Abstract
Background
Arbovirus diseases such as dengue, Zika, and chikungunya are a public health threat in tropical and subtropical areas. In the absence of a vaccine or specific treatment, vector management (in this case the control of the primary vector Aedes aegypti) is the best practice to prevent the three diseases. A good understanding of vector behaviour, ecology, human mobility and water use can help design effective vector control programmes. This study collected baseline information on these factors for identifying the arbovirus transmission risk and assessed the requirements for a large intervention trial in Colombia.
Methods
Baseline surveys were conducted in 5,997 households, randomly selected from 24 clusters (neighbourhoods with on average 2000 houses and 250 households inspected) in the metropolitan area of Cucuta, Colombia. The study established population characteristics including water management and mobility as well as larval-pupal indices which were estimated and compared in all clusters. Additionally, the study estimated disease incidence from two sources: self-reported dengue cases in the household survey and cases notified by the national surveillance system.
Results
In all 24 study clusters similar social and demographic characteristics were found but the entomological indicators and estimated disease incidence rates varied. The entomological indicators showed a high vector infestation: House Index = 25.1%, Container Index = 12.3% and Breteau Index = 29.6. Pupae per person Index (PPI) as an indicator of the transmission risk showed a large range from 0.22 to 2.04 indicating a high transmission risk in most clusters. The concrete ground tanks for laundry –mostly outdoors and uncovered- were the containers with the highest production of Ae. aegypti as 86.3% of all 17,613 pupae were identified in these containers. Also, the annual incidence of dengue was high: 841.6 self-reported cases per 100,000 inhabitants and the dengue incidence notified by the National surveillance system was 1,013.4 cases per 100,000 in 2019. Only 2.2% of the households used container water for drinking. 40.3% of the study population travelled during the day (when Aedes mosquitoes bite) outside their clusters.
Conclusions
The production of Ae. aegypti mosquitoes occurred almost exclusively in concrete ground tanks for laundry (lavadero), the primary intervention target. The baseline study provides necessary evidence for the design and implementation of a cluster randomized intervention trial in Colombia.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-023-15893-4.
Keywords: Dengue, Zika, Chikungunya, Aedes aegypti
Introduction
Arboviral diseases such as dengue, Zika and chikungunya (DZC) are a public health threat in tropical and sub-tropical countries due to the increase of the global burden promoted by the rapid spread of their mosquito vectors, Aedes aegypti (primary vector) and Aedes albopictus (secondary vector) [1, 2]. In Latin America, these diseases are mainly transmitted by Ae. aegypti [3] which breeds particularly in artificial water containers in close proximity to human dwellings [4]. Environmental changes, unplanned urbanization, inadequate sanitization and human activities involving water storage and deficient trash management contribute to the production of this vector [5–7]. Certain socio-demographic characteristics such as level of education [8], gender inequality [9] and crowded living conditions [10] add to the risk of Ae. aegypti vector infestation.
In Colombia, Ae. aegypti is widespread in mainly urban areas below 2300 m above the see level [11]. Suitable habitats favoured by numerous water containers and climate change have caused the proliferation of Ae. aegypti throughout the country [12]. The presence of Ae. aegypti is reflected by the high burden of dengue, Zika and chikungunya in the last years (2014–2019), 504,414 dengue cases was reported [13]. Colombia is now hyper-endemic for the dengue virus (DENV) with the circulation of all four serotypes and outbreaks occurring every 3–4 years [14]. Chikungunya and Zika were detected for the first time in the country in 2014 and 2015, respectively [15, 16], and since their introduction 488,378 cases of chikungunya and 109,995 cases of Zika have been reported until 2019 [11, 17–21]. Moreover, Zika has been associated with the development of several neurological complications including microcephaly and Guillain–Barre syndrome [22]. In the absence of antiviral treatment for arboviral infections and of a dengue vaccine suitable for public health use, the key measure for preventing the transmission of DZC continues to be vector control. Effective vector control depends on a sound understanding of larval and adult vector ecology of which little is known in hyperendemic cities of Colombia.
The transmission risk of arboviral disease outbreaks in endemic regions has traditionally been assessed by larval indices (Stegomyia indices) [23–25], complemented or replaced by pupal indices [26] as pupae are a reliable proxy for adult mosquito abundance [27, 28]. Given the suitability of domestic breeding habitats for Ae. aegypti, mosquito management within households is crucial to the control of DZC. Moreover, understanding factors that drive abundance and persistence of Ae. aegypti in the household environment will contribute valuable information to the design and implementation of efficient and targeted vector control strategies. In Cucuta, a major city in Colombia, where the average annual dengue incidence is 668 per 100.000 persons (it is twice as high as the national average with 282 cases per 100 000) [29], little evidence is known on vector infestation level and its risk factors.
This study describes socio demographic characteristics, identifies breeding habitats and establishes container productivity profiles and level of infestation of Ae. aegypti and provides an epidemiological profile. The purpose of this study is to collect baseline information for a cluster-randomised trial (CRTs) that seeks to reduce DCZ risk in Colombia through the implementation of new novel vector control method (insecticidal coating for domestic containers). CRTs are a robust design for measuring the effect of interventions conducted at community level [30]. Several CRTs have shown an impact on vector densities [31–34], but few studies provided evidence of the impact on dengue incidence. The Camino Verde trial, which assessed the impact of community mobilization on dengue incidence, has been a quite unique example [35]. The development of novel approaches to vector control requires well-designed field trials with a larger number and size of cluster and/or longer study periods [36, 37]. With the main goal of reducing the burden of arbovirus diseases, this study was designed with a large and sufficient number of clusters to assess the effectiveness of insecticidal coating in key domestic water containers for reducing the transmission of DENV, CHIKV, and ZIKV. Findings from the here presented baseline study will show the complexity of preparing a large trial and provide baseline information about different factor influencing the transmission of arbovirus diseases.
Methods
Place of study
The study was conducted within 2.5 months in 2019–20 in Metropolitan Cucuta in the North-East of Colombia, including the city of Cucuta (629,414), and two adjacent municipalities Villa del Rosario (93,735) and Los Patios (81,411 inhabitants) located in the Norte de Santander state [38]. The climate is warm and dry, with a mean annual temperature of 26.7 °C and annual rainfall of 806 mm. There are two rainy seasons from the end of March to the beginning of June and from the end of September to the beginning of December. DZC are endemic, with occasional epidemic outbreaks [39].
Study design and sample size
This is the baseline study (using a cluster design) for a large cluster randomized trial on the impact of treating water containers with a protective paint. The sample size was calculated for detection of a 50% reduction in the House Index with > 99% power at 5% significance level. Given a baseline HI of 30%, an intra-cluster correlation coefficient (ICC) of 0.01 and a cluster size of 2,000 households it was found that a minimum of 12 clusters per study arm was needed [40]. The same result but with a lower power (68%) was found for a baseline DZC incidence of 3% and a 50% reduction in the intervention arm. A large number of households per cluster was needed as Ae. aegypti vectors are day-biters [41–43] and it was assumed that many household members stay within their neighbourhoods during the day (mainly housewives, small children and school children when the school is close to their house) where they are at risk to get infected. All estimations were done through The Shiny CRT Calculator which is a web-based app to determine sample size and power for cluster trials [44].
Sampling procedure for household survey and entomological inspections
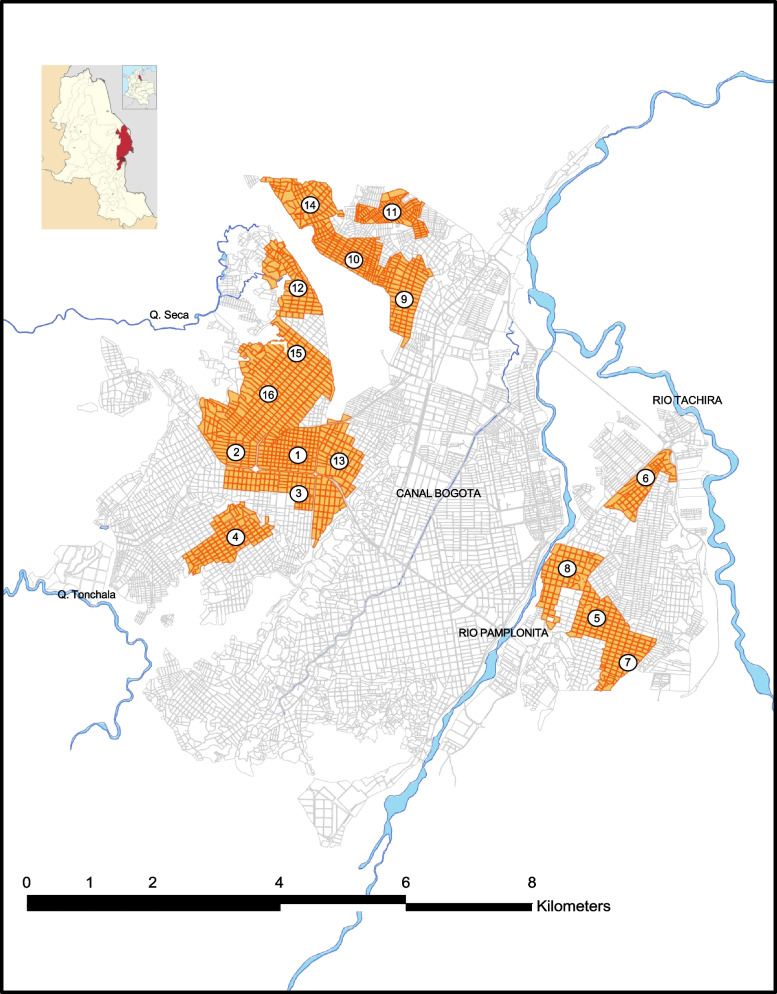
On the basis of available maps, the city was stratified into high, middle and low endemic/infested areas for Ae. aegypti (strata) using the surveillance information from SIVIGILA of the preceding year as well as information obtained from interviews with the vector control coordinators in Cúcuta. By this way, 24 high and medium endemic areas (clusters) were selected in Cucuta (16 clusters), Los patios (4 clusters) and Villa del Rosario (4 clusters). Figure 1 shows the selected clusters in Cucuta to be sampled by staff. For the baseline study they were not yet paired and allocated to the intervention and control arm as this was planned to do with the information resulting from the baseline study enabling us to find pairs of clusters with similar characteristics. The following characteristic should be similar in each pair of clusters: (I) High and middle level endemicity for Aedes-borne diseases; (II) Similar socio-economic conditions; (III) Similar types of the built environment: houses, schools, industry, health centers, workshops, churches and stores; (IV) Similar characteristics of public spaces. Within each cluster, all blocks of houses were inspected including public spaces. For the entomological/socio-demographic household survey, a proportion of 10% of 2000 houses was sampled in this study, resulting in 200 houses in each cluster. This number was rounded to 250 by convenience. Therefore, the study targeted 6000 households (250 households in each cluster) using a systematic random sampling method. For this we first estimated the sampling interval by dividing the total number of households in each cluster (around 2000) by the number of sample households (250), resulting in a sampling interval of 8. All households located within each cluster were mapped (using Google Maps) and a randomly defined starting point was selected and from there every 8th household was visited. Clear replacement rules were given to the interviewers and the compliance was checked in sample of families.
Fig. 1.
Selection of clusters in Cucuta
Map shows the 16 clusters selected in Cucuta which were visited by interviewers and vector control staff
Vector control staff and trained interviewers participated in the survey. They were divided into “couples” of one vector inspector and one interviewer. Each couple was assigned one cluster where they had to visit the sample houses. The interviewer did the interview with the head of household and simultaneously the inspector (local vector control staff) did the inspection of water containers both of them filling a form.
Data collection
The baseline surveys included a household questionnaire and entomological survey (see Additional files 1 and 2). The instruments were discussed in a participatory workshop with experts and pre-tested among the members of vector control staff in the three municipalities leading to some modifications. The first instrument was the questionnaire which was applied by trained interviewers and the other part was the form for the entomological inspections (larvae/pupae survey) which was applied by the vector control staff. As this is part of the national vector control programme only verbal consent of the house owner was obtained to check their water containers. An extensive training was conducted by the research team before starting the field work.
Household questionnaire
Demographic and health data was collected through face-to-face interviews using a standard questionnaire that included both structured and semi- structured questions. Household questionnaire was adapted from published research in Colombia [45]. The questions referred to socio-demographic parameters (number of people living in the house, age, sex, and educational level), self-reported DZC disease acquired during the last months, population mobility during the day, usage of water in tanks and willingness to accept the intervention trial (see Additional file 1).
Entomological survey
The standard entomological survey form was adapted following the guidelines of Standard Operational Procedures (SOPs) by WHO [46]. The questions were adapted to local conditions and survey requirements (see Additional file 2). The following data were recorded from each household: total number of containers (potential breeding sites), number of mosquito larvae positive and negative containers (any species), pupae count per container, container type, and other container characteristics (if they were covered or uncovered, outdoors or indoors).
Dengue surveillance and case definition
Data of the national surveillance system were obtained from SIVIGILA (the national health surveillance system), aggregated by year and setting (study areas) over the study period. Notified dengue cases including those classified as dengue fever (DF) and severe dengue, relying on a clinical case definition or lab confirmed or hospitalized patients. This study used annual population data (from the National Institute of Statistics-DANE; [47]) for calculating the incidence rate.
Data management and analysis
Descriptive analyse and double data entry was practiced (to minimize data entry errors) into a database using Microsoft Office Excel software by an assistant and supervised by the research team. The analysis was done using SPSS software version 28.0.1.1 (15).
Socio-demographic data were entered in a database. We assessed the frequency of variables potentially associated with the outcomes of recent dengue virus infection (sex, age, educational level, peoples` mobility) and self-reported dengue fever in household members. Persons’ Chi-square test (χ2) was applied to determine the differences between population characteristics and DZC self-reported cases, and peoples` mobility across all settings. All statistical analyses were performed at a 0.05 significance level.
Entomological indices were analysed per cluster and overall to show the presence, distribution and abundance of Ae. aegypti and the breeding sites most productive for adult mosquitoes were identified [48].
House index (HI): Percentage of houses infested with larvae and/or pupae.
Container index (CI): Percentage of water-holding containers infested with larvae or pupae.
Breteau index (BI): Number of positive containers per 100 houses inspected.
Pupae per person (PPI): Number of pupae per person in each household.
To estimate the pupal count for large container (more than 20 L) the methodology by Romero-Vivas [49] was used: According to water level, the number of pupae found was multiplied by a calibration factor.
Ethical considerations
The household questionnaire was only applied to adults who provided information related to the purpose of this study. No child or adolescent below the age of 18 was interviewed in this study. All people participating in the study were informed in local language through the study information sheet in a written and oral way. They were asked to sign the informed consent form. All participants were informed that their participation was voluntary and that their responses remained anonymous, therefore the study used numbers which replaced the names of individuals and codes which replaced the address of house. Before examining the domestic and peri-domestic water-holding containers, the field team requested permission to enter the house, did the inspection and collected entomological and sociodemographic data.
The study received approval from local health authorities in Cucuta and Norte de Santander and the study protocol was approved by the ethical committee of the Albert–Ludwigs-Universität (application number 141/19) in Freiburg, Germany and the National Institute of Health in Bogota, Colombia.
Results
In the baseline survey, a total of 5,997 households with 23,408 people in 24 clusters were visited by the field team. Some data were missing, or some questions were not answered by household participants, these values were excluded from the analysis.
Socio-demographic information
Of the household members 53.3% (12,448/23,357) were women (Table 1). The mean number of people living in a household was 3.9 (range 1–12, SD = 1.9). The mean age of the study population was 35 years (range: 2 months-105 years, SD = 21.7). The largest age group was between 35 and 64 years (35.8%; 8,375/23,365). Almost half of the study population above 5 years of age (44.9%; 10,240/22,817) reached secondary education and a significant proportion (31.9%) reached primary education. (The educational level was analysed for 5 years and older as the primary school starts at the age of 5 years in Colombia [50]). Similar characteristics were found in each cluster. (A complete description of all clusters can be found in the Additional file 3).
Table 1.
Population characteristics and self-reported annual incidence of DZC
| Characteristics | Variable | N* | Proportion % | Frequency of DZC during last 12 months % (n/N)** | P*** |
|---|---|---|---|---|---|
| Sex | Female | 12,448 | 53.3% | 2.3% (285/12,448) | 0.001 |
| Male | 10,909 | 46.7% | 1.7% (183/10,909) | ||
| Total (excluding 51 missing values) | 23,357 | ||||
| Age groups | 1 < year | 122 | 0.5% | 0.8% (1/122) | 0.001 |
| 1–4 years | 1,172 | 5.0% | 2.5% (29/1,172) | ||
| 5–9 years | 1,608 | 6.9% | 3.7% (6071,608) | ||
| 10–19 years | 3,849 | 16.5% | 1.9% (73/3,849) | ||
| 20–34 years | 5,677 | 24.3% | 1.7% (99/5,677) | ||
| 35–64 years | 8,375 | 35.8% | 1.8% (147/8,375) | ||
| > 64 years | 2,562 | 11.0% | 2.3% (59/2,562) | ||
| Total (excluding 43 missing values) | 23,365 | ||||
| Educational level | No education | 745 | 3.5% | 2.1% (16/745) | 0.423 |
| Primary education | 7,215 | 33.5% | 2.1% (152/7,215) | ||
| Secondary education | 10,251 | 47.6% | 1.8% (187/10,251) | ||
| Higher education | 3,316 | 15.4% | 2.2% (73/3,316) | ||
| Total (excluding 587 missing values and 1,294 children under 5 years) | 21,527 | ||||
| Mobility during the day | Inside cluster | 13,103 | 59.7% | 2.2% (291/13,103) | 0.03 |
| Outside cluster | 8,842 | 40.3% | 1.7% (146/8,842) | ||
| Total (excluding 1,463 missing values) | 21,945 |
*N number of people responding excluding missing values; **n number of people who reported to have had dengue divided by the total number of people who responded. ***p-values based on Pearson's chi-squared test
Peoples’ mobility
The insect vector Ae. aegypti bites primarily during the day inside or around houses and has a limited flight range; therefore, people’ mobility during the day is important to know when assessing the place of dengue transmission [51]. High mobility will make it less likely to show a protective effect by household interventions in a CRT. Therefore, we decided for a large cluster size where the majority of inhabitants move inside clusters during the day. We found in the household survey that the majority of the study population (59.7%; 13,103/21,945) remains inside the cluster boundaries while a considerable proportion, 40.3% (8,842/21,945) moves to places outside their cluster where they are exposed to vector bites. A Pearson's chi-squared test was performed to compare the proportion of daytime spent outside cluster by each member of household in different population characteristics, which showed there was a statistically significant difference among sex (X2 = 300.27, df = 1, P = 0.001), the age groups (X2 = 1593.58, df = 5, P = 0.000) and the education level of population (X2 = 1575.28, df = 3, P = 0.000). Those who moved outside their cluster were mainly male (53.8%; 4753/8836), young adults and adults between 20 to 64 years old (72.4%; 6,398/8,823) and people in secondary education (50.7%; 4,329/8,537).
Epidemiological information
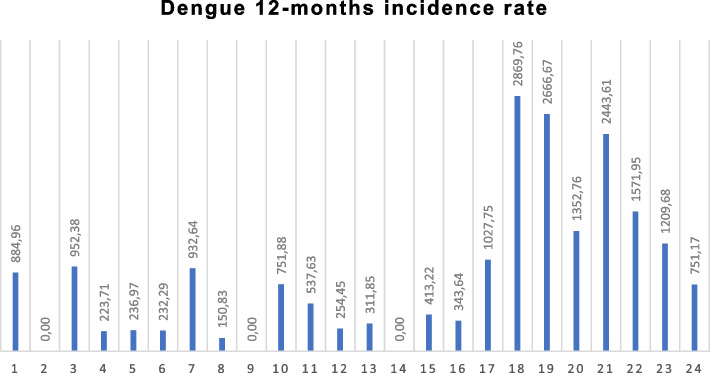
Across all clusters, 2.0% (468/23,093) of household members reported to have had any arboviral disease (DZC) during the last 12 months (Dengue incidence was 0.84%). This ranged from 3.0% (116/3,814) in Los Patios, 2.3% (90/3,948) in Villa del Rosario, to 1.7% (262/15,331) in Cucuta. A small group of respondents (68 persons) assumed to have had mixed infections with two arbovirus diseases. The 12-months self-reported incidence was the following: dengue 841.6 per 100,000 inhabitants, Zika 585.3 per 100,000 inhabitants and chikungunya 572.45 per 100,000 inhabitants. Persons with dengue (83.2%) usually went for diagnosis and treatment to the hospital while persons with Zika or Chikungunya used much less the hospital services (45.3% and 57.5% respectively). Figure 2 shows the reported Dengue incidence rate by study population in 24 clusters. Los Patios (1,965.9 per 100,000 inhabitants) and Villa del Rosario (1,494.4 per 100,000) noticeably had a higher dengue incidence rate than Cucuta (402.9 per 100,000). Cluster 18 had the highest dengue incidence rate (2,869.8 per 100,000 inhabitants) and clusters 2, 9 and 14 did not report any dengue case (Fig. 2).
Fig. 2.
Self-reported dengue per cluster in the previous 12 months
The bar graph shows 12-months incidence rates per 100,000 inhabitants. Cúcuta: cluster 1–16; Los Patios: cluster 17–20; Villa del Rosario: cluster 21–24
Table 1 shows the annual incidence of self-reported DZC related to sociodemographic characteristics (statistically significant differences except for education level). Females had a higher incidence (2.3%; 285/12,448) than males (1.7%; 185/10,909) (X2 = 11.09, df = 1, P = 0.001). Younger age groups, particularly those between 5 and 9 years of age (3.7%; 60/1,608) reported more cases of arbovirus diseases in comparison with other age groups (X2 = 32.66, df = 6, P = 0.001). Educational level (as an indicator for socioeconomic characeristics) was not associated with disease incidence. People who stayed inside their clusters during the day had a higher incidence (2.2%; 291/13,103) than those who move during daytime outside their home DZC (1.7%, 146/8,842; X2 = 8.778, df = 1, P = 0.03).
Dengue incidence notified by the National surveillance system, 2015–2021
During the 7-year period, 2015–2021, 8,190 dengue cases were notified by SIVIGILA in the study areas. The number of annual case notifications varied from a low of 333 cases in 2020 (173.1 per 100,000 population) to a high of 1,949 cases in 2019 (1,013.4 per 100,000 population), with a mean of 1,170 cases per year (incidence 608.4 per 100,000 population). Across all clusters, the number of dengue cases varied with a range of 4 to 193 dengue cases between 2015–2021. Cluster 14 had the lowest dengue incidence rate (51.1 per 100,000 population) in 2020 and Cluster 5 had the highest incidence rate (2,933.8 per population (see Table 2). Patients with fever and other DZC symptoms resort to the health services only when they feel severely sick.
Table 2.
Annual dengue incidence per 100,000 people notified by SIVIGILA
| Cluster | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|---|---|---|
| 1 | 870.7 | 245.3 | 220.8 | 686.8 | 1,410.4 | 245.3 | 1,802.8 |
| 2 | 607.5 | 341.7 | 88.6 | 734.1 | 1,151.8 | 139.2 | 1,316.3 |
| 3 | 564.3 | 435.3 | 322.5 | 645.0 | 1,015.8 | 225.7 | 854.6 |
| 4 | 557.1 | 330.8 | 243.7 | 1,532.0 | 1,410.2 | 139.3 | 1,009.7 |
| 5 | 1,420.1 | 764.7 | 577.4 | 1,201.6 | 2,933.8 | 577.4 | 811.5 |
| 6 | 776.8 | 263.1 | 263.1 | 839.5 | 814.4 | 137.8 | 451.1 |
| 7 | 298.5 | 155.2 | 107.5 | 418.0 | 835.9 | 107.5 | 238.8 |
| 8 | 981.7 | 387.5 | 374.6 | 632.9 | 1,433.7 | 439.2 | 426.2 |
| 9 | 608.6 | 294.5 | 215.9 | 981.5 | 795.1 | 157.0 | 647.8 |
| 10 | 501.6 | 178.4 | 156.1 | 802.6 | 412.4 | 78.0 | 646.5 |
| 11 | 520.8 | 186.7 | 147.4 | 668.2 | 599.4 | 78.6 | 343.9 |
| 12 | 824.8 | 477.5 | 130.2 | 1,128.7 | 803.1 | 238.8 | 2,452.8 |
| 13 | 905.6 | 235.7 | 285.3 | 372.2 | 2,009.7 | 173.7 | 831.2 |
| 14 | 664.5 | 127.8 | 115.0 | 805.0 | 396.1 | 51.1 | 907.2 |
| 15 | 583.3 | 272.2 | 108.9 | 840.0 | 770.0 | 147.8 | 754.5 |
| 16 | 990.2 | 452.1 | 301.4 | 1,819.0 | 2,077.3 | 236.8 | 1,410.0 |
| 17 | 301.2 | 283.4 | 301.2 | 513.7 | 974.3 | 230.3 | 885.7 |
| 18 | 331.0 | 296.8 | 159.8 | 993.0 | 707.7 | 57.1 | 764.8 |
| 19 | 224.4 | 366.2 | 248.1 | 826.8 | 1,169.4 | 141.7 | 685.1 |
| 20 | 555.9 | 465.4 | 245.6 | 1,745.3 | 736.9 | 116.4 | 452.5 |
| 21 | 1,395.8 | 197.2 | 227.6 | 1,395.8 | 880.0 | 197.2 | 1,213.8 |
| 22 | 1,252.1 | 445.5 | 337.1 | 1,131.7 | 517.7 | 96.3 | 565.9 |
| 23 | 496.6 | 264.9 | 242.8 | 1,390.6 | 640.1 | 121.4 | 684.3 |
| 24 | 605.1 | 357.5 | 288.8 | 1,883.9 | 440.0 | 233.8 | 1,265.1 |
| Overall | 688.4 | 316.1 | 230.3 | 989.0 | 1,013.4 | 173.1 | 848.6 |
Table shows the annual dengue incidence from 2015 to 2021 in all study clusters
Entomological information
Entomological inspections were conducted in all 5,997 households using the Standard Operational Procedures (SOPs) of the vector control services. Table 3 presents the larval and pupal indices in the 24 clusters from the three settings. The overall house index (HI) was 25.1% (1,504/5,997), the container index (CI) was 12.3% (1,776/14,386) and the Breteau index (BI) 29.6 per 100 houses (1,776/5,997) which shows high vector infestation and above the 5% assumed by WHO with the potential of epidemic disease transmission [52]. The HI was significantly different among the different clusters (σ2 = 0.0081, SD = 0.09, range = 6%-37.6%). The CIs were generally above 3% except for cluster 22 (2.25%). The BI values were highest in cluster 23 (48.8; 122/250), cluster 12 (46.8; 118/252) and cluster 24 (45.2; 113/250) and the lowest in cluster 18 (6.8; 17/250). The Pupae per person index (PPI) varied among clusters, with a range from 0.22 to 2.04 (SD = 0.49, σ2 = 0.2404), most PPI values being between 0.5 and 1.5, indicating a considerable risk of epidemic transmission [53].
Table 3.
Vector infestation in study clusters
| Cluster | Houses | Persons | deposits | Positive houses | Positive deposits | Pupae count | HI | CI | BI | PPI |
|---|---|---|---|---|---|---|---|---|---|---|
| Cucuta | 3,997 | 15,645 | 8,541 | 1,048 | 1,240 | 13,800.4 | 26.2% | 14.5% | 31.02 | 0.88 |
| 1 | 280 | 1,130 | 600 | 94 | 102 | 1,383.7 | 33.6% | 17.0% | 36.43 | 1.22 |
| 2 | 212 | 905 | 426 | 41 | 47 | 601.2 | 19.3% | 11.0% | 22.17 | 0.66 |
| 3 | 265 | 1,050 | 557 | 72 | 82 | 504.5 | 27.2% | 14.7% | 30.94 | 0.48 |
| 4 | 235 | 894 | 358 | 45 | 49 | 192.9 | 19.1% | 13.7% | 20.85 | 0.22 |
| 5 | 230 | 844 | 554 | 81 | 94 | 985.9 | 35.2% | 17.0% | 40.87 | 1.17 |
| 6 | 250 | 861 | 743 | 72 | 76 | 582.1 | 28.8% | 10.2% | 30.40 | 0.68 |
| 7 | 272 | 965 | 607 | 42 | 43 | 960.5 | 15.4% | 7.1% | 15.81 | 1.00 |
| 8 | 247 | 663 | 563 | 88 | 105 | 1,150.2 | 35.6% | 18.7% | 42.51 | 1.73 |
| 9 | 265 | 1,066 | 342 | 87 | 95 | 1,074.5 | 32.8% | 27.8% | 35.85 | 1.01 |
| 10 | 267 | 1,064 | 358 | 50 | 57 | 1,446.7 | 18.7% | 15.9% | 21.35 | 1.36 |
| 11 | 234 | 930 | 308 | 63 | 83 | 281.2 | 26.9% | 26.9% | 35.47 | 0.30 |
| 12 | 252 | 1,179 | 703 | 82 | 118 | 290.8 | 32.5% | 16.8% | 46.83 | 0.25 |
| 13 | 223 | 962 | 737 | 60 | 92 | 1,238.3 | 26.9% | 12.5% | 41.26 | 1.29 |
| 14 | 235 | 1,000 | 313 | 80 | 89 | 696.7 | 34.0% | 28.4% | 37.87 | 0.70 |
| 15 | 250 | 968 | 735 | 37 | 49 | 724.5 | 14.8% | 6.7% | 19.60 | 0.75 |
| 16 | 280 | 1,164 | 637 | 54 | 59 | 1,686.7 | 19.3% | 9.3% | 21.07 | 1.45 |
| Los Patios | 1000 | 3,815 | 2,525 | 133 | 142 | 2,327.8 | 13.3% | 5.6% | 14.20 | 0.61 |
| 17 | 250 | 973 | 783 | 38 | 40 | 549.8 | 15.2% | 5.1% | 16.00 | 0.57 |
| 18 | 250 | 906 | 754 | 15 | 17 | 580 | 6.0% | 2.3% | 6.80 | 0.64 |
| 19 | 250 | 975 | 591 | 53 | 55 | 774.5 | 21.2% | 9.3% | 22.00 | 0.79 |
| 20 | 250 | 961 | 397 | 27 | 30 | 423.5 | 10.8% | 7.6% | 12.00 | 0.44 |
| Villa del Rosario | 1000 | 3,948 | 3,320 | 323 | 394 | 4,272.4 | 32.3% | 11.9% | 39.40 | 1.08 |
| 21 | 250 | 1,064 | 643 | 58 | 64 | 588.9 | 23.2% | 10.0% | 25.60 | 0.55 |
| 22 | 250 | 827 | 751 | 77 | 95 | 1,686.1 | 30.8% | 12.6% | 38.00 | 2.04 |
| 23 | 250 | 992 | 1,088 | 94 | 122 | 1,498.9 | 37.6% | 11.2% | 48.80 | 1.51 |
| 24 | 250 | 1,065 | 838 | 94 | 113 | 498.5 | 37.6% | 13.5% | 45.20 | 0.47 |
| Total | 5,997 | 23,408 | 14,386 | 1,504 | 1,776 | 20,400.6 | 25.1% | 12.3% | 29.61 | 0.87 |
HI House index, CI Container index, BI Breteau index, PPI Pupae per person index
Most infested container types
A total of 14,386 water holding containers were found in the entomological inspection. These included containers used for water storage as well as some discarded mainly small containers not used for water storage but in which water had accumulated. Overall, the most common containers were concrete ground tanks (48.5%; 6,975/14,386) of which concrete tanks for washing purposes (79.3%; 5,528/6,975) were the most common ones (Fig. 3). Other container types included elevated tanks (24.5%; 3,529/14,386), plastic tanks (11.3%; 3,529/14,386) and buckets (10.1%; 1,446/14,386). Among the positive containers, the most infested water containers with Ae. aegypti larvae were ground tanks for cleaning and washing purposes (71.9%, 1277/14,386). Table 4 shows the distribution of water containers. The distribution of containers was similar in all clusters. (A description of the distribution of containers per cluster and setting can be found in the Additional file 3).
Fig. 3.

A concrete ground tank for washing purpose
Concrete ground tanks for washing (lavadero) are made of cement or ceramic tiles with the shape of a rectangle or square and variable sizes (size measurement in the photo)
Table 4.
Types of water containers inspected and pupae productivity
| Type of containers | # containers | % | Positive container | % | Pupae count (n*) | %* Productivity |
|---|---|---|---|---|---|---|
| Concrete ground laundry tank (lavadero) | 5,528 | 38.4% | 1,277 | 23.1% | 17,613.2 | 86.3% |
| Concrete ground tank for water storing | 1,447 | 10.1% | 119 | 8.2% | 1,661.1 | 8.1% |
| Plastic tank | 1,624 | 11.3% | 119 | 7.3% | 688.8 | 3.4% |
| Buckets | 1,446 | 10.1% | 138 | 9.5% | 238 | 1.2% |
| Elevated tank | 3,529 | 24.5% | 30 | 0.9% | 124 | 0.6% |
| Metal barrel | 137 | 1.0% | 30 | 21.9% | 46.5 | 0.2% |
| Sinks | 67 | 0.5% | 6 | 9.0% | 8 | 0.0% |
| Flowerpot | 95 | 0.7% | 21 | 22.1% | 8 | 0.0% |
| Others (including natural habits) | 72 | 0.5% | 9 | 14.1% | 5 | 0.0% |
| Jars/pots | 118 | 0.8% | 5 | 4.2% | 4 | 0.0% |
| Wheels | 75 | 0.5% | 15 | 20.0% | 4 | 0.0% |
| Fuel jars (pimpinas) | 121 | 0.8% | 3 | 2.5% | 0 | 0.0% |
| Water bottles | 127 | 0.9% | 4 | 3.1% | 0 | 0.0% |
| Total | 14,386 | 1,776 | 12.3% | 20,400.6 |
Pupal productivity: n* = total number of pupae for all containers of that type; %* = (total number of pupae from that container type/overall total number of pupae) × 100
Pupal productivity in different water container types
The total number of pupae collected across all cluster was 20,400. Of these 13,800 pupae were collected from the clusters in Cucuta (271 households) with a median of 50.9 total pupae per household; 2,328 pupae were collected from the 4 clusters of the municipality of Los Patios (80 households) with a median of 29.1 and 4,272 from the 4 clusters in the municipality of Villa del Rosario (190 households) with a median of 22.5 pupae per household.
The water container type with the highest proportion of pupae was the concrete ground tank, producing 94.5% of all pupae as a proxy for the production of adult mosquitoes (Table 4). From these, tanks for washing and cleaning (lavaderos) were noticeably the most productive water containers for Ae. aegypti (86.3%). This is probably related to the fact that most of these ground tanks were uncovered and outside of the house. Less important for pupal production were plastic tanks, producing 3.4% of pupae and least important were buckets, elevated tanks and metal barrels, producing together only 2.0% of pupae (Table 4).
Ground tanks and water use
Characteristics of the concrete ground tanks (lavaderos) were similar in all clusters. Most of them were mainly used for washing and cleaning, without a lid (92.5%; 5,017/5,421) and located outdoors (60.1%; 3,259/5,421). The mean number of ground tanks was 1 per household (SD = 0.41, range = 0–4). Overall, the mean capacity of these containers was 363 L (range 20 L to 5,000 L). Traditionally, these ground tanks have been treated by house owners with another no-standardized vector control method, chlorine tablets, which are self-applied by a member of household. Almost half of participant households (46.4%) reported ever having applied chlorine tablets, however the doses and frequency of application was variable. A small proportion (6.8%) of the population reported to use fish as biological control in these containers.
Regarding the sources of drinking water, only 2.2% (133/5,997) of the houses take drinking water from these ground tanks while the majority uses it for laundry (97.8%) and drink water from the tap provided by the municipal water company. We observed in our survey that people had quite a strict separation of the water sources for drinking and cleaning or washing (water mostly from ground tanks or plastic tanks). Drinking water also is used for cooking, showering and flush toilets.
Willingness to receive a new vector control intervention
The community in all clusters was interested in receiving the proposed novel vector control method (insecticidal coating for water containers). Overall, 88.3% of respondents held this view and there was no significant difference between clusters (5,296/5,997). The experience of the vector control staff (24 inspectors) was assessed through a short questionnaire; they considered that this study was very useful and interesting for improving public health in the city particularly regarding vector borne diseases, but they wanted to receive more training on arbovirus diseases and all of them would like to participate in the next phase of the study.
Discussion
This study is designed as the baseline study for a large Cluster Randomized Trial (CRT) but provides also by itself important information on the: epidemiological, entomological, and socio-demographic characteristics and assesses the transmission risk of arboviral diseases in an endemic area of Colombia.
Vector infestation level
The field team reported similar socio demographic characteristics in all clusters, but entomological characteristics were varied. High vector densities were found throughout all clusters. All clusters had a HI above 5% (range 6.0%-37.5%; Table 3) which is sometimes assumed to be a rough proxy measure of the risk threshold of epidemic transmission. Most clusters had a CI and BI above 5% and 20, respectively [52], similar to other Latin American countries, [8, 54]. Furthermore, the pupae per person index (PPI) as a proxy measure for Ae. aegypti densities was high in most study clusters (range: 0.22 and 2.04) with 18 clusters out of 24 being above 0.5 [53]. These data suggest high risk of arbovirus transmission by Ae. aegypti highlighting the urgent need for an effective intervention.
Main Ae. aegypti breeding sites
Concrete ground tanks were the most common type of water containers in all clusters. The population in Cucuta has historically collected water for multiple uses as a common practice of their daily routine due to the occasional shortage of tap water which has been a continuous problem in the region and other parts of the country [8].
The highest pupae production (i.e. % of all pupae in a special container type) was found in these ground or laundry tanks (86.3%). Pupal counts provide a more precise estimate of vector abundance than larval surveys [55, 56]. Thus, the vast majority of Ae. aegypti mosquitoes are developing in lavaderos, which are filled with water for washing clothes and cleaning purposes. Similar findings were made in other parts of Colombia and Latin America [27, 57–60] where different terms were used such as tanquilla or alberca [61]. Our study supports the recommendation that this type of container has to be targeted for any kind of vector control intervention in those areas where it has a high pupal productivity.
Vector control
In Colombia, vector control, such as the application of the organophosphate temephos in ground water containers, is generally only practiced in epidemic situations. For routine control, communities are recommended to keep containers covered and clean [62]. In our study, almost half of the population (46.4%) reported putting chlorine tablets (which they purchase on the street or on the market) in the water of their laundry tanks as vector control method. Chlorine has been used to clean and eliminate immature stage of mosquitoes in water containers [63, 64] but there is only scarce evidence on the use of chlorine tablets regarding its efficacy against insect larvae in laundry tanks, and there is no information on dosage and the safe use of chlorinated water for drinking.
This study showed that almost half of the population (48.7%) had not received any health education or inspection by vector control staff in their houses. The vector control program should reach more houses particularly where they have not applied any vector control method. Studies have demonstrated a significant benefit of involving adding communities and other stakeholders in the existing government DZC control programme [35, 65, 66]. The human health dimension of ecosystem health (ecohealth) has been a successful eco-bio-social approach used in vector control programs [67, 68]. The application of eco-friendly vector control tools in combination with environmental management practices and community mobilization has been proven effective in reducing infection risk in endemic countries [69]. Studies in India and Bolivia demonstrated that engaging women and community can reduce mosquito abundance and dengue risk [70]. Moreover, the use of information and communication technology (ICT) particularly mobile phones for dengue prevention has increased in the last decade, showing to be useful for introducing behavioural change [71]. These approaches can be implemented by vector control program in Colombia to enhance communication on arboviral diseases, increase acceptance in the use of new vector control tools and reach more people.
Considerations for the forthcoming intervention study
This study provides cluster specific information on socio-economic indicators and cluster size, on the estimated mosquito abundance, the main breeding places and their pupal productivity, the type and size of water containers, water use for drinking and cleaning which should be considered for the planning of the intervention (covering the productive water containers with a protective and transparent coating) and implementation of the CRT in randomly assigned clusters (using incidence rates and entomological indicators) into intervention and control clusters. The intervention in these ground tanks should be safe for human consumption as a small proportion of households (2.2%) uses the water is also for cooking and drinking. Moreover, people’s willingness to accept the insecticidal coating in their laundry tanks was high in all clusters (88.3%) based on the information, that a sub-study with water samples (to be presented in a different paper) showed no toxicity of the paint and will be delivered for free. Previous studies in Nepal and Bangladesh found that the application of insecticidal coating on house walls was safe and well accepted by communities and health workers. High acceptance (94%) of the households’ participants was reported in the control of leishmaniasis and Chagas disease [72, 73]. Household members perceived a decrease in mosquito and sandfly presence in the house [74].
A specific challenge for assessing the effectiveness of vector control interventions is people’s mobility during the day as 40.3% of the people in our sample used to leave the cluster area during the day at least for some hours so that they can get infected in other places and bring the viruses home. Male, adult people (20 years and older) and people in secondary education had higher proportion of time spent outside their clusters. This finding is similar to a study in Mexico which found differences in human mobility according to gender and age [75]. These population groups may play an important role in arbovirus dispersion reducing the effect of household interventions.
The novel insecticidal coating contains two active ingredients pyriproxyfen and alphacypermethrin which have both been widely evaluated for vector control. The concept of coating of surfaces with micro-encapsulated insecticidal/larvicidal products for vector control has gained special attention in comparison with other vector control methods such as insecticide-impregnated bednets, and indoor residual spraying [76, 77]. Its prolonged efficacy (for 9 to 12 months) in vector control and the excellent acceptance by communities [78–80] make it a potential alternative for Ae. aegypti control. It is suggested that this can be also applied by communities themselves and other affordable “do-it-yourself” strategies [76]. The ability and willingness to pay for disease prevention is also reflected in a growing private sector interest to offer such products to private and commercial customers [81]. In Colombia, insecticidal products are registered by the national authority (INVIMA) and some of them have been validated and tested through efficacy and feasibility studies with encouraging results [82]. However, standardized protocols and large-scale trials are needed for evaluating cost-effectiveness of dengue reduction as we are proposing in our study.
Limitations
Although some studies have shown an association between arbovirus infection and occupation [83, 84]. Other studies showed no clear association [85]. Information related to occupation of the head of household was not collected in this study. Many people do not like to be asked for employment and income which has been reported as a challenge in household surveys [86]. Additionally, there was no time for a longer questionnaire due to the different activities to be conducted during the household visit. However, our target population was rather homogeneous in terms of social class.
Supplementary Information
Acknowledgements
The authors would like to thank the participating families in this study. We gratefully acknowledge the team of the Centre for Medicine and Society, Master Programme Global Urban Health at Freiburg University, particularly Dr. Sonia Diaz for her encouragement and support on this work. We thank the vector control team in Cucuta, Villa del Rosario and Los Patios from the Health Secretary (Secretaria municipal de Salud de Cucuta) and the State Health Institute (IDS Norte de Santander) for support and approval of this studies. Hector Anaya, Roque Suarez and Marcos Rojas who led and supervised the field project in each municipality. MAC and RCS also thank Ministerio de Ciencia, Tecnología e Innovación (Minciencias), and the Government of Norte de Santander from Colombia for supporting our doctoral studies.
Authors’ contributions
All authors contributed to the concept and design of the study. MAC, RCS and AK did the interviewer training, and drafted the paper. All authors read and approved the study and the procedures. MAC and RCS prepared the submission to the ethical committee and prepared the logistics. MAC, RCS and JY prepared the field study, contacted the local leaders and organized the logistics. MP contributed to the statistical analysis. MAC, RCS, AK and MP formatted, revised and corrected the article until its final version. All authors read and commented on the draft paper and approved the final version.
Funding
Open Access funding enabled and organized by Projekt DEAL. MAC and RCS received a scholarship for their doctoral studies from the Ministry of Science, Technology and Innovation (Minciencias) and the State of Norte de Santander in Colombia (Grant number: 753, program: “Formación de Capital Humano de Alto Nivel para el Departamento de Norte de Santander”). The MinCiencias and the State of Norte of Santander were not actively involved in the design of the study, the data collection, the analysis, the interpretation of the data or in writing the manuscript.
The article processing charge was funded by the German Research Foundation (DFG) and the University of Freiburg in the funding programme Open Access Publishing.
Availability of data and materials
All data generated or analysed during this study are included in this published article (Additional file 3).
Declarations
Ethics approval and consent to participate
The participation in this study was voluntary, anonymous and did not represent a risk for the participants or their families. This study did not include human participants that are minors. Socio-demographic information was provided by a household participant (the head of house or parent) who had to be at least 18 years old and sign an informed consent. All participants were informed they could withdraw from the study at any time. The study was explained in Spanish by a vector control technician to participants. Participants did not receive any kind of compensation for taking part in the study. For data protection the information collected is anonymized: the questionnaire will not record names or addresses. Household address had an individual code for identification. The originals were kept in a safe place (locker) and were not being shown to anybody else. The originals will be kept for 12 months after publication and then destroyed. Study results or publications will present aggregated data only. Researchers confirm that all methods were carried out in accordance with relevant guidelines and regulations. The ethics committee of the Albert-Ludwigs-Universität Freiburg, Germany approved the project and the intervention was authorized by the local health authorities in Colombia.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wilder-Smith A, Gubler DJ, Weaver SC, Monath TP, Heymann DL, Scott TW. Epidemic arboviral diseases: priorities for research and public health. Lancet Infect Dis. 2017;17(3):e101–e106. doi: 10.1016/S1473-3099(16)30518-7. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer MUG, Reiner RC, Jr, Brady OJ, Messina JP, Gilbert M, Pigott DM, Yi D, Johnson K, Earl L, Marczak LB, Shirude S, Davis Weaver N, Bisanzio D, Perkins TA, Lai S, Lu X, Jones P, Coelho GE, Carvalho RG, Van Bortel W, Marsboom C, Hendrickx G, Schaffner F, Moore CG, Nax HH, Bengtsson L, Wetter E, Tatem AJ, Brownstein JS, Smith DL, Lambrechts L, Cauchemez S, Linard C, Faria NR, Pybus OG, Scott TW, Liu Q, Yu H, Wint GRW, Hay SI, Golding N. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat Microbiol. 2019;4(5):854–863. doi: 10.1038/s41564-019-0376-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heydari N, Larsen DA, Neira M, Beltrán Ayala E, Fernandez P, Adrian J, Rochford R, Stewart-Ibarra AM. Household Dengue Prevention Interventions, Expenditures, and Barriers to Aedes aegypti Control in Machala, Ecuador. Int J Environ Res Public Health. 2017;14(2):196. doi: 10.3390/ijerph14020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan SJ, Mundis SJ, Aguirre A, Lippi CA, Beltrán E, Heras F, Sanchez V, Borbor-Cordova MJ, Sippy R, Stewart-Ibarra AM, Neira M. Seasonal and geographic variation in insecticide resistance in Aedes aegypti in southern Ecuador. PLoS Negl Trop Dis. 2019;13(6):e0007448. doi: 10.1371/journal.pntd.0007448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maciel-de-Freitas R, Marques WA, Peres RC, Cunha SP, de Oliveira RL. Variation in Aedes aegypti (Diptera: Culicidae) container productivity in a slum and a suburban district of Rio de Janeiro during dry and wet seasons. Mem Inst Oswaldo Cruz. 2007;102(4):489–496. doi: 10.1590/S0074-02762007005000056. [DOI] [PubMed] [Google Scholar]
- 6.Rubio A, Cardo MV, Vezzani D. Tire-breeding mosquitoes of public health importance along an urbanisation gradient in Buenos Aires Argentina Mem. Inst Oswaldo Cruz. 2011;106(6):678–684. doi: 10.1590/S0074-02762011000600006. [DOI] [PubMed] [Google Scholar]
- 7.Gubler DJ. Dengue, urbanization and globalization: the unholy trinity of the 21(st) Century. Trop Med Health. 2011;39(4 Suppl):3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quintero J, Carrasquilla G, Suárez R, González C, Olano VA. An ecosystemic approach to evaluating ecological, socioeconomic and group dynamics affecting the prevalence of Aedes aegypti in two Colombian towns. Cad Saude Publica. 2009;25(Suppl 1):S93–103. doi: 10.1590/S0102-311X2009001300009. [DOI] [PubMed] [Google Scholar]
- 9.Danis-Lozano R, Rodríguez MH, Hernández-Avila M. Gender-related family head schooling and Aedes aegypti larval breeding risk in southern Mexico. Salud Publica Mex. 2002;44(3):237–242. doi: 10.1590/S0036-36342002000300007. [DOI] [PubMed] [Google Scholar]
- 10.Koyadun S, Butraporn P, Kittayapong P. Ecologic and sociodemographic risk determinants for dengue transmission in urban areas in Thailand. Interdisc Perspect Infect Dis. 2012;2012:907494. doi: 10.1155/2012/907494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Instituto Nacional de Salud, Ministerio de Salud y Protección Social. Informe de evento. Dengue. 2016. https://www.ins.gov.co/buscador-eventos/Informesdeevento/DENGUE%202017.pdf. Accessed 12 Dec 2021.
- 12.Portilla Cabrera CV, Selvaraj JJ. Geographic shifts in the bioclimatic suitability for Aedes aegypti under climate change scenarios in Colombia. Heliyon. 2019;6(1):e03101. doi: 10.1016/j.heliyon.2019.e03101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sistema Nacional de Vigilancia en Salud Pública. Instituto Nacional de Salud. 2021 https://www.ins.gov.co/Direcciones/Vigilancia/Paginas/SIVIGILA.aspx. Accessed 29 July 2021.
- 14.Castrillón JC, Castaño JC, Urcuqui S. Dengue en Colombia: diez años de evolución [Dengue in Colombia: ten years of database records] Rev Chilena Infectol. 2015;32(2):142–149. doi: 10.4067/S0716-10182015000300002. [DOI] [PubMed] [Google Scholar]
- 15.Martínez M, Gómez S. Chikungunya en Colombia, el inicio de la transmisión autóctona. IQEN. 2014;19:261–79. https://www.ins.gov.co/buscador-eventos/IQEN/IQEN%20vol%2019%202014%20num%2018.pdf.
- 16.Rodriguez-Morales J, Patiño-Valencia S, Villamil-Gómez WE, Alvarado-Socarras JA, Jimenez-Canizales CE. Situación del Zika en Colombia: experiencia de miembros de la Red Colombiana de Colaboración en Zika (RECOLZIKA) Acta Méd Peruana. 2016;33:79–81. doi: 10.35663/amp.2016.331.22. [DOI] [Google Scholar]
- 17.Instituto Nacional de Salud-Ministerio de Salud y Protección Social . Boletín Epidemiológico Semanal. 2014. [Google Scholar]
- 18.Instituto Nacional de Salud-Ministerio de Salud y Protección Social . Boletín Epidemiológico Semanal. 2015. [Google Scholar]
- 19.Instituto Nacional de Salud-Ministerio de Salud y Protección Social . Boletín Epidemiológico Semanal. 2016. [Google Scholar]
- 20.Instituto Nacional de Salud-Ministerio de Salud y Protección Social . Boletín Epidemiológico Semanal. 2017. [Google Scholar]
- 21.Instituto Nacional de Salud-Ministerio de Salud y Protección Social. Boletín Epidemiológico Semanal. 2018a. https://www.ins.gov.co/buscador-eventos/BoletinEpidemiologico/2018%20Bolet%C3%ADn%20epidemiol%C3%B3gico%20semana%2052.pdf. Accessed 12 Dec 2021.
- 22.Parra B, Lizarazo J, Jiménez-Arango JA, Zea-Vera AF, González-Manrique G, Vargas J, Angarita JA, Zuñiga G, Lopez-Gonzalez R, Beltran CL, Rizcala KH, Morales MT, Pacheco O, Ospina ML, Kumar A, Cornblath DR, Muñoz LS, Osorio L, Barreras P, Pardo CA. Guillain-Barré Syndrome Associated with Zika Virus Infection in Colombia. N Engl J Med. 2016;375(16):1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 23.McBride CS, Baier F, Omondi AB, Spitzer SA, Lutomiah J, Sang R, et al. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515:222–227. doi: 10.1038/nature13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Monteiro FJC, Mourão FRP, Ribeiro ESD, Rêgo M, Frances P, Souto RNP, et al. Prevalence of dengue, Zika and chikungunya viruses in Aedes (Stegomyia) aegypti (Diptera: Culicidae) in a medium-sized city, Amazon, Brazil. Rev Inst Med Trop Sao Paulo. 2020;62:e10. doi: 10.1590/s1678-9946202062010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wongkoon S, Jaroensutasinee M, Jaroensutasinee K, Preechaporn W. Development sites of Aedes aegypti and Ae. albopictus in Nakhon Si Thammarat. Thailand. Dengue Bull. 2007;31:141–152. [Google Scholar]
- 26.Jiménez-Alejo A, Morales-Pérez A, Nava-Aguilera E, Flores-Moreno M, Apreza-Aguilar S, Carranza-Alcaraz W, Cortés-Guzmán AJ, Fernández-Salas I, Ledogar RJ, Cockcroft A, Andersson N. Pupal productivity in rainy and dry seasons: findings from the impact survey of a randomised controlled trial of dengue prevention in Guerrero, Mexico. BMC Public Health. 2017;17(Suppl 1):428. doi: 10.1186/s12889-017-4294-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Focks DA, Alexander N. Multicountry study of Aedes aegypti pupal productivity survey methodology: findings and recommendations. Dengue bulletin WHO. 2007;31:192–200. [Google Scholar]
- 28.Barrera R, Amador M, Clark GG. Use of the pupal survey technique for measuring Ae-des aegypti (Diptera: Culicidae) productivity in Puerto Rico. Am J Trop Med Hyg. 2006;74(2):290–302. doi: 10.4269/ajtmh.2006.74.290. [DOI] [PubMed] [Google Scholar]
- 29.Instituto Nacional de Salud-Ministerio de Salud y Protección Social. SISPRO: in Reports ASIS. Datos RIPS. Ministerio de Protección Social. Available from: http://rssvr2.sispro.gov.co/reportesAsis/. Accessed 27 Mar 2021.
- 30.Hayes, R.J., & Moulton, L.H. Cluster Randomised Trials (2nd ed.). Chapman and Hall/CRC. 2017. 10.4324/9781315370286.
- 31.Vanlerberghe V, Toledo ME, Rodríguez M, et al. Community involvement in dengue vector control: cluster randomised trial. BMJ. 2009;338:1959. doi: 10.1136/bmj.b1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arunachalam N, Tyagi BK, Samuel M, et al. Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai City. India Pathog Glob Health. 2012;106:488–496. doi: 10.1179/2047773212Y.0000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castro M, Sanchez L, Perez D, et al. A community empowerment strategy embedded in a routine dengue vector control programme: a cluster randomised controlled trial. Trans R Soc Trop Med Hyg. 2012;106:315–321. doi: 10.1016/j.trstmh.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Espinoza-Gomez F, Hernandez-Suarez CM, Coll-Cardenas R. Educational campaign versus mala-thion spraying for the control of Aedes aegypti in Colima Mexico. J Epidemiol Community Health. 2002;56:148–152. doi: 10.1136/jech.56.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andersson N, Nava-Aguilera E, Arosteguí J, Morales-Perez A, Suazo-Laguna H, Legorreta-Soberanis J, Hernandez-Alvarez C, Fernandez-Salas I, Paredes-Solís S, Balmaseda A, Cortés-Guzmán AJ, Serrano de Los Santos R, Coloma J, Ledogar RJ, Harris E. Evidence based community mobilization for dengue prevention in Nicaragua and Mexico (Camino Verde, the Green Way): cluster randomized controlled trial. BMJ. 2015;351:h3267. doi: 10.1136/bmj.h3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson AL, Courtenay O, Kelly-Hope LA, Scott TW, Takken W, Torr SJ, Lindsay SW. The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl Trop Dis. 2020;14(1):e0007831. doi: 10.1371/journal.pntd.0007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolbers M, Kleinschmidt I, Simmons CP, Donnelly CA. Considerations in the design of clinical trials to test novel entomological approaches to dengue control. PLoS Negl Trop Dis. 2012;6(11):e1937. doi: 10.1371/journal.pntd.0001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DANE. Censo Nacional de Población y vivienda 2018. https://geoportal.dane.gov.co/geovisores/sociedad/cnpv-2018/. Accessed 30 Apr 2021.
- 39.Instituto Departamental de Salud de Norte de Santander (INS Norte de Santander). 2016. Análisis de Situación de Salud con el Modelo de Determinantes Sociales. Coordinación de vigilancia en Salud Pública. Fecha de consulta: 4 de abril de 2018. Available from en: http://salasituacionalidsnds.weebly.com/uploads/1/0/7/1/10714324/asisdepartamental-2016-nortedesantander.pdf. Accessed 19 Mar 2018.
- 40.The Shiny CRT Calculator: Power and Sample size for Cluster Randomised Trials. (2022). https://clusterrcts.shinyapps.io/rshinyapp/. Accessed 20 June 2022.
- 41.Wong J, Astete H, Morrison AC, Scott TW. Sampling considerations for designing Aedes aegypti (Diptera:Culicidae) oviposition studies in Iquitos, Peru: Substrate preference, diurnal periodicity, and gonotrophic cycle length. J Med Entomol. 2011;48(1):45–52. doi: 10.1603/ME10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasuno M, Tonn RJ. A study of biting habits of Aedes aegypti in Bangkok. Thailand Bull World Health Organ. 1970;43(2):319–325. [PMC free article] [PubMed] [Google Scholar]
- 43.Chadee DD. Landing periodicity of the mosquito Aedes aegypti in Trinidad in relation to the timing of insecticidal space-spraying. Med Vet Entomol. 1988;2(2):189–192. doi: 10.1111/j.1365-2915.1988.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 44.Hemming K, Kasza J, Hooper R, Forbes A, Taljaard M. A tutorial on sample size calculation for multiple-period cluster randomized parallel, cross-over and stepped-wedge trials using the Shiny CRT Calculator. Int J Epidemiol. 2020;49(3):979–995. doi: 10.1093/ije/dyz237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higuera-Mendieta DR, Cortés-Corrales S, Quintero J, González-Uribe C. KAP Surveys and Dengue Control in Colombia: Disentangling the Effect of Sociodemographic Factors Using Multiple Correspondence Analysis. PLoS Negl Trop Dis. 2016;10(9):e0005016. doi: 10.1371/journal.pntd.0005016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Special Programme for Research and Training in Tropical Diseases (TDR), World Health Organization. Operational guide for assessing the productivity of Aedes aegypti breeding sites. http://www.who.int/tdr/publications/documents/sop-pupal-surveys.pdf]. http://www.who.int/tdr/publications/documents/sop-pupal-surveys.pdf. Accessed 20 Jul 2018.
- 47.DANE. Geovisor CNPV 2018: Geoportal DANE GEOPORTAL DANE. https://geoportal.dane.gov.co/geovisores/territorio/analisis-cnpv-2018/?lt=4.646075&lg=-74.088605&z=20. Accessed 15 Dec 2021.
- 48.World Health Organization. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever. Revised an. World Health Organization, Regional Office for South-East Asia 2011. Available online at: http://origin.searo.who.int/entity/vector_borne_tropical_diseases/documents/SEAROTPS60/en/.
- 49.Romero-Vivas CM, Llinás H, Falconar AK. Three calibration factors, applied to a rapid sweeping method, can accurately estimate Aedes aegypti (Diptera: Culicidae) pupal numbers in large water-storage containers at all temperatures at which dengue virus transmission occurs. J Med Entomol. 2007;2007(44):930–937. doi: 10.1093/jmedent/44.6.930. [DOI] [PubMed] [Google Scholar]
- 50.Ministerio de Education (2014). Indicadores educativos enero 2014 Available from: https://www.mineducacion.gov.co/1621/articles329021_archivo_pdf_indicadores_educativos_enero_2014.pdf. Accessed 27 July 2019.
- 51.Reiner RC, Jr, Achee N, Barrera R, Burkot TR, Chadee DD, Devine GJ, Endy T, Gubler D, Hombach J, Kleinschmidt I, Lenhart A, Lindsay SW, Longini I, Mondy M, Morrison AC, Perkins TA, Vazquez-Prokopec G, Reiter P, Ritchie SA, Smith DL, Strickman D, Scott TW. Quantifying the Epidemiological Impact of Vector Control on Dengue. PLoS Negl Trop Dis. 2016;10(5):e0004588. doi: 10.1371/journal.pntd.0004588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. Guidelines for Dengue Surveillance and Mosquito Control Ed Manila, Philippines: World Health Organization Regional Office for the Western Pacific Geneva: World Health Organization. 2003. Accessed 19 April 2021. https://apps.who.int/iris/handle/10665/206987.
- 53.Focks DA, Brenner RJ, Hayes J, Daniels E. Transmission thresholds for dengue in terms of Aedes aegypti pupae per person with discussion of their utility in source reduction efforts. Am J Trop Med Hyg. 2000;62(1):11–18. doi: 10.4269/ajtmh.2000.62.11. [DOI] [PubMed] [Google Scholar]
- 54.Morales-Pérez A, Nava-Aguilera E, Balanzar-Martínez A, et al. Aedes aegypti breeding ecology in Guerrero: cross-sectional study of mosquito breeding sites from the baseline for the Camino Verde trial in Mexico. BMC Public Health. 2017;17:450. doi: 10.1186/s12889-017-4293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ngugi HN, Nyathi S, Krystosik A, Ndenga B, Mbakaya JO, Aswani P, Musunzaji PS, Irungu LW, Bisanzio D, Kitron U, Desiree LaBeaud A, Mutuku F. Risk factors for Aedes aegypti household pupal persistence in longitudinal entomological household surveys in urban and rural Kenya. Parasit Vectors. 2020;13(1):499. doi: 10.1186/s13071-020-04378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tun-Lin W, Lenhart A, Nam VS, Rebollar-Téllez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M. Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health. 2009;14(9):1143–1153. doi: 10.1111/j.1365-3156.2009.02341.x. [DOI] [PubMed] [Google Scholar]
- 57.Vásquez-Trujillo A, Cardona-Arango D, Segura-Cardona AM, Portela-Câmara DC, Alves-Honório N, Parra-Henao G. House-Level Risk Factors for Aedes aegypti Infestation in the Urban Center of Castilla la Nueva, Meta State, Colombia. J Trop Med. 2021;23(2021):8483236. doi: 10.1155/2021/8483236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kroeger A, Dehlinger U, Burkhardt S, Atehortua W, Anaya H, Becker N. Community based dengue control in Colombia: people's knowledge and practice and the potential con-tribution of the biological larvicide Bti (Bacilus thuringienesis israelensis) Trop Med Parasi-tol. 1995;46:241–246. [PubMed] [Google Scholar]
- 59.Fernandez EA, Leontsini E, Sherman C, Chan AST, Reyes CE, Lozano RC, et al. Trial of a community-based intervention to decrease infestation of Aedes aegypti mosqui-toes in cement washbasins in El Progreso. Honduras Acta Trop. 1995;70:171–183. doi: 10.1016/S0001-706X(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 60.Alcalá L, Quintero J, González-Uribe C, Brochero H. Productividad de Aedes aegypti (L.) (Diptera: Culicidae) en viviendas y espacios públicos en una ciudad endémica para dengue en Colombia. Biomedica. 2015;35(2):258–268. doi: 10.7705/biomedica.v35i2.2567. [DOI] [PubMed] [Google Scholar]
- 61.Quintero J, Garcia-Betancourt T, Cortes S, Garcia D, Alcala L, Gonzalez-Uribe C, et al. Effectiveness and feasibility of long-lasting insecticide-treated curtains and water container covers for dengue vector control in Colombia: a cluster randomised trial. Trans R Soc Trop Med Hyg. 2015;109(2):116–125. doi: 10.1093/trstmh/tru208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ministerio de la Protección Social: Gestión para la vigilancia entomológica y control de la transmisión del dengue. Bogota, Colombia: Ministerio de la Protección Social - Instituto Nacional de Salud; 2014.
- 63.Mackay AJ, Amador M, Felix G, Acevedo V, Barrera R. Evaluation of Household Bleach as an Ovicide for the Control of Aedes aegypti. J Am Mosq Control Assoc. 2015;31(1):77–84. doi: 10.2987/14-6427R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fernandez EA, Leontsini E, Sherman C, Chan AS, Reyes CE, Lozano RC, et al. Trial of a community-based intervention to decrease infestation of Aedes aegypti mosquitoes in cement washbasins in El Progreso. Honduras Acta Trop. 1998;70(2):171–183. doi: 10.1016/S0001-706X(98)00033-3. [DOI] [PubMed] [Google Scholar]
- 65.Abeyewickreme W, Wickremasinghe AR, Karunatilake K, Sommerfeld J, Axel K. Community mobilization and household level waste management for dengue vector control in Gampaha district of Sri Lanka; an intervention study. Pathog Glob Health. 2012;106(8):479–487. doi: 10.1179/2047773212Y.0000000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arunachalam N, Tyagi BK, Samuel M, Krishnamoorthi R, Manavalan R, Tewari SC, Ashokkumar V, Kroeger A, Sommerfeld J, Petzold M. Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai City. India Pathog Glob Health. 2012;106(8):488–496. doi: 10.1179/2047773212Y.0000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Boischio A, Sánchez A, Orosz Z, Charron D. Health and sustainable development: challenges and opportunities of ecosystem approaches in the prevention and control of dengue and Chagas disease. Cad Saude Publica. 2009;25(1):149–154. doi: 10.1590/S0102-311X2009001300014. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell-Foster K, Ayala EB, Breilh J, Spiegel J, Wilches AA, Leon TO, Delgado JA. Integrating participatory community mobilization processes to improve dengue prevention: an eco-bio-social scaling up of local success in Machala, Ecuador. Trans R Soc Trop Med Hyg. 2015;109(2):126–133. doi: 10.1093/trstmh/tru209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sommerfeld J, Kroeger A. Eco-bio-social research on dengue in Asia: a multicountry study on ecosystem and community-based approaches for the control of dengue vectors in urban and peri-urban Asia. Pathog Glob Health. 2012;106(8):428–435. doi: 10.1179/2047773212Y.0000000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arunachalam N, Tyagi BK, Samuel M, Krishnamoorthi R, Manavalan R, Tewari SC, et al. Community-based control of Aedes aegypti by adoption of eco-health methods in Chennai City. India Pathogens and Global Health. 2012;106(8):488–496. doi: 10.1179/2047773212Y.0000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carrillo MA, Kroeger A, Cardenas Sanchez R, Diaz Monsalve S, Runge-Ranzinger S. The use of mobile phones for the prevention and control of arboviral diseases: a scoping review. BMC Public Health. 2021;21(1):110. doi: 10.1186/s12889-020-10126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Banjara MR, Das ML, Gurung CK, Singh VK, Joshi AB, Matlashewski G, Kroeger A, Olliaro P. Integrating case detection of visceral Leishmaniasis and other febrile illness with vector control in the post-elimination phase in Nepal. Am J Trop Med Hyg. 2019;100(1):108–114. doi: 10.4269/ajtmh.18-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dias JC, Jemmio A. Sobre uma pintura inseticida para o controle de Triatoma infestans, na Bolívia [About an insecticidal paint for controlling Triatoma infestans, in Bolivia] Rev Soc Bras Med Trop. 2008;41(1):79–81. doi: 10.1590/S0037-86822008000100016. [DOI] [PubMed] [Google Scholar]
- 74.Ghosh D, Alim A, Huda MM, Halleux CM, Almahmud M, Olliaro PL, Matlashewski G, Kroeger A, Mondal D. Comparison of Novel Sandfly Control Interventions: A Pilot Study in Bangladesh. Am J Trop Med Hyg. 2021;105(6):1786–1794. doi: 10.4269/ajtmh.20-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Falcón-Lezama JA, Santos-Luna R, Román-Pérez S, Martínez-Vega RA, Herrera-Valdez MA, Kuri-Morales ÁF, Adams B, Kuri-Morales PA, López-Cervantes M, Ramos-Castañeda J. Analysis of spatial mobility in subjects from a Dengue endemic urban locality in Morelos State, Mexico. PLoS One. 2017;12(2):e0172313. doi: 10.1371/journal.pone.0172313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirve S, Kroeger A, Matlashewski G, Mondal D, Banjara MR, Das P, Be-Nazir A, Arana B, Olliaro P. Towards elimination of visceral leishmaniasis in the Indian subcontinent-Translating research to practice to public health. PLoS Negl Trop Dis. 2017;11(10):e0005889. doi: 10.1371/journal.pntd.0005889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Banjara MR, Joshi AB, Singh VK, Das ML, Gurung CK, Olliaro P, Halleux C, Matlashewski G, Kroeger A. Response to visceral Leishmaniasis cases through active case detection and vector control in low-endemic hilly districts of Nepal. Am J Trop Med Hyg. 2022;107(2):349–354. doi: 10.4269/ajtmh.21-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maloney KM, Ancca-Juarez J, Salazar R, Borrini-Mayori K, Niemierko M, Yukich JO, Naquira C, Keating JA, Levy MZ. Comparison of insecticidal paint and deltamethrin against Triatoma infestans (Hemiptera: Reduviidae) feeding and mortality in simulated natural conditions. J Vector Ecol. 2013;38:6–11. doi: 10.1111/j.1948-7134.2013.12003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Llácer E, Dembilio O, Jacas JA. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae) J Econ Entomol. 2010;103:402–408. doi: 10.1603/EC09310. [DOI] [PubMed] [Google Scholar]
- 80.Mosqueira B, Chabi J, Chandre F, Akogbeto M, Hougard JM, Carnevale P, Mas-Coma S. Efficacy of an insecticide paint against malaria vectors and nuisance in West Africa—Part 2: Field evaluation. Malar J. 2010;9:341. doi: 10.1186/1475-2875-9-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schiøler KL, Alifrangis M, Kitron U, Konradsen F. Insecticidal paints: a realistic approach to vector control? PLoS Negl Trop Dis. 2016;10(4):e0004518. doi: 10.1371/journal.pntd.0004518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gual-Gonzalez L, Medina M, Valverde-Castro C, Beltrán V, Caro R, Triana-Chávez O, Nolan MS, Cantillo-Barraza O. Laboratory Evaluation and Field Feasibility of Micro-Encapsulated Insecticide Effect on Rhodnius prolixus and Triatoma dimidiata Mortality in Rural Households in Boyacá, Colombia. Insects. 2022;13(11):1061. doi: 10.3390/insects13111061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Velasco-Salas ZI, Sierra GM, Guzmán DM, Zambrano J, Vivas D, Comach G, et al. Dengue seroprevalence and risk factors for past and recent viral transmission in Venezuela: a comprehensive community-based study. Am J Trop Med Hyg. 2014;91(5):1039–1048. doi: 10.4269/ajtmh.14-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mwanyika GO, Sindato C, Rugarabamu S, Rumisha SF, Karimuribo ED, Misinzo G, Rweyemamu MM, Abdel Hamid MM, Haider N, Vairo F, Kock R, Mboera LEG. Seroprevalence and associated risk factors of chikungunya, dengue, and Zika in eight districts in Tanzania. Int J Infect Dis. 2021;111:271–280. doi: 10.1016/j.ijid.2021.08.040. [DOI] [PubMed] [Google Scholar]
- 85.Dhanoa A, Hassan SS, Jahan NK, Reidpath DD, Fatt QK, Ahmad MP, Meng CY, Ming LW, Zain AZ, Phipps ME, Othman I, Rabu AB, Sirajudeen R, Fatan AABA, Ghafar FA, Ahmad HB, Allotey P. Seroprevalence of dengue among healthy adults in a rural community in Southern Malaysia: a pilot study. Infect Dis Poverty. 2018;7(1):1. doi: 10.1186/s40249-017-0384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Meyer BD, Mok WK, Sullivan JX. Household Surveys in Crisis. J Econ Perspect. 2015;29(4):199–226. doi: 10.1257/jep.29.4.199. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (Additional file 3).