Abstract
Transgenic soybean (Glycine max) culture cells expressing apoaequorin, a Ca2+ indicator, were exposed to glucan fragments derived from Phytophthora sojae or to chitin oligomers. The effects of these elicitors on cytosolic Ca2+ concentrations and on mRNA levels of two β-tubulin isoforms, tubB1 and tubB2, were investigated. The glucan elicitors, to which the cells are known to react with a biphasic cytosolic Ca2+ increase, induced a down-regulation of the tubB1 mRNA levels while the tubB2 mRNA level remained constant. The decrease of tubB1 mRNA level was observed after 1 hour of glucan treatment. In contrast, chitin oligomers, known to provoke a monophasic Ca2+ increase of short duration, did not affect the tubB1 mRNA level. Pre-incubation with 10 mm 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid, an extracellular Ca2+ chelator, blocked the cytosolic Ca2+ increase as well as the decrease of tubB1 mRNA levels induced by glucan elicitors. Likewise, pre-incubation with 1 mm neomycin, which reduced only the second glucan-induced Ca2+ peak, blocked the decrease of tubB1 mRNA level. Experiments with cordycepin, a transcription inhibitor, indicated that glucan fragments induced the degradation of tubB1 mRNA. In conclusion, the glucan-induced cytosolic Ca2+ changes are correlated with a strong increase in tubB1 mRNA degradation.
The cytoskeleton is a dynamic structure composed of microtubules (α- and β-tubulin polymers) and microfilaments (F-actin polymers) and is involved in many cellular processes such as cell division, cytoplasmic streaming, organelle positioning, cellular transport, and signal transduction (Bershadsky and Vasiliev, 1988). Involvement of the cytoskeleton in animal cells responses to pathogens has been extensively studied (for review, see Virji, 1996). It has also been shown that the cytoskeleton is altered when plants are subjected to various stresses (Creelman and Mullet, 1991; Shibaoka, 1994; Blancaflor et al., 1998). For example, during plant-pathogen interactions, microtubules have a role in the spread of tobacco mosaic virus from cell to cell (Heinlein et al., 1995). The dynamic role of the cytoskeleton in signal transduction during fungal infection has been reported by Gross et al. (1993). These authors showed that a local microtubule depolymerization occurred in parsley cells at the infection site of Phytophthora infestans. This phenomenon is accompanied by a re-arrangement in the microfilament array, an increase in cytoplasmic streaming, followed by the movement of the nucleus toward the infection site, and a local induction of the pathogenesis-related PR1 gene. In barley cells infected with a non-pathogen (Erysiphe pisi), the penetration efficiency of this incompatible fungus was enhanced by the application of either cytoskeleton destabilizing or stabilizing agents (Kobayashi et al., 1997). Also, the penetration of Rhizobium into root hairs is followed by a re-arrangement of the microfilament network allowing migration of the bacteria and nodule formation (Timmers et al., 1998; Whitehead et al., 1998).
Other changes in the plant such as changes in ion fluxes occur when plants are attacked by pathogens or exposed to elicitors (for review, see Boller, 1995; Zimmermann et al., 1999), including enhancement of intracellular Ca2+ concentrations (Knight et al., 1991; Ehrhardt et al., 1996; Levine et al., 1996; Mithöfer et al., 1999). For example, Ca2+ is involved in the hypersensitive response during rust-fungal infection leading to cell death (Xu and Heath, 1998). Thus, Ca2+ is thought to be the major second messenger in signal transduction during plant defense responses.
Moreover, Ca2+ is an essential regulator of cytoskeleton dynamics (Cyr, 1994). Most of the microtubule-associated proteins, which influence microtubule stability and interaction with other cellular components are Ca2+-binding proteins (Schellenbaum et al., 1992). It has also been shown that at high cytosolic Ca2+ concentrations, the microtubule polymers are destabilized, whereas at resting levels they are stabilized (Fisher et al., 1996; Moore et al., 1997). In addition, Ca2+-dependent protein kinases have been co-localized with F-actin (Putnam-Evans et al., 1989), indicating that Ca2+ together with protein phosphorylation is involved in cytoskeleton dynamics (Baskin and Wilson, 1997; Drewes et al., 1998).
We are interested in determining the possible effect of Ca2+ as a signaling compound on the dynamics of cytoskeleton changes induced during host-pathogen interactions. For this purpose, we used a glucan elicitor and cultured soybean (Glycine max) cells. The glucan elicitor has a degree of polymerization from 7 to 15 (DP 7–15) and is derived from the specific soybean pathogen Phytophthora sojae. These elicitors have been well studied with respect to their various effects in plant defense responses (for review, see Ebel, 1998), and a putative receptor has been purified and cloned in soybean (Umemoto et al., 1997). To monitor elicitor-mediated Ca2+ signals, we used the Ca2+-binding photoprotein aequorin. Aequorin has been used previously to monitor the Ca2+ responses to different stimuli in different plant cell systems (Knight et al., 1991; Sedbrook et al., 1996; Mazars et al., 1997). We have previously shown, using a transgenic soybean cell line expressing apoaequorin, that glucan elicitors induce biphasic cytosolic Ca2+ increases in soybean cells, which are necessary for the later phytoalexin production (Mithöfer et al., 1999).
In the present work, we report a connection between the Ca2+ responses and a down-regulation of β-tubulin mRNA in soybean cells.
RESULTS
The Expression of the Soybean β-Tubulin 1 Isoform Is Down-Regulated by Glucan Elicitors
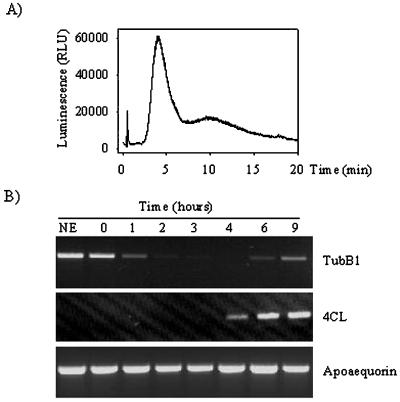
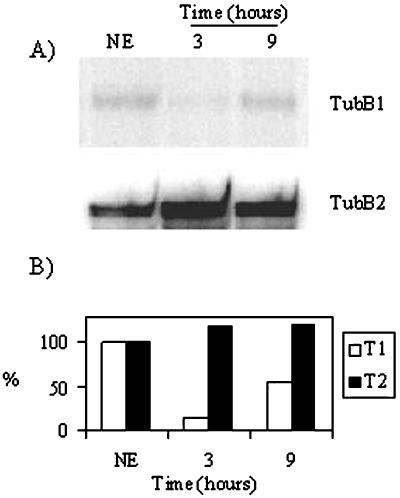
We have previously shown, using a soybean cell line transgenic for aequorin (line 6.6.12), that glucan fragments DP 7–15 induce a biphasic cytosolic Ca2+ rise 2 min after elicitor application (Mithöfer et al., 1999) and that this Ca2+ signal is correlated with the later phytoalexin production. We used the same transgenic cell line to look at other late elicitor effects. We initially checked the response of the aequorin-expressing soybean cells to glucan elicitation at saturating concentrations (60 μm) by determining the Ca2+ response. Treatment with glucans DP 7–15 induced a typical biphasic Ca2+ response lasting approximately 25 min (Mithöfer et al., 1999) in the transgenic cells (Fig. 1A). From these cells, samples were collected for RNA isolation one-half hour before elicitation (no elicitor [NE]) and at 0, 1, 2, 3, 4, 6, and 9 h after elicitation. We performed reverse transcriptase (RT)-PCR reactions using oligonucleotides specific for the soybean β-tubulin isoform tubB1, and observed that the transcript level of the tubB1 mRNA decreased after 1 h of treatment (Fig. 1B). The tubB1 mRNA level continued to decrease to an undetectable level after 3 and 4 h of incubation with glucan elicitors but increased again after 6 and 9 h (Fig. 1B).
Figure 1.
Effect of glucan fragments DP 7–15 on cytosolic Ca2+ increase and transcript levels. A, Cytosolic Ca2+ increase measured by aequorin luminescence after addition of glucan fragments DP 7–15 (60 μm). B, RT-PCR experiments for tubB1, 4CL, and apoaequorin, using RNA preparations from cells without treatment (NE) or treated with 60 μm glucan fragments DP 7–15 for 0, 1, 2, 3, 4, 6, or 9 h. The cDNAs amplified are tubB1 (top), 4CL (middle), and apoaequorin (bottom). The results shown are representative of at least three independent experiments.
To verify that the elicitation effectively induced late defense responses, we looked at the expression of the gene encoding the 4:coumarate coenzyme A ligase (4CL), an enzyme that participates in the phenylpropanoid pathway and whose expression is known to be induced by glucan elicitors (Uhlmann and Ebel, 1993). We observed an induction of the 4CL gene after 4 h of incubation with glucan fragments DP 7–15 (Fig. 1B), indicating that glucan elicitors are inducing not only cytosolic Ca2+ increases but also defense genes and that the tubB1 mRNA down-regulation might be due to the treatment with glucan elicitors. As a control for each RT-PCR, we used the transgene present in the soybean cell line, apoaequorin, whose expression is unaffected by elicitation with DP 7–15 (Fig. 1B).
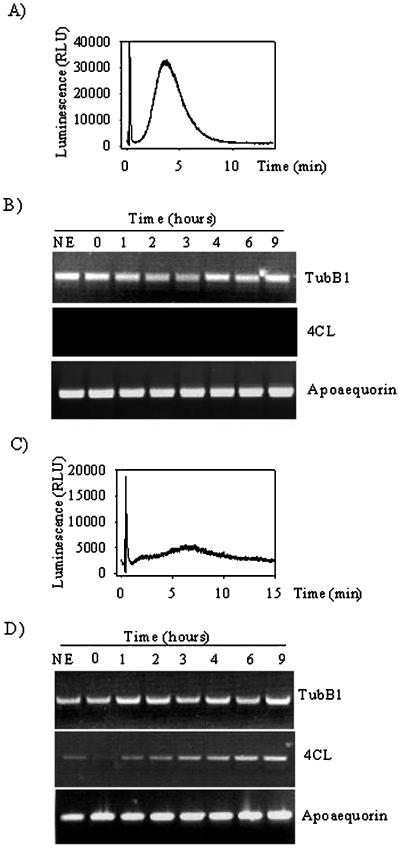
To determine whether the drop of tubB1 transcript level is a general phenomenon, we used a general elicitor derived from fungal cell wall, chitotetraose (CH4), which induces a monophasic Ca2+ increase but no phytoalexin production in soybean cells (Mithöfer et al., 1999). As previously observed, a monophasic Ca2+ increase was induced after 2 min of elicitation with 200 nm CH4 (Fig. 2A), but this treatment did not cause any appreciable change in the tubB1 mRNA level over the time of treatment (Fig. 2B). In addition, no induction of the 4CL gene was observed upon CH4 elicitation (Fig. 2B). These results are consistent with the previous observations in which no late defense responses could be induced by CH4 in soybean cells.
Figure 2.
Effects of CH4 and flg22 on cytosolic Ca2+ increase and transcript levels. Cytosolic Ca2+ increase measured by aequorin luminescence after CH4 (A) and flg22 (C) treatment. RT-PCR experiments for tubB1, 4CL, and apoaequorin from cells without treatment (NE) or treated with 200 nm CH4 (B), 10 μm flg22 (D). The results shown are representative of at least three independent experiments.
In addition, we used a non-fungal elicitor flagellin 22 (flg22) (Felix et al., 1999), derived from bacterial flagella. Treatment of the cells with flg22 induced a small, monophasic Ca2+ increase, which was 18 times smaller than the one induced by glucans DP 7–15 (Fig. 2C). The RT-PCR experiments from cells treated with 10 μm flg22 showed an induction of the 4CL gene expression after 2 h (Fig. 2D). However, the treatment did not down-regulate the level of tubB1 mRNA (Fig. 2D). Taken together, the results suggest that the decrease of tubB1 mRNA is specifically due to glucan elicitors and that other fungal (CH4) or non-fungal elicitors (flg22) do not induce such a down-regulation.
The Microtubular Network Is Unaffected by Glucan Elicitors
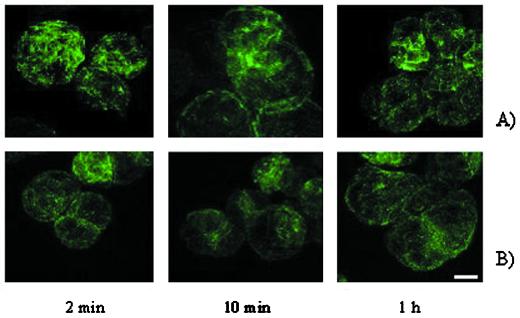
In mammalian cells, the amount of free tubulin dimers in the cytoplasm appears to be closely regulated since an excess of free tubulin dimers triggers the degradation of tubulin mRNA by binding to the nascent tubulin polypeptide (Theodorakis and Cleveland, 1992). Because we observed a down-regulation of tubB1 mRNA level in response to glucan elicitors the effect of glucan elicitors on the cytoskeleton organization was investigated. We treated soybean cells with glucan fragments for 2, 10, and 60 min, fixed them in paraformaldehyde, stained the microtubules using a mouse anti-β-tubulin antibody, and visualized them with a fluorescein-labeled anti-mouse secondary antibody. The microtubule bundles did not exhibit any visible changes at the time points observed (Fig. 3A). In other attempts, an incubation of 4 h with glucan elicitors did not change the microtubule organization (data not shown). The same experiment was performed using CH4 (as a control) and again the microtubules remained intact (Fig. 3B) showing that both elicitors did not affect the microtubule organization under the experimental conditions.
Figure 3.
Microtubule organization is unaffected by elicitor treatment. Fluorescent microtubule immunostaining after treatment of soybean cells with glucan DP 7–15 (A) or CH4 (B) for 2, 10, and 60 min, the cells were visualized by confocal image scanning and stacked images are shown. Scale bar = 10 μm.
Another Divergent β-Tubulin Isoform, tubB2, Is Not Affected by Glucan Elicitors
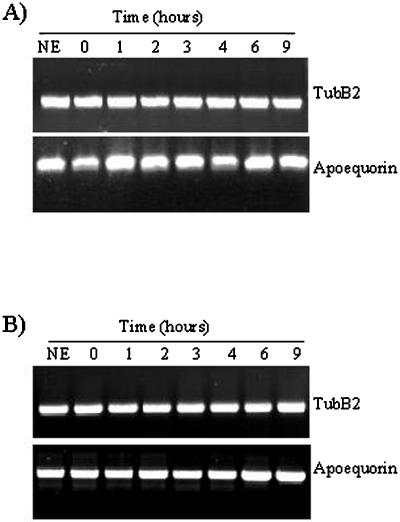
To see if the effect observed on the expression of tubB1 by glucans DP 7–15 is restricted to this isoform, we chose a second β-tubulin isoform available in the database and named tubB2 (Guiltinan et al., 1987). RT-PCR was performed using specific oligonucleotides for a non-conserved region between both isoforms in the 3′ end of the gene. Using the same RNA extracts, we observed that the tubB2 mRNA level remained constant over the 9 h of incubation with glucan fragments (Fig. 4A). The same observation was made when we treated the cells with CH4 for up to 9 h (Fig. 4B).
Figure 4.
TubB2 expression level after elicitor treatments. Glucan fragments DP 7–15 (A) and CH4 (B). RT-PCR experiments performed with oligonucleotides specific for tubB2 (top) and apoaequorin (bottom). The results shown are representative of at least three independent experiments.
RNase protection assays were performed to confirm the differential regulation of the mRNA levels of tubB1 and tubB2 after treatment with glucan fragments and previously monitored by RT-PCR. Using antisense RNA probes to the 3′ end of both tubB1 and tubB2 mRNA, we observed that the tubB1 mRNA level decreased to approximately 14% of the level present in untreated cells after 3 h of treatment and reached 60% of the level present in untreated cells at 9 h (Fig. 5, A and B). As shown previously, there was no decrease in tubB2 mRNA in response to the addition of glucan elicitors (Fig. 5, A and B). Moreover, this experiment confirmed that the tubB2 mRNA level was more abundant than that of the tubB1 mRNA. TubB1 was present at approximately 1% of the tubB2 mRNA in untreated cells. These results indicate that glucan fragments specifically down-regulate the tubB1 mRNA level without affecting the tubB2 mRNA level. In addition, the fact that tubB2 is unaffected by glucan elicitors and expressed at a much higher level could explain why no changes were observed in the microtubule immunostainining experiments.
Figure 5.
Glucan elicitors differentially affect tubB1 and tubB2 mRNA levels. A, RNase protection assay performed on total RNA extracted from soybean cells not treated (NE) and 3 and 9 h after glucan elicitor treatment (60 μm). Only the protected fragments of tubB1 (top) and tubB2 (bottom) are shown. Radioactive bands were quantified and results were plotted in B. The results shown are representative of two independent experiments.
Ca2+ Modulators Suppress the tubB1 mRNA Decrease
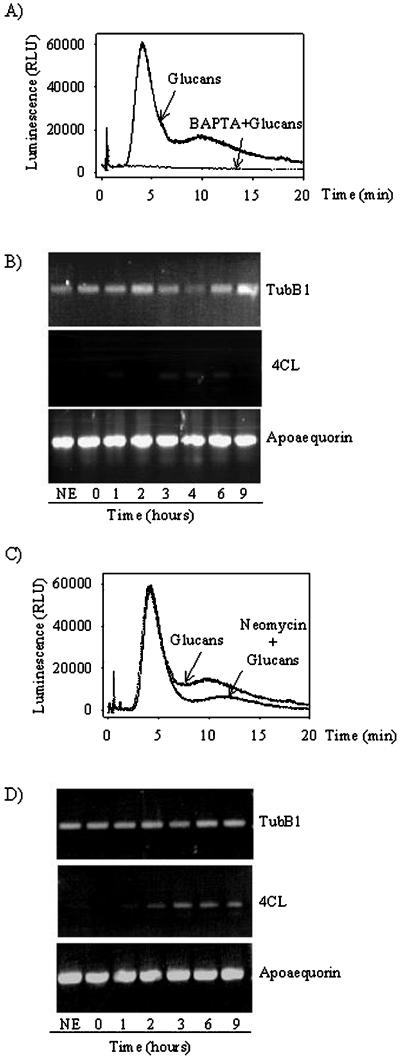
To further correlate the tubB1 mRNA decrease with Ca2+ signaling in glucan elicited soybean cells, we used two different Ca2+ modulators. First, knowing that 1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid (BAPTA), a chelator commonly used to cause extracellular Ca2+ depletion, inhibits the glucan-induced Ca2+ increase (Mithöfer et al., 1999), we investigated the effect of BAPTA on the level of tubB1 mRNA. Soybean cells were pre-incubated with 10 mm BAPTA 10 min prior to addition of glucan fragments DP 7–15 (60 μm) and at the same time checked for their response to the treatment by measuring the cytosolic Ca2+ concentrations (Fig. 6A). After blockage of the Ca2+ influx by BAPTA, no decrease of the tubB1 mRNA level was observed (Fig. 6B) and no induction of the 4CL gene was observed either (Fig. 6B), suggesting that the Ca2+ influx might mediate both the decrease of tubB1 message levels and 4CL induction.
Figure 6.
Ca2+ modulators and their effects on the cytosolic Ca2+ increases and transcript levels after glucan elicitor treatment. Aequorin luminescence measurements from cells treated with 1 mm BAPTA and glucans DP 7–15 (60 μm) (A) or 1 mm neomycin and glucans DP 7–15 (60 μm) (C). RT-PCR experiments performed with oligonucleotides specific for tubB1, 4-CL, and apoaequorin after treatment with 1 mm BAPTA and glucans DP 7–15 (60 μm) (B) or 1 mm neomycin and glucans DP 7–15 (60 μm) (D). The results shown are representative of at least three independent experiments.
The second Ca2+ modulator we used was neomycin, a compound inhibiting phospholipase C that is involved in the phosphatidylinositol (1,4,5)-trisphos-phate turnover responsible for the release of Ca2+ from intracellular stores. Neomycin was successfully used previously as a phospholipase C inhibitor to study Ca2+ metabolism in plant cells (Knight et al., 1997). Neomycin was able to reduce strongly the second elicitor-induced Ca2+ peak (Fig. 6C) as previously described by Mithöfer et al. (1999). On applying 1 mm neomycin 10 min before glucan fragments, we did not observe any down-regulation of tubB1 mRNA levels (Fig. 6D). In contrast, the 4CL gene is still induced after 4 h (Fig. 6D), indicating that the Ca2+ flux still going on after neomycin treatment might be sufficient to activate the 4CL gene but not to induce the tubB1 mRNA degradation.
BAPTA and neomycin by themselves did not induce any of the responses considered here (data not shown) nor did they affect apoaequorin expression, indicating that these two drugs had no side effects.
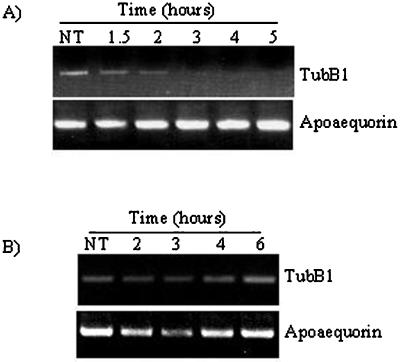
Glucan Fragments DP 7–15 Induce tubB1 mRNA Degradation
Because of the apparent regulation of the tubulin synthesis by tubulin mRNA degradation, we investigated the tubB1 degradation rate, using a transcription inhibitor, cordycepin, that has been successfully applied previously in plants (Holtorf et al., 1998). We applied cordycepin 2 h before addition of either glucan elicitors or water and took samples 1, 2, and 3 h after elicitor addition. Performing RT-PCR, we observed that the tubB1 mRNA was stable within the 2 h of cordycepin treatment but when we added the elicitor the tubB1 mRNA level started to decrease 1 h after elicitor addition (Fig. 7A). The level of tubB1 mRNA did not increase again at later times. Cordycepin combined with water did not induce any repression of the tubB1 expression over the 6 h tested (Fig. 7B). Moreover, in the RNase protection assay, we observed an increase of small tubB1 mRNA protected fragments (approximately 25–50 nucleotides) after 3 h of glucan elicitor treatment, which could be degradation products (data not shown). Taken together, these results indicate that there is an increased degradation of tubB1 mRNA due to elicitor treatment.
Figure 7.
Increased degradation of the tubB1 mRNA. Cells were first treated with 150 μg mL−1 of cordycepin for 2 h, followed by addition of glucan elicitor (60 μm) (A) or an equivalent amount of water (B). The results shown are representative of at least three independent experiments.
DISCUSSION
We demonstrate here that glucan elicitors induce a decrease of the tubB1 transcript level, most likely by enhancing its degradation. This enhanced degradation is observed after 1 h of glucan elicitor treatment and stops after 6 h of incubation with this elicitor. In contrast, we showed that the transcript level of another tubulin isoform, tubB2, was not affected by this elicitor. Moreover, the tubB1 mRNA down-regulation occurs in presence of glucan elicitors deriving from the mycelial wall of P. sojae and neither CH4 nor flg22 induced this degradation. We also show the existence of a correlation between the down-regulation of tubB1 mRNA and the elicitor-induced Ca2+ responses. However, the cytoskeleton dynamics remained similar to control experiments.
The biological significance of the message decrease of what is normally considered a constitutive gene by pathogen-derived molecules is unclear. However, a differential expression of tubulin isoforms was also observed in some other instances, confirming that tubulin genes are regulated by environmental conditions and that their expression is by no means as constitutive as previously thought. Giani et al. (1998) observed in rice that tubulin mRNA levels are reduced upon an external addition of abscisic acid, while the protein amount remained stable. Plant cells (as well as animal cells) express different α- and β-tubulin isoforms. For Arabidopsis up to six different α-tubulin (Kopczak et al., 1992) and nine different β-tubulin genes (Snustad et al., 1992) have been identified. It has been proposed that each isotype may have a specific function either during plant development in a tissue-specific manner (Giani and Breviario, 1996; Stotz and Long, 1999) or in response to environmental conditions (Blancaflor et al., 1998; Giani et al., 1998). For soybean, only three β-tubulin isoforms are available in the database. TubB1 and tubB2 have been released in the database together and have 87% homology at the protein level (Guiltinan et al., 1987). It is interesting that the tubB1 gene is controlled by a promoter containing light-responsive elements (Tonoike et al., 1994) and is expressed only in etiolated hypocotyls, whereas the tubB2 gene is expressed in the whole plant and in any light condition (Han et al., 1991). The soybean cell suspension used here, composed of photoautotrophic cells, is grown under conditions of constant light and has been shown to express phytochrome-regulated genes (Lam et al., 1989). Since all the experiments were performed under the same light conditions, we can exclude any light effect on the tubB1 expression.
The tubB1 mRNA down-regulation is specifically observed in response to glucan elicitors. The down-regulation of the mRNA level induced by these elicitors could result from a general redirection of the available cellular resources to defense-related metabolism or from a specific repression of processes important for growth. Most of the defense genes are induced in the 3 to 4 h following pathogen attack or elicitor treatment (Gross et al., 1993; Uhlmann and Ebel, 1993). Logemann et al. (1995) showed that an elicitor preparation (Pep-25) leads to the activation of Phe ammonia lyase and chalcone synthase genes concomitant with a transcriptional repression of histone, cdc2, and cyclin genes, which participate in regulating the cell cycle. In our case the 4CL gene is induced at the same time as the tubB1 mRNA level decreases. Furthermore, our results suggest that the tubB1 transcript level returns to its basal level after 9 h of elicitation, indicating that after this time the cell has already activated the defense responses and that regular housekeeping functions might start again.
In mammalian cells a tight feedback control of tubulin synthesis exists: tubulin mRNAs are destabilized in the presence of free tubulin heterodimers via their binding to the conserved N-terminal tetrapeptide (MREI) of the nascent tubulin polypeptide (Theodorakis and Cleveland, 1992). This type of regulation has not been conclusively demonstrated in plants yet, but the occurrence of this tetrapeptide at the N terminus of tubB1 suggests that the same type of regulation may take place in plant cells. If this regulation mechanism occurs in plant cells, the tubB1 mRNA degradation could be a consequence of a specific and rapid depolymerization of specific microtubules that is undetectable within microtubular fibers in our immunostaining experiments. In rice, when microtubules are depolymerized with oryzalin the tubulin mRNA level stays constant, indicating the existence of different, additional regulatory mechanisms (Giani et al., 1998). However, the N-terminal tetrapeptide of the tubB2 isotype is divergent (MRES) so the tubB1 and tubB2 mRNAs might be regulated in different ways. Another negatively regulated gene is the Arabidopsis cystathionine γ-synthase coding for a key enzyme in Met biosynthesis. Its message is also destabilized by an autoregulatory mechanism acting certainly via Met (Chiba et al., 1999). Therefore, control of mRNA stability by feedback mechanisms might be an efficient way of regulating specific transcript levels when the cell is subjected to different conditions.
Activation of plant defense-related genes has been extensively studied. Most of them are regulated at the transcriptional level and for some genes, cis-acting promoter elements have been identified (Rushton and Somssich, 1998). However, not much is known about repression of genes during response of plants to pathogens, especially about increased mRNA degradation, as presented here for tubB1. The PvPRP1 mRNA encoding a bean Pro-rich protein has been shown to undergo increased degradation after fungal elicitor treatment (Zhang et al., 1993). This degradation is accompanied by a decrease in the bean β-tubulin mRNA level after 1 h of fungal elicitor treatment. The fact that another fungal elicitor also induces the degradation of tubulin mRNA in bean indicates that the phenomenon might be common during pathogen-induced defense responses. AUUUA motifs present in the 3′-untranslated region (UTR) of PvPRP1 have been proposed to trigger mRNA degradation (Ohme-Takagi et al., 1993; Zhang and Mehdy, 1994). Three AUUUA motifs are present in the 3′-UTR of tubB1 but none has been found in the 3′-UTR of tubB2, which suggests differential regulation via degradation or stabilization of the message for the two different tubulin isoforms.
The cytosolic Ca2+ increases presented here were visualized directly using aequorin-transgenic soybean cell cultures. The raw luminescence values presented here could have been converted to an estimate of changes in cytosolic Ca2+ concentrations. However, the calibration method currently used to convert luminescence values into Ca2+ concentration is based on Ca2+ determination in animal cells and may lead to significant errors in transgenic plant cells (Blinks et al., 1978). In our view, the raw luminescence data presented are well suited to present the results since they accurately reflect the striking differences in the shape and kinetics of the Ca2+ signals induced by the different elicitors (McAinsh and Hetherington, 1998).
By blocking the elicitor-induced changes in Ca2+ currents with BAPTA or reducing the release of Ca2+ from internal stores with neomycin, the decrease in tubB1 mRNA levels was no longer observed suggesting that Ca2+ might be involved in the regulation of the tubB1 message levels. High Ca2+ concentrations are known to destabilize plant microtubules (Fisher et al., 1996) and therefore might induce tubB1 mRNA degradation. Many elicitors induce increases in cytosolic Ca2+ concentrations in our cell culture each with a specific Ca2+ signature (McAinsh and Hetherington, 1998). Only the specific glucan-induced Ca2+ increase is correlated with the down-regulation of tubB1 mRNA levels. In contrast, CH4 and flagellin, although they increase the cytosolic CÇ2+ concentrations, appear to do so in a different manner and no tubB1 degradation is observed. Despite inducing only a small Ca2+ increase, flg22 induces an increase of the 4CL mRNA level. 4CL induction was also observed in response to glucan fragments after a pre-incubation with 1 mm neomycin. The 4CL induction might then be related to the primary Ca2+ increase, which is not affected by neomycin, or to an incomplete blockage of the second Ca2+ increase. Therefore, the signals leading to the induction of the 4CL gene and the tubB1 down-regulation appear to be different and both genes might respond to distinct aspects of the Ca2+ signature (McAinsh and Hetherington, 1998). The Ca2+ channel blocker LaCl3 strongly reduces the glucan-induced Ca2+ signal (Mithöfer et al., 1999). However, it blocks the calcium entry only partially, and its effects on the tubB1 mRNA degradation therefore might be incomplete and difficult to monitor accurately. To decode exactly the Ca2+ signal responsible for the tubB1 repression and the 4CL induction, it would be helpful to be able to visualize the Ca2+ elevation and its spatio-temporal distribution in single cells.
It has been described previously that fungal infection leads to a local microtubule depolymerization (Gross et al., 1993), but we did not observe any effects on the cytoskeleton in soybean cells up to 4 h after applying the elicitor. This does not rule out that glucan elicitors induce changes in the microtubule organization of soybean cells. Only important modifications such as major depolymerization can be observed by the existing technology. Subtle changes might have been missed. Moreover, we used commercially available anti-tubulin antibodies directed against common tubulin epitopes. These antibodies might not be appropriate to detect changes only at the level of tubB1 mRNA. However, Gus-Mayer et al. (1998) showed that elicitor by itself (Pep-25) does not induce microtubule depolymerization in parsley cells and that only mechanical stress mimicking pathogen entry does so. It will be interesting to infect the aequorin-transgenic soybean cells with P. sojae and look at tubB1 expression and microtubule organization together with Ca2+ fluxes in the infected cells.
In conclusion, the data presented report a possible mechanism of targeted mRNA decay induced with specific elicitors that may involve Ca2+ signaling.
MATERIALS AND METHODS
Plant Material
Suspension-cultured soybean (Glycine max) cells, derived from wild-type SB-P (Horn et al., 1983), transgenic for apoaequorin (line 6.6.12), were described earlier by Mithöfer et al. (1999). The cells were grown under constant light conditions (3,000 lux) on a rotary shaker (125 rpm) in Murashige and Skoog medium (Duchefa Biochemie, Haarlem, The Netherlands) supplemented with 5 g L−1 Suc, 1 mg L−1 α-naphthylacetic acid, and 0.2 mg L−1 kinetin.
Aequorin Reconstitution and Luminescence Measurements
Intracellular Ca2+ measurements were performed as described by Mithöfer et al. (1999).
Chemicals
The glucan fragments DP 7–15 were kindly provided by Jürgen Ebel's group (University of Munich). The N,N′,N′′, N′"-tetraacetylchitotetraose (CH4) and cordycepin were purchased from Sigma (Munich). Neomycin sulfate and BAPTA [1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetrasodium salt] were purchased from Fluka (Milwaukee, WI).
Reverse Transcription PCR
Total RNA was extracted from 100 mg (fresh weight) of transgenic soybean cultured cells subjected to the different treatments, using the TRIZOL reagent (Life Technologies/Gibco-BRL, Gaithersburg, MD) according to the manufacturer's protocol.
Five micrograms of total RNA was used for reverse transcription with the Ready-to-Go You-Prime-First strand beads (Pharmacia Biotech, Piscataway, NJ) using oligod(T)15 as primer. PCR was then performed on a 100-fold dilution of the cDNA with the following specific primers: for tubB1, 5′-TCTGAACCACTTGATCTCAAC-3′ and 5′-GCCATGGCTTCGTCCTCG-3′; for tubB2, 5′-CTTGAACCATCTGATCTCAGC-3′ and 5′-GTTGAAGCCATCCT-CAAGCCAG-3′; for 4CL, 5′-GAGGCTCTCCGGAACA-GG-3′ and 5′-CTATAAAC-TCTTTTACAGCCTC-3′; and for apoaequorin, 5′-CCACATCAAATCTCCAGTTGA-3′ and 5′-GGACAGCTCCACCGTAGAGCT-3′. The different PCR reactions were performed in single tubes to avoid primer competition (Knight et al., 1996). The conditions used were the following: 2 min denaturation at 94°C, 35 cycles of 30 s denaturation at 94°C, 30 s annealing at 60°C, 1 min elongation at 72°C, 10 min final elongation at 72°C using Taq DNA polymerase from Qiagen USA (Valencia, CA). The PCR products were then separated by electrophoresis in 1% (w/v) agarose gels stained with ethidium bromide.
RNase Protection Assay
RNase A/T1 protection assay was performed as described by Ausubel et al. (1987). The tubB1 3′-end probe was prepared from a cloned 3′-end cDNA of tubB1 yielding an antisense RNA probe that overlaps the final 154 nucleotides of tubB1. The tubB2 probe was made from a cloned 3′-end cDNA of tubB2 protecting a 343 nucleotides fragment. The linearized plasmids were transcribed in the presence of [α-32P]-UTP with a specific activity of 800 mCi mmol−1 (Amersham-Pharmacia Biotech, Uppsala) using T7 (tubB1 probe) and SP6 (tubB2 probe) RNA polymerase (Roche Molecular Biochemicals, Rotkreuz, Switzerland). Labeled probes (20,000 cpm for 5 μg of target RNA) were hybridized to 50 μg of total RNAs. The resulting hybrids were digested with RNAses A/T1 (80 μg mL−1 RNase A and 12 units mL−1 RNase T1) at 26°C for 50 min. Protected fragments were separated on 6% (v/v) polyacrylamide-8 m urea gels. Radioactivity of protected fragments was quantitated with a Storm 860 PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Immunostaining
Soybean cells subjected to the different treatments were fixed 15 min at RT in fixation buffer (3.7% [w/v] paraformaldehyde, 0.05% [v/v] Triton X-100 in MSB/glycerol buffer (20 mm PIPES [1,4-piperazinediethanesulfonic acid], pH 6.9, 8 mm EGTA, 1 mm MgCl2, 2% [v/v] glycerol), washed 4 times during 30 min in MSB/glycerol containing 0.05% (v/v) Triton X-100. Fixed cells were spread on poly-Lys cover slides, subjected to 10-min enzymatic cell wall digestion at RT {0.02% [w/v] pectolyase, 0.1% [w/v] macerozyme, 0.3% [w/v] caylase in digestion buffer: 25 mm MES [2-(N-morpholino)ethanesulfonic acid], pH 5.5, 8 mm CaCl2, 625 mm mannitol} and washed twice with phosphate-buffered saline, 0.05% (v/v) Triton X-100 for 10 min. After blocking with 5% (w/v) normal goat serum for 20 min at room temperature, the cells were incubated with 1/5,000 dilution of mouse anti-β-tubulin primary antibody (Amersham-Pharmacia Biotech) overnight at 4°C. The secondary goat anti-mouse IgG antibody labeled with fluorescein (1/250 dilution; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) was applied for 1 h at room temperature after washing with phosphate-buffered saline, 0.1% (w/v) bovine serum albumin. The cover slides were mounted in anti-fading agent containing 1 μg mL−1 Hoechst 33342 (Sigma). The cells were observed using an inverted microscope (Leica Microsystems, Wetzlar, Germany) equipped with a TCS confocal system and an Ar/Kr laser and through a 100× objective. Stacks images were analyzed using the Imaris program (Bitplane, Zurich).
ACKNOWLEDGMENTS
We are grateful to Prof. Jürgen Ebel for kindly providing glucan elicitors. We thank Drs. Margaret Collinge, Axel Mithöfer, and Bruno Tinland for critically reading the manuscript. We also thank Prof. Anne-Marie Lambert for helpful discussions.
Footnotes
This work was supported by the Swiss National Science Foundation (grant no. 31–047269.96 to G.N.-U. and T.B.).
LITERATURE CITED
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology. Massachusetts General Hospital Harvard Medical School; 1987. [Google Scholar]
- Baskin TI, Wilson JE. Inhibitors of protein kinases and phosphatases alter root morphology and disorganize cortical microtubules. Plant Physiol. 1997;113:493–502. doi: 10.1104/pp.113.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A, Vasiliev JM. Cytoskeleton. New York: Plenum Publishing; 1988. [Google Scholar]
- Blancaflor EB, Jones DL, Gilroy S. Alterations in the cytoskeleton accompany aluminum-induced growth inhibition and morphological changes in primary roots of maize. Plant Physiol. 1998;118:159–172. doi: 10.1104/pp.118.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks JR, Mattingly PH, Jewell BR, van Leeuwen M, Harrer GC, Allen DG. Practical aspects of the use of aequorin as a calcium indicator: assay, preparation, microinjection and interpretation of signals. Methods Enzymol. 1978;57:292–328. [Google Scholar]
- Boller T. Chemoperception of microbial signals in plant cells. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:189–214. [Google Scholar]
- Chiba Y, Ishikawa M, Kijima F, Tyson RH, Kim J, Yamamoto A, Nambara E, Leustek T, Wallsgrove RM, Naito S. Evidence for autoregulation of cystathionine γ-synthase mRNA stability in Arabidopsis. Science. 1999;286:1371–1374. doi: 10.1126/science.286.5443.1371. [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE. Water deficit modulates gene expression in growing zones of soybean seedlings: analysis of differentially expressed cDNAs, a new β-tubulin gene, and expression of genes encoding cell wall proteins. Plant Mol Biol. 1991;17:591–608. doi: 10.1007/BF00037046. [DOI] [PubMed] [Google Scholar]
- Cyr RJ. Microtubule in plant morphogenesis: role of the cortical array. Annu Rev Cell Biol. 1994;10:153–180. doi: 10.1146/annurev.cb.10.110194.001101. [DOI] [PubMed] [Google Scholar]
- Drewes G, Ebneth A, Mandelkow EM. MAPs, MARKs and microtubule dynamics. Trends Biol Sci. 1998;23:307–311. doi: 10.1016/s0968-0004(98)01245-6. [DOI] [PubMed] [Google Scholar]
- Ebel J. Oligoglucoside elicitor-mediated activation of plant defense. Bioessays. 1998;20:569–576. doi: 10.1002/(SICI)1521-1878(199807)20:7<569::AID-BIES8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Ehrhardt DW, Wais R, Long SR. Calcium spiking in plant root hairs responding to Rhizobium nodulation signals. Cell. 1996;85:673–681. doi: 10.1016/s0092-8674(00)81234-9. [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Fisher DD, Gilroy S, Cyr RJ. Evidence for opposing effects of calmodulin on cortical microtubules. Plant Physiol. 1996;112:1079–1087. doi: 10.1104/pp.112.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giani S, Breviario D. Rice β-tubulins mRNA levels are modulated during flower development and in response to external stimuli. Plant Sci. 1996;116:147–157. [Google Scholar]
- Giani S, Qin X, Faoro F, Breviario D. In rice, oryzalin and abscisic acid differentially affect tubulin mRNA and protein levels. Planta. 1998;205:334–341. doi: 10.1007/s004250050328. [DOI] [PubMed] [Google Scholar]
- Gross P, Julius C, Schmelzer E, Hahlbrock K. Translocation of cytoplasm and nucleus to fungal penetration sites is associated with depolymerization of microtubules and defense gene activation in infected, cultured parsley cells. EMBO J. 1993;12:1735–1744. doi: 10.1002/j.1460-2075.1993.tb05821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiltinan MJ, Ma P, Barker RF, Bustos MM, Cyr RJ, Ramin Y, Fosket DE. The isolation, characterization and sequence of two divergent β-tubulin genes from soybean (Glycine max L.) Plant Mol Biol. 1987;10:171–184. doi: 10.1007/BF00016154. [DOI] [PubMed] [Google Scholar]
- Gus-Mayer S, Naton B, Hahlbrock K, Schmelzer E. Local mechanical stimulation induces components of the pep25 defense response in parsley. Proc Natl Acad Sci USA. 1998;95:8398–8403. doi: 10.1073/pnas.95.14.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han IS, Jongewaard I, Fosket DE. Limited expression of diverged β-tubulin gene during soybean (Glycine max [L.] Merr.) development. Plant Mol Biol. 1991;16:225–234. doi: 10.1007/BF00020554. [DOI] [PubMed] [Google Scholar]
- Heinlein M, Epel BL, Padgett HS, Beachy RN. Interaction of tobamovirus movement proteins with the plant cytoskeleton. Science. 1995;270:1983–1985. doi: 10.1126/science.270.5244.1983. [DOI] [PubMed] [Google Scholar]
- Holtorf H, Schöb H, Kunz C, Waldvogel R, Meins F., Jr Stochastic and nonstochastic post-transcriptional silencing of chitinase and β-1,3-glucanase genes involves increased RNA turnover: possible role for ribosome-independent RNA degradation. Plant Cell. 1998;11:471–483. doi: 10.1105/tpc.11.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn ME, Sherrard JH, Wildholm JM. Photoautotrophic growth of soybean cells in suspension culture. Plant Physiol. 1983;72:426–429. doi: 10.1104/pp.72.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Cold calcium signaling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell. 1996;8:489–503. doi: 10.1105/tpc.8.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Trewavas AJ, Knight MR. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. 1997;12:1067–1078. doi: 10.1046/j.1365-313x.1997.12051067.x. [DOI] [PubMed] [Google Scholar]
- Knight MR, Campbell AK, Smith SM, Trewavas AJ. Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature. 1991;352:524–526. doi: 10.1038/352524a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kobayashi I, Funaki Y, Fujimoto S, Takemoto T, Kunoh H. Dynamic reorganization of microfilaments and microtubules is necessary for the expression of non-host resistance in barley coleoptile cells. Plant J. 1997;11:525–537. [Google Scholar]
- Kopczak SD, Haas NA, Hussey PJ, Silflow CD, Snustad DP. The small genome of Arabidopsis contains at least six expressed α-tubulin genes. Plant Cell. 1992;4:539–547. doi: 10.1105/tpc.4.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E, Benedyk M, Chua NH. Characterization of phytochrome-regulated gene expression in a photoautotrophic cell suspension: possible role for calmodulin. Mol Cell Biol. 1989;9:4819–4823. doi: 10.1128/mcb.9.11.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Penell RI, Alvarez ME, Palmer R, Lamb C. Calcium-mediated apoptosis in a plant hypersensitive disease resistance response. Curr Biol. 1996;1:427–437. doi: 10.1016/s0960-9822(02)00510-9. [DOI] [PubMed] [Google Scholar]
- Logemann E, Wu SC, Schröder J, Schmelzer E, Somssich IE, Hahlbrock K. Gene activation by UV light, fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-regulated genes. Plant J. 1995;8:865–876. doi: 10.1046/j.1365-313x.1995.8060865.x. [DOI] [PubMed] [Google Scholar]
- Mazars C, Thion L, Thuleau P, Graziana A, Knight MR, Moreau M, Ranjeva R. Organization of cytoskeleton controls changes in cytosolic calcium of cold-shocked Nicotiana plumbaginifolia protoplasts. Cell Calcium. 1997;22:413–420. doi: 10.1016/s0143-4160(97)90025-7. [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Hetherington AM. Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 1998;3:32–36. [Google Scholar]
- Mithöfer A, Ebel J, Bhagwat AA, Boller T, Neuhaus-Url G. Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with β-glucan or chitin elicitors. Planta. 1999;207:566–574. [Google Scholar]
- Moore RC, Zhang M, Cassimeris L, Cyr RJ. In vitro assembled plant microtubules exhibit a high state of dynamic instability. Cell Motil Cytoskelet. 1997;38:278–286. doi: 10.1002/(SICI)1097-0169(1997)38:3<278::AID-CM6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi M, Taylor CB, Newman TC, Green PJ. The effect of sequences with high AU content on mRNA stability in tobacco. Proc Natl Acad Sci USA. 1993;90:11811–11815. doi: 10.1073/pnas.90.24.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam-Evans C, Harmon AC, Palevitz BA, Fechheimer M, Cormier MJ. Calcium-dependent protein kinase is localized with F-actin in plant cells. Cell Motil Cytoskelet. 1989;12:12–22. [Google Scholar]
- Rushton PJ, Somssich IE. Transcriptional control of plant genes responsive to pathogens. Curr Opin Plant Biol. 1998;1:311–315. doi: 10.1016/1369-5266(88)80052-9. [DOI] [PubMed] [Google Scholar]
- Schellenbaum P, Vantard M, Lambert AM. Higher plant microtubule-associated proteins (MAPs): a survey. Biol Cell. 1992;76:359–364. [Google Scholar]
- Sedbrook JC, Kronebusch PJ, Borisy GG, Trewavas AJ, Masson PH. Transgenic aequorin reveals organ-specific cytosolic Ca2+ responses to anoxia in Arabidopsis thaliana seedlings. Plant Physiol. 1996;111:243–257. doi: 10.1104/pp.111.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaoka H. Plant hormones-induced changes in the orientation of cortical microtubules. Annu Rev Cell Biol. 1994;45:527–544. [Google Scholar]
- Snustad DP, Haas NA, Kopczak SD, Silflow CD. The small genome of Arabidopsis contains at least nine expressed β-tubulin genes. Plant Cell. 1992;4:549–556. doi: 10.1105/tpc.4.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotz HU, Long SR. Expression of the pea (Pisum sativum L.) α-tubulin gene TubA1 is correlated with cell division activity. Plant Mol Biol. 1999;41:601–614. doi: 10.1023/a:1006338401808. [DOI] [PubMed] [Google Scholar]
- Theodorakis NG, Cleveland DW. Physical evidence for cotranslational regulation of β-tubulin mRNA degradation. Mol Cell Biol. 1992;12:791–799. doi: 10.1128/mcb.12.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmers AC, Auriac MC, De Billy F, Truchet G. Nod factors internalization and microtubular cytoskeleton changes occur concomitantly during nodule differentiation in alfalfa. Development. 1998;125:339–349. doi: 10.1242/dev.125.3.339. [DOI] [PubMed] [Google Scholar]
- Tonoike H, Han IS, Jongewaard I, Doyle M, Guiltinan M, Fosket DE. Hypocotyl expression and light down-regulation of the soybean tubulin gene, tubB1. Plant J. 1994;5:343–351. doi: 10.1111/j.1365-313x.1994.00343.x. [DOI] [PubMed] [Google Scholar]
- Uhlmann A, Ebel J. Molecular cloning and expression of 4-coumarate:coenzyme A ligase, an enzyme involved in the resistance response of soybean (Glycine max L.) against pathogen attack. Plant Physiol. 1993;102:1147–1156. doi: 10.1104/pp.102.4.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto N, Kakitani M, Iwamatsu A, Yoshikawa M, Yamaoka N, Ishida I. The structure and function of a soybean β-glucan-elicitor-binding protein. Proc Natl Acad Sci USA. 1997;94:1029–1034. doi: 10.1073/pnas.94.3.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M. Microbial utilization of human signalling molecules. Microbiology. 1996;142:3319–3336. doi: 10.1099/13500872-142-12-3319. [DOI] [PubMed] [Google Scholar]
- Whitehead LF, Day DA, Hardham AR. Cytoskeletal arrays in the cells of soybean root nodules: the role of actin microfilaments in the organisation of symbiosomes. Protoplasma. 1998;203:194–205. [Google Scholar]
- Xu H, Heath MC. Role of calcium in the signal transduction during the hypersensitive response caused by basidiospore-derived infection of the cowpea rust-fungus. Plant Cell. 1998;10:585–597. doi: 10.1105/tpc.10.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Sheng J, Liu Y, Mehdy MC. Fungal elicitor-induced bean proline-rich protein mRNA down-regulation is due to destabilization that is transcription and translation dependent. Plant Cell. 1993;5:1089–1099. doi: 10.1105/tpc.5.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Mehdy MC. Binding of a 50-kD protein to a U-rich sequence in an mRNA encoding a proline-rich protein that is destabilized by fungal elicitor. Plant Cell. 1994;6:135–145. doi: 10.1105/tpc.6.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann S, Ehrhardt T, Plesch G, Müller-Röber B. Ion channels in plant signaling. Cell Mol Life Sci. 1999;55:183–203. doi: 10.1007/s000180050284. [DOI] [PMC free article] [PubMed] [Google Scholar]