Abstract
Alzheimer's is a chronic neurodegenerative disease where amyloid-beta (Aβ) plaques and neurofibrillary tangles are formed inside the brain. It is also characterized by progressive memory loss, depression, neuroinflammation, and derangement of other neurotransmitters. Due to its complex etiopathology, current drugs have failed to completely cure the disease. Natural compounds have been investigated as an alternative therapy for their ability to treat Alzheimer's disease (AD). Traditional herbs and formulations which are used in the Indian ayurvedic system are rich sources of antioxidant, anti-amyloidogenic, neuroprotective, and anti-inflammatory compounds. They promote quality of life by improving cognitive memory and rejuvenating brain functioning through neurogenesis. A rich knowledge base of traditional herbal plants (Turmeric, Gingko, Ashwagandha, Shankhpushpi, Giloy, Gotu kola, Garlic, Tulsi, Ginger, and Cinnamon) combined with modern science could suggest new functional leads for Alzheimer's drug discovery. In this article Ayurveda, the ancient Indian herbal medicine system based on multiple clinical and experimental, evidence have been reviewed for treating AD and improving brain functioning. This article presents a modern perspective on the herbs available in the ancient Indian medicine system as well as their possible mechanisms of action for AD treatment. The main objective of this research is to provide a systematic review of herbal drugs that are easily accessible and effective for the treatment of AD.
Keywords: Alzheimer's, neurodegenerative, Aβ plaques, cognitive memory, medicinal herbs, neurofibrillary tangles
1. INTRODUCTION
Alzheimer’s disease (AD) causes permanent damage to neurons. The hallmark features of Alzheimer’s disease includes the formation of intracellular neurofibrillary tangles, senile plaques, and loss of neuronal synapses and pyramidal neurons inside the brain [1]. These changes lead to the emergence of the characteristic Alzheimer’s disease symptoms, which include progressive and gross cognitive impairment and frequent behavioral disorders like depression, anxiety, aggression, and wandering [2]. The entorhinal cortex and hippocampus are the foremost areas of the brain where Alzheimer’s disease damages neuronal connections. Later on, it affects areas of the cerebral cortex that controls social interaction, language, and logic. Dementia is often associated with progressive deterioration of intellectual function. It affects individuals over a gradual period of time followed by their lost ability to live and function independently [2-9]. Even after a century of research, we still can not determine the relationship between cognitive decline and proteinaceous deposition of plaques and tangles [10].
Approximately 5 million new cases of dementia are diagnosed every year, affecting more than 25 million people worldwide, the majority of whom have AD [3-5]. Since AD is age-specific and is directly correlated with age its prevalence doubles by 5 years after the age of 65. In the past few years, a lot of interest has been shown in the epidemiologic study of dementia and AD in underdeveloped and developing nations. In Europe, the combined incidence rate of AD among those 65 and older was 19.4 per 1000 person-years. In the US, according to the combined data from two large, community-based studies, the incidence rate for AD was 15 per 1000 person-years for people aged 65+ [6-8].
Currently, only FDA-approved therapeutic options, including acetylcholinesterase (AChE) inhibitors (donepezil, galantamine, rivastigmine, and tacrine), a partial NMDA receptor antagonist (memantine), and Aduhelm (recently) for AD treatment are available [11, 12]. However, they are expensive, have side effects, and approximately 5 to 8 years to a person’s life span. Recently, traditional and complementary modalities of medicine have received a lot of attention. Since the dawn of time, medicinal plants have been used to treat a wide range of illnesses, including schizophrenia, epilepsy, depression, anxiety, and even neuro-regeneration [9, 10, 13, 14]. Numerous herbs with advantageous neurochemical constituents have also been discovered, such as plant molecules with anti-neurodegenerative or neuroprotective properties [15]. Studies have shown that Indian ayurveda had used medicinal plants for improving memory and rejuvenating brain cells since very ancient times. These herbs improve brain activity by enhancing anti-inflammatory and anti-amyloidogenic properties. Several well-known medicinal plants that have been extensively researched by scientists for their nootropic benefits are Curcuma longa (Turmeric), Ginkgo biloba, Withania somnifera (Ashwagandha), Convolvulus pluricaulis (Shankhpushpi), Tinospora cordifolia (Giloy), Centella asiatica (Gotu kola), Allium sativum Linn (Garlic), Ocimum sanctum Linn (Tulsi), Zingiber officinale (Ginger), Cinnamon zeylanicum (Cinnamon) and Azadirachta indica (Neem).
2. ALZHEIMER’S DISEASE (AD) HYPOTHESIS
AD is a multifactorial disorder in which more than 200 proteins or enzymes involved in age-related biological processes have been implicated in the pathogenesis of the disease [16-18]. There is a strong positive correlation between aging and AD risk which cumulatively affect the impact of various risks over the span of a person’s lifetime, including biological factors, psychosocial factors, environmental exposures, and genetic susceptibility [6, 19-21]. The various etiologic hypotheses responsible for elevated AD risk are summarized in Table 1, along with major protective and risk factors. The etiology of Alzheimer’s disease is still not clear, and the current one-drug, one-target treatment strategy for the disease appears to be clinically ineffective. Although, several hypotheses have been linked with AD until today, so far amyloid β (Aβ) hypothesis describes the most acceptable explanation for AD progression [22, 23].
Table 1.
List of various etiological hypotheses and their characteristic features related with Alzheimer’s disease (AD) progression.
|
Etiological
Hypothesis |
Characteristic Features | References |
|---|---|---|
| Aβ cascade | Aβ peptides deposit as senile plaques, intraneuronal neurofibrillary tangles (NFTs), neurodegeneration | [112-114] |
| Tau hypothesis | Neurofibrillary tangles, impairment of axons of neurons | [115] |
| Inflammation hypothesis |
Reactive gliosis and neuroinflammation | [116, 117] |
| Cholinergic and oxidative stress hypothesis | Cholinergic neurons are damage, cellular oxidative stress | [118, 119] |
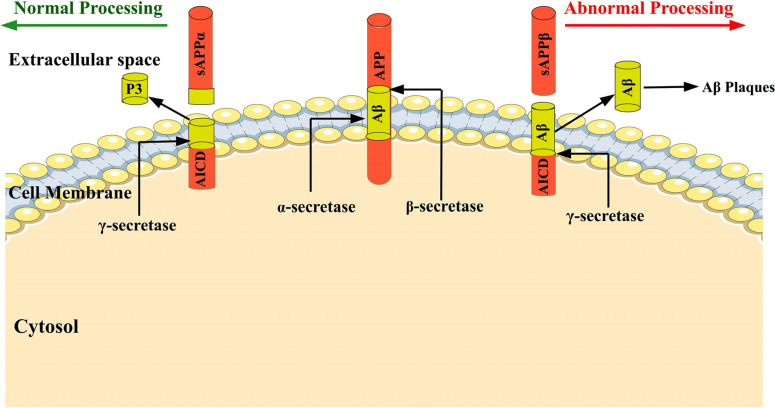
The amyloid precursor protein (APP) is processed through 3 different proteases via α and β-secretase pathways [24, 25]. During the normal physiological condition, 90% of the APP is cleaved through α and γ-secretase and forms soluble p3 and APP intracellular domain (AICD) fragments. When a small percentage of APP molecules enter the β-secretase route, APP is cleaved by β-secretase. It resultsin the formation of β-APPs and membrane-bound C99 peptides. The γ-secretase cleaves the C-terminal membrane-bound C99 peptide within the transmembrane domain to produce two primary isoforms of Αβ with 40 and 42 amino acid lengths [24, 26, 27]. The Αβ 42 is hydrophobic and is the primary cause for plaques formation associated with the early onset of Alzheimer’s disease [28] (EOAD). Since the aggregation of Aβ is thought to be the cause of the disorder, as shown in Fig. (1) no medicine has yet been proven to reverse, stop, or even reduce the progression of the disease.
Fig. (1).
The amyloid precursor protein (APP) is a transmembrane protein, and during normal physiological condition, 90% of the APP is cleaved through α and γ-secretase and forms soluble p3 and APP intracellular domain (AICD) fragments. When a small percentage of APP molecules enter the β-secretase route, APP is cleaved by β-secretase. It results in the formation of β-APPs and membrane-bound C99 peptides. The γ-secretase cleaves the C-terminal membrane-bound C99 peptide within the transmembrane domain to produce two primary isoforms of Αβ with 40 and 42 amino acid lengths which are responsible for Αβ plaques formation.
Herbal plants contain several phytochemically active compounds such as Tannic acid, Quercetin, Kaempferol, Curcumin, Catechin, and Epicatechin. Numerous valuable natural compounds, such as Triterpenes, Flavonoids, Sterols, Lignans, Tannins, Polyphenols and Alkaloids have been discovered through phytochemical studies of various plant parts and they all are known to exhibit a wide range of pharmacological properties (anti-amyloidogenic, anti-inflammatory, antioxidant and anti-cholinesterase activities). These compounds are known to suppress the production and elongation of Amyloid-beta (Aβ) fibrils in a dose-dependent manner [13].
3. HERBAL PLANTS OF INTEREST FOR THERAPEUTIC TREATMENT OF AD
3.1. Curcuma longa (Turmeric)- Spice of Life
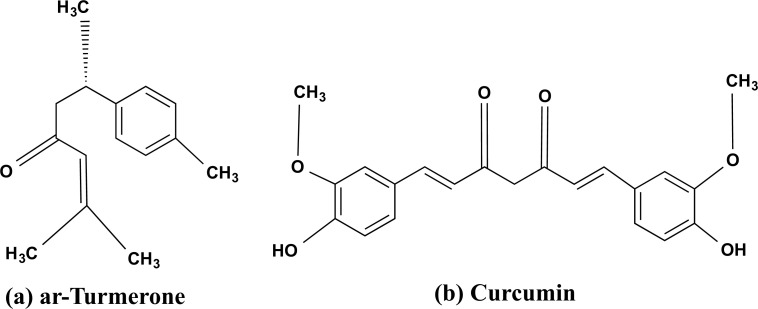
Turmeric, particularly known as “Indian Saffron” in Europe, is a perennial rhizome and belongs to Zingiberaceae family. Turmerone oil and water-soluble curcuminoids such as curcumin are the most important active ingredients of the turmeric plant as shown in Fig. (2a and b) [14]. Despite being considered a common cooking spice turmeric exhibits healing, antioxidant, anti-inflammatory, anti-cancer, and anti-amyloid properties [29-33]. However, since Vedic times, its medicinal properties have been documented to be used in the Ayurveda, Siddha, and Unani systems of medicine.
Fig. (2).
The phytochemically active chemical structure of curcumin (a) ar-Tumerone, and (b) curcumin.
Curcuminoids (2-9% present) are the primary active compounds responsible for the medicinal potential of turmeric. Curcuminoids include curcumin (77%), demethoxycurcumin (17%), and bis-demethoxycurcumin (3%) as the main active phytochemicals [34-36]. The mode of action of curcumin is pleiotropic [37]. Since the accumulation of Aβ is the primary hallmark of Alzheimer’s disease [28]. Curcumin focuses on the two histology indicators of AD, i.e., Tau and Aβ. Studies have found that curcumin doses ranging from 0.1 to 1.0 M had disaggregated fibrillar Aβ40, inhibited Aβ40 aggregation inside the brain, and prevented the development of Aβ42 oligomers [29, 30, 38, 39].
In another in-vivo study, Alzheimer transgenic APPSw mouse model (Tg2576) showed that expression of proinflammatory cytokine IL-1β and the astrocytic inflammatory marker GFAP were significantly reduced at 160 ppm dose of curcumin [40]. The plaque burden was also decreased along with insoluble and soluble amyloids. The low dose of curcumin reduces the production of Aβ plaques and the oligomerization tendency for Aβ formation in Tg2576 mice’s brains [38, 41].
Curcumin is a lipophilic substance that can easily cross the blood-brain barrier [42]. Although, multiple attempts of research had demonstrated curcumin as a future potential drug but its absorption through gastrointestinal metabolism results in its limited bioavailability. Due to its low bioavailability and poor solubility, curcumin’s principal pharmacological properties, such as anti-oxidant, anti-inflammatory, anti-amyloidogenic, anti-bacterial, and anti-tumor are also limited [43, 44]. To enhance the bioavailability and solubility of curcumin, Cur-loaded nanoparticles of various sorts have been engineered for the treatment of various ailments. The enhanced curcumin’s biocompatibility and solubility using different nanoformulations improves its therapeutic potential and lays the groundwork for more clinical research and applications [41, 45].
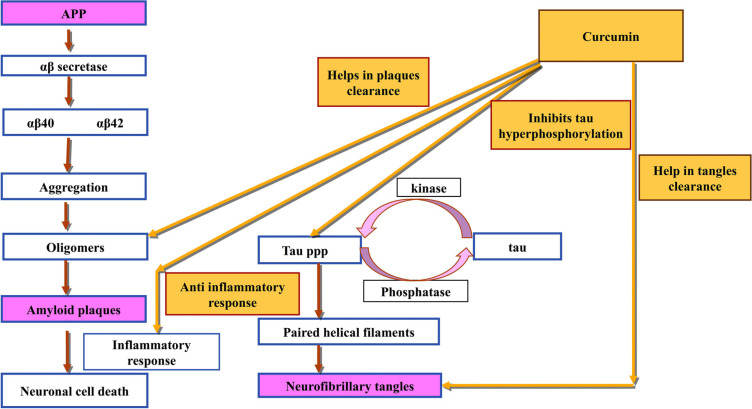
Sun et al., used an anionic polymerization approach to make Cur-loaded polybutylcyanoacrylate (PBCA) nanoparticles (CUR-PBCN) which exhibit improved curcumin transport towards the brain. Within 24 hours, CUR-PBCN had slowly released 75% of the medicine, demonstrating that nanoparticles show sustained-release capability [46]. Various mechanisms of action where curcumin can act as a multitarget drug is described in Fig. (3).
Fig. (3).
This flowchart shows the diverse mechanisms of action by which curcumin provides neuroprotection against Alzheimer’s disease. The active compounds inhibit the development and neurotoxicity of Aβ and hyperphosphorylated tau, which are two histology hallmarks of Alzheimer’s disease.
3.2. Ginkgo biloba
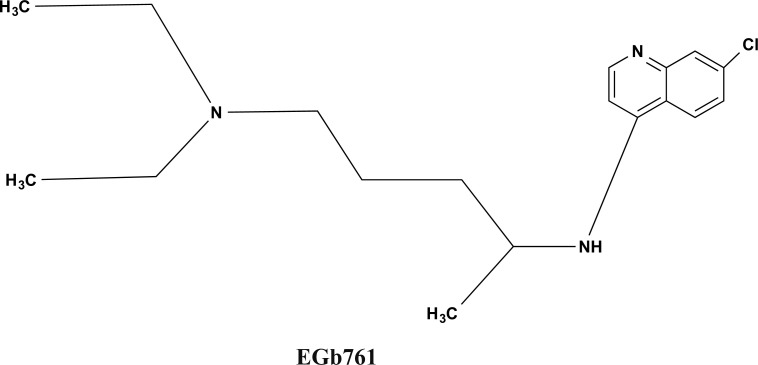
Ginkgo biloba or Maidenhair tree has been used for generations as a traditional medicine. EGb 761 is a well-defined product of Ginkgo biloba leaves extract and contains approximately 6% terpene lactones (2.8-3.4% ginkgolides A, B and C, and 2.6-3.2% bilobalide) and 24% flavone glycosides (primarily Quercetin, Kaempferol, and Isorhamnetin) as shown in Fig. (4) [47].
Fig. (4).
The bioactive chemical structure of Gingko biloba extract Egb761.
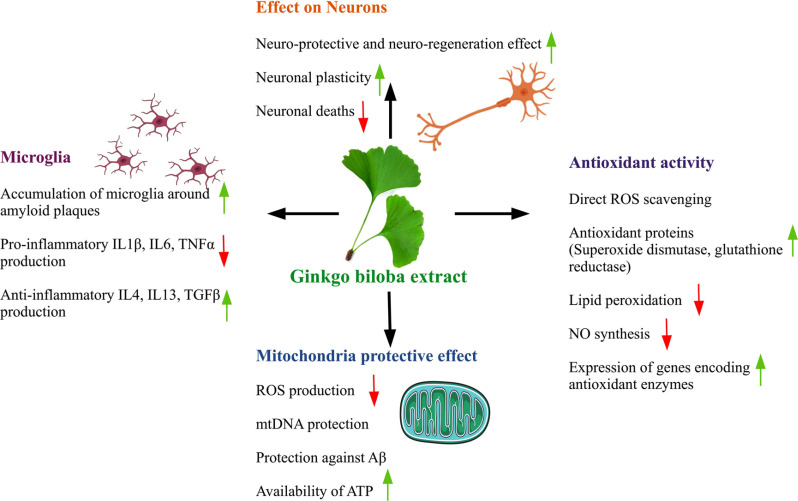
The extract EGb 761 obtained from Gingko biloba leaves helps in enhancing the antioxidant activity of the brain cells (free-radical scavenging action) which has a role in neuroprotection. It protects mitochondria through an anti-apoptotic activity which maintains the integrity of the mitochondrial membrane and prevents the release of cytochrome c, which eventually blocks the formation of the apoptosome and the apoptotic caspase cascade. The active phytochemical compounds present in Ginkgo biloba extracts prevent Aβ aggregation and amyloidogenesis as shown in Fig. (5). Other potential pathways for EGb761’s neuroprotective effects on AD include ion homeostasis, stimulation of growth factor production, and modulation of tau protein phosphorylation [48]. It was hypothesized that Ginkgo biloba extracts exhibit promising efficacy against AD by inhibiting amyloid-beta aggregation [49]. In animals, Ginkgo biloba has been shown to boost cognitive memory. The effect of 10 mg/kg, 20 mg/kg, or 40 mg/kg Ginkgo biloba extract on long-term reference memory retention in rats was investigated in a study. It appears to aid in the acquisition of spatial knowledge [49, 50].
Fig. (5).
The several mechanisms by which Ginkgo extract provides neuroprotection in Alzheimer’s disease.
Moreover, in an extensive study, 622 subjects were considered as control and 41 subjects were 65 years plus in age were administered with an antioxidant combination of lycopene, polyunsaturated fatty acid (PUFA), and Ginkgo biloba extract (one dose/day). Over the span of 3 years, the cognitive function of the test and control groups was examined. It was observed that the antioxidant supplement had improved cognitive function in the test group [50].
3.3. Withania somnifera (Ashwagandha)
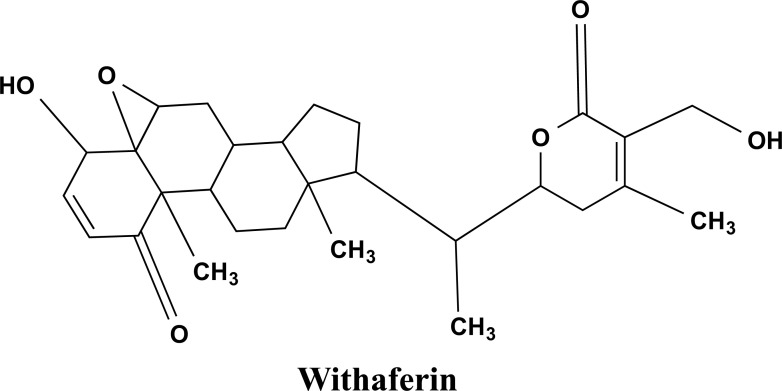
Withania somnifera (WS) is a member of the Solanaceae family. Alkaloid extract of WS roots is utilized for its calming effects on CNS, and it also possesses free radical scavenging and antioxidant activity. The extracts of WS have the potential to maintain a healthy immune system [51]. The ergostane-type steroidal lactones, Withasomniferin A, Dehydrowithanolide R, Withasomidienone, withaferin A as shown in Fig. (6), Withasomniferols A to C, phytosterols Sitoindosides VII to X and beta-sitosterol are among the steroidal chemicals found in WS extracts [51-53]. Withasomniferols A is a Withanolide obtained from the roots of WS. It has been proven to counteract the amyloid β-42 induced toxicity in human neurons [52, 54].
Fig. (6).
The chemical structure of Withaferin A. It is a lactone produced from Withania somnifera with anti-inflammatory, immunomodulatory, anti-metastasis, and anti-carcinogenic activities.
In AD, Aβ plaques formation causes neuronal cell death. This neuronal cell death is blocked by Withanamides present in WS [55]. Molecular modeling and simulation studies have shown that the binding of Withanamides A and C to the active motif of Aβ (25-35) suppresses fibril formation [55]. It was found that axons and dendrites of neurons were greatly regenerated after continuous treatment with a methanol extract of Ashwagandha. In addition to cellular regeneration, Ashwagandha improved mice’s memory by reversing amyloid peptide-induced memory loss. Studies have proven that oral administration of Withania Somnifera extract containing withanolides and Withanosides have halted the progression of AD. It also recovers plaque pathology and the development of Aβ peptides and oligomers in the brains of middle-aged and old-aged APP/PS1 AD transgenic mice [52]. These above-mentioned studies have proved that Withanolides and Withanosides can act as potential therapeutic active compounds against AD [54, 55].
Studies have shown that direct peripheral effects of withanosides/withanolides on liver lipoprotein receptor-related protein (LRP) and soluble form of LRP in plasma (sLRP) have a significant impact on reducing amyloid load. Since the amyloid deposits are poorly cleared from the brain in AD which leads to an earlier onset of the familial forms of AD, therefore, WS can be potentially useful for familial AD treatment [52].
3.4. Convolvulus pluricaulis (Shankhpushpi)
The methanolic extracts of all four verities namely, Convolvulus pluricaulis Choisy, Clitorea ternatea Linn. (CT), Canscora decussata Schult. (CD) and Evolvulus alsinoides Linn. (EA), are considered as sources for Shankhpushpi by Indian practitioners. Shankhpushpi has been used as a nervine tonic for improving cognitive and memory function [56].
Various secondary metabolites including, flavanol glycosides, triterpenoids, steroids, and anthocyanins, have been identified as the principle bioactive compounds of the Shankhpushpi’s isolated extract that is shown in Fig. (7). These compounds can be responsible for various pharmacological effects in addition to enhancing cognitive ability [56]. The ethanolic extract of Shankhpushpi enhances the antioxidant activity to a significant level inside brain cells when tested in-vitro. It functions by scavenging free radicals and acting as an antioxidant. A recent study shows that it lowers - Aβ accumulation in the brain, which protects against memory loss in AD [57]. The neuropharmacological based activity also indicates that E. alsinoides is the most effective of the four Shankhpushpi types [58]. It also boosts antioxidant enzyme activity and controls tau-induced oxidative stress by restoring acetylcholinesterase activity [59]. A considerable improvement in passive avoidance learning and retention was observed in young adult rats that had been incubated with CT aqueous root extract [60]. Age-matched saline controls and CT-treated rats have shown a significant increase in dendritic branching points, intersections, and dendritic processes emanating from the soma of neurons in the Amygdale area, indicating that CT improves memory through promoting the functional growth of neurons [61].
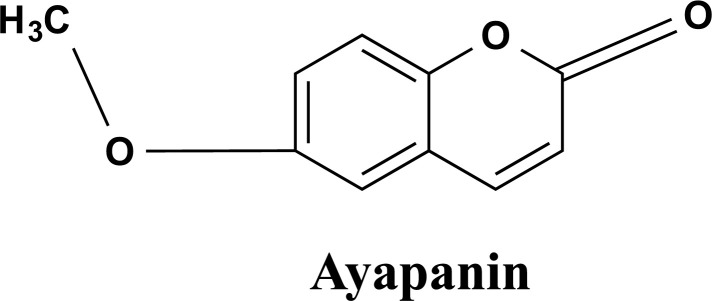
Fig. (7).
The chemical structure of Ayapanin isolated from the sources of Shankhpushpi. It improves scopolamine-induced spatial memory impairment.
3.5. Tinospora cordifolia (Giloy)
Tinospora cordifolia (TC), also known as Giloy, a member of the Menispermaceae family has historically been utilized in Ayurvedic medicine and is referred to as an adaptogen or rejuvenator. It possesses antioxidant, immunomodulatory, anti-inflammatory, antihyperglycemic, antispasmodic, antiulcer, and many other properties. The putative antistress benefits of Tinospora cordifolia have recently come to light in studies involving both humans and animals [62]. TC roots aqueous extract helps in improving verbal learning and logical memory [63, 64]. The most plausible antidepressant mechanisms involve the inhibition of the amines inside the brain from being reabsorbed. In cyclosporine-treated rats, histological analysis of the hippocampus revealed that T. cordifolia protects against neurodegenerative alterations [65, 66].
In mice, extracts of the TC have shown similar anti-stress properties. According to some studies, when given in combination with other plants, it improves memory and spatial learning in rats [65, 67]. Several research groups are working towards protection against neuroinflammation and oxidative stress. Different antioxidant compounds have been studied till now to analyse their potential for protection against neuroinflammation and oxidative stress as they are responsible for altered mitochondrial activity and production of free radicals [67-69].
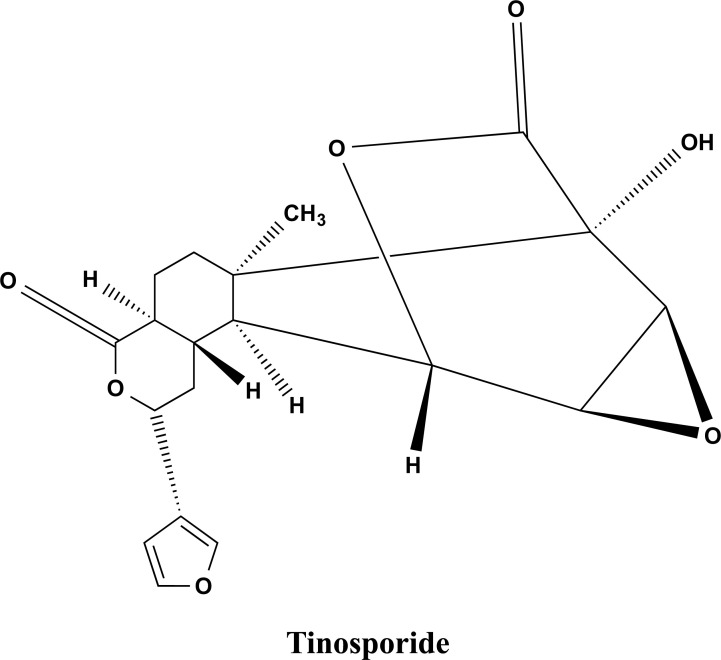
A recent study has demonstrated an antioxidant and anti-inflammatory effect of T. cordifolia leaf extract. The study has reported the attenuation of NF-κB nuclear translocation and upregulation of antioxidant enzymes in activated human monocytic (THP-1) cells [64]. On LPS-activated RAW264.7 cells, the aqueous extract of T. cordifolia dramatically decreased the gene expression of inflammatory cytokines like IL-1β and TNF-α, hepcidin, as well as NO production. Tinosporaside (shown in Fig. 8) was detected by HPLC analysis, which is likely to have contributed to T. cordifolia’s ability to reduce inflammation [63].
Fig. (8).
The chemical structure of Tinosporide extracted from the stem of T. Cordifolia.
3.6. Centella asiatica (Gotu kola)
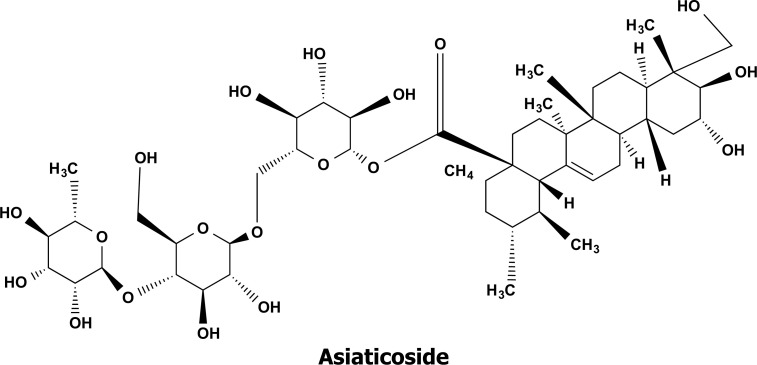
In Asia, Centella asiatica, a plant belonging to the Apiaceae (Umbelliferae) family, has been utilized as a traditional medicine for more than 2000 years. The whole plant and its extract, including the ethanolic and aqueous extracts, of C. asiatica have both been discovered to possess a multitude of therapeutic properties and biological activities. Triterpenoids, such as Asiatic acid and Asiaticoside as shown in Fig. (9), make up the majority of the active compounds of C. asiatica ethanol extract (CAE) [70]. It possesses excellent antioxidant capabilities for activating the antioxidative defence system inside the brain, reducing Fe3+, and scavenging 2,2-diphenyl-1-picrylhydrazyl (DPPH) [71, 72]. It also reduces lipid peroxidation and protects against DNA damage.
Fig. (9).
The bioactive chemical structure of Asiaticoside isolated from Centella asiatica extract.
According to Ayurveda, C. asiatica is a renewing plant for neurons and other brain cells that can boost intelligence and memory [73]. Its compounds were discovered to have a protective effect against Aβ-induced neurotoxicity, which is linked to AD dementia. The antioxidative defence system in cells, including the activities of Catalase, Superoxide Dismutase, Glutathione Reductase, Glutathione Peroxidase, and levels of Glutathione Disulphide, and glutathione is modulated by CAE. It aids in the death of Aβ cells in vitro, making it a promising medicine for the treatment and prevention of AD [74]. Studies have shown that C. asiatica can treat AD-like diseases in rats by inhibiting hyperphosphorylated tau (P-tau) bio-synthetic and anti-apoptosis proteins [74, 75].
3.7. Allium sativum Linn. (Garlic)
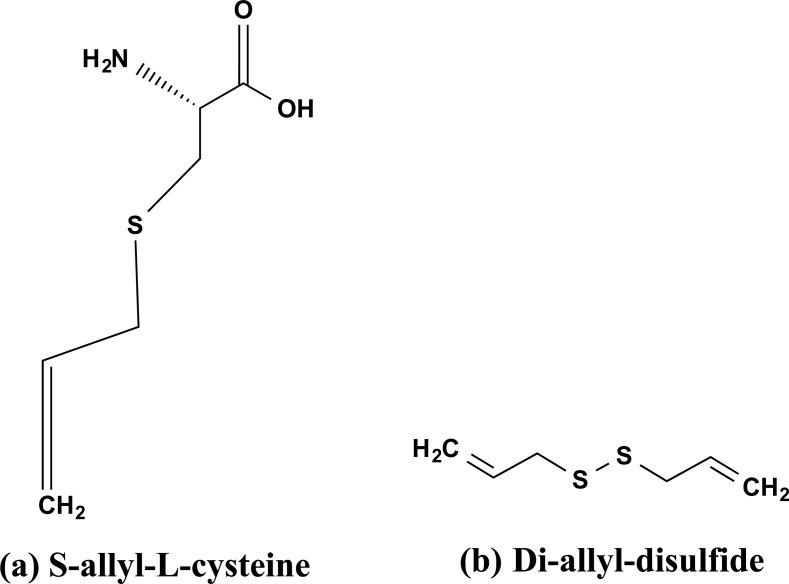
Allium sativum belonging to the Amaryllidaceae family has been utilized as a herbal medicine since ancient times. Aged garlic extract (AGE) is a non-toxic solvent extract of garlic powder. It is derived from an extended extraction period of ≥ 15 months at room temperature. The aging process converts allicin, an unstable compound is transformed into a stable substance. It contains two key compounds Di-allyl-disulfide (DAD) and S-allylcysteine (SAC) as shown in Figs. (10a and b) [76, 77]. Additional phytochemicals found in AGE include Allixin, Ajoene, Polyphenols, Thiosulfinates, and fFavanoids [78, 79]. Recently, there has been some attention to the possibility of AGE to improve cognitive impairment. In AD Aβ induces the expression of the GRP-78 thus potentiates the ER stress-induced neuronal death. The SAC component of AGE was found to have a strong neuroprotective impact on ER stress-induced neuronal death [80, 81].
Fig. (10).
The bioactive chemical structures isolated from Allium sativum. (a) S-allyl-L-cysteine (SAC), and (b) di-allyl-disulfide (DAD).
It was found that AGE had slowed the progressive degradation of hippocampal-based cognitive activities by reducing the accumulation of cerebral Aβ [76]. As cognitive impairment is a typical pathology in AD, and AGE has greatly reduced cognitive impairment in rats by enhancing working memory and reference memory [77]. Fresh garlic extract can defibrillate Aβ fibrils. After 2-3 days of incubation, the highest defibrillation was detected. The anti-amyloidogenic activity of the boiled aqueous garlic extract was retained, indicating that the anti-amyloidogenic activity of the garlic extract isnon-enzymatic in nature [82]. As a result, Allium sativum is potentially a helpful substance, that can be utilized to treat AD, as shown in Fig. (11). Ingesting garlic on daily basis may also suppres the accumulation of Aβ inside the human brain.
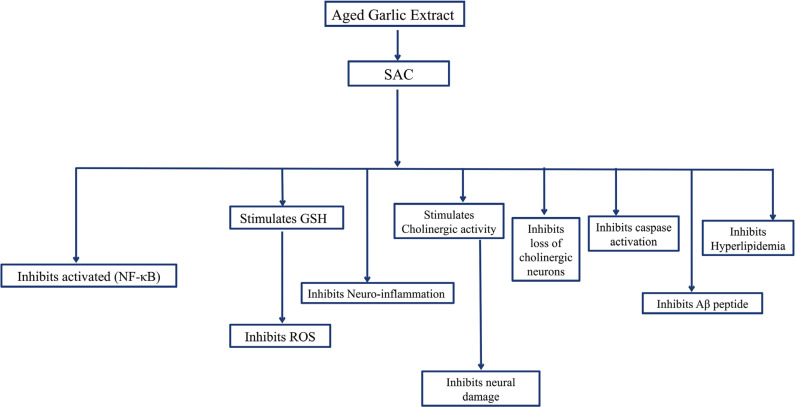
Fig. (11).
There are several mechanisms by which S-allyl-cysteine (SAC) provides neuroprotection against Alzheimer’s disease.
3.8. Ocimum sanctum (Tulsi)
Ocimum sanctum belongs to the Lamiaceae family and has been utilized in Ayurveda since ancient times due to its diverse healing properties. O. sanctum is considered the ‘Incomparable one’ herb of India, and has been cherished for its healing capacity. The chemical composition of O. sanctum is intricate and includes numerous nutrients and biologically active compounds.
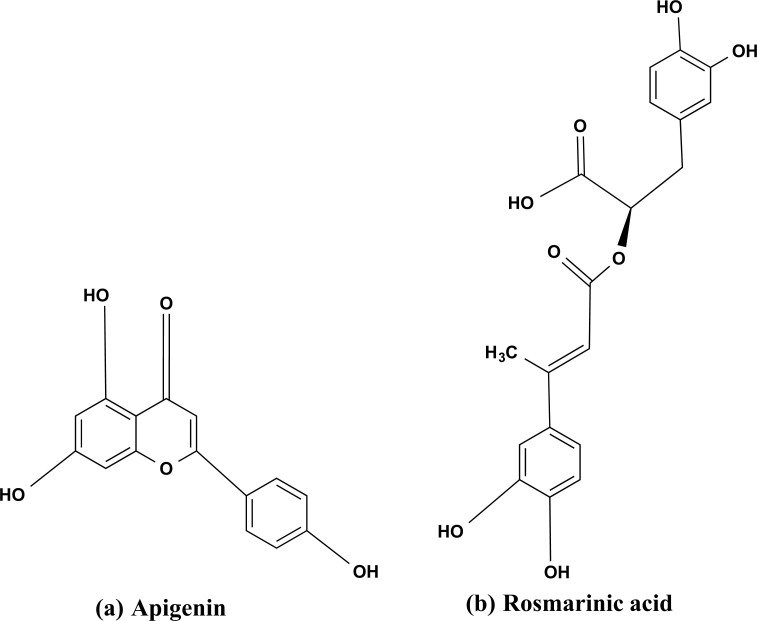
There are numerous biologically active phytochemicals present in holy basil’s stem and leaves, including Triterpenoids, Saponins, Tannins, and Flavonoids [83]. The phenolic group that contains active compounds, exhibits anti-inflammatory and antioxidant activities are apigenin Fig. (12a), rosmarinic acid Fig. (12b), Isothymusin, Isothymonin, and Cirsimaritin. The hypercholesterolemia-induced erythrocyte lipid peroxidation activity was inhibited in male albino rabbits after treating them with an aqueous extract of O. Sanctum in a dose-dependent manner [84]. Additionally, oral feeding significantly reduces the peroxidative damage induced by Hypercholestrolemia to the liver and aortic tissue. The compounds that have been extracted from O. sanctum aqueous extract are Civsimavatine, Eugenol, Civsilineol, and Apigenin, which were studied for their cyclooxygenase inhibitory activity or anti-inflammatory activity [85]. In a study, linoleic acid, which is present in varying amounts in the fixed oil of various O. sanctum species, is able to block both the lipoxygenase and cyclooxygenase pathways of arachidonate metabolism and could be useful in reducing inflammation [86].
Fig. (12).
The bioactive chemical structures isolated from Ocimum sanctum. (a) Apigenin, and (b) Rosmarinic acid.
A study was conducted to analyse the potential of O. sanctum extract as an anti-amnesic and nootropic agent in mice [87]. The amnesic response of mice towards scopolamine (0.04 mg/kg), diazepam (1 mg/kg), and aging were reduced by an aqueous extract of the entire plant of O. sanctum. The comparison of control (piracetam-treated), scopolamine, and elderly groups of mice has shown a significant lowered transfer latency and increased step-down latency when treated with O. sanctum extract. Therefore, preparation of O. Sanctum might be useful for treating cognitive disorders, including AD and dementia. The production of choline acetyltransferase (ChAT) can be induced and maintained in human Cerebral Microvascular Endothelial Cells (HCMECs) by an ethanolic extract from O. sanctum [88, 89]. Therefore, it may be a candidate for a neuroprotective substance, but a lot of in vitro research is still required. In human cerebral microvascular endothelial cells (HCMECs), an ethanolic extract from O. sanctum can induce and sustain the production of Choline Acetyltransferase (ChAT) [86, 88, 90]. Thus, it can be a candidate for neuroprotective substance but further in vitro studies are needed.
3.9. Zingiber officinale (Ginger)
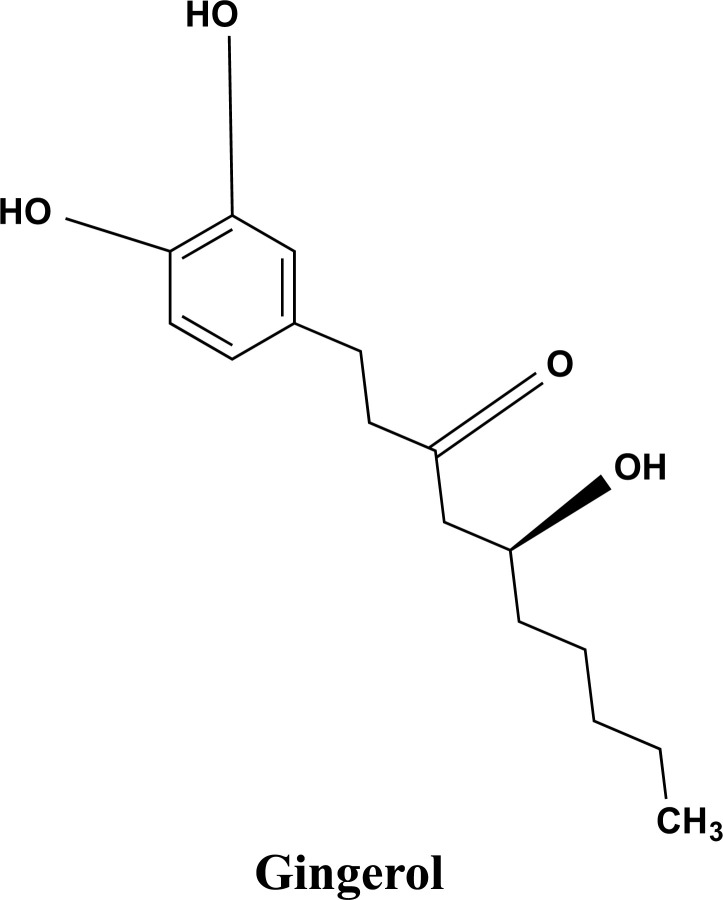
For ages, ginger (Zingiber officinale Roscoe) has been utilized in Indian spices and for a variety of therapeutic uses [91]. Ginger extracts (GE) have anti-inflammatory effects [92-94]. Inflammation plays a key role in the etiology of AD, prompting researchers to investigate the use of anti-inflammatory drugs for the treatment of the disease. A recent study has discovered that a mixture of ginger extracts from Zingiber officinale Roscoe, and Alpinia galanga can prevent THP-1 cells from being activated by TNF, IL-1, LPS, and Aβ [95]. In a molecular dynamic simulation-based study of ginger, its two components, referred to as Mol1 and Mol2 were identified as potential natural inhibitors of HssAChE (acetylcholinesterase), and it was proved to be just as effective as the current medicine of choice(donepezil) for the treatment of AD [96]. The suppression of many inflammatory response markers by GE shows that this herbal preparation could be a promising drug for AD. Many interesting pharmacological and physiological functions of gingerols (structure shown in Fig. 13) and shogaols have been documented, including antipyretic, cardiotonic, chemopreventive, anti-inflammatory, and antioxidant capabilities [91, 97].
Fig. (13).
The bioactive chemical compound gingerol is isolated from ginger.
3.10. Cinnamon zeylanicum (Cinnamon)
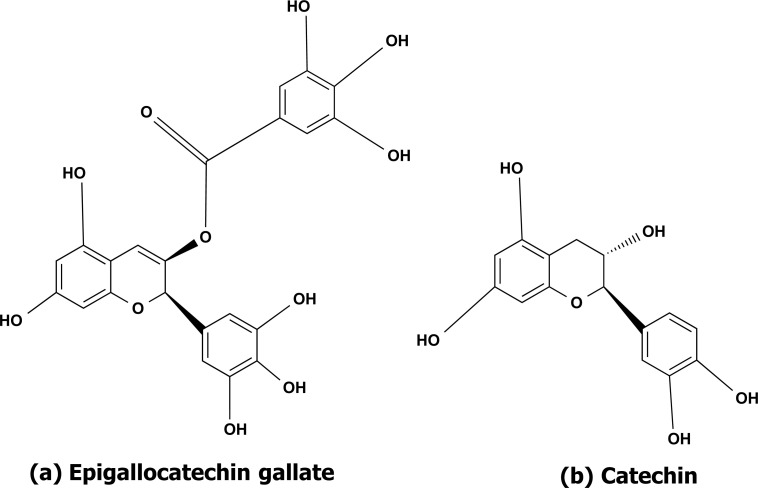
Cinnamon belongs to the Lauraceae family and its important active compounds are made up of cinnamaldehyde and a flavonoid. So far, the main phenolic chemicals isolated from Cinnamon have been catechin and epigallocatechin gallate (EGCG) as shown in Figs. (14a and b) that can cross the bloodbrain barrier and can target all the three AD hallmarks i.e., inhibition of the aggregation of Aβ, inhibition of tau hyperphosphorylation and degradation of plaques [98-100]. Cinnamon reduces the production, accumulation, and toxicity of Aβ plaques in PC12 neuronal cells. In AD fly models, the inhibitory effects are similar [99]. It also aids in tau phosphorylation inhibition and acetylcholinesterase activity suppression. It inhibits the generation of intracellular ROS and the expression of pro-inflammatory cytokines such as NF-kB, IL-6, and TNF [101, 102].
Fig. (14).
The phytochemically active chemical structure of (a) epigallocatechin gallate, and (b) Catechin isolated from Cinnamon.
3.11. Azadirachta indica
Azadirachta indica (Neem) is an evergreen tree found throughout in India and its neighbouring countries. Neem is derived from the Sanskrit word Nimba which means - Nature’s Drugstore’ or Panacea. More than 300 chemically diverse phytochemicals have been isolated from neem. The major phytochemicals present in neem are Glycoproteins, Triterpenes, Limonoids, Flavonoids, Phenols, Tannins, Nimbins, Saponins, Catechins, Azadirachtin, and Gallic acid [103-109].
An experimental study on rats performed by Maiti et al. showed that A. indica Pre-treatment increased the number of ambulations comparable to diazepam. Their study also suggested the antidepressant activity of this medicinal plant [110]. In another experiment, limonoids extracted from the neem fruits decreased the extensive aggregation of tau in vitro and led to the formation of thin, short, and fragile aggregates. Limonoids reverted tau-mediated toxicity at 1µM concentration and were also able to overcome oxidative stress [111].
CONCLUSION
Alzheimer’s disease (AD) is a global health concern due to its rising cases. It causes cognitive impairment and neurodegeneration. A number of evidence collected through clinical, animal, and in vitro studies indicates that the herbal plants reviewed in this research article help in neurogenesis and have many other therapeutic benefits. Traditional medicines with a strong knowledge base combined with modern science and techniques, help in improving the formulations that may be employed in drug development against AD and other neurogenerative diseases.
FUTURE PROSPECTS
Hypertension, depression, and neurodegenerative illnesses are growing at a tremendous and alarming rate while their treatment is often expensive, has side effects, and is generally ineffective. There is a need to investigate our traditional herbs to generate efficient antidisease medicines. The phytochemicals mentioned in this review can be used as a potential drug against AD, as some of these molecules have shown clinically positive results in the suppression of AD. Since multiple factors are involved in the development and progression of AD, hence a considerable shift from a single-target drug development approach to a multi-target drug development approach would result in a more effective drug development strategy, and herbal compounds are best fitted for such circumstances. In that case, herbal plants will undoubtedly produce promising results as they have been practiced since ancient times, they are least likely to have any side effects, and they will also be cost-effective. The novel functional identification for AD could be beneficial in the future.
ACKNOWLEDGEMENTS
All authors contributed to the literature survey and manuscript writing.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- Aβ
Amyloid-beta
- PUFA
Polyunsaturated Fatty Acid
- GE
Ginger Extracts
- EGCG
Epigallocatechin Gallate
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
AM is highly thankful to SERB New Delhi for a research Grant no. (CRG/2018/00912). SK acknowledges a fellowship from SERB New Delhi under Core Research Grant Sponsored project.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Wenk G.L. Neuropathologic changes in Alzheimer’s disease. J. Clin. Psychiatry. 2003;64(Suppl. 9):7–10. [PubMed] [Google Scholar]
- 2.Francis P.T., Palmer A.M., Snape M., Wilcock G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry. 1999;66(2):137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferri C.P., Prince M., Brayne C., Brodaty H., Fratiglioni L., Ganguli M., Hall K., Hasegawa K., Hendrie H., Huang Y., Jorm A., Mathers C., Menezes P.R., Rimmer E., Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wimo A., Winblad B., Aguero-Torres H., von Strauss E. The magnitude of dementia occurrence in the world. Alzheimer Dis. Assoc. Disord. 2003;17(2):63–67. doi: 10.1097/00002093-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Brookmeyer R., Johnson E., Ziegler-Grahamm K., Arrighi H.M. O1-02-01: Forecasting the global prevalence and burden of Alzheimer’s disease. Alzheimer’s Dement. 2007;3(3S_Part_3):S168-S168. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 6.Qiu C., Kivipelto M., von Strauss E. Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387. doi: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- 8.Wiley J. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. doi: 10.1002/alz.12328. [DOI] [PubMed] [Google Scholar]
- 9.Kumar G.P., Anilakumar K.R., Naveen S. Phytochemicals having neuroprotective properties from dietary sources and medicinal herbs. Pharmacogn. J. 2015;7(1):01-17. doi: 10.5530/pj.2015.1.1. [DOI] [Google Scholar]
- 10.Jivad N., Rabiei Z. A review study on medicinal plants used in the treatment of learning and memory impairments. Asian Pac. J. Trop. Biomed. 2014;4(10):780–789. doi: 10.12980/APJTB.4.2014APJTB-2014-0412. [DOI] [Google Scholar]
- 11.Lleó A. Current therapeutic options for Alzheimer’s disease. Curr. Genomics. 2007;8(8):550–558. doi: 10.2174/138920207783769549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glymour M.M., Weuve J., Dufouil C., Mayeda E.R. Aduhelm, the newly approved medication for Alzheimer’s disease: what epidemiologists can learn and what epidemiology can offer. Am. J. Epidemiol. 2022;191(8):1347–1351. doi: 10.1093/aje/kwac063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatta M. Neurogenesis and brain-derived neurotrophic factor levels in herbal therapy. Int. J. Res. Med. Sci. 2016;4(11):4654. [Google Scholar]
- 14.Rao R.V., Descamps O., John V., Bredesen D.E. Ayurvedic medicinal plants for Alzheimer’s disease: A review. Alzheimers Res. Ther. 2012;4(3):22. doi: 10.1186/alzrt125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H. Neuroprotective herbs for stroke therapy in traditional eastern medicine. Neurol. Res. 2005;27(3):287–301. doi: 10.1179/016164105X25234. [DOI] [PubMed] [Google Scholar]
- 16.Iqbal K., Grundke-Iqbal I. Alzheimer’s Disease, a Multifactorial Disorder Seeking Multitherapies. Vol. 6. Amsterdam: Elsevier; 2010. pp. 420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo T., Zhang D., Zeng Y., Huang T.Y., Xu H., Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020;15(1):40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bird T.D. Genetic aspects of Alzheimer disease. Genet. Med. 2008;10(4):231–239. doi: 10.1097/GIM.0b013e31816b64dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson P.T., Alafuzoff I., Bigio E.H., Bouras C., Braak H., Cairns N.J., Castellani R.J., Crain B.J., Davies P., Tredici K.D., Duyckaerts C., Frosch M.P., Haroutunian V., Hof P.R., Hulette C.M., Hyman B.T., Iwatsubo T., Jellinger K.A., Jicha G.A., Kövari E., Kukull W.A., Leverenz J.B., Love S., Mackenzie I.R., Mann D.M., Masliah E., McKee A.C., Montine T.J., Morris J.C., Schneider J.A., Sonnen J.A., Thal D.R., Trojanowski J.Q., Troncoso J.C., Wisniewski T., Woltjer R.L., Beach T.G. Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J. Neuropathol. Exp. Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards G.A., III, Gamez N., Escobedo G., Jr, Calderon O., Moreno-Gonzalez I. Modifiable risk factors for Alzheimer’s disease. Front. Aging Neurosci. 2019;11:146. doi: 10.3389/fnagi.2019.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xia X., Jiang Q., McDermott J., Han J.D.J. Aging and Alzheimer’s disease: Comparison and associations from molecular to system level. Aging Cell. 2018;17(5):e12802. doi: 10.1111/acel.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H. Zheng, Y. β amyloid hypothesis in Alzheimer’s disease: pathogenesis, prevention, and management. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2019;41(5):702–708. doi: 10.3881/j.issn.1000-503X.10875. [DOI] [PubMed] [Google Scholar]
- 23.Barage S.H., Sonawane K.D. Amyloid cascade hypothesis: Pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides. 2015;52:1–18. doi: 10.1016/j.npep.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 24.Chen G., Xu T., Yan Y., Zhou Y., Jiang Y., Melcher K., Xu H.E. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017;38(9):1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien R.J., Wong P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011;34(1):185–204. doi: 10.1146/annurev-neuro-061010-113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haass C., Selkoe D.J. Cellular processing of β-amyloid precursor protein and the genesis of amyloid β-peptide. Cell. 1993;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-E. [DOI] [PubMed] [Google Scholar]
- 27.Coulson E.J., Paliga K., Beyreuther K., Masters C.L. What the evolution of the amyloid protein precursor supergene family tells us about its function. Neurochem. Int. 2000;36(3):175–184. doi: 10.1016/S0197-0186(99)00125-4. [DOI] [PubMed] [Google Scholar]
- 28.Murphy M.P., LeVine H. III Alzheimer’s disease and the amyloid-β peptide. J. Alzheimers Dis. 2010;19(1):311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ak T. Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008;174(1):27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Schaffer M., Schaffer P.M., Zidan J., Sela G.B. Curcuma as a functional food in the control of cancer and inflammation. Curr. Opin. Clin. Nutr. Metab. Care. 2011;14(6):588–597. doi: 10.1097/MCO.0b013e32834bfe94. [DOI] [PubMed] [Google Scholar]
- 31.Kunnumakkara A.B., Bordoloi D., Padmavathi G., Monisha J., Roy N.K., Prasad S., Aggarwal B.B. Curcumin, the golden nutraceutical: Multitargeting for multiple chronic diseases. Br. J. Pharmacol. 2017;174(11):1325–1348. doi: 10.1111/bph.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H.T., Ho Y.S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness. 2018;7(2):134–137. doi: 10.1016/j.fshw.2018.06.001. [DOI] [Google Scholar]
- 33.Akbik D., Ghadiri M., Chrzanowski W., Rohanizadeh R. Curcumin as a wound healing agent. Life Sci. 2014;116(1):1–7. doi: 10.1016/j.lfs.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Amalraj A., Pius A., Gopi S., Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives-a review. J. Tradit. Complement. Med. 2017;7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharifi-Rad J., Rayess Y.E., Rizk A., Sadaka C., Zgheib R., Zam W., Sestito S., Rapposelli S. Neffe-Skocińska, K.; Zielińska, D. Turmeric and its major compound Curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020;11:01021. doi: 10.3389/fphar.2020.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priyadarsini K. The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19(12):20091–20112. doi: 10.3390/molecules191220091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh P., Bhooshan Pandey K., Ibrahim Rizvi S. Curcumin: the yellow molecule with pleiotropic biological effects. Lett. Drug Des. Discov. 2015;13(2):170–177. doi: 10.2174/1570180812666150630184101. [DOI] [Google Scholar]
- 38.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., Cole G.M. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 39.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Tonk S., Kuruva C.S., Bhatti J.S., Kandimalla R., Vijayan M., Kumar S., Wang R., Pradeepkiran J.A., Ogunmokun G., Thamarai K., Quesada K., Boles A., Reddy A.P. Protective effects of Indian spice curcumin against amyloid-β in Alzheimer’s disease. J. Alzheimers Dis. 2018;61(3):843–866. doi: 10.3233/JAD-170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng K.K., Yeung C.F., Ho S.W., Chow S.F., Chow A.H.L., Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. AAPS J. 2013;15(2):324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mishra S., Palanivelu K. The effect of curcumin (turmeric) on Alzheimer's disease: An overview. Ann. Indian Acad. Neurol. 2008;11(1):13–19. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hassanzadeh K., Buccarello L., Dragotto J., Mohammadi A., Corbo M., Feligioni M. Obstacles against the marketing of curcumin as a drug. Int. J. Mol. Sci. 2020;21(18):6619. doi: 10.3390/ijms21186619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dei Cas M., Ghidoni R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients. 2019;11(9):2147. doi: 10.3390/nu11092147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yallapu M.M., Nagesh P.K.B., Jaggi M., Chauhan S.C. Therapeutic applications of curcumin nanoformulations. AAPS J. 2015;17(6):1341–1356. doi: 10.1208/s12248-015-9811-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun M., Gao Y., Guo C., Cao F., Song Z., Xi Y., Yu A., Li A., Zhai G. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J. Nanopart. Res. 2010;12(8):3111–3122. doi: 10.1007/s11051-010-9907-4. [DOI] [Google Scholar]
- 47.Kressmann S., Biber A., Wonnemann M., Schug B., Blume H.H., Müller W.E. Influence of pharmaceutical quality on the bioavailability of active components from Ginkgo biloba preparations. J. Pharm. Pharmacol. 2010;54(11):1507–1514. doi: 10.1211/002235702199. [DOI] [PubMed] [Google Scholar]
- 48.Shi C., Liu J., Wu F., Yew D. Ginkgo biloba extract in Alzheimer’s disease: From action mechanisms to medical practice. Int. J. Mol. Sci. 2010;11(1):107–123. doi: 10.3390/ijms11010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shif O., Gillette K., Damkaoutis C.M., Carrano C., Robbins S.J., Hoffman J.R. Effects of Ginkgo biloba administered after spatial learning on water maze and radial arm maze performance in young adult rats. Pharmacol. Biochem. Behav. 2006;84(1):17–25. doi: 10.1016/j.pbb.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Yasuno F., Tanimukai S., Sasaki M., Ikejima C., Yamashita F., Kodama C., Mizukami K., Asada T. Combination of antioxidant supplements improved cognitive function in the elderly. J. Alzheimers Dis. 2012;32(4):895–903. doi: 10.3233/JAD-2012-121225. [DOI] [PubMed] [Google Scholar]
- 51.Matsuda H., Murakami T., Kishi A., Yoshikawa M. Structures of withanosides I, II, III, IV, V, VI, and VII, new withanolide glycosides, from the roots of Indian Withania somnifera Dunal. and inhibitory activity for tachyphylaxis to clonidine in isolated guinea-pig ileum. Bioorg. Med. Chem. 2001;9(6):1499–1507. doi: 10.1016/S0968-0896(01)00024-4. [DOI] [PubMed] [Google Scholar]
- 52.Sehgal N., Gupta A., Valli R.K., Joshi S.D., Mills J.T., Hamel E., Khanna P., Jain S.C., Thakur S.S., Ravindranath V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA. 2012;109(9):3510–3515. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuboyama T., Tohda C., Zhao J., Nakamura N., Hattori M., Komatsu K. Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. Neuroreport. 2002;13(14):1715–1720. doi: 10.1097/00001756-200210070-00005. [DOI] [PubMed] [Google Scholar]
- 54.Kuboyama T., Tohda C., Komatsu K. Neuritic regeneration and synaptic reconstruction induced by withanolide A. Br. J. Pharmacol. 2005;144(7):961–971. doi: 10.1038/sj.bjp.0706122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jayaprakasam B., Padmanabhan K., Nair M.G. Withanamides in Withania somnifera fruit protect PC-12 cells from β-amyloid responsible for Alzheimer’s disease. Phytother. Res. 2010;24(6):859–863. doi: 10.1002/ptr.3033. [DOI] [PubMed] [Google Scholar]
- 56.Sethiya N.K., Nahata A., Mishra S.H., Dixit V.K. An update on Shankhpushpi, a cognition-boosting Ayurvedic medicine. J. Chin. Integr. Med. 2009;7(11):1001–1022. doi: 10.3736/jcim20091101. [DOI] [PubMed] [Google Scholar]
- 57.Parihar M.S., Hemnani T. Phenolic antioxidants attenuate hippocampal neuronal cell damage against kainic acid induced excitotoxicity. J. Biosci. 2003;28(1):121–128. doi: 10.1007/BF02970142. [DOI] [PubMed] [Google Scholar]
- 58.Sethiya N.K., Nahata A., Singh P.K., Mishra S.H. Neuropharmacological evaluation on four traditional herbs used as nervine tonic and commonly available as Shankhpushpi in India. J. Ayurveda Integr. Med. 2019;10(1):25–31. doi: 10.1016/j.jaim.2017.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kizhakke P. A.; Olakkaran, S.; Antony, A.; Tilagul K, S.; Hunasanahally P, G. Convolvulus pluricaulis (Shankhapushpi) ameliorates human microtubule-associated protein tau (hMAPτ) induced neurotoxicity in Alzheimer’s disease Drosophila model. J. Chem. Neuroanat. 2019;95:115–122. doi: 10.1016/j.jchemneu.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Damodaran T., Cheah P.S., Murugaiyah V., Hassan Z. The nootropic and anticholinesterase activities of Clitoria ternatea Linn. root extract: Potential treatment for cognitive decline. Neurochem. Int. 2020;139:104785. doi: 10.1016/j.neuint.2020.104785. [DOI] [PubMed] [Google Scholar]
- 61.Rai K.S., Murthy K.D., Rao M.S., Karanth K.S. Altered dendritic arborization of amygdala neurons in young adult rats orally intubated with Clitorea ternatea aqueous root extract. Phytother. Res. 2005;19(7):592–598. doi: 10.1002/ptr.1657. [DOI] [PubMed] [Google Scholar]
- 62.Mutalik M., Mutalik M. Tinospora cordifolia: Role in depression, cognition and memory. Aust. J. Med. Herb. 2011;23(4):168–173. [Google Scholar]
- 63.Ghatpande N.S., Misar A.V., Waghole R.J., Jadhav S.H., Kulkarni P.P. Tinospora cordifolia protects against inflammation associated anemia by modulating inflammatory cytokines and hepcidin expression in male Wistar rats. Sci. Rep. 2019;9(1):10969. doi: 10.1038/s41598-019-47458-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reddi K.K., Tetali S.D. Dry leaf extracts of Tinospora cordifolia (Willd.) Miers attenuate oxidative stress and inflammatory condition in human monocytic (THP-1) cells. Phytomedicine. 2019;61:152831. doi: 10.1016/j.phymed.2019.152831. [DOI] [PubMed] [Google Scholar]
- 65.Agarwal A., Malini S., Bairy K., Rao M.S. Effect of Tinospora cordifolia on learning and memory in normal and memory deficit rats. Indian J. Pharmacol. 2002;34(5):339–349. [Google Scholar]
- 66.Upadhyay A., Kumar K., Kumar A., Mishra H. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi) - validation of the Ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. 2010;1(2):112–121. doi: 10.4103/0974-7788.64405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prakash R., Ramya N., Dhivya R., Priyadarshini M. Neuroprotective activity of ethanolic extract of Tinospora cordifolia on LPS induced neuroinflammation. Transl. Biomed. 2017;8(4) [Google Scholar]
- 68.Mishra R., Manchanda S., Gupta M., Kaur T., Saini V., Sharma A., Kaur G. Tinospora cordifolia ameliorates anxiety-like behavior and improves cognitive functions in acute sleep deprived rats. Sci. Rep. 2016;6(1):25564. doi: 10.1038/srep25564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birla H., Keswani C., Singh S.S., Zahra W., Dilnashin H., Rathore A.S., Singh R., Rajput M., Keshri P., Singh S.P. Unraveling the neuroprotective effect of tinospora cordifolia in a parkinsonian mouse model through the proteomics approach. ACS Chem. Neurosci. 2021;12(22):4319–4335. doi: 10.1021/acschemneuro.1c00481. [DOI] [PubMed] [Google Scholar]
- 70.James J., Dubery I. Pentacyclic triterpenoids from the medicinal herb, Centella asiatica (L.). Urban. Molecules. 2009;14(10):3922–3941. doi: 10.3390/molecules14103922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kulkarni O., Mukherjee S., Bhandare R., Jagtap S., Dugad S., Pawar N., Pawar P.K. Evaluation of comparative free-radical quenching potential of Brahmi (Bacopa monnieri) and Mandookparni (Centella asiatica). Ayu. 2011;32(2):258–264. doi: 10.4103/0974-8520.92549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Subathra M., Shila S., Devi M.A., Panneerselvam C. Emerging role of Centella asiatica in improving age-related neurological antioxidant status. Exp. Gerontol. 2005;40(8-9):707–715. doi: 10.1016/j.exger.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Shinomol G.K. Muralidhara; Bharath, M.M. Exploring the role of “Brahmi” (Bacopa monnieri and Centella asiatica) in brain function and therapy. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011;5(1):33–49. doi: 10.2174/187221411794351833. [DOI] [PubMed] [Google Scholar]
- 74.Dhanasekaran M., Holcomb L.A., Hitt A.R., Tharakan B., Porter J.W., Young K.A., Manyam B.V. Centella asiatica extract selectively decreases amyloid β levels in hippocampus of Alzheimer’s disease animal model. Phytother. Res. 2009;23(1):14–19. doi: 10.1002/ptr.2405. [DOI] [PubMed] [Google Scholar]
- 75.Chiroma S.M., Baharuldin M.T.H., Mat Taib C.N., Amom Z., Jagadeesan S., Ilham Adenan M., Mahdi O., Moklas M.A.M. Centella asiatica protects d-galactose/AlCl3 mediated Alzheimer’s disease-like rats via PP2A/GSK-3β signaling pathway in their Hippocampus. Int. J. Mol. Sci. 2019;20(8):1871. doi: 10.3390/ijms20081871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sripanidkulchai B. Benefits of aged garlic extract on Alzheimer’s disease: Possible mechanisms of action. Exp. Ther. Med. 2020;19(2):1560–1564. doi: 10.3892/etm.2019.8390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thorajak P., Pannangrong W., Welbat J.U., Chaijaroonkhanarak W., Sripanidkulchai K., Sripanidkulchai B. Effects of aged garlic extract on cholinergic, glutamatergic and GABAergic systems with regard to cognitive impairment in Aβ-induced rats. Nutrients. 2017;9(7):686. doi: 10.3390/nu9070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Amagase H., Petesch B.L., Matsuura H., Kasuga S., Itakura Y. Intake of garlic and its bioactive components. J. Nutr. 2001;131(3):955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 79.Chauhan N.B. Multiplicity of garlic health effects and Alzheimer’s disease. J. Nutr. Health Aging. 2005;9(6):421–432. [PubMed] [Google Scholar]
- 80.Kosuge Y. Neuroprotective mechanisms of S-allyl-L-cysteine in neurological disease. Exp. Ther. Med. 2020;19(2):1565–1569. doi: 10.3892/etm.2019.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mathew B., Biju R. Neuroprotective effects of garlic a review. Libyan J. Med. 2008;3(1):23–33. doi: 10.4176/071110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta V.B., Indi S.S., Rao K.S.J. Garlic extract exhibits antiamyloidogenic activity on amyloid-beta fibrillogenesis: Relevance to Alzheimer’s disease. Phytother. Res. 2009;23(1):111–115. doi: 10.1002/ptr.2574. [DOI] [PubMed] [Google Scholar]
- 83.Jaggi R.K., Madaan R., Singh B. Anticonvulsant potential of holy basil, Ocimum sanctum Linn., and its cultures. Indian J. Exp. Biol. 2003;41(11):1329–1333. [PubMed] [Google Scholar]
- 84.Geetha R.K., Kedlaya R., Vasudevan D.M. Inhibition of lipid peroxidation by botanical extracts of Ocimum sanctum: In vivo and in vitro studies. Life Sci. 2004;76(1):21–28. doi: 10.1016/j.lfs.2004.05.036. [DOI] [PubMed] [Google Scholar]
- 85.Kelm M.A., Nair M.G., Strasburg G.M., DeWitt D.L. Antioxidant and cyclooxygenase inhibitory phenolic compounds from Ocimum sanctum Linn. Phytomedicine. 2000;7(1):7–13. doi: 10.1016/S0944-7113(00)80015-X. [DOI] [PubMed] [Google Scholar]
- 86.Singh S. Comparative evaluation of antiinflammatory potential of fixed oil of different species of Ocimum and its possible mechanism of action. Indian J. Exp. Biol. 1998;36(10):1028–1031. [PubMed] [Google Scholar]
- 87.Joshi H., Parle M. Evaluation of nootropic potential of Ocimum sanctum Linn. in mice. Indian J. Exp. Biol. 2006;44(2):133–136. [PubMed] [Google Scholar]
- 88.Hening P., Mataram M.B.A., Wijayanti N., Kusindarta D.L., Wihadmadyatami H. The neuroprotective effect of Ocimum sanctum Linn. ethanolic extract on human embryonic kidney-293 cells as in vitro model of neurodegenerative disease. Vet. World. 2018;11(9):1237–1243. doi: 10.14202/vetworld.2018.1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cohen M. Tulsi - Ocimum sanctum: A herb for all reasons. J. Ayurveda Integr. Med. 2014;5(4):251–259. doi: 10.4103/0975-9476.146554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kusindarta D.L., Wihadmadyatami H., Haryanto A. Ocimum sanctum Linn. stimulate the expression of choline acetyltransferase on the human cerebral microvascular endothelial cells. Vet. World. 2016;9(12):1348–1354. doi: 10.14202/vetworld.2016.1348-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Afzal, Μ.; Al-Hadidi, D.; Menon, M.; Pesek, J.; Dhami, M.S.I. Ginger: an ethnomedical, chemical and pharmacological review. Drug Metabol. Drug Interact. 2001;18(3-4):159–190. doi: 10.1515/DMDI.2001.18.3-4.159. [DOI] [PubMed] [Google Scholar]
- 92.Altman R.D., Marcussen K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001;44(11):2531–2538. doi: 10.1002/1529-0131(200111)44:11<2531:AID-ART433>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 93.Kiuchi F., Shibuya M., Sankawa U. Inhibitors of prostaglandin biosynthesis from ginger. Chem. Pharm. Bull. 1982;30(2):754–757. doi: 10.1248/cpb.30.754. [DOI] [PubMed] [Google Scholar]
- 94.Tjendraputra E., Tran V.H., Liu-Brennan D., Roufogalis B.D., Duke C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001;29(3):156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 95.Grzanna R., Phan P., Polotsky A., Lindmark L., Frondoza C.G. Ginger extract inhibits β-amyloid peptide-induced cytokine and chemokine expression in cultured THP-1 monocytes. J. Altern. Complement. Med. 2004;10(6):1009–1013. doi: 10.1089/acm.2004.10.1009. [DOI] [PubMed] [Google Scholar]
- 96.Cuya T., Baptista L., Celmar Costa França T. A molecular dynamics study of components of the ginger (Zingiber officinale) extract inside human acetylcholinesterase: implications for Alzheimer disease. J. Biomol. Struct. Dyn. 2018;36(14):3843–3855. doi: 10.1080/07391102.2017.1401004. [DOI] [PubMed] [Google Scholar]
- 97.Bode A.M., Dong Z. CRC Press: Florida, US, 2nd ed.; Herbal Medicine: Biomolecular and Clinical Aspects. The Amazing and Mighty Ginger. [Google Scholar]
- 98.Kang Y.J., Seo D.G., Park S.Y. Phenylpropanoids from cinnamon bark reduced β-amyloid production by the inhibition of β-secretase in Chinese hamster ovarian cells stably expressing amyloid precursor protein. Nutr. Res. 2016;36(11):1277–1284. doi: 10.1016/j.nutres.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 99.Frydman-Marom A., Levin A., Farfara D., Benromano T., Scherzer-Attali R., Peled S., Vassar R., Segal D., Gazit E., Frenkel D., Ovadia M. Orally administrated cinnamon extract reduces β-amyloid oligomerization and corrects cognitive impairment in Alzheimer’s disease animal models. PLoS One. 2011;6(1):e16564. doi: 10.1371/journal.pone.0016564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Momtaz S., Hassani S., Khan F., Ziaee M., Abdollahi M. Cinnamon, a promising prospect towards Alzheimer’s disease. Pharmacol. Res. 2018;130:241–258. doi: 10.1016/j.phrs.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 101.George R.C., Lew J., Graves D.J. Interaction of cinnamaldehyde and epicatechin with tau: implications of beneficial effects in modulating Alzheimer’s disease pathogenesis. J. Alzheimers Dis. 2013;36(1):21–40. doi: 10.3233/JAD-122113. [DOI] [PubMed] [Google Scholar]
- 102.Gruenwald J., Freder J., Armbruester N. Cinnamon and Health. Crit. Rev. Food Sci. Nutr. 2010;50(9):822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 103.Braga T.M., Rocha L., Chung T.Y., Oliveira R.F., Pinho C., Oliveira A.I., Morgado J., Cruz A. Biological activities of gedunin—A limonoid from the Meliaceae family. Molecules. 2020;25(3):493. doi: 10.3390/molecules25030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fernandes S.R., Barreiros L., Oliveira R.F., Cruz A., Prudêncio C., Oliveira A.I., Pinho C., Santos N., Morgado J. Chemistry, bioactivities, extraction and analysis of azadirachtin: State-of-the-art. Fitoterapia. 2019;134:141–150. doi: 10.1016/j.fitote.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 105.Patel S.M., Venkata K.C.N., Bhattacharyya P., Sethi G., Bishayee A. Potential of neem (Azadirachta indica L.) for prevention and treatment of oncologic diseases. Semin. Cancer Biol. 2016;40-41:100–115. doi: 10.1016/j.semcancer.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Gupta S.C., Prasad S., Tyagi A.K., Kunnumakkara A.B., Aggarwal B.B. Neem (Azadirachta indica): An indian traditional panacea with modern molecular basis. Phytomedicine. 2017;34:14–20. doi: 10.1016/j.phymed.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 107.Saleem S., Muhammad G., Hussain M.A., Bukhari S.N.A. A comprehensive review of phytochemical profile, bioactives for pharmaceuticals, and pharmacological attributes of Azadirachta indica. Phytother. Res. 2018;32(7):1241–1272. doi: 10.1002/ptr.6076. [DOI] [PubMed] [Google Scholar]
- 108.Sandhir R., Khurana M., Singhal N.K. Potential benefits of phytochemicals from Azadirachta indica against neurological disorders. Neurochem. Int. 2021;146:105023. doi: 10.1016/j.neuint.2021.105023. [DOI] [PubMed] [Google Scholar]
- 109.Alzohairy M.A. Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evidence-Based Complement. Alternat. Med. 2016;2016 doi: 10.1155/2016/7382506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maiti R., Raghavendra M., Kumar S., Acharya S.B. Role of aqueous extract of Azadirachta indica leaves in an experimental model of Alzheimer's disease in rats. Int. J. Appl. Basic Med. Res. 2013;3(1):37–47. doi: 10.4103/2229-516X.112239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gorantla N.V., Das R., Mulani F.A., Thulasiram H.V., Chinnathambi S. Neem derivatives inhibits tau aggregation1. J. Alzheimers Dis. Rep. 2019;3(1):169–178. doi: 10.3233/ADR-190118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hardy J.A., Higgins G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 113.Musiek E.S., Holtzman D.M. Three dimensions of the amyloid hypothesis: Time, space and ‘wingmen’. Nat. Neurosci. 2015;18(6):800–806. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang J., Gu B.J., Masters C.L., Wang Y.J. A systemic view of Alzheimer disease-insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017;13(10):612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 115.Brier M.R., Gordon B., Friedrichsen K., McCarthy J., Stern A., Christensen J., Owen C., Aldea P., Su Y., Hassenstab J. Tau and Aβ imaging, CSF measures, and cognition in Alzheimer’s disease. Sci. Translat. Med. 2016;8(338):338ra366-338ra366. doi: 10.1126/scitranslmed.aaf2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jevtic S., Sengar A.S., Salter M.W., McLaurin J. The role of the immune system in Alzheimer disease: Etiology and treatment. Ageing Res. Rev. 2017;40:84–94. doi: 10.1016/j.arr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 117.McGeer P.L., McGeer E.G. Targeting microglia for the treatment of Alzheimer’s disease. Expert Opin. Ther. Targets. 2015;19(4):497–506. doi: 10.1517/14728222.2014.988707. [DOI] [PubMed] [Google Scholar]
- 118.Brinkman S.D., Gershon S. Measurement of cholinergic drug effects on memory in Alzheimer’s disease. Neurobiol. Aging. 1983;4(2):139–145. doi: 10.1016/0197-4580(83)90038-6. [DOI] [PubMed] [Google Scholar]
- 119.Summers W.K., Majovski L.V., Marsh G.M., Tachiki K., Kling A. Oral tetrahydroaminoacridine in long-term treatment of senile dementia, Alzheimer type. N. Engl. J. Med. 1986;315(20):1241–1245. doi: 10.1056/NEJM198611133152001. [DOI] [PubMed] [Google Scholar]