Abstract
Neurodegeneration is the progressive loss of structure or function of neurons, which may ultimately involve cell death. The most common neurodegenerative disorder in the brain happens with Alzheimer's disease (AD), the most common cause of dementia. It ultimately leads to neuronal death, thereby impairing the normal functionality of the central or peripheral nervous system. The onset and prevalence of AD involve heterogeneous etiology, either in terms of genetic predisposition, neuro-metabolomic malfunctioning, or lifestyle. The worldwide relevancies are estimated to be over 45 million people. The rapid increase in AD has led to a concomitant increase in the research work directed towards discovering a lucrative cure for AD. The neuropathology of AD comprises the deficiency in the availability of neurotransmitters and important neurotrophic factors in the brain, extracellular beta-amyloid plaque depositions, and intracellular neurofibrillary tangles of hyperphosphorylated tau protein. Current pharmaceutical interventions utilizing synthetic drugs have manifested resistance and toxicity problems. This has led to the quest for new pharmacotherapeutic candidates naturally prevalent in phytochemicals. This review aims to provide an elaborative description of promising Phyto component entities having activities against various potential AD targets. Therefore, naturopathy may combine with synthetic chemotherapeutics to longer the survival of the patients.
Keywords: Alzheimer’s disease, targets, synthetic drugs, naturopathy, neurodegenerative disorder, synthetic drugs
1. INTRODUCTION
Alois Alzheimer, a German physician, reported the first case of Alzheimer's disease (AD) in 1907 [1]. AD is a progressive neurodegenerative brain disorder affecting millions worldwide [2]. It is characterized by dementia-related symptoms, encompassing loss of memory, learning ability, thinking power, and development of primary progressive aphasia, self-dissociation, and behavioral abnormalities, accounting for difficulties in carrying out simple daily tasks [3]. The disease is associated with other progressive neuropsychiatric symptoms such as apathy, verbal and physical agitation, irritability, anxiety, depression, delusions, and hallucinations [4]. The age of onset of AD lies between ages 40 and 60 [5]. A cohort study displayed a bimodal distribution, which peaks before and after 65 years of age, but there may be age-associated factors unrelated to AD that enhance the vulnerability of older individuals over younger ones [6].
The cause of selective neurodegeneration in AD is not adequately understood, but efforts have been made to fish out the possible molecular targets linked to the disease's onset and progression. Metadata from the past compiled with the recent advances in science following the procurement of sophisticated techniques have validated several candidates that are directly linked to selective neuronal and synaptic function losses in AD, including extracellular neuritic plaques containing the Aβ peptide [7], neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau (τ) proteins [8, 9], neuro-synaptobolomic changes pertaining to the decreased functioning of neurotransmitters (decrease in the availability of GABA [10], ACh [11], dopamine [12] and serotonin [13], generally either by upregulating the neurotransmitter degrading enzymes (AChE, BChE [14]) or by downregulating their binding receptors [12, 15]), and malfunctioning of proteins that maintain the cellular redox state. Apart from these there are many risk factors such as age, genetic predisposition, and coexisting health problems. Alzheimer's disease is most widely seen in the age above 65. Aging induces defects in the mitochondrial electron transport chain. Age-related and/or otherwise acquired defects in protective mechanisms allow the free radicals to damage lipid membranes and DNA causing neuronal degeneration in brain cells. Genetic predisposition may add to a high vulnerability [16]. A gene called Apolipoprotein E for the development of Alzheimer's disease [17]. Some studies have shown that aged people who have other diseases like high blood pressure, heart disease, and high cholesterol, along with age, are prone to Alzheimer's disease [18].
Quest for potential neurological targets of AD includes selective neuronal and synaptic cholinergic function losses, extracellular neuritic plaques containing the β-amyloid peptide, and Neurofibrillary Tangles (NFTs) composed of hyperphosphorylated forms of the tau (τ) protein.
1.1. Cholinergic Target
Acetylcholine (ACh) is a cholinergic neurotransmitter responsible for memory and learning. It was found that the loss of acetylcholinesterase may cause cholinergic function loss correlated to cognitive decline [19]. AD is related to a reduced level of the neurotransmitter Acetylcholine (ACh) at the synaptic gap in the central nervous system in mammals. The AChE, mainly found in neuromuscular junctions and chemical synapses of the cholinergic type, hydrolyses the neurotransmitter ACh in the central nervous system.
1.2. Amyloidosis
Aβ is a normal peptide generated throughout the lifetime, but amyloid plaques are a neuropathological hallmark of AD [7]. Amyloidosis is the abnormal deposition of amyloid proteins in tissues, with the altered amyloid proteins forming an insoluble β-pleated sheet. Reduced tissue and cellular clearance are observed in amyloid protein deposits. The membrane protein Aβ precursor protein (AβPP) is proteolyzed to form Aβ, which is the amyloid form of Aβ that makes up the amyloid plaques (neurotic plaques) found in the brains of Alzheimer's patients [20]. Amyloid fibril formation is more specifically promoted by AChE, the peripheral site of the enzyme is involved in the aggregation of Aβ peptides. The AChE-Amyloid complex is formed because of the interaction of AChE with growing amyloid fibrils that accelerate the assembly of Aβ peptides, leading to mature senile plaque in the AD brain [21, 22], that comprises Aβ40 (a 40 amino acid containing chain) and Aβ42 (a 42 amino acid containing chain), of which Aβ42 is more prone to aggregate and assemble into fibrils [23].
Despite widespread support for Aβ fibrils being the leading cause of pathology seen in AD, it was suggested that oligomerization of Aβ42 plays a more critical role. The oligomerization of Aβ42 produces soluble Aβ oligomers, known as Aβ-derived diffusible ligands (ADDLs). Experiments showed that these ADDLs are potentially more toxic than Aβ fibrils as they target synaptic spines and disrupt synaptic plasticity, thus affecting cognitive function. Their toxicity lies in toxin receptors on cell surfaces and Fyn, a tyrosine kinase receptor overexpressed in AD [24].
1.3. Tau Proteins
The Tau hypothesis revolves around neurofibrillary tangles (NFTs) in AD. As a result of increased phosphorylation of Tau (originally bound to microtubules), there is an increase in free Tau accompanied by loss of functioning microtubules. Phosphorylated Tau is a subunit of paired helical filaments (PHFs), which form NFTs. The impaired microtubules affect axonal transport of proteins and eventually cause neuronal death [25]. Tau formations drastically exacerbate acute and chronic inflammatory processes [26]. Those inflammatory processes are mediated or induced by microglial clusters around the densest regions of beta plaques, high levels of pro-inflammatory cytokines, and microglial activation that precedes the formation of neurofibrillary tangles [27]. Concerning mitochondrial dysfunction, it is believed that the deposit of Aβ fragments and pathological Tau protein affects mitochondrial function in brain cells, specifically concerning the impairment of mitochondrial oxidative metabolism [28].
1.4. Oxidative Stress
The brain is an organ with high energetic activity, and this energy demand is supplied by mitochondrial oxidative phosphorylation, which can lead to the formation of highly reactive oxygen species. Oxidative stress is a result of the excessive production of these species [29, 30]. In this case, the protective mechanisms are compromised, the reactive oxygen species accumulate, and the neurons become susceptible to the excitotoxic lesion. However, this mechanism depends on Aβ fragments which, when accumulated, promote the reduction of iron and brain copper, which are key factors to trigger oxidative stress, which, under these conditions, promote DNA damage [31].
2. SYMPTOMATIC AND DISEASE-MODIFYING THERAPY FOR AD
The Cholinesterase inhibitors and the NMDA receptor antagonists are useful for treating AD. Acetylcholinesterase (AChE) inhibitors such as tacrine, donepezil, rivastigmine, galantamine, and N-methyl-D-aspartate (NMDA) antagonist memantine (Fig. 1) are commonly used to treat mild to moderate Alzheimer’s disease [32]. Recent treatment and disease management strategy includes the development and administration of anti-tau and anti-amyloid antibodies for target-specific clearance of the same. Attempts have been made to develop a proper passive immunization therapy, but humanized monoclonal antibodies including, Solanezumab (Phase 3; adverse effects, minimal brain penetration) [33], Gantenerumab (Phase 3; failed to show efficacy on clinical endpoints, some trials were discontinued) [34], Crenezumab (Phase 3; failed efficacy), Aducanumab (Phase 3; efficacious in some trial data and not so efficacious in others, conflicting evidence for clinical improvements) [35, 36], BAN2401 (Phase 3; increased risk of developing vasogenic edema, especially in patients carriers of APOE4) [37], and Bapineuzumab (Phase 3; No significant clinical benefit), are either still in clinical trial status with evidence of limited efficacies or have failed to achieve their projected success endpoints, with an array of adverse side-effects being their major drawback [38, 39].
Fig. (1).
Chemical structure of cholinesterase inhibitors donepezil, rivastigmine, galantamine, and N-methyl-D-aspartate (NMDA) antagonist memantine.
2.1. Cholinesterase Inhibitors
Acetylcholine performs the function of sending messages between nerve cells. Acetylcholinesterase inhibitors (AChEIs) aim to increase acetylcholine availability in synaptic neurotransmission to treat memory disturbances. The AChEIs preferably bind with an active catalytic site (CAS) and prevent the hydrolysis of Ach and rectify ACh deficiency in the brain. The entire gorge, including CAS and the peripheral site of the AChE enzyme, is blocked by AChEI, so it is known as a dual binding site inhibitor that prevents Ach's hydrolysis and inhibits plaque deposition. These dual binding site AChEIs are proved to be more effective anti –Alzheimer's agents than the single binding site inhibitors.
The first-line synthetic drugs for mild to moderate AD are donepezil, rivastigmine, and galantamine. The donepezil and rivastigmine are selective inhibitors, while galantamine inhibits both, AChE and BChE [40].
2.2. NMDA Receptor Antagonist
Memantine is a non-competitive NMDA receptor antagonist effective in treating moderate to severe Alzheimer's disease. The modulation of NMDA receptors results in reduced glutamate-induced excite-toxicity [41-43].
3. PHYTOTHERAPY IN AD
Synthetic anti-Alzheimer's medicines have many unsatisfactory effects and resistance issues. Therefore, phytochemicals play an alternative to protect the brain neuronal cells from neurodegenerative AD. The present paper attempts to elucidate the clinical effects of many commonly used herbal medicines for the treatment of AD (Table 1).
Table 1.
A list of phytocompounds used against AD, followed by the source of plant species, the part used, its chemical structure and mode of action along with the reference of the cited manuscript/s.
| Plant Species | Part Used |
Bioactive
Constituent |
Chemical Structures | Mode of Study | Mode of Action |
|---|---|---|---|---|---|
|
Withania somnifera (Ashwagandha) [44-72] |
NA |
Withanolides (In general) |

|
In silico | Inhibits AChE (some bind to the aromatic gorge and the catalytic triad of the catalytic site, some are allosteric inhibitors) and BChE (allosteric inhibitors). |
| NA | Withanolide A |

|
In silico | Inhibits hAChE (interacts with Thr78, Trp81, Ser120 and His442 residues of the catalytic site). |
|
| Root (Methanol extract) |
In vitro (SH-SY5Y cell line) |
NGF-independent axonal growth (in combination with withanoside IV and withanoside VI). |
|||
| Root (Methanol extract) |
In vitro (SH-SY5Y cell line) |
Promotes neurite growth (at 1 mM concentration). |
|||
| Purchased (99.3% purity) ChromaDex Incorporation (Irvine, CA) |
In vitro (Primary cortical neurons of Sprague-Dawley rat) |
Down-regulates BACE1 and up-regulates ADAM10. | |||
| Root (Methanol extract) |
Withanoside IV |

|
In vitro (SH-SY5Y cell line) |
NGF-independent dendrite growth (in combination with withanolide A and withanoside VI). |
|
| Root (Methanol extract) |
In vitro (Cortical neurons from Sprague-Dawley rats) In vivo (Male ddY mice) |
Gets metabolized into sominone, which is believed to promote axonal growth in Aβ(25-35) challenged neurons. | |||
| Root (Methanol extract) |
In vitro (Cortical neurons from Sprague-Dawley rats) |
Sominone induced neuronal outgrowth via the phosphorylation of RET (GDNF receptor). |
|||
| Root (Methanol extract) |
In vitro (SH-SY5Y cell line) |
Promotes neurite growth (at 1 mM concentration). |
|||
| Root (Methanol extract) |
Withanoside VI |

|
In vitro (SH-SY5Y cell line) |
NGF-independent dendrite growth (in combination with withanolide A and withanoside IV). |
|
| Root (Methanol extract) |
In vitro (SH-SY5Y cell line) |
Promotes neurite growth (at 1 mM concentration). |
|||
| Root (Methanol extract) |
Coagulin Q |

|
In vitro (SH-SY5Y cell line) |
Promotes neurite growth (at 1 mM concentration). |
|
|
Withania somnifera (Ashwagandha) [44-72] |
Root (Methanol extract) |
(20S,22R)-4β,5β,6α,27-tetrahydroxy-1-oxowitha-2,24-dienolide |

|
In vitro (SH-SY5Y cell line) |
Promotes neurite growth (at 1 mM concentration). |
| Purchased (93.7% purity) ChromaDex Incorporation (Irvine, CA) |
Asiatic Acid |

|
In vitro (Primary cortical neurons of Sprague-Dawley rat) |
Down-regulates BACE1 and up-regulates ADAM10. | |
| Fruit | Withanamide A |
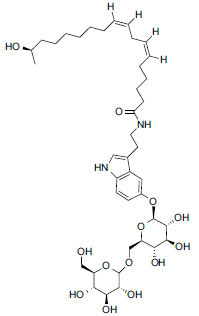
|
In vitro (PC-12 cells) In silico |
Protects against Aβ(39-42) induced cell damage (100% cell survivability at 100 and 50 μg/mL concentrations). Bound to the active motif of Aβ(39-42) peptide between Gly25 and Met35 amino acid residues. |
|
| Fruit | Withanamide C |
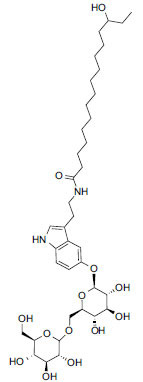
|
In vitro (PC-12 cells) In silico |
Protects against Aβ(39-42) induced cell damage (8% as compared to control). Bound to the active motif of Aβ(39-42) peptide between Gly25 and Met35 amino acid residues. |
|
|
Zingiber officinale; Curcuma amada; Curcuma longa [73-93] |
Rhizome Rhizome Rhizome, Leaves |
α-zingiberene;
α-sesquiphell-andrene; Zingiberene |
|
In vitro (TLC bioautography assay) |
Active AChE inhibitor. |
|
In vitro (TLC bioautography assay) |
Active AChE inhibitor. | ||||
|
Zingiber officinale; Curcuma longa; Curcuma aromatica; Curcuma amada; Curcuma xanthorrhiza [73-93] |
Rhizome Rhizome Rhizome Rhizome Rhizome |
ar-curcumene |

|
In vitro (TLC bioautography assay) |
Active AChE inhibitor. |
|
Curcuma spp. [73-93] |
Purchased (Sigma) | Curcumin |

|
In vitro | Inhibited formation, extension, and destabilization of Aβ40 and Aβ42. |
| Not Specified |
In vitro (PC-12 cells) |
20 μM completely suppresses metal ion mediated BACE1 mRNA levels. | |||
| Purchased (Sigma) |
In vitro (THP-1 monocytic cells) |
Suppresses the activation of Egr-1, downregulates the Aβ40-induced expression of TNF-α, IL-1β, MCP-1, MIP-1b and IL-8. |
|||
| Kancor Flavors, Kerala, India; Arjuna Natural Extracts, Kerala, India |
In vivo (Human trial) |
Prevents and/or reverses the aggregation Aβ at 0.2 to 1 μM (IC50). | |||
| Not Specified | Bisdemethoxycurcumin |
|
In vitro (PBMC isolated from venous blood of AD patients) |
Upregulates the expression of MGAT3 and TLR, and modulates the phagocytic pathway involved in Aβ plaque clearance. | |
|
Curcuma xanthorrhiza (Temulawak, Javanese ginger, Javanese turmeric) [73-93] |
Rhizome | Zedoaraldehyde |

|
In vitro (HEK293 cell lines) In vitro (TLC bioautography assay) |
Promotes the expression of SIRT1. Shows moderate AChE inhibitory activities. |
| Rhizome | 13-hydroxygermacrone |

|
In vitro (HEK293 cell lines) In vitro (TLC bioautography assay) |
Promotes the expression of SIRT1. Shows moderate AChE inhibitory activities. |
|
| Rhizome | Germacrone |

|
In vitro (HEK293 cell lines) In vitro (TLC bioautography assay) |
Promotes the expression of SIRT1. Shows moderate AChE inhibitory activities. |
|
|
Curcuma xanthorrhiza (Temulawak, Javanese ginger, Javanese turmeric) [73-93] |
Rhizome | α-curcumene |

|
In vitro (TLC bioautography assay) |
Shows moderate AChE inhibitory activities. |
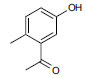
| Rhizome | 3-hydroxy-6-methylacetophenone |
|
In vitro (HEK293 cell lines) |
Promotes the expression of SIRT1. | |
|
Curcuma zedoaria [73-93] |
Rhizome (Methanol extract) |
Curcuzedoalide |

|
In vitro (RAW264.7 cells) |
Inhibites the synthesis of NO in lipopolysaccharide- stimulated RAW 264.7 cells (IC50: 23.44±0.77μg/mL) |
|
Curcuma amada; Curcuma kwangsiensis [73-93] |
Rhizome Rhizome |
Zerumin A |

|
In vitro (HPLC-based activity profiling approach) |
Positive modulator of GABAA receptors. |
|
Ginkgo biloba (Maidenhair tree) [94-119] |
Standardized marketed extract (EGb-761) | Bilobalide |

|
In vivo (Wistar rats) |
Reduced 5-HTIA receptors. |
|
In vivo (Human trial) |
Displayed better values in ADAS-Cog and GERRI. | ||||
|
In vivo (Human trial) |
Displayed better values in ADAS-Cog and GERRI. | ||||
|
In vivo (Human trial) |
Improvement observed in senile primary degenerative DAT and MID, and decrease in theta activity in EEG. | ||||
|
In vivo (gerbil) |
Reduced COX III mRNA levels, thereby protecting ischemia-induced neuronal death. | ||||
|
In vitro (Rat cerebellar neurons) |
Dose-dependent protection against glutamate-induced neuronal death. | ||||
|
In vitro (PC12 cells) |
2-fold increase in the level of ND1 subunit of NADH dehydrogenase mRNA. |
||||
|
In vitro (PC12 cells) |
Protects mitochondria against the uncoupling of OXPHOS, promotes the expression of COX III subunit of cytochrome oxidase. |
||||
| Provided by IPSEN Laboratories |
In vitro (Male Wistar rat hippocampal slice) |
Inhibited NMDA-induced activation of glutamatergic PLA2 and subsequent breakdown of phospholipids and choline efflux (IC50 = 2.3µM). |
|||
|
Ginkgo biloba (Maidenhair tree) [94-119] |
Standardized marketed extract (Egb-761) | Ginkgolide A |

|
In vivo (Wistar rats) In vivo (Human trial) In vivo (Human trial) In vitro (Rat cerebellar neurons) In vivo (gerbil) In vitro (PC12 cells) |
Reduced 5-HTIA receptors. Displayed better values in ADAS-Cog and GERRI. Improvement observed in senile primary degenerative DAT and MID, and decrease in theta activity in EEG. Dose-dependent protection against glutamate-induced neuronal death. Reduced COX III mRNA levels, thereby protecting ischemia-induced neuronal death. 2-fold increase in the level of ND1 subunit of NADH dehydrogenase mRNA. |
| Ginkgolide B |

|
||||
| Ginkgolide C |

|
||||
| Ginkgolide J |

|
||||
| Cyanidin |

|
||||
| Pelargonidin |

|
||||
| Kaempferol |

|
||||
| Isorhamnetin |

|
||||
| Standardized marketed extract (Egb-761) | Delphinidin |

|
In vivo (Wistar rats) |
Reduced 5-HTIA receptors. | |
|
In vivo (Human trial) |
Displayed better values in ADAS-Cog and GERRI. | ||||
|
In vivo (Human trial) |
Improvement observed in senile primary degenerative DAT and MID, and decrease in theta activity in EEG. | ||||
| - | Standardized marketed extract (Egb-761) | Delphinidin | - |
In vivo (gerbil) |
Reduced COX III mRNA levels, thereby protecting ischemia-induced neuronal death. |
|
In vitro (Rat cerebellar neurons) |
Dose-dependent protection against glutamate-induced neuronal death. | ||||
|
In vitro (PC12 cells) |
2-fold increase in the level of ND1 subunit of NADH dehydrogenase mRNA. | ||||
| Not specified |
In vitro (PC12 cells) |
Inhibited tau hyperphosphorylation by inhibition of GSK-3β. | |||
|
Ginkgo biloba (Maidenhair tree) [94-119] Ficus carica (Fig Tree) [117, 118] |
Standardized marketed extract (EGb-761) |
Quercetin;
3,3′,4′,5,7-pentahydroxyflavone |

|
In vivo (Wistar rats) |
Reduced 5-HTIA receptors. |
|
In vivo (Human trial) |
Displayed better values in ADAS-Cog and GERRI. | ||||
|
In vivo (Human trial) |
Improvement observed in senile primary degenerative DAT and MID, and decrease in theta activity in EEG. | ||||
|
In vitro (Rat cerebellar neurons) |
Dose-dependent protection against glutamate-induced neuronal death. | ||||
|
In vivo (gerbil) |
Reduced COX III mRNA levels, thereby protecting ischemia-induced neuronal death. | ||||
|
In vitro (PC12 cells) |
2-fold increase in the level of ND1 subunit of NADH dehydrogenase mRNA. | ||||
| Fruit | In vitro | AChE and BChE inhibitory activity. | |||
| Purchased -Extrasynthese (Genay Cedex, France) |
In vitro (Brain mitochondria fraction isolated from C57BL/6j mice) |
Antidepressant effects by inhibiting MAO-A, responsible the metabolism of 5-HT in brain. | |||
|
Caesalpinia mimosoides (Mimosa Thorn) [252-254] |
Young sprouts and leaves |
In vitro (P19-derived neurons) |
Inhibited AChE, has antioxidant capacity (IC50 of 3.18 ± 0.07 𝜇g/mL) | ||
| Purchased (Wako Pure Chemical (Tokyo, Japan) |
In silico In vitro (fetal rat cerebral cortex (E18)) |
Inhibited BACE1 (IC50 5.4 μM), hydrogen bond with Asp32 residue. | |||
|
Coffea spp. (Coffee) [120-154] |
Procured from R.B. Wetzel and S.J. Wood (MES buffer with 15 mM. β-Cyclodextrin |
β-Cyclodextrin |

|
In vitro (PC12) In vitro (Electrospray Mass spectroscopy study) |
Inhibits fibrillization of Aβ40 peptide, the hydrophobic aromatic moieties of the peptide became inclusive in the hydrophobic cavity of β-Cyclodextrin. |
| Not specified |
3,5-di-O-caffeoylquinic acid;
Isochlorogenic acid A |

|
In vitro (SH-SY5Y cells) In vivo (SAMP8 mice) |
Reverses the Aβ42 induced damage and upregulate phosphoglycerate kinase-1 mRNA expression. Improved learning and memory and increased mRNA expression level of PGK1 in mice brain. |
|
|
Coffea spp. (Coffee) [120-154] |
Purchased (Sigma-Aldrich Chemical Co.) |
Kahweol |

|
In vitro (NIH3T3 cells) |
Dose-dependent protection against H2O2-induced oxidative stress, DNA damage, and removed the superoxide anion. |
| Purchased (Sigma (St. Louis, MO)) |
In vitro (SH-SY5Y cells) |
Protects 6-OHDA challenged SH-SY5Y cells from oxidative stress, downregulates caspase 3, and modulates HO-1 via activation of PI3K/Akt (PKB), p38 and Nrf2. | |||
| Purchased (Sigma-Aldrich Chemical Co.) |
Cafestol |

|
In vitro (NIH3T3 cells) |
Dose-dependent protection against H2O2-induced oxidative stress, DNA damage, and removed the superoxide anion. | |
| Purchased (Sigma-Aldrich Chemical Co.) |
In vivo (Drosophila model for PD and AD) |
Improved the expression of glutathione-s-transferase activity and activation of Nrf2 pathway (dose 0.2 μg/ml). | |||
| Purchased (Sigma-Aldrich Chemical Co.) |
γ-tocopherol.
Vitamin E |

|
In vitro (C3H/10T1/2 murine fibroblast cells) |
Hydroxide group donates a hydrogen atom, which stabilizes free radicals, exerting cytoprotective effect. | |
|
Theobroma cacao; Coffea sp. (Coffee) [120-154] |
Purchased (Katayama Chemical Co., Ltd. Tokyo, Japan) |
Caffeine |

|
In vitro (Xenopus laevis oocyte microinjected with cRNAs of α1 and β1 subunits of the bovine GABAA receptors) |
Inhibited GABAA receptor (competitive inhibition), at a Ki of 15 mM. |
| Purchased (Sigma-Aldrich) |
In vitro (Human blood cell- derived inside-out vesicles) |
Inhibits ABCC4/5 transport, and non-specific PDE inhibitor. (Ki(μM)- 48.2±1.2 for cGMP and 40.7±2.9 for cAMP). | |||
| Purchased (Sigma Chemical Co.) |
In vitro (Homogenized brain from Sprague-Dawley rat) |
Inhibition of PDEs and adenosine receptor agonist. (Substitution of methyl groups of caffeine with n-propyl groups- Ca-independent PDE inhibitor. | |||
| NA | In silico | Inhibits PDE (interacts with Asn 395, Tyr 403, Tyr 406, Thr 407, Ile 410, Phe 414, Met 431, Ser 442, Glu 443, Phe 446 and Phe 506 residues of the enzyme). | |||
| Purchased (Sigma- Aldrich) |
In vitro (Biochemical assay) In vitro (C3H1OT1/2 cells) |
Improves cellular energy crisis via inhibition of PARP-1 mediated NAD+ cleavage, (IC50 (μM) 1,400 ± 240). | |||
| Purchased (Sigma- Aldrich) |
In vitro (Hippocampal CAl neurons from Wistar rat) |
Blocks glycine- gated chloride channels in rat hippocampal neurons, IC50 = 4.5 x 10-4 M. | |||
| Not specified |
In vitro (Sartorius muscles of Rana pipiens) |
0.005 M of caffeine increases both the calcium outflux and influx in muscle fibers. | |||
| Purchased (Sigma- Aldrich) |
In vitro (Cortical neurons of NMNAT2blad mice) In vivo (rTg4510 mice) |
Restores normal level of NMNAT2. | |||
| Not specified |
In vitro (Extensor digitorum longus muscles of Sprague-Dawley rats) |
Stimulation of Na+/ K+-ATPase activity. |
|||
|
Theobroma cacao; Coffea sp. (Coffee) [120-154] Camellia ptilophylla Camellia irrawadiensis |
Purchased (Sigma Chemical Co., St. Louis) |
Theobromine |

|
In vitro (Xenopus laevis oocyte microinjected with cRNAs of α1 and β1 subunits of the bovine GABAA receptors) |
Inhibited GABAA receptor (competitive inhibition), Ki of 3.8 mM when GABA concentration was 0.25 µM. |
| Purchased (Sigma-Aldrich) |
In vitro (Human blood cell- derived inside-out vesicles) |
Inhibits high affinity ATP-dependent cyclic nucleotide transporters (ABCC5). | |||
| Purchased (Sigma- Aldrich) |
In vitro (Biochemical assay) In vitro (C3H1OT1/2 cells) |
Improves cellular energy crisis via inhibition of PARP-1 mediated NAD+ cleavage, (IC50 (μM) 110 ± 19). | |||
| Purchased (Sigma- Aldrich) |
In vitro (Hippocampal CAl neurons from Wistar rat) |
Blocks glycine- gated chloride channels in rat hippocampal neurons, IC50 = 1.49 x 10-3 M. | |||
|
Theobroma cacao; Coffea sp. (Coffee) [120-154] |
Purchased (Sigma Chemical Co., St. Louis) | Theophylline |

|
In vitro (Xenopus laevis oocyte microinjected with cRNAs of α1 and β1 subunits of the bovine GABAA receptors) |
Inhibited GABAA receptor (noncompetitive inhibition), Ki of 0.55 mM. |
| Purchased (Sigma-Aldrich) |
In vitro (Human blood cell- derived inside-out vesicles) |
Inhibits ABCC4/5 transport, and non-specific PDE inhibitor. (Ki(μM)- 68.7±1.5 for cGMP and 85.4±2.6 for cAMP). | |||
| Purchased (Calbiochem) |
In vitro (Homogenized brain from Sprague-Dawley rat) |
Inhibition of PDEs and adenosine receptor agonist. | |||
| Purchased (Sigma- Aldrich) |
In vitro (Biochemical assay) In vitro (C3H1OT1/2 cells) |
Improves cellular energy crisis via inhibition of PARP-1 mediated NAD+ cleavage, (IC50 (μM) 46 ± 15). | |||
| Purchased (Sigma- Aldrich) |
In vitro (Hippocampal CAl neurons from Wistar rat) |
Blocks glycine- gated chloride channels in rat hippocampal neurons, IC50 = 3.9 x 10-4 M. | |||
| Purchased (Sigma Chemical, USA) |
In vitro (Wistar rat diaphragm muscle) |
Stimulated Na+/K+- ATPase activity. | |||
|
Ferula foetida; Coffea spp. (Coffee) [120-154] |
Purchased (Sigma Chemical, USA) |
Ferulic acid;
4-hydroxy-3-methoxycinnamic acid |

|
In vivo (Mongolian gerbils) |
Aβ42 induced synaptosomes isolated from the brain followed by ferulic acid treatment (150 mg/kg i.p. injection) showed a significant decrease in iNOS, ROS levels, protein oxidation and lipid peroxidation and activation of HSP72 and HO-1. |
|
Camellia sinensis (Tea, Cha) [155-178] |
Black tea leaf extract |
Theanine;
L-theanine; Suntheanine |

|
In vivo (Albino Wistar rats) |
Inhibits AChE, BACE1, γ-secretase, reduces Aβ burden, apoptosis (inhibits cytochrome c release, expression of Bax, caspases-3, 8 and 9, enhances Bcl-2 expression) and oxidative stress (promoting GPx, CAT, SOD). |
| Purchased (Taiyo Kahaku Co., Ltd. (Yokkaichi, Japan)) |
In vivo (Slc:ICR mice) |
Attenuated Aβ42-induced memory impairment, inhibited ERK, p38 mitogen-activated protein kinase, NF-κB and reduced oxidative damage. | |||
| Manufactured by ITO EN Ltd. (Tokyo, Japan) |
In vivo (Human trial) |
Improved MMSE-J score (Dose- 2 g/day- 3 months, containing 42 mg theanine and 227 mg catechins). | |||
|
Camellia sinensis (Tea, Cha) [155-178] |
Fermented leaf extract | (-)-catechin gallate |

|
Thioflavin-T fluorescence-based assay and TEM In vitro (SH-SY5Y cells) |
Decreased Aβ aggregation at a concentration of 10μgml−1. |
| Fermented leaf extract | (-)-epicatechin gallate |

|
Thioflavin-T fluorescence-based assay and TEM In vitro (SH-SY5Y cells) |
Decreased Aβ aggregation at a concentration of 10μgml−1. | |
| Tokyo Food Techno Co. Ltd., Japan. |
In vitro (Xenopus laevis oocyte microinjected with cRNAs of α1 and β1 subunits of the bovine GABAA receptors) |
Inhibits GABAA receptor, (IC50 5.5 µM). | |||
| Fermented leaf extract | (-)-epigallocatechin gallate |

|
Thioflavin-T fluorescence-based assay and TEM In vitro (SH-SY5Y cells) |
Decreased Aβ aggregation at a concentration of 10μgml−1. | |
| Tokyo Food Techno Co. Ltd., Japan. |
In vitro (Xenopus laevis oocyte microinjected with cRNAs of α1 and β1 subunits of the bovine GABAA receptors) |
Inhibits GABAA receptor, (IC50 16 µM). | |||
| NA | In silico | Vitiate Fluoride-induced free radicals by reducing the expression of Keap1 (interacting with Gly 343, Thr 595, Leu 578, and Asp 579 residues of the protein) and promoting the translocation of Nrf2 into the nucleus. | |||
| Fermented leaf extract | Theaflavin-3-gallate |

|
In vitro (Erythrocyte ghost prepared from Wistar rat blood) |
In vitro protection of erythrocytes from oxidative stress. Pharmacophores within the molecule that contributes to the antioxidative property. | |
| Fermented leaf extract | Theaflavin-3’-gallate |

|
In vitro (Erythrocyte ghost prepared from Wistar rat blood) |
In vitro protection of erythrocytes from oxidative stress. Pharmacophores within the molecule that contributes to the antioxidative property. | |
| PI & PI Technology, Inc., (China) | Thromboelastometry | Inhibits PAI-1. | |||
|
Camellia sinensis (Tea, Cha) [155-178] |
Fermented leaf extract | Theaflavin 3,3′-digallate |

|
In vitro (Erythrocyte ghost prepared from Wistar rat blood) |
In vitro protection of erythrocytes from oxidative stress. Pharmacophores within the molecule that contributes to the antioxidative property. |
| PI & PI Technology, Inc., (China) | Thromboelastometry | Inhibits PAI-1. | |||
| Black tea leaf extract | Thearubigin |

|
In vivo (Albino Wistar rats) |
Inhibits AchE, BACE1, γ-secretase, reduces Aβ burden, apoptosis (inhibits cytochrome c release, expression of Bax, caspases-3, 8 and 9, enhances Bcl-2 expression) and oxidative stress (promoting GPx, CAT, SOD). | |
|
Myrica rubra; Camellia sinensis (Tea, Cha) [155-178] |
Purchased (Sigma- Aldrich, USA) |
Macamides (General
Structure) |

|
In vivo (C57BL/6 mice) |
Improve the availability of BDNF in hippocampus of the brain (Dose- i.p. 50 mg/kg). |
| Not specified |
In vitro (SH-SY5Y cells) In vivo & Ex vivo (Kunming mice) |
Reversed scopolamine-induced cognitive deficits, by inhibiting AchE (IC50 = 58.9 μM) and reduced brain iron load. | |||
|
Lepidium meyenii (Maca; Peruvian ginseng) [179-197] |
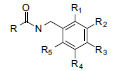
n-Pentane-extract of hypocotyl | Macamides |
|
In vitro (OLGA-PH-J/92 neuronal cells of crayfish, Orconectes limosus) In vivo (Sprague-Dawley rats) |
Reduction of H2O2-induced oxidative stress. Reduction of infarct size in rats subjected to focal ischemic stroke. |
| Macaenes |

|
||||
|
Benzyl
isothiocyanates |

|
||||
| HPLC-UV-guided separation from tuber | (±)-lepithiohydantoins A |

|
In vitro (Corticosterone-induced PC12 cells injury model) |
Provided neuroprotection. | |
| (±)-lepithiohydantoins B |

|
||||
|
Lepidium meyenii (Maca; Peruvian ginseng) [179-197] |
- | (±)-lepithiohydantoins C |

|
- | - |
| (±)-lepithiohydantoins D |

|
||||
| Spray-Dried Maca extracts | Choline |

|
In vivo (Human trial) |
Safe to be administered as oral supplement. | |
| NA | Brassicasterol |

|
In silico | Inhibits tau protein (interacts with Gln351, Lys353 and Ser352 residues) and Aβ protein (interacts with Lys28, Asp23, Val30, His14, Ala211, Ala21 and Ile32 residues). | |
|
Bacopa monnieri (Brahmi; Water Hyssop) [197-218] |
Purchased from Natural Remedies (India) | Bacoside A3 |

|
In vitro (SH-SY5Y cells) |
Inhibitory effects against Aβ42 mediated cytotoxicity (by inhibiting membrane interactions, preventing self-assembly and membrane perturbation). |
| Part not specified |
In vitro (U87MG cells) |
Suppressed the Aβ-mediated nuclear translocation of NF-κB, iNOS, PGE2 (by suppressing the overexpression of COX-2), and reduced ROS. | |||
| Purchased from Natural Remedies (India) | Bacopaside II |

|
In vitro (SH-SY5Y cells) |
Inhibitory effects against Aβ42 mediated cytotoxicity (by inhibiting membrane interactions, preventing self-assembly and membrane perturbation). | |
|
Bacopa monnieri (Brahmi; Water Hyssop) [197-218] |
Purchased from Natural Remedies (India) | Bacopaside X |

|
In vitro (SH-SY5Y cells) |
Inhibitory effects against Aβ42 mediated cytotoxicity (by inhibiting membrane interactions, preventing self-assembly and membrane perturbation). |
| NA | Bacopaside XII |

|
In silico | Inhibits Keap1 (interacts via hydrogen bonds with Arg380, Asn382, Ser383, Pro384, Asn 387, Asp389 and Tyr572 residues). | |
| Purchased from Natural Remedies (India) | Bacopasaponin C |

|
In vitro (SH-SY5Y cells) |
Inhibitory effects against Aβ42 mediated cytotoxicity (by inhibiting membrane interactions, preventing self-assembly and membrane perturbation). | |
| Purchased (Sigma- Aldrich, USA) |
Stigmasterol |

|
In vivo (C57BL/6J mice) In vivo (SH-SY5Y wild type cells and SH-SY5Y cells) |
Reduced Aβ plaque generation, reduced expression of γ-secretase, cholesterol and presenilin distribution in lipid rafts, decreased BACE activity and internalization of BACE1 to endosomal compartments. |
|
|
Bacopa monnieri (Brahmi; Water Hyssop) [197-218] |
Purchased (Sigma- Aldrich, USA) |
Betulinic acid |

|
In vivo (Wistar rats) |
Improves cognition and amplification of hippocampal long- term potentiation, at molar ratio of 1:4 (Aβ to betulinic acid). |
|
Tabebuia aurea
(Found in more than 2500 plant species) |
Ethanolic extract of ground bark |
In vitro (BALB/c, wt C57BL/6, and IL-10−/− C57BL/6 mice) |
Reported to penetrate the BBB and is safe at a dose concentration higher than 500 mg/kg. | ||
|
Rosmarinus officinalis; (Rosemary) [219-232] |
Not Specified | Rosmarinic Acid |

|
In vivo (Tg2576 mice) |
Suppresses Aβ accumulation, and enhanced the levels of dopamine, levodopa, norepinephrine, and 3,4-dihydroxyphenylacetic acid in cerebral cortex. Downregulated MAO-B in the substantia nigra and ventral tegmental area. |
| Purchased (Sigma) | In vitro | Inhibited formation, extension, and destabilization of Aβ40 and Aβ42. | |||
|
Melissa officinalis (Lemon balm) [229-231] |
Maruzen Pharmaceuticals Co., Ltd., Japan |
In vivo (Human trial) |
NPI-Q score improved by 0.5 points. | ||
|
Ocimum tenuiflorum (Tulsi, Holy Basil) [233-251] |
NA | NA | NA. | ||
|
Rosmarinus officinalis (Rosemary) [219-232] |
Not Specified | Carnosic acid |

|
In vitro (1) Primary cerebrocortical neurons and astrocytes of embryonic Sprague-Dawley rats. (2) Mixed neuronal/ glial mesencephalic primary cultures from fetuses of C57BL/6J mice. (3) Human iPSCs generated from fibroblasts. In vivo (NSA mice (Harlan Lab)) |
Protect brain cells from cyanide-induced injury via the activation of the Keap1/Nrf2 pathway via direct S-alkylation of targeted cysteines (ex. cysteine 151) on Keap1. |
| Purchased (Sigma- Aldrich, USA) |
In vitro (SH-SY5Y cells) |
Inhibited ROS-mediated injury of SH-SY5Y cells by the selective expression of HO-1, NQO1, and γ-GCL | |||
| Isolated from dried whole plant, following extraction protocol | Nepitrin |

|
In vivo (Swiss albino mice) In silico |
Improved memory in scopolamine-induced Swiss albino mice, concentration-dependent inhibition of AChE (IC50: 65 μg/mL) and BChE (IC50: 72 μg/mL). Occupies the same binding site (Trp279, Phe330, and Tyr334) and forms the similar set of interactions to those formed by a standard AD drug, donepezil, along with few novel additional interactions (Arg289, and probably Trp84). |
|
|
Calendula
officinalis; (also in Erythroxylum monogynum; Rosmarinus officinalis) |
Dried extract of flowers and leaves, subjected to hydrodistillation | Neophytadiene |

|
In vitro (Biochemical assay) In silico |
Inhibits tyrosinase, BChE and AChE. Inhibits tyrosinase. |
|
Rosmarinus officinalis; Ocimum kenyense; Elettaria cardamomum; Salvia lavandulaefolia; Eucalyptus sp. [219-232] |
Supercritical CO2 extract of small cardamom seeds and pure compound (Sigma) |
1,8-Cineole;
Eucalyptol |

|
In vitro (SH-SY5Y cells) |
Prevents the oligomerization of Aβ42 and inhibiting iron-dependent production of ROS. |
| Not specified |
In vitro (PC12 cells) |
Prevents oxidative damage, inhibits AChE, prevents Aβ42 oligomerization, and restores ChAT expression, decreased mitochondrial membrane potential, NOS-2, COX-2 and Aβ25-35 induced NF-κB activation. | |||
|
Rosmarinus officinalis; Oryza sativa; Zea mays; Piper nigrum [219-232] |
Not specified | β-caryophyllene |

|
In vivo (APP/PS1 AD mice model) |
Reduced neuroinflammatory response by activating cannabinoid receptor 2 and the PPARγ pathway, reduced astrogliosis, microglial activation, levels of COX-2 and the mRNA levels of the proinflammatory cytokines, TNF-α and IL-1β in the cerebral cortex. |
|
Ocimum tenuiflorum (Tulsi, Holy Basil) [233-251] |
Purchased (Sigma- Aldrich, USA) |
Ursolic acid |

|
In vitro (Jurkat cells, HEK- 293 cells, KBM-5, H1299 cells, and U937 cells) |
Suppresses the NF-ĸB, by inhibiting IκB kinase thereby inhibiting degradation and phosphorylation of IκBα. |
|
Ocimum tenuiflorum; Ocimum basilicum; Ocimum gratissimum; Eugenia aromatica; Eugenia caryophyllata; Cinnamomum zeylanicum [233-251] |
Not specified | Eugenol |

|
In vitro (SH-SY5Y cells) In vivo (Swiss Albino mice; Human APP Overexpressing Drosophila transgenic model) |
Eugenol potentiates the effect of Niacin in treating AD, aiding to decrease AB42, its deposition, accumulation, and plaque formation. |
| Purchased pure compound | In silico | Increased ACh level and decreased AChE activity (interacting with Tyr124 by hydrogen bond, Pi-Pi T-shaped interaction with Tyr337, pi-pi staked interaction with Ty341, and pi-alkyl interactions with His447, Trp286, Phe295 and Val294). | |||
| Purchased (Sigma- Aldrich, USA) |
In vivo (Charles-Foster albino rats) |
Increased the total muscarinic receptors, ACh level, decreased AChE activity, attenuated glutamate neurotoxicity (increase in levels of glutamate, calcium, calcium-dependent calpain-2, and BDNF), and mitochondrial dysfunction in scopolamine-induced rats. | |||
| Manufactured by Yueyang Jiazhiyuan Biological Co. Ltd., China. |
In vivo (Wistar rats) |
Reduced NO and 8-OHdG levels, inhibited caspase-3 activity, reduced Bax protein levels and increased the levels of Bcl-2, SOD and GPx in aluminium-induced neurotoxicity in rats. | |||
| Several species of plants | Not specified |
Niacin;
Nicotinic acid; Vitamin B3 |

|
In vitro (SH-SY5Y cells) In vivo (Swiss Albino mice; Drosophila transgenic model) |
Eugenol potentiates the effect of Niacin in treating AD, aiding to decrease AB42, its deposition, accumulation, and plaque formation. |
|
Stachys inflata; Stachys lavandulaefolia; Stachys byzantina; Indian pennywort; Palicourea tomentosa; Salvia syriaca; Ocimum tenuiflorum [233-251] |
NA | Germacrene B |

|
In silico | Inhibit BACE1 (interacts with Val93 residue of BACE1). |
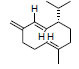
| Leaves of Psychotria tomentosa (hydrodistillation) | Germacrene D |
|
In silico | Inhibits AchE. | |
|
Caesalpinia mimosoides (Mimosa Thorn) [252-254] Camellia sinensis |
Twigs and Leaves (Methanol extract) |
Theogallin;
3-galloylquinic acid |

|
In vitro (Neuro2a cells and Neuro2a/APPSwe cells) |
Inhibited BACE1 (binding energy −8.97 kcal/mol) and promoted neurite outgrowth. |
Abbreviation: NA: Not Applicable.
3.1. Withania somnifera (Ashwagandha; Indian ginseng)
Ashwagandha, a green shrub of the family Solanaceae (ITIS, 2022), has been, for a long time, a preferred staple medication in Indian Ayurvedic treatments for a vast range of diseases under the therapeutic abilities of different bioactive components isolated from different parts of the plant, functioning to encompass [44] the reduction of oxidative stress by a dose-dependent improvement in the expression of vital enzyme profile including SOD, CAT and GPx [44, 45]. It is also found to work as a general neuroprotective drug that promotes dendrite formation by the expression of MAP2 and promotes PSD-95 expression in neurites in a dose-dependent fashion in neuroblastoma cells [46, 47]. The major biochemical constituents include steroidal alkaloids and lactones, collectively termed withanolide. The molecular docking simulation revealed that the withanolides, owing to their bulky skeleton, were buried into the aromatic gorge and to the catalytic triad but remained close to the anionic subsites of the catalytic site of AChE. For example, Withanolide A is found to specifically interact with Thr78, Trp81, Ser120 and His442 residues of the catalytic site of the hAChE and bound to the allosteric sites of BChE and thereby inhibiting their functions [48, 49]. This imparts a high-end therapeutic value to the compound by its potential to decrease Aβ deposition and maintain optimum acetylcholine levels at the neuronal synapse, an important parameter in the Tg2576 transgenic mouse model of AD [50, 51]. The modality of AD operates where the neurodegeneration is partly responsible for the depletion of essential NGF and vice versa, which correlates with the aggressive severity of the disease, often because the BFCN, one of the sources of NGF, is degenerated [52, 53]. This problem can be addressed by the administration of withanolide A and withanosides IV and VI in novel combinations, which are found to induce NGF-independent neuronal development and neurite extension in rat cerebral cortex neurons, where the axonal growth is promoted by withanolide A, while the dendrites are extended by withanosides IV and VI [54]. In the intestine, withanoside IV gets metabolized into sominone, which is believed to be the main bioactive compound promoting axonal growth, principally by the phosphorylation of RET (the receptor for the GDNF) [55, 56]. Other isolated compounds like Coagulin Q, Withanolide A, and (20S, 22R)-4β,5β,6α,27-tetrahydroxy-1-oxowitha-2,24-dienolide was also found to promote neurite growth and positively regulate synapse reconstruction activities [48, 57]. Arc protein, a reliable marker for neuroplastic changes, is upregulated upon administration of leaf extracts of Withania somnifera in the scopolamine-treated group [58, 59]. This may prove to be effective in recovering the neuronal plasticity and combating memory changes in AD, as a similar downregulation of Arc has been observed in AD [60]. Sehgal et al. reported that Withania somnifera extract was found to clear the toxic Aβ peptide from the brain and promoted its sequestration and degradation in the plasma and the peripheral tissue, respectively, by mediating the enhanced expression of LRP and NEP in the liver [61]. The liver LRP mediates endocytosis of Aβ peptides in the plasma, which is further cleared by NEP and other proteases in the liver [62, 63]. Recent data suggest an impaired GABAergic system, the principal inhibitory neurotransmitter in the mammalian CNS, where the levels of GABA decrease, were found to be significantly prevalent during disease progression in AD [10, 64]. The aqueous root extract of W. somnifera is found to act as a GABA receptor agonist; more importantly, the GABAρ1 receptor was found to possess 27-fold more sensitive to the aquous extract than GABAA receptors and thereby acts as a potent tool against GABAergic signaling dysfunction [65]. A direct inhibitory mechanism is being exhibited by withanolide A and Asiatic acid by down-regulating BACE1, the hallmark enzyme that leads to the production of Aβ plaque, and upregulates ADAM10, which is involved in non-amyloidogenic processing of AβPP [66]. The circulating levels of several matrix metalloproteinases, including MMP-1, MMP-3, and MMP-9, are found to be increased in AD and may contribute to disease pathology. An in-silico study performed by Kumar et al. validated 17 phytochemicals isolated from Withania somnifera to bind with high affinity to the S1'-specificity pocket of the MMP-9 catalytic domain and lead to its functional inhibition [67-70]. A direct inhibitory mechanism was observed in an experiment on rat neuronal cells (PC-12) and in silico, with two withanamides A and C, isolated from the fruit, where candidate withanamides were found to uniquely bind to the active motif of Aβ peptide between 25 and 35 amino acid residues. This led to conclusive evidence suggesting that withanamides can prevent plaque formation. The study also suggests that protein-protein hydrophobic interactions may be weaker compared to that between the protein and withanamides thereby weakening the robust fibrillar plaque structure [71]. Few clinical trials have been performed using registered trademarked products Sensoril, a standardized aqueous extract comprising of the leaves and roots of Withania somnifera (Two trials, N = 19, 2 teaspoons x 3/ Day for 12 weeks; and N = 59, 2 teaspoons x 3/ Day for one year; Mentally retarded children) and BR-16A (Mentat; Himalaya Wellness Company, Bengaluru, India) comprising of 24 medicinal plant extracts including Withania somnifera, Bacopa monnieri, Centella asiatica, and Evolvulus alsinoides, (N = 53, ages 18- 65, 250-500 mg/day for 8 weeks, 1,000 mg/day for 12 weeks; N = 66, ages 18- 75; and N = 20, ages 20- 35, 250 mg/day for 14 days) have been found to improve cognitive functions [72]. A note of concern is that two compounds, withaferin A and withanolide D, are found to be neurotoxic at a concentration of 1 mM [54].
3.2. Curcuma longa (Turmeric; Haldi), Curcuma zedoaria (Temu putih), Curcuma xanthorrhiza (Javanese turmeric; Temulawak), Curcuma amada (Amada; Mango ginger), Curcuma aeruginosa, Curcuma heyneana, Curcuma mangga, Curcuma phaeocaulis and Curcuma purpurascens
Curcuma is a rhizomatous perennial plant genus belonging to the family Zingiberaceae (ITIS, 2022). Several species under this genus are of culinary significance around the globe and have been extensively used in ethnomedicine for their therapeutic capabilities [73]. Besides the globally recognized therapeutic compound curcumin, this genus is rich in several phytochemicals that constitute its secondary metabolites. Curcuminoids comprising curcumin, demethoxycurcumin, and bisdemethoxycurcumin, are the active components responsible for most of the medicinal properties of turmeric species [74]. Curcumin, with the highest concentration being observed in C. longa, is a beneficial phytochemicals found in rich quantities in many species of Curcuma, including C. zedoaria, C. angustifolia, and C. leucorrhea [75, 76]. Curcumin, in a dose-dependent fashion, not only inhibited the formation and extensions of Aβ fibrils but also led to the destabilization of pre-formed Aβ fibrils [77]. The mechanistic insight of this inhibition is explained by an experiment where a concentration as low as 20 μM could completely suppress the BACE1 mRNA levels [78]. From the standpoint of AD management and therapy, diminution of inflammation is one of the essential mandates. In experimental setups using PBMC and THP-1 cells, Curcumin proved to be effective in reducing local inflammations by a pathway involving the suppression of Egr-1 and effectively downregulated the Aβ40-induced expression of inflammatory cytokines (TNF-α and IL-1β) and chemokines (MCP-1, MIP-1b and IL-8) [74, 79]. A randomized, double-blind, placebo-controlled clinical trial was undertaken for 6 months with 34 individuals founding that Curcumin prevents and/or reverses the aggregation of Aβ at 0.2 to 1 μM (IC50) [80]. The adequate clearance of Aβ plaque depends on the proper functioning of the protein MGAT3, entailed in the phagocytic pathway, which is downregulated in AD [81]. A natural derivative of Curcumin, Bisdemethoxycurcumin, has been found to upregulate the expression of MGAT3 and TLR genes and positively alter the disease state by promoting proper Aβ plaque clearance [82]. SIRT1, Class III histone deacetylases (sirtuins), is a metabolism and cell cycle regulator and in recent years, has been taken into consideration as a therapeutic target for AD [83]. Treatment of HEK293 cell lines with zedoaraldehyde, 13-hydroxygermacrone, 3-hydroxy-6-methylacetophenone, and germacrone isolated from C. xanthorrhiza was found to promote the expression of SIRT1 and all except 3-Hydroxy-6-methylacetophenone, showed moderate AChE inhibitory activities [84]. Few sesquiterpenoids isolated from C. xanthorrhiza showed AChE inhibitory activities [84]. The highest efficacy was displayed by ar-curcumene, α-sesquiphellandrene, and zingiberene (also found in Zingiber officinale, another plant under the same family) [73, 85]. In terms of reduction of oxidative stress-induced neuronal damage, several Curcuma species have proved to be effective; citing examples, curcuzedoalide from C. zedoaria rhizomes reduced NO-mediated oxidative stress in RAW 264.7 cells [86]. Curcumin, one of the major constituents of Curcuma spp. has a general antioxidant activity which stands large for a vast spectrum of diseases [87-89], eight sesquiterpenes (germacrone, zerumbone epoxide, dehydrocurdione, isoprocurcumenol, procurcumenol, curcumenone, gweicurculactone and zederone) and one labdane diterpene (zerumin A) isolated from C. zedoaria protected cells from oxidative damage [90]. The same labdane diterpene zerumin A has also been isolated from C. amada and has been established to be a potent modulator of GABAA receptors which is relevant for therapeutic management of AD [91, 92] A comparative analysis of a few traditionally used Curcuma species, C. aeruginosa, C. heyneana, C. mangga, C. phaeocaulis and C. purpurascens, revealed that their phenolic compounds, principally curcuminoids, were able to reduce oxidative stress and can manifest their potentiality as a therapeutic antioxidant [93].
3.3. Ginkgo biloba (Maidenhair Tree)
The plant has had an association with mankind that dates back to 2000 years; since the very existence of humankind, several parts of the plant have been used for traditional medicine and later been upgraded for several pharmacological tinctures applicable in modern medicine. Fossil records of this plant date back to the mid-Jurassic and can easily be considered a living fossil. Classified under Ginkgophyta, it is not known to have any close relative in the plant kingdom [94, 95]. The plant extracts have been reported to perform neuroprotective properties and manifest enhancement of cognitive properties [96]. The plant is known to possess sesquiterpenoid (bilobalide) and a unique group of compounds, terpenic lactones (ginkgolide) [97]. A standardized marketed extract of G. biloba, EGb-761, containing 24% flavonoids, 7% proanthocyanidins, and 6% terpenoids, has been extensively used in research encompassing neurodegenerative diseases [97]. The G. biloba constitutes more than 30 genuine flavonoids, of which the flavonoid fraction of EGb-761 consists of kaempferol, quercetin and isorhamnetin conjugated with glucose or rhamnose [98]. The proanthocyanidins comprise epiafzelechin, epicatechin, epifisetinido, epigallocatechin, and epirobinetinidol [99]. The terpenoid fraction of the extract consists mainly of ginkgolides (A, B, C and J), bilobalide, delphinidin, cyanidin, trace amounts of pelargonidin, and an unknown anthocyanidin [100, 101]. Pelargonidin is one of the most common forms of anthocyanidin compound in leaves of several plants and is known to behave as a strong antioxidant with myriad therapeutic potentials, useful for a vast range of diseases, even in toxicant-induced oxidative stress and maintaining cellular integrity by maintaining the optimum functioning of the sub-cellular compartments [102, 103]. Several studies performed with EGb-761 provide promising results of clinical significance, where the treatment groups comprising Alzheimer's disease or multi-infarct dementia displayed better Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) and Geriatric Evaluation by Relative's Rating Instrument (GERRI) values, indicating a significant improvement of cognitive function (Dose = 120 mg/d, N = 309, ADAS-Cog score became 1.4 points better (P = .04) and GERRI score became 0.14 points better (P = .004) than the placebo group [104]; Dose = 40 mg/d, N = 327, the placebo group GERRI score worsened by 0.07 points from its baseline (p = 0.05), whereas the treatment group slightly improved by 0.05 points (p = 0.09), similarly the ADAS-Cog score of the placebo group worsened by 1.1 points (p = 0.04) [105]; 28% of the treatment group showed improvement, N = 156 [106]). The science behind this can be explained by analysis of the reversal of the reduced 5-HTIA receptors upon administration of EGb-761 in a nonspecific way, either by inhibiting the lipid peroxidation or inhibiting aldehyde production [107]. Both flavonoid and ginkgolide constituents are known to participate in this free radical scavenging and antioxidant effects [108]. More specifically, bilobalide improves the cellular ATP levels by protecting mitochondria against the uncoupling of OXPHOS by promoting the expression of mtDNA encoding COX III subunit of cytochrome oxidase and ND1 subunit of NADH dehydrogenase at a dosage of 15.3 or 30.6µM [109, 110] and 30.6µM for 48 or 72 h [111] on rat PC12 cell respectively [112, 113]. The role of the PLA2 enzyme in AD is linked with memory impairment and neurodegeneration and is found to be under the control of both cholinergic and glutamatergic systems thus the selective activation and inhibition of specific isoforms of PLA2 prove to be of therapeutic significance. Inhibition of cPLA2 and iPLA2 contributes to Aβ production via the down-regulation of cholinergic and glutamate receptors. While on the other hand, Aβ-induced up-regulation of PLA2 isoforms (cPLA2 and sPLA2) is found to play crucial roles in mediating inflammation and oxidative stress [114]. An IC50 value of 2.3 µM of Bilobalide inhibits NMDA-induced activation of glutamatergic PLA2 and subsequent breakdown of phospholipids and choline efflux in rat hippocampal slice [115]. Delphinidin, a common anthocyanidin in pigmented fruits and vegetables, attenuated the elevation of intracellular calcium levels and inhibited tau hyperphosphorylation, thereby protecting PC12 cells from Aβ-induced toxicity by the inhibition of GSK-3β [116]. Quercetin (3,3′,4′,5,7-pentahydroxyflavone) (Also found in Ficus carica [117, 118] has been reported to exert antidepressant effects, studied in C57BL/6j mice brain mitochondria fraction by the inhibition of MAO-A, responsible for the metabolism of 5-HT, but does not show side effects on the metabolism of dietary monoamines in the gut [119].
3.4. Coffea arabica (Arabic Coffee), Coffea semen, Coffea robusta (Robusta), Coffea liberica (Liberian coffees), Coffea benghalensis (Bengal coffee)
Coffea is a genus of small trees under the family Rubiaceae (ITIS, 2022). For a considerable time, several species under this genus have been established as a flagbearer of the beverage industry and have a considerable consumptive role in modern lifestyle regimens [120, 121]. Several phytochemicals are found to be prevalent in Coffea sp., which can be categorized into seven broad categories comprising (i) phenolic compounds called chlorogenic acids, (ii) purine derivative called methylxanthine, which comprises the hallmark compound caffeine, (iii) flavonoids, (iv) compound with vitamin-like activity like tocopherol (Vitamin E), (v) diterpene alcohols, (vi) protein-reducing sugar conjugates synthesized from Maillard reaction called melanoidins and (vii) oligosaccharides.
The xanthine derivative is methylxanthines comprising caffeine, theobromine, and theophylline; the diterpene alcohols comprise of cafestol and kahweol, and the phenolic compounds constitute hydroxycinnamic acids (comprising ferulic acid, caffeic acid, p-coumaric acid), caffeoylquinic acids, feruloylquinic acids, p-coumaroylquinic acids collectively called chlorogenic acids, flavonoids include catechins and anthocyanins) [122-124]. For items with routine consumption, the therapeutic ease of administration is vital as much of the data regarding the consequences of daily or routine intake is already available at a scalable demographic level. β-Cyclodextrin is a cyclic oligosaccharide in Coffea sp., which is found to interact with the Aβ40 and inhibits its fibrillization. The β-Cyclodextrin- Aβ40 peptide complex shows the inclusion of hydrophobic aromatic moieties of the peptide in the hydrophobic cavity of β-Cyclodextrin [125]. Methylxanthines (Theobromine, Theophylline, and Caffeine) are known to mobilize intracellular calcium, inhibit PDEs, inhibit GABAA receptors [126], inhibition of high affinity ATP-dependent cyclic nucleotide transporters and function as antagonists of adenosine receptors, all of which are of direct pharmacological significance in AD [127-130]. The inhibition of PDE2, 4 and 5 and ABCC4/5 is found to improve memory performance by enhancing the levels of cyclic second messengers by inhibiting its degradation and efflux, respectively, in models of AD [131]. Caffeine and theophylline (also present in cacao (Theobroma cacao)) is a non-selective inhibitor for PDE5 versus ABCC4/5 and at IC50 values ranging from 100 to 1000 mM, is a non-specific PDE inhibitor with little selectivity among PDEs 1 to 11 [127]. The inhibition of PDE by caffeine requires interaction with Asn 395, Tyr 403, Tyr 406, Thr 407, Ile 410, Phe 414, Met 431, Ser 442, Glu 443, Phe 446, Phe 506 residues of the enzyme [132]. But the challenge faced is that PDE inhibitors must target specific isoforms to restrain unwanted side-effects imposed by global PDE inhibition of the treated subject [131]. Oxidative stress of DNA mediated by peroxynitrite and other ROS leads to overactivation of DNA repair protein PARP and subsequent cell death due to overconsumption of NAD+ [133, 134]. Theophylline and caffeine are weak competitive inhibitors of the enzyme PARP-1 concerning NAD+ cleavage and thereby improve cellular energy crisis [135, 136]. Caffeine is known to protect neurons from the toxic effects of Aβ protein-mediated toxicity, block the action of GABA and glycine-gated chloride channels in rat hippocampal neurons [137], stimulate Na/K-ATPase activity (which is lower in AD and results in neuronal death [138, 139], improve calcium influx and efflux [140], and inhibit adenosine receptors providing an umbrella of protection to neuronal cells [141]. Theophylline has also been found to show a similar stimulatory effect on Na+/K+- ATPase in Wistar rat diaphragm muscle cells [142]. Caffeine is also found to modulate a key neuronal maintenance factor, NMNAT2 (synthesizing NAD), and restore its expression to normal levels in rTg4510 taupathic mice and cortical neurons of NMNAT2blad mice in vitro [143]. The phenolic compound 3,5-di-O-caffeoylquinic acid is found to reverse the Aβ42-induced damage in SH-SY5Y cells, upregulate phosphoglycerate kinase-1 mRNA expression, and improve the expression of the energy state of the cell [144]. A study on gerbil brain synaptosomes reported Ferulic acid (4-hydroxy-3-methoxycinnamic acid) at a concentration of 150 mg/kg (i.p.), to reduce Aβ-induced ROS and RNS and upregulate two neuroprotective factors, HO-1 and HSP70 [145]. Even with a paramount of promising validations for the use of Hydroxycinnamic acid in AD, its efficacy is under perusal owing to the shortcomings like low bioavailability and poor penetration of the BBB [146]. Both the diterpenes kahweol and cafestol exert an antioxidant effect in a dose-dependent manner in NIH3T3 cells and efficiently remove superoxide anion (radical scavenging activity) [147]. More specifically, kahweol protected 6-OHDA challenged SH-SY5Y cells from oxidative stress, downregulated caspase 3, and modulated HO-1 via activation of PI3K/Akt (PKB), p38, and Nrf2 [148], while cafestol improved the expression of glutathione-s-transferase activity and activation of the Nrf2 pathway in the Drosophila model for PD and AD [149]. The γ-tocopherol and not α-tocopherol have been found to exert a neuroprotective effect in the context of AD by stabilizing free radicals by donating a hydrogen atom from its hydroxide group [150, 151]. Moreover, α-tocopherol is found to exhibit contraindication in AD [152]. The significance is as such that α-tocopherol being the primary tocopherol in supplements and possessing its transport protein (hepatic α-tocopherol transfer protein), compiled with its preferential absorption and greater biological activity [153] renders tocopherol treatment perilous, and also the genetic architecture of Vitamin E metabolism and uptake of the subject is suspected of contributing to the clinical failures [154].
3.5. Camellia sinensis (Tea, Cha)
Camellia falls under the family Theaceae (ITIS, 2022), comprising shrub-type plants. Amongst more than 200 species under the genus Camellia, C. sinensis and C. assamica are of the greatest economic importance in global commerce as a consumable products [155]. Being consumed for almost 50 centuries, Camellia leaves is known to constitute more than 700 chemicals, including polyphenols, catechins, flavonoids, amino acids, vitamins, caffeine, and pharmaco-active polysaccharides, [156] of which few compounds like the amino acid theanine (4-6% dry weight) [157, 158], the phenolic acid theogallin (3-galloylquinic acid) (2-3% dry weight), [158, 159] carotenoid derivative theaspirone, [160], quamoreokchaside I-II, kamoreokchaside I [161], and black tea phenolic compounds theaflavins, thearubigins and flavonol glycosides [162], can be considered as unique chemical propositions of tea. Moreover, as processing is an integral part of tea manufacturing, the nutraceutical composition of green tea as compared to processed black tea varies moderately and may be of clinical significance. In exact terms, green tea leaves are rich in catechins, constituting 30% of its dry weight [158], comprising of (−)-epicatechin, (−)-epigallocatechin, (−)-epicatechin gallate, (−)-epigallocatechin gallate, and (−)-gallocatechin gallate [163], whereas during black tea fermentation, quinones are produced under enzymatic oxidations, which further undergoes condensation reactions which directs the formation bisflavanols, theaflavins (possess a benzotropolone skeleton; Pharmacophores within the molecule that contributes to the antioxidative property- 1. 3´4´5´-trihydroxyl (gallate) group in the B ring; 2. an esterified gallate group at the third position of the C ring; 3. hydroxyl groups at 5 and 7 positions of the A ring.), epitheaflavic acids, and thearubigins (formed by the concoction of theaflavins and other oxidation products of catechins) which adds to the uniqueness pool of tea phytochemicals [158, 164]. Catechins are known to undergo biotransformation, including glucuronidation, methylation, sulfation, and ring-fission metabolism forming metabolic intermediates [156], which are further broken down to phenylacetic and phenylpropanoic acids by the gut microflora [165]. The structure of theaflavins constitutes antioxidative pharmacophores that foster their radical scavenging property [166] study performed on SH-SY5Y neuroblastoma cells validates the efficacy of catechins and theaflavins in the reduction of BACE1 mRNA expression induced by Aβ, with epicatechin and epigallocatechin showing the maximal potency [167]. Both (−)-epicatechin gallate and (−)-epigallocatechin gallate has been found to inhibit GABAA receptor with more strong interaction than other derivatives in an experimental setup involving Xenopus laevis oocyte microinjected with cRNAs of α1 and β1 subunits of the Bovine GABAA Receptors to express the same [168]. Serine protease inhibitor (SERPIN) superfamily proteins have been found to play a complex role in neuropathophysiology [169]. SERPINE1 (PAI-1) has a direct role in CNS maintenance by activating the ERK pathway and regulating the levels of plasminogen conversion via uPA and tPA [170]. Elevated levels of PAI-1 are involved in the inhibition of plasmin-mediated proteolysis of AβPP, and the promotion of Aβ plaque accumulation [170]. Two of the theaflavins, theaflavin-3,3'-digallate and theaflavin-3'-gallate, were found to inhibit PAI, of which theaflavin-3'-gallate is found to be more potent [171]. Black tea extract (Polyphenols (442.17 mg/100 g gallic acid equivalent), Theaflavin (2.16%), Thearubigins (19.31%), Catechins (2.04%), Caffeine (1.81%), and Theanine (4.1 mg/100 ml)) were found to modulate and reduce cellular antioxidant profile like GPx, CAT, SOD, and suppressed the expression of hallmark proteins Aβ42, AβPP, β- secretase and γ- secretase, in hippocampus and cortex of aluminium chloride-induced (100 mg/kg, i.p., 60 days) AD model Wistar rats [172]. More precisely, L-Theanine attenuated Aβ42-induced memory impairment, inhibited ERK, p38 mitogen-activated protein kinase, NF-κB and reduced oxidative damage [173]. A clinical trial with 2g/day of green tea extract (containing 227 mg catechins and 42 mg theanine) was carried out with 15 elderly patients with MMSE-J score <28 and found that the treated group's MMSE-J score improved by 1.7 points (p=0.03) [174]. Three of the polyphenols, (–)-catechin gallate, (–)-epicatechin gallate, and (–)-epigallocatechin gallate, were found to significantly decrease the aggregation of Aβ at a concentration of 10 μg ml−1, with (–)-catechin gallate and (–)-epicatechin gallate providing more robust protection to SH-SY5Y cells against the same [161]. A molecular docking study revealed the potential of epigallocatechin gallate to vitiate Fluoride-induced free radicals via the Nrf2/Keap1 signalling pathway by reducing the expression of Keap1 (interacting with Gly 343, Thr 595, Lue 578, and Asp 579 residues of the protein) and promoting the translocation of Nrf2 into the nucleus [175]. Myricetin (3, 5, 7, 3′, 4′, 5′-hexahydroxy flavonol), is a polyhydroxy flavonol compound originally isolated from Myrica rubra, which is found to be present in C. sinensis as well [176]. Myricetin is found to improve the tissue-specific availability of BDNF [177]. Owing to its iron-chelating capability, myricetin reduced iron overload in the AD brain and inhibited the expression of transferrin receptor 1, thereby maintaining the optimum iron level of the brain and reducing iron-induced oxidative damage [178].
3.6. Lepidium meyenii (Maca; Peruvian ginseng)
The Lepidium meyenii, like many of the tuberous members of the family Brassicaceae (ITIS, 2022), is an edible plant native to South America. It is of higher nutritional value (10.2% protein, 8.5% whole fiber, and 59% carbohydrate) than potato [179]. The plant's tuber is a popular food with medicinal and nutraceutical benefits [180]. The plant is known to possess bioactive compounds such as phytosterols (campesterol and β-sitosterol), different types of glucosinolates (β-D-glucopyrano unit and an O-sulfated anomeric (Z)-thiohydroximate which is linked by variable residue to an aglycone) [181] (glucobrassicanapin, glucotropaeolin, glucoalyssin, glucobrassicin, etc.), alkaloids (macaridine, lepidilin A & B, lepidine, tetrahydro-β-carbolines, etc.) [182], and novel compounds, macaene (acyclic polyunsaturated oxoacid), macamides (benzylated amides) and two thiohydantoin derivatives, Macahydantoins A and B, [180]. More importantly, glucosinolates, the relatively biologically inactive compound, have a vast range of biological activity upon being hydrolyzed and biotransformed from glucosinolates to isothiocyanates and other compounds by the gut bacteria [183]. Among several isothiocyanates, phenyl isothiocyanate and its derivatives have been found to exert maximal AChE and BChE inhibitory activity with the best results displayed by 2-methoxyphenyl isothiocyanate with 57% inhibition at 1.14 mM and 3-methoxyphenyl isothiocyanate with 49.2% inhibition at 1.14 mM for AChE and BChE respectively [184]. n-Pentane extracts of L. meyenii at concentrations of 2.8g/mL (in vitro on OLGA-PH-J/92 neuronal cells of crayfish, Orconectes limosus) and 3 mg/kg (in vivo on male Sprague–Dawley rats) has shown statistically significant neuroprotective effects, but in vivo concentrations greater than 3 mg/kg showed certain adverse effects [182]. A recent investigation by HPLC-UV-guided separation of compounds from the tubers of L. meyenii yielded (±)-l epithio hydantoins A to D, which showed neuroprotective activities against corticosterone induced injury in PC12 cells and seeks further investigation [185]. The plant is also known to possess choline as a metabolite [186]. In terms of the “cholinergic hypothesis”, the general assumption lies that the basal forebrain cholinergic dysfunction is related to memory impairment and neurodegeneration found in several forms of dementia [187]. Choline present in foods is available in two major forms, water-soluble form, including free choline, phosphocholine, and glycerophosphocholine, and lipid-soluble forms, including phosphatidylcholine and sphingomyelin, varying in their absorptive and metabolic profiles [188]. The free choline is absorbed in the jejunum and ileum of the small intestine either by sodium-independent carrier-mediated transport into the enterocyte or by passive diffusion (only when the free choline concentrations are high), following which it has two fates, either it can be irreversibly oxidized to betaine, or it can be directly absorbed into the portal circulation, which can further be acetylated to improve the tissue-specific availability of acetylcholine neurotransmitter [189]. The significance of this is as several such studies have confirmed that dietary supplementation of choline improves the cognitive and neurodegenerative profiles of the subject, which becomes relevant in most types of neurodegenerative diseases [190, 191]. But the overall effect of dietary choline supplementation on the tissue-specific availability of choline and its subsequent conversion to acetylcholine is debatable, and available data suggest that it can either be beneficial or show no effect in the context of altered cholinergic neurotransmission [192-194] The brain is completely dependent on in situ steroid synthesis and has no access to the steroid pool of the blood. Brassicasterol, a 28-carbon sterol, is found in several plant species and is capable of successfully crossing the BBB, which adds to its pharmacological significance for therapeutic administration [195, 196]. Brassicasterol has been found to interact with Lys28, Asp23, Val30, His14, Ala211, Ala21and Ile32 residues of Aβ, and Gln351, Lys353, and Ser352 of tau protein forming hydrogen bonds and leading to their inhibition [197]. Thus, the administration of brassicasterol may serve multipartite functions; first, by directly interacting with tau and Aβ; second, by improving the repertoire of cephalic sterols and third, reduced brassicasterol in CSF may act as a biomarker for the progression of AD [195].
3.7. Bacopa monnieri (Brahmi; Water Hyssop)
The plant Bacopa monnieri, a herb under the family Plantaginaceae (ITIS, 2022), is a traditional medicinal plant in Ayurvedic medicine concerning to India and has been deeply rooted in Indian medicine for over 3000 years, owing to its known effects of improving cephalic and cognitive functions [197] including functions such as enhancement of memory, anti-anxiety and anti-depressant [198]. Though several health benefits of the plant have been validated in the published scientific literature, including its role as antiulcerogenic, hepatoprotective, anti-inflammatory, and analgesic, among many [199, 200], considering our aim of this review, we strictly focus on the neuroprotective effects of the plant in context to AD. More than 70 chemical constituents have been identified and isolated from Bacopa monnieri [200]. The main chemical constituents with pharmacotherapeutic values include the alkaloids brahmine, herpestine, and nicotine, the saponins, monnierasides I-III, hersaponin, bacoside A1, A2, A3 and B and four sapogenins bacogenin A1, A2, A3 and A4 [201-205]. The pharmacological benefits are mainly conferred by the alcoholic extract of the glycosidic (saponins) fraction of the phytochemical pool [199], of which Bacoside A and B have extensively been studied [206]. However, rather than being a single chemical entity, bacoside A constitutes bacoside A3 (3-β-[O-β-d-glucopyranosyl (1-3)-O-[α-L-arabinofuranosyl (1-2)]-O-β-d-glucopyranosyl)oxy]), bacopaside II (3-O-[α-L-arabinofuranosyl-(1→2)-{β-D-gluco-pyranosyl-(I→3)}-β-D-glucopyranosyl] pseudojujubogenin), bacopaside X (3-O-α-L-arabinofuranosyl-(1→2)-{β-D-glucopyranosyl-(1→3)}-α-L-arabinopyranosyl] jujubogenin) and bacopasaponin C (3-O-[β-D-glucopyranosyl-(1→3)-{α-L-arabinofuranosyl-(1→2)}-α-L-arabinopyranosyl] pseudojujubogenin) [207]. The major glycoside constituents are a dammarane type of triterpenoid saponins, which has been isolated and characterized from Bacopa, whose aglycone unit constitutes jujubogenin or pseudojujubogenin moieties [198]. In vitro experiment with Bacoside A validates that it exerts inhibitory effects against Aβ42 mediated cytotoxicity in SH-SY5Y cell line, mainly by inhibiting membrane interactions of Aβ42 oligomers by preventing their self-assembly and membrane perturbation through amphiphilic interactions, thereby maintaining the cellular integrity of neuronal cells [208]. Another experiment using U87MG cell showed that pre-treatment with bacoside-A3 displayed suppression of Aβ-mediated nuclear translocation of NF-κB, formation of iNOS, suppressed PGE2 (which is often elevated in AD and is involved in its pathogenesis [209]) by suppressing the overexpression of COX-2, and reduced oxidative radical formation [210]. In silico study revealed that bacopaside-XII binds with Keap1 with high affinity, interacting via hydrogen bonds with Arg380, Asn382, Ser383, Pro384, Asn 387, Asp389, and Tyr572 residues of the protein and leads to its inhibition [211], this leads to the dissociation of Nrf2 from Keap1 and its subsequent translocation to the nucleus, upon exposure to ROS, thereby maintaining the functional integrity of Keap1-Nrf2-ARE pathway [212]. Few clinical trials performed by using B. monnieri found promising results, like the administration of 1050 mg of crude Brahmi per day in children (6- 8 years) strengthened their cognitive functions [213]. Few other essential phytochemicals include betulinic acid, betulinic acid, stigmasterol, wogonin, oroxindin, β-sitosterol, brahamoside, brahmic acid, brahminoside and isobrahmic acid [214]. The pentacyclic lupane-type triterpenoid, Betulinic acid, is a biologically active compound reported approximately in 2500 plant species. With a molar ratio of 1:4 (Aβ to betulinic acid), betulinic acid improves cognition and hippocampal long-term potentiation amplification in Wistar rats [215]. Another important characteristic is that betulinic acid can penetrate the BBB and is safe at a dose concentration higher than 500 mg/kg, making it a very suitable drug candidate for long-term application [216, 217]. The phytosterol stigmasterol reduced Aβ plaque generation by performing a multipartite function, reducing expression of γ-secretase, directly decreasing BACE activity, and reducing cholesterol and presenilin distribution in lipid rafts and decreasing internalization of BACE1 to endosomal compartments [218]. Extract of the plant has shown efficacy in modulating the oxidative profile of cells by modulating SOD, CAT, glutathione, and GPx, and decreasing the level of lipid peroxidation in rat brain [214].
3.8. Rosmarinus officinalis (Rosemary)
Rosemary, a member of the mint family, Lamiaceae (ITIS, 2022), is a common herb of Mediterranean origin with a variety of applications in the food industry. GC-MS of the terpene pool identified 1,8-Cineole, terpinen-4-ol, camphor, and borneol in the monoterpene fraction, 4,14-retro retinol, ferruginol methyl ester, and neophytadiene in the diterpene fraction, and (E)-β-caryophyllene, δ-cadinene and caryophyllene oxide in the sesquiterpene fraction. Amongst several phytocompound constituents of rosemary, two phenolic diterpenes, namely rosmarinic acid and carnosic acid, have gained much attention regarding their interesting biological properties [219]. The flavonoid constituents include cirsimaritin, homoplantaginin, genkwanin, gallocatechin, and nepitrin [220]. Carnosic acid has shown to protect brain cells from cyanide-induced injury via the activation of the Keap1/ Nrf2 pathway via direct S-alkylation of targeted cysteines (ex. cysteine 151) on Keap1, and ROS-mediated injury of PC12h cells by the selective expression of phase 2 antioxidant enzymes, including HO-1, NQO1, and c-GCL, thereby modulating the intracellular redox state [221]. The targeted cysteines act as a sensor for electrophilic compounds; thus, even low concentrations of quinone-type carnosic acid led to the preferential activation of more reactive Keap1 over GSH, activating the cellular defence pathway and providing cytoprotection. However, before considering carnosic acid as a therapeutic molecule, it must be noted that it is the quinone-type carnosic acid, but not the catechol-type carnosic acid, that can activate the Keap1/Nrf2 pathway [222]. As stated earlier, it is important to maintain the optimum functionality of the mitochondria to retain the functional integrity of any cell [223]. It has also been reported to protect mitochondria from ROS mediated damage in SH-SY5Y cells by the selective expression of HO-1, NQO1, and γ-GCL [224]. Several compounds in Rosmarinus officinalis, are also present in other plant species, have been reported to exert a vast range of therapeutic potential, like in silico study with neophytadiene (also present in Red Cedar (Erythroxylum monogynum) and Calendula officinalis) shows that it can bind to vital enzymes like tyrosinase and AChE and inhibit them [225], in vitro 1,8-Cineole (also found in Ocimum kenyense, Cardamom (Elettaria cardamomum), Spanish sage (Salvia lavandulaefolia) and many other species) prevents PC12 cells from oxidative damage, inhibits AChE, prevents Aβ42 oligomerization, restores ChAT expression and decreases Aβ25-35 induced NF-κB activation [226, 227], in vivo study with β-caryophyllene (also found in rice (Oryza sativa) and maize (Zea mays)) on a transgenic APP/PS1 AD mice model beneficial effect of the compound in reducing neuroinflammatory response in context of AD involved cannabinoid receptor 2 activation and the PPARγ pathway [228], in vivo experiments with rosmarinic acid (also found in Lemon balm (Melissa officinalis), perilla (Perilla frutescens) and sage (Salvia officinalis)) on mice found that it suppresses Aβ accumulation and enhanced the levels of monoamines such as dopamine, levodopa, norepinephrine, and 3,4-dihydroxyphenylacetic acid in cerebral cortex and improved dopamine signalling pathway via significant downregulation of MAO-B in the substantia nigra and ventral tegmental area [229]. It also directly inhibited the formation, extension, and destabilization of Aβ40 and Aβ42 [230]. In a randomized, double-blind placebo-controlled study with Melissa officinalis extract containing rosmarinic acid in 23 human patients (12 patients in the treatment group and 11 patients in the placebo group) with mild dementia due to AD, the NPI-Q score improved by 0.5 points, indicating a significant difference (P = 0.012), but disease biomarkers like PET SUVR, FDG PET z-scores, MRI z-scores, CSF- Aβ42, CSF-tau, and CSF-pTau, showed no significant differences [231]. A flavonoid nepitrin isolated from R. officinalis improved memory in scopolamine-induced Swiss albino mice and led to a concentration-dependent inhibition of AChE (IC50 value 65 μg/mL) and BChE (IC50 value 72 μg/mL). Molecular docking revealed that nepitrin occupies the same binding site (Trp279, Phe330, and Tyr334) and forms the similar set of interactions to those formed by a standard AD drug, donepezil, along with few novel additional interactions (Arg289, and probably Trp84) [232].
3.9. Ocimum tenuiflorum (Tulsi; Holy Basil; Syn: Ocimum sanctum)
Ocimum tenuiflorum is an aromatic perennial plant in the family Lamiaceae (ITIS 2022), identified as two common cultivars, Rama Tulsi (large, green-leafed form) and Krishna Tulsi (purple and lanceolate-leafed form) [233]. Being established as a cultivated plant for at least, if not more, three thousand years, several uses of Tulsi have been documented, including in the area of medicine, with particular reference to Indian Ayurveda [234]. Virtually all parts of the plant have been used for their medicinal property. Many scientific studies have elucidated antistress, antioxidant, immunomodulating, anti-inflammatory, antibacterial, antiviral, and antifungal properties, among many, of Tulsi with a wide margin of safety [235]. Several phytochemicals of medical significance are prevalent in leaf extract of Ocimum sp., which comprises secondary metabolites like tannins, flavonoids, alkaloids, saponin, glycosides, terpenoid, and phenols [236, 237]. GC-MS analysis of an alcoholic extract of O. tenuiflorus includes oleanolic acid, rosmarinic acid (also found in Rosemary), ursolic acid, carvacrol, β elemene, β-caryophyllene, germacrene, cyclopropylidene, cyclopentane, cyclohexane, 1, 2, 4-triethenyl, phenylpropanoids mainly Eugenol (1-Methyl eugenol and 2-Eugenol) and Methyl-Isoeugenol (Benzene, 1, 2-dimethoxy-4-(2-propenyl)), and sesquiterpene isoprenoid α-Farnesene [236]. The monoterpene fraction obtained from the volatile oils comprises camphene, linalool, myrcene, sabinene, and borneol [237]. Ursolic acid, a pentacyclic triterpene acid, has been reported to suppress the activities of NF-ĸB activation by inhibiting degradation and phosphorylation of IκBα by activating IκB kinase [238, 239]. In vivo study on Wistar albino rats with Aβ, with O. tenuiflorum extract was found to increase the BDNF expressions and GABA levels, significantly reduce Aβ and tau protein in the hippocampus, and decrease glutamate and AChE activity, thereby ameliorating the cognitive dysfunction and restoring the hippocampal architecture [240, 241]. The phenylpropanoid eugenol (also found in Clove (Eugenia aromatica), True Cinnamon (Cinnamomum zeylanicum) and Basil (Ocimum basilicum) and Clove Basil (Ocimum gratissimum)) has a vast range of pharmaco-activity in context of neurodegeneration and AD, it potentiates the effect of Niacin (Nicotinic acid; Vitamin B3) in treating AD [242, 243], reducing the chances of dementia and onset of AD on account of niacin deficiency [244], Eugenol increased acetylcholine level, decreased AChE activity (interacting with Tyr124 by hydrogen bond, Pi-Pi T-shaped interaction with Tyr337, pi-pi staked interaction with Ty341, and pi-alkyl interactions with His447, Trp286, Phe295 and Val294) [245], and increased the total muscarinic receptor in scopolamine-induced Charles-Foster albino rats [246], in another experiment on Wistar rats with aluminium-induced neurotoxicity followed by eugenol treatment, it resulted in a significant reduction NO and 8-OHdG levels, inhibition of caspase-3 activity, reduction of Bax protein levels and increase in levels of Bcl-2, SOD and GPx [247]. The volatile sesquiterpene compound germacrene (also found in hedgenettles (Stachys inflata, Stachys lavandulaefolia, Stachys byzantina), Indian pennywort (Centella asiatica), Palicourea tomentosa and Salvia syriaca) [248, 249] were found to inhibit BACE1, more specifically germacrene B interacts with Val93 residue of BACE1 as predicted by the in-silico study [250]. In contrast, germacrene D was found to have significant AChE inhibition activity [251-254].
CONCLUSION
The recent global trends showed that several researchers are inclined toward investigating and assessing therapeutic targets for the brain and its associated diseases. Phytocompounds provide a vast pool of novel bioactive compounds of therapeutic potential. Therefore, phytotherapy can be a promising approach that prioritizes the increase of the therapeutic effect of synthetic drugs on the brain by reducing overall toxicity and reduction of less drug dosage, leading to a more effective arsenal to combat neurogenerative diseases with precise and pinpointed patient compliance. The phyto active components such as curcumin, B. monnieri extract, and the dietary supplement containing Ginkgo biloba extract has been shown to have promising hopes under clinical trials against AD-associated cognitive decline and neuroinflammation. The physiological effects of these compounds have shown in vitro and in vivo efficacies. However, clinical validation of the same using modern approaches like CSF biomarkers in AD and the application of Amyloid PET for the understanding of the functional modality of the phytocompounds in terms of physiological state of the cognitive impairments needs to be developed. More importantly, extensive, well-structured clinical studies with greater cohorts are required to understand the efficacy of these phytocompounds and their further validation using modern approaches like amyloid PET and cerebrospinal fluid biomarkers comprising the Alzheimer's disease profile, including Aβ42, total tau and phosphorylated tau.
The Amyloid PET and cerebrospinal fluid examination may permit the diagnosis of Alzheimer's disease before the manifestation of true dementia. In western countries, at least in the excellence centers, this type of evaluation is applied routinely to patients less than 70-75 years old to give them a precise diagnosis and to recruit them for trials with disease-modifying drugs. In most patients with mild cognitive impairment (MCI), clinicians wait for the conversion of MCI to dementia, and in these cases, many substances, including natural phytotherapeutics, are available in the store and pharmacies. The present study aims to provide a detailed overview of the type of phytocompounds that proves to be promising drug candidate in the context of AD and also points out the pharmacodynamics of those compounds, which, to our belief, will be a concise reference guide for all the researchers working in this field of neurodegenerative disease.
ACKNOWLEDGEMENTS
The authors would like to acknowledge their affiliated institution for the infrastructural support.
LIST OF ABBREVIATIONS
- ABCC
ATP-binding Cassette Subfamily C
- AChE
Acetylcholinesterase
- AD
Alzheimer’s Disease
- ADAM10
A Disintegrin and Metalloproteinase Domain-containing Protein 10
- ADAS-Cog
Alzheimer's Disease Assessment Scale-cognitive subscale
- Akt
Stock A Strain k Thymoma/Transforming Protein Kinase
- APP
Amyloid Precursor Protein
- Arc
Activity-regulated Cytoskeleton-associated Protein
- ARE
Antioxidant Response Element
- ATP
Adenosine Triphosphate
- Aβ
β-amyloid
- Aβ42
β-amyloid (1-40)
- Aβ42
β-amyloid (1-42)
- AβPP
Amyloid-beta Precursor Protein
- BACE1
Beta-Secretase 1
- Bax
Bcl-2-associated X Protein
- BBB
Blood-Brain Barrier
- BChE
Butyrylcholinesterase
- Bcl-2
B-cell lymphoma-2
- BDNF
Brain-derived Neurotrophic Factor
- BFCN
Basal Forebrain Cholinergic Neurons
- c-GCL
c-glutamyl Cysteine Ligase
- CAT
Catalase
- ChAT
Choline Acetyltransferase
- CNS
Central Nervous System
- COX
Cyclooxygenase
- CSF
Cerebrospinal Fluid
- CSF-pTau
CSF-phosphorylated tau 181p
- DAT
Dementia of the Alzheimer type
- Egr-1
Early Growth Response-1
- ERK
Extracellular Signal-Regulated Kinase
- FDG
18F-fluorodeoxyglucose
- GABA
γ-Aminobutyric Acid
- GABAA
γ-Aminobutyric Acid type A
- GDNF
Glial Cell Line-derived Neurotrophic Factor
- GERRI
Geriatric Evaluation by Relative's Rating Instrument
- GPx
Glutathione Peroxidase
- GSK3
Glycogen synthase kinase 3
- hAChE
Human Acetylcholinesterase
- HEK293
Human Embryonic Kidney 293 cells
- HO-1
Heme Oxygenase-1
- HSP70
Heat Shock Protein 70
- 5-HT
5-Hydroxytryptamine
- i.p.
Intraperitoneal
- IC50
Half-maximal inhibitory concentration
- IL
Interleukin
- iNOS
Inducible Nitric Oxide Synthase
- ITIS
Integrated Taxonomic Information System
- Keap1
Kelch-like ECH-associated Protein 1
- LRP
Low-density Lipoprotein Receptor-related Protein
- MAO-A
Monoamine Oxidase-A
- MAO-B
Monoamine Oxidase-B
- MAP2
Microtubule Associated Protein 2
- MCP-1
Monocyte Chemoattractant Protein-1
- MGAT
β-1,4-mannosyl-glycoprotein 4-β-N-acetylglucosaminyltransferase
- MID
Multi-infarct Dementia
- MIP-1b
Macrophage Inflammatory Protein-1 Beta
- MMP
Matrix Metalloproteinase
- MMSE-J
Mini-Mental State Examination - Japanese Version
- MRI
Magnetic Resonance Imaging
- mTORC1
Mammalian Target of Rapamycin Complex 1
- NAD+
Nicotinamide Adenine Dinucleotide
- NADH
(reduced) NAD+
- NEP
Neprilysin
- NF-κB
Nuclear Factor Kappa B
- NG108-15
Mouse Neuroblastoma (N18TG-2) and rat glioma (C6BU-1)-hybrid cell line
- NGF
Nerve Growth Factors
- NMDA
N-methyl-d Aspartate
- NMNAT2
Nicotinamide Mononucleotide Adenylyl Transferase 2
- NO
Nitric Oxide
- NPI-Q
Neuropsychiatric Inventory Questionnaire
- NQO1
NADPH quinone oxidoreductase 1
- Nrf2
Nuclear factor-erythroid factor 2-related factor 2
- 6-OHDA
6-hydroxydopamine
- 8-OHdG
8-hydroxy-2-deoxyguanosine
- OXPHOS
Oxidative Phosphorylation
- PAI-1
Plasminogen Activator Inhibitor type-1
- PARP
Poly (ADP-ribose) Polymerase
- PBMC
Peripheral Blood Mononuclear Cell
- PD
Parkinson’s Disease
- PDE
Phosphodiesterase
- PET
Positron Emission Tomography
- PGE2
Prostaglandin E2
- PI3K
Phosphoinositide 3-kinases
- PKB
Protein Kinase B
- PLA2
Phospholipase A2
- PPARγ
Peroxisome Proliferator Activated Receptor-γ
- PSD-95
Postsynaptic Density Protein 95
- RET
Rearranged During Transfection (Protein)
- RNS
Reactive Nitrogen Species
- ROS
Reactive Oxygen Species
- SAMP
Senescence Accelerated-Prone Mice
- SERPIN
Serine Protease Inhibitor
- SERPINE1
SERPIN Family E Member 1
- SIRT1
Silent Mating-type Information Regulation 2 Homolog 1 (aka. sirtuin 1)
- SOD
Superoxide Dismutase
- SUVR
Standardized Uptake Value Ratio
- TEM
Transmission Electron Microscopy
- TH
Tyrosine Hydroxylase
- TLR
Toll-Like Receptor
- TNF-α
Tumor Necrosis Factor-α
- tPA
Tissue-type Plasminogen Activators
- uPA
Urokinase-type Plasminogen Activators
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Yang H.D., Kim D.H., Lee S.B., Young L.D. History of Alzheimer’s disease. Dement. Neurocognitive Disord. 2016;15(4):115–121. doi: 10.12779/dnd.2016.15.4.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jack C.R., Jr, Bennett D.A., Blennow K., Carrillo M.C., Dunn B., Haeberlein S.B., Holtzman D.M., Jagust W., Jessen F., Karlawish J., Liu E., Molinuevo J.L., Montine T., Phelps C., Rankin K.P., Rowe C.C., Scheltens P., Siemers E., Snyder H.M., Sperling R., Elliott C., Masliah E., Ryan L., Silverberg N. NIA‐AA Research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knopman D.S., Amieva H., Petersen R.C., Chételat G., Holtzman D.M., Hyman B.T., Nixon R.A., Jones D.T. Alzheimer disease. Nat. Rev. Dis. Primers. 2021;7(1):33. doi: 10.1038/s41572-021-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cummings J.L., Doody R., Clark C. Disease-modifying therapies for Alzheimer disease: Challenges to early intervention. Neurology. 2007;69(16):1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- 5.Rocca W.A., Amaducci L.A., Schoenberg B.S. Epidemiology of clinically diagnosed Alzheimer’s disease. Ann. Neurol. 1986;19(5):415–424. doi: 10.1002/ana.410190502. [DOI] [PubMed] [Google Scholar]
- 6.Huff F.J., Growdon J.H., Corkin S., Rosen T.J. Age at onset and rate of progression of Alzheimer’s disease. J. Am. Geriatr. Soc. 1987;35(1):27–30. doi: 10.1111/j.1532-5415.1987.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 7.Gouras G.K. Olsson, T.T.; Hansson, O. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics. 2015;12(1):3–11. doi: 10.1007/s13311-014-0313-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iqbal K., Liu F., Gong C.X., Grundke-Iqbal I. Tau in Alzheimer disease and related tauopathies. Curr. Alzheimer Res. 2010;7(8):656–664. doi: 10.2174/156720510793611592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grundke-Iqbal I., Iqbal K., Tung Y.C., Quinlan M., Wisniewski H.M., Binder L.I. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA. 1986;83(13):4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bareggi S.R., Franceschi M., Bonini L., Zecca L., Smirne S. Decreased CSF concentrations of homovanillic acid and γ-aminobutyric acid in Alzheimer’s disease. Age- or disease-related modifications? Arch. Neurol. 1982;39(11):709–712. doi: 10.1001/archneur.1982.00510230035010. [DOI] [PubMed] [Google Scholar]
- 11.Ehrenstein G., Galdzicki Z., Lange G.D. The choline-leakage hypothesis for the loss of acetylcholine in Alzheimer’s disease. Biophys. J. 1997;73(3):1276–1280. doi: 10.1016/S0006-3495(97)78160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan X., Kaminga A.C., Wen S.W., Wu X., Acheampong K., Liu A. Dopamine and dopamine receptors in Alzheimer’s disease: a systematic review and network meta-analysis. Front. Aging Neurosci. 2019;11:175. doi: 10.3389/fnagi.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross A.J. Serotonin in Alzheimer-type dementia and other dementing illnesses. Ann. N. Y. Acad. Sci. 1990;600(1 The Neurophar):405-415. doi: 10.1111/j.1749-6632.1990.tb16897.x. [DOI] [PubMed] [Google Scholar]
- 14.Giacobini E. Cholinesterases: new roles in brain function and in Alzheimer’s disease. Neurochem. Res. 2003;28(3/4):515–522. doi: 10.1023/A:1022869222652. [DOI] [PubMed] [Google Scholar]
- 15.Kihara T., Shimohama S. Alzheimer’s disease and acetylcholine receptors. Acta Neurobiol. Exp. (Warsz.) 2004;64(1):99–105. doi: 10.55782/ane-2004-1495. [DOI] [PubMed] [Google Scholar]
- 16.McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group* under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- 17.Ryman D.C., Acosta-Baena N., Aisen P.S., Bird T., Danek A., Fox N.C., Goate A., Frommelt P., Ghetti B., Langbaum J.B.S., Lopera F., Martins R., Masters C.L., Mayeux R.P., McDade E., Moreno S., Reiman E.M., Ringman J.M., Salloway S., Schofield P.R., Sperling R., Tariot P.N., Xiong C., Morris J.C., Bateman R.J. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology. 2014;83(3):253–260. doi: 10.1212/WNL.0000000000000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzheimer’s Association Report. Alzheimer’s disease facts and figures Alzheimer’s Association. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Thies W., Bleiler L. 2013 Alzheimer’s disease facts and figures. Alzheimers Dement. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Inestrosa N.C., Alvarez A., Pérez C.A., Moreno R.D., Vicente M., Linker C., Casanueva O.I., Soto C., Garrido J. Acetylcholinesterase accelerates assembly of amyloid-beta-peptides into Alzheimer’s fibrils: possible role of the peripheral site of the enzyme. Neuron. 1996;16(4):881–891. doi: 10.1016/S0896-6273(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez A., Opazo C., Alarcón R., Garrido J., Inestrosa N.C. Acetylcholinesterase promotes the aggregation of amyloid-β-peptide fragments by forming a complex with the growing fibrils. J. Mol. Biol. 1997;272(3):348–361. doi: 10.1006/jmbi.1997.1245. [DOI] [PubMed] [Google Scholar]
- 22.De Ferrari G.V., Canales M.A., Shin I., Weiner L.M., Silman I., Inestrosa N.C. A structural motif of acetylcholinesterase that promotes amyloid beta-peptide fibril formation. Biochemistry. 2001;40(35):10447–10457. doi: 10.1021/bi0101392. [DOI] [PubMed] [Google Scholar]
- 23.Iwatsubo T., Odaka A., Suzuki N., Mizusawa H., Nukina N., Ihara Y. Visualization of Aβ42(43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: Evidence that an initially deposited species is Aβ42(43). Neuron. 1994;13(1):45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 24.Lacor P.N., Buniel M.C., Furlow P.W., Sanz Clemente A., Velasco P.T., Wood M., Viola K.L., Klein W.L. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J. Neurosci. 2007;27(4):796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trojanowski J.Q., Lee V.M.Y. The Alzheimer’s brain: finding out what’s broken tells us how to fix it. Rous-Whipple Award Lecture. Am. J. Pathol. 2005;167(5):1183–1188. doi: 10.1016/S0002-9440(10)61206-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman L.G., Qureshi Y.H., Yu W.H. Promoting autophagic clearance: viable therapeutic targets in Alzheimer’s disease. Neurotherapeutics. 2015;12(1):94–108. doi: 10.1007/s13311-014-0320-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benevento C.E. Mitochondrial dysfunction induced by beta-amyloid peptides. Campinas: Universidade Estadual de Campinas; 2011. [Google Scholar]
- 28.Stancu I.C., Vasconcelos B., Terwel D., Dewachter I. Models of β-amyloid induced Tau-pathology: the long and “folded” road to understand the mechanism. Mol. Neurodegener. 2014;9(1):51. doi: 10.1186/1750-1326-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J., Yin F., Liu Z., Zhang Y. A novel antagonistic role of natural compound icariin on neurotoxicity of amyloid β peptide. Indian J. Med. Res. 2015;142(2):190–195. doi: 10.4103/0971-5916.164254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Samadder A., Dey S., Sow P., Das R., Nandi S., Das J., Bhattacharjee B., Chakrovorty A., Biswas M., Guptaroy P. Phyto-chlorophyllin prevents food additive induced genotoxicity and mitochondrial dysfunction via cytochrome c mediated pathway in mice model. Comb. Chem. High Throughput Screen. 2021;24(10):1618–1627. doi: 10.2174/1386207323666201230093510. [DOI] [PubMed] [Google Scholar]
- 31.Schwab C., McGeer P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J. Alzheimers Dis. 2008;13(4):359–369. doi: 10.3233/JAD-2008-13402. [DOI] [PubMed] [Google Scholar]
- 32.Saxena M., Dubey R. Target enzyme in Alzheimer’s disease: acetylcholinesterase inhibitors. Curr. Top. Med. Chem. 2019;19(4):264–275. doi: 10.2174/1568026619666190128125912. [DOI] [PubMed] [Google Scholar]
- 33.Willis B.A., Sundell K., Lachno D.R., Ferguson-Sells L.R., Case M.G., Holdridge K., DeMattos R.B., Raskin J., Siemers E.R., Dean R.A. Central pharmacodynamic activity of solanezumab in mild Alzheimer’s disease dementia. Alzheimers Dement. (N. Y.) 2018;4(1):652–660. doi: 10.1016/j.trci.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostrowitzki S., Lasser R.A., Dorflinger E., Scheltens P., Barkhof F., Nikolcheva T., Ashford E., Retout S., Hofmann C., Delmar P., Klein G., Andjelkovic M., Dubois B., Boada M., Blennow K., Santarelli L., Fontoura P. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res. Ther. 2017;9(1):95. doi: 10.1186/s13195-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Budd-Haeberlein S., Von Hehn C., Tian Y., Chalkias S., Muralidharan K.K., Chen T., Wu S., Li J., Skordos L., Nisenbaum L. EMERGE and ENGAGE topline results: two phase 3 studies to evaluate aducanumab in patients with early Alzheimer’s disease. 2019;16(5a):e047259. [Google Scholar]
- 36.Lalli G., Schott J.M., Hardy J., De Strooper B. Aducanumab: a new phase in therapeutic development for Alzheimer’s disease? EMBO Mol. Med. 2021;13(8):e14781. doi: 10.15252/emmm.202114781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swanson C., Zhang Y., Dhadda S., Wang J., Kaplow J., Bradley H., Rabe M., Totsuka K., Lai R.Y., Gordon R., Kramer L.D. The GAP study of BAN2401 study 201 in early AD. Persistence of BAN2401-mediated amyloid reductions post-treatment: a preliminary comparison of amyloid status between the core phase of BAN2401-g000-201 and baseline of the open-label extension phase in subjects with early Alzheimer’s disease. Neurology. 2020;94(15) Suppl. [Google Scholar]
- 38.Rygiel K. Novel strategies for Alzheimer’s disease treatment: An overview of anti-amyloid beta monoclonal antibodies. Indian J. Pharmacol. 2016;48(6):629–636. doi: 10.4103/0253-7613.194867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tolar M., Abushakra S., Hey J.A., Porsteinsson A., Sabbagh M. Aducanumab, gantenerumab, BAN2401, and ALZ-801—the first wave of amyloid-targeting drugs for Alzheimer’s disease with potential for near term approval. Alzheimers Res. Ther. 2020;12(1):95. doi: 10.1186/s13195-020-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Engelhardt E., Bertolucci P., Brito-Marques P. Efficacy of rivastigmine in the cognitive performance of patients with mild to moderate Alzheimer’s disease: results of the Brazilian arm of an open multicentre study. Arq. Neuropsiquiatr. 2003;61:S54–S55. [Google Scholar]
- 41.Prasher V.P. Review of donepezil, rivastigmine, galantamine and memantine for the treatment of dementia in Alzheimer’s disease in adults with Down syndrome: implications for the intellectual disability population. Int. J. Geriatr. Psychiatry. 2004;19(6):509–515. doi: 10.1002/gps.1077. [DOI] [PubMed] [Google Scholar]
- 42.Hyde C., Peters J., Bond M., Rogers G., Hoyle M., Anderson R., Jeffreys M., Davis S., Thokala P., Moxham T. Evolution of the evidence on the effectiveness and cost-effectiveness of acetylcholinesterase inhibitors and memantine for Alzheimer’s disease: systematic review and economic model. Age Ageing. 2013;42(1):14–20. doi: 10.1093/ageing/afs165. [DOI] [PubMed] [Google Scholar]
- 43.Danysz W., Parsons C.G. The NMDA receptor antagonist memantine as a symptomatological and neuroprotective treatment for Alzheimer’s disease: preclinical evidence. Int. J. Geriatr. Psychiatry. 2003;18(S1) Suppl. 1:S23–S32. doi: 10.1002/gps.938. [DOI] [PubMed] [Google Scholar]
- 44.Kulkarni S.K., Dhir A. Withania somnifera: An Indian ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(5):1093–1105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharya S.K., Satyan K.S., Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J. Exp. Biol. 1997;35(3):236–239. [PubMed] [Google Scholar]
- 46.Tohda C., Kuboyama T., Komatsu K. Dendrite extension by methanol extract of Ashwagandha (roots of Withania somnifera) in SK-N-SH cells. Neuroreport. 2000;11(9):1981–1985. doi: 10.1097/00001756-200006260-00035. [DOI] [PubMed] [Google Scholar]
- 47.Keith D., El-Husseini A. Excitation control: balancing PSD-95 function at the synapse. Front. Mol. Neurosci. 2008;1:4. doi: 10.3389/neuro.02.004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choudhary M.I., Nawaz S.A. Zaheer-ul-Haq; Lodhi, M.A.; Ghayur, M.N.; Jalil, S.; Riaz, N.; Yousuf, S.; Malik, A.; Gilani, A.H.; Atta-ur-Rahman. Withanolides, a new class of natural cholinesterase inhibitors with calcium antagonistic properties. Biochem. Biophys. Res. Commun. 2005;334(1):276–287. doi: 10.1016/j.bbrc.2005.06.086. [DOI] [PubMed] [Google Scholar]
- 49.Grover A., Shandilya A., Agrawal V., Bisaria V.S., Sundar D. Computational evidence to inhibition of human acetyl cholinesterase by withanolide a for Alzheimer treatment. J. Biomol. Struct. Dyn. 2012;29(4):651–662. doi: 10.1080/07391102.2012.10507408. [DOI] [PubMed] [Google Scholar]
- 50.Soreq H., Seidman S. Acetylcholinesterase — new roles for an old actor. Nat. Rev. Neurosci. 2001;2(4):294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe T., Yamagata N., Takasaki K., Sano K., Hayakawa K., Katsurabayashi S., Egashira N., Mishima K., Iwasaki K., Fujiwara M. Decreased acetylcholine release is correlated to memory impairment in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res. 2009;1249:222–228. doi: 10.1016/j.brainres.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 52.Fahnestock M., Michalski B., Xu B., Coughlin M.D. The precursor pro-nerve growth factor is the predominant form of nerve growth factor in brain and is increased in Alzheimer’s disease. Mol. Cell. Neurosci. 2001;18(2):210–220. doi: 10.1006/mcne.2001.1016. [DOI] [PubMed] [Google Scholar]
- 53.Hefti F., Weiner W.J. Nerve growth factor and Alzheimer’s disease. Ann. Neurol. 1986;20(3):275–281. doi: 10.1002/ana.410200302. [DOI] [PubMed] [Google Scholar]
- 54.Kuboyama T., Tohda C., Zhao J., Nakamura N., Hattori M., Komatsu K. Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. Neuroreport. 2002;13(14):1715–1720. doi: 10.1097/00001756-200210070-00005. [DOI] [PubMed] [Google Scholar]
- 55.Kuboyama T., Tohda C., Komatsu K. Withanoside IV and its active metabolite, sominone, attenuate Aβ(25-35)-induced neurodegeneration. Eur. J. Neurosci. 2006;23(6):1417–1426. doi: 10.1111/j.1460-9568.2006.04664.x. [DOI] [PubMed] [Google Scholar]
- 56.Tohda C., Joyashiki E. Sominone enhances neurite outgrowth and spatial memory mediated by the neurotrophic factor receptor, RET. Br. J. Pharmacol. 2009;157(8):1427–1440. doi: 10.1111/j.1476-5381.2009.00313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao J., Nakamura N., Hattori M., Kuboyama T., Tohda C., Komatsu K. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem. Pharm. Bull. (Tokyo) 2002;50(6):760–765. doi: 10.1248/cpb.50.760. [DOI] [PubMed] [Google Scholar]
- 58.Gautam A., Wadhwa R., Thakur M.K. Involvement of hippocampal Arc in amnesia and its recovery by alcoholic extract of Ashwagandha leaves. Neurobiol. Learn. Mem. 2013;106:177–184. doi: 10.1016/j.nlm.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 59.Guzowski J.F., Lyford G.L., Stevenson G.D., Houston F.P., McGaugh J.L., Worley P.F., Barnes C.A. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20(11):3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rudinskiy N., Hawkes J.M., Betensky R.A., Eguchi M., Yamaguchi S., Spires-Jones T.L., Hyman B.T. Orchestrated experience-driven Arc responses are disrupted in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2012;15(10):1422–1429. doi: 10.1038/nn.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sehgal N., Gupta A., Valli R.K., Joshi S.D., Mills J.T., Hamel E., Khanna P., Jain S.C., Thakur S.S., Ravindranath V. Withania somnifera reverses Alzheimer’s disease pathology by enhancing low-density lipoprotein receptor-related protein in liver. Proc. Natl. Acad. Sci. USA. 2012;109(9):3510–3515. doi: 10.1073/pnas.1112209109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanzi R.E., Moir R.D., Wagner S.L. Clearance of Alzheimer’s abeta peptide: The many roads to perdition. Neuron. 2004;43(5):605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 63.Tamaki C., Ohtsuki S., Iwatsubo T., Hashimoto T., Yamada K., Yabuki C., Terasaki T. Major involvement of low-density lipoprotein receptor-related protein 1 in the clearance of plasma free amyloid β-peptide by the liver. Pharm. Res. 2006;23(7):1407–1416. doi: 10.1007/s11095-006-0208-7. [DOI] [PubMed] [Google Scholar]
- 64.Li Y., Sun H., Chen Z., Xu H., Bu G., Zheng H. Implications of GABAergic neurotransmission in Alzheimer’s disease. Front. Aging Neurosci. 2016;8:31. doi: 10.3389/fnagi.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Candelario M., Cuellar E., Reyes-Ruiz J.M., Darabedian N., Feimeng Z., Miledi R., Russo-Neustadt A., Limon A. Direct evidence for GABAergic activity of Withania somnifera on mammalian ionotropic GABAA and GABAρ receptors. J. Ethnopharmacol. 2015;171:264–272. doi: 10.1016/j.jep.2015.05.058. [DOI] [PubMed] [Google Scholar]
- 66.Patil S.P., Maki S., Khedkar S.A., Rigby A.C., Chan C. Withanolide A and asiatic acid modulate multiple targets associated with amyloid-beta precursor protein processing and amyloid-beta protein clearance. J. Nat. Prod. 2010;73(7):1196–1202. doi: 10.1021/np900633j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kumar G., Paliwal P., Patnaik R. Withania somnifera phytochemicals confer neuroprotection by inhibition of the catalytic domain of human matrix metalloproteinase-9. Lett. Drug Des. Discov. 2017;14(6):718–726. doi: 10.2174/1570180814666161121111811. [DOI] [Google Scholar]
- 68.Lorenzl S., Albers D.S., Relkin N., Ngyuen T., Hilgenberg S.L., Chirichigno J., Cudkowicz M.E., Beal M.F. Increased plasma levels of matrix metalloproteinase-9 in patients with Alzheimer’s disease. Neurochem. Int. 2003;43(3):191–196. doi: 10.1016/S0197-0186(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 69.Leake A., Morris C.M., Whateley J. Brain matrix metalloproteinase 1 levels are elevated in Alzheimer’s disease. Neurosci. Lett. 2000;291(3):201–203. doi: 10.1016/S0304-3940(00)01418-X. [DOI] [PubMed] [Google Scholar]
- 70.Yoshiyama Y., Asahina M., Hattori T. Selective distribution of matrix metalloproteinase-3 (MMP-3) in Alzheimer’s disease brain. Acta Neuropathol. 2000;99(2):91–95. doi: 10.1007/PL00007428. [DOI] [PubMed] [Google Scholar]
- 71.Jayaprakasam B., Padmanabhan K., Nair M.G. Withanamides in Withania somnifera fruit protect PC-12 cells from β-amyloid responsible for Alzheimer’s disease. Phytother. Res. 2010;24(6):859–863. doi: 10.1002/ptr.3033. [DOI] [PubMed] [Google Scholar]
- 72.Ng Q.X., Loke W., Foo N.X., Tan W.J., Chan H.W., Lim D.Y., Yeo W.S. A systematic review of the clinical use of Withania somnifera (Ashwagandha) to ameliorate cognitive dysfunction. Phytother. Res. 2020;34(3):583–590. doi: 10.1002/ptr.6552. [DOI] [PubMed] [Google Scholar]
- 73.Sasikumar B. Genetic resources of Curcuma: diversity, characterization and utilization. Plant Genet. Resour. 2005;3(2):230–251. doi: 10.1079/PGR200574. [DOI] [Google Scholar]
- 74.Hamaguchi T., Ono K., Yamada M. REVIEW: Curcumin and Alzheimer’s disease. CNS Neurosci. Ther. 2010;16(5):285–297. doi: 10.1111/j.1755-5949.2010.00147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dutta B. Study of secondary metabolite constituents and curcumin contents of six different species of genus Curcuma. J. Med. Plants Res. 2015;3(5):116–119. [Google Scholar]
- 76.Roy S., Raychaudhuri S.S. In vitro regeneration and estimation of curcumin content in four species of Curcuma. Plant Biotechnol. (Tsukuba) 2004;21(4):299–302. doi: 10.5511/plantbiotechnology.21.299. [DOI] [Google Scholar]
- 77.Ono K., Hasegawa K., Naiki H., Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s? -amyloid fibrils in vitro. J. Neurosci. Res. 2004;75(6):742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 78.Lin R., Chen X., Li W., Han Y., Liu P., Pi R. Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: Blockage by curcumin. Neurosci. Lett. 2008;440(3):344–347. doi: 10.1016/j.neulet.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 79.Giri R.K., Rajagopal V., Kalra V.K. Curcumin, the active constituent of turmeric, inhibits amyloid peptide-induced cytochemokine gene expression and CCR5-mediated chemotaxis of THP-1 monocytes by modulating early growth response-1 transcription factor. J. Neurochem. 2004;91(5):1199–1210. doi: 10.1111/j.1471-4159.2004.02800.x. [DOI] [PubMed] [Google Scholar]
- 80.Yang C., Su X., Liu A., Zhang L., Yu A., Xi Y., Zhai G. Advances in clinical study of curcumin. Curr. Pharm. Des. 2013;19(11):1966–1973. [PubMed] [Google Scholar]
- 81.Cashman J.R., Ghirmai S., Abel K.J., Fiala M. Immune defects in Alzheimer’s disease: new medications development. BMC Neurosci. 2008;9(S2) Suppl. 2:S13. doi: 10.1186/1471-2202-9-S2-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fiala M., Liu P.T., Espinosa-Jeffrey A., Rosenthal M.J., Bernard G., Ringman J.M., Sayre J., Zhang L., Zaghi J., Dejbakhsh S., Chiang B., Hui J., Mahanian M., Baghaee A., Hong P., Cashman J. Innate immunity and transcription of MGAT-III and Toll-like receptors in Alzheimer’s disease patients are improved by bisdemethoxycurcumin. Proc. Natl. Acad. Sci. USA. 2007;104(31):12849–12854. doi: 10.1073/pnas.0701267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Huber K., Superti-Furga G. After the grape rush: Sirtuins as epigenetic drug targets in neurodegenerative disorders. Bioorg. Med. Chem. 2011;19(12):3616–3624. doi: 10.1016/j.bmc.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 84.Zhang C., Ji J., Ji M., Fan P. Acetylcholinesterase inhibitors and compounds promoting SIRT1 expression from Curcuma xanthorrhiza. Phytochem. Lett. 2015;12:215–219. doi: 10.1016/j.phytol.2015.04.007. [DOI] [Google Scholar]
- 85.Czernicka L., Ludwiczuk A., Rój E., Marzec Z., Jarzab A., Kukula-Koch W. Acetylcholinesterase inhibitors among Zingiber officinale terpenes—extraction conditions and thin layer chromatography-based bioautography studies. Molecules. 2020;25(7):1643. doi: 10.3390/molecules25071643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee T.K., Trinh T.A., Lee S.R., Kim S., So H.M., Moon E., Hwang G.S., Kang K.S., Kim J.H., Yamabe N., Kim K.H. Bioactivity-based analysis and chemical characterization of anti-inflammatory compounds from Curcuma zedoaria rhizomes using LPS-stimulated RAW264.7 cells. Bioorg. Chem. 2019;82:26–32. doi: 10.1016/j.bioorg.2018.09.027. [DOI] [PubMed] [Google Scholar]
- 87.Mishra S., Palanivelu K. The effect of curcumin (turmeric) on Alzheimer′s disease: An overview. Ann. Indian Acad. Neurol. 2008;11(1):13–19. doi: 10.4103/0972-2327.40220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Butterfield D.A., Castegna A., Pocernich C.B., Drake J., Scapagnini G., Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002;13(8):444–461. doi: 10.1016/S0955-2863(02)00205-X. [DOI] [PubMed] [Google Scholar]
- 89.Farkhondeh T., Samarghandian S., Pourbagher-Shahri A.M., Sedaghat M. The impact of curcumin and its modified formulations on Alzheimer’s disease. J. Cell. Physiol. 2019;234(10):16953–16965. doi: 10.1002/jcp.28411. [DOI] [PubMed] [Google Scholar]
- 90.Hamdi O.A.A., Ye L.J., Kamarudin M.N.A., Hazni H., Paydar M., Looi C.Y., Awang K. Neuroprotective and Antioxidant Constituents from Curcuma zedoaria Rhizomes. Rec. Nat. Prod. 2015;9(3):349–355. [Google Scholar]
- 91.Alonso-Amelot M.E. Multitargeted bioactive materials of plants in the Curcuma genus and related compounds: recent advances. Studies Nat. Prod. Chem. 2016;47:111–200. doi: 10.1016/B978-0-444-63603-4.00004-8. [DOI] [Google Scholar]
- 92.Schramm A., Ebrahimi S.N., Raith M., Zaugg J., Rueda D.C., Hering S., Hamburger M. Phytochemical profiling of Curcuma kwangsiensis rhizome extract, and identification of labdane diterpenoids as positive GABAA receptor modulators. Phytochemistry. 2013;96:318–329. doi: 10.1016/j.phytochem.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 93.Nurrulhidayah A.F., Rafi M., Lukitaningsih E., Widodo H., Rohman A., Windarsih A. Review on in vitro antioxidant activities of Curcuma species commonly used as herbal components in Indonesia. Food Res. 2020;4(2):286–293. [Google Scholar]
- 94.Kramer K.U., Green P.S., Kubitzki K. The families and genera of vascular plants. V. 1: Pteridophytes and gymnosperms. Wiley: New Jersey; 1990. [Google Scholar]
- 95.Luo Y., Smith J.V. Studies on molecular mechanisms of Ginkgo biloba extract. Appl. Microbiol. Biotechnol. 2004;64(4):465–472. doi: 10.1007/s00253-003-1527-9. [DOI] [PubMed] [Google Scholar]
- 96.Diamond B.J., Shiflett S.C., Feiwel N., Matheis R.J., Noskin O., Richards J.A., Schoenberger N.E. Ginkgo biloba extract: Mechanisms and clinical indications. Arch. Phys. Med. Rehabil. 2000;81(5):668–678. doi: 10.1016/S0003-9993(00)90052-2. [DOI] [PubMed] [Google Scholar]
- 97.Maclennan K., Darlington C.L., Smith P.F. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog. Neurobiol. 2002;67(3):235–257. doi: 10.1016/S0301-0082(02)00015-1. [DOI] [PubMed] [Google Scholar]
- 98.Singh B., Kaur P. Gopichand; Singh, R.D.; Ahuja, P.S. Biology and chemistry of Ginkgo biloba. Fitoterapia. 2008;79(6):401–418. doi: 10.1016/j.fitote.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 99.Stafford H.A., Kreitlow K.S., Lester H.H. Comparison of proanthocyanidins and related compounds in leaves and leaf-derived cell cultures of Ginkgo biloba L., Pseudotsuga menziesii Franco, and Ribes sanguineum Pursh. Plant Physiol. 1986;82(4):1132–1138. doi: 10.1104/pp.82.4.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kulić Ž.; Germer, S.; Ritter, T.; Röck, B.; Elsäßer, J.; Schneider, H. A Detailed View on the Proanthocyanidins in Ginkgo Extract EGb 761. Planta Med. 2022;88(5):398–404. doi: 10.1055/a-1379-4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nishida S., Satoh H. Comparative vasodilating actions among terpenoids and flavonoids contained in Ginkgo biloba extract. Clin. Chim. Acta. 2004;339(1-2):129–133. doi: 10.1016/j.cccn.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Samadder A., Abraham S.K., Khuda-Bukhsh A.R. Nanopharmaceutical approach using pelargonidin towards enhancement of efficacy for prevention of alloxan-induced DNA damage in L6 cells via activation of PARP and p53. Environ. Toxicol. Pharmacol. 2016;43:27–37. doi: 10.1016/j.etap.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 103.Samadder A., Tarafdar D., Abraham S., Ghosh K., Khuda-Bukhsh A. Nano-pelargonidin protects hyperglycemic-induced L6 cells against mitochondrial dysfunction. Planta Med. 2017;83(5):468–475. doi: 10.1055/s-0043-100017. [DOI] [PubMed] [Google Scholar]
- 104.Le Bars P.L., Katz M.M., Berman N., Itil T.M., Freedman A.M., Schatzberg A.F. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278(16):1327–1332. doi: 10.1001/jama.1997.03550160047037. [DOI] [PubMed] [Google Scholar]
- 105.Le Bars P.L., Kieser M., Itil K.Z. A 26-week analysis of a double-blind, placebo-controlled trial of the ginkgo biloba extract EGb 761 in dementia. Dement. Geriatr. Cogn. Disord. 2000;11(4):230–237. doi: 10.1159/000017242. [DOI] [PubMed] [Google Scholar]
- 106.Kanowski S., Herrmann W., Stephan K., Wierich W., Hörr R. Proof of efficacy of the Ginkgo biloba special extract EGb 761 in outpatients suffering from mild to moderate primary degenerative dementia of the Alzheimer type or multi-infarct dementia. Pharmacopsychiatry. 1996;29(2):47–56. doi: 10.1055/s-2007-979544. [DOI] [PubMed] [Google Scholar]
- 107.Huguet F., Drieu K., Piriou A. Decreased cerebral 5-HT1A receptors during ageing: reversal by Ginkgo biloba extract (EGb 761). J. Pharm. Pharmacol. 2011;46(4):316–318. doi: 10.1111/j.2042-7158.1994.tb03802.x. [DOI] [PubMed] [Google Scholar]
- 108.Das S., Das J., Paul A., Samadder A., Khuda-Bukhsh A.R. Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet B-induced reactive oxygen species and DNA damage. J. Acupunct. Meridian Stud. 2013;6(5):252–262. doi: 10.1016/j.jams.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 109.Chandrasekaran K., Mehrabian Z., Spinnewyn B., Chinopoulos C., Drieu K., Fiskum G. Bilobalide, a component of the Ginkgo biloba extract (EGb 761), protects against neuronal death in global brain ischemia and in glutamate-induced excitotoxicity. Cell. Mol. Biol. 2002;48(6):663–669. [PubMed] [Google Scholar]
- 110.Packer L., Christen Y. Ginkgo biloba extract (EGb 761): Lessons from Cell Biology. New York: Elsevier; 1998. p. 7. [Google Scholar]
- 111.Tendi E.A., Bosetti F., DasGupta S.F., Giuffrida Stella A.M., Drieu K., Rapoport S.I. Ginkgo biloba extracts EGb 761 and bilobalide increase NADH dehydrogenase mRNA level and mitochondrial respiratory control ratio in PC12 cells. Neurochem. Res. 2002;27(4):319–323. doi: 10.1023/A:1014963313559. [DOI] [PubMed] [Google Scholar]
- 112.DeFeudis F., Drieu K. Ginkgo biloba extract (EGb 761) and CNS functions: basic studies and clinical applications. Curr. Drug Targets. 2000;1(1):25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- 113.Defeudis F. Bilobalide and neuroprotection. Pharmacol. Res. 2002;46(6):565–568. doi: 10.1016/S1043-6618(02)00233-5. [DOI] [PubMed] [Google Scholar]
- 114.Schaeffer E.L., Gattaz W.F. Cholinergic and glutamatergic alterations beginning at the early stages of Alzheimer disease: participation of the phospholipase A2 enzyme. Psychopharmacology (Berl.) 2008;198(1):1–27. doi: 10.1007/s00213-008-1092-0. [DOI] [PubMed] [Google Scholar]
- 115.Weichel O., Hilgert M., Chatterjee S.S., Lehr M., Klein J. Bilobalide, a constituent of Ginkgo biloba, inhibits NMDA-induced phospholipase A 2 activation and phospholipid breakdown in rat hippocampus. Naunyn Schmiedebergs Arch. Pharmacol. 1999;360(6):609–615. doi: 10.1007/s002109900131. [DOI] [PubMed] [Google Scholar]
- 116.Kim H.S., Sul D., Lim J.Y., Lee D., Joo S.S., Hwang K.W., Park S.Y. Delphinidin ameliorates beta-amyloid-induced neurotoxicity by inhibiting calcium influx and tau hyperphosphorylation. Biosci. Biotechnol. Biochem. 2009;73(7):1685–1689. doi: 10.1271/bbb.90032. [DOI] [PubMed] [Google Scholar]
- 117.Khojah H., Edrada-Ebel R. Identification of bioactive metabolites from Ficus carica and their neuroprotective effects of Alzheimer’s disease. Int. J. Med. Health Sci. 2017;11:2277–4505. [Google Scholar]
- 118.Ahmad S., Bhatti F.R., Khaliq F.H., Younas T., Madni A., Latif A. In vitro enzymatic investigation of Ficus carica (Fruit). Pak. J. Pharm. Sci. 2016;29(5):1541–1544. [PubMed] [Google Scholar]
- 119.Bandaruk Y., Mukai R., Kawamura T., Nemoto H., Terao J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012;60(41):10270–10277. doi: 10.1021/jf303055b. [DOI] [PubMed] [Google Scholar]
- 120.Davis A.P., Govaerts R., Bridson D.M., Stoffelen P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006;152(4):465–512. doi: 10.1111/j.1095-8339.2006.00584.x. [DOI] [Google Scholar]
- 121.do Carmo Carvalho D., Brigagão M.R.P.L., dos Santos M.H., de Paula F.B.A., Giusti-Paiva A., Azevedo L. Organic and conventional Coffea arabica L.: a comparative study of the chemical composition and physiological, biochemical and toxicological effects in Wistar rats. Plant Foods Hum. Nutr. 2011;66(2):114–121. doi: 10.1007/s11130-011-0221-9. [DOI] [PubMed] [Google Scholar]
- 122.Mejia E.G., Ramirez-Mares M.V. Impact of caffeine and coffee on our health. Trends Endocrinol. Metab. 2014;25(10):489–492. doi: 10.1016/j.tem.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 123.Patay É.B., Bencsik T., Papp N. Phytochemical overview and medicinal importance of Coffea species from the past until now. Asian Pac. J. Trop. Med. 2016;9(12):1127–1135. doi: 10.1016/j.apjtm.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 124.Farah A., Donangelo C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006;18(1):23–36. doi: 10.1590/S1677-04202006000100003. [DOI] [Google Scholar]
- 125.Camilleri P. Haskins, N.J.; Hewlett, D.R. β-Cyclodextrin interacts with the Alzheimer amyloid β-A4 peptide. FEBS Lett. 1994;341(2-3):256–258. doi: 10.1016/0014-5793(94)80467-2. [DOI] [PubMed] [Google Scholar]
- 126.Hossain S.J., Aoshima H., Koda H., Kiso Y. Effects of coffee components on the response of GABA(A) receptors expressed in Xenopus oocytes. J. Agric. Food Chem. 2003;51(26):7568–7575. doi: 10.1021/jf0303971. [DOI] [PubMed] [Google Scholar]
- 127.Franco R., Oñatibia-Astibia A., Martínez-Pinilla E. Health benefits of methylxanthines in cacao and chocolate. Nutrients. 2013;5(10):4159–4173. doi: 10.3390/nu5104159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Francis S.H., Sekhar K.R., Ke H., Corbin J.D. Inhibition of cyclic nucleotide phosphodiesterases by methylxanthines and related compounds. Handbook of Experimental Pharmacology: Methylxanthines. 2011;200:93-133. doi: 10.1007/978-3-642-13443-2_4. [DOI] [PubMed] [Google Scholar]
- 129.Aronsen L., Orvoll E., Lysaa R., Ravna A.W., Sager G. Modulation of high affinity ATP-dependent cyclic nucleotide transporters by specific and non-specific cyclic nucleotide phosphodiesterase inhibitors. Eur. J. Pharmacol. 2014;745:249–253. doi: 10.1016/j.ejphar.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 130.Choi O.H., Shamim M.T., Padgett W.L., Daly J.W. Caffeine and theophylline analogues: Correlation of behavioral effects with activity as adenosine receptor antagonists and as phosphodiesterase inhibitors. Life Sci. 1988;43(5):387–398. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- 131.Heckman P.R.A., Wouters C., Prickaerts J. Phosphodiesterase inhibitors as a target for cognition enhancement in aging and Alzheimer’s disease: a translational overview. Curr. Pharm. Des. 2014;21(3):317–331. doi: 10.2174/1381612820666140826114601. [DOI] [PubMed] [Google Scholar]
- 132.Madeswaran A., Umamaheswari M., Asokkumar K., Sivashanmugam T., Subhadradevi V., Jagannath P. In silico docking studies of phosphodiesterase inhibitory activity of commercially available flavonoids. Orient. Pharm. Exp. Med. 2012;12(4):301–306. doi: 10.1007/s13596-012-0071-5. [DOI] [Google Scholar]
- 133.Love S., Barber R., Wilcock G.K. Increased poly(ADP-ribosyl)ation of nuclear proteins in Alzheimer’s disease. Brain. 1999;122(2):247–253. doi: 10.1093/brain/122.2.247. [DOI] [PubMed] [Google Scholar]
- 134.Pacher P., Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am. J. Pathol. 2008;173(1):2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Banasik M., Stedeford T., Strosznajder R.P. Natural inhibitors of poly(ADP-ribose) polymerase-1. Mol. Neurobiol. 2012;46(1):55–63. doi: 10.1007/s12035-012-8257-x. [DOI] [PubMed] [Google Scholar]
- 136.Rankin P.W., Jacobson E.L., Benjamin R.C., Moss J., Jacobson M.K. Quantitative studies of inhibitors of ADP-ribosylation in vitro and in vivo. J. Biol. Chem. 1989;264(8):4312–4317. doi: 10.1016/S0021-9258(18)83741-3. [DOI] [PubMed] [Google Scholar]
- 137.Uneyama H., Harata N., Akaike N. Caffeine and related compounds block inhibitory amino acid-gated Cl− currents in freshly dissociated rat hippocampal neurones. Br. J. Pharmacol. 1993;109(2):459–465. doi: 10.1111/j.1476-5381.1993.tb13591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hattori N. Kitagawa, K.; Higashida, T.; Yagyu, K.; Shimohama, S.; Wataya, T.; Perry, G.; Smith, M.A.; Inagaki, C. Cl−-ATPase and Na+/K+-ATPase activities in Alzheimer’s disease brains. Neurosci. Lett. 1998;254(3):141–144. doi: 10.1016/S0304-3940(98)00654-5. [DOI] [PubMed] [Google Scholar]
- 139.Rogus E.M. Cheng, L.C.; Zierler, K. β-adrenergic effect on Na+-K+ transport in rat skeletal muscle. Biochim. Biophys. Acta Biomembr. 1977;464(2):347–355. doi: 10.1016/0005-2736(77)90009-8. [DOI] [PubMed] [Google Scholar]
- 140.Bianchi C.P. The effect of caffeine on radiocalcium movement in frog sartorius. J. Gen. Physiol. 1961;44(5):845–858. doi: 10.1085/jgp.44.5.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Daly J.W. Caffeine analogs: biomedical impact. Cell. Mol. Life Sci. 2007;64(16):2153–2169. doi: 10.1007/s00018-007-7051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Delbono O., Kotsias B.A. Hyperpolarizing effect of aminophylline, theophylline, and cAMP on rat diaphragm fibers. J. Appl. Physiol. 1988;64(5):1893–1899. doi: 10.1152/jappl.1988.64.5.1893. [DOI] [PubMed] [Google Scholar]
- 143.Ali Y.O., Bradley G., Lu H.C. Erratum: Corrigendum: Screening with an NMNAT2-MSD platform identifies small molecules that modulate NMNAT2 levels in cortical neurons. Sci. Rep. 2017;7(1):46780. doi: 10.1038/srep46780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Han J., Miyamae Y., Shigemori H., Isoda H. Neuroprotective effect of 3,5-di-O-caffeoylquinic acid on SH-SY5Y cells and senescence-accelerated-prone mice 8 through the up-regulation of phosphoglycerate kinase-1. Neuroscience. 2010;169(3):1039–1045. doi: 10.1016/j.neuroscience.2010.05.049. [DOI] [PubMed] [Google Scholar]
- 145.Perluigi M., Joshi G., Sultana R., Calabrese V., De Marco C., Coccia R., Cini C., Butterfield D.A. In vivo protective effects of ferulic acid ethyl ester against amyloid-beta peptide 1-42-induced oxidative stress. J. Neurosci. Res. 2006;84(2):418–426. doi: 10.1002/jnr.20879. [DOI] [PubMed] [Google Scholar]
- 146.Zhang X., He X., Chen Q., Lu J., Rapposelli S., Pi R. A review on the hybrids of hydroxycinnamic acid as multi-target-directed ligands against Alzheimer’s disease. Bioorg. Med. Chem. 2018;26(3):543–550. doi: 10.1016/j.bmc.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 147.Lee K.J., Jeong H.G. Protective effects of kahweol and cafestol against hydrogen peroxide-induced oxidative stress and DNA damage. Toxicol. Lett. 2007;173(2):80–87. doi: 10.1016/j.toxlet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 148.Hwang Y.P., Jeong H.G. The coffee diterpene kahweol induces heme oxygenase-1 via the PI3K and p38/Nrf2 pathway to protect human dopaminergic neurons from 6-hydroxydopamine-derived oxidative stress. FEBS Lett. 2008;582(17):2655–2662. doi: 10.1016/j.febslet.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 149.Trinh K., Andrews L., Krause J., Hanak T., Lee D., Gelb M., Pallanck L. Decaffeinated coffee and nicotine-free tobacco provide neuroprotection in Drosophila models of Parkinson’s disease through an NRF2-dependent mechanism. J. Neurosci. 2010;30(16):5525–5532. doi: 10.1523/JNEUROSCI.4777-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vatassery G.T. Vitamin E and other endogenous antioxidants in the central nervous system. Geriatrics. 1998;53(Suppl. 1):S25–S27. [PubMed] [Google Scholar]
- 151.Cooney R.V., Franke A.A., Harwood P.J., Hatch-Pigott V., Custer L.J., Mordan L.J. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc. Natl. Acad. Sci. USA. 1993;90(5):1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Morris M.C., Schneider J.A., Li H., Tangney C.C., Nag S., Bennett D.A., Honer W.G., Barnes L.L. Brain tocopherols related to Alzheimer’s disease neuropathology in humans. Alzheimers Dement. 2015;11(1):32–39. doi: 10.1016/j.jalz.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Berman K., Brodaty H. Tocopherol (vitamin E) in Alzheimer’s disease and other neurodegenerative disorders. CNS Drugs. 2004;18(12):807–825. doi: 10.2165/00023210-200418120-00005. [DOI] [PubMed] [Google Scholar]
- 154.Browne D., McGuinness B., Woodside J.V., McKay G.J. Vitamin E and Alzheimer’s disease: what do we know so far? Clin. Interv. Aging. 2019;14:1303–1317. doi: 10.2147/CIA.S186760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vijayan K., Zhang W.J., Tsou C.H. Molecular taxonomy of Camellia (Theaceae) inferred from nrITS sequences. Am. J. Bot. 2009;96(7):1348–1360. doi: 10.3732/ajb.0800205. [DOI] [PubMed] [Google Scholar]
- 156.Khan N., Mukhtar H. Tea polyphenols for health promotion. Life Sci. 2007;81(7):519–533. doi: 10.1016/j.lfs.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fernando W.M.A.D.B., Somaratne G., Goozee K.G., Williams S., Singh H., Martins R.N. Diabetes and Alzheimer’s disease: can tea phytochemicals play a role in prevention? J. Alzheimers Dis. 2017;59(2):481–501. doi: 10.3233/JAD-161200. [DOI] [PubMed] [Google Scholar]
- 158.Graham H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992;21(3):334–350. doi: 10.1016/0091-7435(92)90041-F. [DOI] [PubMed] [Google Scholar]
- 159.Stagg G.V., Swaine D. The identification of theogallin as 3-galloylquinic acid. Phytochemistry. 1971;10(7):1671–1673. doi: 10.1016/0031-9422(71)85047-1. [DOI] [Google Scholar]
- 160.Ho C.T., Zheng X., Li S. Tea aroma formation. Food Sci. Hum. Wellness. 2015;4(1):9–27. doi: 10.1016/j.fshw.2015.04.001. [DOI] [Google Scholar]
- 161.Rho T., Choi M.S., Jung M., Kil H.W., Hong Y.D., Yoon K.D. Identification of fermented tea (Camellia sinensis) polyphenols and their inhibitory activities against amyloid-beta aggregation. Phytochemistry. 2019;160:11–18. doi: 10.1016/j.phytochem.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 162.McDowell I., Taylor S., Gay C. The phenolic pigment composition of black tea liquors—part I: Predicting quality. J. Sci. Food Agric. 1995;69(4):467–474. doi: 10.1002/jsfa.2740690411. [DOI] [Google Scholar]
- 163.Kim Y., Goodner K.L., Park J.D., Choi J., Talcott S.T. Changes in antioxidant phytochemicals and volatile composition of Camellia sinensis by oxidation during tea fermentation. Food Chem. 2011;129(4):1331–1342. doi: 10.1016/j.foodchem.2011.05.012. [DOI] [Google Scholar]
- 164.Truong V.L., Jeong W.S. Cellular defensive mechanisms of tea polyphenols: Structure-activity relationship. Int. J. Mol. Sci. 2021;22(17):9109. doi: 10.3390/ijms22179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li C., Lee M.J., Sheng S., Meng X., Prabhu S., Winnik B., Huang B., Chung J.Y., Yan S., Ho C.T., Yang C.S. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chem. Res. Toxicol. 2000;13(3):177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 166.Fatima M., Rizvi S.I. Antioxidative effect of black tea theaflavin on erythrocytes subjected to oxidative stress. Natl. Acad. Sci. Lett. 2015;38(1):25–28. doi: 10.1007/s40009-014-0285-9. [DOI] [Google Scholar]
- 167.Geiser R.J., Chastain S.E., Moss M.A. Regulation of bace1 mRNA expression in Alzheimer’s disease by green tea catechins and black tea theaflavins. Biophys. J. 2017;112(3):362a. doi: 10.1016/j.bpj.2016.11.1965. [DOI] [Google Scholar]
- 168.Hossain S.J., Hamamoto K., Aoshima H., Hara Y. Effects of tea components on the response of GABA(A) receptors expressed in Xenopus Oocytes. J. Agric. Food Chem. 2002;50(14):3954–3960. doi: 10.1021/jf011607h. [DOI] [PubMed] [Google Scholar]
- 169.Silverman G.A., Bird P.I., Carrell R.W., Church F.C., Coughlin P.B., Gettins P.G.W., Irving J.A., Lomas D.A., Luke C.J., Moyer R.W., Pemberton P.A., Remold-O’Donnell E., Salvesen G.S., Travis J., Whisstock J.C. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J. Biol. Chem. 2001;276(36):33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 170.Higgins P.J. The TGF-beta1/upstream stimulatory factor-regulated PAI-1 gene: potential involvement and a therapeutic target in Alzheimer’s disease. J. Biomed. Biotechnol. 2006;2006(3):15792. doi: 10.1155/JBB/2006/15792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jankun J. Skotnicka, M.; Łysiak-Szydłowska, W.; Al-Senaidy, A.; Skrzypczak-Jankun, E. Diverse inhibition of plasminogen activator inhibitor type 1 by theaflavins of black tea. Int. J. Mol. Med. 2011;27(4):525–529. doi: 10.3892/ijmm.2011.615. [DOI] [PubMed] [Google Scholar]
- 172.Mathiyazahan D.B., Justin Thenmozhi A., Manivasagam T. Protective effect of black tea extract against aluminium chloride-induced Alzheimer’s disease in rats: A behavioural, biochemical and molecular approach. J. Funct. Foods. 2015;16:423–435. doi: 10.1016/j.jff.2015.05.001. [DOI] [Google Scholar]
- 173.Kim T.I., Lee Y.K., Park S.G., Choi I.S., Ban J.O., Park H.K., Nam S.Y., Yun Y.W., Han S.B., Oh K.W., Hong J.T. l-Theanine, an amino acid in green tea, attenuates β-amyloid-induced cognitive dysfunction and neurotoxicity: Reduction in oxidative damage and inactivation of ERK/p38 kinase and NF-κB pathways. Free Radic. Biol. Med. 2009;47(11):1601–1610. doi: 10.1016/j.freeradbiomed.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 174.Ide K., Yamada H., Takuma N., Park M., Wakamiya N., Nakase J., Ukawa Y., Sagesaka Y. Green tea consumption affects cognitive dysfunction in the elderly: a pilot study. Nutrients. 2014;6(10):4032–4042. doi: 10.3390/nu6104032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shanmugam T., Selvaraj M., Poomalai S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: An in-vivo and in-silico study. Int. Immunopharmacol. 2016;39:128–139. doi: 10.1016/j.intimp.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 176.Semwal D., Semwal R., Combrinck S., Viljoen A. Myricetin: A dietary molecule with diverse biological activities. Nutrients. 2016;8(2):90. doi: 10.3390/nu8020090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Q.M., Wang G.L., Ma Z.G. Protective effects of myricetin on chronic stress-induced cognitive deficits. Neuroreport. 2016;27(9):652–658. doi: 10.1097/WNR.0000000000000591. [DOI] [PubMed] [Google Scholar]
- 178.Wang B., Zhong Y., Gao C., Li J. Myricetin ameliorates scopolamine-induced memory impairment in mice via inhibiting acetylcholinesterase and down-regulating brain iron. Biochem. Biophys. Res. Commun. 2017;490(2):336–342. doi: 10.1016/j.bbrc.2017.06.045. [DOI] [PubMed] [Google Scholar]
- 179.Dini A., Migliuolo G., Rastrelli L., Saturnino P., Schettino O. Chemical composition of Lepidium meyenii. Food Chem. 1994;49(4):347–349. doi: 10.1016/0308-8146(94)90003-5. [DOI] [Google Scholar]
- 180.Campos D., Chirinos R., Barreto O., Noratto G., Pedreschi R. Optimized methodology for the simultaneous extraction of glucosinolates, phenolic compounds and antioxidant capacity from maca (Lepidium meyenii). Ind. Crops Prod. 2013;49:747–754. doi: 10.1016/j.indcrop.2013.06.021. [DOI] [Google Scholar]
- 181.Fahey J.W., Zalcmann A.T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56(1):5–51. doi: 10.1016/S0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 182.Pino-Figueroa A., Nguyen D., Maher T.J. Neuroprotective effects of Lepidium meyenii (Maca). Ann. N. Y. Acad. Sci. 2010;1199(1):77–85. doi: 10.1111/j.1749-6632.2009.05174.x. [DOI] [PubMed] [Google Scholar]
- 183.Narbad A., Rossiter J.T. Gut glucosinolate metabolism and isothiocyanate production. Mol. Nutr. Food Res. 2018;62(18):1700991. doi: 10.1002/mnfr.201700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Burčul, F.; Generalić Mekinić I.; Radan, M.; Rollin, P.; Blažević I. Isothiocyanates: cholinesterase inhibiting, antioxidant, and anti-inflammatory activity. J. Enzyme Inhib. Med. Chem. 2018;33(1):577–582. doi: 10.1080/14756366.2018.1442832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Peng X.R., Zhang R.R., Liu J.H., Li Z.R., Zhou L., Qiu M.H. Lepithiohydimerins A—D: Four Pairs of neuroprotective thiohydantoin dimers bearing a disulfide bond from maca (Lepidium meyenii Walp.). Chin. J. Chem. 2021;39(10):2738–2744. doi: 10.1002/cjoc.202100353. [DOI] [Google Scholar]
- 186.Gonzales-Arimborgo C., Yupanqui I., Montero E., Alarcón-Yaquetto D., Zevallos-Concha A., Caballero L., Gasco M., Zhao J., Khan I., Gonzales G. Acceptability, safety, and efficacy of oral administration of extracts of black or red maca (Lepidium meyenii) in adult human subjects: A randomized, double-blind, placebo-controlled study. Pharmaceuticals (Basel) 2016;9(3):49. doi: 10.3390/ph9030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Contestabile A., Ciani E., Contestabile A. The place of choline acetyltransferase activity measurement in the “cholinergic hypothesis” of neurodegenerative diseases. Neurochem. Res. 2008;33(2):318–327. doi: 10.1007/s11064-007-9497-4. [DOI] [PubMed] [Google Scholar]
- 188.Wiedeman A., Barr S., Green T., Xu Z., Innis S., Kitts D. Dietary choline intake: current state of knowledge across the life cycle. Nutrients. 2018;10(10):1513. doi: 10.3390/nu10101513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lewis E.D., Field C.J., Jacobs R.L. Should the forms of dietary choline also be considered when estimating dietary intake and the implications for health? Lipid Technol. 2015;27(10):227–230. doi: 10.1002/lite.201500048. [DOI] [Google Scholar]
- 190.Wang Y., Guan X., Chen X., Cai Y., Ma Y., Ma J., Zhang Q., Dai L., Fan X., Bai Y. Choline supplementation ameliorates behavioral deficits and Alzheimer’s disease‐like pathology in transgenic APP/PS1 mice. Mol. Nutr. Food Res. 2019;63(18):1801407. doi: 10.1002/mnfr.201801407. [DOI] [PubMed] [Google Scholar]
- 191.Velazquez R., Ferreira E., Knowles S., Fux C., Rodin A., Winslow W., Oddo S. Lifelong choline supplementation ameliorates Alzheimer’s disease pathology and associated cognitive deficits by attenuating microglia activation. Aging Cell. 2019;18(6):e13037. doi: 10.1111/acel.13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wurtman R.J., Cansev M., Ulus I.H. Handbook of neurochemistry and molecular neurobiology. Amsterdam: Spriger; 2009. Choline and its products acetylcholine and phosphatidylcholine. [DOI] [Google Scholar]
- 193.Wecker L. Influence of dietary choline availability and neuronal demand on acetylcholine synthesis by rat brain. J. Neurochem. 1988;51(2):497–504. doi: 10.1111/j.1471-4159.1988.tb01066.x. [DOI] [PubMed] [Google Scholar]
- 194.Köppen A., Klein J., Erb C., Löffelholz K. Acetylcholine release and choline availability in rat hippocampus: effects of exogenous choline and nicotinamide. J. Pharmacol. Exp. Ther. 1997;282(3):1139–1145. [PubMed] [Google Scholar]
- 195.Vanmierlo T., Popp J., Kölsch H., Friedrichs S., Jessen F., Stoffel-Wagner B., Bertsch T., Hartmann T., Maier W., von Bergmann K., Steinbusch H., Mulder M., Lütjohann D. The plant sterol brassicasterol as additional CSF biomarker in Alzheimer’s disease. Acta Psychiatr. Scand. 2011;124(3):184–192. doi: 10.1111/j.1600-0447.2011.01713.x. [DOI] [PubMed] [Google Scholar]
- 196.Samadder A., Das J., Das S., De A., Saha S.K., Bhattacharyya S.S., Khuda-Bukhsh A.R. Poly(lactic-co-glycolic) acid loaded nano-insulin has greater potentials of combating arsenic induced hyperglycemia in mice: Some novel findings. Toxicol. Appl. Pharmacol. 2013;267(1):57–73. doi: 10.1016/j.taap.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 197.Goyzueta-Mamani L.D., Barazorda-Ccahuana H.L., Chávez-Fumagalli M.A.F., Alvarez F. K.L.; Aguilar-Pineda, J.A.; Vera-Lopez, K.J.; Lino C.C.L. In silico analysis of metabolites from peruvian native plants as potential therapeutics against Alzheimer’s disease. Molecules. 2022;27(3):918. doi: 10.3390/molecules27030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Charoenphon N., Anandsongvit N., Kosai P., Sirisidthi K., Kangwanrangsan N., Jiraungkoorskul W. Brahmi (Bacopa monnieri): Up-to-date of memory boosting medicinal plant: A review. Indian J. Agric. Res. 2016;50(1) doi: 10.18805/ijare.v50i1.8582. [DOI] [Google Scholar]
- 199.Sivaramakrishna C., Rao C.V., Trimurtulu G., Vanisree M., Subbaraju G.V. Triterpenoid glycosides from Bacopa monnieri. Phytochemistry. 2005;66(23):2719–2728. doi: 10.1016/j.phytochem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 200.Ganjewala D., Srivastava A.K. Recent progress on chemical composition and bioactivities of Bacopa monnieri (Linn.) a plant of Ayurveda. Med. Aromat. Plant Sci. Biotechnol. 2011;5:102–108. [Google Scholar]
- 201.Sharma P.C., Yelne M.B., Dennis T.J. Database on medicinal plants used in Ayurved. New Delhi: Central Council for Research in Ayurveda & Siddha; 2005. [Google Scholar]
- 202.Rastogi S., Kulshrestha D.K. Bacoside A2- a triterpenoid saponin from Bacopa monniera. Indian J. Chem. Sect. B. 1999;38:353–356. [Google Scholar]
- 203.Rastogi S., Pal R., Kulshreshtha D.K. Bacoside A3-A triterpenoid saponin from Bacopa monniera. Phytochemistry. 1994;36(1):133–137. doi: 10.1016/S0031-9422(00)97026-2. [DOI] [PubMed] [Google Scholar]
- 204.Basu N., Rastigi R.P., Dhar M.L. Chemical examination of Bacopa monniera. Wettst. Part III: the constitution of bacoside B. Indian J. Chem. 1967;5:84. [Google Scholar]
- 205.Chandel R.S., Kulshreshtha D.K., Rastogi R.P. Bacogenin-A3: A new sapogenin from Bacopa monniera. Phytochemistry. 1977;16(1):141–143. doi: 10.1016/0031-9422(77)83039-2. [DOI] [Google Scholar]
- 206.Chatterji N., Rastogi R.P., Dhar M.L. Chemical examination of Bacopa monniera Wettst.: Part I-Isolation of chemical constituents. Indian J. Chem. 1963;1(5):212–215. [Google Scholar]
- 207.Sekhar V.C., Viswanathan G., Baby S. Bacopaside II nanoparticles inhibit proliferation of C6 glioma cells. Phytomed. Plus. 2021;1(3):100040. [Google Scholar]
- 208.Malishev R., Shaham-Niv S., Nandi S., Kolusheva S., Gazit E., Jelinek R. Bacoside-A, an Indian traditional-medicine substance, inhibits β-amyloid cytotoxicity, fibrillation, and membrane interactions. ACS Chem. Neurosci. 2017;8(4):884–891. doi: 10.1021/acschemneuro.6b00438. [DOI] [PubMed] [Google Scholar]
- 209.Montine T.J., Sidell K.R., Crews B.C., Markesbery W.R., Marnett L.J., Roberts L.J., II, Morrow J.D. Elevated CSF prostaglandin E2 levels in patients with probable AD. Neurology. 1999;53(7):1495–1498. doi: 10.1212/WNL.53.7.1495. [DOI] [PubMed] [Google Scholar]
- 210.Bai Q.K., Zhao Z.G. Isolation and neuronal apoptosis inhibitory property of bacoside‐A3 via downregulation of β‐amyloid induced inflammatory response. Biotechnol. Appl. Biochem. 2021:1–9. doi: 10.1002/bab.2147. [DOI] [PubMed] [Google Scholar]
- 211.Singh B., Pandey S., Rumman M., Kumar S., Kushwaha P.P., Verma R., Mahdi A.A. Neuroprotective and neurorescue mode of action of Bacopa monnieri (L.) Wettst in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced Parkinson’s disease: an in silico and in vivo study. Front. Pharmacol. 2021;12:616413. doi: 10.3389/fphar.2021.616413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.de Vries H.E., Witte M., Hondius D., Rozemuller A.J.M., Drukarch B., Hoozemans J., van Horssen J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008;45(10):1375–1383. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 213.Sharma R., Chaturvedi C., Tewari P. Efficacy of Bacopa monniera in revitalizing intellectual functions in children. J. Res. Educ. Indian Med. 1987;1(2) [Google Scholar]
- 214.Chaudhari K.S., Tiwari N.R., Tiwari R.R., Sharma R.S. Neurocognitive effect of nootropic drug brahmi (Bacopa monnieri) in Alzheimer’s disease. Ann. Neurosci. 2017;24(2):111–122. doi: 10.1159/000475900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Navabi S.P., Sarkaki A., Mansouri E., Badavi M., Ghadiri A., Farbood Y. The effects of betulinic acid on neurobehavioral activity, electrophysiology and histological changes in an animal model of the Alzheimer’s disease. Behav. Brain Res. 2018;337:99–106. doi: 10.1016/j.bbr.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 216.Oliveira Costa J.F., Barbosa-Filho J.M., de Azevedo Maia G.L., Guimarães E.T., Meira C.S., Ribeiro-dos-Santos R., Pontes de Carvalho L.C., Soares M.B.P. Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int. Immunopharmacol. 2014;23(2):469–474. doi: 10.1016/j.intimp.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 217.Bhattacharyya S.S., Paul S., De A., Das D., Samadder A., Boujedaini N., Khuda-Bukhsh A.R. Poly (lactide-co-glycolide) acid nanoencapsulation of a synthetic coumarin: Cytotoxicity and bio-distribution in mice, in cancer cell line and interaction with calf thymus DNA as target. Toxicol. Appl. Pharmacol. 2011;253(3):270–281. doi: 10.1016/j.taap.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 218.Burg V.K., Grimm H.S., Rothhaar T.L., Grösgen S., Hundsdörfer B., Haupenthal V.J., Zimmer V.C., Mett J., Weingärtner O., Laufs U., Broersen L.M., Tanila H., Vanmierlo T., Lütjohann D., Hartmann T., Grimm M.O.W. Plant sterols the better cholesterol in Alzheimer’s disease? A mechanistical study. J. Neurosci. 2013;33(41):16072–16087. doi: 10.1523/JNEUROSCI.1506-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jemia M.B., Tundis R., Maggio A., Rosselli S., Senatore F., Menichini F., Bruno M., Kchouk M.E., Loizzo M.R. NMR-based quantification of rosmarinic and carnosic acids, GC–MS profile and bioactivity relevant to neurodegenerative disorders of Rosmarinus officinalis L. extracts. J. Funct. Foods. 2013;5(4):1873–1882. doi: 10.1016/j.jff.2013.09.008. [DOI] [Google Scholar]
- 220.Habtemariam S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evid. Based Complement. Alternat. Med. 2016;2016:1–14. doi: 10.1155/2016/2680409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang D., Lee B., Nutter A., Song P., Dolatabadi N., Parker J., Sanz-Blasco S., Newmeyer T., Ambasudhan R., McKercher S.R., Masliah E., Lipton S.A. Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J. Neurochem. 2015;133(6):898–908. doi: 10.1111/jnc.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Satoh T., Kosaka K., Itoh K., Kobayashi A., Yamamoto M., Shimojo Y., Kitajima C., Cui J., Kamins J., Okamoto S., Izumi M., Shirasawa T., Lipton S.A. Carnosic acid, a catechol-type electrophilic compound, protects neurons both in vitro and in vivo through activation of the Keap1/Nrf2 pathway via S- alkylation of targeted cysteines on Keap1. J. Neurochem. 2008;104(4):1116–1131. doi: 10.1111/j.1471-4159.2007.05039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Samadder A., Das S., Das J., Khuda-Bukhsh A.R. Relative efficacies of insulin and poly (lactic-co-glycolic) acid encapsulated nano-insulin in modulating certain significant biomarkers in arsenic intoxicated L6 cells. Colloids Surf. B Biointerfaces. 2013;109:10–19. doi: 10.1016/j.colsurfb.2013.03.028. [DOI] [PubMed] [Google Scholar]
- 224.de Oliveira M.R., Peres A., Ferreira G.C., Schuck P.F., Gama C.S., Bosco S.M.D. Carnosic acid protects mitochondria of human neuroblastoma SH-SY5Y cells exposed to paraquat through activation of the Nrf2/HO-1Axis. Mol. Neurobiol. 2017;54(8):5961–5972. doi: 10.1007/s12035-016-0100-3. [DOI] [PubMed] [Google Scholar]
- 225.Ak G., Zengin G., Ceylan R., Fawzi M.M., Jugreet S., Mollica A., Stefanucci A. Chemical composition and biological activities of essential oils from Calendula officinalis L. flowers and leaves. Flavour Fragrance J. 2021;36(5):554–563. doi: 10.1002/ffj.3661. [DOI] [Google Scholar]
- 226.Paul K., Ganguly U., Chakrabarti S., Bhattacharjee P. Is 1,8-cineole-rich extract of small cardamom seeds more effective in preventing Alzheimer’s disease than 1,8-cineole alone? Neuromolecular Med. 2020;22(1):150–158. doi: 10.1007/s12017-019-08574-2. [DOI] [PubMed] [Google Scholar]
- 227.Khan A., Vaibhav K., Javed H., Tabassum R., Ahmed M.E., Khan M.M., Khan M.B., Shrivastava P., Islam F., Siddiqui M.S., Safhi M.M., Islam F. 1,8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: relevance to Alzheimer’s disease. Neurochem. Res. 2014;39(2):344–352. doi: 10.1007/s11064-013-1231-9. [DOI] [PubMed] [Google Scholar]
- 228.Cheng Y. Dong, Z.; Liu, S. β-Caryophyllene ameliorates the Alzheimer-like phenotype in APP/PS1 Mice through CB2 receptor activation and the PPARγ pathway. Pharmacology. 2014;94(1-2):1–12. doi: 10.1159/000362689. [DOI] [PubMed] [Google Scholar]
- 229.Hase T., Shishido S., Yamamoto S., Yamashita R., Nukima H., Taira S., Toyoda T., Abe K., Hamaguchi T., Ono K., Noguchi-Shinohara M., Yamada M., Kobayashi S. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci. Rep. 2019;9(1):8711. doi: 10.1038/s41598-019-45168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yamada M., Ono K., Hamaguchi T., Noguchi-Shinohara M. Natural Compounds as Therapeutic Agents for Amyloidogenic Diseases. Cham: Springer; 2015. Natural phenolic compounds as therapeutic and preventive agents for cerebral amyloidosis. pp. 79–94. [DOI] [PubMed] [Google Scholar]
- 231.Noguchi-Shinohara M., Ono K., Hamaguchi T., Nagai T., Kobayashi S., Komatsu J., Samuraki-Yokohama M., Iwasa K., Yokoyama K., Nakamura H., Yamada M. Safety and efficacy of Melissa officinalis extract containing rosmarinic acid in the prevention of Alzheimer’s disease progression. Sci. Rep. 2020;10(1):18627. doi: 10.1038/s41598-020-73729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Karim N., Khan I., Abdelhalim A., Abdel-Halim H., Hanrahan J.R. Molecular docking and antiamnesic effects of nepitrin isolated from Rosmarinus officinalis on scopolamine-induced memory impairment in mice. Biomed. Pharmacother. 2017;96:700–709. doi: 10.1016/j.biopha.2017.09.121. [DOI] [PubMed] [Google Scholar]
- 233.Darrah H.H. Investigation of the cultivars of the basils (Ocimum). Econ. Bot. 1974;28(1):63–67. doi: 10.1007/BF02861381. [DOI] [Google Scholar]
- 234.Kumar A., Rahal A., Chakraborty S., Tiwari R., Latheef S.K., Dhama K. Ocimum sanctum (Tulsi): a miracle herb and boon to medical science-A Review. Int. J. Agron. Plant Prod. 2013;4(7):1580–1589. [Google Scholar]
- 235.Joshi R.K., Setzer W.N., Da Silva J.K. Phytoconstituents, traditional medicinal uses and bioactivities of Tulsi (Ocimum sanctum Linn.): a review. Am. J. Essent. Oil Nat. Prod. 2017;5(1):18–21. [Google Scholar]
- 236.Borah R., Biswas S.P. Tulsi (Ocimum sanctum), excellent source of phytochemicals. Int. J. Environ. Agric. Biotech. 2018;3(5):265258. [Google Scholar]
- 237.Panchal P., Parvez N. Phytochemical analysis of medicinal herb (Ocimum sanctum). Int. J. Nanomat. Nanotech. 2019;5(2):008-011. [Google Scholar]
- 238.Srinivas N., Sali K., Bajoria A. Therapeutic aspects of Tulsi unraveled: A review. J. Indian Acad. Oral Med. Radiol. 2016;28(1):17. doi: 10.4103/0972-1363.189984. [DOI] [Google Scholar]
- 239.Shishodia S., Majumdar S., Banerjee S., Aggarwal B.B. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003;63(15):4375–4383. [PubMed] [Google Scholar]
- 240.Nandini H.S., Krishna K.L., Apattira C. Combination of Ocimum sanctum extract and Levetiracetam ameliorates cognitive dysfunction and hippocampal architecture in rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2022;120:102069. doi: 10.1016/j.jchemneu.2021.102069. [DOI] [PubMed] [Google Scholar]
- 241.Kandhan T.S., Thangavelu L., Roy A. Acetylcholinesterase activity of Ocimum sanctum leaf extract. J. Adv. Pharm. Educ. Res. 2018;8(1):41–44. [Google Scholar]
- 242.Anuj G., Sanjay S. Eugenol: A potential phytochemical with multifaceted therapeutic activities. Pharmacologyonline. 2010;2:108–120. [Google Scholar]
- 243.Saxena U., Akella V. Niacin compositions for reduction of amyloid beta peptide 42 (abeta 42) production and for treatment of Alzheimer's disease (AD). US Patent No US8541435B2. 2013 [Google Scholar]
- 244.Morris M.C., Evans D.A., Bienias J.L., Scherr P.A., Tangney C.C., Hebert L.E., Bennett D.A., Wilson R.S., Aggarwal N. Dietary niacin and the risk of incident Alzheimer’s disease and of cognitive decline. J. Neurol. Neurosurg. Psychiatry. 2004;75(8):1093–1099. doi: 10.1136/jnnp.2003.025858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Mohd Zamli K., Asari A., Khaw K.Y., Murugaiyah V., al-Rashida M., Mohamad H., Mohd Yusoff H., Abdul Wahab N.H., Osman H. Cholinesterase inhibition activity and molecular docking study of eugenol derivatives. Sains Malays. 2021;50(4):1037–1045. doi: 10.17576/jsm-2021-5004-14. [DOI] [Google Scholar]
- 246.Garabadu D., Sharma M. Eugenol attenuates scopolamine-induced hippocampal cholinergic, glutamatergic, and mitochondrial toxicity in experimental rats. Neurotox. Res. 2019;35(4):848–859. doi: 10.1007/s12640-019-0008-6. [DOI] [PubMed] [Google Scholar]
- 247.Mesole S.B., Alfred O.O., Yusuf U.A., Lukubi L., Ndhlovu D. Apoptotic inducement of neuronal cells by aluminium chloride and the neuroprotective effect of eugenol in wistar rats. Oxid. Med. Cell. Longev. 2020;2020:1–7. doi: 10.1155/2020/8425643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bahadori M.B., Maggi F., Zengin G., Asghari B., Eskandani M. Essential oils of hedgenettles (Stachys inflata, S. lavandulifolia, and S. byzantina) have antioxidant, anti-Alzheimer, antidiabetic, and anti-obesity potential: A comparative study. Ind. Crops Prod. 2020;145:112089. doi: 10.1016/j.indcrop.2020.112089. [DOI] [Google Scholar]
- 249.Bahadori M.B., Dinparast L., Zengin G., Sarikurkcu C., Bahadori S., Asghari B., Movahhedin N. Functional components, antidiabetic, anti-Alzheimer’s disease, and antioxidant activities of Salvia syriaca L. Int. J. Food Prop. 2017;20(8):1761–1772. doi: 10.1080/10942912.2016.1218893. [DOI] [Google Scholar]
- 250.Mawaddani N., Wibowo N.R.K., Nadhira Q.H.H., Pramifta R.A. In silico study of Centella asiatica active compound as BACE1 inhibitor in Alzheimer’s disease. JSMARTech. 2020;1(2):036-040. doi: 10.21776/ub.jsmartech.2020.001.02.3. [DOI] [Google Scholar]
- 251.Formagio A.S.N., Vilegas W., Volobuff C.R.F. kassuya, C.A.L.; Cardoso, C.A.L.; Pereira, Z.V.; Silva, R.M.M.F.; dos Santos Yamazaki, D.A.; de Freitas Gauze, G.; Manfron, J.; Marangoni, J.A. Exploration of essential oil from Psychotria poeppigiana as an anti-hyperalgesic and anti-acetylcholinesterase agent: Chemical composition, biological activity and molecular docking. J. Ethnopharmacol. 2022;296:115220. doi: 10.1016/j.jep.2022.115220. [DOI] [PubMed] [Google Scholar]
- 252.Tangsaengvit N., Kitphati W., Tadtong S., Bunyapraphatsara N., Nukoolkarn V. Neurite outgrowth and neuroprotective effects of quercetin from Caesalpinia mimosoides Lamk. on cultured P19-derived neurons. Evid. Based Complement. Alternat. Med. 2013;2013:1–7. doi: 10.1155/2013/838051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. Flavonols and flavones as BACE-1 inhibitors: Structure–activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta, Gen. Subj. 2008;1780(5):819–825. doi: 10.1016/j.bbagen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 254.Rangsinth P., Duangjan C., Sillapachaiyaporn C., Isidoro C., Prasansuklab A., Tencomnao T. Caesalpinia mimosoides leaf extract promotes neurite outgrowth and inhibits BACE1 activity in mutant APP-overexpressing neuronal neuro2a cells. Pharmaceuticals (Basel) 2021;14(9):901. doi: 10.3390/ph14090901. [DOI] [PMC free article] [PubMed] [Google Scholar]



