Abstract
Bipolar disorders (BDs) are a heterogeneous group of severe affective disorders generally described by the alternation of (hypo)manic, depressive, and mixed phases, with euthymic intervals of variable duration. BDs are burdened with high psychiatric and physical comorbidity, increased suicide risk and reduced life expectancy. In addition, BDs can progress into complicated forms (e.g., mixed states, rapid/irregular cycling), which are more difficult to treat and often require personalized pharmacological combinations. Mood stabilizers, particularly Lithium and Valproic acid (VPA), still represent the cornerstones of both acute and chronic pharmacotherapies of BDs. Lithium is the gold standard in BD-I and BDII with typical features, while VPA seems more effective for atypical forms (e.g., mixed-prevalence and rapid-cycling). However, despite appropriate mood stabilization, many patients show residual symptoms, and more than a half recur within 1-2 years, highlighting the need of additional strategies. Among these, the association of atypical antipsychotics (AAPs) with mood stabilizers is recurrent in the treatment of acute phases, but it is also being growingly explored in the maintenance pharmacotherapy. These combinations are clinically more aggressive and often needed in the acute phases, whereas simplifying pharmacotherapies to mood stabilizers only is preferable in the long-term, whenever possible. When mood stabilizers are not enough for maintenance treatment, Quetiapine and, less consistently, Aripiprazole have been proposed as the most advisable adjunctive strategies, for their safety and tolerability profiles. However, in view of the increased risk of serious adverse effects, a careful patient-centered balance between costs and benefits is mandatory.
Keywords: Bipolar disorders, lithium, valproic acid, antipsychotics, acute treatment, chronic management
1. INTRODUCTION
Bipolar disorder (BD) is an affective disorder described by periods of elevated mood alternated with longer periods of depression and asymptomatic intervals, namely, euthymic intervals. It is a severe medical condition characterized by psychosocial impairment, increased risk of suicide, and high rates of comorbidity with several other medical conditions with an overall reduced life expectancy [1]. BD has a high inheritability of about 70% with a concordance of 40-50% in identical twins. In constitutively vulnerable individuals, negative environmental factors, such as childhood trauma and substance use, can influence the onset and severity of the illness. Although the pathogenesis of BD is unknown, deficits in neuronal/glial cell plasticity, monoaminergic activity, inflammatory responses, cellular metabolic pathways, and mitochondrial function have been proposed [2]. According to recent epidemiological analyses, the prevalence of the diverse phenotypes of the so-called bipolar spectrum in the general population is about 5%. Among the different BD subtypes, BD-I is defined by at least one manic episode, while in BD-II expansive phases do not fulfill diagnostic criteria for mania, do not require hospitalization, and are therefore termed “hypomania”. In addition, patients could develop more complex phenomenologies, such as mixed states, in which depressive and (hypo)manic symptoms co-exist, albeit with a huge variety of combinations (from anxious depression to irritable-dysphoric mania). Disease history could also complicate over time, with a reduction in the number and length of free intervals and a progressive trend toward a chronic unremitting course. These BD subtypes are referred to as rapid cycling and are diagnosed when patients develop at least four mood episodes within a year. Factors that influence the severity of the illness include younger age of onset [3], psychotic features [4], persistent subthreshold symptoms [5], rapid cycling [6], the number of previous episodes [7], the comorbidity with other disorders, and the presence of residual symptoms during the euthymic phase. Interestingly, some evidence indicates that a subgroup of BD patients may have a neurodegenerative course in which recurrences are associated with reductions in grey and white matter volumes, worsening cognitive impairment with a higher rate of relapse, and a reduced rate of response to the pharmacological treatment [8]. In particular, the comorbidity of BD with two or more psychiatric disorders involves more than 50% of patients and is a predictor of poor outcomes [9, 10]. The most common comorbid conditions are substance use disorder (e.g., alcohol use disorder; AUD), anxiety disorder, personality disorders, and attention deficit hyperactivity disorder (ADHD) [11, 12]. Similarly, BD forms characterized by prevalent mixed states or complicated courses (atypical, rapid, continuous, or irregular cycling) are more difficult to manage through the available pharmacological strategies [13-15]. Therefore, the heterogeneity of BD, both inter- and intra-individually, is apparently too broad to be thoroughly embraced within any single didactic definition, and so is its management to be tailored to a unique patient in a unique segment of his life. Taking these considerations for granted, it can be added that the management of BD consists of two different moments: (a) the treatment of acute episodes, whether manic or depressive and (b) the long-term prevention of further episodes, with the purpose to prolong free intervals as much as possible. Long-term relapse prevention is a real challenge in BD treatment, with up to 50-60% of patients relapsing after 1-2 years following remission. Several drugs have been found to be effective in treating BDs, both in the acute and the chronic phases. The most widely employed are lithium salts, which still represent the load-bearing pillar in the long-term treatment of both BD-I and BD-II. Valproic acid (VPA) is another major valuable option for BDs, either in monotherapy or in association with other drugs, including Lithium. Other important anticonvulsants customarily used as mood stabilizers are Carbamazepine, Oxcarbazepine, and Lamotrigine. Moreover, atypical antipsychotics (AAPs) have received much attention in the last two decades, especially in association with Lithium or VPA. Even though combination treatments can be more effective, possibly inducing quicker pharmacological responses, they often cause an increase in adverse reactions that need to be carefully evaluated in order to set a rational balance between costs and benefits [16]. Theoretically, drugs that are effective in the acute phase should be continued for the maintenance treatment, finding the best compromise for each patient between their efficacy and the risk of the onset of adverse effects in the long-term. Clinically, more aggressive combinations of mood stabilizers and AAPs are often needed and useful in the acute phases, whereas simplifying pharmacotherapies to mood stabilization only should be preferable in the long term [17]. Keeping all these premises in mind, with this review we attempt to draw up a rational set of recommendations on the most effective drugs used in the management of BD, with a special focus on Lithium, VPA and AAPs.
2. LITHIUM
Lithium salts are considered the prototypical drugs in the treatment of BDs, given their strong efficacy in inducing remission of acute phases (especially mania/hypomania) and reducing the probability of relapse in both polarities (so-called mood stabilization) [18, 19]. Predictors of response to Lithium include a family history of BD and lithium response, preserved free intervals between mood episodes, classical mania-depression-interval cycles, no overt comorbidities, no mixed features, and a lower number of episodes prior to lithium introduction [13, 20, 21]. On the other hand, a higher number of lifetime episodes, psychotic and mixed features, atypical cycles with mania-depression-interval sequence or irregular cycles, reduced or absent euthymic intervals, rapid-cycling, overt psychiatric comorbidities and substance use disorders (SUDs) are predictors of poor response to Lithium [20].
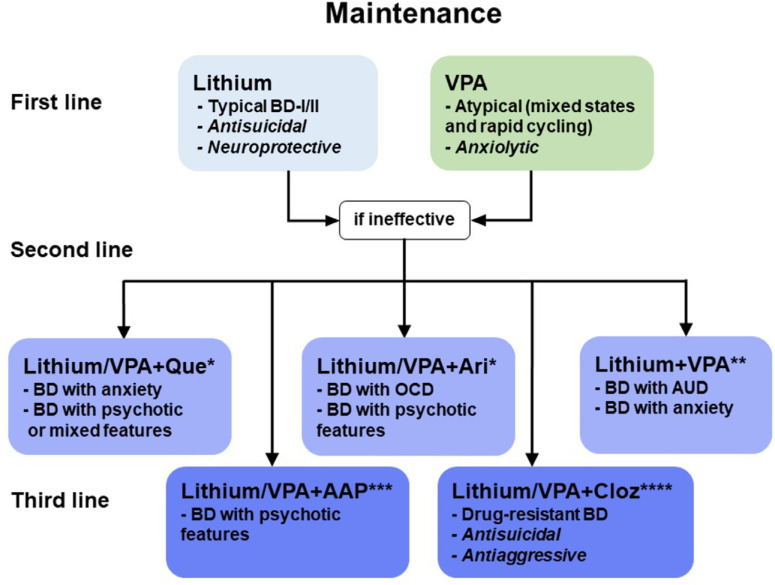
Several randomized controlled trials (RCT) have evaluated Lithium vs. placebo in patients during the acute manic phase, which have demonstrated lithium efficacy, both in terms of response and remission rates [22-24]. For the long-term maintenance treatment, a recent systematic review of international guidelines has confirmed Lithium as a first-line mood-stabilizing agent for mood episodes of prophylaxis in BDs (Fig. 1) [25-27]. In BD-I patients, the BALANCE study has clearly demonstrated Lithium monotherapy superiority over Valproate (VPA) and Quetiapine over time, with prevention of relapse for up to 2 years. This superiority was confirmed in other studies and meta-analysis [28, 29]. Lithium efficacy has also been proven in preventing mood episodes in BD-II where it is still considered a first-line treatment [30]. In three placebo-controlled RCTs conducted in the 80s for 1-2 years, Lithium decreased the frequency and severity of hypomanic and depressive episodes [31, 32].
Fig. (1).
Maintenance of the different types of BDs recommends the use of mood stabilizers (Lithium or VPA) as first-line treatment. When mood stabilizers are not enough, AAPs can be used as an adjunctive strategy, carefully monitoring the increased risk of serious adverse effects. *Quetiapine (Que) and, less consistently, Aripiprazole (Ari) are recommended as second-line treatment for their safety and tolerability compared to the other AAPs. **The association of Lithium and VPA is a second-line strategy in BD with AUD and anxiety. ***Stronger AAPs, like Risperidone and Olanzapine, are mostly recommended for BD with severe psychotic features as a third-line treatment for their risk of serious adverse reactions, especially in the long-term. ****Clozapine (Cloz) is recommended as a third-line treatment for drug-resistant BD, although its rare life-threatening side effects (e.g., agranulocytosis) impose a constant monitoring of patients’ conditions.
The role of Lithium in the treatment of acute depressive episodes is more controversial since its efficacy in monotherapy seems limited, and other classes of drugs, such as antidepressants, Quetiapine and, to some extent, Lamotrigine have been proven more effective in this context [33, 34]. However, there is good evidence for the efficacy of Lithium augmentation strategies in depressed patients poorly responsive to antidepressant monotherapy and some studies have shown that higher Lithium levels, ranging between 0.8-1.2 mM, might be required to increase its potential mood-elevating properties and prevent depressive phases [35, 36].
Apart from its mood episodes preventing effect, Lithium has also other important clinical properties. First of all, it is the only mood stabilizer that has been proven to be effective in preventing suicide, with a 90% decreased risk [37]. Considering that patients with BDs exhibit a 20-fold higher risk of suicide compared to the general population [38], suicide prevention represents a crucial clinical indication for Lithium therapy. Finally, Lithium’s unique neuroprotective and neurotrophic effects have been extensively proven in animal models and in some BD patients and this can be relevant for the treatments of specific forms of BD where a degenerative progression of the illness can be postulated and in late-onset forms that prelude by years the full-blown manifestation of neurodegenerative conditions [39-41].
Lithium has shown some efficacy in the treatment of BD comorbid with AUD [42, 43]. In a small RCT, there was a significant decrease in terms of the number of drinks per day and amount of alcohol intake. Despite its likely benefits, Lithium has to be used with caution in heavy drinkers because of potential electrolyte imbalance. In relation to the other concomitant SUD, Lithium either alone or in combination with other drugs, was proven partially useful in small studies addressing cannabis and cocaine use disorder [44-46]. Lithium might have an anti-abuse/anti-impulsive effect in complex patients with SUD, independently of the stabilizing effect on comorbid psychiatric diagnoses, enhancing self-constructive behaviors and improving psychosocial outcomes [47-50].
Considering that Lithium usually is administrated for many years or throughout the whole patient’s life, its harmful adverse effect on thyroid and kidney function has to be constantly monitored and it can be reduced through Lithium plasma monitoring [51, 52]. Particularly, this latter adverse effect limits its use in patients with significant renal impairment. On this matter, Lithium suspension can be problematic with a risk of recurrence of a manic or depressive episode, especially in the short term, depending on the polarity of the first episode before treatment. A rapid discontinuation of Lithium can increase this risk, whereas a gradual suspension may reduce the reappearance of these episodes [53, 54].
3. VALPROIC ACID (VPA)
VPA’s good tolerability and manageability in clinical practice make this compound a valuable option as an alternative to Lithium in the treatment of BD in general, either alone or in association, especially for patients that do not tolerate Lithium adverse reactions. Consistently, patients treated with VPA show lower discontinuation rates compared to Lithium, even though the effectiveness of VPA monotherapy in mood episode prevention might be weaker [55].
Clinical studies and real-world practice have confirmed the efficacy of VPA as a first-line treatment for both acute manic phases and prophylaxis of mood recurrences of BDs, primarily for manic episodes as Lithium [19, 55]. Clinical-controlled studies have clearly demonstrated VPA superiority over placebo, and meta-analyses have concluded that VPA is effective in preventing relapse of mood episodes [56]. Several RCTs have reported no significant differences in efficacy between VPA and Lithium in the treatment of both acute mood phases and mood stabilization [57, 58], although the data on VPA for maintenance treatment are scant compared to Lithium. VPA efficacy has been confirmed for treating BD-II, despite recent Canadian guidelines recommending it as a second-line treatment for this particular BD subtype [16].
Interestingly, the treatment of acute manic phases with attack dosages of VPA in the order of 20-30 mg/kg/die seems to increase the rapidity of the antimanic effect, similarly to what was observed with AAPs [59].
Due to its sedative and anxiolytic effects, VPA could be particularly useful in the acute management of agitated mixed manic-dysphoric patients [60]. Consistently, if Lithium is the best choice for typical BD-I and BD-II forms, VPA is more indicated for atypical and complicated forms of BD, such as rapid/continuous or irregular cycling courses and mixed states predominance [13, 61], characterized by the coexistence of depressive and hypomanic symptoms simultaneously, irritability and dysphoric mood (Fig. 1). Two meta-analyses have proposed that VPA is slightly more effective than placebo for treating depressive episodes [62, 63], but in this scenario, other drugs such as Quetiapine and antidepressants are preferred, where the latter have to be administrated cautiously due to risk of a manic switch and in association with a mood stabilizer [64]. Overall, VPA seemingly displays more of an anxiolytic rather than an antidepressant effect, both in the treatment of generalized anxiety and panic anxiety [65-67]. On this topic, several authors suggested favorable results with VPA in the case of comorbidity of BD with anxiety disorders [68-70]. One small RCT showed that VPA was more effective than a placebo in improving symptoms of anxiety in BD patients [71]. In a recent study, BD patients in association with comorbid anxiety disorder obtained a better improvement during maintenance treatment by VPA compared to Lithium [72]. In another follow-up study, BD patients with panic disorder comorbidity treated with VPA had a high percentage of remission [73].
In the case of SUD co-occurrent with BD, some studies have shown the usefulness of VPA in the treatment of alcohol abuse, which often complicates the course of BD. Preliminary but substantial evidence suggests the usefulness of VPA in the control of alcohol and sedatives-hypnotics withdrawal symptoms and as an anti-craving agent, contributing to reducing the risk of abuse relapse [12, 74]. In relation to other SUDs, similar to Lithium, there is limited evidence that VPA has some efficacy in cannabis and cocaine use disorder [46]. Relatedly, VPA seems to be effective in the acute management and long-term treatment of both trait and state impulse dyscontrol, mainly aggressive behaviors [75]. The use of VPA is generally discouraged in women of reproductive age, where its deleterious effect on the fetus constrains its use for this category [76].
4. LITHIUM AND VPA IN ASSOCIATION
Considering that Lithium and VPA are the most used mood stabilizers with different pharmacological and clinical profiles, it follows that their association is a valid option in clinical practice, especially in the management of complicated forms of BD, where monotherapies are hardly successful. In addition, this association seems to be sufficiently safe and tolerated by patients [77, 78]. Another point in favor of their use in combination comes from their different mechanism of action, despite some common pharmacodynamic targets being shared [79]. Although widely used in clinical practice, the evidence supporting the Lithium-VPA combination in acute phase treatment and relapse prevention is limited, being based mostly on uncontrolled trials [60, 77, 80].
Two small randomized controlled trials (RCTs) have shown some advantages in terms of time to mood relapse, but these results did not reach statistical significance [29, 45]. The BALANCE trial indicated that combination therapy was more effective than VPA to prevent relapse, but when compared to Lithium monotherapy, it could neither confirm nor refute the benefit of combination therapy. This association seemed more effective to prevent a manic episode, while for depression relapse, Lithium monotherapy was as effective as combined therapy [29].
The combination of these two drugs might be recommended for AUD comorbid with BD, where lithium and VPA seem to be more effective in association than in monotherapy in preventing alcohol abuse behaviors [45, 74, 81]. In addition, this combination has shown some promising clinical advantageousness over monotherapy in cocaine and cannabis use disorders associated with BD [82].
Whether VPA and Lithium association could allow reducing of the minimal effective dose of both drugs, significantly decreasing the risk of toxicity, is still an open question and it lacks solid scientific evidence. Surprisingly, there is an absolute paucity of literature addressing this topic and, to our knowledge, only one study has shown that when Lithium and VPA are used in combination, the therapeutic range for Lithium maintenance treatment reduces to 0.4 - 0.8 mmol/L [83].
5. LITHIUM OR VPA (LITHIUM/VPA) IN ASSOCIATION WITH AAPs
Besides the extensive use of mood stabilizers, such as Lithium and VPA in BDs, about half of patients relapse within 1-2 years, and they still maintain residual symptoms during free intervals, despite being under appropriate pharmacological therapy [5, 84]. In addition, during the manic or hypomanic episode, mood stabilizers might require 2-4 weeks to obtain a clinical response, and, in severe cases, especially with psychotic features, this delay can be detrimental [60, 85].
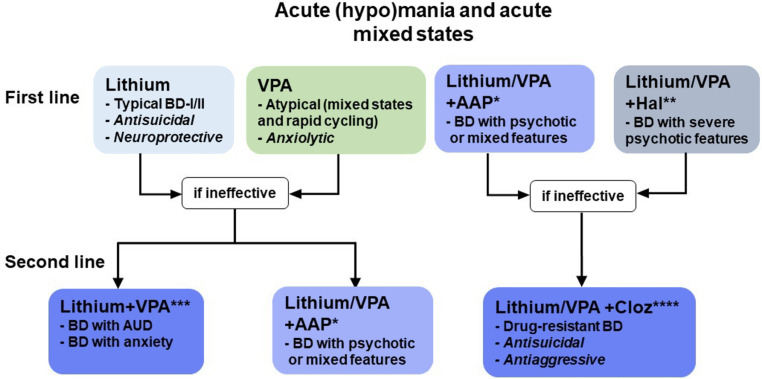
For these reasons, the employment of AAPs or typical antipsychotics (TAPs) in most severe cases, together with a mood stabilizer as add-on therapy is very common in clinical practice in the management of acute phases, although in some severe cases, it might be required also in maintenance treatment, carefully balancing the risk of depressive switches (Fig. 2) [86, 87]. On this topic, epidemiological data report that polypharmacy is prescribed more frequently than theoretically expected, while more studies are required to offer ground for this practice [88-90].
Fig. (2).
Acute (hypo)mania and acute mixed states recommend the use of mood stabilizers (Lithium or VPA) as first-line treatment. *AAPs can be used as first-line or second-line adjunctive treatment, depending on the severity of the episode (manic or hypomanic). Several AAPs can be utilized for these indications according to the patient’s characteristics, both in terms of response and tolerability. **The strong TAP Haloperidol can be used in the most severe cases of BD with psychotic features and constant monitoring of patient’s conditions. ***The association of Lithium and VPA is a second-line strategy in BD with AUD and anxiety, but less effective than Lithium/VPA + AAP for hypo(manic) episodes. ****Clozapine (Cloz) is recommended as a second or third-line treatment for drug-resistant BD, although its rare life-threatening side effects (e.g., agranulocytosis) impose constant monitoring of patients’ conditions.
As a matter of fact, data indicate that AAPs in combination therapies are also increasingly used for long-term maintenance treatments [91-93]. Undoubtedly, these combinations can obtain a rapid clinical remission of psychotic symptoms and agitation/aggressiveness that is hardly observed with classical mood stabilizers [94].
However, the potential benefits of AAPs for chronic use should be weighed very carefully along with the risk of adverse reactions, particularly in terms of metabolic dysregulation [95-97].
A few RCTs have found that strategies combining AAPs with mood stabilizers prolong the time to recurrence of any mood event (mania, depression, or mixed), mostly for manic phases prevention [98, 99]. However, this improvement has not always been confirmed [100, 101]. Among the several AAPs, the most studied in combination with Lithium or VPA in the treatment of BD, in order of relevance, are Quetiapine, Aripiprazole, Risperidone, Olanzapine, and Clozapine.
6. LITHIUM/VPA PLUS QUETIAPINE
Quetiapine is probably the most commonly used AAP in BDs for its good tolerability profile and broad clinical effect. Due to its sedative properties, one of its most common usage is the off-label treatment of insomnia and agitation [102]. Several authors have found evidence that Quetiapine alone or in association with a mood stabilizer improves sleep in BD patients, while in the general population, the evidence is still limited [103, 104]. Indeed, Quetiapine is a very potent antagonist at H1 receptors, and its H1 occupancy is about 90% already at the low dose of 50 mg [105], highlighting its sedative characteristics even at lower doses. Considering the relevance of sleep disturbances in several BD patients, including circadian rhythms dysregulation, several studies highlighted the utility of adjunctive melatonin as a sleep-promoting chronobiotic, together with its potential benefits on metabolic dysregulation [106-109].
In addition, it is considered a second choice for the treatment of resistant depression, although clinical data are only partially convincing on this aspect [110, 111].
For the treatment of BDs in the manic acute phase or maintenance, Quetiapine in combination with Lithium or VPA is generally considered one of the best adjunct options. On this topic, a study found an improvement in the acute clinical response of about 1/3 of patients treated with combination therapy [112]. For maintenance treatment of BDs, a few studies have shown that the addition of Quetiapine to a mood stabilizer improves the efficacy in preventing any mood episodes, either manic, depressive, or mixed, irrespective of the initial episode [95, 96, 98]. Quetiapine putative efficacy as adjunctive treatment in mixed states is worth mentioning considering the difficulty to treat this type of BD [113, 114].
Interestingly, there is preliminary evidence that Quetiapine significantly shows efficacy in the treatment of generalized anxiety disorder (GAD), panic disorder, and anxiety symptoms in patients with bipolar depression [115, 116]. In relation to SUD/BD co-occurrence, Quetiapine is not recommended for the treatment of AUD comorbid to BD because of lack of efficacy [74, 81]. Similarly, despite showing some potential efficacy for psychostimulant abuse treatment in earlier studies [117, 118], more recent accounts do not support a role for Quetiapine administration in this clinical context [119, 120].
The main limitation to long-term Quetiapine employment are metabolic and cardiovascular side effects, which include weight gain, impaired glucose metabolism, dyslipidemia, and hypertension, which could be already observable after 2-3 months of treatment and seem to be only partly dose-dependent and, therefore, hardly predictable (Table 1) [121-123].
Table 1.
Clinical differences among the most used AAPs in terms of adverse reactions (weight gain, T2D, dyslipidemia, and parkinsonism).
| AAPs | Weight Gain | Diabetes (T2D) | Dyslipidemia | Parkinsonism |
|---|---|---|---|---|
| Clozapine | +++ | +++ | +++ | 0 |
| Olanzapine | +++ | +++ | +++ | +/- |
| Quetiapine | ++ | ++ | ++ | +/- |
| Risperidone | ++ | + | + | ++ |
| Aripiprazole | + | + | + | + |
Note: Based mostly on meta-analysis studies (119), values are reported as high (+++), moderate (++), low (+), rare (+/-), and with no effect (0).
7. LITHIUM/VPA PLUS ARIPIPRAZOLE
Aripiprazole, alone or in combination with mood stabilizers, is effective for treating acute manic symptoms in BD patients with classical mania or mixed states [124, 125]. Moreover, Aripiprazole adjunction to Lithium or VPA improves the manic symptoms in shorter times already after 1-2 weeks and this combination seems to have a short-term good tolerability profile [126]. In a multicenter RCT of patients with a current manic or mixed episode and with limited response to Lithium or Valproate monotherapy, the addition of Aripiprazole significantly improved patients’ conditions and increased time to relapse to any mood episode compared with mood stabilizer monotherapy for one year [127].
These data have motivated some authors to think that Aripiprazole, alone or in combination with Lithium/VPA could be considered a valid option for maintenance treatment, especially in those patients that do not respond satisfactorily to classical mood stabilizers [128]. However, long-term studies are required to confirm the evidence of benefits from this combination in maintenance pharmacological schemes [129], and even more so when Aripiprazole is administered through long-acting injections [130]. In fact, most studies consider mania prevention as their main clinical outcome, heedless of antipolar states, general functioning, and global patient’s well-being [27, 131].
In addition, it must be reminded that combinations with Aripiprazole are likely to increase akathisia, tremor, and other extrapyramidal symptoms [132-134]. Finally, it is of interest to remember that a handful of cases of Aripiprazole-induced mania/hypomania are reported in the literature [135-137].
There is evidence that augmentation treatment with Aripiprazole could be beneficial in the management of resistant forms of obsessive-compulsive disorder (OCD) coexisting with BD, in other major psychiatric conditions, or due to iatrogenic mechanisms [138, 139]. Preliminarily, it could be said that low-dose augmentation with Aripiprazole could be beneficial in some cases of resistant OCD, but caution is recommended [140, 141].
Another similar dispute is related to the employment of Aripiprazole in the treatment of impulse control and addictive disorders [142, 143], especially in high-risk patients [144, 145]. There is evidence that Aripiprazole could be associated with the development of hypersexual drives [146], kleptomania [147], pathological gambling, or compulsive eating [148]. Considering the above-mentioned risks, along with the substantially inconclusive results of contrary evidence [149, 150], there seems to be no indication for Aripiprazole in the treatment of impulse control and addiction comorbid with BD [151].
As a matter of fact, nowadays Aripiprazole is one of the most frequently used AAPs in clinical practice for its reduced risk of weight gain and metabolic complications compared to other AAPs [152], because of its different mechanism of action (Table 1).
Among Aripiprazole’s pharmacodynamic effects, we can enumerate the serotonergic antagonism (5-HT2A-C), partial agonism at D2/D3 receptors, and non-negligible histaminergic antagonism, particularly at high doses [153-156]. The absence of antimuscarinic effects likely contributes to the reduced risk of metabolic syndrome relative to dibenzodiazepines, such as Olanzapine, and Clozapine [157-159].
However, it is worth mentioning that the long-term effect on weight gain and metabolic derangements could be relevant, especially in the youth, as shown by recent inquiries [160-162].
One clinical advantage of Aripiprazole compared to other AAPs is that it could represent a valid option when antipsychotic-induced hyperprolactinemia becomes a relevant clinical complication, considering its very low risk to induce prolactin elevation [163]. Some authors even suggest that low-dose Aripiprazole (5 mg/day) could be used as an adjunct for treating antipsychotic-induced hyperprolactinemia, either alone or in combination with other drugs [164, 165].
8. LITHIUM/VPA PLUS RISPERIDONE
Due to its relatively “clean” pharmacodynamic profile, with a strong D2, 3, and 5HT2a, c antagonistic activity and minor effects on other receptors, Risperidone is a commonly used AAP in clinical practice, whose pharmacological properties partly overlap with those of TAPs [166]. Expectedly, there is solid evidence that Risperidone’s strong efficacy in the remission induction of acute mania is comparable to Haloperidol, the former being slightly more associated with weight gain and the latter with extrapyramidal symptoms. On the contrary, the usage of Risperidone in long-term treatment is much more questionable [167-169].
One RCT, involving patients remitted from an acute manic episode, showed that adjunctive Risperidone to a mood stabilizer reduced the risk of recurrence of any mood episode, compared with monotherapy. The time to relapse with add-on Risperidone therapy was significantly longer compared to the mood stabilizer plus placebo during the first 24 weeks of treatment. Nonetheless, the risk of recurrence did not differ between the group of patients that interrupted Risperidone after 24 weeks and those kept on Risperidone for up to 52 weeks, disproving the benefit of protracting combination treatment longer than six months. The weight gain after 52 weeks with adjunctive Risperidone was higher than placebo but lower compared to Olanzapine [99].
Post-hoc analysis of the same study further limited the efficacy of this combination, showing that prolonging Risperidone administration reduced the risk of only manic but not depressive phases during the first 24 weeks. It was also found that those who continued risperidone for up to 52 weeks had an ever greater risk of mood recurrence, confirming that extending adjunct treatment after six months could be more detrimental than beneficial [170]. These results were preceded by consistent experiences, confirming the efficacy of risperidone mainly as an antimanic agent [171, 172].
Among AAPs, Risperidone has the highest risk of EPS and persistent hyperprolactinemia [173, 174]. Furthermore, it is crucial to acknowledge that both preclinical and clinical evidence have shown that chronic risperidone assumption could negatively impact cognitive function and memory [175, 176].
9. LITHIUM/VPA PLUS OLANZAPINE
Olanzapine is a well-known AAP with a very wide pharmacodynamic profile [87, 177]. Its efficacy for the treatment of acute manic and mixed phases is indisputable, either in monotherapy or in combination with mood stabilizers [178, 179]. Moreover, the add-on treatment with Olanzapine to Lithium showed an earlier clinical response in the treatment of BD-I and BD-II (hypo)manic episodes compared to the classical association of VPA to Lithium [180]. Likewise, Olanzapine added to VPA has proven advantageous in the treatment of acute manic or mixed episodes compared to either VPA or Olanzapine alone, despite being associated with more adverse effects, especially rapid weight gain [181, 182].
The indication of Olanzapine for long-term treatment is more controversial. Similarly to Risperidone, the use of Olanzapine for maintenance treatment after 24 weeks has not proven beneficial and is generally not recommended in clinical practice, even more so in light of the heavy risk of metabolic complications and weight gain [99, 183]. In relation to evaluating the clinical efficacy of Olanzapine, as for other AAPs, much confusion derives from the partial clinical outcomes chosen, such as mania prevention or hospitalization risk reduction, rather than terminal outcomes such as life expectancy and quality of life [184]. In fact, Olanzapine is more efficacious than Lithium in the prevention of manic phases, but the same does not seem to be true for depressive phases and for overall physical health [123, 185, 186]. Conversely, other studies point to its efficacy in the long-term prevention of depressive episodes [187].
This could be due to Olanzapine's weaker depressogenic effects compared to other AAPs, although it still appears to be more associated with depressive episodes than Quetiapine [171, 188]. Consistently with this latter position, the combination of Olanzapine and Fluoxetine is one of the few FDA-approved treatments for bipolar depression, along with Quetiapine, Cariprazine, and Lurasidone [189]. In sum, in spite of its apparent efficacy to treat almost all forms and stages of BD, it should be considered only as a second-line treatment in long-term management, because of its chronic safety and tolerability issues (especially the high risk of metabolic toxicity and cardiovascular events), together with the difficulty of discontinuation due to severe withdrawal symptoms [190, 191] (Table 1).
10. LITHIUM/VPA PLUS CLOZAPINE
Clozapine, first introduced in the 70’ and then reintroduced in the 90’ after a long period of market withdrawal due to its association with potentially lethal agranulocytosis, was the first true AAP used in clinical practice [192]. Its therapeutic superiority over the other AAPs is generally recognized, especially in drug-resistant psychoses and in psychotic symptoms associated with Parkinson’s disease [193, 194]. Although still not completely explained, its unique and wide receptor interaction profile may explain its unequaled clinical efficacy [195].
However, it is rare but extremely serious acute adverse reactions, such as leukopenia and cardiomyopathy, have strongly limited Clozapine’s use and downgraded it to a second or third choice treatment, as a sort of “last chance” treatment. In addition, other common and dangerous chronic side effects, such as metabolic syndrome and weight gain, require constant patient monitoring, although this aspect is shared with other AAPs, namely Olanzapine (Table 1) [196]. In fact, Clozapine is not yet officially approved for BD, although it is occasionally administrated off-label in these patients, especially for manic/mixed phases that do not respond to the other treatment strategies [197]. However, Clozapine is still unique in clinical practice for its efficacy in resistant forms and its peculiar anti-suicidal, anti-aggressive, and slightly antidepressant-like effects [198, 199].
Case reports, retrospective and uncontrolled studies have reported that Clozapine may be effective for complicated resistant forms of BD, including rapid cycling and mixed episodes [200-202]. These observations are confirmed by recent systematic reviews, supporting the idea that Clozapine, either in monotherapy or combined with Lithium/VPA, could represent a decisive approach to some forms of treatment-resistance BD [203, 204].
Although it might increase the risk of agranulocytosis [205], the association of Clozapine with VPA has been proven effective and well tolerated [206], concurrently reducing the risk of Clozapine-induced seizures [207]. On the contrary, the association of Clozapine/Li might combine the anti-suicidal properties of both drugs [208]. Moreover, Lithium seems to effectively counteract the risk of lowering white blood cells induced by Clozapine [209, 210]. Interestingly, there is evidence that, in some treatment-resistant cases of BD, Clozapine can also be used at lower dosages in order to reduce its side effects, while maintaining substantial clinical efficacy in mood stabilization, along with its sedative, anti-aggressive and anti-suicidal properties [211, 212].
In conclusion, Clozapine as an add-on therapy is probably an underused option available for treatment-resistant BD, albeit its rare life-threatening side effects impose constant monitoring of patients’ condition and relegate this treatment to a second/third-line choice.
CONCLUSION
In spite of appropriate pharmacotherapies, BDs show high rates of recurrence and can progress into complicated forms associated with atypical features, chronic-unremitting courses, and treatment resistance. Mood stabilizers, such as Lithium and VPA, are the core of BDs pharmacotherapy. Lithium is the gold standard in the treatment of typical forms of BDs, while VPA seems more effective for patients with atypical features. However, clinicians are often confronted with the necessity of additional strategies according to the patient’s characteristics, both in terms of response and tolerability. Among adjunctive approaches, combining AAPs with mood stabilizers is considered one of the best options, especially in the treatment and prevention of (hypo)manic episodes. However, chronic adverse effects (e.g., weight gain and MetS) and the risk of depressive switches restrict the long-term sustainability of these combinations to severe cases that respond unsatisfactorily to mood stabilizers. Among the several AAPs available, Quetiapine and, less consistently, Aripiprazole are the most commonly used in combination with Lithium or VPA for their better tolerability and a weaker association with depressive switches. Indeed, these two AAPs are being increasingly considered as an option for treatment-resistant depression, underlining their peculiar pharmacological characteristics. However, the treatment of acute bipolar depression has been only mentioned in this study and we invite the readers to resort to other reviews on this topic (see, for example, Baldessarini et al., 2020) [189]. Finally, we should remind the unique properties of Clozapine in the treatment of suicidality, aggressiveness, and drug-resistant psychoses, although its rare life-threatening side effects impose constant monitoring of patients’ conditions and relegate its use to a second-line or third-line intervention.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AAPs
Atypical Antipsychotics
- AUD
Alcohol Use Disorder
- BD
Bipolar Disorder
- GAD
Generalized Anxiety Disorder
- OCD
Obsessive-compulsive Disorder
- RCT
Randomized Controlled Trials
- SUDs
Substance Use Disorders
- TAPs
Typical Antipsychotics
- VPA
Valproic Acid
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Vieta E., Berk M., Schulze T.G., Carvalho A.F., Suppes T., Calabrese J.R. Bipolar disorders; Nat. Rev. Dis. Prim; 2018. p. 4. [DOI] [PubMed] [Google Scholar]
- 2.Maletic V., Raison C. Integrated neurobiology of bipolar disorder. Front. Psychiatry. 2014;5:98. doi: 10.3389/fpsyt.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gignac A., McGirr A., Lam R.W., Yatham L.N. Recovery and recurrence following a first episode of mania: a systematic review and meta-analysis of prospectively characterized cohorts. J. Clin. Psychiatry. 2015;76(9):1241–1248. doi: 10.4088/JCP.14r09245. [DOI] [PubMed] [Google Scholar]
- 4.Carlson G.A., Kotov R., Chang S.W., Ruggero C., Bromet E.J. Early determinants of four-year clinical outcomes in bipolar disorder with psychosis. Bipolar Disord. 2012;14(1):19–30. doi: 10.1111/j.1399-5618.2012.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perlis R.H., Ostacher M.J., Patel J.K., Marangell L.B., Zhang H., Wisniewski S.R., Ketter T.A., Miklowitz D.J., Otto M.W., Gyulai L., Reilly-Harrington N.A., Nierenberg A.A., Sachs G.S., Thase M.E. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). Am. J. Psychiatry. 2006;163(2):217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 6.Vázquez G.H., Holtzman J.N., Lolich M., Ketter T.A., Baldessarini R.J. Recurrence rates in bipolar disorder: Systematic comparison of long-term prospective, naturalistic studies versus randomized controlled trials. Eur. Neuropsychopharmacol. 2015;25(10):1501–1512. doi: 10.1016/j.euroneuro.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Kessing L.V., Hansen M.G., Andersen P.K., Angst J. The predictive effect of episodes on the risk of recurrence in depressive and bipolar disorders - a life-long perspective. Acta Psychiatr. Scand. 2004;109(5):339–344. doi: 10.1046/j.1600-0447.2003.00266.x. [DOI] [PubMed] [Google Scholar]
- 8.Vieta E., Reinares M., Rosa A.R. Staging bipolar disorder. Neurotox. Res. 2011;19(2):279–285. doi: 10.1007/s12640-010-9197-8. [DOI] [PubMed] [Google Scholar]
- 9.McIntyre R.S., Konarski J.Z., Yatham L.N. Comorbidity in bipolar disorder: a framework for rational treatment selection. Hum. Psychopharmacol. 2004;19(6):369–386. doi: 10.1002/hup.612. [DOI] [PubMed] [Google Scholar]
- 10.Perugi G., Barbuti M. There are no patients without comorbidity. Eur. Neuropsychopharmacol. 2021;50:104–106. doi: 10.1016/j.euroneuro.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Merikangas K.R., Akiskal H.S., Angst J., Greenberg P.E., Hirschfeld R.M.A., Petukhova M., Kessler R.C. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry. 2007;64(5):543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiss F., Scarselli M., Tidona S., Carli M., Perugi G. Triple diagnosis of attention-deficit/hyperactivity disorder with co-existing bipolar and alcohol use disorders: Clinical aspects and pharmacological treatments. Curr. Neuropharmacol. 2023:21. doi: 10.2174/1570159X20666220830154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swann A.C., Bowden C.L., Morris D., Calabrese J.R., Petty F., Small J., Dilsaver S.C., Davis J.M. Depression during mania. Treatment response to lithium or divalproex. Arch. Gen. Psychiatry. 1997;54(1):37–42. doi: 10.1001/archpsyc.1997.01830130041008. [DOI] [PubMed] [Google Scholar]
- 14.Maj M., Pirozzi R., Starace F. Previous pattern of course of the illness as a predictor of response to lithium prophylaxis in bipolar patients. J. Affect. Disord. 1989;17(3):237–241. doi: 10.1016/0165-0327(89)90005-0. [DOI] [PubMed] [Google Scholar]
- 15.Cole A.J., Scott J., Ferrier I.N., Eccleston D. Patterns of treatment resistance in bipolar affective disorder. Acta Psychiatr. Scand. 1993;88(2):121–123. doi: 10.1111/j.1600-0447.1993.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 16.Yatham L.N., Kennedy S.H., Parikh S.V., Schaffer A., Bond D.J., Frey B.N., Sharma V., Goldstein B.I., Rej S., Beaulieu S., Alda M., MacQueen G., Milev R.V., Ravindran A., O’Donovan C., McIntosh D., Lam R.W., Vazquez G., Kapczinski F., McIntyre R.S., Kozicky J., Kanba S., Lafer B., Suppes T., Calabrese J.R., Vieta E., Malhi G., Post R.M., Berk M. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. doi: 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sani G., Perugi G., Tondo L. Treatment of bipolar disorder in a lifetime perspective: Is lithium still the best choice? Clin. Drug Investig. 2017;37(8):713–727. doi: 10.1007/s40261-017-0531-2. [DOI] [PubMed] [Google Scholar]
- 18.Malhi G.S., Outhred T. Therapeutic mechanisms of lithium in bipolar disorder: recent advances and current understanding. CNS Drugs. 2016;30(10):931–949. doi: 10.1007/s40263-016-0380-1. [DOI] [PubMed] [Google Scholar]
- 19.Miura T., Noma H., Furukawa T.A., Mitsuyasu H., Tanaka S., Stockton S., Salanti G., Motomura K., Shimano-Katsuki S., Leucht S., Cipriani A., Geddes J.R., Kanba S. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: A systematic review and network meta-analysis. Lancet Psychiatry. 2014;1(5):351–359. doi: 10.1016/S2215-0366(14)70314-1. [DOI] [PubMed] [Google Scholar]
- 20.Bowden C.L. Clinical correlates of therapeutic response in bipolar disorder. J. Affect. Disord. 2001;67(1-3):257–265. doi: 10.1016/S0165-0327(98)00160-8. [DOI] [PubMed] [Google Scholar]
- 21.Grof P., Duffy A., Cavazzoni P., Grof E., Garnham J., MacDougall M., O’Donovan C., Alda M. Is response to prophylactic lithium a familial trait? J. Clin. Psychiatry. 2002;63(10):942–947. doi: 10.4088/JCP.v63n1013. [DOI] [PubMed] [Google Scholar]
- 22.Sakurai H., Kato M., Yasui-Furukori N., Suzuki T., Baba H., Watanabe K., Inada K., Kishida I., Sugawara K.Y., Kikuchi T., Katsuki A., Uchida H. Pharmacological management of bipolar disorder: Japanese expert consensus. Bipolar Disord. 2020;22(8):822–830. doi: 10.1111/bdi.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowden C.L., Grunze H., Mullen J., Brecher M., Paulsson B., Jones M., Vågerö M., Svensson K. A randomized, double-blind, placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder. J. Clin. Psychiatry. 2005;66(1):111–121. doi: 10.4088/JCP.v66n0116. [DOI] [PubMed] [Google Scholar]
- 24.Goodwin G.M., Haddad P.M., Ferrier I.N., Aronson J.K., Barnes T.R.H., Cipriani A., Coghill D.R., Fazel S., Geddes J.R., Grunze H., Holmes E.A., Howes O., Hudson S., Hunt N., Jones I., Macmillan I.C., McAllister-Williams H., Miklowitz D.R., Morriss R., Munafò M., Paton C., Sahakian B.J., Saunders K.E.A., Sinclair J.M.A., Taylor D., Vieta E., Young A.H. Evidence-based guidelines for treating bipolar disorder: Revised third edition recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2016;30(6):495-553. doi: 10.1177/0269881116636545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Calabrese J.R., Vieta E., Shelton M.D. Latest maintenance data on lamotrigine in bipolar disorder. Eur. Neuropsychopharmacol. 2003;13(Suppl. 2):57–66. doi: 10.1016/S0924-977X(03)00079-8. [DOI] [PubMed] [Google Scholar]
- 26.Weisler R.H., Nolen W.A., Neijber A., Hellqvist Å., Paulsson B. Continuation of quetiapine versus switching to placebo or lithium for maintenance treatment of bipolar I disorder (Trial 144: A randomized controlled study). J. Clin. Psychiatry. 2011;72(11):1452–1464. doi: 10.4088/JCP.11m06878. [DOI] [PubMed] [Google Scholar]
- 27.Verdolini N., Hidalgo-Mazzei D., Del Matto L., Muscas M., Pacchiarotti I., Murru A., Samalin L., Aedo A., Tohen M., Grunze H., Young A.H., Carvalho A.F., Vieta E. Long‐term treatment of bipolar disorder type I: A systematic and critical review of clinical guidelines with derived practice algorithms. Bipolar Disord. 2021;23(4):324–340. doi: 10.1111/bdi.13040. [DOI] [PubMed] [Google Scholar]
- 28.Kessing L.V., Bauer M., Nolen W.A., Severus E., Goodwin G.M., Geddes J. Effectiveness of maintenance therapy of lithium vs other mood stabilizers in monotherapy and in combinations: A systematic review of evidence from observational studies. Bipolar Disord. 2018;20(5):419–431. doi: 10.1111/bdi.12623. [DOI] [PubMed] [Google Scholar]
- 29.Geddes J.R., Goodwin G.M., Rendell J., Azorin J-M., Cipriani A., Ostacher M.J., Morriss R., Alder N., Juszczak E. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (balance): A randomised open-label trial. Lancet. 2010;375(9712):385–395. doi: 10.1016/S0140-6736(09)61828-6. [DOI] [PubMed] [Google Scholar]
- 30.Nierenberg A.A., McElroy S.L., Friedman E.S., Ketter T.A., Shelton R.C., Deckersbach T., McInnis M.G., Bowden C.L., Tohen M., Kocsis J.H., Calabrese J.R., Kinrys G., Bobo W.V., Singh V., Kamali M., Kemp D., Brody B., Reilly-Harrington N.A., Sylvia L.G., Shesler L.W., Bernstein E.E., Schoenfeld D., Rabideau D.J., Leon A.C., Faraone S., Thase M.E. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J. Clin. Psychiatry. 2016;77(1):90–99. doi: 10.4088/JCP.14m09349. [DOI] [PubMed] [Google Scholar]
- 31.Fieve R.R., Kumbaraci T., Dunner D.L. Lithium prophylaxis of depression in bipolar I, bipolar II, and unipolar patients. Am. J. Psychiatry. 1976;133(8):925–929. doi: 10.1176/ajp.133.8.925. [DOI] [PubMed] [Google Scholar]
- 32.Amsterdam J.D., Lorenzo-Luaces L., Soeller I., Li S.Q., Mao J.J., DeRubeis R.J. Short-term venlafaxine v. lithium monotherapy for bipolar type II major depressive episodes: Effectiveness and mood conversion rate. Br. J. Psychiatry. 2016;208(4):359–365. doi: 10.1192/bjp.bp.115.169375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Young A.H., McElroy S.L., Bauer M., Philips N., Chang W., Olausson B., Paulsson B., Brecher M. A double-blind, placebo-controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (Embolden I). J. Clin. Psychiatry. 2010;71(2):150–162. doi: 10.4088/JCP.08m04995gre. [DOI] [PubMed] [Google Scholar]
- 34.Zhihan G., Fengli S., Wangqiang L., Dong S., Weidong J. Lamotrigine and lithium combination for treatment of rapid cycling bipolar disorder: Results from meta-analysis. Front. Psychiatry. 2022;13:913051. doi: 10.3389/fpsyt.2022.913051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsu C.W., Tsai S.Y., Tseng P.T., Liang C.S., Vieta E., Carvalho A.F., Stubbs B., Kao H.Y., Tu Y.K., Lin P.Y. Differences in the prophylactic effect of serum lithium levels on depression and mania in bipolar disorder: A dose-response meta-analysis. Eur. Neuropsychopharmacol. 2022;58:20–29. doi: 10.1016/j.euroneuro.2022.01.112. [DOI] [PubMed] [Google Scholar]
- 36.Nelson J.C., Baumann P., Delucchi K., Joffe R., Katona C. A systematic review and meta-analysis of lithium augmentation of tricyclic and second generation antidepressants in major depression. J. Affect. Disord. 2014;168:269–275. doi: 10.1016/j.jad.2014.05.053. [DOI] [PubMed] [Google Scholar]
- 37.Smith K.A., Cipriani A. Lithium and suicide in mood disorders: Updated meta-review of the scientific literature. Bipolar Disord. 2017;19(7):575–586. doi: 10.1111/bdi.12543. [DOI] [PubMed] [Google Scholar]
- 38.Cipriani A., Hawton K., Stockton S., Geddes J.R. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013;346(jun27 4):f3646. doi: 10.1136/bmj.f3646. [DOI] [PubMed] [Google Scholar]
- 39.Fries G.R., Pfaffenseller B., Stertz L., Paz A.V.C., Dargél A.A., Kunz M., Kapczinski F. Staging and neuroprogression in bipolar disorder. Curr. Psychiatry Rep. 2012;14(6):667–675. doi: 10.1007/s11920-012-0319-2. [DOI] [PubMed] [Google Scholar]
- 40.Elefante C., Brancati G.E., Torrigiani S., Amadori S., Ricciardulli S., Pistolesi G., Lattanzi L., Perugi G. Bipolar disorder and manic-like symptoms in Alzheimer’s, vascular and frontotemporal dementia: A systematic review. Curr. Neuropharmacol. 2023:21. doi: 10.2174/1570159X20666220706110157. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carli M., Aringhieri S., Kolachalam S., Longoni B., Grenno G., Rossi M., Gemignani A., Fornai F., Maggio R., Scarselli M. Is adult hippocampal neurogenesis really relevant for the treatment of psychiatric disorders? Curr. Neuropharmacol. 2021;19(10):1640–1660. doi: 10.2174/1570159X18666200818194948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinberg G.R., Kemp B.E. AMPK in health and disease. Physiol. Rev. 2009;89(3):1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 43.Salloum I.M., Cornelius J.R., Daley D.C., Kirisci L., Himmelhoch J.M., Thase M.E. Efficacy of valproate maintenance in patients with bipolar disorder and alcoholism: a double-blind placebo-controlled study. Arch. Gen. Psychiatry. 2005;62(1):37–45. doi: 10.1001/archpsyc.62.1.37. [DOI] [PubMed] [Google Scholar]
- 44.Geller B., Cooper T.B., Sun K., Zimerman B., Frazier J., Williams M., Heath J. Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J. Am. Acad. Child Adolesc. Psychiatry. 1998;37(2):171–178. doi: 10.1097/00004583-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Kemp D.E., Gao K., Ganocy S.J., Elhaj O., Bilali S.R., Conroy C., Findling R.L., Calabrese J.R. A 6-month, double-blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid-cycling bipolar disorder and Co-occurring substance abuse or dependence. J. Clin. Psychiatry. 2009;70(1):113–121. doi: 10.4088/JCP.07m04022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Albanese M.J., Clodfelter R.C., Jr, Khantzian E.J. Divalproex sodium in substance abusers with mood disorder. J. Clin. Psychiatry. 2000;61(12):916–921. doi: 10.4088/JCP.v61n1205. [DOI] [PubMed] [Google Scholar]
- 47.McMillan T.M. Lithium and the treatment of alcoholism: A critical review. Addiction. 1981;76(3):245–258. doi: 10.1111/j.1360-0443.1981.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 48.Fawcett J., Clark D.C., Aagesen C.A., Pisani V.D., Tilkin J.M., Sellers D., McGuire M., Gibbons R.D. A double-blind, placebo-controlled trial of lithium carbonate therapy for alcoholism. Arch. Gen. Psychiatry. 1987;44(3):248–256. doi: 10.1001/archpsyc.1987.01800150060008. [DOI] [PubMed] [Google Scholar]
- 49.Clark D.C., Fawcett J. Does lithium carbonate therapy for alcoholism deter relapse drinking? Recent Dev. Alcohol. 1989;7:315-328. doi: 10.1007/978-1-4899-1678-5_16. [DOI] [PubMed] [Google Scholar]
- 50.Gadh S. Low-dose lithium impact in an addiction treatment setting. Pers. Med. Psychiatry. 2020;21-22:100059. doi: 10.1016/j.pmip.2020.100059. [DOI] [Google Scholar]
- 51.Bocchetta A., Ardau R., Fanni T., Sardu C., Piras D., Pani A., Del Zompo M. Renal function during long-term lithium treatment: a cross-sectional and longitudinal study. BMC Med. 2015;13(1):12. doi: 10.1186/s12916-014-0249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carli M., Risaliti E., Francomano M., Kolachalam S., Longoni B., Bocci G., Maggio R., Scarselli M. A 5-year study of lithium and valproic acid drug monitoring in patients with bipolar disorders in an Italian clinical center. Pharmaceuticals. 2022;15(1):105. doi: 10.3390/ph15010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baldessarini R.J., Tondo L., Floris G., Rudas N. Reduced morbidity after gradual discontinuation of lithium treatment for bipolar I and II disorders: a replication study. Am. J. Psychiatry. 1997;154(4):551–553. doi: 10.1176/ajp.154.4.551. [DOI] [PubMed] [Google Scholar]
- 54.Tondo L., Baldessarini R.J., Hennen J., Floris G. Lithium maintenance treatment of depression and mania in bipolar I and bipolar II disorders. Am. J. Psychiatry. 1998;155(5):638–645. doi: 10.1176/ajp.155.5.638. [DOI] [PubMed] [Google Scholar]
- 55.Cipriani A., Reid K., Young A.H., Macritchie K., Geddes J. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Libr. 2013;2013(10):CD003196. doi: 10.1002/14651858.CD003196.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bowden C.L., Calabrese J.R., McElroy S.L., Gyulai L., Wassef A., Petty F., Pope H.G., Jr, Chou J.C., Keck P.E., Jr, Rhodes L.J., Swann A.C., Hirschfeld R.M., Wozniak P.J. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch. Gen. Psychiatry. 2000;57(5):481–489. doi: 10.1001/archpsyc.57.5.481. [DOI] [PubMed] [Google Scholar]
- 57.Tohen M., Chengappa K.N.R., Suppes T., Baker R.W., Zarate C.A., Bowden C.L., Sachs G.S., Kupfer D.J., Ghaemi S.N., Feldman P.D., Risser R.C., Evans A.R., Calabrese J.R. Relapse prevention in bipolar I disorder: 18-month comparison of olanzapine plus mood stabiliser v. mood stabiliser alone. Br. J. Psychiatry. 2004;184(4):337–345. doi: 10.1192/bjp.184.4.337. [DOI] [PubMed] [Google Scholar]
- 58.Calabrese J.R., Pikalov A., Streicher C., Cucchiaro J., Mao Y., Loebel A. Lurasidone in combination with lithium or valproate for the maintenance treatment of bipolar I disorder. Eur. Neuropsychopharmacol. 2017;27(9):865–876. doi: 10.1016/j.euroneuro.2017.06.013. [DOI] [PubMed] [Google Scholar]
- 59.Duffy A., Goodday S., Passos I.C., Kapczinski F. Changing the bipolar illness trajectory. Lancet Psychiatry. 2017;4(1):11–13. doi: 10.1016/S2215-0366(16)30352-2. [DOI] [PubMed] [Google Scholar]
- 60.Reischies F.M., Hartikainen J., Berghöfer A. Initial lithium and valproate combination therapy in acute mania. Neuropsychobiology. 2002;46(Suppl. 1):22–27. doi: 10.1159/000068020. [DOI] [PubMed] [Google Scholar]
- 61.Freeman T.W., Clothier J.L., Pazzaglia P., Lesem M.D., Swann A.C. A double-blind comparison of valproate and lithium in the treatment of acute mania. Am. J. Psychiatry. 1992;149(1):108–111. doi: 10.1176/ajp.149.1.108. [DOI] [PubMed] [Google Scholar]
- 62.Smith L.A., Cornelius V.R., Azorin J.M., Perugi G., Vieta E., Young A.H., Bowden C.L. Valproate for the treatment of acute bipolar depression: Systematic review and meta-analysis. J. Affect. Disord. 2010;122(1-2):1–9. doi: 10.1016/j.jad.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 63.Bond D.J., Lam R.W., Yatham L.N. Divalproex sodium versus placebo in the treatment of acute bipolar depression: A systematic review and meta-analysis. J. Affect. Disord. 2010;124(3):228–234. doi: 10.1016/j.jad.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 64.Yatham L.N., Kennedy S.H., Parikh S.V., Schaffer A., Beaulieu S., Alda M., O’Donovan C., MacQueen G., McIntyre R.S., Sharma V., Ravindran A., Young L.T., Milev R., Bond D.J., Frey B.N., Goldstein B.I., Lafer B., Birmaher B., Ha K., Nolen W.A., Berk M. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. 2013;15(1):1–44. doi: 10.1111/bdi.12025. [DOI] [PubMed] [Google Scholar]
- 65.Bach D.R., Korn C.W., Vunder J., Bantel A. Effect of valproate and pregabalin on human anxiety-like behaviour in a randomised controlled trial. Transl. Psychiatry. 2018;8(1):157. doi: 10.1038/s41398-018-0206-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Van Ameringen M., Mancini C., Pipe B., Bennett M. Antiepileptic drugs in the treatment of anxiety disorders: role in therapy. Drugs. 2004;64(19):2199–2220. doi: 10.2165/00003495-200464190-00004. [DOI] [PubMed] [Google Scholar]
- 67.Aliyev N.A., Aliyev Z.N. Valproate (depakine-chrono) in the acute treatment of outpatients with generalized anxiety disorder without psychiatric comorbidity: Randomized, double-blind placebo-controlled study. Eur. Psychiatry. 2008;23(2):109–114. doi: 10.1016/j.eurpsy.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Bowden C.L. Valproate. Bipolar Disord. 2003;5(3):189–202. doi: 10.1034/j.1399-5618.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 69.Rakofsky J.J., Dunlop B.W. Treating nonspecific anxiety and anxiety disorders in patients with bipolar disorder: a review. J. Clin. Psychiatry. 2011;72(1):81–90. doi: 10.4088/JCP.09r05815gre. [DOI] [PubMed] [Google Scholar]
- 70.Davis L.L., Ryan W., Adinoff B., Petty F. Comprehensive review of the psychiatric uses of valproate. J. Clin. Psychopharmacol. 2000;20(Suppl. 1):1S–17S. doi: 10.1097/00004714-200002001-00001. [DOI] [PubMed] [Google Scholar]
- 71.Davis L.L., Bartolucci A., Petty F. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J. Affect. Disord. 2005;85(3):259–266. doi: 10.1016/j.jad.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 72.Lee J., Baek J.H., Lee D., Ahn S.W., Yang S.Y., Choi Y., Bahk Y.C., Hong K.S. Defining phenotypes of long-term lithium and valproate response, including combination therapy: a modified application of the Alda scale in patients with bipolar disorders. Int. J. Bipolar Disord. 2020;8(1):36. doi: 10.1186/s40345-020-00199-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perugi G., Frare F., Toni C., Tusini G., Vannucchi G., Akiskal H.S. Adjunctive valproate in panic disorder patients with comorbid bipolar disorder or otherwise resistant to standard antidepressants: a 3-year “open” follow-up study. Eur. Arch. Psychiatry Clin. Neurosci. 2010;260(7):553–560. doi: 10.1007/s00406-010-0109-y. [DOI] [PubMed] [Google Scholar]
- 74.Grunze H., Schaefer M., Scherk H., Born C., Preuss U.W. Comorbid bipolar and alcohol use disorder—A therapeutic challenge. Front. Psychiatry. 2021;12:660432. doi: 10.3389/fpsyt.2021.660432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huband N., Ferriter M., Nathan R., Jones H. Antiepileptics for aggression and associated impulsivity. Cochrane Database Syst. Rev. 2010;2010(2):CD003499. doi: 10.1002/14651858.CD003499.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macfarlane A., Greenhalgh T. Sodium valproate in pregnancy: what are the risks and should we use a shared decision-making approach? BMC Pregnancy Childbirth. 2018;18(1):200. doi: 10.1186/s12884-018-1842-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freeman M.P., Stoll A.L. Mood stabilizer combinations: A review of safety and efficacy. Am. J. Psychiatry. 1998;155(1):12–21. doi: 10.1176/ajp.155.1.12. [DOI] [PubMed] [Google Scholar]
- 78.Granneman G.R., Schneck D.W., Cavanaugh J.H., Witt G.F. Pharmacokinetic interactions and side effects resulting from concomitant administration of lithium and divalproex sodium. J. Clin. Psychiatry. 1996;57(5):204–206. [PubMed] [Google Scholar]
- 79.Rapoport S.I., Basselin M., Kim H.W., Rao J.S. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res. Brain Res. Rev. 2009;61(2):185–209. doi: 10.1016/j.brainresrev.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma V., Persad E., Mazmanian D., Karunaratne K. Treatment of rapid cycling bipolar disorder with combination therapy of valproate and lithium. Can. J. Psychiatry. 1993;38(2):137–139. doi: 10.1177/070674379303800213. [DOI] [PubMed] [Google Scholar]
- 81.Preuss U.W., Schaefer M., Born C., Grunze H. Bipolar disorder and comorbid use of illicit substances. Medicina. 2021;57(11):1256. doi: 10.3390/medicina57111256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muti M., Del Grande C., Musetti L., Marazziti D., Pergentini I., Corsi M., Turri M., Umberto Corsini G., Dell’Osso L. Prescribing patterns of lithium or lithium+valproate in manic or mixed episodes. Int. Clin. Psychopharmacol. 2013;28(6):305–311. doi: 10.1097/YIC.0b013e3283642348. [DOI] [PubMed] [Google Scholar]
- 83.Hong J., Reed C., Novick D., Haro J.M., Windmeijer F., Knapp M. The cost of relapse for patients with a manic/mixed episode of bipolar disorder in the EMBLEM study. PharmacoEconomics. 2010;28(7):555–566. doi: 10.2165/11535200-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 84.Bowden C.L., Mosolov S., Hranov L., Chen E., Habil H., Kongsakon R., Manfredi R., Lin H.N. Efficacy of valproate versus lithium in mania or mixed mania: a randomized, open 12-week trial. Int. Clin. Psychopharmacol. 2010;25(2):60–67. doi: 10.1097/YIC.0b013e328333ac1b. [DOI] [PubMed] [Google Scholar]
- 85.Vieta E., Sanchez-Moreno J. Acute and long-term treatment of mania. Dialogues Clin. Neurosci. 2008;10(2):165–179. doi: 10.31887/DCNS.2008.10.2/evieta. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Aringhieri S., Carli M., Kolachalam S., Verdesca V., Cini E., Rossi M., McCormick P.J., Corsini G.U., Maggio R., Scarselli M. Molecular targets of atypical antipsychotics: From mechanism of action to clinical differences. Pharmacol. Ther. 2018;192:20–41. doi: 10.1016/j.pharmthera.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 87.Goldberg J.F. Complex combination pharmacotherapy for bipolar disorder: Knowing when less is more or more is better. Focus Am. Psychiatr. Publ. 2019;17(3):218–231. doi: 10.1176/appi.focus.20190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fountoulakis K.N., Yatham L.N., Grunze H., Vieta E., Young A.H., Blier P., Tohen M., Kasper S., Moeller H.J. The CINP guidelines on the definition and evidence-based interventions for treatment-resistant bipolar disorder. Int. J. Neuropsychopharmacol. 2020;23(4):230–256. doi: 10.1093/ijnp/pyz064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fountoulakis K.N., Vieta E., Young A., Yatham L., Grunze H., Blier P., Moeller H.J., Kasper S. The International college of neuropsychopharmacology (CINP) treatment guidelines for bipolar disorder in adults (CINP-BD-2017), Part 4: Unmet needs in the treatment of bipolar disorder and recommendations for future research. Int. J. Neuropsychopharmacol. 2017;20(2):196–205. doi: 10.1093/ijnp/pyw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bjørklund L., Horsdal H.T., Mors O., Østergaard S.D., Gasse C. Trends in the psychopharmacological treatment of bipolar disorder: a nationwide register-based study. Acta Neuropsychiatr. 2016;28(2):75–84. doi: 10.1017/neu.2015.52. [DOI] [PubMed] [Google Scholar]
- 91.Chang C.M., Wu C.S., Huang Y.W., Chau Y.L., Tsai H.J. Utilization of psychopharmacological treatment among patients with newly diagnosed bipolar disorder From 2001 to 2010. J. Clin. Psychopharmacol. 2016;36(1):32–44. doi: 10.1097/JCP.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 92.Kessing L.V., Vradi E., Andersen P.K. Nationwide and population-based prescription patterns in bipolar disorder. Bipolar Disord. 2016;18(2):174–182. doi: 10.1111/bdi.12371. [DOI] [PubMed] [Google Scholar]
- 93.McElroy S.L., Keck P.E., Jr, Tugrul K.C., Bennett J.A. Valproate as a loading treatment in acute mania. Neuropsychobiology. 1993;27(3):146–149. doi: 10.1159/000118970. [DOI] [PubMed] [Google Scholar]
- 94.Suppes T., Vieta E., Liu S., Brecher M., Paulsson B. Maintenance treatment for patients with bipolar I disorder: results from a north american study of quetiapine in combination with lithium or divalproex (trial 127). Am. J. Psychiatry. 2009;166(4):476–488. doi: 10.1176/appi.ajp.2008.08020189. [DOI] [PubMed] [Google Scholar]
- 95.Vieta E., Suppes T., Eggens I., Persson I., Paulsson B., Brecher M. Efficacy and safety of quetiapine in combination with lithium or divalproex for maintenance of patients with bipolar I disorder (international trial 126). J. Affect. Disord. 2008;109(3):251–263. doi: 10.1016/j.jad.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Bowden C.L., Vieta E., Ice K.S., Schwartz J.H., Wang P.P., Versavel M. Ziprasidone plus a mood stabilizer in subjects with bipolar I disorder: a 6-month, randomized, placebo-controlled, double-blind trial. J. Clin. Psychiatry. 2010;71(2):130–137. doi: 10.4088/JCP.09m05482yel. [DOI] [PubMed] [Google Scholar]
- 97.Suppes T., Vieta E., Gustafsson U., Ekholm B. Maintenance treatment with quetiapine when combined with either lithium or divalproex in bipolar I disorder: analysis of two large randomized, placebo-controlled trials. Depress. Anxiety. 2013;30(11):1089–1098. doi: 10.1002/da.22136. [DOI] [PubMed] [Google Scholar]
- 98.Yatham L.N., Beaulieu S., Schaffer A., Kauer-Sant’Anna M., Kapczinski F., Lafer B., Sharma V., Parikh S.V., Daigneault A., Qian H., Bond D.J., Silverstone P.H., Walji N., Milev R., Baruch P., da Cunha A., Quevedo J., Dias R., Kunz M., Young L.T., Lam R.W., Wong H. Optimal duration of risperidone or olanzapine adjunctive therapy to mood stabilizer following remission of a manic episode: A CANMAT randomized double-blind trial. Mol. Psychiatry. 2016;21(8):1050–1056. doi: 10.1038/mp.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gonzalez-Pinto A., Vieta E., Reed C., Novick D., Barraco A., Aguado J., Haro J.M. Effectiveness of olanzapine monotherapy and olanzapine combination treatment in the long term following acute mania — Results of a two year observational study in bipolar disorder (EMBLEM). J. Affect. Disord. 2011;131(1-3):320–329. doi: 10.1016/j.jad.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 100.Kulkarni J., Filia S., Berk L., Filia K., Dodd S., de Castella A., Brnabic A.J.M., Lowry A.J., Kelin K., Montgomery W., Fitzgerald P.B., Berk M. Treatment and outcomes of an Australian cohort of outpatients with bipolar I or schizoaffective disorder over twenty-four months: implications for clinical practice. BMC Psychiatry. 2012;12(1):228. doi: 10.1186/1471-244X-12-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wine J.N., Sanda C., Caballero J. Effects of quetiapine on sleep in nonpsychiatric and psychiatric conditions. Ann. Pharmacother. 2009;43(4):707–713. doi: 10.1345/aph.1L320. [DOI] [PubMed] [Google Scholar]
- 102.Atkin T., Comai S., Gobbi G. Drugs for insomnia beyond benzodiazepines: Pharmacology, clinical applications, and discovery. Pharmacol. Rev. 2018;70(2):197–245. doi: 10.1124/pr.117.014381. [DOI] [PubMed] [Google Scholar]
- 103.Modesto-Lowe V., Harabasz A.K., Walker S.A. Quetiapine for primary insomnia: Consider the risks. Cleve. Clin. J. Med. 2021;88(5):286–294. doi: 10.3949/ccjm.88a.20031. [DOI] [PubMed] [Google Scholar]
- 104.Stahl S.M. Dopamine system stabilizers, aripiprazole, and the next generation of antipsychotics, part 1, “Goldilocks” actions at dopamine receptors. J. Clin. Psychiatry. 2001;62(11):841–842. doi: 10.4088/JCP.v62n1101. [DOI] [PubMed] [Google Scholar]
- 105.Maruani J., Anderson G., Etain B., Lejoyeux M., Bellivier F., Geoffroy P.A. The neurobiology of adaptation to seasons: Relevance and correlations in bipolar disorders. Chronobiol. Int. 2018;35(10):1335–1353. doi: 10.1080/07420528.2018.1487975. [DOI] [PubMed] [Google Scholar]
- 106.McGowan N.M., Kim D.S., de Andres Crespo M., Bisdounis L., Kyle S.D., Saunders K.E.A. Hypnotic and melatonin/melatonin-receptor agonist treatment in bipolar disorder: A systematic review and meta-analysis. CNS Drugs. 2022;36:345–363. doi: 10.1007/s40263-022-00911-7. [DOI] [PubMed] [Google Scholar]
- 107.Anderson G., Maes M. Melatonin: an overlooked factor in schizophrenia and in the inhibition of anti-psychotic side effects. Metab. Brain Dis. 2012;27(2):113–119. doi: 10.1007/s11011-012-9307-9. [DOI] [PubMed] [Google Scholar]
- 108.Cruz-Sanabria F., Carmassi C., Bruno S., Bazzani A., Carli M., Scarselli M., Faraguna U. Melatonin as a chronobiotic with sleep-promoting properties. Curr. Neuropharmacol. 2022:20. doi: 10.2174/1570159X20666220217152617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chiesa A., Chierzi F., De Ronchi D., Serretti A. Quetiapine for bipolar depression. Int. Clin. Psychopharmacol. 2012;27(2):76–90. doi: 10.1097/YIC.0b013e32834e4c56. [DOI] [PubMed] [Google Scholar]
- 110.Calabrese J.R., Keck P.E., Jr, Macfadden W., Minkwitz M., Ketter T.A., Weisler R.H., Cutler A.J., McCoy R., Wilson E., Mullen J. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am. J. Psychiatry. 2005;162(7):1351–1360. doi: 10.1176/appi.ajp.162.7.1351. [DOI] [PubMed] [Google Scholar]
- 111.Sachs G., Chengappa K.N.R., Suppes T., Mullen J.A., Brecher M., Devine N.A., Sweitzer D.E. Quetiapine with lithium or divalproex for the treatment of bipolar mania: a randomized, double-blind, placebo-controlled study. Bipolar Disord. 2004;6(3):213–223. doi: 10.1111/j.1399-5618.2004.00115.x. [DOI] [PubMed] [Google Scholar]
- 112.Vieta E., Suppes T., Ekholm B., Udd M., Gustafsson U. Long-term efficacy of quetiapine in combination with lithium or divalproex on mixed symptoms in bipolar I disorder. J. Affect. Disord. 2012;142(1-3):36–44. doi: 10.1016/j.jad.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 113.Takeshima M. Treating mixed mania/hypomania: a review and synthesis of the evidence. CNS Spectr. 2017;22(2):177–185. doi: 10.1017/S1092852916000845. [DOI] [PubMed] [Google Scholar]
- 114.Sheehan D.V., Harnett-Sheehan K., Hidalgo R.B., Janavs J., McElroy S.L., Amado D., Suppes T. Randomized, placebo-controlled trial of quetiapine XR and divalproex ER monotherapies in the treatment of the anxious bipolar patient. J. Affect. Disord. 2013;145(1):83–94. doi: 10.1016/j.jad.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 115.Lydiard R.B., Culpepper L., Schiöler H., Gustafsson U., Paulsson B. Quetiapine monotherapy as treatment for anxiety symptoms in patients with bipolar depression: a pooled analysis of results from 2 double-blind, randomized, placebo-controlled studies. Prim. Care Companion J. Clin. Psychiatry. 2009;11(5):215–225. doi: 10.4088/PCC.08m00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ray L.A., Heydari A., Zorick T. Quetiapine for the treatment of alcoholism: Scientific rationale and review of the literature. Drug Alcohol Rev. 2010;29(5):568–575. doi: 10.1111/j.1465-3362.2010.00185.x. [DOI] [PubMed] [Google Scholar]
- 117.Brown E.S., Nejtek V.A., Perantie D.C., Rajan Thomas N., Rush A.J. Cocaine and amphetamine use in patients with psychiatric illness: a randomized trial of typical antipsychotic continuation or discontinuation. J. Clin. Psychopharmacol. 2003;23(4):384–388. doi: 10.1097/01.jcp.0000085412.08426.08. [DOI] [PubMed] [Google Scholar]
- 118.Nejtek V.A., Avila M., Chen L.A., Zielinski T., Djokovic M., Podawlitz A., Kaiser K., Bae S., Rush A.J. Do atypical antipsychotics effectively treat co-occurring bipolar disorder and stimulant dependence? A randomized, double-blind trial. J. Clin. Psychiatry. 2008;69(8):1257–1266. doi: 10.4088/JCP.v69n0808. [DOI] [PubMed] [Google Scholar]
- 119.Bentzley B.S., Han S.S., Neuner S., Humphreys K., Kampman K.M., Halpern C.H. Comparison of treatments for cocaine use disorder among adults. JAMA Netw. Open. 2021;4(5):e218049. doi: 10.1001/jamanetworkopen.2021.8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tapp A., Wood A.E., Kennedy A., Sylvers P., Kilzieh N., Saxon A.J. Quetiapine for the treatment of cocaine use disorder. Drug Alcohol Depend. 2015;149:18–24. doi: 10.1016/j.drugalcdep.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 121.Dubath C., Piras M., Gholam M., Laaboub N., Grosu C., Sentissi O., Gamma F., Solida A., von Gunten A., Conus P., Eap C.B. Effect of quetiapine, from low to high dose, on weight and metabolic traits: Results from a prospective cohort study. Pharmacopsychiatry. 2021;54(6):279–286. doi: 10.1055/a-1525-2820. [DOI] [PubMed] [Google Scholar]
- 122.Leucht S., Cipriani A., Spineli L., Mavridis D., Örey D., Richter F., Samara M., Barbui C., Engel R.R., Geddes J.R., Kissling W., Stapf M.P., Lässig B., Salanti G., Davis J.M. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 123.Carli M., Kolachalam S., Longoni B., Pintaudi A., Baldini M., Aringhieri S., Fasciani I., Annibale P., Maggio R., Scarselli M. Atypical antipsychotics and metabolic syndrome: From molecular mechanisms to clinical differences. Pharmaceuticals. 2021;14(3):238. doi: 10.3390/ph14030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brown R., Taylor M.J., Geddes J. Aripiprazole alone or in combination for acute mania. Cochrane Libr. 2013;(12):CD005000. doi: 10.1002/14651858.CD005000.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suppes T., Eudicone J., McQuade R., Pikalov A., III, Carlson B. Efficacy and safety of aripiprazole in subpopulations with acute manic or mixed episodes of bipolar I disorder. J. Affect. Disord. 2008;107(1-3):145–154. doi: 10.1016/j.jad.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 126.Vieta E., T’joen C., McQuade R.D., Carson W.H., Jr, Marcus R.N., Sanchez R., Owen R., Nameche L. Efficacy of adjunctive aripiprazole to either valproate or lithium in bipolar mania patients partially nonresponsive to valproate/lithium monotherapy: a placebo-controlled study. Am. J. Psychiatry. 2008;165(10):1316–1325. doi: 10.1176/appi.ajp.2008.07101560. [DOI] [PubMed] [Google Scholar]
- 127.Marcus R., Khan A., Rollin L., Morris B., Timko K., Carson W., Sanchez R. Efficacy of aripiprazole adjunctive to lithium or valproate in the long-term treatment of patients with bipolar I disorder with an inadequate response to lithium or valproate monotherapy: a multicenter, double-blind, randomized study. Bipolar Disord. 2011;13(2):133–144. doi: 10.1111/j.1399-5618.2011.00898.x. [DOI] [PubMed] [Google Scholar]
- 128.De Fazio P., Girardi P., Maina G., Mauri M.C., Mauri M., Monteleone P., Perini G.I., Perugi G., Rossi A. Aripiprazole in acute mania and long-term treatment of bipolar disorder: a critical review by an Italian working group. Clin. Drug Investig. 2010;30(12):827–841. doi: 10.2165/11584270-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 129.de Bartolomeis A., Perugi G. Combination of aripiprazole with mood stabilizers for the treatment of bipolar disorder: from acute mania to long-term maintenance. Expert Opin. Pharmacother. 2012;13(14):2027–2036. doi: 10.1517/14656566.2012.719876. [DOI] [PubMed] [Google Scholar]
- 130.Kotzalidis G.D., Rapinesi C., Chetoni C., De Filippis S. Aripiprazole IM depot as an option for the treatment of bipolar disorder. Expert Opin. Pharmacother. 2021;22(11):1407–1416. doi: 10.1080/14656566.2021.1910236. [DOI] [PubMed] [Google Scholar]
- 131.Calabrese J.R., Jin N., Johnson B., Such P., Baker R.A., Madera J., Hertel P., Ottinger J., Amatniek J., Kawasaki H. Aripiprazole once-monthly as maintenance treatment for bipolar I disorder: a 52-week, multicenter, open-label study. Int. J. Bipolar Disord. 2018;6(1):14. doi: 10.1186/s40345-018-0122-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li D-J., Tseng P-T., Stubbs B., Chu C-S., Chang H-Y., Vieta E. Efficacy, safety and tolerability of aripiprazole in bipolar disorder: An updated systematic review and meta-analysis of randomized controlled trials. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;79(Pt B):289-301. doi: 10.1016/j.pnpbp.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 133.Meduri M., Gregoraci G., Baglivo V., Balestrieri M., Isola M., Brambilla P. A meta-analysis of efficacy and safety of aripiprazole in adult and pediatric bipolar disorder in randomized controlled trials and observational studies. J. Affect. Disord. 2016;191:187–208. doi: 10.1016/j.jad.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 134.Fountoulakis K.N., Vieta E. Efficacy and safety of aripiprazole in the treatment of bipolar disorder: a systematic review. Ann. Gen. Psychiatry. 2009;8(1):16. doi: 10.1186/1744-859X-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Viikki M., Valtonen H.M., Leinonen E. Three cases of hypomania and one case of mania during aripiprazole treatment. Vol. 44. Pharmacopsychiatry: Germany: 2011. pp. 78–81. [DOI] [PubMed] [Google Scholar]
- 136.Lin H-Y., Lin C-C., Liu C-C. Hypomania associated with adjunctive aripiprazole in an elder female with recurrent major depressive disorder: dose-related phenomenon? J. Clin. Psychopharmacol. 2012;32(6):836–837. doi: 10.1097/JCP.0b013e318272d2b7. [DOI] [PubMed] [Google Scholar]
- 137.Donohue A. First manic episode in a 55-year-old man after initiation of aripiprazole. Psychiatry (Edgmont (Pa. : Township) 2010;7:37-9. [PMC free article] [PubMed] [Google Scholar]
- 138.Del Casale A., Sorice S., Padovano A., Simmaco M., Ferracuti S., Lamis D.A., Rapinesi C., Sani G., Girardi P., Kotzalidis G.D., Pompili M. Psychopharmacological treatment of obsessive-compulsive disorder (OCD). Curr. Neuropharmacol. 2019;17(8):710–736. doi: 10.2174/1570159X16666180813155017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schönfelder S., Schirmbeck F., Waltereit R., Englisch S., Zink M. Aripiprazole improves olanzapine-associated obsessive compulsive symptoms in schizophrenia. Clin. Neuropharmacol. 2011;34(6):256–257. doi: 10.1097/WNF.0b013e31823429bd. [DOI] [PubMed] [Google Scholar]
- 140.Veale D., Miles S., Smallcombe N., Ghezai H., Goldacre B., Hodsoll J. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and meta-analysis. BMC Psychiatry. 2014;14(1):317. doi: 10.1186/s12888-014-0317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amerio A., Odone A., Ghaemi S.N. Aripiprazole augmentation in treating comorbid bipolar disorder and obsessive-compulsive disorder: A systematic review. J. Affect. Disord. 2019;249:15–19. doi: 10.1016/j.jad.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 142.Brunetti M., Di Tizio L., Dezi S., Pozzi G., Grandinetti P., Martinotti G. Aripiprazole, alcohol and substance abuse: a review. Eur. Rev. Med. Pharmacol. Sci. 2012;16(10):1346–1354. [PubMed] [Google Scholar]
- 143.Martinotti G., Orsolini L., Fornaro M., Vecchiotti R., De Berardis D., Iasevoli F., Torrens M., Di Giannantonio M. Aripiprazole for relapse prevention and craving in alcohol use disorder: current evidence and future perspectives. Expert Opin. Investig. Drugs. 2016;25(6):719–728. doi: 10.1080/13543784.2016.1175431. [DOI] [PubMed] [Google Scholar]
- 144.Mahapatra A., Sharma P., Sagar R. Aripiprazole induced impulse control disorders: Where do we stand? Asian J. Psychiatr. 2016;23:128–130. doi: 10.1016/j.ajp.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 145.Mohan T., Dolan S., Mohan R., Dawson J. Aripiprazole and impulse-control disorders in high-risk patients. Asian J. Psychiatr. 2017;27:67–68. doi: 10.1016/j.ajp.2017.02.015. [DOI] [PubMed] [Google Scholar]
- 146.Bulbena-Cabré A., Bulbena A. Aripiprazole-Induced Hypersexuality. Prim. Care Companion CNS Disord. 2016;18(6):26650. doi: 10.4088/PCC.16l01983. [DOI] [PubMed] [Google Scholar]
- 147.Jones R., Shad M. Aripiprazole-induced kleptomania: Case report. J. Affect. Disord. 2019;244:242–243. doi: 10.1016/j.jad.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 148.Wolfschlag M., Håkansson A. Increased risk for developing gambling disorder under the treatment with pramipexole, ropinirole, and aripiprazole: A nationwide register study in Sweden. PLoS One. 2021;16(6):e0252516. doi: 10.1371/journal.pone.0252516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moran L.M., Phillips K.A., Kowalczyk W.J., Ghitza U.E., Agage D.A., Epstein D.H., Preston K.L. Aripiprazole for cocaine abstinence: a randomized–controlled trial with ecological momentary assessment. Behav. Pharmacol. 2017;28(1):63–73. doi: 10.1097/FBP.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cuomo I., Kotzalidis G.D., de Persis S., Piacentino D., Perrini F., Amici E., De Filippis S. Head-to-head comparison of 1-year aripiprazole long-acting injectable (LAI) versus paliperidone LAI in comorbid psychosis and substance use disorder: impact on clinical status, substance craving, and quality of life. Neuropsychiatr. Dis. Treat. 2018;14:1645–1656. doi: 10.2147/NDT.S171002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sepede G., Lorusso M., Spano M.C., Di Nanno P., Di Iorio G., Di Giannantonio M. Efficacy and safety of atypical antipsychotics in bipolar disorder with comorbid substance dependence: A systematic review. Clin. Neuropharmacol. 2018;41(5):181–191. doi: 10.1097/WNF.0000000000000297. [DOI] [PubMed] [Google Scholar]
- 152.Vancampfort D., Firth J., Correll C.U., Solmi M., Siskind D., De Hert M., Carney R., Koyanagi A., Carvalho A.F., Gaughran F., Stubbs B. The Impact of Pharmacological and Non-Pharmacological Interventions to Improve Physical Health Outcomes in People With Schizophrenia: A Meta-Review of Meta-Analyses of Randomized Controlled Trials. Focus Am. Psychiatr. Publ. 2021;19(1):116–128. doi: 10.1176/appi.focus.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shapiro D.A., Renock S., Arrington E., Chiodo L.A., Liu L.X., Sibley D.R., Roth B.L., Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28(8):1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- 154.Abosi O., Lopes S., Schmitz S., Fiedorowicz J.G. Cardiometabolic effects of psychotropic medications. Horm. Mol. Biol. Clin. Investig. 2018;36(1):20170065. doi: 10.1515/hmbci-2017-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carli M., Kolachalam S., Aringhieri S., Rossi M., Giovannini L., Maggio R., Scarselli M. Dopamine D2 receptors dimers: How can we pharmacologically target them? Curr. Neuropharmacol. 2018;16(2):222–230. doi: 10.2174/1570159X15666170518151127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Scarselli M., Armogida M., Chiacchio S., DeMontis M.G., Colzi A., Corsini G.U., Maggio R. Reconstitution of functional dopamine D2s receptor by co-expression of amino- and carboxyl-terminal receptor fragments. Eur. J. Pharmacol. 2000;397(2-3):291–296. doi: 10.1016/S0014-2999(00)00272-7. [DOI] [PubMed] [Google Scholar]
- 157.Chang S.C., Goh K.K., Lu M.L. Metabolic disturbances associated with antipsychotic drug treatment in patients with schizophrenia: State-of-the-art and future perspectives. World J. Psychiatry. 2021;11(10):696–710. doi: 10.5498/wjp.v11.i10.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Weston-Green K., Huang X.F., Deng C. Second generation antipsychotic-induced type 2 diabetes: a role for the muscarinic M3 receptor. CNS Drugs. 2013;27(12):1069–1080. doi: 10.1007/s40263-013-0115-5. [DOI] [PubMed] [Google Scholar]
- 159.Rosemond E., Rossi M., McMillin S.M., Scarselli M., Donaldson J.G., Wess J. Regulation of M₃ muscarinic receptor expression and function by transmembrane protein 147. Mol. Pharmacol. 2011;79(2):251–261. doi: 10.1124/mol.110.067363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shymko G., Grace T., Jolly N., Dobson L., Hacking D., Parmar A., Kapi P., Waters F. Weight gain and metabolic screening in young people with early psychosis on long acting injectable antipsychotic medication (aripiprazole vs paliperidone). Early Interv. Psychiatry. 2021;15(4):787–793. doi: 10.1111/eip.13013. [DOI] [PubMed] [Google Scholar]
- 161.Parmar A., Hulme D., Hacking D., Shymko G., Dragovic M., Terina G., Waters F. Aripiprazole in young people with early psychosis: a systematic review and meta-analysis of weight gain. Australas. Psychiatry. 2022;30(1):90–94. doi: 10.1177/10398562211037334. [DOI] [PubMed] [Google Scholar]
- 162.Weiss C., Weiller E., Baker R.A., Duffy R.A., Gwin K.K., Zhang P., McQuade R.D. The effects of brexpiprazole and aripiprazole on body weight as monotherapy in patients with schizophrenia and as adjunctive treatment in patients with major depressive disorder. Int. Clin. Psychopharmacol. 2018;33(5):255–260. doi: 10.1097/YIC.0000000000000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu M.L., Shen W.W., Chen C.H. Time course of the changes in antipsychotic-induced hyperprolactinemia following the switch to aripiprazole. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(8):1978–1981. doi: 10.1016/j.pnpbp.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 164.Labad J., Montalvo I., González-Rodríguez A., García-Rizo C., Crespo-Facorro B., Monreal J.A., Palao D. Pharmacological treatment strategies for lowering prolactin in people with a psychotic disorder and hyperprolactinaemia: A systematic review and meta-analysis. Schizophr. Res. 2020;222:88–96. doi: 10.1016/j.schres.2020.04.031. [DOI] [PubMed] [Google Scholar]
- 165.Lu Z., Sun Y., Zhang Y., Chen Y., Guo L., Liao Y., Kang Z., Feng X., Yue W. Pharmacological treatment strategies for antipsychotic-induced hyperprolactinemia: a systematic review and network meta-analysis. Transl. Psychiatry. 2022;12(1):267. doi: 10.1038/s41398-022-02027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Corena-McLeod M. Comparative pharmacology of risperidone and paliperidone. Drugs R D. 2015;15(2):163–174. doi: 10.1007/s40268-015-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cipriani A., Barbui C., Salanti G., Rendell J., Brown R., Stockton S., Purgato M., Spineli L.M., Goodwin G.M., Geddes J.R. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–1315. doi: 10.1016/S0140-6736(11)60873-8. [DOI] [PubMed] [Google Scholar]
- 168.Hirschfeld R.M.A., Keck P.E., Jr, Kramer M., Karcher K., Canuso C., Eerdekens M., Grossman F. Rapid antimanic effect of risperidone monotherapy: a 3-week multicenter, double-blind, placebo-controlled trial. Am. J. Psychiatry. 2004;161(6):1057–1065. doi: 10.1176/appi.ajp.161.6.1057. [DOI] [PubMed] [Google Scholar]
- 169.Rendell J.M., Gijsman H.J., Bauer M.S., Goodwin G., Geddes J. Risperidone alone or in combination for acute mania. Cochrane Libr. 2006;2010(1):CD004043. doi: 10.1002/14651858.CD004043.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Valdes M., Bertolin S., Qian H., Wong H., Lam R.W., Yatham L.N. Risperidone adjunctive therapy duration in the maintenance treatment of bipolar I disorder: A post hoc analysis. J. Affect. Disord. 2019;246:861–866. doi: 10.1016/j.jad.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 171.Popovic D., Reinares M., Goikolea J.M., Bonnin C.M., Gonzalez-Pinto A., Vieta E. Polarity index of pharmacological agents used for maintenance treatment of bipolar disorder. Eur. Neuropsychopharmacol. 2012;22(5):339–346. doi: 10.1016/j.euroneuro.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 172.Vieta E., Montgomery S., Sulaiman A.H., Cordoba R., Huberlant B., Martinez L., Schreiner A. A randomized, double-blind, placebo-controlled trial to assess prevention of mood episodes with risperidone long-acting injectable in patients with bipolar I disorder. Eur. Neuropsychopharmacol. 2012;22(11):825–835. doi: 10.1016/j.euroneuro.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 173.Gao K., Kemp D.E., Ganocy S.J., Gajwani P., Xia G., Calabrese J.R. Antipsychotic-induced extrapyramidal side effects in bipolar disorder and schizophrenia: a systematic review. J. Clin. Psychopharmacol. 2008;28(2):203–209. doi: 10.1097/JCP.0b013e318166c4d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Haddad P.M., Sharma S.G. Adverse effects of atypical antipsychotics: differential risk and clinical implications. CNS Drugs. 2007;21(11):911–936. doi: 10.2165/00023210-200721110-00004. [DOI] [PubMed] [Google Scholar]
- 175.Poddar I., Callahan P.M., Hernandez C.M., Pillai A., Yang X., Bartlett M.G., Terry A.V., Jr Chronic oral treatment with risperidone impairs recognition memory and alters brain-derived neurotrophic factor and related signaling molecules in rats. Pharmacol. Biochem. Behav. 2020;189:172853. doi: 10.1016/j.pbb.2020.172853. [DOI] [PubMed] [Google Scholar]
- 176.Kozicky J.M., Torres I.J., Bond D.J., Lam R.W., Yatham L.N. Comparison of neuropsychological effects of adjunctive risperidone or quetiapine in euthymic patients with bipolar I disorder. Int. Clin. Psychopharmacol. 2012;27(2):91–99. doi: 10.1097/YIC.0b013e32834e3bea. [DOI] [PubMed] [Google Scholar]
- 177.Scarselli M., Annibale P., Gerace C., Radenovic A. Enlightening G-protein-coupled receptors on the plasma membrane using super-resolution photoactivated localization microscopy. Biochem. Soc. Trans. 2013;41(1):191–196. doi: 10.1042/BST20120250. [DOI] [PubMed] [Google Scholar]
- 178.Niufan G., Tohen M., Qiuqing A., Fude Y., Pope E., McElroy H., Ming L., Gaohua W., Xinbao Z., Huichun L., Liang S. Olanzapine versus lithium in the acute treatment of bipolar mania: A double-blind, randomized, controlled trial. J. Affect. Disord. 2008;105(1-3):101–108. doi: 10.1016/j.jad.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 179.Tohen M., Chengappa K.N.R., Suppes T., Zarate C.A., Jr, Calabrese J.R., Bowden C.L., Sachs G.S., Kupfer D.J., Baker R.W., Risser R.C., Keeter E.L., Feldman P.D., Tollefson G.D., Breier A. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch. Gen. Psychiatry. 2002;59(1):62–69. doi: 10.1001/archpsyc.59.1.62. [DOI] [PubMed] [Google Scholar]
- 180.Maina G., Albert U., Salvi V., Mancini M., Bogetto F. Valproate or olanzapine add-on to lithium: An 8-week, randomized, open-label study in Italian patients with a manic relapse. J. Affect. Disord. 2007;99(1-3):247–251. doi: 10.1016/j.jad.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 181.Xu L., Lu Y., Yang Y., Zheng Y., Chen F., Lin Z. Olanzapine-valproate combination versus olanzapine or valproate monotherapy in the treatment of bipolar I mania: a randomized controlled study in a Chinese population group. Neuropsychiatr. Dis. Treat. 2015;11:1265–1271. doi: 10.2147/NDT.S81146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Houston J.P., Tohen M., Degenhardt E.K., Jamal H.H., Liu L.L.L., Ketter T.A. Olanzapine-divalproex combination versus divalproex monotherapy in the treatment of bipolar mixed episodes: a double-blind, placebo-controlled study. J. Clin. Psychiatry. 2009;70(11):1540–1547. doi: 10.4088/JCP.08m04895yel. [DOI] [PubMed] [Google Scholar]
- 183.Huhn M., Nikolakopoulou A., Schneider-Thoma J., Krause M., Samara M., Peter N., Arndt T., Bäckers L., Rothe P., Cipriani A., Davis J., Salanti G., Leucht S. Comparative efficacy and tolerability of 32 Oral antipsychotics for the acute treatment of Adults with multi-episode schizophrenia: A systematic review and network meta-analysis. Focus Am. Psychiatr. Publ. 2020;18(4):443–455. doi: 10.1176/appi.focus.18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wingård L., Bodén R., Brandt L., Tiihonen J., Tanskanen A., Kieler H., Andersen M., Reutfors J. Reducing the rehospitalization risk after a manic episode: A population based cohort study of lithium, valproate, olanzapine, quetiapine and aripiprazole in monotherapy and combinations. J. Affect. Disord. 2017;217:16–23. doi: 10.1016/j.jad.2017.03.054. [DOI] [PubMed] [Google Scholar]
- 185.Cipriani A., Rendell J.M., Geddes J. Olanzapine in long-term treatment for bipolar disorder. Cochrane Libr. 2009;(1):CD004367. doi: 10.1002/14651858.CD004367.pub2. [DOI] [PubMed] [Google Scholar]
- 186.Cipriani A., Rendell J., Geddes J.R. Olanzapine in the long-term treatment of bipolar disorder: A systematic review and meta-analysis. J. Psychopharmacol. 2010;24(12):1729–1738. doi: 10.1177/0269881109106900. [DOI] [PubMed] [Google Scholar]
- 187.Tohen M., Sutton V.K., Calabrese J.R., Sachs G.S., Bowden C.L. Maintenance of response following stabilization of mixed index episodes with olanzapine monotherapy in a randomized, double-blind, placebo-controlled study of bipolar 1 disorder. J. Affect. Disord. 2009;116(1-2):43–50. doi: 10.1016/j.jad.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 188.Popovic D., Reinares M., Amann B., Salamero M., Vieta E. Number needed to treat analyses of drugs used for maintenance treatment of bipolar disorder. Psychopharmacology (Berl.) 2011;213(4):657–667. doi: 10.1007/s00213-010-2056-8. [DOI] [PubMed] [Google Scholar]
- 189.Baldessarini R.J., Vázquez G.H., Tondo L. Bipolar depression: a major unsolved challenge. Int. J. Bipolar Disord. 2020;8(1):1. doi: 10.1186/s40345-019-0160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Karaş H.; Güdük, M.; Saatcioğlu, O. Withdrawal-emergent dyskinesia and supersensitivity psychosis due to olanzapine use. Noro Psikiyatri Arsivi. 2016;53(2):178–180. doi: 10.5152/npa.2015.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chouinard G., Samaha A.N., Chouinard V.A., Peretti C.S., Kanahara N., Takase M., Iyo M. Antipsychotic-induced dopamine supersensitivity psychosis: pharmacology, criteria, and therapy. Psychother. Psychosom. 2017;86(4):189–219. doi: 10.1159/000477313. [DOI] [PubMed] [Google Scholar]
- 192.Crilly J. The history of clozapine and its emergence in the US market. Hist. Psychiatry. 2007;18(1):39–60. doi: 10.1177/0957154X07070335. [DOI] [PubMed] [Google Scholar]
- 193.Young C.R., Longhurst J.G., Bowers M.B., Jr, Mazure C.M. The expanding indications for clozapine. Exp. Clin. Psychopharmacol. 1997;5(3):216–234. doi: 10.1037/1064-1297.5.3.216. [DOI] [PubMed] [Google Scholar]
- 194.Aringhieri S., Kolachalam S., Gerace C., Carli M., Verdesca V., Brunacci M.G., Rossi C., Ippolito C., Solini A., Corsini G.U., Scarselli M. Clozapine as the most efficacious antipsychotic for activating ERK 1/2 kinases: Role of 5-HT 2A receptor agonism. Eur. Neuropsychopharmacol. 2017;27(4):383–398. doi: 10.1016/j.euroneuro.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 195.Khokhar J.Y., Henricks A.M., Sullivan E.D.K., Green A.I. Unique effects of clozapine: A pharmacological perspective. Adv. Pharmacol. 2018;82:137–162. doi: 10.1016/bs.apha.2017.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nucifora F.C., Jr, Mihaljevic M., Lee B.J., Sawa A. Clozapine as a model for antipsychotic development. Neurotherapeutics. 2017;14(3):750–761. doi: 10.1007/s13311-017-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kapczinski F., Pfaffenseller B., Dursun S.M., Azevedo Cardoso T. Clozapine for bipolar disorder: What do we know so far and what next? Bipolar Disord. 2021;23(2):115–116. doi: 10.1111/bdi.13050. [DOI] [PubMed] [Google Scholar]
- 198.Meltzer H.Y., Alphs L., Green A.I., Altamura A.C., Anand R., Bertoldi A., Bourgeois M., Chouinard G., Islam M.Z., Kane J., Krishnan R., Lindenmayer J.P., Potkin S. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch. Gen. Psychiatry. 2003;60(1):82–91. doi: 10.1001/archpsyc.60.1.82. [DOI] [PubMed] [Google Scholar]
- 199.Andreea T., Petru I., Miron A.A., Paula-Simina P., Lorena D. Clozapine for treatment-refractory aggressive behavior. Psychiatr. Q. 2021;92(2):721–733. doi: 10.1007/s11126-020-09839-x. [DOI] [PubMed] [Google Scholar]
- 200.Calabrese J.R., Kimmel S.E., Woyshville M.J., Rapport D.J., Faust C.J., Thompson P.A., Meltzer H.Y. Clozapine for treatment-refractory mania. Am. J. Psychiatry. 1996;153(6):759–764. doi: 10.1176/ajp.153.6.759. [DOI] [PubMed] [Google Scholar]
- 201.Calabrese J.R., Meltzer H.Y., Markovitz P.J. Clozapine prophylaxis in rapid cycling bipolar disorder. J. Clin. Psychopharmacol. 1991;11(6):396–397. doi: 10.1097/00004714-199112000-00026. [DOI] [PubMed] [Google Scholar]
- 202.Suppes T., Phillips K.A., Judd C.R. Clozapine treatment of nonpsychotic rapid cycling bipolar disorder: A report of three cases. Biol. Psychiatry. 1994;36(5):338–340. doi: 10.1016/0006-3223(94)90631-9. [DOI] [PubMed] [Google Scholar]
- 203.Li X.B., Tang Y.L., Wang C.Y., de Leon J. Clozapine for treatment-resistant bipolar disorder: a systematic review. Bipolar Disord. 2015;17(3):235–247. doi: 10.1111/bdi.12272. [DOI] [PubMed] [Google Scholar]
- 204.Delgado A., Velosa J., Zhang J., Dursun S.M., Kapczinski F., de Azevedo Cardoso T. Clozapine in bipolar disorder: A systematic review and meta-analysis. J. Psychiatr. Res. 2020;125:21–27. doi: 10.1016/j.jpsychires.2020.02.026. [DOI] [PubMed] [Google Scholar]
- 205.Jakobsen M.I., Grønborg H., Hansen H.V., Fink-Jensen A. Clozapine-associated neutropenia following augmentation with sodium valproate. SAGE Open Med. Case Rep. 2021:9. doi: 10.1177/2050313X211019791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kando J.C., Tohen M., Castillo J., Centorrino F. Concurrent use of clozapine and valproate in affective and psychotic disorders. J. Clin. Psychiatry. 1994;55(6):255–257. [PubMed] [Google Scholar]
- 207.Williams A.M., Park S.H. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101–111. doi: 10.1007/s40263-014-0222-y. [DOI] [PubMed] [Google Scholar]
- 208.Wilkinson S.T., Trujillo D.D., Rupp Z.W., Kidambi A., Ramirez K.L., Flores J.M., Avila-Quintero V.J., Rhee T.G., Olfson M., Bloch M.H. Pharmacological and somatic treatment effects on suicide in adults: A systematic review and meta‐analysis. Depress. Anxiety. 2022;39(2):100–112. doi: 10.1002/da.23222. [DOI] [PubMed] [Google Scholar]
- 209.Silva E., Higgins M., Hammer B., Stephenson P. Clozapine rechallenge and initiation despite neutropenia- a practical, step-by-step guide. BMC Psychiatry. 2020;20(1):279. doi: 10.1186/s12888-020-02592-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Amerio A., Odone A. Clozapine and lithium as maintenance therapy in a young treatment-resistant bipolar patient. Asian J. Psychiatr. 2018:94–95. doi: 10.1016/j.ajp.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 211.Wilkowska A. Cubała, W. Clozapine as transformative treatment in bipolar patients. Neuropsychiatr. Dis. Treat. 2019;15:2901–2905. doi: 10.2147/NDT.S227196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Fehr B.S., Ozcan M.E., Suppes T. Low doses of clozapine may stabilize treatment? resistant bipolar patients. Eur. Arch. Psychiatry Clin. Neurosci. 2005;255(1):10–14. doi: 10.1007/s00406-004-0528-8. [DOI] [PubMed] [Google Scholar]