Abstract
Black patients suffer higher rates of antibody-mediated rejection and have worse long-term graft survival after heart transplantation. Percent donor-derived cell free DNA (%ddcfDNA) is released into the blood following allograft injury. This study analyzed %ddcfDNA 63 heart transplant recipients categorized as Black and Non-Black race, during the first 200 days after transplant. Immediately after transplant, %ddcfDNA was higher for Black patients (mean [SE]: 8.3% [1.3%] vs 3.2% [1.2%], p=0.001). In the first week post-transplant, the rate of decay in %ddcfDNA was similar (0.7% [0.68] vs. 0.7% [0.11], p=0.78), and values declined in both groups to a comparable plateau at 7 days post-transplant (0.46% [0.03] vs. 0.45% [0.04], p=0.78). The proportion of Black patients experiencing AMR was higher than Non-Black patients (21% vs 9%, [HR of 2.61 [95%CI: 0.651-10.43], p=0.18). Black patients were more likely to receive a race mismatched organ than Non-black patients (69% vs. 35%, p=0.01), which may explain the higher levels of early allograft injury.
Keywords: cell-free DNA, cardiac transplantation, graft injury, allograft rejection, biomarker
Black patients suffer higher rates of acute allograft rejection and have worse long-term survival after heart transplantation.1-3 Although multiple factors have been implicated, such as race specific differences in immune responses,4 immunotherapy pharmacokinetics, and social determinants of health, a direct causal relationship has not been proven. Donor-derived cell free DNA is released into the bloodstream following episodes of allograft injury and recent studies have associated elevations of percent of donor-derived cell free DNA (%ddcfDNA) to identify acute cellular rejection (ACR) and antibody-mediated rejection (AMR) with high accuracy.5 In a multicenter, prospective, cohort study with a high enrollment of Black patients, we studied temporal changes in %ddcfDNA levels to identify race specific differences in early allograft injury.
The present study analyzed 63 adult heart transplant recipients enrolled in the five center Genomic Research Alliance for Transplantation (GRAfT) study. The supplement includes immunosuppression protocols for the centers. We measured %ddcfDNA at serial time points after transplantation. Patients were categorized by self-described race as Black or Non-Black, which included White, Asian, American Indian, and Hispanic patients. Donor-derived cell-free DNA measurements were expressed as a percentage of total donor plus recipient cell-free DNA circulating in the plasma (values ranged from 0-25%) with higher levels indicating worsening allograft injury. An exponential decay model using a non-adaptive first-order likelihood estimation (FIRO) was utilized looking at the %ddcfDNA with three parameters: (1) initial day 1 post-transplant, (2) the level at which they are no longer declining, and (3) the rate of decline, with each parameter assessed by race. Estimated parameter values and standard errors (SE) corresponding to each of these components were reported separately by race. Summary statistics are presented as median (interquartile range), or frequency and percent. Comparisons were made via the Wilcoxon rank sum test, chi-square, or Fisher’s exact tests. An unadjusted Cox model was used to assess the relationship between race and rejection. A p-value < 0.05 was considered significant. This study was approved by the institutional review board at the participating centers and the National Institutes of Health (NIH).
The heart transplant recipients had a median age of 56 years (IQR: 45-64) and the proportion of Black patients was high (46%). There were no significant differences in the baseline patient or donor characteristics between Black and Non-Black patients, this includes age, sex, cardiomyopathy type, insurance, receipt of an increased risk donor or ischemic time (Table). Black patients were more likely to receive a race mismatched organ (69% vs 35%, p=0.01).
Table:
Summary of Patient, Donor and Transplant Characteristics by Race for the Cell-free DNA Cohort
| CHARACTERISTICS AT TRANSPLANT |
TOTAL (N=63) |
NON-BLACK (N=34) |
BLACK (N=29) |
P VALUE |
|---|---|---|---|---|
| PATIENT CHARACTERISTICS | ||||
| MEDIAN AGE, Y | 56.0 (45.0, 63.0) | 56.5 (45.8, 63.2) | 55.0 (43.0, 60.0) | 0.55 |
| SEX, N (%) | 0.39 | |||
| MALE | 47 (74.6%) | 27 (79.4%) | 20 (69.0%) | |
| FEMALE | 16 (25.4%) | 7 (20.6%) | 9 (31.0%) | |
| CARDIOMYOPATHY TYPE | 0.82 | |||
| NONISCHEMIC | 43 (75.4%) | 23 (71.9%) | 20 (80.0%) | |
| ISCHEMIC | 10 (17.5%) | 6 (18.8%) | 4 (16.0%) | |
| HYPERTROPHIC | 2 (3.5%) | 2 (6.2%) | 0 (0.0%) | |
| RESTRICTIVE | 2 (3.5%) | 1 (3.1%) | 1 (4.0%) | |
| UNOS STATUS | 0.77 | |||
| 1A | 42 (73.7%) | 23 (71.9%) | 19 (76.0%) | |
| 1B | 15 (26.3%) | 9 (28.1%) | 6 (24.0%) | |
| INSURANCE | 1.00 | |||
| PRIVATE | 31 (54.4%) | 18 (56.2%) | 13 (52.0%) | |
| MEDICAID | 6 (10.5%) | 3 (9.4%) | 3 (12.0%) | |
| MEDICARE | 18 (31.6%) | 10 (31.2%) | 8 (32.0%) | |
| OTHER | 2 (3.5%) | 1 (3.1%) | 1 (4.0%) | |
| DONOR CHARACTERISTICS | ||||
| MEDIAN DONOR AGE, Y | 30.0 (23.0, 36.0) | 25.5 (21.0, 38.2) | 31.0 (27.0, 36.0) | 0.56 |
| DONOR SEX | 0.40 | |||
| MALE | 40 (70.2%) | 24 (75.0%) | 16 (64.0%) | |
| FEMALE | 17 (29.8%) | 8 (25.0%) | 9 (36.0%) | |
| DONOR RACE | 0.17 | |||
| BLACK | 14 (24.6%) | 5 (15.6%) | 9 (36.0%) | |
| WHITE | 34 (59.6%) | 22 (68.8%) | 12 (48.0%) | |
| HISPANIC | 7 (12.3%) | 4 (12.5%) | 3 (12.0%) | |
| ASIAN | 1 (1.8%) | 1 (3.1%) | 0 (0.0%) | |
| OTHER | 1 (1.8%) | 0 (0.0%) | 1 (4.0%) | |
| DONOR CAUSE OF DEATH | 0.38 | |||
| ANOXIA | 21 (36.8%) | 9 (28.1%) | 12 (48.0%) | |
| CEREBROVASCULAR ACCIDENT | 6 (10.5%) | 3 (9.4%) | 3 (12.0%) | |
| HEAD TRAUMA | 27 (47.4%) | 18 (56.2%) | 9 (36.0%) | |
| OTHER | 3 (5.3%) | 2 (6.2%) | 1 (4.0%) | |
| TRANSPLANT CHARACTERISTICS |
||||
| PHS INCREASED RISK DONOR | 15 (26.3%) | 5 (15.6%) | 10 (40.0%) | 0.07 |
| RACE MISMATCH | 32 (50.8%) | 12 (35.3%) | 20 (69.0%) | 0.01 |
| MEDIAN ISCHEMIC TIME, MIN | 2.5 (2.0, 3.1) | 2.5 (2.0, 3.2) | 2.4 (2.0, 2.9) | 0.44 |
PHS: public health service definition of increased risk donor based on risk of transmission of communicable disease at time of transplant.
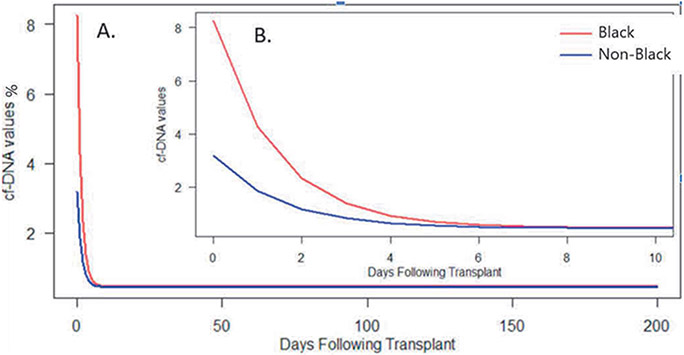
Donor-derived cell-free DNA values were analyzed initially for the first week after heart transplant and then analyzed to 200 days post-transplant and are presented in the Figure. Black recipients had higher %ddcfDNA values than Non-Black recipients for the first five days following transplant. Immediately after transplant, %ddcfDNA was higher for Black patients (mean [SE]: 8.3% [1.3%] vs 3.2% [1.2%], p=0.001). In the first week post-transplant, the rate of decay (⌂%ddcfDNA/day) was similar (0.7%/day [0.68] vs. 0.7%/day [0.11], p=0.78), and values declined in both groups to a comparable plateau at 7 days post-transplant (0.46% [0.03] vs. 0.45% [0.04], p=0.78). On further analysis of the first 200 days post-transplant, 19 (30%) and 9 (14%) heart transplant recipients experienced ACR and AMR, respectively. The risk of Black patients experiencing ACR was similar to Non-Black patients HR 0.842 (95%CI:0.34-2.09, p=0.7). The risk of Black patients experiencing AMR was higher than Non-Black patients HR of 2.61 (95%CI: 0.65-10.43, p=0.18).
Figure.
Graphical depiction of exponential decay model expressing relationship between %ddcfDNA values and Race over the first 200 (A) and 10 (B, inlaid) days following heart transplant.
These data demonstrate that Black patients have higher %ddcfDNA values immediately after heart transplant; and subsequently developed AMR at a higher prevalence compared to Non-black patients. This early injury pattern may help explain the poorer long-term outcomes observed in Black patients. The mechanism by which early elevations in ddcfDNA occur after transplant are unclear and could stem from conditions occurring during graft harvesting and transportation. We found that ischemic time was similar between Black and Non-Black patients, but Black patients were more likely to receive a race-mismatched organ. Although prior studies have suggested no mortality benefit with race matched organs,6 a recent analysis has challenged that notion.7 Residual circulating leukocytes within the transplanted heart may lead to elevations in ddcfDNA, where the cfDNA is not myocardial in origin. These leukocytes may serve as antigen presenting cells, triggering an alloimmune response. In all transplanted hearts there is some element of ischemia reperfusion injury (IRI). The IRI process triggers the development of natural, self-reactive IgM antibodies which can activate the complement system.8 The resultant graft injury could manifest with elevations of ddcfDNA. Finally, cfDNA itself, and specifically mitochondrial cfDNA, has been shown to trigger an immune system response.9, 10 This immune response may be responsible for the higher frequency of AMR seen in Black patients within GRAfT, albeit not statistically significant due to our small sample size.
The mechanisms that trigger higher early allograft injury in Black patients needs to be explored more carefully. Future appropriately powered studies should distinguish whether the allograft injury seen in Black patients occurs only early on or continues long-term after transplant. The long-term consequences of early allograft injury in Black patients may explain the higher risk of AMR and poorer long-term graft survival after heart transplant. These findings support longitudinal cohort studies with a high proportion of Black patients to discern the biological mechanisms driving disparate outcomes after heart transplantation.
Disclosures:
Drs. Agbor-Enoh and Solomon receive research support from the National Institutes of Health Clinical Center intramural research funds. Palak Shah is supported by a National Institutes of Health Career Development Award 1K23HL143179.
References
- 1.Cole RT, Gandhi J, Bray RA, Gebel HM, Yin M, Shekiladze N, Young A, Grant A, Mahoney I, Laskar SR, Gupta D, Bhatt K, Book W, Smith A, Nguyen D, Vega JD and Morris AA. Racial differences in the development of de-novo donor-specific antibodies and treated antibody-mediated rejection after heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2018;37:503–512. [DOI] [PubMed] [Google Scholar]
- 2.Maredia H, Bowring MG, Massie AB, Bae S, Kernodle A, Oyetunji S, Merlo C, Higgins RSD, Segev DL and Bush EL. Better Understanding the Disparity Associated With Black Race in Heart Transplant Outcomes: A National Registry Analysis. Circulation Heart failure. 2021;14:e006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moayedi Y, Fan CS, Miller RJH, Tremblay-Gravel M, Posada JGD, Manlhiot C, Hiller D, Yee J, Woodward R, McCaughan JA, Shullo MA, Hall SA, Pinney S, Khush KK, Ross HJ and Teuteberg JJ. Gene expression profiling and racial disparities in outcomes after heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2019;38:820–829. [DOI] [PubMed] [Google Scholar]
- 4.Khush KK, Pham MX, Teuteberg JJ, Kfoury AG, Deng MC, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Hiller D, Yee J and Valantine HA. Gene expression profiling to study racial differences after heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agbor-Enoh S, Shah P, Tunc I, Hsu S, Russell S, Feller E, Shah K, Rodrigo ME, Najjar SS, Kong H, Pirooznia M, Fideli U, Bikineyeva A, Marishta A, Bhatti K, Yang Y, Mutebi C, Yu K, Kyoo Jang M, Marboe C, Berry GJ, Valantine HA and Investigators GR. Cell-Free DNA to Detect Heart Allograft Acute Rejection. Circulation. 2021;143:1184–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV and Shah AS. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. The Annals of thoracic surgery. 2010;89:1956–63; discussion 1963-4. [DOI] [PubMed] [Google Scholar]
- 7.Cox K, Carter K, Lirette S, Cochran R, Creswell L, Panos A, Baran D and Copeland H. Donor and Recipient Racial Mismatch Impacts Thoracic Organ Transplant Survival. The Journal of Heart and Lung Transplantation. 2019;38:S266–S267. [Google Scholar]
- 8.Atkinson C, Qiao F, Yang X, Zhu P, Reaves N, Kulik L, Goddard M, Holers VM and Tomlinson S. Targeting pathogenic postischemic self-recognition by natural IgM to protect against posttransplantation cardiac reperfusion injury. Circulation. 2015;131:1171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dholakia S, De Vlaminck I and Khush KK. Adding Insult on Injury: Immunogenic Role for Donor-derived Cell-free DNA? Transplantation. 2020;104:2266–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deuse T, Hu X, Agbor-Enoh S, Koch M, Spitzer MH, Gravina A, Alawi M, Marishta A, Peters B, Kosaloglu-Yalcin Z, Yang Y, Rajalingam R, Wang D, Nashan B, Kiefmann R, Reichenspurner H, Valantine H, Weissman IL and Schrepfer S. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat Biotechnol. 2019;37:1137–1144. [DOI] [PubMed] [Google Scholar]