Abstract
The use of exogenous melatonin (exo-MEL) as a sleep-promoting drug has been under extensive debate due to the lack of consistency of its described effects. In this study, we conduct a systematic and comprehensive review of the literature on the chronobiotic, sleep-inducing, and overall sleep-promoting properties of exo-MEL. To this aim, we first describe the possible pharmacological mechanisms involved in the sleep-promoting properties and then report the corresponding effects of exo-MEL administration on clinical outcomes in: a) healthy subjects, b) circadian rhythm sleep disorders, c) primary insomnia. Timing of administration and doses of exo-MEL received particular attention in this work. The exo-MEL pharmacological effects are hereby interpreted in view of changes in the physiological properties and rhythmicity of endogenous melatonin. Finally, we discuss some translational implications for the personalized use of exo-MEL in the clinical practice.
Keywords: Melatonin, sleep-promoting, sleep-inducing, hypnotic, soporific, chronobiotic, circadian rhythm sleep disorders, insomnia
1. INTRODUCTION
Melatonin (MEL) was isolated by Aaron B. Lerner and collaborators in 1958 [1]. Its effect on sleep was first described in 1962 as a state of “mild sedation” produced after an intravenous administration of exogenous melatonin (exo-MEL) [2]. However, almost fifty years later, its nature as a hypnotic, sedative, or soporific drug was - and still is - under intense debate [3, 4]. The early studies of the ’70s and ’80s, using high doses of exo-MEL and employing subjective measures of its effects, demonstrated an increase in sleepiness after its administration in healthy subjects (HS) [5-8]. A decade later, in the studies conducted in the ’90s, the exo-MEL doses were notably reduced, and through the introduction of objective measures of sleep quantity and quality, some authors demonstrated that exo-MEL can reduce the sleep onset latency (SOL) and the number of awakenings, while it may increase the total sleep time (TST) and/or the sleep efficiency (SE) [9-16], thus suggesting its hypnotic properties.
Although some studies have failed to find a sleep-promoting effect of exo-MEL [17-19], a significant effort has been made to explore its therapeutic applications, particularly in the treatment of insomnia. On this subject, contrasting results emerged, with some findings describing the efficacy of exo-MEL in improving sleep initiation, sleep maintenance and/or sleep quality [20-29], and others that did not confirm such effects instead [30-33].
The label of hypnotic has led to the expectation that exo-MEL owns properties comparable to those derived from the typical hypnotics (e.g., benzodiazepines -BDZs-), such as the fast induction of sleep onset independently of the recipient characteristics or the environmental conditions (posture, light exposition, etc.), and a clear dose-response curve characterization [34-36]. On the contrary, the effects of exo-MEL on sleep may change as a function of variables such as the timing of administration, individual receptor responsiveness, levels of endogenous MEL secretion, age, and gender; in addition, variables such as motivated effort to be awake or an upright posture may suppress the sleep-promoting effects of exo-MEL [3, 37]. Even if some of these issues might also be observed in typical hypnotic drugs, the degree of inconsistency in the exo-MEL effects resulted in a controversy about its effects on sleep modulation as a hypnotic [38, 39]. In this perspective, it has been proposed that exo-MEL might better fall under the label of ‘sleep-promoting” drug, underlying its properties as a substance that may increase the sleep propensity and facilitate the transition from wakefulness to sleep. Indeed, in contrast to common hypnotics, MEL induces a behavioral state that resembles quiet wakefulness, which may predispose to normal sleep initiation, rather than sleepiness or drowsiness [4].
Reaffirming the complexity around the characterization of the exo-MEL effect on sleep, in a meta-analysis conducted on this topic, Brzezinski et al., 2005 showed that exo-MEL decreases SOL and increases SE and TST in several clinical conditions such as insomnia, schizophrenia, Alzheimer's Disease, as well as in healthy volunteers [40]. In contrast, in the same year, the group of Buscemi et al., 2005, found that the reduction in SOL seems to be less significant for primary sleep disorders associated with insomnia as compared to patients with Circadian Rhythm Sleep Disorders, particularly for Delayed Sleep Phase Disorder, thus concluding that exo-MEL may act through “a direct resetting of the endogenous circadian pacemaker rather than via a direct action on somnogenic structures of the brain” [41]. Previously, Hughes and colleagues had already highlighted the importance of understanding whether MEL “has robust hypnotic effects independent of its chronobiotic effects” [9]. Currently, some questions about the mechanisms underpinning the sleep-promoting and the chronobiotic effects of exo-MEL are still unsolved.
While the hypnotic effects of exo-MEL lack a general consensus, its role in modulating circadian rhythms has been well established [42, 43]. Indeed, a morning-time administration of exo-MEL produces a delay in the endogenous circadian rhythms, whereas an evening administration induces advances in the endogenous circadian rhythms, resulting in a shift of the sleep/wake patterns, as well as of several readouts of circadian body rhythmicity, such as the minimal core body temperature (CBT), the cortisol release, and the Dim Light Melatonin Onset (DLMO) [44]. These findings support the definition of MEL as a chronobiotic, “a substance that adjusts the timing of the internal biological rhythms” [45]. However, the transduction mechanisms through which MEL can modulate the circadian oscillations in the target cells are still under scrutiny. For instance, the specificity of receptors that modulate the chronobiotic effect has been the subject of investigation [46-52]. Results based on in vivo vs. in vitro studies suggest that the differential role of MT1 and MT2 receptors in melatonin-induced inhibition and phase-shifting effects on the suprachiasmatic nucleus (SCN) remains to be elucidated. Moreover, a seminal study conducted in zebrafish lacking melatonin, due to mutation of arylalkylamine N-acetyltransferase 2 (aanat2), obtained intriguing results that suggest that MEL can contribute to the sleep promotion downstream of the circadian system via adenosine signaling [53].
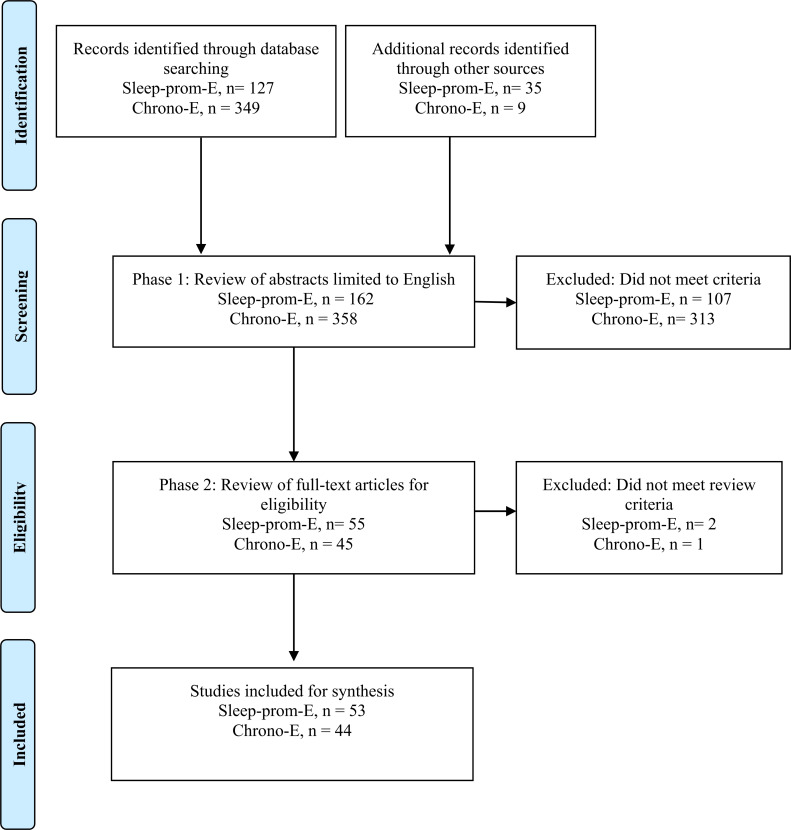
Grounded on the partial understanding of the mechanisms of MEL action, in this study, we conduct a systematic and comprehensive review of the literature on the chronobiotic, sleep-inducing, and overall sleep-promoting properties of exo-MEL. To this aim, we first describe the possible pharmacological mechanisms involved in the sleep-promoting properties and then report the corresponding effects of exo-MEL administration on clinical outcomes in healthy subjects (HS), patients with insomnia and patients with Circadian Rhythm Sleep Disorders (CRSD) such as Non-24-hr sleep-wake disorder (N24SWD) or free/running disorder (FR), Delayed Sleep phase Disorder (DSPD), Advanced Sleep Phase Disorder (ASPD) and Irregular Sleep-Wake Rhythm Disorder (ISWRD). We revised the different approaches regarding doses, timing of administration, and the main outcomes, as well as the possible implications of the current knowledge for the use of exo-MEL in clinical practice. Details about the methods used for the literature search regarding the clinical evidence are provided in Fig. (1).
Fig. (1).
PRISMA Flow diagram of literature search and selection process.
In the following sections, we describe the methods and results of the systematic review. Then is described the MEL physiology, considering its functions, synthesis, receptors, and signal transduction mechanisms. The pharmacokinetics of exo-MEL will also be described. Then, the MEL mechanisms are explored, firstly regarding its chronobiotic effects exerted on both central and peripherical oscillators, and secondly regarding the sleep-promoting effect attributed to its action on the GABAergic system, interaction with thermoregulation processes, and direct action on somnogenic structures. Finally, the clinical evidence regarding the chronobiotic and sleep-promoting effects is reviewed and discussed.
2. METHODS
This review was performed following the PRISMA guidelines, including peer-reviewed, double-blind and single-blind, randomized controlled trials (RCT), case series and case reports on the use of exogenous melatonin (exo-MEL) to treat circadian rhythm sleep disorders, insomnia and healthy adults (i.e., without sleep disturbances). A systematic search on PubMed was performed until March 2021. The search strategy was built on previously published reviews, combining controlled (MeSH terms) and natural vocabulary.
The search terms for the sleep-inducing and sleep-promoting effects (Sleep-prom-E) included:
(((melatonin) OR (“exogenous melatonin”)) AND ((((healthy volunteers) OR (“healthy subjects”)) OR (“healthy adults”)) OR (insomnia))) AND ((((hypnotic) OR (sleep-inducing)) OR (“sleep-promoting”)) OR (soporific))) Filters: Humans, English, Adult: 19+ years.
The search terms for the chronobiotic effect (Chrono-E) included:
(((melatonin) OR (“exogenous melatonin”)) AND (((((((((((((sleep disorders, circadian rhythm) OR (circadian rhythm sleep disorders)) OR (sleep wake disorders)) OR (Delayed Sleep Phase Disorder)) OR (delayed sleep phase syndrome)) OR (Advanced Sleep Phase Disorder)) OR (advanced sleep phase syndrome)) OR (Irregular Sleep-Wake Rhythm Disorder)) OR (Non 24-h sleep-wake disorder)) OR (Free-running disorder)) OR (healthy volunteers)) OR (healthy subjects)) OR (healthy adults))) AND (((Chronobiotic) OR (phase advance)) OR (phase delay)) Filters: Humans, English, Adult: 19+ years.
2.1. Inclusion Criteria
English language, studies in adults 19+ years with an established diagnosis of insomnia, Non-24-h sleep-wake disorder or Free-running disorder, Delayed Sleep Phase Disorder, Advanced Sleep Phase Disorder or Irregular Sleep-Wake Rhythm Disorder as well as Healthy Subjects, empirical studies including randomized controlled trials (RCT) double or single-blinded as well as case series and case reports.
2.2. Exclusion Criteria
Studies in children and/or adolescents, studies in subjects with medical conditions (dementia, intellectual disability or autism, traumatic brain injury, hypertensive patients, medical comorbidity (i.e. coronary infarct), psychiatric comorbidity, shift-work, jet-lag, therapies with a combination of melatonin and light, therapy with melatonin agonists, reviews, letters, and editorials.
No specific criteria were established concerning the outcomes measured due to the broad heterogeneity of the experimental designs used to evaluate the effects of MEL on sleep.
2.3. Selection and Data Collection Process
In the selection phase, two reviewers conducted the screening of the titles and abstracts based on the eligibility criteria through an iterative double screening of a subset of records (to achieve a concordance > 95%), followed by a single screening of the remaining records. Disagreements were discussed by the two reviewers, and a third reviewer was available in case of disagreement between the two reviewers. The two reviewers independently collected data from reports.
3. SEARCH RESULTS
Through the systematic database searching, were found 127 articles about the Sleep-prom-E and 349 articles for the Chrono-E. Additionally, a hand search was performed through an “ancestry approach” including references cited in relevant studies to track down earlier work, thus adding other nine articles for the Chrono-E and 35 articles for the Sleep-prom-E.
In total, 55 items about the Sleep-prom-E and 45 items about the Chrono-E were selected for full-text revision. Based on eligibility criteria, after a phase of full-text assessment, three articles were excluded. Finally, 53 articles were selected for the systematic analysis of the Sleep-prom-E and 44 articles were selected for the Chrono-E, respectively, see the PRISMA flow diagram in Fig. (1).
Variables extracted from each study were: author, year of publication, study design, aim, diagnosis, number of subjects (N), age and gender distribution, circadian and sleep variables measured, dose, type of melatonin (prolonged release (PR) or fast release (FR), timing of administration, treatment duration and general results (Appendix Tables 1, 2). These results are analyzed and discussed in detail in section 7.
4. MELATONIN PHYSIOLOGY
Melatonin (MEL), N-acety1-5-methoxytryptamine, is a hormone primarily synthesized by the pineal gland and secreted with circadian rhythmicity. The cycle of light and darkness influences the release of MEL, that physiologically reaches the maximum peak during the dark phase of the cycle (around 03:00-04:00 a.m.) and is suppressed by the exposure to bright light. Therefore, MEL has been defined as “the hormone of darkness” [45, 54, 55]. One of the functions of MEL is the transduction of photoperiodic information (such as the time and the length of the day), representing the main synchronizer for the circadian organization of multiple internal processes in the organism [56, 57]. In mammals, MEL regulates seasonal changes within the neuroendocrine and reproductive perimeter [4]. In humans, besides the effects on circadian rhythm and sleep propensity, MEL has been associated with pubertal and aging processes [58], blood pressure control [59], free-radical scavenging [60], and regulation of antioxidant processes, particularly in tissues such as the retina, gastrointestinal tract, thymus, spleen, heart, skeletal muscle, liver, stomach, gut, skin, placenta, testes, ovaries, cerebral cortex, and striatum [57,61]. Recent evidence suggests that MEL is associated with the vital counteraction of cellular damage mechanisms responsible for the severe consequences triggered by sleep deprivation [62]. Although MEL is synthesized in multiple tissues, the extra-pineal melatonin is not synthesized with a circadian pattern nor is released into the circulation, whereby it is inferred that it exerts a local action with a limited, if any, contribution to the circadian regulation of biological rhythms [61].
4.1. Melatonin Synthesis
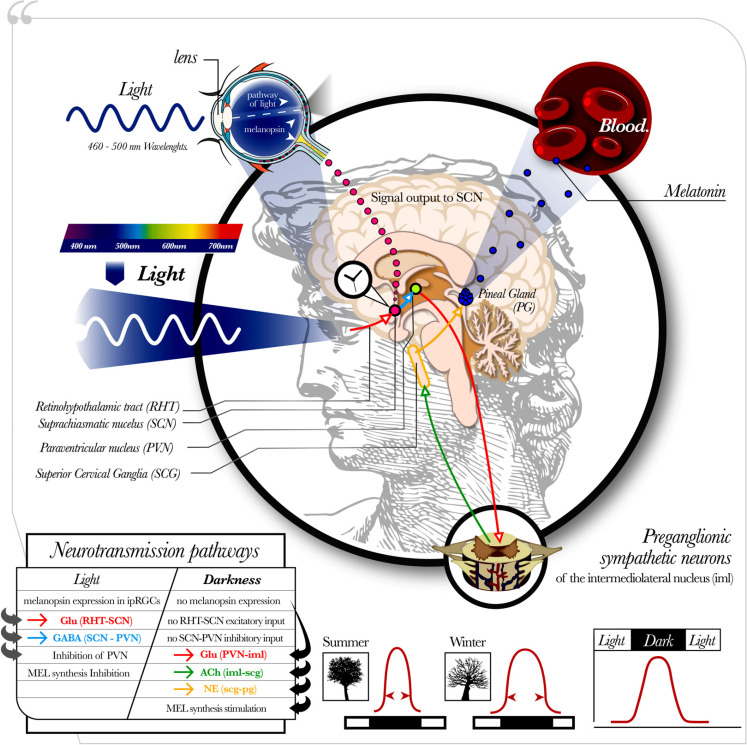
The synthesis pathway of the pineal MEL, Fig. (2), involves the intrinsically photosensitive retinal ganglion cells (ipRGCs), a cellular subgroup corresponding to less than 1% of all retinal ganglion cells [63, 64] expressing the photopigment melanopsin in response to light exposure [65]. When light with a wavelength of 460-500 nm (blue spectrum) reaches the eye, the melanopsin expressed in the ipRGCs [64] depolarizes the target neurons sending action potentials to several brain structures, including the SCN, which is reached through a monosynaptic pathway called the retinohypothalamic tract (RHT) [56]. The RHT stores both glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP). Glutamate has an excitatory effect on SCN neurons, while PACAP appears to potentiate the effects of glutamate [67, 68]. The SCN also receives gamma-aminobutyric acid (GABA) signals from the intergeniculate leaflet (IGL), as well as neuropeptide neurotransmission and serotoninergic signals from the raphe nucleus, which modulates the glutamatergic input from the RHT [69]. The photic activation of the SCN induces the release of GABA inputs from the SCN to the paraventricular nucleus of the hypothalamus (PVN), blocking the glutamatergic activity in the PVN and resulting, ultimately, in an inhibition of the melatonin synthesis. Instead, in the absence of light, with no inhibitory input from the SCN, the PVN is activated [70, 71], inducing glutamatergic signals to the sympathetic preganglionic neurons of the intermediolateral nucleus (iml), from which cholinergic projections stimulate the postganglionic neurons located in the superior cervical ganglion (SCG). The SCG contains norepinephrine (NE) projections able to stimulate the pinealocytes, leading to an increase in cyclic adenosine-3’,5’-monophosphate (cAMP) and in the activation of N-acetyltransferase (NAT), an enzyme that converts serotonin in N- acetylserotonin (NAS), which finally is transformed into MEL by the Hydroxyindole-O-methyltransferase (HIOMT). Finally, MEL is released immediately into circulation [55, 61, 66].
Fig. (2).
Regulatory pathway of the pineal MEL. The initial step in the pineal MEL synthesis involves the intrinsically photosensitive retinal ganglion cells (ipRGCs), expressing the photopigment melanopsin in response to light exposition (particularly within frequencies of 460-500 nm (blue spectrum). It depolarizes the neurons sending action potentials to several brain targets, including the suprachiasmatic nucleus (SCN), which is reached through a monosynaptic pathway called the retinohypothalamic tract (RHT) [56]. The RHT stores both glutamate and pituitary adenylate cyclase-activating polypeptide (PACAP). The photic activation of the SCN induces the release of GABA inputs from the SCN to the paraventricular nucleus of the hypothalamus (PVN), blocking its glutamatergic activity and resulting in an inhibition of the melatonin synthesis. Instead, in the absence of light, with no inhibitory input from the SCN, the PVN is activated, inducing glutamatergic signals to the sympathetic preganglionic neurons of the intermediolateral nucleus (iml), from which cholinergic projections stimulate the postganglionic neurons located in the superior cervical ganglion (SCG). The SCG contains norepinephrine (NE) projections able to stimulate the pinealocytes, resulting in an activation of the MEL synthesis. As such, MEL is released immediately into circulation with a peak during the dark phase of the day [55, 61, 66]. MEL is produced for a longer time in winter when nights are long, than in summer when nights are short.
MEL is released from the pineal gland at night along with varying levels of N-acetylserotonin (NAS). NAS can activate TrkB (Tropomyosin receptor kinase B), a brain-derived neurotrophic factor (BDNF) receptor [72]. The NAS activation of TrkB regulates several processes associated with sleep, such as neurogenesis [73]. Moreover, the NAS/melatonin ratio can be regulated by different factors, including activation of the aryl hydrocarbon receptor (AhR), purinergic P2Y1r and mGluR5. Variations in the expression and activity of these receptors modulate the NAS/melatonin ratio, which may contribute to some of the differential effects of melatonin, both endogenous and exogenous [74]. In addition, inflammatory cytokines can transiently inhibit NAS and melatonin production by the pineal gland, including the increase in tumor necrosis factor (TNF) following cesarean section [75]. Elevations in pro-inflammatory activity may suppress pineal NAS and MEL production under conditions where the immune-dampening effects of MEL may not be desirable, indicating that the regulation of pineal melatonin production is intimately intertwined with wider immune activation, as suggested by the 'immune-pineal axis' model, first developed by Prof Regina P Markus. [76]. Therefore, wider systemic processes can modulate the endogenous melatonergic pathway.
4.2. Melatonin Receptors and Signal Transduction Mechanisms
MEL exerts its effects by two G protein-coupled receptors (GPCRs), MT1 (MEL 1a) and MT2 (MEL 1b). Another binding site (not considered a receptor), initially called the “MT3” and later characterized as the quinone reductase 2, is an enzyme involved in antioxidant activity. Other binding sites of MEL are the retinoid-related orphan nuclear hormone receptor (RZR/RORa) and intracellular proteins like calmodulin, calreticulin, and tubulin [57,77]. MT1 and MT2 are expressed in various tissues of the body, together or independently. MT1 receptors are present in many structures of the nervous system: retina, cerebellum, hippocampus, and central dopaminergic pathways (substantia nigra, ventral tegmental area, nucleus accumbens, caudate, and putamen), cerebral cortex (particularly on layers II, III, V), retrosplenial cortex, occipital cortex, habenula, forebrain, periaqueductal gray, dorsal raphe, pars tuberalis, and pituitary gland. MT1 receptors are also present in the ovary, testis, mammary gland, coronary blood vessels, aorta, liver, kidney, gallbladder, skin, and immune cells. MT2 expression is mostly limited to the brain and, in moderate quantity, to the lung, cardiac aortic and coronary tissues, myometrium and granulosa cells, immune cells, duodenum, and adipocytes. Finally, MT3 is expressed in the liver, kidney, brain, heart, lung, intestine, muscle, and brown adipose tissue and the eye tissue, involving the cornea, the lens, and the retina, particularly in photoreceptors, amacrine cells, and ganglion cells [78]. Expression levels of MEL receptors are affected by light and plasma MEL concentration leading to circadian variations in MEL binding [57]. Other variables such as estradiol levels, aging, neurodegenerative diseases, and individual receptor sensitivity are also related to the variability in the functionality of MEL receptors [57, 61, 77].
The signal transduction mechanisms of MEL receptors involve multiple intracellular signaling pathways that may be tissue-dependent (for an overview on the topic, see [46, 47]. In general terms, both MT1 and MT2 are GPCRs that mainly signal through the heterotrimeric Gi proteins, where the α-subunit mediates the inhibition of adenylyl cyclase and the dampening of intracellular cAMP levels leading to reduced activation of both the protein kinase A (PKA) and the transcription factor cAMP-responsive element-binding (CREB). Furthermore, MEL receptors also interact with the G protein-gated inwardly rectifying potassium channels (GIRK) and with voltage-gated calcium channels [57, 77]. In particular, the MT1 receptor can interact with the G protein subtype Gq, through which it can activate the phospholipase C (PLC) producing the second messengers diacylglycerol (2-DAG) and inositol trisphosphate (IP3). Then, IP3 binds to IP3 receptors on the endoplasmic reticulum leading to Ca2+ increase and PKC stimulation. MT1 receptors also have been associated with the activation of the inward-rectifier potassium channels (Kir, IRK) [48] and with the mitogen-activated protein kinase (MAPK) pathway, an important signaling pathway that participates in the processes of phosphorylation of clock proteins contributing to the rhythmic regulation of gene expression [52]. The MT2 interactions involve, besides the cAMP-mediated pathway, the inhibition of the cyclic guanosine monophosphate (cGMP) formation and the phospholipase C/diacylglycerol signaling pathway, through which it increases the PKC activity [48].
Regarding the MEL receptors’ role in relation with the sleep-promoting and chronobiotic effects, previous studies have attributed a differential action of MT1 and MT2 based on in vitro evidence, where MT1 was associated with the melatonin-induced inhibition of the SCN neuronal activity [47], while MT2 was involved in the melatonin phase-shifting effects [50, 51, 79]. Instead, in vivo studies have shown that the activation of the MT1 receptors was able to induce phase advances in the circadian activity of the SCN [50,79]. Similarly, the modulation of CREB phosphorylation induced by the retinohypothalamic transmitter (PACAP) via the MT1 receptor has been associated with phase shift effects [80]. These results suggest that the MT1 receptor is also involved in the phase-shifting effect. However, the phase-shifting effect has also been associated with the MT2 receptor/protein kinase C dependent pathway [47, 51] and with the inhibition of cGMP pathway mediated by MT2 [66]. The SCN-alerting signal inhibition has been associated with the activation of the PKC pathway through the MT1 [66, 81] and with the activation of the Inward-rectifier potassium channels (Kir, IRK) [48]. Nonetheless, a role for the MT2 receptor in the sleep-promoting mechanism of melatonin has also been reported [56].
The role of MT2 and MT1 receptors on sleep has been studied through selective ligands (MT2 partial agonists: UCM765 and UCM924, and non-selective MT1-MT2 agonist UCM793) using both pharmacological and genetic approaches of knockout (KO) mice for MT1 and/or MT2 receptors, as reviewed by Gobbi & Comai, 2019 [82]. To summarize, pharmacological studies in rats undergoing continuous electroencephalography (EEG) and electromyography (EMG) recordings demonstrated that UCM765 (MT2 partial agonist) decreased the latency to the first episode of Non-rapid eye movement (NREM) sleep and increased the total amount of NREM sleep [83]. Likewise, the MT2 agonist (IIK7) increased the duration of NREM sleep without affecting REM sleep [84]. Moreover, MT1/MT2 nonselective agonist UCM793 did not significantly modify sleep stages [85], suggesting that the MT2 receptor subtype is mainly involved in the regulation of NREM sleep. To test the hypothesis that the promotion of NREM sleep is MT2-mediated, an MT2 receptor antagonist (4P-PDOT) before UCM765 was administered, and it was found that 4P-PDOT blocked the effects of UCM765 on NREM sleep duration [85]. Studies in MT2KO mice showed that, unlike in wild-type (WT) controls, UCM765 did not enhance NREM sleep, thus confirming the important role of MT2 receptors in modulating NREM sleep. The KO of both MT1 and MT2 receptors did not significantly affect the amount of NREM and rapid eye movement (REM) sleep, even when it induced an increase in wakefulness [86]; instead, the inactivation of only one of the two MEL receptor subtypes is able to produce significant effects on sleep stages: on the one hand, MT2KO mice have shown a significant reduction in NREM, while no effects on REM sleep duration have been observed; on the other hand, MT1KO mice have shown a significant reduction of REM [85, 86], thus corroborating the differential role of MT2 and MT1 on NREM and REM sleep.
5. MELATONIN PHARMACOKINETICS
Pharmacokinetic studies of exo-MEL reported that oral immediate-release formulations have time to maximal plasma/serum concentration (Tmax) values of approximately 50 min and an elimination half-life (T1/2) of approximately 45 min administration. In contrast, the oral slow-release formulations showed a Tmax of up to 167 min and T1/2 values of up to 91 min [87]. Great variability was instead reported within- and between-studies regarding variables such as maximal plasma/serum concentration (Cmax), area-under-the-curve plasma/serum concentrations (AUC), clearance (Cl), and volume of distribution (VD), which are related to individual differences in absorption, distribution, metabolism, and elimination of the drug [87]. Doses of 0.1-0.3 mg produced serum MEL levels within the normal nocturnal concentrations (physiological doses). In contrast, doses of 1 mg to 5 mg led to plasma melatonin concentrations from 10 to 100 times higher than the physiological peak, indicated as supraphysiological [88, 89]. The metabolism of MEL takes place mainly in the liver, where more than 90% of the circulating MEL is deactivated, and secondarily in the kidney. The process involves the hydroxylation to 6-hydroxymelatonin by the hepatic cytochrome P450, mainly the CYP1A2 isoform, followed by a conjugation with sulfuric acid (90%) or glucuronic acid (10%) that forms the conjugates 6-sulfatoxymelatonin (aMT6s) and 6-glucuronylmelatonin respectively, which in turn are finally excreted through the urine [88, 90]. The 6-sulfatoxymelatonin (aMT6s) is the major urinary metabolite of MEL, and its excretion may reflect the plasma melatonin profile, frequently used as a circadian marker of the endogenous melatonin levels in humans [90].
6. MELATONIN EFFECTS
Several mechanisms have been proposed to address the exo-MEL sleep-promoting effects. They can schematically be divided into: a) the chronobiotic effects exerted by exo-MEL on both central and peripherical oscillators. b) the sleep-promoting effect attributed to several somnogenic mechanisms, i.e., action on the GABAergic system, interaction with thermoregulation processes, direct action on somnogenic structures within the brain, the induction of adenosine signaling, and the interaction with BK channels. More recently, an action of MEL at the interface between circadian and homeostatic processes has been suggested. These proposed mechanisms are reviewed below.
6.1. Chronobiotic Effect
The chronobiotic effect of melatonin relies upon its action at several locations defining the circadian timing system. The circadian timing system is controlled by the hypothalamic SCN, the central oscillator, coordinating the peripheral oscillators through neural and hormonal signals, which shape phase, amplitude, and period of daily circadian oscillations. At the cellular level, a well-conserved and almost ubiquitous basic mechanism is the molecular oscillatory system represented by the so-called clock genes. The clock genes products regulate the expression of the tissue- specific output of clock-controlled genes (CCGs) to control the cellular circadian function [91]. This molecular mechanism controlling the circadian system involves interconnected negative, positive and regulatory feedback loops that have been well documented. The core of these mechanisms consists in the interplay between two transcription factors: CLOCK (circadian locomotor output cycles kaput) and BMAL1 (Brain and Muscle ARNT-Like 1). The positive feedback refers to the activation of the transcription of other clock genes: three Period (Per 1-3) and two Cryptocrome (Cry 1-2) genes by CLOCK/BMAL1 heterodimers. In the negative feedback, the proteins PER and CRY form complexes that translocate to the nucleus, where they inhibit their own transcription. The inhibition of the PER/CRY repressor is required to restart a new cycle of transcription. A second loop has also been described, in which CLOCK/BMAL1 heterodimers also drive transcription of nuclear receptors of REV-ERB and ROR families, which inhibit and activate the rhythmic transcription of BMAL1. These two loops are regulated by several regulatory elements, including the DBP (D-box binding protein), PARzip protein (proline and acidic amino acid-rich basic leucine zipper), and NAD+-dependent SIRT1 (nicotinamide adenine dinucleotide -dependent deacetylase sirtuin-1 (silent mating type information regulation 2 homolog 1; SIRT1)) [91-94]. The expression of Per1 can be used as a marker of clock-containing SCN neurons since mRNA levels for Per1 are highest during the subjective day, thus showing a circadian variation and providing an indicator of the circadian phase [95].
6.1.1. Central Chronobiotic Effects
The molecular clock genes-CCGs interplay determines the period length of the self-sustained circadian rhythm in SCN cells [93,96-98]. Since the SCN period length may differ slightly from the 24h period, it requires to be entrained by external stimuli such as the light/dark cycle and the feedback loop involving the rhythmic release of MEL [96]. Therefore, besides participating in the pathway signal for the endogenous MEL synthesis, Fig. (2), the SCN also receives a signal from the MEL released by the pineal gland, through which MEL acts as “a feedback that contributes to the adjustment of the pacemaker to the external cycle and, presumably, to the maintenance of high-amplitude oscillations” [99].
The SCN contributes to maintaining wakefulness by producing an alerting signal during the day and helps maintain sleep by producing a reduced signal at night [66], thus, exerting a circadian modulation of sleep and wake propensity [100]. The “Opponent-Process” model of sleep regulation postulates that the SCN generates the circadian rhythm of the sleep-wake cycle by enhancing arousal during the active period [101]. The peak of the alerting signal takes place at the end of the active period and is followed by a period of increased sleep propensity: these periods correspond to the named “forbidden zone” and the “sleep gate”, respectively, where the forbidden zone coincides with the peak of the SCN activity, and the sleep gate is coupled with the release of endogenous MEL [102, 103]. It has been proposed that MEL may influence sleep indirectly by two different effects on the SCN: 1) phase shifting of the SCN activity and/or 2) attenuation/antagonism of the SCN-dependent mechanism that promotes and maintains wakefulness at particular times of day (inhibitory effect) [102].
The mechanisms that allow MEL to shift the SCN phase through MT1 and MT2 receptors involve the inhibition of the cAMP signaling pathway and other pathways that do not couple to the classical MEL receptor signaling mechanisms [96]. Moreover, the SCN has a temporal sensitivity window that is different from day to night, so that in the day, the SCN sensitivity to PACAP increases, activating the cAMP adenyl cyclase signaling pathway, while in the night, the SCN sensitivity to glutamate increases, stimulating the Ca2+/calmodulin signaling pathway; here, MEL seems to inhibit only the PACAP-induced but not the glutamate-induced pathway [96]. In this way, MEL may modulate CREB phosphorylation induced by PACAP via the MT1 receptor, a process that has been associated with its phase shift effects [80]. Moreover, MEL phase-shifting effect has also been associated with the MT2 receptor/protein kinase C dependent pathway [47, 51], and with the inhibition of cGMP pathway mediated by MT2 [66]. At the level of clock genes, an in situ study in rats has demonstrated that a single MEL injection in the SCN, prior to the light-dark transition, acutely modified the expression of RorB and REV-ERB and phase-shifted the expression of bmal1 the following night [104]. Also, an in vitro study in rat SCN showed that exo-MEL alters the SCN clock regulating Per and Clock genes [105].
Regarding the inhibitory effect, seminal studies showed that the primary effect of MEL in the rat SCN is the inhibition of neuronal activity [106, 107]. Later studies in mice and rats have confirmed that MEL hyperpolarizes the resting membrane potential and suppresses spontaneous action potential rate in the SCN [95, 108, 109]. This MEL-induced inhibition is mainly due to the activation of potassium channels that tend to hyperpolarize the membrane potential, making neurons less excitable [108]. Moreover, the decrease in excitability of SCN induced by MEL seems to be independent of the timing of administration [109], thus suggesting that, in contrast to the phase-shifting effects, the inhibitory effects of MEL on SCN excitability are not restricted to a specific time window. The acute inhibitory effect of exo-MEL on SCN has been attributed to the action through the MT1 receptors [47]. Moreover, MT1 and MT2 may differentially regulate GABA-A receptor function, through which MEL can increase the amplitude of GABA-A-mediated synaptic transmission in the rat SCN [110]. Several studies in animals have demonstrated that the modulation of GABAergic function is determinant for the internal synchronization, circadian rhythmicity and attenuation of the SCN activity induced by MEL [111-113]. Evaluating the effect of MEL on cells located in either dorsal or ventral regions of the SCN and targeting both Per1 and non-Per1 neurons, Scott et al., 2010 found that application of MEL induces acute changes in membrane excitability in both Per1 and non-Per1 SCN neurons at all times of the LD cycle. This was related to the fact that the expression of MT1 receptor mRNA in the SCN is not subject to significant circadian variation [95]. The evidence described is consistent regarding an acute inhibitory effect of MEL on SCN, which is independent of the circadian phase and that may be mediated, at least in part, by GABAergic transmission. The SCN has outputs to adrenergic, serotoninergic, histaminergic, and orexinergic neurons that constitute pathways through which it may regulate sleep and arousal centers [100]. Although some mechanisms linking the inhibition of SCN by MEL and the downstream activation of somnogenic structures have been proposed [81,114], the SCN-mediated actions of MEL on sleep only may account for an indirect effect. In the section 6.2.2 direct action mechanisms proposed to explain the sleep-promoting effects of MEL are described.
6.1.2. Chronobiotic Effects Outside the SCN and in Peripheral Clocks
Other brain structures also contribute to the role of MEL in modulating circadian physiology. High density of MEL receptors has been described in the pars tuberalis (PT) of the pituitary gland, a structure playing a key role in seasonal physiology and considered an important component to distribute circadian information to the periphery, in which the time signals received from MEL are transduced into hormonal messages that are spread via the circulation to peripheric target tissues [115]. Seminal studies in ovine showed that MEL acts primarily to attenuate the effects of the cAMP signaling, thus inhibiting the transcription factor CREB and leading in turn to the inactivation of gene transcription in the PT; likewise, MEL demonstrated to modulate gene expression through a serum response element (SRE) via a MAPK-Elk-1 pathway in the PT [115]. MEL can also induce Cry1 mRNA in rats and sheep, synchronizing the PT to the photoperiod [116]. The long-term regulation of mPer1 mRNA and mPER1 protein expression has also been attributed to MEL [96], and several studies in animals have demonstrated that MEL stimulates Cry1 expression, while Per1 expression is inhibited [93], thus, suggesting that rising melatonin levels could reset circadian rhythms of clock genes in the PT [117]. Furthermore, MEL may sensitize the adenylyl cyclase signaling pathway in a time-dependent manner so that the MEL signal opens a temporally distinct time window for the transmembrane activation of gene expression in the PT [96].
MEL can also regulate circadian clock genes-CCGs oscillations peripherally. Indeed, the molecular circadian machinery is also expressed in peripheral tissues as the liver, pancreas, fat, gut, lung, and heart, forming the “multi-oscillatory circadian network” [93]. Circadian rhythms in peripheral tissues depend on SCN signals by which they are considered ‘slave’ oscillators [118]. However, a more balanced relationship between the central and peripheral clock has been proposed within the conceptual framework of the “orchestra” model, in which each peripheral clock can adapt to its own external and internal stimuli but is “conducted” by SCN signals [119]. According to both the “master-slave” and the “orchestra” models, several signaling pathways synchronize the master clock and the peripheral ones, including neurotransmitters, secreted factors, the modulation of the timing for some specific behaviors, and hormones [118]. Among these signals, MEL is the major hormonal output, distributing temporal cues generated by the SCN influencing cells in other areas of the CNS and the peripheral tissue [93]. Outside the SCN, MEL may directly modulate the expression of several core oscillator genes, in the heart of the rat [120], in human prostate cancer cells [121] and the adrenal cortex [122, 123]. Likewise, MEL could be directly responsible for circadian activity modulation in primary isolated adipocytes [124] and rhythmic protein synthesis in hepatocytes in vivo [125]. Thus, MEL plays multiple roles in the multi-oscillatory circadian network, influencing the phases of both the pacemaker and several peripheral oscillators [126]. Moreover, MEL may also act directly on components of the ubiquitin-proteasome system, a mechanism that regulates the timing of transcriptional activators being essential to maintain the precision of the circadian timing mechanism [127]. In this mechanism, MEL works as a proteasome inhibitor that interferes with the destruction of transcription factors, thus modulating the feedback loops that regulate transcription of clock genes and providing in this form a selective stability for proteins when MEL is elevated [117].
Recent evidence shows that MEL may regulate basic cellular metabolism, chromatin integrity, gene transcription and translation, and RNA expression and degradation, thus modulating several components in clock genes and CCGs mechanisms [91]. Moreover, the high density of MEL receptors in the SCN allows MEL to modify the rhythm of the central clock, but simultaneously allows MEL to have an impact on all the peripheral oscillators and tissues, as a “time-giver” of several physiological functions, among which is the sleep-wake cycle [128].
6.2. Hypnotic/Sleep-inducing Actions
Within the medical practice, hypnotics are commonly referred to as drugs capable of inducing sleep. Classical hypnotics (BDZs and z-drugs) act as positive allosteric modulators (PAMs) of GABA-A receptors. Their administration induces a sleep-like state that resembles non-rapid eye-movement (NREM) sleep [129]. It has been proposed that exo-MEL could act with overlapping mechanisms. However, some authors pointed out how defining this effect as hypnotic (or sedative) can be misleading, not only for MEL but also for typical hypnotics such as BDZs, and therefore proposed to replace it with other terms [3]. Hereby, we propose, for the sake of this review, to unify these terms (hypnotic and sedative) under the rubric of sleep-inducing actions, as induction implies causality in the transition from wakefulness to sleep-like states. In this perspective, a sleep-inducing drug or molecule differs from a sleep-promoting one in the ability to cause sleep in a necessary and sufficient manner (sleep-inducing) rather than creating favorable conditions for falling asleep (sleep-promoting).
Early on, it was proposed that the “anxiolytic, anticonvulsant, and other psychotropic actions” of exo-MEL were similar to those exhibited by BDZs, and that these effects may involve the enhancement of GABAergic activity [130], with sleep-inducing effects similar to those of typical hypnotics/sleep-inducing drugs. Although some indirect effects of MEL on GABAergic system have been described [81, 110, 114], very high doses of exo-MEL are required in animal experiments to show a positive allosteric effect on GABA binding to GABA-A receptors, indicating that this mechanism may be relevant only for very high doses also in humans [102]. Indeed, a central-type BDZ antagonist (flumazenil) was not able to disrupt the sleep-promoting effect of exo-MEL (3 mg) in humans [131]. Therefore, at relatively low doses, the exo-MEL effects on sleep do not seem to be mediated by BDZ receptors. Sleep is a complex process that involves an orchestrated neurochemical network. Despite evidence supporting the notion that MEL has sleep-promoting effects [132], the underlying mechanisms remain to be elucidated. Some of them are described below.
6.2.1. Reduction in Core Body Temperature
The sleep-promoting effect of exo-MEL through a thermoregulatory mechanism has been proposed since the increase in sleepiness produced by exo-MEL occurs in conjunction with a decrease in core temperature and an elevation in skin temperature, and because the circadian modulation of temperature seems to be partially regulated by the endogenous MEL release [3, 12, 131, 133]. Although the exact mechanism by which exo-MEL influences body temperature has not been completely established, it has been proposed that exo-MEL may exert a central action on the preoptic area and the anterior hypothalamus (the main thermoregulatory centers), as well as a peripheral action on receptors involved in heat generation and dissipation. Here, the sleep-promoting effects of exo-MEL have been hypothesized to be related to a hypothermic response mediated by vasodilatation processes [134-136]. However, since some studies have found changes in temperature but not in sleep [22], or effects on sleep but not on temperature [10, 137], some authors have suggested that the mechanisms underlying the exo-MEL effects on sleep and thermoregulation might be independent [4].
6.2.2. Direct Action on Somnogenic Structures
A regionally localized interplay between MEL and the GABAergic system has been recently described within the lateral hypothalamus (LH). At this level, in the mouse model, melatonin controls GABAergic neurons via MT1-dependent inhibition of the HCN (Hyperpolarization-Activated Cyclic Nucleotide-Gated) channels. In view of the crucial role of LH GABAergic neurons in maintaining wakefulness, melatonin might have a sleep-inducing effect via a local action exerted within the LH [138].
Along with the LH, the anatomical distribution of MEL receptors within the brain extends to areas involved in regulating arousal, REM, and NREM sleep. Specifically, MT2 receptors have been found in the reticular nucleus of the thalamus (RT), suggesting its involvement in NREM sleep promotion, while the MT1 is expressed in the locus coeruleus (LC) and LH, which suggest its involvement in REM sleep and arousal [82].
In particular, the RT activation promotes NREM sleep by connecting deeper brain structures to the cortex via thalamocortical pathways. During NREM, RT generates rhythmic activity that is transmitted to thalamic nuclei and modulated by corticothalamic inputs that contribute to a widespread synchronization across neuronal networks [82]. In rats, the MT2 partial agonist UCM765 induces a rhythmic, synchronized burst activity separated by periods of silence in the RT neurons. Moreover, the infusion of the MT2 receptor antagonist 4P-PDOT blocked the effects of UCM765 on RT neurons, confirming an MT2 receptor-mediated response. Instead, when UCM765 was injected in brain regions not involved in sleep regulation but containing MT2 receptors, such as substantia nigra and pars reticulata, no effects on NREM sleep were observed [85]. Since the rhythmic activity in RT promotes NREM sleep, MT2 receptors are proposed within this sleep regulatory frame [82].
Another pharmacological experiment conducted in mice by Sharma et al., (2018) showed that orexin neurons in the perifornical lateral hypothalamus (PFH) express MT1 but not MT2 receptors. Moreover, MEL infusion into the PFH at dark onset significantly increased NREM sleep and reduced wakefulness, while the MEL antagonist luzindole, administered at light onset (onset of sleep period), significantly increased wakefulness. The authors also found that local MEL infusion at dark onset inhibited orexin neurons, evidenced by a significant reduction in the number of orexin neurons expressing c-Fos. Based on these results, the authors suggest that MEL may act via the MT1 receptors to inhibit orexin neurons and promote sleep [139].
Other possible targets involved in a putative direct sleep-inducing effect are the dorsal raphe (DR) and the locus coeruleus (LC). The evidence is provided by a study conducted in MT1KO mice that report an impairment in the physiological light-dark fluctuation of DR 5-HT neurons and a blunt in the daily circadian changes in locus coeruleus (LC) neural activity [140]. Since the firing of monoaminergic neurons changes as a function of sleep and wake cycles (firing during wakefulness, decreasing their firing during NREM sleep, and being silent during REM sleep), it has been proposed that future studies should explore the role of MT1 receptors on monoaminergic neurons involved in the modulation of sleep [82].
Based on the research conducted by the group of Gobbi & Comai, the authors hypothesized that the peak of MEL might desensitize or down-regulate its own receptors, generating a differential expression and/or sensitivity of MT1 during REM sleep and MT2 during NREM sleep that may contribute to a rhythmic balance between NREM, REM, and wakefulness [82]. While MT1 receptors show a large variability in their regional expression, MT2 receptor density is lower in SCN and more widespread throughout the brain, indicating that the MT2 receptor could be involved in the homeostatic regulation of sleep and less in the regulation of circadian rhythms. Likewise, several studies suggest that the MT1 receptor is mostly involved in the circadian regulation of behavior [128]. However, although MT2 receptors have been identified in critical areas for sleep, it does not necessarily imply that MEL is a hormone acting per se on sleep. Instead, it is proposed as a clock cue influencing circadian rhythms, including circadian regulation of sleep [128]. Likewise, as hypnotic properties of exo-MEL have been described as “weak,” and considering that endogenous MEL peaks at night in both nocturnal and diurnal animals, it has led to hypothesize that MEL is not per se a sleep-inducing neuromodulator, but rather a pace-maker influencing the circadian expression of sleep-promoting molecules [82]. In this model proposed by Gobbi and Comai., 2019, MEL acts as an “orchestra conductor”, regulating the expression of MT1, MT2, and other non-MEL receptors involved in promoting sleep.
Through experiments conducted in diurnal animals lacking MEL due to a mutation of arylalkylamine N-acetyltransferase 2 (aanat2) [53], it was demonstrated that MEL might promote the initiation and maintenance of nighttime sleep, even in the absence of endogenous circadian rhythms at a cellular level. In zebrafish lacking MEL, indeed, night sleep is dramatically reduced and can be completely rescued by exo-MEL. Therefore MEL, and also exo-MEL, may promote sleep directly and not by modulating the circadian clock [53]. Moreover, the administration of two adenosine agonists was able to partially rescue sleep in this animal model. These results suggest that the sleep-promoting effects of MEL can be partially attributed to the adenosine signaling [141]. Although the specific interactions between adenosine and MEL remain to be elucidated, since adenosine levels decrease as the sleep period progresses during the night, MEL and adenosine may interact mainly at the beginning of the sleep period to promote sleep initiation [142]. Additional evidence about the interaction between MEL and adenosine in the regulation of sleep comes from another animal model. Aiming at investigating the effect of intraperitoneal injections of MEL on the activity of 5′-nucleotidase (the adenosine synthetizing enzyme) in the forebrain of juvenile rats subjected to acute hypobaric hypoxia, the group of Zmorski et al., 2003, found that MEL increased the activity of 5′-nucleotidase; moreover, in animals kept in constant darkness, injections of melatonin into normoxic rats promoted adenosine synthesis, even more intensely [143].
Although it is well established that MT1 and MT2 function through G proteins, the downstream molecular targets leading to the MEL sleep-promoting effect remain to be elucidated. The BK channel is a conductance potassium channel broadly expressed in the nervous system, it participates in the downregulation of neurotransmitter release, and its expression may be regulated by light and dark cycles, playing important roles in circadian behaviors [144]. To identify the role of BK channels (large-conductance, voltage, and calcium-activated potassium channels), on sleep-promoting effects of MEL, the group of Niu et al., 2020 used a forward genetics approach to identify proteins important to in vivo functions of the C. elegans BK channel (SLO-1). They found that PCDR-1 (a putative MEL receptor in C. elegans) is indispensable for SLO-1 physiological function in neurons and that it allows MEL to promote sleep by activating SLO-1. Specifically, they found that KO of pcdr-1, slo-1, or homt-1 (a gene required for MEL synthesis) induced increased neurotransmitter release and shortened sleep duration. Moreover, Exo-MEL was able to inhibit neurotransmitter release and promote sleep in WT but not in pcdr-1 and slo-1 mutants. These results indicate that both PCDR-1 and SLO-1 are required for the sleep-promoting effects of exo-Mel. Furthermore, using a Xenopus oocyte heterologous expression system, the authors found that the human BK channel (hSlo)1 is activated by MEL through MT1 but not through MT2. Moreover, the binding of MEL to the MEL receptor possibly activates the BK channel through the release of Gβγ subunits. The results derived from this study show that BK channels may play an evolutionarily conserved role in mediating the effect of MEL on sleep [144].
Although, as described in detail in the previous paragraphs, some potential direct hypnotic mechanisms of exo-MEL have been identified in a variety of animal models, clinical doses of exo-MEL in humans seem to have an indirect sleep-promoting rather than a direct sleep-inducing effect, as addressed in the following section.
7. CLINICAL EVIDENCE
7.1. Chronobiotic Effects of Exo-MEL
The internal biological time in humans is usually measured through the minimal core body temperature (CBT) and the dim light melatonin onset (DLMO). DLMO is defined as the “initial surge in melatonin release in the early part of the night,” which is measured under dim light conditions and after 17:00-18:00 to eliminate the masking effects of the natural photoperiod [145]. DLMO is operationally determined as the point in time when melatonin levels rise above a set threshold [146]. These markers have a circadian rhythmicity allowing evaluation of the individual circadian phase [66]. Exo-MEL has also demonstrated properties as a time cue that can exert chronobiotic effects [45]. Through the phase response curve (PRC), it is possible to predict the direction and the magnitude of the modification in the circadian rhythms (phase shift) in response to external stimuli (e.g., exo-MEL), according to the timing of administration [45]. The studies evaluating exo-MEL effects on circadian rhythmicity in HS and in CRSDs are summarized in Appendix A (Table 1) and are reviewed below.
Table 1. Chronobiotic effect of exogenous melatonin.
| Author, Year |
Study
Design |
Aim | DX | Subjects | Variables | Dose | Timing | Duration | General Results |
|---|---|---|---|---|---|---|---|---|---|
| Wright et al., 1986 [153] | Double-blind placebo-controlled crossover | To investigate the effects of small doses of MEL administered to normal adults daily at 17:00 | HS | n=12 males 10 age range 22-46 |
MEL onset, cortisol, GH, PRL, T4, LH, and testosterone. Mood and Fatigue (VAS) | 2 mg | 17:00 | 4 weeks | ↑ Tiredness/fatigue, ← MEL onset-offset advanced in 1-3 h in 5/12 subjects (but not significant) |
| Mallo et al., 1988 [159] | Case series | To evaluate the effect of a semi-chronic treatment (with exo-MEL) on the endocrine rhythms | HS | n = 6 males 6 age range 22-26 |
MEL, cortisol, and PRL profiles | 8 mg | 22:00 | 4 days | Third day after exo-MEL withdrawal: ← MEL phase, ↑ PRL between 19:00 and 21:00 h |
| Terzolo et al., 1990 [158] | Case series | To investigate the effects of long-term exo-MEL administration on several hormones characterized by overt daily patterns of secretion | HS | n = 6 males 6 age range 23-32 |
MEL, PRL, cortisol, and testosterone phase. Hormonal levels (LH, FSH, PRL, TSH, Gn-RH, TRH, ACTH, HCG), blood pressure, heart rate | 2 mg | 18:00 | 8 weeks | ← MEL phase, morning acrophase of cortisol and testosterone |
| Lewy et al., 1992 [44] | Placebo-controlled crossover | to determine the DLMO phase in sighted individuals before and after a physiological dose of exo-MEL | HS | n=8 age range 20-48 |
DLMO | 0.5 mg | Trial 1:17/19 Trial 2:13/15, 14/16, 15/17, 16/18, 18/21. Trial 3: 12/14, 20/22, 22/24. |
1 week | ← DLMO exo-MEL CT6-CT12 (clock time: 13:00 to 19:00 h) → DLMO exo-MEL CT20-CT5 (clock time: 03:00 to 12:00) |
| Zaidan et al., 1994 [148] * |
Not specified | To evaluate the phase shift effect of exo-MEL when administered in a physiological rhythm (iv infusion) | HS | n=7 males 7 age range 25-32 |
MEL and cortisol profile (onset, offset, acrophase) | 20 µg (iv) | infusion began at 04:00 or 12:00 or 16:00 or 20:00, administered for 3 h | Single-dose for each protocol | ← MEL acrophase when exo-MEL was given at 16-19 (45±67.7min) and at 20-23 (72.8±66.7min) → MEL acrophase when exo-MEL was given between 4-7 h (23.6±38.6 min) and at 12-15 (40.7±46 min) No changes in cortisol |
| Deacon et al., 1994[147]* | Double-blind placebo-controlled crossover randomized | To describe the effects of an acute administration of exo-MEL on the phase shift of endogenous MEL and CBT | HS | n=8 males 8 age range (23-28) |
MEL onset (acute and delayed), Alertness (VAS), CBT (acute and delayed), SQ | 5 mg | 17:00 | single dose | ← CBT Acrophase ← MEL onset (1.14±0.49h), ↓ alertness, CBT (acute), ↑ SQ |
| Deacon & Arendt, 1995 [134] * | Double-blind, placebo-controlled crossover | To evaluate the dose-dependence effect of exo-MEL on temperature and endogenous MEL secretion | HS | n=6 males 3 age 27.2±3.7 |
CBT (acute and delayed), sleep log (SOT, SOFFT, n° and duration of awakenings), sleep quality (VAS), MEL plasma levels onset, alertness | 0.05, 0.5 or 5 mg | 17:00 | single dose | ↓ CBT (acute), alertness, ← sleep phase and MEL onset (0.36±0.13 h, 0.69± 0.15 h and 1.43± 0.16 h for 0.05 mg, 0.5 mg and 5 mg respectively) ← nadir of CBT, ↑QS |
| Attenburrow et al., 1995 [155]* | Double-Blind, placebo-controlled crossover | To evaluate if MEL administered to HS at 17:00 advances circadian rhythms as determined by a shift in time of onset of endogenous MEL secretion | HS | n=12 males 12 age 30 (range 21-37) |
MEL onset | 0.5 mg | 17:00 | single dose (acute), seven days (subacute) |
← MEL onset (subacute administration induced a MEL onset 50 min earlier than in placebo or acute condition) |
| Kräuchi et al., 1997 [149]* | Double-blind placebo-controlled crossover | To evaluate the phase-shifting and thermoregulatory effects of a single admin of exo-MEL or S-20098 | HS | n=8 males 8 age 27±4 range 23-32 |
DLMO, CBT, Heart rate, distal and proximal skin temperature | 5 mg | 18:00 | Single dose |
← DLMO (49 min), earlier increase in distal skin temperature, earlier decrease in CBT, heart rate, and proximal skin temperature (the day after treatment) |
| Cajochen et al., 1997 [152] | Double-blind placebo-controlled | To distinguish between circadian and non-circadian effects of exo-MEL and its repercussions on sleep architecture and EEG power density. | HS | n=8 males 8 age 27±4 range 23-32 |
Sleepiness and mood self-rating (VAS), CBT, 5-min waking EEG, all night EEG (TST, SE, SL, REM_L, S2, SWS, REM, Arousal) | 5 mg | 18:00 | Single dose | ← CBT ↑ Wakefulness in the latter one-half of the sleep episode on the posttreatment night (earlier termination of sleep period) ↑ REM (longest first REM episode) ↑ theta/alpha in the waking EEG ↑ self-reported sleepiness, |
| Middleton et al., 1997 [154] |
Double-blind placebo-controlled crossover | To investigate the effects of daily exo-MEL administration on sighted individuals kept in continuous very dim light. | HS | n=10 males 10 age 23.9±0.75 |
aMT6s, CBT, actigraphy (SOT, SOFFT) and sleep log (BT, latency, number and duration of night awakenings, wake-up time, SQ). |
5 mg | 20:00 |
(Leg 1): MEL days 1-15 (1st), PLA days 16-30 (2nd) Leg 2: crossover |
MEL1st: stabilization of sleep-wake cycle in 8/10 subjects, PLA1st: 9/10 subjects free-running MEL 2nd: ← sleep-wake cycle in 5/9, → sleep-wake cycle in 2/9 and stabilization in 2/9 subjects. Subsequent synchronization to 24h in 7/9 subjects, CBT continued to run in 4/9 subjects. aMT6s free ran in all subjects with PLA1st, and in 5/9 of MEL 1st. |
| Yang et al., 2001 [150]* | Double-blind placebo-controlled counterbalanced | To test whether a delayed weekend sleep pattern may lead to a phase delay of the endogenous circadian rhythm and whether exo-MEL may counteract the phase delay | HS | n=10 males 2 age 22.1 |
DLMO, PSG (TST, SE, WASO, S1, S2, S3, S4, SWS, NREM, REM, SOL, S2_L, SWS_L, REM_L), sleepiness (SSS), cognition, mood (VAMS) | 6 mg | 5.5 h before habitual BT (mean: 18:04) | Single dose | ← DLMO ↓ SOL and S2_L Improvement in VAMS scores (items: “sleepy”, “overall feeling better” and “alert”, “effort to do anything”) |
| Wirz-Justice et al., 2002 [151] |
Double-blind placebo-controlled randomized | To investigate the phase-shifting potential of MEL on the most probable timing for a delay in the circadian rhythms. | HS | n=9 males 9 age 23.6±2.8 |
CBT, Heart rate, DLMO | 5 mg | 07:00 | Single dose | No changes on circadian markers |
| Rajaratnam et al., 2003 [160] |
Double-blind placebo-controlled crossover | To evaluate whether an artificially prolonged MEL profile influence its own secretion and that of other endocrine parameters | HS | n=8 males 8 age 24.4±4.4 |
MEL plasma onset, cortisol peak, actigraphy (mean activity levels), sleepiness, mood, pituitary and gonadal hormones | 1.5 mg (PR) | 16:00 | 8 days | ← MEL onset and cortisol peak (difference between PLA and exo-MEL advance= 2.9 h) ↓ activity mean during the first sleep interval of an extended sleep opportunity of 16 h |
| Rajaratnam et al., 2004 [161] |
Double-blind placebo-controlled repeated-measures |
To establish whether MEL could advance the sleep timing at a time normally associated with the wake maintenance zone | HS | n=8 males 8 age 24.4±4.4 |
PSG (TST, SE, SOL, sleep timing, duration and percentage of S1,S2,S3,S4,REM, REM:NREM, S1_L, S2_L, REM_L, duration of first REM), Spectral power | 1.5 mg (PR) | 16:00 | 8 days | . ← sleep phase after exo-MEL compared to PLA. During MEL administration, S1, S2, REM longer in the first interval of sleep and shorter in the second interval. SE and TST higher in the first sleep interval and lower in the second interval compared to PLA |
| Burgess et al., 2008 [157] * | Double-blind placebo-controlled counterbalanced | To generate a new PRC to exo-MEL and to determine if any phase shift will occur when EXO-MEL taken close to habitual BT | HS | n=27 males 14 age 28.8±6.9 |
DLMO | 3 mg (FR) | Multiple | Single-dose for 3 consecutive days | ← DLMO 1.8 h (peak when exo-MEL taken 5h before DLMO), → DLMO 1.3 h (peak when exo-MEL taken 11 h after DLMO) |
| Burgess et al., 2010 [156] * | Double-blind placebo-controlled counterbalanced | To generate a PCR to 0.5 mg oral MEL and to compare it to the previously published 3.0 mg PCR using the same protocol | HS | n=34 males16 age 25.3±4.8 |
DLMO | 0.5 mg | Multiple | Single-dose for 3 consecutive days | ← DLMO maximum advance at timing 2-4 h before DLMO or 9-11 h before sleep midpoint, maximum advance of 1.5 h → DLMO maximum delay at timing 12-15 h after the DLMO (13.6 h after the DLMO or within 4 h after wake time, maximum delay of 1.3 h |
| Arendt et al., 1988 [162] | Single-blind placebo-controlled | To evaluate the efficacy of an exo-MEL treatment in a free-running blind patient | BFR | n=1 59-year-old man |
Sleep log (Lights-off, SOL, SOFFT, SOT, AW), SQ, aMT6S (Acrophase). | 5 mg | 21:00-24:00 | 4 weeks | ↓ day sleeps, “some modification of the endogenous rhythm”: aMT6S acrophase delay with respect to the acrophase predicted the end of the treatment, enhanced well-being. |
| Folkard et al., 1990 [163] | Single-blind placebo-controlled | To evaluate the effects of exo-MEL on sleep and circadian rhythms of cortisol, temperature, and MEL in a free-running blind patient | BFR | n=1 60-year-old man |
Rectal temperature, alertness, calmness, elation, sleep log, cortisol, and aMT6S profiles | 5 mg | 23:30 | 4 weeks | ↑ TST and MOOD ↓ naps and variability in sleep onset. No changes in temperature. MEL and cortisol free-run rhythms. |
| Sarrafzadeh et al., 1990 [164] |
Case report | To evaluate the effects of exo-MEL on sleep in a blind patient with delayed cortisol and melatonin rhythms | BFR | n=1 76-year-old man |
Sleep log (lights-off, SOL, SOT, SOFFT, AW), SQ, aMT6S and cortisol acrophase, Mood and fatigue (VAS) | 5-6 mg | 23:30 and 20:00 | 4 and 2 weeks respectively | ← SOT and SOFF ↓ SL, AW, naps, ↑ SQ and mood No changes in MEL and cortisol profiles |
| Sack et al., 1991 [166] | Double-blind placebo-controlled | To test the phase-shifting and entraining effects of MEL in human subjects. | BFR | n=5 males 5 age range 30-41 |
Plasma MEL and cortisol | 0.5 mg 5 mg | 22:00 | 21 days | ← MEL and cortisol rhythms |
| McArthur et al., 1996 [171] |
Case report | To report a Non-24h patient with a baseline circadian period of 25.1 hours whose free-running sleep-wake cycle was treated with exo-MEL | BFR | n=1 41-year-old man |
DLMO, PSG (TST and sleep stages) Actigraphy (TST, SE), sleep log | 0.5 mg | 21:00 (initiated when DLMO near to 21:00) | 4 weeks | Regularization to a circadian period of 24.1 hours, the morning awakening become uniform and was locked to the DLMO. |
| Masaaki et al., 1997 [174] | Case report | To describe a MEL treatment in two patients with non-24 h syndrome | FR | Patient 1: female 34-year-old Patient 2: male 23-year-old |
Subjective difficulty in falling asleep, sleep-wake patterns | 2-10 mg 1-5 mg |
24:00-22:00 23:00 |
-- | Patient 1: Difficult to fall asleep and waking, without changes in sleep-wake patterns. Patient 2: sleep-wake patterns change from free-running to DSPD (transiently) |
| Siebler et al., 1998 [173] | Case report | Clinical case description | FR | n=1 female 23-years-old |
sleep-wake patterns | 5 mg | 21:00-22:00 | 6 years | Stabilization of sleep-wake patterns |
| Lockley et al., 2000 [167] | Single-blind placebo-controlled | To assess the ability of exo-MEL to entrain the circadian system of free-running blind subjects. | BFR | n=7 males 7 age range 33-60 |
Cortisol, aMT6s, CBT | 5 mg | 21:00 | 31-71 days | Entrainment of circadian rhythms to a 24.0 period in 4/7 patients, |
| Sack et al., 2000 [168] | Single-blind placebo-controlled crossover | To investigate whether a daily dose of MEL could entrain the circadian rhythms to a normal 24 hour cycle | BFR | n=7 males 7 age range 42-57 |
MEL profile, PSG (TST, SL, SE, WASO) | 10 mg | 1 h before BT | 3-9 weeks | MEL rhythm entrained to a 24h cycle in 6 of 7 patients, ↓ WASO, ↑ SE |
| Lewy et al., 2001 [170] | Case series | To evaluate if exo-MEL can a de novo (starting) dose of 0.5 mg initially capture free-running rhythms | BFR | n=3 males 1 age range 42-47 |
MEL profiles | 0.5 mg | 1 or 2 h before BT | -- | All three subjects entrained circadian rhythms of MEL onset to the de novo 0.5 mg dose. |
| Lewy et al., 2002 [165] | Case report | To evaluate whether exo-MEL at 20 mg may entrain the circadian rhythm of a free-running blind patient with a long circadian period (24.9h) | BFR | n=1 male 46-year-old |
MEL profiles | 20 mg and 0.5 mg | 1 h before BT | 47 days | Entrained circadian rhythms to a 24h with 05mg treatment |
| Hack et al., 2003 [169] | Single-blind placebo-controlled | To assess further the entraining effects of exo-MEL on the cortisol rhythm and its acute effects on subjective sleep in blind subjects with free-running aMT6s rhythms | BFR | n=10 males 9 age range 32-65 |
Sleep log (SL, SOT, AW, SOFFTT, TST, N of naps), SQ, aMT6S at baseline and cortisol acrophase. | 0.5 mg | 21:00 | 26-81 days | 7/10 subjects treated with exo-MEL at individual CT10 to CT 16: entrainment or shortened of cortisol period, 2/10 subjects treated with exo-MEL at CT22, CT3: continued to free-run. ↑ TST, ↓ naps and AW, → SOFFT |
| Lewy et al., 2004 [172] | Double-blind placebo-controlled | To describe the response to a treatment with low doses of exo-MEL, initiated in the phase delay zone of the PCR. | BFR | n=7 males 4 age range (21-67) |
DLMO | 0.05 to 0.5 mg | 1 h before preferred BT (predicted to be locked with the “delay zone” of the MEL PCR). | Range 54-367 days) | Eventually all subjects entrained their circadian rhythm, reduction in circadian period (from 24.51h to 23.99h). Treatment takes between 37 to 52 days until entrainment |
| Dahlitz et al., 1991 [175] | Double-blind placebo-controlled randomized | To report the action of exo-MEL on sleep-wake cycle in 8 subjects with DSPD | DSPD | n=8 males 8 age range 14-61 |
PSG (REM_L, NREM SL), Sleep Log (BT, SOT, SOFFT, TST), Alertness (self-rating), motor activity, plasma MEL and urinary aMT6s at baseline | 5 mg | 22:00 (5 h before SOT determined by pretrial sleep log) | 4 weeks | ← SOT and SOFFT ↓ SL ↓ TST (slight) |
| Tzischinsky et al., 1992 [184] |
Case report | To evaluate the influence of the exo-MEL timing in the treatment of sleep disturbances in a blind young man. | DSPD Blind | n=1 18-year-old man |
Oral temperature, actigraphy (SOT, TST, SE), subjective alertness | 5 mg | 20:00 | 3 weeks | ← Temperature acrophase (3h) ← SOT (1h) ↑ TST ↑ daytime alertness |
| Alvarez et al., 1992 [176] | Placebo-controlled | To report a series of 14 subjects with a DSPS seen over five-year period in a sleep disorders clinic, presenting with primary complaint of insomnia | DSPD | N=14 (MEL=8) |
Sleep-log (SOT, SOFFT), alertness self-rating scales plasma MEL, aMT6s | 5 mg | 22:00 (Aprox 5h before SOT= 03:11 ± 0:50) | 4 weeks | ←SOT (82 min) and SOFFT (117 min) |
| Oldani et al., 1994 [177] | Case series | To objectively evaluate the efficacy of exo-MEL with an optimal and proper scheduling, in advancing the sleep wake rhythm in patients with DSPD | DSPD | n=7 males 4 age range 14-46 |
PSG (SOT, SOFFT, TST, SL, WASO, AW, % S1, S2, S3-4, REM, REM_L, SS (number of stage shifts per hour of sleep), | 5 mg | Between 17:00 and 19:00 (9h prior to the SOT =216±61 min after midnight) | 4 weeks | ←SOT (115 min) and SOFFT (106 min) |
| Laurant., 1997 [188] | Single blind placebo-controlled crossover randomized | To investigate the influence of 5 mg MLT on vigilance and cognitive processing speed in DSPS | DSPD | n=24 | Cognition | 5 mg | Not specified | 2 weeks | ↑ Cognitive performance |
| Nagtegaal et al., 1998 [180] |
Double-blind placebo-controlled crossover | To establish the effectiveness of exo-MEL administered to DSPS patients 5 h before their individual DLMO, in advancing the timing of sleep and the circadian rhythms | DSPD | n=30 males 14 age 37.3±15.3 |
DLMO, CBT, PSG (SOL, SOT, REM_L, amount of REM sleep, n° and duration of AW, actual sleep time, SWS duration, actigraphy (SOT, SOL), sleep log (SL. SOT, SOFT, WASO, SE, TST) and subjective sleep quality | 5 mg | 5h before individual DLMO (mean DLMO at baseline: 23:17h (mean timing: 18:17). | 2 weeks | ←DLMO (98 ± 69min) ←SOT ↓SL ↑ MOOD |
| Okawa et al., 1998 [185] | Case series | Not reported | DSPD and NON-24 | 9 DSPD, 2 Non24 n=11 males 8 age range 16-46 |
Sleep log, actigraphy, CBT | 1-3 mg | 0.5 to 2 h before BT | not reported | positive effects from exo-MEL treatment in 6/11 patients (variables measured not reported) |
| Dagan et al., 1998 [187] | -- | To examine the efficiency of exo-MEL treatment in DSPD subjects by means of subjective reports | DSPD | n=61 males 37 age 27.38 ±10.67 |
Survey of exo-MEL effectiveness one year after the treatment | 5 mg | 22:00 | 6 weeks | 96.7% of patients report that exo-MEL treatment was helpful. Relapse was reported with variable timing of appearance. Relapse was associated with more delayed baseline patterns |
| Kamei et al., 2000 [186] | Case series | To investigate the effects of exo-MEL on CRSD and the clinical characteristics of MEL responders | DSPD and NON-24 | DSPD n=30, males 22, age 24±7.6 NON-24 n=16 males 11, age 20.05±7.6 |
Sleep log and wrist actigraphy | 0-3-1 mg | 5,3 and 1 h before BT | not reported | 40% of DSPD patients and 31% of Non 24h patients showed positive effects from exo-MEL treatment (variables measured not reported) |
| Kayumov et al., 2001 [178] |
Double-blind placebo-controlled crossover randomized | to investigate the effect of exo-MEL on DSPS | DSPD | n=22 males 15 age 35.6±14 females 7 age 30.8±12.4 |
PSG (SOL, TST, SE, WASO, Arousal, % of S1, S2, S3, S4, REM, REM_L, REM), aMT6s, SSS, Fatigue, alertness, cognition | 5 mg | 19:00 (week1), 19:00-21:00 (week 2-3), timing chosen by the patient (week 4) | 4 weeks | ↓ SOL, SSS, composite fatigue score circadian pattern of MEL secretion was normalized in 3 of 5 patients with an abnormal circadian pattern at baseline |
| Mundey et al., 2005 [181] | Double-blind placebo-controlled parallel Randomized | To assess the effectiveness of exo-MEL (0.3 or 3.0 mg) to advance circadian and sleep phase with timing ranging between 1.5 and 6.5 h prior to DLMO. | DSPD | n=13 (PLA=4, exo-MEL=9) males 8 age range 23--42 |
DLMO, CBT, Actigraphy and sleep log (SO, SOFFT, TST, SE, SL) | 0.3 or 3.0 mg | 1.5 and 6.5 h before DLMO. (1h earlier in weeks 3 and 4) Baseline mean DLMO: 23:46±1.62 | 4 weeks | ← DLMO and CBT (1.75 ± 0.89 hours, and 1.63 ± 1.79 hours, respectively). |
| Rahman et al., 2010 [189] | Double-blind placebo-controlled Crossover randomized | To investigate the role of exo-MEL as a chronobiotic in ameliorating depressive symptomatology in DSPS patients. | DSPD | n=20 With depressive symptoms n=8, males 5, age 31.5±7.2. Without depressive symptoms n=12, males 8, age 36.2±17.7 |
HDRS-17, CES-D, PSG (SOL, TST, SE, WASO, Arousal index, Alpha score, %S1,S2,S3,S4,REM, SWS 1st cycle, SWS 2nd cycle, REM_L, REM episodes), aMT6s (baseline) | 5 mg | 19:00-21:00 h | 4 weeks | ↓ SOL ↓HDRS-17 and CES-D scores |
| Abbas et al., 2010 [183] | Case report | To describe a clinical case of DSPD treated with exo-MEL. | DSPD | n=1 53-year-old man |
aMT6 and sleep patterns | 3 mg | 20:00 | Regularization of sleep pattern and aMT6 excretion | |
| Sletten et al., 2018 [182] | Double-blind placebo-controlled randomized | To test the efficacy of exo-MEL in DSPD using a pragmatic, clinically relevant protocol that included behavioral sleep-wake scheduling (sleeping at desired or required bedtime). | DSPD | n=104 (MEL n=54, males 28, age 29.9±9.63 PLA n=50, males 25, age 28.8±10.46 |
DLMO, actigraphy and sleep log (TST, SE, WASO, SOT, SOFFT), CGI-S, PSQI, ESS, MEQ, ISI, BDI-II, BAI | 0.5 mg (FR) | 1 hour before DBT | 28 days | ←SOT, ←SOFFT ↑ SE ↑ GCI ↓ Sleep disturbance (PSQI) ↓ ISI, ↓ SOL No significant differences in DLMO between exo-MEL and PLA (exo-MEL: DLMO ← 0.73 ± 1.21 h. PLA: DLMO ← 0.24 ± 1.11 h [difference:0.49 min]) |
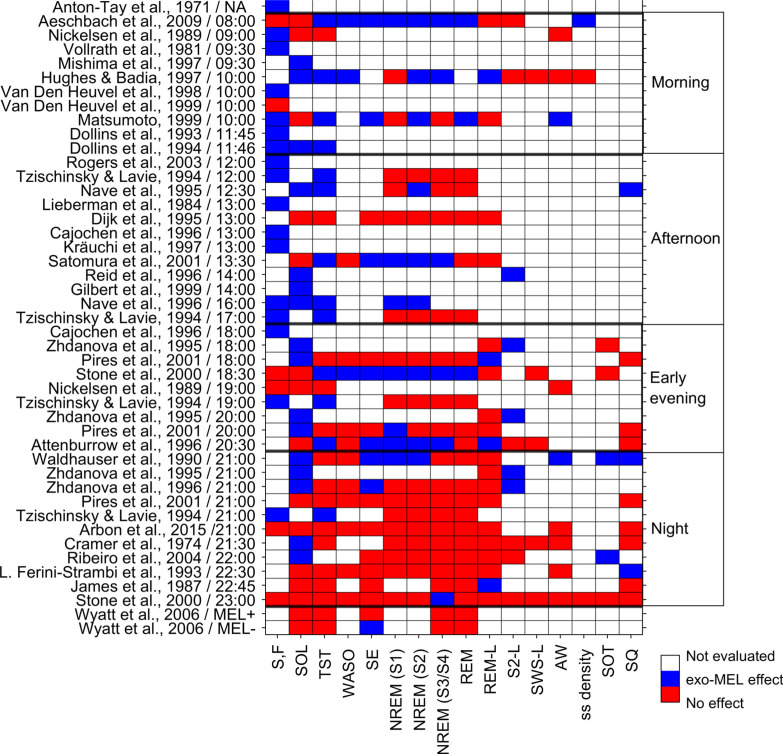
Conventions: ← advance, → delay, ↑ increase, ↓ decrease, * plotted in Fig. (3a).
The investigation of exo-MEL effects on circadian rhythmicity in HS has been approached with a variety of experimental designs in terms of duration of treatments, including single-dose protocols [134, 147-152] and chronic administration of exo-MEL [44, 153-161]. In these studies, exo-MEL has been administered orally at a dose ranging from 0.05 to 8 mg. Only one study included exo-MEL administration as an intravenous infusion (20 µl for three hours) [148]. Even at such low doses, a chronobiotic effect was observed. Although most of the studies referred to measured circadian markers at baseline, generally, they were not used to define the timing of exo-MEL administration. Nevertheless, significant changes in endogenous circadian rhythms in response to exo-MEL were shown in several studies [44, 134, 147-150, 152, 154, 156-161], with only few exceptions. For example, the study of Wright et al., 1986 [153], the first in evaluating the effects of chronic administration of exo-MEL in HS (oral 2 mg at 17:00), found a phase-advance in the endogenous MEL profile in five out of twelve subjects. Even if the phase shift was not significant in the overall sample, the results derived from this study served as a reference for successive studies exploring chronobiotic effects of exo-MEL in humans. Likewise, the study of Attenburrow et al., 1995 comparing the effects of exo-MEL after a single-dose (acute) versus seven-day (subacute) administration found an advance in endogenous MEL only in the subacute condition, suggesting that longer treatment duration may contribute to the phase-shifts [155]. However, other studies using single-dose design were able to identify significant phase shifts [134, 147-150, 152]. In the studies that reported changes in the profile of endogenous MEL, the phase-shift obtained was generally adjusted to the PRC of melatonin, Fig. (3a). Only one study failed to find significant effects of exo-MEL on circadian markers [151]. This study was focused on investigating the phase delay potential of MEL using a modified constant routine (CR) protocol, and showed that, after a single-dose of exo-MEL (5 mg) administered at 07:00 in the morning, none of the circadian markers evaluated were phase-shifted compared to placebo. The authors attributed the lack of a delay effect to the limited one-time point and one dose protocol tested; moreover, the authors argued that possibly “the delay region of a melatonin PRC is less important” due to the natural free-running period >24 h characteristic of humans, which may explain a greater phase advancing action of exo-MEL administered in the evening in comparison to a weaker phase delay effect of exo-MEL at morning.
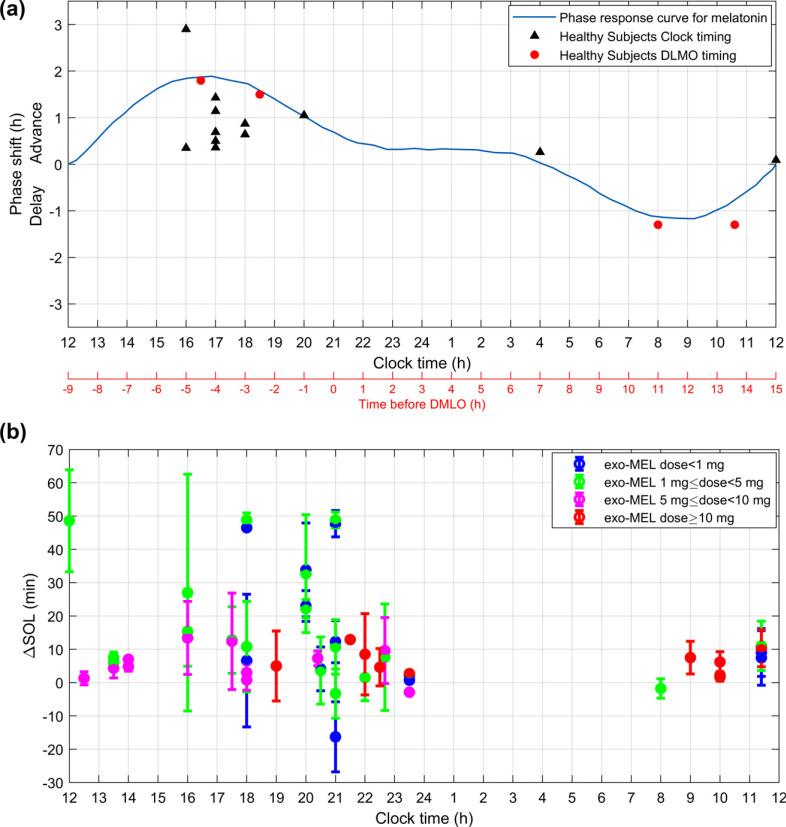
Fig. (3a).
Chronobiotic effect of exogenous melatonin. Studies evaluating the phase-shift effect of exo-MEL on the DLMO in healthy subjects. The results are expressed as the difference (Δ) between the DLMO phase shift induced by the placebo and that induced by exo-MEL. When the Δ phase-shift was not reported directly, the phase shift to exo-MEL reported by each study was corrected by subtracting the phase shift of the placebo condition. Each study Δ phase-shift was plotted against the time of the exo-MEL administration, discriminating by those studies that established the timing of administration based upon the clock-time (black triangles) and those that reported the timing of administration in relation to the baseline DLMO (red circles). Each study contributed one point to the PRC. The studies plotted are described in Appendix Table 1 and highlighted with an asterisk (*). The theoretical PRC of MEL was plotted based on From Eastman CI, Burgess HJ et al. (2009)[190]. Fig. (3b). Sleep-promoting effect of exogenous melatonin. Summary of the results reported by studies evaluating the exo-MEL effect as sleep-promoting as measured through the sleep onset latency (SOL) in healthy subjects. The results are expressed as the difference (Δ) between the SOL induced by the placebo and that induced by exo-MEL. Each study ΔSOL was plotted against the time of the exo-MEL administration. The exo-MEL posology was divided as dose less than 1mg (blue), dose between 1mg and 5 mg (green), a dose between 5 mg and 10mg (magenta), and dose greater than 10mg (red). Each study protocol was plotted separately so that studies using several timings of administration and different dosages were plotted differentially. The studies plotted are described in Appendix Table 2 and highlighted with an asterisk (*).
Regarding the Non-24-hr sleep-wake disorder (or free-running), the first case report of blind free-running patients (BFR) described in the literature showed that exo-MEL contributed to regulating sleep-wake cycle with a partial [162], or absent effect on other circadian markers such as aMT6S, CBT, and cortisol [163, 164]. Instead, significant changes in endogenous circadian rhythms after exo-MEL treatment were reported in both case reports [165] and several case series [165-170], even if the timing of administration of exo-MEL was established without considering the individual circadian rhythm at baseline. In studies where the timing for exo-MEL administration was established based upon the predicted DLMO [171, 172] was also reached entrainment of circadian rhythms. Likewise, clinical reports of patients with free-running without blindness have been described. For example, the group of Siebler et al., 1998 reported the case of a patient with a hypothalamic tumor, who showed stable sleep-wake patterns under treatment with exo-MEL (5 mg at 21:00-22:00 across six years) [173]. Other two cases of free-running patients treated showed that exo-MEL partially changed the SOL and the sleep-wake rhythm [174]. Overall, in most of the studies described, entrainment effects of exo-MEL were observed. Regarding DSPD, clinical evidence has shown that exo-MEL may induce an advance in the sleep-wake cycle when administered at 22:00 (approximately five hours before the habitual sleep onset time: before starting the treatment, participants’ sleep onset time wason average at 03:11 ± 0:50) [175, 176], or when administered between 17:00 and 19:00 (close to nine hours before the baseline sleep onset time) [177]. Likewise, an exo-MEL treatment given at 19:00-21:00 during four weeks induced a regularization of the endogenous MEL secretion in DSPD patients with an abnormal circadian pattern of MEL secretion at the baseline [178]. An advance in DLMO and sleep phase was also obtained when exo-MEL was administered twelve hours after awakening together with a gradual advancement of rising times [179]. Although the studies mentioned did not use the endogenous circadian markers to define the suitable timing for exo-MEL administration, phase shift effects were reported. Likewise, those studies in which the timing for the exo-MEL administration was based on the timing of the endogenous MEL onset also showed exo-MEL efficacy in inducing phase advances when administered five hours before the individual DLMO [180], or between 1.5 and 6.5 hours before the individual DLMO [181]. In the study of Mundey et al., 2005 [181], although the sample size was relatively small and the timing of exo-MEL administration had a broad range, and was progressively anticipated, it was observed that the magnitude of the advance in the DLMO was proportional to the latency between the timing of administration and the individual DLMO, so that the earlier the exo-MEL administration, the greater the phase advance. In a recent study performed in a large sample size [182], although some advance in the DLMO was observed, it was not significant, and this finding might be associated with the timing of exo-MEL administration (1 h before bed time -BT-). However, other markers of clinical improvement were observed, such as an advance in the sleep phase, a decrease in SOL, and an increase in sleep quality (i.e., sleep-promoting effects). In parallel, clinical reports described how the administration of exo-MEL at 20:00 was able to regularize the sleep-wake patterns [183], and advance the temperature acrophase [184]. Other studies reporting case series also suggested positive effects of exo-MEL in DSPD [185,186]. Other findings in DSPD refer to high rates of satisfaction with the exo-MEL treatment (97%) [187], improvements in cognitive performance [188] and reduction of depressive symptoms [189]. In the studies described in this section, the doses used were pretty homogeneous, ranging from 0.3 to 5 mg, suggesting that the efficacy of exo-MEL in DSPD could rely more on the timing of administration than on the dose, as proposed by Mundey et al., 2005 [181].
Unlike DSPS, to the best of our knowledge, clinical evidence concerning the treatment of ASPD and ISWRD with exo-MEL is still lacking. In HS, a few studies suggested a phase delay effect of exo-MEL [44, 148] that could be explored to treat ASPD. More recently, this effect has not been replicated [151], and therefore exo-MEL administration in ASPD patients was not included in clinical recommendations [191]. Similarly, there are no reports regarding the exo-MEL treatment in patients with a diagnosis of ISWRD. So far, there are only a few studies conducted in patients with dementia assessing the effect of exo-MEL on irregular sleep patterns [192, 193]. Such studies did not report diagnostic criteria for ISWRD, nor measured outcomes to evaluate a potential chronobiotic effect of exo-MEL. Therefore, no clinical recommendation for treating ISWRD with exo-MEL has been outlined [191].
7.2. Sleep-inducing and Sleep-promoting Effect of Exo-MEL
A sleep-inducing drug ideally should lead to sleep initiation in a necessary and sufficient manner, as discussed in Section 4.2. According to this definition, the clinical parameter closely describing a sleep-inducing effect is the reduction in sleep onset latency (SOL), Fig. (3b). Moreover, the sleep initiation effect may be measured through other outcomes such as the reduction of the latency to Stage 2 NREM, and the induction of acute sleepiness. On the other hand, the sleep-maintenance effect might be identified as the decrease in WASO and the increase in TST and SE [194]. Other variables have also been reported in the studies evaluating the effects of exo-MEL on sleep, such as the sleep onset time, the sleep offset time, several EEG parameters, and the self-reported sleep quality. The daytime function, sleep inertia, sleep propensity, and quality of life have been evaluated mainly in insomnia as measurements of the perceived improvement in sleep (Fig. (4) and Appendix A, Table 2).
Fig. (4).
Summary of the results reported in studies evaluating the sleep-inducing and the sleep-promoting effect of exogenous melatonin in healthy subjects. Studies are organized as a function of the timing of administration (morning, afternoon, early evening, night). MEL+ refers to sleep episodes scheduled when endogenous melatonin levels were elevated, while MEL- refers to sleep episodes scheduled when endogenous melatonin levels were low to absent. Sleepiness refers to the acute effects of exo-MEL. Abbreviations: S: sleepiness; F: fatigue, SOL, sleep onset latency; TST, total sleep time; WASO, waking after sleep onset; SE, sleep efficiency; REM, rapid eye movements sleep; S1, Stage 1; S2, Stage 2; S3, Stage 3; S4, Stage 4, SWS, slow wave sleep; AW: awakenings; ss: sleep spindles; SOT: sleep onset time; SQ: sleep quality. Cells in blue indicate that exogenous melatonin had a significant effect on the evaluated variable, cells in red indicate the absence of melatonin-induced effect on the evaluated variable.
Table 2. Sleep-promoting effect of exogenous melatonin.
| Author |
Study
Design |
Aim | DX | Subjects |
Variables
Measured |
Dose | Timing | Duration | General Results |
|---|---|---|---|---|---|---|---|---|---|
| Antón-Tay et al., 1971 [8] |
Case series | To study the effects of exo-Mel administration to humans | HS | n=11 males 5 age 18-25 |
EEG Subjective sleepiness |
0.25 mg/K, 1.25 mg/KG (iv) | Not specified | Single dose | EEG activity slight deactivation, ↑ % and amplitude of alpha rhythm, Sleepiness, wellbeing |
| MORNING ADMINISTRATION | |||||||||
| Vollrath et al., 1981 [5] | Double-blind placebo-controlled crossover | To evaluate the effects of exo-MEL at “low dosages using as form of application nasal spray (ns) | HS | n=10 males 6, females 4 age 28.8 and 26.5 respectively |
Subjective sleepiness | 1.7 mg (ns) | 9:00-10:00 | single dose | ↑ Sleepiness (9/10 subjects fell asleep) |
| Dollins et al., 1993 [196] |
Double-blind placebo-controlled Latin square design |
To determine whether lower acute doses of melatonin than have previously been investigated have sedative-like effects on behavior and if such effects are dose-related. | HS | n=20 males 20 age 25 ± 1.47 (range 19-39) |
MEL levels, oral temperature, cognition, SSS, POMS | 10mg, 20mg, 40mg, or 80mg | 11:45 | single dose | All doses: ↓ Oral temperature, correct responses in auditory vigilance and self-reported vigor, ↑ response latency, self-reported fatigue, confusion, sleepiness |
| Dollins et al., 1994 [89] * | Double-blind placebo-controlled Latin square design |
To evaluate if exo-MEL at physiological doses may induce short-term behavioral effects. | HS | n=20 males 20 age 23.05±4.22 (range 18-24) |
MEL levels, oral temperature, TST SSS, SOL, cognition, POMS, | 0.1mg,0.3mg, 1.0mg, 10 mg | 11:45 | single dose | All doses= ↓ SOL, subjective vigor, ↑ TST, Sleepiness, self-reported fatigue. Doses of 1.0-10 mg= ↓ oral temperature, correct responses in auditory vigilance and reaction time |
| Mishima et al., 1997 [198] * | Single-blind randomized crossover | To study acute hypnotic and hypothermic effects induced by daytime administration of exo-MEL with simultaneous monitoring of serum MEL concentration | HS | n= 6 males 6 age 22.5±19 |
CBT, SL (measured during a MSLT), serum MEL concentration | 3mg or 9mg | 09:30 | single dose | ↓ CBT (3 mg, 9 mg) ↓ SL (9 mg) |
| Hughes & Badia, 1997 [9] * | Double-blind placebo-controlled crossover | To investigate the ability of three different doses of melatonin (1 mg, 10 mg, and 40 mg) to promote sleep in a moderate-duration daytime sleep opportunity. | HS | n=8 males 8 age range 18-30 |
CBT, PSG (SL, SWS_L, REM_L, TST, WASO, % of S1, S2. S3, S4 and REM, ss density, movement time, wake time, AW), pulse rate, blood pressure, cognition | 1 mg, 10 mg 40 mg |
10:00 | Single dose (each treatment) | All dosages: ↓ SL, Stage 3-4, WASO and CBT, ↑ Stage 2, TST. Doses of 10 mg and 40 mg: ↓REM latency, ↑ latency to stage 3-4, TST. |
| Van Den Heuvel et al., 1998 [197] | Double-blind placebo-controlled counterbalanced | To evaluate the effects of daytime melatonin infusion in young adults | HS | n= 24 males 12 age range 19-28 (8 subjects for dose) |
Subjective sleepiness, central and peripheric temperature | Iv 0.04, 0.08, 8.00 μg h-1 kg-1 | 10:00 | Single dose (each treatment) | High dose (supraphysiological): ↑ hand temperature, sleepiness ↓ raising of central temperature |
| Van Den Heuvel et al., 1999 [19] | Double-blind randomized placebo-controlled | To exam the effects of exo-MEL levels on body temperatures and sleepiness when administered with a rapid systemic onset. | HS | n = 8 males 4 age 23.9 ±0.7 |
Subjective sleepiness, central and peripheric temperature | iv 3, 10, and 30 μg | 10:00 | Single dose (each treatment) | All doses (physiological levels): ↑ hand temperature ↓ raising of central temperature |
| Matsumoto, 1999 [10]* | Single-blind crossover | To evaluate the effects of 10mg MEL administered at 10:00 on a diurnal sleep episode (from 11:00 to 19:00 following a full night of sleep. | HS | n=6 males 6 age 23.7±1.3 |
PSG (sleep period time, SL, TST, SE, AW, % and duration of W, S1, S2, S3, S4, REM. Wake time, REM_L, N° REM periods, REM period and cycle length) CBT, Sleepiness | 10 mg | 10:00 | single dose | ↑ TST, SE, Sleepiness, %, and duration of REM and S2. ↓ AW, % and duration of W. No changes in CBT |
| Aeschbach, D.,, 2009 [199] * | Double-blind placebo-controlled and crossover | to test an experimental skin patch designed to deliver melatonin such that plasma levels steadily increase for 6-8 h, and thereby counteract the increasing circadian wake drive and improve daytime sleep. | HS | n=8 males 4 age 27.8±3.6, (range 24-34) |
EEG, (TST, SE, SL, SL_REM, WASO, S1, S2, SWS, REM sleep), EEG spectra, plasma MEL, CBT, sleepiness (KSS) and cognition | 2.1 mg (transdermal) | 08:00 | single dose | ↓ CBT and WASO ↑ REM sleep, SE, TST, S2. Spectral analysis: ↓ density in 2.5-6.5 Hz, ↑ density in 13.25-13.5 HZ. |
| Lieberman et al., 1984 [6] | Double-blind counterbalanced | To examine the ability of oral melatonin, given during the daylight hours in pharmacological quantities, to modify various aspects of behavior | HS | n=14 males 14 age range 18-45 |
POMS, SSS, Cognition | 240 mg (three doses of 80 mg) | Split dose at 12.00, 13.00, 14.00 | single dose | ↑ Fatigue, confusion, Sleepiness, response time ↓ Vigor, number of errors in cognitive tests |
| Dijk et al., 1995 [202] * |
Placebo-controlled crossover | To document the immediate and vigilance state-specific effects of exo-MEL on EEG activity during daytime sleep. | HS | n = 8 males 6 age 22.4 (range 20-26) |
PSG (SL, TST, SE, REM_L, percentage of S1, S2, SWS, REM, and spectral analysis. | 5 mg | 12:30 | Single dose | Spectral analysis: ↑ 13.75-14.0 Hz, ↓ 15.25-16.5 Hz and 2.2.5-5.0 Hz (In the first 2 h). No changes in sleep architecture, SL or SE |
| Nave et al., 1995 [11]* | Double-blind balanced Latin square design | To investigate the effects of exo-MEL on napping during early evening hours (before the nocturnal rise in endogenous melatonin when sleep propensity is low) | HS | n= 20 age 24.6 ± 2.7 |
PSG (SL, TST, S1, S2, S3/S4, REM) | 3mg, 6 mg | 16:00- 17:30 | Single dose by protocol | ↑ TST, S2, SQ ↓ SL |
| Nave et al., 1996 [131] | Double-blind, randomized partially repeated Latin square design. |
To investigate whether 10 mg of flumazenil (BDZ antagonist) can block the hypothermic effects of 3 mg melatonin | HS | n=6 males 6 age 24.5±0.9 |
PSG (SL, TST S1, S2), spectral analysis, CBT, Sleepiness | 3mg | 12:00 | Single dose | ↑ S1, S2, TST and power density in frequency bands: 1-3Hz and 4-7 Hz, and sleepiness ↓ SL and CBT (BDZ antagonist does not interfere with the MEL effects) |
| Reid et al., 1996 [12]* | Double-blind placebo-controlled, crossover | To explore the relationship between hypothermic and hypnotic effects of melatonin | HS | n=16 males 6 age 20.3±2.4 |
PSG (SOL1, SOL2) CBT | 5 mg | 14:00 | single dose | ↓ CBT, SOL1, SOL2 |
| Cajochen et al., 1996 [201] | Double-blind crossover | To evaluate the acute effect of exo-MEL on EEG power density during waking. | HS | n = 8, males 8 Experiment 1: age 27 ± 4, Experiment 2: age 24.8 ± 3.5 | Sleepiness (VAS, Akerstedt Sleepiness Scale), waking EEG | 5 mg | Exp 1: 18:00, Exp 2: 13:00 | sigle-dose | ↑ Sleepiness and 5.25-9 Hz density. No changes in SWS, sleep spindle and beta band |
| Kräuchi, Cajochen, & Wirz-Justice, 1997 [135] * | Double-blind placebo-controlled crossover | Both MEL and postural changes have thermoregulator sequelae. To evaluate their relationship to subjective sleepiness. | HS | n = 8 males 8 age 25±4 range 21-31 |
Waking EEG, Temperature (superficial and central), sleepiness (VAS), heart rate | 5 mg | 13:00 | single dose | ↑ Subjective Sleepiness, distal temperature, 5.25-9 HZ power density in the waking EEG ↓ central temperature |
| Gilbert et al., 1999 [13]* | Double-blind counterbalanced | compared the thermoregulatory and soporific effects of temazepam with those of melatonin. |
HS | n =20 males 13 females 7 age 23.5 ± 0.4 |
CBT, peripheral temperature, PSG (SL), heart rate | 5 mg | 14:00 | Single session | ↓ SL and CTB (with both exo-MEL and tamazepam). Temporal relationship between minimum SL and the maximum rate of decline in CBT |
| Satomura et al., 2001 [137] * | Single-blind randomized | To assess the hypnotic action by administering exo-MEL during the day | HS | n =7 males 7 age 23.7±1.7 |
EEG (SL, TST, SE, WASO, REM_L, S1, S2), CBT | 1,3,6 mg | 13:30 | single dose | ↑ TST, SE, S2 (1mg, 3mg, 6 mg) ↓ CBT (1mg,3mg) |
| Rogers et al., 2003 [200] | Double-blind randomized crossover | To compare neurobehavioural performance effects following the daytime administration of exo-MEL and temazepam | HS | n= 16 males 6 females 10 age 21.4 ± 0.6 |
Cognition, sleepiness (VAS) | 5 mg | 12:00 | single -dose | ↑ Sleepiness, ↓ performance in unpredictable tracking, spatial memory and vigilance tasks. |
| Cramer et al., 1974 [203] * | Placebo-controlled crossover | To study the effects of MEL in healthy young male volunteers | HS | n=15 males 15 |
PSG (SOL, S3_L, REM_L, NREM_L. TST, % of S1, S2, S3/4, REM, sleep stages), refreshing sleep (MMQ), mental state, 5-HIAA, adenosine 3’,5’monophosphate | 50 mg iv | 21:30 | single dose | ↓SOL |
| James et al., 1987 [18]* |
Double-blind placebo-controlled randomized | To report the findings of the acute effects of orally administered exo-MEL in HS | HS | n=10 males 7 females 3 age 29.9 |
PSG (SOL, SE, REM_L, TST, duration and % of Delta and REM), DSQ, sleepiness (SSS), anxiety, and mood | 1mg, 5mg | 22:45 | Single dose | ↑ REM_L in the 5mg condition compared to 1mg and placebo condition. |
| Waldhauser et al., 1990 [204] | Double-blind placebo-controlled parallel group | To determine the effects of exo-MEL on sleep induction, sleep maintenance, sleep architecture and objective and subjective awakening quality in induced insomnia | HS | n=20 males 10 females 10 age 26.4 ± 4.8 |
PSG (Awake till sleep onset, SL, TSP, TST, SE, AW, WASO, REM_L, stage shifts), sleep quality, awakening quality and somatic complaints, cognition, mood, drowsiness. | 80 mg | 21:00 | Single dose | ↓ SL, N° awakenings, S1. ↑ SE, S2, cognition and alertness (morning after MEL treatment) |
| Ferini-Strambi et al., 1993 [17] * | Single-blind placebo controlled | To compare the effects of exo-MEL with those of a benzodiazepine hypnotic [triazolam (TRI) 0.125 mg] at a low dose in healthy volunteers. |
HS | n=6 males 6 age 25.3 ± 3.6 |
PSG (TST, SL, WASO, AW, SE, Sleep stages %, REM-L, REM periods, stage shifts/hour CAP variables), DSQ, SQ | 100 mg | 22:30 | single dose | No changes in classical PSG variables ↑ sleep quality. MEL, TRI and MEL + TRI = ↓ CAP cycles, MEL in comparison with baseline= longer Phase B (lesser arousal). |
| Zhdanova et al., 1995 [14]* | Double-blind placebo-controlled counterbalanced | To examine the induction of PSG recorded sleep by MEL given later in the evening close to the times of endogenous melatonin release and habitual sleep onset. | HS | n=6 males 6 age 26.5 ± 1.3 |
PSG (SOL, S2_L, REM_L), | 0.3 or 1.0 mg | 18:00, 20:00, 21:00 | 3 sessions by timing | ↓ SOL, S2_L (all doses and all time points). |
| Zhdanova et al., 1996 [15]* | Double-blind placebo-controlled counterbalanced | To characterize the effects of augmented circulating MEL levels within the physiologic nocturnal range on PSG parameters | HS | n=12 males 12 age 28.5±1.8 |
PSG (W, S1, S2, S3, S4, REM, SOL, REM_L, S2_L, WASO, SE, TST), Sleepiness (SSS), cognition and mood (POMS) | 0.3, 1.0 mg | 21:00 (2-4 h before sleep) | 3 sessions | ↓ SOL, S2L ↑ SE No effect on other sleep parameters, no effects on cognition and sleepiness the day after exo-MEL treatment. |
| Attenburrow et al., 1996 [16] * |
Double-blind placebo-controlled crossover | To investigate the effect of exo-MEL on PSG measured sleep in normal, middle-aged volunteers when administered 2 hours before the habitual sleep time | HS | n=15 males 4 age 53.9 range 41-67 |
PSG (TIB, TSP, actual sleep time, SE, SOL, WASO, total movement time, S2_L, SWS_L, S1, S2, SWS, NREM, REM, REM_L), LSEQ | 0.3 mg 1.0 mg | 2 hours before BT | Single dose | 1 mg = ↑ TST, SE, NREM, REM_L |
| Stone et al., 2000 [208] * | Double-blind placebo-controlled crossover | To establish the effect of melatonin upon nocturnal and evening sleep. | HS | Exp1. n= 8 males 8 age 23.4 (range 20-30) Exp 2. n= 6 males 6, age 26.5 (range 21-31) |
EEG (TST, SE, SOL, REM/non-REM ratio, L_SWS, L_REM, REM periods, AW, stage shifts, Awake (min), Duration and % of S1,S2, S3+4, REM, Awake), SQ, ESS, CBT,DLMO (acute effect), Cognition | 0.1mg, 0.5mg, 1.0mg, 5.0mg, 10 mg | 18:30 and 23:30 | single dose | Exo-MEL at 18:00 (all doses) =↑TST, SE, S2, REM periods, stage shifts ↓ WA Exo-MEL at 23:30 = ↓ S3 (5mg), ↓CBT (0.1 mg), ↑ SL |
| Pires et al., 2001 [207]* | Double-blind placebo-controlled | to evaluate the acute effects of single low doses of exo-MEL given to HS in the evening. | HS | n=6 males 6 age range 22-24 |
PSG (TST, SL, SE, WASO, percentage of S1, S2, S3/4, REM, REM_L), SQ, POMS, SSS, Cognition | 0.3 or 1.0 mg | 18:00, 20:00, 21:00 | single dose | ↓ SL, (18:00 and 20:00), ↑ SL (21:00) ↓ S1 (20: 00) ↑REM_L (0.3 mg, 18:00) ↑ SL (0.3mg, 21:00) |
| Ribeiro et al., 2004 [205] * | placebo-controlled | to evaluate SOT in HS taking exo-MEL using 2 different criteria of sleep latency: the Rechtschaffen and Kales and the 10 minutes of uninterrupted sleep | HS | n=45, males 45 age 28 ± 5 (PLA n=10 MEL n=30) |
PSG (SL, SE, sleep architecture, REM_L and density). SL evaluated through the Rechtschaffen and Kales criteria (1.5 minutes of S1) and the 10 minutes of uninterrupted sleep criteria) | 10 mg | 1 h before BT | 28 days | ↓ SL (using the 10 min of uninterrupted sleep criteria) Advance in SOT |
| Arbon et al., 2015 [206] * |
Double-blind, placebo-controlled, randomized crossover | To study the effects of pro-longed-release exo-MEL, temazepam and zolpidem on the spectral composition of the EEG during nocturnal sleep in healthy middle-aged men and women. | HS | N = 16 Males 12 Age 58.8 ± 2.9 |
PSG (SOL, REM_L, duration of S1, S2, S3, S4, NREM, REM, TST, SPT, SE, WASO, AW), spectral analysis Plasma melatonin and aMT6S (acute), SQQ, KSS | 2 mg (PR) | 21:00 (2 h before BT) | Single dose (each treatment) | Exo-MEL = Minor reduction in SWA in the first third of the night. No changes in PSG measures compared to PLA. No effect on SQQ or KSS |
| MULTIPLE TIMING | |||||||||
| Nickelsen et al., 1989 [7] * | Double-blind placebo-controlled | To study the sedative potency of high doses of exo-MEL at different times of the day. | HS | n=25 males 14 age 30.4±6.2 females 11 age 30±7.9 |
Sleepiness (SSS), sedative effect perception, sleep-log (SOL, TST, AW) | 50 mg | 19.00 or 9:00 | Single dose | ↑ Tiredness and sedating perception only in morning administration |
| Tzischinsky & Lavie, 1994 [210] |
Double-blind placebo-controlled crossover | To evaluate the hypnotic effect of exo-MEL using an ultrashort sleep-wake paradigm | HS | n=8 males 8 age 27.06±3.7 |
PSG (sleep propensity[sp]) = averaging total sleep time (sum of stages 1, 2, 3/4 and REM sleep) at each of the 72 trials) spectral analysis, Temperature, Sleepiness (VAS). |
5 mg | 12:00, 17:00, 19:00, 21:00 | Single dose (each treatment) | ↑ SP time-dependent: 12 h = ↑ midafternoon sp (peak at 16 h) and delayed the nocturnal increase in sp from 22 to 24h. 17 h = ↑ sp (peak:19h) 19:00 h = ↑ sp (peak at 21h) 21h = faster ↑ sp (peak at 22h) All trials = ↑ Subjective sleepiness, ↓ T° at 12,17,19 h |
| Wyatt et al., 2006 [209] | Double-blind placebo-controlled parallel-group | To investigate the effects of a physiologic and pharmacologic dose of exo-MEL on SL and SE in sleep episodes initiated across a full range of circadian phases. | HS | n=36 males 21 age range 18-30 |
EEG (SE, TST, Latency to consolidated sleep -LCS-, REM % and SWS%), CBT, Plasma MEL profile. | 0.3 or 5.0 mg | 30 min before 6.67h sleep period during forced desynchrony | 21 days | ↑ SE during the sleep episodes out of endogenous MEL release, no changes in SL, LCS, CBT, % of SWS or REM sleep. |
| INSOMNIA | |||||||||
| James et al., 1990 [33] |
Double-blind placebo-controlled crossover | To report the finding of the acute effects of orally administered exo-MEL at bedtime in patients with persistent complaints of insomnia | Initiating or maintaining sleep Disorder | n=10, males 4 age= 33.4 (range 20-57) | PSG (REM_L, REM period, duration and % of REM, TST, SE, SWS, % of movement time, SOT), subjective sleepiness (SSS), SQ, DSQ, mood-rating scales | 1mg or 5mg | 22:45 | 1 session | ↑REM latency (1 mg), and SQ |
| MacFarlane et al., 1991 [211] |
Single Blind placebo-controlled crossover | To determine the effectiveness of consecutive single evening oral doses of exo-MEL in the initiation and maintenance of sleep /chronic insomnia. | Chronic Insomnia | n=13 males 8 age range 25-65 |
Subjective TST, awareness of MEL vs. Placebo, alertness |
75 mg | 22:00 | 1 week | ↑TST and Subjective alertness |
| Haimov et al., 1995 [20] | Double-blind placebo-controlled crossover | To investigate the effects of exo-MEL replacement therapy on MEL-deficient elderly insomniacs. | Chronic Insomnia | Independently living n= 8, males 4, age 73.1±3.9 Institutionalized n= 18, males 6, age 81.1±8.9 |
Actigraphic TST, SE, SL, Mean activity level | 2mg, PR (P-1w) FR (F-1w) |
2 h before the desired bedtime | 1 week-2 month | ↑ SE and ↓Mean level activity in PR-MEL vs. placebo (after 2 months of treatment). ↓ SL and ↑ sleep maintenance in FR-MEL and PR-MEL |
| Ellis et al., 1996 [30] | Double-blind placebo-controlled crossover | To evaluate the hypnotic effect of exo-MEL in subjects with psychophysiological insomnia | psychophysiological Insomnia | n= 15 males 9 age 46±11 range 32-67 |
sleep log (BT, SOT, first wake, final wake, get up time, TST, time wake time in bed, SE), SQ, subjective sleep duration, daytime sleepiness, mood | 5 mg | 20:00 | 1 week | No changes |
| Lushington et al., 1997 [29] | Double-blind placebo-controlled, crossover | to explore the short-term effects of exo-MEL on CBT, SL and subjective vigor and affect in aged women. | Sleep maintenance Insomnia | Insomnia patients n=20 HS n= 10 All females age 65.2±7.4 |
CBT, PSG (focused on SL to S1 and S2), sleepiness and mood (VAS) | 5 mg | 14:00 | single dose | ↓ CBT, SL (stage 1 and 2) in both groups |
| Hughes et al., 1998 [21] | Double-blind placebo-controlled crossover | To assess the sleep-promoting effect of exo-MEL replacement delivery strategies in age-related sleep-maintenance insomnia. | Age-related sleep maintenance insomnia | n=14 males 5 age 70.29±1.8 |
DLMO, CBT, PSG (TST, SE, WASO, SL, TBT, SPT, WT, amount of sleep S1, S2, S3, S4 and REM, REM_L) Actigraphy (TST, SE. WASO, SL), sleep log, SQ, POMS, sleepiness and fatigue (VAS) | 0.5 mg PR and IR |
Early: IR 30m before BT Continuous: PR 30m before BT, Late: IR 4h after BT Placebo: 30m before BT 4h after BT |
Four two- week trials with two weeks of washout | ↓ SOL (PSG) ↓ CBT No changes in TST, WASO, SE, other PSG parameters, nor in subjective measurements or in DLMO profiles |
| Dawson et al., 1998 [31] | Double-blind placebo-controlled crossover | To determine whether nocturnal exo-MEL replacement, using a transbuccal delivery system, would lower core temperature and improve sleep in insomniac patients | Sleep maintenance insomnia ICSD criteria | n=12 age 65.67 ±1.68 |
PSG (TST, SOL, REM_L, EMA, percentage time awake, SE, Stage changes, SOT, WASO), spectral analysis, body temperature, aMT6S | 0.5 mg transbuccal patch | 19:00 | 4 consecutive nights | ↑ aMT.6S ↑ WASO in the first half of the night on night 4 and in whole night on night 3. ↓ Body temperature |
| Zhdanova et al., 2001 [22] | Double-blind placebo-controlled randomized | To examine the ability of similar, physiological exo-MEL doses to restore nighttime MEL levels and SE in insomniac subjects over 50 years old. | Chronic Insomnia | Patients n=15 age >50 HS n=15 age >50 |
PSG (SOL, TST, SE, WASO, AW, duration of S1, S2, S3, S4 and REM, REM_L, SWS_L), CBT, MEL | 0.1 mg, 0.3 mg, 3.0 mg |
30 min before BT | 1 week | ↑ SE (3 doses) only in insomnia patients ↓CBT (3mg) |
| Almeida Montes et al., 2003 [32] | Double-blind placebo-controlled crossover | To assess the hypnotic effect of exo-MEL in primary insomnia. | Primary Insomnia DSM-IV criteria | n=10 males 6 age 50±12.7 range 30-72 |
PSG (duration and latency of S1, S2, S3, REM, AW, TST, average length of NREM-REM cycle and SE), subjective quality and amount of sleep, Sleep log | 0.3mg, 1.0 mg PR | 1h before BT | 1 week for each treatment | No changes |
| Baskett et al., 2003 [213] | Double-blind placebo controlled randomized crossover | To determine whether exo-MEL will improve quality of sleep in healthy older people with age-related sleep maintenance problems. |
HS (normal vs. problem sleepers) |
Normal sleepers= n=20 Problem sleepers n=20 Age 71.7 ± 4.9 range 65-84 |
PSQI, LSEQ, sleep log (diary awakenings, quality scale, alertness scale), actigraphy (TIB, Sleep time, SL, AW, SE) | 5 mg | BT | 4 weeks | No changes in outcomes of interest. ↓ AW in normal sleepers |
| Leger et al., 2004 [37] | Placebo-controlled crossover | To examine the excretion of aMT6s in insomnia patients aged 55 years and its relation with the subsequent response to exo-MEL therapy. | Primary Insomnia DSM-IV criteria | n=396 males 330 age 68 ± 8 range 55-93 |
aMT6s, LSEQ domains: GTS, SQ, AFS, BFW | 2 mg | 21:00-23:00 | 3 weeks | Proportion of response in “Low excretors” response to MEL (58% [65/112] was major than in “normal excretors” 47% [122/260] in SQ and BFW |
| Wade et al., 2007 [23] | Double-blind placebo-controlled randomized parallel |
To evaluate the efficacy and safety of a prolonged-release exo-MEL formulation (PR-melatonin; Circadin 2mg) in insomnia patients aged 55 years and older. | Primary Insomnia based on the Sleep History Questionnaire (SHQ) | n = 334 (MEL= 169, PLA = 165) males 113 age aged: 65.7 ± 6.4 |
LSEQ (GTS, SQ, AFS, BFW), PSQI, subjective quality of day and quality of life, CGI, WHO-5 | 2 mg PR | 2 hours before bedtime | 3 weeks | ↑ SQ, BFW, WHO-5. ↓ SL (24.3 min vs. 12.9 min), measured through the PSQI |
| Lemoine et al., 2007 [24] | multi-center randomized placebo-controlled parallel group | To assess the efficacy and safety of exo-MEL in improving sleep quality and morning alertness in insomnia patients 55 years and older | Primary Insomnia DSM-IV criteria | n=170 males 58 age 55-93 MEL-PR n=82 PLA n=88 |
LSEQ, QON, QOD, BWSQ | 2mg PR | 1-2 h before BT (between 21:00-22.00) | 3 weeks | ↑ SQ, BFW, QON. No withdrawal symptoms |
| Luthringer et al., 2009 [25] | Double-blind, placebo-controlled, parallel group | To investigate the effects of PR exo-MEL 2mg on sleep and subsequent daytime psychomotor performance | Primary Insomnia DSM-IV criteria | n=40 males 24 MEL (n=20) Males 13 (65%) Age 59.6 ± 2.9 PLA (n=20) Males 11 (55%) Age 61.9± 4.8 |
PSG (SOL, TST, REM, NREM, SE, Number of awakenings, WASO, duration and % of sleep stages REM, NREM, SWS, REM_L, spectral analysis), LSEQ, subjective improvement SQ (VAS), cognition | 2 mg PR | 2 h before BT (between 21:00 and 23:00) |
3 weeks | ↓SOL, ↑ SQ and cognitive performance |
| Garzón et al., 2009 [28] | Prospective, randomized, double-blind, placebo-controlled, crossover |
to evaluate the effect of melatonin administration on sleep and behavioral disorders in the elderly and the facilitation of the discontinuation of regular hypnotic drugs. |
Primary Insomnia DSM-IV criteria or transient sleep disorder | n=22, males 7, age 75.8, females 15 age 74.3 | NHSMI, GDS, GAS | 5 mg | BT (aprox 23:00) | 8 weeks | ↑ SQ, nine out of 14 subjects receiving hypnotic drugs were able to discontinue this treatment during melatonin but not placebo administration. |
| Wade et al., 2010 [26] | Double-blind placebo-controlled randomized parallel group | To investigate whether exo-MEL PR efficacy is related to low endogenous MEL levels or age. And to investigate the maintenance of efficacy and the safety of exo-MEL PR beyond the acute treatment. | Primary Insomnia (DSM-IV criteria) | n=746 MEL=373 Age>65 (143) Low excretors (88) PLA=373 Age>65 (150) Low excretors (90) |
SL, PSQI, CGI, WHO-5 | 2 mg PR | 2 before BT (between 21:00 and 22:00) | Short-term: 3 weeks Long-term: 26 weeks |
↓ SL (in elderly regardless of the endogenous MEL) Low excretors: aged 18-80 years ↓ PSQIc5; ↑WHO5 Age>65 ↓ PSQI_c2, ↑sleep maintenance and PSQI total score. The effects were maintained over the 6 months protocol. |
| Wade et al., 2011 [27] | Double-blind placebo-controlled randomized | To evaluate the age cut-off for response to exo-MEL PR and the long-term maintenance of efficacy and safety in subsets of patients aged 18-54 and 55-80 years. | Primary Insomnia, DSM-IV criteria | n=722 age 18-54 (n=144) age 55-80 (n=578). |
SL, PSQI, CGI, WHO-5 | 2 mg PR | 2 h before bedtime | 3 weeks-24 weeks | ↓SL (sleep diary) (55-80 range), ↓ SL (PSQI_c2), PSQI total score (whole sample) ↑WHO-5, CGI (whole sample) |
| Chung et al., 2016 [214] |
Retrospective | To investigate patients’ satisfaction rate with exo-MEL PR when they took it after their sleep-wake cycle was set. | Primary Insomnia ICSD criteria 2° edition | n=44 males 27 age 65.9 ± 8.6 |
sleep indices extracted from interview (BT, SOT, SOFFT, SL, TIB) at baseline, satisfaction with treatment | 2 mg PR | 2 h before BT | 4 weeks | Satisfaction with MEL-PR use in 66% of patients. reduction of sleeping pill dosage by at least 50% in 44% of patients. Complete discontinuation of previous sleeping pills in 20% of patients. No significant differences in sleep indices between patients satisfied vs. dissatisfied |
| Xu et al., 2020 [212] | Double-blind placebo-controlled randomized parallel |
To determine the efficacy of exo-MEL for sleep disturbances in patients with middle-aged primary insomnia | Primary insomnia, DSM-IV criteria | MEL (n=29) Males 12 (41.38%) Age 57.24 ± 5.59 PLA (n=32) Males 17 (53.13%) Age 56.53± 4.65 |
PSG (SL, REM_L, TST, SE, Micro-arousal index, WASO, Early wake (min), % of NREM S1, S2, S3, REM), PSQI, ISI, ESS, | 3 mg IR | 1 h before BT | 4 weeks | ↓ Early wake time, S2 sleep |
Conventions: ← advance, → delay, ↑ increase, ↓ decrease, * plotted in Fig. (3b).
As highlighted in the section 5.1 for exo-MEL chronobiotic effect, among the factors that may modify also the sleep-inducing and sleep-promoting effects of exo-MEL, the timing of administration seems the most relevant [195], as summarized in Fig. (3b). When exo-MEL is given in the morning (before noon), it may increase subjective sleepiness at both supraphysiological doses [5, 10, 196, 197] and low doses (< 1 mg) [89]. Also, SOL objectively measured may decrease after exo-MEL administration in the morning [9, 89, 198]. However, a minority of studies did not replicate such findings [19, 199], interpreting this discrepancy in view of the low dose of exo-MEL (3,10,30 µg in a 1-ml intravenous 0.9% saline injection) used [19]. The authors concluded that the soporific effects of exo-MEL could be reached only through supraphysiological doses. However, after administering physiological doses (0.1 mg) of exo-MEL, other authors reported a decrease in SOL, as well as increased subjective sleepiness and TST [89]. An increase in TST, together with an increase in SE and a decrease in WASO, but without an increase in sleepiness, was instead reported by Aeschbach et al., [199]. The authors used a transdermic administration pathway (skin patch). Their findings provide evidence about the effect of slow-release formulations on sleep maintenance. The effects of exo-MEL on sleep maintenance were also described using an immediate release formulation in other studies [9, 10].
When exo-MEL is given in the afternoon (between 12:00 h and 18:00 h), it may increase sleep propensity both at very high (240 mg) [6] and medium doses (3-6 mg) of oral supplementation [11, 200, 201]. Moreover, the sleep-promoting effects have been demonstrated either in conjunction with the hypothermic action [12, 13, 131, 135], or independent of it [137]. Only in one study [202], the effect of exo-MEL on SOL was not significantly different from the placebo. This result could be explained by the sleep deprivation condition involved in the experimental design, which may have induced a decrease in SOL, irrespective of the treatment condition.
Finally, with the administration of exo-MEL at early evening or night (after 18:00 h), sleep-promoting effects, as reflected by reductions in SOL, were described for high, medium and low doses: e.g., 50 mg administered intravenously in an unspecified solution volume [203] or 80 mg administered orally [204], and at lower doses (oral supplementation between 0.3 mg - 10 mg) [14, 15, 205]. Also, positive effects on sleep maintenance variables, such as the increase in SE and TST, were obtained after administering orally 1mg of exo-MEL [16]. However, other studies did not find an effect on sleep propensity after exo-MEL administration at low-medium doses (1 mg - 5 mg) [18, 206], nor at high doses (100 mg) [17]. It is useful to consider that two of these studies [17, 18] used a late timing of administration (22:45 h and 22:30 h, respectively). At this timing exo-MEL could have overlapped with the natural rise of endogenous MEL in HS, possibly explaining the lack of differences in SOL between exo-MEL and placebo conditions. This is in part supported by other studies comparing early evening vs. night administration of exo-MEL, in which significant effects on sleep initiation and maintenance variables were observed in the early evening but not in the late evening [207, 208]. Changes in the MEL receptor responsiveness produced when endogenous MEL is released have been proposed to explain the reduction in the effects of exo-MEL at this timing [207]. Likewise, in a research protocol evaluating the effect of exo-MEL across a full range of circadian phases under a 27-day forced desynchrony paradigm, it was observed that exo-MEL improved the sleep consolidation only in periods that were out of phase with the endogenous MEL secretion, while when exo-MEL was overlapped with endogenous MEL, the effects of exo-MEL were absent, thus confirming that the hypnotic properties of MEL are dependent of the phase of endogenous MEL release, at least in HS with normal endogenous MEL production [209]. Finally, comparisons of exo-MEL effects when administered in morning vs. night have shown opposite results. In one study, more significant sleep-promoting effects were identified in the morning when compared to night administration [7]. On the contrary, a marked decrease in sleep latency was reported by Tzischinsky & Lavie, 1994 [210] for administrations at night (21:00) when compared to ones made in the early evening and morning (12:00, 17:00, 19:00). The significant decrease in SOL at 21:00h in this study [210] may be related to the sleep deprivation condition included, where the cumulative sleep debt may have enhanced the sleep-promoting effects of exo-MEL at that timing.
Out of many sleep-related outcomes measured after exo-MEL administration, Fig. (4), SOL is the most commonly reported. Thus, we graphically summarized studies in HS evaluating SOL, considering the timing of administration and the dose, Fig. (3b). Some conclusions can be drawn:
1) Differentiating the exo-MEL posology used in each study, data show that high dosages (>5 mg) seem not to significantly increase exo-MEL efficacy in reducing sleep latency as compared to medium and low dosages <5 mg.
2) the timing of administration modifies the effect of exo-MEL on SOL: when administered at the morning or early afternoon, results are less variable. Instead, in the early evening and at night, there is great variability in the data, suggesting that the magnitude of the reduction in the SOL reached through exo-MEL at this timing may be less consistent.
The inconsistent effects of exo-MEL on SOL when administered in the early evening and at night could possibly be explained by the variability in the inter-individual circadian typology, affecting the action of exo-MEL in relation to the endogenous MEL profile, as previously reported by Wyatt et al., 2006 [209].
The studies described so far have been conducted in HS and, despite the high variability in the experimental approaches, most studies have demonstrated sleep-promoting effects of exo-MEL. Although the night-time administration of exo-MEL has demonstrated controversial results in HS, the studies conducted in insomnia patients suggest that exo-MEL administered around bedtime may facilitate sleep initiation and/or sleep maintenance, both as objectively [20-22, 25, 29] and subjectively determined [23, 24, 26-28, 37, 211]. A recent trial described how early awakenings were reduced after exo-MEL administration, while perceived sleep quality was not [212]. Other studies have reported no efficacy of exo-MEL neither in insomnia patients [30-33], nor in subjects with age-related sleep maintenance problems [213]. Nevertheless, the significant improvement in variables related to sleep quality and daytime function reported in studies with a large sample size has allowed recommending exo-MEL as a therapy for the treatment of insomnia, especially for over-50 years patients [23, 24]. Some studies even proposed that exo-MEL may contribute to the discontinuation of sleeping pills intake in insomniac patients [28, 214, 215].
7.3. Other Therapeutic Uses of Exo-MEL
This review is primarily focused on primary CRSD and primary insomnia, in order to explore the chronobiotic and sleep promoting effects of exo-MEL in the absence of other comorbidities. However, exo-MEL finds therapeutic applications also in other clinical conditions, taking advantage of its chronobiotic and sleep-promoting effects. An extensive review of this literature is beyond the scope of this paper; however recent comprehensive reviews are available on the use of exo-MEL in secondary circadian rhythm sleep disorders such as jet lag and shift work disorder [216], in children with neurodevelopmental disorders, such as autism spectrum disorder, attention-deficit/hyperactivity disorder, intellectual disability, Rett syndrome, Smith-Magenis syndrome and Angelman syndrome [217-220], as well as in several neuropsychiatric disorders [221].
7.4. Implications for the Use of Exo-MEL in the Clinical Practice
As suggested by Cajochen et al., 2003 [136], an “optimal melatonin treatment should utilize not only its soporific effects by administration close to the desired bedtime but also its chronobiotic properties, to entrain the sleep/wake behavior”. Despite the flourish body of literature demonstrating the undoubtful efficacy of exo-MEL [40, 41, 222, 223], clear recommendations about parameters such as the timing of administration, the posology, the type of formulation (prolonged-release -PR- vs. immediate or fast release -IR/FR-) and the treatment duration for a standardized use in clinical practice remain to be defined. Some general considerations about the implications of the current translational knowledge to the clinical practice are however discussed below.
Regarding the timing of administration, the studies reviewed suggest that in insomnia exo-MEL given 1 or 2 hours before bedtime decreases SOL [20, 21, 23, 27], improves TST [211] and sleep quality, and facilitates the discontinuation of BDZs [214, 215]. In Non-24-h sleep-wake disorder, significant changes in endogenous circadian rhythms after the exo-MEL treatment were obtained at a timing of administration ranging from 21:00 h - 22:00 h [166, 167, 169, 171], and also at a timing described as 1-2 hours before the individual bedtime [168, 170, 172]. The entrainment in endogenous circadian rhythms was reached in all these studies, even if only two of them grounded the timing of exo-MEL administration on the predicted DLMO [171, 172]. Thus, the regular daily administration of exo-MEL can entrain the circadian rhythmicity in free-running subjects whose endogenous rhythms are not in phase with light-dark cycle. In DSPD, an advance in endogenous circadian markers was obtained by several studies [175-177,180,181,184], and it seems to be proportional to the magnitude of the time to which exo-MEL is given before the individual DLMO (the earliest the administration, the higher the phase advance) [181]. Recommendations for the treatment of DSPD are to give exo-MEL during the phase-advance portion of the PRC: from ±8 h before DLMO to ±2 h after it, with a maximum effect at 3-5 hours before DLMO (about 5-7 hours before habitual SOT, since SOT usually occurs two hours after the DLMO) [217]. An important clinical aspect is the need of introducing a progressive advance in the timing of exo-MEL administration [181] due to the fact that with the exo-MEL treatment, the internal circadian phase progressively moves towards the phase advance region of the melatonin PRC. If exo-MEL is given too close to the DLMO, with the initial advance in the endogenous MEL, the exo-MEL treatment timing can quickly fall out of phase of the phase advance region of the PRC. Therefore, both the timing of administration prior to the predicted DLMO and a progressive advance in the timing of administration may contribute to better results in the interventions on DSPD. Information about the individual circadian markers as the DLMO or CBTmin may help improve the efficacy of exo-MEL treatment; however, in the clinical practice is not always possible to obtain a baseline measure of circadian markers [181]. Although some authors have argued that the DLMO cannot be predicted by sleep diary, actigraphy, or polysomnography [217], correlations between SOT and DLMO have been reported [44]. Since it is necessary to explore cost-effective approaches to infer the individual circadian rhythm [181], a feasible alternative might be estimating exo-MEL timing of administration based on the sleep/wake patterns, as measured by sleep log or actigraphy. The investigation of sleep patterns in the natural context and for a long time window (e.g., a seven-day continuous monitoring with a non-invasive wrist actigraph) could provide a valuable tool for practitioners to support their clinical decision-making in order to personalize exo-MEL timing of administration in a simple, accurate, and cost-effective fashion.
Regarding the posology, in general, doses of 0.1-0.5 mg are sufficient to induce physiologic circulating melatonin levels able to promote sleep and entrain circadian rhythms [4]. However, physiological doses have been recommended to treat patients with age-related insomnia associated with low nocturnal melatonin levels [22]. So far, no clear dose-response effects have been established for exo-MEL treatments. Concerning the type of exo-MEL formulation, there is no evidence indicating that PR formulations represent an advantage over IR [217]. One study that compared the two formulations, reported that, after two months of treatment, the PR formulation had comparable effects to the IR formulation, being effective for both reduction of sleep latency and improvement in sleep maintenance in chronic insomnia [20]. PR formulations have been recommended as replacement therapy for insomnia in patients aged 55 and older, as they improve sleep quality, sleep latency, morning alertness, and quality of life [224]. Further studies are required to establish a differential benefit regarding the type of formulation in the treatment of CRSD. Finally, about treatment duration, the studies reviewed here include a maximal duration of 26 weeks in insomnia [26] and four weeks in CRSD, although a clinical case of a free-running patient treated during six years was also reported [173]; however, no systematic studies have evaluated long-term effects of exo-MEL. It is possible that long-term treatments may be required to maintain the effects of exo-MEL [217], particularly in insomnia patients with reduced melatonin secretion, in which exo-MEL is used as a replacement therapy [54]. Also, in CRSD, the discontinuation of exo-MEL may lead to relapse [175], and the long-term use does not seem to have major adverse effects [173, 187]. Moreover, positive effects of MEL as a neuroprotective and antioxidant have been demonstrated [225, 226] in additional support of the exo-MEL long-term use. Although some studies in animals have suggested a reduction in receptor responsiveness after prolonged exo-MEL exposure [227], other studies suggest a sustained receptor sensitivity after MEL exposure [43]. Further studies are required to establish the long-term effects of exo-MEL treatments.
Finally, it is relevant to underline that exo-MEL is a substance that may increase sleep propensity facilitating the transition from a state of quiet wakefulness to normal sleep initiation if the environmental conditions are appropriate [4]. Then, variables such as motivated effort to be awake, exposition to light, or adopting an upright posture may suppress the sleep-promoting effects of exo-MEL. Therapists prescribing exo-MEL should consider these environmental variables and inform patients about them as part of the indications given before initiating the treatment. Strategies of patient education focused on optimizing sleep hygiene habits could also significantly improve the potential efficacy of exo-MEL.
CONCLUSION
Overall, although the studies reviewed here are heterogeneous, evidence supports that exo-MEL can effectively shift the circadian phase, producing an advance or a delay in the sleep-wake patterns and in the endogenous circadian rhythms. In the same way, the evidence supports the sleep-promoting effects of exo-MEL, confirming a double effect that might allow considering MEL as a chronobiotic with sleep-promoting properties.
The underlying mechanisms of exo-MEL are complex. The differential role of the MT1 and MT2 receptors in the melatonin-induced SCN inhibition and in the SCN phase-shifting effects remains to be elucidated, as well as the signaling pathways involved in each effect. Moreover, the action mechanisms that underlie MEL sleep-promoting effects through a direct action on somnogenic structures involving interactions with the GABAergic system, adenosine signaling, and PK channels deserve further investigation.
Specific recommendations about the optimal timing of administration, dose, and other parameters of the exo-MEL treatments remain to be determined. The timing of administration appears to be a determinant variable for the efficacy of exo-MEL treatments. Surprisingly, only a few studies have evaluated the individual circadian markers to establish the timing of the exo-MEL administration, even in the context of CRSD. Despite their paucity, these studies have clearly demonstrated the need to timely administer Exo-MEL, not in phase with the endogenous one. In the clinical practice, this extremely individualized approach will not be easily accessible. Alternative and more practical approaches to determining individual circadian rhythmicity and its disorders are required to support the correct timing of administration of exo-MEL.
Further and more homogeneous studies in terms of experimental design and outcomes are required. The evaluation of objective variables related to sleep initiation, sleep maintenance and circadian markers as well as longitudinal designs in both sleep and CRSD could contribute to a better characterization of exo-MEL role in the treatment of sleep and CRSD, as well as to a better comprehension of the role of MEL in the regulation of sleep and circadian processes.
ACKNOWLEDGEMENTS
The authors would like to thank Santiago Cruz for his excellent graphic design work and Johan Bocanegra for his support in the data plotting.
LIST OF ABBREVIATIONS
- 5-HIAA
5-Hydroxyindoleacetic Acid
- AASD
American Sleep Disorder Association
- ACTH
Adrenocorticotropic Hormone
- AFS
Awakening from Sleep
- aMT6s
6-Sulfatoxymelatonin
- ASPD
Advanced Sleep Phase Disorder
- AW
Number of Awakenings
- BAI
Beck Anxiety Inventory
- BDI-II
Beck Depression Inventory Version 2
- BFR
Blind Free-running
- BFW
Behavior Following Wakefulness
- BT
Bedtime
- BWSQ
Benzodiazepine Withdrawal Symptom Questionnaire
- CBT
Core Body Temperature
- CGI
Clinical Global Impression
- CT
Circadian Time
- CES-D
Center for Epidemiologic Studies Depression Scale
- DLMO
Dim Light Melatonin Onset
- DSPD
Delayed Sleep Phase Disorder
- DSQ
Daily Sleep Questionnaire
- DSM-5
Diagnostic and Statistical Manual of Mental Disorders
- EEG
Electroencephalogram
- EMA
Early Morning Awakening
- ESS
Epworth Sleepiness Scale
- Exo-MEL
Exogenous Melatonin
- F
Fatigue
- FR
Fast Release
- FSH
Follicle Stimulating Hormone
- GAS
Goldberg Anxiety Scale
- GDS
Geriatric Depression Scale
- GH
Growth Hormone
- Gn-RH
Gonadotrophin-releasing Hormone
- GTS
Getting to Sleep
- HCG
Human Chorionic Gonadotrophin
- HS
Healthy Subjects
- HDRS
Hamilton Depression Rating Scale
- ICSD
International Classification of Sleep Disorders
- IR
Immediate Release
- ISI
Insomnia Severity Index
- ISWRD
Irregular Sleep Wake Rhythm Disorder
- KSS
Karolinska Sleepiness Scale
- LH
Luteinizing Hormone
- LSEQ
Leeds Sleep Evaluation Questionnaire
- MEL-onset
Melatonin Onset
- MEQ
Morningness-Eveningness Questionnaire
- MMQ
Maudsley Medical Questionary
- MSLT
Multiple Sleep Latency Test
- N24SWD
Non-24-hour sleep-wake Disorder
- NHSMI
The Northside Hospital Sleep Medicine Institute Test
- PLA
Treatment with Placebo
- POMS
Profile of Mood States
- PR
Prolonged Release
- PRC
Phase Response Curve
- PRL
Prolactin
- PSG
Polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- QOD
Quality of Day
- QON
Sleep Quality the Previous Night
- QOS
Quality of Sleep
- REM
Rapid Eye Movements Sleep
- REM-L
REM Sleep Latency
- S
Sleepiness
- S1, S2, S3, S4
Stage 1, Stage 2, Stage 3, Stage 4
- S2-L
Stage 2 Latency
- SDS
Sheehan Disability Scale
- SE
Sleep Efficiency
- SL
Sleep Latency
- SOFFT
Sleep Offset Time
- SOL
Sleep Onset Latency
- SOT
Sleep Onset Time
- SP
Sleep Propensity
- SQ
Sleep Quality
- SQQ
Sleep Quality Questionnaire
- ss density
Sleep Spindles Density
- SSS
Stanford Sleepiness Scale
- SWS
Slow Wave Sleep
- SWS_L
Slow Wave Sleep Latency
- T4
Thyroxine
- TIB
Time in Bed
- TRH
Thyrotropin-releasing Hormone
- TSH
Thyroid-stimulating Hormone
- TSP
Total Sleep Period
- TST
Total Sleep Time
- TWT
Total Wake Time
- VAS
Visual Analogue Scale
- WASO
Wake after Sleep Onset
- WHO-5
Well-Being Index
AUTHORS’ CONTRIBUTIONS
FCS and UF wrote the first draft of the manuscript; MC and MS complemented the section on melatonin physiology; SB contributed with the data extraction and revision of the manuscript, CC, AB, MS and UF revised the manuscript.
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines were followed for the study.
FUNDING
Fondazione Arpa Onlus, a non profit organization (https://fondazionearpa.it). This work has been partially supported by grant-RC 1.21 (“Monitoraggio e tele-monitoraggio del sonno in età evolutiva e in pazienti adulti”) and the 5 x 1000 voluntary contributions, Italian Ministry of Health.
CONFLICT OF INTEREST
U.F. is co-founder and president of sleepActa S.r.l, a spin-off company of the University of Pisa operating in the field of sleep medicine. M.S. has been a consultant for Pierpaoli Exelyas Srl. All other authors declare that this article content has no conflict of interest.
APPENDIX
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Lerner A.B., Case J.D., Takahashi Y., Lee T.H., Mori W. Isolation of melatonin, the pineal gland factor that lightens melanocytes1. J. Am. Chem. Soc. 1958;80(10):2587. doi: 10.1021/ja01543a060. [DOI] [Google Scholar]
- 2.Lerner Aaron B. CJD. Melatonin. Intersociey Symp New Negleted Horm; 1962. pp. 590–592. [Google Scholar]
- 3.van den Heuvel C.J., Ferguson S.A., Macchi M.M., Dawson D. Melatonin as a hypnotic: Con. Sleep Med. Rev. 2005;9(1):71–80. doi: 10.1016/j.smrv.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Zhdanova I.V. Melatonin as a hypnotic: Pro. Sleep Med. Rev. 2005;9(1):51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Vollrath L., Semm P., Gammel G. Sleep induction by intranasal application of melatonin. Bremen, FRG: Symp. Melatonin; 1981. pp. 327–329. [DOI] [Google Scholar]
- 6.Lieberman H.R., Waldhauser F., Garfield G., Lynch H.J., Wurtman R.J. Effects of melatonin on human mood and performance. Brain Res. 1984;323(2):201–207. doi: 10.1016/0006-8993(84)90290-7. [DOI] [PubMed] [Google Scholar]
- 7.Nickelsen T., Demisch L., Demisch K., Radermacher B., Schöffling K. Influence of subchronic intake of melatonin at various times of the day on fatigue and hormonal levels: A placebo-controlled, double-blind trial. J. Pineal Res. 1989;6(4):325–334. doi: 10.1111/j.1600-079X.1989.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 8.Antón-Tay F., Díaz J.L., Fernández-Guardiola A. On the effect of melatonin upon human brain. Its possible therapeutic implications. Life Sci. I. 1971;10(15):841–850. doi: 10.1016/0024-3205(71)90155-X. [DOI] [PubMed] [Google Scholar]
- 9.Hughes R.J., Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20(2):124–131. doi: 10.1093/sleep/20.2.124. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M. The hypnotic effects of melatonin treatment on diurnal sleep in humans. Psychiatry Clin. Neurosci. 1999;53(2):243–245. doi: 10.1046/j.1440-1819.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- 11.Nave R., Peled R., Lavie P. Melatonin improves evening napping. Eur. J. Pharmacol. 1995;275(2):213–216. doi: 10.1016/0014-2999(94)00769-4. [DOI] [PubMed] [Google Scholar]
- 12.Reid K., Van den Heuvel C., Dawson D. Day-time melatonin administration: Effects on core temperature and sleep onset latency. J. Sleep Res. 1996;5(3):150–154. doi: 10.1046/j.1365-2869.1996.t01-1-00006.x. [DOI] [PubMed] [Google Scholar]
- 13.Gilbert S.S., van den Heuvel C.J., Dawson D. Daytime melatonin and temazepam in young adult humans: Equivalent effects on sleep latency and body temperatures. J. Physiol. 1999;514(Pt 3):905–914. doi: 10.1111/j.1469-7793.1999.905ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhdanova I.V., Wurtman R.J., Lynch H.J., Ives J.R., Dollins A.B., Morabito C., Matheson J.K., Schomer D.L. Sleep-inducing effects of low doses of melatonin ingested in the evening. Clin. Pharmacol. Ther. 1995;57(5):552–558. doi: 10.1016/0009-9236(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 15.Zhdanova I.V., Wurtman R.J., Morabito C., Piotrovska V.R., Lynch H.J. Effects of low oral doses of melatonin, given 2-4 hours before habitual bedtime, on sleep in normal young humans. Sleep. 1996;19(5):423–431. doi: 10.1093/sleep/19.5.423. [DOI] [PubMed] [Google Scholar]
- 16.Attenburrow M.E.J., Cowen P.J., Sharpley A.L. Low dose melatonin improves sleep in healthy middle-aged subjects. Psychopharmacology (Berl.) 1996;126(2):179–181. doi: 10.1007/BF02246354. [DOI] [PubMed] [Google Scholar]
- 17.Ferini-Strambi L., Zucconi M., Biella G., Stankov B., Fraschini F., Oldani A., Smirne S. Effect of melatonin on sleep microstructure: Preliminary results in healthy subjects. Sleep. 1993;16(8):744–747. doi: 10.1093/sleep/16.8.744. [DOI] [PubMed] [Google Scholar]
- 18.James S.P., Mendelson W.B., Sack D.A., Rosenthal N.E., Wehr T.A. The effect of melatonin on normal sleep. Neuropsychopharmacology. 1987;1(1):41–44. doi: 10.1016/0893-133X(87)90008-X. [DOI] [PubMed] [Google Scholar]
- 19.van den Heuvel C.J., Kennaway D.J., Dawson D. Thermoregulatory and soporific effects of very low dose melatonin injection. Am. J. Physiol. 1999;276(2):E249–E254. doi: 10.1152/ajpendo.1999.276.2.E249. [DOI] [PubMed] [Google Scholar]
- 20.Haimov I., Lavie P., Laudon M., Herer P., Vigder C., Zisapel N. Melatonin replacement therapy of elderly insomniacs. Sleep. 1995;18(7):598–603. doi: 10.1093/sleep/18.7.598. [DOI] [PubMed] [Google Scholar]
- 21.Hughes R.J., Sack R.L., Lewy A.J. The role of melatonin and circadian phase in age-related sleep-maintenance insomnia: Assessment in a clinical trial of melatonin replacement. Sleep. 1998;21(1):52–68. doi: 10.1093/sleep/21.1.52. [DOI] [PubMed] [Google Scholar]
- 22.Zhdanova I.V., Wurtman R.J., Regan M.M., Taylor J.A., Shi J.P., Leclair O.U. Melatonin treatment for age-related insomnia. J. Clin. Endocrinol. Metab. 2001;86(10):4727–4730. doi: 10.1210/jcem.86.10.7901. [DOI] [PubMed] [Google Scholar]
- 23.Wade A.G., Ford I., Crawford G., McMahon A.D., Nir T., Laudon M., Zisapel N. Efficacy of prolonged release melatonin in insomnia patients aged 55-80 years: Quality of sleep and next-day alertness outcomes. Curr. Med. Res. Opin. 2007;23(10):2597–2605. doi: 10.1185/030079907X233098. [DOI] [PubMed] [Google Scholar]
- 24.Lemoine P., Nir T., Laudon M., Zisapel N. Prolonged-release melatonin improves sleep quality and morning alertness in insomnia patients aged 55 years and older and has no withdrawal effects. J. Sleep Res. 2007;16(4):372–380. doi: 10.1111/j.1365-2869.2007.00613.x. [DOI] [PubMed] [Google Scholar]
- 25.Luthringer R., Muzet M., Zisapel N., Staner L. The effect of prolonged-release melatonin on sleep measures and psychomotor performance in elderly patients with insomnia. Int. Clin. Psychopharmacol. 2009;24(5):239–249. doi: 10.1097/YIC.0b013e32832e9b08. [DOI] [PubMed] [Google Scholar]
- 26.Wade A.G., Ford I., Crawford G., McConnachie A., Nir T., Laudon M., Zisapel N. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: A randomized placebo controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010;8:51. doi: 10.1186/1741-7015-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade A.G., Crawford G., Ford I., McConnachie A., Nir T., Laudon M., Zisapel N. Prolonged release melatonin in the treatment of primary insomnia: Evaluation of the age cut-off for short- and long-term response. Curr. Med. Res. Opin. 2011;27(1):87–98. doi: 10.1185/03007995.2010.537317. [DOI] [PubMed] [Google Scholar]
- 28.Garzón C., Guerrero J.M., Aramburu O., Guzmán T. Effect of melatonin administration on sleep, behavioral disorders and hypnotic drug discontinuation in the elderly: A randomized, double-blind, placebo-controlled study. Aging Clin. Exp. Res. 2009;21(1):38–42. doi: 10.1007/BF03324897. [DOI] [PubMed] [Google Scholar]
- 29.Lushington K., Pollard K., Lack L., Kennaway D.J., Dawson D. Daytime melatonin administration in elderly good and poor sleepers: Effects on core body temperature and sleep latency. Sleep. 1997;20(12):1135–1144. doi: 10.1093/sleep/20.12.1135. [DOI] [PubMed] [Google Scholar]
- 30.Ellis C.M., Lemmens G., Parkes J.D. Melatonin and insomnia. J. Sleep Res. 1996;5(1):61–65. doi: 10.1046/j.1365-2869.1996.00003.x. [DOI] [PubMed] [Google Scholar]
- 31.Dawson D., Rogers N.L., van den Heuvel C.J., Kennaway D.J., Lushington K. Effect of sustained nocturnal transbuccal melatonin administration on sleep and temperature in elderly insomniacs. J. Biol. Rhythms. 1998;13(6):532–538. doi: 10.1177/074873098129000354. [DOI] [PubMed] [Google Scholar]
- 32.Almeida M.L.G., Ontiveros U.M.P., Cortés Sotres J., Heinze Martin G. Treatment of primary insomnia with melatonin: A double-blind, placebo-controlled, crossover study. J. Psychiatry Neurosci. 2003;28(3):191–196. [PMC free article] [PubMed] [Google Scholar]
- 33.James S.P., Sack D.A., Rosenthal N.E., Mendelson W.B. Melatonin administration in insomnia. Neuropsychopharmacology. 1990;3(1):19–23. [PubMed] [Google Scholar]
- 34.Dikeos D.G., Theleritis C.G., Soldatos C.R. Benzodiazepines: Effects on sleep. Sleep Disord Diagnosis Ther; 2008. [DOI] [Google Scholar]
- 35.Campo-Soria C., Chang Y., Weiss D.S. Mechanism of action of benzodiazepines on GABAA receptors. Br. J. Pharmacol. 2006;148(7):984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Griffin C.E., III, Kaye A.M., Bueno F.R., Kaye A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013;13(2):214–223. [PMC free article] [PubMed] [Google Scholar]
- 37.Leger D., Laudon M., Zisapel N. Nocturnal 6-sulfatoxymelatonin excretion in insomnia and its relation to the response to melatonin replacement therapy. Am. J. Med. 2004;116(2):91–95. doi: 10.1016/j.amjmed.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Mendelson W.B. A critical evaluation of the hypnotic efficacy of melatonin. Sleep. 1997;20(10):916–919. doi: 10.1093/sleep/20.10.916. [DOI] [PubMed] [Google Scholar]
- 39.Zhdanova I.V., Wurtman R.J. Efficacy of melatonin as a sleep-promoting agent. J. Biol. Rhythms. 1997;12(6):644–650. doi: 10.1177/074873049701200620. [DOI] [PubMed] [Google Scholar]
- 40.Brzezinski A., Vangel M.G., Wurtman R.J., Norrie G., Zhdanova I., Ben-Shushan A., Ford I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005;9(1):41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Buscemi N., Vandermeer B., Hooton N., Pandya R., Tjosvold L., Hartling L., Baker G., Klassen T.P., Vohra S. The efficacy and safety of exogenous melatonin for primary sleep disorders. A meta-analysis. J. Gen. Intern. Med. 2005;20(12):1151–1158. doi: 10.1111/j.1525-1497.2005.0243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wirz-Justice A., Armstrong S.M. Melatonin: Nature’s soporific? J. Sleep Res. 1996;5(2):137–141. [PubMed] [Google Scholar]
- 43.Pandi-Perumal S.R., Srinivasan V., Spence D.W., Cardinali D.P. Role of the melatonin system in the control of sleep: Therapeutic implications. CNS Drugs. 2007;21(12):995–1018. doi: 10.2165/00023210-200721120-00004. [DOI] [PubMed] [Google Scholar]
- 44.Lewy A.J., Ahmed S., Jackson J.M.L., Sack R.L. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol. Int. 1992;9(5):380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 45.Arendt J., Skene D.J. Melatonin as a chronobiotic. Sleep Med. Rev. 2005;9(1):25–39. doi: 10.1016/j.smrv.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Liu J., Clough S.J., Hutchinson A.J., Adamah-Biassi E.B., Popovska-Gorevski M., Dubocovich M.L. MT1 and MT2 melatonin receptors: A therapeutic perspective. Annu. Rev. Pharmacol. Toxicol. 2016;56:361–383. doi: 10.1146/annurev-pharmtox-010814-124742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu C., Weaver D.R., Jin X., Shearman L.P., Pieschl R.L., Gribkoff V.K., Reppert S.M. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19(1):91–102. doi: 10.1016/S0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 48.Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P., Olcese J. International union of basic and clinical pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010;62(3):343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jockers R., Delagrange P., Dubocovich M.L., Markus R.P., Renault N., Tosini G., Cecon E., Zlotos D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016;173(18):2702–2725. doi: 10.1111/bph.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dubocovich M.L., Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27(2):101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 51.Hunt A.E., Al-Ghoul W.M., Gillette M.U., Dubocovich M.L. Activation of MT(2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am. J. Physiol. Cell Physiol. 2001;280(1):C110–C118. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- 52.Goldsmith C.S., Bell-Pedersen D. Diverse roles for MAPK signaling in circadian clocks. Adv. Genet. 2013;84:1–39. doi: 10.1016/B978-0-12-407703-4.00001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gandhi A.V., Mosser E.A., Oikonomou G., Prober D.A. Melatonin is required for the circadian regulation of sleep. Neuron. 2015;85(6):1193–1199. doi: 10.1016/j.neuron.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zisapel N. Melatonin and sleep. Open Neuroendocrinol. J. 2010;3:85–95. doi: 10.1080/09291016.2018.1443554. [DOI] [Google Scholar]
- 55.Carpentieri A., Areco V., de Barboza G.D., Rivoira M.A., Guizzardi S., de Talamoni N.T. In: Melatonin, Neuroprotective Agents and Antidepressant Therapy. López-Muñoz F., Srinivasan V., de Berardis D., Álamo C., Kato T., editors. New Delhi: Springer; 2016. Melatonin: Basic and Clinical Aspects. [DOI] [Google Scholar]
- 56.Hastings M.H. Circadian clocks. Curr. Biol. 1997;7(11):R670–R672. doi: 10.1016/S0960-9822(06)00350-2. [DOI] [PubMed] [Google Scholar]
- 57.Pandi-Perumal S.R., Trakht I., Srinivasan V., Spence D.W., Maestroni G.J.M., Zisapel N., Cardinali D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008;85(3):335–353. doi: 10.1016/j.pneurobio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Majidinia M., Reiter R.J., Shakouri S.K., Yousefi B. The role of melatonin, a multitasking molecule, in retarding the processes of ageing. Ageing Res. Rev. 2018;47:198–213. doi: 10.1016/j.arr.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Baker J., Kimpinski K. Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 2018;45(8):755–766. doi: 10.1111/1440-1681.12942. [DOI] [PubMed] [Google Scholar]
- 60.Manchester L.C., Coto-Montes A., Boga J.A., Andersen L.P.H., Zhou Z., Galano A., Vriend J., Tan D.X., Reiter R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015;59(4):403–419. doi: 10.1111/jpi.12267. [DOI] [PubMed] [Google Scholar]
- 61.Claustrat B., Brun J., Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med. Rev. 2005;9(1):11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Vaccaro A., Kaplan Dor Y., Nambara K., Pollina E.A., Lin C., Greenberg M.E., Rogulja D. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell. 2020;181(6):1307–1328.e15. doi: 10.1016/j.cell.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 63.Provencio I., Jiang G., De Grip W.J., Hayes W.P., Rollag M.D. Melanopsin: An opsin in melanophores, brain, and eye. Proc. Natl. Acad. Sci. USA. 1998;95(1):340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foster R.G., Hankins M.W. Non-rod, non-cone photoreception in the vertebrates. Prog. Retin. Eye Res. 2002;21(6):507–527. doi: 10.1016/S1350-9462(02)00036-8. [DOI] [PubMed] [Google Scholar]
- 65.Graham D.M., Wong K.Y. Melanopsin-expressing, Intrinsically Photosensitive Retinal Ganglion Cells Melanopsin-expressing, Intrinsically Photosensitive Retinal Ganglion Cells (ipRGCs). WebVision; 2016. pp. 1–47. [Google Scholar]
- 66.Berry R.B. Fundam. Sleep Med. Saint Louis: Elsevier; 2012. Circadian rhythm sleep disorders. pp. 515–543. [DOI] [Google Scholar]
- 67.Hannibal J., Fahrenkrug J. Target areas innervated by PACAP-immunoreactive retinal ganglion cells. Cell Tissue Res. 2004;316(1):99–113. doi: 10.1007/s00441-004-0858-x. [DOI] [PubMed] [Google Scholar]
- 68.Reghunandanan V., Reghunandanan R. Neurotransmitters of the suprachiasmatic nuclei. J. Circadian Rhythms. 2006;4:2. doi: 10.1186/1740-3391-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Esseveldt K.E., Lehman M.N., Boer G.J. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain Res. Brain Res. Rev. 2000;33(1):34–77. doi: 10.1016/S0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- 70.Kalsbeek A., Cutrera R.A., Van Heerikhuize J.J., Van Der Vliet J., Buijs R.M. GABA release from suprachiasmatic nucleus terminals is necessary for the light-induced inhibition of nocturnal melatonin release in the rat. Neuroscience. 1999;91(2):453–461. doi: 10.1016/S0306-4522(98)00635-6. [DOI] [PubMed] [Google Scholar]
- 71.Borjigin J., Zhang L.S., Calinescu A-A. Circadian regulation of pineal gland rhythmicity. Mol. Cell. Endocrinol. 2012;349(1):13–19. doi: 10.1016/j.mce.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang S.W., Liu X., Pradoldej S., Tosini G., Chang Q., Iuvone P.M., Ye K. N-acetylserotonin activates TrkB receptor in a circadian rhythm. Proc. Natl. Acad. Sci. USA. 2010;107(8):3876–3881. doi: 10.1073/pnas.0912531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anderson G., Maes M. Reconceptualizing adult neurogenesis: Role for sphingosine-1-phosphate and fibroblast growth factor-1 in co-ordinating astrocyte-neuronal precursor interactions. CNS Neurol. Disord. Drug Targets. 2014;13(1):126–136. doi: 10.2174/18715273113126660132. [DOI] [PubMed] [Google Scholar]
- 74.Anderson G. Tumour microenvironment: Roles of the Aryl hydrocarbon receptor, O-GlcNAcylation, Acetyl-CoA and melatonergic pathway in regulating dynamic metabolic interactions across cell types-tumour microenvironment and metabolism. Int. J. Mol. Sci. 2020;22(1):141. doi: 10.3390/ijms22010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pontes G.N., Cardoso E.C., Carneiro-Sampaio M.M.S., Markus R.P. Pineal melatonin and the innate immune response: The TNF-α increase after cesarean section suppresses nocturnal melatonin production. J. Pineal Res. 2007;43(4):365–371. doi: 10.1111/j.1600-079X.2007.00487.x. [DOI] [PubMed] [Google Scholar]
- 76.Markus R.P., Cecon E., Pires-Lapa M.A. Immune-pineal axis: Nuclear factor κB (NF-kB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 2013;14(6):10979–10997. doi: 10.3390/ijms140610979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Comai S., Lopez-Canul M., De Gregorio D., Posner A., Ettaoussi M., Guarnieri F.C., Gobbi G. Melatonin MT1 receptor as a novel target in neuropsychopharmacology: MT1 ligands, pathophysiological and therapeutic implications, and perspectives. Pharmacol. Res. 2019;144:343–356. doi: 10.1016/j.phrs.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 78.Ostrin L.A. Ocular and systemic melatonin and the influence of light exposure. Clin. Exp. Optom. 2019;102(2):99–108. doi: 10.1111/cxo.12824. [DOI] [PubMed] [Google Scholar]
- 79.Dubocovich M.L. Melatonin receptors: Role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl. 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 80.Jin X., von Gall C., Pieschl R.L., Gribkoff V.K., Stehle J.H., Reppert S.M., Weaver D.R. Targeted disruption of the mouse Mel(1b) melatonin receptor. Mol. Cell. Biol. 2003;23(3):1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cardinali D., Pandi-Perumal S., Niles L. Melatonin and its receptors: Biological function in circadian sleep-wake regulation. In: Monti J., Pandi-Perumal S., Sinton C., editors. Neurochemistry of Sleep and Wakefulness. Cambridge: Cambridge University Press; 2008. pp. 283–314. [DOI] [Google Scholar]
- 82.Gobbi G., Comai S. Differential function of melatonin MT1 and MT2 receptors in REM and NREM sleep. Front. Endocrinol. (Lausanne) 2019;10:87. doi: 10.3389/fendo.2019.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ochoa-Sanchez R., Comai S., Spadoni G., Bedini A., Tarzia G., Gobbi G. Melatonin, selective and non-selective MT1/MT2 receptors agonists: Differential effects on the 24-h vigilance states. Neurosci. Lett. 2014;561:156–161. doi: 10.1016/j.neulet.2013.12.069. [DOI] [PubMed] [Google Scholar]
- 84.Fisher S.P., Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci. Lett. 2009;457(2):93–96. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ochoa-Sanchez R., Comai S., Lacoste B., Bambico F.R., Dominguez-Lopez S., Spadoni G., Rivara S., Bedini A., Angeloni D., Fraschini F., Mor M., Tarzia G., Descarries L., Gobbi G. Promotion of non-rapid eye movement sleep and activation of reticular thalamic neurons by a novel MT2 melatonin receptor ligand. J. Neurosci. 2011;31(50):18439–18452. doi: 10.1523/JNEUROSCI.2676-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Comai S., Ochoa-Sanchez R., Gobbi G. Sleep-wake characterization of double MT1/MT2 receptor knockout mice and comparison with MT1 and MT2 receptor knockout mice. Behav. Brain Res. 2013;243:231–238. doi: 10.1016/j.bbr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Harpsøe N.G., Andersen L.P.H., Gögenur I., Rosenberg J. Clinical pharmacokinetics of melatonin: A systematic review. Eur. J. Clin. Pharmacol. 2015;71(8):901–909. doi: 10.1007/s00228-015-1873-4. [DOI] [PubMed] [Google Scholar]
- 88.Tordjman S., Chokron S., Delorme R., Charrier A., Bellissant E., Jaafari N., Fougerou C. Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 2017;15(3):434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dollins A.B., Zhdanova I.V., Wurtman R.J., Lynch H.J., Deng M.H. Effect of inducing nocturnal serum melatonin concentrations in daytime on sleep, mood, body temperature, and performance. Proc. Natl. Acad. Sci. USA. 1994;91(5):1824–1828. doi: 10.1073/pnas.91.5.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zawilska J.B., Skene D.J., Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol. Rep. 2009;61(3):383–410. doi: 10.1016/S1734-1140(09)70081-7. [DOI] [PubMed] [Google Scholar]
- 91.Cipolla-Neto J., Amaral F.G.D. Melatonin as a hormone: New physiological and clinical insights. Endocr. Rev. 2018;39(6):990–1028. doi: 10.1210/er.2018-00084. [DOI] [PubMed] [Google Scholar]
- 92.Takahashi J.S. Finding new clock components: Past and future. J. Biol. Rhythms. 2004;19(5):339–347. doi: 10.1177/0748730404269151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pevet P., Challet E. Melatonin: Both master clock output and internal time-giver in the circadian clocks network. J. Physiol. Paris. 2011;105(4-6):170–182. doi: 10.1016/j.jphysparis.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 94.Nakahata Y., Kaluzova M., Grimaldi B., Sahar S., Hirayama J., Chen D., Guarente L.P., Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scott F.F., Belle M.D.C., Delagrange P., Piggins H.D. Electrophysiological effects of melatonin on mouse Per1 and non-Per1 suprachiasmatic nuclei neurones in vitro . . J. Neuroendocrinol. 2010;22(11):1148–1156. doi: 10.1111/j.1365-2826.2010.02063.x. [DOI] [PubMed] [Google Scholar]
- 96.Stehle J.H., von Gall C., Korf H-W. Melatonin: A clock-output, a clock-input. J. Neuroendocrinol. 2003;15(4):383–389. doi: 10.1046/j.1365-2826.2003.01001.x. [DOI] [PubMed] [Google Scholar]
- 97.Bedont J.L., Blackshaw S. Constructing the suprachiasmatic nucleus: A watchmaker’s perspective on the central clockworks. Front. Syst. Neurosci. 2015;9:74. doi: 10.3389/fnsys.2015.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hamada T., Antle M.C., Silver R. Temporal and spatial expression patterns of canonical clock genes and clock-controlled genes in the suprachiasmatic nucleus. Eur. J. Neurosci. 2004;19(7):1741–1748. doi: 10.1111/j.1460-9568.2004.03275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hardeland R. Chronobiology of melatonin beyond the feedback to the suprachiasmatic nucleus-consequences to melatonin dysfunction. Int. J. Mol. Sci. 2013;14(3):5817–5841. doi: 10.3390/ijms14035817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dijk D-J., Lockley S.W. Integration of human sleep-wake regulation and circadian rhythmicity. J. Appl. Physiol. 2002;92(2):852–862. doi: 10.1152/japplphysiol.00924.2001. [DOI] [PubMed] [Google Scholar]
- 101.Edgar D.M., Dement W.C., Fuller C.A. Effect of SCN lesions on sleep in squirrel monkeys: Evidence for opponent processes in sleep-wake regulation. J. Neurosci. 1993;13(3):1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sack R.L., Hughes R.J., Edgar D.M., Lewy A.J. Sleep-promoting effects of melatonin: At what dose, in whom, under what conditions, and by what mechanisms? Sleep. 1997;20(10):908–915. doi: 10.1093/sleep/20.10.908. [DOI] [PubMed] [Google Scholar]
- 103.Shochat T., Haimov I., Lavie P. Melatonin--the key to the gate of sleep. Ann. Med. 1998;30(1):109–114. doi: 10.3109/07853899808999392. [DOI] [PubMed] [Google Scholar]
- 104.Agez L., Laurent V., Pévet P., Masson-Pévet M., Gauer F. Melatonin affects nuclear orphan receptors mRNA in the rat suprachiasmatic nuclei. Neuroscience. 2007;144(2):522–530. doi: 10.1016/j.neuroscience.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 105.Kandalepas P.C., Mitchell J.W., Gillette M.U.G. Melatonin signal transduction pathways require E-Box-Mediated transcription of Per1 and Per2 to reset the SCN clock at dusk. PLoS One. 2016;11(6):e0157824. doi: 10.1371/journal.pone.0157824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stehle J., Vanecek J., Vollrath L. Effects of melatonin on spontaneous electrical activity of neurons in rat suprachiasmatic nuclei: An in vitro iontophoretic study. . J. Neural Transm. (Vienna) 1989;78(2):173–177. doi: 10.1007/BF01252503. [DOI] [PubMed] [Google Scholar]
- 107.Shibata S., Cassone V.M., Moore R.Y. Effects of melatonin on neuronal activity in the rat suprachiasmatic nucleus in vitro . . Neurosci. Lett. 1989;97(1-2):140–144. doi: 10.1016/0304-3940(89)90153-5. [DOI] [PubMed] [Google Scholar]
- 108.Jiang Z.G., Nelson C.S., Allen C.N. Melatonin activates an outward current and inhibits Ih in rat suprachiasmatic nucleus neurons. Brain Res. 1995;687(1-2):125–132. doi: 10.1016/0006-8993(95)00478-9. [DOI] [PubMed] [Google Scholar]
- 109.van den Top M., Buijs R.M., Ruijter J.M., Delagrange P., Spanswick D., Hermes M.L.H.J. Melatonin generates an outward potassium current in rat suprachiasmatic nucleus neurones in vitro independent of their circadian rhythm. . Neuroscience. 2001;107(1):99–108. doi: 10.1016/S0306-4522(01)00346-3. [DOI] [PubMed] [Google Scholar]
- 110.Wan Q., Man H-Y., Liu F., Braunton J., Niznik H.B., Pang S.F., Brown G.M., Wang Y.T. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat. Neurosci. 1999;2(5):401–403. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- 111.Albus H., Vansteensel M.J., Michel S., Block G.D., Meijer J.H. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr. Biol. 2005;15(10):886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 112.Itri J., Michel S., Waschek J.A., Colwell C.S. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J. Neurophysiol. 2004;92(1):311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu C., Reppert S.M. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25(1):123–128. doi: 10.1016/S0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 114.Cardinali D.P., Pandi-Perumal S.R., Niles L.P., Brown G.M. Melatonin and sleep: Possible involvement of GABAergic mechanisms. GABA Sleep Mol Funct Clin Asp. 2010;9783034602:279–301. doi: 10.1007/978-3-0346-0226-6_13. [DOI] [Google Scholar]
- 115.Morgan P.J., Williams L.M. The pars tuberalis of the pituitary: A gateway for neuroendocrine output. Rev. Reprod. 1996;1(3):153–161. doi: 10.1530/ror.0.0010153. [DOI] [PubMed] [Google Scholar]
- 116.Wagner G.C., Johnston J.D., Tournier B.B., Ebling F.J.P., Hazlerigg D.G. Melatonin induces gene-specific effects on rhythmic mRNA expression in the pars tuberalis of the Siberian hamster (Phodopus sungorus). Eur. J. Neurosci. 2007;25(2):485–490. doi: 10.1111/j.1460-9568.2006.05291.x. [DOI] [PubMed] [Google Scholar]
- 117.Vriend J., Reiter R.J. Melatonin feedback on clock genes: A theory involving the proteasome. J. Pineal Res. 2015;58(1):1–11. doi: 10.1111/jpi.12189. [DOI] [PubMed] [Google Scholar]
- 118.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418(6901):935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 119.Dibner C., Schibler U., Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- 120.Zeman M., Szántóová K., Stebelová K., Mravec B., Herichová I. Effect of rhythmic melatonin administration on clock gene expression in the suprachiasmatic nucleus and the heart of hypertensive TGR(mRen2)27 rats. J. Hypertens. 2009;27(6):S21–S26. doi: 10.1097/01.hjh.0000358833.41181.f6. [DOI] [PubMed] [Google Scholar]
- 121.Jung-Hynes B., Huang W., Reiter R.J., Ahmad N. Melatonin resynchronizes dysregulated circadian rhythm circuitry in human prostate cancer cells. J. Pineal Res. 2010;49(1):60–68. doi: 10.1111/j.1600-079X.2010.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Torres-Farfan C., Richter H.G., Rojas-García P., Vergara M., Forcelledo M.L., Valladares L.E., Torrealba F., Valenzuela G.J., Serón-Ferré M. mt1 Melatonin receptor in the primate adrenal gland: Inhibition of adrenocorticotropin-stimulated cortisol production by melatonin. J. Clin. Endocrinol. Metab. 2003;88(1):450–458. doi: 10.1210/jc.2002-021048. [DOI] [PubMed] [Google Scholar]
- 123.Torres-Farfan C., Mendez N., Abarzua-Catalan L., Vilches N., Valenzuela G.J., Seron-Ferre M. A circadian clock entrained by melatonin is ticking in the rat fetal adrenal. Endocrinology. 2011;152(5):1891–1900. doi: 10.1210/en.2010-1260. [DOI] [PubMed] [Google Scholar]
- 124.Alonso-Vale M.I.C., Andreotti S., Mukai P.Y., Borges-Silva Cd., Peres S.B., Cipolla-Neto J., Lima F.B. Melatonin and the circadian entrainment of metabolic and hormonal activities in primary isolated adipocytes. J. Pineal Res. 2008;45(4):422–429. doi: 10.1111/j.1600-079X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 125.Brodsky V.Y., Zvezdina N.D. Melatonin as the most effective organizer of the rhythm of protein synthesis in hepatocytes in vitro and in vivo . . Cell Biol. Int. 2010;34(12):1199–1204. doi: 10.1042/CBI20100036. [DOI] [PubMed] [Google Scholar]
- 126.Hardeland R., Cardinali D.P., Srinivasan V., Spence D.W., Brown G.M., Pandi-Perumal S.R. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011;93(3):350–384. doi: 10.1016/j.pneurobio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 127.Gatfield D., Schibler U. Physiology. Proteasomes keep the circadian clock ticking. Science. 2007;316(5828):1135–1136. doi: 10.1126/science.1144165. [DOI] [PubMed] [Google Scholar]
- 128.Pevet P., Challet E., Felder-Schmittbuhl M-P. Handb. Clin. Neurol. Elsevier; 2021. Melatonin and the circadian system: Keys for health with a focus on sleep. pp. 331–343. [DOI] [PubMed] [Google Scholar]
- 129.Brickley S.G., Franks N.P., Wisden W. Modulation of GABAA receptor function and sleep. Curr. Opin. Physiol. 2018;2:51–57. doi: 10.1016/j.cophys.2017.12.011. [DOI] [Google Scholar]
- 130.Golombek D.A., Pévet P., Cardinali D.P. Melatonin effects on behavior: Possible mediation by the central GABAergic system. Neurosci. Biobehav. Rev. 1996;20(3):403–412. doi: 10.1016/0149-7634(95)00052-6. [DOI] [PubMed] [Google Scholar]
- 131.Nave R., Herer P., Haimov I., Shlitner A., Lavie P. Hypnotic and hypothermic effects of melatonin on daytime sleep in humans: Lack of antagonism by flumazenil. Neurosci. Lett. 1996;214(2-3):123–126. doi: 10.1016/0304-3940(96)12899-8. [DOI] [PubMed] [Google Scholar]
- 132.Arendt J. Melatonin: Countering chaotic time cues. Front. Endocrinol. (Lausanne) 2019;10:391. doi: 10.3389/fendo.2019.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dawson D., Encel N. Melatonin and sleep in humans. J. Pineal Res. 1993;15(1):1–12. doi: 10.1111/j.1600-079X.1993.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 134.Deacon S., Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688(1-2):77–85. doi: 10.1016/0006-8993(95)96872-I. [DOI] [PubMed] [Google Scholar]
- 135.Kräuchi K., Cajochen C., Wirz-Justice A. A relationship between heat loss and sleepiness: Effects of postural change and melatonin administration. J. Appl. Physiol. 1997;83(1):134–139. doi: 10.1152/jappl.1997.83.1.134. [DOI] [PubMed] [Google Scholar]
- 136.Cajochen C., Kräuchi K., Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J. Neuroendocrinol. 2003;15(4):432–437. doi: 10.1046/j.1365-2826.2003.00989.x. [DOI] [PubMed] [Google Scholar]
- 137.Satomura T., Sakamoto T., Shirakawa S., Tsutsumi Y., Mukai M., Ohyama T., Uchimura N., Maeda H. Hypnotic action of melatonin during daytime administration and its comparison with triazolam. Psychiatry Clin. Neurosci. 2001;55(3):303–304. doi: 10.1046/j.1440-1819.2001.00868.x. [DOI] [PubMed] [Google Scholar]
- 138.Huang Y., Li Y., Leng Z. Melatonin inhibits GABAergic neurons in the hypothalamus consistent with a reduction in wakefulness. Neuroreport. 2020;31(2):92–98. doi: 10.1097/WNR.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 139.Sharma R., Sahota P., Thakkar M.M. Melatonin promotes sleep in mice by inhibiting orexin neurons in the perifornical lateral hypothalamus. J. Pineal Res. 2018;65(2):e12498. doi: 10.1111/jpi.12498. [DOI] [PubMed] [Google Scholar]
- 140.Comai S., Ochoa-Sanchez R., Dominguez-Lopez S., Bambico F.R., Gobbi G. Melancholic-Like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int. J. Neuropsychopharmacol. 2015;18(3):1–10. doi: 10.1093/ijnp/pyu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brown R.E., Basheer R., McKenna J.T., Strecker R.E., McCarley R.W. Control of sleep and wakefulness. Physiol. Rev. 2012;92(3):1087–1187. doi: 10.1152/physrev.00032.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gandhi A.V. The Regulation of Sleep and Circadian Rhythms: The Role of Melatonin and Adenosine in Zebrafish. Dissertation, California Institute of Technology. [DOI]
- 143.Zamorskii I.I., Pishak V.P. Effect of melatonin on the intensity of adenosine production in the rat forebrain under conditions of acute hypoxia and varied photoperiodicity. Neurophysiology. 2003;35:44–47. doi: 10.1023/A:1023998306826. [DOI] [Google Scholar]
- 144.Niu L., Li Y., Zong P., Liu P., Shui Y., Chen B., Wang Z.W. Melatonin promotes sleep by activating the BK channel in C. elegans. . Proc. Natl. Acad. Sci. USA. 2020;117(40):25128–25137. doi: 10.1073/pnas.2010928117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lewy A.J., Sack R.L. The dim light melatonin onset as a marker for circadian phase position. Chronobiol. Int. 1989;6(1):93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 146.Burgess H.J., Fogg L.F. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3(8):e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Deacon S., English J., Arendt J. Acute phase-shifting effects of melatonin associated with suppression of core body temperature in humans. Neurosci. Lett. 1994;178(1):32–34. doi: 10.1016/0304-3940(94)90282-8. [DOI] [PubMed] [Google Scholar]
- 148.Zaidan R., Geoffriau M., Brun J., Taillard J., Bureau C., Chazot G., Claustrat B. Melatonin is able to influence its secretion in humans: Description of a phase-response curve. Neuroendocrinology. 1994;60(1):105–112. doi: 10.1159/000126726. [DOI] [PubMed] [Google Scholar]
- 149.Kräuchi K., Cajochen C., Möri D., Graw P., Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am. J. Physiol. 1997;272(4 Pt 2):R1178–R1188. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- 150.Yang C.M., Spielman A.J., D’Ambrosio P., Serizawa S., Nunes J., Birnbaum J. A single dose of melatonin prevents the phase delay associated with a delayed weekend sleep pattern. Sleep. 2001;24(3):272–281. doi: 10.1093/sleep/24.3.272. [DOI] [PubMed] [Google Scholar]
- 151.Wirz-Justice A., Werth E., Renz C., Müller S., Kräuchi K. No evidence for a phase delay in human circadian rhythms after a single morning melatonin administration. J. Pineal Res. 2002;32(1):1–5. doi: 10.1034/j.1600-079x.2002.10808.x. [DOI] [PubMed] [Google Scholar]
- 152.Cajochen C., Kräuchi K., Möri D., Graw P., Wirz-Justice A. Melatonin and S-20098 increase REM sleep and wake-up propensity without modifying NREM sleep homeostasis. Am. J. Physiol. 1997;272(4 Pt 2):R1189–R1196. doi: 10.1152/ajpregu.1997.272.4.R1189. [DOI] [PubMed] [Google Scholar]
- 153.Wright J., Aldhous M., Franey C., English J., Arendt J. The effects of exogenous melatonin on endocrine function in man. Clin. Endocrinol. (Oxf.) 1986;24(4):375–382. doi: 10.1111/j.1365-2265.1986.tb01641.x. [DOI] [PubMed] [Google Scholar]
- 154.Middleton B., Arendt J., Stone B.M. Complex effects of melatonin on human circadian rhythms in constant dim light. J. Biol. Rhythms. 1997;12(5):467–477. doi: 10.1177/074873049701200508. [DOI] [PubMed] [Google Scholar]
- 155.Attenburrow M.E.J., Dowling B.A., Sargent P.A., Sharpley A.L., Cowen P.J. Melatonin phase advances circadian rhythm. Psychopharmacology (Berl.) 1995;121(4):503–505. doi: 10.1007/BF02246501. [DOI] [PubMed] [Google Scholar]
- 156.Burgess H.J., Revell V.L., Molina T.A., Eastman C.I. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. . J. Clin. Endocrinol. Metab. 2010;95(7):3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burgess H.J., Revell V.L., Eastman C.I. A three pulse phase response curve to three milligrams of melatonin in humans. J. Physiol. 2008;586(2):639–647. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Terzolo M., Piovesan A., Puligheddu B., Torta M., Osella G., Paccotti P., Angeli A. Effects of long-term, low-dose, time-specified melatonin administration on endocrine and cardiovascular variables in adult men. J. Pineal Res. 1990;9(2):113–124. doi: 10.1111/j.1600-079X.1990.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 159.Mallo C., Zaidan R., Faure A., Brun J., Chazot G., Claustrat B. Effects of a four-day nocturnal melatonin treatment on the 24 h plasma melatonin, cortisol and prolactin profiles in humans. Acta Endocrinol. (Copenh.) 1988;119(4):474–480. doi: 10.1530/acta.0.1190474. [DOI] [PubMed] [Google Scholar]
- 160.Rajaratnam S.M.W., Dijk D.J., Middleton B., Stone B.M., Arendt J. Melatonin phase-shifts human circadian rhythms with no evidence of changes in the duration of endogenous melatonin secretion or the 24-hour production of reproductive hormones. J. Clin. Endocrinol. Metab. 2003;88(9):4303–4309. doi: 10.1210/jc.2003-030460. [DOI] [PubMed] [Google Scholar]
- 161.Rajaratnam S.M.W., Middleton B., Stone B.M., Arendt J., Dijk D-J. Melatonin advances the circadian timing of EEG sleep and directly facilitates sleep without altering its duration in extended sleep opportunities in humans. J. Physiol. 2004;561(Pt 1):339–351. doi: 10.1113/jphysiol.2004.073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Arendt J., Aldhous M., Wright J. Synchronisation of a disturbed sleep-wake cycle in a blind man by melatonin treatment. Lancet. 1988;1(8588):772–773. doi: 10.1016/S0140-6736(88)91586-3. [DOI] [PubMed] [Google Scholar]
- 163.Folkard S., Arendt J., Aldhous M., Kennett H. Melatonin stabilises sleep onset time in a blind man without entrainment of cortisol or temperature rhythms. Neurosci. Lett. 1990;113(2):193–198. doi: 10.1016/0304-3940(90)90302-P. [DOI] [PubMed] [Google Scholar]
- 164.Sarrafzadeh A., Wirz-Justice A., Arendt J., English J. Melatonin stabilises sleep onset in a blind man. Sleep. 1990;90:51–54. [Google Scholar]
- 165.Lewy A.J., Emens J.S., Sack R.L., Hasler B.P., Bernert R.A. Low, but not high, doses of melatonin entrained a free-running blind person with a long circadian period. Chronobiol. Int. 2002;19(3):649–658. doi: 10.1081/CBI-120004546. [DOI] [PubMed] [Google Scholar]
- 166.Sack R.L., Lewy A.J., Blood M.L., Stevenson J., Keith L.D. Melatonin administration to blind people: Phase advances and entrainment. J. Biol. Rhythms. 1991;6(3):249–261. doi: 10.1177/074873049100600305. [DOI] [PubMed] [Google Scholar]
- 167.Lockley S.W., Skene D.J., James K., Thapan K., Wright J., Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J. Endocrinol. 2000;164(1):R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 168.Sack R.L., Brandes R.W., Kendall A.R., Lewy A.J. Entrainment of free-running circadian rhythms by melatonin in blind people. N. Engl. J. Med. 2000;343(15):1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 169.Hack L.M., Lockley S.W., Arendt J., Skene D.J. The effects of low-dose 0.5-mg melatonin on the free-running circadian rhythms of blind subjects. J. Biol. Rhythms. 2003;18(5):420–429. doi: 10.1177/0748730403256796. [DOI] [PubMed] [Google Scholar]
- 170.Lewy A.J., Bauer V.K., Hasler B.P., Kendall A.R., Pires M.L.N., Sack R.L. Capturing the circadian rhythms of free-running blind people with 0.5 mg melatonin. Brain Res. 2001;918(1-2):96–100. doi: 10.1016/S0006-8993(01)02964-X. [DOI] [PubMed] [Google Scholar]
- 171.McArthur A.J., Lewy A.J., Sack R.L. Non-24-hour sleep-wake syndrome in a sighted man: Circadian rhythm studies and efficacy of melatonin treatment. Sleep. 1996;19(7):544–553. doi: 10.1093/sleep/19.7.544. [DOI] [PubMed] [Google Scholar]
- 172.Lewy A.J., Emens J.S., Bernert R.A., Lefler B.J. Eventual entrainment of the human circadian pacemaker by melatonin is independent of the circadian phase of treatment initiation: Clinical implications. J. Biol. Rhythms. 2004;19(1):68–75. doi: 10.1177/0748730403259670. [DOI] [PubMed] [Google Scholar]
- 173.Siebler M., Steinmetz H., Freund H-J. Therapeutic entrainment of circadian rhythm disorder by melatonin in a non-blind patient. J. Neurol. 1998;245(6-7):327–328. doi: 10.1007/s004150050228. [DOI] [PubMed] [Google Scholar]
- 174.Kato M., Kajimura N., Sekimoto M., Watanabe T., Takahashi K. Melatonin treatment for rhythm disorder. Psychiatry Clin. Neurosci. 1998;52(2):262–263. doi: 10.1111/j.1440-1819.1998.tb01065.x. [DOI] [PubMed] [Google Scholar]
- 175.Dahlitz M., Alvarez B., Vignau J., English J., Arendt J., Parkes J.D. Delayed sleep phase syndrome response to melatonin. Lancet. 1991;337(8750):1121–1124. doi: 10.1016/0140-6736(91)92787-3. [DOI] [PubMed] [Google Scholar]
- 176.Alvarez B., Dahlitz M.J., Vignau J., Parkes J.D. The delayed sleep phase syndrome: Clinical and investigative findings in 14 subjects. J. Neurol. Neurosurg. Psychiatry. 1992;55(8):665–670. doi: 10.1136/jnnp.55.8.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oldani A., Ferini-Strambi L., Zucconi M., Stankov B., Fraschini F., Smirne S. Melatonin and delayed sleep phase syndrome: Ambulatory polygraphic evaluation. Neuroreport. 1994;6(1):132–134. doi: 10.1097/00001756-199412300-00034. [DOI] [PubMed] [Google Scholar]
- 178.Kayumov L., Brown G., Jindal R., Buttoo K., Shapiro C.M. A randomized, double-blind, placebo-controlled crossover study of the effect of exogenous melatonin on delayed sleep phase syndrome. Psychosom. Med. 2001;63(1):40–48. doi: 10.1097/00006842-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 179.Saxvig I.W., Wilhelmsen-Langeland A., Pallesen S., Vedaa O., Nordhus I.H., Bjorvatn B. A randomized controlled trial with bright light and melatonin for delayed sleep phase disorder: Effects on subjective and objective sleep. Chronobiol. Int. 2014;31(1):72–86. doi: 10.3109/07420528.2013.823200. [DOI] [PubMed] [Google Scholar]
- 180.Nagtegaal J.E., Kerkhof G.A., Smits M.G., Swart A.C.W., Van Der Meer Y.G. Delayed sleep phase syndrome: A placebo-controlled cross-over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J. Sleep Res. 1998;7(2):135–143. doi: 10.1046/j.1365-2869.1998.00102.x. [DOI] [PubMed] [Google Scholar]
- 181.Mundey K., Benloucif S., Harsanyi K., Dubocovich M.L., Zee P.C. Phase-dependent treatment of delayed sleep phase syndrome with melatonin. Sleep. 2005;28(10):1271–1278. doi: 10.1093/sleep/28.10.1271. [DOI] [PubMed] [Google Scholar]
- 182.Sletten T.L., Magee M., Murray J.M., Gordon C.J., Lovato N., Kennaway D.J., Gwini S.M., Bartlett D.J., Lockley S.W., Lack L.C., Grunstein R.R., Rajaratnam S.M.W. Efficacy of melatonin with behavioural sleep-wake scheduling for delayed sleep-wake phase disorder: A double-blind, randomised clinical trial. PLoS Med. 2018;15(6):e1002587. doi: 10.1371/journal.pmed.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Abbas A., Raju J., Milles J., Ramachandran S. A circadian rhythm sleep disorder: Melatonin resets the biological clock. J. R. Coll. Physicians Edinb. 2010;40(4):311–313. doi: 10.4997/JRCPE.2010.406. [DOI] [PubMed] [Google Scholar]
- 184.Tzischinsky O., Pal I., Epstein R., Dagan Y., Lavie P. The importance of timing in melatonin administration in a blind man. J. Pineal Res. 1992;12(3):105–108. doi: 10.1111/j.1600-079X.1992.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 185.Okawa M., Uchiyama M., Ozaki S., Shibui K., Kamei Y., Hayakawa T., Urata J. Melatonin treatment for circadian rhythm sleep disorders. Psychiatry Clin. Neurosci. 1998;52(2):259–260. doi: 10.1111/j.1440-1819.1998.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 186.Kamei Y., Hayakawa T., Urata J., Uchiyama M., Shibui K., Kim K., Kudo Y., Okawa M. Melatonin treatment for circadian rhythm sleep disorders. Psychiatry Clin. Neurosci. 2000;54(3):381–382. doi: 10.1046/j.1440-1819.2000.00724.x. [DOI] [PubMed] [Google Scholar]
- 187.Dagan Y., Yovel I., Hallis D., Eisenstein M., Raichik I. Evaluating the role of melatonin in the long-term treatment of delayed sleep phase syndrome (DSPS). Chronobiol. Int. 1998;15(2):181–190. doi: 10.3109/07420529808998682. [DOI] [PubMed] [Google Scholar]
- 188.Laurant M., Nagtegaal J.E., Smits M.G., Kerkhof G.A., Coenen A.M.L. Influence of melatonin on vigilance and cognitive processing speed in delayed sleep phase syndrome. Int Chronobiol; 1997. pp. 79–81. [Google Scholar]
- 189.Rahman S.A., Kayumov L., Shapiro C.M. Antidepressant action of melatonin in the treatment of Delayed Sleep Phase Syndrome. Sleep Med. 2010;11(2):131–136. doi: 10.1016/j.sleep.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 190.Eastman C.I., Burgess H.J. How To Travel the World Without Jet lag. Sleep Med. Clin. 2009;4(2):241–255. doi: 10.1016/j.jsmc.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Auger R.R., Burgess H.J., Emens J.S., Deriy L.V., Thomas S.M., Sharkey K.M. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2015;11(10):1199–1236. doi: 10.5664/jcsm.5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Singer C., Tractenberg R.E., Kaye J., Schafer K., Gamst A., Grundman M., Thomas R., Thal L.J. A multicenter, placebo-controlled trial of melatonin for sleep disturbance in Alzheimer’s disease. Sleep. 2003;26(7):893–901. doi: 10.1093/sleep/26.7.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Serfaty M., Kennell-Webb S., Warner J., Blizard R., Raven P. Double blind randomised placebo controlled trial of low dose melatonin for sleep disorders in dementia. Int. J. Geriatr. Psychiatry. 2002;17(12):1120–1127. doi: 10.1002/gps.760. [DOI] [PubMed] [Google Scholar]
- 194.Roth T., Richardson G. Commentary: Is melatonin administration an effective hypnotic? J. Biol. Rhythms. 1997;12(6):666–669. doi: 10.1177/074873049701200623. [DOI] [PubMed] [Google Scholar]
- 195.Lavie P. Melatonin: Role in gating nocturnal rise in sleep propensity. J. Biol. Rhythms. 1997;12(6):657–665. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- 196.Dollins A.B., Lynch H.J., Wurtman R.J., Deng M.H., Kischka K.U., Gleason R.E., Lieberman H.R. Effect of pharmacological daytime doses of melatonin on human mood and performance. Psychopharmacology (Berl.) 1993;112(4):490–496. doi: 10.1007/BF02244899. [DOI] [PubMed] [Google Scholar]
- 197.Van Den Heuvel C.J., Kennaway D.J., Dawson D. Effects of daytime melatonin infusion in young adults. Am. J. Physiol. 1998;275(1):E19–E26. doi: 10.1152/ajpendo.1998.275.1.E19. [DOI] [PubMed] [Google Scholar]
- 198.Mishima K., Satoh K., Shimizu T., Hishikawa Y. Hypnotic and hypothermic action of daytime-administered melatonin. Psychopharmacology (Berl.) 1997;133(2):168–171. doi: 10.1007/s002130050387. [DOI] [PubMed] [Google Scholar]
- 199.Aeschbach D., Lockyer B.J., Dijk D-J., Lockley S.W., Nuwayser E.S., Nichols L.D., Czeisler C.A. Use of transdermal melatonin delivery to improve sleep maintenance during daytime. Clin. Pharmacol. Ther. 2009;86(4):378–382. doi: 10.1038/clpt.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rogers N.L., Kennaway D.J., Dawson D. Neurobehavioural performance effects of daytime melatonin and temazepam administration. J. Sleep Res. 2003;12(3):207–212. doi: 10.1046/j.1365-2869.2003.00360.x. [DOI] [PubMed] [Google Scholar]
- 201.Cajochen C., Kräuchi K., von Arx M.A., Möri D., Graw P., Wirz-Justice A. Daytime melatonin administration enhances sleepiness and theta/alpha activity in the waking EEG. Neurosci. Lett. 1996;207(3):209–213. doi: 10.1016/0304-3940(96)12517-9. [DOI] [PubMed] [Google Scholar]
- 202.Dijk D-J., Roth C., Landolt H-P., Werth E., Aeppli M., Achermann P., Borbély A.A. Melatonin effect on daytime sleep in men: Suppression of EEG low frequency activity and enhancement of spindle frequency activity. Neurosci. Lett. 1995;201(1):13–16. doi: 10.1016/0304-3940(95)12118-n. [DOI] [PubMed] [Google Scholar]
- 203.Cramer H., Rudolph J., Consbruch U., Kendel K. On the effects of melatonin on sleep and behavior in man. Adv. Biochem. Psychopharmacol. 1974;11(0):187–191. [PubMed] [Google Scholar]
- 204.Waldhauser F., Saletu B., Trinchard-Lugan I. Sleep laboratory investigations on hypnotic properties of melatonin. Psychopharmacology (Berl.) 1990;100(2):222–226. doi: 10.1007/BF02244410. [DOI] [PubMed] [Google Scholar]
- 205.Pinto L.R., Jr Seabra, Mde.L.; Tufik, S. Different criteria of sleep latency and the effect of melatonin on sleep consolidation. Sleep. 2004;27(6):1089–1092. doi: 10.1093/sleep/27.6.1089. [DOI] [PubMed] [Google Scholar]
- 206.Arbon E.L., Knurowska M., Dijk D.J. Randomised clinical trial of the effects of prolonged-release melatonin, temazepam and zolpidem on slow-wave activity during sleep in healthy people. J. Psychopharmacol. 2015;29(7):764–776. doi: 10.1177/0269881115581963. [DOI] [PubMed] [Google Scholar]
- 207.Pires M.L., Benedito-Silva A.A., Pinto L., Souza L., Vismari L., Calil H.M. Acute effects of low doses of melatonin on the sleep of young healthy subjects. J. Pineal Res. 2001;31(4):326–332. doi: 10.1034/j.1600-079X.2001.310407.x. [DOI] [PubMed] [Google Scholar]
- 208.Stone B.M., Turner C., Mills S.L., Nicholson A.N. Hypnotic activity of melatonin. Sleep. 2000;23(5):663–669. doi: 10.1093/sleep/23.5.1i. [DOI] [PubMed] [Google Scholar]
- 209.Wyatt J.K., Dijk D.J., Ritz-de Cecco A., Ronda J.M., Czeisler C.A. Sleep-facilitating effect of exogenous melatonin in healthy young men and women is circadian-phase dependent. Sleep. 2006;29(5):609–618. doi: 10.1093/sleep/29.5.609. [DOI] [PubMed] [Google Scholar]
- 210.Tzischinsky O., Lavie P. Melatonin possesses time-dependent hypnotic effects. Sleep. 1994;17(7):638–645. doi: 10.1093/sleep/17.7.638. [DOI] [PubMed] [Google Scholar]
- 211.MacFarlane J.G., Cleghorn J.M., Brown G.M., Streiner D.L. The effects of exogenous melatonin on the total sleep time and daytime alertness of chronic insomniacs: A preliminary study. Biol. Psychiatry. 1991;30(4):371–376. doi: 10.1016/0006-3223(91)90293-U. [DOI] [PubMed] [Google Scholar]
- 212.Xu H., Zhang C., Qian Y., Zou J., Li X., Liu Y., Zhu H., Meng L., Liu S., Zhang W., Yi H., Guan J., Chen Z., Yin S. Efficacy of melatonin for sleep disturbance in middle-aged primary insomnia: A double-blind, randomised clinical trial. Sleep Med. 2020;76:113–119. doi: 10.1016/j.sleep.2020.10.018. [DOI] [PubMed] [Google Scholar]
- 213.Baskett J.J., Broad J.B., Wood P.C., Duncan J.R., Pledger M.J., English J., Arendt J. Does melatonin improve sleep in older people? A randomised crossover trial. Age Ageing. 2003;32(2):164–170. doi: 10.1093/ageing/32.2.164. [DOI] [PubMed] [Google Scholar]
- 214.Chung S., Youn S., Park B., Lee S., Kim C. The Effectiveness of Prolonged-Release Melatonin in Primary Insomnia Patients with a Regular Sleep-Wake Cycle. Sleep Med. Res. 2016;7:16–20. doi: 10.17241/smr.2016.00017. [DOI] [Google Scholar]
- 215.Garfinkel D., Zisapel N., Wainstein J., Laudon M. Facilitation of benzodiazepine discontinuation by melatonin: A new clinical approach. Arch. Intern. Med. 1999;159(20):2456–2460. doi: 10.1001/archinte.159.20.2456. [DOI] [PubMed] [Google Scholar]
- 216.Li T., Jiang S., Han M., Yang Z., Lv J., Deng C., Reiter R.J., Yang Y. Exogenous melatonin as a treatment for secondary sleep disorders: A systematic review and meta-analysis. Front. Neuroendocrinol. 2019;52:22–28. doi: 10.1016/j.yfrne.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 217.Bruni O., Alonso-Alconada D., Besag F., Biran V., Braam W., Cortese S., Moavero R., Parisi P., Smits M., Van der Heijden K., Curatolo P. Current role of melatonin in pediatric neurology: Clinical recommendations. Eur. J. Paediatr. Neurol. 2015;19(2):122–133. doi: 10.1016/j.ejpn.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 218.Abdelgadir I.S., Gordon M.A., Akobeng A.K. Melatonin for the management of sleep problems in children with neurodevelopmental disorders: A systematic review and meta-analysis. Arch. Dis. Child. 2018;103(12):1155–1162. doi: 10.1136/archdischild-2017-314181. [DOI] [PubMed] [Google Scholar]
- 219.Schwichtenberg A.J., Malow B.A. Melatonin treatment in children with developmental disabilities. Sleep Med. Clin. 2015;10(2):181–187. doi: 10.1016/j.jsmc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lalanne S., Fougerou-Leurent C., Anderson G.M., Schroder C.M., Nir T., Chokron S. Molecular sciences melatonin: From pharmacokinetics to clinical use in autism spectrum disorder melatonin: From pharmacokinetics to clinical use in autism spectrum. Disord. Int. J. Mol. Sci. 2021;22:1490. doi: 10.3390/ijms22031490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Palagini L., Manni R., Aguglia E., Amore M., Brugnoli R., Bioulac S., Bourgin P., Micoulaud Franchi J.A., Girardi P., Grassi L., Lopez R., Mencacci C., Plazzi G., Maruani J., Minervino A., Philip P., Royant Parola S., Poirot I., Nobili L., Biggio G., Schroder C.M., Geoffroy P.A. International expert opinions and recommendations on the use of melatonin in the treatment of insomnia and circadian sleep disturbances in adult neuropsychiatric disorders. Front. Psychiatry. 2021;12:688890. doi: 10.3389/fpsyt.2021.688890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Auld F., Maschauer E.L., Morrison I., Skene D.J., Riha R.L. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med. Rev. 2017;34:10–22. doi: 10.1016/j.smrv.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 223.Ferracioli-Oda E., Qawasmi A., Bloch M.H. Meta-analysis: Melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8(5):e63773. doi: 10.1371/journal.pone.0063773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lemoine P., Zisapel N. Prolonged-release formulation of melatonin (Circadin) for the treatment of insomnia. Expert Opin. Pharmacother. 2012;13(6):895–905. doi: 10.1517/14656566.2012.667076. [DOI] [PubMed] [Google Scholar]
- 225.Gonzalez R. In: Melatonin, Neuroprotective Agents and Antidepressant Therapy. Melatonin, Neuroprotective Agents Antidepressant Ther; 2016. pp. 297–317. [DOI] [Google Scholar]
- 226.Govitrapong P., Ekthuwapranee K., Ruksee N., Boontem P. Melatonin, a neuroprotective agent: Relevance for stress-induced neuropsychiatric disorders. In: López-Muñoz F., Srinivasan V., de Berardis D., Álamo C., Kato T., editors. Melatonin, Neuroprotective Agents and Antidepressant Therapy. New Delhi: Springer; 2016. pp. 101–115. [DOI] [Google Scholar]
- 227.Gerdin M.J., Masana M.I., Dubocovich M.L. Melatonin-mediated regulation of human MT(1) melatonin receptors expressed in mammalian cells. Biochem. Pharmacol. 2004;67(11):2023–2030. doi: 10.1016/j.bcp.2004.01.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.