Abstract
Alzheimer’s disease (AD) is a devastating neurodegenerative disease that mostly affects the elderly population. Mechanisms underlying AD pathogenesis are yet to be fully revealed, but there are several hypotheses regarding AD. Even though free radicals and inflammation are likely to be linked with AD pathogenesis, still amyloid-beta (Aβ) cascade is the dominant hypothesis. According to the Aβ hypothesis, a progressive buildup of extracellular and intracellular Aβ aggregates has a significant contribution to the AD-linked neurodegeneration process. Since Aβ plays an important role in the etiology of AD, therefore Aβ-linked pathways are mainly targeted in order to develop potential AD therapies. Accumulation of Aβ plaques in the brains of AD individuals is an important hallmark of AD. These plaques are mainly composed of Aβ (a peptide of 39-42 amino acids) aggregates produced via the proteolytic cleavage of the amyloid precursor protein. Numerous studies have demonstrated that various polyphenols (PPHs), including cyanidins, anthocyanins, curcumin, catechins and their gallate esters were found to markedly suppress Aβ aggregation and prevent the formation of Aβ oligomers and toxicity, which is further suggesting that these PPHs might be regarded as effective therapeutic agents for the AD treatment. This review summarizes the roles of Aβ in AD pathogenesis, the Aβ aggregation pathway, types of PPHs, and distribution of PPHs in dietary sources. Furthermore, we have predominantly focused on the potential of food-derived PPHs as putative anti-amyloid drugs.
Keywords: Alzheimer’s disease, Aβ, amyloid precursor protein, neuroprotection, polyphenols, neurodegenerative disease
1. INTRODUCTION
Alzheimer’s disease (AD) is one of the most devastating neurodegenerative diseases (NDs) and the most common form of dementia. Unfortunately, there are still no drugs available to cure or prevent AD. AD characteristics include extracellular plaque deposits of amyloid beta (Aβ) peptides, intracellular neurofibrillary tangles (NFTs) of misfolded and hyperphosphorylated tau protein, and the loss of synapses and neurons [1]. The amyloid cascade hypothesis is considered the most important hypothesis regarding AD pathogenesis. As per this hypothesis, Aβ deposition into plaques in brain tissues is the major causative factor of AD [2]. Aβ peptides are produced from the proteolytic cleavage of amyloid precursor protein (APP). Since Aβ is a partly folded and amphiphilic molecule, therefore Aβ is prone to self-aggregation and generates protofibrils or intermediate oligomers, and eventually insoluble fibrils [3]. Furthermore, Aβ can impair the functions of some membrane transporters, elevate cellular OS, and result in neuroinflammation, which can further lead to enormous synaptic dysfunction and loss of neurons [4]. Aβ-mediated neurotoxicity is strongly linked with the aggregated states of Aβ, where protofibrils and oligomers are more detrimental as compared to mature fibrils and soluble monomers. Protofibrils of Aβ and Aβ oligomers have a high capacity to interact with cell membranes, form pores, induce intraneuronal Aβ accumulation, alter membrane features, and disturb membrane receptors [5]. Nonetheless, the precise mechanisms of these processes are still not known. Therefore, it is important to find out the exact factors that mediate Aβ aggregation for AD prevention and treatment. However, many studies have already been carried out to detect and characterize intermediates that are associated with the Aβ aggregation pathway. Various techniques emphasized the isolation of Aβ aggregates from body fluids, including cerebrospinal fluid (CSF) and postmortem tissues, which were mainly purified via ultracentrifugation or size exclusion chromatography, and perhaps characterize the in vivo condition in the most dependable manner [6, 7]. Many in vitro studies, including structural and kinetic studies with recombinantly or synthetically expressed Aβ were carried out [8]. It was observed that stabilization of transient intermediates during in vitro Aβ assembly is highly challenging [9]. In Table 1, we have summarized the Aβ species that have been identified to date.
Table 1.
Amyloid beta (Aβ) species and their characteristics.
| Aβ Species | Properties | Study Models | Refs. |
|---|---|---|---|
| Amyloid fibrils | Stable and filamentous Aβ aggregates with fibrillar structure and common structural features | In vitro; patients with AD; mouse models | [34-36] |
| Aβ protofibrils | Flexible, short, rod-like structure; <200 nm in length; 6-8 nm in diameter; toxic; precursor of mature fibrils |
In vitro | [37-40] |
| Aβ Plaques | Mainly composed of fibrils; not toxic; large extracellular Aβ deposits; surrounded by reactive astrocytes, activated microglia, axons, and dystrophic dendrites |
In vivo; patients with AD; mouse models | [41, 42] |
| Aβ derived diffusible ligands (ADDLs) | Neurotoxic; nonfibrillar; an estimated mass of 17-42 kDa; trimers to 24 mers | In vitro; brain extracts of humans and murine models | [43, 44] |
| Small oligomers | Toxic; mostly unstable and transient; comprised of 3-50 monomers; heteromorphous | In vivo; AD individuals; mouse models | [6, 9, 45-48] |
| Annular Aβ oligomers | Play significant roles in membrane-disrupting ion channels or pores |
In vitro; cell culture | [49-53] |
| Aβ Monomers | Mainly α-helical and random coil in structure; produced from APP; soluble amphipathic molecule | In vitro; in vivo; human brain extracts | [54-56] |
| Aβ Dimers | Diameter of around 35 nm; hydrophobic core | In vitro; in vivo; human brain extracts | [57-59] |
| Aβ Trimers | Act as a subunit of toxic oligomers | In vivo; mouse models | [6, 60] |
Polyphenols (PPHs) are secondary metabolites of plants that are abundantly found in vegetables, oils, seeds, fruits, and many other foods [10]. PPHs have a number of roles, including facilitating plant reproduction and growth, imparting plant pigmentation, and protecting plants from aggression by pathogens [11]. So far, over 8000 natural PPHs have been detected. PPHs are grouped into 6 major categories according to the nature of their carbon skeletons, including flavonoids, tannins, phenolic acids and derivatives, lignans, curcuminoids, and stilbenes [12]. In ortho or para positions, all PPHs contain at least one phenolic ring bearing one or more hydroxyl groups [13]. PPHs act as strong antioxidants because of their ability to chelate redox-active metal ions, including iron [14] and to scavenge free radicals produced via reactive oxygen species (ROS) [15]. Collectively, these properties indicate a protective effect of PPHs against oxidative stress (OS) [16]. Dietary intake of PPHs is markedly higher than other dietary antioxidants, such as- carotenoids, vitamin C, and vitamin E [17, 18]. Additionally, PPHs also show anti-inflammatory properties [19]. It has been demonstrated that PPHs exert various beneficial effects in preventing various OS-linked diseases, including NDs, inflammation, atherosclerosis, and cancer [20-23].
The modulating or suppressive activities on Aβ aggregation have already been observed in 44 PPHs [24]. In a study, Ono et al. [25] revealed that wine-associated phenols, including (-)-epicatechin, (+)-catechin, kaempferol, quercetin, morin, and myricetin destabilized preformed Aβ fibrils and suppressed the generation of Aβ fibrils from Aβ40 and Aβ42 in a dose-dependent manner. In addition, similar effects were also achieved with resveratrol, olive tree-derived PPHs, epigallocatechin gallate (EGCG), tannic acids, rosmarinic acid, curcumin, and other stilbenes [26-30]. It has been reported by in vivo studies that several PPHs, including curcumin, resveratrol, and EGCG, reduced Aβ levels and their plaque formations in the brains of mouse models [31-33]. Indeed, the capacity of PPHs to avert the polymerizations of Aβ was found to be facilitated via their binding with ions or via direct interaction with Aβ, which further mediates Aβ aggregations [24]. In this review, we have summarized the effects of Aβ in the pathogenesis of AD, the Aβ aggregation pathway, types of PPHs, and distribution of PPHs in dietary sources. Moreover, we have particularly focused on the potential of PPHs as anti-amyloid molecules.
2. ROLES OF Aβ IN THE PATHOGENESIS OF ALZHEIMER’S DISEASE
AD is fatal progressive dementia and its characteristics include extracellular accumulation of Aβ plaques and intracellular NFTs in human brain tissues [61, 62]. Aβ peptides mainly make up the extracellular deposits, whereas hyperphosphorylated tau proteins make up the NFTs [36]. Aβ peptide is a normal product of the cellular metabolism derived from the APP [63]. Aβ peptides that are composed of 39-42 amino acids are sequentially cleaved from the APP’s C-terminal region [63], along with various isoforms that are generated via alternative splicing [64-66]. As per the amyloid hypothesis, aggregation of Aβ peptides is the cause instead of an AD effect [67]. There are several facts that strongly favor the amyloid hypothesis, including elevated in vitro aggregation tendency of familial AD (FAD)-linked Aβ variants, the link of amino acid substitutions in Aβ with early-onset FAD, the colocalization of Aβ aggregates along with dying neurons, and the production of an AD phenotype in transgenic mouse models overproducing Aβ or overexpressing APP via enhanced APP cleavage [68-71]. AD is linked with a rise in the Aβ1-40/Aβ1-42 ratio. Although the abundance of Aβ1-40 is 10 times more than that of Aβ1-42, but it has been observed that Aβ1-42 is the predominant toxic and/or amyloidogenic species [71-75]. Indeed, Aβ aggregation takes place before the generation of NFTs [76]. Furthermore, in contrast with deposits of Aβ peptides, NFTs are not inevitably present in the brain tissues of AD individuals [76]. Moreover, inherited mutations in tau proteins can result in frontotemporal dementia with parkinsonism, instead of inducing AD [77, 78].
Aβ exerts various neurotoxic effects, including OS, the generation of ion channels and membrane disruptions, employment of various cellular factors, or activation of multiple cellular mechanisms, including inflammation and apoptosis [79-81]. Although attention was initially paid to Aβ fibrils, however growing evidence over the past years indicated that lower-order Aβ oligomers mainly exert neurotoxic effects [82, 83]. In the brains of AD individuals and mouse models, AD symptoms, including cognitive dysfunction, memory impairment, and synaptic loss, are better correlated with the levels of soluble Aβ oligomers as compared to the presence of insoluble Aβ plaques [84-86]. In addition to this, initial symptoms of AD might even take place before the accumulation of Aβ plaques [87]. An increased level of lower-order and soluble Aβ oligomers has been detected in human AD brain tissues [88, 89]. It was also observed that the generation of soluble Aβ oligomers takes place before AD development [90]. Oligomeric species derived from cell cultures and murine AD brains showed toxic effects [69, 91]. Owing to the extracellular location of Aβ plaques, it is assumed that toxic effects can occur from the extracellular attack of neurons via Aβ [69, 91]. Nonetheless, Aβ also exists intracellularly in rat brain tissues and cell cultures [92, 93]. It has been demonstrated that nonfibrillar and intracellular Aβ oligomers can exert strong cytotoxic effects and can even exceed extracellular Aβ species [94, 95].
3. THE PATHWAY OF Aβ AGGREGATION
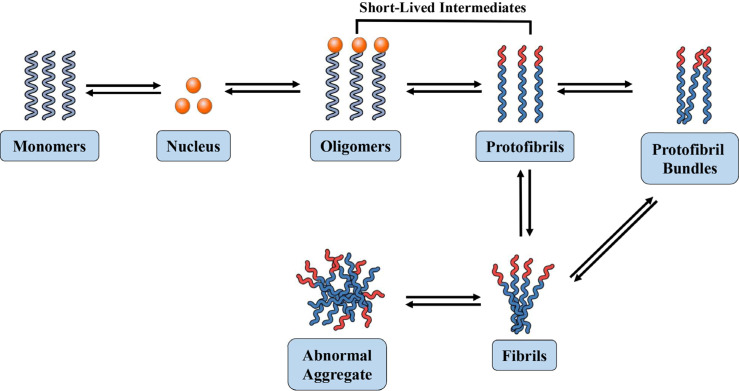
It is a very complex task to elucidate the Aβ aggregation pathway [96, 97]. The Aβ fibrillization model is mediated by a nucleation-dependent polymerization process that necessitates seeding via an ordered nucleus. Subsequently, the growth of the oligomers occurs through the incorporation of Aβ. Indeed, in this model, nuclei formation with seeding function is considered as the rate-limiting step. This observation is in line with the detected lag phase in the generation of Aβ fibrils that can be removed via adding preformed seeds [37, 98-100]. Aβ seeding is very specific, while seeds derived from other amyloidogenic proteins cannot effectively induce Aβ fibrillization [101]. Furthermore, Aβ fibril formation needs a minimum Aβ concentration. This required concentration is between 10 and 40 µM for the fibrillization of Aβ1-40 and approximately 5-times lower for Aβ1-42. Aβ concentration was found to be inversely proportional to the time Aβ stays soluble in vitro [102]. Seeding does not induce Aβ fibril generation and non-specific aggregation of Aβ becomes dominant at 50 times supersaturated Aβ levels, which surpass the physiological Aβ level around 10,000 times [103]. Interestingly, at around 10-fold lower concentrations, Aβ1-40 and Aβ1-39 remained soluble for several days, while Aβ1-42 rapidly aggregated into fibrils [104-106]. Collectively, these findings suggest that a minimum concentration of Aβ is needed for ordered aggregation, whereas a maximum Aβ concentration occurs above which non-specific Aβ aggregation averts certain Aβ polymerization [107].
It has been observed that the aggregation mechanisms for the same Aβ might differ at different initial Aβ levels [108-110]. It has also been confirmed that Aβ1-40 and Aβ1-42 aggregation occur through different mechanisms [9, 54]. In general, the common characteristics of the concluded assembly models involve Aβ oligomer formation as a nucleus which is considerably smaller as compared to Aβ fibrils. In addition, the in vivo nuclei growth mediated by fibrils and protofibrils ultimately bind with plaques, which eventually results in the generation of off-pathway intermediates [37, 111, 112] (Fig. 1). Studies on full-length, C- and N-terminally truncated Aβ suggest that Aβ residues 17-21 and structural alterations in this Aβ segment are important for the early phases of assembly [113, 114]. Models for the transition of monomer-to-oligomer also exist. Aβ monomers occur in equilibrium between the β-sheet and α-helical conformations, wherein solely the β-sheet structure exists in self-assembly-competent form and is pulled from the equilibrium. The monomer conformation is essential for fibrillization, which is induced by the formation of β-turn in the Aβ segment 24-28 [54]. Studies involving high-resolution atomic force microscopy indicated the generation of Aβ octamers, tetramers, and dimers containing β-sheet conformation as early assembly intermediates [115].
Fig. (1).
Schematic representation of Aβ fibrillization process.
Another model is based on the evidence that α-helix-rich oligomeric intermediates accumulate in case of fibrillization [116]. In addition, the shift to a β-sheet may therefore take place at the oligomer levels, as low-molecular-weight and soluble oligomers contain α-helical structure, while insoluble and higher-order only exhibit β-sheet conformation [117-119]. Fibril formation from protofibrils might be entropically driven via hydrophobic contacts between three and six protofibrils that are assumed to form a fibril [120]. It has been indicated by real-time monitoring of fibril growth that the reaction is a cooperative process with a constant elongation rate [121]. Molecular recycling also occurs in fibrils, wherein Aβ can get dissociated from the end of a fibril and get reassociated with another fibril [122]. These results indicate that fibril formation is a reversible process. On the other hand, real equilibria between soluble Aβ monomers and fibrils only occur at the fibril end. It has been estimated that an equilibrium concentration for the soluble Aβ1-40 monomer is 0.7-1.0 µM, which is not dependent on the total Aβ1-40 concentration in fibrillization processes [111]. In healthy people, Aβ is generated intracellularly and then secreted into the extracellular spaces. Aβ is usually produced in endocytic vesicles, which has been identified in the Golgi apparatus, endoplasmic reticulum, as well as at the plasma membrane and in recycling endosomes [123-125]. In vivo seeding has been detected in marmosets following inoculation with brain tissues derived from an individual with FAD [126]. Furthermore, the intracellular presence of Aβ aggregates in human brain tissues and cultured neurons has also been observed [127]. However, intracellular Aβ aggregates were not detected in late AD stages [128, 129].
4. TYPES OF POLYPHENOLS
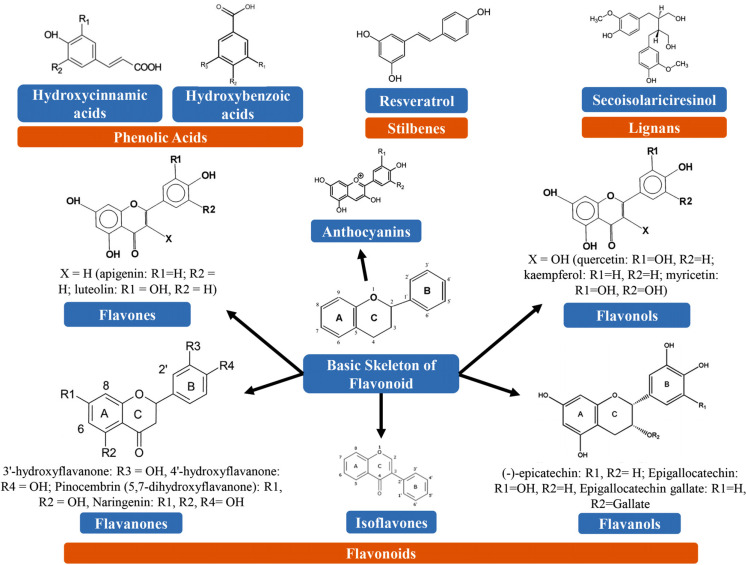
Over 8000 plant-derived polyphenolic compounds have already been detected. There is a close precursor (shikimic acid) or a common intermediate (phenylalanine) in the case of all plant-derived phenolic compounds. They are mainly found in conjugated forms, along with one or more sugar residues attached to hydroxyl groups, even though direct linkages of the sugar (monosaccharide or polysaccharide) to an aromatic carbon also occur. Links with other compounds, including lipids, amines, organic acids, and connections with other phenols, have also been observed [130]. PPHs could be categorized into different groups on the basis of the number of phenol rings that they possess and based on the structural elements that bind these rings to one another. The major classes of PPHs include flavonoids, phenolic acids, lignans, and stilbenes [131]. Chemical structures of different types of PPHs are illustrated in Fig. (2). In Table 2, we have summarized the class and subclasses of PPHs.
Fig. (2).
Chemical structures of various types of polyphenols.
Table 2.
Classification of polyphenols.
| Class | Subclass | Polyphenolic Compounds | Refs. |
|---|---|---|---|
| Phenolic Acids | Hydroxycinnamic acids | Rosmarinic acid, caffeoylquinic acid, sinapic acid, ferulic acid, caffeic acid, and p-coumaric acid |
[132-135] |
| Hydroxybenzoic acids | Ellagic acid and gallic acid | [136, 137] | |
| Flavonoids | Flavanols | Epicatechin, epigallocatechin, epicatechin, and catechins | [138, 139] |
| Anthocyanidins | Cyanidin, malvidin, petunidin, peonidin, delphinidin, and pelargonidin | [140-142] | |
| Flavanones | Eriodictyol, hesperidin, hesperetin, and naringenin | [143, 144] | |
| Isoflavones | Genistein and daidzein | [145-147] | |
| Flavones | Luteolin and apigenin | [148] | |
| Flavonols | Rutin, kaempferol, and quercetin | [149-151] | |
| Stilbenes | Stilbenes | e-Viniferin, piceatannol, and resveratrol | [152, 153] |
| Lignans | Lignans | Matairesinol, lariciresinol, pinoresinol, secoisolariciresinol, enterolactone, enterodiol, arctigenin, and sesamin |
[154, 155] |
| Other polyphenols | Curcuminoids | Curcumin | [156] |
| Hydroxyphenylpropnes | 6-Gingerol | [157] |
4.1. Phenolic Acids
Phenolic acids are most commonly found in foods. Furthermore, they can be grouped into two types, including cinnamic acid derivatives and benzoic acid derivatives. However, the level of hydroxybenzoic acids is usually low in edible plants, along with the exception of certain onions, black radish, and red fruits, which can possess concentrations of several tens of milligrams per kg in fresh weight [12]. As compared to hydroxybenzoic acids, hydroxycinnamic acids are more abundant and composed mainly of sinapic, ferulic, caffeic, and p-coumaric acids.
4.2. Flavonoids
Flavonoids are the most extensively studied type of PPHs. Flavonoids possess a common basic structure containing 2 aromatic rings attached with 3 carbon atoms that produce an oxygenated heterocycle (Fig. 2). So far, over 4000 flavonoids have been detected and most of them are accountable for imparting attractive colors to the leaves, fruits, and flowers [158, 159]. On the basis of the different types of heterocycles involved, flavonoids can be divided into 6 major subclasses, including isoflavones, anthocyanins (ANTs), flavanols, flavanones, flavones, and flavonols. Individual differences within each group can emerge from their level of alkylation and/or glycosylation and their variation in the arrangement and number of the hydroxyl groups [131]. Some of the most common flavonoids include catechins, myricetin, and quercetin.
4.3. Stilbenes
Stilbenes are phenolic compounds and their 2 phenyl moieties are linked through a two-carbon methylene bridge. These phenolic compounds are less commonly found in the human diet. In plants, most stilbenes play a role as antifungal phytoalexins, where these compounds are generated following the pathogenic attack. Resveratrol (3,5,4′-trihydroxy-trans-stilbene) is one of the most prominent PPHs which is widely found in grapes [160].
4.4. Lignans
Lignans are diphenolic compounds that have a 2,3-dibenzylbutane structure. Furthermore, these diphenolic compounds are generated via the dimerization of 2 cinnamic acid residues (Fig. 2). Various lignans, including secoisolariciresinol, are regarded as phytoestrogens. Linseed is one of the richest dietary sources of plant lignans, which has low quantities of matairesinol and secoisolariciresinol (up to 3.7 g/kg dry weight) [161].
5. DISTRIBUTION OF POLYPHENOLS IN DIETARY SOURCES
In plants, the distribution of phenolics at the subcellular, cellular, and tissue levels can vary. Soluble phenolics are found in cell vacuoles, whereas insoluble phenolics are found within the plant cell walls [162]. Various PPHs, including quercetin, are present in all plant products, such as tea, fruit juices, cereals, vegetables, and fruits, while isoflavones and flavanones are limited to certain foods [163, 164]. Complex mixtures of PPHs are present in various dietary sources. It has been observed that an increased level of phenolics is found in their outer parts as compared to those found in their inner layers [165]. There are many factors that can affect the polyphenolic contents of plants, including environmental factors, storage, processing, and the extent of ripeness during the time of harvest. Environmental factors and edaphic factors, including rainfall, sun exposure, and soil type, can also greatly affect the polyphenolic content of the foods. Indeed, the extent of ripeness can significantly affect the proportions and levels of several PPHs [166]. An increased level of anthocyanin and a decreased level of phenolic acid have been reported during ripening. Numerous PPHs, including phenolic acids, are directly linked with the reaction of plants to various types of stress. For instance, PPHs play a role in healing via lignifications of injured regions and possess antimicrobial via elevating their levels following pathogenic attack [165, 167, 168].
Storage also directly affects the polyphenolic contents of the foods. It has been confirmed that the polyphenol content of the foods can alter due to storage, owing to the easy oxidation of these PPHs [166]. Furthermore, oxidation reactions can further lead to the generation of polymerized substances, which can further result in alterations in the quality of foods, specifically in terms of organoleptic characteristics and color. Such alterations might be beneficial in the case of black tea, whereas they can be detrimental in the case of fruit browning. Interestingly, storing wheat flour could lead to a significant loss of phenolic acids [169]. It was also observed that when flour was stored for 6 months, qualitatively, it possessed the same phenolic acids, however, their concentrations were decreased by 70% than fresh flour. In contrast, cold storage has a minor effect on the level of PPHs in onions, pears, or apples [169]. Moreover, cooking has a significant effect on the concentration of PPHs. Tomatoes and onions lose around 75% to 80%, 65%, and 30% of their initial content of quercetin after boiling (for 15 minutes), microwave oven cooking, and frying, respectively [170].
6. POLYPHENOLS AS ANTI-AMYLOID MOLECULES
6.1. Anthocyanins
Along with other PPHs, a high level of ANTs is present in pomegranate juice [171]. In a study, supplementation with pomegranate juice resulted in enhanced task learning and decreased the load of Aβ plaque in the hippocampus of transgenic mouse models (Tg2576/APPsw) [172]. Furthermore, in this study, the researchers revealed that pomegranate reduced the Aβ deposition and soluble Aβ1-42 accumulation by around 50% more than that of control mouse models [172]. Several in vitro studies have also confirmed the suppressive property of 2 pomegranate-derived molecules, including punicalagin and ellagic acid (Fig. 3), on β-secretase activity [38]. The beneficial activities of pomegranate might be responsible for its many PPHs, particularly ANTs, condensed tannins, and ellagitannins (i.e., hydrolyzable tannins) [173]. Tannic acid and gallic acid suppressed the Aβ fibril formations and showed neuroprotective properties [174, 175]. In a study, Joseph et al. [176] showed that supplementation with blueberries (a fruit that contains a high level of ANTs) prevented cognitive impairments in APP/PS1 transgenic mouse models along with no changes in Aβ deposits and reversed the harmful effects of aging on neuronal signaling pathways in senescence-accelerated rodents [176]. Pycnogenol® (PYC) (a French maritime pine bark extract) contains a high concentration of ANTs and this compound provided protection to vascular endothelial cells against Aβ-induced damage and neurons against Aβ-mediated apoptosis [177, 178]. Moreover, in a dose-dependent manner, PYC treatment (10-60 μg/ml) before the exposure of Aβ25-35 markedly reduced the proportion of apoptotic cells. On the other hand, PYC administration at the dose of 40-μg/ml reduced the proportion of apoptotic cells by 80% [177]. It has also been reported that following consumption of an anthocyanin-rich diet, anthocyanin has the ability to enter the brain within minutes [179, 180].
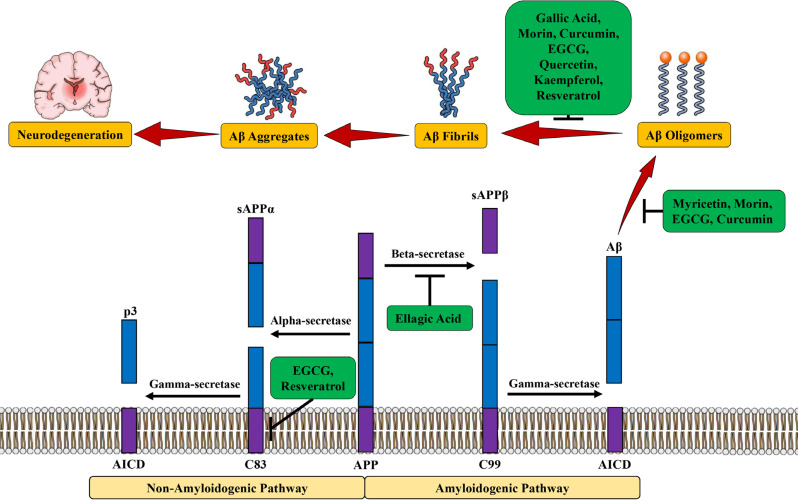
Fig. (3).
Anti-amyloid potential of various polyphenols. Abbreviations: AICD, amyloid precursor protein intracellular domain; APP, amyloid precursor protein; Aβ, amyloid beta; EGCG, epigallocatechin gallate; sAPPα, soluble APPα; sAPPβ, soluble APPβ.
Multiple studies have indicated that berries that contain higher levels of ANTs, cyanidins and their glycosides have the ability to suppress the formation of amyloid filaments [181, 182]. Therefore, it is important to identify potent antioxidants that have the capacity to provide protection to astrocytes and brain tissues against OS. Date-palm fruit contains increased levels of ANTs, dietary fiber, and phenolic acids (including caffeic acid, protocatechuic acid, and ferulic acid). Tg2576 mouse models (that received diet rich in date palm fruits) showed a markedly lower level of Aβ as compared to the Tg2576 mouse models without the diet supplement. In AD mouse models, neuroprotective property showed by 4% date palm fruits is greater than 2% date palm fruits [183]. Supplementation with date fruits also resulted in a decreased Aβ level in rats suffering from severe anxiety behavior and AD, which further lowered AD risk [184]. Several berries contain potent ANTs that may provide protection against AD via various mechanisms [181, 185]. In this regard, for example, ANTs present in green tea and red raspberry are useful in reversing the AD effects [186, 187]. Intake of bilberries caused a marked decrease in the levels of soluble Aβ40 and Aβ42 in transgenic AD mouse models, as compared to the mouse models fed with blackcurrant. Administration of bilberry and blackcurrant extracts decreased APP levels in the cerebral cortex of AD mice, however, alterations in the tau phosphorylation and expression were not seen [188].
ANTs can penetrate the blood-brain barrier and provide protection to brain tissues against OS-induced apoptosis, mitochondrial dysfunction, and Aβ toxicity [189]. Interestingly, a formulation containing ANTs/anthocyanidins reduced Aβ1-42-induced tau-phosphorylation [187]. In a study, Isaak et al. [190] showed that lingonberry ANTs (cyanidin-3-arabinoside, cyanidin-3-glucoside, and cyanidin-3-galactoside) protected the cells from hydrogen-peroxide-mediated apoptosis in H9c2 cells at the concentration of 10 ng/mL (20 nmol/L). In a different study, Badshah et al. [191] reported that neurodegeneration induced by Aβ1-42 in case of AD might be reversed via using potent antioxidants, such as black soybean ANTs. In addition, AD could be reversed via ANTs by the mitochondrial apoptotic pathway by controlling BACE-1, tau, caspase-3, caspase-9, cytochrome c, and Bax [191, 192]. Pacheco et al. [193] and Gutierres et al. [194] estimated the levels of acetylcholinesterase (AChE), Ca2+ ATPase, Na+/ K+-ATPase, and nitrite/nitrate functions in the hippocampus and cerebral cortex of an AD mouse model. These researchers also revealed that ANTs can regulate cholinergic neurotransmission and ion pump activity, which can further lead to enhanced memory. ANTs derived from black chokeberry protected the SH-SY5Y cells from Aβ1-42-induced apoptosis via controlling calcium homeostasis. These ANTs also reduced ROS and intracellular calcium levels, however, they caused elevated mitochondrial potential and ATP.
The aglycone forms of ANTs, including malvidin and malvidin-3-glucoside preserved calcium homeostasis, and showed protective properties against neurotoxicity induced by Aβ1-40 and Aβ25-35, and improved neurological dysfunction [195]. Grapes also serve as a very rich source of ANTs [186, 196]. Grape seeds also serve as a rich source of pro-ANTs, which protect from DNA fragmentation, OS, and lipid peroxidation. It has been observed that grape seeds obtained from nine grapevines (Vitis vinifera L.) varieties are found to possess 22 distinct ANTs, where the ANT levels vary between 0.5 and 4.99 g/kg. The concentration and type of ANTs depend on the cultivation season and variety. Grapes also contain several major aglycone forms of ANTs, including malvidin, peonidin, and delphinidin [186, 197]. There is a growing use of berries in health products owing to the claim that berries can provide protection against several diseases and promote mental health [187, 198]. Furthermore, the nutrients that are present in berries may even slow down AD progression [199]. AD progression and mitochondrial dysfunction may be subdued via ANTs because of their capacity to decrease intracellular calcium levels, Aβ-induced apoptosis, and ROS. In addition to this, ANTs can increase mitochondrial membrane potential and ATP levels. Collectively, these findings suggest that ANTs have the potential to suppress ROS and AD [200].
6.2. Curcumin
Curcumin (a major PPH and turmeric component) decreased in vivo Aβ accumulation (Table 3) [201], however, this PPH failed to decrease in vitro Aβ1-42 generation [202]. Curcumin also significantly suppressed Aβ aggregation and averted the formation of Aβ oligomers and toxicity [202]. Curcumin was found to suppress the level of disaggregated fibrillar Aβ40 (IC50 = 1 μm) and aggregation (IC50 = 0.8 μm). As compared to naproxen and ibuprofen, curcumin significantly suppressed the level of Aβ40 aggregation, and averted the formation of Aβ42 oligomers and toxicity between 0.1 and 1.0 μm [175]. Furthermore, it has been revealed by in vivo and in vitro studies that curcumin decreased the amyloid plaque burden and amyloid concentrations in aged Tg2576 mouse models with progressive Aβ accumulation [175]. Collectively, these findings suggest the clinical use of a low dose of curcumin to treat or even prevent AD. Curcumin also decreased in vitro Aβ concentrations via decreasing the expression of beta-secretase 1 (BACE1) and the maturation of APP [203]. Demethoxycurcumin showed potent inhibitory BACE-1 function (IC50 = 17 μM) in vivo in a drosophila AD model, which further rescued behavioral and morphological impairments induced via overexpression of BACE1 and APP maturation [204]. Curcumin also suppressed the transcription of BACE-1 via activation of Wnt/β-catenin signaling [205, 206]. It has also been suggested that curcumin can bind with Aβ and prevent in vitro and in vivo Aβ aggregation. Curcumin showed a higher affinity for binding with Aβ aggregates (Kd = 0.20 nM), where EC50 of curcumin for the destabilization of Aβ was around 1 µM [28]. In a study, Garcia-Alloza et al. [207] reported that intravenous administration of curcumin at the dose of 7.5 mg/kg for 7 days cleared or decreased the size of Aβ plaques in APPswe/PS1dE9 mouse models. Oral administration of a daily single-dose (500 ppm) of curcumin for 5 months markedly decreased the concentrations of Aβ plaques (by 32.50%) and insoluble Aβ (by 85%) [208].
Table 3.
Summary of the purported effects of polyphenols in models of toxicity related to amyloid peptides.
| Polyphenols | Study Model | Treatment Duration | Study Outcomes | Refs. |
|---|---|---|---|---|
| Curcumin | Aged APPsw Tg2576 mice | 5 months | Reduced Aβ levels and plaque burden | [27] |
| Parts of Tg2576 mouse brain | 3-6 days | Suppressed Aβ aggregation; mediated disaggregation of Aβ | ||
| Neuronal hippocampal cultures | 1 to 24 hours | Suppressed the generation of Aβ fibrils and fibril-destabilizing activities |
||
| Resveratrol | Neuronal hippocampal cultures | 2 hours | Blocked Aβ-induced toxicity | [30, 245, 246] |
| Cells transfected with human APP695 | 24 hours | Mediated Aβ degradation | ||
| Neuronal hippocampal cultures | 24 hours | Showed anti-amyloidogenic properties | ||
| Myricetin | Neuronal hippocampal cultures | 1 to 24 hours | Showed fibril-destabilizing properties; blocked Aβ(1-40)-induced toxicity; suppressed the generation of Aβ fibrils | [25] |
| EGCG | Neuronal hippocampal cultures | 24-48 hours | Suppressed soluble forms of Aβ and Aβ fibrilization; blocked Aβ(1-42)-induced toxicity | [219, 263, 264] |
| Tg APPsw mouse models | 2 months | Reduced Aβ levels and Aβ plaques | ||
| C57/BL mouse models; human neuroblastoma cells | 7-14 days | Elevated the levels of sAPP release; mediated non-amyloidogenic pathway |
||
| Ellagic acid and Punicalagin | Tg APPsw/Tg2576 mouse models | 6-8 months | Ameliorated AD-like pathology and behavior along with a reduced level of Aβ(1-42) and Aβ deposition | [172] |
| Gallic acid and tannic acid |
Neuronal hippocampal cultures | 1 to 24 hours | Showed fibril-destabilizing properties; blocked Aβ-induced toxicity; suppressed the generation of Aβ fibrils | [265] |
Comprehensive structure-activity relationship studies have revealed that substitution conformation of the phenol rings, rigidity and length of the linker, and coplanarity of two phenol rings influence the inhibitory potential of curcumin [209]. By utilizing molecular dynamics simulations and molecular docking, Rao et al. [210] confirmed that binding of curcumin with Aβ aggregates can result in considerable amino acid variations and cause a shift in equilibrium towards non-toxic Aβ aggregates. Curcumin also has the ability to bind strongly with Aβ via hydrogen bond and hydrophobic interactions, which can further lead to the prevention of oligomerization and disruption of preformed fibrils [211]. Furthermore, curcumin can block Aβ aggregation via chelating various metal ions, including Fe3+, Cu2+, and Zn2+, that are responsible for inducing OS and Aβ aggregation [212, 213]. In a study, Kozmon [214] revealed that curcumin has the capacity to chelate Cu2+ ions and directly bind with Aβ, which further results in curcumin-Aβ and curcumin-Cu2+-Aβ complexes that eventually reduce toxic β-sheet structure [214]. Along with the activities of curcumin to regulate Aβ aggregation and generation, it also induces Aβ clearance. Moreover, curcumin increased expressions of lysosome- and autophagy-linked protein markers including beclin-1, LC3A/ B-II, and heat shock proteins, which are crucial for the phagocytosis of Aβ peptides in neurons [215]. CNB-001 is a derivative of curcumin that acts as an inhibitor of 5-lipoxygenase. CNB-001 activates PERK-eIF2-ATF4 of the unfolded protein response (UPR) and induces Aβ degradation [216]. Collectively, these findings suggest that curcumin not only regulates the Aβ cascade but also plays an important role in identifying various novel targets for AD treatment, including PERK-eIF2-ATF4 of the UPR and Wnt/β-catenin pathway.
6.3. Tea-Derived Catechins
In an epidemiological study, it was reported that increased green tea consumption (and, to a lesser degree, black tea consumption) was linked with a lower level of cognitive deficits in 1003 Japanese people aged 70 [217]. Extracts of black and green teas also provided protection to hippocampal glial/neuronal cells against Aβ toxicity [14]. In addition, these activities were also observed with gallic acid, epicatechin gallate, and epigallocatechin gallate (EGCG), whereas epigallocatechin and epicatechin could not provide protection to cells. Catechins gallate esters that are most commonly found in black and green teas play a role in the beneficial properties of teas [218]. Catechins and their gallate esters have the capacity to suppress the generation of Aβ fibrils and Aβ’s soluble forms, including Aβ-oligomers. EGCG reduced Aβ plaques and levels in Tg APPsw transgenic mouse models [219]. Numerous in vitro, in vivo, and in silico studies have been carried out to assess the effects of catechins in AD [220-223]. Since catechins possess antioxidant properties, therefore they might provide protection against OS-linked neurodegeneration in the case of late-onset NDs [224, 225]. It is well known that levels of oxidized DNA, proteins, and peroxidized lipids are elevated in AD individuals [226]. In a study, Haque et al. [227] observed that the administration of catechins that occur in green tea averted Aβ-induced cognitive deficits in rat models [227]. In addition to this, both plasma and hippocampal ROS and lipid peroxide levels were decreased by 20% as compared to controls, which indicates a marked reduction [227].
In another study, Biasibetti et al. [228] assessed the activities of EGCG in streptozotocin-induced dementia rat models. It was revealed by the Morris water maze test that oral administration of EGCG at the dose of 10 mg/kg/day for a month reversed cognitive deficits, decreased nitric oxide (NO) generation, and reduced the levels of ROS [228]. Metal iron-chelating and free radical scavenging properties of catechins might play roles in these antioxidant effects [229-231]. Various metal ions, including iron (III) and copper (II), could be chelated via catechins. Furthermore, iron chelation decreases ROS generation by suppressing the Fenton reaction [232]. It has already been observed that iron (III) and copper (II) can accumulate in the brains of AD individuals [233]. Collectively, these results indicate that tea catechins have the capacity to decrease OS in the brain and peripheral tissues. Furthermore, they also can inhibit behavioral changes linked with cognitive impairment. Catechins exert anti-inflammatory activities, which might also indicate the mechanism of their activities on AD. It has been observed that neuronal injury can result in the release of various pro-inflammatory elements, including cytotoxic elements and cytokines, which can eventually result in neuronal death [234]. In lipopolysaccharide (LPS)-injected mouse models, Lee et al. [235] confirmed that EGCG preadministration at the doses of 1.5 and 3 mg/kg for 3 weeks averted LPS-mediated memory deficits and inhibited the rise of inflammatory proteins and cytokines observed in nontreated controls [235].
In a study with BV-2 microglia, EGCG suppressed the reactions linked with LPS-induced inflammation, such as expressions of inducible NO synthase, NO generation, and cyclooxygenase-2 expressions [236]. PKC-linked processes might also play roles in the activities of catechins in AD. Moreover, PKC plays a role in cell survival and generation of soluble nontoxic Aβ (sAPP) [237]. Levites et al. [238] showed that EGCG at a lower concentration (1-5 µM) induced sAPP generation from human neuroblastoma and PC12 cells, whereas oral EGCG administration at the dose of 2 mg/kg/day increased the levels of PKCα and PKCε in the hippocampus of mouse models than control-treated animals [238]. In a study, Kaur et al. [239] used the passive avoidance test and reported that green tea extract (0.5%) administration for 8 weeks markedly ameliorated memory and learning of aged Wister rats. Effects of AChE in the cerebrum were found to be reduced in treated aged rat models than young rat models [239]. Kim et al. [240] observed that administration of 0.2% (w/w) tea PPH through diet reversed scopolamine-induced amnesia. In addition, tea PPHs also significantly suppressed the AChE activity [240].
6.4. Grape-Derived Polyphenols
Wine is the main product of grapes [241]. In Tg2576 mouse models, supplementation with Cabernet Sauvignon (a red wine) (had a very low level of resveratrol (0.2 mg/L)) markedly attenuated the Aβ neuropathology and AD-type impairment of spatial memory function as compared to control Tg2576 mouse models that received a comparable amount of water or ethanol alone [242]. Moderate consumption of this type of red wine also can increase α-secretase activity and αCTF level, which can eventually prevent the Aβ production and deposition [243]. It was reported that the levels of Aβ1-40 and Aβ1-42 were reduced in the hippocampus and neocortex of mouse models exposed to red wine [244]. Cabernet Sauvignon contains various PPHs, including catechins, tannic acid, and myricetin. These Cabernet Sauvignon-derived PPHs have strong fibril-destabilizing and anti-amyloidogenic activities, which indicate that these PPHs have a significant contribution to the neuroprotective properties of red wine. Resveratrol (a red wine-derived PPH) shows anti-amyloidogenic properties that might include its capacity to mediate Aβ clearance, instead of inhibition of Aβ generation [30]. Marambaud et al. [245] demonstrated that resveratrol did not affect the functions of β-and γ-secretases and did not block the generation of Aβ. Furthermore, these researchers observed that the capacity of resveratrol to destroy Aβ might include proteasome [245]. Cell death induced by Aβ was found to be dose-dependently decreased in the presence of co- and pre-treatments of resveratrol (15-40 µM) [13]. GF 109203X (a highly selective and potent inhibitor of protein kinase C (PKC)) pre-treatment markedly decreased the neuroprotective properties of resveratrol against Aβ25-35-induced cytotoxicity, whereas PD98059 (a highly selective MAP kinase inhibitor) and LY294002 (a potent inhibitor of PI3 kinase) did not modify the neuroprotective properties of resveratrol [246]. It has been indicated by western blot analysis that resveratrol (20-30 µM) triggered PKC phosphorylation and also eliminated the suppressive activity of Aβ25-35, which further indicates the function of PKC in the neuroprotective action of resveratrol [246]. Collectively, these results suggest that moderate consumption of red wine can decrease the risk of dementia and AD [247].
Quercetin (a wine-related PPH) significantly inhibited the in vitro formation of Aβ fibrils [248]. Ferulic acid (another wine-related PPH) did not avert the generation of fibrils, however, it altered the Aβ fibril lengths and provided protection against Aβ-induced toxicity in transgenic Caenorhabditis elegans [249]. Moreover, quercetin reversed Aβ-induced neurotoxicity and showed fibril-destabilizing properties on preformed Aβ fibrils in cells overexpressing APP Swedish mutation (APPswe), which is linked with early-onset FAD [250]. Quercetin-3-O-glucuronide (a polyphenol metabolite) has the capacity to affect the generation of neurotoxic oligomeric Aβ species. Consumption of Cabernet Sauvignon resulted in the accumulation of quercetin-3-O-glucuronide in the brains of rats and improvement in AD-related impairments via inducing neuroplasticity mechanisms [251]. In transgenic Tg2576 mouse models, Cabernet Sauvignon-derived wine consumption markedly inhibited AD phenotypes via averting the generation of Aβ [252].
Muscadine wine (derived from muscadine grapes) inhibited memory deficits in transgenic Tg2576 mice by interfering with the process of Aβ oligomerization [253]. Furthermore, the researchers observed in a Morris water maze (MWM) test that treatment with muscadine for 10 months markedly decreased the spatial memory impairment in around 14-month-old Tg2576 mouse models as compared to gender- and age-matched control, non-treated Tg2576 mouse models [253]. Resveratrol mainly acts via directly binding with Aβ and interfering with the aggregation of Aβ [254]. Resveratrol and its derivatives (found in wine), including ε-viniferin glucoside and piceid, significantly suppressed Aβ fibrillization and provided protection to PC12 cells against toxicities induced by Aβ [255]. In cell culture, ellagic acid (a wine-related PPH) reduced toxic intermediate oligomeric species and also decreased neurotoxicity induced by Aβ via inducing fibril formation [256].
In order to assess the protective properties of red wine, various studies have utilized the commercial grape seed polyphenolic extract (GSPE), which is a rich source of proanthocyanidins, gallic acid, and catechins. It was observed that GSPE markedly suppressed in vitro Aβ aggregation. Moreover, oral administration of GSPE reduced AD-type cognitive deficit and decreased Aβ plaques in an AD mouse model [257, 258]. It was suggested by the structural-activity relationships of GSPE compounds that the GSPE mixture contains the most effective PPHs that act as potent inhibitors of Aβ aggregation [259]. GSPE also inhibited the aggregations of tau and detachment of preformed tau aggregates, perhaps via non-covalent interactions of PPHs (derived from GSPE) with tau residues [260]. It was also reported that GSPE administration through drinking water markedly decreased the ameliorated motor phenotype and concentrations of toxic hyperphosporylated tau of transgenic mouse models expressing a human tau protein with P301L mutation [261]. Bioactive dietary polyphenol preparation (BDPP) is another grape-derived PPH that contains a combination of resveratrol, grape seed extract, and Concord grape juice. BDPP was found to mitigate cognitive deficit, loss of synaptic plasticity, and amyloid load in AD mice. Collectively, these findings suggest that combination treatment with extract preparation is more effective as compared to single PPH treatment [262].
7. STRUCTURE-ACTIVITY RELATIONSHIPS (SARs) OF Aβ-AGGREGATION INHIBITORS
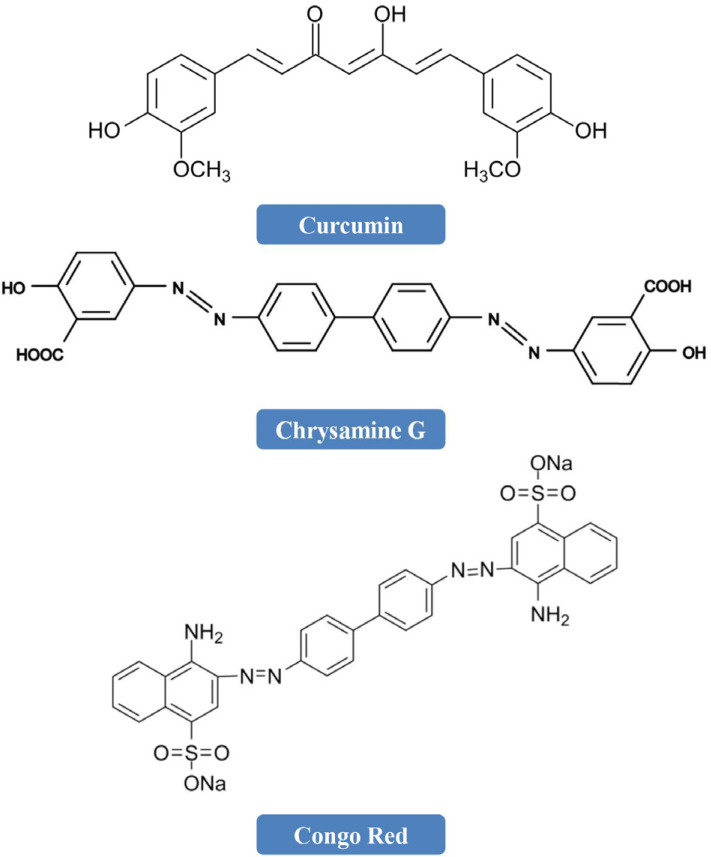
A method of identification of potent inhibitors could be designing them according to natural compounds [266]. Various organic dyes, including curcumin, chrysamine G (CG), and congo red (CR) have the ability to strongly bind with Aβ [208, 267]. It has been reported that curcumin (widely found in turmeric) has the ability to suppress the formation of Aβ(1-40) and reduce its toxicity [267, 268]. CG, CR, and curcumin have a common chemical scaffold and they possess 2 substituted aromatic groups divided by a planar, rigid backbone (Fig. 4).
Fig. (4).
Chemical structures of curcumin, chrysamine G, and congo red.
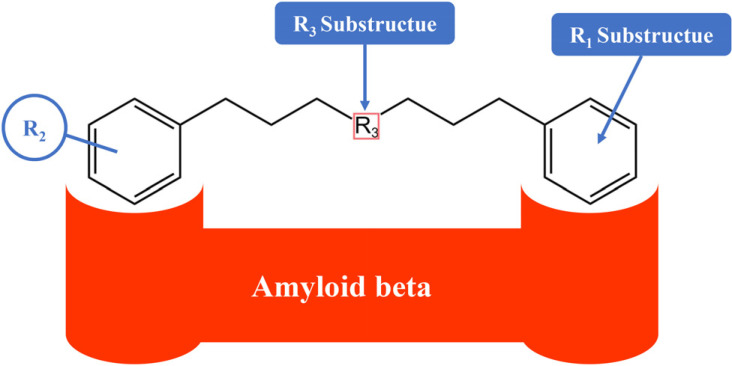
Numerous studies have revealed that other curcumin-like ligands also suppress the aggregation of Aβ [269-277]. In order to identify the chemical properties crucial for inhibition, Reinke and Gestwicki [266] developed a library of small molecules. In order to develop the library, they gave importance to the molecules that look like CR and curcumin. The developed library addressed 3 components anticipated to influence activity (Fig. 5). Firstly, curcumin’s chemical scaffold, along with 2 aromatic end groups, is optimum for suppression and the R1 portion deals with whether compounds that have the deficiency of the second aromatic group preserve function. Secondly, substitutions on the phenyl groups are vital for the functions, thus the R2 study deals with the activity of changing the hydrogen-bonding characteristics of these substitutions. Finally, the R3 substructure assesses the functions of flexibility and linker length. Collectively, even a slight alteration in any of the R1, R2, or R3 substructures has significant effects on function [266].
Fig. (5).
Structural components that are essential for the function of Aβ-aggregation inhibitors [209]. [R1 Substructue= a phenyl group; R2 Substructue= a hydroxyl group; R3 Substructue= linker length and flexibility [limited to 8 Å - 16 Å].
8. FUTURE DIRECTIONS
Various mechanisms have already been indicated that are linked with PPH-induced protection from cytotoxicity mediated by amyloid assemblies. In this regard, for instance, resveratrol shows potent free radical scavenging activities in multiple cellular types [278, 279]. Various other PPHs also show antioxidant properties against NO-induced toxicity [25]. Along with the antioxidant properties of PPHs, multiple intracellular signaling pathways are also linked (at least partly) with their neuroprotective properties [25]. For instance, resveratrol induces MAP kinase activation in cells [280]. However, several researchers showed that in vitro suppression of Aβ generation is not reliant on oxidative environments. In addition, several researchers also revealed that structural properties of PPHs ought to be regarded as influencing their inhibitory function. Therefore, the antioxidant properties of PPHs might not offer a central mechanistic view for the suppressive mechanism by means of in vitro conditions. Interestingly, no correlation was detected between the antioxidant properties of the reported PPHs and in vitro inhibitory IC50 values. For instance, epicatechin is a potent antioxidant (2.54 times more potent than vitamin C) [281], however this PPH cannot effectively inhibit amyloid generation. Although morin exhibits relatively lower antioxidant properties (1.65 times higher as compared to vitamin C), however, morin can effectively inhibit Aβ [281]. Various studies have revealed that PPHs do not suppress cell death mediated via hydrogen peroxide or other OS factors [282-284]. Thus, a novel mechanistic technique is required, which will be reliant on structural similarities between multiple highly potent inhibitors of PPHs, and compared with the Congo red (an amyloidogenic dye). It has been observed that all the effective inhibitors of PPHs contain minimum 2 phenolic rings along with 2 to 6 atom linkers, and at least 3 OH groups attached to the aromatic rings. Collectively, these structural similarities indicate three-dimensional shapes that are vital for the non-covalent interaction with β-sheet structures, which are commonly observed in the case of all amyloidogenic structures. Furthermore, this interaction might only take place when the native conformation of the amyloidogenic proteins converts to the assembly conformation and cannot thus take place with the folded native protein [285-287].
CONCLUSION
There is growing evidence indicating that dietary intake of PPHs have the potential to decrease the occurrence of age-linked neurological disorders. Epidemiological studies have also indicated that there is an inverse link between the AD risk or cognitive deficit and the intake of PPHs-enriched vegetables, fruits, and beverages. Mechanisms of actions underlying the neuroprotective properties of PPHs are still not fully revealed. However, various PPHs, including turmeric-derived curcumin, tea-derived catechins, and grape-derived PPHs, have the capacity to inhibit the generation of Aβ fibrils and Aβ-oligomers. In addition, PPHs might also mediate Aβ clearance via playing roles on Aβ-degrading enzymes, including metallopeptidases or proteasomes. Collectively, the aforesaid results suggest that these PPHs might play roles as neuroprotective agents to prevent cognitive impairments. Moreover, future clinical trials are also required to assess the effectiveness of PPHs.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- APP
Amyloid Precursor Protein
- Aβ
Amyloid Beta
- BDPP
Bioactive Dietary Polyphenol Preparation
- CSF
Cerebrospinal Fluid
- NDs
Neurodegenerative Diseases
- OS
Oxidative Stress
- ROS
Reactive Oxygen Species
- UPR
Unfolded Protein Response
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Blennow K., de Leon M.J., Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 2.Karran E., Mercken M., Strooper B.D. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 3.Ow S.Y., Dunstan D.E. A brief overview of amyloids and Alzheimer’s disease. Protein Sci. 2014;23(10):1315–1331. doi: 10.1002/pro.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson M.P. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430(7000):631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L.N., Long H.W., Mu Y., Chew L.Y. The toxicity of amyloid ß oligomers. Int. J. Mol. Sci. 2012;13(6):7303–7327. doi: 10.3390/ijms13067303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesné S., Koh M.T., Kotilinek L., Kayed R., Glabe C.G., Yang A., Gallagher M., Ashe K.H. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440(7082):352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 7.Cleary J.P., Walsh D.M., Hofmeister J.J., Shankar G.M., Kuskowski M.A., Selkoe D.J., Ashe K.H. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat. Neurosci. 2005;8(1):79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 8.Walsh D.M., Hartley D.M., Condron M.M., Selkoe D.J., Teplow D.B. In vitro studies of amyloid β-protein fibril assembly and toxicity provide clues to the aetiology of Flemish variant (Ala692→Gly) Alzheimer’s disease. Biochem. J. 2001;355(3):869–877. doi: 10.1042/bj3550869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitan G., Kirkitadze M.D., Lomakin A., Vollers S.S., Benedek G.B., Teplow D.B. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc. Natl. Acad. Sci. USA. 2003;100(1):330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pérez-Jiménez J., Neveu V., Vos F., Scalbert A. Systematic analysis of the content of 502 polyphenols in 452 foods and beverages: an application of the phenol-explorer database. J. Agric. Food Chem. 2010;58(8):4959–4969. doi: 10.1021/jf100128b. [DOI] [PubMed] [Google Scholar]
- 11.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126(4):1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cieślik, E.; Gręda, A.; Adamus, W. Contents of polyphenols in fruit and vegetables. Food Chem. 2006;94(1):135–142. doi: 10.1016/j.foodchem.2004.11.015. [DOI] [Google Scholar]
- 14.Mira L., Fernandez M.T., Santos M., Rocha R., Florêncio M.H., Jennings K.R. Interactions of flavonoids with iron and copper ions: A mechanism for their antioxidant activity. Free Radic. Res. 2002;36(11):1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- 15.Royer M., Diouf P.N., Stevanovic T. Polyphenol contents and radical scavenging capacities of red maple (Acer rubrum L.) extracts. Food Chem. Toxicol. 2011;49(9):2180–2188. doi: 10.1016/j.fct.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh D., McGhie T.K., Zhang J., Adaim A., Skinner M. Effects of anthocyanins and other phenolics of boysenberry and blackcurrant as inhibitors of oxidative stress and damage to cellular DNA in SH-SY5Y and HL-60 cells. J. Sci. Food Agric. 2006;86(5):678–686. doi: 10.1002/jsfa.2409. [DOI] [Google Scholar]
- 17.Reboul E., Thap S., Tourniaire F., André M., Juhel C., Morange S., Amiot M.J., Lairon D., Borel P. Differential effect of dietary antioxidant classes (carotenoids, polyphenols, vitamins C and E) on lutein absorption. Br. J. Nutr. 2007;97(3):440–446. doi: 10.1017/S0007114507352604. [DOI] [PubMed] [Google Scholar]
- 18.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H., Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. doi: 10.1016/j.cofs.2016.02.002. [DOI] [Google Scholar]
- 20.Candiracci M., Piatti E., Dominguez-Barragán M., García-Antrás D., Morgado B., Ruano D., Gutiérrez J.F., Parrado J., Castaño A. Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J. Agric. Food Chem. 2012;60(50):12304–12311. doi: 10.1021/jf302468h. [DOI] [PubMed] [Google Scholar]
- 21.Cheng Y.C., Sheen J.M., Hu W.L., Hung Y.C. Polyphenols and oxidative stress in atherosclerosis-related ischemic heart disease and stroke. Oxid. Med. Cell. Longev. 2017;2017:1–16. doi: 10.1155/2017/8526438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebrahimi A., Schluesener H. Natural polyphenols against neurodegenerative disorders: Potentials and pitfalls. Ageing Res. Rev. 2012;11(2):329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y., Zheng J., Li Y., Xu D-P., Li S., Chen Y-M., Li H-B. Natural polyphenols for prevention and treatment of cancer. Nutrients. 2016;8(8):515. doi: 10.3390/nu8080515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Velander P., Wu L., Henderson F., Zhang S., Bevan D.R., Xu B. Natural product-based amyloid inhibitors. Biochem. Pharmacol. 2017;139:40–55. doi: 10.1016/j.bcp.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ono K., Yoshiike Y., Takashima A., Hasegawa K., Naiki H., Yamada M. Potent anti-amyloidogenic and fibril-destabilizing effects of polyphenols in vitro: implications for the prevention and therapeutics of Alzheimer’s disease. J. Neurochem. 2003;87(1):172–181. doi: 10.1046/j.1471-4159.2003.01976.x. [DOI] [PubMed] [Google Scholar]
- 26.Korshavn K.J., Jang M., Kwak Y.J., Kochi A., Vertuani S., Bhunia A., Manfredini S., Ramamoorthy A., Lim M.H. Reactivity of metal-free and metal-associated amyloid-β with glycosylated polyphenols and their esterified derivatives. Sci. Rep. 2015;5(1):17842. doi: 10.1038/srep17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ono K., Hasegawa K., Naiki H., Yamada M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s? -amyloid fibrils in vitro. J. Neurosci. Res. 2004;75(6):742–750. doi: 10.1002/jnr.20025. [DOI] [PubMed] [Google Scholar]
- 28.Ono K., Hasegawa K., Naiki H., Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer’s β-amyloid fibrils in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2004;1690(3):193–202. doi: 10.1016/j.bbadis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Palhano F.L., Lee J., Grimster N.P., Kelly J.W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013;135(20):7503–7510. doi: 10.1021/ja3115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivière C., Richard T., Quentin L., Krisa S., Mérillon J.M., Monti J.P. Inhibitory activity of stilbenes on Alzheimer’s β-amyloid fibrils in vitro. Bioorg. Med. Chem. 2007;15(2):1160–1167. doi: 10.1016/j.bmc.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 31.Karuppagounder S.S., Pinto J.T., Xu H., Chen H.L., Beal M.F., Gibson G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Int. 2009;54(2):111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezai-Zadeh K., Arendash G.W., Hou H., Fernandez F., Jensen M., Runfeldt M., Shytle R.D., Tan J. Green tea epigallocatechin-3-gallate (EGCG) reduces β-amyloid mediated cognitive impairment and modulates tau pathology in Alzheimer transgenic mice. Brain Res. 2008;1214:177–187. doi: 10.1016/j.brainres.2008.02.107. [DOI] [PubMed] [Google Scholar]
- 33.Fernandes L., Cardim-Pires T.R., Foguel D., Palhano F.L. Green tea polyphenol epigallocatechin-gallate in amyloid aggregation and neurodegenerative diseases. Front. Neurosci. 2021;15:718188. doi: 10.3389/fnins.2021.718188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stromer T., Serpell L.C. Structure and morphology of the Alzheimer’s amyloid fibril. Microsc. Res. Tech. 2005;67(3-4):210–217. doi: 10.1002/jemt.20190. [DOI] [PubMed] [Google Scholar]
- 35.Lührs T., Ritter C., Adrian M., Riek-Loher D., Bohrmann B., Döbeli H., Schubert D., Riek R. 3D structure of Alzheimer’s amyloid-β(1–42) fibrils. Proc. Natl. Acad. Sci. USA. 2005;102(48):17342–17347. doi: 10.1073/pnas.0506723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross C.A., Poirier M.A. What is the role of protein aggregation in neurodegeneration? Nat. Rev. Mol. Cell Biol. 2005;6(11):891–898. doi: 10.1038/nrm1742. [DOI] [PubMed] [Google Scholar]
- 37.Arimon M., Díez-Pérez I., Kogan M.J., Durany N., Giralt E., Sanz F., Fernández-Busquets X. Fine structure study of Aβ 1–42 fibrillogenesis with atomic force microscopy. FASEB J. 2005;19(10):1344–1346. doi: 10.1096/fj.04-3137fje. [DOI] [PubMed] [Google Scholar]
- 38.Kheterpal I., Lashuel H.A., Hartley D.M., Walz T., Lansbury P.T., Jr, Wetzel R. Abeta protofibrils possess a stable core structure resistant to hydrogen exchange. Biochemistry. 2003;42(48):14092–14098. doi: 10.1021/bi0357816. [DOI] [PubMed] [Google Scholar]
- 39.Williams A.D., Sega M., Chen M., Kheterpal I., Geva M., Berthelier V., Kaleta D.T., Cook K.D., Wetzel R. Structural properties of Aβ protofibrils stabilized by a small molecule. Proc. Natl. Acad. Sci. USA. 2005;102(20):7115–7120. doi: 10.1073/pnas.0408582102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicoll A.J., Panico S., Freir D.B., Wright D., Terry C., Risse E., Herron C.E., O’Malley T., Wadsworth J.D.F., Farrow M.A., Walsh D.M., Saibil H.R., Collinge J. Amyloid-β nanotubes are associated with prion protein-dependent synaptotoxicity. Nat. Commun. 2013;4(1):2416. doi: 10.1038/ncomms3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selkoe D.J. Cell biology of protein misfolding: The examples of Alzheimer’s and Parkinson’s diseases. Nat. Cell Biol. 2004;6(11):1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 42.Müller-Hill B., Beyreuther K. Molecular biology of Alzheimer’s disease. Annu. Rev. Biochem. 1989;58:287–307. doi: 10.1146/annurev.bi.58.070189.001443. [DOI] [PubMed] [Google Scholar]
- 43.Chromy B.A., Nowak R.J., Lambert M.P., Viola K.L., Chang L., Velasco P.T., Jones B.W., Fernandez S.J., Lacor P.N., Horowitz P., Finch C.E., Krafft G.A., Klein W.L. Self-assembly of Abeta(1-42) into globular neurotoxins. Biochemistry. 2003;42(44):12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- 44.Klein W.L., Stine W.B., Jr, Teplow D.B. Small assemblies of unmodified amyloid β-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol. Aging. 2004;25(5):569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Dahlgren K.N., Manelli A.M., Stine W.B., Jr, Baker L.K., Krafft G.A., LaDu M.J. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J. Biol. Chem. 2002;277(35):32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 46.Walsh D.M., Klyubin I., Fadeeva J.V., Cullen W.K., Anwyl R., Wolfe M.S., Rowan M.J., Selkoe D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416(6880):535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 47.Walsh D.M., Klyubin I., Fadeeva J.V., Rowan M.J., Selkoe D.J. Amyloid-β oligomers: their production, toxicity and therapeutic inhibition. Biochem. Soc. Trans. 2002;30(4):552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- 48.Shea D., Daggett V. Amyloid-β oligomers: multiple moving targets. Biophysica. 2022;2(2):91–110. doi: 10.3390/biophysica2020010. [DOI] [Google Scholar]
- 49.Kagan B.L., Hirakura Y., Azimov R., Azimova R., Lin M.C. The channel hypothesis of Alzheimer’s disease: current status. Peptides. 2002;23(7):1311–1315. doi: 10.1016/S0196-9781(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 50.Lashuel H.A., Hartley D., Petre B.M., Walz T., Lansbury P.T. Neurodegenerative disease: Amyloid pores from pathogenic mutations. Nature. 2002;418(6895):291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 51.Quist A., Doudevski I., Lin H., Azimova R., Ng D., Frangione B., Kagan B., Ghiso J., Lal R. Amyloid ion channels: A common structural link for protein-misfolding disease. Proc. Natl. Acad. Sci. USA. 2005;102(30):10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mattson M.P., Chan S.L. Neuronal and glial calcium signaling in Alzheimer’s disease. Cell Calcium. 2003;34(4-5):385–397. doi: 10.1016/S0143-4160(03)00128-3. [DOI] [PubMed] [Google Scholar]
- 53.Le Y., Gong W., Tiffany H.L., Tumanov A., Nedospasov S., Shen W., Dunlop N.M., Gao J.L., Murphy P.M., Oppenheim J.J., Wang J.M. Amyloid (β)42 activates a G-protein-coupled chemoattractant receptor, FPR-like-1. J. Neurosci. 2001;21(2):RC123–RC123. doi: 10.1523/JNEUROSCI.21-02-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazo N.D., Grant M.A., Condron M.C., Rigby A.C., Teplow D.B. On the nucleation of amyloid β-protein monomer folding. Protein Sci. 2005;14(6):1581–1596. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y., Shen J., Luo X., Zhu W., Chen K., Ma J., Jiang H. Conformational transition of amyloid β-peptide. Proc. Natl. Acad. Sci. USA. 2005;102(15):5403–5407. doi: 10.1073/pnas.0501218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Masters C.L., Selkoe D.J. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2012;2(6):a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayden E.Y., Teplow D.B. Amyloid β-protein oligomers and Alzheimer’s disease. Alzheimers Res. Ther. 2013;5(6):60. doi: 10.1186/alzrt226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walsh D.M., Selkoe D.J.A. Oligomers? a decade of discovery. J. Neurochem. 2007;101(5):1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 59.Wang H., Kulas J.A., Wang C., Holtzman D.M., Ferris H.A., Hansen S.B. Regulation of beta-amyloid production in neurons by astrocyte-derived cholesterol. Proc. Natl. Acad. Sci. USA. 2021;118(33):e2102191118. doi: 10.1073/pnas.2102191118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Townsend M., Shankar G.M., Mehta T., Walsh D.M., Selkoe D.J. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J. Physiol. 2006;572(2):477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14(1):32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen G., Xu T., Yan Y., Zhou Y., Jiang Y., Melcher K., Xu H.E. Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017;38(9):1205–1235. doi: 10.1038/aps.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mittendorf K.F., Deatherage C.L., Ohi M.D., Sanders C.R. Tailoring of membrane proteins by alternative splicing of pre-mRNA. Biochemistry. 2012;51(28):5541–5556. doi: 10.1021/bi3007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y., Liu J., Huang B., Xu Y.M., Li J., Huang L.F., Lin J., Zhang J., Min Q.H., Yang W.M., Wang X.Z. Mechanism of alternative splicing and its regulation. Biomed. Rep. 2015;3(2):152–158. doi: 10.3892/br.2014.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ren P., Lu L., Cai S., Chen J., Lin W., Han F. Alternative splicing: A new cause and potential therapeutic target in autoimmune disease. Front. Immunol. 2021;12:713540. doi: 10.3389/fimmu.2021.713540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: Progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 68.Zhang L., Trushin S., Christensen T.A., Tripathi U., Hong C., Geroux R.E., Howell K.G., Poduslo J.F., Trushina E. Differential effect of amyloid beta peptides on mitochondrial axonal trafficking depends on their state of aggregation and binding to the plasma membrane. Neurobiol. Dis. 2018;114:1–16. doi: 10.1016/j.nbd.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8(6):595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bayer T.A., Wirths O. Focusing the amyloid cascade hypothesis on N-truncated Abeta peptides as drug targets against Alzheimer’s disease. Acta Neuropathol. 2014;127(6):787–801. doi: 10.1007/s00401-014-1287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wong P.C., Cai H., Borchelt D.R., Price D.L. Genetically engineered mouse models of neurodegenerative diseases. Nat. Neurosci. 2002;5(7):633–639. doi: 10.1038/nn0702-633. [DOI] [PubMed] [Google Scholar]
- 72.Irie K., Murakami K., Masuda Y., Morimoto A., Ohigashi H., Ohashi R., Takegoshi K., Nagao M., Shimizu T., Shirasawa T. Structure of β-amyloid fibrils and its relevance to their neurotoxicity: Implications for the pathogenesis of Alzheimer’s disease. J. Biosci. Bioeng. 2005;99(5):437–447. doi: 10.1263/jbb.99.437. [DOI] [PubMed] [Google Scholar]
- 73.Murphy M.P., LeVine H. III Alzheimer’s disease and the amyloid-β peptide. J. Alzheimers Dis. 2010;19(1):311–323. doi: 10.3233/JAD-2010-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tamagno E., Guglielmotto M., Monteleone D., Manassero G., Vasciaveo V., Tabaton M. The unexpected role of Aβ1-42 monomers in the pathogenesis of Alzheimer’s disease. J. Alzheimers Dis. 2018;62(3):1241–1245. doi: 10.3233/JAD-170581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Michno W., Nyström S., Wehrli P., Lashley T., Brinkmalm G., Guerard L., Syvänen S., Sehlin D., Kaya I., Brinet D., Nilsson K.P.R., Hammarström P., Blennow K., Zetterberg H., Hanrieder J. Pyroglutamation of amyloid-βx-42 (Aβx-42) followed by Aβ1–40 deposition underlies plaque polymorphism in progressing Alzheimer’s disease pathology. J. Biol. Chem. 2019;294(17):6719–6732. doi: 10.1074/jbc.RA118.006604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang X., Fu Z., Meng L., He M., Zhang Z. The early events that initiate β-amyloid aggregation in Alzheimer’s disease. Front. Aging Neurosci. 2018;10:359. doi: 10.3389/fnagi.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stanford P.M., Shepherd C.E., Halliday G.M., Brooks W.S., Schofield P.W., Brodaty H., Martins R.N., Kwok J.B., Schofield P.R. Mutations in the tau gene that cause an increase in three repeat tau and frontotemporal dementia. Brain. 2003;126(4):814–826. doi: 10.1093/brain/awg090. [DOI] [PubMed] [Google Scholar]
- 78.Goedert M., Jakes R. Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta Mol. Basis Dis. 2005;1739(2-3):240–250. doi: 10.1016/j.bbadis.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003;4(1):49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 80.Roberson E.D., Mucke L. 100 Years and counting: Prospects for defeating Alzheimer’s disease. Science. 2006;314(5800):781–784. doi: 10.1126/science.1132813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sciaccaluga M., Megaro A., Bellomo G., Ruffolo G., Romoli M., Palma E., Costa C. An unbalanced synaptic transmission: Cause or consequence of the amyloid oligomers neurotoxicity? Int. J. Mol. Sci. 2021;22(11):5991. doi: 10.3390/ijms22115991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Amin L. Harris, D.A. Aβ receptors specifically recognize molecular features displayed by fibril ends and neurotoxic oligomers. Nat. Commun. 2021;12(1):3451. doi: 10.1038/s41467-021-23507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moir R.D., Lathe R., Tanzi R.E. The antimicrobial protection hypothesis of Alzheimer’s disease. Alzheimers Dement. 2018;14(12):1602–1614. doi: 10.1016/j.jalz.2018.06.3040. [DOI] [PubMed] [Google Scholar]
- 84.Guerrero-Muñoz M.J., Gerson J., Castillo-Carranza D.L. Tau oligomers: The toxic player at synapses in Alzheimer’s disease. Front. Cell. Neurosci. 2015;9:464. doi: 10.3389/fncel.2015.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hector A., Brouillette J. Hyperactivity induced by soluble amyloid-β oligomers in the early stages of Alzheimer’s disease. Front. Mol. Neurosci. 2021;13:600084. doi: 10.3389/fnmol.2020.600084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sadigh-Eteghad S., Sabermarouf B., Majdi A., Talebi M., Farhoudi M., Mahmoudi J. Amyloid-beta: a crucial factor in Alzheimer’s disease. Med. Princ. Pract. 2015;24(1):1–10. doi: 10.1159/000369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Palmqvist S., Schöll M., Strandberg O., Mattsson N., Stomrud E., Zetterberg H., Blennow K., Landau S., Jagust W., Hansson O. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat. Commun. 2017;8(1):1214. doi: 10.1038/s41467-017-01150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferreira S.T., Lourenco M.V., Oliveira M.M., De Felice F.G. Soluble amyloid-Î2 oligomers as synaptotoxins leading to cognitive impairment in Alzheimer’s disease. Front. Cell. Neurosci. 2015;9:191. doi: 10.3389/fncel.2015.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Esparza T.J., Wildburger N.C., Jiang H., Gangolli M., Cairns N.J., Bateman R.J., Brody D.L. Soluble amyloid-beta aggregates from human Alzheimer’s disease brains. Sci. Rep. 2016;6(1):38187. doi: 10.1038/srep38187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sengupta U., Nilson A.N., Kayed R. The role of amyloid-β Oligomers in toxicity, propagation, and immunotherapy. EBioMedicine. 2016;6:42–49. doi: 10.1016/j.ebiom.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Noguchi A., Matsumura S., Dezawa M., Tada M., Yanazawa M., Ito A., Akioka M., Kikuchi S., Sato M., Ideno S., Noda M., Fukunari A., Muramatsu S., Itokazu Y., Sato K., Takahashi H., Teplow D.B., Nabeshima Y., Kakita A., Imahori K., Hoshi M. Isolation and characterization of patient-derived, toxic, high mass amyloid β-protein (Abeta) assembly from Alzheimer disease brains. J. Biol. Chem. 2009;284(47):32895–32905. doi: 10.1074/jbc.M109.000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan S.D., Stern D.M. Mitochondrial dysfunction and Alzheimer’s disease: role of amyloid-β peptide alcohol dehydrogenase (ABAD). Int. J. Exp. Pathol. 2005;86(3):161–171. doi: 10.1111/j.0959-9673.2005.00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maynard C.J., Bush A.I., Masters C.L., Cappai R., Li Q.X. Metals and amyloid-β in Alzheimer’s disease. Int. J. Exp. Pathol. 2005;86(3):147–159. doi: 10.1111/j.0959-9673.2005.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tomiyama T., Shimada H. APP osaka mutation in familial Alzheimer’s disease—Its discovery, phenotypes, and mechanism of recessive inheritance. Int. J. Mol. Sci. 2020;21(4):1413. doi: 10.3390/ijms21041413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ding Y., Zhao J., Zhang X., Wang S., Viola K.L., Chow F.E., Zhang Y., Lippa C., Klein W.L., Gong Y. Amyloid beta oligomers target to extracellular and intracellular neuronal synaptic proteins in Alzheimer’s disease. Front. Neurol. 2019;10:1140. doi: 10.3389/fneur.2019.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh D.M., Tseng B.P., Rydel R.E., Podlisny M.B., Selkoe D.J. The oligomerization of amyloid β-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39(35):10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 97.Teplow D.B. Structural and kinetic features of amyloid β-protein fibrillogenesis. Amyloid. 1998;5(2):121–142. doi: 10.3109/13506129808995290. [DOI] [PubMed] [Google Scholar]
- 98.Caughey B., Lansbury P.T. Jr Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu. Rev. Neurosci. 2003;26(1):267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 99.Petkova A.T., Leapman R.D., Guo Z., Yau W-M., Mattson M.P., Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer’s β-amyloid fibrils. Science. 2005;307(5707):262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 100.Harper J.D., Lansbury P.T. Models of amyloid seeding in Alzheimer’s disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 1997;66:385–407. doi: 10.1146/annurev.biochem.66.1.385. [DOI] [PubMed] [Google Scholar]
- 101.O’Nuallain B., Williams A.D., Westermark P., Wetzel R. Seeding specificity in amyloid growth induced by heterologous fibrils. J. Biol. Chem. 2004;279(17):17490–17499. doi: 10.1074/jbc.M311300200. [DOI] [PubMed] [Google Scholar]
- 102.Harper J.D., Wong S.S., Lieber C.M., Lansbury P.T. Jr Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem. Biol. 1997;4(2):119–125. doi: 10.1016/S1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 103.Emendato A., Milordini G., Zacco E., Sicorello A., Dal Piaz F., Guerrini R., Thorogate R., Picone D., Pastore A. Glycation affects fibril formation of Aβ peptides. J. Biol. Chem. 2018;293(34):13100–13111. doi: 10.1074/jbc.RA118.002275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abedin F., Kandel N., Tatulian S.A. Effects of Aβ-derived peptide fragments on fibrillogenesis of Aβ. Sci. Rep. 2021;11(1):19262. doi: 10.1038/s41598-021-98644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hoshi M., Sato M., Matsumoto S., Noguchi A., Yasutake K., Yoshida N., Sato K. Spherical aggregates of β-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3β. Proc. Natl. Acad. Sci. USA. 2003;100(11):6370–6375. doi: 10.1073/pnas.1237107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sahoo B., Nag S., Sengupta P., Maiti S. On the stability of the soluble amyloid aggregates. Biophys. J. 2009;97(5):1454–1460. doi: 10.1016/j.bpj.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ranganathan S., Ghosh D., Maji S.K., Padinhateeri R. A minimal conformational switching-dependent model for amyloid self-assembly. Sci. Rep. 2016;6(1):21103. doi: 10.1038/srep21103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ladiwala A.R.A., Litt J., Kane R.S., Aucoin D.S., Smith S.O., Ranjan S., Davis J., Van Nostrand W.E., Tessier P.M. Conformational differences between two amyloid β oligomers of similar size and dissimilar toxicity. J. Biol. Chem. 2012;287(29):24765–24773. doi: 10.1074/jbc.M111.329763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Quartey M.O., Nyarko J.N.K., Maley J.M., Barnes J.R., Bolanos M.A.C., Heistad R.M., Knudsen K.J., Pennington P.R., Buttigieg J., De Carvalho C.E., Leary S.C., Parsons M.P., Mousseau D.D. The Aβ(1–38) peptide is a negative regulator of the Aβ(1–42) peptide implicated in Alzheimer disease progression. Sci. Rep. 2021;11(1):431. doi: 10.1038/s41598-020-80164-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Novo M., Freire S., Al-Soufi W. Critical aggregation concentration for the formation of early Amyloid-β (1–42) oligomers. Sci. Rep. 2018;8(1):1783. doi: 10.1038/s41598-018-19961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.O’Nuallain B., Shivaprasad S., Kheterpal I., Wetzel R. Thermodynamics of A β(1-40) amyloid fibril elongation. Biochemistry. 2005;44(38):12709–12718. doi: 10.1021/bi050927h. [DOI] [PubMed] [Google Scholar]
- 112.Pallitto M.M., Murphy R.M. A mathematical model of the kinetics of β-amyloid fibril growth from the denatured state. Biophys. J. 2001;81(3):1805–1822. doi: 10.1016/S0006-3495(01)75831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nasica-Labouze J., Nguyen P.H., Sterpone F., Berthoumieu O., Buchete N.V., Coté S., De Simone A., Doig A.J., Faller P., Garcia A., Laio A., Li M.S., Melchionna S., Mousseau N., Mu Y., Paravastu A., Pasquali S., Rosenman D.J., Strodel B., Tarus B., Viles J.H., Zhang T., Wang C., Derreumaux P. Amyloid β protein and Alzheimer’s disease: When computer simulations complement experimental studies. Chem. Rev. 2015;115(9):3518–3563. doi: 10.1021/cr500638n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.König A.S., Rösener N.S., Gremer L., Tusche M., Flender D., Reinartz E., Hoyer W., Neudecker P., Willbold D., Heise H. Structural details of amyloid β oligomers in complex with human prion protein as revealed by solid-state MAS NMR spectroscopy. J. Biol. Chem. 2021;296:100499. doi: 10.1016/j.jbc.2021.100499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mastrangelo I.A., Ahmed M., Sato T., Liu W., Wang C., Hough P., Smith S.O. High-resolution atomic force microscopy of soluble Abeta42 oligomers. J. Mol. Biol. 2006;358(1):106–119. doi: 10.1016/j.jmb.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 116.Kirkitadze M.D., Condron M.M., Teplow D.B. Identification and characterization of key kinetic intermediates in amyloid β-protein fibrillogenesis11Edited by F. Cohen. J. Mol. Biol. 2001;312(5):1103-1119. doi: 10.1006/jmbi.2001.4970. [DOI] [PubMed] [Google Scholar]
- 117.Naldi M., Fiori J., Pistolozzi M., Drake A.F., Bertucci C., Wu R., Mlynarczyk K., Filipek S., De Simone A., Andrisano V. Amyloid β-peptide 25-35 self-assembly and its inhibition: a model undecapeptide system to gain atomistic and secondary structure details of the Alzheimer’s disease process and treatment. ACS Chem. Neurosci. 2012;3(11):952–962. doi: 10.1021/cn3000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tew D.J., Bottomley S.P., Smith D.P., Ciccotosto G.D., Babon J., Hinds M.G., Masters C.L., Cappai R., Barnham K.J. Stabilization of neurotoxic soluble β-sheet-rich conformations of the Alzheimer’s disease amyloid-β peptide. Biophys. J. 2008;94(7):2752–2766. doi: 10.1529/biophysj.107.119909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shea D. Hsu, C.C.; Bi, T.M.; Paranjapye, N.; Childers, M.C.; Cochran, J.; Tomberlin, C.P.; Wang, L.; Paris, D.; Zonderman, J.; Varani, G.; Link, C.D.; Mullan, M.; Daggett, V. α-Sheet secondary structure in amyloid β-peptide drives aggregation and toxicity in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 2019;116(18):8895–8900. doi: 10.1073/pnas.1820585116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iannuzzi C., Maritato R., Irace G., Sirangelo I. Misfolding and amyloid aggregation of apomyoglobin. Int. J. Mol. Sci. 2013;14(7):14287–14300. doi: 10.3390/ijms140714287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ban T., Hoshino M., Takahashi S., Hamada D., Hasegawa K., Naiki H., Goto Y. Direct observation of Abeta amyloid fibril growth and inhibition. J. Mol. Biol. 2004;344(3):757–767. doi: 10.1016/j.jmb.2004.09.078. [DOI] [PubMed] [Google Scholar]
- 122.Carulla N., Caddy G.L., Hall D.R., Zurdo J., Gairí M., Feliz M., Giralt E., Robinson C.V., Dobson C.M. Molecular recycling within amyloid fibrils. Nature. 2005;436(7050):554–558. doi: 10.1038/nature03986. [DOI] [PubMed] [Google Scholar]
- 123.O’sullivan M.J., Lindsay A.J. The endosomal recycling pathway-at the crossroads of the cell. Int. J. Mol. Sci. 2020;21(17):6074. doi: 10.3390/ijms21176074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaether C., Schmitt S., Willem M., Haass C. Amyloid precursor protein and Notch intracellular domains are generated after transport of their precursors to the cell surface. Traffic. 2006;7(4):408–415. doi: 10.1111/j.1600-0854.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- 125.Tancini B., Buratta S., Delo F., Sagini K., Chiaradia E., Pellegrino R.M., Emiliani C., Urbanelli L. Lysosomal exocytosis: The extracellular role of an intracellular organelle. Membranes (Basel) 2020;10(12):406. doi: 10.3390/membranes10120406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Baker H.F., Ridley R.M., Duchen L.W., Crow T.J., Bruton C.J. Experimental induction of β-amyloid plaques and cerebral angiopathy in primates. Ann. N. Y. Acad. Sci. 1993;695(1):228–231. doi: 10.1111/j.1749-6632.1993.tb23057.x. [DOI] [PubMed] [Google Scholar]
- 127.Bückig A., Tikkanen R., Herzog V., Schmitz A. Cytosolic and nuclear aggregation of the amyloid β-peptide following its expression in the endoplasmic reticulum. Histochem. Cell Biol. 2002;118(5):353–360. doi: 10.1007/s00418-002-0459-2. [DOI] [PubMed] [Google Scholar]
- 128.Long J.M., Holtzman D.M. Alzheimer disease: An update on pathobiology and treatment strategies. Cell. 2019;179(2):312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bharadwaj P.R. Dubey, A.K.; Masters, C.L.; Martins, R.N.; Macreadie, I.G. Aβ aggregation and possible implications in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 2009;13(3):412–421. doi: 10.1111/j.1582-4934.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kondratyuk T.P., Pezzuto J.M. Natural product polyphenols of relevance to human health. Pharma. Biol. 2009;42(sup1):46-63. doi: 10.3109/13880200490893519. [DOI] [Google Scholar]
- 131.Spencer J.P.E., Abd El Mohsen M.M., Minihane A.M., Mathers J.C. Biomarkers of the intake of dietary polyphenols: strengths, limitations and application in nutrition research. Br. J. Nutr. 2008;99(1):12–22. doi: 10.1017/S0007114507798938. [DOI] [PubMed] [Google Scholar]
- 132.Sova M., Saso L. Natural sources, pharmacokinetics, biological activities and health benefits of hydroxycinnamic acids and their metabolites. Nutrients. 2020;12(8):2190. doi: 10.3390/nu12082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Taofiq O., González-Paramás A.M., Barreiro M.F., Ferreira I.C.F.R. Hydroxycinnamic acids and their derivatives: Cosmeceutical significance, challenges and future perspectives, a review. Molecules. 2017;22(1):281. doi: 10.3390/molecules22020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.El-Seedi H.R., Taher E.A., Sheikh B.Y., Anjum S., Saeed A., AlAjmi M.F., Moustafa M.S., Al-Mousawi S.M., Farag M.A., Hegazy M-E.F., Khalifa S.A.M., Göransson U. Hydroxycinnamic acids: Natural sources, biosynthesis, possible biological activities, and roles in islamic medicine. Stud. Nat. Prod. Chem. 2018;55:269–292. doi: 10.1016/B978-0-444-64068-0.00008-5. [DOI] [Google Scholar]
- 135.Silveira A.C., Dias J.P., Santos V.M., Oliveira P.F., Alves M.G., Rato L., Silva B.M. The Action of polyphenols in diabetes mellitus and Alzheimer’s disease: A common agent for overlapping pathologies. Curr. Neuropharmacol. 2019;17(7):590–613. doi: 10.2174/1570159X16666180803162059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tomás-Barberán F.A., Clifford M.N. Dietary hydroxybenzoic acid derivatives – nature, occurrence and dietary burden. J. Sci. Food Agric. 2000;80:1024–1032. doi: 10.1002/(SICI)1097-0010(20000515)80:7<1024:AID-JSFA567>3.0.CO;2-S. [DOI] [Google Scholar]
- 137.Sarker U., Oba S. Phenolic profiles and antioxidant activities in selected drought-tolerant leafy vegetable amaranth. Sci. Rep. 2020;10(1):18287. doi: 10.1038/s41598-020-71727-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bernatoniene J., Kopustinskiene D.M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23(4):965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arts I.C.W., van de Putte B., Hollman P.C.H. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food Chem. 2000;48(5):1752–1757. doi: 10.1021/jf000026+. [DOI] [PubMed] [Google Scholar]
- 140.Márquez-Rodríguez A.S., Grajeda-Iglesias C., Sánchez-Bojorge N.A., Figueroa-Espinoza M. -.C.; Rodríguez-Valdez, L-.M.; Fuentes-Montero, M.E.; Salas, E. Theoretical characterization by density functional theory (DFT) of delphinidin 3-O-sambubioside and its esters obtained by chemical lipophilization. Molecules. 2018;23(7):1587. doi: 10.3390/molecules23071587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wu X., Prior R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J. Agric. Food Chem. 2005;53(7):2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 142.Almeida S., Alves M.G., Sousa M., Oliveira P.F., Silva B.M. Are Polyphenols Strong Dietary Agents Against Neurotoxicity and Neurodegeneration? Neurotox. Res. 2016;30(3):345–366. doi: 10.1007/s12640-015-9590-4. [DOI] [PubMed] [Google Scholar]
- 143.A Centum of Valuable Plant Bioactives. 1st Ed.; Elsevier Academic; 2021. Hesperidin and naringenin. p. 403-429. [DOI] [Google Scholar]
- 144.Matsumoto H., Ikoma Y., Sugiura M., Yano M., Hasegawa Y. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004;52(21):6653–6659. doi: 10.1021/jf0491411. [DOI] [PubMed] [Google Scholar]
- 145.Wei J., Bhatt S., Chang L.M., Sampson H.A., Masilamani M. Isoflavones, genistein and daidzein, regulate mucosal immune response by suppressing dendritic cell function. PLoS One. 2012;7(10):e47979. doi: 10.1371/journal.pone.0047979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pan W., Ikeda K., Takebe M., Yamori Y. Genistein, daidzein and glycitein inhibit growth and DNA synthesis of aortic smooth muscle cells from stroke-prone spontaneously hypertensive rats. J. Nutr. 2001;131(4):1154–1158. doi: 10.1093/jn/131.4.1154. [DOI] [PubMed] [Google Scholar]
- 147.Poschner S., Maier-Salamon A., Zehl M., Wackerlig J., Dobusch D., Pachmann B., Sterlini K.L., Jäger W. The impacts of genistein and daidzein on estrogen conjugations in human breast cancer cells: a targeted metabolomics approach. Front. Pharmacol. 2017;8:699. doi: 10.3389/fphar.2017.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hostetler G.L., Ralston R.A., Schwartz S.J. Flavones: Food sources, bioavailability, metabolism, and bioactivity. Adv. Nutr. 2017;8(3):423–435. doi: 10.3945/an.116.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Choy K.W., Murugan D., Leong X.F., Abas R., Alias A., Mustafa M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-kappa B (NFκB) signaling in cardiovascular diseases: A mini review. Front. Pharmacol. 2019;10:1295. doi: 10.3389/fphar.2019.01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nijveldt R.J., van Nood E., van Hoorn D.E.C., Boelens P.G., van Norren K., van Leeuwen P.A.M. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001;74(4):418–425. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 151.Buchner N., Krumbein A., Rohn S., Kroh L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006;20(21):3229–3235. doi: 10.1002/rcm.2720. [DOI] [PubMed] [Google Scholar]
- 152.Suprun A.R., Dubrovina A.S., Tyunin A.P., Kiselev K.V. Profile of stilbenes and other phenolics in fanagoria white and red russian wines. Metabolites. 2021;11(4):231. doi: 10.3390/metabo11040231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Błaszczyk, A.; Sady, S.; Sielicka, M. The stilbene profile in edible berries. Phytochem. Rev. 2019;18(1):37–67. doi: 10.1007/s11101-018-9580-2. [DOI] [Google Scholar]
- 154.Rodríguez-García C., Sánchez-Quesada C., Toledo E., Delgado-Rodríguez M., Gaforio J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules. 2019;24(5):917. doi: 10.3390/molecules24050917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Milder I.E.J., Kuijsten A., Arts I.C.W., Feskens E.J.M., Kampman E., Hollman P.C.H., Van ’t Veer P. Relation between plasma enterodiol and enterolactone and dietary intake of lignans in a Dutch endoscopy-based population. J. Nutr. 2007;137(5):1266–1271. doi: 10.1093/jn/137.5.1266. [DOI] [PubMed] [Google Scholar]
- 156.Amalraj A., Pius A., Gopi S., Gopi S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives – A review. J. Tradit. Complement. Med. 2017;7(2):205–233. doi: 10.1016/j.jtcme.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ahmadifar E., Yousefi M., Karimi M., Raieni R.F., Dadar M., Yilmaz S., Dawood M.A.O., Abdel-Latif H.M.R. Benefits of dietary polyphenols and polyphenol-rich additives to aquatic animal health: An overview. Rev. Fish. Sci. 2020;29(4):478–511. doi: 10.1080/23308249.2020.1818689. [DOI] [Google Scholar]
- 158.Patel K., Kumar V., Rahman M., Verma A., Patel D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni. Suef Univ. J. Basic Appl. Sci. 2018;7(1):31–42. doi: 10.1016/j.bjbas.2017.05.009. [DOI] [Google Scholar]
- 159.Pandey K., Rizvi S. Current understanding of dietary polyphenols and their role in health and disease. Curr. Nutr. Food Sci. 2009;5(4):249–263. doi: 10.2174/157340109790218058. [DOI] [Google Scholar]
- 160.Diniz C., Suliburska J., Ferreira I.M.P.L.V.O. New insights into the antiangiogenic and proangiogenic properties of dietary polyphenols. Mol. Nutr. Food Res. 2017;61(6):1600912. doi: 10.1002/mnfr.201600912. [DOI] [PubMed] [Google Scholar]
- 161.Adlercreutz H., Mazur W. Phyto-oestrogens and western diseases. Ann. Med. 1997;29(2):95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 162.Shahidi F., Yeo J.D. Insoluble-bound phenolics in food. Molecules. 2016;21(9):1216. doi: 10.3390/molecules21091216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J. Nutr. Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shahidi F., Ambigaipalan P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects – A review. J. Funct. Foods. 2015;18:820–897. doi: 10.1016/j.jff.2015.06.018. [DOI] [Google Scholar]
- 165.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 166.Bibi N., Shah M.H., Khan N., Al-Hashimi A., Elshikh M.S., Iqbal A., Ahmad S., Abbasi A.M. Variations in total phenolic, total flavonoid contents, and free radicals’ scavenging potential of onion varieties planted under diverse environmental conditions. Plants. 2022;11(7):950. doi: 10.3390/plants11070950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mansoor S., Sharma V., Mir M.A., Mir J.I. un Nabi, S.; Ahmed, N.; Alkahtani, J.; Alwahibi, M.S.; Masoodi, K.Z. Quantification of polyphenolic compounds and relative gene expression studies of phenylpropanoid pathway in apple (Malus domestica Borkh) in response to Venturia inaequalis infection. Saudi J. Biol. Sci. 2020;27(12):3397–3404. doi: 10.1016/j.sjbs.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang Q., Cao Y., Zhou L., Jiang C.Z., Feng Y., Wei S. Effects of postharvest curing treatment on flesh colour and phenolic metabolism in fresh-cut potato products. Food Chem. 2015;169:246–254. doi: 10.1016/j.foodchem.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 169.Arfaoui L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules. 2021;26(10):2959. doi: 10.3390/molecules26102959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kondakova V., Tsvetkov I., Batchvarova R., Badjakov I., Dzhambazova T., Slavov S. Phenol compounds-qualitative index in small fruits. Biotechnol. Biotechnol. Equip. 2014;23(4):1444–1448. doi: 10.2478/V10133-009-0024-4. [DOI] [Google Scholar]
- 171.Ravichandran K., Ahmed A.R., Knorr D., Smetanska I. The effect of different processing methods on phenolic acid content and antioxidant activity of red beet. Food Res. Int. 2012;48(1):16–20. doi: 10.1016/j.foodres.2012.01.011. [DOI] [Google Scholar]
- 172.Bar-Ya’akov I., Tian L., Amir R., Holland D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the pomegranate fruit. Front. Plant Sci. 2019;10:620. doi: 10.3389/fpls.2019.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hartman R.E., Shah A., Fagan A.M., Schwetye K.E., Parsadanian M., Schulman R.N., Finn M.B., Holtzman D.M. Pomegranate juice decreases amyloid load and improves behavior in a mouse model of Alzheimer’s disease. Neurobiol. Dis. 2006;24(3):506–515. doi: 10.1016/j.nbd.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 174.Miguel G., Fontes C., Antunes D., Neves A., Martins D. Anthocyanin concentration of “Assaria” pomegranate fruits during different cold storage conditions. J. Biomed. Biotechnol. 2004;2004(5):338–342. doi: 10.1155/S1110724304403076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Menard C., Bastianetto S., Quirion R. Neuroprotective effects of resveratrol and epigallocatechin gallate polyphenols are mediated by the activation of protein kinase C gamma. Front. Cell. Neurosci. 2013;7:281. doi: 10.3389/fncel.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yang F., Lim G.P., Begum A.N., Ubeda O.J., Simmons M.R., Ambegaokar S.S., Chen P.P., Kayed R., Glabe C.G., Frautschy S.A., Cole G.M. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005;280(7):5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 177.Joseph J.A., Denisova N.A., Arendash G., Gordon M., Diamond D., Shukitt-Hale B., Morgan D. Blueberry supplementation enhances signaling and prevents behavioral deficits in an Alzheimer disease model. Nutr. Neurosci. 2013;6(3):153–162. doi: 10.1080/1028415031000111282. [DOI] [PubMed] [Google Scholar]
- 178.Peng Q.L. Buz’Zard, A.R.; Lau, B.H.S. Pycnogenol® protects neurons from amyloid-β peptide-induced apoptosis. Brain Res. Mol. Brain Res. 2002;104(1):55–65. doi: 10.1016/S0169-328X(02)00263-2. [DOI] [PubMed] [Google Scholar]
- 179.Maimoona A., Naeem I., Saddiqe Z., Jameel K. A review on biological, nutraceutical and clinical aspects of French maritime pine bark extract. J. Ethnopharmacol. 2011;133(2):261–277. doi: 10.1016/j.jep.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 180.Talavéra S., Felgines C., Texier O., Besson C., Gil-Izquierdo A., Lamaison J.L., Rémésy C. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. J. Agric. Food Chem. 2005;53(10):3902–3908. doi: 10.1021/jf050145v. [DOI] [PubMed] [Google Scholar]
- 181.Passamonti S., Vrhovsek U., Vanzo A., Mattivi F. Fast access of some grape pigments to the brain. J. Agric. Food Chem. 2005;53(18):7029–7034. doi: 10.1021/jf050565k. [DOI] [PubMed] [Google Scholar]
- 182.Vepsäläinen S., Koivisto H., Pekkarinen E., Mäkinen P., Dobson G., McDougall G.J., Stewart D., Haapasalo A., Karjalainen R.O., Tanila H., Hiltunen M. Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2013;24(1):360–370. doi: 10.1016/j.jnutbio.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 183.Yamakawa M.Y., Uchino K., Watanabe Y., Adachi T., Nakanishi M., Ichino H., Hongo K., Mizobata T., Kobayashi S., Nakashima K., Kawata Y. Anthocyanin suppresses the toxicity of Aβ deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer’s disease. Nutr. Neurosci. 2016;19(1):32–42. doi: 10.1179/1476830515Y.0000000042. [DOI] [PubMed] [Google Scholar]
- 184.Essa M.M., Braidy N., Awlad-Thani K., Vaishnav R., Al-Asmi A., Guillemin G.J., Al-Adawi S., Subash S. Diet rich in date palm fruits improves memory, learning and reduces beta amyloid in transgenic mouse model of Alzheimer's disease. J. Ayurveda Integr. Med. 2015;6(2):111–120. doi: 10.4103/0975-9476.159073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Afzal M., Redha A., AlHasan R. Anthocyanins potentially contribute to defense against Alzheimer’s disease. Molecules. 2019;24(23):4255. doi: 10.3390/molecules24234255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Burton-Freeman B.M., Sandhu A.K., Edirisinghe I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016;7(1):44–65. doi: 10.3945/an.115.009639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fernando W.M.A.D.B., Somaratne G., Goozee K.G., Williams S., Singh H., Martins R.N. Diabetes and Alzheimer’s disease: can tea phytochemicals play a role in prevention? J. Alzheimers Dis. 2017;59(2):481–501. doi: 10.3233/JAD-161200. [DOI] [PubMed] [Google Scholar]
- 189.Vitaglione P., Donnarumma G., Napolitano A., Galvano F., Gallo A., Scalfi L., Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007;137(9):2043–2048. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 190.Belkacemi A., Ramassamy C. Innovative anthocyanin/anthocyanidin formulation protects SK-N-SH cells against the amyloid-β peptide-induced toxicity: Relevance to Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2015;16(1):37–49. doi: 10.2174/1871524915666150730125532. [DOI] [PubMed] [Google Scholar]
- 191.Isaak C.K., Petkau J.C., Blewett H., Karmin O., Siow Y.L. Lingonberry anthocyanins protect cardiac cells from oxidative-stress-induced apoptosis. Can. J. Physiol. Pharmacol. 2017;95(8):904–910. doi: 10.1139/cjpp-2016-0667. [DOI] [PubMed] [Google Scholar]
- 192.Badshah H., Kim T.H., Kim M.O. Protective effects of Anthocyanins against Amyloid beta-induced neurotoxicity in vivo and in vitro. Neurochem. Int. 2015;80:51–59. doi: 10.1016/j.neuint.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 193.Wu Y., Chen M., Jiang J. Mitochondrial dysfunction in neurodegenerative diseases and drug targets via apoptotic signaling. Mitochondrion. 2019;49:35–45. doi: 10.1016/j.mito.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 194.Pacheco S.M., Soares M.S.P., Gutierres J.M., Gerzson M.F.B., Carvalho F.B., Azambuja J.H., Schetinger M.R.C., Stefanello F.M., Spanevello R.M. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J. Nutr. Biochem. 2018;56:193–204. doi: 10.1016/j.jnutbio.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 195.Gutierres J.M., Carvalho F.B., Schetinger M.R.C., Marisco P., Agostinho P., Rodrigues M., Rubin M.A., Schmatz R., da Silva C.R. de P Cognato, G.; Farias, J.G.; Signor, C.; Morsch, V.M.; Mazzanti, C.M.; Bogo, M.; Bonan, C.D.; Spanevello, R. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014;96(1-2):7–17. doi: 10.1016/j.lfs.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 196.Shih P.H., Wu C.H., Yeh C.T., Yen G.C. Protective effects of anthocyanins against amyloid β-peptide-induced damage in neuro-2A cells. J. Agric. Food Chem. 2011;59(5):1683–1689. doi: 10.1021/jf103822h. [DOI] [PubMed] [Google Scholar]
- 197.Fraige K., Pereira-Filho E.R., Carrilho E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC–DAD–MS and exploratory analysis by principal component analysis. Food Chem. 2014;145:395–403. doi: 10.1016/j.foodchem.2013.08.066. [DOI] [PubMed] [Google Scholar]
- 198.Sun Q., Jia N., Li X., Yang J., Chen G. Grape seed proanthocyanidins ameliorate neuronal oxidative damage by inhibiting GSK-3β-dependent mitochondrial permeability transition pore opening in an experimental model of sporadic Alzheimer’s disease. Aging (Albany NY) 2019;11(12):4107–4124. doi: 10.18632/aging.102041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Galvano F., La Fauci L., Lazzarino G., Fogliano V., Ritieni A., Ciappellano S., Battistini N.C., Tavazzi B., Galvano G. Cyanidins: metabolism and biological properties. J. Nutr. Biochem. 2004;15(1):2–11. doi: 10.1016/j.jnutbio.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 200.Celik E., Sanlier N. Effects of nutrient and bioactive food components on Alzheimer’s disease and epigenetic. Crit. Rev. Food Sci. Nutr. 2019;59(1):102–113. doi: 10.1080/10408398.2017.1359488. [DOI] [PubMed] [Google Scholar]
- 201.Afzal M., Safer A.M., Menon M. Green tea polyphenols and their potential role in health and disease. Inflammopharmacology. 2015;23(4):151–161. doi: 10.1007/s10787-015-0236-1. [DOI] [PubMed] [Google Scholar]
- 202.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sagi S.A., Weggen S., Eriksen J., Golde T.E., Koo E.H. The non-cyclooxygenase targets of non-steroidal anti-inflammatory drugs, lipoxygenases, peroxisome proliferator-activated receptor, inhibitor of κB kinase, and NFκB, do not reduce amyloid β42 production. J. Biol. Chem. 2003;278(34):31825–31830. doi: 10.1074/jbc.M303588200. [DOI] [PubMed] [Google Scholar]
- 204.Liu H., Li Z., Qiu D., Gu Q., Lei Q., Mao L. The inhibitory effects of different curcuminoids on β-amyloid protein, β-amyloid precursor protein and β-site amyloid precursor protein cleaving enzyme 1 in swAPP HEK293 cells. Neurosci. Lett. 2010;485(2):83–88. doi: 10.1016/j.neulet.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 205.Wang X., Kim J.R., Lee S.B., Kim Y.J., Jung M.Y., Kwon H.W., Ahn Y.J. Effects of curcuminoids identified in rhizomes of Curcuma longa on BACE-1 inhibitory and behavioral activity and lifespan of Alzheimer’s disease Drosophila models. BMC Complement. Altern. Med. 2014;14(1):88. doi: 10.1186/1472-6882-14-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhang X., Yin W., Shi X., Li Y. Curcumin activates Wnt/β-catenin signaling pathway through inhibiting the activity of GSK-3β in APPswe transfected SY5Y cells. Eur. J. Pharm. Sci. 2011;42(5):540–546. doi: 10.1016/j.ejps.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 207.Parr C., Mirzaei N., Christian M., Sastre M. Activation of the Wnt/β‐catenin pathway represses the transcription of the β‐amyloid precursor protein cleaving enzyme (BACE1) via binding of T‐cell factor‐4 to BACE1 promoter. FASEB J. 2015;29(2):623–635. doi: 10.1096/fj.14-253211. [DOI] [PubMed] [Google Scholar]
- 208.Garcia-Alloza M., Borrelli L.A., Rozkalne A., Hyman B.T., Bacskai B.J. Curcumin labels amyloid pathology in vivo, disrupts existing plaques, and partially restores distorted neurites in an Alzheimer mouse model. J. Neurochem. 2007;102(4):1095–1104. doi: 10.1111/j.1471-4159.2007.04613.x. [DOI] [PubMed] [Google Scholar]
- 209.Farkhondeh T., Samarghandian S., Pourbagher-Shahri A.M., Sedaghat M. The impact of curcumin and its modified formulations on Alzheimer’s disease. J. Cell. Physiol. 2019;234(10):16953–16965. doi: 10.1002/jcp.28411. [DOI] [PubMed] [Google Scholar]
- 210.Reinke A.A., Gestwicki J.E. Structure-activity relationships of amyloid beta-aggregation inhibitors based on curcumin: influence of linker length and flexibility. Chem. Biol. Drug Des. 2007;70(3):206–215. doi: 10.1111/j.1747-0285.2007.00557.x. [DOI] [PubMed] [Google Scholar]
- 211.Rao P.P.N., Mohamed T., Teckwani K., Tin G. Curcumin binding to beta amyloid: A computational study. Chem. Biol. Drug Des. 2015;86(4):813–820. doi: 10.1111/cbdd.12552. [DOI] [PubMed] [Google Scholar]
- 212.Kundaikar H.S., Degani M.S. Insights into the interaction mechanism of ligands with A β 42 based on molecular dynamics simulations and mechanics: Implications of role of common binding site in drug design for Alzheimer’s disease. Chem. Biol. Drug Des. 2015;86(4):805–812. doi: 10.1111/cbdd.12555. [DOI] [PubMed] [Google Scholar]
- 213.Perrone L., Mothes E., Vignes M., Mockel A., Figueroa C., Miquel M.C., Maddelein M.L., Faller P. Copper transfer from Cu-Abeta to human serum albumin inhibits aggregation, radical production and reduces Abeta toxicity. ChemBioChem. 2010;11(1):110–118. doi: 10.1002/cbic.200900474. [DOI] [PubMed] [Google Scholar]
- 214.Banerjee P., Sahoo A., Anand S., Ganguly A., Righi G., Bovicelli P., Saso L., Chakrabarti S. Multiple mechanisms of iron-induced amyloid beta-peptide accumulation in SHSY5Y cells: protective action of negletein. Neuromol. Med. 2014;16(4):787–798. doi: 10.1007/s12017-014-8328-4. [DOI] [PubMed] [Google Scholar]
- 215.Kozmon S., Tvaroška I. Molecular dynamic studies of amyloid-beta interactions with curcumin and Cu2+ ions. Chem. Pap. 2015;69(9):1262–1276. doi: 10.1515/chempap-2015-0134. [DOI] [Google Scholar]
- 216.Kolli N., Lu M., Maiti P., Rossignol J., Dunbar G.L. Application of the gene editing tool, CRISPR-Cas9, for treating neurodegenerative diseases. Neurochem. Int. 2018;112:187–196. doi: 10.1016/j.neuint.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 217.Valera E., Dargusch R., Maher P.A., Schubert D. Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer’s disease. J. Neurosci. 2013;33(25):10512–10525. doi: 10.1523/JNEUROSCI.5183-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kuriyama S., Hozawa A., Ohmori K., Shimazu T., Matsui T., Ebihara S., Awata S., Nagatomi R., Arai H., Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project. Am. J. Clin. Nutr. 2006;83(2):355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 219.Del Rio D., Stewart A.J., Mullen W., Burns J., Lean M.E.J., Brighenti F., Crozier A. HPLC-MSn analysis of phenolic compounds and purine alkaloids in green and black tea. J. Agric. Food Chem. 2004;52(10):2807–2815. doi: 10.1021/jf0354848. [DOI] [PubMed] [Google Scholar]
- 220.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., Hardy J., Town T., Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25(38):8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Weinreb O., Mandel S., Amit T., Youdim M.B.H. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. J. Nutr. Biochem. 2004;15(9):506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 222.Mandel S.A., Amit T., Weinreb O., Reznichenko L., Youdim M.B.H. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci. Ther. 2008;14(4):352–365. doi: 10.1111/j.1755-5949.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ide K., Yamada H. Clinical benefits of green tea consumption for cognitive dysfunction. PharmaNutrition. 2015;3(4):136–145. doi: 10.1016/j.phanu.2015.07.001. [DOI] [Google Scholar]
- 224.Ali B., Jamal Q.M., Shams S., Al-Wabel N.A., Siddiqui M.U., Alzohairy M.A., Al Karaawi M.A., Kesari K.K., Mushtaq G., Kamal M.A. In silico analysis of green tea polyphenols as inhibitors of AChE and BChE enzymes in Alzheimer’s disease treatment. CNS Neurol. Disord. Drug Targets. 2016;15(5):624–628. doi: 10.2174/1871527315666160321110607. [DOI] [PubMed] [Google Scholar]
- 225.Bennett S., Grant M.M., Aldred S. Oxidative stress in vascular dementia and Alzheimer’s disease: a common pathology. J. Alzheimers Dis. 2008;17(2):245–257. doi: 10.3233/JAD-2009-1041. [DOI] [PubMed] [Google Scholar]
- 226.Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24(4):325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Praticò D. Evidence of oxidative stress in Alzheimer’s disease brain and antioxidant therapy: lights and shadows. Ann. N. Y. Acad. Sci. 2008;1147(1):70–78. doi: 10.1196/annals.1427.010. [DOI] [PubMed] [Google Scholar]
- 228.Haque A.M., Hashimoto M., Katakura M., Hara Y., Shido O. Green tea catechins prevent cognitive deficits caused by Aβ1–40 in rats. J. Nutr. Biochem. 2008;19(9):619–626. doi: 10.1016/j.jnutbio.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 229.Biasibetti R., Tramontina A.C., Costa A.P., Dutra M.F., Quincozes-Santos A., Nardin P., Bernardi C.L., Wartchow K.M., Lunardi P.S., Gonçalves C.A. Green tea (−)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2013;236(1):186–193. doi: 10.1016/j.bbr.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 230.Sang S., Tian S., Wang H., Stark R.E., Rosen R.T., Yang C.S., Ho C.T. Chemical studies of the antioxidant mechanism of tea catechins: radical reaction products of epicatechin with peroxyl radicals. Bioorg. Med. Chem. 2003;11(16):3371–3378. doi: 10.1016/S0968-0896(03)00367-5. [DOI] [PubMed] [Google Scholar]
- 231.Seeram N.P., Henning S.M., Niu Y., Lee R., Scheuller H.S., Heber D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006;54(5):1599–1603. doi: 10.1021/jf052857r. [DOI] [PubMed] [Google Scholar]
- 232.Mandel S., Youdim M.B.H. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004;37(3):304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 233.Weinreb O., Amit T., Mandel S., Youdim M.B.H. Neuroprotective molecular mechanisms of (−)-epigallocatechin-3-gallate: a reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009;4(4):283–296. doi: 10.1007/s12263-009-0143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ward R.J., Zucca F.A., Duyn J.H., Crichton R.R., Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13(10):1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Morales I., Guzmán-Martínez L., Cerda-Troncoso C., Farías G.A., Maccioni R.B. Neuroinflammation in the pathogenesis of Alzheimer’s disease. A rational framework for the search of novel therapeutic approaches. Front. Cell. Neurosci. 2014;8:112. doi: 10.3389/fncel.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lee Y.J., Choi D.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J. Nutr. Biochem. 2013;24(1):298–310. doi: 10.1016/j.jnutbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 237.Wu K.J., Hsieh M.T., Wu C.R., Wood W.G., Chen Y.F. Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuroinflammation. Evid. Based Complement. Alternat. Med. 2012;2012:1–11. doi: 10.1155/2012/163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Alkon D.L., Sun M.K., Nelson T.J. PKC signaling deficits: a mechanistic hypothesis for the origins of Alzheimer’s disease. Trends Pharmacol. Sci. 2007;28(2):51–60. doi: 10.1016/j.tips.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 239.Levites Y., Amit T., Mandel S., Youdim M.B.H. Neuroprotection and neurorescue against Aβ toxicity and PKC‐dependent release of non‐amyloidogenic soluble precursor protein by green tea polyphenol (‐)‐epigallocatechin‐3‐gallate. FASEB J. 2003;17(8):1–23. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 240.Kaur T., Pathak C.M., Pandhi P., Khanduja K.L. Effects of green tea extract on learning, memory, behavior and acetylcholinesterase activity in young and old male rats. Brain Cogn. 2008;67(1):25–30. doi: 10.1016/j.bandc.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 241.Kim H.K., Kim M., Kim S., Kim M., Chung J.H. Effects of green tea polyphenol on cognitive and acetylcholinesterase activities. Biosci. Biotechnol. Biochem. 2004;68(9):1977–1979. doi: 10.1271/bbb.68.1977. [DOI] [PubMed] [Google Scholar]
- 242.Snopek L., Mlcek J., Sochorova L., Baron M., Hlavacova I., Jurikova T., Kizek R., Sedlackova E., Sochor J. Contribution of Red Wine Consumption to Human Health Protection. Molecules. 2018;23(7):1684. doi: 10.3390/molecules23071684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wang J., Ho L., Zhao Z., Seror I., Humala N., Dickstein D.L., Thiyagarajan M., Percival S.S., Talcott S.T., Maria Pasinetti G. Moderate consumption of Cabernet Sauvignon attenuates A neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006;20(13):2313–2320. doi: 10.1096/fj.06-6281com. [DOI] [PubMed] [Google Scholar]
- 244.Li C., Wu X., Liu S., Zhao Y., Zhu J., Liu K. Roles of Neuropeptide Y in Neurodegenerative and Neuroimmune Diseases. Front. Neurosci. 2019;13:869. doi: 10.3389/fnins.2019.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Sánchez-Muniz F.J., Macho-González A., Garcimartín A., Santos-López J.A., Benedí J., Bastida S., González-Muñoz M.J. The nutritional components of beer and its relationship with neurodegeneration and Alzheimer’s disease. Nutrients. 2019;11(7):1558. doi: 10.3390/nu11071558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Silva P., Vauzour D. Wine polyphenols and neurodegenerative diseases: An update on the molecular mechanisms underpinning their protective effects. Beverages. 2018;4(4):96. doi: 10.3390/beverages4040096. [DOI] [Google Scholar]
- 247.Marambaud P., Zhao H., Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 2005;280(45):37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 248.Han Y.S., Zheng W.H., Bastianetto S., Chabot J.G., Quirion R. Neuroprotective effects of resveratrol against β -amyloid-induced neurotoxicity in rat hippocampal neurons: involvement of protein kinase C. Br. J. Pharmacol. 2004;141(6):997–1005. doi: 10.1038/sj.bjp.0705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Luchsinger J.A., Tang M.X., Siddiqui M., Shea S., Mayeux R. Alcohol intake and risk of dementia. J. Am. Geriatr. Soc. 2004;52(4):540–546. doi: 10.1111/j.1532-5415.2004.52159.x. [DOI] [PubMed] [Google Scholar]
- 250.Kim H., Park B.S., Lee K.G., Choi C.Y., Jang S.S., Kim Y.H., Lee S.E. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J. Agric. Food Chem. 2005;53(22):8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 251.Jagota S., Rajadas J. Effect of phenolic compounds against Aβ aggregation and Aβ-induced toxicity in transgenic C. elegans. Neurochem. Res. 2012;37(1):40–48. doi: 10.1007/s11064-011-0580-5. [DOI] [PubMed] [Google Scholar]
- 252.Jiménez-Aliaga K., Bermejo-Bescós P., Benedí J., Martín-Aragón S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci. 2011;89(25-26):939–945. doi: 10.1016/j.lfs.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 253.Ho L., Ferruzzi M.G., Janle E.M., Wang J., Gong B., Chen T.Y., Lobo J., Cooper B., Wu Q.L., Talcott S.T., Percival S.S., Simon J.E., Pasinetti G.M. Identification of brain‐targeted bioactive dietary quercetin‐3‐ O ‐glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. 2013;27(2):769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Caruana M., Cauchi R., Vassallo N. Putative role of red wine polyphenols against brain pathology in Alzheimer’s and Parkinson’s disease. Front. Nutr. 2016;3:31. doi: 10.3389/fnut.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ho L., Chen L.H., Wang J., Zhao W., Talcott S.T., Ono K., Teplow D., Humala N., Cheng A., Percival S.S., Ferruzzi M., Janle E., Dickstein D.L., Pasinetti G.M. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J. Alzheimers Dis. 2009;16(1):59–72. doi: 10.3233/JAD-2009-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Feng Y., Wang X., Yang S., Wang Y., Zhang X., Du X., Sun X., Zhao M., Huang L., Liu R. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology. 2009;30(6):986–995. doi: 10.1016/j.neuro.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 257.Richard T., Poupard P., Nassra M., Papastamoulis Y., Iglésias M.L., Krisa S., Waffo-Teguo P., Mérillon J.M., Monti J.P. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorg. Med. Chem. 2011;19(10):3152–3155. doi: 10.1016/j.bmc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 258.Feng Y., Yang S., Du X., Zhang X., Sun X., Zhao M., Sun G., Liu R. Ellagic acid promotes Aβ42 fibrillization and inhibits Aβ42-induced neurotoxicity. Biochem. Biophys. Res. Commun. 2009;390(4):1250–1254. doi: 10.1016/j.bbrc.2009.10.130. [DOI] [PubMed] [Google Scholar]
- 259.Ono K., Condron M.M., Ho L., Wang J., Zhao W., Pasinetti G.M., Teplow D.B. Effects of grape seed-derived polyphenols on amyloid β-protein self-assembly and cytotoxicity. J. Biol. Chem. 2008;283(47):32176–32187. doi: 10.1074/jbc.M806154200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Wang J., Ho L., Zhao W., Ono K., Rosensweig C., Chen L., Humala N., Teplow D.B., Pasinetti G.M. Grape-derived polyphenolics prevent Abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008;28(25):6388–6392. doi: 10.1523/JNEUROSCI.0364-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Hayden E.Y., Yamin G., Beroukhim S., Chen B., Kibalchenko M., Jiang L., Ho L., Wang J., Pasinetti G.M., Teplow D.B. Inhibiting amyloid β-protein assembly: Size-activity relationships among grape seed-derived polyphenols. J. Neurochem. 2015;135(2):416–430. doi: 10.1111/jnc.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ho L., Yemul S., Wang J., Pasinetti G.M. Grape seed polyphenolic extract as a potential novel therapeutic agent in tauopathies. J. Alzheimers Dis. 2009;16(2):433–439. doi: 10.3233/JAD-2009-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Santa-Maria I., Diaz-Ruiz C., Ksiezak-Reding H., Chen A., Ho L., Wang J., Pasinetti G.M. GSPE interferes with tau aggregation in vivo: implication for treating tauopathy. Neurobiol. Aging. 2012;33(9):2072–2081. doi: 10.1016/j.neurobiolaging.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang J., Bi W., Cheng A., Freire D., Vempati P., Zhao W., Gong B., Janle E.M., Chen T.Y., Ferruzzi M.G., Schmeidler J., Ho L., Pasinetti G.M. Targeting multiple pathogenic mechanisms with polyphenols for the treatment of Alzheimer’s disease-experimental approach and therapeutic implications. Front. Aging Neurosci. 2014;6:42. doi: 10.3389/fnagi.2014.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Choi Y.T., Jung C.H., Lee S.R., Bae J.H., Baek W.K., Suh M.H., Park J., Park C.W., Suh S.I. The green tea polyphenol (−)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70(5):603–614. doi: 10.1016/S0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- 266.Singh N.A., Mandal A.K.A., Khan Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2015;15(1):60. doi: 10.1186/s12937-016-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mori T., Koyama N., Yokoo T., Segawa T., Maeda M., Sawmiller D., Tan J., Town T. Gallic acid is a dual α/β-secretase modulator that reverses cognitive impairment and remediates pathology in Alzheimer mice. J. Biol. Chem. 2020;295(48):16251–16266. doi: 10.1074/jbc.RA119.012330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Ryu E.K., Choe Y.S., Lee K.H., Choi Y., Kim B.T. Curcumin and dehydrozingerone derivatives: synthesis, radiolabeling, and evaluation for β-amyloid plaque imaging. J. Med. Chem. 2006;49(20):6111–6119. doi: 10.1021/jm0607193. [DOI] [PubMed] [Google Scholar]
- 269.Cohen A., Ikonomovic M., Abrahamson E., Paljug W., DeKosky S., Lefterov I., Koldamova R., Shao L., Debnath M., Mason N., Mathis C., Klunk W. Anti-amyloid effects of small molecule Aβ-binding agents in PS1/APP mice. Lett. Drug Des. Discov. 2009;6(6):437–444. doi: 10.2174/157018009789057526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhang K., Chen M., Du Z-Y., Zheng X., Li D-L., Zhou R-P. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018;13(4):742–752. doi: 10.4103/1673-5374.230303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Nakagami Y., Nishimura S., Murasugi T., Kaneko I., Meguro M., Marumoto S., Kogen H., Koyama K., Oda T. A novel β -sheet breaker, RS-0406, reverses amyloid β -induced cytotoxicity and impairment of long-term potentiation in vitro. Br. J. Pharmacol. 2002;137(5):676–682. doi: 10.1038/sj.bjp.0704911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Moss M.A., Varvel N.H., Nichols M.R., Reed D.K., Rosenberry T.L. Nordihydroguaiaretic acid does not disaggregate beta-Amyloid(1-40) protofibrils but does inhibit growth arising from direct protofibril association. Mol. Pharmacol. 2004;66(3):592–600. doi: 10.1124/mol.66.3. [DOI] [PubMed] [Google Scholar]
- 273.Masuda M., Suzuki N., Taniguchi S., Oikawa T., Nonaka T., Iwatsubo T., Hisanaga S., Goedert M., Hasegawa M. Small molecule inhibitors of α-synuclein filament assembly. Biochemistry. 2006;45(19):6085–6094. doi: 10.1021/bi0600749. [DOI] [PubMed] [Google Scholar]
- 274.Taniguchi S., Suzuki N., Masuda M., Hisanaga S., Iwatsubo T., Goedert M., Hasegawa M. Inhibition of heparin-induced tau filament formation by phenothiazines, polyphenols, and porphyrins. J. Biol. Chem. 2005;280(9):7614–7623. doi: 10.1074/jbc.M408714200. [DOI] [PubMed] [Google Scholar]
- 275.Porat Y., Abramowitz A., Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006;67(1):27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 276.Lee K.H., Shin B.H., Shin K.J., Kim D.J., Yu J. A hybrid molecule that prohibits amyloid fibrils and alleviates neuronal toxicity induced by β-amyloid (1–42). Biochem. Biophys. Res. Commun. 2005;328(4):816–823. doi: 10.1016/j.bbrc.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 277.Kung H.F., Lee C.W., Zhuang Z.P., Kung M.P., Hou C., Plössl K. Novel stilbenes as probes for amyloid plaques. J. Am. Chem. Soc. 2001;123(50):12740–12741. doi: 10.1021/ja0167147. [DOI] [PubMed] [Google Scholar]
- 278.Necula M., Kayed R., Milton S., Glabe C.G. Small molecule inhibitors of aggregation indicate that amyloid β oligomerization and fibrillization pathways are independent and distinct. J. Biol. Chem. 2007;282(14):10311–10324. doi: 10.1074/jbc.M608207200. [DOI] [PubMed] [Google Scholar]
- 279.Reddy P.H., Manczak M., Yin X., Grady M.C., Mitchell A., Tonk S., Kuruva C.S., Bhatti J.S., Kandimalla R., Vijayan M., Kumar S., Wang R., Pradeepkiran J.A., Ogunmokun G., Thamarai K., Quesada K., Boles A., Reddy A.P. Protective effects of Indian spice curcumin against Amyloid-ß in Alzheimer’s disease. J. Alzheimers Dis. 2018;61(3):843–866. doi: 10.3233/JAD-170512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Griner S.L., Seidler P., Bowler J., Murray K.A., Yang T.P., Sahay S., Sawaya M.R., Cascio D., Rodriguez J.A., Philipp S., Sosna J., Glabe C.G., Gonen T., Eisenberg D.S. Structure-based inhibitors of amyloid beta core suggest a common interface with tau. eLife. 2019;8:e46924. doi: 10.7554/eLife.46924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pandey K.B., Rizvi S.I. Anti-oxidative action of resveratrol: Implications for human health. Arab. J. Chem. 2011;4(3):293–298. doi: 10.1016/j.arabjc.2010.06.049. [DOI] [Google Scholar]
- 282.Salehi B., Mishra A., Nigam M., Sener B., Kilic M., Sharifi-Rad M., Fokou P., Martins N., Sharifi-Rad J. Resveratrol: A double-edged sword in health benefits. Biomedicines. 2018;6(3):91. doi: 10.3390/biomedicines6030091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zong Y., Sun L., Liu B., Deng Y.S., Zhan D., Chen Y.L., He Y., Liu J., Zhang Z.J., Sun J., Lu D. Resveratrol inhibits LPS-induced MAPKs activation via activation of the phosphatidylinositol 3-kinase pathway in murine RAW 264.7 macrophage cells. PLoS One. 2012;7(8):e44107. doi: 10.1371/journal.pone.0044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Kim D-O., Lee C.Y. Comprehensive study on Vitamin C Equivalent Antioxidant Capacity (VCEAC) of various polyphenolics in scavenging a free radical and its structural relationship. Crit. Rev. Food Sci. Nutr. 2004;44(4):253–273. doi: 10.1080/10408690490464960. [DOI] [PubMed] [Google Scholar]
- 285.Savaskan E., Olivieri G., Meier F., Seifritz E., Wirz-Justice A., Müller-Spahn F. Red wine ingredient resveratrol protects from β-amyloid neurotoxicity. Gerontology. 2003;49(6):380–383. doi: 10.1159/000073766. [DOI] [PubMed] [Google Scholar]
- 286.Lançon A., Delma D., Osman H., Thénot J.P., Latruffe B.J.N., Latruffe N. Human hepatic cell uptake of resveratrol: involvement of both passive diffusion and carrier-mediated process. Biochem. Biophys. Res. Commun. 2004;316(4):1132–1137. doi: 10.1016/j.bbrc.2004.02.164. [DOI] [PubMed] [Google Scholar]
- 287.Conte A., Pellegrini S., Tagliazucchi D. Synergistic protection of PC12 cells from β-amyloid toxicity by resveratrol and catechin. Brain Res. Bull. 2003;62(1):29–38. doi: 10.1016/j.brainresbull.2003.08.001. [DOI] [PubMed] [Google Scholar]