Abstract
The aim of this study was to explore the effects of herbal drug pharmacokinetic interactions on the biotransformation of molnupiravir and its metabolite β-D-N4-hydroxycytidine (NHC) in the blood and brain. To investigate the biotransformation mechanism, a carboxylesterase inhibitor, bis(4-nitrophenyl)phosphate (BNPP), was administered. Not only molnupiravir but also the herbal medicine Scutellaria formula-NRICM101 is potentially affected by coadministration with molnupiravir. However, the herb-drug interaction between molnupiravir and the Scutellaria formula-NRICM101 has not yet been investigated. We hypothesized that the complex bioactive herbal ingredients in the extract of the Scutellaria formula-NRICM101, the biotransformation and penetration of the blood—brain barrier of molnupiravir are altered by inhibition of carboxylesterase. To monitor the analytes, ultrahigh-performance liquid chromatography tandem mass spectrometry (UHPLC—MS/MS) coupled with the microdialysis method was developed. Based on the dose transfer from humans to rats, a dose of molnupiravir (100 mg/kg, i.v.), molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.), and molnupiravir (100 mg/kg, i.v.) + the Scutellaria formula-NRICM101 extract (1.27 g/kg, per day, for 5 consecutive days) were administered. The results showed that molnupiravir was rapidly metabolized to NHC and penetrated into the brain striatum. However, when concomitant with BNPP, NHC was suppressed, and molnupiravir was enhanced. The blood-to-brain penetration ratios were 2% and 6%, respectively. In summary, the extract of the Scutellaria formula-NRICM101 provides a pharmacological effect similar to that of the carboxylesterase inhibitor to suppress NHC in the blood, and the brain penetration ratio was increased, but the concentration is also higher than the effective concentration in the blood and brain.
Keywords: Molnupiravir, β-D-N4-hydroxycytidine, Herb-drug interaction, NRICM101, Pharmacokinetics
Graphical Abstract
1. Introduction
The antiviral drug molnupiravir is an orally bioavailable prodrug that effectively reduces the risk of hospitalization and death in individuals with mild to moderate COVID-19 infection. The active form, β-D-N4-hydroxycytidine (N4-hydroxycytidine; NHC), is the metabolite of molnupiravir. NHC acts as a substrate of cytidine triphosphate and uridine triphosphate in viral RNA and impairs severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The active form of NHC showed poor bioavailability; however, molnupiravir can increase bioavailability by more than 90% and is metabolized to NHC in vivo [2], [3], [4]. The half maximal inhibitory concentration (IC50) of NHC in vitro is reported to range from 0.08 μM for SARS-CoV-2 in Calu-3 cells to 0.3 μM for SARS-CoV-2 in Vero cells (equivalent to 20.8–78 ng/mL) [5].
The Scutellaria formula-NRICM101 (NRICM101; Taiwan Chingguan Yihau) is a traditional Chinese medicine designed by the National Research Institute of Chinese Medicine (NRICM). The composition of NRICM101 includes several Chinese herbal medicines, such as Scutellaria baicalensis, Houttuynia cordata, Magnolia officinalis, and Satis indigotica Fort. [6]. Pharmacological studies have demonstrated the effects of Scutellaria in inhibiting spike protein, angiotensin converting enzyme-2 (ACE2), 3CL protease activity, viral plaque formation, and production of the cytokines interleukin (IL)− 6 and tumor necrosis factor (TNF)-α [6], [7], [8]. Clinical outcomes have also been evaluated, showing that Scutellaria prevents and treats COVID-19 [9]. The active ingredients of this extract include baicalin and baicalein, which act by inhibiting SARS-CoV-2 RNA-dependent RNA polymerase [10], [11].
The conversion of molnupiravir to NHC is catalyzed by carboxylesterase, an enzyme expressed widely in the liver, gastrointestinal tract, brain, and possibly blood [12], [13]. Previous reports suggest that some Chinese herbal medicines, including Scutellaria baicalensis, contain bioactive components that act to inhibit carboxylesterase activity [10]. However, to date, there are no reports on the herb-drug interaction of molnupiravir.
Based on the scientific background above, we hypothesized that Scutellaria formula-NRICM101 potentially interacts with molnupiravir to influence its mechanism of action. To investigate this hypothesis, the biodistribution and pharmacokinetic interaction of Scutellaria formula-NRICM101 with molnupiravir in rat blood and brain was developed. Dual-site microdialysis coupled with an ultrahigh-performance liquid chromatography tandem mass spectrometry (UHPLC—MS/MS) analytical system was developed to monitor molnupiravir and NHC in these regions of interest. To investigate the mechanism underlying this interaction, we evaluated the deposition of (i) molnupiravir (100 mg/kg, i.v.) alone, (ii) molnupiravir (100 mg/kg, i.v.) concomitant with a carboxylesterase inhibitor BNPP (50 mg/kg, i.v.), or (iii) molnupiravir (100 mg/kg, i.v.) concomitant with the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, per day, for 5 consecutive days).
2. Materials and methods
2.1. Chemicals and reagents
Molnupiravir and NHC were purchased from ChemScene (Monmouth Junction, NJ, USA). Bis(4-nitrophenyl) phosphate (BNPP) was obtained from TCI (Tokyo, Japan). MS-grade acetonitrile was purchased from J.T. Baker, Inc. (Phillipsburg, NJ). Ammonium acetate was obtained from Sigma—Aldrich Chemicals (St. Louis, MO). Ultrapure water (Millipore, Bedford, MA) was used for sample preparation. A standard stock solution of molnupiravir and NHC (1 mg/mL) was dissolved in acetonitrile and stored at − 20 ℃ prior to experimental use. The Scutellaria formula-NRICM101, which includes Scutellaria baicalensis, Houttuynia cordata, Satis indigotica Fort., Frichosanthes kirilowii, Schizonepeta tenuifolia, Mentha haplocaly, Morus alba, Glycyrrhiza uralensis, Magnolia officinalis, and Saposhnikovia divaricata, was purchased from the traditional drugstore Tai-chang-tang in Shilin District, Taipei. The herbal origin was identified by the Chinese physician Dr. W.-Y. Peng.
2.2. Preparation of the extract of Scutellaria formula-NRICM101
The Scutellaria formula-NRICM101 consisted of the following herbs: Scutellaria root (Scutellaria baicalensis, 18.75 g), Heartleaf Houttuynia (Houttuynia cordata Thunb., 18.75 g), Mongolian snakefruit (Trichosanthes kirilowii Maxim., 18.75 g), Indigowoad root (Isatis indigotica Fortune ex Lindl., 18.75 g), Mulberry leaf (Morus alba L., 11.25 g), Magnolia bark (Magnolia officinalis Rehder & E.H. Wilson, 11.25 g), peppermint herb (Mentha haplocalyx Briq., 11.25 g), fineleaf Nepeta (Nepeta tenuifolia Benth., 11.25 g), Saposhnikovia root (Saposhnikovia divaricate (Turcz.) Schischk, 7.5 g), and liquorice root (Glycyrrhiza glabra L., 7.5 g). Based on the above weight ratio and optimized extraction efficacy, a full set of herbs and 75% ethanol (1 L) was boiled for 40 min and then cooled to room temperature. After extraction, the resulting solution was filtered, centrifuged at 5000 rpm for 10 min, and then freeze dried to obtain the dried herbal extract. The extraction rate was 9.2%. The content of the herbal extracts was verified based on the Quality Analysis of the Department of Traditional Chinese Medicines of the Ministry of Health and Welfare of Taiwan. The herbal ingredients baicalin, quercetin, epigoitrin, rutin, and magnolol were used for the quality control analysis.
2.3. UHPLC—MS/MS conditions
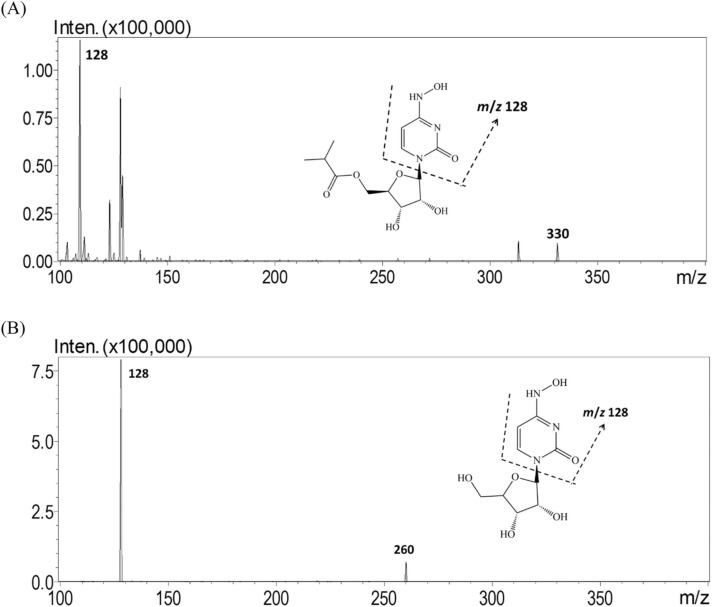
A UHPLC—MS/MS system (UHPLC—MS/MS 8030, Shimadzu, Kyoto, Japan) comprising a quadrupole mass spectrometer equipped with an electrospray ionization (ESI) source was used for molnupiravir and NHC analysis. An Acquity C-18 analytical column (50 mm × 2.1 mm, particle size 1.7 µm, Waters, MA, USA) was used to separate the analytes and dialysates. The mobile phase consisted of 2 mM ammonium acetate (adjusted to pH 4.3 by acetic acid) and acetonitrile with gradient elution at 98:2 (v/v) 2 mM ammonium acetate/acetonitrile for 0–2 min and 70:30 (v/v) 2 mM ammonium acetate/acetonitrile for 2–6 min. During 6–15 min, isocratic elution was used at 98:2 (v/v) 0.2% 2 mM ammonium acetate/acetonitrile to re-equilibrate the column, and the flow rate was 0.3 mL/min. The mobile phase was filtered through a 0.22 µm filter and degassed by an ultrasonic bath sonicator for 60 min before instrumental analysis. The injection volume of each sample was 5 μL, and the total run time was 15 min. LabSolution software (version 1.1; Shimadzu, Kyoto, Japan) was used to optimize the analyzed conditions of molnupiravir and NHC for UHPLC—MS/MS analysis. Both molnupiravir and NHC were monitored in positive ionization mode and multiple reaction monitoring (MRM) mode. The ion transitions monitored for molnupiravir and NHC were 329.0 → 128.0 m/z and 260.0 → 128.0 m/z, respectively, and the collision energies (CEs) were 15 V and 11 V, respectively ( Fig. 1). The mass spectrometric conditions were as follows: ESI, positive mode; interface voltage, 4.5 kV; nebulizing gas flow, 3.0 L/min; drying gas flow, 15.0 L/min; desolvation temperature, 250 °C; heat block temperature, 400 °C; and collision-induced dissociation gas, 230 kPa.
Fig. 1.
Multiple reaction monitoring (MRM) mode product ion mass spectra of (A) molnupiravir at m/z 330.2→128.2 and (B) NHC at m/z 260.1→128.1.
2.4. Method validation
The method validation was based on the bioanalytical method validation guidelines released by the US Food and Drug Administration guidance in 2018 [1], and the method was fully validated by linearity, precision, accuracy, stability, and recovery tests.
The calibration curves of blood and brain were generated with peak integration and nominal concentration data, and the standard solutions were diluted from the standard solution of molnupiravir (1 mg/mL). The diluted concentrations were 2.5, 5, 10, 25, 50, 100, and 250 ng/mL using blank dialysate. The stock solution of NHC (1 mg/mL) was diluted to 10, 50, 100, 250, 500, and 1000 ng/mL by blank dialysates. The linearity of the calibration curve was determined as a coefficient correlation (r2) greater than 0.995 for all the standard curves.
To assess precision and accuracy, the blank blood dialysates or blank brain dialysates were spiked with molnupiravir and NHC at the lower limit of quantification (LLOQ) and low, medium and high concentrations and analyzed on the same day (intraday) or five different days (interday) with five replicates. The accuracy was determined as the closeness between the nominal concentration (Cnom) and observed concentration (Cobs), and the formula was as follows: accuracy (bias %) = [(Cobs-Cnom)/Cnom] × 100. The precision was calculated as the relative standard deviation (RSD), which means that closeness within each Cobs measure to the standard deviation (S.D.) value and was obtained using the following formula (% RSD) = [standard deviation (SD)/Cobs] × 100%. Both the deviation values of R.S.D. (%) and bias (%) should be within ± 15% to be considered acceptable according to the bioanalytical method validation guidelines, and an LLOQ within ± 20% was acceptable.
The stability was evaluated with spiked blank blood and brain dialysates, and the conditions included autosampler stability, bench-top stability, long-term stability and freeze—thaw stability, with three replicates of low and high concentrations. All stability determinations were compared with freshly prepared samples from the standard stock solution. Autosampler stability was demonstrated by placing spiked samples at 10 °C for 6 h; bench-top stability was determined by the stability of samples at room temperature for 6 h; long-term stability was determined by the spiked sample stability at − 20 °C over 4 weeks; and freeze—thaw stability assessed the stability of samples after freeze—thaw for three cycles. The sample was frozen and thawed for at least 12 hr in each cycle. To calculate stability, the formula was as follows: Stability (%) = (the peak integration of processed sample/the peak integration of the freshly prepared sample) × 100.
The in vitro recovery of the microdialysis probe was determined by measuring the increment in molnupiravir and NHC through the semipermeable dialysis membrane. The microdialysis probes were put into anticoagulant citrate dextrose (ACD) solution spiked with the standard solution in 1.5 mL Eppendorf tubes containing molnupiravir at concentrations of 250, 500 and 1000 ng/mL and NHC at concentrations of 500, 1000 and 2000 ng/mL. The microdialysis recovery (Rdial) was calculated by comparing the concentration in the dialysate (Cdial) with the Cnom of three replicates: Rd i a l (%) = (Cdial/Cnom) × 100.
The matrix effect was prepared for two sets of samples to assess the validity of the bioanalytical method as follows:
Set 1. The samples were prepared at target concentrations of 5 and 250 ng/mL for molnupiravir and concentrations of 25 and 1000 ng/mL for NHC in the mobile phase (2% acetonitrile in water), with a total volume of 50 μL in a 1.5-mL Eppendorf tube. After mixing, the solutions were transferred to autosampler vial inserts, and 5 μL was injected into the UHPLC–MS/MS system.
Set 2. The samples were prepared at target concentrations of 5 and 250 ng/mL for molnupiravir and concentrations of 25 and 1000 ng/mL for NHC in the blood and brain dialysates, with a total volume of 50 μL. After mixing, the solutions were transferred from Eppendorf tubes into autosampler vial inserts, and 5 μL was injected into the UHPLC–MS/MS system.
The results obtained in this manner allow determination of the matrix effect (ME) by comparing the absolute peak area obtained in set 1 and set 2 (Table). One depicts the peak areas obtained in mobile phase standards in set 1 as A and the corresponding peak areas for standards spiked in dialysates as B (set 2), and the values can be calculated as follows: ME (%) = B/A × 100.
2.5. Animal experiments
Surgical procedures were approved by the Institutional Animal Care and Use Committee of the National Yang Ming Chiao Tung University (IACUC no. 1110210). Male Sprague—Dawley rats with a weight range of 200 ± 50 g were supplied by the Laboratory Animal Centre of National Yang Ming Chiao Tung University, Taipei, Taiwan. The animals received food (Laboratory Rodent Diet 5001, PMI Feeds, Richmond, IN, USA) and water ad libitum. The animal facilities were controlled with a 12 h light/dark cycle throughout all feeding processes. Before surgery, food was removed for 12 h. Anesthesia was achieved using a single injection of urethane (1 g/kg, i.p.). A polyethylene tube-50 (PE-50) was placed into the femoral vein to administer the drugs.
2.6. Oral administration of the extract of Scutellaria formula-NRICM101
The Scutellaria formula-NRICM101 extract dose was determined from the dose of NRICM101 used clinically. The single dose for the crude herbs of the Scutellaria formula-NRICM101 is 135 g for clinical practice, which equates to a 1.27 g/kg dose p.o. when multiplied by the extraction ratio (9.2%) and normalized to an equivalent dose in rats. The extract of the Scutellaria formula-NRICM101 was dissolved in water for drug administration. The extract of the Scutellaria formula-NRICM101 was prepared in 0.2 g/mL aqueous solution and administered by gastric gavage at a dose of 1.27 g/kg for five consecutive days. On the fifth day, the Scutellaria formula-NRICM101 extract was administered 1 h before molnupiravir administration.
2.7. In vivo microdialysis
The microdialysis system was composed of a microinjection pump (CMA/400; CMA, Stockholm, Sweden), a microfraction collector (CMA/142, CMA) and the dialysate collection probe that were designed and applied in our laboratory [14], [15]. The microdialysis probe was composed of a concentric silica capillary with a semipermeable dialysis membrane (Spectrum, New Brunswick, NJ, USA), a fiberinner diameter of 200 µm and a molecular weight cutoff of 13 kDa. The active lengths of the blood and brain dialysis probes were 1.2 cm and 0.7 cm, respectively. Initially, the blood microdialysis probe was inserted into the left side of the jugular vein in the direction of the heart, and the perfusate was maintained with an anticoagulant citrate dextrose (ACD) solution composed of 3.5 mM citric acid, 7.5 mM sodium citrate and 13.6 mM D-(+)-glucose at a flow rate of 2.0 μL/min. The right femoral vein was catheterized with PE-50 for intravenous drug injection. For brain microdialysis sampling, rats were fixed on a stereotaxic instrument (David Kopf Instruments, Tujunga, CA, USA) for the implantation of the microdialysis probe. After mounting with a stereotaxic instrument, the hole was drilled in the skull with a pen-type grinder, and a microdialysis probe was implanted at the striatum site (+0.2 mm anteroposterior, +3.0 mm mediolateral and −7.5 mm dorsoventral to bregma) according to the book “The Rat Brain in Stereotaxic Coordinates” [16]. The perfusate was an ACD solution with a flow rate of 2.0 μL/min. After a postoperative stabilization period, molnupiravir (100 mg/kg, i.v.) and molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.) were administered through the femoral vein. Blood and brain samples were collected every 20 min for 6 h using a microfraction collector (CMA/142) and stored at − 20 °C for further UHPLC-MS/MS analysis.
2.8. Data analysis and statistics
Profiling solution software (version 1.1; Shimadzu, Kyoto, JPN) was used to evaluate the chromatogram data. WinNonlin Standard Edition software (version 1.1; Scientific Consulting Inc., Apex, NC, USA) was used to calculate the main pharmacokinetic parameters with a noncompartmental model of molnupiravir and NHC in blood and brain, including the area under the concentration curve (AUC), maximum drug concentration (Cmax), half-life (t1/2), clearance (CL), and mean residential time (MRT). The biotransformation ratio of molnupiravir to NHC, the biodistribution ratio of blood to brain, and the efficacy of BNPP were represented by the AUC ratios (AUCNHC/AUCmolnupiravir, AUCbrain/AUCblood and AUCmolnupiravir+BNPP/AUCmolnupiravir), respectively. The drug concentration-time curves were drawn by SigmaPlot (version 10.0; Systat Statistics, London, UK). Statistical analyses were performed using SPSS Statistics (version 22.0, IBM Corp., Armonk, NY, USA). Statistical contrasts were determined using one-way analysis of variance (ANOVA) and Tukey’s post hoc test. The statistically significant criterion was set at 0.05. Data are expressed as the mean ± standard error of the mean.
3. Results
3.1. Quantification of the extract of Scutellaria formula-NRICM101
For the extraction of the Scutellaria formula-NRICM101, 75% ethanol was the optimal solution. Fig. 2A shows a chromatogram of authentic compounds of epigoitrin, rutin, baicalin, quercetin, and magnolol with retention times of 3.50, 9.48, 10.11, 15.10 and 30.60 min, respectively. Fig. 2B shows the chromatogram of the extract of the Scutellaria formula-NRICM101. The contents of epigoitrin, rutin, baicalin, quercetin, and magnolol in the freeze-dried extract of Scutellaria formula-NRICM101 were 1.45, 12.18, 107.97, 1.40, and 57.70 mg/g, respectively.
Fig. 2.
UHPLC chromatograms of (A) water spiked with 25 μg/mL epigoitrin, rutin, baicalin, quercetin, and magnolol and (B) the herbal extract Scutellaria (2.5 mg/mL), including 1.45 mg/g epigoitrin, 12.18 mg/g rutin, 107.9 mg/g baicalin, 1.40 mg/g quercetin, and 57.7 mg/g magnolol in extraction; peak 1 = epigoitrin, peak 2: rutin, peak 3: baicalin, peak 4: quercetin, peak 5: magnolol.
3.2. UHPLC—MS/MS condition optimization
The UHPLC—MS/MS system was optimized to analyze the dialysates of molnupiravir and NHC using the positive MRM-ESI mode. To improve chromatographic separation, the aqueous phase used 2 mM ammonium acetate, and the pH value was adjusted to 4.3 by acetic acid. The organic phase chosen was acetonitrile, which shows superior intensity and separation performance compared with methanol. The retention times for molnupiravir and NHC were 1.25 and 5.70 min, respectively, and the representative MRM chromatograms did not show an interference signal in the blank matrix during the retention time in the molnupiravir and NHC analyses ( Fig. 3). The mass transitions of molnupiravir and NHC were observed at 330.2–128 (m/z) and 260–128.05 (m/z), respectively. The collision energies were − 15 eV and − 11 eV for molnupiravir and NHC, respectively (Fig. 1). The quantitative determination limits (LLOQs) of molnupiravir and NHC in rat dialysates were 2.5 and 10 ng/mL, respectively. The linear range of molnupiravir was 2.5–250 ng/mL, the calibration curve in blood dialysate was y = 520.4x+ 342 (r2 =0.999), and the calibration curve in brain dialysate was y = 754x-522.8 (r2 =0.999). The linear range of NHC is 10–1000 ng/mL, and the calibration curve in blood dialysate is y = 1430x-1736 (r2 =0.999) and in brain dialysate is y = 925x-794.3 (r2 =0.999). The method validation results are presented in Table 1, Table 2, Table 3, Table 4. Due to the high dose of molnupiravir (100 mg/kg, i.v.), the blood concentration is also large. Therefore, during analysis, we diluted the sample until the concentrations of molnupiravir and NHC fell within our linear range before analysis.
Fig. 3.
Representative multiple reaction monitoring (MRM) mode chromatograms of (A) blank blood dialysate, (B) blank blood dialysate spiked with NHC (500 ng/mL) and molnupiravir (100 ng/mL), (C) blood dialysate sample containing NHC (963 ng/mL) and molnupiravir (49.2 ng/mL) collected at 140 min after molnupiravir administration, (D) blank brain dialysate, (E) blank brain dialysate spiked with NHC (250 ng/mL) and molnupiravir (50 ng/mL), (F) brain dialysate sample containing NHC (352.8 ng/mL) and molnupiravir (103.1 ng/mL) collected at 140 min after molnupiravir administration (100 mg/kg, i.v.); peak 1, NHC; peak 2, molnupiravir.
Table 1.
Intra- and interday precision (% RSD) and accuracy (% Bias) of the UHPLC—MS/MS method for the determination of molnupiravir and NHC in rat blood and brain dialysates.
| Nominal concentration (ng/mL) | Intraday |
Interday |
||||
|---|---|---|---|---|---|---|
| Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) | Observed concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) | |
| Molnupiravir | ||||||
| Blood | ||||||
| 2.5 | 2.78 ± 0.13 | 4.68 | 11.34 | 2.40 ± 0.28 | 12.02 | -3.83 |
| 5 | 5.47 ± 0.40 | 7.34 | 9.30 | 4.98 ± 0.26 | 5.23 | -0.33 |
| 25 | 25.06 ± 0.78 | 3.13 | 0.25 | 26.35 ± 2.15 | 8.22 | 5.41 |
| 250 | 252.3 ± 1.50 | 0.59 | 0.90 | 251.11 ± 1.72 | 0.68 | 0.44 |
| Brain | ||||||
| 2.5 | 2.23 ± 0.09 | 4.22 | -10.82 | 2.61 ± 0.31 | 11.93 | 4.52 |
| 5 | 5.68 ± 0.65 | 11.65 | 13.59 | 5.20 ± 0.18 | 3.44 | 4.07 |
| 25 | 23.94 ± 0.36 | 1.49 | -4.22 | 23.51 ± 0.34 | 1.47 | -5.97 |
| 250 | 255.6 ± 2.19 | 0.86 | 2.25 | 248.5 ± 3.29 | 1.32 | -0.60 |
| NHC | ||||||
| Blood | ||||||
| 10 | 9.93 ± 0.88 | 8.93 | -0.66 | 10.63 ± 0.69 | 6.55 | 6.33 |
| 25 | 24.12 ± 1.74 | 7.24 | 3.51 | 25.39 ± 0.48 | 1.87 | 1.54 |
| 100 | 103.19 ± 2.89 | 2.81 | 3.19 | 100.29 ± 1.50 | 1.49 | 0.29 |
| 1000 | 1009 ± 3.30 | 0.33 | 0.73 | 1000 ± 7.33 | 0.73 | 0.10 |
| Brain | ||||||
| 10 | 10.80 ± 0.45 | 4.21 | 7.99 | 10.50 ± 0.30 | 2.90 | 4.95 |
| 25 | 25.62 ± 0.98 | 3.85 | 2.48 | 25.75 ± 0.65 | 2.52 | 3.00 |
| 100 | 102.55 ± 2.78 | 2.71 | 2.55 | 100.73 ± 1.83 | 1.82 | 0.73 |
| 1000 | 1000 ± 0.85 | 0.08 | 0.08 | 1003 ± 4.88 | 0.49 | 0.32 |
Data are expressed as the mean ± SD (n = 5).
Table 2.
Stability of molnupiravir and NHC in rat blood and brain dialysates.
| Nominal concentration (ng/mL) | Autosampler stability (%) | Bench-top stability (%) | Freeze—thaw stability (%) | Long-term stability (%) |
|---|---|---|---|---|
| Molnupiravir | ± | |||
| Blood | ||||
| 5 | 98.57 ± 4.35 | 105.56 ± 1.73 | 105.23 ± 0.92 | 107.23 ± 0.74 |
| 250 | 100.31 ± 0.99 | 108.26 ± 3.29 | 103.92 ± 5.39 | 95.69 ± 0.04 |
| Brain | ||||
| 5 | 99.25 ± 0.36 | 100.99 ± 0.99 | 99.82 ± 1.63 | 99.641 ± 1.56 |
| 250 | 100.85 ± 0.70 | 103.61 ± 1.44 | 104.38 ± 1.35 | 99.17 ± 0.69 |
| NHC | ||||
| Blood | ||||
| 25 | 97.14 ± 1.02 | 105.16 ± 11.27 | 104.53 ± 8.98 | 102.09 ± 5.18 |
| 1000 | 99.52 ± 0.06 | 101.52 ± 1.26 | 101.02 ± 0.33 | 100.45 ± 0.54 |
| Brain | ||||
| 25 | 103.59 ± 5.26 | 103.03 ± 3.99 | 97.35 ± 5.21 | 112.26 ± 5.03 |
| 1000 | 102.58 ± 1.05 | 101.02 ± 0.81 | 101.49 ± 0.54 | 101.69 ± 0.49 |
Data are expressed as the mean ± SD (n = 3).
Table 3.
In vitro microdialysis recovery (%) of molnupiravir and NHC using ACD solution as the perfusate solution. Data are expressed as the mean ± SEM (n = 3).
| Concentration (ng/mL) | Recovery (%) in blood probe | Recovery (%) in brain probe |
|---|---|---|
| Molnupiravir | ||
| 100 | 12.92 ± 0.13 | 7.60 ± 0.13 |
| 250 | 12.74 ± 0.12 | 7.30 ± 0.07 |
| 500 | 12.69 ± 0.16 | 7.48 ± 0.34 |
| average | 12.78 ± 0.10 | 7.46 ± 0.12 |
| NHC | ||
| 500 | 11.91 ± 0.10 | 7.13 ± 0.12 |
| 1000 | 11.27 ± 0.07 | 7.23 ± 0.16 |
| 2000 | 11.33 ± 0.11 | 7.13 ± 0.05 |
| average | 11.51 ± 0.29 | 7.16 ± 0.05 |
The probe in vitro recovery was determined by comparing the concentration of input (Cin) and output (Cout) solution of the analyte according to the equation of recovery (%) = (Cout / Cin) × 100%.
Table 4.
The matrix effects of molnupiravir and NHC in male rat dialysates.
| Nominal concentration (ng/mL) | Blood | Brain |
|---|---|---|
| Molnupiravir | ||
| 5 | 97.01 ± 1.19 | 93.01 ± 5.15 |
| 250 | 95.71 ± 2.38 | 96.38 ± 1.25 |
| NHC | ||
| 25 | 55.35 ± 0.27 | 58.58 ± 0.07 |
| 1000 | 57.10 ± 1.86 | 60.18 ± 1.59 |
Data were calculated as the ratio (%) of the mean peak area of a dialysate spiked with the standard solution to the mean peak area of the standard solution prepared in ACD solution and are as expressed the mean ± SD (n = 3).
3.3. Method validation
The calibration curve of molnupiravir and NHC showed good linearity (r2) in the UHPLC—MS/MS analysis range, and both r2 values were higher than 0.995. The LLOQ was determined when the signal-to-noise ratio was more than 10. The LLOQs were 2.5 ng/mL and 10 ng/mL for molnupiravir and NHC, respectively. The precision (% RSD) and accuracy (% bias) ranged from − 0.41–8.76% and − 0.16–10.32% for molnupiravir and NHC, respectively (Table 1). All LLOQ values were within ± 20%, and those of the other concentrations were within ± 15%. These results indicate that our UHPLC—MS/MS analytical method was reproducible, repeatable, and reliable.
As shown in Table, the stability data of molnupiravir and NHC in rat blood and brain dialysates at low and high concentrations were assessed in triplicate for the following: bench-top stability, autosampler stability, freeze—thaw stability, and long-term stability. The experimental results demonstrated that the RSDs of molnupiravir and NHC were within ± 15%, and the results indicated that the analytes in blood and brain dialysates were stable under these conditions (Table 3).
The in vitro recoveries of molnupiravir and NHC were assessed using low, medium, and high concentrations of molnupiravir (100, 250, and 500 ng/mL) and NHC (500, 1000, and 2000 ng/mL). The recoveries of molnupiravir and NHC were 12.78 ± 0.10% and 11.51 ± 0.29% for blood probes and 7.46 ± 0.12% and 7.16 ± 0.05% for brain probes (n = 3). These results indicated that the recovery depends on the length of the semipermeable membrane and not on the ambient concentration ( Table 5). The analytical results were corrected using these recovery values by dividing by 0.12 and 0.07 for molnupiravir in blood and brain tissue, respectively, and 0.11 and 0.07 for NHC in blood and brain tissue, respectively.
Table 5.
Pharmacokinetic parameters of molnupiravir and NHC in the rat blood and brain after treatment with molnupiravir (100 mg/kg, i.v.) alone, molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.), or molnupiravir (100 mg/kg, i.v.) + the herbal extract Scutellaria formula-NRICM101 (1.27 g/kg, p.o.).
| Parameter | Molnupiravir (100 mg/kg, i.v.) |
|||
|---|---|---|---|---|
| Blood |
Brain |
|||
| Molnupiravir | NHC | Molnupiravir | NHC | |
| AUC (min μg/mL) | 3432 ± 397.1b | 8025 ± 652.7 | 19.07 ± 3.62b | 64.68 ± 6.90 |
| Cmax (μg/mL) | 191.5 ± 24.11 | 153.9 ± 8.15b | 0.39 ± 0.10 | 0.65 ± 0.03 |
| t1/2 (min) | 9 ± 2b | 88 ± 14 | 10 ± 1b | 84 ± 21c |
| Tmax (min) | - | 40 | 40 | 43 ± 7 |
| CL (mL/min/kg) | 32.07 ± 4.45 | - | - | - |
| MRT (min) | 18 ± 2b | 66 ± 1b | 35 ± 1b | 102 ± 14c |
| AUCNHC/AUCmolnupiravir | - | 2.33 ± 0.19b | - | 4.77 ± 0.87 |
| AUCbrain/AUCblood | - | - | 0.005 ± 0.001b | 0.008 ± 0.0003 |
| Molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.) | ||||
| AUC (min μg/mL) | 6578 ± 667.6a | 3476 ± 157.9a | 107.43 ± 23.45a | 195.2 ± 47.67a |
| Cmax (μg/mL) | 199.4 ± 16.95 | 39.84 ± 4.0a | 0.79 ± 0.22 | 1.28 ± 0.34 |
| t1/2 (min) | 132 ± 32a | 104 ± 14 | 148 ± 34a | 105 ± 14c |
| Tmax (min) | - | 40 | 40 | 40 |
| CL (mL/min/kg) | 15.96 ± 1.28 | - | - | - |
| MRT (min) | 77 ± 16a | 89.65 ± 3.19 | 238 ± 56 | 176 ± 24c |
| AUCNHC/AUCmolnupiravir | - | 0.53 ± 0.02a | - | 1.82 ± 0.44 |
| AUCbrain/AUCblood | - | - | 0.02 ± 0.004a | 0.06 ± 0.01a |
| AUCmolnupiravir+ BNPP/AUCmolnupiravir | 1.92 ± 0.19 | 0.49 ± 0.04 | 5.63 ± 1.23 | 3.02 ± 0.74 |
| Molnupiravir (100 mg/kg, i.v.) + the Scutellaria formula-NRICM101 (1.27 g/kg, p.o.) | ||||
| AUC (min μg/mL) | 4645 ± 151.7b | 3776 ± 246.4a | 45.27 ± 11.92 | 232.2 ± 16.68a |
| Cmax (μg/mL) | 184.7 ± 18.66 | 71.24 ± 2.98a,b | 0.50 ± 0.09 | 1.13 ± 0.33 |
| t1/2 (min) | 35 ± 10b | 189 ± 45 | 68 ± 22 | 386 ± 57a,b |
| Tmax (min) | - | 40 | 40 | 50 ± 4 |
| CL (mL/min/kg) | 21.66 ± 0.66 | - | - | - |
| MRT (min) | 29 ± 7b | 73 ± 3b | 53 ± 15b | 445 ± 64b |
| AUCNHC/AUCmolnupiravir | - | 0.83 ± 0.06a | - | 5.13 ± 0.37 |
| AUCbrain/AUCblood | - | - | 0.01 ± 0.003 | 0.06 ± 0.004a |
| AUCmolnupiravir+ Herbs/AUCmolnupiravir | 1.35 ± 0.04 | 0.47 ± 0.03 | 2.37 ± 0.63 | 3.59 ± 0.26 |
Abbreviations: AUC, area under curve the concentration-time curve; t1/2, half-life; Cmax, concentration maximum; CL, clearance; MRT, mean residence time; Herbs, the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o., for five consecutive days).
AUCbrain/AUCblood represents the rat blood-to-brain transfer ratio. Data have been expressed as the mean ± SEM (n = 6).
ap < 0.05 compared with the molnupiravir (100 mg/kg, i.v.) alone group by one-way ANOVA with post hoc Tukey HSD test.
bp < 0.05 compared with the molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.) group by one-way ANOVA with post hoc Tukey HSD test.
cp < 0.05 compared with the molnupiravir (100 mg/kg, i.v.) + the herbal extract Scutellaria formula-NRICM101 (1.27 g/kg, p.o.) group by one-way ANOVA with post hoc Tukey HSD test.
The average matrix effect values for molnupiravir in blood and brain dialysate ranged from 95.71% to 97.01% and 93.01–96.38%, respectively, and NHC ranged from 54.20% to 57.10% and 57.03–60.18%, respectively (Table 4). The matrix effect data showed the effect of interfering ions on the analytical target signal. This result revealed that a matrix effect was found for NHC, which was collected for the concentration calculation, and molnupiravir was not significantly affected by the matrix within the analytical range.
3.4. Pharmacokinetics of molnupiravir and NHC
Pharmacokinetic analysis of the blood samples revealed a rapid biotransformation of molnupiravir metabolism to NHC within 20 min. NHC was detected in the brain with Cmax at 40 min after molnupiravir administration (100 mg/kg, i.v.) ( Fig. 4 A and 4B). Consistent with a fast elimination and rapid metabolism, the elimination half-life (t1/2) of molnupiravir was 9 ± 2 min in blood. The elimination t1/2 of NHC in the blood and brain was 88 ± 14 and 84 ± 21 min, respectively (Table 5). The area under the concentration curve (AUC) of molnupiravir and NHC in the blood was 3432 ± 397.1 and 8025 ± 652.7 min μg/mL, respectively. The AUCs of molnupiravir and NHC in the brain were 19.07 ± 3.62 and 64.68 ± 6.90 min μg/mL, respectively. The biotransformation ratio of molnupiravir to NHC (AUCNHC/AUCmolnupiravir) in the blood and brain was 2.33 ± 0.19 and 4.77 ± 0.87, suggesting a high biotransformation ratio in the blood and brain, respectively (Table 5). The brain distribution ratio (AUCbrain/AUCblood) of molnupiravir and NHC transfer into the brain was 0.005 ± 0.001 and 0.008 ± 0.0003, respectively, suggesting that less than 1% of molnupiravir and NHC crossed into the brain (Table 5) (Fig. 4 A and 4B).
Fig. 4.
Protein unbound molnupiravir and NHC in rat blood and brain. Concentration-time curves of molnupiravir and β-D-N4-hydroxycytidine (NHC) in rat blood (A) and brain (B) after molnupiravir administration (100 mg/kg, i.v.) alone, administration of molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v. and administered molnupiravir (100 mg/kg, i.v.) + the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o., for five consecutive days). Data are expressed as the mean ± SEM (n = 6). The symbols • and ○ represent the concentrations of molnupiravir and HNC, respectively, in administered molnupiravir (100 mg/kg, i.v.) alone. The symbols ▼ and △ represent the concentrations of molnupiravir and HNC, respectively, in concomitantly administered molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.); the symbols □ and ■ represent the concentrations of molnupiravir and NHC, respectively, in administered molnupiravir (100 mg/kg, i.v.) + the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o., for five consecutive days). BNPP, bis(4-nitrophenyl) phosphate; a specific inhibitor of carboxylesterase; MPV, molnupiravir; herbs, the herbal extract Scutellaria formula-NRICM101. The IC50 of NHC in vitro is reported to range from 0.08 μM for SARS-CoV-2 in Calu-3 cells to 0.3 μM for SARS-CoV-2 in Vero cells (equivalent to 20.8–78 ng/mL) [5].
3.5. Biotransformation mechanism of molnupiravir
Carboxylesterase is an important enzyme that metabolizes molnupiravir to NHC [13]. To verify the biotransformation mechanism of molnupiravir, a carboxylesterase inhibitor, bis(4-nitrophenyl)phosphate (BNPP), was used. The pharmacokinetic results showed that the AUCs of molnupiravir and NHC in blood were 6578 ± 667.6 and 3476 ± 157.9 min μg/mL, respectively, and the AUCs in brain were 107.43 ± 23.45 and 195.2 ± 47.67 min μg/mL, respectively, after administration of molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.). The biotransformation ratio (AUCNHC/AUCmolnupiravir) of molnupiravir metabolism to NHC in the blood and brain was 0.53 ± 0.02 and 1.82 ± 0.44, respectively (Table 5). The brain distribution ratio (AUCbrain/AUCblood) of molnupiravir and NHC was 0.02 ± 0.004 and 0.06 ± 0.01, respectively, after administration of molnupiravir (100 mg/kg, i.v.) + BNPP (50 mg/kg, i.v.). Compared to that of the group administered molnupiravir (100 mg/kg, i.v.) alone, the AUC ratio (AUCmolnupiravir+BNPP/AUCmolnupiravir) in the blood was 1.92 ± 0.19 and 0.49 ± 0.04 for molnupiravir and NHC, respectively, suggesting that carboxylesterase was effectively inhibited by BNPP. The AUC ratio (AUCmolnupiravir+BNPP/AUCmolnupiravir) in the brain was 5.63 ± 1.23 and 3.02 ± 0.74 for molnupiravir and NHC, respectively (Table 5) (Fig. 4A and 4B).
3.6. Herbal drug interaction of molnupiravir and Scutellaria extract
To investigate the herb-drug interaction of molnupiravir and NHC, the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o.) was provided for five consecutive days. On the fifth day, the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o.) was administered one hour before molnupiravir administration (100 mg/kg, i.v.). The pharmacokinetic results showed that the AUCs of molnupiravir and NHC in the blood were 4645 ± 151.7 and 3776 ± 264.4 min μg/mL, respectively, and the AUCs in the brain were 45.27 ± 11.92 and 232.2 ± 16.68 min μg/mL, respectively. The Cmax of the NHC in the blood was 71.24 ± 2.98 μg/mL, and the Cmax of molnupiravir and the NHC in the brain was 45.27 ± 11.92 and 232.2 ± 16.68 μg/mL, respectively. The biotransformation ratio (AUCNHC/AUCmolnupiravir) of molnupiravir that metabolizes to NHC in blood and brain was 0.83 ± 0.06 and 5.13 ± 0.37, respectively (Table 5). The brain distribution ratio (AUCbrain/AUCblood) of molnupiravir and NHC was 0.01 ± 0.003 and 0.06 ± 0.004, respectively, after administration of molnupiravir (100 mg/kg, i.v.) + the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o., for five consecutive days). Compared to molnupiravir (100 mg/kg, i.v.) administration alone, the AUC ratio (AUCmolnupiravir+Herb/AUCmolnupiravir) in the blood was 1.35 ± 0.04 and 0.47 ± 0.03 for molnupiravir and NHC, respectively, suggesting that NHC was blocked by the herbal extract. However, the AUC ratio (AUCmolnupiravir+ Herb/AUCmolnupiravir) in the brain was 2.37 ± 0.63 and 3.59 ± 0.26 for molnupiravir and NHC, respectively (Table 5) (Fig. 4 A and 4B).
4. Discussion
In this study, we demonstrated that the concentration of molnupiravir declines rapidly in blood, consistent with a previous report in which molnupiravir was rapidly hydrolyzed to NHC by carboxylesterases [13]. Here, intravenous administration was used to avoid problems caused by the first-pass effect after oral administration and focus on the main topic, which was to study the herb-drug interaction. The dose of this experiment molnupiravir (100 mg/kg, i.v.) was based on the single clinical dose of molnupiravir (800 mg, p.o.), multiplied by the bioavailability [4], [17] and the transfer constant [18].
Our pharmacokinetic analysis revealed that the active metabolite NHC reaches a maximum concentration of 153.9 ± 8.15 μg/mL at Tmax 40 min in the blood after molnupiravir administration (100 mg/kg, i.v.) (Table 5). Both molnupiravir and NHC rapidly cross the blood—brain barrier (BBB) and reach Cmax values of 0.39 ± 0.10 and 0.65 ± 0.03 μg/mL at 40 min, respectively (Table 5). A previous report showed that the average median inhibitory concentration of NHC antivirals (IC50) is 0.30 μM (equivalent to 0.078 μg/mL) for SARS-CoV-2 in Vero cells [5]. Our data showed that NHC levels were above the effective concentration of IC50 0.3 μM (equivalent to 78 ng/mL) for 6 h in the blood (Fig. 4A), which represented an effective therapeutic dose regimen. The Cmax of molnupiravir and NHC was 0.39 ± 0.10 and 0.65 ± 0.03 μg/mL, respectively. The NHC concentration slowly decreased and was lower than 0.3 μM (equivalent to 78 ng/mL) after approximately 3 hr in the brain (Fig. 4B).
To investigate the metabolic pathway of molnupiravir, a carboxylesterase inhibitor, BNPP, was administered concomitantly with molnupiravir (100 mg/kg, i.v.) and BNPP (50 mg/kg, i.v.). Compared to the AUC of molnupiravir (100 mg/kg, i.v.) alone and the concomitant administration of molnupiravir (100 mg/kg, i.v.) and BNPP (50 mg/kg, i.v.), the blood concentration increased from 3432 ± 397.1–6578 ± 667.6 min μg/mL. In contrast, the AUC of NHC in the blood decreased from 8025 ± 652.7–3476 ± 157.9 min. The biotransformation ratio (represented by the AUC ratio of AUCNHC/AUCmolnupiravir) decreased significantly from 2.47 ± 0.24–0.55 ± 0.05 in the blood (Table 5), suggesting that carboxylesterase was inhibited by BNPP, consistent with previous reports [19], [20], [21].
Our data demonstrated that both molnupiravir and NHC were detectable in the striatum with low penetration ratios (AUCbrain/AUCblood) of 0.005 ± 0.001 and 0.008 ± 0.0003, respectively (Table 5). However, the AUC level of NHC (64.68 ± 6.90 min μg/mL) was higher than that of molnupiravir (19.07 ± 3.62 min μg/mL) (Table 5), which may result from the different penetration capacities of molnupiravir and NHC into the BBB. A low level of carboxylesterase is present in the brain [22], [23], but it did not significantly affect the biotransformation of molnupiravir to NHC. In addition, no in vivo studies support the possibility that BNPP can cross the BBB into the brain to block carboxylesterase activity. The penetration capacity of molnupiravir to NHC may be due to passive diffusion. However, a previous report showed that slight enhancement in the drug BBB penetration by BNPP may be due to plasma butyrylcholinesterase and carboxylesterase inhibition [24].
Due to the complex herbal ingredients and the multiple pharmacological targets of the herbal formula, the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o.) was administered five consecutive days before molnupiravir was administered (100 mg/kg, i.v.) to investigate the pharmacokinetic interaction of herbal drugs. After concomitant administration of molnupiravir and the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o.), the AUC of molnupiravir increased from 3432 ± 397.1–4645 ± 151.7 min μg/mL, and the AUC of NHC decreased from 8025 ± 652.7–3776 ± 246.4 min μg/mL. Additionally, the Cmax of NHC also decreased from 153.9 ± 8.15–71.24 ± 2.98 μg/mL (Table 5). The AUC ratio (AUCmolnupiravir+ Herbs/AUCmolnupiravir) was used to evaluate the efficacy of herbal medicine on the pharmacokinetics of molnupiravir. The results demonstrated that molnupiravir was suppressed and NHC was enhanced after concomitant administration of molnupiravir and the extract of the Scutellaria formula-NRICM101. These data suggested that the metabolic pathway of molnupiravir in the blood was inhibited by the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o.), which is similar to the group in which BNPP blocks carboxylesterase (Table 5). In a previous study, carboxylesterase was reported to be inhibited by the extract of Radix Scutellariae flavones [25]. Our data are consistent with previous reports, and the main herb of the Scutellaria formula-NRICM101 contains Radix Scutellariae, which provides a significant effect of inhibition of carboxylesterase. The enzymatic mechanism of the extract of the Scutellaria formula-NRICM101 also supported the group administered molnupiravir and BNPP (Table 5).
After pretreatment with the extract of the Scutellaria formula-NRICM101 (1.27 g/kg, p.o.), the AUC of molnupiravir and NHC in the brain increased from 19.07 ± 3.62–45.27 ± 11.92 min μg/mL and 64.68 ± 6.90–232.2 ± 16.68 min μg/mL, and the Cmax of molnupiravir and NHC increased from 0.39 ± 0.10–0.50 ± 0.09 μg/mL and 0.65 ± 0.03–1.13 ± 0.33 min μg/mL, respectively. The blood-to-brain AUC ratio (AUCbrain/AUCblood) of molnupiravir and NHC was increased from 0.005 ± 0.001 and 0.008 ± 0.0003–0.01 ± 0.003 and 0.06 ± 0.004, respectively, suggesting that the BBB penetration of molnupiravir and NHC was enhanced (Table 5). These data are supported by a previous report, which indicated that baicalin (a bioactive ingredient in the Scutellaria formula-NRICM101) increases BBB permeability, leading to a better therapeutic effect on cerebral ischemia—reperfusion injury in mice [26].
5. Conclusion
In conclusion, considering the dosage regimen and therapeutic antiviral effect, the half maximal inhibitory concentration (IC50) of NHC in the in vitro study ranges from 0.08 μM to 0.3 μM (equivalent to 20.8–78 ng/mL) in different cell lines, such as SARS-CoV-2 in Calu-3 cells and SARS-CoV-2 in Vero cells [5]. The blood level of NHC was above the IC50 (78 ng/mL), indicative of an effective therapeutic dosage regimen. Both molnupiravir and NHC penetrated the BBB with a low level of NHC in the brain, but the concentration of NHC was still higher than the IC50. Our findings show that Scutellaria extract (1.27 g/kg, p.o.) increased the level of molnupiravir and suppressed the level of NHC in the blood, but both molnupiravir and NHC were increased in the brain, which may be due to inhibition of carboxylesterase and improved permeability of the blood to the brain. However, whether combined with the extract Scutellaria formula-NRICM101 or not, the level of NHC was above the effective concentration of both blood and brain in the groups treated with molnupiravir alone and molnupiravir plus the extract Scutellaria formula-NRICM101.
CRediT authorship contribution statement
Chun-Hao Chang: Methodology, Investigation, Writing – original draft. Wen-Ya Peng: Investigation, Visualization. Wan-Hsin Lee: Methodology, Investigation. Tung-Yi Lin: Investigation, Visualization, Funding acquisition. Muh-Hwa Yang: Funding acquisition, Project administration. Jeffrey W. Dalley: Writing – review & editing. Tung-Hu Tsai: Funding acquisition, Project administration, Supervision, Writing – original draft. All authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
This study was conducted as part of the PhD dissertation of Chun-Hao Chang. This study was supported in part by research grants from the National Science and Technology Council Taiwan (NSTC111-2113-M-A49-018 and NSTC111-2321-B-A49-007) and a graduate student scholarship from the College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
References
- 1.Gordon C.J., Tchesnokov E.P., Schinazi R.F., Götte M. Molnupiravir promotes SARS-CoV-2 mutagenesis via the RNA template. J. Biol. Chem. 2021;297(1) doi: 10.1016/j.jbc.2021.100770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toots M., Yoon J.J., Cox R.M., Hart M., Sticher Z.M., Makhsous N., Plesker R., Barrena A.H., Reddy P.G., Mitchell D.G. Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia. Sci. Transl. Med. 2019;11(515):eaax5866. doi: 10.1126/scitranslmed.aax5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.FitzGerald R., Dickinson L., Else L., Fletcher T., Hale C., Amara A., Walker L., Penchala S.D., Lyon R., Shaw V., Greenhalf W., Bullock K., Lavelle-Langham L., Reynolds H., Painter W., Holman W., Ewings S., Griffiths G., Khoo S. Pharmacokinetics of ß-d-N4-Hydroxycytidine, the parent nucleoside of prodrug molnupiravir, in nonplasma compartments of patients with severe acute respiratory syndrome coronavirus 2 Infection. Clin. Infect. Dis. 2022;ciac199:e525–e528. doi: 10.1093/cid/ciac199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.USFDA, Molnupiravir, Oral Treatment of COVID-19, EUA #000108 (2021).
- 5.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., III, Stevens L.J. An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci. Transl. Med. 2020;12(541):eabb5883. doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai K.C., Huang Y.C., Liaw C.C., Tsai C.I., Chiou C.T., Lin C.J., Wei W.C., Lin S.J., Tseng Y.H., Yeh K.M., Lin Y.L., Jan J.T., Liang J.J., Liao C.C., Chiou W.F., Kuo Y.H., Lee S.M., Lee M.Y., Su Y.C. A traditional Chinese medicine formula NRICM101 to target COVID-19 through multiple pathways: a bedside-to-bench study. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.111037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y.D., Lu C.C., Hsu Y.M., Tsai F.J., Bau D.T., Tsai S.C., Cheng C.C., Lin J.J., Huang Y.Y., Juan Y.N., Chiu Y.J., Kuo S.C., Yang J.S., Wu L.T. In Silico and In Vitro studies of taiwan chingguan yihau (NRICM101) on TNF-alpha/IL-1beta-induced human lung cells. Biomed. (Taipei) 2022;12(3):56–71. doi: 10.37796/2211-8039.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei W.C., Liaw C.C., Tsai K.C., Chiou C.T., Tseng Y.H., Chiou W.F., Lin Y.C., Tsai C.I., Lin C.S. Targeting spike protein-induced TLR/NET axis by COVID-19 therapeutic NRICM102 ameliorates pulmonary embolism and fibrosis. Pharmacol. Res. 2022;184 doi: 10.1016/j.phrs.2022.106424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tseng Y.H., Lin S.J., Hou S.M., Wang C.H., Cheng S.P., Tseng K.Y., Lee M.Y., Lee S.M., Huang Y.C., Lin C.J., Lin C.K., Tsai T.L., Lin C.S., Cheng M.H., Fong T.S., Tsai C.I., Lu Y.W., Lin J.C., Huang Y.W., Hsu W.C., Kuo H.H., Wang L.H., Liaw C.C., Wei W.C., Tsai K.C., Shen Y.C., Chiou W.F., Lin J.G., Su Y.C. Curbing COVID-19 progression and mortality with traditional Chinese medicine among hospitalized patients with COVID-19: a propensity score-matched analysis. Pharmacol. Res. 2022;184 doi: 10.1016/j.phrs.2022.106412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song J., Zhang L., Xu Y., Yang D., Yang S., Zhang W., Wang J., Tian S., Yang S., Yuan T. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem. Pharmacol. 2021;183 doi: 10.1016/j.bcp.2020.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zandi K., Musall K., Oo A., Cao D., Liang B., Hassandarvish P., Lan S., Slack R.L., Kirby K.A., Bassit L., Amblard F., Kim B., Abubakar S., Sarafianos S.G., Schinazi R.F. Baicalein and baicalin inhibit SARS-CoV-2 RNA-dependent-RNA polymerase. Microorganisms. 2021;9(5):893. doi: 10.3390/microorganisms9050893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosokawa M. Structure and catalytic properties of carboxylesterase isozymes involved in metabolic activation of prodrugs. Molecules. 2008;13(2):412–431. doi: 10.3390/molecules13020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Medicines Agency, Issues Advice on Use of Lagevrio (Molnupiravir) for the Treatment of COVID-19., 2022.
- 14.Tsai T.H., Lee C.Y., Yeh P.H. Effect of P‐glycoprotein modulators on the pharmacokinetics of camptothecin using microdialysis. Br. J. Pharm. 2001;134(6):1245–1252. doi: 10.1038/sj.bjp.0704363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsai T.H. Pharmacokinetics of pefloxacin and its interaction with cyclosporin A, a P‐glycoprotein modulator, in rat blood, brain and bile, using simultaneous microdialysis. Br. J. Pharm. 2001;132(6):1310–1316. doi: 10.1038/sj.bjp.0703927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paxinos G., Watsons C. Academic Press; 1982. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 17.Painter W.P., Holman W., Bush J.A., Almazedi F., Malik H., Eraut N.C.J.E., Morin M.J., Szewczyk L.J., Painter G.R. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021;65(5) doi: 10.1128/AAC.02428-20. e02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reagan‐Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 19.Qian X.K., Zhang J., Song P.F., Zhao Y.S., Ma H.Y., Jin Q., Wang D.D., Guan X.Q., Li S.Y., Bao X. Discovery of pyrazolones as novel carboxylesterase 2 inhibitors that potently inhibit the adipogenesis in cells. Bioorg. Med. Chem. 2021;40 doi: 10.1016/j.bmc.2021.116187. [DOI] [PubMed] [Google Scholar]
- 20.Eng H., Niosi M., McDonald T., Wolford A., Chen Y., Simila S., Bauman J., Warmus J., Kalgutkar A. Utility of the carboxylesterase inhibitor bis-para-nitrophenylphosphate (BNPP) in the plasma unbound fraction determination for a hydrolytically unstable amide derivative and agonist of the TGR5 receptor. Xenobiotica. 2010;40(6):369–380. doi: 10.3109/00498251003706598. [DOI] [PubMed] [Google Scholar]
- 21.Heymann E., Krisch K., Büch H., Buzello W. Inhibition of phenacetin-and acetanilide-induced methemoglobinemia in the rat by the carboxylesterase inhibitor bis-[p-nitrophenyl] phosphate. Biochem. Pharmacol. 1969;18(4):801–811. doi: 10.1016/0006-2952(69)90050-1. [DOI] [PubMed] [Google Scholar]
- 22.Taketani M., Shii M., Ohura K., Ninomiya S., Imai T. Carboxylesterase in the liver and small intestine of experimental animals and human. Life Sci. 2007;81(11):924–932. doi: 10.1016/j.lfs.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Satoh T., Taylor P., Bosron W.F., Sanghani S.P., Hosokawa M., La Du B.N. Current progress on esterases: from molecular structure to function. Drug Metab. Dispos. 2002;30(5):488–493. doi: 10.1124/dmd.30.5.488. [DOI] [PubMed] [Google Scholar]
- 24.Khan D., Gilmer J., Carolan C., Gaynor J., Ryder S. Pharmacological effects of a novel isosorbide-based butyrylcholinesterase inhibitor. Chem. Biol. Interact. 2008;175(1–3):231–234. doi: 10.1016/j.cbi.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J., Xiao M., Ji X., Lai Y.S., Song Q., Zhang Y., Ip C.M., Ng W.L., Zuo Z. Inhibition of Radix Scutellariae flavones on carboxylesterase mediated activations of prodrugs. Life Sci. 2022;305 doi: 10.1016/j.lfs.2022.120743. [DOI] [PubMed] [Google Scholar]
- 26.Long Y., Liu S., Wan J., Zhang Y., Li D., Yu S., Shi A., Li N., He F. Brain targeted borneol-baicalin liposome improves blood-brain barrier integrity after cerebral ischemia-reperfusion injury via inhibiting HIF-1α/VEGF/eNOS/NO signal pathway. Biomed. Pharmacother. 2023;160 doi: 10.1016/j.biopha.2023.114240. [DOI] [PubMed] [Google Scholar]