Abstract
Significant knowledge gaps regarding the effectiveness and safety of medical cannabis (MC) create clinical challenges for MC physicians, making treatment recommendations and patients choosing treatment among the growing number of options offered in dispensaries. Additionally, data describing the characteristics of people who use MC and the products and doses they receive are lacking. The Medical Marijuana and Me (M3) Study was designed to collect patient-centered data from MC users. We aim to describe preferred MC use patterns that patients report as “most effective” for specific health conditions and symptoms, identify user characteristics associated with such use patterns, characterize adverse effects, including cannabis use disorder, identify products and patient characteristics associated with adverse effects, describe concurrent prescription medication use, and identify concomitant medication use with potential drug-MC interaction risk. Among MC initiators, we also aim to quantify MC use persistence and identify reasons for discontinuation, assess MC utilization pattern trajectories over time, describe outcome trajectories of primary reasons for MC use and determine factors associated with different trajectories, track changes in concomitant substance and medication use after MC initiation, and identify factors associated with such changes. M3 is a combined study comprised of: (1) a prospective cohort of MC initiators completing surveys at enrollment, 3 months, and 9 months after MC initiation and (2) a cross-sectional study of current MC users. A multidisciplinary committee including researchers, physicians, pharmacists, patients, and dispensary personnel designed and planned study protocols, established study measures, and created survey questionnaires. M3 will recruit 1,000–1,200 participants aged ≥18 years, with ∼50% new and ∼50% current MC patients from MC clinics across Florida, USA. Study enrollment started in May 2022 and will continue until the target number of patients is achieved. Survey domains include sociodemographic characteristics, physical and mental health, cannabis use history, reasons for MC use and discontinuation, MC products and use patterns, concurrent use of prescription medications and other substances, and side effects. Data collected in the M3 Study will be available for interested researchers affiliated with the Consortium for Medical Marijuana Clinical Outcomes Research. The M3 Study and Databank will be the largest cohort of current and new MC users in Florida, USA, which will provide data to support MC-related health research necessary to inform policy and clinical practice and ultimately improve patient outcomes.
Keywords: Medical marijuana, MMJ, Medical cannabis, Clinical outcomes, Effectiveness
Introduction
With over 700,000 qualified patients and 2,000 certifying physicians in 2022, Florida is growing into one of the largest medical cannabis (MC) programs in the USA [1]. In Florida, USA, MC is available with a physician's certification and recommendation for qualifying medical conditions [2]. However, the limited evidence regarding the effectiveness and safety of MC [3, 4] imposes challenges for clinicians making MC treatment recommendations and patients choosing treatment among the growing number of options offered in dispensaries [5]. These challenges range from finding effective doses, products, and regimens considering indications, patient comorbidities, or other modalities that may determine treatment approaches to comparing the outcomes of different treatment recommendations and modalities [5−8]. Problems in relying on anecdotal evidence (rather than formal controlled studies) are further exacerbated by a lack of data on patients who leave the program or abstain from follow-up visits due to reasons often unknown to physicians. These challenges create a knowledge gap regarding the characteristics of patients who may or may not benefit from MC or those who experience MC side effects. Little is known regarding the types of MC products, doses, regimens, and dosage forms and their variation in terms of safety and efficacy outcomes. Additionally, it is unclear whether health and safety outcomes differ between MC-naïve patients and experienced patients joining the MC program, given the lack of data assessing the trajectories of health outcomes in these groups.
In 2021, we surveyed 116 MC physicians in Florida, USA, to establish research priorities related to MC [5]. The top communicated priorities were drug interactions with prescription drugs, comparing different components of MC, serious side effects, effects of varying potency levels of tetrahydrocannabinol (THC) products, and comparing different dosage forms [5]. The Medical Marijuana and Me (M3) Study was designed to address these identified research priorities and support high-quality, impactful research to: (a) inform state policy and clinical practice and improve patient outcomes, (b) provide data resources and infrastructure for researchers affiliated with the Consortium, (c) support pilot studies, papers, and grants, and collect patient-centered data relevant to the most common health conditions among a diverse group of MC users in Florida, USA. The M3 Study includes both current and new patients in Florida's MC program to assess the following objectives:
Persistence of MC use: to quantify persistence among MC initiators at 3 months and 9 months after MC initiation and identify reasons for discontinuation.
Outcome trajectories: to describe outcome trajectories for the most cited primary reasons for MC use (e.g., pain, anxiety, insomnia, and posttraumatic stress disorder [PTSD]) and determine factors associated with differences in trajectories among MC initiators.
Preferred MC use patterns: to describe the modes of MC consumption, dosing, and frequency of use that patients report to be “most effective” and describe patient characteristics associated with these preferences.
MC utilization pattern trajectories: to describe changes in products, modes of consumption, and dosing over time.
Adverse effects: to characterize the types of adverse effects and identify the specific products and patient characteristics associated with different types of MC-related side effects, including symptoms of cannabis use disorder.
Change in other substance use: to document the use of alcohol, tobacco, and other substances and assess any changes in their consumption among MC initiators.
Concurrent use of prescription medications: to describe the simultaneous use of prescription medications and factors associated with changes in prescription medications/substance use after initiating MC.
MC drug interactions: to characterize concomitant medication use and assess the potential risk for drug interactions with MC.
Methods
Ethics Approval and Participant Compensation
The M3 Study and Databank was approved by the University of Florida (UF) Institutional Review Board (IRB202200068). Eligible participants view a presentation explaining the study steps and procedures on the study website before agreeing to participate. Participants sign an online consent form before completing the surveys and are compensated with a $20 gift card for each survey completion. Survey responses are saved as deidentified data on ResVault, a highly secure computing environment at the UF for protecting restricted and confidential data [9].
Study Design and Population
The M3 Study consists of two components, including a longitudinal follow-up of a prospective cohort of MC initiators and a cross-sectional survey of a sample of current MC users in Florida, USA, in 2022. Cohort participants complete three online surveys: at enrollment (before starting MC), then at 3 months, and 9 months after MC initiation. Participants of the cross-sectional study complete a single online survey. Data from both designs will be deposited into the M3 Databank, a repository intended to house the data and allow data sharing for research purposes.
Recruitment
Participant recruitment was launched on May 1, 2022, and was still ongoing as of October 2022. Most participants are recruited through ten geographically dispersed MC clinics and clinic networks in Florida, USA. Involved clinics help facilitate enrollment through various combinations of flyers, posters, info sheets, survey invitations attached to appointment reminder emails, and text message invitations for new patients. These clinics also host an on-site M3 Study iPad for participants to access the study website/consent/survey and host M3 research assistants in their offices to enroll participants on-site. Medical assistants and physicians help recruit participants by distributing study materials, providing verbal information about the study, and facilitating patients' contact with the study research assistants. Interested patients can immediately enroll and complete the survey using their mobile device or a study tablet or receive the study promotional material containing the study web address and contact information to enroll later. The study web page is publicly accessible via an internet search, and participants can enroll without being approached or directly recruited by study personnel. To help capture a diverse group of MC patients, we aim to recruit 1,000–1,200 participants aged 18 years or older across Florida, USA, with 500–600 (50%) new MC users in the cohort study and another 500–600 (50%) current MC users in the cross-sectional study. Distribution of recruitment materials aims at patients of both sexes, different age groups, race/ethnicity, and qualifying conditions, and patients from different geographical locations/counties and types of MC clinics to include patients from different socioeconomic backgrounds.
Additionally, in-person recruiters target patients from older age groups who may be less experienced with internet use [10, 11]. Along with recruitment, we perform descriptive analyses of demographic characteristics to identify groups requiring further targeting. For example, to broaden age ranges and the representation of qualifying conditions based on preliminary analyses, we sent targeted email invitations to under-represented individuals in the MC Contact Registry (a contact database of participants interested in MC research hosted by the consortium) to participate in the cross-sectional study.
Study Surveys
The study protocol, measures, and surveys were designed by a multidisciplinary planning committee that included researchers, physicians, pharmacists, patients, and dispensary personnel. Survey domains include sociodemographic characteristics, physical and mental health, cannabis use history, reasons for MC use and discontinuation, MC products and use patterns, concurrent use of prescription medications and other substances, and side effects. Validated research measures and tools were used when available for a specific domain. We pilot-tested the surveys on 20 randomly selected participants from the MC Contact Registry who provided feedback to improve the understandability of the surveys before launching the study. Surveys are completed via Qualtrics. The surveys are available upon request from the authors.
Study Measures and Data Collection
All M3 Study measures are self-reported and collected via online surveys, with an optional telephone survey to collect data on concurrent medication use. Table 1 summarizes the M3 Study measures and their corresponding time of data collection.
Table 1.
Medical Marijuana and Me (M3) measures by study design and timepoint of data collection
| Study measure | Study type and collection timepoint |
|||
|---|---|---|---|---|
| cohort study | cross-sectional study | |||
| baseline | 3 months | 9 months | ||
| Demographics | ||||
| Age in years | × | × | ||
| Race/ethnicity | × | × | ||
| Sex at birth | × | × | ||
| Gender identity | × | × | ||
| Highest level of education | × | × | ||
| Employment status | × | × | ||
| Veteran status | × | × | ||
| Health insurance | × | × | ||
| Annual family income in the last year | × | × | ||
| ZIP code | × | × | ||
| General health | ||||
| Overall health status (Short Form [SF-8] Health Survey) [12, 13] | × | × | × | × |
| Depression (PHQ-8) [14, 15] | × | × | × | × |
| Anxiety (GAD-7) [16] | × | × | × | × |
| PTSD (adapted from DSM-5 [PC-PTSD-5]) [17, 18] | × | × | × | × |
| Pain (BPI) [19] | × | × | × | × |
| Quality of sleep (single item adapted from PSQI) [20] | × | × | × | × |
| Positive affect (PROMIS) [21, 22] | × | × | × | |
| Anger (PROMIS) [21, 22] | × | × | × | |
| History of cannabis use (including cannabis obtained without a medical card in Florida) | ||||
| Ever used during a lifetime | × | |||
| Age when used for the first time | × | × | ||
| Weekly use | × | × | ||
| Number of years used weekly | × | × | ||
| Daily use | × | × | ||
| Number of years used daily | × | × | ||
| Cannabis use experience level | × | |||
| Cannabis products ever tried before medical cannabis | × | |||
| Cannabis use in the last 6 months | × | |||
| Frequency of cannabis use in past 6 months (from CUDIT-R) [23] | × | × | × | |
| Frequency of cannabis use in past 3 months (from CUDIT-R) [23] | × | |||
| Frequency of cannabis use per day in an average use day | × | |||
| Flower use in the past 30 days | × | |||
| Frequency of flower use in the past 30 days | × | |||
| Frequency of flower use per day in an average use day | × | |||
| Ever used CBD | × | |||
| Frequency of CBD use in past 6 months | × | |||
| Amount of money spent on cannabis in a typical month | × | |||
| Alcohol and other substance use | ||||
| Alcohol use (AUDIT-C) [24] | × | × | × | × |
| Cigarette smoking use (from BRFSS) [25] | × | × | × | |
| E-cigarette/vape use (from BRFSS) [25] | × | × | × | |
| Frequency of opioid use (with prescription) in past 30 days | × | × | × | |
| Frequency of opioid use (without prescription) in past 30 days | × | × | × | |
| Frequency of benzodiazepine use (with prescription) in past 30 days | × | × | × | |
| Frequency of benzodiazepine use (without prescription) in past 30 days | × | × | × | |
| Frequency of amphetamine use (with prescription) in past 30 days | × | × | × | |
| Frequency of amphetamine use (with prescription) in past 30 days | × | × | × | |
| Frequency of cocaine/crack use in past 30 days | × | × | × | |
| Frequency of hallucinogens use in past 30 days | × | × | × | |
| Frequency of synthetic cannabis use in past 30 days | × | × | × | |
| Frequency of kratom use in past 30 days | × | × | × | |
| Use of recreational substance to track | × | |||
| Substance name to track (up to 3 substances) | × | |||
| Change in substance tracked | × | × | ||
| Use of (alcohol, cigarettes, vapes, cocaine, hallucinogens, club drugs, synthetic cannabis, and kratom) in past 5 years | × | |||
| Change in (alcohol, cigarettes, vapes, cocaine, hallucinogens, club drugs, synthetic cannabis, kratom) since starting medical cannabis | × | |||
| Is change in use (alcohol, cigarettes, vapes, cocaine, hallucinogens, club drugs, synthetic cannabis, kratom) related to medical cannabis? | × | |||
| Use of (opioids with and without prescription, amphetamines with and without prescription, benzodiazepines with and without prescription) in the past 5 years | × | |||
| Change in (opioids with and without prescription, amphetamines with and without prescription, benzodiazepines with and without prescription) since starting medical cannabis | × | |||
| Is change in use (opioids with and without prescription, amphetamines with and without prescription, benzodiazepines with and without prescription) related to medical cannabis? | × | |||
| Medications | ||||
| List of current medications (medication name, dose, frequency of use, and route of administration) | × | × | × | × |
| Use of medications to track | × | |||
| Medications names to track (up to 3) | × | |||
| Change in medications tracked | × | × | ||
| Tried to reduce specific medications in past 5 years | × | |||
| Name of 3 medications tried to reduce in past 5 years | × | |||
| Overall change of medication use since starting medical cannabis | × | |||
| Is a change in medication use related to medical cannabis? | × | |||
| Medical conditions | ||||
| Medical conditions diagnosed by healthcare professional | × | × | ||
| Cancer: type of cancer | × | × | ||
| Cancer: metastasis | × | × | × | × |
| Cancer: current cancer status | × | × | × | × |
| Cancer: current cancer treatment | × | × | × | × |
| Medical cannabis effect on medical conditions | × | × | × | |
| Method of cannabis use that was most effective for each condition | × | × | × | |
| Name of product that works best for each condition | × | × | × | |
| Other health benefits of medical cannabis | × | × | × | |
| Knowledge of care providers about medical cannabis use | × | × | × | |
| Cancer: knowledge of oncologist/provider about use/intent to use | × | × | × | × |
| Reasons for using or stopping medical cannabis | ||||
| Medical conditions that are reasons for medical cannabis use | × | × | ||
| Certified medical condition | × | × | ||
| Cancer: cancer-specific reason for use | × | × | ||
| Cannabis use motive (recreational vs. medical) | × | × | × | × |
| Reasons for stopping | × | × | ||
| Thinking about stopping use | × | × | ||
| Reasons for thinking about stopping use | × | × | ||
| Expectations | ||||
| Expected effectiveness | ||||
| Concern about addiction/dependence on medical cannabis | × | × | × | × |
| Likelihood to be taking cannabis in a year | × | × | × | × |
| Duration of medical cannabis use | ||||
| Still using medical cannabis | × | × | ||
| Duration of medical cannabis use | × | × | × | |
| Duration of use before stopping | × | × | ||
| Year first obtained medical cannabis card in Florida | × | |||
| Medical cannabis products | ||||
| Products tried from the medical cannabis program in Florida (flower, vaporizer cartridges or vape pen (liquid, not flower), concentrates (for vaping or smoking), topicals, oral tinctures (with a dropper), oral concentrates, oral capsules or edibles (chews, lozenges, chocolates, or gels), other | × | × | × | |
| Products used in the past 30 days (all products) | × | × | × | |
| Reasons for not using the product (all products) | × | × | × | |
| Frequency of product use in past 30 days | × | × | × | |
| Frequency of product use in a typical day (all products) | × | × | × | |
| THC concentration (all products) | × | × | × | |
| Duration to effect onset (all products) | × | × | × | |
| Duration of effects to last (all products) | × | × | × | |
| CBD: THC ratio (all products) | × | × | × | |
| Number of strains tried (all products) | × | × | × | |
| Strain used most (all products) | × | × | × | |
| Duration to consume one flower container | × | × | × | |
| Number of flower containers used per month | × | × | × | |
| Frequency of methods to consume flower in past 30 days (smoking, vaping, cooking) | × | × | × | |
| Number of hits, tokes, or puffs per session when smoking/vaping flower/cartridges/concentrates | × | × | × | |
| Number of seconds when inhaling smoke or vape | × | × | × | |
| Frequency of using concentrate methods in past 30 days (shatter, rosin, wax, keif, crumble, dab tab, hash) | × | × | × | |
| Frequency of using at least 25 mg (rice-sized) of concentrate in past 30 days | × | × | × | |
| Frequency of using topical methods in past 30 days (patch, cream, lotion, balms, salves, spray, transdermal gel, oil) | × | × | × | |
| Milliliters of tincture consumed per occasion | × | × | × | |
| Average milligrams of THC and CBD consumed when using tinctures | ||||
| Frequency of using oral concentrate methods in past 30 days (distillate syringe, RSO syringe) | × | × | × | |
| Amount of oral concentrate per occasion | × | × | × | |
| Frequency of oral methods in past 30 days (capsule/tablets, gel/gummies, brownie/cookie) | × | × | × | |
| Amount spent on products per month in USD | × | × | × | |
| Overall preferred medical cannabis and/or a product type or strain | × | × | × | |
| Preferred method of using medical cannabis | × | × | × | |
| Name of overall preferred strain/product | × | × | × | |
| Amount of used cannabis from a Florida dispensary | × | × | ||
| Side effects | ||||
| Symptoms bothered by in the past 2 weeks | × | × | × | × |
| Were symptoms caused by medical cannabis? | × | × | × | |
| Severe side effects requiring an emergency room visit, seeing a physician, being hospitalized, or feeling extremely sick for a few hours | × | × | × | |
| Describe severe side effects | × | × | × | |
| Frequency of severe side effects | × | × | × | |
| Concurrent use of substance/medication when severe side effect occurred | × | × | × | |
| Mode of consumption when severe side effect occurred | × | × | × | |
| Cannabis use disorder (CUDIT-R) [23] | × | × | × | × |
| Beliefs and opinions | ||||
| Agree that cannabis products with high THC will be more effective | × | × | × | × |
| Preference to try low-THC products | × | × | × | × |
| Importance of CBD in the medical effects of medical cannabis | × | × | × | × |
| Importance of including terpenes in medical cannabis products | × | × | × | × |
| Factors influencing products tried | × | × | × | |
| Interest in growing own medical cannabis if it becomes legal in Florida | × | × | × | |
| Additional topics that should be researched | × | × | ||
AUDIT-C, Alcohol Use Disorders Identification Test-Concise [24]; BPI, Brief Pain Inventory [19]; BRFSS, Behavioral Risk Factor Surveillance System [25]; CBD, cannabidiol; CUDIT-R, Cannabis Use Disorders Identification Test-Revised [23]; DSM-5 (PC-PTSD-5), Primary Care Posttraumatic Stress Disorder Screen for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [17, 18]; GAD-7, General Anxiety Disorder-7 [16]; PHQ-8, eight-item Patient Health Questionnaire depression scale [14, 15]; PTSD, posttraumatic stress disorder [17, 18]; PROMIS, Patient-Reported Outcomes Measurement Information System [21, 22]; PSQI, Pittsburgh Sleep Quality Index [20]; SF-8, Short Form 8 [12, 13]; THC, tetrahydrocannabinol.
Demographics
Demographic data are collected in the cross-sectional survey and at the baseline of the cohort study. Demographic variables include age, sex at birth, gender identity, race, ethnicity, the highest level of education, employment status, veteran status, kind of health insurance coverage, combined yearly family income before taxes in the last year, and five-digit ZIP code.
Change in General Health Status
We use the Short Form (SF-8) Health Survey to assess overall health status in the past 4 weeks [12, 13]. We also used the eight-item Patient Health Questionnaire depression scale (PHQ-8) for depression in the past 2 weeks [14, 15], the General Anxiety Disorder-7 (GAD-7) for anxiety in the past 2 weeks [16], and the Primary Care Posttraumatic Stress Disorder Screen for the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition DSM-5 (PC-PTSD-5) for PTSD in the past 30 days [17, 18]. We used the Brief Pain Inventory (BPI) for pain level in the past 24 h and pain interference with physical activities in the past 7 days [19]. Finally, we used a single item adapted from the Pittsburgh Sleep Quality Index (PSQI) for sleep quality in the past 30 days [20]. The listed general health measures are assessed in the cross-sectional study and at baseline, 3 months, and 9 months of the cohort study. The cohort study also employs the Patient-Reported Outcomes Measurement Information System (PROMIS) tools for positive affect and anger in the past 7 days at the three timepoints [21, 22].
History of Cannabis Use (Including Cannabis Obtained without a Medical Card in Florida, USA)
To help understand the cumulative effects of cannabis use and its role in MC-related outcomes, we assess pre-MC and lifetime cannabis exposure among new and current users, respectively. Among MC initiators (cohort study), we ask about lifetime cannabis use, the age of cannabis use for the first time, ever using cannabis on a weekly or daily basis, the number of years of weekly and daily use, and the level of experience with cannabis use. We also ask about any cannabis products used before MC, the frequency of cannabis use in the past 6 months (first item of the Cannabis Use Disorders Identification Test-Revised [CUDIT-R]) [23], daily frequency of cannabis use, any flower use and the frequency of flower use in the past 30 days, and the frequency of flower use per day in an average use day. Moreover, we ask about ever using cannabidiol (CBD) and the frequency of CBD use in the past 6 months. In addition, we ask the participants to estimate the cost of cannabis in a typical use month. To assess the overall change in the frequency of cannabis use after MC initiation, we also use the first item of the CUDIT-R at 3 and 9 months [23].
Among current MC users (cross-sectional study), we assess the age of use for the first time, ever using cannabis on a weekly or daily basis, the number of years of weekly and daily use, and the level of experience they have with cannabis use. We also assess the frequency of cannabis use in the past 6 months using the first item of the CUDIT-R [23].
Change in Alcohol and Other Substance Use
We assess alcohol use in both the cohort (at baseline, 3 months, and 9 months) and cross-sectional studies using the Alcohol Use Disorders Identification Test-Concise (AUDIT-C) [24]. In the cohort study, we ask the participants to report the use of any recreational substances they wish to track changes in after MC initiation and to type the names of up to three such substances. We then assess self-reported changes in the use of the substances listed at baseline in the 3-month and 9-month follow-up surveys. For a more objective assessment, we also collect repeated measures to assess substance use at different timepoints. We use the Behavioral Risk Factor Surveillance System (BRFSS) at baseline, 3 months, and 9 months to assess changes in cigarette smoking and e-cigarette/vape use [25]. We also assess the frequency of use of opioids, benzodiazepines, and amphetamine (with and without a prescription), and cocaine/crack, hallucinogens, synthetic cannabis, and kratom in the past 30 days, at baseline, 3 months, and 9 months.
In the cross-sectional study, we ask the participants about the use of opioids, benzodiazepines, and amphetamine (with and without a prescription), and alcohol, cigarettes, vapes, cocaine, hallucinogens, club drugs, synthetic cannabis, and kratom in the past 5 years. We also ask about changes in their substance use since starting MC and if the change was related to MC.
Concurrent Medications
At baseline, we ask MC initiators if they are currently using any medications to track changes after starting MC initiation and to type the names of up to three such medications. We then assess self-reported changes in the use of these medications in the 3-month and 9-month surveys. In the cross-sectional study, we ask the participants if they have tried to reduce specific medications in the past 5 years, the names of up to three such medications, and the overall change in medication use since starting MC.
For a more objective and general assessment of medication use, respondents are provided with an additional survey to provide a comprehensive list of all current prescription and nonprescription medications. Respondents can independently enter medication information, including medication name, dose, frequency of use, and route of administration, or opt for a phone survey. In the phone survey, a team of trained research assistants at the University of Florida conducts phone interviews to document a list of current medications used. Medication data are collected at baseline, 3 months, and 9 months of the cohort study and once in the cross-sectional study. The list of medications will be assessed for including medications with potential interactions with MC. Repeated sampling of the current medication list in the cohort study will allow additional assessments of medication use and changes during follow-up.
Effects on Medical Conditions
We provide a list of medical conditions and ask the participants to check all conditions they have been diagnosed with by a healthcare professional at baseline in the cohort study and cross-sectional studies. The condition list includes anxiety, depression, PTSD, attention-deficit/hyperactivity disorder, bipolar disorder, schizophrenia, insomnia/sleeping problems, migraine/headache, fibromyalgia, chronic pain, cancer, amyotrophic lateral sclerosis, asthma, chronic lung disease, hypertension, heart disease, diabetes, kidney disease/dialysis, Crohn's disease/ulcerative colitis, stroke, multiple sclerosis, Parkinson's disease, epilepsy/seizure disorder, Alzheimer's disease/dementia, glaucoma, HIV/AIDS, other, and none. When patients report having cancer, we ask them to specify the type of cancer they have. We also ask if their cancer is metastatic, their current cancer status, and what treatments they receive at the cohort baseline and in the cross-sectional study. We then ask the participants at 3 months and 9 months of the cohort study and in the cross-sectional study to report the effect of MC on each condition. When the participants report improvement in their medical conditions, we ask them to specify the methods of MC use that were most effective and the best products for each condition. At the same timepoints, we also ask the participants to report any other health benefits of MC and about the knowledge of their other care providers (those not involved in certifying them for MC use) about their MC use.
Reasons for Using and Stopping MC
From the medical conditions list, we ask the participants to identify the main reasons for their MC use (baseline cohort and cross-sectional study). We then ask the participants to specify the health condition they were certified for MC use by their MC physician based on the list of approved conditions in Florida, USA. When cancer is reported as a reason for MC use, we ask the participants to choose the cancer-specific reasons for using MC. At all study timepoints, we ask the participants to rate their MC use motives across a spectrum of completely recreational to completely medical. Among MC initiators, if the participants report stopping or thinking about stopping MC use, we ask about the reasons for stopping or thinking about stopping MC.
Outcome Expectations
To assess the presence of expectation bias, we ask MC initiators about their expectations regarding MC effectiveness, their concerns regarding developing or having addiction/dependence on MC, and the likelihood of maintaining MC use a year from the time of taking the survey.
Duration of MC Use
We ask MC initiators if they are still using MC and the total duration of MC use at 3 months and 9 months. We also ask current users in the cross-sectional study about the total duration of MC use and the year they first obtained an MC card in Florida, USA.
MC Products
All the following measures are collected at 3 months and 9 months of the cohort study and in the cross-sectional study. We first present a list of MC products, including flower, vaporizer cartridges or vape pens (liquid, not flower), concentrates (for vaping or smoking), topicals, oral tinctures (with a dropper), oral concentrates, oral capsules or edibles (chews, lozenges, chocolates, or gels), and other. The list is accompanied by a picture of each product that serves as a visual prompt. We ask the participants to check all products they have tried from the Florida MC program from the list. We then ask them to check all products they used in the past 30 days. For each product they did not use in the past 30 days, we ask about the reasons for not using that product. For each product used in the past 30 days, we ask the participants to report the frequency of use in the past 30 days, frequency of use in a typical use day, THC concentration, CBD:THC ratio, the time between administration and onset of effects and between start and end of effects, number of strains/products used from each product category, and strain that was used the most. For each product category, we also ask about the frequency of using different administration methods where applicable. Using these measures, we aim to calculate the daily THC dose and study the association between daily THC dose and reported safety and effectiveness outcomes.
Additionally, we ask all study participants about the amount spent on products per month in US dollars. We further ask if they found a specific method of using MC and/or a product type or strain that they prefer overall, what their preferred method of administration is, and to type the name of their overall preferred product or strain. Finally, we ask MC initiators to indicate the percent amount of cannabis used from a Florida, USA, dispensary relative to their overall cannabis consumption in the past month.
Side Effects
We present a list of 28 physical and psychological symptoms that represent potential side effects of MC and ask participants to check any symptoms they have experienced in the past 2 weeks at baseline, 3 months, and 9 months within the cohort study and in the cross-sectional study. In the cross-sectional study and at 3 months and 9 months of the cohort study, we ask participants if MC caused each checked symptom. We also ask if participants experienced any severe side effects requiring an emergency room visit, seeing a physician, being hospitalized, or feeling extremely sick for a few hours. If the participants answer with “yes,” we ask them to describe these side effects, the mode of MC consumption, and any concurrent use of substances or medications when the severe side effect occurred. Finally, we assess the presence of symptoms of cannabis use disorder at all the cohort study timepoints and in the cross-sectional study using the CUDIT-R [23].
Beliefs and Opinions
At the end of each survey, we ask the participants about their beliefs regarding THC, CBD, and terpene contributions to MC effects. We also ask the participants how influential their physician, family, online sources, and other sources have been on which MC products they have tried so far. Finally, we ask if participants are interested in growing their own MC if it were legal in Florida, USA, and then to suggest any additional topics that should be included in future MC research.
Data Sharing Plan
Data collected in the M3 Study will be housed in the M3 Databank and available for research. Interested investigators can submit data request applications through the Consortium M3 Study page at https://mmjoutcomes.org/m3study/. Applications will be reviewed by the M3 Scientific Oversight Committee and approved based on scientific merit, clinical significance, the rigor of the proposed study and analysis plan, appropriateness of the data for the suggested purpose, and potential overlap with other approved or planned studies. Applicants are required to submit a detailed analysis plan for their proposed study. The plan must provide the research question/hypothesis, a rationale for performing the study, a list of M3 measures needed to perform the proposed analysis, and a clear analytical framework outlining the study's independent variable(s), dependent variable(s), potential confounders, mediators, effect modifiers, and others. The plan must also include the biostatistical analyses that will be performed, including power analysis, where applicable. Applicants must also provide a data management protocol and security plan. Upon application approval, investigators with approved applications will have a 12-month access period to a set of deidentified data relevant to their proposed research. Linkages with other databases are possible within the databank.
Discussion
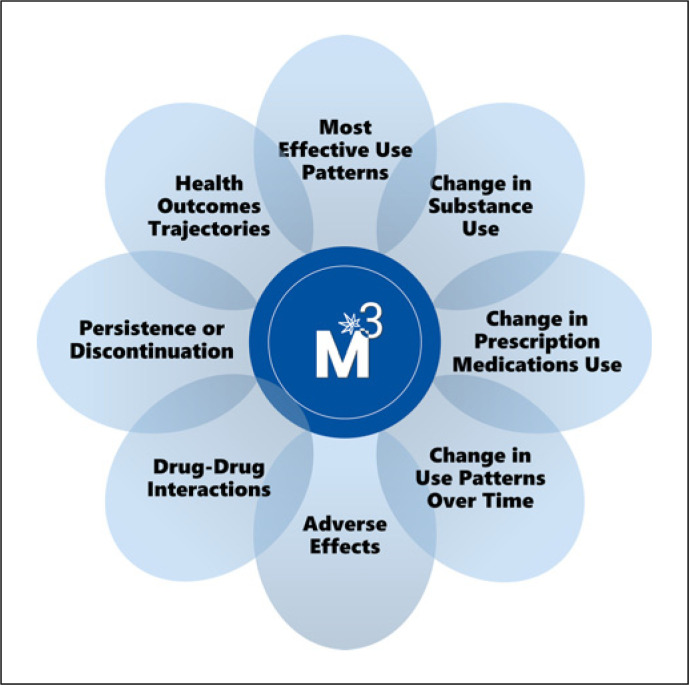
The M3 Study is a combined longitudinal cohort of MC initiators and a cross-sectional study of current MC users in Florida, USA. M3 collects patient-centered data to support research on MC effectiveness and safety outcomes relevant to the most common health conditions among a diverse group of MC users. M3 will provide data resources and infrastructure for researchers interested in using M3 data to address specific research questions in eight broad domains shown in Figure 1.
Fig. 1.
The Medical Marijuana and Me (M3) data domains.
While current evidence regarding the effectiveness and safety of MC to treat the approved qualifying medical conditions remains limited, further research is urged [4, 26, 27]. The M3 Study's longitudinal design will allow for assessing the temporal changes among MC initiators, including those who discontinue MC use, potentially due to lack of effectiveness or experiencing negative outcomes and side effects. The study will also assess patient characteristics, product types, use patterns, reported MC health outcomes, and reasons for use continuation or discontinuation. Such findings will help guide treatment approaches, especially among new patients, and deliver important hypotheses for future study within controlled designs. Additionally, previous research has shown that many patients seek MC for other currently non-approved indications, including anxiety, depression, sleep disturbances, nausea and vomiting, substance use-related disorders, including opioid use and dependence, and to improve overall well-being and quality of life [3, 28−30]. Information on patients' motives to use MC is relevant to understand patients' engagement in the Florida MC program and can inform future regulatory decisions, such as the definition of qualifying conditions.
Furthermore, the M3 measures were designed to address some of the challenges of cannabis use quantification in previous observational studies, mainly those caused by the wide variety of available MC products, methods and routes of administration, and product composition, including THC and CBD concentrations and ratios [31, 32]. We developed a comprehensive set of questions to quantify MC use and THC exposure across nine main product categories commonly available at dispensaries and used by MC patients. Different outcome trajectories will be compared based on the type, quantity, and frequency of MC exposure and used products' THC and CBD composition.
Finally, while many countries that openly legalized cannabis for medical and recreational use, like Canada, collect and publish data on cannabis used for medical purposes at the national level [33−35], no such data yet exist in the USA. The latter is because cannabis remains a Schedule I substance in the USA, and state laws vary regarding its use for medical and recreational purposes. As such, efforts to collect prospective and registry-type data on MC use in states with approved MC programs are highly needed. There have been several studies on MC use in different parts of the USA [30, 36−41]. These studies have mostly focused on studying the association between MC use and change in opioid use [30, 36, 37, 39−41]. The M3 Study builds on previous studies by adding a patient-centered perspective to a spectrum of health outcomes, focusing on adverse effects and drug interactions, and tracking changes in substance and medication use after MC initiation. Our study is the first and largest prospective cohort of MC users in Florida, the third largest state in the USA, and will provide a large and diverse sample of persons using MC for a variety of qualifying medical indications. The proposed study has limitations. First, the observational nature of this study will not allow the assessment of MC's efficacy in treating health conditions and symptoms and is prone to bias when making inferences about treatment effects. Moreover, the lack of control groups will limit the comparability of MC-related health and safety outcomes to other therapies used for the same conditions or symptoms. Nevertheless, as clinical trials involving THC-based cannabis products remain limited, comprehensive assessments of utilization patterns and self-reported experiences can enhance anecdotal data on patient experiences and generate important hypotheses for future research. Second, many MC initiators included in the study are expected to have a previous history of cannabis use from other sources before joining the MC program since no exclusion criteria restricted their participation. The potential change in some of the proposed outcomes among these patients may not be as apparent as in cannabis-naïve patients, if any, because it is unclear if their exposure to cannabis would change compared to that before starting MC. Third, while our study is designed to include a diverse population of MC users, the generalizability of this population to the overall MC patient population in Florida, USA, is limited. The limited generalizability of our study is due to the lack of available data describing Florida's MC patient population's characteristics and the fact that our sample is a convenient sample of volunteering MC patients from different MC clinics across Florida, USA. Nevertheless, this study will resemble the largest research cohort of MC users in Florida, USA, including patients from diverse backgrounds and age groups across a broad spectrum of health conditions.
Conclusions
This manuscript presents a novel protocol that aims to collect MC-related patient-centered data, capture patient-reported experiences with MC use, and support hypothesis generation for future research on MC effectiveness and safety outcomes. M3 will provide data for researchers interested in addressing specific research questions in eight broad domains and support pilot studies, papers, and grants.
Acknowledgments
The authors acknowledge the contributions of the Planning Committee to the M3 study design and surveys: Dinese C. Vidot, PhD, Dushyantha T. Jayaweera, MD, Jason Ford, MA, PhD, Jennifer Jean-Jacques, MPH, Jessica Walters, John M. Crump, MD, Jonathan Quinonez, DO, Justin Davis, MD, Martha Rosenthal, PhD, Michelle Weiner, DO, Michelle Wilson, MBA, Patricia A. Green-Powell, PhD, and MC clinic networks in Florida, USA, who helped with patient recruitment: Affordable Marijuana License, Dr. Bob's Compassion Clinic, CannaMD, DocMJ, Green Health & Wellness, John M. Crump, MD, with Releafe Now, the practice of Justin Davis, MD, the practice of Kelly King, MD, the practice of Melanie Bone, MD, and Michelle Weiner, DO, with Spine and Wellness Centers of America.
Statement of Ethics
This study was approved by the University of Florida Institutional Review Board (IRB202002925). All study participants provided signed online consent forms. Survey responses are saved as deidentified data on ResVault, a highly secure computing environment at the University of Florida for protecting restricted and confidential data.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study was funded by the Consortium for Medical Marijuana Clinical Outcomes Research, a state-funded research consortium of nine universities in the state of Florida.
Author Contributions
Ruba Sajdeya, Robert L. Cook, Yan Wang, Hannah J. Fechtel, Gabe Spandau, Almut G. Winterstein, and Amie J. Goodin conceptualized the manuscript. Ruba Sajdeya, Robert L. Cook, Hannah J. Fechtel, Gabe Spandau, and Joshua D. Brown drafted the initial manuscript. Ruba Sajdeya, Robert L. Cook, Yan Wang, Hannah J. Fechtel, Gabe Spandau, Almut G. Winterstein, Amie J. Goodin, Joshua D. Brown, Sebastian Jugl, and Nicole E. Smolinski revised, edited, and approved the final draft. Robert L. Cook and Yan Wang developed the initial survey. Ruba Sajdeya, Hannah J. Fechtel, Almut G. Winterstein, Amie J. Goodin, Joshua D. Brown, and Sebastian Jugl contributed substantively to survey development.
Data Availability Statement
Study surveys are available upon request from the authors. Data collected in the M3 Study will be available for researchers interested in using it to answer MC-related specific research questions upon application and approval by the M3 Databank.
Funding Statement
This study was funded by the Consortium for Medical Marijuana Clinical Outcomes Research, a state-funded research consortium of nine universities in the state of Florida.
References
- 1.2022 OMMU Updates Office Of Medical Marijuana Use [Internet] [cited 2022 Jul 31]. Available from: https://knowthefactsmmj.com/2022/01/05/2022-ommu-updates/
- 2.Statutes and Constitution View Statutes Online Sunshine [Internet] Available from: http://www.leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0300-0399/0381/Sections/0381.986.html.
- 3.National academies of sciences E division H and M practice B on PH and PH agenda C on the HE of MAER and R . The health effects of cannabis and cannabinoids the current state of evidence and recommendations for research. US: National Academies Press; 2017. Therapeutic effects of cannabis and cannabinoids [internet] [cited 2021 Mar 11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425767/ [PubMed] [Google Scholar]
- 4.Jugl S, Okpeku A, Costales B, Morris EJ, Alipour-Haris G, Hincapie-Castillo JM, et al. A mapping literature Review of medical cannabis clinical outcomes and quality of evidence in approved conditions in the USA from 2016 to 2019. Med Cannabis Cannabinoids. 2021 Feb 25;4:21–42. doi: 10.1159/000515069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sajdeya R, Shavers A, Jean-Jacques J, Costales B, Jugl S, Crump C, et al. Practice patterns and training needs among physicians certifying patients for medical Marijuana in Florida. J Prim Care Community Health. 2021 Jan 1;12:21501327211042790. doi: 10.1177/21501327211042790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleber HD, Dupont RL. Physicians and medical Marijuana. Am J Psychiatry. 2012 Jun 1;169(6):564–568. doi: 10.1176/appi.ajp.2012.12030373. [DOI] [PubMed] [Google Scholar]
- 7.Gundersen DC. The legalization of Marijuana implications for regulation and practice. J Nurs Regul. 2015 Oct 1;6(3):34–38. [Google Scholar]
- 8.Bridgeman MB, Abazia DT. Medicinal cannabis history, pharmacology, and implications for the acute care setting. Pharm Ther. 2017 Mar;42(3):180–188. [PMC free article] [PubMed] [Google Scholar]
- 9.ResVault: Research Computing [Internet] [cited 2023 Jan 31]. Available from: https://gravity.rc.ufl.edu/services/restricted-data/researchvault/ [Google Scholar]
- 10.Czaja SJ, Charness N, Fisk AD, Hertzog C, Nair SN, Rogers WA, et al. Factors predicting the use of technology findings from the center for research and education on aging and technology enhancement (CREATE) Psychol Aging. 2006 Jun;21(2):333–352. doi: 10.1037/0882-7974.21.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaportzis E, Clausen MG, Gow AJ. Older adults perceptions of technology and barriers to interacting with tablet computers a focus group study. Front Psychol. 2017 Oct 4;8:1687. doi: 10.3389/fpsyg.2017.01687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yiengprugsawan V, Kelly M, Tawatsupa B. SF-8TM Health Survey. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research [internet] Dordrecht: Springer Netherlands; 2014. pp. p. 5940–5942. cited 2022 Aug 2] [Google Scholar]
- 13.Ware JE, Kosinski M, Dewey JE, Gandek B, Kisinski M, Ware JE, et al. How to score and interpret single-item health status measures a manual for users of the SF-8TM Health Survey. 2001 Jan 1; [cited 2022 Aug 2]. Available from: https://www.scinapse.io. [Google Scholar]
- 14.Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009 Apr;114(1–3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Razykov I, Ziegelstein RC, Whooley MA, Thombs BD. The PHQ-9 versus the PHQ-8 — is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the Heart and Soul Study. J Psychosom Res. 2012 Sep 1;73(3):163–168. doi: 10.1016/j.jpsychores.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder the GAD-7. Arch Intern Med. 2006 May 22;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 17.Bovin MJ, Kimerling R, Weathers FW, Prins A, Marx BP, Post EP, et al. Diagnostic accuracy and acceptability of the primary care posttraumatic stress disorder screen for the diagnostic and statistical manual of mental disorders (Fifth edition) among US veterans. JAMA Netw Open. 2021 Feb 4;4(2):e2036733. doi: 10.1001/jamanetworkopen.2020.36733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prins A, Bovin MJ, Smolenski DJ, Marx BP, Kimerling R, Jenkins-Guarnieri MA, et al. The primary care PTSD screen for DSM-5 (PC-PTSD-5) development and evaluation within a veteran primary care sample. J Gen Intern Med. 2016 Oct;31(10):1206–1211. doi: 10.1007/s11606-016-3703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in pain research and therapy: issues in pain measurement. Vol. 12. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 20.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index a new instrument for psychiatric practice and research. Psychiatry Res. 1989 May;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 21.Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks 2005–2008. J Clin Epidemiol. 2010 Nov 1;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothrock NE, Amtmann D, Cook KF. Development and validation of an interpretive guide for PROMIS scores. J Patient Rep Outcomes. 2020 Feb 28;4(1):16. doi: 10.1186/s41687-020-0181-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adamson SJ, Kay-Lambkin FJ, Baker AL, Lewin TJ, Thornton L, Kelly BJ, et al. An improved brief measure of cannabis misuse the Cannabis Use Disorders Identification Test-Revised (CUDIT-R) Drug Alcohol Depend. 2010 Jul 1;110(1–2):137–143. doi: 10.1016/j.drugalcdep.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C) an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998 Sep 14;158(16):1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 25.CDC: BRFSS–Questionnaires [Internet] 2022. [cited 2022 Aug 2]. Available from: https://www.cdc.gov/brfss/questionnaires/index.htm.
- 26.National academies of sciences E division H and M practice B on PH and PH agenda C on the HE of MAER and R . The health effects of cannabis and cannabinoids the current state of evidence and recommendations for research. US: National Academies Press; 2017. Recommendations to support and improve the cannabis research agenda [Internet] [cited 2021 Mar 30]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425745/ [PubMed] [Google Scholar]
- 27.Sajdeya R, Goodin AJ, Tighe PJ. Cannabis use assessment and documentation in healthcare priorities for closing the gap. Prev Med. 2021 Dec 1;153:106798. doi: 10.1016/j.ypmed.2021.106798. [DOI] [PubMed] [Google Scholar]
- 28.Azcarate PM, Zhang AJ, Keyhani S, Steigerwald S, Ishida JH, Cohen BE. Medical reasons for Marijuana use, forms of use, and patient perception of physician attitudes among the US population. J Gen Intern Med. 2020 Jul;35(7):1979–1986. doi: 10.1007/s11606-020-05800-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sajdeya R, Joseph V, Setten N, Ibañez G, Wang Y, Powell L, et al. Reasons for Marijuana use and its perceived effectiveness in therapeutic and recreational Marijuana users among people living with HIV in Florida. Cannabis. 2021 Feb 18;4(1):40–52. doi: 10.26828/cannabis/2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucas P, Baron EP, Jikomes N. Medical cannabis patterns of use and substitution for opioids and other pharmaceutical drugs, alcohol, tobacco, and illicit substances; results from a cross-sectional survey of authorized patients. Harm Reduct J. 2019 Jan 28;16(1):9. doi: 10.1186/s12954-019-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jugl S, Sajdeya R, Morris EJ, Goodin AJ, Brown JD. Much ado about dosing the needs and challenges of defining a standardized cannabis unit. Med Cannabis Cannabinoids. 2021;4(2):121–124. doi: 10.1159/000517154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sajdeya R, Cook RL. Need to improve dose measurements in studies of Marijuana use for pain. J Acquir Immune Defic Syndr. 2020 Mar 1;83(3):e23. doi: 10.1097/QAI.0000000000002238. [DOI] [PubMed] [Google Scholar]
- 33.Canada H. Cannabis research and data [Internet] 2020 [cited 2022 Oct 14]. Available from https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data.html. [Google Scholar]
- 34.Canada H. Data on cannabis for medical purposes [Internet] 2020 [cited 2022 Oct 14]. Available from https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/medical-purpose.html. [Google Scholar]
- 35.Canada H. Cannabis market data [Internet] 2020 [cited 2022 Oct 14]. Available from https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/research-data/market.html. [Google Scholar]
- 36.Ross J, Slawek DE, Zhang C, Starrels JL, Levin FR, Sohler NL, et al. First-year trajectories of medical cannabis use among adults taking opioids for chronic pain an observational cohort study. Pain Med. 2021 Aug 19;22(12):3080–3088. doi: 10.1093/pm/pnab257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vigil JM, Stith SS, Adams IM, Reeve AP. Associations between medical cannabis and prescription opioid use in chronic pain patients a preliminary cohort study. PLoS One. 2017 Nov 16;12(11):e0187795. doi: 10.1371/journal.pone.0187795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matson TE, Carrell DS, Bobb JF, Cronkite DJ, Oliver MM, Luce C, et al. Prevalence of medical cannabis use and associated health conditions documented in electronic health records among primary care patients in Washington state. JAMA Netw Open. 2021 May 6;4(5):e219375. doi: 10.1001/jamanetworkopen.2021.9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boehnke KF, Litinas E, Clauw DJ. Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain. 2016 Jun 1;17(6):739–744. doi: 10.1016/j.jpain.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Pritchett CE, Flynn H, Wang Y, Polston JE. Medical cannabis patients report improvements in health functioning and reductions in opiate use. Subst Use Misuse. 2022 Nov 10;57(13):1883–1892. doi: 10.1080/10826084.2022.2107673. [DOI] [PubMed] [Google Scholar]
- 41.Bachhuber MA, Saloner B, Cunningham CO, Barry CL. Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med. 2014 Oct;174(10):1668–1673. doi: 10.1001/jamainternmed.2014.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Study surveys are available upon request from the authors. Data collected in the M3 Study will be available for researchers interested in using it to answer MC-related specific research questions upon application and approval by the M3 Databank.