Abstract
Background:
The role of statin therapy in the development of kidney disease in patients with type 2 diabetes mellitus (DM) remains uncertain. We aimed to determine the relationships between statin initiation and kidney outcomes in patients with type 2 DM.
Methods:
Through a new-user design, we conducted a multicentre retrospective cohort study using the China Renal Data System database (which includes inpatient and outpatient data from 19 urban academic centres across China). We included patients with type 2 DM who were aged 40 years or older and admitted to hospital between Jan. 1, 2000, and May 26, 2021, and excluded those with pre-existing chronic kidney disease and those who were already on statins or without follow-up at an affiliated outpatient clinic within 90 days after discharge. The primary exposure was initiation of a statin. The primary outcome was the development of diabetic kidney disease (DKD), defined as a composite of the occurrence of kidney dysfunction (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73 m2 and > 25% decline from baseline) and proteinuria (a urinary albumin-to-creatinine ratio ≥ 30 mg/g and > 50% increase from baseline), sustained for at least 90 days; secondary outcomes included development of kidney function decline (a sustained > 40% decline in eGFR). We used Cox proportional hazards regression to evaluate the relationships between statin initiation and kidney outcomes, as well as to conduct subgroup analyses according to patient characteristics, presence or absence of dyslipidemia, and pattern of dyslipidemia. For statin initiators, we explored the association between different levels of lipid control and outcomes. We conducted analyses using propensity overlap weighting to balance the participant characteristics.
Results:
Among 7272 statin initiators and 12 586 noninitiators in the weighted cohort, statin initiation was associated with lower risks of incident DKD (hazard ratio [HR] 0.72, 95% confidence interval [CI] 0.62–0.83) and kidney function decline (HR 0.60, 95% CI 0.44–0.81). We obtained similar results to the primary analyses for participants with differing patterns of dyslipidemia, those prescribed different statins, and after stratification according to participant characteristics. Among statin initiators, those with intensive control of high-density lipoprotein cholesterol (LDL-C) (< 1.8 mmol/L) had a lower risk of incident DKD (HR 0.51, 95% CI 0.32–0.81) than those with inadequate lipid control (LDL-C ≥ 3.4 mmol/L).
Interpretation:
For patients with type 2 DM admitted to and followed up in academic centres, statin initiation was associated with a lower risk of kidney disease development, particularly in those with intensive control of LDL-C. These findings suggest that statin initiation may be an effective and reasonable approach for preventing kidney disease in patients with type 2 DM.
Statins are among the most commonly prescribed medications, administered to 146 million people worldwide.1 Statin therapy reduces the risk of cardiovascular disease in patients with type 2 diabetes mellitus (DM) and in patients with hypertension. 2–5 Current guidelines from the Canadian Cardiovascular Society and the American Diabetes Association recommend statin therapy for patients with diabetes aged 40 years or older.6–9 However, some recent studies have shown that treatment with a statin alters glucose metabolism and affects glycemic control in such patients.10–15 Given that a worsening of glycemic control is associated with the development or progression of microvascular disease,16 patients with diabetes who are undergoing statin treatment might be at higher risk of developing microvascular complications.
Diabetic kidney disease (DKD) is a common microvascular complication in patients with type 2 DM, is the leading cause of end-stage kidney disease, and imposes enormous health care and financial burdens in both low- and high-income countries.17,18 Although multiple experimental and epidemiologic studies have shown that dyslipidemia is a risk factor for kidney disease in patients with diabetes, the role of lipid-lowering therapy in the development of kidney disease in patients with type 2 DM remains unclear.19–21 Previous studies suggested that statins might have protective effects against diabetes-induced oxidative stress and podocyte injury in the kidney. 22,23 However, several population-based studies have shown that the use of statins does not reduce the risk of kidney disease24,25 and, as noted earlier, may even have adverse effects in patients with diabetes.26,27 Thus, it is uncertain whether the administration of statins represents an appropriate means of preventing DKD.
To address this knowledge gap, we performed a retrospective observational cohort study of patients with type 2 DM across China to determine the effect of statin initiation on the development of DKD and kidney function decline.
Methods
Study design and setting
This is a multicentre retrospective cohort study using a new-user design and de-identified data collected from the China Renal Data System (CRDS) database from Jan. 1, 2000, to May 26, 2021.28 At present, the government’s basic medical insurance provides coverage for more than 95% of the population in China, including access to care at academic health care centres.29
We reported the study according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.30
Data source
The CRDS database is a joint initiative of the National Clinical Research Center for Kidney Disease and the China Center for Disease Control and Prevention. This database contains data for more than 7 million inpatients and outpatients from 19 large, urban academic centres that cover the major geographic regions across China (Appendix 1, Supplementary Methods 1, available at www.cmaj.ca/lookup/doi/10.1503/cmaj.230093/tab-related-content). The accuracy and completeness of this database have been verified in our previous studies31,32 and by other validation activities (Appendix 1, Supplementary Methods 1).
Participants
We selected patients with type 2 DM admitted to hospital between Jan. 1, 2000, and May 26, 2021, and aged 40 years or older, for inclusion in the present study. The diagnosis of type 2 DM was based on the International Classification of Diseases, 10th Revision (ICD-10) code E11,33 and all hospital admissions for diabetes-related complications or general admissions with diabetes as a comorbidity were included.
We assigned the date of the first statin prescription as the index date for statin initiators. For noninitiators, we assigned the index date as a randomly selected date of any admission (i.e., not limited to hospital admissions for diabetes or related complications). All study participants (both initiators and noninitiators) had not received a statin prescription within the previous year before the index date.
We excluded patients for whom baseline serum creatinine or urinary protein concentrations were not available, and those with a diagnosis of chronic kidney disease (as defined by ICD-10 code N18) or an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2 or proteinuria (defined as a urinary albumin-to-creatinine ratio ≥ 30 mg/g) before the index date.34 We also excluded patients with identified or suspected acute kidney injury at the index date. We based the diagnosis of acute kidney injury on ICD-10 codes N10/N17/O90.4 or Kidney Disease Improving Global Outcomes creatinine criteria.35 We defined suspected acute kidney injury as a 50% or greater change in serum creatinine within 1 month.36
We defined a 1-year observational period before the index date and excluded those who did not have any records of prescriptions from an affiliated outpatient clinic or during a hospital admission during this period. We also excluded patients without follow-up at an affiliated outpatient clinic within 90 days after discharge or who were prescribed a statin and 1 or more other lipid-lowering drugs. The follow-up period started on the index date and continued until any outcome occurred, the participant was lost to follow-up, or the date of the final serum creatinine measurement or urinary protein test, whichever came first.
Exposure
The primary exposure was the initiation of statin treatment (Appendix 1, Supplementary Methods 2), as defined by a statin being prescribed. We extracted details of statin prescriptions — including dose, usage, and starting and ending times of statins during inpatient and outpatient periods — from the CRDS database and further validated them by accessing the relevant individual academic centre’s information system to extract the electronic medical records data. For comparison, we defined noninitiators as those who did not receive a statin prescription or a prescription for any nonstatin lipid-lowering drugs (e.g., fibrate, ezetimibe and nicotine acid).
Outcomes
The primary outcome was the development of DKD, defined as a composite of the occurrence of kidney dysfunction37 (defined as an eGFR < 60 mL/min/1.73 m2 and > 25% decline from baseline) and proteinuria (defined as a urinary albumin-to-creatinine ratio ≥ 30 mg/g and > 50% increase from baseline), sustained for at least 90 days. The secondary outcomes were individual indices indicating the development of DKD and the development of kidney function decline (defined as a sustained > 40% decline in eGFR).38 We calculated the eGFR using serum creatinine and the Chronic Kidney Disease Epidemiology Collaboration equation.39
Covariates
We collected demographic information (age, sex) and comorbidities determined using ICD-10 codes at baseline (defined as within a 3-month period before the index date). We calculated the age-adjusted Charlson Comorbidity Index to quantify the overall comorbidity status.40 We identified antihypertensive drugs and glucose-lowering drugs during the observational period (i.e., within 1 year before the index date). The relevant Anatomic Therapeutic Chemical codes are summarized in Appendix 1, Supplementary Table 1.
We extracted physical examination and laboratory test results, including blood pressure measurement, body mass index, serum lipid concentrations (total cholesterol [TC], low-density lipoprotein cholesterol [LDL-C], high-density lipoprotein cholesterol [HDL-C] and triglyceride [TG]), eGFR, glycosylated hemoglobin (HbA1c), serum uric acid, alanine aminotransaminase and aspartate aminotransaminase, hemoglobin and leukocytes. We included the most recent measurements of these parameters within 3 months before the index date. All covariates were considered clinically relevant based on biological mechanism or evidence from previously published data.10,41
Statistical analysis
We summarized the clinical parameters and baseline characteristics of the statin initiator and noninitiator groups and used standardized mean differences (SMDs) to evaluate the balance of the 2 groups.
We used propensity score overlap weighting to balance the characteristics of the statin initiators and noninitiators to mimic randomized clinical trials.42 We identified a total of 38 covariates and modelled their hypothetical causal pathways using a directed acyclic graph (Appendix 1, Supplementary Figure 1); we included these in the propensity score overlap weighting. Overlap weight for each participant was calculated by multivariable logistic regression and assigned to each participant proportionally to the probability of that patient belonging to the opposite treatment group. This method created balance between the exposure groups with regard to all the covariates included in the propensity score. After weighting, parameters with an SMD higher than 0.1 were regarded as unbalanced between the groups.43
We evaluated the relationships between statin initiation and kidney outcomes using Cox proportional hazards regression after weighting. Hazard ratios (HRs) and the corresponding 95% confidence intervals (CIs) were reported. We tested the proportional hazards assumption using Schoenfeld residuals.
We conducted a predefined subgroup analysis according to the presence or absence of dyslipidemia (defined as LDL-C ≥ 3.4 mmol/L or TC ≥ 5.2 mmol/L or TG ≥ 1.7 mmol/L) and the pattern of dyslipidemia (high TC or LDL-C, high TG, or both).44 We also determined the potential effect modification associated with statin initiation on the development of DKD using the same Cox model after weighting and after stratification according to age, sex, HbA1c, comorbidity, use of insulin, use of metformin, and treatment with antihypertensive drugs (renin–angiotensin system inhibitors, β-blockers, calcium channel blockers and diuretics).
Additional analyses
We performed additional analyses to compare the effects of statin initiation on kidney outcomes in patients with different levels of lipid control. We evaluated the first serum LDL-C concentration recorded for statin initiators between 90 and 365 days after the index date. Those whose serum LDL-C concentrations were less than 1.8 mmol/L, 1.8 to less than 3.4 mmol/L, and 3.4 mmol/L or higher were defined as belonging to the intensive lipid control, moderate lipid control and inadequate lipid control groups, respectively.44,45 Furthermore, we evaluated the relationships between the various types of statins with kidney outcomes. We also conducted a multivariable logistic regression to assess the association between statin initiation and the use of glucose-lowering drugs during follow-up, after weighting.
Sensitivity analyses
We performed a series of sensitivity analyses to evaluate the robustness of the findings. We developed a long-term follow-up cohort from our study population, which excluded those with loss of follow-up (i.e., not seen at an affiliated outpatient clinic) within the first 3 years. We fitted propensity score matching and inverse probability treatment weighting (stable weighting) models to evaluate the relationship between statin initiation and kidney outcomes, in place of overlap weighting (Appendix 1, Supplementary Methods 3). We used a time-varying Cox model with statin initiation treated as a time-varying exposure. To evaluate the bias associated with reverse causality, we excluded participants who developed a kidney outcome within the first year of the study.
Finally, to account for the bias introduced by unmeasured confounders, we calculated the E-value for the kidney outcomes. The E-value represents the minimum magnitude of association required for an unmeasured confounder to reverse the observed association toward a null. In brief, if the relative risk between unmeasured confounders, kidney outcomes and statin initiation is greater than the estimated E-value, residual confounders may be sufficient to explain the identified association.46
We managed missing data in all analyses using multiple imputation, with an assumption of missing at random. We performed all statistical analyses using SAS version 9.4 (SAS Institute, Cary, NY, USA) and R 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria) and with a significance level of 0.05 (2-sided).
Ethics approval
The study was approved by the China Office of Human Genetic Resources for Data Preservation Application (approval no.: 2021-BC0037). The protocol was approved by the Medical Ethics Committee of Nanfang Hospital, Southern Medical University (approval no.: NFEC-2019–213), and the requirement for informed consent was waived.
Results
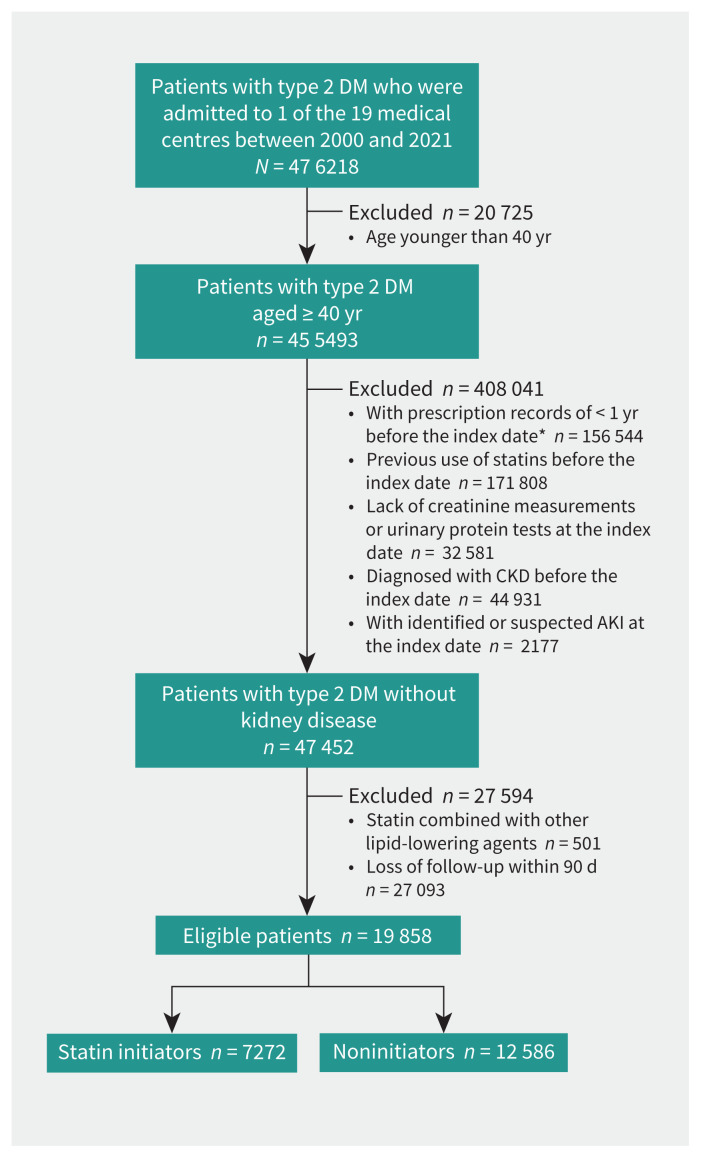
We selected a total of 19 858 participants from the original cohort of 455 493 patients with type 2 DM admitted to a participating hospital during the study period (Figure 1). The baseline characteristics and weighting of the groups are summarized in Table 1 and Appendix 1, Supplementary Table 2. Among 7272 statin initiators and 12 586 noninitiators, 11 012 (55.5%) were male and their median age was 62.2 years (interquartile range 54.5–69.4 yr). The mean duration of follow-up was 1.6 years.
Figure 1:
Flow chart of study population. *We assigned the date of the first statin prescription as the index date for statin initiators. For noninitiators, we assigned the index date as a randomly selected date of any admission. AKI = acute kidney injury, CKD = chronic kidney disease, DM = diabetes mellitus.
Table 1:
Baseline characteristics of study population*
| Characteristic | No. (%)† of total patients n = 19 858 |
Before weighting | After weighting | ||||
|---|---|---|---|---|---|---|---|
| No. (%)† of noninitiators n = 12 586 |
No. (%)† of statin initiators n = 7272 |
SMD‡ | Percentage† of noninitiators | Percentage† of statin initiators | SMD‡ | ||
| Age, yr, median (IQR) | 62.2 (54.5–69.4) | 60.7 (53.5–67.4) | 65.0 (57.1–72.7) | 0.40 | 62.7 (55.3–69.9) | 62.8 (54.7–70.5) | < 0.001 |
| Male | 11 012.0 (55) | 7140.0 (56) | 3872.0 (53) | 0.07 | 53.0 | 53.0 | < 0.001 |
| BMI, median (IQR) | 23.6 (21.5–26.0) | 23.4 (21.2–25.8) | 24.1 (22.0–26.4) | 0.22 | 23.8 (21.6–26.2) | 23.8 (21.7–26.1) | < 0.001 |
| Blood pressure, median (IQR), mm Hg | |||||||
| SBP | 131 (120–144) | 129 (118–141) | 135 (123–149) | 0.03 | 131 (120–145) | 132 (120–145) | < 0.001 |
| DBP | 79 (71–86) | 78 (70–85) | 80 (72–87) | 0.02 | 79 (71–86) | 80 (71–87) | < 0.001 |
| Baseline eGFR, median (IQR), mL/min/1.73 m2 § | 94.2 (83.7–103.1) | 96.6 (86.8–105.1) | 89.6 (79.2–98.8) | 0.46 | 93.5 (82.8–101.9) | 93.3 (82.9–101.9) | < 0.001 |
| Dyslipidemia | |||||||
| LDL-C ≥ 3.4 mmol/L | 4086.0 (20.6) | 1753.0 (13.9) | 2333.0 (32.1) | 0.44 | 24.9 | 24.9 | < 0.001 |
| TC ≥ 5.1 mmol/L | 5583.0 (28.1) | 2747.0 (21.8) | 2836.0 (39.0) | 0.38 | 33.4 | 33.4 | < 0.001 |
| TG ≥ 1.7 mmol/L | 4324.0 (21.8) | 2378.0 (18.9) | 1946.0 (26.8) | 0.19 | 24.7 | 24.7 | < 0.001 |
| Albumin, g/L, median (IQR) | 40.5 (37.6–43.5) | 40.2 (37.1–43.3) | 40.9 (38.2–43.7) | 0.21 | 40.8 (38.0–43.8) | 40.7 (38.0–43.6) | < 0.001 |
| HbA1c, %, median (IQR) | 7.0 (6.2–8.3) | 6.9 (6.2–8.1) | 7.0 (6.3–8.5) | 0.15 | 7.0 (6.3–8.5) | 7.0 (6.2–8.4) | < 0.001 |
| Glucose-lowering drugs | |||||||
| Insulin | 9159.0 (46.1) | 6410.0 (50.9) | 2749.0 (37.8) | 0.27 | 43.9 | 43.9 | < 0.001 |
| Metformin | 6851.0 (34.5) | 3604.0 (28.6) | 3247.0 (44.7) | 0.34 | 39.2 | 39.2 | < 0.001 |
| SGLT2 inhibitors | 82.0 (0.4) | 24.0 (0.2) | 58.0 (0.8) | 0.09 | 0.5 | 0.5 | < 0.001 |
| DPP4 inhibitors | 1345.0 (6.8) | 607.0 (4.8) | 738.0 (10.1) | 0.20 | 8.6 | 8.6 | < 0.001 |
| Sulfonylureas | 4358.0 (21.9) | 2476.0 (19.7) | 1882.0 (25.9) | 0.15 | 24.0 | 24.0 | < 0.001 |
| Antihypertensive drugs | |||||||
| RASi | 4872.0 (24.5) | 1827.0 (14.5) | 3045.0 (41.9) | 0.64 | 27.4 | 27.4 | < 0.001 |
| β-blockers | 3759.0 (18.9) | 1522.0 (12.1) | 2237.0 (30.8) | 0.47 | 19.2 | 19.2 | < 0.001 |
| CCB | 5939.0 (29.9) | 2908.0 (23.1) | 3031.0 (41.7) | 0.41 | 32.0 | 32.0 | < 0.001 |
| Diuretic | 3186.0 (16.0) | 2370.0 (18.8) | 816.0 (11.2) | 0.21 | 12.7 | 12.7 | < 0.001 |
| Comorbidities | |||||||
| Diabetic complications** | 4297.0 (21.6) | 2297.0 (18.3) | 2000.0 (27.5) | 0.22 | 24.9 | 24.9 | < 0.001 |
| Myocardial infarction | 218.0 (1.1) | 24.0 (0.2) | 194.0 (2.7) | 0.21 | 0.6 | 0.6 | < 0.001 |
| Arrhythmia | 1899.0 (9.6) | 770.0 (6.1) | 1129.0 (15.5) | 0.31 | 10.1 | 10.1 | < 0.001 |
| Hypertension | 9049.0 (45.6) | 4550.0 (36.2) | 4499.0 (61.9) | 0.53 | 48.6 | 48.6 | < 0.001 |
| Hyperuricemia | 2154.0 (10.8) | 948.0 (7.5) | 1206.0 (16.6) | 0.28 | 12.8 | 12.8 | < 0.001 |
| Cerebral bleeding | 236.0 (1.2) | 103.0 (0.8) | 133.0 (1.8) | 0.09 | 1.6 | 1.6 | < 0.001 |
| Cerebral infraction | 2492.0 (12.5) | 548.0 (4.4) | 1944.0 (26.7) | 0.65 | 12.3 | 12.3 | < 0.001 |
| CCI, median (IQR)¶ | 6 (5–7) | 6 (5–7) | 6 (4–7) | 0.23 | 6 (4–7) | 5 (4–7) | < 0.001 |
Note: BMI = body mass index, CCB = calcium channel blocker, CCI = Charlson Comorbidity Index, DBP = diastolic blood pressure, DPP4 = dipeptidyl peptidase-4, eGFR = estimated glomerular filtration rate, HbA1c = glycosylated hemoglobin, IQR = interquartile range, LDL-C = low-density lipoprotein cholesterol, RASi = renin–angiotensin system inhibitor, SBP = systolic blood pressure, SGLT2 = sodium–glucose co-transporter 2, SMD = standardized mean difference, TC = total cholesterol, TG = triglyceride.
We defined the time from within 3 months before the index date as the baseline period. We assigned the date of the first statin prescription as the index date for statin initiators. For noninitiators, we assigned the index date as a randomly selected date of any admission.
Unless stated otherwise.
Covariates with SMD > 0.1 were regarded as unbalanced between the groups.43
We calculated eGFR using serum creatinine and the Chronic Kidney Disease Epidemiology Collaboration equation.39
We calculated the CCI to quantify the overall comorbidity status.40
Diabetic complications including diabetic retinopathy, diabetic peripheral neuropathy, diabetic ketosis, diabetic foot and other diabetic angiopathies.
Association of statin initiation with risk of kidney outcomes
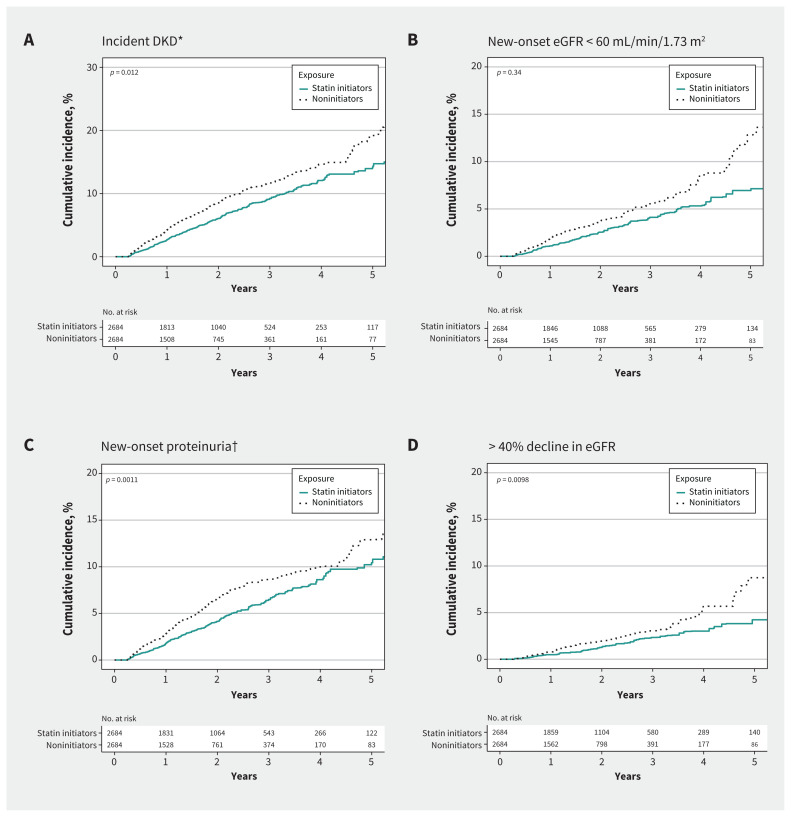
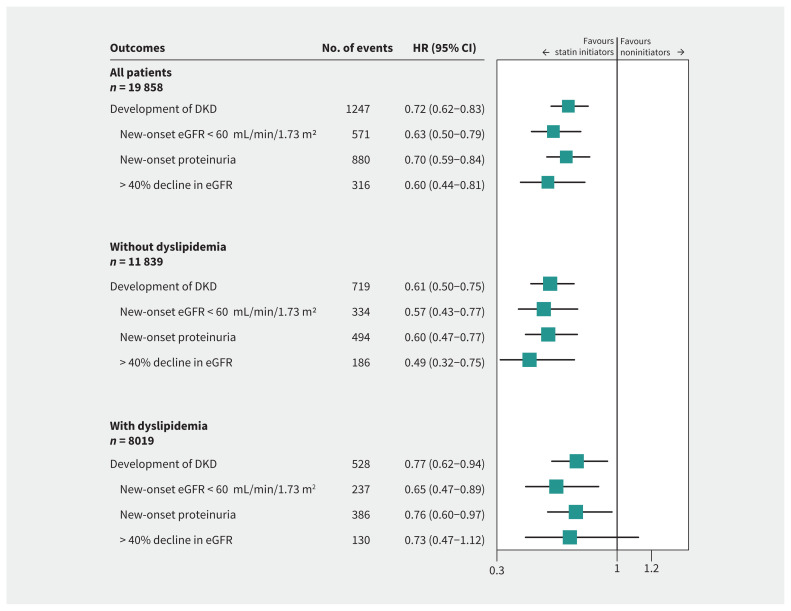
The weighted cumulative incidences of kidney outcomes among statin initiators and noninitiators are shown in Figure 2 and Appendix 1, Supplementary Table 3. Statin initiation was significantly associated with lower cumulative incidences of incident DKD, new-onset proteinuria and more than 40% decline in eGFR (p < 0.05 for all), but not with new-onset eGFR lower than 60 mL/min/1.73 m2 (p = 0.34). After weighting, the risk of incident DKD was significantly lower for statin initiators than for noninitiators (HR 0.72, 95% CI 0.62–0.83) (Figure 3).
Figure 2:
Statin initiation and weighted cumulative incidence of kidney outcomes in patients with type 2 diabetes mellitus. *We defined incident diabetic kidney disease (DKD) as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 and > 25% decline from baseline and new-onset proteinuria. 37 †We defined new-onset proteinuria as a urinary albumin-to-creatinine ratio ≥ 30 mg/g and > 50% increase from baseline).
Figure 3:
Adjusted hazard ratio (HR) of statin initiation associated with kidney outcomes among all patients and patients with or without dyslipidemia. We defined dyslipidemia as low-density lipoprotein cholesterol (LDL-C) ≥ 3.4 mmol/L or total cholesterol (TC) ≥ 5.2 mmol/L or triglyceride (TG) ≥ 1.7 mmol/L.44 We estimated HRs by Cox proportional model after propensity score overlap weighting with confounders adjusted for all characteristics in Table 1. Schoenfeld residuals validated the proportional hazards assumption (p = 0.35). Note: CI = confidence interval, DKD = diabetic kidney disease, eGFR = estimated glomerular filtration rate.
Using Cox proportional hazards regression after weighting, statin initiation was found to be associated with lower risks of new-onset eGFR lower than 60 mL/min/1.73 m2 (HR 0.63, 95% CI 0.50–0.79), new-onset proteinuria (HR 0.70, 95% CI 0.59–0.84) and kidney function decline (HR 0.60, 95% CI 0.44–0.81) (Figure 3).
In subgroup analyses, we also obtained consistent findings for participants with or without dyslipidemia and with different patterns of dyslipidemia (high TG, high TC or LDL-C, or both) (Figure 3 and Appendix 1, Supplementary Figure 2). We did not find any significant effect modifiers among the other clinical characteristics (all pinteraction > 0.05) (Appendix 1, Supplementary Figure 3).
Additional analyses
We performed additional analyses to compare the effects of statin initiation on kidney outcomes in those with different levels of LDL-C control (Appendix 1, Supplementary Figure 4). By comparison with the inadequate lipid control group (LDL-C ≥ 3.4 mmol/L), patients with intensive control (LDL-C < 1.8 mmol/L) had the lowest risk of developing DKD (HR 0.51, 95% CI 0.32–0.81). Similar results were obtained for the risk of new-onset eGFR lower than 60 mL/min/1.73 m2 and proteinuria. Among those with moderate control (LDL-C 1.8 to < 3.4 mmol/L), there was no significant association with the development of DKD when compared with the inadequate lipid control group (HR 0.84, 95% CI 0.61–1.11).
Atorvastatin (n = 4424 [60.8%]) and rosuvastatin (n = 1135 [15.6%]) were the most frequently used statins (Appendix 1, Supplementary Table 4). The hazard ratios for DKD ranged from 0.65 to 0.88 and from 0.46 to 0.75 for patients taking lipophilic (simvastatin, pitavastatin, fluvastatin and atorvastatin) and hydrophilic (pravastatin and rosuvastatin) statins, respectively.
With respect to glucose metabolism and glycemic control (Appendix 1, Supplementary Table 5), there was a significant positive association between statin initiation and an increase in the use of oral glucose-lowering drugs (OR 1.75, 95% CI 1.50–2.05).
Sensitivity analyses
About 1 in 5 study participants (4107/19 858, 20.7%) were followed up for longer than 3 years, with a mean follow-up of 3.5 years. The associations of statin initiation with kidney outcomes remained in these patients (Appendix 1, Supplementary Table 6). We also found similar results for the associations of statin initiation with the risks of kidney outcomes when using the stable weighting, propensity score matching and time-varying Cox models (Appendix 1, Supplementary Table 7 and 8).
With respect to the reverse causality (Appendix 1, Supplementary Table 9), we found consistent results after excluding participants who developed DKD within 1 year. The E-value for kidney outcomes ranged from 2.1 to 2.7 in the primary analyses.
Interpretation
In this multicentre cohort study of patients with type 2 DM admitted to and followed up in an academic centre in China, we found that statin initiation was associated with significantly lower risks of developing DKD and kidney function decline. These associations were robust, being unaffected by differences in clinical characteristics or the pattern of dyslipidemia. The similar results in multiple sensitivity analyses that evaluated reverse causality and unmeasured confounders also suggest that the findings of the present study are robust.
Several animal and epidemiologic studies have shown that dyslipidemia plays a role in the development and progression of DKD.47,48 Because dyslipidemia is both a risk factor and potential target for the treatment of DKD, further research into the clinical benefits of lipid-lowering drugs is required. However, given that the cardiovascular benefits of statins in patients with diabetes have been well established,2,49 it is difficult to conduct a randomized controlled trial (RCT) to compare the kidney outcomes of patients undergoing statin therapy with placebo-treated controls. Therefore, the use of medical data obtained from real-world clinical practice represents a rational means of studying the effect of statins to prevent kidney disease in patients with diabetes. Our findings provide evidence that statins may be reno-protective in patients with type 2 DM in a real-world setting and may help physicians to optimize disease management.
The results of previous studies assessing the reno-protective effect of statins in patients with diabetes have been contradictory. Two large population-based studies (n = 43 438 and 62 716)24,27 showed that statin use did not have beneficial effects on kidney outcomes and may possibly have had adverse effects. However, the kidney outcomes used in these studies were based on ICD codes, which lack sensitivity, and therefore the incidence of the outcomes may have been underestimated. Also, given the lack of inclusion of laboratory data, such as eGFR, proteinuria (urinary albumin-to-creatinine ratio), HbA1c and cholesterol, the baseline characteristics of the participants who were or were not taking a statin could not be well matched, and this represents a major limitation of these studies.
Consistent with our findings, several previous studies have shown that statins may be reno-protective in patients with diabetes. 50–52 However, these studies were limited by small sample sizes, inconsistent effects on proteinuria and renal function, or the use of an ICD-based method of diagnosis of DKD. More importantly, one of the basic principles of examining effectiveness of medications in cohort studies is excluding prevalent users.53 The exposure in these observational studies including prevalent users of a statin raises concern for bias toward better outcomes.
The strengths of the present study include its real world–based data source, new-user design, large sample size, inclusion of individuals with a wide range of disease phenotypes, and use of hard kidney outcomes. The comprehensive patient-level data with time stamps ensure that thorough weighting of the groups was possible. In addition, we adjusted for important potential confounders, such as comorbidities, concomitant drug administration, the type of statin used and the level of lipid control. Additional strengths include the use of sophisticated statistical methods to reduce the risk of confounding and indication bias. Given the E-values for kidney outcomes in the primary analyses (2.1–2.7), the robustness of the study results do not appear to be substantially affected by the presence of unassessed confounders.46
In our study, we found that various specific statins may have variable effects on kidney outcomes in patients with type 2 DM. A previous RCT that compared the renal effects of atorvastatin and rosuvastatin in patients with diabetes who had proteinuria showed that atorvastatin might be more reno-protective.54 Recently, a real-world study also found that rosuvastatin was associated with increased risk of proteinuria compared with atorvastatin. 55 However, these studies did not include a placebo control; therefore, it could not be determined whether atorvastatin protected the kidney or rosuvastatin harmed the kidney. In the present study, atorvastatin appeared to be most reno-protective, across all kidney outcomes.
Whether the potential reno-protective effect of statins is independent of their lipid-lowering effects remains unclear.56,57 In animal models of diabetes, statin therapy has been shown to cause increases in antioxidant enzyme levels, reduce the accumulation of advanced glycation end products, and reverse diabetes-related podocyte injury, which may prevent or slow the development of kidney disease, independent of the effects of reducing lipid concentrations.58–60 In a post hoc analysis of data from randomized trials,61 the effect of statin treatment on proteinuria was shown to be inconsistent with the degree of control of hypercholesterolemia in individual patients. However, in the present study, intensive control of LDL-C tended to be associated with a better kidney outcome, implying a reno-protective effect driven at least partly by the lipid-lowering effects. Considering the relatively few participants taking other lipid-lowering drugs, which limited the feasibility and statistical power of the comparison between statin and other lipid-lowering drugs, we could not preclude the potential reno-protective effect of statins independent of their lipid-lowering effect.
Although the management of type 2 DM has improved substantially in recent decades, patients with diabetes in China are still at substantial risk of kidney disease and progressive loss of renal function. Our study found that only 36.6% of people with type 2 DM who were age 40 years or older were prescribed statins during the study period, which is lower than that in Canada (54.0% for males and 45.3% for females)62 and the United States (41.6%).63 The current national guideline in China recommends statin therapy for patients with diabetes who are aged 40 years or older.64 The suboptimal accordance with this recommendation might contribute to the higher risk of kidney disease progression we observed in our study population. Our findings suggest that there is an urgent need to promote guideline-concordant care in real-world clinical practice in China.65
Limitations
Although we performed propensity score overlap weighting to balance the baseline characteristics of the statin initiators and noninitiators, other uncontrolled factors could have affected the kidney outcomes. We performed multiple sensitivity analyses to adjust for these residual confounders.
Statin initiation may have represented a marker of atherosclerosis, high health awareness or high frequency of hospital visits, all of which could have resulted in ascertainment bias and influenced the results. However, the number of outpatient visits by the weighted cohort during the follow-up period was similar for statin initiators and noninitiators. We selected our study population from patients with type 2 DM who were admitted to 1 of the 19 urban academic centres in the CRDS and who received follow-up at 1 of the affiliated clinics. These patients might have been sicker and had poorer glycemic control and higher risk of diabetic complications than those who were not admitted to hospital and those without follow-up. As such, caution is needed when generalizing our results to all patients with type 2 DM.
Finally, patients whose data are included in the CRDS are predominantly Chinese; therefore, whether there are ethnic differences in the potential reno-protective effect of statins warrants further research. Additionally, we found that only one-third of inpatients used metformin and statins in our study, reflecting poor adherence to guidelines in China.65 Our findings need to be validated in other countries in which a higher proportion of patients with type 2 DM are receiving guideline-concordant care.
Conclusion
We found that statin initiation was associated with significantly lower risks of incident DKD and kidney function decline among patients with type 2 DM admitted to and followed up in academic centres. We obtained similar results for participants with differing patterns of dyslipidemia, those prescribed different statins, and after stratification according to participant characteristics. Among statin initiators, these associations were more pronounced in those with intensive LDL-C control (< 1.8 mmol/L).
These findings suggest that statin initiation may be an effective approach for preventing kidney disease in patients with type 2 DM. Further research is needed to compare the reno-protective effects of specific statins and newer lipid-lowering drugs, such as proprotein convertase subtilisin/kexin type 9 inhibitors, on kidney outcomes.
Supplementary Material
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Xin Xu and Sheng Nie conceptualized and designed the study. Sheng Nie and Shiyu Zhou drafted the manuscript. Licong Su, Ruqi Xu, Yanqin Li and Ruixuan Chen contributed to data collection. Shiyu Zhou, Shengli An, Peiyan Gao and Ye Cao contributed to the statistical analysis. Xiaodong Zhang, Fan luo, Qi Gao, Shengli An, Wenyi Cai, Lilong Lin, Hong Xu, Bicheng Liu, Jianping Weng, Chunbo Chen, Huafeng Liu, Qiongqiong Yang, Hua Li, Yaozhong Kong, Guisen Li, Qijun Wan, Yan Zha, Ying Hu, Gang Xu, Yongjun Shi, Yilun Zhou, Guobin Su, Ying Tang and Mengchun Gong contributed to the data acquisition. All of the authors revised the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work. Shiyu Zhou, Licong Su and Ruqi Xu contributed equally to this work.
Funding: This study is supported by grants from the National Key R&D Program of China (2021YFC2500200 and 2021YFC2500204 to Xin Xu) and the National Natural Science Foundation of China (81970586 to Xin Xu and 81900626 to Sheng Nie). This work was also supported by the Outstanding Youths Development Scheme of Nanfang Hospital, Southern Medical University (2020J005 to Sheng Nie).
Data sharing: Please contact Sheng Nie at niesheng0202@126.com or Xu Xin at xux007@163.com for access to the de-identified individual-level data.
References
- 1.Blais JE, Wei Y, Yap KKW, et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis 2021;328:44–51. [DOI] [PubMed] [Google Scholar]
- 2.Collins R, Armitage J, Parish S, et al. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–16. [DOI] [PubMed] [Google Scholar]
- 3.Adhyaru BB, Jacobson TA. Safety and efficacy of statin therapy. Nat Rev Cardiol 2018;15:757–69. [DOI] [PubMed] [Google Scholar]
- 4.O’Malley PG, Arnold MJ, Kelley C, et al. Management of dyslipidemia for cardiovascular disease risk reduction: synopsis of the 2020 updated U.S. Department of Veterans Affairs and U.S. Department of Defense clinical practice guideline. Ann Intern Med 2020;173:822–9. [DOI] [PubMed] [Google Scholar]
- 5.Krane V, Schmidt KR, Gutjahr-Lengsfeld LJ, et al. Long-term effects following 4 years of randomized treatment with atorvastatin in patients with type 2 diabetes mellitus on hemodialysis. Kidney Int 2016;89:1380–7. [DOI] [PubMed] [Google Scholar]
- 6.American Diabetes Association Professional Practice Committee. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45(Suppl 1):S144–S174. [DOI] [PubMed] [Google Scholar]
- 7.Greenland P, Bonow RO. Interpretation and use of another statin guideline. JAMA 2016;316:1977–9. [DOI] [PubMed] [Google Scholar]
- 8.Mangione CM, Barry MJ, Nicholson WK, et al. Statin use for the primary prevention of cardiovascular disease in adults: US Preventive Services Task Force recommendation statement. JAMA 2022;328:746–53. [DOI] [PubMed] [Google Scholar]
- 9.Pearson GJ, Thanassoulis G, Anderson TJ, et al. 2021 Canadian Cardiovascular Society guidelines for the management of dyslipidemia for the prevention of cardiovascular disease in adults. Can J Cardiol 2021;37:1129–50. [DOI] [PubMed] [Google Scholar]
- 10.Mansi IA, Chansard M, Lingvay I, et al. Association of statin therapy initiation with diabetes progression: a retrospective matched-cohort study. JAMA Intern Med 2021;181:1562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erlandson KM, Jiang Y, Debanne SM, et al. Rosuvastatin worsens insulin resistance in HIV-infected adults on antiretroviral therapy. Clin Infect Dis 2015;61:1566–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liew SM, Lee PY, Hanafi NS, et al. Statins use is associated with poorer glycaemic control in a cohort of hypertensive patients with diabetes and without diabetes. Diabetol Metab Syndr 2014;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holman RR, Paul S, Farmer A, et al. Atorvastatin in Factorial with Omega-3 EE90 Risk Reduction in Diabetes (AFORRD): a randomised controlled trial. Diabetologia 2009;52:50–9. [DOI] [PubMed] [Google Scholar]
- 14.Koh KK, Quon MJ, Han SH, et al. Atorvastatin causes insulin resistance and increases ambient glycemia in hypercholesterolemic patients. J Am Coll Cardiol 2010;55:1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbasi F, Reaven GM. Statin-induced diabetes: how important is insulin resistance? J Intern Med 2015;277:498–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas MC, Brownlee M, Susztak K, et al. Diabetic kidney disease. Nat Rev Dis Primers 2015;1:15018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zagkotsis G, Markou M, Paschou E, et al. Preventing the development and progression of diabetic kidney disease: Where do we stand? Diabetes Metab Syndr 2018;12:585–90. [DOI] [PubMed] [Google Scholar]
- 18.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012;55:1577–96. [DOI] [PubMed] [Google Scholar]
- 19.Chen SC, Tseng CH. Dyslipidemia, kidney disease, and cardiovascular disease in diabetic patients. Rev Diabet Stud 2013;10:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelsey MD, Nelson AJ, Green JB, et al. Guidelines for cardiovascular risk reduction in patients with type 2 diabetes: JACC guideline comparison. J Am Coll Cardiol 2022;79:1849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai T, Abel L, Langford O, et al. Associations between statins and adverse events in primary prevention of cardiovascular disease: systematic review with pairwise, network, and dose-response meta-analyses. BMJ 2021;374:n1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aktay G, Gürsoy S, Uyumlu U, et al. Protective effect of atorvastatin on oxidative stress in streptozotocin-induced diabetic rats independently their lipid-lowering effects. J Biochem Mol Toxicol 2019;33:e22295. [DOI] [PubMed] [Google Scholar]
- 23.Vlad A, Vlad M, Petrica L, et al. Therapy with atorvastatin versus rosuvastatin reduces urinary podocytes, podocyte-associated molecules, and proximal tubule dysfunction biomarkers in patients with type 2 diabetes mellitus: a pilot study. Ren Fail 2017;39:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol 2014;2:894–900. [DOI] [PubMed] [Google Scholar]
- 25.Bangalore S, Fayyad R, Hovingh GK, et al. Statin and the risk of renal-related serious adverse events: analysis from the IDEAL, TNT, CARDS, ASPEN, SPARCL, and other placebo-controlled trials. Am J Cardiol 2014;113:2018–20. [DOI] [PubMed] [Google Scholar]
- 26.Hanai K, Babazono T, Uchigata Y. Effects of statins on the kidneys in patients with type 2 diabetes. Clin Exp Nephrol 2017;21:633–42. [DOI] [PubMed] [Google Scholar]
- 27.Acharya T, Huang J, Tringali S, et al. Statin use and the risk of kidney disease with long-term follow-up (8.4-year study). Am J Cardiol 2016;117:647–55. [DOI] [PubMed] [Google Scholar]
- 28.The CRDS investigators. China Renal Data System. China; 2022. Available: http://www.crds-network.org.cn/#/database (accessed 2023 Apr. 24).
- 29.Statistical bulletin on the development of medical security in 2020. China: National Healthcare Security Administration. Available: http://www.nhsa.gov.cn/art/2023/3/9/art_7_10250.html (accessed 2023 Apr. 24). [Google Scholar]
- 30.von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X, Nie S, Xu H, et al. Detecting Neonatal. Acute Kidney Injury by Serum Cystatin C.J Am Soc Nephrol 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su G, Xiao C, Cao Y, et al. Piperacillin/tazobactam and risk of acute kidney injury in adults hospitalized with infection without vancomycin: a multi-centre real-world data analysis. Int J Antimicrob Agents 2023;61:106691. [DOI] [PubMed] [Google Scholar]
- 33.International Statistical Classification of Diseases and Related Health Problems, 10th rev. (Vol 2: instruction manual). Geneva: World Health Organization; 2010. [Google Scholar]
- 34.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2022 Clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int 2022;102(5S):S1–S127. [DOI] [PubMed] [Google Scholar]
- 35.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int 2012;2:1–138. [Google Scholar]
- 36.Diamantidis CJ, Zepel L, Smith VA, et al. Epidemiology of community-acquired acute kidney injury among US veterans. Am J Kidney Dis 2023. Mar 23; S0272-6386(23)00580-2. doi: 10.1053/j.ajkd.2023.01.448. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin A, Stevens PE, Bilous RW, et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD work group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013;3:1–150. [Google Scholar]
- 38.Levin A, Agarwal R, Herrington WG, et al. International consensus definitions of clinical trial outcomes for kidney failure: 2020. Kidney Int 2020;98:849–59. [DOI] [PubMed] [Google Scholar]
- 39.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlson M, Szatrowski TP, Peterson J, et al. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen SF, Nordestgaard BG. Statin use before diabetes diagnosis and risk of microvascular disease: a nationwide nested matched study. Lancet Diabetes Endocrinol 2014;2:894–900. [DOI] [PubMed] [Google Scholar]
- 42.Thomas LE, Li F, Pencina MJ. Overlap weighting: a propensity score method that mimics attributes of a randomized clinical trial. JAMA 2020;323:2417–8. [DOI] [PubMed] [Google Scholar]
- 43.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu JR, Gao RL, Zhao SP, et al. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol 2018;15:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. JAMA 2019;321:602–3. [DOI] [PubMed] [Google Scholar]
- 47.Russo GT, De Cosmo S, Viazzi F, et al. Plasma triglycerides and HDL-C levels predict the development of diabetic kidney disease in subjects with type 2 diabetes: The AMD Annals Initiative. Diabetes Care 2016;39:2278–87. [DOI] [PubMed] [Google Scholar]
- 48.Macisaac RJ, Ekinci EI, Jerums G. Markers of and risk factors for the development and progression of diabetic kidney disease. Am J Kidney Dis 2014;63(Suppl 2):S39–62. [DOI] [PubMed] [Google Scholar]
- 49.Reston JT, Buelt A, Donahue MP, et al. Interventions to improve statin tolerance and adherence in patients at risk for cardiovascular disease: a systematic review for the 2020 U.S. Department of Veterans Affairs and U.S. Department of Defense Guidelines for Management of Dyslipidemia. Ann Intern Med 2020;173:806–12. [DOI] [PubMed] [Google Scholar]
- 50.Gaede P, Lund-Andersen H, Parving HH, et al. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008;358:580–91. [DOI] [PubMed] [Google Scholar]
- 51.Luk AO, Yang X, Ma RC, et al. Association of statin use and development of renal dysfunction in type 2 diabetes — the Hong Kong Diabetes Registry. Diabetes Res Clin Pract 2010;88:227–33. [DOI] [PubMed] [Google Scholar]
- 52.Colhoun HM, Betteridge DJ, Durrington PN, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). Am J Kidney Dis 2009;54:810–9. [DOI] [PubMed] [Google Scholar]
- 53.Danaei G, Tavakkoli M, Hernán MA. Bias in observational studies of prevalent users: lessons for comparative effectiveness research from a meta-analysis of statins. Am J Epidemiol 2012;175:250–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Zeeuw D, Anzalone DA, Cain VA, et al. Renal effects of atorvastatin and rosuvastatin in patients with diabetes who have progressive renal disease (PLANET I): a randomised clinical trial. Lancet Diabetes Endocrinol 2015;3:181–90. [DOI] [PubMed] [Google Scholar]
- 55.Shin JI, Fine DM, Sang Y, et al. Association of rosuvastatin use with risk of hematuria and proteinuria. J Am Soc Nephrol 2022;33:1767–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He Y, Li X, Gasevic D, et al. Statins and multiple noncardiovascular outcomes: umbrella review of meta-analyses of observational studies and randomized controlled trials. Ann Intern Med 2018;169:543–53. [DOI] [PubMed] [Google Scholar]
- 57.Agarwal R. Effects of statins on renal function. Mayo Clin Proc 2007;82:1381–90. [DOI] [PubMed] [Google Scholar]
- 58.Lu L, Peng WH, Wang W, et al. Effects of atorvastatin on progression of diabetic nephropathy and local RAGE and soluble RAGE expressions in rats. J Zhejiang Univ Sci B 2011;12:652–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei P, Grimm PR, Settles DC, et al. Simvastatin reverses podocyte injury but not mesangial expansion in early-stage type 2 diabetes mellitus. Ren Fail 2009;31:503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu B, Shen H, Zhou J, et al. Effects of simvastatin on oxidative stress in streptozotocin-induced diabetic rats: a role for glomeruli protection. Nephron, Exp Nephrol 2005;101:e1–8. [DOI] [PubMed] [Google Scholar]
- 61.Idzerda NMA, Pena MJ, Parving HH, et al. Proteinuria and cholesterol reduction are independently associated with less renal function decline in statin-treated patients; a post hoc analysis of the PLANET trials. Nephrol Dial Transplant 2019;34:1699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nandiwada S, Manca DP, Yeung RO, et al. Achievement of treatment targets among patients with type 2 diabetes in 2015 and 2020 in Canadian primary care. CMAJ 2023;195:E1–E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chobufo MD, Regner SR, Zeb I, et al. Burden and predictors of statin use in primary and secondary prevention of atherosclerotic vascular disease in the US: from the National Health and Nutrition Examination Survey 2017–2020. Eur J Prev Cardiol 2022;29:1830–8. [DOI] [PubMed] [Google Scholar]
- 64.Chinese Diabetes Society; National Office for Primary Diabetes Care. National handbook for the prevention and control of diabetes in primary care (2022) [article in Chinese]. Zhonghua nei ke za zhi 2022;61:717–48. [DOI] [PubMed] [Google Scholar]
- 65.Wang L, Peng W, Zhao Z, et al. Prevalence and treatment of diabetes in China, 2013–2018. JAMA 2021;326:2498–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.