Abstract
Background
Persistent critical illness is a recognisable clinical syndrome defined conceptually as when the patient’s reason for being in the intensive care unit (ICU) is more related to their ongoing critical illness than their original reason for admission. Our objectives were: (1) to assess the day in ICU on which chronic factors (e.g., age, gender and comorbidities) were more predictive of survival than acute factors (e.g. admission diagnosis, physiological derangements) measured on the day of admission; (2) to assess the consistency of this finding across major patient subgroups and over time and (3) to compare case mix characteristics and outcomes for patients determined to develop persistent critical illness (based on ICU length of stay) with other patients.
Methods
Observational cohort study using a high-quality clinical database from the national clinical audit of adult critical care. 217 adult ICUs in England, Wales and Northern Ireland. 835,946 adult patients admitted to participating ICUs between 1 April 2009 and 31 March 2016. The main outcome measure was mortality at discharge from acute hospital.
Results
We fitted two statistical models (‘chronic’ and ‘acute’) and updated these based upon patients with an ICU length of stay of at least 1, 2, etc., up to 28 days. The discrimination of the chronic model first exceeded that of the acute model on day 11. Patients with longer stays (>10 days) comprised 9% of admissions but used 45% of ICU bed-days. After a mean ICU length of stay of 22 days and a subsequent 28 days in hospital, 30% died.
Conclusions
Persistent critical illness is commonly encountered in clinical practice and is associated with increased healthcare utilisation and adverse outcomes. Improvements in our understanding of the longer term outcomes and in the development of tools to aid prognostication are urgently required – for humane as well as health economic reasons.
Keywords: Prognosis, critical illness, critical care, hospital mortality, respiration, artificial
Introduction
Critical care is an expensive resource. 1 10–20% of patients die in the intensive care unit (ICU) 2 and there are, commonly, burdens of survivorship with longer term, adverse physical and psychological consequences. 3 It is, therefore, appropriate that the use of critical care is examined to maximise benefit, minimise harm and facilitate judicious use of a limited, expensive resource. It is in this context that critical care staff recognise a phenomenon termed persistent critical illness (PerCI). Conceptually, PerCI has been defined as ‘patients whose reason for being in the ICU is now more related to their ongoing critical illness than their original reason for admission to the ICU 4 ’.
To explore this conceptual definition empirically, Iwashyna and colleagues analysed data from over one million patients in the Australian and New Zealand Intensive Care Society Adult Patient Database (ANZICS-APD). They reported that the ‘acute’ factors (reason for admission/diagnosis and acute physiological derangements during the first 24 hours following admission to ICU) become progressively less predictive of survival as time evolves. By day 10 in the ICU, the more ‘chronic’ factors (age, gender and chronic comorbidities) became more predictive of survival. 5 These empirical results were consistent with subjective results from a survey of critical care clinicians in Australia and New Zealand when asked to estimate the day in ICU on which persistent critical illness was perceived to become apparent (median 10 days, IQR 7–14). 6 The empirical analyses indicated that patients determined to have PerCI (i.e., with an ICU stay of 10 or more days) accounted for 5% of patients admitted to ICU, but consumed a disproportionate proportion (33%) of ICU bed-days, with only 46% discharged home. Bagshaw and colleagues conducted a similar analysis on a smaller (17,783 patients), provincial cohort of patients admitted to ICUs in Alberta, Canada, and reported that chronic factors were more predictive of survival than acute factors on day 9 in ICU. Patients staying at least 9 days in ICU accounted for 16% of admissions and 54% of ICU bed-days. 7 Most recently, Kerckhoffs and colleagues reported a similar analysis of over 400,000 ICU patients in the Netherlands, and reported that chronic factors were more predictive of hospital survival than acute factors on day 7 in ICU. Patients staying at least 7 days in ICU accounted for 8% of ICU admissions and 52% of ICU bed-days. 8
Using a high-quality clinical database of patients admitted to ICU in the UK, our objectives were: (1) to assess the day in ICU on which chronic factors were more predictive of survival than acute factors measured on the day of admission; (2) to assess the consistency of this finding across major patient subgroups and over time and (3) to compare case mix characteristics and outcomes for patients determined to develop PerCI (based on ICU length of stay) with non-PerCI patients.
Methods
Data source
The Case Mix Programme (CMP) is the national clinical audit of adult ICUs in England, Wales and Northern Ireland. Data on consecutive admissions are recorded prospectively and abstracted retrospectively by trained data collectors according to precise rules and definitions. Data collected include raw physiological (lowest and highest values) and diagnostic data during the first 24 h following admission to ICU, together with demographic, outcome and activity data. CMP data undergo extensive validation, both locally and centrally, before being pooled into the central CMP Database. Details of data collection and validation have been reported previously, and the CMP Database has been independently assessed to be of high quality. 9
Study population and selection criteria
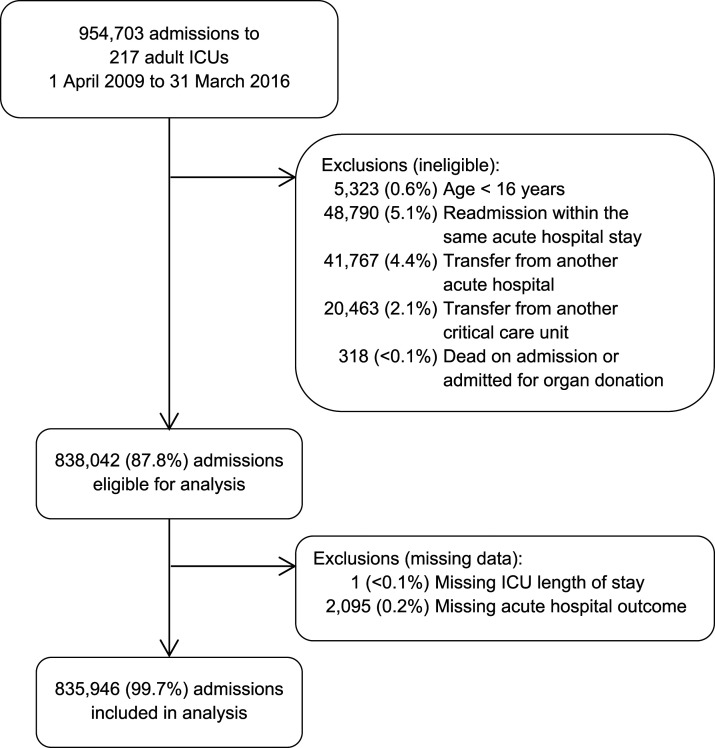
Patients were included in the analysis if they were admitted to an NHS adult, general ICU in England, Wales or Northern Ireland (participating in the CMP) between 1 April 2009 and 31 March 2016 and were aged 16 years or over at ICU admission. The CMP covers >90% of all ICUs. The following admissions were excluded from the analyses: readmission to ICU of the same patient in the same acute hospital stay; patients transferred directly from another acute hospital or from another ICU in the same hospital; patients who were dead or had withdrawal of all active treatment at ICU admission or were admitted solely to facilitate organ donation and admissions missing date of discharge from ICU or acute hospital outcome.
Statistical analyses
All analyses followed an a priori statistical analysis plan. The approach mirrored that of Iwashyna et al. 5
Definition of exposure variable
The ICU length of stay was defined as the number of days from the date of admission to ICU to the earliest of: date of being declared fully ready for discharge from ICU; date of discharge from ICU or date of death. Admission and discharge/death on the same day counted as 0 days.
Primary outcome
The primary outcome was acute hospital mortality, defined as death before ultimate discharge from acute hospital (including deaths occurring in another acute hospital following transfer).
Handling of missing data
Missing data for physiological parameters during the first 24 h following admission to ICU were imputed using multiple imputation by fully conditional specification implemented using the MICE (multivariate imputation by chained equations) algorithm. The multiple imputation model included all variables to be imputed (with the same structure as in the planned evaluative models), the exposure and outcome variables, and additional relevant auxiliary variables. Five multiply imputed datasets were created and results of analyses were combined with Rubin’s rules. A separate binary indicator was fitted for admissions with no evidence available to assess past medical history. Missing body mass index (BMI) was singly imputed to the population mean of 27.
Modelling
Eligible patients were randomly divided into development and validation cohorts of equal size, stratified by year of admission. The following characteristics: age; gender; presence of any severe comorbidity (defined by APACHE II) 10 ; main diagnostic category (body system affected by primary reason for admission to ICU coded using the ICNARC Coding Method) 11 ; APACHE II Acute Physiology Score and total score 10 ; ICNARC Physiology Score 12 ; ICNARCH-2015 predicted risk of acute hospital mortality 13 ; ICU length of stay; total length of acute hospital stay and acute hospital outcome (alive/dead) were summarised overall and for the development and validation cohorts.
Two statistical models, ‘chronic’ and ‘acute’, were fitted using logistic regression with robust standard errors clustered by ICU employing acute hospital mortality as the dependent variable. Discrimination was assessed by the area under the receiver operating characteristic curve (AUC). 14 Calibration was assessed, graphically, by plotting observed against predicted mortality in ten equal-sized groups by predicted acute hospital mortality.
The chronic model included the following covariates:
(1) age (restricted cubic splines with four knots);
(2) gender;
(3) BMI (restricted cubic splines with four knots);
(4) dependency prior to admission to acute hospital (based on activities of daily living – able, no assistance, minor or major assistance, total assistance);
(5) severe comorbidities evident in the 6 months prior to admission to ICU (cardiovascular, respiratory, renal, hepatic, haematological malignancy, metastatic cancer and immunocompromise);
(6) no evidence available to assess past medical history;
(7) residence prior to admission (home-including residential place of work/education and non-health-related institution; nursing home or health-related institution; hospice; no fixed abode or temporary abode);
(8) quintile of deprivation (assessed using the Index of Multiple Deprivation 2015 for England, Welsh Index of Multiple Deprivation 2014 for Wales, and Multiple Deprivation Measure 2010 for Northern Ireland, additional categories for patients normally resident outside England, Wales and Northern Ireland and for those with unknown address);
(9) date of admission to ICU (linear time in years since 1 April 2009); and
(10) month of year (categorical).
The acute model included the following covariates:
(1) Source of admission to ICU (ED or not in hospital, theatre as planned admission following elective/scheduled surgery, theatre as unplanned admission following elective/scheduled surgery, theatre as admission following emergency/urgent surgery, ward or intermediate care area);
(2) CPR within 24 hours prior to admission to ICU (no CPR, in-hospital CPR and out-of-hospital CPR);
(3) acute hospital length of stay prior to admission to ICU (0, 1, 2–7, 8 or more days);
(4) primary reason for admission to ICU (categorised by body system and pathological/physiological process or individual conditions as included in the ICNARCH-2015 risk prediction model);
(5) highest level of care during the first 24 hours in ICU (Level 3-intensive care, Level 2-high dependency care or lower);
(6) mechanical ventilation during the first 24 hours in ICU;
- (7) physiological parameters during the first 24 hours in ICU-
- (1) highest heart rate (restricted cubic splines with four knots),
- (2) lowest systolic blood pressure (restricted cubic splines with four knots),
- (3) highest central temperature or non-central temperature +1°C if no central temperature available (restricted cubic splines with four knots),
- (4) lowest respiratory rate, ventilated or non-ventilated (right-restricted cubic splines with four knots),
- (5) PaO2/FiO2 with lowest PaO2 (restricted cubic splines with four knots),
- (6) lowest arterial pH (restricted cubic splines with four knots),
- (7) PaCO2 associated with lowest arterial pH (restricted cubic splines with three knots),
- (8) highest blood lactate (restricted cubic splines with four knots),
- (9) 24-hours urine output, scaled for admissions staying less than 24 hours (restricted cubic splines with four knots),
- (10) highest serum urea (restricted cubic splines with four knots),
- (11) highest creatinine (right-restricted cubic splines with four knots),
- (12) highest sodium (restricted cubic splines with three knots),
- (13) lowest white blood cell count (restricted cubic splines with four knots),
- (14) lowest platelet count (restricted cubic splines with four knots),
- (15) lowest total Glasgow Coma Score (3, 4–6, 7–13, 14, 15, sedated, or paralysed and sedated).
Both models, chronic and acute, were initially fitted in all eligible patients and subsequently refitted restricting the cohort of patients included to those with an ICU length of stay of at least 1 day, 2 days, 3 days, etc., up to 28 days. At each step, both models were fitted in the development cohort and assessed in the validation cohort. The discrimination (AUC) of both chronic and acute models for each ICU length of stay cut-off, in the validation cohorts, were plotted and the point at which the chronic and acute model-based curves crossed was assessed. A 95% confidence interval for the day on which the discrimination of the chronic model first exceeded that of the acute model was calculated by repeating the entire process (including random split into development and validation samples and repeated model fitting) in 1000 bootstrap samples of the original data.
The above process of model fitting was repeated for the major patient subgroups (diagnostic categories defined by the body system of the primary reason for admission to ICU) and for each fiscal year (April–March) from 2009–2010 to 2015–2016.
Patient characteristics (as listed above) were compared for longer stay ICU patients (defined according to the day on which the discrimination of the chronic model first exceeded that of the acute model) and shorter stay ICU patients. No statistical testing or modelling of these differences was undertaken.
Results
Between 1 April 2009 and 31 March 2016, there were a total of 954,703 admissions to 217 adult, general critical care units in England, Wales and Northern Ireland participating in the Case Mix Programme. After exclusions, 835,946 admissions (87.6%) were included in the analyses (Figure 1). The characteristics of the cohort, overall and following the random split into development and validation cohorts, are presented in Table 1. Information on missing data is presented in the Supplementary Table S1.
Figure 1.
Selection of patients.
Table 1.
Patient characteristics and outcome.
| Characteristic | Entire cohort | Development cohort | Validation cohort |
|---|---|---|---|
| Number of admissions | 835,946 | 417,972 | 417,974 |
| Age (years), mean (SD) | 61.5 (17.8) | 61.5 (17.8) | 61.5 (17.8) |
| Gender, n (%) | |||
| Female | 375,998 (45.0) | 187,459 (44.8) | 188,539 (45.1) |
| Male | 459,948 (55.0) | 230,513 (55.2) | 229,435 (54.9) |
| Presence of any severe comorbidity, n (%) | 146,809 (17.7) | 73,620 (17.7) | 73,189 (17.6) |
| Main diagnostic category, n (%) | |||
| Gastrointestinal | 232,857 (27.9) | 116,485 (27.9) | 116,372 (27.8) |
| Respiratory | 171,675 (20.5) | 85,714 (20.5) | 85,961 (20.6) |
| Cardiovascular | 127,103 (15.2) | 63,741 (15.3) | 63,362 (15.2) |
| Neurological | 91,809 (11.0) | 45,818 (11.0) | 45,991 (11.0) |
| Genitourinary | 86,671 (10.4) | 43,313 (10.4) | 43,358 (10.4) |
| Haematological | 9419 (1.1) | 4728 (1.1) | 4691 (1.1) |
| Endocrine | 66,106 (7.9) | 33,151 (7.9) | 32,955 (7.9) |
| Musculoskeletal | 41,244 (4.9) | 20,457 (4.9) | 20,787 (5.0) |
| Dermatological | 9032 (1.1) | 4549 (1.1) | 4483 (1.1) |
| APACHE II Acute Physiology Score, mean (SD) | 11.4 (6.1) | 11.4 (6.1) | 11.4 (6.1) |
| APACHE II Score, mean (SD) | 15.6 (7.0) | 15.6 (7.0) | 15.6 (7.0) |
| ICNARC Physiology Score, mean (SD) | 16.8 (9.3) | 16.8 (9.4) | 16.8 (9.3) |
| ICNARCH-2015 predicted risk of acute hospital mortality (%) | |||
| Mean (SD) | 20.7 (26.2) | 20.7 (26.3) | 20.7 (26.2) |
| Median (IQR) | 8.1 (2.1, 29.9) | 8.1 (2.1, 29.9) | 8.1 (2.1, 29.9) |
| ICU LOS (days) | |||
| Mean (SD) | 4.5 (7.6) | 4.4 (7.6) | 4.5 (7.7) |
| Median (IQR) | 2.1 (1.0, 4.8) | 2.1 (1.0, 4.8) | 2.1 (1.0, 4.8) |
| Total acute hospital LOS (days) | |||
| Mean (SD) | 20.4 (29.3) | 20.3 (29.0) | 20.5 (29.6) |
| Median (IQR) | 11 (6, 24) | 11 (5, 24) | 11 (6, 24) |
| Acute hospital outcome, n (%) | |||
| Alive | 656,020 (78.5) | 328,100 (78.5) | 327,920 (78.5) |
| Dead | 179,926 (21.5) | 89,872 (21.5) | 90,054 (21.5) |
APACHE: acute physiology and chronic health evaluation; LOS: length of stay; ICNARC: intensive care national audit & research centre; ICU: intensive care unit; IQR: interquartile range; SD: standard deviation.
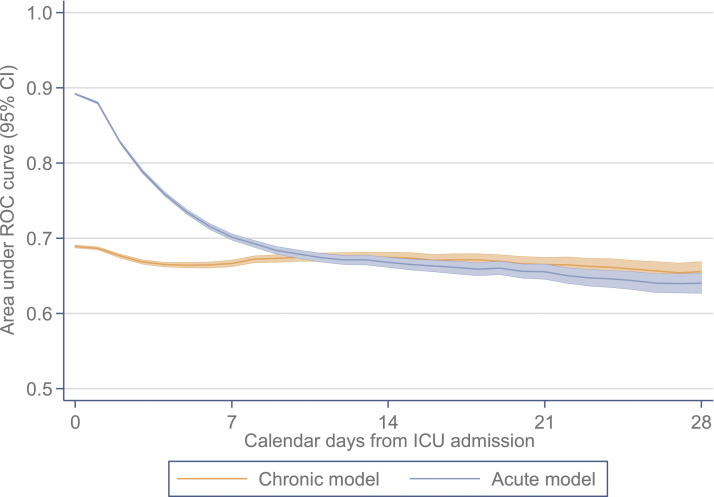
When fitted in the full development cohort, and assessed in the validation cohort, the chronic model had an AUC of 0.689 (95% confidence interval 0.687–0.691) compared with 0.892 (0.891–0.893) for the acute model. The coefficients for both models are reported in the Supplementary Tables S2 and S3. Both models were well calibrated (Supplementary Figures S1 and S2). The change in discrimination, as the cohort was restricted to those with longer stays in ICU, is presented in Figure 2 with full details in the Supplementary Table S4. The discrimination of the chronic model first exceeded that of the acute model on day 11 in ICU (chronic model AUC 0.675 [0.669–0.680] versus acute model 0.674 [0.669–0.680]), with 95% confidence interval from day 10 to day 13.
Figure 2.
Change in the AUC for acute versus chronic models by number of days since ICU admission; AUC, area under the ROC curve; CI, confidence interval; ICU, intensive care unit; ROC, receiver operating characteristic.
When repeated across major patient subgroups, the day on which the discrimination of the chronic model first exceeded that of the acute model varied from day 6 (neurological) to day 17 (gastrointestinal) and, when repeated across individual years, it varied from day 8 to day 10 (Table 2). This was earlier in all subgroups than the 11 days found in the primary analysis, reflecting the contribution of reason for admission to the discrimination of the acute model.
Table 2.
Summary of results.
| Subgroup | N | AUC (95% CI) for models fitted in all eligible patients a | Crossover day b (95% CI) | |
|---|---|---|---|---|
| Chronic model | Acute model | |||
| Overall | 835,946 | 0.689 (0.687, 0.691) | 0.892 (0.891, 0.893) | 11 (10, 13) |
| By diagnostic group | ||||
| Gastrointestinal | 232,857 | 0.709 (0.705, 0.713) | 0.880 (0.877, 0.883) | 17 (10, 26) |
| Respiratory | 171,675 | 0.712 (0.709, 0.716) | 0.843 (0.840, 0.846) | 8 (7, 9) |
| Cardiovascular | 127,103 | 0.639 (0.635, 0.644) | 0.893 (0.890, 0.895) | 10 (8, 11) |
| Neurological | 91,809 | 0.636 (0.630, 0.642) | 0.873 (0.869, 0.877) | 6 (5, 7) |
| Genitourinary | 86,671 | 0.741 (0.735, 0.747) | 0.893 (0.889, 0.897) | 11 (9, 21) |
| Other | 125,801 | 0.749 (0.743, 0.755) | 0.913 (0.910, 0.916) | 10 (8, 14) |
| By year | ||||
| 2009–2010 | 86,195 | 0.685 (0.679, 0.691) | 0.870 (0.866, 0.873) | 10 (7, 14) |
| 2010–2011 | 100,404 | 0.684 (0.679, 0.690) | 0.872 (0.868, 0.875) | 8 (7, 12) |
| 2011–2012 | 115,754 | 0.691 (0.686, 0.696) | 0.877 (0.874, 0.881) | 8 (7, 10) |
| 2012–2013 | 118,914 | 0.692 (0.687, 0.697) | 0.878 (0.875, 0.881) | 8 (7, 9) |
| 2013–2014 | 126,345 | 0.687 (0.682, 0.692) | 0.882 (0.879, 0.885) | 10 (8, 13) |
| 2014–2015 | 139,554 | 0.691 (0.687, 0.696) | 0.887 (0.884, 0.890) | 10 (8, 13) |
| 2015–2016 | 148,780 | 0.683 (0.678, 0.688) | 0.886 (0.883, 0.889) | 10 (8, 16) |
AUC: area under the receiver operating characteristic curve; CI: confidence interval; N: total sample size (development and validation samples combined).
aAUC for models fitted in the development sample and evaluated in the validation sample.
bDay on which the AUC for the chronic model first exceeded that for the acute model (95% CI calculated using nonparametric bootstrap).
Case mix, outcomes and activity for longer stay patients (ICU length of stay of 11 or more days) compared with shorter stay patients (ICU length of stay <11 days) are presented in Table 3. Longer stay patients comprised 9% of admissions to ICU but consumed 45% of ICU bed-days. The two groups were similar in terms of age and presence of severe comorbidities, but longer stay patients were more likely to be male (60% vs 54%), more likely to have a respiratory condition as their primary reason for admission to ICU (38% vs 19%) and had greater acute severity of illness, as assessed by both the APACHE II and ICNARC scores and acute hospital mortality risk predictions.
Table 3.
Patient characteristics and outcome for longer stay (11 days or longer) versus shorter stay admissions.
| Characteristic | Length of stay in ICU | |
|---|---|---|
| 11 days or longer | <11 days | |
| Number of admissions (%) | 78,536 (9.4) | 757,410 (90.6) |
| Age (years), mean (SD) | 60.7 (16.1) | 61.6 (18.0) |
| Gender, n (%) | ||
| Female | 31,166 (39.7) | 344,832 (45.5) |
| Male | 47,370 (60.3) | 412,578 (54.5) |
| Presence of any severe comorbidity, n (%) | 13,640 (17.5) | 133,169 (17.7) |
| Main diagnostic category, n (%) | ||
| Gastrointestinal | 17,290 (22.0) | 215,567 (28.5) |
| Respiratory | 28,990 (36.9) | 142,685 (18.8) |
| Cardiovascular | 11,053 (14.1) | 116,050 (15.3) |
| Neurological | 9199 (11.7) | 82,610 (10.9) |
| Genitourinary | 4970 (6.3) | 81,701 (10.8) |
| Haematological | 1144 (1.5) | 8275 (1.1) |
| Endocrine | 2947 (3.8) | 63,159 (8.3) |
| Musculoskeletal | 1978 (2.5) | 39,266 (5.2) |
| Dermatological | 964 (1.2) | 8068 (1.1) |
| APACHE II Acute Physiology Score, mean (SD) | 14.4 (5.8) | 11.0 (6.0) |
| APACHE II Score, mean (SD) | 18.6 (6.5) | 15.3 (6.9) |
| ICNARC Physiology Score, mean (SD) | 23.2 (8.0) | 16.1 (9.2) |
| ICNARCH-2015 predicted risk of acute hospital mortality (%) | ||
| Mean (SD) | 32.9 (24.4) | 19.5 (26.1) |
| Median (IQR) | 27.1 (12.4, 49.3) | 6.7 (1.9, 26.4) |
| ICU LOS (days) | ||
| Mean (SD) | 21.7 (15.5) | 2.7 (2.3) |
| Median (IQR) | 16.9 (13.0, 24.8) | 1.9 (0.9, 3.8) |
| Total acute hospital LOS (days) | ||
| Mean (SD) | 50.2 (46.9) | 17.3 (24.9) |
| Median (IQR) | 36 (23, 60) | 10 (5, 20) |
| Acute hospital outcome, n (%) | ||
| Alive | 55,269 (70.4) | 600,751 (79.3) |
| Dead | 23,267 (29.6) | 156,659 (20.7) |
APACHE: acute physiology and chronic health evaluation; LOS: length of stay; ICNARC: intensive care national audit & research centre; ICU: intensive care unit; IQR: interquartile range; SD: standard deviation.
Discussion
We studied patient records from over 800,000 patients treated on 217 ICUs across England, Wales and Northern Ireland to reproduce the analysis of the ANZICS-APD study of Iwashyna et al. 5 We wished to ascertain if there was a point beyond which acute characteristics measured on the day of admission were no more predictive of in-hospital mortality than chronic characteristics. Consistent with the findings of Iwashyna et al., we found that such a transition point does occur. As anticipated, the predictive ability of a model built on acute characteristics from the day of admission deteriorated as time since admission increased. By day 11, performance was similar to a model using only chronic characteristic. This was similar to the findings from the ANZICS-APD analysis of day 10, and the Canadian analysis of day 9.5,7
Patients who remained in critical care for 11 days or longer (deemed PerCI) accounted for 45% of ICU bed-days and, after a mean ICU length of stay of 22 days and a further 28 days in hospital, 30% died. The long-term adverse consequences of ICU (the ‘post-intensive care syndrome’) are associated with prolonged stay in ICU 3 and therefore those who survive PerCI are likely to experience significant morbidity.
Strengths and limitations of the study
These analyses provide objective justification of the validity of the PerCI concept in a large, nationally representative clinical database but are not without limitations. Most obviously, a patient requiring multiple organ support at day 10 or 11 is dissimilar to one who is close to liberation from mechanical ventilation, although both would meet these criteria for PerCI. Failure to be discharged from critical care by day 10 or 11 might reflect organisational or logistic factors and not relate to the patient. Recent data from Australasia showed that discharge delays (>6 h) affect up to 50% of patients. 15 A strength of our study is the ability to base the calculations on the time the patient was deemed clinically ready for discharge rather than the time at which the discharge actually took place, negating any adverse effect of delays.
Comparison with other studies
By contrast to the ANZICS-APD analysis of Iwashyna et al., 5 longer stay patients comprised 9% of ICU admissions (compared with 5%) and used a larger proportion of ICU bed-days (45% compared with 33%), indicating that PerCI is a more substantial resource burden in the UK. In this respect, our findings were more similar to those from Canada 7 and the Netherlands, 8 in which longer stay patients accounted for more than half of ICU bed-days. The variation among the different studies on the exact day on which chronic factors became more predictive than acute factors may reflect variations in patient groups represented in the datasets, available predictors, complexity of modelling approaches, and whether the models were refitted to each successive cohort of patients.
Although our analysis plan was based on the original approach by Iwashyna et al., there are some differences in our analyses. Iwashyna et al. included covariates for day of the week and hour of the day in their chronic model. 5 We opted not to include these covariates in either the chronic or acute models: in the chronic model as, in the absence of adjustment for severity, these covariates are most likely to reflect the urgency of admission, which is an acute characteristic; and in the acute model as, once adjusted for severity, we have previously demonstrated that neither of these characteristics affects outcome. 16 We included time trend and seasonality in both models (unlike Iwashyna et al., who considered these to be chronic). We have also not included any ICU characteristics in either model to ensure a focus on the predictive performance of patient factors alone, although we note previous work from the US that identified hospital-level variation in the proportion of patients with PerCI. 17
Implications for clinicians and policymakers
Clinicians should be aware that, in critically ill patients who remain on the ICU after day 10, the influence of the acute presentation on their recovery is similar to the impact of the patient’s health status before the onset of their acute illness. Such patients spend a long time in hospital and, ultimately, only 70% will survive their acute hospital stay. Awareness of these data may be important considerations for clinicians’ perspectives, decision-making and discussions with patients and their families.
Unanswered questions and future research
These data compel us to prioritise the following areas of research:
(1) What are the outcomes for patients with PerCI (other than acute hospital mortality)? From a patient’s perspective, avoiding acute hospital mortality is important but may not be the most important outcome. In addition to understanding effects of PerCI on longer term mortality, there is a pressing need to understand the associated longer term morbidity. It is plausible that those who survive following an episode of PerCI will almost universally experience long-term complications including physical, cognitive and psychiatric impairment.
(2) Are there superior definitions of PerCI? Intuitively, the number of days of receipt of multi-organ support may better reflect the identifiable clinical phenomenon and this alternative definition needs to be evaluated.
(3) How can we best prognosticate for these patients to inform discussions with the patients and their families about likely outcomes? The burden of continued multi-organ support is borne by the patient in the hope of a ‘good’ outcome. All tools currently available for prognostication are based upon a combination of the acute physiological disturbance, which we have found to lack predictive capabilities in these patients, and chronic health characteristics. The development of tools that provide insight into likely outcomes in these patients would inform discussions with patients and their families, and potentially influence the treatment that patients receive. Continued provision of ‘futile’ care adversely affects the care of acutely ill patients requiring ICU admission. 18
(4) Are there modifiable pathophysiological mechanisms that mediate PerCI and the failure to recover?
Conclusions
Whether considering Australasia, Canada, the Netherlands or the UK, PerCI is encountered in clinical practice and, when studied empirically, is associated with adverse outcome and increased healthcare utilisation.
Improvements in our understanding of the longer term outcomes and in the development of tools to aid prognostication are required – for humane as well as health economic reasons.
Supplemental Material
Supplemental Material, sj-pdf-1-inc-10.1177_17511437211047180 for Timing and burden of persistent critical illnessin UK intensive care units: An observational cohort study by David A Harrison, Ben C Creagh-Brown and Kathryn M Rowan in Journal of the Intensive Care Society
Acknowledgements
We thank the patients and staff in ICUs participating in the Case Mix Programme.
Authors’ contribution: All authors contributed to the conception and design, and interpretation of data; contributed towards drafting the article and revising it critically for important intellectual content; and approved the final version to be published. DAH performed the analysis and wrote the first draft of the manuscript and is the guarantor. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval: The Case Mix Programme has approval under Section 251 of the NHS Act 2006 to process patient data without consent (approval number PIAG 2–10(f)/2005). This approval is reviewed annually by the Health Research Authority’s Confidentiality Advisory Group. This study was reviewed and approved by ICNARC’s independent Data Access Advisory Group. Under Health Research Authority guidance, review by a Research Ethics Committee is not required for studies limited to secondary analysis of anonymised data.
Data sharing: Requests to access Case Mix Programme data can be made via https://www.icnarc.org/Our-Audit/Audits/Cmp/Reports/Access-Our-Data.
Transparency: The manuscript’s guarantor (DAH) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; and there were no discrepancies from the study as planned.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
David A Harrison https://orcid.org/0000-0002-9002-9098
Ben C Creagh-Brown https://orcid.org/0000-0002-4397-1232
Kathryn M Rowan https://orcid.org/0000-0001-8217-5602
References
- 1.Adhikari NK, Fowler RA, Bhagwanjee S, et al. Critical care and the global burden of critical illness in adults. Lancet 2010; 376: 1339–1346. DOI: 10.1016/s0140-6736(10)60446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Intensive Care National Audit & Research Centre (ICNARC) . Key statistics from the case mix programme-adult, general critical care units. 1 april 2017 to 31 march 2018. London, UK: ICNARC, 2018. [Google Scholar]
- 3.Harvey MA, Davidson JE. Postintensive care syndrome. Crit Care Med 2016; 44: 381–385. DOI: 10.1097/ccm.0000000000001531. [DOI] [PubMed] [Google Scholar]
- 4.Iwashyna TJ, Hodgson CL, Pilcher D, et al. Towards defining persistent critical illness and other varieties of chronic critical illness. Crit Care Resusc 2015; 17: 215–218. [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Hodgson CL, Pilcher D, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: a retrospective, population-based, observational study. Lancet Respir Med 2016; 4: 566–573. DOI: 10.1016/s2213-2600(16)30098-4. [DOI] [PubMed] [Google Scholar]
- 6.Iwashyna TJ, Hodgson CL, Pilcher D, et al. Persistent critical illness characterised by Australian and New Zealand ICU clinicians. Crit Care Resusc 2015; 17: 153–158. [PubMed] [Google Scholar]
- 7.Bagshaw SM, Stelfox HT, Iwashyna TJ, et al. Timing of onset of persistent critical illness: a multi-centre retrospective cohort study. Intensive Care Med 2018; 44: 2134–2144. DOI: 10.1007/s00134-018-5440-1. [DOI] [PubMed] [Google Scholar]
- 8.Kerckhoffs MC, Brinkman S, de Keizer N, et al. The performance of acute versus antecedent patient characteristics for 1-year mortality prediction during intensive care unit admission: a national cohort study. Crit Care 2020; 24: 330. DOI: 10.1186/s13054-020-03017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harrison DA, Brady AR, Rowan K. Case mix, outcome and length of stay for admissions to adult, general critical care units in England, Wales and Northern Ireland: the intensive care national audit & research centre case mix programme database. Crit Care 2004; 8: R99–R111. DOI: 10.1186/cc2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, et al. Apache Ii. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 11.Young JD, Goldfrad C, Rowan K. Development and testing of a hierarchical method to code the reason for admission to intensive care units: the ICNARC Coding Method. Br J Anaesth 2001; 87: 543–548. [DOI] [PubMed] [Google Scholar]
- 12.Harrison DA, Parry GJ, Carpenter JR, et al. A new risk prediction model for critical care: the intensive care national audit & research centre (ICNARC) model*. Crit Care Med 2007; 35: 1091–1098. DOI: 10.1097/01.ccm.0000259468.24532.44. [DOI] [PubMed] [Google Scholar]
- 13.Ferrando-Vivas P, Jones A, Rowan KM, et al. Development and validation of the new ICNARC model for prediction of acute hospital mortality in adult critical care. J Crit Care 2017; 38: 335–339. DOI: 10.1016/j.jcrc.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982; 143: 29–36. DOI: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 15.Tiruvoipati R, Botha J, Fletcher J, et al. Intensive care discharge delay is associated with increased hospital length of stay: a multicentre prospective observational study. PLoS One 2017; 12: e0181827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arulkumaran N, Harrison DA, Brett SJ. Association between day and time of admission to critical care and acute hospital outcome for unplanned admissions to adult general critical care units: cohort study exploring the ‘weekend effect’. Br J Anaesth 2017; 118: 112–122. DOI: 10.1093/bja/aew398. [DOI] [PubMed] [Google Scholar]
- 17.Viglianti EM, Bagshaw SM, Bellomo R, et al. Hospital-level variation in the development of persistent critical illness. Intensive Care Med 2020; 46: 1567–1575. DOI: 10.1007/s00134-020-06129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh TN, Kleerup EC, Raj PP, et al. The opportunity cost of futile treatment in the ICU*. Crit Care Med 2014; 42: 1977–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-inc-10.1177_17511437211047180 for Timing and burden of persistent critical illnessin UK intensive care units: An observational cohort study by David A Harrison, Ben C Creagh-Brown and Kathryn M Rowan in Journal of the Intensive Care Society