Abstract
Patients with insulin-dependent diabetes mellitus are at high risk for vascular disorders such as hypertension, nephropathy, and retinopathy. The most common cause of morbidity and mortality in patients with insulin-dependent diabetes is vascular disease. Despite ongoing research, the pathogenesis of vascular disease in diabetes remains unclear. In recent years, numerous investigators have examined the role of the endothelium-derived relaxing factor, nitric oxide, in the disease state of hypertension and its complications. We review the role of nitric oxide in the development of diabetes-related vascular disease and discuss findings suggesting that nitric oxide metabolism and vascular responsiveness to nitric oxide are altered in diabetes. Patients with diabetes may benefit from therapy that addresses this pathogenic deficiency.
Full text
PDF
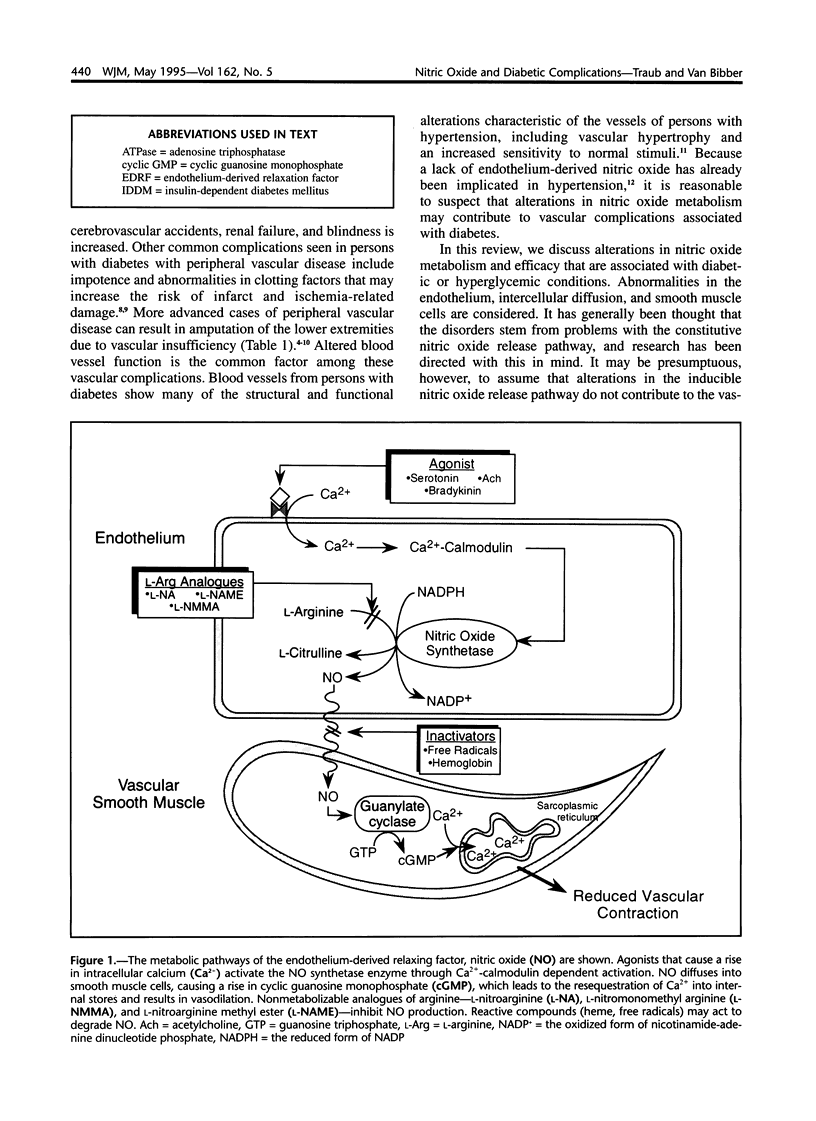

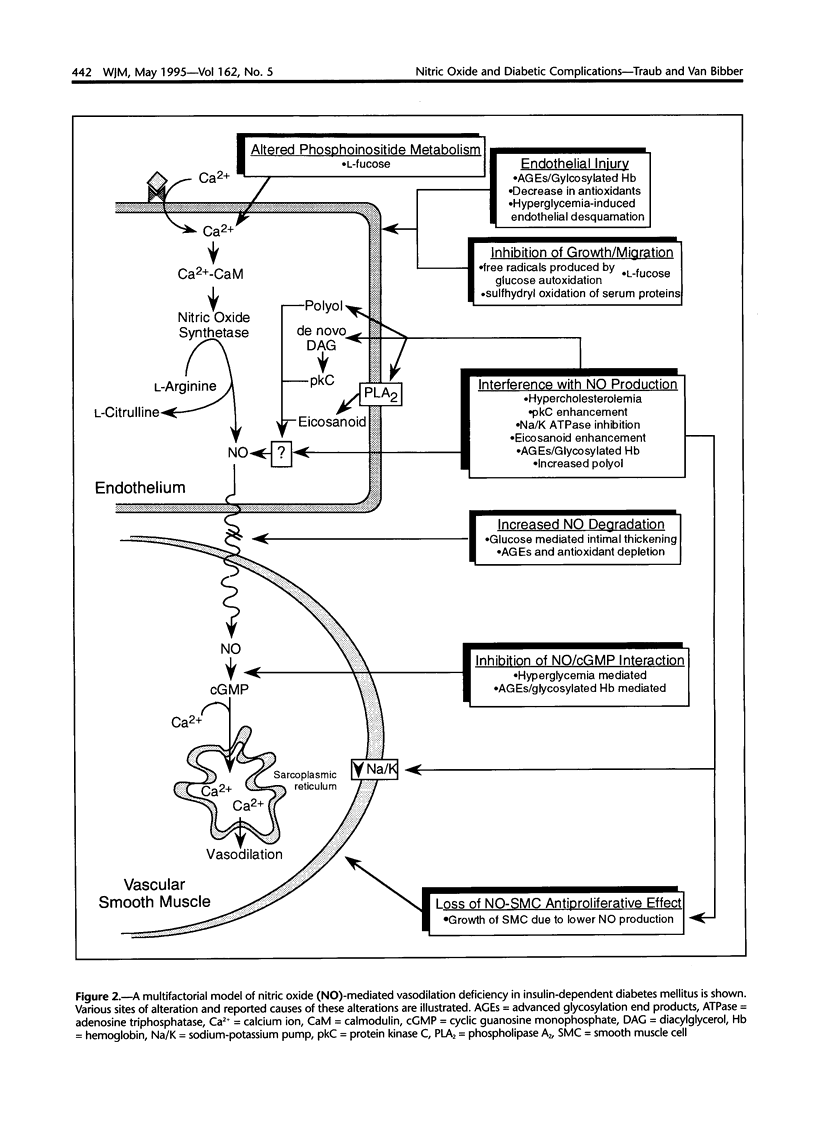



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Rocchini A. P. Hypertension in individuals with insulin-dependent diabetes mellitus. Pediatr Clin North Am. 1993 Feb;40(1):93–104. doi: 10.1016/s0031-3955(16)38483-8. [DOI] [PubMed] [Google Scholar]
- Azadzoi K. M., Saenz de Tejada I. Diabetes mellitus impairs neurogenic and endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J Urol. 1992 Nov;148(5):1587–1591. doi: 10.1016/s0022-5347(17)36975-6. [DOI] [PubMed] [Google Scholar]
- Azadzoi K. M., Saenz de Tejada I. Hypercholesterolemia impairs endothelium-dependent relaxation of rabbit corpus cavernosum smooth muscle. J Urol. 1991 Jul;146(1):238–240. doi: 10.1016/s0022-5347(17)37759-5. [DOI] [PubMed] [Google Scholar]
- Bassenge E. Clinical relevance of endothelium-derived relaxing factor (EDRF). Br J Clin Pharmacol. 1992;34 (Suppl 1):37S–42S. doi: 10.1111/j.1365-2125.1992.tb04147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen H. G., Lash J. M. Topical hyperglycemia rapidly suppresses EDRF-mediated vasodilation of normal rat arterioles. Am J Physiol. 1993 Jul;265(1 Pt 2):H219–H225. doi: 10.1152/ajpheart.1993.265.1.H219. [DOI] [PubMed] [Google Scholar]
- Breyer J. A. Diabetic nephropathy in insulin-dependent patients. Am J Kidney Dis. 1992 Dec;20(6):533–547. doi: 10.1016/s0272-6386(12)70215-9. [DOI] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliero E., Maiello M., Boeri D., Roy S., Lorenzi M. Increased expression of basement membrane components in human endothelial cells cultured in high glucose. J Clin Invest. 1988 Aug;82(2):735–738. doi: 10.1172/JCI113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron N. E., Cotter M. A. Contraction and relaxation of aortas from galactosaemic rats and the effects of aldose reductase inhibition. Eur J Pharmacol. 1993 Oct 12;243(1):47–53. doi: 10.1016/0014-2999(93)90166-f. [DOI] [PubMed] [Google Scholar]
- Ceriello A., Giugliano D., Quatraro A., Lefebvre P. J. Anti-oxidants show an anti-hypertensive effect in diabetic and hypertensive subjects. Clin Sci (Lond) 1991 Dec;81(6):739–742. doi: 10.1042/cs0810739. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Tilton R. G., Chang K., Hasan K. S., Ido Y., Wang J. L., Sweetland M. A., Lancaster J. R., Jr, Williamson J. R., McDaniel M. L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992 Apr;41(4):552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Studer R. K., DeRubertis F. R. Impaired nitric oxide-dependent cyclic guanosine monophosphate generation in glomeruli from diabetic rats. Evidence for protein kinase C-mediated suppression of the cholinergic response. J Clin Invest. 1994 Jan;93(1):311–320. doi: 10.1172/JCI116961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio F., Ceriello A. Decreased cultured endothelial cell proliferation in high glucose medium is reversed by antioxidants: new insights on the pathophysiological mechanisms of diabetic vascular complications. In Vitro Cell Dev Biol. 1992 Nov-Dec;28A(11-12):787–790. doi: 10.1007/BF02631069. [DOI] [PubMed] [Google Scholar]
- Elliott T. G., Cockcroft J. R., Groop P. H., Viberti G. C., Ritter J. M. Inhibition of nitric oxide synthesis in forearm vasculature of insulin-dependent diabetic patients: blunted vasoconstriction in patients with microalbuminuria. Clin Sci (Lond) 1993 Dec;85(6):687–693. doi: 10.1042/cs0850687. [DOI] [PubMed] [Google Scholar]
- Falkenberg M. Metabolic control and amputations among diabetics in primary health care--a population-based intensified programme governed by patient education. Scand J Prim Health Care. 1990 Mar;8(1):25–29. doi: 10.3109/02813439008994925. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. Introduction to EDRF research. J Cardiovasc Pharmacol. 1993;22 (Suppl 7):S1–S2. [PubMed] [Google Scholar]
- Garg U. C., Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest. 1989 May;83(5):1774–1777. doi: 10.1172/JCI114081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilcrease M. Z., Hoover R. L. Examination of monocyte adherence to endothelium under hyperglycemic conditions. Am J Pathol. 1991 Nov;139(5):1089–1097. [PMC free article] [PubMed] [Google Scholar]
- Graier W. F., Wascher T. C., Lackner L., Toplak H., Krejs G. J., Kukovetz W. R. Exposure to elevated D-glucose concentrations modulates vascular endothelial cell vasodilatory response. Diabetes. 1993 Oct;42(10):1497–1505. doi: 10.2337/diab.42.10.1497. [DOI] [PubMed] [Google Scholar]
- Gupta S., Sussman I., McArthur C. S., Tornheim K., Cohen R. A., Ruderman N. B. Endothelium-dependent inhibition of Na(+)-K+ ATPase activity in rabbit aorta by hyperglycemia. Possible role of endothelium-derived nitric oxide. J Clin Invest. 1992 Sep;90(3):727–732. doi: 10.1172/JCI115944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger I. O., Flammer J., Lüscher T. F. Nitric oxide and endothelin-1 are important regulators of human ophthalmic artery. Invest Ophthalmol Vis Sci. 1992 Jun;33(7):2340–2343. [PubMed] [Google Scholar]
- Hattori Y., Kawasaki H., Abe K., Kanno M. Superoxide dismutase recovers altered endothelium-dependent relaxation in diabetic rat aorta. Am J Physiol. 1991 Oct;261(4 Pt 2):H1086–H1094. doi: 10.1152/ajpheart.1991.261.4.H1086. [DOI] [PubMed] [Google Scholar]
- Hogan M., Cerami A., Bucala R. Advanced glycosylation endproducts block the antiproliferative effect of nitric oxide. Role in the vascular and renal complications of diabetes mellitus. J Clin Invest. 1992 Sep;90(3):1110–1115. doi: 10.1172/JCI115928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Levine S. N., Duett J., Hollier B. Reduced vitamin E and increased lipofuscin products in erythrocytes of diabetic rats. Diabetes. 1991 Oct;40(10):1241–1244. doi: 10.2337/diab.40.10.1241. [DOI] [PubMed] [Google Scholar]
- Kamata K., Miyata N., Abiru T., Kasuya Y. Functional changes in vascular smooth muscle and endothelium of arteries during diabetes mellitus. Life Sci. 1992;50(19):1379–1387. doi: 10.1016/0024-3205(92)90256-o. [DOI] [PubMed] [Google Scholar]
- Kiff R. J., Gardiner S. M., Compton A. M., Bennett T. Selective impairment of hindquarters vasodilator responses to bradykinin in conscious Wistar rats with streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1991 Jun;103(2):1357–1362. doi: 10.1111/j.1476-5381.1991.tb09793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. Nitric oxide: a pathogenetic factor in autoimmunity. Immunol Today. 1992 May;13(5):157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- Lawrence E., Brain S. D. Altered microvascular reactivity to endothelin-1, endothelin-3 and NG-nitro-L-arginine methyl ester in streptozotocin-induced diabetes mellitus. Br J Pharmacol. 1992 Aug;106(4):1035–1040. doi: 10.1111/j.1476-5381.1992.tb14452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Dinerman J. L., Snyder S. H. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994 Feb 1;120(3):227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- Maatman T. J., Montague D. K., Martin L. M. Erectile dysfunction in men with diabetes mellitus. Urology. 1987 Jun;29(6):589–592. doi: 10.1016/0090-4295(87)90097-5. [DOI] [PubMed] [Google Scholar]
- Maiello M., Boeri D., Bonadonna R., Odetti P., Saccarello A., Adezati L. Platelet and clotting activities after cold stress in diabetic patients. Thromb Res. 1988 Jun 15;50(6):885–894. doi: 10.1016/0049-3848(88)90348-9. [DOI] [PubMed] [Google Scholar]
- Mascardo R. N. The effects of hyperglycemia on the directed migration of wounded endothelial cell monolayers. Metabolism. 1988 Apr;37(4):378–385. doi: 10.1016/0026-0495(88)90139-4. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nakamura Y., Horii Y., Nishino T., Shiiki H., Sakaguchi Y., Kagoshima T., Dohi K., Makita Z., Vlassara H., Bucala R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol. 1993 Dec;143(6):1649–1656. [PMC free article] [PubMed] [Google Scholar]
- Nakao-Hayashi J., Ito H., Kawashima S. An oxidative mechanism is involved in high glucose-induced serum protein modification causing inhibition of endothelial cell proliferation. Atherosclerosis. 1992 Nov;97(1):89–95. doi: 10.1016/0021-9150(92)90054-k. [DOI] [PubMed] [Google Scholar]
- Pelligrino D. A., Miletich D. J., Albrecht R. F. Diminished muscarinic receptor-mediated cerebral blood flow response in streptozotocin-treated rats. Am J Physiol. 1992 Apr;262(4 Pt 1):E447–E454. doi: 10.1152/ajpendo.1992.262.4.E447. [DOI] [PubMed] [Google Scholar]
- Popova G., Protich M., Glavanakova V. Prouchvane na endotelnata sŭdova deskvamatsiia pri diabetno bolni. Vutr Boles. 1988;27(4):19–21. [PubMed] [Google Scholar]
- Rodríguez-Mañas L., Arribas S., Girón C., Villamor J., Sánchez-Ferrer C. F., Marín J. Interference of glycosylated human hemoglobin with endothelium-dependent responses. Circulation. 1993 Nov;88(5 Pt 1):2111–2116. doi: 10.1161/01.cir.88.5.2111. [DOI] [PubMed] [Google Scholar]
- Rosenbloom A. L. Long-term complications of type I (insulin-dependent) diabetes mellitus. Pediatr Ann. 1983 Sep;12(9):668–684. [PubMed] [Google Scholar]
- Roy S., Sala R., Cagliero E., Lorenzi M. Overexpression of fibronectin induced by diabetes or high glucose: phenomenon with a memory. Proc Natl Acad Sci U S A. 1990 Jan;87(1):404–408. doi: 10.1073/pnas.87.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Stefani M. R., Dunlap J. A., Yorek M. A. Effect of L-fucose on proliferation and myo-inositol metabolism in cultured cerebral microvessel and aortic endothelial cells. J Cell Physiol. 1992 Nov;153(2):321–331. doi: 10.1002/jcp.1041530212. [DOI] [PubMed] [Google Scholar]
- Taylor P. D., McCarthy A. L., Thomas C. R., Poston L. Endothelium-dependent relaxation and noradrenaline sensitivity in mesenteric resistance arteries of streptozotocin-induced diabetic rats. Br J Pharmacol. 1992 Oct;107(2):393–399. doi: 10.1111/j.1476-5381.1992.tb12757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Brown M. L., Cohen R. A. Elevated glucose impairs endothelium-dependent relaxation by activating protein kinase C. J Clin Invest. 1991 May;87(5):1643–1648. doi: 10.1172/JCI115179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Palacino J. J., Weisbrod R. M., Cohen R. A. Aldose reductase inhibition restores endothelial cell function in diabetic rabbit aorta. J Cardiovasc Pharmacol. 1993 Feb;21(2):205–211. doi: 10.1097/00005344-199302000-00004. [DOI] [PubMed] [Google Scholar]
- Walker W. G. Relation of lipid abnormalities to progression of renal damage in essential hypertension, insulin-dependent and non insulin-dependent diabetes mellitus. Miner Electrolyte Metab. 1993;19(3):137–143. [PubMed] [Google Scholar]
- Weisbrod R. M., Brown M. L., Cohen R. A. Effect of elevated glucose on cyclic GMP and eicosanoids produced by porcine aortic endothelium. Arterioscler Thromb. 1993 Jun;13(6):915–923. doi: 10.1161/01.atv.13.6.915. [DOI] [PubMed] [Google Scholar]



