Abstract
Background
In superficial tumors of the breast, it is necessary to plan the thickness of surgical skin flaps, and whether skin can be preserved for esthetics results. This study aimed to find an ultrasound-measured cut-off distance between tumor and skin (TSD) that allows patients to have the skin over the tumor spared.
Methods
This is a diagnostic accuracy study comparing preoperative ultrasound TSD with pathological TSD and the thickness of the skin flaps. We recruited all consecutive women diagnosed with breast cancer between January 2017 and December 2019 whose surgical planning allowed to have the tumor and overlying skin to be removed in bloc (reconstruction procedures, situations where skin removal would not lead to esthetic problems, and superficially located tumors). Measurements were made: preoperatively (by ultrasound), during surgery (using a metal caliper to obtain the thickness of surgical skin flap), and after surgery (pathological). A pathological tumor-skin distance greater than surgical skin flap thickness would indicate preservation of skin above the tumor.
Results
We evaluated 95 consecutive patients with 102 lesions. The average surgical flap thickness was 5.5 mm (3–10 mm). The ultrasound-measured cut-off TSD of 2.1 mm obtained 96.0% accuracy in predicting free anterior margin, considering a 5-mm-thick surgical flap.
Conclusion
In breast superficial tumors, a cut-off distance of 2.1 mm or more measured preoperatively by ultrasound allows safe preservation of the skin above the tumor. Future studies need to follow up for longer the women submitted to skin preservation surgeries, especially those not undergoing radiotherapy.
Keywords: Breast cancer, Resection margins, Breast reconstruction, Skin preservation, Skin sparing
Introduction
Skin involvement occurs in 4.4–11.3% of breast cancer cases [1, 2]. However, superficial tumors can extend to the dermis without macroscopic evidence of skin involvement [3]. Although the first publications on skin-sparing mastectomies (MPP) describe the removal of the tumor together with the overlying skin, currently studies suggest incisions distant from the lesion, with preservation of the entire cutaneous envelope [4, 5].
However, the skin just above the tumor may contain residual cancer tissue, especially in palpable and superficial tumors, thereby increasing the risk of local recurrence if not resected [6]. Thus, it is necessary to carefully assess the relationship between the tumor and the skin to properly plan the surgical incision, in a safe and cosmetic manner at the same time. Figure 1 shows the distances to consider when assessing the possibility of skin preservation in breast cancer surgery: the tumor-skin distance, the surgical skin flap, and skin (epidermis and dermis) thickness.
Fig. 1.
Schematic illustration of the breast including the tumor (round dotted shape) and the skin. A = Distance between the tumor and the epidermis. B = Surgical flap thickness. C = Skin thickness including dermis and epidermis.
Ultrasound scans allow the evaluation of the tumoral size and location, and its relationship with adjacent structures such as the skin, the muscles, and axilla. However, because the patient is usually lying down with the arms extended behind the head during the exam, the breast may be flattened, and the distance measured by ultrasound may be different than the same measurement on a surgical or pathological specimen. A previous study has shown that the distance between the tumor and the skin by pathology could be 3.1 times that of ultrasound, i.e., 0.5 cm measured by ultrasound would be equivalent of 1.5 cm in a pathological specimen [7]. However, no cut-off has ever been determined to guide the surgeons. This study aimed to determine a useful ultrasound measurement cut-off for the distance between tumor and skin that could allow patients to have the skin over the tumor preserved.
Methods
Study Design, Ethics, and Reporting
This is a diagnostic accuracy study, in which we explored a cut-off value for the distance between the breast tumor and the anterior skin, measured by ultrasound preoperatively, that would indicate safety for skin preservation above the tumor. A safe ultrasound measurement was investigated through the correlation with the same distance in pathology. The ultrasound exam comes with a flattening effect on measurements that needs to be robustly determined. The pathological tumor-skin distance must, therefore, be greater than the thickness of the surgical skin flap to result in free anterior margin.
This project was approved by Universidade Federal de São Paulo (UNIFESP) Ethics Committee (CAAE: 70471817.9.0000.5505), and patients signed informed consent forms for this study. This report is in accordance with the recommendations of the STARD 2015 (Standards for Reporting Diagnostic Accuracy Studies) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statements when appropriate [8, 9].
Participants and Settings
Initially, we prospectively recruited for this study all patients consecutively admitted in two institutions for breast cancer evaluation and with an indication for surgery between January 2017 and December 2019 (a convenience series). The first institution is a private clinic specialized in breast diseases (mastology) in Jundiaí, a city with around 400,000 inhabitants. The clinic receives patients with access to private health insurance. The other institution is a large university hospital (http://www.hospitalsaopaulo.org.br) serving the Brazilian public health system (Sistema Único de Saúde [SUS]) in São Paulo city (more than 12 million inhabitants).
The decision on inclusion or exclusion of participants was made during surgical planning and involved the healthcare team and the patients. The participants of this study would be those whose surgical planning was to have the tumor and the anterior skin removed altogether in reconstruction procedures or in situations where the location of the tumor allowed skin removal without significant aesthetic problems (unaesthetic scar, for example, or asymmetry between breasts). We excluded patients who would not have the tumor removed surgically together with the adjacent anterior skin (either because this would not be needed or for aesthetical reasons), patients with carcinoma in situ and with non-nodular lesions (such as microcalcifications and architectural distortions).
Test Methods
All patients underwent ultrasonography with a linear 12 MHz transducer (Logic 6, GE Medical Systems, Milwaukee, WI, USA). The same equipment was used in the whole cohort. One specialized physician (the first author) did all evaluations for the two institutions. The evaluator obtained tumor-skin distances with the transducer aligned to the largest axis of the lesion, after the scan included the longitudinal, transverse, radial, and anti-radial planes, using the least breast manual compression possible (none or mild). The distance between the most anterior hypoechoic border of the tumor and the epidermis was obtained. This distance is reported here as TSD-USG (tumor-skin distance by ultrasound), and it is expressed in millimeters.
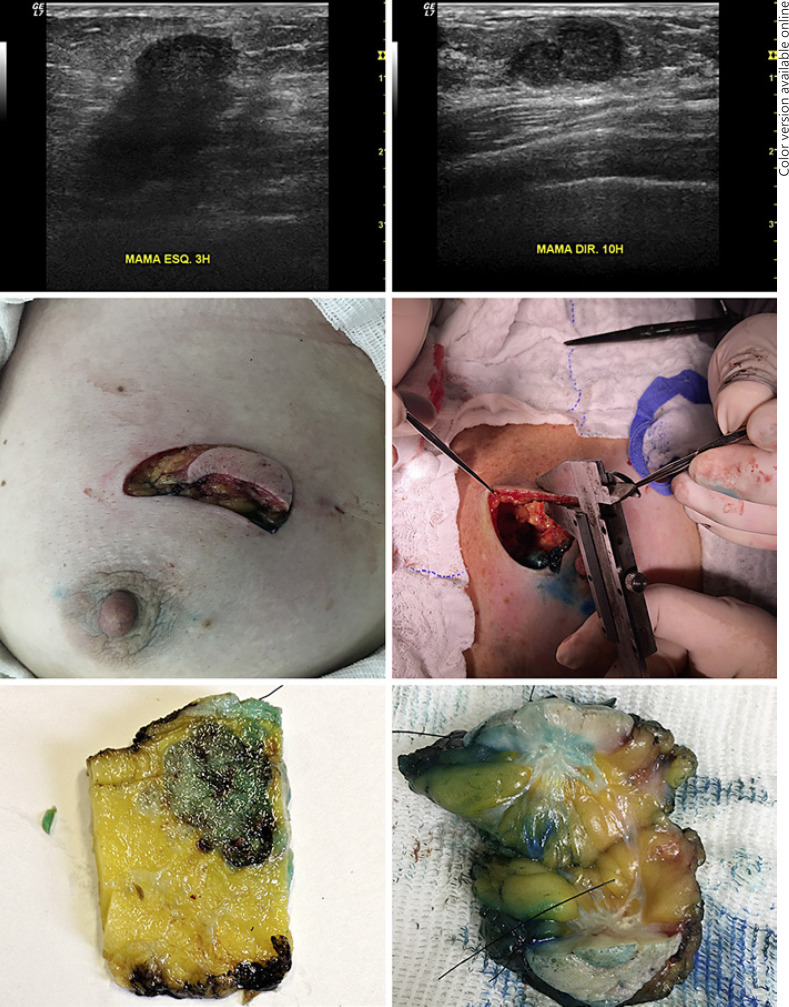
The same surgeon (also the first author), with more than 10 years of experience, performed the operations for the whole cohort. He removed the tumor in block, together with adjacent skin, and produced skin flaps surrounding the tumor as usually performed in skin-sparing procedures. The material used to obtain the thickness of surgical skin flap was a 150-mm analog universal caliper (6" Digimess 100.001). Three metallic calipers were purchased for this study and sent to the Brazilian National Institute of Metrology, Quality, and Technology, which confirmed the accuracy of the instruments. The auxiliary surgeon prepared the skin flap with lifting forceps, and the main surgeon (author) obtained the measurement (skin plus subcutaneous fat) with the caliper. Figure 2 shows the measurement technique.
Fig. 2.
Preoperative ultrasound measurements of the distance between the breast tumor and the skin. Intraoperative photo of the tumor, together with skin, and the measurement of the surgical flap with the caliper. Postoperative photo of the surgical specimen with the pathological measurement of the distance between the tumor and the skin.
The surgical specimen was sent to pathological evaluation preserved in 10% formalin for at least 24 h. Three laboratories were involved in this study, two working for the private clinic and one in the university hospital. The pathologists were informed about the study objectives but were blind for the intraoperative measurements. They were asked to provide the distance between the most anterior border of the tumor and the epidermis (TSD, now called TSD-PAT), the skin thickness (epidermis and dermis until the irregular dermal-fat junction), and information on the involvement of the skin by neoplasia (carcinoma reaching the reticular dermis). They obtained the measurements using a millimetric ruler for distances above 10 mm, and a microscopic ruler coupled to the microscopic equipment for those below that value. This full-thickness vertical tissue was submitted in one to two cassettes and processed with the routine pathology cases. On microscopic examination, an eyepiece micrometer was used to obtain precise measurements. We calculated the rate between the pathology measurement (TSD-PAT) and the ultrasonographic measurement (TSD-USG).
Statistical Analysis
Aiming ultimately to determine a cut-off ultrasound measurement of the distance between tumor and skin that could allow patients to have the skin over the tumor preserved, we considered the pathological TSD above 5 mm (equivalent to the average thickness of a skin flap) as the ground truth and calculated sensitivity, specificity, and area under the receiver operator characteristic (ROC) curve of the ultrasound exam. We used logistic regression considering as a dependent variable the possibility of skin preservation and as predictors the classification by the ultrasound and other patients' characteristics.
We verified the data distribution using Kolmogorov-Smirnov test. Linear associations between two continuous variables were examined using the Pearson or Spearman coefficients. Initially, we built the model using all possible predictors of the risk of skin preservation need. However, due to the large number of predictors, considering the sample size, the initial, only those presenting p values of 0.10 were selected. Then, the variables with no significant associations (at 0.05) were excluded one by one per significancy (backward method). After this adjustment, we conducted the diagnostic analysis of the remaining items, using the standardized Student's t test and Cook D distance.
We considered as significant values of less than 0.05 in this study. We used STATA12 and SPSS 20.0 software in the analyses.
Results
In the study period, 56 patients in one institution and 39 patients in the other were considered eligible for this study. The age of these 95 patients was 62.5 years in average (varying from 35 to 92 years).
Mastectomy was carried out in 11 patients (9.8%), and 3 patients (2.9%) received neoadjuvant therapy. In 12 patients, there were signs of skin involvement, and in 10 of these the skin was confirmed as affected in the pathological analysis. General patients' characteristics are shown in Table 1.
Table 1.
Characteristics of patients with breast cancer included in the study
| Patients' characteristics (N = 95) | |
| Age (N = 95), years | |
| Mean±SD | 62.5±14.5 |
| Median (minimum-maximum) | 62.0 (35.0–92.0) |
| Age category (N = 95) | |
| Up to 40 years old | 5 (5.3) |
| From 41 to 60 years old | 36 (37.9) |
| 61 years old or older | 54 (56.8) |
| Race/ethnicity (n = 56)* | |
| White | 43 (76.7%) |
| Black | 10 (17.8%) |
| Mixed | 2 (3.5%) |
| Asian | 1 (1.7%) |
|
| |
| Tumor characteristics (N = 102) | |
| Neoadjuvant treatment, N (%) | |
| No | 99 (97.1) |
| Yes | 3 (2.9) |
| Staging, N (%) | |
| T1 | 68 (66.7) |
| T2 | 23 (22.5) |
| T3 | 11 (10.8) |
| Lymph nodes (N) | |
| Positive | 19 (18.6) |
| Negative | 83 (81.4) |
| Type of surgery, N (%) | |
| Conservative | 91 (89.2) |
| Mastectomy | 11 (10.8) |
| Skin affected, N (%) | |
| No | 92 (90.2) |
| Yes | 10 (9.8) |
| Clinical signs that skin is affected, N (%) | |
| No | 90 (88.2) |
| Yes | 12 (11.8) |
| Final tumor size, cm | |
| Mean ± SD | 1.85±1.26 |
| Median (minimum-maximum) | 1.50 (0.5–8.0) |
SD, standard deviation.
Information on race was not available for all patients.
The measurement made by the pathologists (TSD-PAT) was always larger than the ultrasound value (TSD-USG). The average rate between TSD-PAT and TSD-USG was 2.64. When we excluded patients with affected skin, this rate was 2.88 (±1.26). The skin was 3.57 mm thick in average (1.6–8.0 mm), and the skin flap was 5.55 mm (3–10 mm). In 27 (44.3%) patients, the value was greater than 5 mm, and in 34 (55.7%), it was lower.
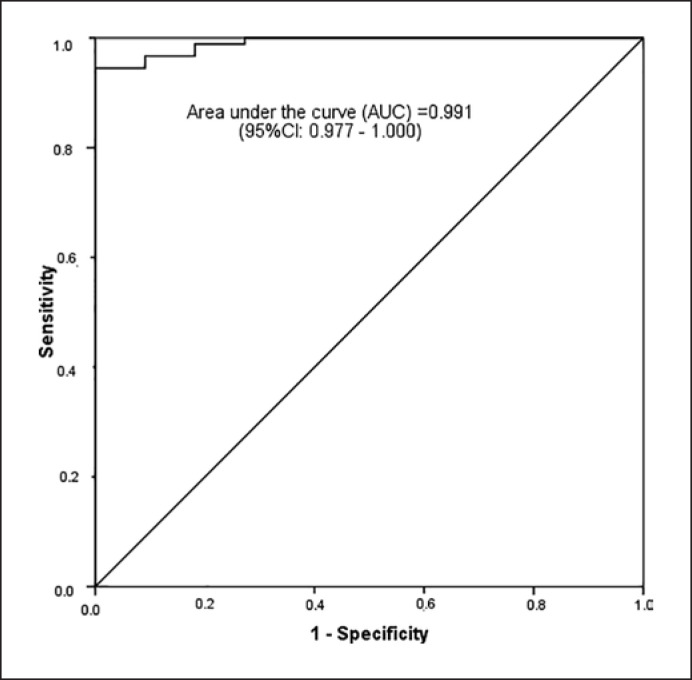
The ROC curve (Fig. 3) has shown that the cut-off point of 2.1 mm in the ultrasound measurement is associated with a sensitivity of 96.7% and specificity of 90.9% of a case with skin preservation possibility (Table 2). The positive predictive value was 98.9%. This is the same as saying that, by classifying patients as having measurements equal to or above 2.1 mm in the ultrasound as those that will probably present a measurement equal to or above 5 mm in the pathological exam, 98.9% of them will be correctly classified.
Fig. 3.
Receiver operator characteristic (ROC) curve for a distance of 5 mm or higher between the tumor and the skin in the pathological exam.
Table 2.
Accuracy of a cut-off value of 2.1 mm ultrasound measurement of the distance between tumor and skin for the classification of patients with breast cancer who could have the skin over the tumor preserved (n = 102)
| Cut-off ≥2.1 mm (ultrasound) Estimate (95% CI) |
|
|---|---|
| Global accuracy (%)* | 96.1 |
| Sensitivity | 96.7 (90.7–99.3) |
| Specificity | 90.9 (58.7–99.8) |
| Positive predictive value | 98.9 (93.9–100.0) |
| Negative predictive value | 76.9 (46.2–95.0) |
| ROC (95% CI) | 0.991 (0.977–1.000) |
Percentage of correct classifications
The correlation between ultrasound and pathology exam was high (r = 0.798; p < 0.001), indicating a linear relationship. When we excluded cases where the skin was affected, the pathological measurements were always superior than ultrasound (Fig. 4).
Fig. 4.
Dispersion plot of the distance between skin and tumor according to the pathology and ultrasound measurements.
We tried to evaluate other factors that could affect the pathological measurement. For that, we used logistical regression models (Table 3). In the multivariate model, we considered as predictors (simultaneously) the ultrasound measurement, a palpable lesion, and the neoadjuvant treatment (significant at 10% in the univariate models). Only the measurement by ultrasound remained significant (at p < 0.001).
Table 3.
Logistic regression model results
| Pathological measurement of tumorskin distance ≥ 5 mm | Multivariate model |
|
|---|---|---|
| Adjusted odds ratio (95% CI) | p value | |
| Ultrasound measurement ≥2.1 mm | 183.52 (10.24–3,290.6) | <0.001 |
| Palpable lesion (reference = absent) | 0.87 (0.05–15.19) | 0.921 |
| Neoadjuvant treatment (reference = no) | 0.40 (0.00–52.04) | 0.712 |
95% CI, 95% confidence interval.
Discussion
We found in this study a cut-off value of 2.1 mm in the ultrasound measurement as a safe minimum distance to consider skin preservation above the tumor − patients with lower measurements should probably have the overlying skin removed as well. This result should be tested in robust randomized clinical trials. This study also confirmed that, as expected and reported before [7], TSD measurements made by pathologists on the final specimen are always larger than the preoperative ultrasound measurements.
Skin preservation surgeries are the standard in the surgical treatment of breast cancer, especially skin-sparing mastectomy, which allows superior cosmetic results with recurrence rates similar to that of more radical surgeries [10, 11]. When started in the 90s, the mastectomy technique involved mandatory removal of skin and subcutaneous tissue overlying cancer [4, 12]. After 30 years, the incision technique evolved, and the skin removal was reduced or even eliminated. However, this is not always possible, and in some cases, especially in superficial tumors, it may be necessary to remove the tumor in bloc with the skin. The incision is made over the lesion, regardless of where it is located. In our series, 21/102 (20.5%) tumors were superficially located, i.e., a TSD-USG ≤3 mm − a definition proposed by Eom et al. [13]. Of these, 11 (10.7%) had the skin affected by carcinoma. Superficial tumors without clinical signs of skin involvement (thickening, skin tethering, or focal color changes) with TSD-USG greater than 2.1 mm could have the skin overlying the lesion preserved, assuming a thickness of surgical skin flap no larger than 5 mm. According to our data, 98.9% of the cases would be properly selected for this approach, i.e., they would have a free anterior margin.
Fijii et al. [14] retrospectively evaluated the tumor-dermis distance of 171 patients who underwent skin-sparing mastectomy and total mastectomy. However, the authors excluded the skin (dermis and epidermis) from the ultrasound measurement trying to eliminate the “compression” factor of the transducer. They analyzed the distance between the anterior limit of the tumor and the most caudal region of the dermis. According to them, a tumor-dermis distance of less than 2.0 mm would indicate the removal of the skin overlying the lesion. The value was obtained after a mean follow-up of 47.1 months and no cases of skin flap recurrence in both groups. The study did not evaluate cases of skin preservation at distances less than 2.0 mm, as all patients in this condition had the tumor removed in bloc with the dermis. The data are relevant and provide a reference for the surgeon, but the measurement was obtained from retrospectively digitized images, which carries important bias, as the recorded image was not necessarily the one intended by the radiologist to demonstrate the shortest distance between the tumor and the skin. Additionally, skin flap thickness was not evaluated. Thus, the tumor-dermis distance of 2.0 mm may be overestimated.
According to Fiji et al. [14], ultrasound may not be the ideal test to evaluate the absolute distance between tumor and skin surface because of confounding from the compression of the transducer on the breast during examination. Chammings et al. [15] in 2021 (therefore after we finished our data collection) looked at this issue by evaluating the level of compression applied in elastography of the breast. Although their primary outcome was different from ours (stiffness, not distance), the authors recommended “a minimal compression should be applied” for the evaluation (as opposed to none, moderate, and marked). This is exactly what we did in our study, and we used the minimum pressure possible, trying to not compress the breast during the examination. However, the effect of different levels of compression on the distance measured should be specifically examined in future studies.
We plotted the ROC curve to assess the minimum cut-off point to predict the free anterior margin in superficial breast tumors. The higher the TSD-USG cut-off value, the greater the probability of a free anterior margin, and also the greater the number of women having the removal of the skin overlying the tumor recommended. According to our data, with a TSD-USG cut-off point of 1.8 mm, 2/92 (2.2%) cases would have the anterior margin compromised (TSD-USG < TSD-PAT of 5 mm), while with TSD-USG of 2.1 mm, 1/88 (1.1%) cases would present this condition and with TSD-USG of 2.6 mm no case. On the other hand, with a cut-off point of 1.8 mm, 1/10 (10%) cases would have the skin removed unnecessarily, 3/13 (23%) of them with a cut-off of 2.1 mm, and 5/16 (31.3%) with a cut-off of 2.6 mm. When excluding patients with compromised skin, a TSD-USG greater than 2.1 mm would result in 100% of the cases with a free anterior margin (TSD-PAT >5 mm) and 3/4 cases would have had the skin overlying the lesion removed unnecessarily. We observed a sensitivity of 98.9%, 96.7%, and 94.5%, respectively, for TSD-USG cut-off points of 1.8 mm, 2.1 mm, and 2.6 mm. The specificity for the same cut-off points was, respectively, 81.8%, 90.9%, and 100%. Thus, the 2.1-mm TSD-USG had the best accuracy in predicting the free anterior margin and at the same time preventing many women from removing the skin overlying the tumor unnecessarily.
Kataoka et al. [16] investigated the cut-off value of the tumor-skin distance on magnetic resonance imaging (TSD-MRI) to obtain a free anterior margin in skin-sparing mastectomy. The authors analyzed 42 patients, and cut-off point obtained was 5 mm. We believe that this TSD-RM was superior to TSD-USG due to a change in tumor-skin distance due to the flattening of the breast when the women are in the supine position, as observed by Carbonaro et al. [17] and Pallone et al. [18]. We confirmed, in our study, that the measurements made by ultrasonography are lower than the pathology measurement (TSD-PAT). The comparison between TSD-MRI and TSD-PAT, however, has never been studied.
Cao et al. [19] observed that 8.3% (14/168) of cases submitted to skin preservation mastectomy had a compromised anterior margin, even after undergoing additional margin sampling. In their study, the local recurrence rate reached 10% compared to 4% when the margins were free. Vaughan et al. retrospectively studied 206 skin preservation mastectomies and observed 5.3% (11/206) of local recurrences, with 72% of them in the subcutaneous tissue in the same quadrant of the initial lesion. The authors pointed out that local resection of the skin and subcutaneous tissue overlying the high-grade tumor may further decrease the rates of local recurrence in skin preservation mastectomies [19].
An anterior margin with cancer can be a determinant of local recurrence rates, especially in cases where radiotherapy is not performed [20]. Torresan et al. [21] evaluated the skin flaps of 42 mastectomies and observed a residual tumor in 9.5% of the cases (4/42). According to them, both terminal ductolobular units and residual lesions in the skin flaps were associated with a thickness greater than 5 mm. Therefore, a compromised anterior margin should be considered an important risk factor for local recurrence, regardless of stage or biological characteristics of the tumor, especially in patients not undergoing chemotherapy or radiation therapy.
The production of skin flaps is a fundamental step in skin-preserving mastectomy. The flap, including dermis and subcutaneous tissue, needs to be thin enough to properly separate the glandular tissue from the skin, and thick enough to avoid damage to vascularization without producing a noticeable depression [22]. This balance has been so far a lot based on the surgeon's experience, with little evidence to support surgical planning. The superficial fascia is an important anatomical reference during surgery. However, using it routinely can result in thin flaps because, as observed by Beer et al. [23], the distance between the fascia and the dermis varies between 0.2 and 4.0 mm. Thus, the subcutaneous tissue has been the best reference − however, there is no consensus regarding the ideal thickness to be preserved in each patient. In our service, the thickness of surgical skin flaps has usually been planned to be around 5 mm thick, for oncologic safety.
One limitation of this study is that we suppose that the thickness of the surgical flap (5 mm) would be the same as that of the surgical specimen after the action of formaldehyde, but this is lacking verification. In this context, intraoperative frozen section pathological assessment of margins could be useful to recommend the excision of the skin overlying the tumor.
Another potential limitation is that ultrasonography can fail to identify the involvement of the margins by ductal carcinoma in situ, present in up to one third of the cases [24]. The long-term follow-up of patients with superficial lesions selected for skin preservation with TSD-USG above 2.1 mm could provide data about this.
Conclusion
In superficial tumors of the breast, tumor-skin distance of 2.1 mm or more, measured preoperatively by ultrasound (TSD-USG), allows a safe preservation of the skin above the lesion. Future studies need to follow up for longer the women submitted to skin preservation surgeries especially those not undergoing radiotherapy.
Statement of Ethics
This study protocol was approved by Universidade Federal de São Paulo (UNIFESP) Ethics Committee (CAAE: 70471817.9.0000.5505), and patients signed informed consent forms for this study.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This project received no financial support of any kind.
Author Contributions
Rodrigo Gregório Brandão designed the study and contributed with data collection and interpretation, and manuscript writing and editing. Simone Elias, Ângela Flavia Logullo Waitzberg, and Gil Facina contributed with data collection and interpretation/analysis and evaluated the manuscript critically. Afonso Celso Pinto Nazário and Vanessa Monteiro Sanvido contributed with study design, general supervision, and critical evaluation of the manuscript. All authors approved the final version to be submitted and are accountable for all aspects of the work.
Data Availability Statement
The data that support the findings of this study are not publicly available on legal grounds for patients' privacy/confidentiality.
Funding Statement
This project received no financial support of any kind.
References
- 1.Fisher ER, Gregorio RM, Fisher B, Redmond C, Vellios F, Sommers SC. The pathology of invasive breast cancer. A syllabus derived from findings of the National Surgical Adjuvant Breast Project (protocol no. 4) Cancer. 1975;36:1–85. doi: 10.1002/1097-0142(197507)36:1<1::aid-cncr2820360102>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Wertheim U, Ozzello L. Neoplastic involvement of nipple and skin flap in carcinoma of the breast. Am J Surg Pathol. 1980;4((6)):543–549. doi: 10.1097/00000478-198012000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Ho CM, Mak CK, Lau Y, Cheung WY, Chan MC, Hung WK. Skin involvement in invasive breast carcinoma safety of skin-sparing mastectomy. Ann Surg Oncol. 2003;10((2)):102–107. doi: 10.1245/aso.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Toth BA, Lappert P. Modified skin incisions for mastectomy the need for plastic surgical input in preoperative planning. Plast Reconstr Surg. 1991;87((6)):1048–1053. [PubMed] [Google Scholar]
- 5.Papassotiropoulos B, Güth U, Chiesa F, Rageth C, Amann E, Baege A, et al. Prospective evaluation of residual breast tissue after skin- or nipple-sparing mastectomy results of the SKINI-trial. Ann Surg Oncol. 2019;26((5)):1254–1262. doi: 10.1245/s10434-019-07259-1. [DOI] [PubMed] [Google Scholar]
- 6.Cunnick GH, Mokbel K. Skin-sparing mastectomy. Am J Surg. 2004 Jul;188((1)):78–84. doi: 10.1016/j.amjsurg.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Brandão RG, Alves KBF, Elias S, Waitzberg AFL, Nazario ACP. Assessment of skin involvement in breast cancer preoperative ultrasound and anatomopathological correlation. Indian J Appl Res. 2017;7:602–605. [Google Scholar]
- 8.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement guidelines for reporting observational studies. PLoS Med. 2007 Oct 16;4((10)):e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig L, et al. Stard 2015 an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015 Oct 28;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilliland MD, Larson DL, Copeland EM. Appropriate timing for breast reconstruction. Plast Reconstr Surg. 1983 Sep;72((3)):335–340. doi: 10.1097/00006534-198309000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Carlson GW. Local recurrence after skin-sparing mastectomy a manifestation of tumor biology or surgical conservatism? Ann Surg Oncol. 1998 Oct-Nov;5((7)):571–572. doi: 10.1007/BF02303823. [DOI] [PubMed] [Google Scholar]
- 12.Carlson GW. Skin sparing mastectomy anatomic and technical considerations. Am Surg. 1996;62((2)):151–155. [PubMed] [Google Scholar]
- 13.Eom YH, Kim EJ, Chae BJ, Song BJ, Jung SS. The distance between breast cancer and the skin is associated with axillary nodal metastasis. J Surg Oncol. 2015 Jun;111((7)):824–828. doi: 10.1002/jso.23898. [DOI] [PubMed] [Google Scholar]
- 14.Fujii T, Nakazawa Y, Ogino M, Obayashi S, Yajima R, Honda C, et al. Oncological safety of immediate breast reconstruction with skin- or nipple-sparing mastectomy the value of tumor-to-dermis distance measured by preoperative ultrasonography. World J Surg Oncol. 2021 Mar 12;19((1)):72. doi: 10.1186/s12957-021-02185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chamming's F, Hangard C, Gennisson JL, Reinhold C, Fournier LS. Diagnostic accuracy of four levels of manual compression applied in supersonic shear wave elastography of the breast. Acad Radiol. 2021 Apr;28((4)):481–486. doi: 10.1016/j.acra.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Kataoka A, Sawaki M, Okumura S, Onishi S, Iwase M, Sugino K, et al. Prediction of pathological margin status using preoperative contrast-enhanced MRI in patients with early breast cancer who underwent skin-sparing mastectomy. Breast J. 2019;25((2)):202–206. doi: 10.1111/tbj.13194. [DOI] [PubMed] [Google Scholar]
- 17.Carbonaro LA, Tannaphai P, Trimboli RM, Verardi N, Fedeli MP, Sardanelli F. Contrast enhanced breast MRI spatial displacement from prone to supine patient's position. Preliminary results. Eur J Radiol. 2012 Jun;81((6)):771–774. doi: 10.1016/j.ejrad.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Pallone MJ, Poplack SP, Avutu HB, Paulsen KD, Barth RJ., Jr Supine breast MRI and 3D optical scanning a novel approach to improve tumor localization for breast conserving surgery. Ann Surg Oncol. 2014 Jul;21((7)):2203–2208. doi: 10.1245/s10434-014-3598-5. [DOI] [PubMed] [Google Scholar]
- 19.Cao D, Tsangaris TN, Kouprina N, Wu LSF, Balch CM, Vang R, et al. The superficial margin of the skin-sparing mastectomy for breast carcinoma factors predicting involvement and efficacy of additional margin sampling. Ann Surg Oncol. 2008 May;15((5)):1330–1340. doi: 10.1245/s10434-007-9795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burdge EC, Yuen J, Hardee M, Gadgil PV, Das C, Henry-Tillman R, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol. 2013 Oct;20((10)):3294–3302. doi: 10.1245/s10434-013-3174-4. [DOI] [PubMed] [Google Scholar]
- 21.Torresan RZ, dos Santos CC, Okamura H, Alvarenga M. Evaluation of residual glandular tissue after skin-sparing mastectomies. Ann Surg Oncol. 2005 Dec;12((12)):1037–1044. doi: 10.1245/ASO.2005.11.027. [DOI] [PubMed] [Google Scholar]
- 22.Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg. 2014 Jul;101((8)):899–911. doi: 10.1002/bjs.9470. [DOI] [PubMed] [Google Scholar]
- 23.Beer GM, Varga Z, Budi S, Seifert B, Meyer VE. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer. 2002 Mar 15;94((6)):1619–1625. doi: 10.1002/cncr.10429. [DOI] [PubMed] [Google Scholar]
- 24.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, et al. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002 Oct 16;94((20)):1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available on legal grounds for patients' privacy/confidentiality.