Abstract
Alzheimer's and Parkinson's are neurodegenerative disorders that affect a great number of people around the world, seriously compromising the quality of life of individuals, due to motor and cognitive damage. In these diseases, pharmacological treatment is used only to alleviate symptoms. This emphasizes the need to discover alternative molecules for use in prevention. Using Molecular Docking, this review aimed to evaluate the anti-Alzheimer’s and anti-Parkinson’s activity of linalool and citronellal, as well as their derivatives. Before performing Molecular Docking simulations, the compounds’ pharmacokinetic characteristics were evaluated. For Molecular Docking, 7 chemical compounds derived from citronellal, and 10 compounds derived from linalool, and molecular targets involved in Alzheimer's and Parkinson's pathophysiology were selected. According to the Lipinski rules, the compounds under study presented good oral absorption and bioavailability. For toxicity, some tissue irritability was observed. For Parkinson-related targets, the citronellal and linalool derived compounds revealed excellent energetic affinity for α-Synuclein, Adenosine Receptors, Monoamine Oxidase (MAO), and Dopamine D1 receptor proteins. For Alzheimer disease targets, only linalool and its derivatives presented promise against BACE enzyme activity. The compounds studied presented high probability of modulatory activity against the disease targets under study, and are potential candidates for future drugs.
Keywords: Neuroprotection, monoterpenes, natural products, neurodegeneration, molecular docking, Alzheimer’s disease, Parkinson’s disease
1. INTRODUCTION
Neurodegenerative diseases (NDD) affect a large number of individuals worldwide. These disorders result from the progressive neuronal loss, and affect important brain regions, causing neurophysiological changes with direct impacts on motor and cognitive mechanisms [1]. The etiological basis of these diseases is multifactorial. In addition to the expression of specific genes responsible for NDD, exposure to environmental agents also influences the extent and severity of the resulting neurodegenerative processes [1-4].
The pathophysiological mechanisms that culminate in programmed cell death over time are due to molecular changes in protein dynamics, and dysfunctions in the ubiquitin-proteasome-autophagy system, with exacerbation of oxidative and neuroinflammatory processes [5].
Neurodegeneration in Alzheimer's disease begins with proteolytic cleavage of amyloid precursor protein (APP), and results in the production, aggregation, and deposition of β-amyloid substance (Aβ), and senile plaques. Alzheimer's disease (AD) presents a series of clinical manifestations, including impairments in cognition, learning, memory and reasoning. Parkinson's disease (PD) is characterized by motor disorders, bradykinesia, rigidity, resting tremors, and in more advanced cases, postural instability. Both diseases are marked by a high incidence of morbidity and mortality [6, 7]. Pharmacological therapy for these comorbidities is unable to completely delay or reverse their progression, being effective only for the relief of some of the symptoms involved in dementia, depression, and autonomic dysfunction [8].
Pharmacological therapy for these comorbidities remains unable to completely delay or reverse their progression, and is only effective for symptomatic relief. Given their high prevalence, AD and PD are considered principal causes of psychosocial disability; they grow exponentially and are associated with a lack of treatment [6-8]. Though investigations into natural substances (such as monoterpenes) which can modulate neurodegenerative disease are extremely important, developing effective treatments remains difficult.
Linalool and citronellal derivatives (monoterpene phytochemicals) present neuroprotective effect with anti-inflammatory and antioxidant related activity [9-11]. This makes these compounds potentially useful as pharmacological agents in preventing neurodegenerative mechanisms. Our study sought to evaluate the anti-Alzheimer’s and anti-Parkinson’s potential of linalool and citronellal derivatives through a review of the literature, together with computational prospection and molecular docking techniques. Finally, pharmacokinetic predictions were developed using in silico methodologies to optimize the discovery process, while identifying potential ADMET failures.
1.1. Essential Oils and Monoterpenes as Potential Pharmacological Agents
The use of essential oils (EO) is widespread; phytocomplexes (originating from primary or secondary plant metabolism) are present in most of the products used today, being principal ingredients in cosmetics, body and hair perfumes, hygiene products, oral antiseptic solutions, and toothpaste. Phytocomplexes are also used in aromatherapy, which is based on the use of essential oils and their ability to be easily absorbed by the skin and relieve many disease symptoms especially those that affect the central nervous system [12].
EOs are odorific substances formed during plant secondary metabolism to protect against predatory attacks and pollinating agents. Their components are formed through three biosynthetic pathways: (I) the methylerythritol phosphate (MEP) pathway, yielding monoterpenes and diterpenes; (II) the mevalonic acid pathway, giving rise to sesquiterpenes; and (III) the shikimic acid pathway, leading to the formation of phenylpropanoids. These compounds are responsible for the majority of biological properties found in aromatic and medicinal plants [13].
Monoterpenes, constituting 90% of essential oils, are volatile and aromatic, and belong to a diverse group of chemical compounds which are the subject of many studies. They present a huge variety of structures and, thus, biological activities, among them: sedative, anticonvulsant, hypnotic, hypothermic actions, antispasmodic, vasorelaxant, and antinociceptive effects [14].
Monoterpenes such as limonene, citral, citronellal, eugenol, menthol, safrole and linalool are known to have anxiolytic [15], antidepressant [16], anticonvulsant [15, 17] and antinociceptive effects [18], and therefore are the focus of many studies to develop new drugs with greater efficacy, selectivity, and safety [19].
1.1.1. Linalool and Derivatives
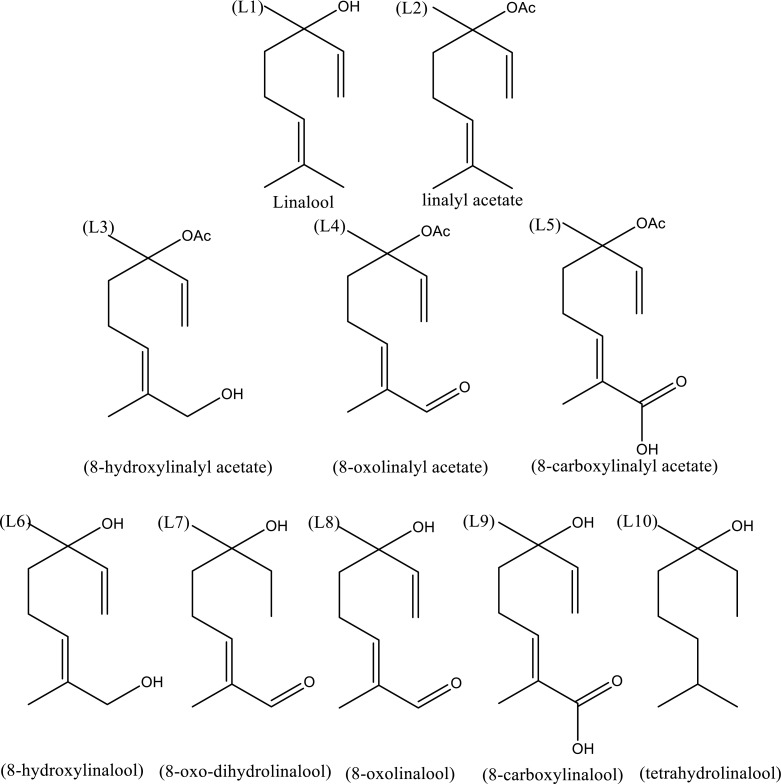
Linalool is an open-chain monoterpene existing in two enantiomer forms (R)-(-)-linalool and (S)-(+)-linalool. Enantiomers are molecules that are mirror images of each other and are not superimposable, either by rotation or translation. Therefore, enantiomers often present differences in pharmacological activity [20]. Linalool is a common point in the biosynthesis of alcohol and aldehyde derivatives formed by the enzymatic action of linalool synthase-(linalool acetate and linalool oxides), and cytochrome P-450 enzymes-(8-oxolinalol, 8-hydroxylinalool and 8-carboxylinalool) [15, 21]. Linalool also undergoes acetylation processes that when followed by oxidations, give rise to metabolites such as 8-hydroxylinalyl acetate, 8-oxolinalyl acetate, and 8-carboxylyl acetate [16]. Whether by synthesis or biosynthesis, tetrahydrolinalool can be obtained as a final product through linalool dehydrogenation reactions [17, 19] (Fig. 1).
Fig. (1).
Linalool and derivatives. L1 - a monoterpene alchohol (3,7-dimethyl-1,6-octadiene-3-ol), L2 - linalyl acetate (3,7-dimethylocta-1,6-dien-3-yl acetate); L3 - L5 - linalyl acetate derivatives (acetylated at carbon 3). L3 - 8-hydroxylinalyl acetate (E) -8-hydroxy-3,7-dimethylocta-1,6-dien-3-yl acetate); L4 - 8-oxolinalyl acetate, (E) -3,7-dimethyl-8-oxoocta-1,6-dien-3-yl acetate); L5 - 8-carboxylinalyl acetate (E) -6-acetoxy-2,6-dimethylocta-2,7-dienoic acid). Compounds L6-L10 are linalool derivatives substituted at carbon atom 8. L6- 8-hydroxylinalool (E)-2,6-dimethylocta-2,7-diene-1,6-diol); L7-8-oxo-dihydrolinalool (E)-6-hydroxy-2,6-dimethyloct-2-enal; L8 - 8-oxolinalool (E)-6-hydroxy-2,6-dimethylocta-2,7-dienal); L9- 8-carboxylinalool (E)-6-hydroxy-2,6-dimethylocta-2,7-dienoic acid) and L10 -Tetrahydrolinalool (3,7-dimethyloctan-3-ol).
Linalool can be extracted from aromatic plants, such as those of the genera Lavandula, Ocimum, and Eucalyptus, among others. Due to its fragrance, linalool and its derivatives are commonly used in the perfume industry [19, 22]. Currently, research has shown that due to their low molecular weight and high lipophilicity, these compounds cross the blood brain barrier and, therefore, can be used in pharmaceutical therapy to promote behavioral and cognitive changes and sleep. Linalool and its derivatives have become potential agents for the treatment of neurological and psychiatric diseases by modulating different brain circuits [9, 23-25].
Evidence has shown that linalool plays neuroprotective roles, minimizing neuroinflammation and oxidative stress in neurodegenerative disorders such as Parkinson's and Alzheimer's [9, 26]. Studies suggest that linalool increases mitochondrial respiration by protecting against glutamatergic hyperstimulation and reduces apoptotic processes [27, 28]. In vitro research with linalool reinforces its central anti-inflammatory potential, suppressing inflammatory signals induced by the NF-kB pathway, and reducing the production of cytokines, nitric oxide, and reactive oxygen species [29, 30].
In Alzheimer's models using transgenic mice, it was possible to show that linalool at a dose of 25 mg/kg improved cognitive parameters such as learning and memory in the Morris water maze test. In the same study, the animals treated with linalool presented significantly decreased amyloid burden, tautopathy, and neuroinflammation in areas such as the hippocampus and amygdala [31]. Similar research corroborates these findings; treatment with linalool improves characteristic symptoms of AD, decreasing oxidative stress markers induced by chronic administration of D-galactose and aluminum trichloride [32]. Mechanisms also involve decreased acetylcholinesterase activity, increased expression of BDNF, and the tropomyosin kinase B (TrkB) receptor [30]. All these examples suggest the potential of linalool in improving behavioral parameters in models of AD-like cognitive impairment. Linalool and its derivatives can act in processes related to neuroplasticity and cholinergic signaling, as in these neurodegenerative diseases, both pathways are compromised.
1.1.2. Citronellal and Derivatives
Citronellal monoterpene (3,7-dimethyl-6-octen-1-al) is the main component in the essential oils of many aromatic species including Cymbopogon winterianus Jowitt (Java citronella), Corymbia citriodora (Hook) KDHill (lemon eucalyptus), and Cymbopogon nardus L. (citronella). Citronellal is a racemic mixture of its enantiomers and is often used in folk medicine and aromatherapy. It is also widely used in the cosmetics and soap industries [33, 34].
There are no studies in the literature that evaluate the activity and effectiveness of citronellal or its derivatives (Fig. 2) against either Parkinson's or Alzheimer's disease. However, there has been a growing number of studies evaluating the activity of these compounds at the level of the central nervous system, mainly neuroprotective [35] and anti-inflammatory [34] activities with evidence of their actions on the glutamatergic system [36].
Fig. (2).
Citronellal and derivatives. C1 - Metoxycitronellal -(R)-7-methoxy-3,7-dimethyloctanal; C2- D-citronellal - (R)-3,7-dimethyloct-6-enal; C3 - S- citronellal -(S)-3,7-dimethyloct-6-enal; C4 - Hidroxycitronellal - (R)-7-hydroxy-3,7-dimethyloctanal; C5 - citronellic acid -(R)-3,7-dimethyloct-6-enoic acid; C6 - Isopulegol - (5R)-5-methyl-2-(prop-1-en-2-yl)cyclohexanol; C7 - menthol -(5R)-2-isopropyl-5-methylcyclohexanol.
In contrast, due to its action as a GABAA agonist, the neuroprotective effect of citronellal has been noted for being able to negatively modulate glutamatergic receptors, to block voltage-gated Na+ channels, activate potassium channels, and attenuate inflammation and neuronal oxidative stress [13]. Due to its antioxidant effects, isopulegol also presents neuroprotective activity [37].
Studies suggest that menthol has therapeutic effects on neuroinflammatory diseases, protecting dopaminergic neurons as well as inhibiting lipopolysaccharide-induced microglial activation. Menthol can also inhibit the expression of pro-inflammatory enzymes. Studies involving mechanisms of action, in vitro and in vivo, have revealed that menthol inhibits neuroinflammatory response through MAPK, NF-κB and AKT signaling pathways [38].
1.2. Parkinson’s Disease
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the world, affecting from 1 to 2% of people over 65 years of age, and with a prevalence set to double by 2030 [39]. Despite the large number of studies on PD, its cause has not yet been determined, nor is there any evidence of a cure. The possible etiological bases that can explain the pathophysiology of the disease involve genetic factors, toxins, environmental agents, oxidative stress, and mitochondrial abnormalities [40, 41].
The motor manifestations of PD (akinesia or bradykinesia, rigidity, tremor, and postural instability) begin focally when there is a reduction in dopamine (DA) concentrations in the contralateral striatum and posterior putamen (striatal motor region) [42]. PD is related to the selective loss of DA neurons in the substantia nigra parte compacta (SNpc) region, with the formation of Lewy bodies, and in the striatal corpus of the nigrostriatal pathway in the brain. This loss of DA causes dysregulation in basal ganglia circuits and leads to the appearance of both motor and non-motor symptoms, such as sleep disorders, cognitive deficits, and depression [43].
At the cellular level, PD involves changes in mitochondrial electron transporter function, changes in catecholamine metabolism [44, 45], and excess production of cytotoxic factors such as inflammatory cytokines (IL-1, IL-6, TNF-α), and reactive oxygen species [46]. Current therapeutic strategies for managing PD are aimed at providing symptomatic relief and minimizing the progression of the disease. However, few pharmacological advances have been made which can protect dopaminergic neurons and, consequently, the motor circuit. It is known that cranial stimulation, stem cells, and gene editing are promising treatment areas for progressive neurodegenerative disorders [47, 48].
Experimental models have revealed pathogenic molecular mechanisms and targets for pharmacological intervention. The findings imply the involvement of targetable protein structures such as D1 and D2 dopaminergic receptors, Adenosine A2, α-synuclein, Catechol-o-methyltransferase, and Type B Monoamine oxidase receptors, described in Fig. (3).
Fig. (3).
Potential pharmacological targets of PD.
These targets influence important dopaminergic circuits, impacting protein phosphorylation, mitochondrial dysfunction, and oxidative stress. These targets also influence the normal function and survival of dopaminergic neurons and the onset of PD [47]. Here, we will discuss findings concerning the mechanisms of action that involve PD, the biological potential of linalool and citronellal derivatives facing these structures and their influence on cell survival and neuroprotection mechanisms.
1.2.1. Potential Targets in Parkinson's Disease
1.2.1.1. D1 and D2 Dopaminergic Receptors
Dopamine is involved in neural mechanisms related to mood control, emotional stability, learning, movement, and memory [49]. Dopaminergic responses are triggered by activation of dopaminergic receptors [50] present in the nuclei base and limbic system; D 1-like (D1 and D5-receptors coupled to a G protein - type Gs; result in increased cAMP levels), and D2-like (D2, D3, and D4-receptors coupled to a Gi-type G protein; result in decreased cAMP levels). D1 and D2 receptors accumulate in the dorsal region of the striatum and are involved in the pathophysiology of PD. The receptor subtypes D3 and D4 are more concentrated in the mesolimbic region, with the D5 receptor in the hippocampus and hypothalamus [51]. In the hippocampus, alterations in memory function involve altered signaling via the D2R, whose dopamine acts to increase cortical excitability. The catecholamine D1R increases the release of cortical acetylcholine [52, 53].
Studies have revealed the importance of the dopaminergic D2 receptor in the control of abnormal and dyskinetic involuntary movements in rats with hemi-Parkinsonism [54]. Striatum neuron groups are stimulated by the activation of D1 and D2 receptors. Neurons that are stimulated by the D1 receptor, directly signal the internal globus pallidus (direct pathway). These neurons tonically inhibit the thalamus, which in turn sends excitatory projections to the cortex that initiate movement. Thus, activation of the direct pathway disinhibits the thalamus, that is, the direct pathway stimulates movement. Neurons that express the D2 receptor signal the external globus pallidus which inhibits the subthalamic nucleus, glutamatergic neurons that project to the internal globus pallidus (indirect pathway). Activation of the indirect pathway de-inhibits neurons in the subthalamic nucleus, which in turn stimulate neurons in the inner segment of the globus pallidus and inhibit the thalamus, i.e., the indirect pathway inhibits movement. Under normal conditions, the presence of dopamine activates neurons that express D1 in the direct pathway, while inhibiting neurons of the indirect pathway that express D2; these effects promote movement. In PD, a state of dopamine deficiency, the opposite effect is observed: the direct pathway presents a reduction in activity, while the indirect pathway is hyperactive, resulting in reduced movement [55].
Pharmacological treatments for the motor symptoms of PD are mainly based on dopamine. Levodopa remains the most effective drug for PD treatment. Another strategy to increase dopaminergic neurotransmission is to use dopamine receptor agonists. Agonists such as bromocriptine (D2 agonist) and pergolide (D1 and D2) have been successfully used as adjuvants in levodopa treatment [56, 57]. Studies have shown that both enantiomer of linalool [30], and citronellal [30] present antinociceptive properties, with dopaminergic transmission via D2 receptors. Given the participation of these monoterpenoids in the dopaminergic pathway and the absence of studies concerning their antiparkinsonian effects, there is a need for more biological studies with linalool and citronellal derivatives and neurodegenerative disease.
1.2.1.2. Adenosine A2A Receptors
Adenosine receptors, belonging to the GPCR family, are composed of four subtypes,: A1, A1A, A 2B, and A3 [58]. The adenosine A2A receptor is located mainly in the basal ganglia, more specifically in the striatum [59], as are dopamine D2 receptors [59, 60]. D2 receptors present in the striatum help regulate motor activity. Decreased D2 receptor activation results in increasing GABAergic signaling from the substantia nigra and the internal pallidal segment. This inhibits thalamocortical projection neurons, causing reduced activation in the cortex responsible for motor activity regulation [61-63].
Parkinson's disease, when causing dopamine depletion, culminates in weaker response, triggering theabove-mentioned signaling, and the consequent motor symptoms such as bradykinesia (slowness in the execution of voluntary movements), dyskinesia (involuntary movements), tremor, and rigidity [63]. In motor activity, D2 and A2A receptor co-participation is evidenced with antagonism of the A2A receptor, which causes simultaneous activation of D2, which attenuates PD dyskinesia [64]. Further, an increase in A2A receptor expression is evidenced in patients suffering from Parkinson's disease [60].
Two theories are postulated for this simultaneous hyperactivity of the A2A receptor and hypoactivity of the D2 receptor. Either the A2A receptors act as a dimer with the D2 receptor [65]; or the A2A receptors located in the cell bodies and terminals of GABAergic receptors, together with the D2 receptors, present interactions between them [60, 65]. The way in which A2A receptor antagonism acts to improve dyskinesia is not yet fully elucidated [66], but it is known that in addition to greater activation of thalamocortical neurons, receptor antagonism acts by suppressing neuroinflammation and restoring dopamine levels [67].
In some countries, receptor antagonism is already used in the clinical management of the disease as adjunctive therapy together with levodopa and MAO-B inhibitors [58, 65]. In Japan, which approved the drug istradefyline, reduction in dyskinesia “off time” in patients with PD is reported [59]. Various studies associate receptor antagonists such as xanthine (i.e. caffeine) with the reduction of Parkinson disease symptoms [66, 67]. It was demonstrated in vivo, that aged mice (16-18 months) ingesting low amounts of caffeine (0.3 g/L) for a long time presented a lower response to the A2A receptor. This was quantified using an immunoassay (immunoblot) to detect proteins related to the receptor [41, 66, 68]. The A2A receptor association with nociception, evidenced in the activities of linalool and citronellal [69], and in animal tests, demonstrates activity involving A2AR. However, little is known about how A2A R modulation acts on PD.
1.2.1.3. α-Synuclein
α-synuclein, formed by 140 amino acids [70], and found in presynaptic terminals, is a protein involved in exocytosis regulation [71, 72], anchoring, traffic, and vesicle fusion, as well as axonal transport. Its structure is characterized by three domains: a positively charged N-terminus, an acidic C-terminal domain, and a central domain which tends to undergo aggregation resulting in oligomerization. Normally, the oligomeric and monomeric states are in dynamic equilibrium [72, 73], however, in Parkinson's disease, there is a mutation in the α-synuclein gene, which makes the insoluble oligomeric state the most prevalent [74].
The insoluble oligomeric form of α-synuclein is the main component of the Lewis bodies and Lewis neurites typically found in PD [75, 76]. Accumulation of α-synuclein promotes a series of cellular damages, such as increased oxidative stress, and mitochondrial abnormalities in neuronal cells [77], with lysosomal, mitochondrial and vesicular damage resulting from increased membrane permeability [78, 79]. Further, it is thought that excess protein causes greater permeability in dopamine-containing vesicles and generates reactive oxygen species in dopaminergic neurons, the cause of the neurotoxicity and neurodegeneration reported in PD [80].
Various treatment strategies have been devised to decrease concentrations of the α-synuclein oligomer and protein production through RNA interference, whether mediated by a lentivirus, or by promotion of intracellular degradation of aggregated proteins through molecules that increase lysosomal activity, by stimulating macroautophagy [81], or promoting extracellular degradation using monoclonal antibodies [71, 72], by preventing aggregation through the induction of overexpression and activation of chaperones [81], or use of phytochemicals such as geraniol (an acyclic monoterpene associated with α-synuclein in mice that can decrease mRNA), to reduce fibrillation and aggregation of α-synuclein [11, 77].
Stress and protein homeostasis is linked to the health and aging process of the body, since it is controlled by an important protective network, called the vitagene network. Vitagene regulates the response to cellular stress by activating pro-survival pathways responsible for the production of molecules such as thermal shock proteins (Hsps), glutathione and bilirubin, all with antioxidant and anti-apoptotic activity. In transgenic animal models, it was observed that overexpression of Hsps may decrease the risks of neuronal dysfunction and degeneration due to decreased aggregation of α -synuclein, a protein that causes neurotoxicity and neurodegeneration [74]. Glutathione is one of the most abundant antioxidant agents present in the body and its levels tend to decrease with advancing age, leading to a greater chance of developing neurodegenerative diseases. Its protective activity is observed against changes in protein conformation, damage caused by peroxidonytrite-induced sinaptossomas, and maintenance of glutamine synthesis activity [70]. Bilirubin, on the other hand, functions as a scavenger (cleaner/scanner) of NO and reactive nitrogen species, thus reducing the cellular damage caused by these molecules [73]. Hormetic nutrients (e.g., Nrf2 pathway activators) are endowed with antioxidant activity, minimizing the associated neurodegenerative processes [82].
1.2.1.4. Catechol -O-Methyltransferase (COMT)
Catechol-O-methyl transferase (COMT) is a magnesium-dependent catabolic enzyme which presents two isoforms: soluble or membrane-bound [83]. The enzyme transfers methyl groups to catecholamines, or to their hydroxylated derivatives, or even to drugs that have catechol in their structure. This activity is important to regulate catecholamine neurotransmission. In PD there is a reduction of dopaminergic neurons and, consequently, dopamine. Treatment strategies to increase dopamine have been formulated, but dopamine does not cross the blood-brain barrier, and management of the disease is now done through the use of a dopamine precursor, Levodopa [84]. This precursor, however, undergoes the action of metabolic enzymes, such as COMT, which cause a smaller fraction of L-dopa to cross the blood-brain barrier, a factor that limits treatment [85].
An alternative to increase L-DOPA availability in the nervous system is COMT inhibition, which in addition to increasing the availability of L-dopa, would also decrease the formation of its O-methylated metabolite, levodopa. Levodopa competes with L-Dopa for neutral amino acid transporters at the blood-brain barrier, and thus, a decrease in the metabolite favors better Levodopa absorption [85]. In Parkinson's disease, COMT inhibitors improve motor symptoms [86], with evidence of a reduction in “off time” in these patients with the use of entalcapone and tolcapone, both drugs are used in the current clinical management of the disease [56]. Other substances, including those of natural origin, are being investigated for potential COMT inhibition. Z-vallesiachotamine, a monoterpene indole alkaloid derived from Vallesia glabra, presents considerable COMT inhibition, which has been evaluated using an in vitro fluorescence method, which estimated the activation of the enzyme [87]. There are no studies involving linalool, citronellal, or their derivatives for their influence on α-synuclein or COMT.
1.2.1.5. Monoamine Oxidase B (MAO-B)
Monoamine oxidase (MAO) is a flavoenzyme located in the outer membrane of mitochondria that acts as a catalyst for the oxidation of monoaminergic neurotransmitters. Thus, MAO is an important target in the treatment of neurodegenerative diseases [88, 89]. It presents two isoforms (MAO-A and MAO-B), which differ mainly with respect to their tissue distribution and substrate preferences; both are important therapeutic targets [90, 91]. The two isoforms show a different expression pattern with respect to time. MAO-A seems to present a maximum level of expression during childhood. MAO-B expression increases by approximately 7.1% (±1.3%) every 10 years [92].
MAO-B is a two-domain enzyme, anchored to the outer mitochondrial membrane via a C-terminal helix. It has a domain for flavin-adenine dinucleotide (FAD), which acts as a cofactor, and another domain for the substrate [93]. MAO-B is the predominant isoform of MAO in the brain, mainly in the basal ganglia. It is responsible for converting dopamine into 3,4-dihydroxyphenylacetic acid and homovalinic acid [90]. MAO-B, together with catechol-O-methyltransferase (COMT), is involved in the inactivation of dopamine through metabolism, this makes it an important therapeutic target in Parkinson's Disease (PD) [91].
MAO-B inhibitor drugs (MAO-Bis) are widely used in PD because of their ability to increase the activity of endogenous dopamine and levodopa, favoring the use of lower doses of levodopa in more advanced stages of PD. The neuroprotective properties of MAO-Bis, like Selegiline, have been demonstrated. The effect seems to be related to the ability of the drug to inhibit neurotoxicity induced by the NMDA receptor, and induce the synthesis of both neurotrophic factors and antioxidant enzymes [94, 95]. The first preclinical studies with a selective MAO-B inhibitor (MAO-Bi), deprenyl (Selegiline), were undertaken by Knoll et al. (1965) and by Knoll and Magyar (1972). Subsequently, Birkmayer et al., (1975) presenting the anti-Parkinson effect of Selegiline in their studies [96]. Currently, MAO-Bis are widely used to treat PD, with clinically proven efficacy against PD, selegiline, rasagiline, and safinamide are their main clinical representatives [90]. As MAO-B (inhibition) is an important therapeutic target in PD, various pharmacological studies have been performed to develop new drugs with inhibitory action on MAO-B [88]. However, there are still no studies evaluating the action of linalool and citronellal derived monoterpenes on MAO-B. Thus, it is essential to perform in silico and in vitro studies with these substances in order to evaluate their pharmacological potential.
1.3. Alzheimer’s Disease
Alzheimer’s Disease (AD) is one of the most common age-related neurodegenerative pathologies. It is characterized by beta-amyloid deposits (in senile plaques) and neurofibrillary tangles (paired helical filaments) in the cerebral cortex and subcortical gray matter. As a result, massive synaptic loss and severe neuronal death occur in brain regions that are responsible for important cognitive functions, such as the cerebral cortex, hippocampus, and ventral striatum [97, 98].
The histopathological particularities that are present in the brain parenchyma of patients affected by AD include amyloid fibril deposits in blood vessel walls, associated with a variety of different types of senile plaques, accumulation of abnormal filaments of the tau protein, and consequent formation of neurofibrillary tangles (NFT), neuronal and synaptic loss, glial activation, and inflammation [99].
Based on these neuropathological markers, two hypotheses were proposed in order to explain the etiology of the disease. According to the amyloid cascade hypothesis, neurodegeneration in Alzheimer's disease begins with the proteolytic cleavage of the amyloid precursor protein (APP), and results in the production, aggregation, and deposition of β-amyloid substance (Aβ) and senile plaques. There is also the Tau hypothesis, or phosphorylation of the Tau protein, which forms tangles in nerve cells and prevents cellular proteins from exercising their normal functions [97, 100, 101].
There is also the cholinergic hypothesis, which reports that the dysfunction of the cholinergic system is sufficient to produce memory deficiency in animal models, this is similar to Alzheimer's disease, and highlights the importance of acetylcholine in memory and learning. Acetylcholine deficit in the brain is mediated by the enzyme choline acetyltransferase and causes the symptoms of AD [41, 102]. According to Sereniki & Vital (2008) [100], brains of patients with Alzheimer's disease present cholinergic neuron degeneration, as well as a reduction in cholinergic markers, thus, choline acetyltransferase and acetylcholinesterase present reduced activity in the cerebral cortex of patients with Alzheimer disease [102]. As a result, attempts were made to therapeutically stimulate cholinergic activity through cholinesterase inhibitors, which degrade acetylcholine in the synaptic cleft, enhancing cholinergic transmission.
In addition to the aforementioned mechanisms, there are many other important factors that may be related to the etiology of AD that have been explored over the last few years [41]. Alzheimer's disease was named after a 51-year-old patient who developed dementia, predominantly with language impairment and behavioral change [103]. Research [97, 104] reveals that this pathology affects about 10% of people over the age of 65, and 40% of those over 80 years old. The authors emphasize that AD is considered the third leading cause of death in developed countries, the first being cancer, followed by cardiovascular disease. It is assumed that, in the year 2050, more than 25% of all society will be old, which could well increase the prevalence of AD [101].
The disease begins with episodic memory deficits, accompanied by the progressive transformation of other cognitive domains, some patients present cognitive alterations, but without memory-related alterations [97]. The established risk factors for the development of AD are related to age and family history. The etiology of AD is not fully clarified, although there are many promising studies concerning its biochemistry and genetics [67].
Its main characteristic is progressive dementia with the predominant involvement of episodic memory. One of the most common risk factors for developing AD is age; the risk of developing this condition increases with age. Generally speaking, the first clinical sign is the failure of recent memory, although remote memories are preserved until the disease reaches a later stage [105].
In addition to attention difficulties and verbal fluency impairment, other cognitive functions deteriorate as the pathology evolves. Among them are the ability to make calculations, visual and spatial skills, and the ability to use common objects and tools. The patient's degree of wakefulness and lucidity is not compromised until the disease reaches a more advanced stage. Motor weakness is not observed, although muscle contractures are an almost universal feature in the advanced stages of the pathology [105].
Besides these symptoms, patients also present behavioral disorders such as aggression, hallucinations, hyperactivity, irritability, and depression. At some point in the evolution of AD, these mood disorders affect a considerable percentage of patients who develop the disease [100]. Most AD research in recent years has been directed towards finding a modifying therapy that will change the course of the disease. The previous focus was on symptoms. However, finding a modifying therapy has not yet been achieved; there is a lack of drugs capable of modifying the complex mechanisms of this disease. Management needs to be tailored to the individual patient and their specific circumstances, adapting as the disease progresses [106].
To date, drug therapy is performed through the use of conventional acetylcholinesterase inhibitors which treat mild and moderate AD: (Donepezil, rivastigmine, galantamine), and N-methyl-d-aspartate (NMDA) receptor blockers (Memantine) for moderate to severe cases. Although these drugs represent the best pharmacological treatments currently available for AD, they have a relatively low overall effect and do not interfere with the course of the neurodegenerative process. It is likely that the downregulation of cholinergic transmission occurs too late for treatments such as cholinesterase inhibitors to have any effect [107]. It is therefore important to encourage the development of new drugs with anti-PD potential, capable of improving symptoms and containing neurodegenerative mechanisms. Targets related to AD (Fig. 4) and the behavior of molecules and derivatives of linalool and citronellal were investigated in computational models for later validation in in vitro and in vivo tests.
Fig. (4).
Potential pharmacological targets of AD.
1.3.1. Potential Targets in Alzheimer's Disease
1.3.1.1. Glycogen Synthase Kinase 3 (GSK3)
Glycogen Synthase Kinase 3 (GSK-3) is a multifunctional serine-threonine kinase protein belonging to the GCMC family and ubiquitously expressed. Initially, it was identified as a regulator of glucose metabolism [108], and over time, it was discovered that it participates in several key pathways of cell biology, often involved in neurodegenerative processes [109-111]. GSK-3 is involved in several fundamental physiological processes and an abnormality in this protein can lead to a number of disorders in the body [112]. GSK-3 has two isoforms (GSK-3α and GSK-3β), which are encoded by different genes. GSK-3β is predominantly expressed in the Central Nervous System, and it is already known that levels of this isoform increase with the aging process [112].
GSK-3β is activated by the amyloid cascade and is a critical point for tau protein hyperphosphorylation, representing a link between the two main biomarkers of Alzheimer's disease (AD), the tau protein and the β -amyloid peptide [97, 113]. GSK-3β is formed through the action of γ-secretase, which cuts the fragment of transmembrane C99 into Aβ and AICD. The AICD binds to the Fe65 and, directly or indirectly, activates the GSK-3β. GSK-3β is inhibited and activated by post-translational phosphorylation of Ser9 and Tyr216 residues, respectively [108].
Once activated, GSK-3β phosphorylates microtubule-associated tau proteins (MAPT), which are involved in microtubule stabilization and axonal transport of signaling molecules and trophic factors. Physiologically, the tau protein binds to microtubules and is then phosphorylated by kinases, causing a momentary shutdown. Subsequently, phosphatases dephosphorylate tau, and therefore rebinding of the protein to the microtubules occurs. These dynamic (on and off) microtubule cycles are critical to the normal function of axonal transport. In the pathological process, GSK-3β hyper-phosphorylates tau proteins which lose their affinity for microtubules, resulting in paired helical filaments (PHFs) and neurofibrillary tangles (NFTs) which are more easily aggregated within neurons. As a result, axonal transport fails through the degradation of microtubules and, consequently of the cytoskeleton, which causes neuronal damage [29, 113, 114].
Several studies demonstrate hyperactivation of GSK-3β in the brains of patients with Alzheimer's Disease (AD), with much evidence that supports its participation in AD pathophysiology [111, 115]. Both isoforms are somehow related to AD pathology since GSK-3α participates in APP processing and Aβ formation, while GSK-3β participates in tau protein phosphorylation. Further, GSK-3 favors the production of inflammatory molecules and promotes microglia activation, which leads to neuroinflammation [29].
As a way of demonstrating its participation in the pathological process of AD, recent in vitro and in vivo studies have revealed that inhibition of GSK-3β promotes decreases in both Tau phosphorylation and Aβ levels, important biomarkers of AD. Thus, GSK-3β is an important therapeutic target for AD and many recent studies seek to evaluate the potential and efficacy of agents that can inhibit GSK-3β to develop new drugs for the treatment and prevention of AD [116].
In this context, it is already known that monoterpenes and monoterpenoids prevent the aggregation of hyper-phosphorylated tau proteins by inhibiting the active form of GSK-3β through down regulation of the PI3K/Akt-dependent pathway [29, 117]. Studies that evaluate the action of linalool and citronellal derivatives on GSK-3β are needed to evaluate this important pharmacological potential.
1.3.1.2. TNF-α Converting Enzyme (TACE)
Tumor Necrosis Factor alpha (TNF-α) is a type II monomeric transmembrane protein widely expressed in activated immune cells. Its converting enzyme, called TNF-α Converting Enzyme (TACE), is responsible for making the molecule soluble and therefore favoring its binding to TNFR1 (CD120a) and TNFR2 (CD120b) receptors [118, 119]. Deregulation of TNF-α is directly related to neuronal degeneration and inflammation, and can trigger pathological processes such as Alzheimer's disease, in which TNF-α levels are augmented [119, 120]. The type 1 receptor (TNFR1) predominantly exerts pro-inflammatory effects; type 2 (TNFR2) is neuroprotective and promotes tissue homeostasis and regeneration [120].
The serum and plasma levels of TNF-α are elevated in AD, and this is often due to chronic activation of resident microglia, which does not efficiently phagocytize beta-amyloid (Aβ) due to high levels of pro-inflammatory cytokines, including TNF-α. Further, studies at the cellular level have revealed that TNF increases apoptosis of Aβ-treated neurons [120]. Studies in human brain tissues with post-mortem AD have shown that TNFR1 levels are augmented and TNFR2 levels are lessened [121]. Another study has demonstrated that in the AD brain, TNF was more likely to bind to type 1 than type 2 receptors, which may explain the predominant role of TNRF1 in AD pathophysiology [121]. Corroborating these results, a study in a mouse model has demonstrated that inducing TNRF2 deletions exacerbates AD pathology, while overexpression prevents disease progression [122, 123].
Thus, authors have demonstrated 30% to 40% reductions in enzymatic levels of inflammatory cytokines such as TNF-α [124]. Yet another study evaluating the expression of cytokines and inflammatory proteins during exposure to UVB rays has demonstrated that linalool was able to significantly reduce overexpression of TNF-α, IL-6, IL-10, and COX-2 in skin cells [27].
1.3.1.3. Human Angiotensin-Converting Enzyme (ACE)
ACE is a zinc chloride-dependent dipeptidase responsible for regulating blood pressure and body fluids. This is done through the conversion of angiotensin I to angiotensin II, which is a potent vasoconstrictor [119]. There are two forms found in humans, the somatic form, found in various tissues, composed of 1277 amino acids and a molecular mass of approximately 146 kDa; and the germinal form, a minor isoenzyme, found exclusively in the testes, which is composed of 711 amino acids and presents a molecular mass of around 80 kDa [125].
Three different studies on the relationship between ACE and Alzheimer's have produced corroborating results. The first, performed with mice, demonstrated the influence of macrophages with ACE overexpression in the preservation of cognition and synapses, reduction of neuroinflammation, and amplified resistance against the pathognomonic amyloid-β forms of Alzheimer's disease [126]. Another study, also performed with (transgenic) mice, revealed that cognitive decline in Alzheimer's can be prevented through overexpression of ACE in myelocytes [125]. Further, a recent investigation based on genetic analysis of ACE polymorphisms has revealed that a reduction of ACE serum levels is associated with a higher risk of Alzheimer's disease progression [125].
1.3.1.4. BACE1 Inhibitor
One of the main causal hypotheses for the emergence of Alzheimer's Disease (AD) is the amyloid cascade, and an important therapeutic target for AD is β-secretase [127]. The hypothesis is based on the aggregation of β-amyloid peptide (Aβ), which is a hydrophobic peptide with 40 (Aβ40) or 42 (Aβ42) residues. The accumulation of this peptide may be due to overproduction or decreased degradation. Its oligomers are aggregated in the form of diffuse plaques in the amyloid fibrils, which cause neurotoxicity, leading to cell death and, thus, neurodegeneration [128]. β-secretase is involved in the initiation of the amyloid cascade upon production of Aβ42; accumulation of Aβ42 oligomers initiates the events [129]. Accumulation of Aβ may be related to environmental influences, stressors during aging, or during the progression of the disease [130, 131].
β-secretase (belonging to a subgroup of the A1 aspartyl protease family) is responsible for cleaving the amyloid precursor protein (APP) at two sites: the β site (Met671-Asp672) or the β' site (Tyr681-Glu682). Its action on APP results in the products β s-APP and C-terminal peptide C99. The fragment which remains bound to the membrane will be converted into Aβ40 and Aβ42 by γ-secretase, and may initiate cascade events [131, 132]. A huge number of studies suggest that BACE1 is the main β-secretase in the brain responsible for the production and aggregation of Aβ. Rodent studies show that selective inhibition of BACE1 prevents both Aβ formation and aggregation [133].
In an attempt to develop a new drug capable of treating or preventing AD, inhibition of this enzyme has been extensively studied [130]. Marumoto, et al. (2017) [134] studied 80 aromatic compounds, including monoterpenes such as citronellyl acetate, to find various compounds which can inhibit β-secretase (BACE1) in vitro with a similar potency compared to sesquiterpenes and C13 norisoprenoid compounds, and superior to most of the other sesquiterpenes were also evaluated. Such preliminary studies demonstrate the inhibitory potential of monoterpenes, but further studies are needed. There are still no studies involving linalool, citronellal derivatives, and β-secretase in the literature. As an important therapeutic target in AD, it is important to evaluate compounds with possible inhibitory potential against β-secretase.
1.3.1.5. Acetylcholinesterase (AChe)
Acetylcholinesterase (AChE) is involved in nerve impulse propagation [135] across cholinergic synapses [136]. It is a serine hydrolase responsible for terminating the activity of the neurotransmitter acetylcholine by hydrolyzing it into acetic acid and choline [137]. It is present in neurons of the central and peripheral nervous system, and its targets include skeletal muscle, and endocrine and exocrine glands [136].
The cholinergic hypothesis for the pathogenesis of AD suggests that the progressive degeneration of cholinergic neurons is the principal contributing factor to the disease. AChE inhibition has thus become a promising therapeutic strategy for the treatment of AD. Cholinesterase inhibitors (ChEIs) increase the concentration of acetylcholine in the brain, which in turn improves the patient's memory and cognitive [135].
The AChE inhibition rate of six Algerian plant essential oils (EOs) has been demonstrated and ranged from 40.57% to 73.60%. The greatest inhibition was by Lavandula officinalis (73.6%) which is composed of 35.8% linalool [138]. The inhibition was also proven in an animal model in zebrafish and resulted in the accumulation of acetylcholine in nerve endings [139]. Another study evaluated the anticholinesterase activity (76.41%) of Coriandrum sativum (which contains high levels of linalool). The results were comparable to the reference drug galanthamine, and demonstrated significant inhibition [140]. Similar work has also reported the ability of Stachys terpenes lavandulifolia Vahl. (Lamiaceae) to inhibit AChE which is composed of 0.5% linalool [141, 142].
2. MATERIALS AND METHODS
2.1. Data Set
The molecules under study were 17 compounds of the monoterpene class, 7 of which were chemical compounds derived from Citronellal and 10 compounds derived from Linalool. The structures were obtained by reviewing the literature, having monoterpenes with potential action against neurodegenerative diseases as the object of the search.
2.2. Molecular Modelling
The structures were designed in MarvinSketch software (version 18.14-2018) [143]. These were then saved in .MOL Files format, and imported into the HyperChem software for windows v.8.0.5 (HyperChem, 2009) [144]. The compounds underwent geometry optimization using molecular mechanics MM+ force field, without restrictions for aromatic form conversions, and clean molecular graphing in three dimensions. The optimized structures were subjected to conformational analysis using a random search method with 1000 interactions, 100 cycles of optimization, and the 10 lowest minimum energy conformers. The compounds were saved in the MOL format.
2.3. Pharmacokinetic Properties
Prior to carrying out the Molecular Docking simulations, the compounds were submitted to an assessment of toxicity risks, bioavailability, and oral absorption. Analyses were performed using OSIRIS Data Warrior 4.7.3 software [145, 146]. The cytotoxic effects assessed were mutagenicity, carcinogenicity, irritability to the skin and reproductive system toxicity. The TPSA (Topological Polar Surface Area) values were used to calculate the rate of absorption (%) of both the monoterpenes and the positive controls.
%ABS = 109 - (0.345 X TPSA)
The calculation proceeded with the insertion of the compound under study, saved in 3D structures in the Spatial Data File (SDF) format. Lipinski's rule consists of four parameters that influence the bioavailability of a possible drug candidate, commonly a drug can only violate up to a single parameter [147]. The rule prescribes that the bioactive may not present a molecular weight below 500 Da, LogP greater than or equal to 5, less than or equal to 10 hydrogen bond acceptors, and up to 5 hydrogen bond donors [148].
2.4. Molecular Docking
Molecular Docking simulations, searching for target proteins and the respective ligands for Parkinson's Disease and Alzheimer's Disease, were performed. For Parkinson’s Disease the study targets were Human Adenosine A2A Receptor (PDB: 3UZA, at a resolution of 3.2 Å, complexed with 6-(2,6-dimethylpyridin-4-yl)-5-phenyl-1,2,4-triazin-3-amine) [149], α-synuclein (PDB: 1XQ8, at a resolution of 2 Å) [150], COMT Catechol o-methyl transferase (PDB: 1H1D, up to a resolution of 2 Å complexed with 1-(3,4,Dihydroxy-5-nitrophenyl)-3-{4-[3-(trifluoromethyl) phenyl] piperazin-1-yl}propan-1-one) [151], Monoamine Oxidase B MAO-B (PDB: 2C65, up to a resolution of 1.7 Å complexed with (1R)-4-({[Ethyl(methyl)amino]carbonyl}oxy)-n-methyl-n-[(1e)-prop-2-en-1-ylidene]indan-1-aminium) [93], Dopamine D1 Receptor (PDB: 7JOZ, up to a resolution of 3.80 Å complexed with 6-{4-[(furo [3,2-c]pyridin-4-yl)oxy]-2-methylphenyl}-1,5-dimethylpyrimidine-2,4(1H,3H)-dione) [152], and Dopamine D2 Receptor (PDB: 6CM4, up to a resolution of 2.87 Å complexed with 3-[2-[4-(6-fluoranyl-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido [1,2-a]pyrimidin-4-one) [153].
To evaluate the affinity of the compounds under study with each of the chosen targets, a comparison was made with the PDB ligand and positive control (a drug already used with activity at the targets reported in the literature). The positive controls used were respectively: L-DOPA (α-synuclein) [154], Pramipexole (D2 Dopamine Receptor) [155], Bromocriptine (D1 Dopamine Receptor) [156], Istradefylline (Adenosine A2A Receptor) [157], Entacapone (COMT Catechol O-methyltransferase) [158], and Rasagiline (Monoamine Oxidase MAO) [95].
For the investigation of a possible mechanism of action for Alzheimer's Disease, 4 mechanisms were studied, and included: Acetylcholinesterase ACE co-crystal structure (PDB: 3BKL, at a resolution of 2.18 Å) [159], in this enzyme, the drug Donepezil was used as a positive control [160], the crystalline structure of BACE bound to 2-imino-3-methyl-5,5-diphenylimidazolidin-4-one (PDB: 4DJU, at a resolution of 1.8 Å) [161], the crystal structure of glycogen synthase kinase 3(GSK-3) in complex with 3-amino-6-{4-[(4-methylpiperazin-1-yl)sulfonyl]phenyl}-n-pyridin-3 ylpyrazine-2-carboxamide (PDB: 4ACD, at a resolution of 2.60 Å) [162], and the TACE crystal structure complexed with IK682 (PDB: 2FV5, at a resolution of 2.18 Å) [163].
We used Molegro Virtual Docker v.6.0.1 (MVD) software [164] with the parameters predefined in the software. The complexed ligand was used to define the active site. The compounds were imported to analyze the stability of the system through the interactions identified with the active site of the enzyme, taking the energy value of the MolDock Score as a reference [164-166].
The MolDock SE (Simplex Evolution) algorithm was used with the following parameters: A total of twenty runs with a maximum of 1500 iterations using a population of 50 individuals, 2000 minimization steps for each flexible residue, and 2000 global minimization steps per run. The MolDock Score (GRID) scoring function was used to calculate the snap energy values. A GRID was set at 0.3 Å and the search sphere was set at 15 Å radius.
To analyze ligand energy, internal electrostatic interactions, internal hydrogen bonds and sp2-sp2 torsions were evaluated. For each enzyme under study, the energy score was calculated using the MolDock Score and the Rerank Score (Supplementary Material Tables S1-S20). The probability was calculated by dividing the score of the molecule under study by the lowest energy score for each algorithm, and at the end, an overall average was calculated between the algorithms to generate the mean and the total mean of each enzyme.
3. RESULTS AND DISCUSSION
3.1. Computational Pharmacokinetics Studies
3.1.1. Oral Absorption
Drug absorption can occur by simple or facilitated diffusion, and mediation by plasma proteins can occur either with or against concentration gradients [167]. The 17 molecules were evaluated as to their oral absorption percentages, based on their Total Polar Topological Surface Area (TPSA) values. TPSA, according to Prasana and Doerksen (2020), involves contributions from polar functional groups such as oxygen and nitrogen (and their bonded hydrogens), and verifies the sum of contributions from molecular interactions relevant to the compound [168]. TPSA and compound absorption values are presented in Table 1.
Table 1.
Bioavailability and absorption of the Citronelal and Linalool derivatives.
| Citronelal Derivatives | ||
|---|---|---|
| Compound | TPSA (Å) | % Absorption |
| C1-Methoxycitronellal | 26.3 | 99.92 |
| C2-D-Citronellal | 17.07 | 100.00 |
| C3-Hydroxycitronellal | 37.3 | 96.13 |
| C4- Citronelic Acid | 37.3 | 96.13 |
| C5-Isopulegol | 20.23 | 100.00 |
| C6-Menthol | 20.23 | 100.00 |
| C7-S-Citronellal | 17.07 | 100.00 |
| Linalool Derivatives | ||
| Compound | TPSA (Å) | % Absorption |
| L1- Linalool | 20.23 | 100.00 |
| L2- Linalyl | 26.3 | 99.92 |
| L3 - 8-hydroxylinalyl acetate | 46.53 | 92.94 |
| L4 - 8-oxolinalyl acetate | 43.37 | 94.03 |
| L5 - 8-carboxylinalyl acetate | 63.6 | 87.05 |
| L6 - 8-hydroxylinalool | 40.46 | 95.04 |
| L7 - 8-oxo-dihydrolinalool | 37.3 | 96.13 |
| L8 - 8-oxolinalool | 37.3 | 96.13 |
| L9 - 8-carboxylinalool | 57.53 | 89.15 |
| L10 - Tetrahydrolinalool | 20.23 | 100.00 |
Note: Compounds in bold present the highest probability.
Absorption is an important parameter for planning drugs and medications. All of the compounds under study displayed high percentages for this parameter. The values obtained were between 96.13% and 100.00%. For the citronelal derivative series, the best-performing compounds were C2, C5, C6, and C7 at 100.00%. The lowest absorption rates were obtained by compounds C3 and C4, at 96.13%. The compounds of the linalool series also displayed high absorption percentages, with the best rates obtained by compounds L1 and L10 at 100.00%. The lowest rate was 87.05%, obtained by compound L5.
3.1.2. Bioavailability
Bioavailability represents the amount of drug available to a given target organ and is also called the absorbed fraction [169]. To determine the bioavailability of a drug, its route of administration, chemical form, and other patient-specific factors such as gastrointestinal and hepatic enzymes and transporters must be combined [170]. Lipinski's rule violations are considered important when determining bioavailability [171]. As a result, both positive controls and test compounds must present: no more than 5 hydrogen bond donors, fewer than 10 hydrogen bond acceptors, no greater than 5 log P, and a molecular weight of less than 500 Da [171]. Violations of Lipinski's rule and compound bioavailability evaluations are presented in Table 2.
Table 2.
Data on Lipinski's rule violations.
| Citronelal Derivatives | |||||
|---|---|---|---|---|---|
| ID | Molecular Weight | Num. H-bond Acceptors | Num. H-bond Donnors | Log P | Violations |
| C1-Methoxycitronellal | 186.294 | 2 | 0 | 2.5696 | 0 |
| C2-D-Citronellal | 154.252 | 1 | 0 | 3.1371 | 0 |
| C3-Hydroxycitronellal | 172.267 | 2 | 1 | 2.1417 | 0 |
| C4-Citronelic Acid | 170.251 | 2 | 1 | 2.9998 | 0 |
| C5-Isopulegol | 154.252 | 1 | 1 | 2.6138 | 0 |
| C6-Menthol | 156.268 | 1 | 1 | 2.4112 | 0 |
| C7-S-Citronellal | 154.252 | 1 | 0 | 3.1371 | 0 |
| Linalool Derivatives | |||||
| ID | Molecular Weight | Num. H-bond Acceptors | Num. H-bond Donnors | Log P | Violations |
| L1- Linalool | 154.252 | 1 | 1 | 3.2311 | 0 |
| L2- Linalyl | 210.316 | 2 | 0 | 4.2143 | 0 |
| L3 - 8-hydroxylinalyl acetate | 226.315 | 3 | 1 | 3.2876 | 0 |
| L4 - 8-oxolinalyl acetate | 224.299 | 3 | 0 | 3.0753 | 0 |
| L5 - 8-carboxylinalyl acetate | 240.298 | 4 | 1 | 2.938 | 0 |
| L6 - 8-hydroxylinalool | 170.251 | 2 | 2 | 2.3044 | 0 |
| L7 - 8-oxo-dihydrolinalool | 170.251 | 2 | 1 | 2.2776 | 0 |
| L8 - 8-oxolinalool | 168.235 | 2 | 1 | 2.0921 | 0 |
| L9 - 8-carboxylinalool | 184.234 | 3 | 2 | 1.9548 | 0 |
| L10 -Tetrahydrolinalool | 158.284 | 1 | 1 | 3.2807 | 1 |
The 17 potentially active terpenes were subjected to various analyses to assess their ADMET properties. Using the physicochemical properties presented, we tried to identify and evaluate whether the compounds displayed good absorption and bioavailability, considering the Lipinski rule as a parameter. According to Shimohama and Collaborators [172, 173], compounds with molecular weights below 500 Da, with partition coefficient values of less than five, with less than five hydrogen bonds, and no more than ten hydrogen acceptors, will present excellent levels of bioavailability and absorption.
There were no violations of Lipinski's rule for any parameter under study, indicating that these compounds will likely present good bioavailability. Factors such as lipophilicity and solubility contribute to drug distribution, an important parameter in drug discovery processes [174]. The most common descriptor for evaluation of lipophilicity is the n-octane-water Partition Coefficient (Log P), ideal values for lipophilicity should be < 5 [175].
3.1.3. Toxicity
Toxicity corresponds to the ability of a substance to produce harmful effects, whether in a living organism or in an ecosystem. Toxic risk is characterized as the probability that a harmful or toxic effect will occur depending on the conditions of the use of the substance [176]. The compounds under study were evaluated for toxicity in the OSIRIS Data Warrior 5.0 program [145, 146].
The parameters evaluated included: mutagenicity, that is, the ability of the substance to cause mutations in the DNA of the organism in which the bioactive is inserted - by severity [177]; tumorigenicity, which is the ability of the bioactive to cause tumors [178]; skin irritability, which corresponds to sensitivity that the compound can cause in the skin or in internal organ tissues such as the esophagus, larynx, stomach, and intestine; and toxicity in the reproductive system, which corresponds to the toxic effects that a substance can have on sexual function, and male and female fertility [179, 180]. The results are shown in Table 3 [181, 182]. The OSIRIS Data Warrior analysis, based on similarities between the fragments of each molecule was not sensitive enough to explain the effects nor the real toxicity.
Table 3.
Toxicity data for the compounds under study.
| Citronelal | ||||
|---|---|---|---|---|
| ID | Mutagenic | Tumorigenic | Reproductive | Irritant |
| C1-Methoxycitronellal | none | none | none | high |
| C2-D-Citronellal | none | none | none | high |
| C3-Hydroxycitronellal | none | none | none | high |
| C4- Citronelic Acid | none | none | none | high |
| C5-Isopulegol | none | none | none | none |
| C6-Menthol | none | none | none | none |
| C7-S-Citronellal | none | none | none | high |
| Linalool Derivatives | ||||
| ID | Mutagenic | Tumorigenic | Reproductive | Irritant |
| L1- Linalool | none | none | none | high |
| L2- Linalyl | none | none | none | high |
| L3 - 8-hydroxylinalyl acetate | none | none | none | high |
| L4 - 8-oxolinalyl acetate | none | none | none | high |
| L5 - 8-carboxylinalyl acetate | none | none | none | high |
| L6 - 8-hydroxylinalool | none | none | none | high |
| L7 - 8-oxo-dihydrolinalool | none | none | none | high |
| L8 - 8-oxolinalool | none | none | none | high |
| L9 - 8-carboxylinalool | none | none | none | high |
| L10 -Tetrahydrolinalool | none | none | none | Low |
Of the citronelal derivatives, only compounds C1, C2, C3, C4, and C7 presented toxicity risks, with a high risk of tissue irritability. Compounds C5 and C6 did not present toxicity risks in any of the parameters studied, indicating their promise. All of the compounds derived from Linalool, presented toxicity risks in the tissue irritability parameter, with compounds L1, L2, L3, L4, L5, L6, L7, L8, and L9 classified as high risk. Compound 10 was classified as low risk.
Despite the presence of toxicity risk, the compounds were maintained, since the analysis performed by the software used evaluates only parts of the molecules in isolation, and also since the risk of toxicity presented did not extend to genetic material, which would be more serious. Further, the toxicity risk was detected in only one parameter.
3.2. Molecular Docking
The 17 monoterpene molecules under study were subjected to Molecular Docking simulations with 10 proteins; six targets for Parkinson's disease and four targets for Alzheimer's disease. The results of the Molecular Docking simulations were generated using three algorithms that involved a Moldock scoring function, a Rerank scoring function, and a consensus calculation made with the two functions under study. When more negative values occur, this leads to a conclusion of better interactions in the scoring functions. A protein in which the compound obtained binding energy values greater than or close to the standard drug in at least one scoring function is considered active.
The docking results generated by the scoring function were validated by redocking the crystallographic ligand with all investigated proteins. The root mean square deviations (RMSDs) of the obtained fit poses were calculated against the crystal structure. RMSD values of less than 2 Å indicate an optimal degree of screening reliability [183, 184]. Information about the starting structures and redocking validation results are presented in Table 4. During the redocking analysis, most of the RMSD values were below 2.0 Å, that is, the generated poses correctly positioned the ligand in the active site. For docking validation in general, the programs provided satisfactory values.
Table 4.
RMSD values for the proteins selected in the study.
| Parkinson’s Disease | ||
|---|---|---|
| Protein | Binder PDB ID | RMSD |
| Adenosin Receptor (PDB: 3UZA) | 6-(2,6-dimethylpyridin-4-yl)-5-phenyl-1,2,4-triazin-3-amine - T4G | 0.1471 |
| Alpha Synuclein (PDB: 1XQ8) | - | - |
| COMT (PDB: 1H1D) | 1-(3,4,Dihydroxy-5-nitrophenyl)-3-{4-[3-(trifluoromethyl) phenyl] piperazin-1-yl}propan-1-one - BIA | 1.3867 |
| MAO (PDB: 2C65) | (1R)-4-({[Ethyl(methyl)amino]carbonyl}oxy)-n-methyl-n-[(1e)-prop-2-en-1-ylidene]indan-1-aminium - 4CR | 4.6072 |
| D1 (PDB: 7JOZ) | 6-{4-[(furo [3,2-c]pyridin-4-yl)oxy]-2-methylphenyl}-1,5-dimethylpyrimidine-2,4(1H,3H)-dione - VFP | 0.0993 |
| D2 (PDB: 6CM4) | 3-[2-[4-(6-fluoranyl-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido [1,2-a]pyrimidin-4-one - 8NU | 0.1423 |
| Alzheimer’s Disease | ||
| Protein | Binder PDB ID | RMSD |
| Acetylcholinesterase (PDB: 3BKL) | N-{(5S)-4,4-dihydroxy-6-phenyl-5-[(phenylcarbonyl)amino]hexanoyl}-L-tryptophan - KAW | 0.3335 |
| BACE (PDB: 4DJU) | (2E)-2-imino-3-methyl-5,5-diphenylimidazolidin-4-one - 0KK | 0.1312 |
| GSK3 (PDB: 4ACD) | 3-amino-6-{4-[(4-methylpiperazin-1-yl)sulfonyl]phenyl}-n-pyridin-3-ylpyrazine-2-carboxamide - GR9 | 0.1702 |
| TACE (PDB: 2FV5) | (2r)-N-hydroxy-2-[(3s)-3-methyl-3-{4-[(2-methylquinolin-4-yl)methoxy]phenyl}-2-oxopyrrolidin-1-yl]propanamide - 541 | 1.4912 |
The only enzyme that did not present an RMSD within the acceptable limit was the MAO enzyme (PDB: 2C65), its RMSD value was 4.6072. The ligand in question is a high molecular weight compound (M = 275 g/Mol), which may have resulted in a high mean standard deviation. The α-synuclein protein does not present a complexed ligand. However, in the literature, Monteiro and Collaborators (2018) [185] reported validating this macromolecule. The option to detect 10 possible cavities, admitted as possible active sites, on which to run the molecular docking was chosen.
The docking results are presented in Table 5. According to the results, the compounds of the series under study obtained negative energies for all enzymes under study, thus demonstrating an interaction with all study targets. The consensus calculations were performed using the scores of each analyzed protein calculated using algorithms obtained in the Molegro Virtual Doker software, namely: MoldokScore and RerankScore.
Table 5.
Consensus values calculated from the Score energies of the Citronelal derivatives under study and their Alzheimer’s and Parkinson’s disease target enzymes.
| Citronelal Derivatives | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Parkinson’s Disease Targets | Alzheimer’s Disease Targets | ||||||||
| α-Sinuclein | Adenosin A2A | COMT | MAO | D1 | D2 | AChE | BACE | GSK3 | TACE | |
| C1 -Methoxycitronellal | 0.9041 | 0.7119 | 0.7225 | 0.6931 | 0.6570 | 0.5919 | 0.4480 | 0.7889 | 0.4990 | 0.5665 |
| C2 D-Citronellal |
0.8824 | 0.6529 | 0.7029 | 0.6355 | 0.6472 | 0.5489 | 0.4044 | 0.6583 | 0.5277 | 0.5218 |
| C3 Hydroxycitronellal |
0.8762 | 0.6645 | 0.7376 | 0.6686 | 0.6329 | 0.5547 | 0.4290 | 0.7201 | 0.3772 | 0.5325 |
| C4 Citronelic Acid |
0.9796 | 0.6630 | 0.8521 | 0.6891 | 0.6376 | 0.5781 | 0.4944 | 0.6009 | 0.5547 | 0.5273 |
| C5 Isopulegol |
0.7749 | 0.4468 | 0.6870 | 0.5416 | 0.5646 | 0.5566 | 0.2802 | 0.5456 | 0.4604 | 0.4965 |
| C6 Menthol |
0.7837 | 0.5039 | 0.7161 | 0.5785 | 0.5676 | 0.5358 | 0.3533 | 0.6146 | 0.4601 | 0.4850 |
| C7 S-Citronellal |
0.8883 | 0.6416 | 0.7251 | 0.6564 | 0.5926 | 0.5551 | 0.4154 | 0.6538 | 0.5053 | 0.5118 |
| Positive Control | 0.9718 | 0.5 | 0.8113 | 0.6163 | 0.6853 | 0.6586 | 0.6911 | - | - | - |
| PDB Ligand | - | 0.9386 | 1 | 0.5 | 0.8026 | 1 | 1 | 1 | 1 | 1 |
Note: The molecule with the highest probability is in bold. Compounds with a higher probability than the ligand are in yellow.
According to the results (presented in Table 6), the compounds derived from citronellal demonstrated greater affinity (than the ligand) for the α-Synuclein, Adenosine receptor, Monoamine Oxidase (MAO), and Dopamine D1 receptor proteins; targets related to Parkinson. For Alzheimer’s Disease related targets, the compounds under study did not demonstrate greater potency than the ligand for the study targets. Table 6 presents results obtained by the linalool derivatives for the study enzymes, being Parkinson's and Alzheimer’s disease targets.
Table 6.
Consensus values calculated from the score energies of the Linalool derivatives under study and their Alzheimer's and Parkinson's disease target enzymes.
| Linalool Derivatives | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ID | Parkinson’s Disease Targets | Alzheimer’s Disease Targets | ||||||||
| α-Sinuclein | Adenosin A2A | COMT | MAO | D1 | D2 | AChE | BACE | GSK3 | TACE | |
| L1 Linalool |
0.6982 | 0.5122 | 0.7371 | 0.5515 | 0.5352 | 0.5348 | 0.3884 | 0.6826 | 0.5088 | 0.5223 |
| L2 Linalyl |
0.8525 | 0.6915 | 0.9459 | 0.6701 | 0.7950 | 0.6706 | 0.4968 | 0.8919 | 0.6519 | 0.6514 |
| L3 8-hydroxylinalyl acetate |
0.8164 | 0.8706 | 0.7698 | 0.6997 | 0.6847 | 0.6711 | 0.4965 | 0.9615 | 0.4677 | 0.6358 |
| L4 8-oxolinalyl acetate |
0.8421 | 0.7228 | 0.8037 | 0.7334 | 0.6465 | 0.6804 | 0.4672 | 0.8779 | 0.5782 | 0.6615 |
| L5 8-carboxylinalyl acetate |
1 | 0.8108 | 1 | 0.7250 | 0.6873 | 0.6261 | 0.4815 | 0.9456 | 0.3289 | 0.7393 |
| L6 8-oxo-dihydrolinalool |
0.7350 | 0.6019 | 0.7679 | 0.6011 | 0.5844 | 0.5740 | 0.4016 | 0.8214 | 0.6054 | 0.5580 |
| L7 8-oxo-dihydrolinalool |
0.6384 | 0.5901 | 0.7560 | 0.5857 | 0.5929 | 0.5667 | 0.4282 | 0.7627 | 0.2970 | 0.5540 |
| L8 8-oxolinalool |
0.7212 | 0.5947 | 0.6594 | 0.5845 | 0.5707 | 0.5607 | 0.3800 | 0.7277 | 0.5610 | 0.5597 |
| L9 8-carboxylinalool |
0.8377 | 0.5146 | 0.9325 | 0.6282 | 0.6559 | 0.5390 | 0.4186 | 0.6873 | 0.3308 | 0.5518 |
| L10 Tetrahydrolinalool |
0.6532 | 0.5331 | 0.7345 | 0.5608 | 0.4906 | 0.5363 | 0.3769 | 0.6300 | 0.5164 | 0.5162 |
| Positive Control | 0.8199 | 0.5 | 0.8206 | 0.5356 | 0.6564 | 0.6586 | 0.6660 | - | - | - |
| PDB Ligand | - | 0.9386 | 0.9268 | 0.5 | 0.7246 | 1 | 1 | 0.9684 | 1 | 1 |
Note: The molecule with the highest probability is in bold. Compounds with a higher probability than the ligand are in yellow.
As to the linalool derivatives, for targets related to Parkinson's disease, and similarly for the citronellal derivatives, these proved to be more potent for the α-Synuclein, Adenosine Receptor, Monoamine Oxidase, Dopamine D1 Receptor and Dopamine D2 Receptor enzymes. For the Alzheimer's disease targets, these compounds displayed more promise for the BACE enzyme. These will be discussed in more detail, in addition to demonstrating the molecular interactions of the most promising compounds with the target enzymes.
3.4. Parkinson’s Disease
3.4.1. α-Synuclein
All of the compounds in the citronellal series presented probabilities above 50% for the α-synuclein protein. The highest probability obtained was for compound C4 (Citronelic acid), p = 0.9796, followed by the positive control with a value of 0.9718. For the linalool derivatives, all compounds presented probability values above 50%. The most likely compound was compound L5 (8-carboxylinalyl acetate, p = 1.0, followed by compound L2 (Linalyl) with a p-value of 0.8525. The positive control presented a probability of 0.8199, and we noted that all five compound probabilities were either higher or close to this value, respectively compounds L5 (8-carboxylinalyl acetate, p = 1.0), L2 (Linalyl, p = 0.8525), L4 (8-oxolinalyl acetate, p = 0.8421), L9 (8-carboxylinalool, p = 0.8377), and L3 (8-hydroxylinalyl acetate, p = 0.8164). (Supplementary Material- Fig. S1 (15.3MB, pdf) ) presents the interactions of compounds C4 - Citronelic acid (Citronellal Derivative), L5 - 8-carboxylinalyl acetate (Linalool Derivative), and the positive control L-Dopa with α-synuclein.
Molecular coupling with the α-synuclein enzyme demonstrated a prevalence of hydrophobic and hydrogen interactions. Compound C4 (Citronelic Acid, derived from Citronellal) presented mostly hydrophobic interactions. The residues involved in the interaction were: Leu 100 (two interactions), Lys 97 (three interactions), and Lys 96. Hydrogen interactions occurred at only two residues, Lys 96 and Lys 97 established with the oxygen atom (O) of the carboxyl group. However, compound L5 (8-carboxylinalyl acetate, derived from Linalool), established equal numbers of hydrophobic and hydrogen interactions. These hydrogen interactions were respectively formed at residues Lys 96 and Val 95, with oxygen atoms of the carboxyl group and the ester carbonyl.
Hydrophobic interactions occurred at residues Lys 97, Lys 96, Phe 94, and Val 95 involving carbon (C) atoms of the monoterpene. Similar interactions occurred with the hydroxyl groups (OH) at residues Lys 97 and Lys 96. In addition, similar hydrophobic interactions were observed at residues Lys 97 and Lys 96. The positive control presented hydrogen interactions involving residues Phe 94 and Lys 96. Hydrophobic interactions were also observed with amino acids Lys 97 and Lys 96. Similar interactions also occurred between the three compounds, involving a hydrogen bond established at residue Lys 96 and hydrophobic interactions at amino acids Lys 96 and Lys 97.
3.4.2. A2A Receptor
For the A2A Adenosine Receptor macromolecule (with the exception of compound C5 - Isopulegol), the compounds of the study presented probabilities much higher than the results presented by the positive control Istradefylline. The PDB ligand presented the highest probability. For the Citronellal derivatives, six compounds displayed higher probabilities than the positive control. Compound C6 (Menthol) presented a probability of 0.5039. Compounds C2 (D-Citronellal), C3 (Hydroxycitronellal), C4 (Citronelic acid), and C7 (S-Citronellal) presented probabilities above 60% and compound C1 (Methoxycitronellal) presented a probability of p = 0.7119. The linalool derivatives (in their entirety) displayed probabilities superior to the positive controls. The PDB ligand presented a probability of p = 0.9386, while the positive control presented a probability of only 0.5.
Compounds L1 (Linalool), L7 (8-oxo-dihydrolinalool), L8 (8-oxolinalool), L9 (8-carboxylinalool) and L10 (Tetrahydrolinalool) presented probabilities above 50%. Compounds L2 (Linalyl), and L6 (8-oxo-dihydrolinalool) presented probabilities above 60%. Compound L4 (8-oxolinalyl acetate) obtained probability values above 70%, totaling p = 0.7228. Compounds L5 (8-carboxylinanalyl acetate) and L3 (8-hydroxylinalyl acetate) presented probabilities above 80%, respectively (p = 0.8108) and (p = 0.8706). Compound L3 presented probabilities above the positive control and presented affinity values close to those presented by the PDB ligand of the enzyme under study. The molecular couplings of: Compound C1-Methoxycitronellal (Citronellal Derivative), Compound L5-8-carboxylinalyl acetate (Linalool Derivative), the positive control Istradefylline, and the PDB ligand with the Adenosine Receptor are presented (Supplementary Material- Fig. S2 (15.3MB, pdf) ).
Molecular coupling with the Adenosine Receptor demonstrated the three types of interaction: steric interactions, hydrophobic interactions, and hydrogen bonds. Compound C1 Methoxycitronellal (Citronellal Derivative) presented only hydrophobic interactions, established with the carbon atoms (C). Interactions were established at residues Phe 108 (3 interaction), Ala 63 (1 interaction), Ile 66 (1 interaction), His 278 (1 interaction), Val 84 (2 interactions), Ile 274 (2 interactions), Leu 249 (2 interactions), and Met 177 (1 interaction). Bonding to carbon (C) and Hydrogen (H) atoms of the carbon chain of the monoterpene was also observed for compound L5 (8-carboxylinalyl acetate), but unlike compound C1 Methoxycitronellal, which only displayed hydrophobic interactions, compound C3 also displayed hydrogen interactions.
Hydrogen interactions occurred at Asn 253 (1 interaction), and Ala 63 (1 interaction). Hydrophobic interactions occurred at Val 84 (3 interactions), Ile 274 (2 interactions), Phe 168 (1 interaction), Ala 277 (1 interaction), Leu 249 (3 interactions), His 278 (1 interaction), and Trp 246 (2 interactions). The two monoterpenes under study displayed coincidences between hydrophobic interaction residues at Leu 249, Ile 274, Val 84, and Phe 168.
The positive control Istradefylline was the only compound to present steric interactions. The hydrogen interactions involved residues His 250 (1 interaction) and Asn 253 (1 interaction). Hydrophobic interactions occurred at Ile 92 (1 interaction), Ala 88 (1 interaction), Leu 85 (1 interaction), Ile 274 (3 interactions), Phe 168 (1 interaction), Met 270 (1 interaction), Leu 249 (3 interactions), Met 177 (2 interactions), His 250 (1 interaction), Val 186 (3 interactions), Trp 246 (1 interaction), and Phe 242 (2 interactions). Steric interactions occurred at Asn 253 (1 interaction) and Ala 88 (2 interactions). The positive control displayed interactions similar to the citronellal derivative, with hydrophobic interactions forming at residues Phe 168, Ile 274, Leu 249, and Met 177. In relation to the Linalool derivative, hydrophobic interactions also occurred at Ile 274, Phe 168, Leu 249, and Trp 246. Coincident hydrogen interactions occurred at residue Asn 253.
The PDB ligand presented both hydrophobic interactions and hydrogen bonds. The hydrogen interactions visualized involved the Asn residue 253. Hydrophobic interactions were the most prevalent and involved the amino acids Ala 63 (1 interaction), Ile 274 (2 interactions), His 278 (1 interaction), Ala 277 (1 iteration), Met 177 (1 interaction), His 250 (1 interaction), Leu 249 (4 interactions), Met 270 (1 interaction), Phe 168 (2 interactions), Ile 66 (1 interaction), and Ala 63 (1 interaction). The Citronellal-derived compound presented similar interactions with the PDB ligand that involved hydrophobic interactions at Met 177, Phe 168, Leu 249, Ile 274, His 278, Ile 66, and Ala 63. As for the linalool derivative, the PDB ligand presented similarity in the hydrogen interaction at the Asn 253 residue, as well as for hydrophobic interactions involving the amino acids Ile 274, Phe 168, Leu 249, Ala 277, and His 278.
3.4.3. Catechol-O-methyltransferase (COMT)
For the enzyme Catechol-O-methyltransferase (COMT), four compounds presented higher affinity than demonstrated by either the positive control or the PDB ligand. For Citronellal derivatives, the highest probability value was obtained by the PDB ligand (p = 1.0). Compound C4 (Citronelic Acid) presented probability values equivalent to 0.8521, being higher than the values obtained by the positive control, which corresponded to 0.8113. For the Linalool derivatives, compound L5 (8-carboxylinalyl acetate) presented the highest probability value, equivalent to 1.0, followed by compounds L2-Linalyl (p = 9459) and L9-8-carboxylinalool (p = 0.9325). The positive control presented probability values corresponding to 0.8206 and the PDB ligand presented a probability of 0.9268. (Supplementary Material- Fig. S3 (15.3MB, pdf) ) presents the molecular coupling that occurred between the compounds C4 (Citronelic Acid), L5 (8-carboxylinalyl acetate), the positive control, and the PDB ligand with Catechol-O-methyltransferase (COMT).
Molecular coupling with Catechol-O-methyltransferase (COMT) demonstrated the occurrence of hydrophobic interactions and hydrogen bonds. The C4-Citronelic Acid compound presented only one hydrogen interaction, which was established at the Lys 144 residue. Hydrophobic interactions occurred at the amino acids Leu 198 (1 interaction), Pro 174 (2 interactions) and Trp 38 (2 interactions). The compound L5 (8-carboxylinalyl acetate), presented hydrogen bonds at residue Lys 144 and hydrophobic interactions at the amino acids Val 173 (1 interaction), Leu 198 (2 interactions), Pro 174 (2 interactions). interactions), and Trp 38 (2 interactions). The monoterpenes under study coincided in most of the observed residues, the only residue that did not coincide between them was a hydrophobic interaction established at Val 173, which occurred only with the Linalool derivative.
The positive control presented only three hydrophobic interactions that were established at residues Trp 38 (2 interactions), Pro 174 (1 interaction), and Met 40 (1 interaction). Hydrogen interactions were more prevalent, being observed at amino acids Pro 174 (1 interaction), Glu 199 (1 interaction), Asp 141 (1 interaction), Asn 170 (1 interaction), and Lys 144 (2 interactions). The positive control presented interactions coincident with the monoterpenes, a hydrogen interaction at Lys 144 and hydrophobic interactions at residues Trp 38, and Pro 174.
The PDB ligand presented great similarity in interactions with the positive control, with hydrogen interactions at residues Glu 199 (1 interaction), Asp 141 (1 interaction), Asn 170 (1 interaction), and Lys 144 (2 interactions). The hydrophobic interactions corresponded to amino acids Trp 38 (2 interactions), Pro 174 (1 interaction), and Met 40 (1 interaction). As was observed with the positive control, coincident residues for hydrogen interactions were observed at Lys 144, and for hydrophobic interactions at Trp 38, and Pro 174.
3.4.4. Monoamine Oxidase (MAO)
For monoamine oxidase, all compounds under study presented higher probabilities than either the positive control or the PDB ligand. The highest probability value obtained in the series of Citronellal derivatives was obtained by the compound C1-Methoxycitronellal (p = 0.6931). The compounds C5-Isopulegol and C6-Menthol presented probabilities above 50%, and the compounds C1-Methoxycitronellal, C2-D-Citronellal, C3- Hydroxycitronellal, C4-Citronelic Acid, and C7-S-Citronellal presented probability values above 60%. The positive control obtained probability values of 0.6163, while the PDB ligand presented probability values of around 0.5. With regard to Linalool derivatives, similarly to the Citronellal derivatives, all presented greater affinities than either the positive control or the PDB ligand, such that the compounds L1-Linalool, L7-8-oxo-dihydrolinalool, L8-8-oxolinalool and L10- Tetrahydrolinalool all presented probabilities above 50%, while the compounds L2-Linalyl, L3- 8-hydroxylinalyl acetate, L6-8-oxo-dihydrolinalool, L6-8-carboxylinalool presented probabilities above 60%. Probability values above 70% were obtained by compounds L5-8-carboxylinalyl acetate and L4-8-oxolinalyl acetate, which presented the highest probability of p = 0.7334. The positive control presented a probability of 0.5356 and the PDB ligand presented a probability of 0.5. Molecular coupling with compounds C1-Methoxycitronellal (Citronellal Derivative), Compound L4- 8-oxolinalyl acetate (Linalool Derivative), Rasagiline (positive control), the PDB ligand, and MAO are presented in (Supplementary Material- Fig. S4 (15.3MB, pdf) ).
The interactions established with the MAO enzyme were hydrophobic, hydrogen, and steric. For compound C1- methoxycitronellal, hydrogen interactions were formed with the oxygen atoms (O) at residue Ser 59. Hydrophobic interactions were established with the atoms of the carbon chain, at amino acids Met 436 (2 interactions), Arg 42 (2 interactions), Tyr 398 (2 interactions), Cys 397 (2 interactions), and Trp 388 (1 interaction), Lys 296 (1 interaction) and Val 294 (1 interaction). For compound L4- 8-oxolinalyl acetate (Linalool derivative), hydrogen interactions were established with the oxygen atoms (O) of the ester group, and with hydrogen atoms (H) of the carbon chain, which were present at Gly 57 (1 interaction), Cys 397 (1 interaction), Gly 58 (1 interaction), Arg 42 (1 interaction), Tyr 398 (1 interaction), Ser 15 (1 interaction), and Ile 14 (1 interaction). Hydrophobic interactions were located in the carbon chain as with the citronellal derivative. Hydrophobic interactions occurred at residues Met 436 (2 interactions) and Arg 42 (1 interaction). The monoterpenes under study displayed coincident interactions, with hydrophobic interactions at the Met 436 residue and Arg 42. The positive control Rasagiline presented hydrogen interactions with the nitrogen (N) and hydrogen (H) atoms of the compound at residues Tyr 398 (1 interaction), Gly 434 (1 interaction), and Gly 58 (1 interaction). Hydrophobic interactions were more numerous, being established at residues Met 436 (1 interaction), Arg 42 (1 interaction), Tyr 398 (1 interaction), and Gly 57 (1 interaction). The occurrence of a steric interaction at the Cys 397 residue was also noted.
The control displayed coincidence with the citronellal derivative in interactions that involved hydrophobic interactions at Met 436 and Arg 42. For the linalool derivative, the positive control coincided with the interaction of hydrogen at Gly 58 and Tyr 398. In addition to coincident hydrophobic interactions at Arg 42 and Met 436.
The PDB ligand presented the greatest number of interactions; this is due to its high molar mass and connectivity, which allow multiple interactions. The hydrogen interactions involved residues Tyr 60 (2 interactions), Ser 59 (1 interaction), Gly 58 (2 interactions), Gly 434 (1 interaction), Thr 426 (1 interaction), Arg 42 (1 interaction), Thr 426 (1 interaction), Ser 15 (1 interaction), Gly 41 (1 interaction), Ala 263 (2 interactions), Gly 11 (1 interaction), and Glu 34 (1 interaction). The hydrophobic interactions involved residues Ile 199 (1 interaction), Phe 168 (1 interaction), Leu 171 (2 interactions), Tyr 326 (1 interaction), Phe 343 (3 interactions), Val 294 (2 interactions), Gly 57 (3 interactions), Trp 988 (3 interaction), Cys 997 (1 interaction), Tyr 398 (2 interaction), Pro 265 (1 interaction) and Ile 264 (1 interaction). Steric interactions were the last type of interactions visualized and these involved residues Glu 94 (1 interaction), Arg 96 (2 interactions), Ala 439 (1 interaction), Arg 42 (2 interactions), Ile 14 (2 interactions), Tyr 398 (2 interactions), Cys 397 (1 interaction), Lys 296 (1 interaction), Phe 348 (3 interactions), Cys 172 (2 interactions), and Phe 168 (1 interaction). The PDB ligand also presented an interaction similar to C1-Methoxycitronellal, referring to the hydrogen bond established at the Ser 59 residue. As for the hydrophobic interactions, similarities were observed at the residues Val 294, Cys 397, Trp 388, and Tyr 398, for the Linalool derivative.The compound L4-8-oxolinalyl acetate presented coincidence only in the interactions of the amino acid residues referring to hydrogen interactions at the Arg 42, Gly 58 and Ser 15 residues.
3.4.5. Dopamine D1 Receptor
With respect to the Dopamine D1 receptor, no compound related to Citronellal derivatives presented higher probability values than the positive control or the PDB ligand under study. The positive control presented probability values corresponding to 0.6853, while the PDB ligand presented the highest probability at p = 0.8026. Of the compounds derived from Linalool, four compounds displayed higher probabilities than the standard Bromocriptine positive control and PDB ligand. The highest probability compound was compound L2-Linalyl with p = 0.7950. Compound L3-8-hydroxylinalyl acetate (p = 0.6847), L5-8-carboxylinalyl acetate (0.6873) and L9-8-carboxylinalool (p = 0.6559). The positive control Bromocriptine presented a probability of 0.6564, while the PDB ligand presented probability values corresponding to 0.7276. (Supplementary Material- Fig. S5 (15.3MB, pdf) ) presents the molecular coupling of L2-Linalyl (a Linalool Derivative), Bromocriptine (the positive control), and the PDB ligand with the Dopamine D1 receptor.
Molecular coupling with the Dopamine D1 receptor displayed three types of interactions: steric interactions, hydrogen interactions, and hydrophobic interactions. Compound L2-Linalyl (Linalool derivative) displayed hydrophobic interactions, steric interactions and hydrogen bonds. The hydrophobic interactions occurred at the following residues: Phe 289 (2 interactions), Leu 190 (1 interaction), Phe 288 (2 interactions), and Ile 104 (2 interactions).
There was only one hydrogen interaction, which occurred at Ser 198 (1 interaction). Steric interactions were observed only once at the Ser 195 residue. The positive control Bromocriptine formed the greatest number of interactions, and was the only compound to present steric interactions. Its hydrogen interactions occurred at: Ser 189 (1 interaction), Ser 107 (1 interaction) and Cys 186 (2 interactions). Bromocriptine´s hydrophobic interactions occurred at amino acids: Ile 104 (3 interactions), Phe 289 (2 interactions), Phe 288 (2 interactions), Phe 313 (1 interaction), Val 317 (1 interaction) and Val 100 (1 interaction). Steric interactions occurred at two specific residues: Asp 103 (1 interaction) and Val 317 (1 interaction). Coincident interactions were observed between the L2-Linalyl compound (Linalool derivative) and the positive control Bromocriptine, which involved hydrophobic interactions at residues Phe 289, Phe 288, and Ile 104.
The PDB ligand presented only one hydrogen interaction formed by the Cys residue 186 and three hydrophobic interactions at amino acids Ile 104 (2 interactions) and Phe 289 (1 interaction). Similar residues were observed for the PDB ligand and the Linalool-derived compound through hydrophobic interactions at Ile 104 and Phe 289.
3.4.6. Dopamine D2 Receptor
The Dopamine D2 receptor results were similar to those obtained for the D1 receptor, in which the Citronellal derivatives did not present higher probability values than the positive control or the PDB ligand under study. The positive control presented probability values of 0.6586, while the PDB ligand, being the compound with the highest probability, presented values corresponding to 1.0. Regarding Linalool derivatives, the PDB ligand presented the highest probability, corresponding to the value 1.0, while the positive control presented values corresponding to 0.6586. In this series, three compounds presented higher probabilities than the positive control, corresponding to L2-Linalyl (p = 0.6706), L3-8-hydroxylinalyl acetate (p = 0.6711) and L4-8-oxolinalyl acetate (0.6804). Supplementary Material Fig. (S6 (15.3MB, pdf) ) presents the molecular coupling that occurred between the L4-8-oxolinalyl acetate compounds, the positive control, and the PDB ligand with the Dopamine D2 Receptor.
In molecular coupling with the Dopamine D2 Receptor, three types of interaction were visualized: steric interactions, hydrophobic interactions, and hydrogen bonds. For the compound L4-8-oxolinalyl acetate, only one hydrogen interaction was observed, at the amino acid Asp 114. The hydrophobic interactions corresponded to Cys 118 (1 interaction), Val 115 (2 interactions), Phe 390 (2 interactions) and Trp 386 (2 interactions).
The positive control Pramipexole was the only compound to present steric interactions, these at the residues: Trp 386 (1 interaction), Phe 390 (1 interaction) and Cys 118 (1 interaction). Three hydrogen interactions were stabilized by residues Ser 197 (2 interactions) and Asp 114 (1 interaction). Hydrophobic interactions were the most prevalent Tyr 416 (1 interaction, Phe (1 interaction), Trp 386 (3 interactions), Cys 118 (2 interactions), Val 115 (1 interaction) and Phe 390 (1 interaction). In addition, coincidences were observed in the residues of the hydrophobic interactions between the positive control Pramipexole and the monoterpene, Asp 114, Trp 386, Phe 390, Cys 118, and Val 115.
The PDB ligand presented hydrogen interactions at residues Val 115 (1 interaction), and Ser 197 (2 interactions), Cys 118 (1 interaction), Tyr 416 (1 interaction) and Thr 412 (1 interaction). The hydrophobic interactions were more prevalent and included the amino acids Tyr 408 (1 interaction), Trp 386 (2 interactions), Cys 118 (3 interactions), Phe 390 (1 interaction), Val 115 (1 interaction), Trp 100 (1 interaction) and Phe 110 (1 interaction). The PDB ligand presented similarities with the interactions demonstrated by the monoterpene under study in relation to the hydrophobic interactions at Trp 386, Cys 118, Val 115 and Phe 390.
3.5. Alzheimer’s Disease
3.5.1. BACE
The BACE enzyme was the only Alzheimer's disease target in which the compounds displayed higher affinity than the ligands under study. For the BACE enzyme, only the Linalool derivatives presented higher affinity than the PDB ligand under study. Compound L3 8-hydroxylinalyl acetate (Linalool Derivative) presented the best performance (p = 0.9615). The PDB ligand presented a probability of 0.9684. The molecular coupling of compounds L3- 8-hydroxylinalyl acetate (Linalool Derivative) and the PDB ligand with the BACE enzyme are presented in Supplementary Material- Fig. (S7 (15.3MB, pdf) ).
Molecular coupling with the BACE enzyme involved only hydrophobic or hydrogen bonding interactions. Compound L3- 8-hydroxylinalyl acetate (Linalool Derivative) displayed the highest number of hydrophobic interactions, at Ile 79 (2 interactions), Trp 137 (2 interactions), Val 130 (1 interaction). The hydrogen bonds occurred at the amino acids Phe 169 (1 interaction), Tyr 132 (1 interaction), and Asp 93 (1 interaction).
The PDB ligand presented a prevalence of hydrogen interactions, which were stabilized through residues Asp 289 (2 interactions) and Asp 93 (2 interactions). Only one hydrophobic interaction occurred at residue Tyr 132. The PDB ligand coincided with the Linalool derivative for hydrogen bonds established at residue Asp 93.
For the citronellal derivatives, it was observed that the compounds C1- Methoxycitronellal, C2- D-Citronellal, C3- Hydroxycitronellal, C4- Citronelic Acid, C6- Menthol, and C7- S-Citronellal were able to modulate two or even three targets, revealing multitarget potential, or a capability to modulate several enzymes [186]. In addition, compounds C1 and C4 proved to be the most likely compounds for the targets under study(the α-synuclein enzyme for Compound C4, and the MAO enzyme for Compound C1). For the Linalool derivatives, all ten compounds under study displayed multitarget potential. Compound L5 displayed the highest probabilities for more than one target under study, presenting greater potencies than the other compounds. Compound L5 targeted α-synuclein and COMT enzymes.
CONCLUSION
Considering the methodologies applied, the monoterpenes studied presented satisfactory results. In general, the compounds presented low toxicity and, as suggested by Lipinski's rule of five, high oral absorption and bioavailability values. In the molecular docking tests, citronellal and linalool derivatives presented binding energies close to (or greater than) the binding energy of the endogenous ligand, and greater affinities for targets related to Parkinson's disease: α-Synuclein proteins, the Adenosine receptor, Monoamine Oxidase (MAO), the Dopamine D1 receptor and the Dopamine D2. For Alzheimer's disease, linalool and its derivatives alone presented promising activation energy against the BACE enzyme target. The studied compounds reveal promising activity with satisfactory pharmacokinetics, both of which are essential for new drug development.
ACKNOWLEDGEMENTS
The authors acknowledge the Coordination for the Improvement of Higher Education Personnel (Capes) - Brazil, and the National Council for Scientific and Technological Development (CNPq) (for granting scholarships), and the Federal University of Paraíba (UFPB).
LIST OF ABBREVIATIONS
- α-Syn
α-Synuclein
- A1, A1A, A2B,A3
Adenosine Receptor Subtypes
- Aβ
β-amyloid
- ACE
Human Angiotensin Converting Enzyme
- AChE
Acetylcholinesterase
- AD
Alzheimer Disease
- ADMET
Absorption, Distribution, Metabolism, Excretion, and Toxicity
- AICD
APP Intracellular Domain
- AKT
Protein Kinase B
- APP
Protein Amyloid Precursor
- BACE 1
β-site APP Cleaving Enzyme 1
- BDNF
Brain-derived Neurotrophic Factor
- cAMP
Cyclic Adenosine 3,5-Monophosphate
- ChEIs
Cholinesterase Inhibitors
- CMGC
CMGC Kinase Family Proteins
- COMT
Catechol-O-methyltransferase
- COX-2
Cyclooxygenase 2 Enzyme
- D1 - D5
Dopaminergic Receptors
- DNA
Deoxyribonucleic Acid
- DP
Dopamine
- FAD
Flavin Adenine Dinucleotide
- FE65s
Family of Adaptor/Scaffolding Proteins
- GABAA
Gamma Aminobutyric Acid
- Gi
G-protein Inhibitory (Subtype)
- GPCR
G Protein-coupled Receptors
- Gs
G-protein Stimulating (Subtype)
- GSK-3
Glycogen Synthase Kinase 3
- GSK-3α
Glycogen Synthase Kinase 3α
- GSK-3β
Glycogen Synthase Kinase 3β
- IL-I
Interleukin I
- IL-6
Interleukin 6
- IL-10
Interleukin 10
- MAPK
Mitogen Activated Protein Kinases
- MAO
Monoamino Oxidase
- MAO A
Monoamino Oxidase A
- MAO B
Monoamino Oxidase B
- MAO-Bis
Monoamine Oxidase Type B Inhibitors
- MAPT
Microtubule-associated Tau Protein
- MEP
Methylerythritol Phosphate
- MVD
Molegro Virtual Docker
- Na+
Sodium Ion
- NDD
Neurodegenerative Diseases
- NFκB
Nuclear Factor-κB
- NFT
Neurofibrillary Tangles
- Nrf2
Erythroid-derived Nuclear Factor 2
- NMDA
N-methyl-d-aspartate
- OE
Essencial Oils
- OH
Hydroxyl Groups
- PD
Parkinson Disease
- PDB
Protein Data Bank
- PHFs
Paired Helical Filaments
- PI3K/Akt
Protein kinase B
- RMSDs
Root Mean Square Deviations
- RNA
Ribonucleic Acid
- RNAm
Messenger Ribonucleic Acid
- SDF
Spatial Data File
- SE
Simplex Evolution
- Ser9
GSK-3β Phosphate
- SNpc
Black Substance Compact Part
- TACE
TNF-α Converting Enzyme
- TNF-α
Tumor Necrosis Factor alpha
- TNFR1
Tumor Necrosis Factor Receptor type 1
- TNFR2
Tumor Necrosis Factor Receptor type 2
- TPSA
Topological Polar Surface Area
- TrkB
Tropomyosin Kinase B Receptor
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s website along with the published article.
REFERENCES
- 1.Lamptey R.N.L., Chaulagain B., Trivedi R., Gothwal A., Layek B., Singh J. A review of the common neurodegenerative disorders: current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022;23(3):1851. doi: 10.3390/ijms23031851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu H., Hu Y., Zhang Y., Zhang H., Gao S., Wang L., Wang T., Han Z., Sun B., Liu G. Mendelian randomization highlights significant difference and genetic heterogeneity in clinically diagnosed Alzheimer’s disease GWAS and self-report proxy phenotype GWAX. Alzheimers Res. Ther. 2022;14(1):17. doi: 10.1186/s13195-022-00963-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain N., Chen-Plotkin A.S. Genetic modifiers in neurodegeneration. Curr. Genet. Med. Rep. 2018;6(1):11–19. doi: 10.1007/s40142-018-0133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain V., Baitharu I., Barhwal K., Prasad D., Singh S.B., Ilavazhagan G. Enriched environment prevents hypobaric hypoxia induced neurodegeneration and is independent of antioxidant signaling. Cell. Mol. Neurobiol. 2012;32(4):599–611. doi: 10.1007/s10571-012-9807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jellinger K.A. Basic mechanisms of neurodegeneration: a critical update. J. Cell. Mol. Med. 2010;14(3):457–487. doi: 10.1111/j.1582-4934.2010.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajiah K., Maharajan M.K., Yeen S.J., Lew S. Quality of life and caregivers’ burden of Parkinson’s Disease. Neuroepidemiology. 2017;48(3-4):131–137. doi: 10.1159/000479031. [DOI] [PubMed] [Google Scholar]
- 7.Henderson C., Knapp M., Nelis S.M., Quinn C., Martyr A., Wu Y.T., Jones I.R., Victor C.R., Pickett J.A., Hindle J.V., Jones R.W., Kopelman M.D., Matthews F.E., Morris R.G., Rusted J.M., Thom J.M., Clare L. Use and costs of services and unpaid care for people with mild‐to‐moderate dementia: Baseline results from the IDEAL cohort study. Alzheimers Dement. (N. Y.) 2019;5(1):685–696. doi: 10.1016/j.trci.2019.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui L., Hou N.N., Wu H.M., Zuo X., Lian Y.Z., Zhang C.N., Wang Z.F., Zhang X., Zhu J.H. Prevalence of Alzheimer’s disease and Parkinson’s disease in China: An updated systematical analysis. Front. Aging Neurosci. 2020;12(December):603854. doi: 10.3389/fnagi.2020.603854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lucena J.D., Gadelha-Filho C.V.J., da Costa R.O., de Araújo D.P., Lima F.A.V., Neves K.R.T., de Barros Viana G.S. L-linalool exerts a neuroprotective action on hemiparkinsonian rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020;393(6):1077–1088. doi: 10.1007/s00210-019-01793-1. [DOI] [PubMed] [Google Scholar]
- 10.Javed H., Azimullah S., Meeran M.F., Ansari S., Ojha S. Neuroprotective effects of thymol, a dietary monoterpene against dopaminergic neurodegeneration in rotenone-induced rat model of Parkinson’s Disease. Int. J. Mol. Sci. 2019;20(7):1538. doi: 10.3390/ijms20071538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rekha K.R., Inmozhi S.R. Geraniol protects against the protein and oxidative stress induced by rotenone in an in vitro model of Parkinson’s Disease. Neurochem. Res. 2018;43(10):1947–1962. doi: 10.1007/s11064-018-2617-5. [DOI] [PubMed] [Google Scholar]
- 12.Sakkas H., Papadopoulou C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017;27(3):429–438. doi: 10.4014/jmb.1608.08024. [DOI] [PubMed] [Google Scholar]
- 13.Pina L.T.S., Guimarães A.G., Santos W.B.R., Oliveira M.A., Rabelo T.K., Serafini M.R. Monoterpenes as a perspective for the treatment of seizures: A Systematic Review. Phytomedicine. 2021;81:153422. doi: 10.1016/j.phymed.2020.153422. [DOI] [PubMed] [Google Scholar]
- 14.Despinasse Y., Fiorucci S., Antonczak S., Moja S., Bony A., Nicolè F., Baudino S., Magnard J.L., Jullien F. Bornyl-diphosphate synthase from Lavandula angustifolia: A major monoterpene synthase involved in essential oil quality. Phytochemistry. 2017;137:24–33. doi: 10.1016/j.phytochem.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Chen X., Yauk Y.K., Nieuwenhuizen N.J., Matich A.J., Wang M.Y., Perez R.L., Atkinson R.G., Beuning L.L. Characterisation of an (S)-linalool synthase from kiwifruit (Actinidia arguta) that catalyses the first committed step in the production of floral lilac compounds. Funct. Plant Biol. 2010;37(3):232–243. doi: 10.1071/FP09179. [DOI] [Google Scholar]
- 16.Milanos S., Elsharif S.A., Janzen D., Buettner A., Villmann C. Metabolic products of linalool and modulation of GABAA receptors. Front Chem. 2017;5:46. doi: 10.3389/fchem.2017.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belsito D., Bickers D., Bruze M., Calow P., Greim H., Hanifin J.M., Rogers A.E., Saurat J.H., Sipes I.G., Tagami H. A safety assessment of non-cyclic alcohols with unsaturated branched chain when used as fragrance ingredients. Food Chem. Toxicol. 2010;48(Suppl. 3):S1–S42. doi: 10.1016/j.fct.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 18.Rayff da Silva P. Anxiolytic and antidepressant-like effects of monoterpene tetrahydro-linalool and in silico approach of new potential targets. Curr. Top. Med. Chem. 2022;22(18):1515–1537. doi: 10.2174/1568026622666220505104726. [DOI] [PubMed] [Google Scholar]
- 19.Maggini V., Calvi L., Pelagatti T., Gallo E.R., Civati C., Privitera C., Squillante F., Maniglia P., Di Candia D., Spampatti R., Firenzuoli F. An optimized terpene profile for a new medical cannabis oil. Pharmaceutics. 2022;14(2):298. doi: 10.3390/pharmaceutics14020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R. Effects of stereoisomers on drug activity. Am. J. Biomed. Sci. Res. 2021;13(3):220–222. doi: 10.34297/AJBSR.2021.13.001861. [DOI] [Google Scholar]
- 21.Yang T., Stoopen G., Thoen M., Wiegers G., Jongsma M.A. Chrysanthemum expressing a linalool synthase gene ‘smells good’, but ‘tastes bad’ to western flower thrips. Plant Biotechnol. J. 2013;11(7):875–882. doi: 10.1111/pbi.12080. [DOI] [PubMed] [Google Scholar]
- 22.Sugawara Y., Hara C., Aoki T., Sugimoto N., Masujima T. Odor distinctiveness between enantiomers of linalool: difference in perception and responses elicited by sensory test and forehead surface potential wave measurement. Chem. Senses. 2000;25(1):77–84. doi: 10.1093/chemse/25.1.77. [DOI] [PubMed] [Google Scholar]
- 23.López V., Nielsen B., Solas M., Ramírez M.J., Jäger A.K. Exploring pharmacological mechanisms of lavender (Lavandula angustifolia) essential oil on central nervous system targets. Front. Pharmacol. 2017;8(MAY):280. doi: 10.3389/fphar.2017.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.dos Santos É.R.Q., Maia C.S.F., Fontes E.A., Junior, Melo A.S., Pinheiro B.G., Maia J.G.S. Linalool-rich essential oils from the Amazon display antidepressant-type effect in rodents. J. Ethnopharmacol. 2018;212(212):43–49. doi: 10.1016/j.jep.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 25.Harada H., Kashiwadani H., Kanmura Y., Kuwaki T. Linalool odor-induced anxiolytic effects in mice. Front. Behav. Neurosci. 2018;12:241. doi: 10.3389/fnbeh.2018.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan C., Shin M., Park Y., Choi B., Jang S., Lim C., Yun H.S., Lee I.S., Won S.Y., Cho K.S. Linalool alleviates Aβ42-induced neurodegeneration via suppressing ros production and inflammation in fly and rat models of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2021;2021:1–10. doi: 10.1155/2021/8887716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gunaseelan S., Balupillai A., Govindasamy K., Ramasamy K., Muthusamy G., Shanmugam M., Thangaiyan R., Robert B.M., Prasad Nagarajan R., Ponniresan V., Rathinaraj P. Linalool prevents oxidative stress activated protein kinases in single UVB-exposed human skin cells. PLoS One. 2017;12(5):e0176699. doi: 10.1371/journal.pone.0176699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabogal-Guáqueta A.M., Hobbie F., Keerthi A., Oun A., Kortholt A., Boddeke E., Dolga A. Linalool attenuates oxidative stress and mitochondrial dysfunction mediated by glutamate and NMDA toxicity. Biomed. Pharmacother. 2019;118(May):109295. doi: 10.1016/j.biopha.2019.109295. [DOI] [PubMed] [Google Scholar]
- 29.Wojtunik-Kulesza K., Rudkowska M., Kasprzak-Drozd K., Oniszczuk A., Borowicz-Reutt K. Activity of selected group of monoterpenes in alzheimer’s disease symptoms in experimental model studies—a non-systematic review. Int. J. Mol. Sci. 2021;22(14):7366. doi: 10.3390/ijms22147366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weston-Green K., Clunas H., Jimenez Naranjo C. A review of the potential use of pinene and linalool as terpene-based medicines for brain health: discovering novel therapeutics in the flavours and fragrances of cannabis. Front. Psychiatry. 2021;12(August):583211. doi: 10.3389/fpsyt.2021.583211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sabogal-Guáqueta A.M., Osorio E., Cardona-Gómez G.P. Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology. 2016;102:111–120. doi: 10.1016/j.neuropharm.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu P., Wang K., Lu C., Dong L., Gao L., Yan M., Aibai S., Yang Y., Liu X. The protective effect of lavender essential oil and its main component linalool against the cognitive deficits induced by d-galactose and aluminum trichloride in mice. Evid. Based Complement. Alternat. Med. 2017;2017:1–11. doi: 10.1155/2017/7426538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quintans-Júnior L.J., Melo M.S., De Sousa D.P., Araujo A.A.S., Onofre A.C.S., Gelain D.P., Gonçalves J.C.R., Araújo D.A.M., Almeida J.R.G.S., Bonjardim L.R. Antinociceptive effects of citronellal in formalin-, capsaicin-, and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. J. Orofac. Pain. 2010;24(3):305–312. [PubMed] [Google Scholar]
- 34.de Santana M.T., de Oliveira M.G.B., Santana M.F., De Sousa D.P., Santana D.G., Camargo E.A., de Oliveira A.P., Almeida J.R.G.S., Quintans-Júnior L.J. Jr Citronellal, a monoterpene present in Java citronella oil, attenuates mechanical nociception response in mice. Pharm. Biol. 2013;51(9):1144–1149. doi: 10.3109/13880209.2013.781656. [DOI] [PubMed] [Google Scholar]
- 35.Quintans-Júnior L., Rocha R.F., Caregnato F.F., Moreira J.C.F., Silva F.A., Araújo A.A.S., Santos J.P.A., Melo M.S., de Sousa D.P., Bonjardim L.R., Gelain D.P. Antinociceptive action and redox properties of citronellal, an essential oil present in lemongrass. J. Med. Food. 2011;14(6):630–639. doi: 10.1089/jmf.2010.0125. [DOI] [PubMed] [Google Scholar]
- 36.Melo M.S., Sena L.C.S., Barreto F.J.N., Bonjardim L.R., Almeida J.R.G.S., Lima J.T., De Sousa D.P., Quintans-Júnior L.J. Antinociceptive effect of citronellal in mice. Pharm. Biol. 2010;48(4):411–416. doi: 10.3109/13880200903150419. [DOI] [PubMed] [Google Scholar]
- 37.Silva M.I.G., Silva M.A.G., de Aquino Neto M.R., Moura B.A., de Sousa H.L., de Lavor E.P.H., de Vasconcelos P.F., Macêdo D.S., de Sousa D.P., Vasconcelos S.M.M., de Sousa F.C.F. Effects of isopulegol on pentylenetetrazol-induced convulsions in mice: Possible involvement of GABAergic system and antioxidant activity. Fitoterapia. 2009;80(8):506–513. doi: 10.1016/j.fitote.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Du J., Liu D., Zhang X., Zhou A., Su Y., He D., Fu S., Gao F. Menthol protects dopaminergic neurons against inflammation-mediated damage in lipopolysaccharide (LPS)-Evoked model of Parkinson’s disease. Int. Immunopharmacol. 2020;85:106679. doi: 10.1016/j.intimp.2020.106679. [DOI] [PubMed] [Google Scholar]
- 39.Aarsland D., Batzu L., Halliday G.M., Geurtsen G.J., Ballard C., Ray Chaudhuri K., Weintraub D. Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Primers. 2021;7(1):47. doi: 10.1038/s41572-021-00280-3. [DOI] [PubMed] [Google Scholar]
- 40.Noyce A.J., Lees A.J., Schrag A.E. The prediagnostic phase of Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry. 2016;87(8):871–878. doi: 10.1136/jnnp-2015-311890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naghavi M., Wang H., Lozano R., Davis A., Liang X., Zhou M., Vollset S.E., Abbasoglu Ozgoren A., Abdalla S., Abd-Allah F. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Oroz M.C., Jahanshahi M., Krack P., Litvan I., Macias R., Bezard E., Obeso J.A. Initial clinical manifestations of Parkinson’s disease: features and pathophysiological mechanisms. Lancet Neurol. 2009;8(12):1128–1139. doi: 10.1016/S1474-4422(09)70293-5. [DOI] [PubMed] [Google Scholar]
- 43.Dias V., Junn E., Mouradian M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013;3(4):461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez M., Morales I., Rodriguez-Sabate C., Sanchez A., Castro R., Brito J.M., Sabate M. The degeneration and replacement of dopamine cells in Parkinson’s disease: the role of aging. Front. Neuroanat. 2014;8:80. doi: 10.3389/fnana.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schapira A.H.V., Olanow C.W., Greenamyre J.T., Bezard E. Slowing of neurodegeneration in Parkinson’s disease and Huntington’s disease: future therapeutic perspectives. Lancet. 2014;384(9942):545–555. doi: 10.1016/S0140-6736(14)61010-2. [DOI] [PubMed] [Google Scholar]
- 46.Gao H-M., Tu D., Gao Y., Liu Q., Yang R., Liu Y., Guan T., Hong J-S. Roles of Microglia in Inflammation-Mediated Neurodegeneration: Models, Mechanisms, and Therapeutic Interventions for Parkinson’s Disease. 1st ed. Elsevier Inc.; 2017. p. 1. [DOI] [Google Scholar]
- 47.Raza C., Anjum R., Shakeel N.A. Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 2019;226:77–90. doi: 10.1016/j.lfs.2019.03.057. [DOI] [PubMed] [Google Scholar]
- 48.Liu Z., Cheung H. Stem cell-based therapies for Parkinson Disease. Int. J. Mol. Sci. 2020;21(21):8060. doi: 10.3390/ijms21218060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zang X., Cheng Z.Y., Sun Y., Hua N., Zhu L.H., He L. The ameliorative effects and underlying mechanisms of dopamine D1-like receptor agonist SKF38393 on Aβ1–42-induced cognitive impairment. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2018;81(September):250–261. doi: 10.1016/j.pnpbp.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Perreault M.L., Hasbi A., O’Dowd B.F., George S.R. Heteromeric dopamine receptor signaling complexes: emerging neurobiology and disease relevance. Neuropsychopharmacology. 2014;39(1):156–168. doi: 10.1038/npp.2013.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bamford N.S., Robinson S., Palmiter R.D., Joyce J.A., Moore C., Meshul C.K. Dopamine modulates release from corticostriatal terminals. J. Neurosci. 2004;24(43):9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan X., Kaminga A.C., Wen S.W., Wu X., Acheampong K., Liu A. Dopamine and dopamine receptors in alzheimer’s disease: a systematic review and network meta-analysis. Front. Aging Neurosci. 2019;11:175. doi: 10.3389/fnagi.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donthamsetti P., Gallo E.F., Buck D.C., Stahl E.L., Zhu Y., Lane J.R., Bohn L.M., Neve K.A., Kellendonk C., Javitch J.A. Arrestin recruitment to dopamine D2 receptor mediates locomotion but not incentive motivation. Mol. Psychiatry. 2020;25(9):2086–2100. doi: 10.1038/s41380-018-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caro Aponte P.A., Otálora C.A., Guzmán J.C., Turner L.F., Alcázar J.P., Mayorga E.L. Correlation between dopamine receptor D2 expression and presence of abnormal involuntary movements in Wistar rats with hemiparkinsonism and dyskinesia. Neurologia (English Edition) 2021;36(3):191–200. doi: 10.1016/j.nrleng.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 55.Brito G.M.R., Souza S.R.G. Distúrbios motores relacionados ao mal de parkinson e a dopamina. Revista Uningá. 2019;56(3):95–105. doi: 10.46311/2318-0579.56.eUJ2866. [DOI] [Google Scholar]
- 56.Connolly B.S., Lang A.E. Pharmacological treatment of Parkinson disease: a review. JAMA. 2014;311(16):1670–1683. doi: 10.1001/jama.2014.3654. [DOI] [PubMed] [Google Scholar]
- 57.Armstrong M.J., Okun M.S. Diagnosis and treatment of Parkinson disease. JAMA. 2020;323(6):548–560. doi: 10.1001/jama.2019.22360. [DOI] [PubMed] [Google Scholar]
- 58.Al-Attraqchi O.H.A., Attimarad M., Venugopala K.N., Nair A., Al-Attraqchi N.H.A. Adenosine A2A Receptor as a potential drug target - current status and future perspectives. Curr. Pharm. Des. 2019;25(25):2716–2740. doi: 10.2174/1381612825666190716113444. [DOI] [PubMed] [Google Scholar]
- 59.Zheng J., Zhang X., Zhen X. Development of adenosine A2A receptor antagonists for the treatment of parkinson’s disease: a recent update and challenge. ACS Chem. Neurosci. 2019;10(2):783–791. doi: 10.1021/acschemneuro.8b00313. [DOI] [PubMed] [Google Scholar]
- 60.Kulisevsky J., Poyurovsky M. Adenosine A2A-receptor antagonism and pathophysiology of Parkinson’s disease and drug-induced movement disorders. Eur. Neurol. 2012;67(1):4–11. doi: 10.1159/000331768. [DOI] [PubMed] [Google Scholar]
- 61.Borroto-Escuela D.O., Fuxe K. Adenosine heteroreceptor complexes in the basal ganglia are implicated in Parkinson’s disease and its treatment. J. Neural Transm. (Vienna) 2019;126(4):455–471. doi: 10.1007/s00702-019-01969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borroto-Escuela D.O. Perez De La Mora, M.; Manger, P.; Narváez, M.; Beggiato, S.; Crespo-Ramírez, M.; Navarro, G.; Wydra, K.; Díaz-Cabiale, Z.; Rivera, A.; Ferraro, L.; Tanganelli, S.; Filip, M.; Franco, R.; Fuxe, K. Brain dopamine transmission in health and parkinson’s disease: modulation of synaptic transmission and plasticity through volume transmission and dopamine heteroreceptors. Front. Synaptic Neurosci. 2018;10:20. doi: 10.3389/fnsyn.2018.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wichmann T. Changing views of the pathophysiology of Parkinsonism. Mov. Disord. 2019;34(8):1130–1143. doi: 10.1002/mds.27741. [DOI] [PubMed] [Google Scholar]
- 64.Waggan I., Rissanen E., Tuisku J., Matilainen M., Helin S., Parkkola R., Rinne J.O., Airas L. Effect of dopaminergic medication on adenosine 2A receptor availability in patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2021;86:40–44. doi: 10.1016/j.parkreldis.2021.03.030. [DOI] [PubMed] [Google Scholar]
- 65.Fredholm B.B., Svenningsson P. Why target brain adenosine receptors? A historical perspective. Parkinsonism Relat. Disord. 2020;80(Suppl. 1):S3–S6. doi: 10.1016/j.parkreldis.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 66.Garcez M.L., Damiani A.P., Pacheco R., Rodrigues L., de Abreu L.L., Alves M.C., de Andrade V.M., Boeck C.R. Caffeine neuroprotection decreases A2A adenosine receptor content in aged mice. Neurochem. Res. 2019;44(4):787–795. doi: 10.1007/s11064-018-02710-3. [DOI] [PubMed] [Google Scholar]
- 67.Ikram M., Park T.J., Ali T., Kim M.O. Antioxidant and neuroprotective effects of caffeine against alzheimer’s and parkinson’s disease: insight into the role of Nrf-2 and A2AR signaling. Antioxidants. 2020;9(9):902. doi: 10.3390/antiox9090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Peana A.T., Rubattu P., Piga G.G., Fumagalli S., Boatto G., Pippia P., De Montis M.G. Involvement of adenosine A1 and A2A receptors in (−)-linalool-induced antinociception. Life Sci. 2006;78(21):2471–2474. doi: 10.1016/j.lfs.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 69.Pourtaqi N., Imenshahidi M., Razavi B.M., Hosseinzadeh H. Effect of linalool on the acquisition and reinstatement of morphine-induced conditioned place preference in mice. Avicenna J. Phytomed. 2017;7(3):242–249. doi: 10.22038/ajp.2016.15567.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drake J., Kanski J., Varadarajan S., Tsoras M., Butterfield D.A. Elevation of brain glutathione by? -glutamylcysteine ethyl ester protects against peroxynitrite-induced oxidative stress. J. Neurosci. Res. 2002;68(6):776–784. doi: 10.1002/jnr.10266. [DOI] [PubMed] [Google Scholar]
- 71.Jankovic J., Goodman I., Safirstein B., Marmon T.K., Schenk D.B., Koller M., Zago W., Ness D.K., Griffith S.G., Grundman M., Soto J., Ostrowitzki S., Boess F.G., Martin-Facklam M., Quinn J.F., Isaacson S.H., Omidvar O., Ellenbogen A., Kinney G.G. Safety and tolerability of multiple ascending doses of prx002/rg7935, an anti–α-synuclein monoclonal antibody, in patients With Parkinson disease. JAMA Neurol. 2018;75(10):1206–1214. doi: 10.1001/jamaneurol.2018.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jankovic J., Tan E.K. Parkinson’s disease: etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry. 2020;91(8):795–808. doi: 10.1136/jnnp-2019-322338. [DOI] [PubMed] [Google Scholar]
- 73.Mancuso C., Pani G., Calabrese V. Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11(5):207–213. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- 74.Calabrese V., Cornelius C., Dinkova-Kostova A.T., Calabrese E.J., Mattson M.P. Cellular stress responses, the hormesis paradigm, and vitagenes: novel targets for therapeutic intervention in neurodegenerative disorders. Antioxid. Redox Signal. 2010;13(11):1763–1811. doi: 10.1089/ars.2009.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wakabayashi K. Where and how alpha‐synuclein pathology spreads in Parkinson’s disease. Neuropathology. 2020;40(5):415–425. doi: 10.1111/neup.12691. [DOI] [PubMed] [Google Scholar]
- 76.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 77.Rekha K.R., Selvakumar G.P., Santha K., Inmozhi Sivakamasundari R. Geraniol attenuates α-synuclein expression and neuromuscular impairment through increase dopamine content in MPTP intoxicated mice by dose dependent manner. Biochem. Biophys. Res. Commun. 2013;440(4):664–670. doi: 10.1016/j.bbrc.2013.09.122. [DOI] [PubMed] [Google Scholar]
- 78.Dehay B., Bourdenx M., Gorry P., Przedborski S., Vila M., Hunot S., Singleton A., Olanow C.W., Merchant K.M., Bezard E., Petsko G.A., Meissner W.G. Targeting α-synuclein for treatment of Parkinson’s disease: mechanistic and therapeutic considerations. Lancet Neurol. 2015;14(8):855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rocha E.M., De Miranda B., Sanders L.H. Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 2018;109(Pt B):249-257. doi: 10.1016/j.nbd.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Mehra S. Sahay, S.; Maji, S.K. α-Synuclein misfolding and aggregation: Implications in Parkinson’s disease pathogenesis. Biochim. Biophys. Acta. Proteins Proteomics. 2019;1867(10):890–908. doi: 10.1016/j.bbapap.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Brundin P., Dave K.D., Kordower J.H. Therapeutic approaches to target alpha-synuclein pathology. Exp. Neurol. 2017;298(Pt B):225-235. doi: 10.1016/j.expneurol.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calabrese E.J., Iavicoli I., Calabrese V. Hormesis: why it is important to biogerontologists. Biogerontology. 2012;13(3):215–235. doi: 10.1007/s10522-012-9374-7. [DOI] [PubMed] [Google Scholar]
- 83.Katsaiti I., Nixon J. Are there benefits in adding catechol-o methyltransferase inhibitors in the pharmacotherapy of Parkinson’s disease patients? A systematic review. J. Parkinsons Dis. 2018;8(2):217–231. doi: 10.3233/JPD-171225. [DOI] [PubMed] [Google Scholar]
- 84.Cacabelos R. Parkinson’s disease: From pathogenesis to pharmacogenomics. Int. J. Mol. Sci. 2017;18(3):551. doi: 10.3390/ijms18030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.de Beer J., Petzer J.P., Lourens A.C.U., Petzer A. Design, synthesis and evaluation of 3-hydroxypyridin-4-ones as inhibitors of catechol-O-methyltransferase. Mol. Divers. 2021;25(2):753–762. doi: 10.1007/s11030-020-10053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Müller T. Catechol-O-methyltransferase inhibitors in Parkinson’s disease. Drugs. 2015;75(2):157–174. doi: 10.1007/s40265-014-0343-0. [DOI] [PubMed] [Google Scholar]
- 87.dos Santos Passos C., Klein-Júnior L.C., de Mello Andrade J.M., Matté C., Henriques A.T. The catechol-O-methyltransferase inhibitory potential of Z-vallesiachotamine by in silico and in vitro approaches. Rev. Bras. Farmacogn. 2015;25(4):382–386. doi: 10.1016/j.bjp.2015.07.002. [DOI] [Google Scholar]
- 88.dos Santos Passos C., Soldi T.C., Torres Abib R., Anders Apel M., Simões-Pires C., Marcourt L., Gottfried C., Henriques A.T. Monoamine oxidase inhibition by monoterpene indole alkaloids and fractions obtained from Psychotria suterella and Psychotria laciniata. J. Enzyme Inhib. Med. Chem. 2013;28(3):611–618. doi: 10.3109/14756366.2012.666536. [DOI] [PubMed] [Google Scholar]
- 89.Carradori S., D’Ascenzio M., Chimenti P., Secci D., Bolasco A. Selective MAO-B inhibitors: a lesson from natural products. Mol. Divers. 2014;18(1):219–243. doi: 10.1007/s11030-013-9490-6. [DOI] [PubMed] [Google Scholar]
- 90.Dezsi L., Vecsei L. Monoamine Oxidase B Inhibitors in Parkinson’s Disease. CNS Neurol. Disord. Drug Targets. 2017;16(4):425–439. doi: 10.2174/1871527316666170124165222. [DOI] [PubMed] [Google Scholar]
- 91.Sampaio T.F., dos Santos E.U.D., de Lima G.D.C., dos Anjos R.S.G., da Silva R.C., Asano A.G.C., Asano N.M.J., Crovella S., de Souza P.R.E. MAO-B and COMT Genetic Variations Associated With Levodopa Treatment Response in Patients With Parkinson’s Disease. J. Clin. Pharmacol. 2018;58(7):920–926. doi: 10.1002/jcph.1096. [DOI] [PubMed] [Google Scholar]
- 92.Chamoli M., Chinta S.J., Andersen J.K. An inducible MAO-B mouse model of Parkinson’s disease: a tool towards better understanding basic disease mechanisms and developing novel therapeutics. J. Neural Transm. (Vienna) 2018;125(11):1651–1658. doi: 10.1007/s00702-018-1887-z. [DOI] [PubMed] [Google Scholar]
- 93.Binda C., Hubálek F., Li M., Herzig Y., Sterling J., Edmondson D.E., Mattevi A. Crystal structures of monoamine oxidase B in complex with four inhibitors of the N-propargylaminoindan class. J. Med. Chem. 2004;47(7):1767–1774. doi: 10.1021/jm031087c. [DOI] [PubMed] [Google Scholar]
- 94.Unzeta M., Sanz E. Novel MAO-B Inhibitors. Potential Therapeutic Use of the Selective MAO-B Inhibitor PF9601N in Parkinson’s Disease. 1st ed. Elsevier Inc.; 2011. p. 100. [DOI] [PubMed] [Google Scholar]
- 95.Szökő É.; Tábi, T.; Riederer, P.; Vécsei, L.; Magyar, K. Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline, in Parkinson’s disease. J. Neural Transm. (Vienna) 2018;125(11):1735–1749. doi: 10.1007/s00702-018-1853-9. [DOI] [PubMed] [Google Scholar]
- 96.Finberg J.P.M. Inhibitors of MAO-B and COMT: their effects on brain dopamine levels and uses in Parkinson’s disease. J. Neural Transm. (Vienna) 2019;126(4):433–448. doi: 10.1007/s00702-018-1952-7. [DOI] [PubMed] [Google Scholar]
- 97.Tellechea P., Pujol N., Esteve-Belloch P., Echeveste B., García-Eulate M.R., Arbizu J., Riverol M. Early- and late-onset Alzheimer disease: Are they the same entity? Neurologia (English Edition) 2018;33(4):244–253. doi: 10.1016/j.nrleng.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 98.Fulgêncio J. F. A. da S. Evolution of diagnostic methods in Alzheimer's Disease. 2017:1-114. [Google Scholar]
- 99.Selkoe D. J. Alzheimer’s Disease. Genes, Proteins, and Therapy. 2001;2018(81):4110. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 100.Sereniki A., Vital M.A.B.F. A doença de Alzheimer: aspectos fisiopatológicos e farmacológicos. Rev. Psiquiatr. Rio Gd. Sul. 2008;30(1 suppl)(Suppl.) doi: 10.1590/S0101-81082008000200002. [DOI] [Google Scholar]
- 101.Fonseca B.S., Araujo J.K., Borges J.P.M., Mota L.D.J., Miranda L.V.E., Fernandes T.B., Santos T.P.P., Barbosa S.S.S., Santos M.C., Souza C.L.S. Análise da influência dos hormônios sexuais na Doença de Alzheimer: revisão integrativa de literatura. Revista Eletrônica Acervo Saúde. 2021;13(9):e8815. doi: 10.25248/reas.e8815.2021. [DOI] [Google Scholar]
- 102.Briggs R., Kennelly S. P., O’neill D. CMJv16n3-Briggs.Indd. Clin. Med. (Northfield. Il). 2016;16(3):247-253. doi: 10.7861/clinmedicine.16-3-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mendez M.F. Early-onset Alzheimer’s disease: nonamnestic subtypes and type 2 AD. Arch. Med. Res. 2012;43(8):677–685. doi: 10.1016/j.arcmed.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Raupp I.M., Sereniki A., Virtuoso S., Ghislandi C., Cavalcanti e Silva E.L., Trebien H.A., Miguel O.G., Andreatini R. Anxiolytic-like effect of chronic treatment with Erythrina velutina extract in the elevated plus-maze test. J. Ethnopharmacol. 2008;118(2):295–299. doi: 10.1016/j.jep.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 105.Lindeboom J., Weinstein H. Neuropsychology of cognitive ageing, minimal cognitive impairment, Alzheimer’s disease, and vascular cognitive impairment. Eur. J. Pharmacol. 2004;490(1-3):83–86. doi: 10.1016/j.ejphar.2004.02.046. [DOI] [PubMed] [Google Scholar]
- 106.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25(1):59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 107.Caramelli P., Teixeira A.L., Buchpiguel C.A., Lee H.W., Livramento J.A., Fernandez L.L., Anghinah R. Diagnosis of Alzheimer’s disease in Brazil: Supplementary exams. Dement. Neuropsychol. 2011;5(3):167–177. doi: 10.1590/S1980-57642011DN05030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saraswati A.P., Ali Hussaini S.M., Krishna N.H., Babu B.N., Kamal A. Glycogen synthase kinase-3 and its inhibitors: Potential target for various therapeutic conditions. Eur. J. Med. Chem. 2018;144:843–858. doi: 10.1016/j.ejmech.2017.11.103. [DOI] [PubMed] [Google Scholar]
- 109.Balaraman Y., Limaye A.R., Levey A.I., Srinivasan S. Glycogen synthase kinase 3β and Alzheimer’s disease: pathophysiological and therapeutic significance. Cell. Mol. Life Sci. 2006;63(11):1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Phukan S., Babu V.S., Kannoji A., Hariharan R., Balaji V.N. GSK3β role in therapeutic landscape and development of modulators. Br. J. Pharmacol. 2010;160(1):1–19. doi: 10.1111/j.1476-5381.2010.00661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lauretti E., Dincer O., Praticò D. Glycogen synthase kinase-3 signaling in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867(5):118664. doi: 10.1016/j.bbamcr.2020.118664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lee S.J., Chung Y.H., Joo K.M., Lim H.C., Jeon G.S., Kim D., Lee W.B., Kim Y.S., Cha C.I. Age-related changes in glycogen synthase kinase 3β (GSK3β) immunoreactivity in the central nervous system of rats. Neurosci. Lett. 2006;409(2):134–139. doi: 10.1016/j.neulet.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 113.Lin R., Jones N.C., Kwan P. Unravelling the role of glycogen synthase kinase-3 in alzheimer’s disease-related epileptic seizures. Int. J. Mol. Sci. 2020;21(10):3676. doi: 10.3390/ijms21103676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Dunning C.J., McGauran G., Willén K., Gouras G.K., O’Connell D.J., Linse S. Direct high affinity interaction between Aβ42 and GSK3α stimulates hyperphosphorylation of tau. A new molecular link in Alzheimer’s disease? ACS Chem. Neurosci. 2016;7(2):161–170. doi: 10.1021/acschemneuro.5b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Leroy K., Yilmaz Z., Brion J.P. Increased level of active GSK-3? in Alzheimer’s disease and accumulation in argyrophilic grains and in neurones at different stages of neurofibrillary degeneration. Neuropathol. Appl. Neurobiol. 2007;33(1):43–55. doi: 10.1111/j.1365-2990.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 116.Matsunaga S., Fujishiro H., Takechi H. Efficacy and safety of glycogen synthase kinase 3 inhibitors for Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2019;69(4):1031–1039. doi: 10.3233/JAD-190256. [DOI] [PubMed] [Google Scholar]
- 117.Sotolongo K., Ghiso J., Rostagno A. Nrf2 activation through the PI3K/GSK-3 axis protects neuronal cells from Aβ-mediated oxidative and metabolic damage. Alzheimers Res. Ther. 2020;12(1):13. doi: 10.1186/s13195-019-0578-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Varfolomeev E., Vucic D. Intracellular regulation of TNF activity in health and disease. Cytokine. 2018;101:26–32. doi: 10.1016/j.cyto.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 119.Ortí-Casañ N., Wu Y., Naudé P.J.W., De Deyn P.P., Zuhorn I.S., Eisel U.L.M. Targeting TNFR2 as a novel therapeutic strategy for Alzheimer’s disease. Front. Neurosci. 2019;13:49. doi: 10.3389/fnins.2019.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cheng X., Yang L., He P., Li R., Shen Y. Differential activation of tumor necrosis factor receptors distinguishes between brains from Alzheimer’s disease and non-demented patients. J. Alzheimers Dis. 2010;19(2):621–630. doi: 10.3233/JAD-2010-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang H., He P., Xie J., Staufenbiel M., Li R., Shen Y. Genetic deletion of TNFRII gene enhances the Alzheimer-like pathology in an APP transgenic mouse model via reduction of phosphorylated I B. Hum. Mol. Genet. 2014;23(18):4906–4918. doi: 10.1093/hmg/ddu206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steeland S., Gorlé N., Vandendriessche C., Balusu S., Brkic M., Van Cauwenberghe C., Van Imschoot G., Van Wonterghem E., De Rycke R., Kremer A., Lippens S., Stopa E., Johanson C.E., Libert C., Vandenbroucke R.E. Counteracting the effects of TNF receptor‐1 has therapeutic potential in Alzheimer’s disease. EMBO Mol. Med. 2018;10(4):e8300. doi: 10.15252/emmm.201708300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mohamed M.E., Abduldaium M.S., Younis N.S. Cardioprotective effect of linalool against isoproterenol-induced myocardial infarction. Life (Basel) 2021;11(2):120. doi: 10.3390/life11020120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Riordan J.F. Angiotensin-I-converting enzyme and its relatives. Genome Biol. 2003;4(8):225. doi: 10.1186/gb-2003-4-8-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Khurana V., Goswami B. Angiotensin converting enzyme (ACE). Clin. Chim. Acta. 2022;524:113–122. doi: 10.1016/j.cca.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 126.Koronyo-Hamaoui M., Sheyn J., Hayden E.Y., Li S., Fuchs D.T., Regis G.C., Lopes D.H.J., Black K.L., Bernstein K.E., Teplow D.B., Fuchs S., Koronyo Y., Rentsendorj A. Peripherally derived angiotensin converting enzyme-enhanced macrophages alleviate Alzheimer-related disease. Brain. 2020;143(1):336–358. doi: 10.1093/brain/awz364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Uddin M.S., Kabir M.T., Rahman M.S., Behl T., Jeandet P., Ashraf G.M., Najda A., Bin-Jumah M.N., El-Seedi H.R., Abdel-Daim M.M. Revisiting the amyloid cascade hypothesis: from anti-aβ therapeutics to auspicious new ways for Alzheimer’s disease. Int. J. Mol. Sci. 2020;21(16):5858. doi: 10.3390/ijms21165858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mouchlis V.D., Melagraki G., Zacharia L.C., Afantitis A. Computer-aided drug design of β-secretase, γ-secretase and anti-tau inhibitors for the discovery of novel alzheimer’s therapeutics. Int. J. Mol. Sci. 2020;21(3):703. doi: 10.3390/ijms21030703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ahmad S.S., Khan S., Kamal M.A., Wasi U. The structure and function of α β and γ-secretase as therapeutic target enzymes in the development of alzheimer’s disease: A review. CNS Neurol. Disord. Drug Targets. 2020;18(9):657–667. doi: 10.2174/1871527318666191011145941. [DOI] [PubMed] [Google Scholar]
- 130.Ohno M. Alzheimer’s therapy targeting the β-secretase enzyme BACE1: Benefits and potential limitations from the perspective of animal model studies. Brain Res. Bull. 2016;126(Pt 2):183–198. doi: 10.1016/j.brainresbull.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Moussa C.E.H. Beta-secretase inhibitors in phase I and phase II clinical trials for Alzheimer’s disease. Expert Opin. Investig. Drugs. 2017;26(10):1131–1136. doi: 10.1080/13543784.2017.1369527. [DOI] [PubMed] [Google Scholar]
- 132.Citron M. β-Secretase inhibition for the treatment of Alzheimer’s disease – promise and challenge. Trends Pharmacol. Sci. 2004;25(2):92–97. doi: 10.1016/j.tips.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 133.Stockley J.H., O’Neill C. The proteins BACE1 and BACE2 and β-secretase activity in normal and Alzheimer’s disease brain. Biochem. Soc. Trans. 2007;35(3):574–576. doi: 10.1042/BST0350574. [DOI] [PubMed] [Google Scholar]
- 134.Marumoto S., Okuno Y., Miyazawa M. Inhibition of β-secretase activity by monoterpenes, sesquiterpenes, and C13 norisoprenoids. J. Oleo Sci. 2017;66(8):851–855. doi: 10.5650/jos.ess16188. [DOI] [PubMed] [Google Scholar]
- 135.Mota W.M., Barros M.L., Cunha P.E.L., Santana M.V.A., Stevam C.S., Leopoldo P.T.G., Fernandes R.P.M. Avaliação da inibição da acetilcolinesterase por extratos de plantas medicinais. Rev. Bras. Plantas Med. 2012;14(4):624–628. doi: 10.1590/S1516-05722012000400008. [DOI] [Google Scholar]
- 136.Rotundo R.L. Biogenesis, assembly and trafficking of acetylcholinesterase. J. Neurochem. 2017;142(Suppl. 2):52–58. doi: 10.1111/jnc.13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thapa S., Lv M., Xu H. Acetylcholinesterase: A primary target for drugs and insecticides. Mini Rev. Med. Chem. 2017;17(17):1665–1676. doi: 10.2174/1389557517666170120153930. [DOI] [PubMed] [Google Scholar]
- 138.Cheraif K., Bakchiche B., Gherib A., Bardaweel S.K., Çol Ayvaz M., Flamini G., Ascrizzi R., Ghareeb M.A. Chemical composition, antioxidant, anti-tyrosinase, anti-cholinesterase and cytotoxic activities of essential oils of six algerian plants. Molecules. 2020;25(7):1710. doi: 10.3390/molecules25071710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wang X., Bey A.L., Katz B.M., Badea A., Kim N., David L.K., Duffney L.J., Kumar S., Mague S.D., Hulbert S.W., Dutta N., Hayrapetyan V., Yu C., Gaidis E., Zhao S., Ding J.D., Xu Q., Chung L., Rodriguiz R.M., Wang F., Weinberg R.J., Wetsel W.C., Dzirasa K., Yin H., Jiang Y. Altered mGluR5-Homer scaffolds and corticostriatal connectivity in a Shank3 complete knockout model of autism. Nat. Commun. 2016;7(1):11459. doi: 10.1038/ncomms11459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hajlaoui H., Arraouadi S., Noumi E., Aouadi K., Adnan M., Khan M.A., Kadri A., Snoussi M. Antimicrobial, Antioxidant, Anti-Acetylcholinesterase, Antidiabetic, and Pharmacokinetic Properties of Carum carvi L. and Coriandrum sativum L. Essential Oils Alone and in Combination. Molecules. 2021;26(12):3625. doi: 10.3390/molecules26123625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tundis R., Bonesi M., Pugliese A., Nadjafi F., Menichini F., Loizzo M.R. Tyrosinase, acetyl-and butyryl-cholinesterase inhibitory activity of stachys lavandulifolia vahl (lamiaceae) and its major constituents. Rec. Nat. Prod. 2015;9(1):81. [Google Scholar]
- 142.Javidnia K., Mojab F., Mojahedi S.A. Chemical constituents of the essential oil of Stachys lavandulifolia Vahl from Iran. J. Essent. Oil-Bear. Plants. 2003;6(3):174–178. doi: 10.1080/0972-060X.2003.10643347. [DOI] [Google Scholar]
- 143. Available from: ChemAxon. Marvin Sketch.
- 144. Available from: Hypercube Int. HyperChem. MakoLab, 1.,
- 145. OSIRIS 5.0 DATA WARRIOR Program.
- 146.Rorije E., Aldenberg T., Buist H., Kroese D., Schüürmann G. The OSIRIS weight of evidence approach: ITS for skin sensitisation. Regul. Toxicol. Pharmacol. 2013;67(2):146–156. doi: 10.1016/j.yrtph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 147.de Sousa Luis J.A., da Silva Souza H.D., Lira B.F., da Silva Alves F., de Athayde-Filho P.F., de Souza Lima T.K., Rocha J.C., Mendonça Junior F.J.B., Scotti L., Scotti M.T. Combined structure- and ligand-based virtual screening aiding discovery of selenoglycolicamides as potential multitarget agents against Leishmania species. J. Mol. Struct. 2019;1198:126872. doi: 10.1016/j.molstruc.2019.126872. [DOI] [Google Scholar]
- 148.Lipinski C.F., Maltarollo V.G., Oliveira P.R., da Silva A.B.F., Honorio K.M. Advances and perspectives in applying deep learning for drug design and discovery. Front. Robot. AI. 2019;6(November):108. doi: 10.3389/frobt.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Congreve M., Andrews S.P., Doré A.S., Hollenstein K., Hurrell E., Langmead C.J., Mason J.S., Ng I.W., Tehan B., Zhukov A., Weir M., Marshall F.H. Discovery of 1,2,4-triazine derivatives as adenosine A(2A) antagonists using structure based drug design. J. Med. Chem. 2012;55(5):1898–1903. doi: 10.1021/jm201376w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ulmer T.S., Bax A., Cole N.B., Nussbaum R.L. Structure and dynamics of micelle-bound human α-synuclein. J. Biol. Chem. 2005;280(10):9595–9603. doi: 10.1074/jbc.M411805200. [DOI] [PubMed] [Google Scholar]
- 151.Bonifácio M.J., Archer M., Rodrigues M.L., Matias P.M., Learmonth D.A., Carrondo M.A., Soares-da-Silva P. Kinetics and crystal structure of catechol-o-methyltransferase complex with co-substrate and a novel inhibitor with potential therapeutic application. Mol. Pharmacol. 2002;62(4):795–805. doi: 10.1124/mol.62.4.795. [DOI] [PubMed] [Google Scholar]
- 152.Sun B., Feng D., Chu M.L.H., Fish I., Lovera S., Sands Z.A., Kelm S., Valade A., Wood M., Ceska T., Kobilka T.S., Lebon F., Kobilka B.K. Crystal structure of dopamine D1 receptor in complex with G protein and a non-catechol agonist. Nat. Commun. 2021;12(1):3305. doi: 10.1038/s41467-021-23519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang S., Che T., Levit A., Shoichet B.K., Wacker D., Roth B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature. 2018;555(7695):269–273. doi: 10.1038/nature25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Deffains M. Canron, M.H.; Teil, M.; Li, Q.; Dehay, B.; Bezard, E.; Fernagut, P.O. L‐DOPA regulates α‐synuclein accumulation in experimental parkinsonism. Neuropathol. Appl. Neurobiol. 2021;47(4):532–543. doi: 10.1111/nan.12678. [DOI] [PubMed] [Google Scholar]
- 155.Antonini A., Calandrella D. Pharmacokinetic evaluation of pramipexole. Expert Opin. Drug Metab. Toxicol. 2011;7(10):1307–1314. doi: 10.1517/17425255.2011.614232. [DOI] [PubMed] [Google Scholar]
- 156.Zhuang Y., Xu P., Mao C., Wang L., Krumm B., Zhou X.E., Huang S., Liu H., Cheng X., Huang X.P., Shen D.D., Xu T., Liu Y.F., Wang Y., Guo J., Jiang Y., Jiang H., Melcher K., Roth B.L., Zhang Y., Zhang C., Xu H.E. Structural insights into the human D1 and D2 dopamine receptor signaling complexes. Cell. 2021;184(4):931–942.e18. doi: 10.1016/j.cell.2021.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jenner P., Mori A., Aradi S.D., Hauser R.A. Istradefylline – a first generation adenosine A 2A antagonist for the treatment of Parkinson’s disease. Expert Rev. Neurother. 2021;21(3):317–333. doi: 10.1080/14737175.2021.1880896. [DOI] [PubMed] [Google Scholar]
- 158.Hansen R.N., Suh K., Serbin M., Yonan C., Sullivan S.D. Cost-effectiveness of opicapone and entacapone in reducing OFF-time in Parkinson’s disease patients treated with levodopa/carbidopa. J. Med. Econ. 2021;24(1):563–569. doi: 10.1080/13696998.2021.1916750. [DOI] [PubMed] [Google Scholar]
- 159.Watermeyer J.M., Kröger W.L., O’Neill H.G., Sewell B.T., Sturrock E.D. Probing the basis of domain-dependent inhibition using novel ketone inhibitors of Angiotensin-converting enzyme. Biochemistry. 2008;47(22):5942–5950. doi: 10.1021/bi8002605. [DOI] [PubMed] [Google Scholar]
- 160.Giacomini A.C.V.V., Bueno B.W., Marcon L., Scolari N., Genario R., Demin K.A., Kolesnikova T.O., Kalueff A.V., de Abreu M.S. An acetylcholinesterase inhibitor, donepezil, increases anxiety and cortisol levels in adult zebrafish. J. Psychopharmacol. 2020;34(12):1449–1456. doi: 10.1177/0269881120944155. [DOI] [PubMed] [Google Scholar]
- 161.Cumming J.N., Smith E.M., Wang L., Misiaszek J., Durkin J., Pan J., Iserloh U., Wu Y., Zhu Z., Strickland C., Voigt J., Chen X., Kennedy M.E., Kuvelkar R., Hyde L.A., Cox K., Favreau L., Czarniecki M.F., Greenlee W.J., McKittrick B.A., Parker E.M., Stamford A.W. Structure based design of iminohydantoin BACE1 inhibitors: Identification of an orally available, centrally active BACE1 inhibitor. Bioorg. Med. Chem. Lett. 2012;22(7):2444–2449. doi: 10.1016/j.bmcl.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 162.Berg S., Bergh M., Hellberg S., Högdin K., Lo-Alfredsson Y., Söderman P., von Berg S., Weigelt T., Ormö M., Xue Y., Tucker J., Neelissen J., Jerning E., Nilsson Y., Bhat R. Discovery of novel potent and highly selective glycogen synthase kinase-3β (GSK3β) inhibitors for Alzheimer’s disease: design, synthesis, and characterization of pyrazines. J. Med. Chem. 2012;55(21):9107–9119. doi: 10.1021/jm201724m. [DOI] [PubMed] [Google Scholar]
- 163.Niu X., Umland S., Ingram R., Beyer B.M., Liu Y.H., Sun J., Lundell D., Orth P. IK682, a tight binding inhibitor of TACE. Arch. Biochem. Biophys. 2006;451(1):43–50. doi: 10.1016/j.abb.2006.03.034. [DOI] [PubMed] [Google Scholar]
- 164. CLC Bio Company. Mollegro Virtual Docker 6.0.
- 165.De Azevedo W., Jr, Walter F. MolDock applied to structure-based virtual screening. Curr. Drug Targets. 2010;11(3):327–334. doi: 10.2174/138945010790711941. [DOI] [PubMed] [Google Scholar]
- 166.Thomsen R., Christensen M.H. MolDock: a new technique for high-accuracy molecular docking. J. Med. Chem. 2006;49(11):3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]
- 167.Wu F., Zhou Y., Li L., Shen X., Chen G., Wang X., Liang X., Tan M., Huang Z. Computational approaches in preclinical studies on drug discovery and development. Front Chem. 2020;8:726. doi: 10.3389/fchem.2020.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Prasanna S., Doerksen R. Topological polar surface area: a useful descriptor in 2D-QSAR. Curr. Med. Chem. 2009;16(1):21–41. doi: 10.2174/092986709787002817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Price G., Patel D.A. Drug bioavailability. 2022. Statpearls Publishing; 2022. [PubMed] [Google Scholar]
- 170.Alagga A.A., Gupta V. Drug Absorption. StatPearls; 2021. [PubMed] [Google Scholar]
- 171.Benet L.Z., Hosey C.M., Ursu O., Oprea T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016;101:89–98. doi: 10.1016/j.addr.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shimohama S., Tanino H., Kawakami N., Okamura N., Kodama H., Yamaguchi T., Hayakawa T., Nunomura A., Chiba S., Perry G., Smith M.A., Fujimoto S. Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 2000;273(1):5–9. doi: 10.1006/bbrc.2000.2897. [DOI] [PubMed] [Google Scholar]
- 173.Ansari M.A., Scheff S.W. NADPH-oxidase activation and cognition in Alzheimer disease progression. Free Radic. Biol. Med. 2011;51(1):171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tambosi G., Coelho P.F., Luciano S., Lenschow I.C.S., Zétola M., Stulzer H.K., Pezzini B.R. Challenges to improve the biopharmaceutical properties of poorly water-soluble drugs and the application of the solid dispersion technology. Materia (Rio J.) 2018;23(4):23. doi: 10.1590/s1517-707620180004.0558. [DOI] [Google Scholar]
- 175.Lobo S. Is there enough focus on lipophilicity in drug discovery? Expert Opin. Drug Discov. 2020;15(3):261–263. doi: 10.1080/17460441.2020.1691995. [DOI] [PubMed] [Google Scholar]
- 176.FIOCRUZ. Risco Químico. 2018.
- 177.Sushko I., Novotarskyi S., Körner R., Pandey A.K., Cherkasov A., Li J., Gramatica P., Hansen K., Schroeter T., Müller K.R., Xi L., Liu H., Yao X., Öberg T., Hormozdiari F., Dao P., Sahinalp C., Todeschini R., Polishchuk P., Artemenko A., Kuz’min V., Martin T.M., Young D.M., Fourches D., Muratov E., Tropsha A., Baskin I., Horvath D., Marcou G., Muller C., Varnek A., Prokopenko V.V., Tetko I.V. Applicability domains for classification problems: Benchmarking of distance to models for Ames mutagenicity set. J. Chem. Inf. Model. 2010;50(12):2094–2111. doi: 10.1021/ci100253r. [DOI] [PubMed] [Google Scholar]
- 178.Sato Y., Bando H., Di Piazza M., Gowing G., Herberts C., Jackman S., Leoni G., Libertini S., MacLachlan T., McBlane J.W., Pereira Mouriès L., Sharpe M., Shingleton W., Surmacz-Cordle B., Yamamoto K., van der Laan J.W. Tumorigenicity assessment of cell therapy products: The need for global consensus and points to consider. Cytotherapy. 2019;21(11):1095–1111. doi: 10.1016/j.jcyt.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 179.Chandra S.A., Stokes A.H., Hailey R., Merrill C.L., Melich D.H., DeSmet K., Furst S.M., Peterson R.A., Mellon-Kusibab K., Adler R.R. Dermal toxicity studies: factors impacting study interpretation and outcome. Toxicol. Pathol. 2015;43(4):474–481. doi: 10.1177/0192623314548765. [DOI] [PubMed] [Google Scholar]
- 180.Rousseau C. F., Sabbah-Petrover E., Revaud D., Voisin E. M., Ruthsatz M., Chiavaroli C. Toxicological aspects in the regulation of gene therapy medicinal products. Regul. Toxicol. 2021:1431M-1458. [Google Scholar]
- 181.Vonk J.A., Benigni R., Hewitt M., Nendza M., Segner H., van de Meent D., Cronin M.T.D. The use of mechanisms and modes of toxic action in integrated testing strategies: the report and recommendations of a workshop held as part of the European Union OSIRIS Integrated Project. Altern. Lab. Anim. 2009;37(5):557–571. doi: 10.1177/026119290903700512. [DOI] [PubMed] [Google Scholar]
- 182.Khan T., Lawrence A.J., Azad I., Raza S., Joshi S., Khan A.R. Computational drug designing and prediction of important parameters using in silico methods- a review. Curr. Computeraided Drug Des. 2019;15(5):384–397. doi: 10.2174/1573399815666190326120006. [DOI] [PubMed] [Google Scholar]
- 183.Koes D.R., Baumgartner M.P., Camacho C.J. Lessons learned in empirical scoring with smina from the CSAR 2011 benchmarking exercise. J. Chem. Inf. Model. 2013;53(8):1893–1904. doi: 10.1021/ci300604z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Daligaux P., Bernadat G., Tran L., Cavé C., Loiseau P.M., Pomel S., Ha-Duong T. Comparative study of structural models of Leishmania donovani and human GDP-mannose pyrophosphorylases. Eur. J. Med. Chem. 2016;107:109–118. doi: 10.1016/j.ejmech.2015.10.037. [DOI] [PubMed] [Google Scholar]
- 185.Monteiro A.F.M., Viana J.D.O., Nayarisseri A., Zondegoumba E.N., Mendonça Junior F.J.B., Scotti M.T., Scotti L. Computational studies applied to flavonoids against Alzheimer’s and Parkinson’s diseases. Oxid. Med. Cell. Longev. 2018;2018:1–21. doi: 10.1155/2018/7912765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Albohy A., Zahran E.M., Abdelmohsen U.R., Salem M.A., Al-Warhi T., Al-Sanea M.M., Abelyan N., Khalil H.E., Desoukey S.Y., Fouad M.A. Multitarget in silico studies of ocimum menthiifolium, family lamiaceae against sars-cov-2 supported by molecular dynamics simulation. J. Biomol. Struct. Dyn. 2020;0(0):1–11. doi: 10.1080/07391102.2020.1852964. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material is available on the publisher’s website along with the published article.