Abstract
Aims
Familial ST-segment Depression Syndrome (Fam-STD) is a novel inherited cardiac disease associated with arrhythmias and sudden cardiac death. This study aimed at investigating the cardiac activation pathway in patients with Fam-STD, modelling the electrocardiogram (ECG) phenotype, and performing in-depth ST-segment analyses.
Methods and results
CineECG analysis of patients with Fam-STD and age- and sex-matched controls. The groups were compared using the CineECG software which included the trans-cardiac ratio and the electrical activation pathway. We simulated the Fam-STD ECG phenotype by adjusting action potential duration (APD) and action potential amplitude (APA) in specific cardiac regions. High-resolution ST-segment analyses were performed per lead by dividing the ST-segment into nine 10 ms subintervals. Twenty-seven Fam-STD patients (74% females, mean age 51.6 ± 6.2 years) and 83 matched controls were included. Among Fam-STD patients, electrical activation pathway analysis in the anterior-basal orientation showed significantly abnormal direction toward the basal areas of the heart starting from QRS 60–89 ms until Tpeak-Tend (all P < 0.001). Simulations with shortened APD and reduced APA in the left ventricle basal regions recapitulated the Fam-STD ECG phenotype. Detailed ST-segment analyses showed significant differences in all nine 10 ms subintervals (all P < 0.01), with the most prominent findings during the 70–79/80–89 ms intervals.
Conclusion
CineECG analyses indicated abnormal repolarization with basal directions, and the Fam-STD ECG phenotype was simulated by reducing APD and APA in the left ventricle basal regions. Detailed ST-analysis showed amplitudes consistent with the proposed diagnostic criteria for Fam-STD patients. Our findings provide new insight into the electrophysiological abnormalities of Fam-STD.
Keywords: Familial ST-segment Depression Syndrome, CineECG, Electrocardiogram, Arrhythmia, Electrical activation pathway, ECGsim
Graphical Abstract
Graphical Abstract.
What’s new?
Familial ST-segment Depression Syndrome (Fam-STD) patients have significantly increased electrical activation pathways towards the base of the heart during the entire repolarization phase.
The Fam-STD electrocardiogram (ECG) phenotype can be simulated by reducing the action potential amplitude and action potential duration in the left ventricular basal areas.
The most pronounced ST-depressions in Fam-STD were found in leads V5, V4, and II at 70–89 ms after the nadir of the S-wave.
Introduction
Familial ST-segment Depression Syndrome (Fam-STD), first reported in 2018, is a recently identified inherited cardiac arrhythmia, characterized by persistent, non-ischaemic ST-segment depressions and risk of atrial fibrillation, ventricular arrhythmias, and sudden cardiac death.1–3 The disease adds to the spectrum of cardiogenetic disorders diagnosed based on specific electrocardiographic patterns4–6— however, the pathophysiology of Fam-STD is still unknown.
The electrocardiogram (ECG) has been used in clinical cardiology for more than a century and has continuously been refined to increase resolution and allow a three-dimensional evaluation.7,8 A recently developed ECG modality, termed CineECG,9 enables computation of the electrical activation pathway (previously termed mean Temporal Spatial Isochrones) derived from the ECG and anatomical localization of the electrical activity in the ventricular myocardium.10 The electrical activation pathways are derived from the vectorcardiogram and applied to a standard model of the cardiac anatomy.11 CineECG is thus able to relate clinical notable components of cardiac activation to the cardiac anatomy12 and is rather robust to electrode misplacement.12 CineECG has previously found increased electrical activity in the right ventricular outflow tract, during the end of the QRS complex, in spontaneous and ajmaline-induced Brugada Syndrome patients11 and accurately differentiate between normal depolarization and bundle branch blocks.9 An important benefit of the CineECG modality is the easy application and reliance on the standard 12-lead ECG. Indeed, advanced analyses of the 12-lead ECG have recently been proved to provide a similar diagnostic yield as multi-electrode body surface mapping for assessing the standard deviation of activation time.13 CineECG analysis may therefore reveal new features or traits of Fam-STD syndrome that could potentially improve the understanding of the underlying electrophysiological abnormalities and improve the diagnostic process.
In this study, we examined the cardiac electrical activity pathway in patients with Fam-STD. We furthermore performed modelling of the ECG phenotype and performed an in-depth analysis of the ST-segment in Fam-STD patients.
Methods
Study population
All patients included in the current study were consecutive patients from Eastern Denmark and fulfilled the proposed criteria for Familial ST-segment Depression Syndrome.3 Patients had to display constant, concave-shaped, unexplained ST-depression in multiple leads and have proved familial occurrence of the ECG phenotype, consistent with proposed diagnostic criteria.3 Both probands and affected relatives were eligible for inclusion if a digital ECG was available. The control group was gathered from the open online Physionet-XL-PTB-Diagnostic ECG dataset,14 matched to the patient group by age and gender. The dataset contains 21 837 clinical 12-lead ECG 10 s recordings. The database was split into five coarse superclasses with healthy ECG recordings constituting ≈25% of the database; this subset was used for the current project.
Clinical investigations and handling of Fam-STD patients have been previously described.3 The current study complied with the Helsinki Declaration-II, and The Patient Safety Authority (3–3013–2380/1) approved the study.
Selection of electrocardiograms for analysis
All available electronic 12-lead ECGs from identified patients were evaluated. All ECGs were 10 s recordings obtained at a sampling rate of 500 Hz. For the primary analysis, the most recent ECG for each individual was included if the following criteria were met: (i) sufficient signal quality, (ii) no bundle branch block or ventricular pacing, and (iii) no ongoing arrhythmia (atrial fibrillation/flutter, premature ventricular contractions or ventricular tachycardia/fibrillation). All ECGs were manually inspected and selected by cardiologists with extensive experience in ECG interpretation. The study and control groups were matched by age and sex.
Standard electrocardiographic features
Initially, standard ECG features were studied: heart rate (beats per minute, bpm), QRS duration (ms), QT-interval (ms), Bazett corrected QT (QTc (Bazett)), time of peak T-wave (time from QRS onset to peak of T-wave, ms) and R- and S-wave maximum amplitudes in leads V1 to V6 (mV).
CineECG analytic setup
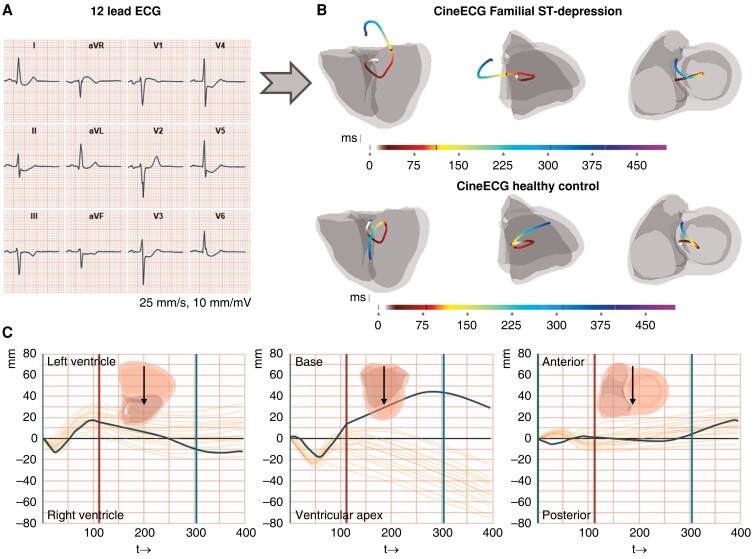
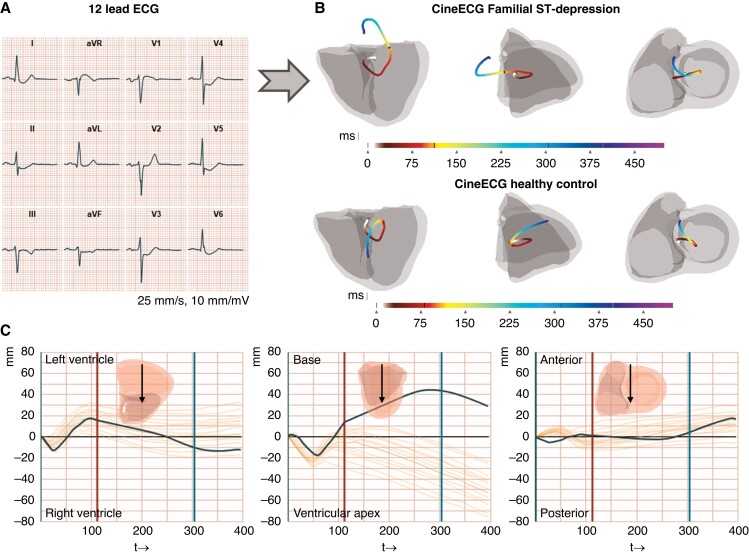
The ECGs were analysed utilizing the software ‘CineECG’, version 0.1.05862 M (pre-release), created by van Dam et al.10 CineECG is a computational model system that analyses standard 12 lead ECGs and calculates a range of quantitative parameters based on a mean representative QRS complex to the end of the T-wave. CineECG also produces a graphical 3D output that directly visualizes the temporo-spatial features of the cardiac activation and recovery, and integrates the electrical features with a standardized cardiac anatomical model;9 the standard cardiac X-ray projections (excluding the atria) four-chamber and right anterior and left anterior oblique views are used. The start of the QRS complex was defined as a time point of 0 ms, and QRS and ST-intervals were relative to this starting point. A constant velocity of 0.7 m/s in the 3D direction was used for myocardial electrical activation velocity in the model system.9 For a general overview of the CineECG computations, see Figure 1.
Figure 1.
Graphical abstract. CineECG analysis in Familial ST-segment Depression Syndrome. A) A representative median heartbeat of a Familial ST-segment Depression Syndrome (Fam-STD) patient with large ST-depressions in I–II, aVF, and V3–V6. B) Visualization of the electrical pathway derived from a representative Fam-STD patient and a healthy control individual. The electrical activation pathway is shown by the coloured lines starting at 0 ms (start-QRS) and ending at end of the T-wave, units in milliseconds. Four-chamber, right, and left anterior oblique projections are shown. C) Electrical activation pathway analysis of a representative Fam-STD tracing. The co-ordinate system with time (ms) on the x-axis and direction (millimetres) on the y-axis includes a 95% normal range based on the control group.
CineECG parameters
Trans-cardiac ratio
The trans-cardiac ratio (TCR) described the length between the start and endpoints in a straight line relative to the length of the electrical activation pathway in the heart model. The TCR result is given as a ratio of the total distance travelled within the given interval. When the ratio is low, the distance between the start and endpoints is small relative to the total length of the electrical activation pathway in the given interval (see Supplementary material online, Figure S1). A normal cardiac activation is usually associated with a TCR below 40% for the QRS complex.11 Two measurements of TCR were studied: TCR-QRS (distance ratio from 0 ms to nadir of S-wave) and TCR ST–T (distance ratio from the nadir of S-wave to end of T-wave).
Electrical activation pathway—direction
The electrical activation pathway direction described the direction of activation and recovery of the electrical activity throughout the ECG, in segments ranging from start-QRS to the end of the T-wave. The electrical activation pathway directions were determined every millisecond for each of the three orientations: posterior–anterior (PA), right–left (RL), and apex–base (AB). A positive value denoted an electrical activation pathway towards the anterior, left, or base of the heart. The included parameters were three QRS intervals (0–29, 30–59, and 60–89 ms), two ST-segment intervals (90–129 and 130–169 ms), 170 ms-Tpeak (170 ms to 1 ms before the peak of T-wave), and Tpeak-Tend (interval from the peak of T-wave to end of T-wave).
Electrical activation pathway—location
The electrical activation pathway location was used to describe the average location of all cardiac electric activity during the activation and repolarization phases.15 In a healthy heart, the electrical activation pathway starts in the left ventricular septal location, moves transseptal, and then towards the apex of the left ventricle (LV). The electrical activation pathway location parameter described the percentage of time spent by the electrical activation pathway in each of the three cardiac regions [septum, LV, and right ventricle (RV)] in a given time interval. A detailed description of the electrical activation pathway computation has been described thoroughly.15 Seven specific parameters were applied for the electrical activation pathway location; covering the ECG complex from QRS-start to the end of the T-wave allowing precise pinpointing of differences in time spent, per location, between groups. The seven analysed parameters were identical to those described for electrical activation pathway direction: QRS 0–29, 30–59, and 60–89 ms, ST-segment 90–129 and 130–169 ms, 170 ms-Tpeak, and Tpeak-Tend.
ECGsim modelling
ECGsim, an open-source simulation software (www.ecgsim.org) version 3.1.0.5039 (pre-release), was used for modelling regional interactive changes in action potential parameters, i.e. action potential amplitude (APA) and action potential duration (APD) on the ventricular surface.16 The model was based on recordings from a healthy 22-year-old male and uses an equivalent dipole layer source model on the myocardial surface to simulate local electrical currents from the myocardium. The resulting potentials within the volume conductor of the body were computed using a boundary element-derived transfer matrix. The ECGsim software and methodology have previously been described thoroughly.17,18 In the current study, ECGsim was used to simulate which changes in APA and APD were necessary to achieve ECG patterns similar to the Fam-STD ECG phenotype.
Detailed ST-segment analysis
Detailed investigation of the ST-segment amplitudes [in millivolts (mV)] was performed in the Fam-STD and control groups. The ST-segment was divided into nine subintervals each of 10 ms duration for all 12 leads, starting at the nadir of the S-wave. We chose the nadir of the S-wave as our reference point for these analyses as this is often used as a point of measurement to the J-point in ECG software and is a more reproducible point of measurement. A positive value indicated ST-segment elevation while a negative value indicated ST-segment depression.
Statistics
Microsoft Excel (Redmond, Washington, USA) and R studio (v.4.1.2; RStudio IDE, Boston, MA, USA) software were used for the analyses. Continuous variables are presented as means ± standard deviation and categorical data as absolute numbers (percentages). Histograms and the Shapiro–Wilk test were used to test for normal distribution and comparisons between groups were performed with unpaired two-sample t-test and Mann–Whitney U test, when appropriate. Differences in patient characteristics and the detailed ST-segment analysis had to reach a P-value of <0.05 to be considered statistically significant. For the standard ECG and CineECG results, we corrected for multiple comparisons using the Bonferroni correction. With 17 standard ECG results and alpha set at 0.05, the standard ECG results, therefore, had to reach a P-value of <0.003 (0.05/17) to be considered statistically significant. For the 44 investigated CineECG parameters and alpha set at 0.05, the CineECG results had to reach a P-value of <0.001 (0.05/44) to be considered statistically significant.
Results
Baseline characteristics of the study population
A total of 27 Fam-STD patients (20 females, 74%) were included with a mean age of 51.6 ± 6.2 years at the time of ECG recording. The mean age for females and males was 54.1 ± 6.7 and 44.4 ± 14.0 years, respectively. A total of 83 controls (60 females, 72%) were included with a mean age of 51.4 ± 1.0 years (54.1 ± 0.3 and 44.4 ± 0.3 years, for females and males, respectively, Table 1). In the Fam-STD group, the mean left ventricular ejection fraction was 54.1 ± 5.6%, six received beta-blockers, one was treated with digoxin, and none was treated with class 1C antiarrhythmic agents or amiodarone.
Table 1.
Baseline characteristics of the study population
| Fam-STD (n = 27) | Controls (n = 83) | P-value | |
|---|---|---|---|
| Sex, female | 20 (74%) | 60 (72%) | 0.861 |
| Age, years | 51.6 ± 6.2 | 51.4 ± 1.0 | 0.203 |
| Height, cm | 171.5 ± 8.0 | 166.5 ± 9.0a | 0.011 |
| Weight, kg | 69.9 ± 15.8 | 74.3 ± 14.7 | 0.206 |
Continuous data are presented as mean ± standard deviation and categorical data presented as absolute numbers (percentages). Statistically significant P-values are marked in bold.
Data available in 67 individuals.
Standard electrocardiographic features
Analysis of standard ECG features showed significant differences in heart rate (63 ± 12 vs. 71 ± 9 bpm, P < 0.003) and Bazett corrected QT (QTc (Bazett)) (396 ± 21 vs. 414 ± 16, P < 0.003), but no significant differences in QRS duration, uncorrected QT interval, or time to Peak T wave between patients and controls, respectively (all P > 0.05, Supplementary material online, Table S1). In R-wave maximum amplitudes, no significant differences were found, while S-wave maximum amplitudes were significantly different in V4–V6 (all P < 0.003, Supplementary material online, Table S1).
CineECG findings
Trans-cardiac ratio
We observed a significant difference in TCR ST–T (54.4 ± 22.7 vs. 80.6 ± 15.6, P < 0.001; threshold 0.05/44 = 0.001) between Fam-STD patients and controls, indicating a reduced relative distance travelled between nadir of the S-wave to the end of the T-wave. This shows that for the ST–T segment in Fam-STD patients, the linear distance between the start and endpoints was smaller relative to the total length of the electrical activation pathway. No difference in TCR QRS was found (P > 0.001; Table 2).
Table 2.
CineECG results
| Fam-STD | Controls | P-value | |
|---|---|---|---|
| Trans-cardiac ratio, QRS, % | 28.8 ± 13.7 | 35.7 ± 17.2 | 0.070 |
| Trans-cardiac ratio, ST–T, % | 54.4 ± 22.7 | 80.6 ± 15.6 | <0.001 |
| QRS 0–29 ms, direction PA | 0.156 ± 0.237 | 0.282 ± 0.176 | 0.014 |
| QRS 0–29 ms, direction RL | −0.196 ± 0.469 | −0.29 ± 0.353 | 0.347 |
| QRS 0–29 ms, direction AB | −0.764 ± 0.303 | −0.79 ± 0.245 | 0.518 |
| QRS 30–59 ms, direction PA | −0.089 ± 0.275 | −0.109 ± 0.203 | 0.728 |
| QRS 30–59 ms, direction RL | 0.825 ± 0.145 | 0.864 ± 0.143 | 0.304 |
| QRS 30–59 ms, direction AB | −0.053 ± 0.473 | −0.234 ± 0.356 | 0.088 |
| QRS 60–89 ms, direction PA | −0.126 ± 0.173 | −0.263 ± 0.318 | 0.001 |
| QRS 60–89 ms, direction RL | 0.13 ± 0.395 | 0.413 ± 0.359 | 0.001 |
| QRS 60–89 ms, direction AB | 0.865 ± 0.204 | 0.621 ± 0.386 | <0.001 |
| ST 90–129 ms, direction PA | 0.006 ± 0.12 | 0.085 ± 0.396 | 0.178 |
| ST 90–129 ms, direction RL | −0.461 ± 0.222 | −0.422 ± 0.45 | 0.492 |
| ST 90–129 ms, direction AB | 0.806 ± 0.28 | −0.018 ± 0.681 | <0.001 |
| ST 130–169 ms, direction PA | 0.031 ± 0.143 | 0.275 ± 0.224 | <0.001 |
| ST 130–169 ms, direction RL | −0.523 ± 0.219 | −0.572 ± 0.287 | 0.224 |
| ST 130–169 ms, direction AB | 0.762 ± 0.287 | −0.474 ± 0.494 | <0.001 |
| 170 ms-Tpeak, direction PA | 0.308 ± 0.334 | 0.346 ± 0.133 | 0.682 |
| 170 ms-Tpeak, direction RL | −0.515 ± 0.388 | −0.233 ± 0.192 | <0.001 |
| 170 ms-Tpeak, direction AB | 0.364 ± 0.515 | −0.873 ± 0.103 | <0.001 |
| Tpeak-Tend, direction PA | 0.22 ± 0.34 | 0.22 ± 0.1 | 0.928 |
| Tpeak-Tend, direction RL | 0.08 ± 0.51 | 0.16 ± 0.16 | 0.928 |
| Tpeak-Tend, direction AB | −0.47 ± 0.61 | −0.94 ± 0.03 | <0.001 |
Continuous data are presented as mean ± standard deviation. Significance level set at P < 0.001 (0.05/44 = 0.001 after correction for multiple testing). Statistically significant P-values are marked in bold.
NS, non-significant.
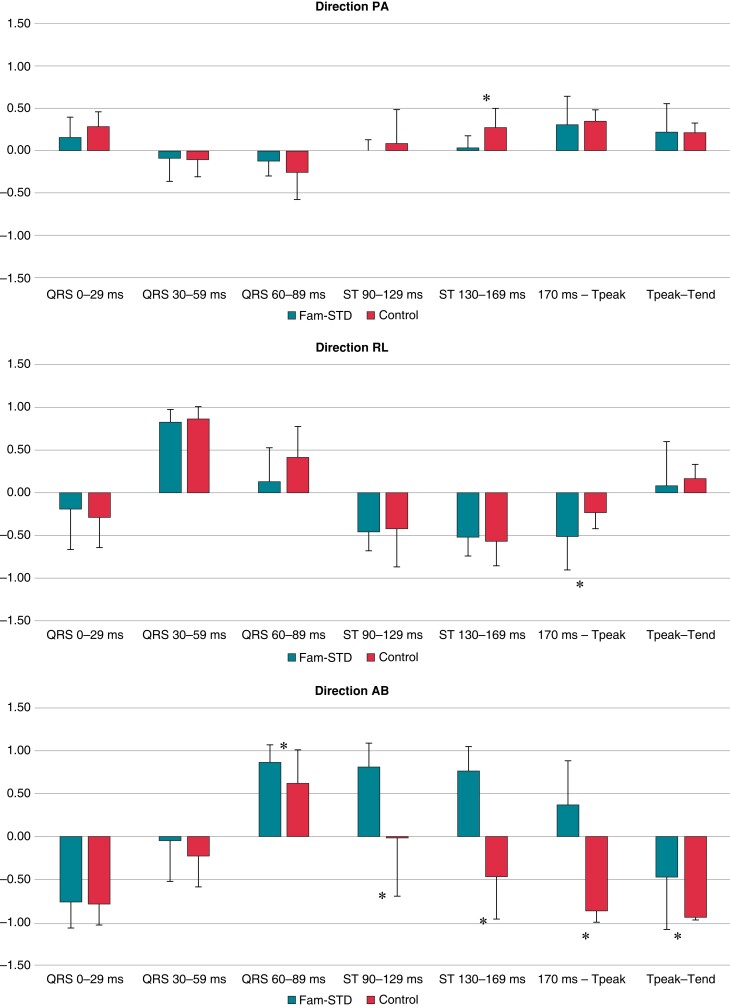
Electrical activation pathway—directions
Direction posterior to anterior
Analyses of QRS 0–29/30–59/60–89 ms and ST 90–129 ms intervals did not show any significant differences between patients and controls (all P > 0.001; Table 2 and Figure 2). A significantly abnormal direction was found in the late ST-segment phase 130–169 ms (0.031 ± 0.143 vs. 0.275 ± 0.224, P < 0.001) that was consistent with that of the Fam-STD patients having reduced distance in the electrical activation pathway in the posterior–anterior direction during the early phase of repolarization. No significant findings were observed for 170 ms-Tpeak and Tpeak-Tend measurements (both P > 0.001).
Figure 2.
Analysis of the electrical activation pathway directions. Significant differences for direction PA (ST 90–129 ms), direction RL (170 ms-Tpeak), and direction AB (QRS 60–89, ST 90–129, ST 130–169 ms, 170 ms-Tpeak, and Tpeak-Tend) were observed. Data are presented as means with error bars illustrating standard deviation. *P < 0.001 (0.05/44 = 0.001 after correction for multiple testing). Fam-STD, Familial ST-segment Depression Syndrome.
Direction right to left
Comparable with the findings from direction PA we found, no significant differences for the analysed QRS 0–29/30–59/60–89 ms intervals (all P > 0.001) consistent with the overall depolarization was similar between groups in the right–left direction. Furthermore, no significant differences were seen during ST 90–129/130–169 ms intervals (all P > 0.001), indicative of no differences in amplitudes in the period between depolarization and repolarization. A significantly different direction occurred during 170 ms-Tpeak (−0.515 ± 0.388 vs. −0.233 ± 0.192, P < 0.001) between Fam-STD and controls indicative of an increased distance in the electrical activation pathway towards the right side compared to controls indicative of abnormal repolarization. No significant differences between groups for the Tpeak-Tend measurement were found (P > 0.001).
Direction apex to base
Comparable with PA and RL directions, we did not find any significant direction differences in QRS 0–29/30–59 ms (both P > 0.001). However, we observed significant differences, however small, in the direction of the late QRS (60–89 ms), and significant differences in early ST (90–129/130–169 ms), and even in the T-wave (170 ms-Tpeak and Tpeak-Tend) (all P < 0.001). The results from the AB direction indicate that Fam-STD patients, starting from the terminal part of the QRS complex, have an increased distance in the electrical activation pathway towards the base of the heart compared to controls, suggesting that the abnormalities occur from early repolarization onwards. Notably, the AB direction during ST-segment 90–129/130–169 and 170 ms-Tpeak was opposite between the two groups, with Fam-STD patients pointing toward the base while controls were consistently moving toward the apex (Figure 1B).
Electrical activation pathway—locations
For the electrical activation pathway septal location, there were trends towards differences for 170 ms-Tpeak and Tpeak-Tend, but the differences were not significant after correction for multiple testing (P = 0.0015 and 0.0294; threshold 0.05/44 = 0.001). For the electrical activation pathway, location LV, no significant or major trends between groups were observed. For the electrical activation pathway RV location, there were trends towards differences for QRS 0–29 ms, ST 90–129/130–169 ms, 170 ms-Tpeak, and Tpeak-Tend, but none remained significant after correction for multiple testing (all P > 0.001). Taken together, the electrical activation pathway location results did not support any relative difference between patients and controls for time spent in the septum, LV, or RV in the CineECG model system. All data are shown in Supplementary material online, Table S2 and Supplementary material online, Figure S2.
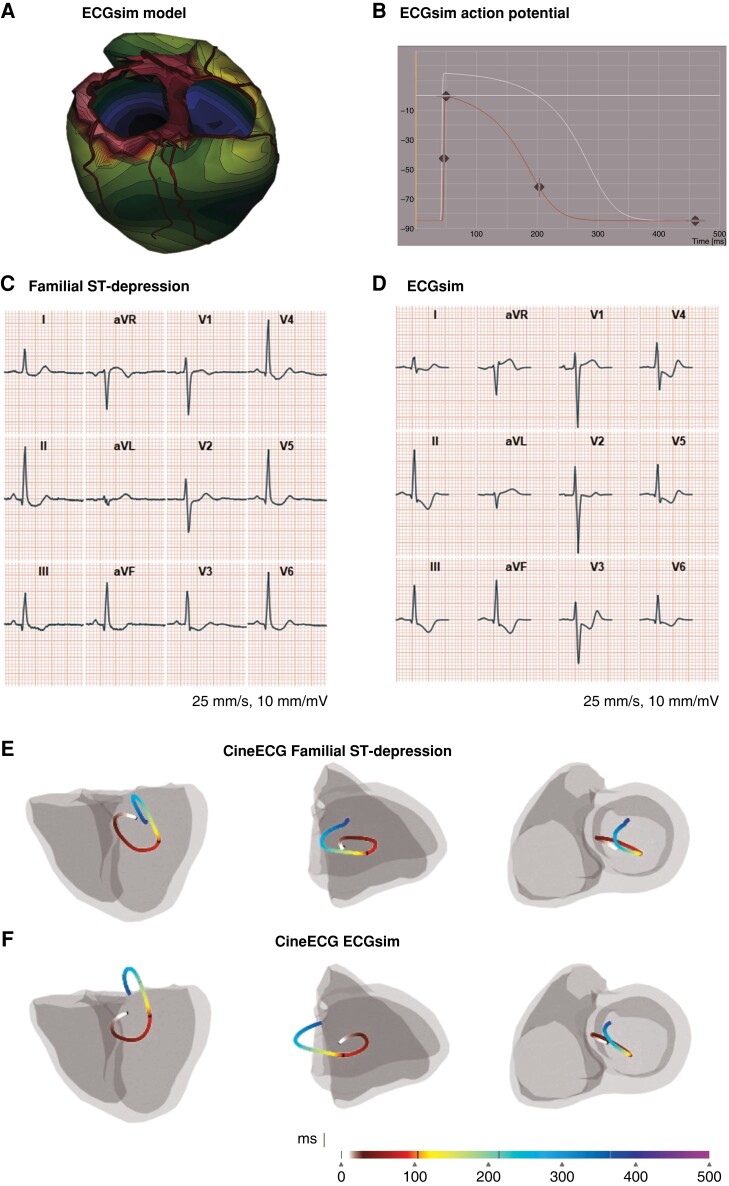
ECGsim findings
The observed electrical activation pathway direction of the ST-segment was found towards the base, and we, therefore, hypothesized that abnormalities in the basal regions of the heart were important for the observed ECG phenotype. For the selected region, the APA was reduced by ∼15 mV, and the APD was shortened by 100 ms (Figure3A and B). We tested multiple action potential settings in the left ventricle’s basal areas, including increasing the APA by 15 mV and keeping the APD at 300 ms, which resulted in modest ST-elevation in multiple leads (see Supplementary material online, Figure S3A). Furthermore, we applied the optimal settings from the basal areas to the left and right ventricles, respectively. However, this resulted in marked ST-elevation in leads II, III, aVF and variable degrees of ST-depression in aVR, aVL, and V1–V4, clearly not representative of the Fam-STD ECG phenotype (see Supplementary material online, Figure S3B and C). Only when the left basal region was selected (Figure 3A), including the basal areas around the aortic valve and mitral valve, the typical Fam-STD ECG was reproduced (Figure3C and D; the ECG presented as a comparator was recorded in a 27-year-old female). The modelled ECG had similar features as the human ECG with marked ST-segment depressions in leads I–III, V3–V6, and ST-segment elevation in aVR and V1 although the downward slope of the initial ST-segment depression was steeper in the modelled ECG. When analysing the simulated ECG using the CineECG algorithm, the electrical activation pathways were very similar (Figure3E and F).
Figure 3.
Simulation of the Familial ST-segment Depression Syndrome ECG phenotype. A) ECGsim, the purple areas have reduced the action potential amplitude (APA, mV) and shortened action potential duration (APD, ms). B) ECGsim, showing the adjustments to the action potential in the selected purple area from panel A. Action potential duration (APD, ms) is shown on the x-axis, and the action potential amplitude (APA, mV) on the y-axis. C) ECG complex from a 27-year-old female Familial ST-segment Depression Syndrome (Fam-STD) patient. D) ECG complex from the simulated ECG. E) Electrical activation pathway, derived from 27-year-old female Fam-STD ECG complex. The electrical activation pathway is shown by the coloured lines starting at 0 ms (start-QRS) and ending at end of the T-wave, units in milliseconds. F) The electrical activation pathway, derived from a simulated Fam-STD ECG, showed a very similar pattern as observed in panel E.
Detailed ST-segment analysis
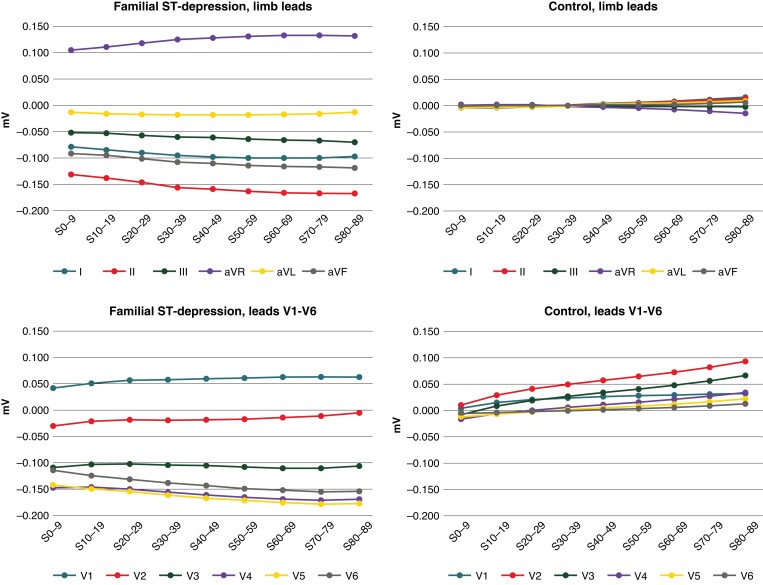
Systematic ST-segment analysis in the Fam-STD group showed the largest ST-deviations in leads V5, V4, and II, and the smallest changes in leads aVL, III, and V2 (Figure 4 and Supplementary material online, Table S3). Temporal analysis showed a gradual increase in ST-deviations with increasing distance from the nadir of the S-wave. The largest ST-deviations were observed in the 70–79 and 80–89 ms time intervals where the mean ST-deviations were −0.178/−0.177 mV in V5, −0.171/−0.169 mV in V4, and −0.167/−0.167 mV in II.
Figure 4.
Detailed ST-segment analyses. Detailed ST-segment analyses of Familial ST-segment Depression Syndrome patients and controls with the ST-segment divided into 10 ms intervals. Time intervals are shown on the x-axis (ms; 0 marks the nadir of the S-wave) and ST-segment amplitudes (in millivolts) on the y-axis. Significant differences (P < 0.01) between patients and controls were found across all nine investigated time intervals.
In the control group, analysis of leads I–aVF showed that the ST-segments were generally isoelectric with small deviations at the end of the ST-segment, towards the start of the T-wave (Figure 4 and Supplementary material online, Table S3). For leads V1–V6, there was discrete ST-elevation in the final phase of the interval towards the T-wave, specifically at the 70–79 and 80–89 ms intervals of the ST-segment, with 0.082/0.093 mV of ST-elevation in V2 and 0.056/0.066 mV in V3. Comparison of ST-deviations per time intervals between Fam-STD patients and controls showed significant differences for all nine investigated time intervals (all P < 0.01).
Discussion
This is the first study using the CineECG analytic pipeline in Fam-STD. The analyses have provided new insight not visible from the standard 12-lead ECG, through a three-dimensional analysis of the electrical activation pathway, an analysis of the temporal abnormalities in the electrical activation pathway, and the high-resolution analysis of ST-segment deviations. We found marked differences in the electrical activation pathway in all directions, most prominent in the apex–base direction from the early repolarization to the end of repolarization. However, the differences found in the terminal part of the QRS complex were small, and the Fam-STD phenotype is therefore primarily confined to the repolarization phase. Combined, these findings illustrate electrical abnormalities during the entire repolarization phase in the Fam-STD group. ECG simulations indicated that reduced APD and APA in the basal regions of the left ventricle were necessary to recapitulate the Fam-STD ECG phenotype. Finally, the detailed ST-segment analysis showed significant differences across all leads with the largest differences in leads V5, V4, and II.
We found multiple significant differences in the CineECG data among Fam-STD patients compared with controls. The TCR ST–T value was significantly lower indicating a reduced relative travelled distance of the electrical impulse in this phase of the ECG, likely caused by the abnormal directions of the electrical activity during the repolarization. The reduced TCR values in the Fam-STD patients indicate that the early ST–T segment was in the opposite direction compared to the terminal T-wave. We hypothesized that the Fam-STD ECG phenotype was caused by changes in the electrical behaviour of the basal parts of the heart. For this purpose, we used ECGsim to simulate changes in the basal regions of the left ventricle. We were able to recreate ECG patterns with very similar features, although not completely identical to those observed in Fam-STD patients—one limitation of the ECGsim model is that it is based on a single 22-year-old healthy male. Large localized electrical differences, such as displayed in the ECGsim model, likely require areas of electrical isolation in order to maintain electrical gradients. When comparing the electrical activation pathway data, the Fam-STD and simulated ECGs were very similar, showing comparative patterns in electrical activity. By applying the modifications of the action potential to other areas of the heart, we saw vast differences in the simulated ECGs underlining the notion of the electrical abnormalities being located to the left ventricle’s basal areas. Furthermore, we recently reported invasive electrophysiological data (EPS) from Fam-STD patients3 and documented ventricular arrhythmias originating from the left ventricular basal areas, consistent with the in silico data presented in the current study. However, in the later phases of Fam-STD, significant left ventricular systolic dysfunction has been noted in several patients suggesting either progression to a more generalized myocardial involvement and/or myocardial dysfunction secondary to longstanding abnormal electrical activation, a mechanism previously described for left bundle branch block (LBBB)-induced cardiomyopathy.19
CineECG analyses in both LBBB and Brugada Syndrome (BrS) have previously shown strong electrical activation pathway directions towards the location of abnormal conduction.11 In BrS, the electrical activation pathway was directed towards the right ventricular outflow tract during the late QRS, and part of the ST-segment, to return to the apex of the heart during the T-wave. The difference between the normal and BrS electrical activation pathway trajectories occurred in the same time intervals as in this present study; however, Fam-STD patients had abnormal trajectories until the end of the T-wave. In LBBB, the electrical activation pathway was directed towards the left ventricular wall and inside the LV cavity.9 Furthermore, the CineECG method was able to differentiate between different intraventricular conduction defects, which suggests that CineECG can be used as a non-invasive mapping tool and may improve early recognition of conduction defects.9 The results from the BrS11 and bundle branch block9 studies, combined with the current data and simulations, indicate the electrical activation pathway points toward the location of abnormalities within the heart. It is important to underline that CineECG analysis is not suggested, nor recommended, as a diagnostic test of Fam-STD, but is a research tool for gaining further insight into the underlying electrophysiological abnormalities.
In BrS, loss of action potential (AP) dome occurs only in the epicardium in a localized area, which generates a transmural voltage gradient, manifesting as a ST-segment elevation.20 If the opposite occurs, a loss of AP dome caused by reduced AP dome in the endocardium, the likely result would be ST-segment depression. In line with these previous results, the loss of AP dome could also explain the reduced APA required in the ECG simulations to recapitulate the Fam-STD ECG phenotype. The reduced APA, and thereby the AP dome, may be caused by altered ionic homeostasis in e.g. Na+, Ca2+, and/or K+ currents. Specifically, in BrS, the loss of the AP dome largely depends on the intrinsic Ito amplitude which is prominent in the right ventricular epicardium.20 If similar imbalances occurred in the endocardium of the left ventricular basal regions, it may result in reduced AP dome causing ST-segment depressions. Structural discontinuities at the right ventricle’s subepicardium have been shown to result in excitation failure and activation delay through current-to-load mismatch, which also presents as the Brugada pattern on the ECG.21 It is possible that a similar mechanism could exist in Fam-STD, possibly affecting the left ventricle, however, we have yet to find significant proof of structural abnormalities, in particular in the early phases of the disease. We cannot exclude that a reduced resting membrane potential could contribute to the observed ST-depression, however, it is important to emphasize that from the clinical perspective, ischaemic heart disease, myocardial hypertrophy, and myocardial fibrosis all have been very uncommon findings in Fam-STD patients, leading us to conclude that other unrelated mechanisms are at play in Fam-STD. In BrS, conduction delay is associated with accumulation of adipocytes and interstitial fibrosis in the RVOT area, which is seen as a predictor of major arrhythmic events in patients with BrS.22 It is possible that similar mechanisms exist in Fam-STD, however, additional evidence from e.g. comprehensive histological cardiac examination, advanced mapping and imaging, in vitro modelling, etc. is required for substantiating these theories. Future knowledge on the underlying molecular genetic cause, myocardial tissue analyses, etc. in Fam-STD will likely provide important insight into these mechanisms.
The ST-segment analysis is a cornerstone in the diagnosis of Fam-STD and proposed diagnostic criteria include unexplained, constant, concave-upward, ST-depressions ≥0.1 mV in at least four leads (V3–V6 and I–III) measured 80 ms after the J-point.3 Thorough ST-segment analysis showed significant ST-segment differences across all leads and in all investigated time intervals. These findings confirm and elaborated previous findings:2,3 (i) the largest ST-depressions were indeed in leads V5, V4, and II, (ii) discrete ST-elevation in V1 occurred consistently, and (iii) temporal analyses showed the largest ST-deviations were in the last part of the ST-segment, 70–89 ms after the nadir of the S-wave. In the current project we measured from the nadir of the S-wave but it is important to note that the recommended measurement point of 80 ms after the J-point includes the 70–89 ms interval in the current paper (with measurements performed from the S-nadir).
Study limitations
Fam-STD is a rare condition, and the number of patients was limited and was predominantly female, and we did not investigate the physiology of the atria. Furthermore, the CineECG model system utilized a standard heart model system that did not consider individual variation in heart size and orientation, thoracic form and size, etc.
Conclusions
In conclusion, this study contributes with new electrophysiological insights on the novel, autosomal dominantly inherited Familial ST-segment Depression Syndrome in form of electrical activation pathway patterns and values, ECG modelling, and a detailed ST-segment analysis. Based on the simulations, the reduction of APA and APD in the left ventricle basal areas reproduces the Fam-STD ECG phenotype. Future studies should focus on comparing electrical activation pathways between Fam-STD and diseases with related ECG features, such as ST-depressions due to coronary heart disease, left ventricular strain, digoxin treatment, etc., and correlation of CineECG findings with invasively obtained EPS data from a larger number of patients.
Supplementary Material
Contributor Information
Rasmus Frosted, Department of Cardiology, The Heart Centre, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; Department of Cardiology, Copenhagen University Hospital—Herlev-Gentofte Hospital, Borgmester Ib Juuls Vej 11, 2730 Herlev, Denmark.
Christian Paludan-Müller, Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark; Laboratory for Molecular Cardiology, Department of Cardiology, The Heart Centre, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark.
Oliver Bundgaard Vad, Laboratory for Molecular Cardiology, Department of Cardiology, The Heart Centre, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Morten Salling Olesen, Department of Cardiology, The Heart Centre, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; Laboratory for Molecular Cardiology, Department of Cardiology, The Heart Centre, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Henning Bundgaard, Department of Cardiology, The Heart Centre, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Peter van Dam, Department of Cardiology, Division Heart & Lungs, University Medical Center Utrecht, Utrecht University, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; ECG Excellence BV, Weijland 38, 2415 BC Nieuwerbrug, The Netherlands.
Alex Hørby Christensen, Department of Cardiology, The Heart Centre, Copenhagen University Hospital—Rigshospitalet, Blegdamsvej 9, 2100 Copenhagen, Denmark; Department of Cardiology, Copenhagen University Hospital—Herlev-Gentofte Hospital, Borgmester Ib Juuls Vej 11, 2730 Herlev, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Blegdamsvej 3B, 2200 Copenhagen, Denmark.
Supplementary material
Supplementary material is available at Europace online.
Funding
The work was supported by The Independent Research Fund Denmark (grant 0134-00363B, A.H.C.), The Novo Nordisk Foundation, Denmark (NNF20OC0065799, A.H.C.; NNF17OC0031204, M.S.O.), NordForsk (H.B.U.), The Research Foundation at Rigshospitalet (C.P.-M., O.B.V.), The Department of Clinical Medicine (University of Copenhagen) (O.B.V.), and Nederlandse Hartstichting (QRS-Vision 2018B007).
Data Availability
The data underlying this study cannot be made publicly available due to privacy/ethical concerns.
Translational perspective
The current study sheds new light on the underlying electrophysiological abnormalities in Familial ST-depression Syndrome by demonstrating an electrocardiographic vector with a severely basal-shifted direction and a likely arrhythmic substrate in the basal regions of the left ventricle. This knowledge is important for the optimal planning of therapeutic interventions. Furthermore, an in-depth analysis of the ST-segment deviations diagnostic for this syndrome provides detailed reference data that may facilitate optimal handling of patients and their relatives. Understanding the molecular pathophysiology of Familial ST-depression Syndrome is crucial for optimizing the clinical care for these patients. There is a large unmet need in the handling of arrhythmias and left ventricular systolic dysfunction in these patients and elucidating the underlying action potential abnormalities, ionic imbalances, or dysfunctional myocardial structural proteins would be important stepping stones for optimizing patient care.
References
- 1. Bundgaard H, Jøns C, Lodder EM, Izarzugaza JMG, Romero Herrera JA, Pehrson Set al. A novel familial cardiac arrhythmia syndrome with widespread ST-segment depression. N Engl J Med 2018;379:1780–1. [DOI] [PubMed] [Google Scholar]
- 2. Christensen AH, Nyholm BC, Vissing CR, Pietersen A, Tfelt-Hansen J, Olesen MSet al. Natural history and clinical characteristics of the first 10 Danish families with familial ST-depression syndrome. J Am Coll Cardiol 2021;77:2617–9. [DOI] [PubMed] [Google Scholar]
- 3. Christensen AH, Vissing CR, Pietersen A, Tfelt-Hansen J, Hartvig Lindkær Jensen T, Pehrson Set al. Electrocardiographic findings, arrhythmias, and left ventricular involvement in familial ST-depression syndrome. Circ Arrhythm Electrophysiol 2022;15:e010688. doi: 10.1161/CIRCEP.121.010688 [DOI] [PubMed] [Google Scholar]
- 4. Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. J Am Coll Cardiol 1992;20:1391–6. [DOI] [PubMed] [Google Scholar]
- 5. Brugada R, Hong K, Cordeiro JM, Dumaine R. Short QT syndrome. CMAJ Can Med Assoc J 2005;173:1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jervell A, Lange-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J 1957;54:59–68. [DOI] [PubMed] [Google Scholar]
- 7. Frank E. An accurate. Clinically practical system for spatial vectorcardiography. Circulation 1956;13:737–49. [DOI] [PubMed] [Google Scholar]
- 8. Shah A J, Hocini M, Pascale P, Roten L, Komatsu Y, Daly Met al. Body surface electrocardiographic mapping for non-invasive identification of arrhythmic sources. Arrhythmia Electrophysiol Rev 2013;2:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Boonstra M, Hilderink B, Locati E, Asselbergs F, Loh P, Dam P. Novel CineECG enables anatomical 3D localization and classification of bundle branch blocks. EP Eur 2021;23:i80–7. [DOI] [PubMed] [Google Scholar]
- 10. van Dam PM. A new anatomical view on the vector cardiogram: the mean temporal–spatial isochrones. J Electrocardiol 2017;50:732–8. [DOI] [PubMed] [Google Scholar]
- 11. van Dam PM, Locati ET, Ciconte G, Borrelli V, Heilbron F, Santinelli Vet al. Novel CineECG derived from standard 12-lead ECG enables right ventricle outflow tract localization of electrical substrate in patients with Brugada syndrome. Circ Arrhythm Electrophysiol 2020;13:e008524. [DOI] [PubMed] [Google Scholar]
- 12. Boonstra MJ, Brooks DH, Loh P, van Dam PM. CineECG: a novel method to image the average activation sequence in the heart from the 12-lead ECG. Comput Biol Med 2022;141:105128. [DOI] [PubMed] [Google Scholar]
- 13. Sedova KA, van Dam PM, Sbrollini A, Burattini L, Necasova L, Blahova Met al. Assessment of electrical dyssynchrony in cardiac resynchronization therapy: 12-lead electrocardiogram vs. 96-lead body surface map. EP Eur 2023;25:554–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.https://www.nature.com/articles/s41597-020-0495-6 PTB-XL, a large publicly available electrocardiography dataset | Scientific Data [Internet]. [cited 2022 Mar 25]; Available from: [DOI] [PMC free article] [PubMed]
- 15. van Dam P, Boonstra M, Locati E, Loh P. The relation of 12 lead ECG to the cardiac anatomy: the normal CineECG. J Electrocardiol 2021;69:67–74. [DOI] [PubMed] [Google Scholar]
- 16. Dam P, Oostendorp T, Oosterom A. ECGSIM: interactive simulation of the ECG for teaching and research purposes 2010;37:841–4. [Google Scholar]
- 17. van Oosterom A, Oostendorp TF. ECGSIM: an interactive tool for studying the genesis of QRST waveforms. Heart 2004;90:165–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Oosterom A, Oostendorp TF, van Dam PM. Potential applications of the new ECGSIM. J Electrocardiol 2011;44:577–83. [DOI] [PubMed] [Google Scholar]
- 19. Sanna GD, Merlo M, Moccia E, Fabris E, Masia SL, Finocchiaro Get al. Left bundle branch block-induced cardiomyopathy: a diagnostic proposal for a poorly explored pathological entity. Int J Cardiol 2020;299:199–205. [DOI] [PubMed] [Google Scholar]
- 20. Yan G-X, Lankipalli RS, Burke JF, Musco S, Kowey PR. Ventricular repolarization components on the electrocardiogram: cellular basis and clinical significance. J Am Coll Cardiol 2003;42:401–9. [DOI] [PubMed] [Google Scholar]
- 21. Hoogendijk MG, Potse M, Linnenbank AC, Verkerk AO, den Ruijter HM, van Amersfoorth SCMet al. Mechanism of right precordial ST-segment elevation in structural heart disease: excitation failure by current-to-load mismatch. Heart Rhythm 2010;7:238–48. [DOI] [PubMed] [Google Scholar]
- 22. Iqbal M, Putra ICS, Pranata R, Budiarso MN, Pramudyo M, Goenawan Het al. Electrocardiographic markers indicating right ventricular outflow tract conduction delay as a predictor of major arrhythmic events in patients with Brugada syndrome: a systematic review and meta-analysis. Front Cardiovasc Med 2022;9:931622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study cannot be made publicly available due to privacy/ethical concerns.