Abstract
Aims
Atrial fibrillation (AF) is a risk factor for brain infarction, which can lead to epilepsy. We aimed to investigate whether treatment of AF with direct oral anticoagulants (DOACs) affects the risk of epilepsy in comparison to treatment with the vitamin K antagonist phenprocoumon (PPC).
Methods and results
We performed an active comparator, nested case-control study based on the German Pharmacoepidemiological Research Database that includes claims data from statutory health insurance providers of about 25 million persons since 2004. In 2011–17, 227 707 AF patients initiated treatment with a DOAC or PPC, of which 1828 cases developed epilepsy on current treatment with an oral anticoagulant. They were matched to 19 084 controls without epilepsy. Patients with DOAC treatment for AF had an overall higher risk of epilepsy with an odds ratio of 1.39, 95% CI (1.24; 1.55) compared to current PPC treatment. Cases had higher baseline CHA2DS2-VASc scores and more frequently a history of stroke than controls. After excluding patients with ischaemic stroke prior to the diagnosis of epilepsy, the risk of epilepsy was still higher on DOACs than on PPC. In contrast, within a cohort of patients with venous thromboembolism, the risk of epilepsy on treatment with DOACs was less elevated [adjusted odds ratio 1.15, 95% CI (0.98; 1.34)].
Conclusion
In patients with AF initiating oral anticoagulation, treatment with a DOAC was associated with an increased risk of epilepsy compared to the vitamin K antagonist PPC. Covert brain infarction may explain the observed elevated risk of epilepsy.
Keywords: Atrial fibrillation, Anticoagulation, Direct oral anticoagulants, Vitamin K antagonist, Silent stroke, Epilepsy
Graphical Abstract
Graphical Abstract.
Created by BioRender.com.
What’s new?
In patients with atrial fibrillation (AF), treatment with direct oral anticoagulants (DOACs) was associated with a higher risk of epilepsy and seizures than a vitamin K antagonist.
Epilepsy and seizures predominantly occurred in patients with AF who were at high thromboembolic risk but had no clinically apparent stroke.
One potential explanation would be that in patients with AF, a vitamin K antagonist protects better against covert brain infarction than DOACs.
Introduction
Atrial fibrillation (AF) is associated with significant morbidity, mortality and increased healthcare costs. The main complication of AF is stroke. As oral anticoagulants markedly reduce the risk of stroke, they are a central pillar of the guideline-based therapy for AF. Routine application of this therapeutic strategy has become easier with direct oral anticoagulants (DOACs), the use of which is steadily increasing while vitamin K antagonists (VKA) tend to become less important. Clinical trials and post-marketing surveillance programmes have indicated that, overall, DOACs have a comparable efficacy to VKAs in preventing thromboembolic events but cause less intracranial haemorrhages.1,2
A caveat, however, relates to the fact that studies on oral anticoagulation in AF have focused on acute ischaemic stroke or haemorrhage as outcome parameter but have neglected covert brain infarction, also known as ‘silent’ stroke. With covert brain infarction, imaging studies show lesions typical for ischaemic stroke but patients do not have a clinical stroke history.3 A meta-analysis reported covert brain infarction as detected by magnetic resonance imaging in 40% of AF patients.4 These numbers are worrisome as covert brain infarction is associated with dementia and epileptic seizures.5,6 Brain infarction is known to be the most frequent cause of acquired epilepsy in elderlies,7 with 8% of stroke patients developing epilepsy over 5 years after the ischaemic insult.8 Thus, covert brain infarction may be one potential explanation why AF is associated with a higher epilepsy rate.9,10 However, until now, it is still unclear whether anticoagulant drugs would reduce covert brain infarction or its clinical manifestations.11,12
In a previous pharmacovigilance study, we applied methods for signal detection in claims data to explore potential side effects of DOAC) treatment.13 The aim of the current study was to further examine a signal suggesting an association between DOAC dispensations and international classification of diseases (ICD) codes of epilepsy or seizures using a study design that specifically addresses the research question and minimizes potential sources of bias (e.g. temporal relationship between covariates and exposure, confounding). Following patients with a defined indication for anticoagulation from the time point of treatment initiation, we applied an active comparator design to assess the risk of epilepsy from DOACs vs. VKA. In addition, we aimed to explore potential underlying mechanisms of the observed association between DOACs and epilepsy.
Methods
Data source
This study was performed on data from the German Pharmacoepidemiological Research Database (GePaRD). The GePaRD is based on claims data from four statutory health insurance providers in Germany and currently includes more than 25 million subjects who have been insured with one of the participating providers since 2004 or later. In addition to demographic data, GePaRD contains information on all reimbursable drug dispensations and all reimbursable outpatient (i.e. from general practitioners and specialists) and inpatient services and diagnoses. Per data year, there is information on ∼20% of the general population covering all geographical regions of Germany. The GePaRD has been used for various drug utilization and safety studies, including studies on oral anticoagulants.14 All involved health insurance providers as well as the German Federal Office for Social Security and the Senator for Health, Women and Consumer Protection in Bremen as their responsible authorities approved the use of GePaRD data for this study according to current legal conditions. According to the Ethics Committee of the University of Bremen, studies based on GePaRD are exempt from institutional review board review.
Study design and cohort of patients with atrial fibrillation
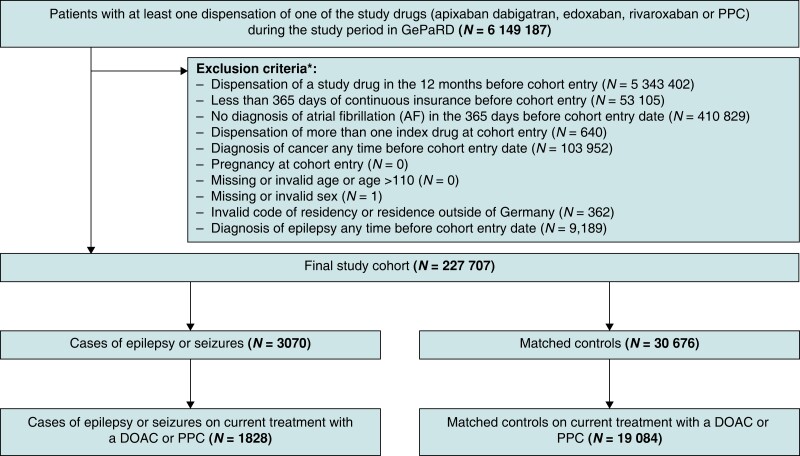
The study was designed as an active comparator case-control study nested in a cohort of patients with AF initiating DOAC or phenprocoumon (PPC) treatment after a period of 365 days with no use of the respective anticoagulant drug (‘new users’). The PPC is the VKA predominantly prescribed in Germany. The study period started on 1 January 2011 and ended on 31 December 2017. To be included into the study, individuals had to have (i) at least one dispensation of one of the index drugs (PPC, rivaroxaban, apixaban, dabigatran, and edoxaban) during the study period with no dispensation of any index drug in the 365 days before cohort entry, (ii) at least 365 days of continuous active insurance before the cohort entry, and (iii) at least one outpatient or hospital diagnosis of atrial fibrillation (ICD-10-GM I48, including sub-codes) in the 365 days before cohort entry. Patients with a diagnosis of epilepsy/seizures or cancer at any time before cohort entry and women who were pregnant at cohort entry were excluded (Figure 1).
Figure 1.
Flowchart of the study cohort according to the defined inclusion and exclusion criteria. The diagnosis of epilepsy at any time before cohort entry (exclusion criteria) was based on the International Classification of Diseases, 10th revision, German modification (ICD-10-GM) codes listed in Supplementary material online, Table S7.
Incident cases of epilepsy or seizures were identified by searching for the first ICD-10-GM codes G40 (epilepsy, including sub-codes) or R56.8 (other and unspecified convulsions) as either outpatient diagnosis (classified as certain) or main hospital diagnosis. We defined the index date as the date of diagnosis (outpatient diagnoses) or the admission date (hospital diagnoses).
Up to 10 controls were matched to each case with epilepsy/seizures by sex, age at index date (±1 year), and statutory health insurance provider, using risk set sampling (with replacement) with time in cohort as the time axis. Eligible patients hospitalized for any reason at the index date of the case were excluded from the set of potential controls. Cases were eligible to be selected as a control before their index day.15 We categorized patients as current users if the supply of the respective drug overlapped the index date. The supply of index drugs was estimated by dividing the dispensed amount of active ingredient by the defined daily dose of the respective index drug.
Based on their potential to confound the association between oral anticoagulation and epilepsy or seizures, we selected numerous variables, including components of the CHA2DS2-VASc risk score for stroke, the HAS-BLED score for bleeding, additional risk factors of stroke and epilepsy, and data on other chronic diseases. Confounder variables were obtained from in- and outpatient diagnoses and procedures. See Supplementary material online, Table S1 for a detailed list of all potential confounders.
Exploratory analyses—ischaemic stroke and intracranial haemorrhage
To characterize the cases of epilepsy/seizures on anticoagulant therapy and to explore mechanisms that may explain the association of DOAC treatment and epilepsy/seizures, we examined whether the diagnosis of epilepsy/seizures was preceded by a diagnosis of ischaemic stroke or intracranial bleeding (non-traumatic and traumatic). We therefore described the proportion of patients with at least one diagnosis code of ischaemic stroke or intracranial bleeding after cohort entry and before the diagnosis of epilepsy/seizures. Similarly, we described the proportion of patients with such diagnoses after cohort entry and before the matched index date among controls. Patients with a history of (covert) stroke are at increased risk of recurrent cerebrovascular events. In order to evaluate the occurrence of cerebrovascular events with high sensitivity even in patients with no reported stroke diagnosis prior to the diagnosis of epilepsy/seizures and considering seizures as potential first symptoms of stroke, we also report the proportions of patients with a subsequent diagnosis of ischaemic stroke or intracranial bleeding within 365 days after the first diagnosis of epilepsy/seizures and the matched index date among cases and controls, respectively.
As patients with AF are at increased risk of stroke and stroke is an important cause of epilepsy, we also repeated our main analysis excluding patients with a history of ischaemic stroke or intracranial bleeding (non-traumatic and traumatic) from the study cohort.
Exploratory analysis in a cohort of patients with venous thromboembolism
To evaluate whether a potential association depends on the treatment indication, we repeated the study in patients who started anticoagulation for the treatment of venous thromboembolism (VTE) between 1 January 2011 and 31 December 2018. Since a lower prevalence was anticipated for VTE than for atrial fibrillation and 2018 data had become available by the time this exploratory analysis was conducted, the study period was extended. To be included in the VTE cohort, patients had to fulfil the inclusion criteria (i) and (ii) defined for the atrial fibrillation cohort, must not have a diagnosis of atrial fibrillation any time prior to cohort entry, and had (iiia) at least one hospital diagnosis of deep vein thrombosis or pulmonary embolism [ICD-10-GM I26.x, I80.1, I80.2x, I80.3, I80.81, I80.88, I80.9, I81, I82.x (excluding I82.1)] in the 90 days before the cohort entry date or (iiib) at least one outpatient diagnosis of deep vein thrombosis in the quarter before or the quarter of the cohort entry date with a dispensation of an oral anticoagulant in the same quarter as the diagnosis. Patients with a history of cancer prior to cohort entry were not excluded from this cohort. In line with the main analysis, patients with a diagnosis of epilepsy/seizures at any time before cohort entry and women who were pregnant at cohort entry were excluded.
Statistical analysis
Conditional logistic regression was used to estimate matched crude and confounder-adjusted odds ratios (ORs and aORs), with 95% confidence intervals (95% CIs), comparing current users of any DOAC and current users of PPC. Patients were censored for the occurrence of death, which corresponds to the estimation of the so-called ‘direct effect’, i.e. the effect of DOAC vs. PPC treatment on epilepsy/seizures under the elimination of the competing event death. In the model specification, we included all potential confounders to reduce the likelihood of residual confounding (see Supplementary material online, Table S1). All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA) and R 4.0.1.
Results
Study population
Among 20 656 171 individuals represented in GePaRD during the study period, we identified a study cohort of 227 707 patients with a diagnosis of AF who between 2011 and 2017 initiated anticoagulant treatment with a DOAC (n = 133 881) or with the VKA PPC (n = 93 826) (Figure 1). Rivaroxaban (n = 69 276) was the most commonly used DOAC at treatment initiation, followed by apixaban (n = 40 133), dabigatran (n = 17 258), and edoxaban (n = 7214). Patients initiating treatment with a DOAC were slightly younger, had overall less comorbidities, and took fewer co-medications than patients initiating PPC treatment. Moreover, new DOAC users had lower CHA2DS2-VASc scores than new PPC users. Table 1 summarizes clinical and demographic characteristics of the study cohort.
Table 1.
Baseline characteristics of the study cohort (patients with atrial fibrillation who started oral anticoagulation with a DOAC or PPC)
| Characteristic | PPC, N = 93 826 |
DOAC, N = 133 881 |
Apixaban, N = 40 133 |
Dabigatran, N = 17 258 |
Edoxaban, N = 7214 |
Rivaroxaban, N = 69 276 |
|---|---|---|---|---|---|---|
| Male | 48 394 (51.6%) | 70 929 (53.0%) | 20 707 (51.6%) | 9291 (53.8%) | 4154 (57.6%) | 36 777 (53.1%) |
| Age | 75 (69, 81) | 74 (65, 80) | 75 (66, 81) | 73 (65, 79) | 74 (65, 79) | 73 (65, 79) |
| CHA2DS2VASca,b | 5.00 (4.00, 6.00) | 4.00 (3.00, 6.00) | 5.00 (3.00, 6.00) | 4.00 (3.00, 6.00) | 4.00 (3.00, 5.00) | 4.00 (3.00, 6.00) |
| CHA2DS2VAScb | ||||||
| 0 | 637 (0.7%) | 3066 (2.3%) | 792 (2.0%) | 351 (2.0%) | 163 (2.3%) | 1760 (2.5%) |
| 1 | 2689 (2.9%) | 8526 (6.4%) | 2239 (5.6%) | 1048 (6.1%) | 498 (6.9%) | 4741 (6.8%) |
| 2 | 6707 (7.1%) | 14 611 (10.9%) | 3950 (9.8%) | 1850 (10.7%) | 907 (12.6%) | 7904 (11.4%) |
| 3 | 12 442 (13.3%) | 20 462 (15.3%) | 5595 (13.9%) | 2567 (14.9%) | 1244 (17.2%) | 11 056 (16.0%) |
| 4 | 17 544 (18.7%) | 24 617 (18.4%) | 6963 (17.3%) | 3243 (18.8%) | 1396 (19.4%) | 13 015 (18.8%) |
| 5 | 20 106 (21.4%) | 24 796 (18.5%) | 7629 (19.0%) | 3154 (18.3%) | 1346 (18.7%) | 12 667 (18.3%) |
| 6 | 17 106 (18.2%) | 19 580 (14.6%) | 6503 (16.2%) | 2523 (14.6%) | 949 (13.2%) | 9605 (13.9%) |
| 7 | 9844 (10.5%) | 10 772 (8.0%) | 3708 (9.2%) | 1496 (8.7%) | 457 (6.3%) | 5111 (7.4%) |
| 8 | 4821 (5.1%) | 5348 (4.0%) | 1954 (4.9%) | 772 (4.5%) | 184 (2.6%) | 2438 (3.5%) |
| 9 | 1930 (2.1%) | 2103 (1.6%) | 800 (2.0%) | 254 (1.5%) | 70 (1.0%) | 979 (1.4%) |
| HAS-BLEDa,c | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) |
| Intracerebral bleeding | 369 (0.4%) | 758 (0.6%) | 328 (0.8%) | 141 (0.8%) | 28 (0.4%) | 261 (0.4%) |
| Ischaemic stroke | 13 020 (13.9%) | 17 552 (13.1%) | 6330 (15.8%) | 3124 (18.1%) | 677 (9.4%) | 7421 (10.7%) |
| Transient ischaemic attack | 3867 (4.1%) | 5700 (4.3%) | 1884 (4.7%) | 1040 (6.0%) | 224 (3.1%) | 2552 (3.7%) |
| Deep vein thrombosis | 1190 (1.3%) | 1198 (0.9%) | 250 (0.6%) | 107 (0.6%) | 47 (0.7%) | 794 (1.1%) |
| Pulmonary embolism | 1741 (1.9%) | 1918 (1.4%) | 490 (1.2%) | 129 (0.7%) | 55 (0.8%) | 1244 (1.8%) |
| Coronary artery disease incl. myocardial infarction | 55 755 (59.4%) | 66 283 (49.5%) | 20 273 (50.5%) | 8793 (51.0%) | 3284 (45.5%) | 33 933 (49.0%) |
| Hypertension | 87 897 (93.7%) | 120 163 (89.8%) | 36 343 (90.6%) | 15 576 (90.3%) | 6436 (89.2%) | 61 808 (89.2%) |
| Heart failure | 50 073 (53.4%) | 56 840 (42.5%) | 18 166 (45.3%) | 7234 (41.9%) | 2715 (37.6%) | 28 725 (41.5%) |
| Peripheral arterial disease | 33 934 (36.2%) | 41 565 (31.0%) | 13 430 (33.5%) | 5127 (29.7%) | 2192 (30.4%) | 20 816 (30.0%) |
| Diabetes | 36 287 (38.7%) | 44 071 (32.9%) | 13 695 (34.1%) | 5633 (32.6%) | 2311 (32.0%) | 22 432 (32.4%) |
| COPD | 22 871 (24.4%) | 28 696 (21.4%) | 9019 (22.5%) | 3502 (20.3%) | 1486 (20.6%) | 14 689 (21.2%) |
| Liver disease | 27 995 (29.8%) | 38 037 (28.4%) | 11 749 (29.3%) | 4819 (27.9%) | 2124 (29.4%) | 19 345 (27.9%) |
| Renal disease | 29 299 (31.2%) | 30 556 (22.8%) | 10 619 (26.5%) | 3307 (19.2%) | 1555 (21.6%) | 15 075 (21.8%) |
| Chronic kidney disease (≥stadium 3) | 25 817 (27.5%) | 25 690 (19.2%) | 9076 (22.6%) | 2709 (15.7%) | 1270 (17.6%) | 12 635 (18.2%) |
| Alcohol-related disorders | 3264 (3.5%) | 4789 (3.6%) | 1466 (3.7%) | 575 (3.3%) | 268 (3.7%) | 2480 (3.6%) |
| Smoking | 5471 (5.8%) | 8366 (6.2%) | 2631 (6.6%) | 1039 (6.0%) | 440 (6.1%) | 4256 (6.1%) |
| Antiarrhythmic drugs | 17 096 (18.2%) | 21 263 (15.9%) | 5914 (14.7%) | 3167 (18.4%) | 927 (12.9%) | 11 255 (16.2%) |
| Antihypertensive drugs | 77 603 (82.7%) | 101 475 (75.8%) | 31 117 (77.5%) | 13 093 (75.9%) | 5427 (75.2%) | 51 838 (74.8%) |
| Antiplatelets | 34 205 (36.5%) | 43 621 (32.6%) | 13 825 (34.4%) | 5851 (33.9%) | 2125 (29.5%) | 21 820 (31.5%) |
| NSAID | 75 558 (80.5%) | 110 358 (82.4%) | 33 505 (83.5%) | 13 954 (80.9%) | 6101 (84.6%) | 56 798 (82.0%) |
| Proton pump inhibitor | 37 387 (39.8%) | 51 677 (38.6%) | 16 197 (40.4%) | 6555 (38.0%) | 2440 (33.8%) | 26 485 (38.2%) |
| Corticosteroids | 10 984 (11.7%) | 15 424 (11.5%) | 4697 (11.7%) | 1877 (10.9%) | 712 (9.9%) | 8138 (11.7%) |
Values are numbers (percentages) unless stated otherwise.
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug; PPC, Phenprocoumon.
Median (IQR).
CHA2DS2VASc score: congestive heart failure (1 Point); hypertension (1 Point); aged ≥75 years (2 Points); diabetes mellitus (1 Point); stroke/transient ischaemic attack (2 Points); vascular disease (1 Point), aged 65–74 years (1 Point), female sex (1 Point).
HAS-BLED score: hypertension (1 Point), abnormal renal or liver function (each 1 Point), previous stroke (1 Point), bleeding history or predisposition (anaemia) (1 Point), elderly (> 65 years) (1 Point), drugs (other antiplatelet agents or NSAIDs) or alcohol (each 1 Point), labile INR not included.
Main analysis—risk of epilepsy or seizures in patients with atrial fibrillation treated with a direct oral anticoagulant vs. phenprocoumon
During the study period, 3070 patients of the study cohort were diagnosed with epilepsy/seizures (cases). The cases were matched to 30 676 patients in the study cohort who had not developed epilepsy/seizures by the matched index day (controls). A total of 1828 cases were currently treated with a DOAC (n = 1265) or with PPC (n = 563) on the index day. The remaining 1242 cases received either no longer anticoagulant treatment or more than one anticoagulant on the index day. In the control group, 19 084 patients were on current treatment with a DOAC or with PPC on the matched index day. In what follows, we only refer to cases and controls who were on current anticoagulant treatment at the time when epilepsy/seizures were diagnosed (cases) or at the matched index date (controls).
Median time from initiating DOAC or PPC treatment to the first diagnosis of epilepsy/seizures was 343 days (interquartile range, 121–757) and 353 days (81–782), respectively. In comparison to controls, cases with epilepsy/seizures had overall higher baseline CHA2DS2-VASc scores (see Supplementary material online, Figure S1) but CHA2DS2-VASc scores and age were similar between DOAC or PPC groups (Table 1, Table 2).
Table 2.
Baseline characteristics of cases (patients with atrial fibrillation who were diagnosed with epilepsy or seizures after starting oral anticoagulation) with current use of PPC or a DOAC
| Characteristic | PPC N = 563 |
Any DOAC N = 1265 |
Apixaban N = 433 |
Dabigatran N = 188 |
Edoxaban N = 27 |
Rivaroxaban N = 617 |
|---|---|---|---|---|---|---|
| Male | 280 (49.7%) | 592 (46.8%) | 207 (47.8%) | 92 (48.9%) | 15 (55.6%) | 278 (45.1%) |
| Age1 | 76 (70, 81) | 77 (70, 82) | 77 (71, 83) | 75 (70, 82) | 76 (67, 80) | 76 (70, 82) |
| CHA2DS2VASca,b | 6.00 (4.00, 7.00) | 6.00 (4.00, 7.00) | 6.00 (4.00, 7.00) | 6.00 (4.00, 7.00) | 5.00 (4.00, 7.00) | 5.00 (4.00, 7.00) |
| CHA2DS2VAScb | ||||||
| 0 | 1 (0.2%) | 2 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.3%) |
| 1 | 3 (0.5%) | 16 (1.3%) | 5 (1.2%) | 1 (0.5%) | 0 (0.0%) | 10 (1.6%) |
| 2 | 20 (3.6%) | 65 (5.1%) | 25 (5.8%) | 9 (4.8%) | 3 (11.1%) | 28 (4.5%) |
| 3 | 53 (9.4%) | 94 (7.4%) | 31 (7.2%) | 13 (6.9%) | 2 (7.4%) | 48 (7.8%) |
| 4 | 75 (13.3%) | 195 (15.4%) | 60 (13.9%) | 28 (14.9%) | 4 (14.8%) | 103 (16.7%) |
| 5 | 126 (22.4%) | 238 (18.8%) | 62 (14.3%) | 41 (21.8%) | 6 (22.2%) | 129 (20.9%) |
| 6 | 108 (19.2%) | 245 (19.4%) | 98 (22.6%) | 29 (15.4%) | 4 (14.8%) | 114 (18.5%) |
| 7 | 92 (16.3%) | 219 (17.3%) | 83 (19.2%) | 35 (18.6%) | 5 (18.5%) | 96 (15.6%) |
| 8 | 55 (9.8%) | 136 (10.8%) | 46 (10.6%) | 23 (12.2%) | 2 (7.4%) | 65 (10.5%) |
| 9 | 30 (5.3%) | 55 (4.3%) | 23 (5.3%) | 9 (4.8%) | 1 (3.7%) | 22 (3.6%) |
| HAS-BLEDa,c | 4.00 (3.00, 5.00) | 4.00 (3.00, 5.00) | 4.00 (3.00, 5.00) | 4.00 (3.00, 5.00) | 4.00 (3.00, 5.00) | 4.00 (3.00, 5.00) |
| Intracerebral bleeding (I61x) | 17 (3.0%) | 53 (4.2%) | 21 (4.8%) | 13 (6.9%) | 0 (0.0%) | 19 (3.1%) |
| Ischaemic stroke | 186 (33.0%) | 570 (45.1%) | 207 (47.8%) | 107 (56.9%) | 9 (33.3%) | 247 (40.0%) |
| Transient ischaemic attack | 51 (9.1%) | 113 (8.9%) | 40 (9.2%) | 23 (12.2%) | 2 (7.4%) | 48 (7.8%) |
| Deep vein thrombosis | 9 (1.6%) | 18 (1.4%) | 2 (0.5%) | 1 (0.5%) | 0 (0.0%) | 15 (2.4%) |
| Pulmonary embolism | 7 (1.2%) | 22 (1.7%) | 5 (1.2%) | 0 (0.0%) | 0 (0.0%) | 17 (2.8%) |
| Coronary artery disease incl. myocardial infarction | 372 (66.1%) | 678 (53.6%) | 232 (53.6%) | 103 (54.8%) | 13 (48.1%) | 330 (53.5%) |
| Hypertension | 541 (96.1%) | 1204 (95.2%) | 412 (95.2%) | 181 (96.3%) | 27 (100.0%) | 584 (94.7%) |
| Heart failure | 346 (61.5%) | 638 (50.4%) | 220 (50.8%) | 87 (46.3%) | 15 (55.6%) | 316 (51.2%) |
| Peripheral arterial disease | 245 (43.5%) | 497 (39.3%) | 172 (39.7%) | 75 (39.9%) | 13 (48.1%) | 237 (38.4%) |
| Diabetes | 260 (46.2%) | 518 (40.9%) | 183 (42.3%) | 76 (40.4%) | 14 (51.9%) | 245 (39.7%) |
| COPD | 145 (25.8%) | 309 (24.4%) | 121 (27.9%) | 44 (23.4%) | 3 (11.1%) | 141 (22.9%) |
| Liver disease | 190 (33.7%) | 389 (30.8%) | 129 (29.8%) | 51 (27.1%) | 10 (37.0%) | 199 (32.3%) |
| Renal disease | 223 (39.6%) | 419 (33.1%) | 157 (36.3%) | 48 (25.5%) | 11 (40.7%) | 203 (32.9%) |
| Chronic kidney disease (≥stadium 3) | 204 (36.2%) | 368 (29.1%) | 143 (33.0%) | 40 (21.3%) | 10 (37.0%) | 175 (28.4%) |
| Alcohol-related disorders | 33 (5.9%) | 73 (5.8%) | 26 (6.0%) | 6 (3.2%) | 1 (3.7%) | 40 (6.5%) |
| Smoking | 39 (6.9%) | 78 (6.2%) | 28 (6.5%) | 4 (2.1%) | 2 (7.4%) | 44 (7.1%) |
| Antiarrhythmic drugs | 82 (14.6%) | 166 (13.1%) | 52 (12.0%) | 27 (14.4%) | 2 (7.4%) | 85 (13.8%) |
| Antihypertensive drugs | 481 (85.4%) | 1074 (84.9%) | 367 (84.8%) | 158 (84.0%) | 24 (88.9%) | 525 (85.1%) |
| Antiplatelets | 232 (41.2%) | 541 (42.8%) | 187 (43.2%) | 85 (45.2%) | 11 (40.7%) | 258 (41.8%) |
| NSAID | 453 (80.5%) | 1010 (79.8%) | 348 (80.4%) | 150 (79.8%) | 22 (81.5%) | 490 (79.4%) |
| Proton pump inhibitor | 239 (42.5%) | 609 (48.1%) | 203 (46.9%) | 89 (47.3%) | 13 (48.1%) | 304 (49.3%) |
| Corticosteroids | 57 (10.1%) | 121 (9.6%) | 42 (9.7%) | 18 (9.6%) | 2 (7.4%) | 59 (9.6%) |
Values are numbers (percentages) unless stated otherwise.
CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DOAC, direct oral anticoagulant; IQR, interquartile range; NSAID, non-steroidal anti-inflammatory drug; PPC, phenprocoumon.
Median (IQR).
CHA2DS2VASc score: congestive heart failure (1 Point); hypertension (1 Point); aged ≥ 75 years (2 Points); diabetes mellitus (1 Point); stroke/transient ischaemic attack (2 Points); vascular disease (1 Point), aged 65–74 years (1 Point), female sex (1 Point).
HAS-BLED sum score: hypertension (1 Point), abnormal renal or liver function (each 1 Point), previous stroke (1 Point), bleeding history or predisposition (anaemia) (1 Point), Elderly (> 65 years) (1 Point), Drugs (other antiplatelet agents or NSAIDs) or alcohol (each 1 Point), Labile INR not included.
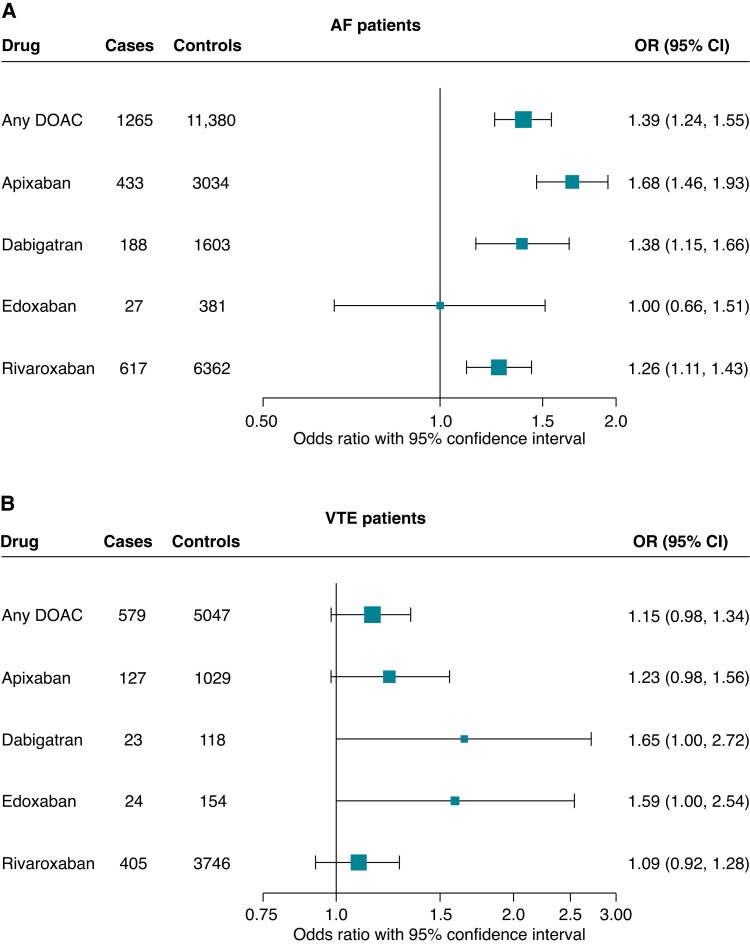
Use of a DOAC was associated with an increased risk of developing epilepsy/seizures compared to PPC [OR 1.54, 95% CI (1.38; 1.70)]. After adjusting for multiple relevant confounders (see Supplementary material online, Table S1), the increased risk of epilepsy/seizures persisted [aOR 1.39, 95% CI (1.24; 1.55)]. The observed association was most pronounced for use of apixaban vs. PPC [aOR 1.68, 95% CI (1.46; 1.93)], followed by dabigatran and rivaroxaban. We saw no association in patients taking edoxaban vs. PPC [aOR 1.00, 95% CI (0.66; 1.51)], but the number of cases using edoxaban was too low (n = 27) for meaningful analysis (Figure 2A, Table 3).
Figure 2.
Forest plot of the estimated risk of epilepsy or seizures comparing current use of a direct oral anticoagulant with current use of phenprocoumon in patients with atrial fibrillation (A) and in patients with venous thromboembolism (B), respectively. Results are presented as matched adjusted odds ratios with corresponding 95% confidence intervals. AF, atrial fibrillation; DOAC, direct oral anticoagulant; PPC, phenprocoumon; VTE, venous thromboembolism.
Table 3.
Estimated risk of epilepsy or seizures comparing current use of a single DOAC with current use of PPC in patients with atrial fibrillation
| Cases N = 1828 |
Controls N = 19 084 |
Crude odds ratio (95% CI) |
Adjusteda odds ratio (95% CI) |
|
|---|---|---|---|---|
| PPC | 563 (30.8) | 7704 (40.4) | Reference | Reference |
| Any DOAC | 1265 (69.2) | 11 380 (59.6) | 1.54 (1.38; 1.70) | 1.39 (1.24; 1.55) |
| Apixaban | 433 (23.7) | 3034 (15.9) | 1.97 (1.72; 2.25) | 1.68 (1.46; 1.93) |
| Dabigatran | 188 (10.3) | 1603 (8.4) | 1.62 (1.36; 1.92) | 1.38 (1.15; 1.66) |
| Edoxaban | 27 (1.5) | 381 (2.0) | 0.98 (0.66; 1.46) | 1.00 (0.66; 1.51) |
| Rivaroxaban | 617 (33.8) | 6362 (33.3) | 1.34 (1.19; 1.51) | 1.26 (1.11; 1.43) |
Cases were diagnosed with epilepsy or seizures. Results are presented as matched crude and adjusted odds ratios with corresponding 95% confidence intervals.
Values are numbers (percentages) unless stated otherwise.
DOAC, direct oral anticoagulants; PPC, Phenprocoumon.
Adjusted for comorbidities (alcohol and drug abuse, acute coronary syndrome, heart failure, cardiovascular disease, diabetes mellitus, hypertension, hyperlipidaemia, obesity, ischaemic stroke, transient ischaemic attack, other cerebral lesions, dementia, liver disease, hepatitis, mild chronic kidney disease, moderate/severe chronic kidney disease, other chronic kidney disease, hyperglycaemia, hypoglycaemia, electrolyte abnormalities, rare disorders), co-medications (antipsychotics/antidepressants, benzodiazepine derivatives, antiepileptic drugs, antibiotics, virustatics, other antiinfectives, cardiovascular system, immunosuppressants, CYP/P-glycoprotein inhibitors, CYP inducers, other drugs).
See Supplementary Results 1 through 4 for findings of sensitivity analyses regarding the data source, unmeasured confounding, DOAC dosing, and AF ablation procedures.
Exploratory analyses—ischaemic stroke and intracranial haemorrhage
At study entry, cases with epilepsy/seizures had more often a history of ischaemic stroke than the overall cohort (Tables 1 and 2). After study entry and before the diagnosis of epilepsy/seizures or the matched index date, 11.7% of cases and 1.6% of controls with current anticoagulant treatment had a new diagnosis of ischaemic stroke. As for intracranial bleeding, 4.6% of cases and 0.2% of controls on current anticoagulant treatment experienced an event after cohort entry and before the (matched) index date. When restricting the analysis to non-traumatic intracranial bleeding, 3.1% of cases on current treatment with a DOAC or PPC experienced an event after cohort entry and prior to the diagnosis of epilepsy/seizures, while only 0.1% of controls on current treatment with a DOAC or PPC had a diagnosis of non-traumatic intracranial bleeding after cohort entry and prior to the matched index date (Table 4).
Table 4.
Exploratory analysis on the occurrence of stroke (ischaemic stroke and intracranial bleeding) after cohort entry and prior to the diagnosis of epilepsy/seizures (cases) or the matched index date (controls)
| Cases (N = 1828) | Controls (N = 19 084) | |||
|---|---|---|---|---|
| Type of stroke | N | % | N | % |
| Ischaemic stroke | 213 | 11.7 | 297 | 1.6 |
| Any intracranial bleeding (traumatic and non-traumatic) | 85 | 4.7 | 39 | 0.2 |
| Non-traumatic intracranial bleeding | 56 | 3.1 | 17 | 0.1 |
| Traumatic intracranial bleeding | 38 | 2.1 | 24 | 0.1 |
Thus, epilepsy/seizures particularly occurred in patients with preceding ischaemic cerebrovascular events. The notion that patients with a high cerebrovascular risk are affected was supported by the observation that cases with epilepsy/seizures more frequently experienced subsequent ischaemic strokes within up to 365 days after the diagnosis of epilepsy/seizures. Also, intracranial bleedings were more frequent in cases after the diagnosis of epilepsy/seizure than in controls during the same period (Table 5).
Table 5.
Exploratory analysis on the occurrence of stroke diagnoses within up to 365 days after the diagnosis of epilepsy or seizures (cases) or the matched index date (controls) among patients on current treatment with a DOAC or PPC
| Cases (N = 1828) |
Controls (N = 19 084) |
|||
|---|---|---|---|---|
| Type of stroke | N | % | N | % |
| Ischaemic stroke | 56 | 3.06 | 189 | 0.99 |
| Any intracranial bleeding (traumatic and non-traumatic) |
39 | 2.13 | 81 | 0.42 |
| Non-traumatic intracranial bleeding (I60, I61, I62) | 27 | 1.48 | 61 | 0.32 |
| Traumatic intracranial bleeding (S06-) | 17 | 0.93 | 25 | 0.13 |
Excluding patients with a diagnosis of ischaemic stroke any time prior to the (matched) index day resulted in an exploratory study cohort of 197 135 new users of a DOAC or PPC with a diagnosis of AF. A total of 1072 cases and 16 467 matched controls were on current treatment with a DOAC or PPC on the (matched) index day. Higher risk of epilepsy/seizures was also observed in this population for current use of a DOAC in comparison to PPC according to adjusted analysis [aOR 1.25, 95% CI (1.09; 1.44)]. Thus, even in patients with AF who were not diagnosed with a prior ischaemic stroke, DOAC treatment in comparison to PPC treatment was associated with an increased risk of epilepsy/seizures (Table 6A). Exclusion of patients with a history of intracranial bleeding prior to the index day led to results consistent with those of the main analysis: aOR 1.45, 95% CI (1.30; 1.62) (Table 6B).
Table 6.
Exploratory and sensitivity analyses: estimated risk of epilepsy or seizures comparing current use of a single DOAC with current use of PPC in subsamples
| A. Without history of ischaemic stroke prior to the (matched) index day | Cases N = 1072 |
Controls N = 16 467 |
Crude odds ratio (95% CI) |
Adjusteda odds ratio (95% CI) |
|---|---|---|---|---|
| PPC | 377 (35.2) | 6744 (41.0) | Reference | Reference |
| Any DOAC | 695 (64.8) | 9723 (59.0) | 1.29 (1.13; 1.47) | 1.25 (1.09; 1.44) |
| Apixaban | 226 (21.1) | 2549 (15.5) | 1.61 (1.35; 1.92) | 1.50 (1.25; 1.80) |
| Dabigatran | 81 (7.6) | 1319 (8.0) | 1.11 (0.87; 1.43) | 1.09 (0.84; 1.41) |
| Edoxaban | 18 (1.7) | 339 (2.1) | 1.01 (0.61; 1.65) | 1.05 (0.63; 1.74) |
| Rivaroxaban | 370 (34.5) | 5516 (33.5) | 1.21 (1.04; 1.40) | 1.19 (1.02; 1.39) |
| B. Without history of intracranial bleeding prior to the (matched) index day | Cases N = 1709 |
Controls N = 18 743 |
Crude odds ratio (95% CI) |
Adjusteda odds ratio (95% CI) |
|---|---|---|---|---|
| PPC | 529 (31.0) | 7583 (40.5) | Reference | Reference |
| Any DOAC | 1180 (69.0) | 11 160 (59.5) | 1.54 (1.38; 1.71) | 1.45 (1.30; 1.62) |
| Apixaban | 399 (23.3) | 2980 (15.9) | 1.94 (1.69; 2.23) | 1.76 (1.53; 2.04) |
| Dabigatran | 171 (10.0) | 1563 (8.3) | 1.58 (1.31; 1.89) | 1.50 (1.25; 1.81) |
| Edoxaban | 26 (1.5) | 374 (2.0) | 1.03 (0.69; 1.56) | 1.04 (0.68; 1.58) |
| Rivaroxaban | 584 (34.2) | 6243 (33.3) | 1.36 (1.20; 1.54) | 1.31 (1.15; 1.48) |
| C. VTE cohort | Cases N = 871 |
Controls N = 8374 |
Crude odds ratio (95% CI) |
Adjusteda odds ratio (95% CI) |
|---|---|---|---|---|
| PPC | 292 (33.5) | 3327 (39.7) | Reference | Reference |
| Any DOAC | 579 (66.5) | 5047 (60.3) | 1.31 (1.13; 1.52) | 1.15 (0.98; 1.34) |
| Apixaban | 127 (14.6) | 1029 (12.3) | 1.41 (1.13; 1.76) | 1.23 (0.98; 1.56) |
| Dabigatran | 23 (2.6) | 118 (1.4) | 2.21 (1.39; 3.51) | 1.65 (1.00; 2.72) |
| Edoxaban | 24 (2.8) | 154 (1.8) | 1.80 (1.15; 2.83) | 1.59 (1.00; 2.54) |
| Rivaroxaban | 405 (46.5) | 3746 (44.7) | 1.24 (1.06; 1.45) | 1.09 (0.92; 1.28) |
| D. Without TK data since 2015 | Cases N = 1293 |
Controls N = 13 481 |
Crude odds ratio (95% CI) |
Adjusteda odds ratio (95% CI) |
|---|---|---|---|---|
| PPC | 442 (34.2) | 5956 (44.2) | Reference | Reference |
| Any DOAC | 851 (65.8) | 7525 (55.8) | 1.54 (1.37; 1.74) | 1.35 (1.19; 1.54) |
| Apixaban | 247 (19.1) | 1679 (12.5) | 2.00 (1.70; 2.37) | 1.66 (1.39; 1.99) |
| Dabigatran | 146 (11.3) | 1193 (8.8) | 1.67 (1.37; 2.03) | 1.41 (1.15; 1.74) |
| Edoxaban | 7 (0.5) | 111 (0.8) | 0.85 (0.39; 1.83) | 0.96 (0.43; 2.14) |
| Rivaroxaban | 451 (34.9) | 4542 (33.7) | 1.35 (1.18; 1.55) | 1.22 (1.06; 1.41) |
Cases were diagnosed with epilepsy or seizures. Results are presented as matched crude and adjusted odds ratios with corresponding 95% confidence intervals.
Values are numbers (percentages) unless stated otherwise.
DOAC, direct oral anticoagulants; PPC, phenprocoumon; VTE, venous thromboembolism.
Adjusted for comorbidities (alcohol and drug abuse, acute coronary syndrome, heart failure, cardiovascular disease, diabetes mellitus, hypertension, hyperlipidaemia, obesity, ischaemic stroke, transient ischaemic attack, other cerebral lesions, dementia, liver disease, hepatitis, mild chronic kidney disease, moderate/severe chronic kidney disease, other chronic kidney disease, hyperglycaemia, hypoglycaemia, electrolyte abnormalities, rare disorders), co-medications (antipsychotics/antidepressants, benzodiazepine derivatives, antiepileptic drugs, antibiotics, virustatics, other antiinfectives, cardiovascular system, immunosuppressants, CYP/P-glycoprotein inhibitors, CYP inducers, other drugs).
Exploratory analysis—risk of epilepsy or seizures in patients with venous thromboembolism
To assess whether the association of DOAC treatment with epilepsy/seizures is specific for patients with AF, we repeated our main analysis in a cohort of new DOAC or PPC users with a diagnosis of VTE, representing another indication of anticoagulation with DOACs or PPC at comparable doses. In 2011–18, a total of 149 518 eligible individuals with a history of VTE initiated treatment with a DOAC (n = 89 030) or PPC (n = 60 488). Rivaroxaban (n = 65 579) was the most commonly used DOAC at treatment initiation, followed by apixaban (n = 17 807), edoxaban (n = 3759), and dabigatran (n = 1885). Among VTE patients, new users of a DOAC were younger, had fewer comorbidities, and took fewer co-medications. In addition, VTE patients starting DOAC treatment had lower CHA2DS2-VASc scores than those on PPC (see Supplementary material online, Table S2). During the study period, 1879 VTE patients were diagnosed with epilepsy/seizures (cases), of which 871 were currently on anticoagulant treatment with either a DOAC (n = 579) or PPC (n = 292). Cases were matched to 18 771 VTE patients who had not developed epilepsy/seizures by the matched index date (controls), 8374 of whom were on current treatment with a DOAC or PPC on the matched index day. Compared to PPC users, DOAC users were at increased risk of epilepsy/seizures in the matched analysis [OR 1.31, 95% CI (1.13; 1.52)]. After confounder adjustment, the risk of epilepsy/seizures was attenuated [aOR 1.15, 95% CI (0.98; 1.34)] (Table 6C). Thus, the risk of epilepsy/seizures from DOAC vs. PPC treatment differed between cohorts of patients with AF and VTE (Figure 2B) and was most pronounced in patients with a diagnosis of AF who are characterized by a high underlying risk of stroke.
Discussion
In a cohort of patients with AF, the risk of developing epilepsy was higher if treated with a DOAC than PPC. The higher risk persisted after adjusting for various potential confounders that may influence treatment and the risk for seizures or epilepsy. In a different cohort of patients with a diagnosis of VTE, there was no difference in risk of epilepsy/seizures between current use of a DOAC vs. PPC.
Atrial fibrillation itself is associated with an increased risk of epilepsy/seizures.9,10 The CHADS2 score is a good indicator for the risk of epilepsy/seizures in AF.9 Indeed, patients who developed epilepsy/seizures during anticoagulant treatment of AF had a higher CHA2DS2-VASc score, an advanced form of the CHADS2 score, than controls, confirming the value of this score as a risk indicator for epilepsy/seizures. Of note, patients taking DOACs at study entry had a lower CHA2DS2-VASc score but developed more often epilepsy/seizures than patients taking PPC, arguing that, if anything, we have underestimated the risk of epilepsy associated with DOAC treatment in AF.
As substrates of P-glycoprotein, DOACs hardly permeate the brain.16 Even if they were to cross the blood–brain barrier under special conditions, antiepileptic effects would be expected because DOACs act as upstream or direct antagonists of the pro-convulsive thrombin.17 In line with this, DOACs were not associated with a significantly higher risk of epilepsy/seizures than PPC if administered in patients with VTE. Direct pro-convulsive effects are therefore unlikely.
Indirect mechanisms that may link anticoagulant treatment of AF with epilepsy/seizures include intracerebral bleedings and ischaemic strokes. Stroke is the most common cause of acquired epilepsy.7 In accordance with other studies, DOACs caused fewer intracranial haemorrhages than VKA but a higher rate of gastrointestinal haemorrhages as shown by a parallel analysis of the GePaRD database.13,18 Thus, it seems unlikely that intracerebral haemorrhages have markedly contributed to the risk of epilepsy/seizures under DOAC treatment of AF.
Notably, ischaemic strokes often preceded epilepsy/seizures. At baseline before starting anticoagulant therapy, cases who later developed epilepsy/seizures had a higher CHA2DS2-VASc score and more frequently a history of ischaemic stroke (Table 2) than the overall cohort (Table 1). The higher rate of ischaemic stroke persisted in cases compared to controls after initiating anticoagulant therapy (Table 4). Apparently, epilepsy/seizures occurred in patients with a high risk for cardioembolism. This notion was supported by the observation that cases with epilepsy/seizures more frequently had a diagnosis of ischaemic stroke within 365 days after the diagnosis of epilepsy/seizures (Table 5). The later diagnosis of stroke may reflect independent consecutive events or epilepsy/seizures being the first symptom of a cerebrovascular event.
Even when we excluded patients with diagnosed ischaemic or haemorrhagic stroke from the main analysis, the higher risk for epilepsy/seizures persisted in patients treated with DOACs compared to PPC. We propose that covert brain infarction may be considered one mechanism potentially underlying epilepsy/seizures in patients without clinically diagnosed stroke during anticoagulant treatment. Atrial fibrillation ablation procedures have been found to increase the risk of covert brain infarction and to be performed in a selected patient population.19,20 In our data, however, the proportion of individuals undergoing AF ablation procedures was overall low with no meaningful difference between cases and controls (Supplementary Results 4, Supplementary material online, Table S6). So far, the differential activity of DOACs and VKAs in preventing covert brain infarction have not been investigated.3 Likewise, it is unclear whether anticoagulants differ in their efficacy to prevent long-term consequences of covert brain infarction, such as dementia and epilepsy.11,12 Vitamin K antagonists act by inhibiting more coagulation factors than DOACs, which may explain their better efficacy in preventing arterial thromboembolism in mechanical heart valves, left ventricular thrombi, and the antiphospholipid syndrome. Transesophageal echocardiography has documented that DOAC treatment leads to the reduction and resolution of thrombi in the left atrium.21 However, the effect on microscopic thrombi in the left atrium, which have been known for a long time and may cause small cortical infarcts and subsequently epileptic seizures, is less clear.11 Although DOACs are at least as effective at preventing stroke in AF as VKAs, the assumption that they are similarly active against covert brain infarction may not be justified.
This study is subject to several limitations that are due to the nature of data from insurance claims. The analysis was based on dispensing drug information which entails the assumption that patients who claim their prescriptions are actually taking their treatment at the recommended dosage. The outcome, epilepsy/seizures, was assessed based on outpatient diagnoses coded as ‘certain’ and main hospital diagnoses. As it was our aim to capture new-onset epilepsy/seizures, patients with a history of these conditions were excluded from the analysis. A potential misdiagnosis would be considered non-differential by exposure status and, therefore, expected to cause bias towards the null. Lastly, claims data do not include diagnostic findings or information on lifestyle factors, such as diet or life events, which might have led to residual confounding. Given the lack of laboratory results, we were not able to evaluate whether patients taking PPC were treated within the therapeutic range. Both over- and underdosing of VKA is expected to increase the risk of cerebrovascular events, which are associated with the occurrence of epilepsy/seizures. Therefore, in our real-world setting, VKA treatment was likely associated with a higher risk of epilepsy/seizures than it would have occurred with optimal VKA dosing. This would result in an underestimation of the risk of epilepsy/seizures from a DOAC compared to optimally dosed VKA.
A major strength of this study is the extensive data source, GePaRD, which covers ∼20% of the German population. As a result, our study included an unselected population of 227 707 patients who had started anticoagulant treatment for AF between 2011 and 2017. Prescriptions and dispensations for the assessment of treatment were electronically processed by pharmacies, and information on dispensing date, product, and dispensed strength are accurate and free of recall bias. Also, adherence to oral anticoagulation was found to be overall high.14 Lastly, an active comparator design and multivariable analysis adjusted for multiple potential confounders was applied to minimize the risk for residual confounding.
This study was designed to investigate the risk of an outcome while under current treatment, i.e. the immediate risk of epilepsy/seizures while being treated with a DOAC vs. PPC. This design was chosen following the detection of a signal for epilepsy/seizures diagnoses in close time proximity to DOAC dispensations in a previous pharmacovigilance study conducted by our group. However, we do not know whether the medication was continuously taken between study inclusion and index date.
In conclusion, anticoagulant treatment with DOACs in AF was associated with an increased risk of epilepsy compared to a VKA. Epilepsy occurred in patients with a higher risk of cerebrovascular events. In patients without preceding stroke, covert brain infarction may be a potential explanation of the observed elevated risk of epilepsy. Future studies are needed to better understand a potential indirect outcome mechanism involving the occurrence of ischaemic cerebrovascular events prior to the diagnosis of epilepsy/seizures. Prospective trials investigating the efficacy of DOACs for preventing covert brain infarction are under way.22 These may help to better understand the risk-benefit balance of this important class of drugs.
Supplementary Material
Acknowledgements
The authors would like to thank all statutory health insurance providers which provided data for this study, namely AOK Bremen/Bremerhaven, DAK-Gesundheit, Die Techniker (TK), and hkk Krankenkasse. The authors would also like to thank Bianca Kollhorst, Susann Grill, Inga Schaffer, and Anja Gabbert for their support.
Contributor Information
Katharina Platzbecker, Leibniz Institute for Prevention Research and Epidemiology—BIPS, Achterstraße 30, 28359 Bremen, Germany.
Helge Müller-Fielitz, Institute for Experimental and Clinical Pharmacology and Toxicology, University of Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany.
Ronja Foraita, Leibniz Institute for Prevention Research and Epidemiology—BIPS, Achterstraße 30, 28359 Bremen, Germany.
Matthias J Koepp, Department of Clinical and Experimental Epilepsy, University College London Queen Square Institute of Neurology, Queen Square, Box 29, London WC1N 3BG, United Kingdom.
Annemarie Voss, Leibniz Institute for Prevention Research and Epidemiology—BIPS, Achterstraße 30, 28359 Bremen, Germany.
René Pflock, Institute for Experimental and Clinical Pharmacology and Toxicology, University of Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany.
Roland Linder, Techniker Krankenkasse, Bramfelder Straße 140, 22305 Hamburg, Germany.
Iris Pigeot, Leibniz Institute for Prevention Research and Epidemiology—BIPS, Achterstraße 30, 28359 Bremen, Germany; Faculty of Mathematics and Computer Science, University of Bremen, Bibliothekstraße 5, 28334 Bremen, Germany.
Tania Schink, Leibniz Institute for Prevention Research and Epidemiology—BIPS, Achterstraße 30, 28359 Bremen, Germany.
Markus Schwaninger, Institute for Experimental and Clinical Pharmacology and Toxicology, University of Lübeck, Ratzeburger Allee 160, 23562 Lübeck, Germany; DZHK (German Research Centre for Cardiovascular Research), Hamburg-Lübeck-Kiel, Germany.
Supplementary material
Supplementary material is available at Europace online.
Funding
This study was supported from the innovation fund (‘Innovationsfonds’) of the Federal Joint Committee in Germany (acronym: PV-Monitor; grant number: 01VSF16020).
Data availability
As we are not the owners of the data, we are not legally entitled to grant access to the data of the German Pharmacoepidemiological Research Database. In accordance with German data protection regulations, access to the data is granted only to employees of the Leibniz Institute for Prevention Research and Epidemiology—BIPS on the BIPS premises and in the context of approved research projects. Third parties may only access the data in cooperation with BIPS and after signing an agreement for guest researchers at BIPS.
References
- 1. Lopez-Lopez JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GNet al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ 2017;359:j5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vinogradova Y, Coupland C, Hill T, Hippisley-Cox J. Risks and benefits of direct oral anticoagulants versus warfarin in a real world setting: cohort study in primary care. BMJ 2018;362:k2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meinel TR, Kaesmacher J, Roten L, Fischer U. Covert brain infarction: towards precision medicine in research, diagnosis, and therapy for a silent pandemic. Stroke 2020;51:2597–606. [DOI] [PubMed] [Google Scholar]
- 4. Kempster PA, Gerraty RP, Gates PC. Asymptomatic cerebral infarction in patients with chronic atrial fibrillation. Stroke 1988;19:955–7. [DOI] [PubMed] [Google Scholar]
- 5. Maxwell H, Hanby M, Parkes LM, Gibson LM, Coutinho C, Emsley HCA. Prevalence and subtypes of radiological cerebrovascular disease in late-onset isolated seizures and epilepsy. Clin Neurol Neurosurg 2013;115:591–6. [DOI] [PubMed] [Google Scholar]
- 6. Conen D, Rodondi N, Müller A, Beer JH, Ammann P, Moschovitis Get al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol 2019;73:989–99. [DOI] [PubMed] [Google Scholar]
- 7. Brodie MJ, Elder AT, Kwan P. Epilepsy in later life. Lancet Neurol 2009;8:1019–30. [DOI] [PubMed] [Google Scholar]
- 8. Galovic M, Dohler N, Erdelyi-Canavese B, Felbecker A, Siebel P, Conrad J, et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol 2018;17:143–52. [DOI] [PubMed] [Google Scholar]
- 9. Hsu CY, Chen TH, Su YW, Chang C-C, Chen M-H, Leu H-Bet al. Usefulness of the CHADS2 score for determining risk of seizure in patients with atrial fibrillation. Am J Cardiol 2016;118:1340–4. [DOI] [PubMed] [Google Scholar]
- 10. Doege C, Luedde M, Kostev K. Atrial fibrillation is associated with a subsequent epilepsy diagnosis independent of stroke: a retrospective matched administrative cohort study on 149,632 patients. Epilepsy Behav 2022;132:108721. [DOI] [PubMed] [Google Scholar]
- 11. Katsanos AH, Kamel H, Healey JS, Hart RG. Stroke prevention in atrial fibrillation: looking forward. Circulation 2020;142:2371–88. [DOI] [PubMed] [Google Scholar]
- 12. Kuhne M, Krisai P, Coslovsky M, Rodondi N, Müller A, Beer JHet al. Silent brain infarcts impact on cognitive function in atrial fibrillation. Eur Heart J 2022;43:2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dijkstra L, Schink T, Linder R, Schwaninger M, Pigeot I, Wright MNet al. A discovery and verification approach for pharmacovigilance using electronic health care data. medRxiv 2022:2022.2005.2010.22274885. [Google Scholar]
- 14. Komen JJ, Pottegard A, Mantel-Teeuwisse AK, Forslund T, Hjemdahl P, Wettermark Bet al. Persistence and adherence to non-vitamin K antagonist oral anticoagulant treatment in patients with atrial fibrillation across five Western European countries. Europace 2021;23:1722–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 16. König J, Müller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev 2013;65:944–66. [DOI] [PubMed] [Google Scholar]
- 17. Maggio N, Blatt I, Vlachos A, Tanne D, Chapman J, Segal M. Treating seizures and epilepsy with anticoagulants? Front Cell Neurosci 2013;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei Bet al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016;37:2893–962. [DOI] [PubMed] [Google Scholar]
- 19. Nakamura T, Okishige K, Kanazawa T, Yamashita M, Kawaguchi N, Kato Net al. Incidence of silent cerebral infarctions after catheter ablation of atrial fibrillation utilizing the second-generation cryoballoon. Europace 2017;19:1681–8. [DOI] [PubMed] [Google Scholar]
- 20. Tilz RR, Dagres N, Arbelo E, Blomström-Lundqvist C, Crijns HJ, Kirchhof Pet al. Which patients with atrial fibrillation undergo an ablation procedure today in Europe? A report from the ESC-EHRA-EORP atrial fibrillation ablation long-term and atrial fibrillation general pilot registries. Europace 2020;22:250–8. [DOI] [PubMed] [Google Scholar]
- 21. Lip GY, Hammerstingl C, Marin F, Cappato R, Meng IL, Kirsch Bet al. Left atrial thrombus resolution in atrial fibrillation or flutter: results of a prospective study with rivaroxaban (X-TRA) and a retrospective observational registry providing baseline data (CLOT-AF). Am Heart J 2016;178:126–34. [DOI] [PubMed] [Google Scholar]
- 22. Sharma M, Hart RG, Smith EE, Bosch J, Yuan F, Casanova Aet al. Rationale, design, and baseline participant characteristics in the MRI and cognitive substudy of the cardiovascular outcomes for people using anticoagulation strategies trial. Int J Stroke 2019;14:270–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
As we are not the owners of the data, we are not legally entitled to grant access to the data of the German Pharmacoepidemiological Research Database. In accordance with German data protection regulations, access to the data is granted only to employees of the Leibniz Institute for Prevention Research and Epidemiology—BIPS on the BIPS premises and in the context of approved research projects. Third parties may only access the data in cooperation with BIPS and after signing an agreement for guest researchers at BIPS.