Abstract
Induction of reactive oxygen species (ROS) was observed within seconds of the addition of exogenous tobacco mosaic virus (TMV) to the outside of tobacco (Nicotiana tabacum cv Samsun NN, EN, or nn) epidermal cells. Cell death was correlated with ROS production. Infectivity of the TMV virus was not a prerequisite for this elicitation and isolated coat protein (CP) subunits could also elicit the fast oxidative burst. The rapid induction of ROS was prevented by both inhibitors of plant signal transduction and inhibitors of NAD(P)H oxidases, suggesting activation of a multi-step signal transduction pathway. Induction of intracellular ROS by TMV was detected in TMV-resistant and -susceptible tobacco cultivars isogenic for the N allele. The burst was also detected with strains of virus that either elicit (ToMV) or fail to elicit (TMV U1) N′ gene-mediated responses. Hence, early ROS generation is independent or upstream of known genetic systems in tobacco that can mediate hypersensitive responses. Analysis of other viruses and TMV CP mutants showed marked differences in their ability to induce ROS showing specificity of the response. Thus, initial TMV-plant cell interactions that lead to early ROS induction occur outside the plasma membrane in an event requiring specific CP epitopes.
Tobacco mosaic virus (TMV) is a single-stranded plus-sense RNA molecule packaged inside a rigid rod of coat protein (CP). The virus probably enters a plant passively through damaged cells in a process that involves attachment, entry, and un-coating (Shaw, 1999). Upon cell entry rapid cotranslational disassembly occurs and viral replication ensues (Wu and Shaw, 1997). These processes can be traced to specific sites that suggest particle or RNA entry through ectodesma or by pinocytosis (Gaard and De Zoeten, 1979; Hills et al., 1987; Shaw, 1999). The latter mechanism of entry suggests receptor-mediated transfer, but attempts to isolate or even define such receptors have not been successful. Although initial virus-plant cell interaction remains a mystery, much is known about systemic movement. Viral RNA moves from cell-to-cell as a complex with the viral 30-K movement protein, and long-distance movement through the phloem to uninfected leaves is facilitated by a functional CP (Deom et al., 1992; Hilf and Dawson, 1993). Thus, once inside a viable cell viral RNA or particles are transferred systemically by symplastic movement through plasmadesmatal connections (Carrington et al., 1996; Citovsky, 1999).
Plant perception of pathogen ingress involves the presence of resistance genes. Genetic resistance to TMV infection is well characterized in tobacco (Nicotiana tabacum cv Samsun NN, EN, or nn). N gene-mediated TMV resistance was first described in Nicotiana glutinosa. The N gene sequence predicts a protein with similarities to the Drosophila Toll protein and contains nucleotide binding sites and C-terminal Leu-rich repeats (Whitham et al., 1994). Initiation of the resistance response is proposed to involve interaction of the N gene product with the virus-encoded replicase (Padgett and Beachy, 1993; Padgett et al., 1997; Erickson et al., 1999).
A second genetic source of recognition between the plant cell and TMV is termed N′ and involves the interaction of the N′ gene with nascent viral CP synthesized within the cell (Culver et al., 1994; Taraporewala and Culver, 1996; Erickson et al., 1999). The N′ gene product has not yet been determined. However, an array of viable TMV CP mutants have been generated that either elicit or fail to elicit the N′-mediated response, suggesting that the N′ gene product has the specificity attributes of a functional resistance gene (Taraporewala and Culver, 1996).
The interaction of resistance genes with pathogen-induced products initiates a series of signaling cascades leading to disease resistance (Baker et al., 1997). Among the cellular events that characterize local resistance are both rapid and late oxidative bursts, cell wall strengthening, induction of pathogenesis-related defense gene expression, and frequently hypersensitive response (HR). ROS induction by fungal or bacterial elicitors often occurs in two distinct phases. Very rapid responses (within minutes or hours), termed phase I, are not always correlated with plant disease resistance, whereas later ROS production (hours or days) is termed phase II and correlates with resistance/susceptibility of the plant to the pathogen. Whereas phase I and II bursts differ kinetically, they may also differ as to the source of ROS and/or the type of ROS produced. The exact kinetics of ROS induction is a function of the biology of the invading pathogen. Phase I responses may well be important in defense as a priming mechanism for latter phase II responses (Baker and Orlandi, 1995).
ROS may be produced at the plasma membrane by an NAD(P)H oxidase analogous to the mammalian O2− producing enzyme (Levine et al., 1994; Desikan et al., 1996; Groom et al., 1996; Jabs et al., 1996; Murphy and Auh, 1996). An alternative source of ROS, elicited during pathogen attack, are cell wall located peroxidases, amine oxidases, or aldehyde oxidases (Allan and Fluhr, 1997; Ori et al., 1997; Bolwell et al., 1998). ROS bursts can have direct anti-pathogen effects. In addition, ROS can serve as intra- and inter-cellular messengers. Hydrogen peroxide (H2O2) and superoxide (O2−) have been implicated as the agents of this burst (for reviews, see Mehdy, 1994; Baker and Orlandi, 1995; Bolwell et al., 1995). However, it is H2O2 that is the most attractive candidate for signaling via ROS because of its relative stability and greater membrane permeability.
Little is known about the very first stages of virus/plant interactions. We have sought to further examine the initial tobacco cell-TMV interaction using the H2O2−-sensitive fluorophore dichlorofluorescein (DCF). DCFH-DA loads readily into epidermal cells of tobacco, where it has been shown to be a sensitive reporter of intra-cellular increases in ROS following addition of pro-oxidants or elicitors of plant pathogen responses (Allan and Fluhr, 1997). Here we show that intact TMV as well as isolated TMV CP can trigger a rapid phase I-like oxidative burst when added to the apoplast of tobacco epidermal cells. The elicitor appears to be the virus CP, which stimulates a plant NAD(P)H oxidase-like activity via an active signal transduction pathway. Exposure of the wounded epidermal cells to TMV results in cell death. The requirements of certain CP secondary structure for a ROS burst and the inability of specific mutant CP to elicit the burst suggests that specific perception of the virus occurs before infection of the cell.
RESULTS
DCF Reports a Rapid TMV-Dependent Oxidative Burst in Specific Cell Types
DCF enters cells in the diacetate form (DCFH-DA) where it is hydrolyzed and trapped as DCFH, a non-fluorescent compound. Subsequent oxidation of DCFH by H2O2, catalyzed by peroxidases, yields the highly fluorescent DCF (Cathcart et al., 1983). Tobacco epidermal cells were loaded with DCFH and monitored by fluorescence spectroscopy. ROS transients were readily detected when the cells were treated with purified TMV virions (Fig. 1). Due to the speed of the response, the increases in fluorescence are unlikely to be the result of changes in peroxidase activity but rather reflect increases in cytosolic H2O2 as shown in Allan and Fluhr (1997).
Figure 1.
Elicitation of rapid intracellular ROS transients by purified TMV. A, Epidermal peels from N. tabacum cv Samsun NN were loaded with DCFH-DA and monitored using fluorometry. As a control for the ability of the tissue to mount a ROS transient, l-Arg was added. B, As in A, but epidermal peels were from N. tabacum cv Samsun nn. C, As in A, but the virus added was CMV. As a control for the ability of the tissue to mount an ROS transient, TMV virus was added. D, As in C, but virus added was CGMMV. E, Dose response curve of ROS elicitation versus virus concentration using TMV or CMV and peels from N. tabacum cv Samsun NN or N. tabacum cv Samsun nn. Maximal response was recorded by the addition of 5 mm H2O2. Error bars are the mean and se of five experiments.
The response was proportional to the amount of virions added, which at its low titer point is equivalent to 75 to 100 lesions/leaf (Fig. 1E). The shape of the ROS transients varied between replicates and tended to reach a peak 4 to 8 min after the addition of TMV. The elicitation of ROS occurred in cells from plants both resistant and susceptible to TMV (cv Samsun NN and cv Samsun nn; Fig. 1, A and B, respectively). In addition, the TMV U1 strain used here does not trigger HR mediated by the N′ genotype that may be present in some lines of cv Samsun (Piccirillo and Porrone, 1995; Taraporewala and Culver, 1996). Thus, the oxidative burst detected does not correlate with any of the previously characterized genetic resistances to TMV and is perhaps unrelated to known resistance responses to TMV.
Both distant and closely related viruses were examined to assess the specificity of the response. Similar concentrations of cucumber mosaic cucumovirus (CMV), a virus that infects tobacco but has a completely unrelated structure (consisting of icosahedral virions), did not elicit a fast oxidative transient (Fig. 1, C and E). Cucumber green mottle mosaic tobamovirus has a CP that shares 45% identity to the CP of the TMV U1 strain (Meshi et al., 1983). This rod-shaped tobamovirus does not infect the cultivar of tobacco used in our study and did not elicit a ROS response (CGMMV; Fig. 1D). However, two other tobamoviruses, Ob and ToMV (tomato mosaic virus, L strain) whose CPs share 59% and 84% identity, respectively, to CP from TMV U1 did elicit the fast oxidative burst (data not shown).
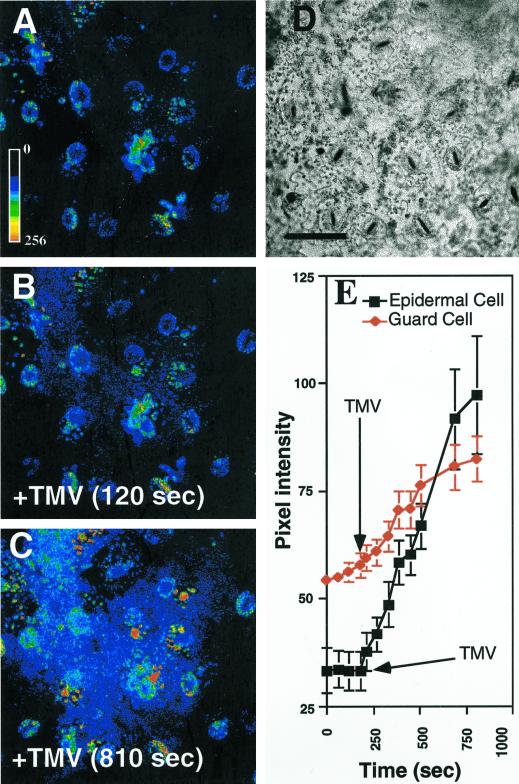
Confocal microscopy imaging was used to examine cellular features of the TMV-elicited oxidative burst. A rapid burst was observed in most loaded epidermal cells within the confocal focal slice (Fig. 2, B, C, and E). Increased fluorescence could be recorded in many of the epidermal cells within 120 s of addition of virions (compare Fig. 2, A and B). Analysis of changes in pixel intensities in epidermal cells and guard cells (Fig. 2E) showed that the TMV-elicited burst was more prominent in epidermal cells, whereas guard cells remained less affected.
Figure 2.
Laser scanning confocal imaging of the TMV-elicited oxidative burst in epidermal cells. Epidermal tissue was loaded with DCFH-DA, washed, and examined by laser scanning confocal microscopy. TMV was added during the time course of image acquisition. A, Epidermal cells loaded with DCFH-DA. The pseudocolor key is included and was applied to pixel intensity values for all three fluorescence images. B, Cells shown in A 120 s after the addition of 100 ng TMV (+TMV). C, Epidermal cells shown in A and B 810 s after addition of TMV. D, Bright field of cells shown in A through C. E, Time course of pixel intensities of selected cells. At 240 s (arrow) 100 ng of TMV was added and the pixel intensities (mean over the whole cell) of epidermal (▪) or guard (♦) cells were analyzed over the next eight captured images. Each time point represents the mean pixel intensity and se of six cells. Experiments were repeated at least six times with similar results. A through D, Scale bar = 50 μm.
TMV Induction of ROS Involves a Flavin-Containing Oxidase
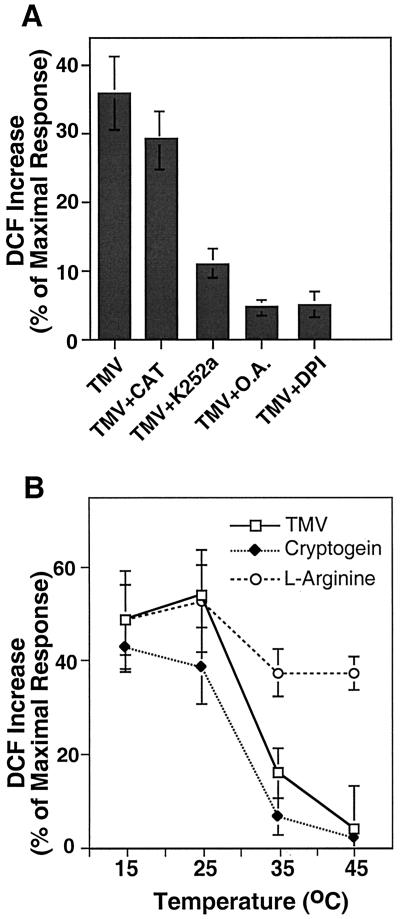
The addition of exogenous catalase to the tissue can be used to distinguish between possible extracellular or intracellular sources of the ROS transients. For example, cryptogein, a secreted polypeptide from Phytophthora cryptogea (Ricci et al., 1989; Milat et al., 1991; Viard et al., 1994), elicits fast intracellular ROS transients that are catalase insensitive. In contrast, catalase has been shown to dissipate H2O2 transients generated in the apoplast by amine oxidase-like activity after addition of l-Arg and other amines (Allan and Fluhr, 1997). The elicitation of ROS by the TMV virions was found to be insensitive to the addition of exogenous catalase (Fig. 3A), or SOD (data not shown), establishing that the source of TMV-induced ROS is intracellular. Furthermore the inhibition of ROS elicitation by application of diphenylene iodonium (DPI; Fig. 3A) suggests that the source of the generated ROS is a flavin-containing oxidase such as NAD(P)H oxidase.
Figure 3.
The sensitivity of TMV-elicited ROS to pharmacological agents and temperature. The increase in DCF fluorescence in tobacco epidermal cells for each treatment is shown as the percentage of maximal response after the addition of 5 mm H2O2. A, DCF increase after addition of TMV (100 ng) in the presence (+) of the indicated pharmacological agents; CAT, catalase (100 units mL−1); K252a, (25 μm); O.A., okadaic acid (100 nm); DPI, diphenyleneiodonium (10 μm). B, ROS transients elicited by TMV (100 ng), cryptogein (25 ng mL−1), and l-Arg (1 mm) were carried out at temperatures ranging from 15°C to 45°C. Results are the mean and se for three replicate experiments.
Tissue HR can occur during or after the phase-II stage of pathogenesis response. In the case of TMV, HR was shown to involve the presence of a Ser/Thr phosphatase and include a temperature sensitive component (Doke and Ohashi, 1988; Dunigan and Madlener, 1995). It was of interest to examine whether the phase I-type ROS transients have similar attributes. Application of kinase inhibitor K252a and the phosphatase 2A inhibitor okadaic acid were found to inhibit ROS production (Fig. 3A), suggesting that phosphorylation and dephosphorylation events play a role in signaling the intracellular build-up of TMV-induced ROS. It was previously shown that application of inhibitors of signal transduction to l-Arg treated peels had no effect (Allan and Fluhr, 1997). TMV-induced bursts in ROS have been shown to be temperature sensitive and do not occur in leaf discs above 30°C (Doke and Ohashi, 1988). The temperature dependence of the ROS transients was followed in TMV, cryptogein, and l-Arg-treated tissue. Raising the temperature from 25°C to 35°C caused a 70% reduction in the burst elicited by TMV and cryptogein (Fig. 3B). No significant burst occurred at 45°C. In contrast, l-Arg-driven H2O2 production, shown previously to be mediated by cell wall peroxidases, was only slightly affected by elevated temperature (Fig. 3B).
Specific Quaternary Structure of the Virus, But Not Infectivity, Is Required to Elicit the ROS Response
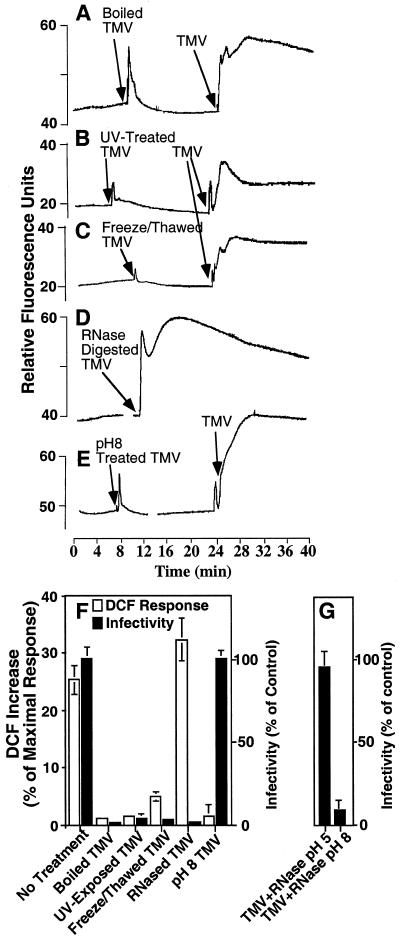
The nature of the elicitor of the ROS response was ascertained by pretreating the virus particle in a number of ways. These treatments included boiling, UV irradiation, and repeated freeze-thawing. The treatments that simultaneously destroy virus structure and infectivity greatly reduced or completely prevented its ability to elicit ROS (Fig. 4). However, pretreatment of the virions with high concentrations of RNase A, that abrogates infectivity without affecting rod structure, had no negative effect on induction of ROS (Fig. 4, D and F). We conclude that an infectious virus is not a prerequisite for the elicitation of the oxidative burst. Treatment of the virus at pH 8.0 ôhas been shown to “swell” the tight rod-like structure relative to that observed at pH 5.0 (Wilson, 1984). We confirmed the influence of pH on the virions structure by pH treatment and concomitant processing of the virions with dilute concentrations of RNase A. Virus infectivity was eliminated by this RNase treatment at pH 8.0 but not at pH 5.0 apparently due to the increased nuclease accessibility (Fig. 4G). When virions were pre-incubated at pH 8.0 (without RNase) infectivity was unaffected, however, the oxidative burst was completely inhibited (Fig. 4, E and F). Therefore, at low pH a virus with tight rod structure can elicit the ROS burst, whereas at alkaline pH a swollen virus is ineffective as an elicitor.
Figure 4.
Elicitation of ROS by modified virus particles. Epidermal peels were loaded with DCFH-DA and fluorescence was monitored during in-flight additions of pretreated virus (100 ng), subsequently followed by the addition of untreated TMV (100 ng, except in D). A, Boiled TMV (10 min at 105°C). B, UV-exposed TMV (30 mJ). C, Freeze-thawed TMV (20 cycles). D, RNase-treated TMV (10-min treatment; 10 μg mL−1). E, TMV held at pH 8.0 (10 mm Tris for 30 min). F and G, Increase in fluorescence after the addition of modified TMV virus. The results of three experiments are expressed as a percentage of the mean maximal response and se obtained after the addition of H2O2 (white bars). The infectivity of the virus for each treatment (black bars) is expressed as the number of lesions produced on N. tabacum cv Samsun NN leaves relative to untreated virion control.
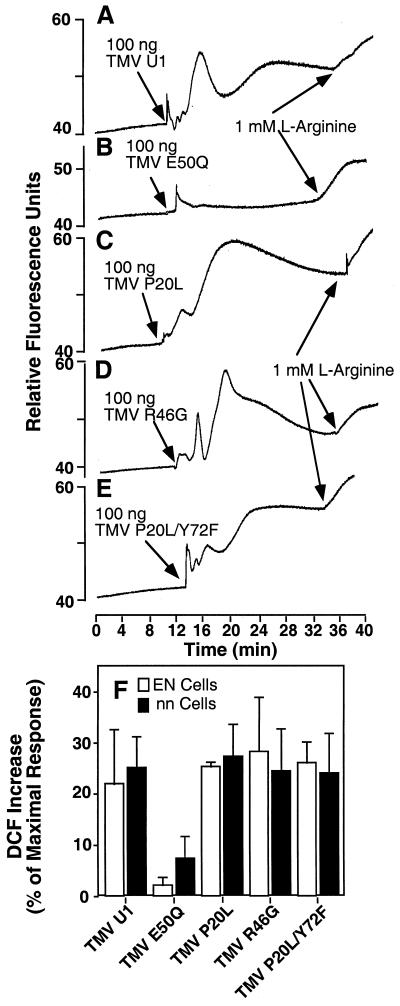
The sensitivity of the ROS burst to structural integrity suggests that CP topography plays a role in the interaction. To further probe the nature of this interaction we took advantage of U1 strain CP mutants that have been characterized in the N′ genetic system (Taraporewala and Culver, 1996). Mutants P20L and R46G are, respectively, strong and moderate elicitors of HR in cultivars carrying the N′ allele. P20L/Y72F is a temperature sensitive HR elicitor. E50Q is not an elicitor of HR and has a modification that removes a carboxyl-carboxylate pair that normally occurs between position E50 and D77 of axially adjacent CP subunits (Culver et al., 1994). It is interesting that all mutant virions except the E50Q replacement were able to elicit ROS in the DCF assay system (Fig. 5, A–E). The E50Q mutation stabilizes the helical aggregates inhibiting viral disassembly, so apparently induction of ROS requires some degree of disassembly. In addition, there was no apparent difference in response between tobacco tissue that carried the N′ gene (cultivar EN; Fig. 5F) and nn/n′n′, as well as no significant difference between the magnitude of wild type and P20L elicited bursts. All CP mutants, except E50Q, caused characteristic fast spikes in fluorescence, followed by a more general increase over the next 10 to 20 min. The exact shape of each transient varied between experiments. The results suggest that CP-based mutations influence ROS induction and N′-type mediated responses differentially and they are likely independent genetic systems.
Figure 5.
Elicitation of ROS by mutant virus particles. Epidermal peels from N. tabacum cv Samsun nn (A–F) or N. tabacum cv Samsun EN (F) were loaded with DCFH-DA and monitored using fluorometry. Peels were exposed to 100 ng of TMV virus containing wild-type CP (U1) or mutant type CP as indicated. l-Arg (1 mm) was added as a control at the end of each experiment. Viruses used wereas follows: A, wild-type CP; B, CP E50Q; C, CP P20L; D, CP R46G; and E, CP P20L/Y72F. F, The mean increase in DCF fluorescence is shown after elicitation with each CP mutant in either N. tabacum cv Samsun NN or EN cells. The increase is expressed as the percentage of the maximal response obtained after the addition of 5 mm H2O2.
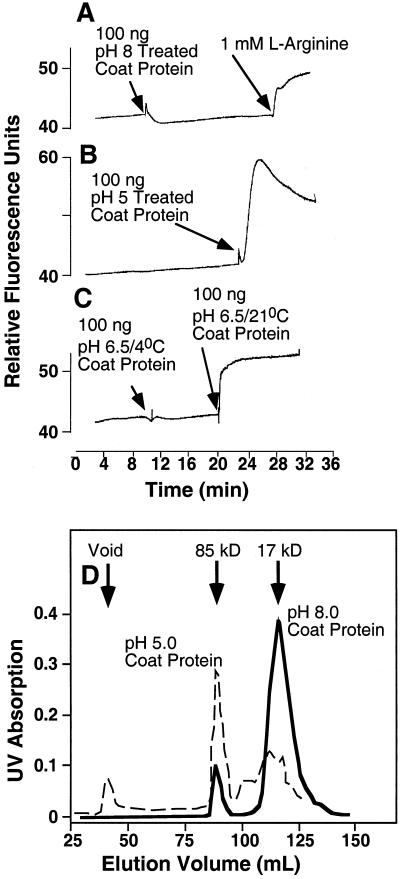
To further examine structural requirements for recognition, CP was isolated from infectious U1 strain virus by acid-induced un-coating (Fraenkel-Conrat, 1957). Isolated CP has been shown to form a mixture of low Mr aggregates at high pH/low salt concentrations, whereas incubation in acidic pH allows large helical aggregates to form (Durham et al., 1971; Durham and Klug, 1971). The aggregation states of isolated CP were established by gel filtration as shown in Figure 6D. Isolated CP pretreated at pH 8.0 showed single CP subunits of 17.5 kD and some aggregates of 85 kD (Fig. 6D). This preparation elicited no oxidative response (Fig. 6A). Allowing the CP to further aggregate to stacks or helices of higher molecular mass by pretreatment at pH 5.0 shifted the molecular mass to a preponderance of 85 kD and higher molecular mass that eluted in the column void volume (Fig. 6D). These fractions were active in ROS induction (Fig. 6B). Near-neutral pH (pH 6.5) allows aggregates of CP to form but only at room temperature (Durham and Klug, 1971). CP pretreated under these conditions was an effective elicitor, whereas preparations held at 2°C to 3°C were not (Fig. 6C). This suggests that CP structure itself is the agent of elicitation, rather than protons. We conclude that to elicit the oxidative response, isolated CP must have some degree of aggregation or at least be potentiated to aggregate.
Figure 6.
Elicitation of ROS by pH-treated TMV CP. Fluorescence of epidermal tissue was monitored during a time course in which in-flight additions were made of pretreated TMV CP (100 ng). A, TMV CP were pretreated at pH 8.0 (3 h in 10 mm Tris buffer), followed by addition of l-Arg (1 mm). B, TMV CP were pretreated at pH 5.0 (3 h in 10 mm MES buffer). C, TMV CP was treated as in A, then neutralized to pH 6.5 (3 h) at either room temperature or 4°C. D, Elution pattern of CP fractions used in A and B. The TMV CP was pretreated at pH 5.0 or 8.0 for 4 h and then resolved on a FPLC column at the same pH as the pretreatment. Calculated molecular masses of the peaks are shown.
Phases of ROS Induction after Elicitation with Exogenous or Endogenous TMV
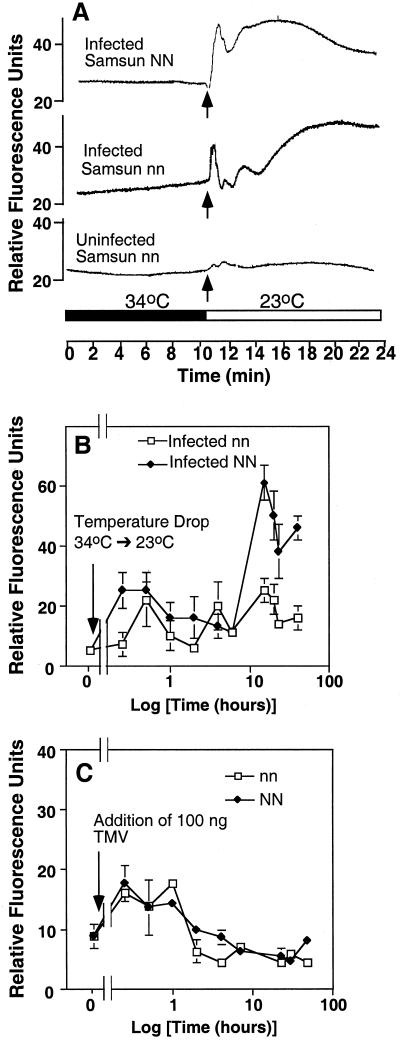
To relate ROS elicitation by exogenous TMV to the multiple phases of ROS induction exhibited during plant-pathogen interactions, we examined intracellular H2O2 accumulation in TMV-pre-infected tissue. Tobacco leaves, held at elevated temperatures (>30°C), show no N gene-mediated HR in response to infection. Peels obtained from the infected leaves were loaded with DCFH-DA at 34°C and after 10 min subjected to a temperature drop to 23°C. A ROS burst was detected in both Samsun NN and nn tissue but not in uninfected tissue (Fig. 7A). The early induction of ROS suggests phase-I kinetics. When tissue was held for progressively longer times (12–24 h) at the temperature permissive to N gene-dependent HR, oxidative responses were detected in infected NN tissue that were consistent with a bi-phasic reaction (Fig. 7B). Infected nn peels do not exhibit the later increase in ROS. In contrast to ROS production in the presence of endogenous TMV, the addition of exogenous TMV to uninfected peel tissue elicited only phase-I kinetics (Fig. 7C).
Figure 7.
ROS transients in pre-infected epidermal peels. Plants were inoculated (or uninfected controls) with TMV and held for 1 week at 30°C to 35°C. Peels were prepared and loaded with DCFH-DA, washed, and affixed to the peel holder at 34°C, then monitored using fluorometry. A temperature drop to 23°C was achieved at the indicated time point. A, Short-term time course of TMV infected N. tabacum cv Samsun NN, N. tabacum cv Samsun nn, and control uninfected peel tissue. B, Long-term time course (time shown on a log scale) of ROS induction after transfer of peels to room temperature. Three replicate epidermal peels from N. tabacum cv Samsun NN and N. tabacum cv Samsun nn plants treated as in A and examined at the specified time points. C, TMV addition (at arrow), during a time course (time shown on a log scale), to uninfected N. tabacum cv Samsun NN or N. tabacum cv Samsun nn peel tissue. Three replicate peels were assayed for each time point.
Cell Death as a Result of Exogenous Application of TMV
Long-term aspects of epidermal cell/TMV interaction were examined by first establishing if exogenously added TMV can infect peel tissue. Peels that had been exposed to TMV were homogenized and then the supernatant applied to N. tabacum Samsun NN leaves, as a bioassay of infectivity (Sulzinski and Zaitlin, 1982). As shown in Figure 8, exposure to virions did not lead to the peel tissue becoming infected to any extent. The lack of TMV increase is not due to inability of peel tissue to support viral replication as TMV application in the presence of polyethylene glycol (PEG)-promoted infection (Fig. 8).
Figure 8.
TMV infection of isolated epidermal cells. Epidermal peels were isolated from N. tabacum cv Samsun nn plants and floated on Suc supplemented buffer. Peels were then exposed to TMV by either floating on the virus (750 ng mL−1) constantly for the next 3 d (●), floating on the same concentration of virus for 30 min then washed before refloating on fresh buffer (▪), or exposing to TMV and PEG at the same time (□) as described in the experimental procedures. Infectivity of the peels over a time course was then tested by bioassay on N. tabacum cv Samsun NN leaves.
Epidermal peels treated with exogenous TMV for 2 d showed considerable cell death (Table I), despite the observation that no infection occurs of these peels (Fig. 8). This death was unrelated to the N gene, occurring in both NN and nn tissue. Cell death was specific for the presence of TMV as application of CGMMV, which does not elicit ROS responses from epidermal cells, had no significant effect (Fig. 1; Table I). This suggests that ROS induced by extracellular TMV is sufficient to drive the death of isolated epidermal cells. Guard cells were also sensitive to the presence of TMV, despite the fact that ROS responses from these cells were less than those of epidermal cells (Fig. 2). To investigate this further, epidermal peels were pretreated at pH 3.0 for 1 h, a treatment that selectively kills all epidermal cells leaving only viable guard cells and trichomes (Squire and Mansfield, 1972). As shown in Table I, peels that were floated on neutral pH after acid-induced death of the epidermal cells showed good guard cell viability. However, in this case, the addition of TMV to acid-treated peels did not result in guard cell death. In contrast, exposure of “acid isolated” guard cells to cryptogein resulted in substantial guard cell death (Table I). We conclude that guard cells are not directly affected by TMV but are influenced by the physiological status of the adjoining cells.
Table I.
Viability of epidermal tissue after exposure to TMV, CGMMV, or cryptogein
| Tissue Type and Treatmenta | Viability of Cell Typeb
|
|
|---|---|---|
| Epidermal | Guard | |
| % | ||
| NN | 82 ± 5 | 96 ± 10 |
| NN + TMV | 3 ± 1 | 48 ± 10 |
| NN + CGMMV | 75 ± 8 | 98 ± 2 |
| nn | 80 ± 4 | 93 ± 10 |
| nn + TMV | 5 ± 2 | 33 ± 5 |
| nn + CGMMV | 78 ± 5 | 80 ± 10 |
| Acid-treated nn | 0 ± 0 | 95 ± 7 |
| Acid-treated nn + TMV | 0 ± 0 | 92 ± 5 |
| Acid-treated nn + cryptogein | 0 ± 0 | 10 ± 8 |
Epidermal peels were floated on Suc-supplemented loading buffer with or without TMV or CGMMV (750 ng mL−1) or cryptogein (100 ng mL−1). After 48 h, peels were removed and loaded with fluorescein diacetate, and viability was scored.
Nos. represent the percent of total cells counted. At least 100 cells were counted for each experiment. The experiments were conducted in triplicate.
DISCUSSION
Plant Cell/TMV Interaction
It has been shown that TMV particles are visible within wounded tobacco cells almost immediately upon inoculation (Plaskitt et al., 1987). However, little more is known about the very first stages of the interaction between TMV and the plant cell. We have found that rapid ROS elicitation occurs after TMV addition in most tobacco epidermal cells (Fig. 2). This indicates that the reaction is likely to be a cell autonomous event and not limited to serendipitous TMV access to singular cells through localized wounding. Furthermore, epidermal peels show no significant TMV infection unless a membrane interaction enhancing factor such as PEG is added (Fig. 8). If virus internalization was prevalent then global infection would have been observed. The lack of virus intracellular access suggests that TMV induction of ROS takes place extracellularly. It is interesting that ROS accumulation is prominent in epidermal cells. The lower ROS induction response in guard cells may be due to the heightened capacity of those cells to scavenge ROS due to the presence of active chloroplasts. Guard cells alternatively may lack the viral perception mechanism and react through apoplastic diffusion of ROS.
Induction of ROS by TMV manifests a degree of specificity. CMV, which has a completely unrelated structure to the tobamoviruses, is fully infective toward tobacco yet failed to elicit the ROS burst. Related tobamoviruses viruses can either elicit (TMV U1, Ob strain TMV, ToMV) or fail to elicit (CGMMV) the perception process that leads to both ROS induction and cell death. Furthermore, specific single residue substitutions in the CP of TMV (E50Q) can destroy its ability to be an elicitor of the burst. Changing the quaternary structure of either the intact virus or of isolated CP by pH pretreatments, modifies the induction of ROS transients (Figs. 5 and 6). Thus, only certain conformations of CP or aggregated CP can trigger the ROS burst. These observations, together with the lack of infectivity, are consistent with epidermal cells exhibiting a receptor-like apoplastic perception for the TMV CP.
Based on genetic considerations, the early TMV-induced oxidative response detected here is both N and N′-gene independent. No difference in ROS induction was detected between tissue containing N or n, N′ or n′ genetic backgrounds. In addition, ROS was elicited by the TMV U1 virus strain that normally does not elicit N′-type responses. Furthermore, the site of ROS elicitation appears to be extracellular, whereas elicitation via the N and N′ genes would necessitate time for cytoplasmic accumulation of the putative elicitors, TMV replicase, or CP, respectively (Pfitzner and Pfitzner, 1992; Whitham et al., 1994; Baker et al., 1997; Padgett et al., 1997).
Certain features of ROS induction reported here mimic the N′-like requirement for certain configurations of CP quaternary structure (Culver et al., 1994; Taraporewala and Culver, 1996). In the intact virus, carboxylate groups from CP residue E50 and the adjacent subunit residue D77 are predicted to interact in the axial direction (Namba et al., 1989). Their charge repulsion may be neutralized in the TMV rod by a proton. Upon cell entry the proton is lost, destabilizing the rod and facilitating co-translational release of CP units. The E50Q mutation abrogates charge repulsion and was shown to stabilize helical protein aggregates as well as inhibit viral disassembly (Culver et al., 1995; Lu et al., 1998). Our observation that E50Q virions show reduced ROS induction parallels the loss of HR elicitation in this mutation in the context of the N′ response (Culver et al., 1994). This result indicates that a certain amount of rod disassembly is necessary for ROS elicitation. However, under conditions that favor the complete nonaggregate state of individual CP (high pH) no induction is observed. We conclude that the active molecule, which is capable of ROS elicitation, is CP in an intermediate state of assembly or CP poised to aggregate. The inability of E50Q to elicit a response would then be a result of high molecular mass TMV particles that are inaccessible to the cell surface (Lu et al., 1998).
TMV-Dependent ROS Induction and Cell Death
Phase-I plant responses to pathogen ingress are considered a necessary prelude but insufficient indication of the final plant resistance response. The appearance of later phase-II response marks true resistance and is associated with resistance genes that recognize pathogen ingress and activate concomitant cellular signaling systems (Baker and Orlandi, 1995). The rapid oxidative response to TMV detected here may be compared with phase-I bursts. In this case, the attenuation of TMV-dependent ROS induction brought about by application of general molecular inhibitors of signal transduction, as well as inhibitors of flavin-containing enzymes, suggest that phase-I and phase-II responses have common complex pathways for activation that culminate in a NAD(P)H-like oxidase activation (Fig. 3).
Phase-II responses can be detected in peels but only in tissue pre-infected with TMV and harboring the N gene. Similarly, phase-II bursts can be seen in infected protoplasts carrying the N gene (infected with TMV U1) and the N′ gene (infected with TMV U5), but not in nn protoplasts (Allan et al., unpublished data). Our results are consistent with measurements carried out previously in pre-infected tobacco leaf discs transferred to permissive temperature (Doke and Ohashi, 1988). It has been suggested that oxidative phase-II response, but not phase-I response, is correlated with HR or cell death (Baker and Orlandi, 1995). Thus, the epidermal cell death that ensued after application of exogenous TMV was unexpected. This death may be related to a potentiation of defense responses after peel-induced wounding. Evidence suggests that wounding enhances pathogen responses through increases in systemin (Stennis et al., 1998), salicylic acid (Kauss and Jeblick, 1995), or jasmonic acid (Graham and Graham, 1996). It is interesting that epidermal cell death also influenced guard cell viability. The elimination of adjoining epidermal cells by acid treatment of the tissue promoted guard cell viability. This result indicates that a “death factor” of unknown nature is apparently transferred to the otherwise symplastically-isolated cells. These results may be related to findings showing that TMV-infected NN cells produce toxins that elicit cell death of uninfected protoplasts (Hooley and McCarthy, 1980).
The rapid response produced by extracellular TMV cannot be shown to be directly associated with disease resistance but may well be of biological significance. For example, rapid changes in the alternative splicing patterns of N resistance gene transcripts were measured within 3 h of TMV infection well preceding both phase II and the HR (Dinesh-Kumar and Baker, 2000). In this case, the rapid oxidative burst shown here exemplifies an early cellular detection device for viral presence and can serve as a priming step in readying latter resistance responses. Additional components of early sensing of “non-self” elicitors have been recently characterized in which a specific domain of the bacterial flagellin protein was found to elicit fast cellular responses from a variety of plant cells (Felix et al., 1999). Thus, the presence of sensitive general chemopreception mechanisms for pathogen components (e.g. bacterial flagellin or viral CP) may facilitate plant protection. The question of how the virus initially penetrates the plant cell remains largely unanswered. Our observations with TMV particles lend credence to the existence of inherent cellular structures situated outside of the cell that can interact with TMV. The detection of the apoplastic sites for TMV/plant cell interaction may further our understanding of cellular routes for viral penetration.
MATERIALS AND METHODS
Chemicals
Dichlorofluorescin diacetate (DCFH-DA; Molecular Probes, Eugene, OR) was dissolved in dimethyl sulfoxide to produce a 100 mm stock, which was frozen as aliquots. Cryptogein was a kind gift of Drs. P. Ricci and H. Keller (Institut National de la Recherche Agronomique, Antibes, France). The signal transduction inhibitors K-252a, okadaic acid, and diphenylene iodonium were purchased from Calbiochem (La Jolla, CA), and H2O2 from Merck (Darmstadt, Germany). Unless stated otherwise other chemicals were of analytical grade purchased from Sigma (St. Louis).
Virus Preparation and Infectivity Tests
TMV (U1 strain) and ToMV were propagated in Nicotiana tabacum cv Samsun nn tobacco plants, whereas Ob was propagated in cv Samsun NN plants. Leaves were mechanically inoculated using carborandum as an abrasive with TMV, ToMV, or Ob that was diluted to the appropriate concentration in inoculation buffer (20 mm sodium phosphate, pH 7.2, 1 mm EDTA).
Virus was purified to a final concentration of 2.6 mg mL−1 (Bruening et al., 1976). TMV CP mutants were prepared as described (Taraporewala and Culver, 1996). Viral titer was checked both by protein assay and scoring lesions after re-infecting cv Samsun NN plants. Purified TMV pretreatments included: incubation with RNAseA, (10 μg mL−1) for 10 min, and exposure to UV irradiation using a UV cross linker (Startalinker 1800; Stratagene, La Jolla, CA; set at 1,000 μJ s−1).
The infectivity of TMV-exposed epidermal peels relied on a bioassay of epidermal extracts rub inoculated on to cv Samsun NN leaves (see above). Epidermal peels (6–12 individual peels, depending on experiment) were exposed to TMV (750 ng mL−1) either for 30 min in loading buffer (Tris-KCl at 10 and 50 mm, respectively, pH 7.2), or for 1 min while floating on 40% (v/v) PEG, followed by 30 min at 4% (v/v) PEG. Peels were then washed by floating on fresh loading buffer then pre-incubated for up to 3 d on a Petri dish containing 10 mL of loading buffer supplemented with 100 mm Suc at room temperature. Dishes were placed on an orbital shaker at 30 rpm to ensure aeration. Some peels were exposed to TMV (750 ng mL−1) for longer periods of up to 3 d. Peels were then removed, washed by reflotation on 10 mL of fresh loading buffer, then immediately homogenized in 250 μL of loading buffer, centrifuged (10,000g for 5 min), and the supernatant (200 μL per leaf) applied to leaves. Three replicate leaves were inoculated per treatment. Lesions were scored 5 to 7 d postinoculation.
Other types of virus included a field isolate of CMV (Lapidot et al., 1997), propagated in cucumber plants and purified as described (Palukaitis et al., 1992). CGMMV was a kind gift of Dr. Y. Antignus (Department of Virology, The Volcani Center, Israel).
CP Isolation and Treatment
CP was isolated as described (Fraenkel-Conrat, 1957). Briefly cold virus stock (0.5 mL at 2.6 mg mL−1) was exposed to 1.5 mL of ice cold glacial acetic acid for 60 min. The precipitate was then centrifuged off (10 min at 10,000g, 3°C), and the supernatant diluted in 1.5 mL of cold distilled water, which was dialyzed for 24 h at 3°C. The resulting aqueous stock was diluted in buffers at pH 8.0 and 6.5 (Tris/KCl, both at 50 mm), or pH 5.0 (MES/KCl, both at 50 mm) to give solutions of 6 μg mL−1, and pre-incubated for 3 h before use in assays to test the induction of an oxidative burst. A fraction of undiluted CP stock was taken for analysis by chromatography. Samples were buffered at pH 5.0 or 8.0 (both 20 mm MES or Tris, respectively) for 4 h before loading on to a Superdex 200 HiLoad 16/60 FPLC column (Amersham Pharmacia, Piscataway, NJ). Samples were run either at pH 5.0 or 8.0, according to pretreatment, at 0.3 mL min−1 flow rate. Fractionation was on a BioLogic FPLC (Bio-Rad Laboratories, Hercules, CA).
Laser Scanning Confocal Microscopy and Fluorometry
The first fully expanded leaves were removed from greenhouse-grown tobacco (Nicotiana tabacum cv Samsun NN, EN, or nn) plants. Epidermal peels were then removed from the abaxial surface of each leaf and placed into a small Petri dish containing 10 mL of loading buffer and 5 μL of DCFH-DA from a 100 mm stock in dimethyl sulfoxide. Peels were loaded, in the dark, for 10 min, then removed and floated on a dish of fresh buffer to wash off excess dye. Individual peels were affixed to a glass coverslip with silicon grease (high vacuum, heavy; Merck, Rahway, NJ) on which the peel remained immersed in 0.5 mL of loading buffer. Examination of peels was carried out immediately using a MRC-1024 laser scanning confocal microscope (Bio-Rad Laboratories). A green argon-ion laser (488 nm) set on 3% power was used for excitation, with 525-nm emission. The viability of the cells within the epidermis under these media conditions was greater than 95% for guard cells and 80% for epidermal cells, as tested by fluorescein diacetate staining. Images were captured over a time course with laser scanning at set time points to avoid photoactivation of the dye. Elicitors (no greater than a 50-μL volume) were added directly to the buffer during the time course. Analysis of images was performed on a Power Macintosh 7200 computer (Cupertino, CA) using the public domain National Institute of Health image program (National Technical Information Service, Springfield, VA).
For fluorometry of whole tissue, a single peel loaded as described above was placed flat onto a polyacrylate plastic holder and affixed at both ends with silicon grease. The holder was inserted into a 3-mL polyacrylate fluorometer cuvette containing 2 mL of aerated loading buffer. The fluorometer (model LS-5B, Perkin-Elmer, Buckinghamshire, UK) was set to an excitation of 488 nm and an emission of 525 nm, with slit widths at 5 nm. The cuvette was then placed into the fluorometer and, after establishing a stable baseline, elicitors, enzymes, or pharmacological agents were added. For longer time courses (24–48 h, e.g. Fig. 7, B and C), three replicate peels were taken for each time point, loaded with DCF-DA (as above), and a reading of fluorescence was taken immediately. Experiments involving changes in temperature were achieved with a water-jacketed cuvette. For statistical purposes, fluorometry experiments were performed in triplicate, and fluorescence increases (over 10 min) are expressed as a percentage of the maximal increase possible from the tissue (determined by exposing the tissue to 5 mm H2O2 for 30 min at the end of each experiment).
Cell Viability Assays
Peels were floated on 10 mL of loading buffer supplemented with 100 mm Suc in covered Petri dishes as described above for infectivity assays. Accidental infection of cells and media over long time courses was kept to a minimum by prewashing leaves, before peeling, with 0.5% (w/v) NaOCl. Peels were exposed to a number of treatments including TMV (750 ng mL−1), and cryptogein (100 ng mL−1). For some experiments (“acid isolation”) peels were pre-exposed to pH 3.5 buffer (citrate/phosphate buffer at 30 mOsM) for 1 h to kill epidermal cells and leave guard cells viable (Squire and Mansfield, 1972). At set time points, of up to 48 h, peels were removed and loaded with the viability stain fluorescein diacetate (5 μL of a 100 mm stock in acetone added to 10 mL of loading buffer) for 10 min, washed, and examined using an epifluorescence microscope (450- to 490-nm band-pass excitation filter, 515-nm long-pass emission filter). Viability was scored for both guard cells and epidermal cells. At least 100 cells were counted in each of three replicate peels.
ACKNOWLEDGMENTS
We thank Drs. Pierre Ricci and Harald Keller for supplying us with cryptogein. We also thank Shlomit Bleichman of blessed memory for her excellent technical assistance and Dr. Ian Ferguson for assistance with the manuscript.
Footnotes
This work was supported by the Israel Ministry of Culture, Science, and Sport within a cooperative program with the Ministry of Science and Technology of South Korea, by the German Minerva Foundation, by the European Commission Project (grant no. BIO4–96–0101), and by a long-term European Molecular Biology Organization postdoctoral fellowship (to A.C.A.).
LITERATURE CITED
- Allan AC, Fluhr R. Two distinct sources of elicited reactive oxygen species in tobacco epidermal cells. Plant Cell. 1997;9:1559–1572. doi: 10.1105/tpc.9.9.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B, Zambryski P, Staskawicz B, Dinesh-Kumar SP. Signaling in plant-microbe interactions. Science. 1997;276:726–733. doi: 10.1126/science.276.5313.726. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Orlandi EW. Active oxygen in plant pathogenesis. Annu Rev Phytopathol. 1995;33:299–321. doi: 10.1146/annurev.py.33.090195.001503. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Buti VS, Davies DR, Zimmerlin A. The origin of the oxidative burst in plants. Free Radic Res. 1995;23:517–532. doi: 10.3109/10715769509065273. [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh CK, Murphy TM. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms. Plant Physiol. 1998;116:1379–1385. doi: 10.1104/pp.116.4.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening G, Beachy RN, Scalla R, Zaitlin M. In vitro and in vivo translation of the ribonucleic acids of a cowpea strain of tobacco mosaic virus. Virology. 1976;71:498–517. doi: 10.1016/0042-6822(76)90377-9. [DOI] [PubMed] [Google Scholar]
- Carrington JC, Kasschau KD, Mahajan SK, Schaad MC. Cell-to-cell and long-distance transport of viruses in plants. Plant Cell. 1996;8:1669–1681. doi: 10.1105/tpc.8.10.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal Biochem. 1983;134:111–116. doi: 10.1016/0003-2697(83)90270-1. [DOI] [PubMed] [Google Scholar]
- Citovsky V. Tobacco mosaic virus: a pioneer of cell-to-cell movement. Philos Trans R Soc Lond Ser B Biol Sci. 1999;354:637–643. doi: 10.1098/rstb.1999.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver JN, Dawson WO, Plonk K, Stubbs G. Site-directed mutagenesis confirms the involvement of carboxylate groups in the disassembly of tobacco mosaic virus. Virology. 1995;206:724–730. doi: 10.1016/s0042-6822(95)80096-4. [DOI] [PubMed] [Google Scholar]
- Culver JN, Stubbs G, Dawson WO. Structure-function relationship between tobacco mosaic-virus coat protein and hypersensitivity in Nicotiana sylvestris. J Mol Biol. 1994;242:130–138. doi: 10.1006/jmbi.1994.1564. [DOI] [PubMed] [Google Scholar]
- Deom CM, Lapidot M, Beachy RN. Plant-virus movement proteins. Cell. 1992;69:221–224. doi: 10.1016/0092-8674(92)90403-y. [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Coffey MJ, Neill SJ. Generation of active oxygen in elicited cells of Arabidopsis thaliana is mediated by a NADPH oxidase-like enzyme. FEBS Lett. 1996;382:213–217. doi: 10.1016/0014-5793(96)00177-9. [DOI] [PubMed] [Google Scholar]
- Dinesh-Kumar SP, Baker BJ. Alternatively spliced N resistance gene transcripts: their possible role in tobacco mosaic virus resistance. Proc Natl Acad Sci USA. 2000;97:1908–1913. doi: 10.1073/pnas.020367497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doke N, Ohashi Y. Involvement of an O2− generating system in the induction of necrotic lesions on tobacco-leaves infected with tobacco mosaic virus. Physiol Mol Plant Pathol. 1988;32:163–175. [Google Scholar]
- Dunigan DD, Madlener JC. Serine threonine protein phosphatase is required for tobacco mosaic virus-mediated programmed cell-death. Virology. 1995;207:460–466. doi: 10.1006/viro.1995.1105. [DOI] [PubMed] [Google Scholar]
- Durham ACH, Finch JT, Klug A. States of aggregation of tobacco mosaic virus protein. Nature. 1971;229:37–42. doi: 10.1038/newbio229037a0. [DOI] [PubMed] [Google Scholar]
- Durham ACH, Klug A. Polymerization of tobacco mosaic virus protein and its control. Nature. 1971;229:42–46. doi: 10.1038/newbio229042a0. [DOI] [PubMed] [Google Scholar]
- Erickson FL, Holzberg S, Calderon-Urrea A, Handley V, Axtell M, Corr C, Baker B. The helicase domain of the TMV replicase proteins induces the N-mediated defense response in tobacco. Plant J. 1999;18:67–75. doi: 10.1046/j.1365-313x.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999;18:265–276. doi: 10.1046/j.1365-313x.1999.00265.x. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H. Degradation of tobacco mosaic virus with acetic acid. Virology. 1957;4:1–4. doi: 10.1016/0042-6822(57)90038-7. [DOI] [PubMed] [Google Scholar]
- Gaard G, De Zoeten GA. Plant virus uncoating as a result of virus-cell wall interactions. Virology. 1979;96:21–31. doi: 10.1016/0042-6822(79)90168-5. [DOI] [PubMed] [Google Scholar]
- Graham TL, Graham MY. Signaling in soybean phenylpropanoid responses: dissection of primary, secondary, and conditioning effects of light, wounding, and elicitor treatments. Plant Physiol. 1996;110:1123–1133. doi: 10.1104/pp.110.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom QJ, Torres MA, Fordham-Skelton AP, Hammond-Kosack KE, Robinson NJ, Jones JDG. rbohA a rice homologue of the mammalian gp91phox respiratory burst oxidase gene. Plant J. 1996;10:515–522. doi: 10.1046/j.1365-313x.1996.10030515.x. [DOI] [PubMed] [Google Scholar]
- Hilf ME, Dawson WO. The tobamovirus capsid protein functions as a host-specific determinant of long-distance movement. Virology. 1993;193:106–114. doi: 10.1006/viro.1993.1107. [DOI] [PubMed] [Google Scholar]
- Hills GJ, Plaskitt KA, Wilson TMA, Zaitlin M. Immunogold localization of the site of replication of tobacco mosaic virus RNA. Micron Microsc Acta. 1987;18:336–336. [Google Scholar]
- Hooley R, McCarthy D. Extracts from virus infected hypersensitive tobacco leaves are detrimental to protoplast survival. Physiol Plant Pathol. 1980;16:25–38. [Google Scholar]
- Jabs T, Dietrich RA, Dangl JL. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science. 1996;273:1853–1856. doi: 10.1126/science.273.5283.1853. [DOI] [PubMed] [Google Scholar]
- Kauss H, Jeblick W. Pretreatment of parsley suspension-cultures with salicylic-acid enhances spontaneous and elicited production of H2O2. Plant Physiol. 1995;108:1171–1178. doi: 10.1104/pp.108.3.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot M, Paran I, BenJoseph R, BenHarush S, Pilowsky M, Cohen S, Shifriss C. Tolerance to cucumber mosaic virus in pepper: development of advanced breeding lines and evaluation of virus level. Plant Dis. 1997;81:185–188. doi: 10.1094/PDIS.1997.81.2.185. [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Lu B, Taraporewala ZF, Stubbs G, Culver JN. Intersubunit interactions allowing a carboxylate mutant coat protein to inhibit tobamovirus disassembly. Virology. 1998;244:13–19. doi: 10.1006/viro.1998.9099. [DOI] [PubMed] [Google Scholar]
- Mehdy MC. Active oxygen species in plant defense against pathogens. Plant Physiol. 1994;105:467–472. doi: 10.1104/pp.105.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T, Kiyama R, Ohno T, Okada Y. Nucleotide sequence of the coat protein cistron and the 3′-noncoding region of cucumber green mottle mosaic virus (watermelon strain) RNA. Virology. 1983;127:54–64. doi: 10.1016/0042-6822(83)90370-7. [DOI] [PubMed] [Google Scholar]
- Milat ML, Ricci P, Bonnet P, Blein JP. Capsidiol and ethylene production by tobacco cells in response to cryptogein, an elicitor from Phytophthora cryptogea. Phytochemistry. 1991;30:2171–2173. [Google Scholar]
- Murphy TM, Auh CK. The superoxide synthases of plasma membrane preparations from cultured rose cells. Plant Physiol. 1996;110:621–629. doi: 10.1104/pp.110.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba K, Pattanayek R, Stubbs G. Visualization of protein-nucleic acid interactions in a virus—refined structure of intact tobacco mosaic virus at 2.9—a resolution by x-ray fiber diffraction. J Mol Biol. 1989;208:307–325. doi: 10.1016/0022-2836(89)90391-4. [DOI] [PubMed] [Google Scholar]
- Ori N, Eshed Y, Pinto P, Paran I, Zamir D, Fluhr R. TAO1, a representative of the molybdenum cofactor containing hydroxylases from tomato. J Biol Chem. 1997;272:1019–1025. doi: 10.1074/jbc.272.2.1019. [DOI] [PubMed] [Google Scholar]
- Padgett HS, Beachy RN. Analysis of a tobacco mosaic-virus strain capable of overcoming N gene-mediated resistance. Plant Cell. 1993;5:577–586. doi: 10.1105/tpc.5.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett HS, Watanabe Y, Beachy RN. Identification of the TMV replicase sequence that activates the N gene-mediated hypersensitive response. Mol Plant-Microbe Interact. 1997;10:709–715. [Google Scholar]
- Palukaitis P, Roossinck MJ, Dietzgen RG, Francki RIB. Cucumber mosaic virus. Adv Virus Res. 1992;41:281–348. doi: 10.1016/s0065-3527(08)60039-1. [DOI] [PubMed] [Google Scholar]
- Pfitzner UM, Pfitzner AJP. Expression of a viral avirulence gene in transgenic plants is sufficient to induce the hypersensitive defense reaction. Mol Plant-Microbe Interact. 1992;5:318–321. doi: 10.1094/mpmi-5-318. [DOI] [PubMed] [Google Scholar]
- Piccirillo P, Porrone F. The presence of the N′ gene in Nicotiana tabacum L. cv Samsun NN. J Phytopathol. 1995;143:381–383. [Google Scholar]
- Plaskitt KA, Watkins PAC, Sleat DE, Gallie DR, Shaw JG, Wilson TMA. Immunogold labeling locates the site of disassembly and transient gene expression of tobacco mosaic virus-like pseudovirus particles in vivo. Mol Plant-Microbe Interact. 1987;1:10–16. [Google Scholar]
- Ricci P, Bonnet P, Huet JC, Sallantin M, Beauvaiscante F, Bruneteau M, Billard V, Michel G, Pernollet JC. Structure and activity of proteins from the pathogenic fungi Phytophthora eliciting necrosis and acquired-resistance in tobacco. Eur J Biochem. 1989;183:555–563. doi: 10.1111/j.1432-1033.1989.tb21084.x. [DOI] [PubMed] [Google Scholar]
- Shaw JG. Tobacco mosaic virus and the study of early events in virus infections. Philos Trans R Soc Lond Ser B Biol Sci. 1999;354:603–611. doi: 10.1098/rstb.1999.0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire GR, Mansfield TA. A simple method of isolating stomata on detached epidermis by low pH treatment: observations on the importance of the subsidiary cells. New Phytol. 1972;71:1033–1043. [Google Scholar]
- Stennis MJ, Chandra S, Ryan CA, Low PS. Systemin potentiates the oxidative burst in cultured tomato cells. Plant Physiol. 1998;117:1031–1036. doi: 10.1104/pp.117.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzinski MA, Zaitlin M. Tobacco mosaic virus replication in resistant and susceptible plants: in some resistant species virus is confined to a small number of initially infected cells. Virology. 1982;121:12–19. doi: 10.1016/0042-6822(82)90114-3. [DOI] [PubMed] [Google Scholar]
- Taraporewala ZF, Culver JN. Identification of an elicitor active site within the three-dimensional structure of the tobacco mosaic tobamovirus coat protein. Plant Cell. 1996;8:169–178. doi: 10.1105/tpc.8.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard MP, Martin F, Pugin A, Ricci P, Blein JP. Protein-phosphorylation is induced in tobacco cells by the elicitor cryptogein. Plant Physiol. 1994;104:1245–1249. doi: 10.1104/pp.104.4.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitham S, Dineshkumar SP, Choi D, Hehl R, Corr C, Baker B. The product of the tobacco mosaic virus resistance gene N similarity to Toll and the interleukin-1 receptor. Cell. 1994;78:1101–1115. doi: 10.1016/0092-8674(94)90283-6. [DOI] [PubMed] [Google Scholar]
- Wilson TMA. Cotranslational disassembly increases the efficiency of expression of TMV RNA in wheat germ cell-free extracts. Virology. 1984;138:353–356. doi: 10.1016/0042-6822(84)90360-x. [DOI] [PubMed] [Google Scholar]
- Wu XJ, Shaw JG. Evidence that a viral replicase protein is involved in the disassembly of tobacco mosaic virus particles in vivo. Virology. 1997;239:426–434. doi: 10.1006/viro.1997.8870. [DOI] [PubMed] [Google Scholar]