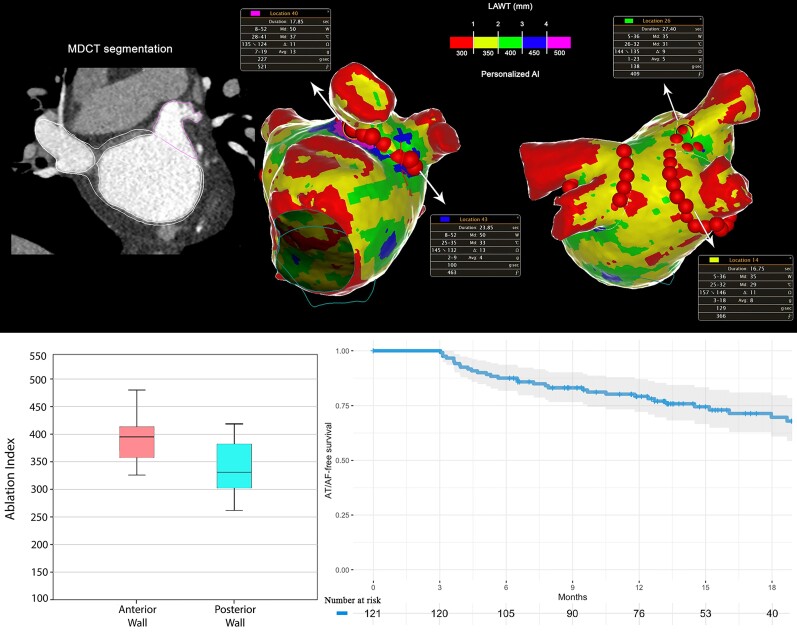
Abstract
Aims
Pulmonary vein (PV) antrum isolation proved to be effective for treating persistent atrial fibrillation (PeAF). We sought to investigate the results of a personalized approach aimed at adapting the ablation index (AI) to the local left atrial wall thickness (LAWT) in a cohort of consecutive patients with PeAF.
Methods and results
Consecutive patients referred for PeAF first ablation were prospectively enrolled. The LAWT three-dimensional maps were obtained from pre-procedure multidetector computed tomography and integrated into the navigation system. Ablation index was titrated according to the local LAWT, and the ablation line was personalized to avoid the thickest regions while encircling the PV antrum. A total of 121 patients (69.4% male, age 64.5 ± 9.5 years) were included. Procedure time was 57 min (IQR 50–67), fluoroscopy time was 43 s (IQR 20–71), and radiofrequency (RF) time was 16.5 min (IQR 14.3–18.4). The median AI tailored to the local LAWT was 387 (IQR 360–410) for the anterior wall and 335 (IQR 300–375) for the posterior wall. First-pass PV antrum isolation was obtained in 103 (85%) of the right PVs and 103 (85%) of the left PVs. Median LAWT values were higher for PVs without first-pass isolation as compared to the whole cohort (P = 0.02 for left PVs and P = 0.03 for right PVs). Recurrence-free survival was 79% at 12 month follow-up.
Conclusion
In this prospective study, LAWT-guided PV antrum isolation for PeAF was effective and efficient, requiring low procedure, fluoroscopy, and RF time. A randomized trial comparing the LAWT-guided ablation with the standard of practice is in progress (ClinicalTrials.gov, NCT05396534).
Keywords: Persistent atrial fibrillation, Left atrial wall thickness, Catheter ablation, Pulmonary vein isolation, Multidetector cardiac tomography
Graphical Abstract
Graphical Abstract.
What’s new?
The present study is the first reporting the feasibility of a pulmonary vein antrum isolation-only approach using a local left atrial wall thickness-guided titration of ablation index for persistent atrial fibrillation ablation.
This approach permits to avoid the thickest left atrial regions and to titrate radiofrequency delivery by increasing ablation index in the thicker segments and avoiding unnecessary high amounts of radiofrequency delivery in the thinnest ones, lowering procedural requirements in terms of radiofrequency delivery, fluoroscopy, and procedure time, while maintaining a recurrence-free survival comparable to that reported in literature.
Absence of first-pass during first procedure and reconnection sites found at redo procedure were mostly located in the thickest antral regions.
Introduction
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia in adults1 and is associated with significant morbidity and overall mortality.2–5 Radiofrequency (RF) catheter ablation is an established and widely adopted strategy for rhythm control.6–8 Pulmonary vein isolation (PVI) is the cornerstone of catheter-based strategies for AF ablation9 and has proven to be effective in treating persistent atrial fibrillation (PeAF), although long-term outcomes have been significantly less favourable than in paroxysmal AF. In a recent meta-analysis,10 PVI for PeAF ablation achieved an arrhythmia-free survival rate of 66.7% at 12 months. Ablation strategies combining PVI with additional substrate ablation, such as either linear ablation, complex fractionated atrial electrogram ablation, or MRI-guided fibrosis ablation, failed to demonstrate better outcomes than PVI-only approach.11–13 Pulmonary vein (PV) reconnection after AF ablation is the main cause of AF recurrence and results from non-contiguous or non-transmural RF lesions. The latter are often the consequence of insufficient RF delivery in the thickest myocardial areas.14 On the other hand, excessive RF delivery in thin regions of the left atrial (LA) wall is associated to increased complication rate.15
Advances in technology and innovative ablation protocols resulted in a remarkable improvement in the outcomes after PVI. Indeed, contact force-sensing catheters, novel indices for the estimation of lesion size, and ensuring ≤ 6 mm inter-lesion distance have markedly improved the arrhythmia-free survival after PVI.16,17
Recently, a personalized PVI approach using ablation index (AI) titration according to the local left atrial wall thickness (LAWT) as assessed with multidetector cardiac tomography (MDCT)-data postprocessing18 demonstrated to achieve a high arrhythmia-free survival rate at 12 months in paroxysmal AF. This approach reported high efficiency, while maintaining good outcomes at long-term follow-up.
The present study sought to investigate the feasibility, safety, and efficacy of a personalized PVI approach based on LAWT-guided titration of the AI in a cohort of consecutive patients with PeAF.
Methods
Patient sample
We conducted a single-centre, experimental, and prospective study. Consecutive patients who underwent first PeAF ablation were prospectively enrolled between April 2019 and June 2021. All patients had documented symptomatic PeAF, non-response or intolerance to ≥1 antiarrhythmic drug (AAD) (Class I or III), and indication for first ablation in accordance with the ESC guidelines.19 The PeAF was defined in the presence of at least one AF episode that was continuously sustained beyond 7 days, including episodes terminated by cardioversion after > 7 days; long-standing PeAF was defined as continuous AF of >12 months duration when a rhythm control strategy was adopted. In all patients, a MDCT study was obtained prior to the ablation procedure, and post-processing aimed for the reconstruction of MDCT-derived maps with LAWT information was performed, as previously described.18 Exclusion criteria were age <18, impossibility to perform a MDCT and any clinical condition contraindicating general anaesthesia or high-frequency low-tidal volume (HFLTV) ventilation.
Written informed consent was obtained from all patients. The study complied with the Declaration of Helsinki and was approved by the Institutional Ethics Committee.
Pre-procedural cardiac multidetector computed tomography and image post-processing
A pre-procedural cardiac MDCT was obtained with a RevolutionTM CT scanner (General Electric Healthcare). The images were acquired during an inspiratory breath-hold using retrospective ECG-gating technique with tube current modulation set between 50% and 100% of the cardiac cycle. The MDCT images were analysed with ADAS 3D™ software (ADAS3D Medical, Barcelona, Spain) to obtain 3D LAWT maps. To compute the LAWT, a three-step algorithm was applied: the endocardial layer was delineated by means of a semi-automatic segmentation based on pixel signal intensity thresholds; the epicardial layer was defined manually; finally, LAWT was automatically computed at each point as the distance between each endocardial point and its projection to the epicardial shell. The resulting 3D LAWT map was then imported into the CARTO navigation system (Biosense Webster Inc., Irvine, CA, USA). The LAWT maps were colour-coded as follows: red < 1 mm, 1 mm ≤ yellow < 2 mm, 2 mm ≤ green < 3 mm, 3 mm ≤ blue < 4 mm, and purple ≥ 4 mm.18 Data on the reproducibility of the segmentation method have been reported previously.20 The reproducibility agreement of LAWT maps derived by the described semi-automatic threshold-based segmentation was also recently analysed.21 Results of the analysis reported a colour agreement between LAWT maps of 95.5% and 92.9% intra- and inter-observer, respectively; the titration of the AI showed an average difference lower than 25 units in all cases. For all analyses, the concordance increased with user experience. These results suggest that LAWT measurements are reproducible; moreover, variability has a negligible impact in the target AI. The distance between the epicardial LA posterior wall and the oesophagus was also computed, and an oesophageal isodistance colour-coded map was created and imported into CARTO.22 This information was used to personalize the ablation line and avoid, as far as possible, RF application on the posterior atrial wall region closest to the oesophagus.
Left atrial wall thickness-guided pulmonary vein isolation
All procedures were performed using CARTO3 mapping system (Biosense Webster, Johnson & Johnson Medical S.p.A., CA, USA). All procedures were performed under general anaesthesia and utilizing HFLTV ventilation protocol.23,24 Transseptal puncture was guided by transoesophageal echocardiography (TOE), performed by cardiologists experienced in cardiac imaging.25 All procedures were performed with single catheter technique,26 using a contact force-sensing irrigated ablation catheter (Thermocool Smarttouch, Biosense Webster Inc., CA, USA). A fast-anatomical map (FAM) of the entire LA anatomy and the PVs was acquired and then merged with the imported 3D LAWT map within the spatial reference coordinates of the CARTO system. Before starting ablation, a multi-thermocouple temperature probe (SensiTherm, St Jude Medical, Inc., St Paul, MN, USA) was always advanced into the oesophagus under fluoroscopic guidance to monitor the oesophageal temperature rises. Endocardial voltage mapping data were acquired in sinus rhythm. Low-voltage areas were defined as areas of endocardial bipolar voltage < 0.5 mV. The PVI was performed with point-by-point RF lesion pattern with a maximal inter-lesion distance of 6 mm.17 The personalized ablation line was depicted on the merged 3D LAWT map around the PV antrum according to a wide antral circumferential ablation (WACA) pattern > 10 mm outside the PV ostia, where the local electrograms did not show near-field PV signals. The line was moved in or moved out with respect to the strict PV antrum to avoid the thickest LAWT areas; moreover, the line is also personalized according to the oesophageal isodistance colour-coded map in order to avoid, as far as possible, RF application on the LA posterior wall where the MDCT-derived atrio-oesophageal distance is the shortest (<1 mm, red area of the colour-coded isodistance map). The AI targets were defined by the local LAWT on the thickness colour-coded map, as previously described18 (Table 1). The power output was set at 35 W for the posterior wall, 40 W for anterior wall at zones with LAWT between <1 and 2 mm (red-yellow zones on thickness map), and 50 W anterior wall at zones with LAWT ≥2 mm (green, blue, and purple zones on thickness map) (Table 1). If the AI target was not reached at a given point for any reason, another lesion reaching the target was applied. A carina line was mandatory for the right pulmonary veins (RPVs), except if a common trunk was found, as RPVs intervenous carina is a common site for both acute and late reconnections.27 Regardless of the local thickness of the 3D LAWT map, AI was down-titrated to 300 whenever the RF point was at < 1 mm distance from the oesophagus footprint on the posterior LA wall.22
Table 1.
Protocol for ablation parameters adapted to left atrial wall thickness and for high-frequency low-tidal volume ventilation
Table 1a Protocol for ablation parameters adapted to left atrial wall thickness
| LAWT | Colour code | Ablation index | RF energy (Watts) | ||
|---|---|---|---|---|---|
| Anterior | Posterior | Anterior | Posterior | ||
| < 1mm | Red | 300 | 300 | 40 | 35 |
| 1–2 mm | Yellow | 350 | 350 | 40 | 35 |
| 2–3 mm | Green | 400 | 400 | 50 | 35 |
| 3–4 mm | Blue | 450 | 450 | 50 | 35 |
| > 4 mm | Purple | 500 | 500 | 50 | 35 |
Table 1b.
Protocol for standard high-frequency low-tidal volume ventilation
| Parameter | Value |
|---|---|
| FiO2 (%) | 60 |
| Ventilation rate (breath/min) | 45–50 |
| Inspiration/expiration ratio | 1–2 |
| Tidal volume (mL/kg) | 3.5–4 |
FiO2, fraction of inspired oxygen; LAWT, left atrial wall thickness; RF, radiofrequency.
Acute PVI was confirmed by demonstrating bidirectional block: entry block was demonstrated by the absence of PV potentials inside the vein with the ablation catheter placed sequentially in each segment inside the circumferential PV line and exit block by proving absence of electric capture of the atrium during high-output pacing from inside the circumferential PV line, at each segment sequentially. In case of ongoing AF at the end of the RF applications, electrical cardioversion was performed to demonstrate bidirectional block during sinus rhythm.26 Additional ablation was delivered at connected sites until PVI was achieved. The procedure was not terminated until confirming the absence of visual gaps between VisiTags.17
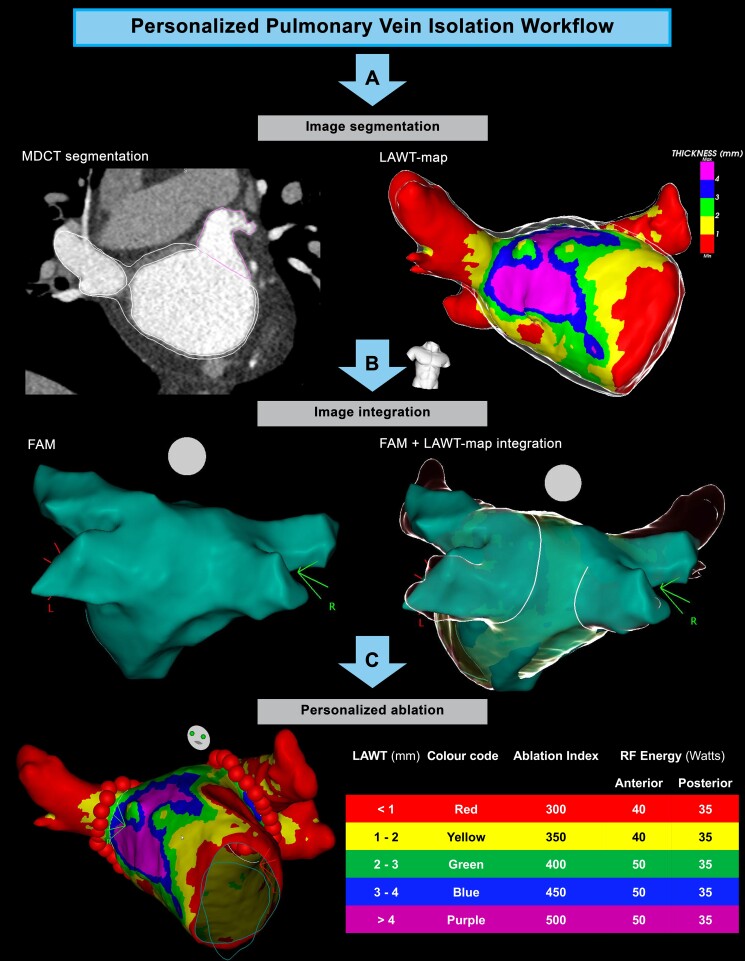
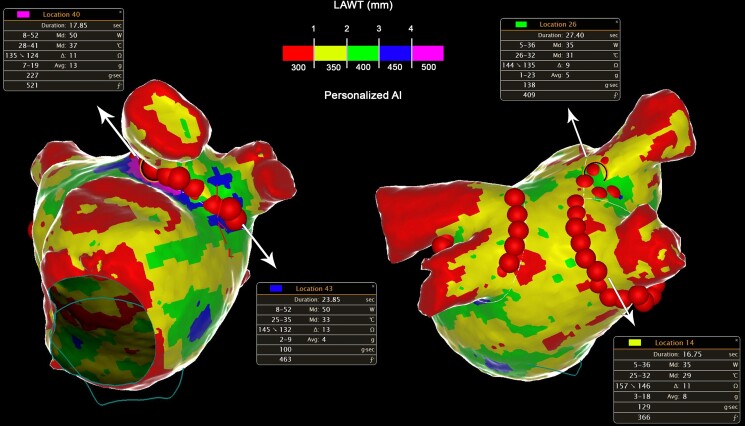
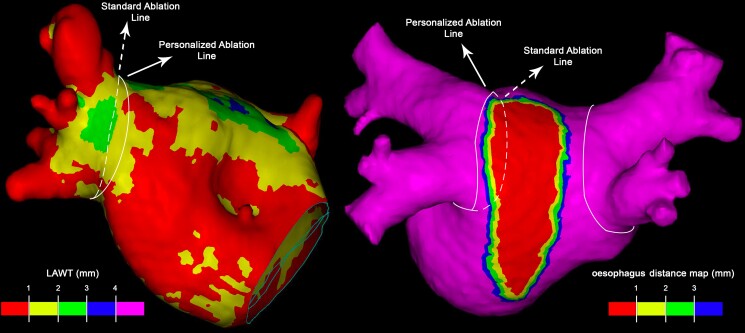
The procedural workflow of LAWT-guided PeAF ablation is represented in Figure 1, Figure 2, and Figure 3.
Figure 1.
Workflow of left atrial wall thickness-guided persistent atrial fibrillation ablation. (A) The first step is multidetector computed tomography-derived image segmentation and 3D left atrial wall thickness-map rendering. (B) Image integration in the navigation system after performing left atrial fast anatomical map. (C) Pulmonary vein isolation with the AI targets adapted to left atrial wall thickness-information.
Figure 2.
Personalized persistent atrial fibrillation ablation with ablation index adapted to left atrial wall thickness.
Figure 3.
Personalized ablation lines in order to avoid antral thickest regions and left atrial posterior wall segments closest to the oesophagus.
Follow-up
Patients were scheduled for follow-up at the outpatient clinic at 1, 3, 6, 12, and every 12 months thereafter, or in case of symptoms. Each evaluation included an ECG and 24 h Holter ECG monitoring. The primary outcome was freedom from recurrence after a blanking period of 3 months. Recurrence was defined as any documented AF, atrial flutter, or atrial tachycardia (AT) episode lasting more than 30 s, regardless of symptoms.28 In the absence of other indications, all AADs were stopped at the end of the blanking period; however, the final decision on whether to withdraw all AADs was left to medical choice. Patients with recurrence were also scheduled for a redo ablation, according to the decision of the treating physician; there was no blanking period after a second procedure.
Statistical analysis
Continuous variables were presented as mean ± standard deviation or median (interquartile range) as appropriate. Categorical variables were presented as total number (percentage). The Student’s t-test or Wilcoxon test were used to compare continuous variables, as appropriate; χ2 or Fisher’s exact test were used to compare categorical variables, as appropriate. Kaplan–Meier curves and the log-rank test were used to assess cumulative AF/AT-free survival. Logistic regression models were performed to identify predictors of AF/AT recurrence during the follow-up, while Youden index analysis was used to calculate the optimal cut-off value. A level of P < 0.05 was considered for statistical significance. Data were analysed with R version 3.6.2 software (R Foundation for Statistical Computing, Vienna, Austria) and Matlab statistics toolbox (Matlab R2010a, The Mathworks, Inc., Natick, MA, USA).
Results
Baseline population
Between April 2019 and June 2021, a total of 125 consecutive patients meeting the inclusion criteria underwent PeAF ablation. Four patients had a minimum follow-up of less than 6 months and were excluded for analysis. The remaining 121 patients were included in the analysis. Baseline clinical characteristics are summarized in Table 2 and Table 3. Mean age was 64.5 ± 9.5 years, 69.4% were male, and 34 patients (28.1%) had a diagnosis of long-standing PeAF. The 37.2% of the population had a diagnosis of prior structural heart disease, being hypertensive cardiomyopathy the most prevalent entity (15.7%). Mean LA diameter was greater in patients with long-standing PeAF diagnosis (42.5 ± 6.0 mm vs. 46.1 ± 4.5 mm, P = 0.005). The LV function was impaired in 26 patients (21.5%).
Table 2.
Patients’ baseline characteristics according to persistent atrial fibrillation classification
| PeAF (n = 87) | Long-standing PeAF (n = 34) | Total patients (n = 121) | P-value | |
|---|---|---|---|---|
| Age (years) | 64.1 ± 9.7 | 65.5 ± 9.1 | 64.5 ± 9.5 | 0.52 |
| Male | 58 (66.7) | 26 (76.5) | 84 (69.4) | 0.38 |
| BMI (kg/m2) | 29.1 ± 6.4 | 30.6 ± 5.1 | 29.6 ± 6.0 | 0.06 |
| Hypertension | 46 (52.8) | 24 (70.6) | 70 (57.8) | 0.10 |
| Dyslipidaemia | 25 (28.7) | 14 (41.2) | 39 (32.2) | 0.28 |
| Smoke history | 5 (5.71) | 1 (2.9) | 6 (5.0) | 0.67 |
| Type 2 diabetes | 13 (14.9) | 8 (23.5) | 21 (17.3) | 0.28 |
| LVEF > 55% | 66 (75.9) | 29 (85.3) | 95 (78.5) | 0.33 |
| LA diameter (mm) | 42.5 ± 6.0 | 46.1 ± 4.5 | 43.5 ± 5.8 | <0.01 |
| CHA2DS2-VASc score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.88 |
| HAS-BLED score | 1.0 (0.0–2.0) | 1.0 (0.2–1.0) | 1.0 (0.0–2.0) | 0.35 |
| Longest AF episode (months) | 2.0 (1.0–2.0) | 16.0 (14.2–17.0) | 2.0 (1.0–13.0) | <0.01 |
| Time since diagnosis (months) | 8.0 (7.0–9.0) | 22.0 (21.0–24.0) | 9.0 (8.0–20.0) | <0.01 |
| Baseline Medications | ||||
| Beta-blocker | 31 (38.3) | 14 (43.8) | 45 (37.2) | 0.67 |
| Flecainide | 15 (17.2) | 6 (17.6) | 21 (17.4) | 0.99 |
| Propafenone | 2 (2.3) | 1 (2.9) | 3 (2.5) | 0.99 |
| Amiodarone | 42 (48.3) | 22 (64.7) | 64 (52.9) | 0.11 |
| Underlying cardiomyopathy | 0.64 | |||
| None | 55 (63.2) | 21 (61.8) | 76 (62.8) | |
| Hypertensive | 15 (17.6) | 4 (11.8) | 19 (15.7) | |
| Ischaemic | 4 (4.7) | 3 (8.8) | 7 (5.8) | |
| Valvular | 2 (2.4) | 2 (5.9) | 4 (3.3) | |
| Hypertrophic | 1 (1.2) | 1 (2.9) | 2 (1.7) | |
| Other | 9 (10.6) | 3 (8.8) | 12 (9.9) |
Results are reported as n (%) for categorical variables and median (interquartile range) or mean ± standard deviation for continuous variables. Statistically significant differences between the two groups are shown in bold (P < 0.05). BMI, body mass index; LA, left atrium; LVEF, left ventricle ejection fraction; PeAF, persistent atrial fibrillation; PVs, pulmonary veins.
Table 3.
Patients’ baseline characteristics according to atrial arrhythmia recurrence during the follow-up
| No recurrence (n = 92) | Arrhythmia recurrence (n = 29) | Total patients (n = 121) | P-value | |
|---|---|---|---|---|
| Age (years) | 64.0 ± 9.4 | 66.0 ± 10.0 | 64.5 ± 9.5 | 0.37 |
| Male | 64 (69.6) | 20 (69.0) | 84 (69.4) | 0.99 |
| BMI (kg/m2) | 29.8 ± 6.1 | 29.1 ± 5.5 | 29.6 ± 6.0 | 0.62 |
| Hypertension | 52 (56.5) | 18 (62.1) | 70 (57.8) | 0.66 |
| Dyslipidaemia | 29 (31.5) | 10 (34.5) | 39 (32.2) | 0.64 |
| Smoke history | 5 (5.4) | 1 (3.4) | 6 (5.0) | 0.99 |
| Type 2 diabetes | 15 (16.3) | 6 (20.7) | 21 (17.3) | 0.56 |
| LVEF >55% | 70 (76.1) | 25 (86.2) | 95 (78.5) | 0.31 |
| LA diameter (mm) | 42.9 ± 6.0 | 45.4 ± 4.7 | 43.5 ± 5.8 | 0.02 |
| CHA2DS2-VASc score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.32 |
| HAS-BLED score | 1.0 (0.0–2.0) | 1.0 (0.0–1.0) | 1.0 (0.0–2.0) | 0.85 |
| Longest AF episode (months) | 2.0 (1.0–13.0) | 3.0 (2.0–15.0) | 2.0 (1.0–13.0) | 0.14 |
| Time since diagnosis (months) | 9.0 (8.0–18.2) | 10.0 (8.0–21.0) | 9.0 (8.0–20.0) | 0.32 |
| Baseline medications | ||||
| Beta-blocker | 32 (34.8) | 13 (44.8) | 45 (37.2) | 0.17 |
| Flecainide | 13 (14.1) | 8 (27.6) | 21 (17.4) | 0.16 |
| Propafenone | 3 (3.3) | 0 (0.0) | 3 (2.5) | 0.99 |
| Amiodarone | 52 (56.5) | 12 (41.4) | 64 (52.9) | 0.20 |
| Underlying cardiomyopathy | 0.68 | |||
| None | 58 (63.0) | 18 (62.1) | 76 (62.8) | |
| Hypertensive | 14 (15.2) | 5 (17.2) | 19 (15.7) | |
| Ischaemic | 5 (5.4) | 2 (6.9) | 7 (5.8) | |
| Valvular | 3 (3.3) | 1 (3.4) | 4 (3.3) | |
| Hypertrophic | 1 (1.1) | 1 (3.4) | 2 (1.7) | |
| Other | 11 (11.9) | 1 (3.4) | 12 (9.9) |
Results are reported as n (%) for categorical variables and median (interquartile range) or mean ± standard deviation for continuous variables. Statistically significant differences between the two groups are shown in bold (P < 0.05).
BMI, body mass index; LA, left atrium; LVEF, left ventricle ejection fraction; PeAF, persistent atrial fibrillation; PVs, pulmonary veins.
The mean time between MDCT acquisition and the procedure was 24 ± 12 h.
Procedural characteristics
Procedural characteristics are summarized in Table 4.
Table 4.
Acute procedural data according to persistent atrial fibrillation classification
| PeAF (n = 87) | Long-standing PeAF (n = 34) | Total patients (n = 121) | P-value | |
|---|---|---|---|---|
| AF at the beginning of the procedure | 30 (34.4) | 13 (38.2) | 43 (35.5) | 0.99 |
| Procedure time skin-to-skin (min) | 57.0 (50.0–65.5) | 60.0 (52.5–71.0) | 57.0 (50.0–67.0) | 0.15 |
| FAM time (min) | 12.0 (11.0–14.0) | 12.5 (11.0–14.0) | 12.0 (11.0–14.0) | 0.42 |
| Fluoroscopy time (s) | 40.0 (18.0–68.0) | 52.5 (33.0–71.2) | 43.0 (20.0–71.0) | 0.07 |
| Fluoroscopy dose (mGy) | 3.5 (1.2–7.5) | 4.7 (3.2–10.2) | 4.2 (1.3- 8.4) | 0.17 |
| <0.5 mV points during SR (%) | 6.0 (1.0–11.0) | 16.0 (13.0–20.0) | 7.0 (2.0–14.0) | <0.01 |
| Low-voltage area > 1cm2 during SR | 17 (19.5) | 13 (38.2) | 30 (24.8) | 0.04 |
| Total RF time (min) | 15.8 (14.1–18.4) | 16.7 (15.5–18.9) | 16.5 (14.3–18.4) | 0.17 |
| Total VisiTags | 59 (53–69) | 64 (58–71) | 60 (54–70) | 0.14 |
| Mean anterior AI | 390 (365–408) | 380 (360–405) | 387 (360–410) | 0.40 |
| Mean posterior AI | 337 (310–375) | 330 (305–370) | 335 (300–375) | 0.45 |
| Right pulmonary veins | ||||
| RF time (min) | 8.6 (7.6–9.7) | 9.0 (8.0–10.9) | 8.8 (7.7–10.0) | 0.12 |
| VisiTags | 31 (27–38) | 33 (31–41) | 31 (28–39) | 0.10 |
| Mean VisiTag time (s) | 14.9 (13.0–18.9) | 14.8 (12.7–18.1) | 14.9(12.8–18.5) | 0.22 |
| Mean anterior AI | 382 (357–398) | 379 (354–396) | 380 (355–397) | 0.41 |
| Mean posterior AI | 343 (325–361) | 339 (320–350) | 341 (320–360) | 0.39 |
| First-pass isolation | 75 (86.2) | 28 (82.4) | 103 (85.1) | 0.58 |
| Acute reconnection | 2 (2.3) | 2 (5.9) | 4 (3.3) | 0.19 |
| Anterior right PVs antrum WT (mm) | 1.5 (1.3–1.9) | 1.4 (1.1–1.9) | 1.4 (1.1–1.9) | 0.40 |
| Posterior right PVs antrum WT (mm) | 1.1 (0.9–1.2) | 0.98 (0.8–1.2) | 1.0 (0.7–1.2) | 0.37 |
| Left pulmonary veins | ||||
| RF time (min) | 7.5 (6.5–8.6) | 7.7 (6.5–9.1) | 7.7 (6.5–8.6) | 0.42 |
| VisiTags | 28 (23–33) | 31 (27–33) | 28 (24–33) | 0.40 |
| Mean VisiTag time (s) | 15.0 (13.0–19.0) | 14.8 (12.7–18.2) | 15.0 (12.7–19.0) | 0.22 |
| Mean anterior AI | 408 (382–431) | 403 (379–428) | 405 (380–430) | 0.36 |
| Mean posterior AI | 348 (330–376) | 342 (320–370) | 345 (320–375) | 0.29 |
| First-pass isolation | 73 (83.9) | 30 (88.2) | 103 (85.1) | 0.78 |
| Acute reconnection | 2 (2.3) | 1 (2.9) | 3 (2.5) | 0.99 |
| Anterior left PVs antrum WT (mm) | 2.04 (2.0–2.4) | 1.98 (1.8–2.2) | 2.1 (1.9–2.4) | 0.45 |
| Posterior left PVs antrum WT (mm) | 1.13 (1.0–1.3) | 0.99 (0.8–1.1) | 1.1 (0.9–1.3) | 0.31 |
| Overall acute first-pass | 65 (74.7) | 24 (70.6) | 89 (73.6) | 0.65 |
| Overall acute reconnection | 3 (3.4) | 3 (8.8) | 6 (5.0) | 0.13 |
| Acute procedural complication | 2 (2.3) | 2 (5.9) | 4 (3.3) | 0.45 |
Results are reported as n (%) for categorical variables and median (interquartile range) or mean ± standard deviation for continuous variables. Statistically significant differences between the two groups are shown in bold (P < 0.05).
AI, ablation index; FAM, fast anatomical map; RF, radiofrequency; SR, sinus rhythm; WT, wall thickness.
At the time of ablation, 43 (35.5%) of patients were in spontaneous AF rhythm.
The mean ventilation rate was 48 ± 7 rpm with a mean tidal volume of 263 ± 47 mL. The median total procedure skin-to-skin time was 57 min (IQR 50–67), and the FAM acquisition time was 12 min (IQR 11–14). The fluoroscopy time was 43 s (IQR 20–71), and the fluoroscopy dose was 4.2 mGy (IQR 1.3–8.4). The proportion of patients with low-voltage area >1 cm2 during sinus rhythm mapping was higher in long-standing PeAF patients (19.5% vs. 38.2%, P = 0.04). The median number of VisiTags was 28 (IQR 24–33) for the left pulmonary veins (LPVs) and 31 (IQR 28–39) for the RPVs, including the right carina. The median RF time was 7.7 min (IQR 6.5–8.6) for the LPVs and 8.8 min (IQR 7.7–10.0) for the RPVs. Acute PVI was achieved in all procedures. First-pass PVI was obtained in 103 (85%) of the RPVs and 103 (85%) of the LPVs, with an overall rate of complete first-pass of 73.6%. Acute reconnections were observed in 3 out of 250 (1.2%) LPVs and in 4 out of 250 (1.6%) RPVs. Complications occurred in four patients: two patients had intraprocedural severe pericardial effusion related to the transseptal puncture and required pericardiocentesis; two patients experienced pseudoaneurysm at inguinal access requiring percutaneous treatment. No patients undergoing personalized PeAF ablation experienced pericarditis. No neurological complications were observed. No patient died peri-procedurally nor during the follow-up.
Left atrial wall thickness and ablation index
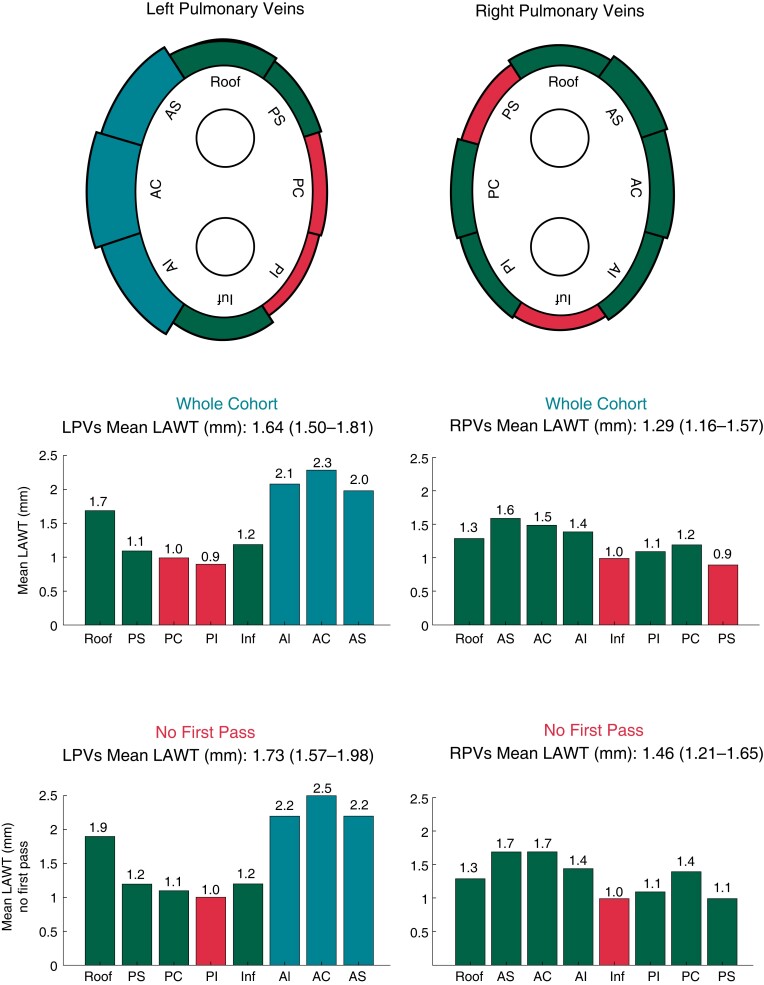
The wall thickness (WT) of the circumferential PV line was thicker in the LPVs as compared to the RPVs [1.64 mm (1.5–1.81) vs. 1.31 mm (1.16–1.57), P < 0.01]. More specifically, anterior wall segments were thicker in the LPVs as compared to the RPVs [2.1 mm (1.9–2.4) vs. 1.4 mm (1.1–1.9), P < 0.01], while posterior wall segments had similar WT in LPVs and RPVs [1.1 mm (0.9–1.3) vs. 1.0 mm (0.7–1.2), P = NS]. Median WT values were higher for PVs with no first-pass during RF ablation with respect to the whole cohort [1.73 mm (1.57–1.98) vs. 1.64 mm (1.50–1.81) P = 0.02 for LPVs; 1.46 mm (1.21–1.65) vs. 1.29 mm (1.16–1.57) P = 0.03, for RPVs]. No statistically significant differences in the WT of the PVs were found between PeAF and long-standing PeAF patients. Ladybug and histogram plots of the WT values for each PV segment are represented in Figure 4.
Figure 4.
Ladybug and histogram plots of the wall thickness values for each pulmonary vein segment in the whole cohort and in the no first-pass subgroup.
The median AI applied was 387 (IQR 360–410) for the anterior wall and 335 (IQR 300–375) for the posterior wall. More specifically, for the LPVs, the median AI applied was 405 (IQR 380–430) for the anterior wall and 345 (IQR 320–375) for the posterior wall, while for the RPVs was 380 (IQR 355–397) for the anterior wall and 341 (IQR 320–360) for the posterior wall.
Clinical outcomes
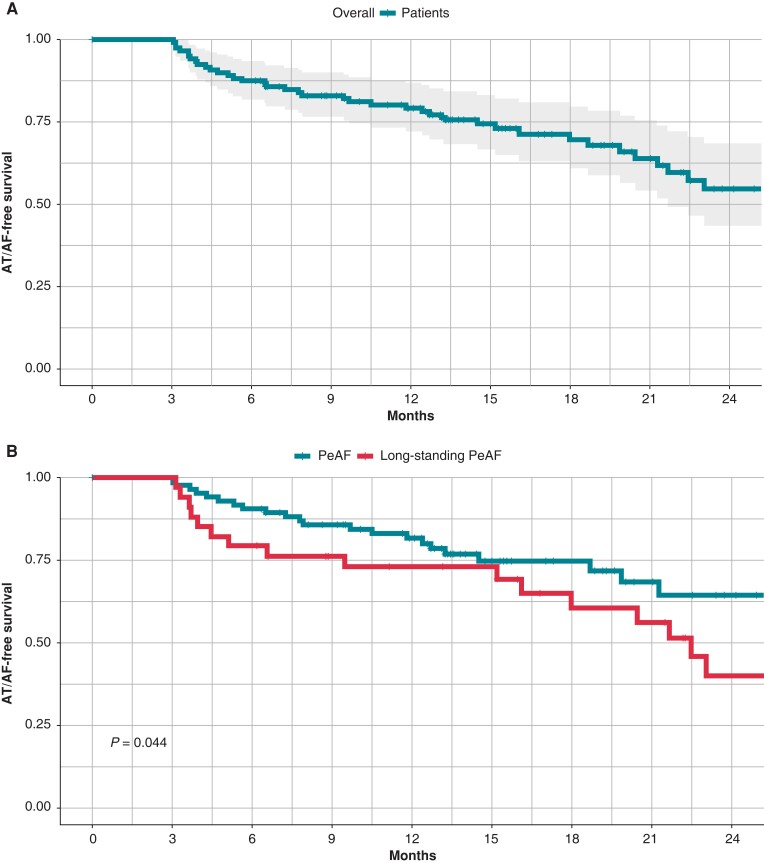
All patients were maintained on AADs during the blanking period (3 months); 26 patients (21%) continued taking AADs after the end of the blanking period. At 12-month follow-up, primary outcome has been reached in the 79% of patients. At the median follow-up of 16 months (IQR 8–21), primary outcome has been reached in the 73% (95% IC: 65–82) of patients. The primary outcome rate was significantly higher in patients with PeAF with respect to those with long-standing PeAF diagnosis (P = 0.044). The primary outcome rate was significantly higher in patients presenting sinus rhythm at the beginning of the ablation procedure with respect to those presenting spontaneous AF (P < 0.05). The presence of low-voltage area >1 cm2 during sinus rhythm mapping was more frequent in patients reporting AF/AT recurrence during the follow-up (20% vs. 41%, P = 0.03). Moreover, at logistic regression analysis, the presence of low-voltage area >1 cm2 during sinus rhythm mapping resulted an independent predictor of AF/AT recurrence during the follow-up (OR = 2.9, 95%CI 1.2–7.2, P = 0.02). There was no significant difference in AF/AT recurrence between the group of patients who discontinued AADs at the end of blanking period and the group with prolonged AADs intake (P = 0.32). Left PVs WT values were significantly higher in patients with AF/AT recurrence during the follow-up with respect to the patient group without recurrences (1.80 ± 0.17 mm vs. 1.63 ± 0.27 mm; P = 0.01). More specifically, a significant difference of WT values was reported for the left anterior carina (2.40 ± 0.28 mm vs. 2.22 ± 0.25 mm; P = 0.01), while a trend towards higher values of WT was reported for left roof (P = 0.07) and anterior left superior (P = 0.09) segments (Table 5). At the logistic regression analysis, left PVs WT >1.75 mm resulted an independent predictor of AF/AT recurrence during the follow-up (OR = 12.2, 95% CI 1.9–96.4, P = 0.01). Patients’ baseline characteristics according to LPVs WT are reported in Table 6. At the median follow-up, a second ablation was performed in 18 (62%) out of 29 patients with AF/AT recurrence. Specifically, 10 (56%) out of 18 patients presented reconnections in both RPVs and LPVs, only 3 patients in RPVs, and only 1 patient in LPVs, while 4 (22%) out of 18 patients undergoing redo procedure presented isolated PVs. Median WT values were higher for PVs with reconnection found in the redo procedures as compared to the median WT of the corresponding PVs of the whole cohort [1.77 mm (1.65–2.14) vs. 1.64 mm (1.50–1.81) P = 0.01 for LPVs; 1.43 mm (1.25–1.61) vs. 1.29 mm (1.16–1.57) P = 0.05, for RPVs].
Table 5.
Left atrial wall thickness values in the total cohort of patients and according to AF/AT recurrence during the follow-up
| No recurrence (n = 92) | Arrhythmia recurrence (n = 29) | Total patients (n = 121) | P-value | |
|---|---|---|---|---|
| PVs antrum | 1.65 ± 0.17 | 1.72 ± 0.29 | 1.66 ± 0.19 | 0.44 |
| Left PVs antrum | 1.63 ± 0.27 | 1.80 ± 0.17 | 1.66 ± 0.25 | 0.01 |
| Left roof | 1.68 ± 0.31 | 1.76 ± 0.32 | 1.71 ± 0.29 | 0.07 |
| Anterior left superior | 1.95 ± 0.28 | 2.11 ± 0.35 | 2.02 ± 0.31 | 0.09 |
| Posterior left superior | 1.09 ± 0.27 | 1.13 ± 0.33 | 1.10 ± 0.27 | 0.22 |
| Anterior left carina | 2.22 ± 0.25 | 2.40 ± 0.28 | 2.31 ± 0.25 | 0.01 |
| Posterior left carina | 0.98 ± 0.32 | 1.01 ± 0.25 | 1.00 ± 0.26 | 0.36 |
| Anterior left inferior | 2.08 ± 0.26 | 2.11 ± 0.41 | 2.09 ± 0.29 | 0.31 |
| Posterior left inferior | 0.90 ± 0.18 | 0.92 ± 0.28 | 0.91 ± 0.24 | 0.45 |
| Left floor | 1.19 ± 0.21 | 1.20 ± 0.33 | 1.20 ± 0.22 | 0.55 |
| Right PVs antrum | 1.31 ± 0.29 | 1.36 ± 0.35 | 1.32 ± 0.30 | 0.42 |
| Right roof | 1.30 ± 0.28 | 1.34 ± 0.34 | 1.33 ± 0.23 | 0.25 |
| Anterior right superior | 1.61 ± 0.31 | 1.65 ± 0.40 | 1.62 ± 0.35 | 0.15 |
| Posterior right superior | 0.90 ± 0.19 | 0.91 ± 0.27 | 0.91 ± 0.23 | 0.56 |
| Anterior right carina | 1.51 ± 0.25 | 1.57 ± 0.29 | 1.54 ± 0.27 | 0.35 |
| Posterior right carina | 1.22 ± 0.26 | 1.21 ± 0.28 | 1.22 ± 0.27 | 0.55 |
| Anterior right inferior | 1.40 ± 0.24 | 1.41 ± 0.32 | 1.41 ± 0.28 | 0.62 |
| Posterior right inferior | 1.10 ± 0.21 | 1.08 ± 0.22 | 1.10 ± 0.20 | 0.35 |
| Right floor | 1.00 ± 0.22 | 1.01 ± 0.25 | 1.01 ± 0.23 | 0.39 |
Results are reported as mean ± standard deviation for continuous variables. Statistically significant differences between the two groups are shown in bold (P < 0.05). PV, pulmonary vein.
Table 6.
Patients’ baseline characteristics according to the left pulmonary veins wall thickness
| Left PVs LAWT ≤ 1.75 mm (n = 76) | Left PVs LAWT > 1.75 mm (n = 45) | Total patients (n = 121) | P-value | |
|---|---|---|---|---|
| Age (years) | 65.1 ± 9.9 | 63.4 ± 8.8 | 64.5 ± 9.5 | 0.34 |
| Male | 46 (60.5) | 38 (84.4) | 84 (69.4) | 0.01 |
| BMI (kg/m2) | 29.2 ± 6.1 | 30.6 ± 5.7 | 29.6 ± 6.0 | 0.25 |
| Hypertension | 42 (55.2) | 28 (62.2) | 70 (57.8) | 0.24 |
| Dyslipidaemia | 26 (34.2) | 13 (28.9) | 39 (32.2) | 0.54 |
| Smoke history | 4 (5.3) | 2 (4.4) | 6 (5.0) | 0.99 |
| Type 2 diabetes | 13 (17.1) | 8 (17.8) | 21 (17.3) | 0.80 |
| LVEF >55% | 61 (80.3) | 34 (75.6) | 95 (78.5) | 0.65 |
| LA diameter (mm) | 43.2 ± 5.8 | 44.2 ± 5.9 | 43.5 ± 5.8 | 0.47 |
| CHA2DS2-VASc score | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 2.0 (1.0–3.0) | 0.74 |
| HAS-BLED score | 1.0 (0.0–1.2) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 0.56 |
| Longest AF episode (months) | 2.0 (2.0–14.0) | 2.0 (1.0–8.0) | 2.0 (1.0–13.0) | 0.13 |
| Time since diagnosis (months) | 9.0 (8.0–20.2) | 9.0 (8.0–14.0) | 9.0 (8.0–20.0) | 0.25 |
| Baseline medications | ||||
| Beta-blocker | 28 (36.8) | 17 (37.8) | 45 (37.2) | 0.69 |
| Flecainide | 11 (14.5) | 10 (22.2) | 21 (17.4) | 0.32 |
| Propafenone | 2 (2.6) | 1 (2.2) | 3 (2.5) | 0.99 |
| Amiodarone | 43 (56.6) | 21 (46.7) | 64 (52.9) | 0.35 |
| Underlying cardiomyopathy | 0.31 | |||
| None | 52 (68.4) | 24 (53.3) | 76 (62.8) | |
| Hypertensive | 8 (10.5) | 11 (24.4) | 19 (15.7) | |
| Ischaemic | 4 (5.3) | 3 (6.7) | 7 (5.8) | |
| Valvular | 3 (3.9) | 1 (2.2) | 4 (3.3) | |
| Hypertrophic | 1 (1.3) | 1 (2.2) | 2 (1.7) | |
| Other | 8 (10.5) | 4 (8.9) | 12 (9.9) |
Results are reported as n (%) for categorical variables and median (interquartile range) or mean ± standard deviation for continuous variables. Statistically significant differences between the two groups are shown in bold (P < 0.05). BMI, body mass index; LA, left atrium; LAWT, left atrial wall thickness; LVEF, left ventricle ejection fraction; PeAF, persistent atrial fibrillation; PVs, pulmonary veins.
Kaplan–Meier curves for primary outcome are shown in Figure 5.
Figure 5.
Atrial arrhythmia-free survival Kaplan–Meier curves. Overall curve (4a) and according to persistent atrial fibrillation classification (4b).
Discussion
Main findings
To our knowledge, this is the first study reporting the feasibility, the efficacy, and the efficiency of a PVI-only approach using a personalized local LAWT-guided titration of AI for PeAF ablation. The main findings of the study are the following: (i) local LAWT-guided ablation is a feasible approach for PeAF ablation leading to a high first-pass rate of PVI; (ii) the presented approach is safe and reported no RF-related adverse events; (iii) the procedure is efficient, reporting low procedural requirements in terms of procedure, fluoroscopy, and RF time; (iv) LAWT-guided PVI-only strategy for PeAF ablation was associated with a high recurrence-free survival at a median follow-up of 16 months, probably due to durable lesions; (v) no first-pass during the first procedure and reconnection sites at redo ablation are mostly encountered in PVs with the thickest antral regions. Our study is the first proof-of-concept demonstration that a LAWT-guided ablation approach is feasible, safe, and effective also in patients with PeAF, who differ from paroxysmal AF patients, being characterized by an increased LA remodelling and a higher prevalence of comorbidities such as hypertension, diabetes, and left ventricular dysfunction.29 Moreover, the reported single-centre prospective study is an initial experience of a novel approach, and its results support the study design and the patient sample calculation of the ongoing multicentre randomized PeAF-by-LAWT trial (NCT05396534) which have a control arm (AI-guided PVI approach according to the CLOSE protocol) and could allow to compare the results of the two procedural approaches.
LAWT-guided pulmonary vein isolation approach
Non-transmural ablation is considered a major determinant of post-ablation arrhythmia recurrence; El Haddad et al.30 showed that PV reconnection could be due to an insufficient lesion depth. Several studies demonstrated that AI accurately predicts the depth of the ablation lesions; therefore, AI has been proposed as a novel marker of ablation lesion quality. Previous analysis has also identified that minimum AI values of 540 and 380 were predictive of freedom from acute reconnection in the anterior/roof and the posterior/inferior segments of PVs antrum, respectively.17 However, growing evidence support the complex anatomical reality of the LA and encompasses the intra- and inter-patient regional variability of LAWT distribution for both anterior and posterior segments of PV–LA junction.31,32 In the present study, LAWT ranged from 0.51 to 2.21 mm in the posterior wall and from 0.86 to 4.43 mm in the anterior wall. Therefore, fixed AI targets and a dichotomized anterior/posterior ablation approach could be an oversimplification and may not fully reflect this variability, leading to non-transmural lesions in the thickest LA regions and, on the other hand, to excessive RF applications in the thinnest ones.
The MDCT-derived LAWT measurements have been reliably validated on a porcine model,31 and integrating 3D LAWT maps into the navigation system allows to be aware of the real-time local LAWT in contact with the ablation catheter tip during the procedure. The use of this tool has been already described by our group in order to aid paroxysmal AF ablation18 and AF reablation procedures.20 The aim of the proposed personalized ablation is that modifying the ablation line to avoid the thickest atrial areas and rising the AI targets in the thickest segments may likely improve the lesion transmurality and consequently the PVI durability. Conversely, lowering the AI targets in the thinnest segments may reduce the procedure duration and improve the safety by avoiding unnecessary RF application. In the recent large non-randomized Surpoint trial,33 Di Biase et al. reported the excellent safety and effectiveness outcomes of a standardized AI-guided ablation protocol for paroxysmal AF ablation; the LAWT-guided approach may add complexity to the procedure in terms of pre-procedural scanning, segmentation, and map integration, but the potential benefits of a personalized approach may outweigh these challenges, particularly in terms of minimizing the procedure and the RF times, in order to maximize the procedural efficiency. Moreover, the small increase in overall complexity due to LA segmentation will be further reduced in the near future because of the progressive increase in the use of artificial intelligence that could enable a full-automatic segmentation process, thereby reducing complexity and enabling a more streamlined approach to a personalized AF ablation.
Long-term clinical outcomes
The presented results are in line with the long-term clinical outcomes reported in literature about PeAF patients undergoing PVI-only ablation strategy. In the recent PRAISE trial,14 clinical recurrence was documented in the 20% of patients at the end of the 12-month follow-up. The STAR AF II trial11 reported a recurrence-free survival of 59% at the 18-month follow-up within the PVI-only ablation arm. In the present study, we reported a recurrence-free survival rate of 79% at the 12-month follow-up and of 73% at the median 16-month follow-up. In line with the previous findings,9 long-term outcomes in the case of long-standing PeAF were significantly worse than those of patients with PeAF episodes lasting less than 1 year.
Berruezo et al.34 showed that hypertension and LA diameter are independent pre-procedural predictors of AF recurrence after PVI to treat AF. A meta-analysis published by D’Ascenzo et al.35 has shown that a PVI-only approach for PeAF patients might present a more favourable long-term outcome in case of LA diameter < 50 mm and non-valvular AF. In addition, it has been shown that the absence of early recurrence during the classical 3-month blanking period is associated with a higher rate of recurrence-free survival at long-term follow-up.10 Moreover, McCready et al.36 reported that in a population of mainly long-standing PeAF, LA size was the major determinant of recurrence after ablation. In line with these previous findings, the presented study reported higher values of LA diameter in patients with arrhythmia recurrence during the follow-up (P = 0.02). Furthermore, in our PeAF cohort, patients with large low-voltage areas demonstrated a higher recurrence rate after PVI-only ablation.37–39
Safety outcomes
Overdosing may contribute to increase the risk of serious complications such as atrial perforation and atrioesophageal fistula9; for this reason, the experts recommend the use of lower energy when RF is applied in thinnest areas and in the posterior aspect of LA.9 An imaging modality that allows to localize the areas of decreased wall thickness more accurately may facilitate a safer RF energy delivery. Notably, we reported no RF-related adverse events, such as deaths, strokes, atrioesophageal fistulas, or cases of symptomatic PV stenosis.
Moreover, MDCT images post-processing permits to obtain the oesophageal fingerprinted isodistance map and to integrate it into the navigation system22: this information permits to personalize the ablation line moving it away from the areas of the LA posterior wall where the atrioesophageal distance is the shortest and to reduce AI delivery when ablation through these areas is unavoidable. This information combined with the use of the oesophageal temperature probe may reduce the possibility of oesophageal heating, therefore increasing procedural safety.22
Procedural efficiency
The goal of PVI is to deliver enough energy to prevent PV reconnection and, on the other hand, to avoid unnecessary and potentially harmful excess of RF. In the PRAISE trial,14 PeAF patients were treated with PVI-only RF ablation according to the CLOSE protocol; they reported a procedure time of 158 ± 34 min, a RF time of 36 ± 9 min, and a fluoroscopy time of 12 min. In the PVI-only subgroup of the STAR AF II trial,11 procedural outcomes showed a procedure time of 167 ± 55 min and a fluoroscopy time of 29 ± 16 min. In the present study, low procedural requirements and low median AI values for both posterior and anterior walls of PV antrum were reported. This was due to the LAWT-guided AI titration and to the personalized ablation line aiming to avoid the thickest LA regions whenever possible. The use of a standardized stepwise near zero-fluoroscopy approach with TOE guidance25 and the use of the pre-procedural imaging integration may explain the low fluoroscopy requirements. As reported by Marrouche et al.,12 procedure duration is an important issue, especially keeping in mind that a higher rate of complications has been generally observed in ablations requiring longer procedural times, regardless of the strategy used.
LAWT information
In line with the literature,40 mean LAWT values encountered in the presented cohort of PeAF patients are higher with respect to those of a previous published study reporting the outcomes of LAWT-guided ablation in patients with paroxysmal AF. Notably, direct measurements of LA on human myocardial specimens performed by Hall et al.41 showed mean thickness values (1.86 mm for anterior and 1.4 mm for posterior segments) comparable to that reported by our group. Moreover, in keeping with previous findings,42 our data showed a trend towards thinner values of LAWT in the long-standing PeAF subgroup. The present study agrees with the data by Inoue et al.,43 that reported a higher AF recurrences rate in patients with higher LAWT values (P < 0.001) and that reconnection segments were found at the thickest antral regions (P = 0.038). Moreover, Mulder et al.44 reported that AI, force-time integral, and impedance drop did not correlate with PV reconnection, whereas AI adjusted to wall thickness had predictive value for PV reconnection. These issues further support that having a real-time local myocardial thickness information available during the ablation may aid to create transmural ablation lesions and prevent PV reconnection after PVI.
Limitations
This is a proof-of-concept single-centre pilot study. The main limitations of the study are the single-centre design and the lack of a control group, so that we cannot draw definitive conclusions or compare the presented approach to a standard AI-guided PVI ablation: a randomized multicentre trial is ongoing to overcome these limitations. Secondly, the AI targets for each LAWT range were based on data already used in two studies18,20; further research is needed to evaluate the optimal parameters of AI titration according to the LAWT. Thirdly, despite the low complication rate, the study size is too small to provide a definitive safety assessment of personalized LAWT-guided ablation strategy for PeAF. Fourthly, all procedures were performed under general anaesthesia, using mechanical ventilation with HFLTV, and with CARTO3 mapping system; thus, the present study results could not be the same if other procedural settings are used. Fifthly, antiarrhythmic therapy was not systematically withdrawn after the blanking period and may have improved clinical success; however, the percentage of patients taking AADs is comparable to that of studies in the literature.17 Sixthly, we adopted the classical 3-month blanking period, but we cannot exclude that a shorter blanking period might be preferable.45,46 Seventhly, slightly different LAWT values have been reported by other groups; this could partly depend on different MDCT acquisition protocols and on differences between segmentation tools and LAWT rendering. Finally, in the absence of continuous ECG monitoring or periodic single-lead ECG monitoring by wearable devices,47–49 it is possible that brief asymptomatic episodes were missed, thereby overestimating the success rates.
Conclusions
The real-time intraprocedural knowledge of the local LAWT could guide PeAF ablation permitting to move away the ablation line from the thickest LA regions and to titrate RF delivery by increasing AI in the thickest segments of the PV antrum; at the same time, this novel approach allow to avoid unnecessary high amounts of RF energy delivery in the thinnest part of the PV antrum, improving procedural efficiency while maintaining a recurrence-free survival comparable to that reported in literature. No first-pass during the first procedure and reconnection sites at redo ablation are mostly encountered in PVs with the thickest antral regions. A multicentre randomized trial comparing the LAWT-guided and the standard AI-guided PVI for PeAF ablation is in progress (ClinicalTrials.gov, NCT05396534). Patient recruitment started on June 2022 and the enrolment completion is estimated by April 2023.
Author’s contributions
G.F.: study concept and design, manuscript drafting, critical revision. D.P.: study concept and design, manuscript drafting, critical revision. D.S.-I.: study concept and design, manuscript drafting, critical revision. P.F.: manuscript drafting, critical revision. C.T.: manuscript drafting, critical revision. A.S.: contributed to major revision answers, critical revision. B.J.: manuscript drafting, critical revision. D.V.: manuscript drafting, critical revision. A.B.: manuscript drafting, critical revision. J.A.: manuscript drafting, critical revision. J.M.-S.: manuscript drafting, critical revision. P.F.: manuscript drafting, critical revision. C.G.: manuscript drafting, critical revision. A.C.: manuscript drafting, critical revision. J.M.C.: manuscript drafting, critical revision. R.S.: manuscript drafting, critical revision. A.O.: manuscript drafting, critical revision. M.H.: manuscript drafting, critical revision. O.C.: manuscript drafting, critical revision. J.-T.O.-P.: manuscript drafting, critical revision. J.M.A.: manuscript drafting, critical revision. A.B.: study concept and design, manuscript drafting, critical revision.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local ethics committee.
Contributor Information
Giulio Falasconi, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain; Campus Clínic, University of Barcelona, C/Villarroel 170, 08024 Barcelona, Spain.
Diego Penela, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
David Soto-Iglesias, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Pietro Francia, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain; Cardiology, Department of Clinical and Molecular Medicine, St. Andrea Hospital, Sapienza University, Via di Grottarossa 1035, 00189 Rome, Italy.
Cheryl Teres, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Andrea Saglietto, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain; Department of Medical Sciences, University of Turin, Corso Dogliotti 14, 10126 Turin, Italy.
Beatriz Jauregui, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Daniel Viveros, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain; Campus Clínic, University of Barcelona, C/Villarroel 170, 08024 Barcelona, Spain.
Aldo Bellido, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Jose Alderete, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain; Campus Clínic, University of Barcelona, C/Villarroel 170, 08024 Barcelona, Spain.
Julia Meca-Santamaria, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Paula Franco, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Carlo Gaspardone, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Rodolfo San Antonio, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Marina Huguet, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Óscar Cámara, Department of Information and Communication Technologies, Pompeu Fabra University, C/Tànger 122-140, 08018 Barcelona, Spain.
José-Tomás Ortiz-Pérez, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Julio Martí-Almor, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Antonio Berruezo, Arrhythmia Department, Heart Institute, Teknon Medical Centre, C/Vilana 12, 08022 Barcelona, Spain.
Data Availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- 1. Dong XJ, Wang BB, Hou FF, Jiao Y, Li HW, Lv SPet al. . Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019. Europace 2023;25:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fabritz L, Crijns H, Guasch E, Goette A, Hausler KG, Kotecha Det al. . Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: the 7th AFNET/EHRA consensus conference. Europace 2021;23:329–44. [DOI] [PubMed] [Google Scholar]
- 3. Lee G, Baker E, Collins R, Merino JL, Desteghe L, Heidbuchel H. The challenge of managing multimorbid atrial fibrillation: a pan-European European heart rhythm association (EHRA) member survey of current management practices and clinical priorities. Europace 2022;24:2004–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nabauer M, Oeff M, Gerth A, Wegscheider K, Buchholz A, Haeusler KGet al. . Prognostic markers of all-cause mortality in patients with atrial fibrillation: data from the prospective long-term registry of the German atrial fibrillation NETwork (AFNET). Europace 2021;23:1903–12. [DOI] [PubMed] [Google Scholar]
- 5. Arbelo E, Aktaa S, Bollmann A, D'Avila A, Drossart I, Dwight Jet al. . Quality indicators for the care and outcomes of adults with atrial fibrillation. Europace 2021;23:494–5. [DOI] [PubMed] [Google Scholar]
- 6. Tonchev IR, Nam MCY, Gorelik A, Kumar S, Haqqani H, Sanders Pet al. . Relationship between procedural volume and complication rates for catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace 2021;23:1024–32. [DOI] [PubMed] [Google Scholar]
- 7. Iliodromitis K, Lenarczyk R, Scherr D, Conte G, Farkowski MM, Marin Fet al. . Patient selection, peri-procedural management, and ablation techniques for catheter ablation of atrial fibrillation: an EHRA survey. Europace 2022;25:667–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Demarchi A, Neumann L, Rordorf R, Conte G, Sanzo A, Ozkartal Tet al. . Long-term outcome of catheter ablation for atrial fibrillation in patients with severe left atrial enlargement and reduced left ventricular ejection fraction. Europace 2021;23:1751–6. [DOI] [PubMed] [Google Scholar]
- 9. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga Let al. . 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Voskoboinik A, Moskovitch JT, Harel N, Sanders P, Kistler PM, Kalman JM. Revisiting pulmonary vein isolation alone for persistent atrial fibrillation: A systematic review and meta-analysis. Heart Rhythm 2017;14:661–7. [DOI] [PubMed] [Google Scholar]
- 11. Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan Ret al. . Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med 2015;372:1812–22. [DOI] [PubMed] [Google Scholar]
- 12. Marrouche NF, Wazni O, McGann C, Greene T, Dean JM, Dagher Let al. . Effect of MRI-guided fibrosis ablation vs conventional catheter ablation on atrial arrhythmia recurrence in patients with persistent atrial fibrillation: the DECAAF II randomized clinical trial. JAMA 2022;327:2296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mulder MJ, Kemme MJB, Allaart CP. Radiofrequency ablation to achieve durable pulmonary vein isolation. Europace 2022;24:874–86. [DOI] [PubMed] [Google Scholar]
- 14. Hussein A, Das M, Riva S, Morgan M, Ronayne C, Sahni Aet al. . Use of ablation Index-guided ablation results in high rates of durable pulmonary vein isolation and freedom from arrhythmia in persistent atrial fibrillation patients: the PRAISE study results. Circ Arrhythm Electrophysiol 2018;11:e006576. [DOI] [PubMed] [Google Scholar]
- 15. Friedman DJ, Overmann JA, Fish JM, Gaeta SA, Tranter JH, Thao Ret al. . Continuous and discontinuous radiofrequency energy delivery on the atrial free wall: lesion transmurality, width, and biophysical characteristics. Heart Rhythm O2 2021;2:635–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mansour M, Calkins H, Osorio J, Pollak SJ, Melby D, Marchlinski FEet al. . Persistent atrial fibrillation ablation with contact force-sensing catheter: the prospective multicenter PRECEPT trial. JACC Clin Electrophysiol 2020;6:958–69. [DOI] [PubMed] [Google Scholar]
- 17. Taghji P, El Haddad M, Phlips T, Wolf M, Knecht S, Vandekerckhove Yet al. . Evaluation of a strategy aiming to enclose the pulmonary veins with contiguous and optimized radiofrequency lesions in paroxysmal atrial fibrillation: A pilot study. JACC Clin Electrophysiol 2018;4:99–108. [DOI] [PubMed] [Google Scholar]
- 18. Teres C, Soto-Iglesias D, Penela D, Jauregui B, Ordonez A, Chauca Aet al. . Personalized paroxysmal atrial fibrillation ablation by tailoring ablation index to the left atrial wall thickness: the ‘ablate by-LAW’ single-centre study-a pilot study. Europace 2022;24:390–9. [DOI] [PubMed] [Google Scholar]
- 19. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist Cet al. . ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J 2020;2021:373–498. [DOI] [PubMed] [Google Scholar]
- 20. Teres C, Soto-Iglesias D, Penela D, Jauregui B, Ordonez A, Chauca Aet al. . Left atrial wall thickness of the pulmonary vein reconnection sites during atrial fibrillation redo procedures. Pacing Clin Electrophysiol 2021;44:824–34. [DOI] [PubMed] [Google Scholar]
- 21. Valles-Colomer A, Rubio Forcada B, Soto-Iglesias D, Planes X, Trueba R, Teres Cet al. . Reproducibility analysis of the computerized tomography angiography-derived left atrial wall thickness maps. J Interv Card Electrophysiol 2023. doi: 10.1007/s10840-023-01472-5. Epub ahead of print. PMID: 36802003 [DOI] [PubMed] [Google Scholar]
- 22. Teres C, Soto-Iglesias D, Penela D, Falasconi G, Viveros D, Meca-Santamaria Jet al. . Relationship between the posterior atrial wall and the esophagus: esophageal position and temperature measurement during atrial fibrillation ablation (AWESOME-AF). A randomized controlled trial. J Interv Card Electrophysiol 2022;65:651–61. [DOI] [PubMed] [Google Scholar]
- 23. Gabriels J, Donnelly J, Khan M, Anca D, Beldner S, Willner Jet al. . High-frequency, low tidal volume ventilation to improve catheter stability during atrial fibrillation ablation. JACC Clin Electrophysiol 2019;5:1224–6. [DOI] [PubMed] [Google Scholar]
- 24. Garcia R, Waldmann V, Vanduynhoven P, Nesti M , Jansen de Oliveira Figueiredo M, Narayanan Ket al. . Worldwide sedation strategies for atrial fibrillation ablation: current status and evolution over the last decade. Europace 2021;23:2039–45. [DOI] [PubMed] [Google Scholar]
- 25. Falasconi G, Penela D, Soto-Iglesias D, Jauregui B, Chauca A, Antonio RSet al. . A standardized stepwise zero-fluoroscopy approach with transesophageal echocardiography guidance for atrial fibrillation ablation. J Interv Card Electrophysiol 2022;64:629–39. [DOI] [PubMed] [Google Scholar]
- 26. Pambrun T, Combes S, Sousa P, Bloa ML, El Bouazzaoui R, Grand-Larrieu Det al. . Contact-force guided single-catheter approach for pulmonary vein isolation: feasibility, outcomes, and cost-effectiveness. Heart Rhythm 2017;14:331–8. [DOI] [PubMed] [Google Scholar]
- 27. Udyavar AR, Chang SL, Tai CT, Lin YJ, Lo LW, Tuan TCet al. . The important role of pulmonary vein carina ablation as an adjunct to circumferential pulmonary vein isolation. J Cardiovasc Electrophysiol 2008;19:593–8. [DOI] [PubMed] [Google Scholar]
- 28. Kirchhof P, Auricchio A, Bax J, Crijns H, Camm J, Diener HCet al. . Outcome parameters for trials in atrial fibrillation: recommendations from a consensus conference organized by the German atrial fibrillation competence NETwork and the European Heart Rhythm Association. Europace 2007;9:1006–23. [DOI] [PubMed] [Google Scholar]
- 29. Gunawardene MA, Willems S. Atrial fibrillation progression and the importance of early treatment for improving clinical outcomes. Europace 2022;24:ii22–8. [DOI] [PubMed] [Google Scholar]
- 30. El Haddad M, Taghji P, Phlips T, Wolf M, Demolder A, Choudhury Ret al. . Determinants of acute and late pulmonary vein reconnection in contact force-guided pulmonary vein isolation: identifying the weakest link in the ablation chain. Circ Arrhythm Electrophysiol 2017;10:e004867. doi: 10.1161/CIRCEP.116.004867. PMID: 28381417 [DOI] [PubMed] [Google Scholar]
- 31. Bishop M, Rajani R, Plank G, Gaddum N, Carr-White G, Wright Met al. . Three-dimensional atrial wall thickness maps to inform catheter ablation procedures for atrial fibrillation. Europace 2016;18:376–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cabrera JA, Ho SY, Climent V, Sanchez-Quintana D. The architecture of the left lateral atrial wall: a particular anatomic region with implications for ablation of atrial fibrillation. Eur Heart J 2008;29:356–62. [DOI] [PubMed] [Google Scholar]
- 33. Di Biase L, Monir G, Melby D, Tabereaux P, Natale A, Manyam Het al. . Composite Index tagging for PVI in paroxysmal AF: a prospective, multicenter postapproval study. JACC Clin Electrophysiol 2022;8:1077–89. [DOI] [PubMed] [Google Scholar]
- 34. Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges Met al. . Pre-procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J 2007;28:836–41. [DOI] [PubMed] [Google Scholar]
- 35. D'Ascenzo F, Corleto A, Biondi-Zoccai G, Anselmino M, Ferraris F, di Biase Let al. . Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation?: a meta-analysis. Int J Cardiol 2013;167:1984–9. [DOI] [PubMed] [Google Scholar]
- 36. McCready JW, Smedley T, Lambiase PD, Ahsan SY, Segal OR, Rowland Eet al. . Predictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace 2011;13:355–61. [DOI] [PubMed] [Google Scholar]
- 37. Masuda M, Fujita M, Iida O, Okamoto S, Ishihara T, Nanto Ket al. . Influence of underlying substrate on atrial tachyarrhythmias after pulmonary vein isolation. Heart Rhythm 2016;13:870–8. [DOI] [PubMed] [Google Scholar]
- 38. Huo Y, Gaspar T, Schönbauer R, Wójcik M, Fiedler L, Roithinger Franz Xet al. . Low-voltage myocardium-guided ablation trial of persistent atrial fibrillation. NEJM Evidence 2022;1:EVIDoa2200141. [DOI] [PubMed] [Google Scholar]
- 39. Jadidi AS, Lehrmann H, Keyl C, Sorrel J, Markstein V, Minners Jet al. . Ablation of persistent atrial fibrillation targeting low-voltage areas with selective activation characteristics. Circ Arrhythm Electrophysiol 2016;9:e002962. doi: 10.1161/CIRCEP.115.002962. [DOI] [PubMed] [Google Scholar]
- 40. Takahashi K, Okumura Y, Watanabe I, Nagashima K, Sonoda K, Sasaki Net al. . Relation between left atrial wall thickness in patients with atrial fibrillation and intracardiac electrogram characteristics and ATP-provoked dormant pulmonary vein conduction. J Cardiovasc Electrophysiol 2015;26:597–605. [DOI] [PubMed] [Google Scholar]
- 41. Hall B, Jeevanantham V, Simon R, Filippone J, Vorobiof G, Daubert J. Variation in left atrial transmural wall thickness at sites commonly targeted for ablation of atrial fibrillation. J Interv Card Electrophysiol 2006;17:127–32. [DOI] [PubMed] [Google Scholar]
- 42. Nakamura K, Funabashi N, Uehara M, Ueda M, Murayama T, Takaoka Het al. . Left atrial wall thickness in paroxysmal atrial fibrillation by multislice-CT is initial marker of structural remodeling and predictor of transition from paroxysmal to chronic form. Int J Cardiol 2011;148:139–47. [DOI] [PubMed] [Google Scholar]
- 43. Inoue J, Skanes AC, Gula LJ, Drangova M. Effect of left atrial wall thickness on radiofrequency ablation success. J Cardiovasc Electrophysiol 2016;27:1298–303. [DOI] [PubMed] [Google Scholar]
- 44. Mulder MJ, Kemme MJB, Hagen AMD, Hopman L, van de Ven PM, Hauer HAet al. . Impact of local left atrial wall thickness on the incidence of acute pulmonary vein reconnection after ablation index-guided atrial fibrillation ablation. Int J Cardiol Heart Vasc 2020;29:100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Saglietto A, Ballatore A, Xhakupi H, Rubat Baleuri F, Magnano M, Gaita Fet al. . Evidence-based insights on ideal blanking period duration following atrial fibrillation catheter ablation. Europace 2022;24:1899–908. [DOI] [PubMed] [Google Scholar]
- 46. Bordignon S, Barra S, Providencia R, de Asmundis C, Marijon E, Farkowski MMet al. . The blanking period after atrial fibrillation ablation: an European Heart Rhythm Association survey on contemporary definition and management. Europace 2022;24:1684–90. [DOI] [PubMed] [Google Scholar]
- 47. Kalarus Z, Mairesse GH, Sokal A, Boriani G, Sredniawa B, Casado-Arroyo Ret al. . Searching for atrial fibrillation: looking harder, looking longer, and in increasingly sophisticated ways. An EHRA position paper. Europace 2023;25:185–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar Pet al. . How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PubMed] [Google Scholar]
- 49. Manninger M, Zweiker D, Svennberg E, Chatzikyriakou S, Pavlovic N, Zaman JABet al. . Current perspectives on wearable rhythm recordings for clinical decision-making: the wEHRAbles 2 survey. Europace 2021;23:1106–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.