Abstract
The assembly and maintenance of eucaryotic flagella and cilia depend on the microtubule motor, kinesin-II. This plus end-directed motor carries intraflagellar transport particles from the base to the tip of the organelle, where structural components of the axoneme are assembled. Here we test the idea that kinesin-II also is essential for signal transduction. When mating-type plus (mt+) and mating-type minus (mt−) gametes of the unicellular green alga Chlamydomonas are mixed together, binding interactions between mt+ and mt− flagellar adhesion molecules, the agglutinins, initiate a signaling pathway that leads to increases in intracellular cAMP, gamete activation, and zygote formation. A critical question in Chlamydomonas fertilization has been how agglutinin interactions are coupled to increases in intracellular cAMP. Recently, fla10 gametes with a temperature-sensitive defect in FLA10 kinesin-II were found to not form zygotes at the restrictive temperature (32°C). We found that, although the rates and extents of flagellar adhesion in fla10 gametes at 32°C are indistinguishable from wild-type gametes, the cells do not undergo gamete activation. On the other hand, fla10 gametes at 32°C regulated agglutinin location and underwent gamete fusion when the cells were incubated in dibutyryl cAMP, indicating that their capacity to respond to the cAMP signal was intact. We show that the cellular defect in the fla10 gametes at 32°C is a failure to undergo increases in cAMP during flagella adhesion. Thus, in addition to being essential for assembly and maintenance of the structural components of flagella, kinesin-II/intraflagellar transport plays a role in sensory transduction in these organelles.
INTRODUCTION
Much as animals use cilia as sensory transducers to perceive light, odorants, and chemotactic agents in their environment, gametes of the green alga Chlamydomonas use their two flagella as sensory organelles (Solter and Gibor, 1977) to perceive and respond to gametes of the opposite sex in their environment (reviewed by Pan and Snell, 2000b). When Chlamydomonas cells are in the vegetative, asexual phase of their life cycle, as they swim through their medium, they undergo frequent, transient collisions with the flagella and cell bodies of other cells in the culture. With vegetative cells, the transient collisions are of no consequence. On the other hand, when vegetatively growing cells are induced to undergo gametogenesis, and the resulting gametes of opposite mating types are mixed together, the random encounters have a different outcome. Collisions between flagella of mating-type plus (mt+) and mating-type minus (mt−) gametes allow interactions between gamete-specific flagellar adhesion molecules, the mt+ and mt− agglutinins (Adair, 1985). Not only do the agglutinin interactions cause the flagella on cells of opposite mating types to adhere to each other but the receptor/ligand-like interactions between the agglutinins induce increases in intracellular cAMP (Goodenough, 1989; Saito et al., 1993) via a protein kinase-dependent pathway (Zhang et al., 1991; Zhang and Snell, 1994). Much as olfactory epithelial cells respond to the odorant-induced increases in intracellular cyclic nucleotides in their cilia (Sklar et al., 1986; Bakalyar and Reed, 1990; Schild and Restrepo, 1998), the interacting Chlamydomonas gametes undergo gamete activation in response to the increase in cAMP. The now activated gametes of both mating types undergo cell wall loss, agglutinin synthesis is induced (Snell and Moore, 1980), agglutinins (Goodenough, 1989; Hunnicutt et al., 1990) and an aurora-like protein kinase (Pan and Snell, 2000a) are translocated from the cell body to the flagella, and cell-cell fusion organelles are activated (Goodenough et al., 1982; Wilson et al., 1997). In the final step of fertilization, the activated gametes that had been adhering only via their flagella begin to adhere to each other via the apically localized fusion organelles on their cell bodies. This cell body adhesion is followed rapidly by cell-cell fusion and formation of a quadriflagellated zygote. Although we have learned much about the responses of Chlamydomonas gametes to flagellar adhesion during fertilization, we still know little about the underlying mechanisms of signal transduction that couple agglutinin interactions to increases in cAMP.
Over the past few years, studies of a newly discovered cellular phenomenon termed intraflagellar transport (IFT) have begun to offer new insights into ciliary and flagellar assembly and maintenance and have provided an inroad to learning more about flagellar signal transduction during fertilization. IFT is a motility process, first discovered in Chlamydomonas (Kozminski et al., 1993), in which nonmembrane-bound particles (IFT particles) are ferried along ciliary/flagellar microtubules, from the base to the tip of the organelle, and then back (reviewed by Cole, 1999; Rosenbaum et al., 1999; Marszalek and Goldstein, 2000). The plus-end–directed microtubule motor protein kinesin-II has been shown to be essential for movement of particles toward the tip (anterograde transport; Walther et al., 1994; Kozminski et al., 1995; Piperno et al., 1996; Cole et al., 1998), and the cycle is completed through the action of a cytoplasmic dynein that carries IFT particles back to the cell body (retrograde transport; Pazour et al., 1998, 1999; Porter et al., 1999; Iomini et al., 2001). Studies of Chlamydomonas as well as several other organisms have shown that IFT delivers structural components of the microtubular axoneme, including inner dynein arms (Piperno et al., 1996), to the tips of the flagella, where they are involved in flagellar assembly and maintenance.
Much of our understanding of IFT and its role in cilia and flagella has come from studies of cells with genetic lesions in IFT components. For example, Chlamydomonas mutants with defects in the heavy and light chains of cytoplasmic dynein form short flagella that are filled with 10 times the normal amounts of IFT particle proteins, indicating that cytoplasmic dynein is essential for retrograde IFT (Pazour et al., 1999, 2000; Porter et al., 1999). Similar experiments documented the role of Chlamydomonas kinesin-II in anterograde transport. Chlamydomonas fla10-1 cells, which express a temperature-sensitive defect in the 90-kDa, kinesin-II motor subunit FLA10 because of a single amino acid substitution in the motor domain (Walther et al., 1994), have normal flagella and are fully motile at the permissive temperature but are unable to form flagella at the restrictive temperature (Huang et al., 1977; Lux and Dutcher, 1991). Moreover, when fla10 cells previously maintained at the permissive temperature are shifted to the restrictive temperature, anterograde IFT particle movement ceases and IFT particle proteins are depleted from the flagella (Kozminski et al., 1995; Piperno and Mead, 1997; Cole et al., 1998; Iomini et al., 2001). Within 1–2 h after the temperature shift, the flagella gradually begin to shorten, and after several hours most of the cells are aflagellate (Kozminski et al., 1995; Piperno et al., 1996). Lesions in kinesin-II and IFT particle proteins in ciliated cells of multicellular organisms also lead to the absence of cilia and are associated with apoptotic photoreceptor cell death (Marszalek et al., 2000), polycystic kidney disease (Moyer et al., 1994; Pazour et al., 2000; Haycraft et al., 2001), and situs inversus (Nonaka et al., 1998; Marszalek et al., 1999; Okada et al., 1999). Related studies of Caenorhabditis elegans chemosensation mutants with defects in formation of sensory cilia revealed that many of the lesions are in genes encoding proteins of the IFT system (Perkins et al., 1986; Shakir et al., 1993; Scholey, 1996; Collet et al., 1998; Signor et al., 2000; Wicks et al., 2000; Haycraft et al., 2001; Qin et al., 2001).
A potentially interesting confluence between sensory transduction during fertilization in Chlamydomonas and flagellar motor proteins/IFT emerged from studies by Piperno et al. (1996) of flagellar assembly using the temperature-sensitive, kinesin-II mutant fla10. These workers reported that fla10 gametes lost the ability to form zygotes soon after being shifted to the restrictive temperature, well before flagella were lost. Because the block to zygote formation was incidental to the primary focus of the manuscript, the authors noted only that the phenotype could not be ascribed to flagellar loss. More recently it was suggested that the requirement for the kinesin-II motor protein in cell fusion could be due to the need to properly localize and transport flagellar agglutinins (Rosenbaum et al., 1999; Iomini et al., 2001).
Because Chlamydomonas fla10 gametes with a temperature-sensitive defect in kinesin-II fail to form zygotes at the restrictive temperature, we wanted to gain a better understanding of the possible role of kinesin-II in sensory transduction during Chlamydomonas fertilization. To do this, we studied the consequences of loss of kinesin-II function on distinct steps in fertilization and tested the hypothesis that, in addition to its role in ferrying structural molecules required for flagellar assembly and for maintaining flagella length, kinesin-II is involved in cellular signaling. Here, we demonstrate that gametes with a conditional defect in FLA10 kinesin-II fail to undergo proper flagellar sensory transduction during fertilization. Although gametes after 40 min at 32°C undergo flagellar adhesion that is indistinguishable from that of wild-type gametes, Chlamydomonas kinesin-II mutants do not form zygotes at the restrictive temperature because the cells fail to couple agglutinin interactions to increases in cAMP.
MATERIALS AND METHODS
Cells and Cell Culture
Chlamydomonas reinhardtii strains 21gr (mt+; CC-1690), 6145C (mt−; CC-1691), imp1-15 (mt+; CC-462), fla10-1 (mt−; CC-1919), available from the Chlamydomonas Genetic Center, Duke University (Durham, NC), were cultured with either medium I or medium II (Sager and Granick, 1954) at 23°C on a 13:11 h light:dark cycle as described previously (Pan and Snell, 2000a). Vegetative cells were induced to become gametes by incubation in medium without nitrogen (N-free medium) followed by culturing in continuous light at room temperature (Pan and Snell, 2000a).
Cell Adhesion and Fusion Assays
mt+ and mt− gametes (1 × 107 cells/ml in N-free medium) were mixed together, and at the indicated times cell-cell adhesion was quantified using an electronic particle counter (Coulter, Palo Alto, CA) as previously described (Snell and Roseman, 1979; Snell and Moore, 1980). Zygote formation was assessed by mixing fla10 gametes with wild-type mt+ gametes for 15 min at the indicated temperatures followed by fixation in 0.5% glutaraldehyde and determination of the number of biflagellated and quadriflagellated cells by examination in a phase contrast microscope. For each determination, 200 cells were counted. The percentage of cells forming zygotes was calculated from the following formula: % zygotes = 100 × 2 quadriflagellated cells/(2 × quadriflagellated cells + single cells). Flagellar loss was determined by counting the numbers of cells with and without flagella in glutaraldehyde-fixed samples. Two hundred cells were counted for each determination.
Incubation of Cells with Dibutyryl cAMP
For experiments with dibutyryl cAMP, gametes in N-free medium and vegetative cells in medium II were incubated in 15 mM dibutyryl cAMP and 0.15 mM papaverine for 30 min as previously described (Pasquale and Goodenough, 1987; Pan and Snell, 2000a). The papaverine was from a freshly made 15 mM stock solution in dimethyl sulfoxide (Sigma, St. Louis, MO). Cell wall loss, which is a measure of gamete activation, was assessed by determining whether cells became sensitive to disruption by incubation in 0.075% Triton 100-X, 0.5 mM EDTA, 10 mM Tris, pH 8.0, as described earlier (Snell, 1982).
Cell Fractionation
Flagella were isolated essentially as described by Zhang et al. (1991). Typically, 3–4 l of cells were concentrated to 30 ml by centrifugation at 3500 × g for 5 min at 4°C, and ice-cold 25% sucrose in 10 mM Tris, pH 7.2, was added to yield a final concentration of 7% sucrose. While stirring the suspension, its pH was rapidly decreased to 4.5 by addition of 0.5 M acetic acid; after the flagella were detached (which typically required ∼20 s) the pH was raised to 7.2 with 0.5 M KOH. All subsequent steps were carried out at 4°C. The suspension of cell bodies and flagella was underlayed with 25% sucrose in 10 mM Tris, pH 7.2, and centrifuged for 10 min at 2500 × g. The upper phase, which contained flagella and a few remaining cell bodies, was underlayed again with 25% sucrose, 10 mM Tris, pH 7.2, and centrifuged as above. The upper phase containing purified flagella was carefully removed and centrifuged at 9000 × g for 8 min to harvest the flagella. The sedimented flagella were resuspended in buffer A (20 mM HEPES, pH 7.2, 5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 25 mM KCl) (Cole et al., 1998) containing a 1:100 dilution of the Sigma protease inhibitor cocktail for plant cells (Sigma catalogue number P9599) and flash frozen in liquid nitrogen.
SDS-PAGE and Immunoblot Analysis
Samples for SDS-PAGE were mixed with one-third volume of 4× SDS sample buffer (0.25 M Tris, pH 6.8, 40% glycerol, 16% SDS, 0.4 mM dithiothreitol, 0.1% bromophenol blue) and boiled for 5 min (Pan and Snell, 2000a). In some experiments sample buffer was used at a final concentration of 2×. The samples were subjected to electrophoresis in 9% acrylamide minislab gels at 30 mA in buffer containing 25 mM Tris, 192 mM glycine, 0.1% SDS and then transferred for immunoblot analysis (see below). Typically 15–30 μg of protein was loaded in each lane. The protein concentration was determined with a protein assay kit (Bio-Rad, Hercules, CA) with bovine serum albumin (albumin standard, Pierce, Rockford, IL) as a standard. The immunoblot analysis was essentially as described by Pan and Snell (2000a). After SDS-PAGE, proteins were transferred to a polyvinylidene difluoride membrane (Immobilon P, Millipore, Bedford, MA) in buffer containing 25 mM Tris, 192 mM glycine, 20% methanol at 100 V for 1 h or at 35 V overnight at 4°C. The membrane was blocked with 5% Carnation dry milk (Nestles, Solon, Ohio) in 20 mM Tris, pH 7.6, 137 mM NaCl, 0.05% Tween-20 (TBST) for 1 h and then incubated with primary antibody in 3% Carnation dry milk in TBST for 1 h. The membrane was washed three times for 5 min each with TBST, followed by incubation for 1 h with a horseradish peroxidase-conjugated goat anti-rabbit IgG antibody (Bio-Rad) diluted 1:10,000 in TBST containing 3% Carnation dry milk. The membrane was washed as before and incubated in ECL immunoblotting reagents (Amersham Pharmacia Biotech, Piscataway, NJ) for 1 min as described by the manufacturer, exposed to Hyperfilm ECL (Amersham Pharmacia Biotech), and developed in an automatic film processor. Doug Cole (University of Idaho, Moscow, ID) kindly provided polyclonal anti-FLA10 antibody and monoclonal anti-IFT particle protein antibodies.
Radioimmunoassay of cAMP
To measure cellular levels of cAMP formed during adhesion, mt+ and mt− gametes (1 × 107 cells/ml in N-free medium) were mixed together and at the indicated times aliquots were mixed with 1 volume of 1 N perchloric acid at room temperature. The acidified extracts were analyzed for cAMP by a radioimmunoassay (Domino et al., 1991) with duplicate samples, which typically differed by 5% or less. The results shown are typical of at least two independent experiments.
RESULTS
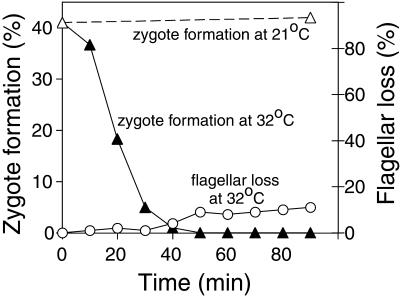
To establish the time that was required for fla10 mt− gametes to lose their ability to form zygotes after being changed to the restrictive temperature, we shifted gametes from the permissive temperature (21°C) to the restrictive temperature (32°C) and at various times assessed their ability to fuse with wild-type mt+ gametes to form quadriflagellated zygotes at 32°C. Although the mt+ gametes used as tester cells in our experiments to assess zygote formation of fla10 mt− gametes were wild type and not the ida4fla10 mt+ gametes used by Piperno et al. (1996), we obtained results similar to those originally reported by those investigators. At the permissive temperature the fla10 gametes formed zygotes when mixed with tester cells (Figures 1 and 2B, left). After being transferred to 32°C, however, the fla10 gametes gradually lost the ability to form zygotes, and by 40 min after transfer, the ability to form zygotes had been completely abrogated (Figure 1). As expected, fla10 gametes kept at 21°C showed no loss in their ability to form zygotes during the 90-min course of the experiment (Figure 1), and control experiments showed that wild-type mt− gametes preincubated for 40 min at 32°C underwent zygote formation with wild-type mt+ gametes similarly to wild-type mt− gametes at 21°C. Importantly, at 40 min after transfer, when zygote formation was blocked, <3% of the fla10 gametes had lost their flagella (Figure 1). Even at 90 min after the temperature shift, only 10% of the cells had lost their flagella, results consistent with previous studies of these mutants (Kozminski et al., 1995; Piperno et al., 1996). Thus, as reported by Piperno et al. (1996), even in cells that are fully flagellated, FLA10 is essential for zygote formation.
Figure 1.
Loss of zygote-forming ability of fla10 gametes at the restrictive temperature. fla10 gametes maintained at 21°C were shifted to 32°C at T0 and at the indicated times were analyzed for their ability to form zygotes after being mixed with mt+ gametes (▴). ▵, zygote formation by fla10 gametes maintained at the permissive temperature (21°C). At the same times, the number of cells that had lost flagella also was determined by examination in the phase contrast microscope (○).
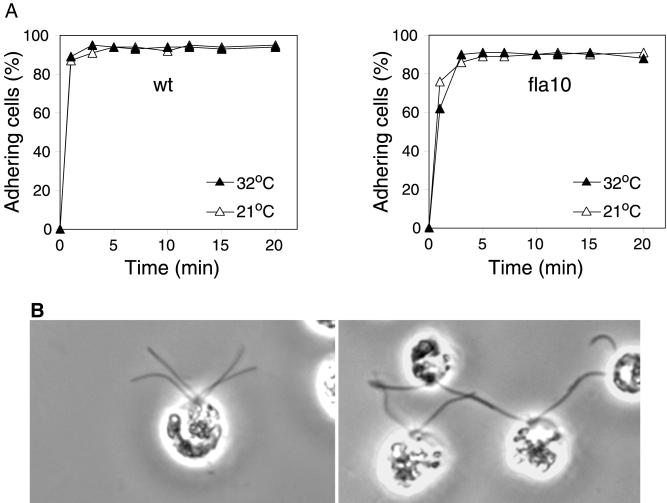
Figure 2.
Adhesion of wild-type (wt) and fla10 gametes at the permissive and restrictive temperatures. (A) Wild-type (left) and fla10 mt− gametes (right) maintained at 21°C (▵) or preincubated at 32°C for 40 min (▴) were mixed with imp1 mt+ gametes at 21 or 32°C, respectively, and cell adhesion was determined by use of an electronic particle counter. (B) fla10 gametes maintained at 21°C (left) or preincubated at 32°C (right) were mixed with wild-type mt+ gametes at 21 or 32°C, respectively, fixed with osmium fumes (Hunnicutt and Snell, 1991), and examined by phase contrast microscopy.
Having confirmed that the fla10 mutant cells were unable to undergo fertilization after being shifted to the restrictive temperature, we began to characterize the fertilization-related phenotype of the cells. Examination by light microscopy indicated that the motility of the cells was indistinguishable from that of the fla10 cells at 21°C or wild-type cells at either temperature. Thus, the failure to fuse could not be attributed to the inability to move or to undergo collisions with cells of the opposite mating type. It was also possible that kinesin-II was required for maintenance of the differentiated gametic phenotype and that loss of kinesin-II function caused the gametes to dedifferentiate into vegetative cells, which are nonadhesive. Also, a functional kinesin-II might have been required for the presence of agglutinins on the flagella, and after 40 min at the restrictive temperature, agglutinins might have been lost from the flagella.
To test whether the fla10 gametes shifted to 32°C still retained their gametic properties, we pretreated fla10 gametes for 40 min at 32°C, mixed them with mt+ gametes at 32°C, and then assessed flagellar adhesion. Because zygotes rapidly become nonadhesive and, therefore, zygote formation interferes with quantitative evaluation of adhesion (Snell and Roseman, 1979), we used an impotent mutant strain, imp1, as the mt+ gametes for these experiments. imp1 gametes undergo normal flagellar adhesion and gamete activation with mt− gametes (Snell and Moore, 1980; Goodenough et al., 1982) but are unable to fuse because of a lesion in the fus1 gene, which is required for adhesion and fusion of the tips of mating structures during the final steps in fertilization (Ferris et al., 1996). In the experiments described below, qualitatively similar results were obtained with wild-type and imp1 mt+ gametes. Examination by phase contrast microscopy indicated that the fla10 gametes at 32°C underwent vigorous adhesion when mixed with the mt+ gametes (Figure 2B, right). This qualitative assessment was confirmed by a quantitative electronic particle counter assay that determines the number of cells adhering by measuring the loss of single cells from the suspension (Snell and Roseman, 1979; Snell and Moore, 1980). As shown in Figure 2A, the initial rates and extents of adhesion of fla10 gametes with imp1 mt+ gametes were the same for the 21 and 32°C cells (Figure 2A, right) and were essentially indistinguishable from the results for wild-type mt− gametes at the two temperatures (Figure 2A, left). In all cases adhesion was rapid and ∼90% of the cells adhered. These results documented that, even though the fla10 gametes were unable to fuse after 40 min at 32°C, they retained the ability to undergo flagellar adhesion. Thus, the cells indeed were gametes and their flagella contained functional agglutinins.
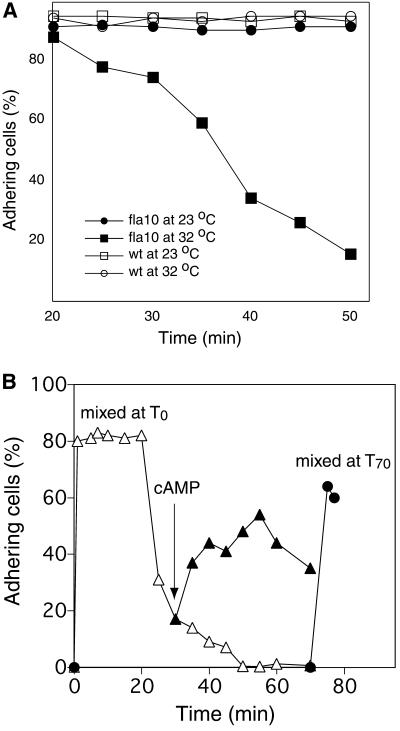
In the course of these experiments we noticed that, while fla10 gametes at the restrictive temperature showed wild-type levels of flagellar adhesion for 20 min after being mixed with gametes of the opposite mating type (Figure 2), when we examined the fla10, 32°C samples after they had been mixed together for longer than 20 min, they began to lose their adhesiveness (Figure 3A, ▪). Loss of adhesiveness occurred only in the fla10 samples and only at the restrictive temperature; fla10 samples at 21°C (Figure 3A, ●) and wild-type mixtures at both 21°C (□) and 32°C (○) retained their adhesiveness for at least 50 min, observations consistent with previous studies (Snell and Moore, 1980; Pasquale and Goodenough, 1987). By 50 min after mixing, all adhesiveness had been lost in the fla10, 32°C sample.
Figure 3.
Loss and restoration of flagellar agglutinins in fla10 gametes at the restrictive temperature. (A) Cell adhesion from the samples in Figure 2A was followed for an additional 30 min. Only the fla10 samples at 32°C lost their adhesiveness. wt, wild type. (B) fla10 gametes preincubated at 32°C for 40 min were mixed with imp1 mt+ gametes at 32°C at T0, and cell adhesion was determined using an electronic particle counter (▵). After 30 min, when the majority of the cells had deadhered, a portion of the sample was incubated with dibutyryl cAMP (▴). ●, cell adhesion results from a control sample in which fla10 gametes that had been preincubated at 32°C for a total of 70 min were mixed with imp1 gametes.
Because previous studies of Chlamydomonas gametes in which flagellar adhesiveness was experimentally impaired have shown that flagellar adhesion can be restored by incubating cells in dibutyryl cAMP (Goodenough, 1989; Hunnicutt et al., 1990), it became possible to take advantage of this new observation to determine whether fla10 cells at 32°C retained their ability to respond to cAMP. To test for responsiveness to cAMP, we preincubated fla10 gametes at 32°C for 40 min, mixed them with mt+ imp1 gametes until adhesiveness was lost, added dibutyryl cAMP, and measured flagellar adhesiveness using the electronic particle counter assay. As above, the cells underwent rapid flagellar adhesion, remained adhesive for 20 min after mixing, and then began to deadhere (Figure 3B, ▵). By 50 min flagellar adhesion had been lost completely. That the loss of adhesiveness was a consequence of adhesion and not just due to prolonged incubation at 32°C was demonstrated by the experiment shown in Figure 3B (●). In this experiment instead of mixing the 40 min, 32°C pretreated fla10 gametes with imp1 gametes immediately after the 40-min pretreatment (T0), we kept the cells at the restrictive temperature for an additional 70 minutes before mixing them with the tester cells (T70). As expected, the cells adhered to essentially the same extent as the samples mixed at T0 (Figure 3B, ●). Finally, we tested whether the T0 samples that had deadhered were able to respond to cAMP by incubating them with dibutyryl cAMP. As shown in Figure 3B (▴), flagellar adhesiveness was restored.
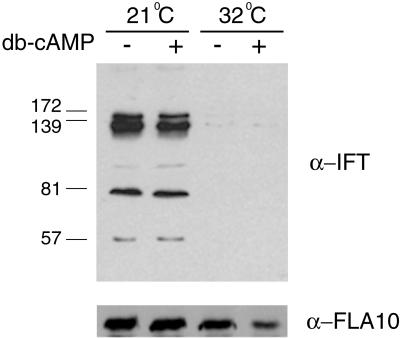
Although the simplest explanation for the restoration of flagellar adhesiveness was that the cellular response to dibutyryl cAMP was independent of kinesin-II and was due to a direct effect on agglutinin mobilization, it was also possible that the dibutyryl cAMP incubation restored kinesin function and IFT. We tested this possibility by using immunoblotting to assay for IFT particle proteins in flagella isolated from gametes at the permissive and restrictive temperatures that were incubated with or without dibutyryl cAMP. Consistent with previous studies (Kozminski et al., 1995; Piperno and Mead, 1997; Cole et al., 1998), we found that IFT particle proteins were present in flagella isolated from 21°C fla10 gametes and greatly diminished in flagella isolated from fla10 gametes that had been shifted to the restrictive temperature (Figure 4A). Moreover, dibutyryl cAMP treatment of fla10 gametes at either 21 or 32°C failed to increase the amount of IFT particles proteins in the flagella (Figure 4A). Similarly, immunoblotting of these samples with an anti–kinesin-II antibody showed that, as expected, the kinesin-II protein was present in flagella isolated from cells at both the permissive and restrictive temperatures (Kozminski et al., 1995; Cole et al., 1998) and the levels were not increased by incubation with dibutyryl cAMP (Figure 4B). Thus, the restoration of flagellar adhesiveness by dibutyryl cAMP was not associated with restoration of IFT particle transport.
Figure 4.
Incubation in cAMP does not induce IFT particle movement into flagella fla10 gametes incubated either at 21 or 32°C for 40 min were incubated with dibutyryl (db) cAMP for 30 min, flagella were isolated from non-cAMP–treated and -treated gametes, and the presence of IFT particle proteins and FLA10 protein was examined by SDS-PAGE and immunoblotting. Monoclonal antibodies against 172-, 139-, 81-, and 57-kDa IFT particle proteins were mixed together for the blot shown in the top panel and the blot was stripped and reprobed with a polyclonal antibody against the 90-kDa FLA10 protein for the blot shown in the bottom panel.
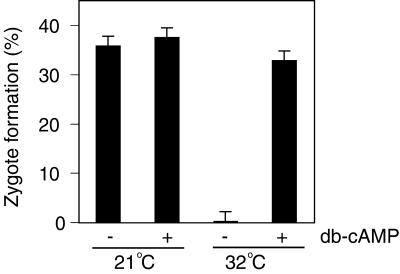
Having documented that the fla10 gametes at 32°C retained their ability to respond to dibutyryl cAMP similarly to wild-type gametes, we next wanted to determine whether their failure to fuse was due to a direct requirement for kinesin-II in cell-cell fusion itself. To test whether the cell fusion machinery was functional in the fla10 gametes at 32°C, we incubated fla10 gametes at 21 and 32°C for 40 min, followed by an additional incubation for 40 min at 32°C with and without dibutyryl cAMP (Pasquale and Goodenough, 1987). Then, we tested the cells for their ability to undergo cell fusion with wild-type mt+ gametes. As before, fusion was completely abrogated in the nondibutyryl cAMP-treated, 32°C fla10 gametes (Figure 5). On the other hand, treatment of the 32°C fla10 gametes with dibutyryl cAMP restored their ability to form zygotes to nearly the levels seen with control and dibutyryl cAMP-treated, 21°C cells (Figure 5). Thus, failure of the cells to fuse was not due to a kinesin-II–related defect in the cell-cell fusion machinery.
Figure 5.
Rescue of fla10 gamete fusion at 32°C by incubation in dibutyryl (db) cAMP. fla10 gametes maintained at 21°C or 40 min after being shifted to 32°C were incubated with or without dibutyryl cAMP for 30 min, and zygote formation was measured.
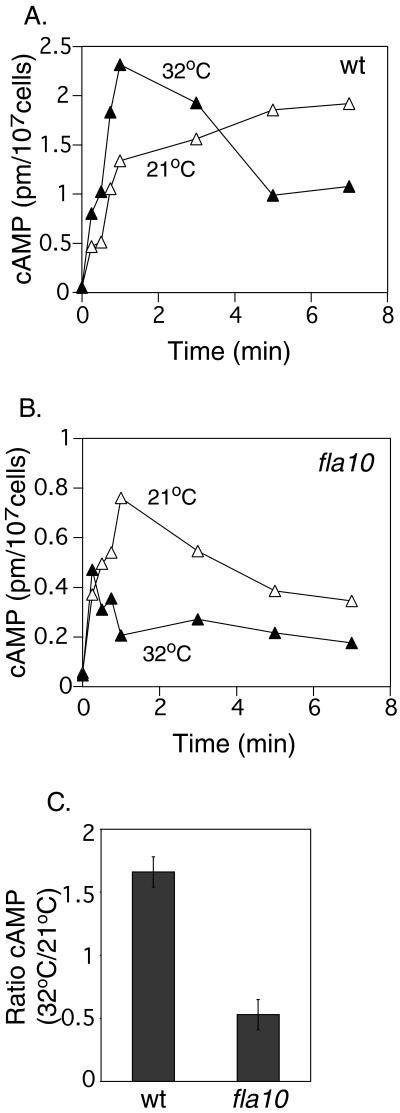
Taken together the results presented above suggested that gametes required a functional kinesin-II to carry out an early step in gamete activation downstream of flagellar adhesion but upstream of increases in cAMP. To test this idea, we used a radioimmunoassay to measure cAMP levels during adhesion of gametes at the permissive and restrictive temperatures. For consistency, imp1 cells were used at the mt+ gametes in these assays. Similar to previous reports (Pasquale and Goodenough, 1987), when wild-type mt− gametes were mixed with imp1 mt+ gametes at 21°C, cAMP levels increased from <0.1 pm/107 cells in the unmixed gametes to nearly 1.5 pm/107 cells within 1 min after the cells were mixed (Figure 6A). When wild-type mt− gametes were preincubated at 32°C before mixing, the cAMP reached nearly 2.3 pm/107 cells within 1 min, which was ∼1.7-fold higher than the 1.3 pm/107 reached at 21°C (Figure 6A). After 3 min the level began to decrease.
Figure 6.
Changes in cAMP levels during adhesion of wild-type (wt) and fla10 gametes at the permissive and restrictive temperatures. Wild-type (A) and fla10 mt− (B) gametes maintained at 21°C or preincubated at 32°C for 40 min were mixed with imp1 gametes at the indicated temperatures, and at the indicated times after mixing, samples were analyzed for cAMP. C, ratios of cAMP present at 32/21°C, 1 min after mixing wild-type or fla10 gametes from the panel A/B experiment and from two separate experiments, in one of which the mixing was carried out in the presence of the phosphodiesterase inhibitor, papaverine (Pasquale and Goodenough, 1987; Hunnicutt et al., 1990).
Whereas the results at 21°C with fla10 gametes were similar to those of wild-type mt− gametes at 21°C, much different results were obtained with fla10 gametes at the restrictive temperature. In the experiments with fla10 gametes that had been preincubated at the restrictive temperature for 40 min, rather than being higher than the level of cAMP present in the 21°C samples, the amount of cAMP present at 1 min was dramatically lower (Figure 6B). A small increase in cAMP (to a level slightly more than one-half of that observed at the permissive temperature) occurred immediately after mixing, and at 1 min, the level was ∼0.2 pm/107 cells (Figure 6B). Figure 6C summarizes results on cAMP present at 1 min after mixing from this experiment and two comparable experiments that showed similar kinetics, one of which was carried out in the presence of a phosphodiesterase inhibitor. Although the absolute amounts of cAMP differed in the three experiments, the 32:21°C ratios of cAMP levels at 1 min were much higher in the experiments with wild-type gametes than for those with fla10 gametes (Figure 6C). These results, in combination with the observations above that fla10 gametes at the restrictive temperature are capable of responding to exogenously added dibutyryl cAMP, indicated that gametes require a functional kinesin-II to undergo the adhesion-induced increase in cAMP that normally accompanies flagellar adhesion.
DISCUSSION
A Role for Kinesin-II in Sensory Transduction in Intact Flagella
We have shown that cell-cell fusion fails in kinesin-II, temperature-sensitive mutants of Chlamydomonas at the restrictive temperature because the gametes require a functional kinesin-II for coupling flagellar adhesion to increases in cAMP. Results obtained after using several approaches support this idea. First, microscopic examination and quantitative flagellar adhesion assays demonstrated that the rate and extent of flagellar adhesion of fla10 gametes after 40 min at the restrictive temperature were indistinguishable from those of wild-type gametes at both temperatures and of fla10 gametes at 21°C (Figure 2); yet, the fla10, 32°C gametes were incapable of cell-cell fusion (Figure 1). Second, fla10 32°C gametes retained the ability to respond to cAMP as assessed by their ability to mobilize flagellar agglutinins (Figure 3) and undergo cell fusion after incubation in dibutyryl cAMP (Figure 5). Finally, direct assays of cAMP showed that the samples containing fla10 gametes at 32°C did not undergo the substantial flagellar adhesion-dependent increase in cAMP that was observed with wild-type gametes at both temperatures (Figure 6) and with fla10 gametes at 21°C.
Although the requirements for kinesin-II in assembly of cilia and flagella as well as in maintenance of organelle length are well documented (reviewed by Scholey, 1996; Cole, 1999; Rosenbaum et al., 1999; Marszalek and Goldstein, 2000), the availability of the temperature-sensitive fla10 mutant made it possible to examine the role of kinesin-II in sensory transduction in a structurally intact cilium/flagellum. The cells used in the experiments reported here, i.e., fla10 cells at 40 min after shift to the restrictive temperature, have distinctive properties. Although IFT particles are absent from the flagella, no structural defects are detectable in the flagellar axoneme (Kozminski et al., 1995); the cells are fully flagellated (Figure 1; Kozminski et al., 1995; Piperno et al., 1996), and they are motile and contain functional flagellar surface adhesion molecules as evidenced by their wild-type rates and extents of flagellar adhesion (Figure 2). The only two functional defects detected so far in these cells are the absence of IFT particle movement (Kozminski et al., 1995; Piperno et al., 1996; Piperno and Mead, 1997) and the failure of flagellar adhesion to induce signal transduction.
Several IFT mutants in C. elegans were first identified by their defects in sensory transduction (Perkins et al., 1986; Shakir et al., 1993; Cole, 1999; Orozco et al., 1999; Signor et al., 1999, 2000; Wicks et al., 2000; Qin et al., 2001; Haycraft et al., 2001). These defects, however, are all due to an indirect consequence of the requirement of IFT for production of a structurally intact cilium. Each of these sensory-defective IFT worm mutants, whether they are kinesin-II (or heteromeric kinesin) mutants, IFT particle protein mutants, or mutants in genes encoding proteins that have been shown to move via IFT, produce abnormal cilia (Perkins et al., 1986). In most cases the cilia are completely absent or shorter than wild-type organelles. For example, osm-3 worms, with lesions in the gene that encodes the heteromeric kinesin protein OSM-3, fail to assemble the distal segments of sensory cilia (Perkins et al., 1986; Signor et al., 1999). Worms with lesions in the OSM-1 and OSM-6 molecules, which are cargoes for CeKinesin-II, lack both the middle and distal segments of sensory cilia (Cole et al., 1998; Collet et al., 1998). The che-11 and daf-10 mutants, with defects in IFT particle proteins (Qin et al., 2001), form nearly full-length cilia, but the organelles are of abnormal structure, being irregular in contour or containing amorphous material in their centers (Albert et al., 1981; Perkins et al., 1986). Thus, the sensory transduction lesions in these C. elegans mutants have underlying structural correlates, which is not the case for the Chlamydomonas fla10 gametes used in our experiments.
Kinesin-II/IFT: Direct Participant in Signaling?
Given that an obvious defect in axonemal structure fails to explain the inability of the fla10 gametes to undergo flagellar adhesion-dependent increases in cAMP, what could be the role of kinesin-II in coupling agglutinin interactions to increases in cAMP? One idea is that kinesin-II participates directly in sensory transduction by moving molecules or molecular complexes within the flagella after the initial interactions between mt+ and mt− agglutinins occur. According to this idea, once mt+ and mt− flagellar agglutinins interact with each other, coupling of the interaction to increases in cAMP would require that the flagellar agglutinins undergo kinesin-II-dependent movement within the flagellar membrane. For example, clustering of the interacting flagellar agglutinins could be required for increases in cAMP, or possibly agglutinins must be moved from the membrane along the shaft of the flagella to the flagellar tips to signal maximally (Mesland et al., 1980; Goodenough, 1993; Piperno et al., 1996). We should note in this regard that Reese and Haimo (2000) demonstrated that cAMP-dependent protein kinase activates kinesin-II binding to microtubules in Xenopus melanophores. Interestingly, Saito et al. (1993) reported that gametes of the Chlamydomonas imp3 mutant, which can adhere but not fuse, undergo only small adhesion-dependent increases in cAMP compared with wild-type gametes. The molecular lesion associated with the imp3 mutation has not been identified. Future studies should indicate whether the imp3 mutation is related to kinesin-II function or IFT.
Another possibility is that the IFT particle itself participates in signaling, because by 40 min after the shift to 32°C, particle proteins no longer are detectable in the flagella (Figure 4). To date, none of the characterized IFT particle proteins is reported to exhibit properties that make it an obvious candidate for a signaling molecule. On the other hand, only a few of the ∼16 IFT particle proteins have been characterized and it could be that one of them is directly involved in signal transduction. One idea that emerges from this speculation is that IFT particles could play a dual role in flagella: one as cargo transporters dependent on kinesin-II and another as supramolecular signaling complexes in close association with the flagellar membrane and the agglutinin molecules (Rosenbaum et al., 1999).
We also cannot not rule out that kinesin-II has roles in the flagella that are independent of IFT particles and possibly even independent of its role as one of the subunits of heterotrimeric kinesin-II (Signor et al., 1999). Recently, several reports have linked cellular signaling events and molecular signaling complexes in nonciliated cells to members of the kinesin superfamily, including kinesin-II (reviewed by Goldstein, 2001; Hollenbeck, 2001; Verhey and Rapoport, 2001). For example, Shimizu et al. (1998) showed that SMAP [SMAP (Smg GDS-associated protein; Smg GDS: small G protein GDP dissociation stimulator)], a proposed mammalian homologue of the nonmotor subunit of sea urchin kinesin-II, binds to a regulator of small G proteins called Smg GDS. SMAP has armadillo repeats and is phosphorylated by Src tyrosine kinase (Shimizu et al., 1996). Also, Nagata et al. (1998) reported that the MAP kinase kinase kinase MLK2 interacts with mammalian members of the kinesin-II/KIF3 family of kinesin-related proteins and with KAP3A, a nonmotor protein subunit of the kinesin-II/KIF3 motor complex. Additionally, the conventional kinesin-related protein, COS2, encoded by the costal2 gene, was shown to be an essential component of a multiprotein signaling complex that is regulated by the hedgehog gene product in Drosophila embryos (Robbins et al., 1997; Sisson et al., 1997). Although COS2 has not been shown to exhibit motor activity, its regulated microtubule binding activity is implicated in determining the cytoplasmic versus nuclear localization of key molecules in the hedgehog-signaling pathway. Finally, JIP scaffolding proteins and associated signaling molecules that are part of the c-jun NH2-terminal kinase (JNK)-signaling pathway bind to rat conventional kinesin light chain proteins (Bowman et al., 2000; Verhey et al., 2001) and this motor protein is important for concentration of the JIP complex in nerve terminals (Verhey et al., 2001).
Finally, the importance of kinesin-II in IFT particle movement is well documented, and one straightforward explanation for the failure of the fla10 gametes to undergo increases in cAMP during adhesion is that kinesin-II/IFT plays an indirect role in sensory transduction by maintaining proper levels of signaling components in the flagella. Another possibility is that IFT particles and cytoplasmic dynein might carry adhesion-activated signals from the flagella to the cell body. These ideas can be tested in future experiments by use of newly described IFT mutants available in the collection of Iomini et al. (2001).
Kinesin-II Is Not Required for Flagellar Mobilization of Active Agglutinins
One surprising result from our experiments was that fla10 gametes at the restrictive temperature underwent dibutyryl cAMP-induced mobilization of flagellar agglutinins. In previous experiments we and others have shown that gametes are able to translocate flagellar agglutinins from their cell bodies onto their flagella (Goodenough, 1989; Hunnicutt et al., 1990). In experiments from our laboratory, flagellar agglutinins were inactivated by an anti-agglutinin mAb under conditions in which the inactive agglutinins on the surface of the cell body were unaffected (Hunnicutt et al., 1990). Incubation of the nonadhesive cells with dibutyryl cAMP restored active agglutinins onto the flagella. Similarly, Goodenough (1989) has shown that incubation of gametes with dibutyryl cAMP led to an increase in flagellar agglutinins. Once the existence of kinesin-II-dependent IFT was reported, IFT became the best candidate for the motility process responsible for agglutinin translocation. Thus, our result that flagellar adhesiveness was restored by incubation of the newly nonadhesive fla10 gametes in dibutyryl cAMP (Figure 3B) was unexpected and argues against the model that kinesin-II/IFT is essential for agglutinin movement from the cell body to the flagella (Piperno et al., 1996; Rosenbaum et al., 1999; Iomini et al., 2001).
Another possible mechanism for moving agglutinins onto the flagella is flagellar surface motility, a poorly understood process visualized in the laboratory as the movement of latex microspheres up and down the surfaces of flagella (Bloodgood, 1995). Additionally, agglutinins might move via the as yet unidentified mechanism responsible for movement of outer dynein arm components into flagella. Piperno et al. (1996), showed that the outer dynein arm IC protein IC69 translocated into flagella of fla10 gametes at the restrictive temperature in a manner indistinguishable from that observed at the permissive temperature. In the context of our experiments, the result that flagellar adhesiveness was restored by dibutyryl cAMP treatment demonstrated that the cells still were functional gametes whose flagella were capable of responding to and participating in a complex signaling event in the absence of a functional kinesin-II. Nevertheless, it will be interesting to learn more about the mechanisms responsible for this kinesin-II–independent agglutinin mobilization.
ACKNOWLEDGMENTS
We thank Dr. Mike Misamore for help with photomicroscopy and Dr. Fred Grinnell for helpful discussions and comments on the manuscript. We are indebted to Dr. Ted Chrisman and Dr. David Garbers' laboratory for assistance with radioimmunoassay of cyclic AMP. This work was supported by grant GM25661 from the National Institutes of Health to W.J.S.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–11–0531. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–11–0531.
REFERENCES
- Adair WS. Characterization of Chlamydomonas sexual agglutinins. J Cell Sci Suppl. 1985;2:233–260. doi: 10.1242/jcs.1985.supplement_2.13. [DOI] [PubMed] [Google Scholar]
- Albert PS, Brown SJ, Riddle DL. Sensory control of dauer larva formation in Caenorhabditis elegans. J Comp Neurol. 1981;198:435–451. doi: 10.1002/cne.901980305. [DOI] [PubMed] [Google Scholar]
- Bakalyar HA, Reed RR. Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science. 1990;250:1403–1406. doi: 10.1126/science.2255909. [DOI] [PubMed] [Google Scholar]
- Bloodgood RA. Flagellar surface motility: gliding and microsphere movements. Methods Cell Biol. 1995;47:273–279. doi: 10.1016/s0091-679x(08)60820-1. [DOI] [PubMed] [Google Scholar]
- Bowman AB, Kamal A, Ritchings BW, Philp AV, McGrail M, Gindhart JG, Goldstein LS. Kinesin-dependent axonal transport is mediated by the sunday driver (SYD) protein. Cell. 2000;103:583–594. doi: 10.1016/s0092-8674(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Cole DG. Kinesin-II, coming and going. J Cell Biol. 1999;147:463–466. doi: 10.1083/jcb.147.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet J, Spike CA, Lundquist EA, Shaw JE, Herman RK. Analysis of osm-6, a gene that affects sensory cilium structure and sensory neuron function in Caenorhabditis elegans. Genetics. 1998;148:187–200. doi: 10.1093/genetics/148.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino SE, Tubb DJ, Garbers DL. Assay of guanylyl cyclase catalytic activity. Methods Enzymol. 1991;195:345–355. doi: 10.1016/0076-6879(91)95179-n. [DOI] [PubMed] [Google Scholar]
- Ferris PJ, Woessner JP, Goodenough UW. A sex recognition glycoprotein is encoded by the plus mating-type gene fus1 of Chlamydomonas reinhardtii. Mol Biol Cell. 1996;7:1235–1248. doi: 10.1091/mbc.7.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein LS. Transduction. When worlds collide: trafficking in JNK. Science. 2001;291:2102–2103. doi: 10.1126/science.1059766. [DOI] [PubMed] [Google Scholar]
- Goodenough UW. Cyclic AMP enhances the sexual agglutinability of Chlamydomonas flagella. J Cell Biol. 1989;109:247–252. doi: 10.1083/jcb.109.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW. Tipping of flagellar agglutinins by gametes of Chlamydomonas reinhardtii. Cell Motil Cytoskeleton. 1993;25:179–189. doi: 10.1002/cm.970250207. [DOI] [PubMed] [Google Scholar]
- Goodenough UW, Detmers PA, Hwang C. Activation for cell fusion in Chlamydomonas: analysis of wild-type gametes and nonfusing mutants. J Cell Biol. 1982;92:378–386. doi: 10.1083/jcb.92.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Swoboda P, Taulman PD, Thomas JH, Yoder BK. The C. elegans homolog of the murine cystic kidney disease gene Tg737 functions in a ciliogenic pathway and is disrupted in osm-5 mutant worms. Development. 2001;128:1493–1505. doi: 10.1242/dev.128.9.1493. [DOI] [PubMed] [Google Scholar]
- Hollenbeck PJ. Kinesin delivers: identifying receptors for motor proteins. J Cell Biol. 2001;152:F25–F28. doi: 10.1083/jcb.152.5.f25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Rifkin MR, Luck DJ. Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J Cell Biol. 1977;72:67–85. doi: 10.1083/jcb.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol. 1990;111:1605–1616. doi: 10.1083/jcb.111.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunnicutt GR, Snell WJ. Rapid and slow mechanisms for loss of cell adhesiveness during fertilization in Chlamydomonas. Dev Biol. 1991;147:216–224. doi: 10.1016/s0012-1606(05)80019-3. [DOI] [PubMed] [Google Scholar]
- Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux F G, 3rd, Dutcher SK. Genetic interactions at the FLA10 locus: suppressors and synthetic phenotypes that affect the cell cycle and flagellar function in Chlamydomonas reinhardtii. Genetics. 1991;128:549–561. doi: 10.1093/genetics/128.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marszalek JR, Goldstein LS. Understanding the functions of kinesin-II. Biochim Biophys Acta. 2000;1496:142–150. doi: 10.1016/s0167-4889(00)00015-x. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LS. Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell. 2000;102:175–187. doi: 10.1016/s0092-8674(00)00023-4. [DOI] [PubMed] [Google Scholar]
- Marszalek JR, Ruiz-Lozano P, Roberts E, Chien KR, Goldstein LS. Situs inversus and embryonic ciliary morphogenesis defects in mouse mutants lacking the KIF3A subunit of kinesin-II. Proc Natl Acad Sci USA. 1999;96:5043–5048. doi: 10.1073/pnas.96.9.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesland DA, Hoffman JL, Caligor E, Goodenough UW. Flagellar tip activation stimulated by membrane adhesions in Chlamydomonas gametes. J Cell Biol. 1980;84:599–617. doi: 10.1083/jcb.84.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer JH, Lee-Tischler MJ, Kwon HY, Schrick JJ, Avner ED, Sweeney WE, Godfrey VL, Cacheiro NL, Wilkinson JE, Woychik RP. Candidate gene associated with a mutation causing recessive polycystic kidney disease in mice. Science. 1994;264:1329–1333. doi: 10.1126/science.8191288. [DOI] [PubMed] [Google Scholar]
- Nagata K, Puls A, Futter C, Aspenstrom P, Schaefer E, Nakata T, Hirokawa N, Hall A. The MAP kinase kinase kinase MLK2 co-localizes with activated JNK along microtubules and associates with kinesin superfamily motor KIF3. EMBO J. 1998;17:149–158. doi: 10.1093/emboj/17.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- Okada Y, Nonaka S, Tanaka Y, Saijoh Y, Hamada H, Hirokawa N. Abnormal nodal flow precedes situs inversus in iv and inv mice. Mol Cell. 1999;4:459–468. doi: 10.1016/s1097-2765(00)80197-5. [DOI] [PubMed] [Google Scholar]
- Orozco JT, Wedaman KP, Signor D, Brown H, Rose L, Scholey JM. Movement of motor and cargo along cilia. Nature. 1999;398:674. doi: 10.1038/19448. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Regulated targeting of a protein kinase into an intact flagellum: an aurora/Ipl1p-like protein kinase translocates from the cell body into the flagella during gamete activation in Chlamydomonas. J Biol Chem. 2000a;275:24106–24114. doi: 10.1074/jbc.M002686200. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Signal transduction during fertilization in the unicellular green alga. Chlamydomonas. Curr Opin Microbiol. 2000b;3:596–602. doi: 10.1016/s1369-5274(00)00146-6. [DOI] [PubMed] [Google Scholar]
- Pasquale SM, Goodenough UW. Cyclic AMP functions as a primary sexual signal in gametes of Chlamydomonas reinhardtii. J Cell Biol. 1987;105:2279–2292. doi: 10.1083/jcb.105.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, Witman GB. A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT) J Cell Biol. 1998;141:979–992. doi: 10.1083/jcb.141.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc Natl Acad Sci USA. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Henderson S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1(FLA10) to reach the distal part of flagella in Chlamydomonas. J Cell Biol. 1996;133:371–379. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Rosenbaum JL, Barr MM. An autosomal recessive polycystic kidney disease gene homolog is involved in intraflagellar transport in C. elegans ciliated sensory neurons. Curr Biol. 2001;11:457–461. doi: 10.1016/s0960-9822(01)00122-1. [DOI] [PubMed] [Google Scholar]
- Reese EL, Haimo LT. Dynein, dynactin, and kinesin II's interaction with microtubules is regulated during bidirectional organelle transport. J Cell Biol. 2000;151:155–166. doi: 10.1083/jcb.151.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Robbins DJ, Nybakken KE, Kobayashi R, Sisson JC, Bishop JM, Therond PP. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein, costal2. Cell. 1997;90:225–234. doi: 10.1016/s0092-8674(00)80331-1. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Cole DG, Diener DR. Intraflagellar transport: the eyes have it. J Cell Biol. 1999;144:385–388. doi: 10.1083/jcb.144.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R, Granick S. Nutritional control of sexuality in Chlamydomonas reinhardtii. J Gen Physiol. 1954;3:729–742. doi: 10.1085/jgp.37.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Small L, Goodenough UW. Activation of adenylyl cyclase in Chlamydomonas reinhardtii by adhesion and by heat. J Cell Biol. 1993;122:137–147. doi: 10.1083/jcb.122.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Scholey JM. Kinesin-II, a membrane traffic motor in axons, axonemes, and spindles. J Cell Biol. 1996;133:1–4. doi: 10.1083/jcb.133.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakir MA, Fukushige T, Yasuda H, Miwa J, Siddiqui SS. C. elegans osm-3 gene mediating osmotic avoidance behavior encodes a kinesin-like protein. Neuroreport. 1993;4:891–894. doi: 10.1097/00001756-199307000-00013. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kawabe H, Minami S, Honda T, Takaishi K, Shirataki H, Takai Y. SMAP, an Smg GDS-associating protein having arm repeats and phosphorylated by Src tyrosine kinase. J Biol Chem. 1996;271:27013–27017. doi: 10.1074/jbc.271.43.27013. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Shirataki H, Honda T, Minami S, Takai Y. Complex formation of SMAP/KAP3, a KIF3A/B ATPase motor-associated protein, with a human chromosome-associated polypeptide. J Biol Chem. 1998;273:6591–6594. doi: 10.1074/jbc.273.12.6591. [DOI] [PubMed] [Google Scholar]
- Signor D, Rose LS, Scholey JM. Analysis of the roles of kinesin and dynein motors in microtubule-based transport in the Caenorhabditis elegans nervous system. Methods. 2000;22:317–325. doi: 10.1006/meth.2000.1084. [DOI] [PubMed] [Google Scholar]
- Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM. Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol. 1999;147:519–530. doi: 10.1083/jcb.147.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JC, Ho KS, Suyama K, Scott MP. Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell. 1997;90:235–245. doi: 10.1016/s0092-8674(00)80332-3. [DOI] [PubMed] [Google Scholar]
- Sklar PB, Anholt RR, Snyder SH. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986;261:15538–15543. [PubMed] [Google Scholar]
- Snell WJ. Study of the release of cell wall degrading enzymes during adhesion of Chlamydomonas gametes. Exp Cell Res. 1982;138:109–119. doi: 10.1016/0014-4827(82)90096-9. [DOI] [PubMed] [Google Scholar]
- Snell WJ, Moore WS. Aggregation-dependent turnover of flagellar adhesion molecules in Chlamydomonas gametes. J Cell Biol. 1980;84:203–210. doi: 10.1083/jcb.84.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell WJ, Roseman S. Kinetics of adhesion and de-adhesion of Chlamydomonas gametes. J Biol Chem. 1979;254:10820–10829. [PubMed] [Google Scholar]
- Solter KM, Gibor A. Evidence for role of flagella as sensory transducers in mating of Chlamydomonas reinhardi. Nature. 1977;265:444–445. doi: 10.1038/265444a0. [DOI] [PubMed] [Google Scholar]
- Verhey KJ, Meyer D, Deehan R, Blenis J, Schnapp BJ, Rapoport TA, Margolis B. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152:959–970. doi: 10.1083/jcb.152.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey KJ, Rapoport TA. Kinesin carries the signal. Trends Biochem Sci. 2001;26:545–550. doi: 10.1016/s0968-0004(01)01931-4. [DOI] [PubMed] [Google Scholar]
- Walther Z, Vashishtha M, Hall JL. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J Cell Biol. 1994;126:175–188. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks SR, de Vries CJ, van Luenen HG, Plasterk RH. CHE-3, a cytosolic dynein heavy chain, is required for sensory cilia structure and function in Caenorhabditis elegans. Dev Biol. 2000;221:295–307. doi: 10.1006/dbio.2000.9686. [DOI] [PubMed] [Google Scholar]
- Wilson NF, Foglesong MJ, Snell WJ. The Chlamydomonas mating type plus fertilization tubule, a prototypic cell fusion organelle: isolation, characterization, and in vitro adhesion to mating type minus gametes. J Cell Biol. 1997;137:1537–1553. doi: 10.1083/jcb.137.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Snell WJ. Flagellar adhesion-dependent regulation of Chlamydomonas adenylyl cyclase in vitro: a possible role for protein kinases in sexual signaling. J Cell Biol. 1994;125:617–624. doi: 10.1083/jcb.125.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Ross EM, Snell WJ. ATP-dependent regulation of flagellar adenylylcyclase in gametes of Chlamydomonas reinhardtii. J Biol Chem. 1991;266:22954–22959. [PubMed] [Google Scholar]