Graphical Abstract
Graphical Abstract.
Ablation-related oesophageal injury is an important cause of serious complications from ablations for atrial fibrillation (AF).1–3 Methods of oesophageal protection originally started simply by reduction of ablation power and contact force and recent studies continue to show that refinement in ablation lesion application remains an important aspect of safeguarding against collateral injury.4–6 However, these methods alone are limited as these run the risk of an inefficient procedure or ineffective lesion application. Alternative ablation methods or modalities have also been developed and advanced over recent years. Pulsed field ablation seems to provide effective ablation lesions whilst remaining tissue selective, but clinical evaluations are ongoing, whilst further experience is gained from this ablation modality.7 Amongst these developments in ablation methods, tools, and technology, dedicated devices made or repurposed for oesophageal protection during left atrial ablations have also been explored.
Recent randomized trial evidence suggests that active control of local temperature can significantly reduce thermal injury to the oesophagus during left atrial ablation compared to standard care.8,9 Other methods of oesophageal protection have been attempted. Detailed monitoring of oesophageal temperature to minimize local heating and deviation of the oesophagus away from the site of danger are both intuitively appealing and form the rationale for products that are marketed commercially, though unsupported by trial evidence.10
The IMPACT study showed that active thermal protection of the oesophagus by the ensoETM (Attune Medical, Chicago, IL, USA)8 is effective in preventing oesophageal injury during radiofrequency ablation. This is a commercially available device used for body temperature control in a critical care setting and repurposed for oesophageal protection during left atrial ablations. Following the IMPACT trial, it is increasingly used in an off-licence manner in left atrial ablation procedures whilst a multicentre study is currently in progress (NCT04577859).
The ensoETM device is a multi-lumen orogastric probe made of medical-grade silicone, 73 cm in length and 1.2 cm outer diameter. The lumens are arranged to allow distilled water to be pumped into and out of the probe in a closed-loop system. The method of use in clinical practice was previously described.11 It is placed after induction of anaesthesia and after the use of trans-oesophageal echo, usually by the attending anaesthetist. The radio-opaque tip should be confirmed to lie well below the diaphragm on fluoroscopy. The proximal end of the probe is connected to a mobile console that sets the irrigated water at the desired temperature. A mouth guard is used to protect it from the teeth in the same manner as a trans-oesophageal echo probe. When irrigating, the volume of water in the probe at all times is 55 mL, and it flows at 2.4 L/min exerting a maximum pressure of 103 kPa. Leakage of water or blockage of flow is detected by the console and provokes an alarm. The purpose of this type of device is to heat extract and therefore limit or prevent local thermal tissue damage and avoid inflammatory processes that lead to fistula formation.
Device-related safety data in the USA are collected by subsidiary bodies of the Food and Drug Administration. The well-established Manufacturer and User Facility Device Experience (MAUDE) database and the Medical and Radiation Emitting Device Recalls database have more recently been supplemented by the more comprehensive Total Product Life Cycle (TPLC) database. In Europe, the Medicines and Healthcare products Regulatory Agency (MHRA) in the UK and the Swissmedic records of Field Safety Corrective Actions (FSCA) in Switzerland fulfil a similar role. We sought to understand the safety of the ensoETM device when used in a real-world setting by reviewing these device databases. The TPLC database was the single most comprehensive database, incorporating pre- and post-market data.
A systematic search was made using all databases to identify:
All adverse events associated with devices used in oesophageal protection during AF ablation (using product names and codes) from the time period of 2015–2022, the period in which the ensoETM has been available.
All oesophageal injuries including atrio-oesophageal fistulas from cardiac ablation procedures within the same time period.
By reviewing the narrative of each event, duplicate and irrelevant reports were manually filtered from the list. The date of access was 26 January 2023 with last update on the database on 9 January 2023 on the ensoETMs used to determine device usage to the same date.
23 089 recorded complications or adverse events related to left atrial ablations were recorded from 2015 to 2022, of which 2.3% were related to oesophageal injury of some kind. Oesophageal injury ranged from oesophageal tears and haematomas to atrio-oesophageal fistulas. Atrio-oesophageal fistulas alone accounted for 1.8% of the total adverse events. There are no data on the total number of ablations performed during this same time period from all the centres that contributed to the databases used, but in the UK alone, it is estimated that over 10 000 AF ablations are performed in 2019, pre-COVID-19.12
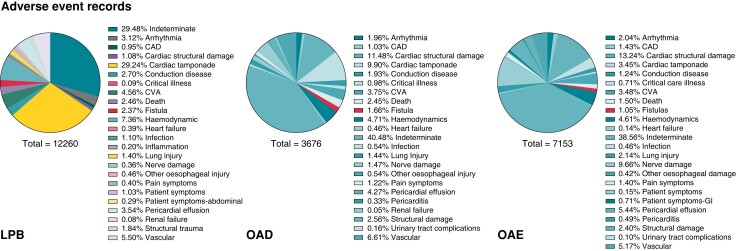
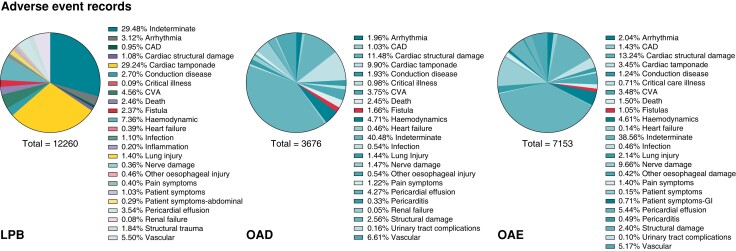
Atrio-oesophageal fistulas were most often linked to the product codes denoting ablation catheters or generators rather than codes linked to protection devices. LPB, OAD, and OAE were the main ablation product codes, but each search yielded a different percentage of oesophageal injuries reflecting the dependence of the databases on manual input of data (Figure 1).
Figure 1.
Atrio-oesophageal fistulas reported within the time period 2015–2022, listed under product codes LPB, OAD, and OAE.
Most reports on cases complicated by an atrio-oesophageal fistula failed to disclose which oesophageal protection device was used, if any (Table 1). In those where there were details, the majority did have an oesophageal temperature monitoring probe (n = 80). The details on which type of temperature monitoring probes were used during these cases of fistulas were even more limited. In a previous review of the medical literature involving temperature monitoring probes during left atrial ablations, eight different commercially available probes were found.10 Of these, only three were listed in the adverse event reports. The most common one listed was the S-Cath, Circa Scientific, an S-shaped multi-sensor oesophageal temperature probe with insulated thermocouples and 12 electrodes. There were four fistula cases where a deviation device was used. Although not specified, there are only three known deviation devices that underwent some form of clinical use or investigation—the DV8, EsoSure and Esolution. The physical profile of these deviation devices and method of use were previously described.10 The DV8 is a balloon retractor and the EsoSure involves nitinol stylets placed into a plastic tube within the oesophagus so that the tube takes the form of the pre-shaped stylet, which then deviates the oesophagus. The Esolution device (not yet available commercially) differs in that there are stacking plates that only enable the device to deviate the oesophagus medial-laterally and with a suctioning component to overcome the trailing edge effect. There’s no randomized evidence for this type of device as yet. From a search of the international databases to date, there have been no cases of atrio-oesophageal fistulas in 25 216 uses of the ensoETM for oesophageal protection during left atrial ablations.
Table 1.
Adverse events associated with left atrial ablation procedures with product codes LPB, OAD, and OAE denoting the ablation products from 2015 to 2022
| Product code by ablation | Fistula (n, %) | Other oesophageal injury (n) | Total oesophageal injury (n, %) | Total adverse events | Protective device used | |||
|---|---|---|---|---|---|---|---|---|
| Oesophageal monitoring probe | Oesophageal temperature control device | Retractor | No details | |||||
| LPB | 282 (2.3) | 56 | 338 (2.8) | 12 260 | 73 | 0 | 3 | 206 |
| OAD | 58 (1.6) | 20 | 78 (2.1) | 3676 | 5 | 0 | 0 | 53 |
| OAE | 81 (1.1) | 30 | 111 (1.6) | 7153 | 2 | 0 | 1 | 78 |
| Total | 421 (1.8) | 106 | 527 (2.3) | 23 089 | 80 | 0 | 4 | 337 |
| Device detailsa | S-Cath- Circa Scientific, Level 1 and ER 400-9 made by Smiths Medical; Esotherm/Sensitherm, Fiab | ensoETM, Attune Medical | DV8, EsoSure | |||||
A search was conducted for the number of atrio-oesophageal fistulas within that time period and if additional devices were used for oesophageal protection in these fistula cases. An attempt was made to review for which specific devices were used within the reported fistula cases, but results were limited.
Most fistula cases reported in these databases lacked in-depth clinical information including the protective method used, whether that was limitation of ablation power or contact force or if a protective device was used. Of the oesophageal monitoring probes specified in the reports, only S-Cath by Circa Scientific was linked to six cases of fistulas. The rest were linked to other oesophageal injury; most remain unknown. There was no other device apart from the ensoETM, used for oesophageal temperature control. The listed deviation devices, DV8, EsoSure are the only devices known to have been available during 2015–2022, but these are not specifically described in the reports.
Attune Medical (Chicago, IL, USA)’s registry identified that 25 216 ensoETMs were used for the purpose of oesophageal protection in left atrial ablations with over 39 000 devices used in total for all indications during the period of interest. No other manufacturer of a device intended for oesophageal protection responded to our request for the number of devices used during this period.
From the international databases, there were six complications or adverse events related to the ensoETM. None of the adverse events led to significant patient harm. The reports mostly detail improper use of the probe due to insufficient training of staff. As an example, an attempt to place the ensoETM when there was already an orogastric tube in situ or lack of use of a mouth guard, which caused damage to the ensoETM probe (and likely damage to the endotracheal tube) and a subsequent water leak. Although not yet appearing in the database search, there is one confirmed case of oesophago-pericardial fistula from 25 216 uses.13
From 2015 to 2022, there have been more than 20 000 uses of the ensoETM device for the purpose of left atrial ablations, but no reports of atrio-oesophageal fistula to date. Based on the recognized prevalence of this complication, 10–50 cases would have been expected. This is robust validation of the findings from the IMPACT study, in that controlling the oesophageal temperature via the ensoETM provides significant oesophageal protection.
Although there are clear limitations in review of these databases, it still yields important information on the safety of a device during its continuing use in clinical practice.
Contributor Information
Lisa W M Leung, Department of Cardiology, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK.
Pavandeep Toor, Department of Cardiology, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK.
Zaki Akhtar, Department of Cardiology, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK.
Abhay Bajpai, Department of Cardiology, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK.
Anthony Li, Department of Cardiology, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK.
Manav Sohal, Department of Cardiology, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK.
Mark M Gallagher, Department of Cardiology, St George’s University Hospitals NHS Foundation Trust, Blackshaw Road, London SW17 0QT, UK.
Data Availability
Data are available on request to the corresponding editor.
References
- 1. Loring Z, Holmes DN, Matsouaka RA, Curtis AB, Day JD, Desai Net al. . Procedural patterns and safety of atrial fibrillation ablation. Circ Arrhythm Electrophysiol 2020;13:e007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation 2017;136:1247–55. [DOI] [PubMed] [Google Scholar]
- 3. Gillinov AM, Pettersson G, Rice TW. Esophageal injury during radiofrequency ablation for atrial fibrillation. J Thorac Cardiovasc Surg 2001;122:1239–40. [DOI] [PubMed] [Google Scholar]
- 4. Müller J, Nentwich K, Berkovitz A, Ene E, Sonne K, Zhuravlev Vet al. . Acute oesophageal safety and long-term follow-up of AI-guided high-power short-duration with 50 W for atrial fibrillation ablation. Europace 2023:euad053. doi: 10.1093/europace/euad053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chieng D, Segan L, Sugumar H, Al-Kaisey A, Hawson J, Moore BMet al. . Higher power short duration vs. lower power longer duration posterior wall ablation for atrial fibrillation and oesophageal injury outcomes: a prospective multi-centre randomized controlled study (Hi-Lo HEAT trial). Europace 2023;25:417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Halbfass P, Wielandts JY, Knecht S, Le Polain de Waroux JB, Tavnernier R, De Wilde Det al. . Safety of very high-power short-duration radiofrequency ablation for pulmonary vein isolation: a two-centre report with emphasis on silent oesophageal injury. Europace 2022;24:400–5. [DOI] [PubMed] [Google Scholar]
- 7. Ekanem E, Reddy VY, Schmidt B, Reichlin T, Neven K, Metzner Aet al. . Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF). Europace 2022;24:1256–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung LW, Bajpai A, Zuberi Z, Li A, Norman M, Kaba RAet al. . Randomized comparison of oesophageal protection with a temperature control device: results of the IMPACT study. Europace 2021;23:205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leung LW, Akhtar Z, Elbatran AI, Bajpai A, Li A, Norman Met al. . Effect of esophageal cooling on ablation lesion formation in the left atrium: insights from ablation Index data in the IMPACT trial and clinical outcomes. J Cardiovasc Electrophysiol 2022;33:2546–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leung LWM, Akhtar Z, Sheppard MN, Louis-Auguste J, Hayat J, Gallagher MM. Preventing esophageal complications from atrial fibrillation ablation: a review. Heart Rhythm O2. 2021;2:651–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zagrodzky J, Gallagher MM, Leung LWM, Sharkoski T, Santangeli P, Tschabrunn Cet al. . Cooling or warming the esophagus to reduce esophageal injury during left atrial ablation in the treatment of atrial fibrillation. J Vis Exp 2020:e60733. doi: 10.3791/60733 [DOI] [PubMed] [Google Scholar]
- 12. National Institute for Health and Care Excellence (NICE) . 2021. Atrial fibrillation: diagnosis and management. NG196. https://www.nice.org.uk/guidance/ng196 (30 January 2023, date last accessed). [PubMed]
- 13. Sanchez J, Woods C, Zagrodzky J, Nazari J, Singleton M, Schricker Aet al. . Multicenter analysis of atrioesophageal fistula rates before and after adoption of active esophageal cooling during atrial fibrillation ablation. medRxiv 2023.02.21.23286267. 10.1101/2023.02.21.23286267 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on request to the corresponding editor.