients with normal BMI are needed. Lastly, while there is evidence that regadenoson may not achieve the same degree of hyperemia compared to other vasodilators when using a standard protocol(Johnson and Gould, 2015), our institution has adapted a delayed 82Rb injection protocol to minimize any technical reduction of CFR due to the use of regadenoson.(Sinusas, 2015)
Conclusions:
Peripheral RHI is insufficient as a clinical screening tool for low CFR as measured by cardiac PET/CT. RHI as measured with EndoPAT2000 did not correlate with CFR as measured by 82Rb PET/CT, limiting its use to detect patients with low CFR identified by adenosine pathway. Different mechanisms may be involved in these measurements and future research should focus on identifying them, making way for newer non-invasive tests to screen for CMD.
Supplementary Material
Highlights:
EndoPAT measures peripheral reactive hyperemia index (RHI) and low RHI (<1.67) is associated with adverse cardiovascular outcomes.
Positron emission tomography/computed tomography (PET/CT) measures coronary flow reserve (CFR), a composite measure of epicardial and microvascular flow.
Low CFR (<2.5) is also associated with adverse cardiovascular outcomes including readmissions.
In our study, RHI did not correlate with CFR, and the distribution of cardiac risk factors differed in patients with low CFR and low RHI.
We found peripheral RHI to be an insufficient clinical screening tool for identifying low CFR as measured by PET/CT.
Further work is required to assess the role of RHI in diagnosing coronary microvascular dysfunction.
Acknowledgements:
Funding: The publication was made possible by the Yale CTSA grant UL1TR000142 from the National Center for Advancing Translational Science (NCATS), NIH. Research reported in this publication was supported by National Heart, Lung and Blood Institute of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health under Award Number T35HL007649. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- ED
emergency department
- CAD
coronary artery disease
- CMD
coronary microvascular dysfunction
- PET
positron emission tomography
- MRI
magnetic resonance imaging
- CFR
coronary flow reserve
- RHI
reactive hyperemia index
- PAT
peripheral arterial tonometry
- NO
nitric oxide
Footnotes
Author Statement:
Marina Gaeta – investigation, formal analysis, funding acquisition, writing – original draft, as well as revisions and editing; Armin Nowroozpoor – investigation, validation, writing – review and editing; James Dziura - conceptualization, methodology, writing, review and editing; Gail D’Onofrio - conceptualization, methodology, resources, review and editing; Albert J. Sinusas – conceptualization, methodology; writing – review and editing; Basmah Safdar – conceptualization, methodology, resources, supervision, funding acquisition, writing – review and editing.
Disclosures: The authors report no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- Allan RB, et al. , 2013. A comparison of flow-mediated dilatation and peripheral artery tonometry for measurement of endothelial function in healthy individuals and patients with peripheral arterial disease. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 45, 263–269. [DOI] [PubMed] [Google Scholar]
- Arsyad A, Dobson GP, 2016. Adenosine relaxation in isolated rat aortic rings and possible roles of smooth muscle Kv channels, KATP channels and A2a receptors. BMC pharmacology & toxicology. 17, 23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axtell AL, et al. , 2010. Assessing endothelial vasodilator function with the Endo-PAT 2000. Journal of visualized experiments : JoVE. 2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bairey Merz CN, et al. , 2017. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 135, 1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonetti PO, et al. , 2004. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. Journal of the American College of Cardiology. 44, 2137–2141. [DOI] [PubMed] [Google Scholar]
- Buus NH, et al. , 2001. Influence of Nitric Oxide Synthase and Adrenergic Inhibition on Adenosine-Induced Myocardial Hyperemia. Circulation. 104, 2305–2310. [DOI] [PubMed] [Google Scholar]
- Camici PG, et al. , 2015. Coronary microvascular dysfunction: mechanisms and functional assessment. Nature Reviews Cardiology. 12, 48–62. [DOI] [PubMed] [Google Scholar]
- Crea F, et al. , 2014. Coronary microvascular dysfunction: an update. Eur Heart J. 35, 1101–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, et al. , 2015. Risk Factors and Markers for Acute Myocardial Infarction With Angiographically Normal Coronary Arteries. The American journal of cardiology. 116, 838–844. [DOI] [PubMed] [Google Scholar]
- Fahim M, et al. , 2001. Role of endothelium in adenosine receptor-mediated vasorelaxation in hypertensive rats. Fundam Clin Pharmacol. 15, 325–34. [DOI] [PubMed] [Google Scholar]
- Feher A, et al. , 2020. Serial Assessment of Coronary Flow Reserve by Rubidium-82 Positron Emission Tomography Predicts Mortality in Heart Transplant Recipients. JACC Cardiovasc Imaging. 13, 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill AG, et al. , 2017. Regulation of Coronary Blood Flow. Comprehensive Physiology. 7, 321–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R, et al. , 2019. Prognostic value of coronary flow reserve in patients with suspected or known coronary artery disease referred to PET myocardial perfusion imaging: A meta-analysis. Journal of Nuclear Cardiology. [DOI] [PubMed] [Google Scholar]
- Hamburg NM, et al. , 2011. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension (Dallas, Tex. : 1979). 57, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TW, et al. , 1999. Adenosine A(2A) receptors mediate coronary microvascular dilation to adenosine: role of nitric oxide and ATP-sensitive potassium channels. The Journal of pharmacology and experimental therapeutics. 291, 655–664. [PubMed] [Google Scholar]
- Hess EP, et al. , 2016. Shared decision making in patients with low risk chest pain: prospective randomized pragmatic trial. BMJ. 355, i6165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski M, et al. , 2020. Profiling the endothelial function using both peripheral artery tonometry (EndoPAT) and Laser Doppler Flowmetry (LD) - Complementary studies or waste of time? Microvascular Research. 130, 104008. [DOI] [PubMed] [Google Scholar]
- Johnson NP, Gould KL, 2015. Regadenoson versus dipyridamole hyperemia for cardiac PET imaging. JACC Cardiovasc Imaging. 8, 438–447. [DOI] [PubMed] [Google Scholar]
- Konttinen J, et al. , 2013. Association between lowered endothelial function measured by peripheral arterial tonometry and cardio-metabolic risk factors - a cross-sectional study of Finnish municipal workers at risk of diabetes and cardiovascular disease. BMC Cardiovasc Disord. 13, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuvin JT, et al. , 2003. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 146, 168–74. [DOI] [PubMed] [Google Scholar]
- Lee CR, et al. , 2012. Relation between digital peripheral arterial tonometry and brachial artery ultrasound measures of vascular function in patients with coronary artery disease and in healthy volunteers. The American journal of cardiology. 109, 651–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y, et al. , 2015. Prognostic Value of Flow-Mediated Vasodilation in Brachial Artery and Fingertip Artery for Cardiovascular Events: A Systematic Review and Meta-Analysis. J Am Heart Assoc. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa Y, et al. , 2010. Digital assessment of endothelial function and ischemic heart disease in women. Journal of the American College of Cardiology. 55, 1688–1696. [DOI] [PubMed] [Google Scholar]
- Michelsen MM, et al. , 2016. Peripheral Reactive Hyperemia Index and Coronary Microvascular Function in Women With no Obstructive CAD: The iPOWER Study. JACC. Cardiovascular imaging. 9, 411–417. [DOI] [PubMed] [Google Scholar]
- Murthy VL, et al. , 2018. Clinical Quantification of Myocardial Blood Flow Using PET: Joint Position Paper of the SNMMI Cardiovascular Council and the ASNC. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 59, 273–293. [DOI] [PubMed] [Google Scholar]
- Mustafa SJ, et al. , Adenosine Receptors and the Heart: Role in Regulation of Coronary Blood Flow and Cardiac Electrophysiology. In: Wilson CN, Mustafa SJ, Eds.), Adenosine Receptors in Health and Disease. Springer Berlin Heidelberg, Berlin, Heidelberg, 2009, pp. 161–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardone M, et al. , 2020. Noninvasive Microvascular Indices Reveal Peripheral Vascular Abnormalities in Patients With Suspected Coronary Microvascular Dysfunction. Can J Cardiol. 36, 1289–1297. [DOI] [PubMed] [Google Scholar]
- Nohria A, et al. , 2006. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985). 101, 545–8. [DOI] [PubMed] [Google Scholar]
- Orbaek M, et al. , 2017. Comparison of the Peripheral Reactive Hyperemia Index with Myocardial Perfusion Reserve by (82)Rb PET/CT in HIV-Infected Patients. Diagnostics (Basel). 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui P KK, National Hospital Ambulatory Medical Care Survey: 2017 emergency department summary tables. National Center for Health Statistics, 2017. [Google Scholar]
- Safdar B, et al. , 2020. Prevalence and characteristics of coronary microvascular dysfunction among chest pain patients in the emergency department. Eur Heart J Acute Cardiovasc Care. 9, 5–13. [DOI] [PubMed] [Google Scholar]
- Safdar B, et al. , 2014. Gender-specific research for emergency diagnosis and management of ischemic heart disease: proceedings from the 2014 Academic Emergency Medicine Consensus Conference Cardiovascular Research Workgroup. Acad Emerg Med. 21, 1350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel RB, et al. , 2011. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging. 4, 371–80. [DOI] [PubMed] [Google Scholar]
- Sinusas AJ, 2015. Does a shortened hyperemia with regadenoson stress pose a concern for quantitative Rb-82 PET imaging? Optimization of regadenoson PET imaging. JACC Cardiovasc Imaging. 8, 448–450. [DOI] [PubMed] [Google Scholar]
- Takase B, et al. , 2018. Disparity between EndoPAT measurement and brachial artery flow-mediated vasodilatation in hypertensive patients. Vascular Failure. 2, 61–65. [Google Scholar]
- Taqueti VR, et al. , 2017. Excess Cardiovascular Risk in Women Relative to Men Referred for Coronary Angiography Is Associated With Severely Impaired Coronary Flow Reserve, Not Obstructive Disease. Circulation. 135, 566–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R., RStudio: Integrated Development Environment for R. RStudio, Inc., Boston, MA, 2016. [Google Scholar]
- Team, R. D. C., R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2010. [Google Scholar]
- Truschel E, et al. , 2009. High-throughput ambulatory assessment of digital reactive hyperemia: concurrent validity with known cardiovascular risk factors and potential confounding. Preventive medicine. 49, 468–472. [DOI] [PubMed] [Google Scholar]
- Venturi E, et al. , 2016. Clinical Phenotype and Microvascular Dynamics of Subjects with Endothelial Dysfunction as Assessed by Peripheral Tonometry. Microcirculation. 23, 230–9. [DOI] [PubMed] [Google Scholar]
- Venuraju S, et al. , 2019. Predicting Severity of Coronary Artery Disease in Patients With Diabetes Using Endothelial Function Measured With Peripheral Arterial Tonometry: PROCEED Study. Angiology. 70, 613–620. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.