Graphical Abstract
Graphical Abstract.
Atrial fibrosis is considered to be a key driver to the initiation and perpetuation of atrial fibrillation (AF). Histological studies evaluating human atrial fibrosis are largely restricted to tissue samples that can be collected during cardiac surgery such as atrial appendage tissue.1,2 Consequently, left atrial (LA) appendage (LAA) tissue is often used as a surrogate marker for the entire LA to assess processes of structural remodelling related to AF. Animal studies suggest that LAA tissue may act as a decent representation of the LA as the cellular composition of murine LAA and LA-body tissue was found to be rather similar.3 To the best of our knowledge however, it remains unclear whether the fibrosis content assessed in human appendage samples accurately represents the fibrosis content of the entire LA.
In recent years, late gadolinium enhancement (LGE) cardiac magnetic resonance (CMR) imaging has matured as a clinically applicable, non-invasive tool to identify both degree and localization of atrial hyper-enhancement, assumed to reflect atrial fibrosis.4 Therefore, this study evaluates the relation between LAA fibrosis and LA-body fibrosis content using LGE-CMR.
This study was approved by the local institutional ethics committee (VU University Medical Center, Amsterdam, The Netherlands). Data from 47 AF patients scheduled for a first pulmonary vein isolation (PVI) procedure were retrospectively analysed. Prior to PVI, all patients underwent a CMR scan including an electrocardiogram-gated free-breathing 3D contrast-enhanced MR angiogram (CE-MRA) and high resolution 3D LGE acquisition. A detailed scan protocol and description of LA-LGE quantification using CEMRG software (King’s College, London, UK) has been described previously.4 In short, the LA wall and LAA were semi-automatically segmented on the 3D CE-MRA, which provides a high contrast for identifying the LA and LAA endocardium. Subsequently, the 3D CE-MRA was co-registered with the 3D LGE images. From this co-registered dataset, a 3D reconstruction was generated (Figure 1 panels A and B). The LAA was separated from the LA-body at the point of deflection from the LA wall. This enabled the separate calculation of the fibrosis extent (in %) and volume (in mL) for both the LA-body and LAA. The LA-body fibrosis extent was compared to LAA fibrosis extent using a paired samples t-test and Pearson correlation. The reproducibility of quantifying LAA fibrosis was evaluated by assessing inter- and intra-observer variability in 10 randomly selected patients. Images were analysed by two experienced readers (L.H. and S.M.). The agreement between LAA fibrosis measurements was determined using intra-class correlation coefficients (ICCs). The ICCs for absolute agreement of single measurements were estimated using a two-way random effect model.
Figure 1.
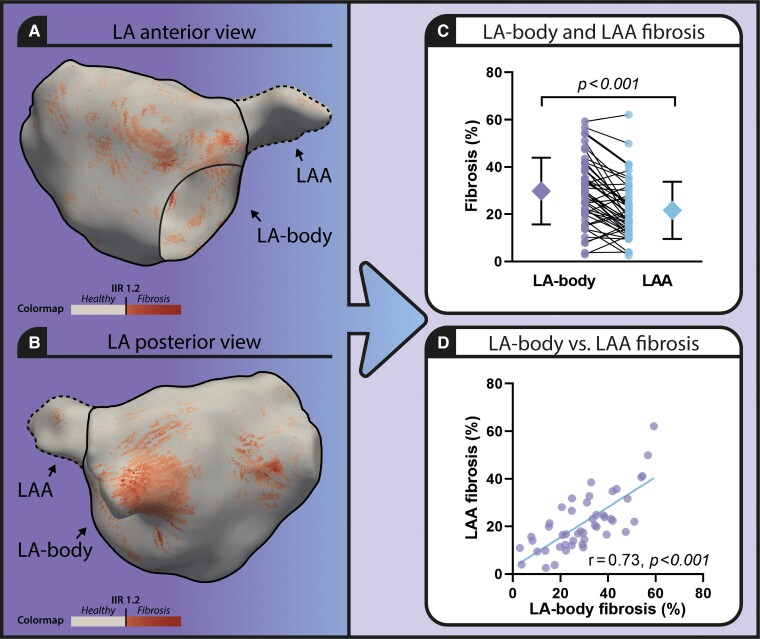
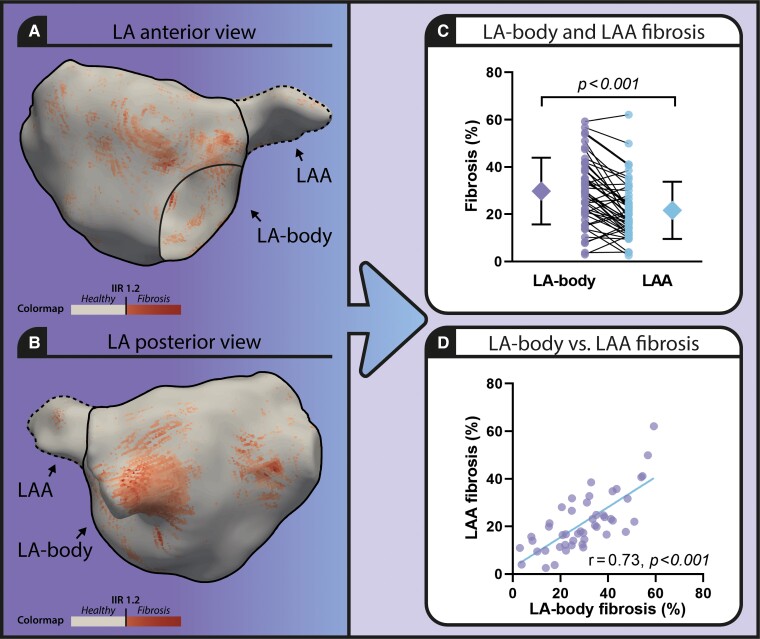
Comparison of left atrial body and left atrial appendage fibrosis extent in atrial fibrillation patients using 3D late gadolinium enhanced cardiac magnetic resonance imaging. Left panel: 3D model of the atrial fibrillation (LA) including the LAA (LA appendage, dotted line) visualizing healthy atrial tissue (beige) and fibrosis (red) (A. anterior view, B. posterior view). (C) Graph comparing fibrosis extent in LA-body and LAA. (D) Scatterplot presenting LA-body and LAA fibrosis extent.
Baseline characteristics are described in our previously published paper.4 Mean LA-body fibrosis extent was significantly different from LAA fibrosis extent (29.80 ± 14.15% vs. 21.62 ± 12.08%, P < 0.001) (Figure 1 panel C). The correlation between LA-body fibrosis extent and LAA fibrosis extent was high (r = 0.74, P < 0.001) (Figure 1 panel D).
Volume of the LA-body segmentation was 107.21 ± 28.96 mL and volume of the LAA segmentation was 6.89 ± 4.34 mL. For both the LA-body volume and LAA volume, no relation with the extent of fibrosis was found (r = −0.02, P = 0.90 and r = −0.26, P = 0.08, respectively).
The ICC for inter-reader variability of LAA fibrosis measurements was 0.92 [95% confidence interval (CI) 0.74–0.98] and the ICC for intra-reader variability of LAA fibrosis measurements was 0.97 (95% CI 0.89–0.99), which was similar to the reproducibility of LA fibrosis quantification.4
The strong correlation between LA-body and LAA fibrosis as assessed by 3D LGE-CMR supports that in histological studies, LAA fibrosis could be used as a clinical useful surrogate to investigate LA tissue characteristics in AF patients. However, histological analysis of the LAA may still misrepresent AF disease state, as the amount of LAA fibrosis was found to be on average one-third lower than the amount of LA-body fibrosis.
Moreover, the regional distribution of LA fibrosis may fluctuate substantially across the LA and might be related not only to a varying atrial wall thickness but also to associated differences in wall stress.5 In addition, a varying composition and dispersion of fibrotic tissue, contributing to the underlying condition for AF, may not be accurately represented by a LAA sample.
Limitations to this work include the challenging segmentation of the thin LA wall and capricious LAA anatomy. Therefore, we performed the segmentation of the atrial wall on the 3D CE-MRA, which provides higher contrast for identifying the atrial endocardium than the 3D LGE. Also, LA-LGE may not always reflect definite atrial fibrosis, but may also represent areas of increased extracellular space caused by inflammation and/or focal oedema. Therefore, a comparison between LA and LAA characteristics using invasive endocardial voltage mapping as a reference standard can provide complemental insights to our findings.6 Furthermore, by augmenting the assessment of LAA fibrotic tissue with a combination of 3D LGE-CMR and endocardial voltage mapping, along with histologic examination, a more comprehensive understanding of the relationship between atrial LGE and low voltage areas can be obtained.7 Additionally, the impact of LAA fibrosis on clinical outcome, in particular its potential role as a thromboembolic risk factor, has to be studied in future research.
To conclude, CMR offers unique features allowing virtual histology to study both LA and LAA structure as a non-invasive alternative to animal studies. However, more research combining non-invasive CMR-based tissue characterization, invasive electro-anatomic mapping techniques, and atrial appendage histology is required to determine whether the LAA is a decent surrogate for the assessment of LA structural remodelling and electro-pathology in patients suffering from AF.
Contributor Information
Luuk H G A Hopman, Department of Cardiology, Amsterdam UMC, De Boelelaan 1118, Amsterdam 1081 HZ, The Netherlands.
Irene M Frenaij, Department of Cardiology, Amsterdam UMC, De Boelelaan 1118, Amsterdam 1081 HZ, The Netherlands.
José A Solís-Lemus, Division of Imaging Sciences and Biomedical Engineering, King’s College London, London, UK.
Sulayman el Mathari, Department of Cardiothoracic Surgery, Amsterdam UMC, Amsterdam, The Netherlands.
Steven A Niederer, Division of Imaging Sciences and Biomedical Engineering, King’s College London, London, UK.
Cornelis P Allaart, Department of Cardiology, Amsterdam UMC, De Boelelaan 1118, Amsterdam 1081 HZ, The Netherlands.
Marco J W Götte, Department of Cardiology, Amsterdam UMC, De Boelelaan 1118, Amsterdam 1081 HZ, The Netherlands.
Funding
None.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Miyauchi S, Nishi H, Ouhara K, Tokuyama T, Okubo Y, Okamura Set al. Relationship between periodontitis and atrial fibrosis in atrial fibrillation: histological evaluation of left atrial appendages. JACC Clin Electrophysiol 2023;9:43–53. [DOI] [PubMed] [Google Scholar]
- 2. Ramos KS, Pool L, van Schie MS, Wijdeveld LFJM, van der Does WFB, Baks Let al. Degree of fibrosis in human atrial tissue is not the hallmark driving AF. Cells 2022;11:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Colazzo F, Castiglioni L, Sironi L, Fontana L, Nobili E, Franzosi Met al. Murine left atrium and left atrial appendage structure and function: echocardiographic and morphologic evaluation. PLoS One 2015;10:e0125541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hopman LHGA, Bhagirath P, Mulder MJ, Eggink IN, van Rossum AC, Allaart CPet al. Quantification of left atrial fibrosis by 3D late gadolinium-enhanced cardiac magnetic resonance imaging in patients with atrial fibrillation: impact of different analysis methods. Eur Heart J Cardiovasc Imaging 2021;23:1182–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benito EM, Cabanelas N, Nuñez-Garcia M, Alarcón F, Figueras IVRM, Soto-Iglesias Det al. Preferential regional distribution of atrial fibrosis in posterior wall around left inferior pulmonary vein as identified by late gadolinium enhancement cardiac magnetic resonance in patients with atrial fibrillation. Europace 2018;20:1959–65. [DOI] [PubMed] [Google Scholar]
- 6. Caixal G, Alarcón F, Althoff TF, Nuñez-Garcia M, Benito EM, Borràs Ret al. Accuracy of left atrial fibrosis detection with cardiac magnetic resonance: correlation of late gadolinium enhancement with endocardial voltage and conduction velocity. Europace 2021;23:380–8. [DOI] [PubMed] [Google Scholar]
- 7. Chen J, Arentz T, Cochet H, Muller-Edenborn B, Kim S, Moreno-Weidmann Zet al. Extent and spatial distribution of left atrial arrhythmogenic sites, late gadolinium enhancement at magnetic resonance imaging, and low-voltage areas in patients with persistent atrial fibrillation: comparison of imaging vs. electrical parameters of fibrosis and arrhythmogenesis. Europace 2019;21:1484–93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.