Abstract
Inflammatory bowel disease (IBD) is a chronic recurrent inflammation of the gastrointestinal tract (GIT). IBD patients are susceptible to various infections such as viral infections due to the long-term consumption of immunosuppressive drugs and biologics. The antiviral and IBD protective traits of flavonoids have not been entirely investigated. This study objective included an overview of the protective role of flavonoids quercetin and silymarin in viral-associated IBD. Several viral agents such as cytomegalovirus (CMV), Epstein–Barr virus (EBV), varicella zoster virus (VZV) and enteric viruses can be reactivated and thus develop or exacerbate the IBD conditions or eventually facilitate the disease remission. Flavonoids such as quercetin and silymarin are non-toxic and safe bioactive compounds with remarkable anti-oxidant, anti-inflammatory and anti-viral effects. Mechanisms of anti-inflammatory and antiviral effects of silymarin and quercetin mainly include immune modulation and inhibition of caspase enzymes, viral binding and replication, RNA synthesis, viral proteases and viral assembly. In the nutraceutical sector, natural flavonoids low bioavailability and solubility necessitate the application of delivery systems to enhance their efficacy. This review study provided an updated understanding of the protective role of quercetin and silymarin against viral-associated IBD.
Keywords: Inflammatory bowel diseases, Viral infections, Quercetin, Silymarin, Antiviral traits
Introduction
Inflammatory bowel disease (IBD) includes the persistent/chronic or recurrent inflammation of the GIT epithelium mainly including ulcerative colitis (UC), involving the sigmoid and colon, and Crohn’s disease (CD), involving the entire GIT (Song and Wu 2022). Another heterogeneous disease process has been identified and classified as IBD unclassified (IBDU) with periods of remission and exacerbation (Malik 2015). The disease mostly affects individuals aged 20–40 years and influences all living aspects posing a considerable socioeconomic burden (Burisch et al. 2023). The relapse and remission of IBD is a great concern among patients with a rate of 50–80% (Cohen et al. 2010). The chronic trend of the IBD needs long-term protection which in turn increases the costs. The supposed pathophysiological mechanism of the disease includes severe immune responses in the gut epithelium particularly among genetically predisposed individuals (Su et al. 2019; Pavel et al. 2021b). The IBD has four stages (emergence, acceleration in incidence, compounding prevalence, prevalence equilibrium) according to the developing conditions of societies worldwide (Molodecky and Kaplan 2010; Kaplan and Windsor 2021). Major etiological agents of IBD include genetic basis, aberrant immune responses and epigenetic factors (Sheehan et al. 2015; Dudzińska et al. 2018). Single-nucleotide polymorphisms (SNPs) and different expression levels of immune-responsive genes such as G protein-coupled receptor 183 (GPR183), IL23/Th1/Th17 pathway (IL23R, DLG5, JAK2, STAT3 and TYK2), predispose individuals to the IBD development (Fransen et al. 2014; Tillack et al. 2014; Dudzińska 2020; Ruiz et al. 2021).
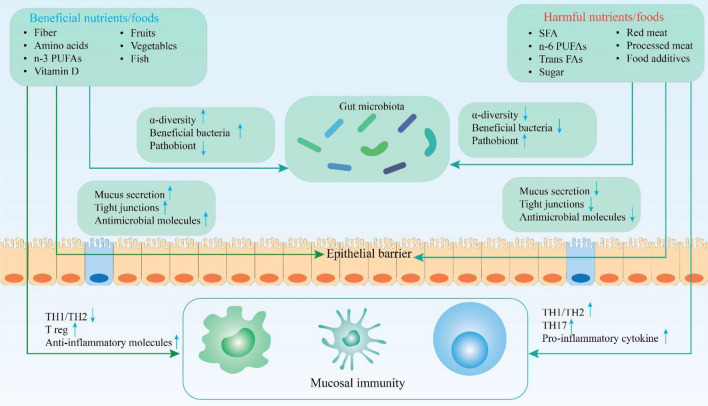
The human gut microbiota plays a crucial role in GIT health through the regulation of inflammatory responses and hence control of the IBD pathophysiology (Wallace et al. 2014; Andreou et al. 2020; Kumari et al. 2022) (Figs. 1 and 2). Various conditions which disrupt the balance and diversity of gut microbiota (dysbiosis) and intestinal homeostasis lead to the growth of pathogens and the consequent initiation of systemic inflammation. Lifestyle, diet, antibiotic consumption, environmental factors and stress conditions include potential risk factors (Jostins et al. 2012; Sheehan et al. 2015; Statovci et al. 2017; Cui and Yuan 2018; Piovani et al. 2019; Andreou et al. 2020; Miri et al. 2022). The gut microbiota dysbiosis in the IBD can be translated into a decrease in Suterella, Bacteroides and Saccharomyces cervisiae, Faecalibacterium prausnitzii, Roseburia species, Bifidobacterium spp and Groups IV and XIVA of Clostridium (Rivière et al. 2016; Pavel et al. 2021a). Additionally, a related increase in Caudovirales, Clavispora lusitaniae, Pasteurellaceae, Veillonellaceae, Fusobacterium species, Ruminococcus gnavusa, Proteobacteria and adherent invasive Escherichia coli pathogens has been documented (Sokol et al. 2017). In this context, probiotics can be also of help. The protective role of probiotics includes inhibition of pro-inflammatory cytokines and interleukins release, Toll-like receptors (TLR)-mediated inflammation, regulation of T helper (Th) cells and increase in mucin genes expression (e Silva et al. 2020; Pavel et al. 2021a). In addition, probiotics mitigate the risk of hereditary IBD. Lactobacillus and Bifidobacterium species figure out between the major probiotics play key roles in GIT health, applied to heal IBD or UC complications (Pavel et al. 2021a; Miri et al. 2022). Figures 1 and 2 describe the variety of mechanisms by which the gut microbiota contribute to protect the mucosal barrier and to prevent epithelial inflammation.
Fig. 1.
The role of gut microbiota in GIT (gastrointestinal tract) health and IBD (inflammatory bowel disease) prevention; the consumption of vegetables and fruits, fiber, vitamins and n-3 PUFAs (polyunsaturated fatty acids) results in the gut microbiota prospering and proliferation exerting anti-inflammatory effects and offering the GIT protection. Additionally, these healthy foods directly regulate the Th1/Th2 cells and increase Treg and anti-inflammatory cytokines providing health conditions for the epithelial barrier. On the contrary, unhealthy foods such as SFAs, n-6 PUFAs, trans-FAs, sugar, red meat, processed meat and food additives decrease the microbial diversity and also beneficial bacteria. These conditions lead to the pathobionts population increase and subsequent development of epithelial inflammation (Jostins et al. 2012; Wallace et al. 2014; Cui and Yuan 2018; Andreou et al. 2020; Li et al. 2022; Liu et al. 2022). PUFAs poly-unsaturated fatty acids, TH1 T helper 1 or cytotoxic T cells, TH2 T helper 2 cells, Treg regulatory T cells, FAs fatty acids, SFAs saturated fatty acids
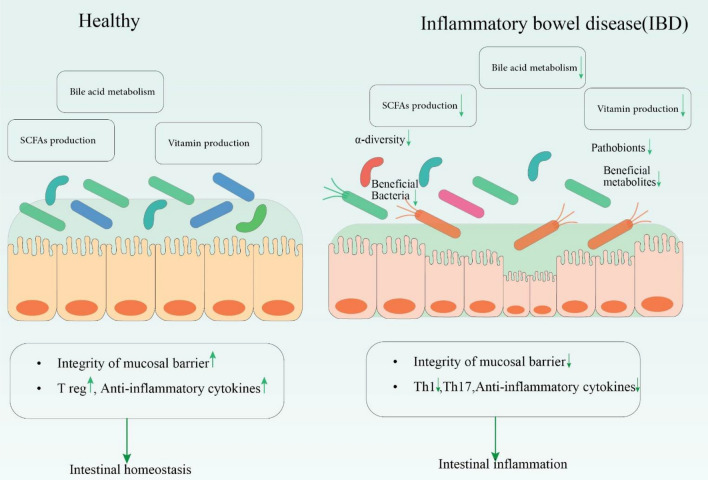
Fig. 2.
The imbalance of Gut microbiota causing the IBD progression; a healthy gut preserves intestinal homeostasis via sufficient production of short-chain fatty acids (SCFAs) and vitamins and bile acid metabolism which maintain the balance within the gut microbiota and lessens the inflammation. However, in IBD conditions, these products are decreased leading to a low diversity of beneficial bacteria and their metabolites, resulting in gut inflammation. Th T helper cell, Treg regulatory T cell
It is worth mentioning that the toxicity and costs of chemotherapy are much higher than those of herbal medicines (HMs) bioactive compounds, both eliciting equal anti-inflammatory effects (Hess et al. 2016; Yajima et al. 2016; Assiry et al. 2022; Safarpour et al. 2022; Sun et al. 2022; Vazifeh et al. 2022). As secondary metabolites, flavonoids confer a myriad of health benefits such as antiviral, antimicrobial, anti-inflammatory, antioxidant, anticancer, antidiabetic and cardioprotective effects (Roy et al. 2022). In a review study, the antiviral effects of naringenin, rutin, quercetin, vitexin and apigenin were assessed. Their main mechanisms of action included viral protein modifications and inactivation of viral DNA/RNA polymerases, proteases and neuraminidase (Ninfali et al. 2020). The non-toxicity of flavonoids and antiviral effects of quercetin (inhibiting influenza A neuraminidase) and silymarin (against hepatitis C virus) have been demonstrated (Ferenci et al. 2008; Wu et al. 2015; Badshah et al. 2021a). Although numerous flavonoids have demonstrated anti-inflammatory and antiviral effects, the current study offered insights on the efficacy of quercetin (a flavanol) and silymarin (also flavo-lignane). Quercetin and silymarin (Fig. 3) flavonoids applied in various formulations have proved numerous health-related effects such as antioxidant, anti-inflammatory and antiviral properties, while exerting low side effects and being available at competitive price (Maisuthisakul et al. 2008; Badshah et al. 2021b; Jannat et al. 2021).
Fig. 3.
Structure of quercetin and silymarin
Figure 4 describes anti-inflammatory effects of quercetin via regulation of various signaling pathways.
Fig. 4.
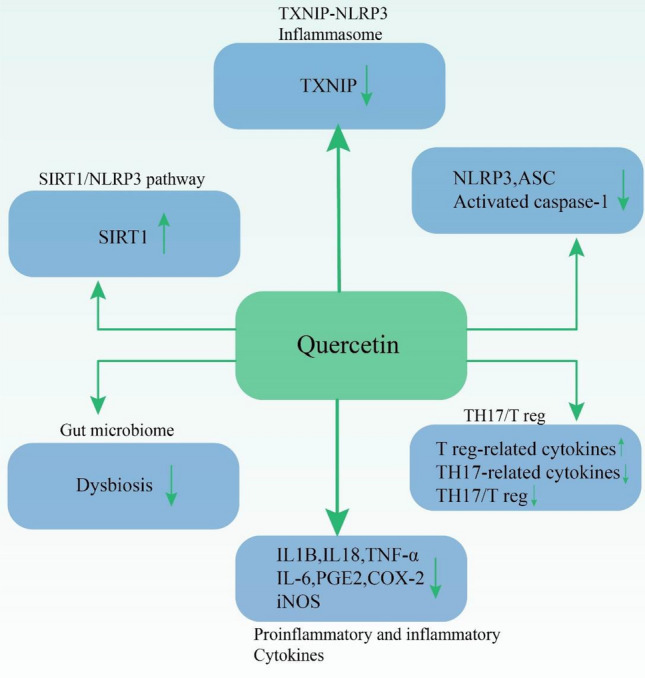
The anti-inflammatory effects of quercetin via regulation of various signaling pathways such as TXNIP-NLRP3 inflammasome, SIRT1-NLRP3 pathway, NLRP3/ASC caspase activating, gut microbiome maintenance and T cells and cytokines/chemokines regulation; TXNIP thioredoxin (TRX)-interacting protein, NLRP3 NOD-like receptor family pyrin domain-containing 3, SIRT1 sirtuin (silent mating-type information regulation 2 homolog) 1, ASC apoptotic speck-like protein containing a caspase recruitment domain, Th T helper cell, Treg regulatory T cell, IL interleukin, TNF-α tumor necrosis factor α, PGE-2 prostaglandin E2, COX-2 cyclooxygenase-2, iNOS inducible nitric oxide synthase. Silibinin is the major active component of silymarin which also exhibits antioxidative, anticancer and antimicrobial traits (Ravichandran et al. 2010; Mariño et al. 2013; Rendina et al. 2014)
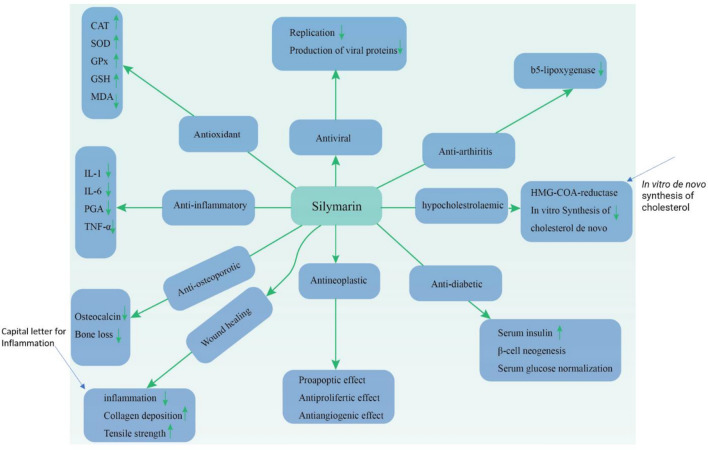
Silymarin has numerous health benefits and exerts its effects via various molecular mechanisms. Figure 5 describes the antiviral, antioxidant, anti-arthritis, anti-inflammatory, antidiabetic, protective and wound healing mechanisms of action of silymarin.
Fig. 5.
The protective and effects of silymarin including antiviral, antioxidant, anti-arthritis, anti-inflammatory, antidiabetic protective and wound healing mechanisms; GPx glutathione peroxidase, CAT catalase, MDA malondialdehyde, GSH glutathione, SOD superoxide dismutase, PGA prostaglandin, IL interleukin, TNF-α tumor necrosis factor α, HMG-CoA 3-hydroxy-3-methylglutaryl coenzyme A (Saeed et al. 2017). Details of association of various viral agents in IBD and each flavonoid antiviral effects have not been evaluated. The objective of this review was to provide an update regarding the effects of viral agents on IBD development and antiviral properties of quercetin and silymarin considering their anti-inflammatory effects
Methods
In this review, the viral agents causing the IBD as well as the antiviral and IBD-protective role of quercetin and silymarin were evaluated according to previous published data. Search engines including Google Scholar, PubMed, Scopus and Web of Science were used. Key words included “inflammatory bowel disease,” “viruses,” “viral agents,” “inflammation,” “immunosuppression,” “silymarin” and “quercetin.” Only related scientific publications were included during 2010–2023. Those publications without full-text availability and non-English papers were excluded. All publications with in vitro, in vivo and clinical trial surveys were included but in silico studies were excluded (Fig. 6).
Fig. 6.
Flowchart of the methodology applied in this review
Viral agents and the IBD
The IBD occurs and develops as a consequence of genetic and environmental factors which further develop the intestinal epithelium inflammation (Molodecky and Kaplan 2010; Sheehan et al. 2015; Kaplan and Windsor 2021). The suppressed or low immune responses following drug consumption play a crucial role in the proliferation of microbial agents leading to viral infections (Masclee et al. 2013; Dudzińska et al. 2018). Among IBD patients, viral infections mostly occur at ages < 35 years and include 40% of total opportunistic infections (Kirchgesner et al. 2018; Wisniewski et al. 2020). Several treatments such as corticosteroids (prednisolone above 20 mg), anti-TNF + thiopurine combination and azathioprine and mercaptopurine (AZA/6MP) have developed viral infections supposedly by suppression of T cells and other immunity responses (Craviotto et al. 2021). Major viral agents causing opportunistic infections in these conditions include cytomegalovirus (CMV), herpes simplex virus (HSV), Epstein–Barr virus (EBV) and varicella zoster virus (VZV) (Craviotto et al. 2021). Other viral agents reported in the IBD have included severe acute respiratory syndrome coronavirus 2 (SARS- CoV-2), influenza virus types A and B, hepatitis A, B and C viruses (HAV, HBV and HCV, respectively), human papilloma virus (HPV) and human immunodeficiency virus (HIV) (Masclee et al. 2013; Tarris et al. 2021). These infections are consequent to alterations in host responses, including neutropenia and lymphopenia following utilization of immunomodulators. Inadequate immunization of IBD patients is another predisposing factor which leads to excess of hospitalization and increases the burden of costs in the health system. Accordingly, gastroenterologists should be aware of viral infections which are difficult to diagnose posing a high rate of morbidity and mortality. A combination regimen of thiopurines and antitumor necrosis factor α (TNF-α) agents and lower doses of systemic steroids are more effective to restrict opportunistic viral infections. Main mechanisms of IBD development following viral infections include induction of pro-inflammatory cytokines and chemokines, cytokine storm and Th cells multiplication leading to inflammation and epithelium damage (Masclee et al. 2013; Dudzińska et al. 2018; Tarris et al. 2021).
Cytomegalovirus and varicella zoster and IBD
The cytomegalovirus (CMV) is a member of Herpes viridae family which has a high rate of infection with lifelong dormant phase around the world in particular among those individuals with compromised immune system (Craviotto et al. 2021). Regarding the CMV pathophysiology, the virus enters monocytes, endothelial cells, fibroblasts or granulocyte stem cells and remains in epithelial cells due to the activity of immune compartments, particularly of natural killer (NK) cells (Jentzer et al. 2020; Mourad et al. 2020). In conditions of impaired NK activity and mucosal immunity or inherent impairment, UC patients are predisposed to the infection. Immunosuppression (impairing T lymphocytes), corticosteroids (causing CMV reactivation), cytokines, especially TNF-α (inducing viral replication) and pro-inflammatory cytokines (IL-6) production (exacerbating the colitis conditions) include mechanisms promoting the CMV-mediated IBD pathophysiology (Lawlor and Moss 2010; Lee and Eun 2022). Conversely, CD4 + T cells produce interferon alpha (IFN-α) in the CD which precludes the CMV reactivation resulting in lower rate of infection and consequent inflammation (Nakase et al. 2010; McCurdy et al. 2015). The CMV and IBD association which can exacerbate the IBD conditions has been described for long years, though debates remains open on the development of severe disease (Powell et al. 1961; Luangsirithanya et al. 2021; O’Connor 2021). The CMV rate of infection in China has been higher in the UC than CD (Yang and Qian 2022). The inflammation of the epithelium is consequent to the stimulation of immune responses by the CMV proteins (Lv et al. 2017). In IBD patients, the proinflammatory cytokines such as TNF-α, interferon-γ and interleukin-2 (IL-2) released by inflammatory or epithelial cells result in the enhancement of chemokines and transcription molecules and subsequent CMV reactivation (Luangsirithanya et al. 2021). The CMV existence in colonic biopsies and its clinical relevance is controversial in spite of observations of refractory illness in pediatric patients (Hommes et al. 2004). However, immunosuppression is associated with the Herpes simplex colitis or UC (Temtem et al. 2021). CMV-associated colitis or UC has been linked to active illness, immunosuppressive medicine, steroid therapy, steroid-refractory disease progression and higher colectomy rates in IBD patients (with high-grade CMV infection) (Kjaer et al. 2018; Mourad et al. 2020; Hazır-Konya et al. 2021; Leal et al. 2022; Ouali and Achkar 2022). CMV has been also associated with early relapse of colitis. Several questions still need to be addressed such as the lack of a global standard definition for this association, the accurate diagnostic test, the exact timing of treatment and the potential role of CMV in the disease (Mourad et al. 2020). Several factors such as advanced age, pancolitis, female gender, low blood leukocytes count, steroids and azathioprine, infliximab (anti-TNF-α) and disease duration less than six months have been associated with the IBD induced by CMV infection (Kishore et al. 2004; Gauss et al. 2015; Campos et al. 2017; Shukla et al. 2017).
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes a severe respiratory disease (Bezzio et al. 2020; Jena et al. 2022) and novel variants of concern (VOCs) have emerged, conveying different clinical symptoms (Brodin 2021; Kennedy et al. 2021; Yang and Rao 2021). Considering the existence of angiotensin-converting enzyme 2 (ACE-2) receptor onto the respiratory and epithelial cells, the SARS-CoV-2 enters into the enterocytes (Puoti et al. 2021). Therefore, etiopathologic aspects mainly include, impaired ACE-2, absorption loss and GIT inflammation. The cytokine storm induced by SARS-CoV-2 is similar to that of the IBD (such as IL-1, IL-6 and TNF-α) (Aziz et al. 2020; Bezzio et al. 2020; Bodini et al. 2020; Barbalho et al. 2021; Hanrahan et al. 2021; Gray‐Rodriguez et al. 2022). Notably, consumption of the anti-TNF drug possibly downregulates the anti-SARS-CoV-2 immune responses which increase the pathologic consequences of various agents such as SARS-CoV-2, hepatitis B virus (HBV) and human immunodeficiency virus (HIV) (Abdullah et al. 2020; Neurath 2020; Wisniewski et al. 2020; Lee et al. 2022). A study demonstrated the microbiota dysbiosis following the SARS-CoV-2 infection which leads to IBD progress. They assumed that probiotics supplementation is promising to prevent and heal the IBD (Din et al. 2021). Other studies have not observed such an association regarding the SARS-CoV-2 (Aziz et al. 2020; Bodini et al. 2020; Jena et al. 2022). It is worth mentioning that the expression of trypsin-like proteases being responsible for the spike protein cleavage is increased among IBD patients and facilitate the infection initiation (Neurath 2020; Hanrahan et al. 2021). The ustekinumab drug (anti-p40 mAb) as the IL-12/IL-23 inhibitor has exhibited a therapeutic effect on IBD and SARS-CoV-2 eradication (Monteleone and Ardizzone 2020; Schmidt et al. 2021). As SARS-CoV-2 enhances the inflammation and develops the IBD, flavonoids inhibit various signaling pathways and encounter the cytokine storm following SARS-CoV-2 infection {Liskova et al. 2021). However, their effects on IBD were discussed via effects on NLRP3 inflammasome (Wang et al. 2018).
Epstein–Barr virus
As another member of the herpesvirus family, Epstein–Barr virus (EBV) has infected most of the world’s population (90%), in a form of latent lifelong infection, as an asymptomatic disease in childhood and as infectious mononucleosis (IM) in adulthood, a self-limiting disease (Jefremow and Neurath 2020; Zhang et al. 2022). In a systematic review and meta-analysis, using 11 metagenomic studies, the EBV plus CMV was determined as a significant cause of IBD (Marongiu et al. 2022). The EBV infecting B cells has been involved in a variety of autoimmune diseases and cancers (Çolak et al. 2018; Caetano et al. 2021; Wallaschek et al. 2021). Fatal lymphoproliferative disease is caused following viral reactivation mainly among immunosuppressed and vulnerable populations (Vockerodt et al. 2015). The virus has been associated with inflammatory responses, lymphoproliferative disorders (LPD), IBD, CD and UC detected within the epithelial cells (Spieker and Herbst 2000; Ryan et al. 2012; Li et al. 2019b; Wu et al. 2019; Xu et al. 2020; Miura et al. 2021). Long-term use of immunosuppressants or biologics and the inflammation of the epithelium due to the EBV infection result in EBV transformation (mostly within B cells and NK cells) from latent to lytic form, colitis and related lymphoproliferative diseases (Ciccocioppo et al. 2015; Nissen et al. 2015; Münz 2019). The EBV DNA provokes pro-inflammatory responses via toll-like receptor (TLR) signaling (Salloum et al. 2018). The EBV DNA can also enhance the expression of IFN-γ, IL-17A and TNF-α in the in vivo colitis model, exacerbating the colon damage (Wu et al. 2019; Andari et al. 2021) and leading to increase of disease severity (Kato et al. 2021). T cells, B cells and NK cells infections also occur, impairing cellular immunity. Additionally, the spread of infection into enterocytes causes epithelial or mucosal tissue damage (Zhang et al. 2022). The misdiagnosis may occur due to the similarity of IBD and EBV-associated lymphoproliferative disorders (LPDs) (Patton et al. 2018; Zhou et al. 2019).
Enteric viruses and the IBD
Viral enteric pathogens including Picornaviridae, Astroviridae, rotaviruses, enteroviruses, Calisiviridae, sapovirus, adenovirus and norovirus are human and animal pathogens replicating in the GIT (Khetsuriani et al. 2006; Craviotto et al. 2021). Some studies have demonstrated higher number of these enteric viruses in the IBD and CD. These viruses interact with the HBGA-like molecules and increase the inflammatory responses via IFN-β, TNF-α, IFN-γ IL-8, IL-17, IL-10, IL-25, TLR3, Th1 and Th17 enhancement during the IBD, CD and UC (Kolho et al. 2012; Yang et al. 2016). These agents have been increased during immunosuppression such as HIV patients. Rotavirus (RV) particularly species A causes infection of intestinal epithelial cells (IECs) in children. An immunosuppressive drug, 6-thioguanine (6-TG) restricts the RV replication in IBD patients via effect on the Ras-related C3 botulinum toxin substrate 1 (Rac1). The imbalance of gut microbiota such as Lactobacillus spp in the ileum has been associated with the increased number of RV. Noroviruses and Adenoviruses bind to α4β1 and α4β7 integrins receptors for leukocytes binding and can increase the expression of sialylated Lewis x (sLex) and Lewis a (sLea) antigens. However, enteric viruses have also reduced the intestinal inflammation via an increase in the IFNβ levels through TLR3 and TLR7 (Ilan et al. 2017; Reddy et al. 2018; Wang et al. 2021).
Hepatitis B and C and the IBD
The existence of HBV among IBD patients has been reported using hepatitis B core antigen (anti-HBc) and hepatitis B surface antigen (HBsAg/anti-HBs). A higher rate of HBsAg has been reported in previous studies (Ilan et al. 2017; Reddy et al. 2018). Due to the lack of sufficient vaccination against HBV among developing countries and higher levels of viral infection, the associated IBD risk has increased (Loras et al. 2010; Childs et al. 2018). The virus status and type of treatment determine the recurrence of HBV. The antiviral therapy should be continued for up to 6 months after immunosuppressive drugs intake (Park et al. 2020). The immunosuppressive treatment (anti-integrin drugs, infliximab, anti-TNF) of IBD patients is a risk factor for the recurrence of infection and poor response to HBV vaccination, resulting in hepatic fibrosis (through WNT-signaling PI3K/Akt/mTOR Ras/ERK1/2 and p53 pathways), impairment and death (Dougan et al. 2021). Therefore, sufficient immunity stimulation through vaccination prior to the treatment is pivotal for infection control. Notably, the anti-HBV response is mitigated among IBD patients even during vaccination.
The rate of HCV is estimated to be 0.7–2.5% worldwide (Lin et al. 2013; Blach et al. 2022). Drug injection, dialysis, Hansen’s disease or hemophilia, and maternal transmission are risk factors. HCV-positive IBD patients have developed auto-antibody responses which destroy the liver. It was found that INF therapy has no significant effect on the progress of IBD in HCV-positive patients. Pyoderma gangrenosum (PG) is also associated with HCV (Croitoru et al. 2021). Interestingly, age does not seem to be a determinant factor in the infection since a recent study in Italy revealed that older age is not affecting viral hepatitis–IBD association (Losurdo et al. 2020). Patients infected with genotype-1 and genotype-3 HCV have been more predisposed to develop colitis with higher ghrelin concentrations (Pavlidis et al. 2011). The decreased rate of publications regarding the HCV and the IBD association reflects the decreased HCV risk due to higher adherence to preventative measures and better aseptic perioperative rules (Flores et al. 2022; Giri et al. 2022).
Other viral agents in the IBD
The mechanism of influenza virus types A and B involved in the IBD progression has not been fully understood. Apparently, the virus effects on the GIT are mediated by the inhibition of gut microbiota-associated immune responses. In addition to the respiratory infection, mainly due to lower immune responses in IBD patients, other clinical signs are associated with the influenza virus. Furthermore, respiratory infection causes the migration of Th17 cells into the intestine and the first secretion of INF-γ followed by IL-15 which affects the microbiome (Zullow and Farraye 2019). TNFα and CD8 + T cells increase too. A study on 140,480 patients outlined a significant higher rate of influenza virus in IBD patients compared to the controls (Tinsley et al. 2019; Caldera et al. 2020). The effect of the influenza vaccination among IBD patients is also controversial advocating the use of repetitive doses of influenza vaccine or new strategies (Froggatt and Heaton 2022). In a study in Germany, on 99 IBD patients, 11 percent were positive for human papilloma virus (HPV) (Brunner et al. 2022). Treatment with thiopurines in IBD patients increases the viral infection and skin warts. Among 58,979 IBD patients, 145 of them were infected with HIV. Patients with UC and HIV had higher hospitalization rates, with consequent minor charges and costs compared to those HIV-negative patients (Then et al. 2021). It was stated that management of comorbid IBD and HIV in patients is possible using immunosuppressive drugs. However, their effect on CD4+ levels should be assessed (Hunt et al. 2021). It was also concluded that HIV persistence in UC could be challenged through a strong gut barrier integrity and reduction of inflammation (Peng et al. 2021).
Quercetin protective effects on the viral-associated IBD
Quercetin (Fig. 5) is a polyphenol, naturally found in different fruits, vegetables, seeds, red onions, berries, teas and kale (Ju et al. 2018). The IBD protective role of quercetin is due to PI3K inhibition and decrease of proinflammatory cytokines and chemokines, MMP-9, TNF-α, iNOS, ICAM-1 and CD4 + T cells (Nair et al. 2002; Comalada et al. 2005; Yu et al. 2008; Huang et al. 2010; Di Petrillo et al. 2022). Quercetin also contributes to inhibiting mast cells degranulation, Th2 cells, neutrophils, eosinophils and macrophages proliferation. Various mechanisms of action have been mentioned such as maintenance or increasing the gut microbiota diversity, nitric oxide (NO) or iNOS reduction via NF-κB downregulation, mitogen-activated protein kinase (MAPK) pathway (via toll-like receptor 4) and suppression of proinflammatory mediators or inflammatory proteins production via antioxidant effects. Additionally, anti-apoptotic effects, heme oxygenase-1 (Hmox1, HO-1) in colitis, downregulation of MMP-9, TNF-α and ICAM-1, increase in anti-viral type I interferon, inhibition of cytokine storm, senescence and cell cycle arrest have been also demonstrated (Yang et al. 2018; Cheng et al. 2019; Li et al. 2019a).
Quercetin enhances the colonic glutathione (GSH) reservoirs and nuclear factor erythroid 2-related factor 2 (Nrf2), protecting the intestinal porcine epithelial cell line 1 (IPEC-1) cells (Jia et al. 2021). It was also stated that quercetin activates anti-viral macrophages (dsRNA‐induced) via the calcium‐STAT pathway in vivo. Quercetin is metabolized by the gut microbiota into various beneficial compounds such as SCFA (short-chain fatty acids), homoprocatechuic acid, bacteriocins, amino acids, propionic acid, vitamins and bile acids. Moreover, quercetin has inferred anti-viral effects against hepatitis B and C, SARS-CoV-2, dengue virus (DENV), H1N1, H3N2, poliovirus, rhinovirus, chikungunya virus (CHIKV), MERS-CoV, herpes virus and Epstein–Barr virus (Cheng et al. 2015; Rojas et al. 2016; Frederico et al. 2017; Zakaryan et al. 2017; Colunga Biancatelli et al. 2020) (Table 1). An in vitro study exhibited that quercetin exerted virucidal effects against equid herpesvirus 1 (EHV-1) at 0 and 1 h of replication (Gravina et al. 2011). Considering low levels of diet quercetin absorption in the GIT or IBD, effective formulations are needed to increase its bioavailability (Salaritabar et al. 2017). Another derivative of quercetin, rutin, a flavonoid quite abundant in the human diet (fruits and vegetables), exerts antimicrobial and antioxidant effects. Glycosylated forms of quercetin and rutin exhibit enhanced intestinal absorption in animal models (Patel and Patel 2019; Zhou et al. 2021). The quercetin plus quercitrin but not rutin exhibited synergistic activity against the DENV (Chiow et al. 2016).
Table 1.
The antiviral effects of quercetin
| Concentration | Virus | Mechanism or effect | References |
|---|---|---|---|
| 1.1 µg/mL | DENV | NS5 protein binding | Dewi et al. (2019) |
| 0.2 μg/mL | Rhinovirus | Inhibition of replication | Ganesan et al. (2012) |
| Network pharmacology | Epstein–Barr virus, hepatitis C, measles, herpesvirus, | Bcl-2, PTGS2 and caspase 3 effect, ACE2 binding, replication and assembly | Jimilihan et al. (2020) |
| 2 µg/mL | Poliovirus | RNA synthesis inhibition | Castrillo et al. (1986) |
| 1 mM | CHIKV | HSP 70 inhibition | Ghosh et al. (2017) |
| Molecular docking, Network and traditional | SARS-CoV-2 | Caspase 3, MAPK, interleukins, CLR signaling pathway, spike, replication, PI3K/Akt signaling pathway, JAK-STAT, TLRs, cyclooxygenase 2, g PI3K-Akt signaling pathway, | Huang et al. (2020), Sun et al. (2020), Vijayakumar et al. (2020), Wang et al. (2020) |
| 2 μg/mL | Poliovirus | Blocking RNA synthesis | Castrillo et al. (1986) |
| 7 µM | MERS-CoV | 3Clpro protease inhibition | Jo et al. (2019) |
NS5 non-structural protein 5, PTGS2 prostaglandin-endoperoxide synthase 2, ACE2 angiotensin converting enzyme 2, HSP heat shock protein, MAPK mitogen-activated protein kinase, CLR c-type lectin receptor, CHIKV chikungunya virus, SGIV Singapore grouper iridovirus, PI3K/Akt or PKB phosphatidylinositol 3-kinase/protein kinase B, MERS-CoV Middle East respiratory syndrome coronavirus
Quercetin has given satisfactory clinical outcomes for individuals with IBD, colitis and CD conferring anti-inflammatory and antioxidant effects. However, the compound has poor oral bioavailability and absorption (Diniz et al. 2020). Quercetin safety dose is 1000 mg/m2 (Hollman et al. 1997). Anti-SARS CoV-2 effects of quercetin included reduced hospitalization incidence and intensive care unit residence (Cheema et al. 2023). Quercetin restores immune homeostasis and colonic microbiota and consolidates the intestinal barrier (Lyu et al. 2022). The metabolic regulatory and senolytic activity of quercetin have been described. Satisfactory outcomes have been reported following the consumption of quercetin and drugs in kidney disease, metabolic syndrome following obesity and pulmonary fibrosis (Guo et al. 2019; Hickson et al. 2019; Dziąbowska-Grabias et al. 2021; Hosseini et al. 2021; Gonzales et al. 2022). The clinical outcomes of the quercetin on the IBD remain to be fully understood needing further investigations (Lyu et al. 2022).
Silymarin protective effects on the viral-associated IBD
Silymarin bioactive compound is obtained from Silybum marianum seeds with high anti-inflammatory activity. Silibinin or silybin is the major and active compound of silymarin that is a mixture of two diastereomers, containing silibinin A and silibinin B, in approximately equimolar ratio (Nikonov et al. 2017). Silymarin has been shown to possess strong antiviral activities against various viral agents by targeting several steps of the viral replication cycle. Its hepatoprotective effects are well documented. Some researchers have confirmed that silymarin has a special effect on the control of immune-based murine colitis by healing the bowel histology in vitro and promoting the decrease of bowel inflammatory cytokines, especially TNF-α, interleukin-1β (IL-1β) and nuclear factor κB (NF-κB) (Davis-Searles et al. 2005; Zou et al. 2021). The in vitro, in vivo and in silico studies of silymarin as attractive antiviral candidates against different viruses are described in Table 2.
Table 2.
The antiviral effects of silymarin
| Concentration | Virus | Mechanism or effect | References |
|---|---|---|---|
| 10 and 20 μg/mL | Hepatitis C | Potentiation of the JAK-STAT antiviral signaling pathway | Polyak et al. (2007) |
| 75−100 μM | Hepatitis C | Inhibition of NS5B polymerase activity and blocking viral entry and transmission | Wagoner et al. (2010) |
| 75−100 μM | Hepatitis C | Inhibition of the NS5B RNA-dependent RNA polymerase | Ahmed–Belkacem et al. (2010) |
| 50 or 80 μM | Hepatitis C | Silibinin impeded HCV endosomal tracking and blocked CME | Blaising et al. (2013) |
| 67.6 mM | Hepatitis C | Inhibition of HCV NS4B and hence the membranous web morphogenesis | Esser‐Nobis et al. (2013) |
| Nanoscale spherical particles (< 200 nm) encapsulating amorphous silibinin at > 97% efficiency | Hepatitis C | Inhibition of HCV cell-to-cell spread and attenuation of HCV infection of PHHs | Liu et al. (2017) |
| 469, 265 or 61.5 mg/kg chimeric mice |
Hepatitis C (in vivo) Intravenous |
Blocked HCV production and increased anti-inflammatory and anti-proliferative gene expressions without affecting serum albumin levels | DebRoy et al. (2016) |
| 6.25–100 μM |
Influenza A virus (IAV) |
S0 and S3 inhibited IAV replication and disrupted the formation of the Atg5-Atg12/Atg16L complex | Dai et al. (2013) |
| 100 μg/ml |
Influenza A virus (IAV) |
Inhibition of late viral RNA synthesis | Song and Choi (2011) |
| 25 mg/kg/day Oral | AV infection of BALB/c mice (in vivo) | S0 and S3 increased the survival rate of mice (40% and 60% respectively) and S3 decreased virus titers in the lungs (100-fold) | |
| Docking to NS4B | DENV | All three silymarin derivatives docked with high binding affinity (≥ − 8 kal/mol) to DENV NS4B | Qaddir et al. (2017) |
| 100 μg/ml | CHIKV | Inhibition of CHIKV replication and protein synthesis | Lani et al. (2015) |
| 2 µg/ml | Human immunodeficiency virus (HIV) | Attenuating cellular functions involved in T cell activation, proliferation and HIV infection | McClure et al. (2012) |
| 125 μM | Human immunodeficiency virus (HIV) | Perturbation of T cell metabolism in vitro; Legalon® SIL additionally blocked HIV infection of T cells | McClure et al. (2014) |
| 0–200 μM | Hepatitis B virus (HBV) | Blockade of clathrin-mediated endocytosis | Umetsu et al. (2018) |
| 30, 100 and 300 mg/kg/d (Oral) | HBV X protein (HBx) transgenic mice (in vivo) | Silymarin had no effect on HBx expression and late stage carcinogenesis, but recovered fatty acid change and liver pathology in the early stages of liver damage | Wu et al. (2008) |
| 6.25–25 μg/ml | Mayaro virus (MAYV) | Inhibition of replication and ROS induction | Camini et al. (2018) |
JAK-STAT Janus kinase-signal transducer and activator of transcription, NS5B nonstructural protein 5B, DENV dengue virus, CHIKV chikungunya virus, HCV hepatitis C virus, PHHs primary human hepatocytes, ROS reactive oxygen species
Recently, clinical trial studies (Table 3) on silymarin and its derivatives have been focusing on HCV infections and particularly the antiviral activity of silymarin against chronic HCV.
Table 3.
Clinical trials of silymarin antiviral effects against HCV
| Virus | Concentration | Country | Year | Age | Number | References |
|---|---|---|---|---|---|---|
| HCV | 140–700 mg, 3 times per day (for 24 weeks) 12 months | Egypt | 2004 | Children less than 18 years of age | 141 infected patients | Tanamly et al. (2004) |
| HCV | 250 mg once daily or twice a week for the first 10 week for 24 week | Israel | 2007 | Age of 18–75 year | 100 chronic infected patients | Gabbay et al. (2007) |
| HCV | 420 mg silymarin, 700 mg silymarin, or matching placebo administered 3 times per day for 24 weeks | United States | 2011 | Median age was 54 years, | 154 chronic HCV infected patients | Fried et al. (2012) |
| HCV | Silybinvitamin E-phospholipids in patients with chronic hepatitis C, 1.5 mg/kg per week, Silybin 47 mg + vitamin E 15 mg + phospholipids 97 mg in two pill for 12 months. after 6, 12 months and at follow up | United States | 2015 | 45 year | 32 chronic infected patients | Malaguarnera et al. (2015) |
| HCV | 1.5 mg/kg per week of Peg–IFN plus RBV and placebo, while Group B received the same dosage of Peg–IFN plus RBV plus association of Silybin 94 mg + vitamin E 30 mg + phospholipids 194 mg in pills for 12 months | Italy | 2013 | 62 patients | Malaguarnera et al. (2016) | |
| HCV |
10 mg/kg/day Silibinin IV 16 patients received 10 mg/kg/day SIL IV (Legalon Sil; Madaus, Köln, Germany) for 7 days. In a subsequent dose-finding study, 20 patients received 5, 10, 15, or 20 mg/kg/day SIL for 14 days |
Germany | 2008 | 20 patients | Ferenci et al. (2008) | |
| HCV | 25 patients receiving 10, 15, or 20 mg/kg/day of SIL | USA | 2012 | 25 patients | Guedj et al. (2012) | |
| HCV | 7-day course of 5, 10, 15, or 20 mg/kg per day of silibinin | Germany | 2009 | 65-year-old | Six patients | Biermer and Berg (2009) |
| HCV | Silibinin 20 mg/kg/day intravenously for 14 or 21 days | Italy | 2011 | 27 treatment-naive patients | Rutter et al. (2011) | |
| HCV | Sofosbuvir and Ribavirin 400 and 800 mg per day along with silymarin (400 mg per day) | Pakistan | 2022 | 8–50 years of age | 30 patients | Ahmed et al. (2022) |
HCV hepatitis C virus, INF interferon, RBV ribavirin, SIL silibinin, IV intravenous
Silymarin has demonstrated antioxidant and anti-inflammatory properties, protecting hepatic cells from damage. Clinical outcomes of silymarin have been delineated in UC in which 35/38 of patients recovered satisfactorily compared to the control group after 6 months (Rastegarpanah et al. 2015). Clinical improvement of UC and CD has been demonstrated in some studies (Hagan et al. 2021). Another study revealed the antiviral effects of silymarin against COVID-19 (Palit et al. 2021).
Future prospects
Considering the pivotal role of inflammation in IBD development, the study and application of anti-inflammatory quercetin and silymarin bioactive compounds is greatly encouraged. The combination of these bioactive compounds with antivirals is also a proper strategy for antiviral therapy (Ninfali et al. 2020). However, due to low GIT bioavailability, stability and absorption concerns, their chemical modification and formulation and application of drug delivery systems need further investigation. On the other, immune suppression by chemotherapeutic drugs leads to the reactivation of infections such as existing or latent viral agents. Therefore, application of safe natural bioactive compounds with anti-inflammatory and antiviral effects is promising. Protein targeting and destroying the major viral agents causing epithelial cells inflammation (IBD development) are also promising, as well as future ligand-receptor interaction assessing studies. Natural databases are also available to be screened for the selection of potential bioactive compounds with anti-inflammatory and antiviral properties prior to the implementation of experimental studies. Another aspect of bioactive compounds utilization in IBD treatment is their stability in the GIT which deserves further in-depth investigation (Chang et al. 2022). Proper drug delivery systems can be applied for a more efficient treatment by targeting the right dose of compounds to the precise destination sites (Chai et al. 2018; Gonçalves et al. 2021).
Conclusion
The immunosuppression during the IBD treatment is associated with the proliferation of various viral agents such as CMV, EBV, HIV, HPV, HBV, HAV, HCV and influenza virus. Various genetic, epigenetic or environmental factors also contribute to exacerbate IBD severity. The exact mechanisms of enteric viruses’ pathophysiology in the IBD development remain to be clarified. Herein, we determined potential viral agents increasing the risk of IBD and unveiled their mechanisms using published data. HMs-derived flavonoids are promising in this context to preclude inflammation and virucidal effects. Quercetin and silymarin have shown IBD protective effects such as antiviral traits and immunomodulation. Mechanisms of protection and antiviral effects of these bioactive compounds include decrease of oxidative damage, inhibition of viral binding and replication, RNA synthesis, caspase enzymes, viral proteases and viral assembly. The consumption of quercetin and silymarin is promising for viral-associated IBD prevention and treatment. Other natural bioactive compounds could be also considered as potential natural remedies to fight against IBD. Further studies are warranted regarding the formulation of these bioactive compounds in antimicrobial and anti-inflammatory treatment for IBD.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- GIT
Gastrointestinal tract
- IBD
Inflammatory bowel disease
- MERS-CoV
Middle East respiratory syndrome coronavirus
- SARS-CoV
Severe acute respiratory syndrome coronavirus
- CD
Crohn’s disease
- UC
Ulcerative colitis
- HBc
Hepatitis B core protein
- HBs
Hepatitis B surface protein
- HBs Ag
Hepatitis B surface antigen
- Th
Helper T cell
- Treg
Regulatory T cell
- SCFAs
Short-chain fatty acids
- TXNIP
Thioredoxin (TRX)-interacting protein
- NLRP3
NOD-like receptor family pyrin domain-containing 3
- SIRT1
Sirtuin (silent mating-type information regulation 2 homolog) 1
- ASC
Apoptotic speck-like protein containing a caspase recruitment domain
- IL
Interleukin
- TNF- α
Tumor necrosis factor α
- PGE-2
Prostaglandin E2
- COX-2
Cyclooxygenase-2
- iNOS
Inducible nitric oxide synthase
- GPx
Glutathione peroxidase
- CAT
Catalase
- MDA
Malondialdehyde
- GSH
Glutathione
- SOD
Superoxide dismutase
- PGA
Prostaglandin
- HMG-CoA
3-Hydroxy-3-methylglutaryl coenzyme A
Author contributions
EZ, SA, AK, SK, SB, MS, SN, AM and AG have participated in data collection, drafting, writing and edition of the manuscript and also support of the work. All authors reviewed the manuscript.
Funding
Not applicable.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors confirm that this article content has no conflict of interest.
Ethical approval and consent to publication
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdullah A, Neurath MF, Atreya R. Mild COVID-19 symptoms in an infliximab-treated ulcerative colitis patient: can ongoing anti-TNF therapy protect against the viral hyperinflammatory response and avoid aggravated outcomes? Visc Med. 2020;36:338–342. doi: 10.1159/000508740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, et al. Effect of silymarin as an adjunct therapy in combination with sofosbuvir and ribavirin in hepatitis C patients: a miniature clinical trial. Oxid Med Cell Longev. 2022;2022:9199190. doi: 10.1155/2022/9199190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed-Belkacem A, et al. Silibinin and related compounds are direct inhibitors of hepatitis C virus RNA-dependent RNA polymerase. Gastroenterol. 2010;138:1112–1122. doi: 10.1053/j.gastro.2009.11.053. [DOI] [PubMed] [Google Scholar]
- Andari S, et al. Epstein-Barr virus DNA exacerbates colitis symptoms in a mouse model of inflammatory bowel disease. Viruses. 2021;13:1272. doi: 10.3390/v13071272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou N-P, Legaki E, Gazouli M. Inflammatory bowel disease pathobiology: the role of the interferon signature. Ann Gastroenterol. 2020;33:125. doi: 10.20524/aog.2020.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assiry AA, Bhavikatti SK, Althobaiti FA, Mohamed RN, Karobari MI. Evaluation of in vitro antiprotease activity of selected traditional medicinal herbs in dentistry and its in silico PASS prediction. BioMed Res Int. 2022;2022:5870443. doi: 10.1155/2022/5870443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The incidence and outcomes of COVID-19 in IBD patients: a rapid review and meta-analysis. Inflamm Bowel Dis. 2020;26:e132–e133. doi: 10.1093/ibd/izaa170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badshah SL, Faisal S, Muhammad A, Poulson BG, Emwas AH, Jaremko M. Antiviral activities of flavonoids. Biomed Pharmacother. 2021;140:111596. doi: 10.1016/j.biopha.2021.111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badshah SL, Faisal S, Muhammad A, Poulson BG, Emwas AH, Jaremko M. Antiviral activities of flavonoids. Biomed Pharmacother. 2021;140:111596. doi: 10.1016/j.biopha.2021.111596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbalho SM, et al. What do influenza and COVID-19 represent for patients with inflammatory bowel disease? Gastroenterol Res. 2021;14:1. doi: 10.14740/gr1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzio C, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69:1213–1217. doi: 10.1136/gutjnl-2020-321411. [DOI] [PubMed] [Google Scholar]
- Biermer M, Berg T. Rapid suppression of hepatitis C viremia induced by intravenous silibinin plus ribavirin. Gastroenterol. 2009;137:390–391. doi: 10.1053/j.gastro.2009.02.087. [DOI] [PubMed] [Google Scholar]
- Blach S, et al. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol Hepatol. 2022;7:396–415. doi: 10.1016/S2468-1253(21)00472-6. [DOI] [PubMed] [Google Scholar]
- Blaising J, et al. Silibinin inhibits hepatitis C virus entry into hepatocytes by hindering clathrin-dependent trafficking. Cell Microbiol. 2013;15:1866–1882. doi: 10.1111/cmi.12155. [DOI] [PubMed] [Google Scholar]
- Bodini G, et al. Concerns related to COVID-19 pandemic among patients with inflammatory bowel disease and its influence on patient management. Eur J Clin Invest. 2020;50(5):e13233. doi: 10.1111/eci.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat Med. 2021;27:28–33. doi: 10.1111/eci.13233. [DOI] [PubMed] [Google Scholar]
- Brunner A, et al. Prevalence of abnormal Pap smear results in inflammatory bowel disease: a prospective study. J Cancer Res Clin Oncol. 2022 doi: 10.1007/s00432-021-03909-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burisch J, et al. The cost of inflammatory bowel disease in high-income settings: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2023;8(5):458–492. doi: 10.1016/S2468-1253(23)00003-1. [DOI] [PubMed] [Google Scholar]
- Caetano BFR, Jorge BAS, Mueller-Coan BG, de Oliveira DE. Epstein-Barr virus microRNAs in the pathogenesis of human cancers. Cancer Lett. 2021;499:14–23. doi: 10.1016/j.canlet.2020.11.019. [DOI] [PubMed] [Google Scholar]
- Caldera F, et al. Immunogenicity of high dose influenza vaccine for patients with inflammatory bowel disease on anti-TNF monotherapy: a randomized clinical trial. Inflamm Bowel Dis. 2020;26:593–602. doi: 10.1093/ibd/izz164. [DOI] [PubMed] [Google Scholar]
- Camini FC, et al. Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress. Antiviral Res. 2018;158:8–12. doi: 10.1016/j.antiviral.2018.07.023. [DOI] [PubMed] [Google Scholar]
- Campos ST, Portela FA, Tomé L. Cytomegalovirus, inflammatory bowel disease, and anti-TNFα. Int J Colorectal Dis. 2017;32:645–650. doi: 10.1007/s00384-017-2752-5. [DOI] [PubMed] [Google Scholar]
- Castrillo J, Berghe DV, Carrasco L. 3-Methylquercetin is a potent and selective inhibitor of poliovirus RNA synthesis. Virol. 1986;152:219–227. doi: 10.1016/0042-6822(86)90386-7. [DOI] [PubMed] [Google Scholar]
- Chai J, et al. The intelligent delivery systems for bioactive compounds in foods: physicochemical and physiological conditions, absorption mechanisms, obstacles and responsive strategies. Trends Food Sci Technol. 2018;78:144–154. doi: 10.1016/j.tifs.2018.06.003. [DOI] [Google Scholar]
- Chang Y, et al. The metabolic profile elucidation of Lonicera japonica flos water extract and the metabolic characteristics evaluation of bioactive compounds in human gastrointestinal tract in vitro. J Pharm Biomed Anal. 2022;219:114906. doi: 10.1016/j.jpba.2022.114906. [DOI] [PubMed] [Google Scholar]
- Cheema HA, et al. Quercetin for the treatment of COVID-19 patients: a systematic review and meta-analysis. Rev Med Virol. 2023;33:e2427. doi: 10.1002/rmv.2427. [DOI] [PubMed] [Google Scholar]
- Cheng Z, et al. Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virol Sin. 2015;30:261–268. doi: 10.1007/s12250-015-3584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-C, Wu Y-H, Huang W-C, Pang J-HS, Huang T-H, Cheng C-Y. Anti-inflammatory property of quercetin through downregulation of ICAM-1 and MMP-9 in TNF-α-activated retinal pigment epithelial cells. Cytokine. 2019;116:48–60. doi: 10.1016/j.cyto.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia Region, 1992–2015. Vaccine. 2018;36:6–14. doi: 10.1016/j.vaccine.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiow K, Phoon M, Putti T, Tan BK, Chow VT. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pac J Trop Med. 2016;9:1–7. doi: 10.1016/j.apjtm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, et al. Human cytomegalovirus and Epstein-Barr virus infection in inflammatory bowel disease: need for mucosal viral load measurement. World J Gastroenterol. 2015;21:1915. doi: 10.3748/wjg.v21.i6.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R, Yu A, Wu E, Xie J, Mulani P, Chao J. Systematic review: the costs of ulcerative colitis in Western countries. Aliment Pharmacol Ther. 2010;31:693–707. doi: 10.1111/j.1365-2036.2010.04234.x. [DOI] [PubMed] [Google Scholar]
- Çolak M, et al. Relationship between Epstein-Barr virus (EBV) infection and viral load in immunosuppressive patients. Istanbul Med J. 2018;19:7–13. doi: 10.5152/imj.2018.93357. [DOI] [Google Scholar]
- Colunga Biancatelli RML, Berrill M, Catravas JD, Marik PE. Quercetin and vitamin C: an experimental, synergistic therapy for the prevention and treatment of SARS-CoV-2 related disease (COVID-19) Front Immunol. 2020 doi: 10.3389/fimmu.2020.01451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comalada M, et al. In vivo quercitrin anti-inflammatory effect involves release of quercetin, which inhibits inflammation through down-regulation of the NF-κB pathway. Eur J Immunol. 2005;35:584–592. doi: 10.1002/eji.200425778. [DOI] [PubMed] [Google Scholar]
- Craviotto V, et al. Viral infections in inflammatory bowel disease: tips and tricks for correct management. World J Gastroenterol. 2021;27:4276. doi: 10.3748/wjg.v27.i27.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croitoru D, Sibbald C, Alavi A, Brooks S, Piguet V. Challenging the association of hepatitis C and pyoderma gangrenosum. Br J Dermatol. 2021;185:1047–1048. doi: 10.1111/bjd.20566. [DOI] [PubMed] [Google Scholar]
- Cui G, Yuan A. A systematic review of epidemiology and risk factors associated with Chinese inflammatory bowel disease. Front Med. 2018;5:183. doi: 10.3389/fmed.2018.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J-P, et al. Identification of 23-(s)-2-amino-3-phenylpropanoyl-silybin as an antiviral agent for influenza A virus infection in vitro and in vivo. Antimicrob Agents Themother. 2013;57:4433–4443. doi: 10.1128/AAC.00759-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Searles PR, et al. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- DebRoy S, et al. Hepatitis C virus dynamics and cellular gene expression in uPA-SCID chimeric mice with humanized livers during intravenous silibinin monotherapy. J Viral Hepat. 2016;23:708–717. doi: 10.1111/jvh.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewi BE, Ratningpoeti E, Desti H, Angelina M (2019) In vitro and in silico study to evaluate the effectiveness of quercitrin as antiviral drug to dengue virus. In: AIP Conference Proceedings. AIP Publishing LLC, p 030004. 10.1063/1.5139341
- Di Petrillo A, Orrù G, Fais A, Fantini MC. Quercetin and its derivates as antiviral potentials: a comprehensive review. Phytother Res. 2022;36:266–278. doi: 10.1002/ptr.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Din AU, et al. SARS-CoV-2 microbiome dysbiosis linked disorders and possible probiotics role. Biomed Pharmacother. 2021;133:110947. doi: 10.1016/j.biopha.2020.110947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz LRL, Souza MTS, Duarte ABS, Sousa DP. Mechanistic aspects and therapeutic potential of quercetin against COVID-19-associated acute kidney injury. Molecules. 2020;25(23):5772. doi: 10.3390/molecules25235772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: expert review. Gastroenterol. 2021;160:1384–1393. doi: 10.1053/j.gastro.2020.08.063. [DOI] [PubMed] [Google Scholar]
- Dudzińska E (2020) Single-nucleotide polymorphisms in inflammatory bowel disease. In: The Recent Topics in Genetic Polymorphisms. IntechOpen. 10.1016/j.trsl.2011.10.006
- Dudzińska E, Gryzinska M, Kocki J. Single nucleotide polymorphisms in selected genes in inflammatory bowel disease. BioMed Res Int. 2018;2018:6914346. doi: 10.1155/2018/6914346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziąbowska-Grabias K, et al. Antioxidant therapy in inflammatory bowel diseases. Antioxidants (Basel) 2021;10(3):412. doi: 10.3390/antiox10030412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e Silva NO, de Brito BB, da Silva FAF, Santos MLC, de Melo FF. Probiotics in inflammatory bowel disease: does it work? World J Meta-Anal. 2020;8:54–66. doi: 10.13105/wjma.v8.i2.54. [DOI] [Google Scholar]
- Esser-Nobis K, et al. Analysis of hepatitis C virus resistance to silibinin in vitro and in vivo points to a novel mechanism involving nonstructural protein 4B. Hepatol. 2013;57:953–963. doi: 10.1002/hep.26260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenci P, et al. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterol. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- Flores GL, Mota JC, da Silva Andrade LT, Lopes RS, Bastos FI, Villar LM. Performance of HCV antigen testing for the diagnosis and monitoring of antiviral treatment: a systematic review and meta-analysis. BioMed Res Int. 2022 doi: 10.1155/2022/7348755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransen K, et al. Correlation of genetic risk and messenger RNA expression in a Th17/IL23 pathway analysis in inflammatory bowel disease. Inflamm Bowel Dis. 2014;20:777–782. doi: 10.1097/MIB.0000000000000013. [DOI] [PubMed] [Google Scholar]
- Frederico ÉHFF, et al. Anti-viral effects of medicinal plants in the management of dengue: a systematic review. Afr J Tradit Complement Altern Med. 2017;14:33–40. doi: 10.1093/ibd/izad076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried MW, et al. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA. 2012;308:274–282. doi: 10.1001/jama.2012.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt HM, Heaton NS. Nonrespiratory sites of influenza-associated disease: mechanisms and experimental systems for continued study. FEBS J. 2022 doi: 10.1111/febs.16363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay E, et al. Antioxidant therapy for chronic hepatitis C after failure of interferon: results of phase II randomized, double-blind placebo controlled clinical trial. World J Gastroenterol. 2007;13:5317. doi: 10.3748/wjg.v13.i40.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, et al. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antivir Res. 2012;94:258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss A, et al. Intestinal cytomegalovirus infection in patients hospitalized for exacerbation of inflammatory bowel disease: a 10-year tertiary referral center experience. Eur J Gastroenterol Hepatol. 2015;27:712–720. doi: 10.1097/meg.0000000000000361. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Desai A, Ravi V, Narayanappa G, Tyagi BK. Chikungunya virus interacts with heat shock cognate 70 protein to facilitate its entry into mosquito cell line. Intervirology. 2017;60:247–262. doi: 10.1159/000489308. [DOI] [PubMed] [Google Scholar]
- Giri S, et al. Prevalence of hepatitis B virus and hepatitis C virus infection in patients with inflammatory bowel disease: a systematic review and meta-analysis. Intest Res. 2022 doi: 10.5217/ir.2022.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves A, Estevinho BN, Rocha F. Methodologies for simulation of gastrointestinal digestion of different controlled delivery systems and further uptake of encapsulated bioactive compounds. Trend Food Sci Technol. 2021;114:510–520. doi: 10.1016/j.tifs.2021.06.007. [DOI] [Google Scholar]
- Gonzales MM, et al. Senolytic therapy to modulate the progression of Alzheimer’s disease (SToMP-AD): a pilot clinical trial. J Prev Alzheimers Dis. 2022;9:22–29. doi: 10.1428/jpad.2021.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravina H, et al. In vitro assessment of the antiviral potential of trans-cinnamic acid, quercetin and morin against equid herpesvirus 1. Res Vet Sci. 2011;91:e158–e162. doi: 10.1016/j.rvsc.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Gray-Rodriguez S, et al. Multisystem screening reveals SARS-CoV-2 in neurons of the myenteric plexus and in megakaryocytes. J Pathol. 2022;257:198–217. doi: 10.1002/path.5878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedj J, Dahari H, Pohl RT, Ferenci P, Perelson AS. Understanding silibinin’s modes of action against HCV using viral kinetic modeling. J Hepatol. 2012;56:1019–1024. doi: 10.1016/j.jhep.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Gong X, Li M. Quercetin actions on lipid profiles in overweight and obese individuals: a systematic review and meta-analysis. Curr Pharm Des. 2019;25:3087–3095. doi: 10.2174/1381612825666190829153552. [DOI] [PubMed] [Google Scholar]
- Hagan M, Hayee BH, Rodriguez-Mateos A. (Poly)phenols in inflammatory bowel disease and irritable bowel syndrome: a review. Molecules. 2021 doi: 10.3390/molecules26071843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan TP, Lubel JS, Garg M. Lessons from COVID-19, ACE2, and intestinal inflammation: could a virus trigger chronic intestinal inflammation? Clin Gastroenterol Hepatol. 2021;19:206. doi: 10.1016/j.cgh.2020.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazır-Konya H, Avkan-Oğuz V, Akpınar H, Sağol Ö, Sayıner A. Investigation of cytomegalovirus in intestinal tissue in a country with high CMV seroprevalence. Turk J Gastroenterol. 2021;32:123. doi: 10.5152/tjg.2021.191008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess LM, Michael D, Mytelka DS, Beyrer J, Liepa AM, Nicol S. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: a retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer. 2016;19:607–615. doi: 10.1007/s10120-015-0486-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson LJ, et al. Senolytics decrease senescent cells in humans: preliminary report from a clinical trial of dasatinib plus quercetin in individuals with diabetic kidney disease. EBioMed. 2019;47:446–456. doi: 10.1016/j.ebiom.2019.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman PC, et al. Relative bioavailability of the antioxidant flavonoid quercetin from various foods in man. FEBS Lett. 1997;418:152–156. doi: 10.1016/s0014-5793(97)01367-7. [DOI] [PubMed] [Google Scholar]
- Hommes DW, Sterringa G, van Deventer SJ, Tytgat GN, Weel J. The pathogenicity of cytomegalovirus in inflammatory bowel disease: a systematic review and evidence-based recommendations for future research. Inflamm Bowel Dis. 2004;10:245–250. doi: 10.1097/00054725-200405000-00011. [DOI] [PubMed] [Google Scholar]
- Hosseini A, Razavi BM, Banach M, Hosseinzadeh H. Quercetin and metabolic syndrome: a review. Phytother Res. 2021;35:5352–5364. doi: 10.1002/ptr.7144. [DOI] [PubMed] [Google Scholar]
- Huang R-Y, Yu Y-L, Cheng W-C, OuYang C-N, Fu E, Chu C-L. Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol. 2010;184:6815–6821. doi: 10.4049/jimmunol.0903991. [DOI] [PubMed] [Google Scholar]
- Huang Y-F, Bai C, He F, Xie Y, Zhou H. Review on the potential action mechanisms of Chinese medicines in treating coronavirus disease 2019 (COVID-19) Pharm Res. 2020;158:104939. doi: 10.1016/j.phrs.2020.104939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RH, et al. COVID-19 and gastrointestinal disease: implications for the gastroenterologist. Digest Dis. 2021;39:119–139. doi: 10.1159/000512152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilan Y, et al. A plant cell-expressed recombinant anti-TNF fusion protein is biologically active in the gut and alleviates immune-mediated hepatitis and colitis. Immunobiol. 2017;222:544–551. doi: 10.1016/j.imbio.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Jannat K, et al. Nanotechnology applications of flavonoids for viral diseases. Pharmaceutics. 2021;13:1895. doi: 10.3390/pharmaceutics13111895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefremow A, Neurath MF. All are equal, some are more equal: targeting IL 12 and 23 in IBD–a clinical perspective. ImmunoTargets Ther. 2020;9:289. doi: 10.2147/itt.s282466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena A, Mishra S, Singh AK, Sekar A, Sharma V. Cytomegalovirus in ulcerative colitis: an evidence-based approach to diagnosis and treatment. Expert Rev Gastroenterol Hepatol. 2022;16:109–120. doi: 10.1080/17474124.2022.2032662. [DOI] [PubMed] [Google Scholar]
- Jentzer A, et al. Cytomegalovirus and inflammatory bowel diseases (IBD) with a special focus on the link with ulcerative colitis (UC) Microorganisms. 2020;8:1078. doi: 10.3390/microorganisms8071078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia H, et al. Quercetin alleviates oxidative damage by activating nuclear factor erythroid 2-related factor 2 signaling in porcine enterocytes. Nutrients. 2021;13:375. doi: 10.3390/nu13020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimilihan S, et al. Study on the active components in the adjuvant treatment of novel coronavirus pneumonia (COVID-19) with Jinhua Qinggan granules based on network pharmacology and molecular docking. J Chin Med Mater. 2020;43:1275–1283. [Google Scholar]
- Jo S, Kim H, Kim S, Shin DH, Kim MS. Characteristics of flavonoids as potent MERS-CoV 3C-like protease inhibitors. Chem Biol Drug Des. 2019;94:2023–2030. doi: 10.1111/cbdd.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jostins L, et al. Host–microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491:119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S, et al. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle. 2018;17:53–63. doi: 10.1080/15384101.2017.1387701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GG, Windsor JW. The four epidemiological stages in the global evolution of inflammatory bowel disease. Nat Rev Gastroenterol Hepatol. 2021;18:56–66. doi: 10.1038/s41575-020-00360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, et al. Substantial Epstein-Barr virus reactivation in a case of severe refractory ulcerative colitis: a possible role in exacerbation. Clin J Gastroenterol. 2021;14:584–588. doi: 10.1007/s12328-020-01319-w. [DOI] [PubMed] [Google Scholar]
- Kennedy NA, et al. Anti-SARS-CoV-2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70:865–875. doi: 10.1136/gutjnl-2021-324388. [DOI] [PubMed] [Google Scholar]
- Khetsuriani N, LaMonte-Fowlkes A, Oberst S, Pallansch MA, Control CfD, Prevention Enterovirus surveillance—United States, 1970–2005. MMWR Surveill Summ. 2006;55:1–20. [PubMed] [Google Scholar]
- Kirchgesner J, Lemaitre M, Carrat F, Zureik M, Carbonnel F, Dray-Spira R. Risk of serious and opportunistic infections associated with treatment of inflammatory bowel diseases. Gastroenterol. 2018;155:337–346.e310. doi: 10.1053/j.gastro.2018.04.012. [DOI] [PubMed] [Google Scholar]
- Kishore J, et al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004;53:1155–1160. doi: 10.1099/jmm.0.45629-0. [DOI] [PubMed] [Google Scholar]
- Kjaer ASL, Ribberholt I, Thomsen K, Ibsen PH, Markova E, Graff J. 18F-FDG PET/CT findings in cytomegalovirus colitis. Diagnostics. 2018;9:3. doi: 10.3390/diagnostics9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolho KL, Klemola P, Simonen-Tikka ML, Ollonen ML, Roivainen M. Enteric viral pathogens in children with inflammatory bowel disease. J Med Virol. 2012;84:345–347. doi: 10.1002/jmv.23193. [DOI] [PubMed] [Google Scholar]
- Kumari A, Bhawal S, Kapila S, Kapila R. Strain-specific effects of probiotic Lactobacilli on mRNA expression of epigenetic modifiers in intestinal epithelial cells. Arch Microbiol. 2022;204:411. doi: 10.1007/s00203-022-03027-0. [DOI] [PubMed] [Google Scholar]
- Lani R, et al. Antiviral activity of silymarin against chikungunya virus. Sci Rep. 2015;5:1–10. doi: 10.1038/srep11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor G, Moss AC. Cytomegalovirus in inflammatory bowel disease: pathogen or innocent bystander? Inflamm Bowel Dis. 2010;16:1620–1627. doi: 10.1038/srep11421. [DOI] [PubMed] [Google Scholar]
- Leal T, Arroja B, Costa D, Ferreira C, Soares JB, Gonçalves R. Colitis due to cytomegalovirus and herpes simplex type 2 as a complication of a first presentation of inflammatory bowel disease. GE Port J Gastroenterol. 2022;29:56–60. doi: 10.1159/000514715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Eun CS. Inflammatory bowel disease in Korea: epidemiology and pathophysiology. Korean J Intern Med. 2022;37:885–894. doi: 10.3904/kjim.2022.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, et al. Clinical course of hepatitis B viral infection in patients undergoing anti-tumor necrosis factor α therapy for inflammatory bowel disease. Gut Liver. 2022;16:396. doi: 10.5009/gnl210081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Li F, Liu X, Liu J, Li D. Synergistic anti-inflammatory effects of quercetin and catechin via inhibiting activation of TLR4–MyD88-mediated NF-κB and MAPK signaling pathways. Phytother Res. 2019;33:756–767. doi: 10.1002/ptr.6268. [DOI] [PubMed] [Google Scholar]
- Li X, et al. The status of Epstein-Barr virus infection in intestinal mucosa of Chinese patients with inflammatory bowel disease. Digest. 2019;99:126–132. doi: 10.1159/000489996. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhou S, Wang Y, Cong J. Changes of intestinal microbiota and microbiota-based treatments in IBD. Arch Microbiol. 2022;204:442. doi: 10.1007/s00203-022-03069-4. [DOI] [PubMed] [Google Scholar]
- Lin MV, Blonski W, Buchner AM, Reddy KR, Lichtenstein GR. The influence of anti-TNF therapy on the course of chronic hepatitis C virus infection in patients with inflammatory bowel disease. Digest Dis Sci. 2013;58:1149–1156. doi: 10.1007/s10620-012-2457-0. [DOI] [PubMed] [Google Scholar]
- Liskova A, Samec M, Koklesova L, Samuel SM, Zhai K, Al-Ishaq RK. Flavonoids against the SARS-CoV-2 induced inflammatory storm. Biomed Pharmacother. 2021;138:111430. doi: 10.1016/j.biopha.2021.111430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C-H, et al. Highly bioavailable silibinin nanoparticles inhibit HCV infection. Gut. 2017;66:1853–1861. doi: 10.1136/gutjnl-2016-312019. [DOI] [PubMed] [Google Scholar]
- Liu M, Mao J, Zhang S. Effect of intervention of probiotics in advance on Treg/Th17 in premature mice. BioMed Res Int. 2022;2022:6131069. doi: 10.1136/gutjnl-2016-312019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Loras C, et al. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010;59:1340. doi: 10.1136/gut.2010.208413. [DOI] [PubMed] [Google Scholar]
- Losurdo G, et al. Chronic viral hepatitis in a cohort of inflammatory bowel disease patients from Southern Italy: a case-control study. Pathogens. 2020;9:870. doi: 10.3390/pathogens9110870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luangsirithanya P, Treewaree S, Pongpaibul A, Pausawasdi N, Limsrivilai J. Cytomegalovirus enterocolitis with subsequent diagnosis of coexisting new-onset inflammatory bowel disease: two case reports and review of the literature. Medicine. 2021 doi: 10.1097/md.0000000000024914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y-l, et al. Is cytomegalovirus infection related to inflammatory bowel disease, especially steroid-resistant inflammatory bowel disease? A meta-analysis. Infect Drug Resist. 2017;10:511. doi: 10.2147/idr.s149784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu YL, Zhou HF, Yang J, Wang FX, Sun F, Li JY. Biological activities underlying the therapeutic effect of quercetin on inflammatory bowel disease. Mediators Inflamm. 2022;2022:5665778. doi: 10.1155/2022/5665778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisuthisakul P, Pasuk S, Ritthiruangdej P. Relationship between antioxidant properties and chemical composition of some Thai plants. J Food Compos Anal. 2008;21:229–240. doi: 10.1016/j.jfca.2007.11.005. [DOI] [Google Scholar]
- Malaguarnera M, et al. Silybin-vitamin E-phospholipids complex reduces liver fibrosis in patients with chronic hepatitis C treated with pegylated interferon α and ribavirin. Am J Transl Res. 2015;7:2510. [PMC free article] [PubMed] [Google Scholar]
- Malaguarnera G, et al. Silybin supplementation during HCV therapy with pegylated interferon-α plus ribavirin reduces depression and anxiety and increases work ability. BMC Psychiatr. 2016;16:1–10. doi: 10.1186/s12888-016-1115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik TA. Inflammatory bowel disease: historical perspective, epidemiology, and risk factors. Surg Clin North Am. 2015;95:1105–1122. doi: 10.1016/j.suc.2015.07.006. [DOI] [PubMed] [Google Scholar]
- Mariño Z, et al. Intravenous silibinin monotherapy shows significant antiviral activity in HCV-infected patients in the peri-transplantation period. J Hepatol. 2013;58:415–420. doi: 10.1016/j.jhep.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Marongiu L, Venturelli S, Allgayer H. Involvement of HHV-4 (Epstein–Barr virus) and HHV-5 (cytomegalovirus) in inflammatory bowel disease and colorectal cancer: a meta-analysis. Cancers. 2022;14:5085. doi: 10.3390/cancers14205085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masclee GM, Penders J, Pierik M, Wolffs P, Jonkers D. Enteropathogenic viruses: triggers for exacerbation in IBD? A prospective cohort study using real-time quantitative polymerase chain reaction. Inflamm Bowel Dis. 2013;19:124–131. doi: 10.1002/ibd.22976. [DOI] [PubMed] [Google Scholar]
- McClure J, et al. Silibinin inhibits HIV-1 infection by reducing cellular activation and proliferation. PLoS One. 2012;7:e41832. doi: 10.1371/journal.pone.0041832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure J, Margineantu DH, Sweet IR, Polyak SJ. Inhibition of HIV by Legalon-SIL is independent of its effect on cellular metabolism. Virology. 2014;449:96–103. doi: 10.1016/j.virol.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy JD, et al. A model for identifying cytomegalovirus in patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2015;13:131–137. doi: 10.1016/j.cgh.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Miri ST, Sotoodehnejadnematalahi F, Amiri MM, Pourshafie MR, Rohani M. The impact of Lactobacillus and Bifidobacterium probiotic cocktail on modulation of gene expression of gap junctions dysregulated by intestinal pathogens. Arch Microbiol. 2022;204:417. doi: 10.1007/s00203-022-03026-1. [DOI] [PubMed] [Google Scholar]
- Miura M, et al. Multicenter, cross-sectional, observational study on Epstein-Barr viral infection status and thiopurine use by age group in patients with inflammatory bowel disease in Japan (EBISU study) J Gastroenterol. 2021;56:1080–1091. doi: 10.1007/s00535-021-01832-w. [DOI] [PubMed] [Google Scholar]
- Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol. 2010;6:339. [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Ardizzone S. Are patients with inflammatory bowel disease at increased risk for Covid-19 infection? J Crohns Colitis. 2020;14:1334–1336. doi: 10.1093/ecco-jcc/jjaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad FH, Hashash JG, Kariyawasam VC, Leong RW. Ulcerative colitis and cytomegalovirus infection: from A to Z. J Crohns Colitis. 2020;14:1162–1171. doi: 10.1093/ecco-jcc/jjaa036. [DOI] [PubMed] [Google Scholar]
- Münz C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis. Nat Rev Microbiol. 2019;17:691–700. doi: 10.1038/s41579-019-0249-7. [DOI] [PubMed] [Google Scholar]
- Nair MP, et al. The flavonoid, quercetin, differentially regulates Th-1 (IFNγ) and Th-2 (IL4) cytokine gene expression by normal peripheral blood mononuclear cells. Biochim Biophys Acta. 2002;1593:29–36. doi: 10.1016/s0167-4889(02)00328-2. [DOI] [PubMed] [Google Scholar]
- Nakase H, Yoshino T, Honzawa Y, Chiba T. Low prevalence of CMV infection in patients with Crohn’s disease in comparison with ulcerative colitis: effect of different immune response on prevalence of CMV infection. Digest Dis Sci. 2010;55:1498–1499. doi: 10.1007/s10620-010-1162-0. [DOI] [PubMed] [Google Scholar]
- Neurath MF. COVID-19 and immunomodulation in IBD. Gut. 2020;69:1335–1342. doi: 10.1136/gutjnl-2020-321269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov O, Chernykh E, Garber M, Nikonova EY. Enteroviruses: classification, diseases they cause, and approaches to development of antiviral drugs. Biochemistry (mosc) 2017;82:1615–1631. doi: 10.1134/s0006297917130041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfali P, Antonelli A, Magnani M, Scarpa ES. Antiviral properties of flavonoids and delivery strategies. Nutrients. 2020;12(9):2534. doi: 10.3390/nu12092534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen LH, et al. Epstein-Barr virus in inflammatory bowel disease: the spectrum of intestinal lymphoproliferative disorders. J Crohns Colitis. 2015;9:398–403. doi: 10.1093/ecco-jcc/jjv040. [DOI] [PubMed] [Google Scholar]
- O’Connor C (2021) Cytomegalovirus (CMV) Infection and Latency. Pathogens 2021, 10, 342. In. s Note: MDPI stays neutral with regard to jurisdictional claims in published. [DOI] [PMC free article] [PubMed]
- Ouali SE, Achkar J-P (2022) Management of hospitalized patients with inflammatory bowel disease and CMV infection or Clostridium difficile infection. Management of inpatient inflammatory bowel disease. Springer, pp 161–180. 10.1007/978-1-0716-1987-2_8
- Palit P, Mukhopadhyay A, Chattopadhyay D. Phyto-pharmacological perspective of silymarin: a potential prophylactic or therapeutic agent for COVID-19, based on its promising immunomodulatory, anti-coagulant and anti-viral property. Phytother Res. 2021;35:4246–4257. doi: 10.1002/ptr.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-K, et al. Prevention and management of viral hepatitis in inflammatory bowel disease: a clinical practice guideline by the Korean Association for the Study of Intestinal Diseases. Intest Res. 2020;18:18. doi: 10.5217/ir.2019.09155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel K, Patel DK (2019) The beneficial role of rutin, a naturally occurring flavonoid in health promotion and disease prevention: a systematic review and update. Bioactive food as dietary interventions for arthritis and related inflammatory diseases. Elsevier, 457–479.10.1016/B978-0-12-813820-5.00026-X
- Patton K, et al. Epstein-barr virus associated colitis: another mimic of IBD? Pathol. 2018;50:S142. doi: 10.1159/000233301. [DOI] [Google Scholar]
- Pavel FM, et al. Highlighting the relevance of gut microbiota manipulation in inflammatory bowel disease. Diagnostics. 2021;11:1090. doi: 10.3390/diagnostics11061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavel FM, et al. Highlighting the relevance of gut microbiota manipulation in inflammatory bowel disease. Diagnostics. 2021;11(6):1090. doi: 10.3390/diagnostics11061090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis C, Panoutsopoulos GI, Tiniakos D, Koutsounas S, Vlachogiannakos J, Zouboulis-Vafiadis I. Serum leptin and ghrelin in chronic hepatitis C patients with steatosis. World J Gastroenterol. 2011;17:5097. doi: 10.3748/wjg.v17.i46.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng X, et al. Differences in HIV burden in the inflamed and non-inflamed colon from a person living with HIV and ulcerative colitis. J Virus Erad. 2021;7:100033. doi: 10.1016/j.jve.2021.100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovani D, Danese S, Peyrin-Biroulet L, Nikolopoulos GK, Lytras T, Bonovas S. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterol. 2019;157:647–659.e644. doi: 10.1053/j.gastro.2019.04.016. [DOI] [PubMed] [Google Scholar]
- Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DYW. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-κB signaling, and HCV infection by standardized silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- Powell RD, Warner NE, Levine RS, Kirsner JB. Cytomegalic inclusion disease and ulcerative colitis: report of a case in a young adult. Am J Med. 1961;30:334–340. doi: 10.1016/0002-9343(61)90105-x. [DOI] [PubMed] [Google Scholar]
- Puoti MG, Rybak A, Kiparissi F, Gaynor E, Borrelli O. SARS-CoV-2 and the gastrointestinal tract in children. Front Pediatr. 2021;9:617980. doi: 10.3389/fped.2021.617980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qaddir I, Rasool N, Hussain W, Mahmood S. Computer-aided analysis of phytochemicals as potential dengue virus inhibitors based on molecular docking, ADMET and DFT studies. J Vector Borne Dis. 2017;54:255. doi: 10.4103/0972-9062.217617. [DOI] [PubMed] [Google Scholar]
- Rastegarpanah M, et al. A randomized, double blinded, placebo-controlled clinical trial of silymarin in ulcerative colitis. Chin J Integr Med. 2015;21:902–906. doi: 10.1007/s11655-012-1026-x. [DOI] [PubMed] [Google Scholar]
- Ravichandran K, Velmurugan B, Gu M, Singh RP, Agarwal R. Inhibitory effect of silibinin against azoxymethane-induced colon tumorigenesis in A/J mice. Clin Cancer Res. 2010;16:4595–4606. doi: 10.1158/1078-0432.ccr-10-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy HG, Schneider BJ, Tai AW. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol. 2018;9(9):180. doi: 10.1038/s41424-018-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendina M, et al. Antiviral activity and safety profile of silibinin in HCV patients with advanced fibrosis after liver transplantation: a randomized clinical trial. Transpl Int. 2014;27:696–704. doi: 10.1111/tri.12324. [DOI] [PubMed] [Google Scholar]
- Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L. Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol. 2016;7:979. doi: 10.3389/fmicb.2016.00979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas Á, et al. Effect of quercetin on hepatitis C virus life cycle: from viral to host targets. Sci Rep. 2016;6:1–9. doi: 10.1038/srep31777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, et al. Flavonoids a bioactive compound from medicinal plants and its therapeutic applications. BioMed Res Int. 2022;2022:5445291. doi: 10.1155/2022/5445291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz F, et al. A single nucleotide polymorphism in the gene for GPR183 increases its surface expression on blood lymphocytes of patients with inflammatory bowel disease. Br J Pharmacol. 2021;178:3157–3175. doi: 10.1111/bph.15395. [DOI] [PubMed] [Google Scholar]
- Rutter K, et al. Intravenous silibinin as ‘rescue treatment’for on-treatment non-responders to pegylated interferon/ribavirin combination therapy. Antivir Ther. 2011;16:1327–1333. doi: 10.3851/imp1942. [DOI] [PubMed] [Google Scholar]
- Ryan JL, et al. Epstein-Barr virus infection is common in inflamed gastrointestinal mucosa. Dig Dis Sci. 2012;57:1887–1898. doi: 10.1007/s10620-012-2116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed M, et al. Silymarin: a potent hepatoprotective agent in poultry industry. World’s Poultry Sci J. 2017;73:483–492. doi: 10.1017/S0043933917000538. [DOI] [Google Scholar]
- Safarpour S, et al. Protective effect of kaempferol and its nanoparticles on 5-fluorouracil-induced cardiotoxicity in rats. BioMed Res Int. 2022;2022:2273000. doi: 10.1155/2022/2273000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salaritabar A, et al. Therapeutic potential of flavonoids in inflammatory bowel disease: a comprehensive review. World J Gastroenterol. 2017;23:5097. doi: 10.3748/wjg.v23.i28.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum N, et al. Epstein-Barr virus DNA modulates regulatory T-cell programming in addition to enhancing interleukin-17A production via Toll-like receptor 9. PLoS One. 2018;13:e0200546. doi: 10.1371/journal.pone.0200546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Grunert PC, Stallmach A. An update for pharmacologists on new treatment options for inflammatory bowel disease: the clinicians’ perspective. Front Pharmacol. 2021;12:655054. doi: 10.3389/fphar.2021.655054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D, Moran C, Shanahan F. The microbiota in inflammatory bowel disease. J Gastroenterol. 2015;50:495–507. doi: 10.1007/s00535-015-1064-1. [DOI] [PubMed] [Google Scholar]
- Shukla T, Singh S, Tandon P, McCurdy JD. Corticosteroids and thiopurines, but not tumor necrosis factor antagonists, are associated with cytomegalovirus reactivation in inflammatory bowel disease. J Clin Gastroenterol. 2017;51:394–401. doi: 10.2147/jir.s116088. [DOI] [PubMed] [Google Scholar]
- Sokol H, et al. Fungal microbiota dysbiosis in IBD. Gut. 2017;66:1039–1048. doi: 10.1136/gutjnl-2015-310746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Choi H. Silymarin efficacy against influenza A virus replication. Phytomedicine. 2011;18:832–835. doi: 10.1016/j.phymed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Song K, Wu D. Shared decision-making in the management of patients with inflammatory bowel disease. World J Gastroenterol. 2022;28:3092–3100. doi: 10.3748/wjg.v28.i26.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieker T, Herbst H. Distribution and phenotype of Epstein-Barr virus-infected cells in inflammatory bowel disease. Am J Pathol. 2000;157:51–57. doi: 10.1016/s0002-9440(10)64516-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statovci D, Aguilera M, MacSharry J, Melgar S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front Immunol. 2017;8:838. doi: 10.3389/fimmu.2017.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H-J, et al. Inflammatory bowel disease and its treatment in 2018: Global and Taiwanese status updates. J Formos Med Assoc. 2019;118:1083–1092. doi: 10.1016/j.jfma.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhang Y, Liu Y, Wang G. Study on mechanism of Reduning Injection in treating novel coronavirus pneumonia based on network pharmacology. J Chin Med Mater. 2020;7:1797–1798. [Google Scholar]
- Sun D, Li C, Chen S, Zhang X. Emerging role of dendritic cell intervention in the treatment of inflammatory bowel disease. BioMed Res Int. 2022 doi: 10.1155/2022/7025634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanamly M, et al. Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: study description and 12-month results. Dig Liver Dis. 2004;36:752–759. doi: 10.1016/j.dld.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Tarris G, de Rougemont A, Charkaoui M, Michiels C, Martin L, Belliot G. Enteric viruses and inflammatory bowel disease. Viruses. 2021;13:104. doi: 10.3390/v13010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temtem T, Whitworth J, Zhang J, Bagga B. Cytomegalovirus in pediatric inflammatory bowel disease patients with acute severe colitis. Clin Res Hepatol Gastroenterol. 2021;45:101625. doi: 10.1016/j.clinre.2021.101625. [DOI] [PubMed] [Google Scholar]
- Then E, et al. The impact of human immunodeficiency virus (HIV) on inflammatory bowel disease (IBD): a retrospective nationwide study. Curr HIV Res. 2021;19:411–419. doi: 10.2174/1570162x19666210611145635. [DOI] [PubMed] [Google Scholar]
- Tillack C, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-γ-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63:567–577. doi: 10.1136/gutjnl-2012-302853. [DOI] [PubMed] [Google Scholar]
- Tinsley A, et al. Increased risk of influenza and influenza-related complications among 140,480 patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25:369–376. doi: 10.1093/ibd/izy243. [DOI] [PubMed] [Google Scholar]
- Umetsu T, et al. Inhibitory effect of silibinin on hepatitis B virus entry. Biochem Biophys Rep. 2018;14:20–25. doi: 10.1016/j.bbrep.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazifeh S, Kananpour P, Khalilpour M, Eisalou SV, Hamblin MR. Anti-inflammatory and immunomodulatory properties of Lepidium sativum. BioMed Res Int. 2022;2022:3645038. doi: 10.1155/2022/3645038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar BG, Ramesh D, Joji A, Kannan T. In silico pharmacokinetic and molecular docking studies of natural flavonoids and synthetic indole chalcones against essential proteins of SARS-CoV-2. Eur J Pharmacol. 2020;886:173448. doi: 10.1016/j.ejphar.2020.173448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vockerodt M, et al. The Epstein-Barr virus and the pathogenesis of lymphoma. J Pathol. 2015;235:312–322. doi: 10.1002/path.4459. [DOI] [PubMed] [Google Scholar]
- Wagoner J, et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010;51:1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace KL, Zheng L-B, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6. doi: 10.3748/wjg.v20.i1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallaschek N, et al. Ephrin receptor A2, the epithelial receptor for Epstein-Barr virus entry, is not available for efficient infection in human gastric organoids. PLoS pathog. 2021;17:e1009210. doi: 10.1371/journal.ppat.1009210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Lv Q, Miao YM, Qiao SM, Dai Y, Wei ZF. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol. 2018;155:494–509. doi: 10.1016/j.bcp.2018.07.039. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. Study on the network pharmacology and preliminary evidence of Lianhua Qingwen in the treatment of novel coronavirus (2019-nCoV) pneumonia. J Chinese Med Mater. 2020;43:772–778. [Google Scholar]
- Wang Z, Guo K, Liu Y, Huang C, Wu M. Dynamic impact of virome on colitis and colorectal cancer: immunity, inflammation, prevention and treatment. Semin Cancer Biol. 2021;86(Part 2):943–954. doi: 10.1016/j.semcancer.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski A, et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur Gastroenterol J. 2020;8:303–313. doi: 10.1177/2050640619889763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-F, et al. Chemopreventive effect of silymarin on liver pathology in HBV X protein transgenic mice. Cancer Res. 2008;68:2033–2042. doi: 10.1158/0008-5472.can-07-2450. [DOI] [PubMed] [Google Scholar]
- Wu W, et al. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses. 2015;8(1):6. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, He C, Tang T-Y, Li Y-Q. A review on co-existent Epstein-Barr virus-induced complications in inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2019;31:1085–1091. doi: 10.1097/meg.0000000000001474. [DOI] [PubMed] [Google Scholar]
- Xu W, et al. Chronic active Epstein-Barr virus infection involving gastrointestinal tract mimicking inflammatory bowel disease. BMC Gastroenterol. 2020;20:1–7. doi: 10.1186/s12876-020-01395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima S, Shimizu H, Sakamaki H, Ikeda S, Ikegami N, Murayama JI. Real-world cost analysis of chemotherapy for colorectal cancer in Japan: detailed costs of various regimens during the entire course of chemotherapy. BMC Health Serv Res. 2016;16:2. doi: 10.1186/s12913-015-1253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Qian J. Current status of cytomegalovirus colitis among patients with inflammatory bowel disease in China: a questionnaire-based multicenter study. Inflamm Bowel Dis. 2022;28:S45–S51. doi: 10.1093/ibd/izab358. [DOI] [PubMed] [Google Scholar]
- Yang H, Rao Z. Structural biology of SARS-CoV-2 and implications for therapeutic development. Nat Rev Microbiol. 2021;19:685–700. doi: 10.1038/s41579-021-00630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-Y, et al. Enteric viruses ameliorate gut inflammation via toll-like receptor 3 and toll-like receptor 7-mediated interferon-β production. Immunity. 2016;44:889–900. doi: 10.1016/j.immuni.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Yang Y, Zhang X, Xu M, Wu X, Zhao F, Zhao C. Quercetin attenuates collagen-induced arthritis by restoration of Th17/Treg balance and activation of Heme Oxygenase 1-mediated anti-inflammatory effect. Int Immunopharmacol. 2018;54:153–162. doi: 10.1016/j.intimp.2017.11.013. [DOI] [PubMed] [Google Scholar]
- Yu ES, Min HJ, An SY, Won HY, Hong JH, Hwang ES. Regulatory mechanisms of IL-2 and IFNγ suppression by quercetin in T helper cells. Biochem Pharmacol. 2008;76:70–78. doi: 10.1016/j.bcp.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Zakaryan H, Arabyan E, Oo A, Zandi K. Flavonoids: promising natural compounds against viral infections. Arch Virol. 2017;162:2539–2551. doi: 10.1007/s00705-017-3417-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Zhao S, Cao Z. Impact of Epstein-Barr virus infection in patients with inflammatory bowel disease. Front Immunol. 2022;13:1001055. doi: 10.3389/fimmu.2022.1001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, et al. EBV-associated lymphoproliferative disorder involving the gastrointestinal tract which mimic IBD in immunocompetent patients: case reports and literature review. Int J Colorectal Dis. 2019;34:1989–1993. doi: 10.1007/s00384-019-03400-4. [DOI] [PubMed] [Google Scholar]
- Zhou L, et al. Electrospun chitosan oligosaccharide/polycaprolactone nanofibers loaded with wound-healing compounds of rutin and quercetin as antibacterial dressings. Int J Biol Macromol. 2021;183:1145–1154. doi: 10.1016/j.ijbiomac.2021.05.031. [DOI] [PubMed] [Google Scholar]
- Zou H, Ye H, Kamaraj R, Zhang T, Zhang J, Pavek P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine. 2021;92:153736. doi: 10.1016/j.phymed.2021.153736. [DOI] [PubMed] [Google Scholar]
- Zullow S, Farraye FA. Updates on vaccinating the inflammatory bowel disease patient. Expert Rev Gastroenterol Hepatol. 2019;13:229–239. doi: 10.1080/17474124.2019.1565993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.